-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHeterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
Monoamines, such as serotonin (5-HT) and tyramine (TA), paralyze both free-living and parasitic nematodes when applied exogenously. Since nematode cell lines are not available and animal screening options are limited, we have developed a screening platform to identify monoamine receptor agonists that involves the heterologous expression of key receptors from parasitic nematodes in chimeric, genetically-engineered mutant C. elegans, at sites likely to yield robust phenotypes upon agonist stimulation. Specifically, we have demonstrated that agonist dependent activation of Gαo-coupled 5-HT receptors or monoamine-gated Cl - channels in key interneurons, cholinergic motor neurons or body wall muscle inhibited locomotion and caused paralysis. This approach includes nematode-specific accessory proteins and the nematode cuticle, and appears to preserve the unique pharmacologies of the individual receptors. Together these data highlight the utility of these transgenic C. elegans for agonist identification and their potential for anthelmintic screening.
Published in the journal: . PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004794
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004794Summary
Monoamines, such as serotonin (5-HT) and tyramine (TA), paralyze both free-living and parasitic nematodes when applied exogenously. Since nematode cell lines are not available and animal screening options are limited, we have developed a screening platform to identify monoamine receptor agonists that involves the heterologous expression of key receptors from parasitic nematodes in chimeric, genetically-engineered mutant C. elegans, at sites likely to yield robust phenotypes upon agonist stimulation. Specifically, we have demonstrated that agonist dependent activation of Gαo-coupled 5-HT receptors or monoamine-gated Cl - channels in key interneurons, cholinergic motor neurons or body wall muscle inhibited locomotion and caused paralysis. This approach includes nematode-specific accessory proteins and the nematode cuticle, and appears to preserve the unique pharmacologies of the individual receptors. Together these data highlight the utility of these transgenic C. elegans for agonist identification and their potential for anthelmintic screening.
Introduction
Nematode infections cause significant morbidity and contribute significantly to a loss of Disability Adjusted Life Years (DALYs) [1–4]. For example, soil-transmitted nematodes, including Necator americanus, Trichuris trichuris and Ascaris lumbricoides infect nearly 2 billion worldwide and are a source of disease in over 400 million children [5]. More importantly, in many cases, such as filarial infection, effective chemotherapy is still not available [6]. Parasitic nematodes also have a devastating economic impact in agricultural settings that, at least secondarily, contributes significantly to a decline in human welfare, especially in areas where good nutrition is already compromised. For example, parasitic nematodes infect livestock and major crops (corn and soybeans) and cause billions in economic losses yearly in the US alone [7]. Importantly, most commercially available anthelmintics have become increasingly ineffective because of growing resistance (benzimidazoles, levamisole and, most recently, ivermectin) and most nematicides (DCBP (1,2-dibromo-3-chloropropane), methyl bromide), to control plant nematodes, have been banned by the EPA because of human toxicity [8–13]. New drugs, new drug targets and new, more effective screening protocols are desperately needed in all settings.
Most anthelmintics in use today act as agonists at key receptors and cause paralysis by interfering with muscle contraction and/or locomotion [14–17]. Since receptor “activation” is essential for anthelmintic activity, receptor knockout is not necessarily the “gold standard” for target validation; in fact knockout may not be lethal. Five molecular targets have been used for drug discovery, two nicotinic cholinergic receptor subunits (tetrahydropyrimidines/imidathiazoles and amino-acetonitriles), glutamate-/GABA-gated Cl- channels (macrocyclic lactones and piperazine, respectively) and Ca++-gated K+ channels (emodepside) [14–17]. Importantly, each of these anthelmintics is active in the free-living nematode, Caenorhabditis elegans and our understanding of their modes of action has, in large part, resulted from our ability to genetically manipulate their putative targets in receptive C. elegans mutant backgrounds [18–21]. Importantly, the identification of new targets has been limited by the lack of useful information about the identity, function and localization of the additional receptors regulating muscle contraction and locomotion. In addition to identifying new targets, we also need new screening protocols that preserve the unique pharmacologies of the receptors from the different parasites and maintain a nematode-specific context that includes the cuticle and appropriate accessory proteins, especially given that no nematode cells lines are available and that the parasites themselves are extremely difficult and expensive to culture.
In the present study, we have developed a heterologous, ectopic over-expression approach to provide a unique nematode screening platform for selective agonist identification, exploiting the unique experimental advantages of the C. elegans model system. Previously, we and others have demonstrated that exogenous monoamines, such as serotonin (5-HT), dopamine (DA) and tyramine (TA), each paralyze C. elegans and, where examined, parasitic nematodes [22–33]. In each case, the key C. elegans receptors mediating this locomotory inhibition have been identified and functionally localized, with each operating at a different level within the locomotory circuit: 5-HT in a few key interneurons, including the two AIB interneurons, DA in the cholinergic motor neurons and TA in head muscle and additional interneurons associated with locomotory decision-making [24, 28, 30]. We have previously constructed quintuple 5-HT receptor null C. elegans (5-HT quint) that do not express any previously identified 5-HT receptors and do not respond to exogenous 5-HT, to identify essential roles for the Gαo-coupled 5-HT1-like SER-4 and the unique 5-HT-gated Cl- channel, MOD-1 in 5-HT-dependent locomotory paralysis [23, 24]. Importantly, SER-4 agonists appear to function as anthelmintics in vivo and have been used to clear Haemonchus contortus infections from gerbils [34, 35]. In the present study, we ectopically expressed SER-4 and MOD-1 orthologues from parasitic nematodes, insects and humans in either the cholinergic motor neurons or body wall muscles of quintuple C. elegans 5-HT receptor null animals that lack all known C. elegans 5-HT receptors, on the assumption that agonist-dependent receptor activation at these sites will cause robust phenotypes that can be readily adapted for agonist screening. For example, the activation of a ligand-gated Cl- channel in body wall muscles would be predicted to hyperpolarize the muscle and significantly inhibit locomotion, while the activation of a Cl- channel or Gαo-coupled GPCR on the cholinergic motor neurons would significantly inhibit acetylcholine (ACh) release from the motor neurons and inhibit both muscle contraction and thus, locomotion. Importantly, as noted below, both hypotheses have proven to be correct.
Materials and Methods
Strains and reagents
bus-8 (e2968), bus-16 (e2802) and bus-17 (e2800) were obtained from Caenorhabditis Genetics Center (CGC). ser-5 (tm2654);ser-4 (ok512);mod-1 (ok103);ser-7 (tm1325) ser-1 (ok345) (5-HT quint), ser-5 (tm2654);mod-1 (ok103);ser-7 (tm1325) ser-1 (ok345) (SER-4 quad) and lgc-55 (tm2913);tyra-3 (ok325) tyra-2 (tm1846) ser-2 (pk1357) (TA quad) were generated as described previously [23, 24]. All strains were maintained on NGM plates with OP50 at 16°C. The cDNA clone of Drosophila melanogaster 5-HT1A (RE57708) was ordered from the Drosophila Genomics Resource Center (DGRC), the cDNA clone Human HTR1A (MGC: 167873; clone ID: 9020250) from GE Healthcare Dharmacon Inc. and cDNA clones of Haemonchus contortus (Hco) lgc-55 and mod-1 orthologues were kindly provided by Dr. Sean Forrester [33, 36]. The unc-17ß promoter, RM#621p, was obtained from Dr. James Rand. The integrated AIB::HisCl1 in N2 (cx15457) animals were a kind gift from Dr. Cornelia Bargmann [37].
Serotonin (5-HT) (H7752-25G), tyramine (TA) (T2879-25G), 8-OH DPAT (H141-25MG), sumatriptan succinate (S1198-10MG), PAPP (S009-25MG) and histamine dihydrochloride (H7250-5G) were purchased from Sigma Life Sciences. Stock solutions (50 mM) of 5-HT, TA, 8-OH-DPAT, sumatriptan and histamine were made up in distilled water, PAPP in 100% ethanol. The constituent of for nematode growth media (NGM), potassium phosphate monobasic (KH2PO4; P285-3), sodium chloride (NaCl; S271-3), calcium chloride dehydrate (CaCl2.2H2O; C79-500), magnesium sulfate heptahydrate (MgSO4.7H2O; BP213-1), tryptone (BP1421-2) and agar (DF0812071) were purchased from Thermo Fisher Scientific Inc., cholesterol (C3045-5G) purchased from Sigma Life Science.
Fusion PCR and transgenic lines
All transgenic constructs were created by overlap fusion PCR [38]. All transgenes contain a GFP marker (with unc-54 3′-UTR) at the 3’-end. Primers used are listed in S1 Text. PCR products from multiple reactions were pooled and co-injected with coelomocyte-RFP screening marker into the appropriate null backgrounds [39]. Once generated, transgenic animals are frozen in liquid nitrogen and thawed fresh weekly for assay. Multiple transgenic lines from each construct were examined.
Paralysis assay
Fresh agar plates (without NaCl, KH2PO4, MgSO4, CaCl2, tryptone and cholesterol) containing 5-HT, TA, PAPP, sumatriptan or 8-OH DPAT at desired concentrations were made daily. For assays involving bus mutants, fresh NGM agar plates (with NaCl, KH2PO4, MgSO4, CaCl2, tryptone and cholesterol) containing 5-HT were used for all assays. For assays with AIB::HisCl1 (cx15457) animals, freshly poured NGM agar or agar only plates containing 10 mM and 2 mM histamine were used. NGM agar plates were prepared as described in WormBook [40].
For all paralysis assays, well-fed, transgenic young adults expressing RFP screening markers were picked 2 hrs prior to assay and maintained on NGM plates with E. coli OP50. For assay, 10 animals are transferred to assay plates (agar only for all assays and NGM agar for assays with bus mutants) containing the appropriate drug and motility was assessed at intervals of 5 min for 30 min. Experiments with sumatriptan were carried out for 60 min, with motility assessed every 5 min. All assays were conducted in the absence of food, i.e. OP50. Animals that moved less than 1 body bend/20 s were counted as paralyzed. Each transgenic line was assayed at least 3 times with 10 animals/assay for each agonist concentration. Data is presented as % paralyzed ± SE over drug exposure time (min). Dose-response curves and EC50s were then generated using a variable slope nonlinear regression model with GraphPad Prism 6 software. Drug concentrations were log10-transformed prior to analysis.
Accession numbers
The accession numbers of the proteins involved in our study are C. elegans SER-4 (accession no. NP_497452), C. elegans LGC-55 (accession no. NP_507870), C. elegans MOD-1 (accession no. CCD72364), D. melanogaster 5-HT1A (accession no. NM_166322.2), D. melanogaster HisCl1 (accession no. Q9VGI0), human HTR1A (accession no. BC136263), H. contortus LGC-55 (accession no. ACZ57924.1) and H. contortus MOD-1 (accession no. ADM53350.1).
Results
Rationale
The monoamines, 5-HT, DA and TA each dramatically inhibit locomotion in C. elegans when applied exogenously at concentrations high enough to overcome the permeability barrier of the nematode cuticle, ultimately resulting in paralysis [24, 25, 27, 30]. Using the C. elegans model, the receptors involved in monoamine-dependent locomotory inhibition have been identified and localized [22–30]. Interestingly, the key receptors involved in 5-HT, DA and TA inhibition each function at a different level in the locomotory circuit with 5-HT-dependent paralysis requiring the expression of the Gαo-coupled, 5-HT1-like receptor, SER-4, and the 5-HT-gated Cl- channel, MOD-1 in a limited number of interneurons, including the two AIBs [24, 25]. Unfortunately, since nematode cell lines are not available and the maintenance of parasitic nematodes outside their hosts is problematic, screening platforms for anti-nematodal activity have been limited and do not usually incorporate the nematode cuticle or potentially important nematode accessory proteins.
The present study was designed to develop a screening platform for nematode monoamine receptor agonists in “chimeric” genetically-engineered C. elegans by heterologously expressing 5-HT and TA receptors at sites likely to yield robust phenotypes upon agonist stimulation. Previously, many investigators have rescued a range of behaviors in C. elegans null animals with the expression of proteins from the parasites, validating this approach [41–43]. We chose to examine locomotion as an endpoint for heterologous, ectopic expression, as the neurons and circuits modulating locomotion in C. elegans and parasitic nematodes appear to be conserved, can be readily assessed by established screening assays, and have always been the primary target for the majority of existing anthelmintics. Specifically, we expressed 1) Gαo-coupled, 5-HT1-like receptors, or 5-HT/ TA-gated Cl- channels in the cholinergic motor neurons of C. elegans mutants lacking any 5-HT or TA receptors, respectively on the assumption that robust agonist-dependent Gαo signaling or potential hyperpolarization, respectively, would dramatically inhibit ACh release and locomotion and 2) 5-HT or TA-gated Cl- channels in body muscle of C. elegans mutants lacking any 5-HT or TA receptors, respectively, on the assumption that agonist-dependent muscle hyperpolarization would cause paralysis.
5-HT inhibits locomotion in 5-HT receptor null animals expressing 5-HT1-like receptors in the AIB interneurons or cholinergic motor neurons
The role of the C. elegans 5-HT1-like receptor, SER-4, in 5-HT-dependent paralysis is well documented [23–25, 44]. Indeed, the utility of the H. contortus SER-4 orthologue, 5-HT1HC as an anthelmintic target has been validated previously both in vivo and in vitro [34, 35]. Locomotion in C. elegans has been assessed previously using a number of different assays, many of which can be readily adapted for screening [45–50]. For example, automated thrashing assays allow thousands of compounds to be easily screened per day [48]. Monoamine-dependent locomotory inhibition and paralysis has been quantified on agar plates (sinusoidal body bends) and in liquid medium (C-shaped “swimming”), containing either M9 buffer or water [22, 24, 25, 27, 29, 30]. The permeability of the C. elegans cuticle appears to vary depending on incubation conditions, with much less 5-HT apparently required in water, than in salt-containing media (M9), possibly because of an increased cuticular permeability under hypotonic conditions [25].
Previously, we assayed locomotion under standard C. elegans culture conditions on NGM agar plates. Under these conditions, 15 mM 5-HT initiated a rapid paralysis in wild type animals, and ser-5;mod-1;ser-7 ser-1 quadruple null (SER-4 quad) animals [24, 44]. As predicted, 5-HT had no effect on locomotion in 5-HT quint animals that lack all previously identified 5-HT receptors (Fig 1A and 1B) [24]. This 5-HT-dependent paralysis was not the classical spastic paralysis associated with cholinergic agonists, such as levamisole, or the flaccid paralysis associated with glutamatergic agonists, such as ivermectin, but instead appeared to result more from “locomotory confusion,” with animals unable to effectively integrate conflicting sensory inputs to initiate and sustain forward/backward locomotion. The C. elegans cuticle appears to be more impermeable than those of some of the parasitic nematodes [51–53]. Therefore, since the concentration of 5-HT required for maximal paralysis was quite high (15 mM) in these short term assays, presumably to overcome cuticular permeability, we re-assayed these animals under hypotonic conditions on agar plates without salt (non-NGM) (Fig 1C and 1D). Attempts to repeat published data from others on 5-HT paralysis in water were unsuccessful, as majority of the animals burst soon (within 5 min) after exposure to water [25]. However, in a hypotonic environment (agar alone without NGM), much lower concentrations of 5-HT were required for inhibition of wild type animals, with 1 mM 5-HT yielding 50% paralysis after 10 min exposure (EC50 about 0.4 mM) (Fig 1C and 1D).
Fig. 1. C. elegans mutants with increased cuticular permeability are hypersensitive to 5-HT-dependent paralysis. 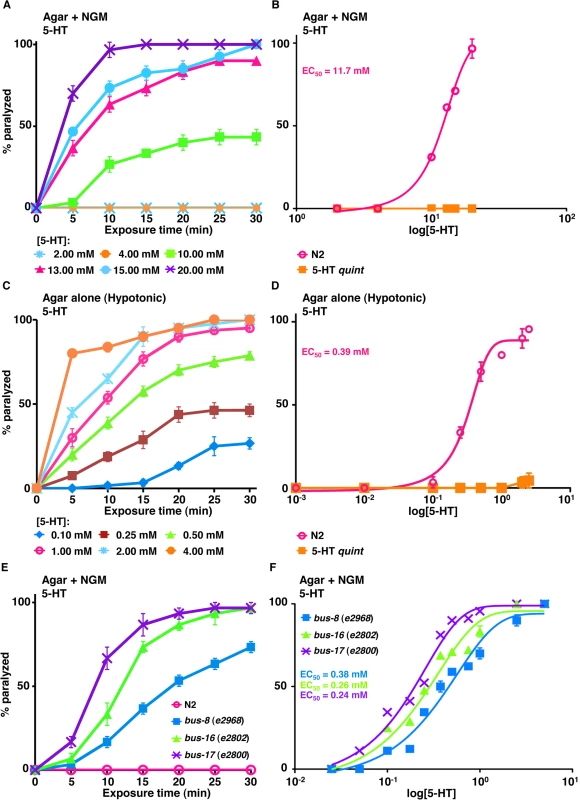
A-B. Paralysis of wild type and mutant C. elegans on NGM agar plates. A. Wild type animals examined for 5-HT-dependent paralysis as outlined in Methods. Data are presented as mean ± SE (n = 3). B. Dose-response curves for 5-HT-dependent paralysis on NGM plates at 10 min exposure for wild type and 5-HT quint animals. C-D. Paralysis of wild type and mutant C. elegans on non-NGM agar (hypotonic) plates. C. Wild type animals were examined for 5-HT-dependent paralysis as outlined in Methods. Data are presented as mean ± SE (n = 3). D. Dose-response curves for 5-HT-dependent paralysis in hypotonic conditions at 15 min exposure for wild type and 5-HT quint animals. E-F. 5-HT-dependent paralysis of wild type and mutant C. elegans on NGM agar plates. E. 5-HT (0.25 mM)-dependent paralysis of wild-type, bus-8 (e2968), bus-16 (e2802) and bus-17 (e2800) mutants. Data are presented as mean ± SE (n = 3). F. Dose-response curves for 5-HT-dependent paralysis at 10 min exposure for wild type and bus mutants. In addition to hypotonic incubation, we also examined 5-HT-dependent paralysis in a number of C. elegans mutants that exhibit increased cuticular permeability. For example, the Hodgkin group previously identified a series of bus mutants that exhibit increased cuticular permeability that have been hypothesized to be excellent vehicles for small molecule screening [54]. Indeed, as noted in Fig 1E and 1F, many of the bus mutants are hypersensitive to 5-HT-dependent paralysis, even under isotonic assay conditions (on NGM agar plates). For example, bus-17 mutants are acutely paralyzed after 10 min on 5-HT with an EC50 of about 0.24 mM, which is substantially lower than that observed in wild-type animals incubated under the same conditions (EC50 = 11.5 mM) (Fig 1F). These results suggest that these mutants might be useful for agonist identification, especially when only limited amounts of compound are available. Indeed, it may even be possible to select mutants that exhibit cuticular permeabilities that mimic those of individual parasites. Unfortunately, these mutants are also sensitive to hypotonicity and burst under the hypotonic conditions used in the present study, so that they could not be used in combination with hypotonicity to further increase sensitivity. Therefore, unless specified, hypotonic conditions were used to assay the transgenic animals described below.
A ser-4::gfp transgene is expressed in a limited number of neurons, including the AIBs [25]. Therefore, SER-4::GFP was specifically expressed in either the AIB interneurons (Pnpr-9) or ectopically, in the cholinergic motor neurons (Punc-17β) of the 5-HT quint. Expression was confirmed by GFP fluorescence (Fig 2A). As predicted, 5-HT quint animals expressing SER-4 in either the AIBs or cholinergic motor neurons were rapidly paralyzed by 5-HT (Fig 2B). Interestingly, on 5-HT, although 5-HT quint animals expressing SER-4 in the AIBs alone moved only infrequently, they initiated backward locomotion for a short distance when prodded with a blunt platinum wire at the tail, suggesting that they were probably unable to process conflicting locomotory signals, as hypothesized above. In contrast, animals expressing SER-4 in the cholinergic motor neurons were fully paralyzed and did not move when prodded.
Fig. 2. The 5-HT/SER-4-dependent inhibition of either the AIB interneurons or cholinergic motor neurons causes locomotory paralysis. 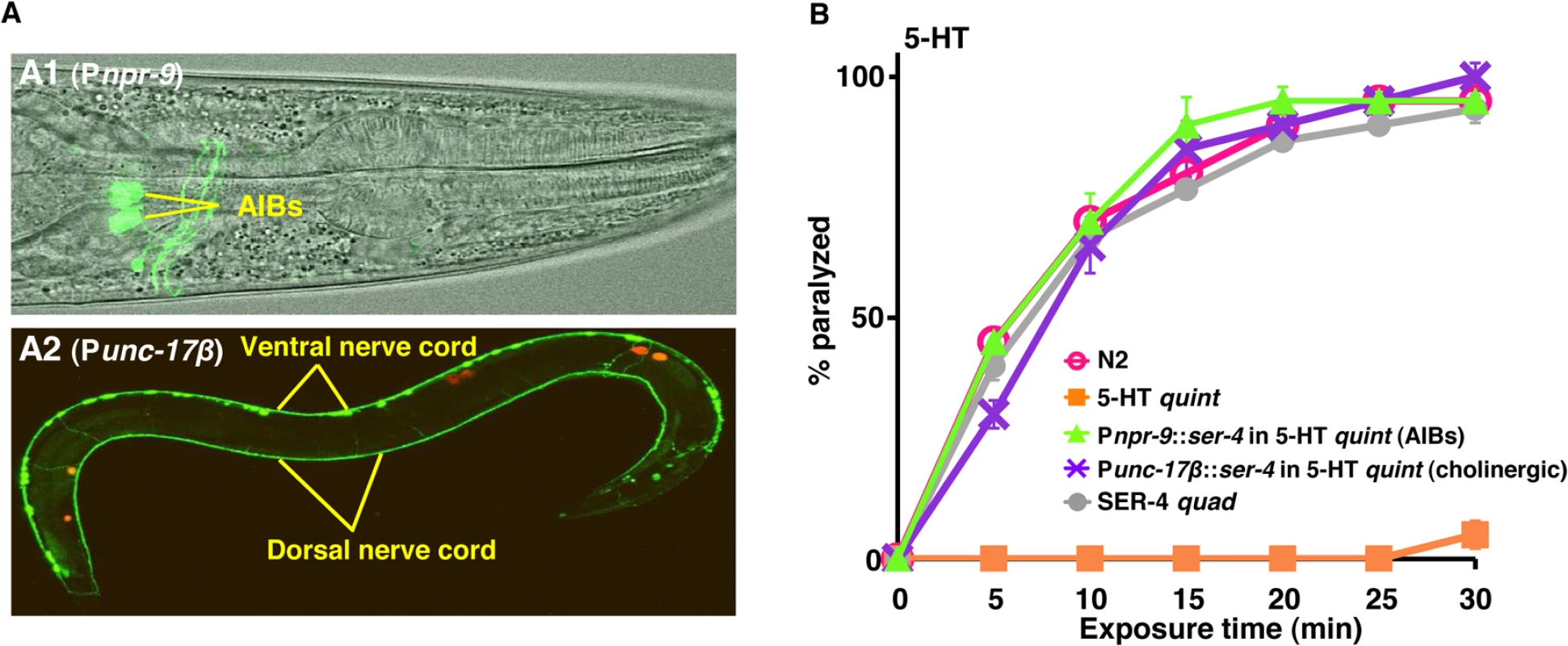
A. Confocal images of 5-HT quint expressing SER-4::GFP in the AIB interneurons (Pnpr-9)(A1) or cholinergic motor neurons (Punc-17β)(A2). GFP fluorescence (A2) or GFP fluorescence overlaid on DIC image (A1). The red stain in A2 is coelomocyte-specific RFP screening marker. B. Paralysis of wild type, mutant and transgenic C. elegans on hypotonic, non-NGM agar plates. Wild type, quadruple 5-HT receptor null animals expressing only SER-4 (SER-4 quad) or 5-HT quint expressing the C. elegans 5-HT1-like receptor, SER-4, in either the cholinergic motor neurons (Punc-17β) or the two AIB interneurons (Pnpr-9) were examined for 5-HT (1 mM)-dependent paralysis as outlined in Methods. Data are presented as mean ± SE (n = 3). Use of heterologous expression for agonist identification
To demonstrate the utility of this screening approach, the Drosophila 5-HT1 orthologue (5HT1A) or the human 5-HT-1A receptor (HTR1A) were also expressed specifically in the cholinergic motor neurons (Punc-17β) of 5-HT quint animals. Locomotion in animals from both transgenic lines was dramatically inhibited by exogenous 5-HT, demonstrating that the receptors were functionally expressed (Fig 3A). To demonstrate the specificity of these chimeric C. elegans for agonist identification, we examined the effect of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT), a subtype-selective agonist for the human 5-HT1A receptor, sumatriptan succinate, a selective mammalian 5-HT1B/D agonist, and p-amino-phenethyl-m-trifluoromethylphenyl piperazine (PAPP). As predicted, 8-OH-DPAT rapidly paralyzed the 5-HT quint animals expressing the human 5-HT1A receptor (Fig 3B). In contrast, 8-OH-DPAT, even at 2 mM, had no effect on locomotion 5-HT quint animals expressing either Drosophila or C. elegans 5-HT1 receptor orthologues, suggesting the conservation of ligand-receptor specificity in chimeric C. elegans (Fig 3B). Sumatriptan, at low concentrations, is a selective mammalian 5-HT1B/D agonist, and, indeed in the present study, sumatriptan was much less effective than 8-OH-DPAT in initiating paralysis [55]. For example, 0.5 mM sumatriptan had no effect on locomotion in either wild type or transgenic animals expressing 5-HT1A receptor orthologues in cholinergic motor neurons and, even at higher concentrations, failed to fully paralyze animals expressing the human 5-HT1A receptor. In addition, although animals expressing the human 5-HT1A receptor responded to increased sumatriptan concentrations more rapidly, these locomotory effects were transient and reduced dramatically after 25 min, presumably due to receptor desensitization (Fig 3C). In contrast, paralysis increased with prolonged sumatriptan exposure in animals expressing either the C. elegans or Drosophila receptors, demonstrating kinetic differences between the orthologous receptors.
Fig. 3. 5-HT and 5-HT receptor agonists selectively paralyze C. elegans 5-HT receptor mutant animals expressing nematode, insect or human 5-HT1-like receptors in the cholinergic motor neurons. 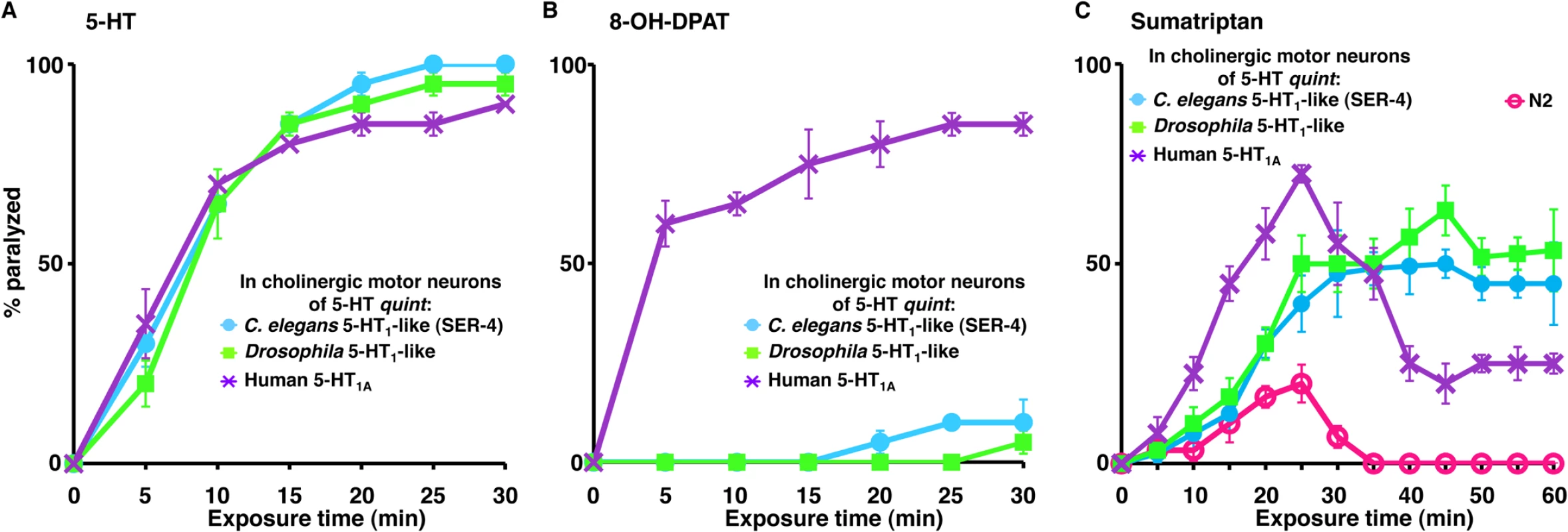
A-C. Paralysis of wild type, mutant and transgenic C. elegans on hypotonic, non-NGM agar plates. A. 5-HT (1 mM)-dependent paralysis of 5-HT quint animals expressing either C. elegans 5-HT1-like (SER-4), Drosophila 5-HT1-like, or human 5-HT1A receptor in cholinergic motor neurons (Punc-17β). Data are presented as mean ± SE (n = 3). B. 8-OH-DPAT (2 mM)-dependent paralysis of 5-HT quint animals expressing either C. elegans 5-HT1-like (SER-4), Drosophila 5-HT1-like, or human 5-HT1A receptor in cholinergic motor neurons (Punc-17β). Data are presented as mean ± SE (n = 3). C. Sumatriptan (1 mM)-dependent paralysis of wild type, 5-HT quint animals expressing either C. elegans 5-HT1-like (SER-4), Drosophila 5-HT1-like, or human 5-HT1A receptor in cholinergic motor neurons (Punc-17β). Data are presented as mean ± SE (n = 3). PAPP, a high affinity agonist for the H. contortus 5-HT1-like receptor, paralyzes H. contortus L3s in vitro and clears experimental H. contortus infections from gerbils [34, 35]. As predicted, PAPP initiated a rapid paralysis in wild type animals (EC50 = 0.37 mM) and, even more rapidly, in 5-HT quint animals expressing the C. elegans SER-4 in the cholinergic motor neurons (EC50 = 0.17 mM), supporting the previous identification of PAPP as a 5-HT1-like receptor agonist (Fig 4A and 4B). In contrast, and somewhat surprisingly, at higher concentrations (≥0.5 mM), PAPP also paralyzed 5-HT quint animals (EC50 = 0.68 mM) that were unaffected by 5-HT, suggesting that, in addition to acting as a 5-HT1-like receptor (SER-4) agonist, PAPP also acted at second target(s) (Fig 4A and 4B). Since exogenous TA and DA also paralyze C. elegans, we surmised that, at higher concentrations, PAPP might be activating additional monoamine receptors. DA-dependent paralysis requires the expression of the Gαo-coupled DA receptor, DOP-3 in the cholinergic motor neurons [26]. Therefore, dop-3 expression was knocked down in the 5-HT quint animals using dop-3 RNAi driven by the dop-3 promoter. As noted in Fig 4C, dop-3 RNAi knockdown in this background significantly reduced PAPP-dependent paralysis, suggesting that DOP-3 is a secondary PAPP target. Screening is in progress to identify additional target(s). Together, these data highlight the utility of this approach in preliminary drug screening and suggest that it may also be useful for the identification of nematode-specific agonists.
Fig. 4. PAPP paralyzes C. elegans via SER-4 and DOP-3. 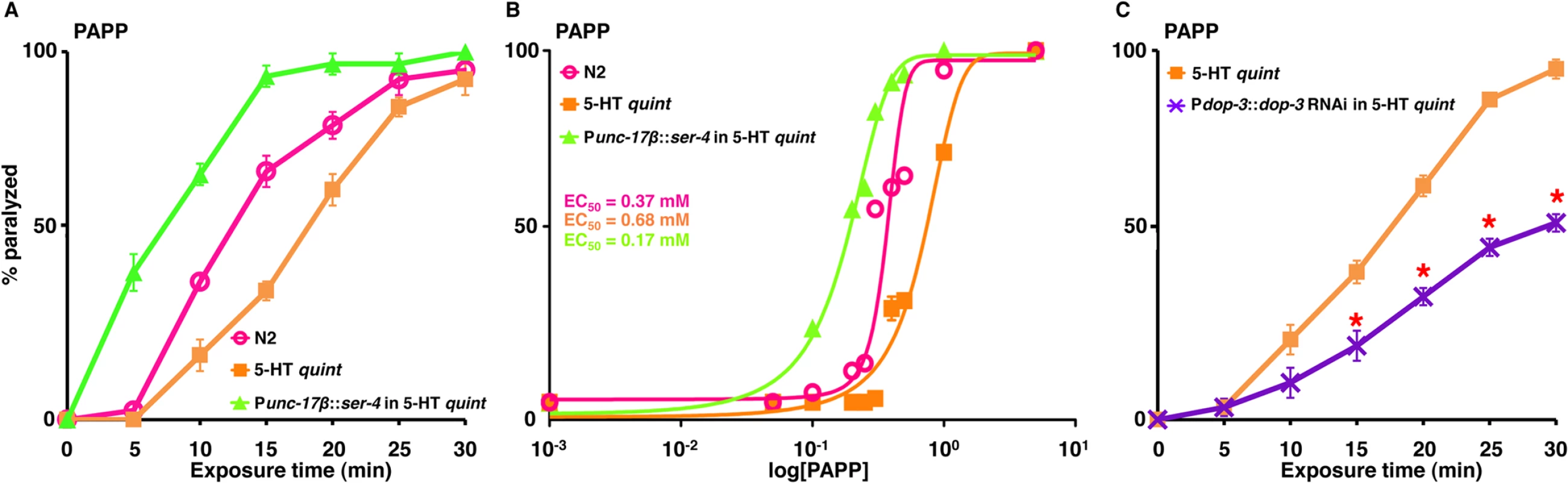
A-C. Paralysis of wild type, mutant and transgenic C. elegans on hypotonic non-NGM agar plates. A. PAPP (0.5 mM)-dependent paralysis of wild-type, 5-HT quint and 5-HT quint animals expressing SER-4 in the cholinergic motor neurons (Punc-17β). Data are presented as mean ± SE (n = 3). B. Dose-response curves for PAPP-dependent paralysis at 15 min exposure for wild type, 5-HT quint and 5-HT quint animals expressing SER-4 in the cholinergic motor neurons (Punc-17β). C. PAPP (0.5 mM)-dependent paralysis of 5-HT quint and 5-HT quint animals expressing Pdop-3::dop-3 RNAi. Data are presented as mean ± SE (n = 3). ‘*’ p≤0.001, significantly different from 5-HT quint animals assayed under identical conditions. The activation of monoamine-gated Cl- channels in cholinergic motor neurons or body wall muscles causes locomotory paralysis
Nematodes also express a unique family of monoamine-gated Cl- channels that appear to be highly conserved within the phylum, including the C. elegans 5-HT - and TA-gated Cl- channels, MOD-1 and LGC-55, that play key roles in 5-HT - and TA-dependent muscle paralysis, respectively. The C. elegans MOD-1 and its H. contortus orthologue were expressed directly in either cholinergic motor neurons (Punc-17β) or body wall muscles (Pmyo-3) of 5-HT quint animals and 5-HT-dependent paralysis was assayed as described above. Muscle expression was confirmed by GFP fluorescence (Fig 5A). As previously noted, 5-HT had no effect on locomotion in 5-HT quint animals, but rapidly paralyzed the 5-HT quint animals expressing either the C. elegans MOD-1 in the cholinergic motor neurons or the H. contortus (Hco) MOD-1 orthologue in cholinergic motor neurons or body wall muscle, with EC50s of about 0.3 mM, 0.2 mM and 0.2 mM, respectively (Fig 5B and 5C). Interestingly, 5-HT-dependent paralysis was more rapid in the transgenic animals expressing MOD-1 orthologues in the cholinergic motor neurons than in wild type animals.
Fig. 5. Exogenous monoamines paralyze C. elegans expressing monoamine-gated Cl- channels in either cholinergic motor neurons or body wall muscles. 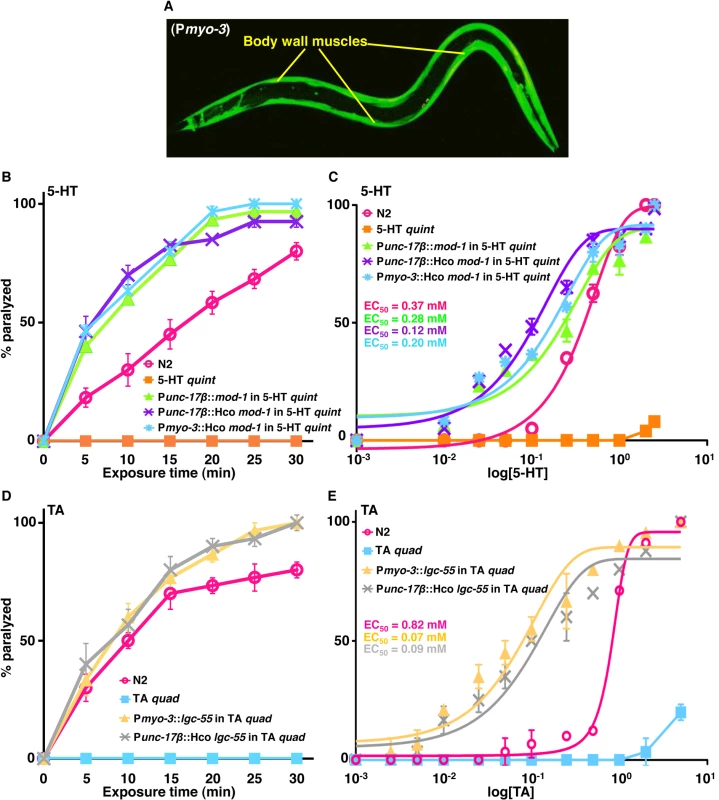
A. Confocal image of 5-HT quint animals expressing H. contortus (Hco) MOD-1::GFP in body wall muscles (Pmyo-3). GFP-fluorescence image. B-E. Paralysis of wild type, mutant and transgenic C. elegans on non-NGM agar plates. B. 5-HT (0.5 mM)-dependent paralysis of wild type, 5-HT quint and 5-HT quint animals expressing either the C. elegans or H. contortus (Hco) MOD-1 orthologues in the cholinergic motor neurons (Punc-17β) or the H. contortus (Hco) MOD-1 orthologue in body wall muscle (Pmyo-3). Data are presented as mean ± SE (n = 4). C. Dose-response curves for 5-HT-dependent paralysis at 15 min exposure for wild type, 5-HT quint and 5-HT quint animals expressing either the C. elegans or H. contortus (Hco) MOD-1 orthologues in the cholinergic motor neurons (Punc-17β) or the H. contortus (Hco) MOD-1 orthologue in body wall muscle (Pmyo-3). D. Tyramine (1 mM)-dependent paralysis of wild type, TA quad and TA quad animals expressing either the C. elegans LGG-55 in body wall muscle (Pmyo-3) or the H. contortus (Hco) LGC-55 orthologue in the cholinergic motor neurons (Punc-17β). Data are presented as mean ± SE (n = 3). E. Dose-response curves for TA-dependent paralysis at 15 min exposure for wild type, TA quad and TA quad animals expressing either LGC-55 in the body wall muscles (Pmyo-3), or H. contortus (Hco) LGC-55 orthologue in cholinergic motor neurons (Punc-17β). Similarly, LGC-55 was expressed in the body wall muscles (Pmyo-3) or its H. contortus orthologue in the cholinergic motor neurons (Punc-17β) of lgc-55;tyra-3 tyra-2 ser-2 quadruple TA receptor null (TA quad) animals. TA quad animals lack all previously identified TA receptors and fail to respond to TA in a range of behavioral assays, including locomotion. As predicted, TA had no effect on locomotion in the TA quad animals, but significantly inhibited locomotion in TA quad animals expressing either C. elegans LGC-55 in body wall muscles or H. contortus (Hco) LGC-55 orthologue in cholinergic motor neurons, each with EC50 of about 0.1 mM (Fig 5D and 5E). Together, these data suggest that monoaminergic activation of these Cl- channels hyperpolarizes either the cholinergic motor neurons or body wall muscles and inhibits muscle contraction, as well as highlighting the utility of chimeric C. elegans as a functional expression platform to identify ligand-gated Cl- channels agonists for use as anthelmintics.
The inhibition of AIB signaling causes “locomotory confusion” and paralysis
Our results suggest that inhibiting AIB signaling by the expression of a Gαo-coupled 5-HT receptor in the AIBs of the 5-HT quint can cause paralysis (Fig 2B). Similarly, the AIB-specific expression (Pinx-1) of the 5-HT-gated Cl- channel, MOD-1 can also cause paralysis (Fig 6A). In contrast, ablation of the AIBs does not cause paralysis [56, 57]. Interestingly, the activation of a Drosophila histamine-gated Cl- channel (HisCl1) expressed ectopically in the AIBs (cx15457) with 2 mM exogenous histamine (His) caused AIB hyperpolarization and locomotory phenotypes, but not paralysis [37]. In contrast, increasing the histamine concentration to 10 mM caused paralysis that persisted for up to 24 hrs in the presence of histamine [37]. Similarly, in the present study, 2 mM histamine did not cause paralysis in wild type animals or in transgenic animals expressing HisCl1 in the AIBs (cx15457) on NGM plates (Fig 6B). However, 2 mM histamine caused significance paralysis under the modified hypotonic assay conditions used in the present study or when the histamine concentration was raised to 10 mM on NGM plates (Fig 6B and 6C). Since the ablation of the AIBs does not cause paralysis, these results support our previous hypothesis that the partial inhibition of AIB signaling by partial hyperpolarization or the activation of Gαo signaling causes an imbalance in the locomotory circuit that results in a state of decision-making “confusion,” an inability to execute and sustain unidirectional movement and ultimately, in cessation of locomotion (paralysis). Theoretically, any ligand that selectively unbalances AIB signaling has the potential to yield a similar locomotory phenotype and its target a potential site for anthelmintic development.
Fig. 6. Inhibiting signaling from the two AIB interneurons causes “locomotory confusion” and paralysis. 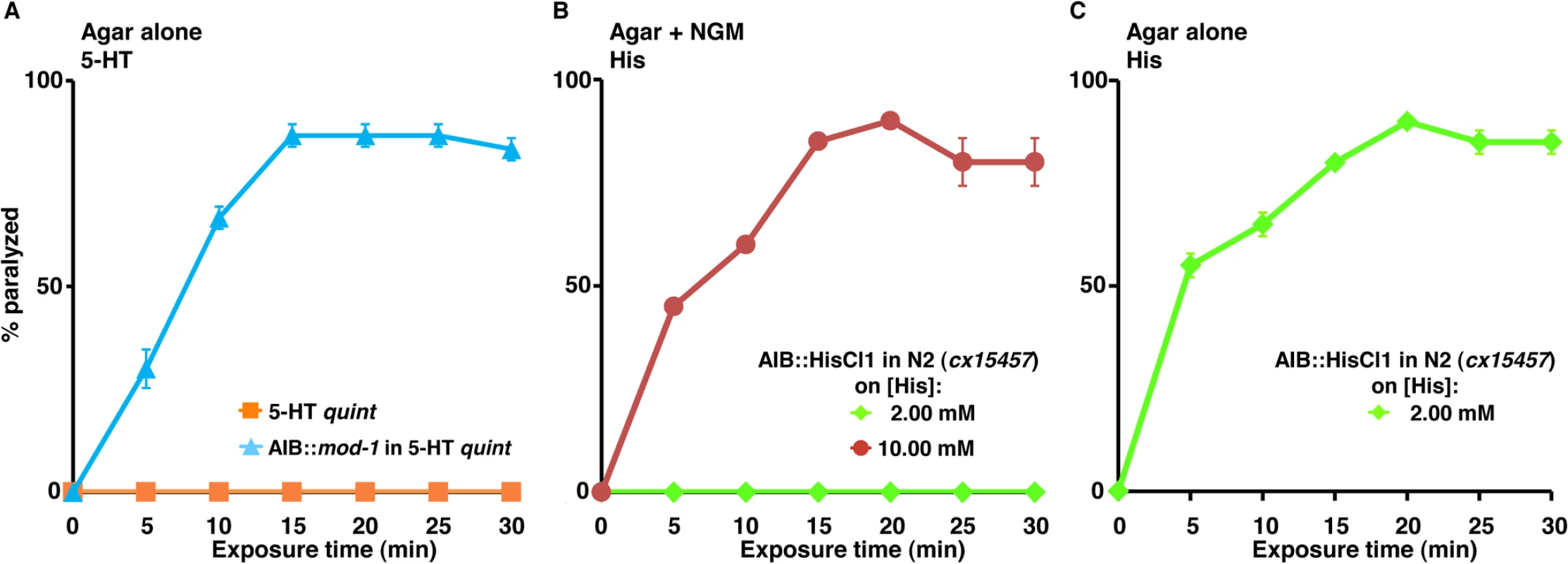
A-C. Paralysis of wild type, mutant and transgenic C. elegans on either NGM or non-NGM agar plates. A. 5-HT quint and 5-HT quint animals expressing MOD-1 in the AIBs (Pinx-1) were examined for 5-HT (1 mM)-dependent paralysis on non-NGM agar plates, as outlined in Methods. Data are presented as mean ± SE (n = 3). B and C. Wild type animals expressing HisCl1 in the AIBs (cx15457) were examined for histamine (2 or 10 mM)-dependent paralysis on NGM (B) and non-NGM (C) agar plates. Data are presented as mean ± SE (n = 3). Discussion
The monoamines, 5-HT, DA and TA each dramatically inhibit locomotion in C. elegans when applied exogenously at concentrations high enough to overcome the permeability barrier of the nematode cuticle, ultimately resulting in paralysis [24, 25, 27, 30]. In addition, monoamine-dependent locomotory paralysis is also observed in many parasitic nematodes, including Ascaris suum and Heterodera glycines [31, 32]. Using the C. elegans model, the receptors involved in monoamine-dependent locomotory inhibition have been identified and localized [22–30]. Interestingly, the key receptors involved in 5-HT, DA and TA inhibition each function at a different level in the locomotory circuit [24, 28, 30]. For example, 5-HT-dependent paralysis in C. elegans involves the expression of the Gαo-coupled, 5-HT1-like receptor, SER-4, and the 5-HT-gated Cl- channel, MOD-1 in a limited number of interneurons, including the two AIBs [25]. Importantly, 5-HT1-like agonists appear to have anti-nematodal activity in vivo [34, 35]. Indeed, the results of the present study suggest that partial inhibition of the AIBs by activation of an endogenously expressed Gαo-coupled 5-HT1-like receptor or 5-HT-gated Cl- channel, or a heterologously expressed histamine-gated Cl- channel, interferes with AIB signaling, causes “locomotory confusion” and ultimately paralysis. Interestingly, animals with ablated AIBs are still motile and move efficiently, although their rates of spontaneous reversal are dramatically altered, suggesting either that this partial inhibition differentially affects AIBs signaling to cause locomotory paralysis or that the ablated animals have compensated for the loss of the AIBs [56, 57].
The present study provides further support to the use of “chimeric” C. elegans, created by the heterologous, ectopic expression of potential key drug targets from parasitic nematodes, for use as a platform for agonist identification and potential anthelmintic screening. Although the present is focused on inhibitory monoamine GPCRs and monoamine-gated ion channels, it can potentially be expanded to any signaling molecules for which the appropriate mutant backgrounds can be prepared. Specific promoters are available for C. elegans muscles and most neurons; alternatively, specific promoters to other neurons can be generated using a Cre-Lox approach [58]. This screening system has the potential to combine the individual pharmacologies of the receptors from different parasitic nematodes with the environment and accessory proteins necessary for functional expression. This becomes especially important because nematode-specific cells lines are not available and the expression of nematode receptors in mammalian cells is quite variable and can require a host of additional modifications, including temperature shock to achieve expression [59, 60]. In fact, few studies have compared receptor pharmacologies in vivo with those of the nematode receptors heterologously expressed in mammalian cells. The functional reconstitution of nematode receptors in heterologous systems (Xenopus oocytes, etc.) often requires additional accessory proteins and/ or subunits that might not have been identified previously, hindering the further development of potential drug targets [61–64]. Not only do transgenic C. elegans provide a promiscuous expression platform for distantly-related receptors: these ectopically-expressed receptors are functional and appear to maintain their ligand-receptor specificity, as highlighted above where only the transgenic animals expressing the human receptor were paralyzed by 8-OH-DPAT. The identification of DOP-3 as a secondary target in PAPP-dependent paralysis also validates the utility and convenience of transgenic C. elegans as a platform for drug target identification and potential anthelmintic screening. Although the current study uses transgenic animals expressing the desired receptor as an extra-chromosomal array, stable lines can be readily constructed if required [65].
This screening platform also includes the nematode cuticle, a potential barrier to the entry of any anthelmintic, as well as a wide array of ABC transporters involved in drug efflux and resistance [66]. The cuticle is made up of six layers, the epicuticle, external cortical, internal cortical, medial, fiber and basal, as well as a carbohydrate-rich surface coat external to the epicuticle [67]. The lipid-rich epicuticle layer might be the key barrier to externally-applied drugs, especially water-soluble molecules (5-HT, TA, 8OH-DPAT etc.) and the reason for the high concentration required to cause paralysis under isotonic environment, i.e. on NGM agar plates [52, 67, 68]. As mentioned, although C. elegans cuticle appears to be more impermeable than those of some parasitic nematodes, the permeability of the C. elegans cuticle can be manipulated by modifying incubation conditions and the availability of various mutant backgrounds. By incubating the animals in a salt-free, hypotonic environment, 5-HT paralyzes wild-type animals with an EC50 of about 0.5 mM, in contrast with an EC50 of about 12 mM on isotonic NGM agar plates. In addition, a number of C. elegans mutations that appear to have increase cuticular permeability may also be useful for enhancing small molecule screening against an array of medically-important targets, including those involved in locomotory paralysis [54, 69]. For example, many of the bus (bacterially swollen) mutations appear to alter the cuticle and increase permeability [54]. Indeed, as shown in Fig 1E and 1F, it might be possible to select specific cuticle mutants with permeabilities that mimic those of individual parasitic nematodes, providing a mean to bypass complicated and expensive process of culturing live parasites, at least during preliminary stages of agonist screening. In fact, C. elegans has been used in the past for large-scale small molecule screens and chemical genomics and predictive models for drug accumulation and bioactivity have been developed that may be used to bias preliminary screening [70, 71]. This ability to alter cuticular permeability will certainly be useful for agonist and potential anthelmintic identification, but in the case of the monoamines examined, relatively high concentrations of ligand are still required and, ultimately, any potential agonists identified using this approach will have to be validated in the target of choice.
In summary, this study has identified two key AIB interneurons that play a role in 5-HT-dependent paralysis and suggests that partial inhibition of signaling from the neurons has the potential to cause “locomotory confusion,” and paralysis. In addition, these studies have demonstrated and validated the utility of these “chimeric” C. elegans as a platform for agonist identification and potential anthelmintic screening.
Supporting Information
Zdroje
1. Awasthi S, Bundy D (2007) Intestinal nematode infection and anaemia in developing countries. BMJ. 334 : 1065–66. 17525401
2. Hotez PJ, Brindley PJ, Bethony JM, et al. (2008) Helminth infections: the great neglected tropical diseases. J Clin Invest. 118 : 1311–21. doi: 10.1172/JCI34261 18382743
3. Hotez PJ, Kamath A (2009) Neglected tropical diseases in sub-Saharan Africa: review of their prevalence, distribution and disease burden. PLoS Negl Trop Dis 3: e412. doi: 10.1371/journal.pntd.0000412 19707588
4. Brooker S (2010) Estimating the global distribution and disease burden of intestinal nematode infections: adding up the numbers—a review. Int J Parasitol. 40 : 1137–1144. doi: 10.1016/j.ijpara.2010.04.004 20430032
5. WHO (2012) Eliminating soil-transmitted helminthiases as a public health problem in children: progress report 2001–2010 and strategic plan 2011–2020. Available: http://whqlibdoc.who.int/publications/2012/9789241503129_eng.pdf?ua=1 Accessed: 18 September 2014
6. Keating J, Yukich JO, Mollenkopf S, Tediosi F (2014) Lymphatic filariasis and onchocerciasis prevention, treatment and control costs across diverse settings: a systematic review. Acta Trop. 135C: 86–95.
7. Jones JT, Haegeman A, Danchin EGJ, et al. (2013) Top 10 plant-parasitic nematodes in molecular plant pathology. Mol Plant Pathol. 14 : 946–961. doi: 10.1111/mpp.12057 23809086
8. Reynoldson JA, Behnke JM, Pallant LJ, et al. (1997) Failure of pyrantel in treatment of human hookworm infections (Ancylostoma duodenale) in the Kimberley region of north west Australia. Acta Trop. 68 : 301–312. 9492915
9. Gill JH, Kerr CA, Shoop WL, Lacey E (1998) Evidence of multiple mechanisms of avermectin resistance in Haemonchus contortus—comparison of selection protocols. Int J Parasitol 28 : 783–9. 9650059
10. Albonico M, Bickle Q, Haji HJ, et al. (2002) Evaluation of the efficacy of pyrantel-oxantel for the treatment of soil-transmitted nematode infections. Trans R Soc Trop Med Hyg. 96 : 685–90. 12625151
11. Lustigman S, McCarter JP (2007) Ivermectin resistance in Onchocerca volvulus: toward a genetic basis. PLoS Negl Trop Dis 1: e76. 17989789
12. Kaplan RM (2004) Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 20 : 477–481. 15363441
13. Geary TG, Woo K, McCarthy JS, et al. (2010) Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol 40 : 1–13. doi: 10.1016/j.ijpara.2009.11.001 19932111
14. Martin RJ (1985) gamma-Aminobutyric acid - and piperazine-activated single-channel currents from Ascaris suum body muscle. Br J Pharmacol 84 : 445–61. 2579701
15. Geary TG, Sims SM, Thomas EM, et al. (1993) Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp Parasitol 77 : 88–96. 8344410
16. Sheriff JC, Kotze AC, Sangster NC, Martin RJ (2002) Effects of macrocyclic lactone anthelmintics on feeding and pharyngeal pumping in Trichostrongylus colubriformis in vitro. Parasitology 125 : 477–484. 12458832
17. Martin RJ, Buxton SK, Neveu C, et al. (2012) Emodepside and SLO-1 potassium channels: a review. Exp Parasitol. 132 : 40–6. doi: 10.1016/j.exppara.2011.08.012 21910990
18. Holden-Dye L, Crisford A, Welz C, et al. (2012) Worms take to the slo lane: a perspective on the mode of action of emodepside. Invert Neurosci 12 : 29–36. doi: 10.1007/s10158-012-0133-x 22539031
19. Krucken J, Harder A, Jeschke P, et al. (2012) Anthelmintic cyclcooctadepsipeptides: complex in structure and mode of action. Trends Parasitol. 28 : 385–94. doi: 10.1016/j.pt.2012.06.005 22858281
20. Hernando G, Bouzat C (2014) Caenorhabditis elegans neuromuscular junction: GABA receptors and ivermectin action.PLoS One 9: e95072. doi: 10.1371/journal.pone.0095072 24743647
21. Miltsch SM, Krucken J, Demeler J, et al. (2013) Interactions of anthelmintic drugs in Caenorhabditis elegans neuro-muscular ion channel mutants. Parasitol Int. 62(6): 591–8. doi: 10.1016/j.parint.2013.05.006 23707730
22. Ranganathan R, Cannon SC, Horvitz HR (2000) MOD-1 is a serotonin-gated chloride channel that modulates locomotory behaviour in C. elegans. Nature. 408 : 470–475. 11100728
23. Hobson RJ, Hapiak VM, Xiao H, Buehrer KL, Komuniecki R (2006) SER-7: a Caenorhabditis elegans 5-HT7-like receptor is essential for the 5-HT stimulation of pharyngeal pumping and egg-laying. Genetics. 172 : 159–169. 16204223
24. Hapiak V, Hobson R, Hughes L, et al. (2009) Dual excitatory and inhibitory serotonergic inputs modulate egg-laying in Caenorhabditis elegans. Genetics. 181 : 153–163. doi: 10.1534/genetics.108.096891 19001289
25. Gurel G, Gustafson MA. Pepper JS, et al. (2012) Receptors and other signaling proteins required for serotonin control of locomotion in Caenorhabditis elegans. Genetics. 192 : 1359–71. doi: 10.1534/genetics.112.142125 23023001
26. Chase DL, Pepper JS, Koelle MR (2004) Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci. 7 : 1096–1103. 15378064
27. McDonald PW, Hardie SL, Jessen TN, et al. (2007) Vigorous motor activity in Caenorhabditis elegans requires efficient clearance of dopamine mediated by synaptic localization of the dopamine transporter DAT-1. J Neurosci. 27 : 14216–27. 18094261
28. Allen AT, Maher KN, Wani KA, et al. (2011) Coexpressed D1 - and D2-like dopamine receptors antagonistically modulate acetylcholine release in Caenorhabditis elegans. Genetics. 188 : 579–590. doi: 10.1534/genetics.111.128512 21515580
29. Rex E, Molitor SC, Hapiak V, et al. (2004) Tyramine receptor (SER-2) isoforms are involved in the regulation of pharyngeal pumping and foraging behavior in Caenorhabditis elegans. J Neurochem. 91 : 1104–15. 15569254
30. Donnelly JL, Clark CM, Leifer AM, et al. (2013) Monoaminergic orchestration of motor programs in a complex C. elegans behavior. PLoS Biol. 11: e1001529. doi: 10.1371/journal.pbio.1001529 23565061
31. Reinitz CA, Stretton AO (1996) Behavioral and cellular effects of serotonin on locomotion and male mating posture in Ascaris suum (Nematoda). J Comp Physiol A. 178 : 655–667. 8618217
32. Masler EP (2007) Responses of Heterodera glycines and Meloidogyne incognitato exogenously applied neuromodulators. J Helminthol. 81 : 421–427. 18005465
33. Beech RN, Callanan MK, Rao VT et al. (2013) Characterization of cys-loop receptor genes involved in inhibitory amine neurotransmission in parasitic and free living nematodes. Parasitol Int. 62 : 599–605. doi: 10.1016/j.parint.2013.03.010 23602737
34. Smith MW, Borts TL, Emkey R et al. (2003) Characterization of a novel G-protein coupled receptor from the parasitic nematode H. contortus with high affinity for serotonin. J Neurochem. 86 : 255–66. 12807445
35. White WH, Gutierrez JA, Naylor SA, et al. (2007) In vitro and in vivo characterization of p-amino-phenethyl-m-trifluoromethylphenyl piperazine (PAPP), a novel serotonergic agonist with anthelmintic activity against Haemonchus contortus, Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet Parasitol. 146 : 58–65. 17383823
36. Rao VT, Accardi MV, Siddiqui SZ, et al. (2010) Characterization of a novel tyramine-gated chloride channel from Haemonchus contortus. Mol Biochem Parasitol. 173 : 64–68. doi: 10.1016/j.molbiopara.2010.05.005 20471431
37. Pokala N, Liu Q, Gordus A, Bargmann CI. (2014) Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proc Natl Acad Sci U S A. 111 : 2770–5. doi: 10.1073/pnas.1400615111 24550306
38. Hobert O (2002) PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques. 32 : 728–30. 11962590
39. Mello C, Fire A (1995) DNA Transformation. Methods Cell Biol. 48 : 451–82. 8531738
40. Stiernagle T (2006) Maintenance of C. elegans. In: WormBook: The online review of C. elegans biology. Available: http://www.ncbi.nlm.nih.gov/books/NBK19649/ Accessed: 8 January 2015
41. Kaminsky R, Ducray P, Jung M, Clover R, Rufener L, Bouvier J, et al. (2008) A new class of anthelmintics effective against drug-resistant nematodes. Nature. 452 : 176–180. doi: 10.1038/nature06722 18337814
42. Crisford A, Murray C, O’Connor V, et al. (2011) Selective toxicity of the anthelmintic emodepside revealed by heterologous expression of human KCNMA1 in Caenorhabditis elegans. Mol Pharmacol. 79 : 1031–1043. doi: 10.1124/mol.111.071043 21415309
43. Welz C, Kruger N, Schniederjans M, et al. (2011) SLO-1-channels of parasitic nematodes reconstitute locomotor behaviour and emodepside sensitivity in Caenorhabditis elegans slo-1 loss of function mutants. PLoS Pathog. 7: e1001330. doi: 10.1371/journal.ppat.1001330 21490955
44. Komuniecki R, Law WJ, Jex A, et al. (2012) Monoaminergic signaling as a target for anthelmintic drug discovery: receptor conservation among the free-living and parasitic nematodes. Mol Biochem Parasitol. 183 : 1–7. doi: 10.1016/j.molbiopara.2012.02.001 22343182
45. Ramot D, Johnson BE, Berry TL Jr, et al. (2008) The Parallel Worm Tracker: a platform for measuring average speed and drug-induced paralysis in nematodes. PloS One. 3: e2208. doi: 10.1371/journal.pone.0002208 18493300
46. Smout MJ, Kotze AC, McCarthy JS, Loukas A (2010) A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PloS Negl Trop Dis. 4: e885. doi: 10.1371/journal.pntd.0000885 21103363
47. Wang SJ, Wang ZW (2013) Track-a-worm, an open-source system for quantitative assessment of C. elegans locomotory and bending behavior. PloS One. 8: e69653. doi: 10.1371/journal.pone.0069653 23922769
48. Buckingham SD, Sattelle DB (2009) Fast, automated measurement of nematode swimming (thrashing) without morphometry. BMC Neurosci. 10 : 84. doi: 10.1186/1471-2202-10-84 19619274
49. Chen B, Deutmeyer A, Carr J, et al. (2011) Microfluidic bioassay to characterize parasitic nematode phenotype and anthelmintic resistance. Parasitology. 138 : 80–8. doi: 10.1017/S0031182010001010 20663251
50. Carr JA, Parashar A, Gibson R, et al. (2011) A microfluidic platform for high-sensitivity, real-time drug screening on C. elegans and parasitic nematodes. Lab Chip. 11 : 2385–96. doi: 10.1039/c1lc20170k 21647497
51. Ho NF, Geary TG, Barsuhn CL, et al. (1992) Mechanistic studies in the transcuticular delivery of antiparasitic drugs. II: Ex vivo/in vitro correlation of solute transport by Ascaris suum. Mol Biochem Parasitol. 52 : 1–13. 1625697
52. Page AP, Johnstone IL (2007) The cuticle. In: WormBook: The online review of C. elegans biology.Available: http://www.ncbi.nlm.nih.gov/books/NBK19745/ Accessed: 10 January 2015
53. Ruiz-Lancheros E, Viau C, Walther TN, et al. (2011) Activity of novel nicotinic anthelmintics in cut preparations of Caenorhabditis elegans. Int J Parasitol. 41 : 455–61. doi: 10.1016/j.ijpara.2010.11.009 21195075
54. Partridge FA, Tearle AW, Gravato-Nobre MJ, Schafer WR, Hodgkin J (2008) The C. elegans glycosyltransferase BUS-8 has two distinct and essential roles in epidermal morphogenesis. Dev Biol. 317 : 549–559. doi: 10.1016/j.ydbio.2008.02.060 18395708
55. Razzaque Z, Heald MA, Pickard JD, et al. (1999) Vasoconstriction in human isolated middle meningeal arteries: determining the contribution of 5-HT1B - and 5-HT1F-receptor activation. Br J Clin Pharmacol. 47(1): 75–82. 10073743
56. Gray JM, Hill JJ, Bargmann CI (2005) A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 102(9): 3184–91. 15689400
57. Piggott BJ, Liu J, Feng Z, et al. (2011) The neural circuits and synaptic mechanisms underlying motor initiation in C. elegans. Cell. 147 : 922–33. doi: 10.1016/j.cell.2011.08.053 22078887
58. Zhang Y, Nash L, Fisher AL (2008) A simplified, robust, and streamlined procedure for the production of C. elegans transgenes via recombineering. BMC Dev Biol. 8 : 119. doi: 10.1186/1471-213X-8-119 19116030
59. Kubiak TM, Larsen MJ, Zantello MR, et al. (2003) Functional annotation of the putative orphan Caenorhabditis elegans G-protein-coupled receptor C10C6.2 as a FLP15 peptide receptor. J Biol Chem. 278 : 42115–20. 12937167
60. Kubiak TM, Larsen MJ, Bowman JW, et al. (2008) FMRFamide-like peptides encoded on the flp-18 precursor gene activate two isoforms of the orphan Caenorhabditis elegans G-protein-coupled receptor Y58G8A.4 heterologously expressed in mammalian cells. Biopolymers. 90 : 339–48. 17879267
61. Larsen MJ, Lancheros ER, Williams T, et al. (2012) Functional expression and characterization of the C. elegans G-protein-coupled FLP-2 Receptor (T19F4.1) in mammalian cells and yeast. Int J Parasitol Drugs Drug Resist. 15 : 1–7.
62. Boulin T, Gielen M, Richmond JE, et al. (2008) Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc Natl Acad Sci U S A. 105 : 18590–5. doi: 10.1073/pnas.0806933105 19020092
63. Boulin T, Fauvin A, Charvet CL, et al. (2011) Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br J Pharmacol. 164 : 1421–32. doi: 10.1111/j.1476-5381.2011.01420.x 21486278
64. Bennett HM, Lees K, Harper KM, et al. (2012) Xenopus laevis RIC-3 enhances the functional expression of the C. elegans homomeric nicotinic receptor, ACR-16, in Xenopus oocytes. J Neurochem. 123 : 911–8. doi: 10.1111/jnc.12013 22970690
65. Mariol MC, Walther L, Bellemin S, Gieseler K (2013) A rapid protocol for integrating extrachromosomal arrays with high transmission rate into the C. elegans genome. J Vis Exp. 82: e50773. doi: 10.3791/50773 24379027
66. Ardelli BF (2013) Transport proteins of the ABC systems superfamily and their role in drug action and resistance in nematodes. Parasitol Int. 62(6): 639–46. doi: 10.1016/j.parint.2013.02.008 23474412
67. Riddle DL, Blumenthal T, Meyer BJ, et al. editors (1997) Section II, cuticle. In: C. elegans II. 2nd edition. Available: http://www.ncbi.nlm.nih.gov/books/NBK20029/ Accessed: 7 January 2015
68. Riddle DL, Blumenthal T, Meyer BJ, et al. editors (1997) Section IV, the nematode surface. In: C. elegans II. 2nd edition. Available: http://www.ncbi.nlm.nih.gov/books/NBK20022/ Accessed: 7 January 2015
69. Schultz RD, Bennett EE, Ellis EA, Gumienny TL (2014) Regulation of Extracellular Matrix Organization by BMP Signaling in Caenorhabditis elegans. PLoS One. 9: e101929. doi: 10.1371/journal.pone.0101929 25013968
70. Burns AR, Kwok TC, Howard A, et al. (2006) High-throughput screening of small molecules for bioactivity and target identification in Caenorhabditis elegans. Nat Protoc. 1 : 1906–14. 17487175
71. Burns AR, Wallace IM, Wildenhain J, et al. (2010) A predictive model for drug bioaccumulation and bioactivity in Caenorhabditis elegans. Nat Chem Biol. 6 : 549–57. doi: 10.1038/nchembio.380 20512140
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



