-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
Despite advances in developing flavivirus live attenuated vaccine (LAV) candidates, a concern exists that they might not be safe in the environment due to their intrinsic genetic instability leading to potential reversion back to wild-type that could be associated with possible dissemination of these mutated viruses by mosquitoes. Here, we describe a miRNA targeting approach that can be adapted to support the design of environmentally-safe LAV restricted in their ability to infect and be transmitted by competent vectors, thereby limiting the possibility of subsequent viral evolution and unpredictable consequences. A combined co-targeting of flavivirus genome with mosquito - and vertebrate brain - specific miRNAs resulted in simultaneous restriction of dengue virus infection and replication in mosquitoes and in brains of newborn mice indicating that the miRNA-mediated approach for virus attenuation represents an alternative to non-specific strategies for the control of viral tissue tropism and pathogenesis in the vertebrate host and replicative efficacy in permissive vectors.
Published in the journal: . PLoS Pathog 11(4): e32767. doi:10.1371/journal.ppat.1004852
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004852Summary
Despite advances in developing flavivirus live attenuated vaccine (LAV) candidates, a concern exists that they might not be safe in the environment due to their intrinsic genetic instability leading to potential reversion back to wild-type that could be associated with possible dissemination of these mutated viruses by mosquitoes. Here, we describe a miRNA targeting approach that can be adapted to support the design of environmentally-safe LAV restricted in their ability to infect and be transmitted by competent vectors, thereby limiting the possibility of subsequent viral evolution and unpredictable consequences. A combined co-targeting of flavivirus genome with mosquito - and vertebrate brain - specific miRNAs resulted in simultaneous restriction of dengue virus infection and replication in mosquitoes and in brains of newborn mice indicating that the miRNA-mediated approach for virus attenuation represents an alternative to non-specific strategies for the control of viral tissue tropism and pathogenesis in the vertebrate host and replicative efficacy in permissive vectors.
Introduction
Mosquito-borne flaviviruses such as dengue, yellow fever, Japanese encephalitis and West Nile viruses (genus, Flavivirus; family, Flaviviridae) are among the most significant arboviral pathogens of humans and domestic animals in many regions of the world. Depending on the particular virus, clinical manifestations can vary from asymptomatic or self-limited flu-like illness to a life threatening jaundice, hemorrhagic fever and shock syndrome, or severe meningitis and encephalitis. Outbreaks and epidemics of flavivirus diseases typically coincide with warm rainy seasons, when the population of competent mosquito vectors reaches sufficient density for sustainable virus transmission between vertebrate hosts. Historically, mosquito control programs were considered as the most effective measure to prevent outbreaks and limit the spread of flavivirus diseases. However, reduction of funding allocated to mosquito control campaigns during the last decades, accompanied by an increase in human population density and global travel [1,2] resulted in an increase in the number of flavivirus-associated illnesses [1–3]. This emphasizes the need for development of effective vaccines or therapeutics as an alternative means for protection against flavivirus diseases.
A number of approaches are being pursued for development of effective vaccine candidates against flaviviruses, including RNA, DNA, inactivated, and subunit vaccines as well as vaccine candidates based on single-round replicated viral particles (reviewed in [4,5]). Although proven to be safe, they typically fail to provide long-lasting immunity after a single dose, and some may not be cost effective. This suggests a considerable advantage to vaccines based on live attenuated viruses that are relatively inexpensive to manufacture and provide a durable and potent immunity after a single dose of vaccination [6]. However, despite advances in developing live attenuated vaccine (LAV) candidates, a concern has been raised that they might not be safe in the environment due to their intrinsic genetic instability, potential reversion back to wild-type, and possible dissemination by mosquito vectors after feeding on vaccinees [7]. The possibility of mosquito transmission is further supported by the fact that many LAV candidates against diseases caused by mosquito-borne viruses can replicate in mosquito-derived cell lines and some are capable of infecting principle mosquito vectors in the laboratory [8–19]. For example, a vaccine strain of Venezuelan equine encephalitis virus (arbovirus, genus Alphavirus, family Togaviridae) was isolated outside its typical range from wild mosquitoes collected during a 1971 horse vaccination campaign in Louisiana [20], highlighting the risk of introduction and dissemination of potentially dangerous viral strains in new geographic locations.
Flaviviruses are enveloped single-stranded, positive-sense RNA viruses with genomes of approximately 11,000 nucleotide bases that contain 5’ and 3’-terminal non-coding regions (NCR) flanking a single open reading frame (ORF) encoding a polyprotein. During cap-dependent translation, the polyprotein is processed by viral and cellular proteases to yield three structural proteins (capsid [C], premembrane [prM], and envelope [E]) followed by at least seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) [21]. In recent years, an approach based on targeting of viral genomes for number of cellular microRNAs (miRNAs) has been proven to be a simple and efficient method to restrict replication and pathogenesis of DNA and RNA viruses in a cell-, tissue - or species-specific manner [22–33]. Moreover, we demonstrated that insertion of targets for several brain-specific microRNAs (miRNAs) into the 3’ NCR and/or ORF of neurotropic chimeric tick-borne encephalitis virus/dengue type 4 virus (TBEV/DEN4) was sufficient to selectively inhibit viral replication in neurons and to constrain the development of lethal encephalitis in adult and newborn mice after intracerebral or intraperitoneal infection [34–36]. In this study using a similar miRNA-targeting approach, we sought to design a mosquito-borne flavivirus that would be selectively restricted for replication in its invertebrate host. As a model system to investigate the effect of miRNA targeting on flavivirus fitness in arthropod vector, we selected a dengue type 4 virus (DEN4) that efficiently replicates in widely distributed Aedes (A.) aegypti and A. albopictus mosquitoes. DEN4 is not neuroinvasive in vertebrate hosts and is currently being used as a genetic background for development of live attenuated vaccines against neurotropic and non-neurotropic flaviviruses [8–12]. However, the DEN4-based chimeric viruses, as well as the DEN4 parent, are able to replicate in the central nervous system (CNS) of neonatal mice infected intracerebrally, causing lethal encephalitis. Therefore, we also explored if combined targeting of the DEN4 genome with mosquito-specific and mouse brain-expressed miRNAs can simultaneously restrict DEN4 infection and replication in the mosquito host and attenuate virus neurovirulence in newborn mice.
Results
Insertion of a single copy of miRNA target into the 3’NCR attenuates DEN4 replication in mosquito cells and live mosquitoes
Recently, miRNA expression profiles have been identified for several mosquito species [37–39]. Based on this data, three mosquito-specific miRNAs (mir-184, mir-275 and mir-1) were selected for DEN4 genome targeting because they satisfy the following criteria: 1) they are highly expressed in different mosquito organs and mosquito-derived cell lines, and also remain abundant during flaviviruses infection [37]; 2) these miRNAs are evolutionarily conserved among insect species including mosquitoes, but they are different from their miRNA analogs in mammals.
To investigate if miRNA targeting of DEN4 genome results in selective restriction of DEN4 replication in mosquitoes, a single copy of mir-184, mir-275, or mir-1 target sequence was introduced into the genome of DEN4 strain 814669 [40] (abbreviated D4s) between nucleotides (nts) 10277 and 10278 (15 nts downstream of the TAA stop codon preceding the 3’NCR). This particular strain of DEN4 was chosen because it has been extensively characterized in the laboratory and is currently being used as a genetic background for construction of LAV candidates against flaviviruses [8–12]. DEN4 viruses carrying miRNA target sequences were generated by electroporation of in vitro transcribed full-length genomic RNA into Vero cells. Parental and each modified DEN4 virus (designated D4s, D4-184s, D4-275s, and D4-1s; Fig 1) were harvested on day 5 post-electroporation and amplified by one additional passage in Vero cells. Specific infectivity values of synthesized RNA and viral titers in Vero cells supernatants for D4-184s, D4-275s, and D4-1s were comparable to the unmodified D4s clone (S1 Table), indicating that insertion of target sequences into the 3’NCR did not result in substantial attenuation of DEN4 in Vero cells. The effect of miRNA targeting on the DEN4 replication in mosquito cell lines was analyzed by comparing growth kinetics of viruses in Aag2 and C710 cells isolated from A. aegypti and A. albopictus mosquitoes, respectively. In both cell lines infected at multiplicity of infection (MOI) of 0.01, parental D4s and D4-1s viruses replicated efficiently with nearly identical kinetics reaching titers of ~6 log10 pfu/ml by 3 dpi (Fig 2A and 2B). In contrast, the replication of viruses D4-184s and D4-275s carrying a target for mir-184 or mir-275 was significantly impaired (p<0.001; 2-way ANOVA). In both cell lines, these viruses exhibited a 1000-fold or higher reduction in virus titer at 3 days post-infection (dpi) compared to the D4s virus, correlating with mir-184 and mir-275 expression levels in Aag2 and C710 cell lines (S1 Fig) [37]. Based on these data, we selected D4-184s and D4-275s for further evaluation of their replication in adult A. aegypti mosquitoes.Female mosquitoes were infected intrathoracically with 100 pfu of D4s, D4-184s, or D4-275s virus. After 7 days of incubation, whole body homogenates of each mosquito were prepared individually, and titers were determined on Vero cell monolayers (Fig 2C). Insertion of either miRNA target sequence in the 3’NCR of DEN4 genome led to a significant restriction of virus replication in mosquito bodies (p< 0.001, one-tailed t-test), however, it was not sufficient to completely suppress the DEN4 infection.
Fig. 1. Schematic representation of viral genomes used in this study. 
Positions of miRNA targets for brain-expressed mir-124 and mosquito-specific mir-1, mir-184, or mir-275 in the ORF and 3’NCR of DEN4 genome are indicated by blue and red boxes, respectively. Gray area represents duplicated, codon optimized DEN4 E/NS1 sequence (nts from 2130 to 2451 of DEN4 genome) encoding 98 amino acids from the C-terminal end of the DEN4 E protein and 7 amino acids from the N-terminal end of the NS1 protein. TM1 and TM2 are transmembrane helixes in the C-terminal anchor region of protein E. Red arrows indicate signalase cleavage sites. Asterisk in the table indicates that miRNA target sequence has been altered using synonymous codons. Fig. 2. Effect of a single copy of miRNA target in the 3’NCR on DEN4 replication in mosquito cells and live mosquitoes. 
(A and B) Growth kinetics of D4s, D4-1s, D4-184s and D4-275s viruses in mosquito Aag2 (A) and C710 (B) cells. Cells were infected at an MOI of 0.01. Each time point represents an average of two replicates ± standard deviation (shown as brackets). The dashed line indicates the limit of virus detection [0.7 log10 pfu/ml]. (C) A. aegypti mosquitoes were infected intrathoracically with 100 pfu of the indicated viruses and incubated for 7 days. Virus titer in each whole mosquito body suspension was determined in Vero cells. Each point represents the virus titer of an individual mosquito. Horizontal line represents the mean virus titer for all mosquitoes. The dashed line indicates the limit of virus detection [0.2 log10 pfu/mosquito]. P-values were calculated using unpaired one-tailed Student's t-test and adjusted using Bonferroni correction method to account for multiple comparisons. Combined targeting for mir-184 and mir-275 miRNAs in the 3’NCR greatly reduced the DEN4 replication in mosquito cells and Aedes mosquitoes
Previously, we demonstrated that multiple miRNA-targeting of flavivirus genome for brain-specific miRNAs in the 3’NCR results in increased efficiency of miRNA-mediated virus suppression in the CNS of mice compared to that observed for viruses containing only a single copy of the miRNA target [34,35]. Accordingly, we reasoned that a similar strategy should be applied to achieve a more reliable attenuation for DEN4 virus replication in cell culture and adult mosquitoes. Two additional constructs were developed based on D4-275s that contained additional target sequence for either mir-184 or mir-275 at nt position 212 of DEN4 3’NCR (Fig 1). Unfortunately, titers that these viruses achieved after recovery were not sufficient for their biological evaluation in mosquitoes (S1 Table).
To improve virus recovery, we modified the DEN4 cDNA clone in the following ways: substituted the SP6 promoter (for in vitro transcription) with the eukaryotic RNA polymerase II cytomegalovirus (CMV) promoter; introduced two intron sequences at nt positions 3922 and 8836 of the DEN4 genome to minimize plasmid DNA toxicity during propagation in E. coli; inserted the hepatitis delta virus ribozyme sequence at the 3’-end of the DEN4 virus genome to ensure correct release of authentic 3’-terminated RNA during transcription; and introduced the previously identified Vero cell-adaptive mutation (L122→F) into the NS4B protein gene to enhance replication in Vero cells [41–44]. The modified parental and mir-184 - or mir-275-targeted DEN4 viruses (designated D4, D4-184, and D4-275; Fig 1) were re-generated by cDNA transfection into Vero cells (S2 Table). Two additional viruses (D4-275-184 and D4-275x2) were developed based on the D4-275 genome and contained a second target sequence for either mir-184 or mir-275 at nt position 212 of the 3’NCR (Fig 1). Viruses were biologically cloned by terminal dilution and amplified by one additional passage in Vero cells (S2 Table). Viruses were genetically stable and contained the miRNA target insertions as assessed by sequence analysis of viral genomes after 5 additional passages in Vero cells. All viruses containing miRNA targets were significantly attenuated compared to D4 virus for growth in A. aegypti-derived Aag2 cells (Fig 3A, p<0.001; 2-way ANOVA) and in A. albopictus-derived C6/36 cells, which replaced the previously used C710 cells (Fig 3B, p<0.001; 2-way ANOVA). As anticipated, the combined expression of miRNA targets at two distant positions of the 3’NCR (D4-275-184 and D4-275x2) led to a more effective restriction of the virus replication in mosquito cells and resulted in at least a 3.5 or 6.0 log10 pfu/ml reduction in virus titer compared to D4 virus in Aag2 and C6/36 cells, respectively. In contrast, all viruses bearing one or two targets for mosquito-specific miRNAs replicated efficiently in Vero cells and the virus yield of each miRNA-targeted virus did not differ significantly from that of parental D4 virus (Fig 3C).
Fig. 3. Effect of combined mir-184 and mir-275 co-targeting of DEN4 genome in the 3’NCR on virus replication in mosquito and Vero cells. 
Confluent monolayers of Aag2 (A), C6/36 (B) or Vero (C) cells were infected with D4, D4-184, D4-275, D4-275x2 and D4-275-184 viruses at an MOI of 0.01. Each time point represents an average of two replicates ± standard deviation (shown as brackets). The dashed line indicates the limit of virus detection [0.7 log10 pfu/ml]. The maintenance of dengue viruses in the environment relies on the ability of the virus to infect, replicate, and develop a disseminated infection within two critical vectors: A. aegypti and A. albopictus mosquitoes. To investigate how targeting of the viral genome for mosquito-specific miRNA affects DEN4 fitness in female mosquitoes, A. aegypti and A. albopictus were fed with blood meals containing 7.1–7.4 log10 pfu/ml of recombinant DEN4 viruses. Viral titers in mosquito bodies, viral infectivity, and dissemination into heads of orally infected mosquitoes were assayed on day 14 after feeding. D4 virus infected 90% of both mosquito species by day 14, reaching a mean virus titer of ~2.5 log10 pfu/mosquito body, and disseminated to the heads of 71% of A. aegypti and 55% of A. albopictus (Fig 4). Introduction of a single copy of miRNA target for either mir-184 or mir-275 miRNA resulted in a significant reduction of the DEN4 titer in mosquito bodies (Fig 4A and 4B; p≤0.002 one-tailed Student's t-test) in both mosquito species as well as viral infectivity and ability of virus to develop a disseminated infection in A. aegypti (Fig 4C; p<0.05 one-tailed Fisher’s exact test). Infectivity of D4-184 and D4-275 viruses in A. albopictus were only slightly reduced as compared to D4 virus (Fig 4D; p = 0.144 and p = 0.0728), however both viruses were significantly attenuated in the ability to develop a disseminated infection (Fig 4D; p<0.05 one-tailed Fisher’s exact test). All D4-184 and D4-275 viruses recovered from A. albopictus and the majority of viruses recovered from infected A. aegypti contained deletions or point mutations in the miRNA target sequences (S2 Fig), indicating that targeting of the DEN4 genome by a single copy of miRNA target was not sufficient to prevent virus transmission by mosquitoes. In contrast, the D4-275-184 virus was unable to infect the midgut and thus failed to disseminate in both mosquito species indicating that a combined expression of mir-184 and mir-275 targets in D4-275-184 was sufficient to completely block DEN4 replication in the principal mosquito vectors (Fig 4C and 4D, p<0.001; Fisher’s exact test). These results are consistent with our previous observations made for viruses targeted by brain-specific miRNAs [34–36], suggesting that targeting of the viral genome in the same sites for two different miRNAs is a more efficient approach for flavivirus attenuation in the mosquitoes and the CNS of mice compared to a monotypic miRNA-targeting. Interestingly, a duplication of the target sequence for mir-275 miRNA alone resulted in a virus that was more infectious for A. aegypti (but not to A. albopictus) as compared to the D4-275-184 virus.
Fig. 4. Effect of combined mir-184 and mir-275 co-targeting of DEN4 genome in the 3’NCR on virus fitness in A. aegypti and A. albopictus. 
A. aegypti (A and C) and A. albopictus (B and D) were orally infected with blood meals (BM) containing 7.1 to 7.4 log10 pfu/ml of recombinant D4 or D4-275-184 virus, respectively, and processed at 14 dpi. (A and B) Each point represents virus titer in individual mosquito (n = 18 for A. aegypti exposed to D4, and n = 24 mosquitoes for each other virus). Horizontal line represents an average titer for all mosquitoes. The dashed line indicates the limit of the assay detection [0.2 log10 pfu/mosquito]. P-values comparing mean titers of D4 and miRNA targeted viruses were calculated using unpaired one-tailed Student's t-test and adjusted using Bonferroni correction method to account for multiple comparisons. (C and D) Percentage of mosquitoes that became infected (blue) or developed a disseminated infection (orange) with each virus. Differences in infection and dissemination frequencies were compared between D4 and miRNA targeted viruses using one-tailed Fisher’s exact test. P-values were adjusted using Bonferroni correction method to account for multiple comparisons. Expression of mosquito-specific miRNA targets in the ORF region also results in a restriction of the mosquito vector range of the DEN4 virus
Previous studies of miRNA-regulated gene expression have demonstrated that the majority of mRNAs are post-transcriptionally regulated by targeting in the 3’NCR, and although regulation of mRNAs through miRNA-targeting in the coding region is less frequent, it is well documented (reviewed in [45,46]). Therefore, to explore if targeting of an ORF region of DEN4 by mosquito-specific miRNAs can result in specific viral attenuation in mosquitoes, targets for mosquito-expressed mir-184 and mir-275 as well as three targets for human neuron-specific mir-124 miRNA were introduced in the DEN4 genome between sequences encoding the two C-terminal stem-anchor domains of DEN4 E protein (D4-E virus; Fig 1). We designed these insertions of miRNA targets in this region using an approach that has been described previously [34,47]. As a control, we generated a D4-E* virus, in which mir-184 and mir-275 target sequences of D4-E were synonymously mutated in the third base position of each codon. The levels of replication for both control D4-E* virus and D4-E-mutant-bearing miRNA targets were similar in Vero cells indicating that the presence of miRNA targets or their scrambled sequences in the ORF of the viral genome does not affect viral fitness in cells that do not express the corresponding miRNAs (Fig 5C). Genome regions containing miRNA targets in D4-E or scrambled sequences in D4-E* virus remained stable for at least 5 subsequent passages in Vero cells as demonstrated by sequence analysis (S3 Fig). Replication of D4-E, but not D4-E*, was strongly attenuated in both Aag2 and C6/36 cells as compared to the D4 virus (Fig 5A and 5B, p<0.001; 2-way ANOVA), but the level of a residual replication of D4-E in either cell line was higher than that observed for the D4-275-184 virus carrying miRNA targets in the 3’NCR (Figs 3 and 5). Studies in orally infected A. aegypti mosquitoes demonstrated that infection rates of D4-E but not D4-E* were significantly decreased as compared to D4 (Fig 6B, p<0.001 and p = 0.151, respectively; one-tailed Fisher’s exact test). Infectivity of D4-E to A. aegypti was also significantly lower than that of D4-E* (1/24 versus 11/24; p = 0.0009; Fisher’s exact test) and none of the mosquitoes (0/24) had disseminated infection with D4-E virus compared to 20.1% (5/24) for D4-E* (p = 0.025; Fisher’s exact test). These results indicate that miRNA targeting of the D4-E genome within the ORF can specifically attenuate the virus for both mosquito-derived cells and mosquitoes due to the presence of authentic miRNA target sequences.
Fig. 5. miRNA targeting of the DEN4 genome within the ORF and 3’NCR greatly attenuates virus replication in Aag2 and C6/36 cells but not in Vero cells. 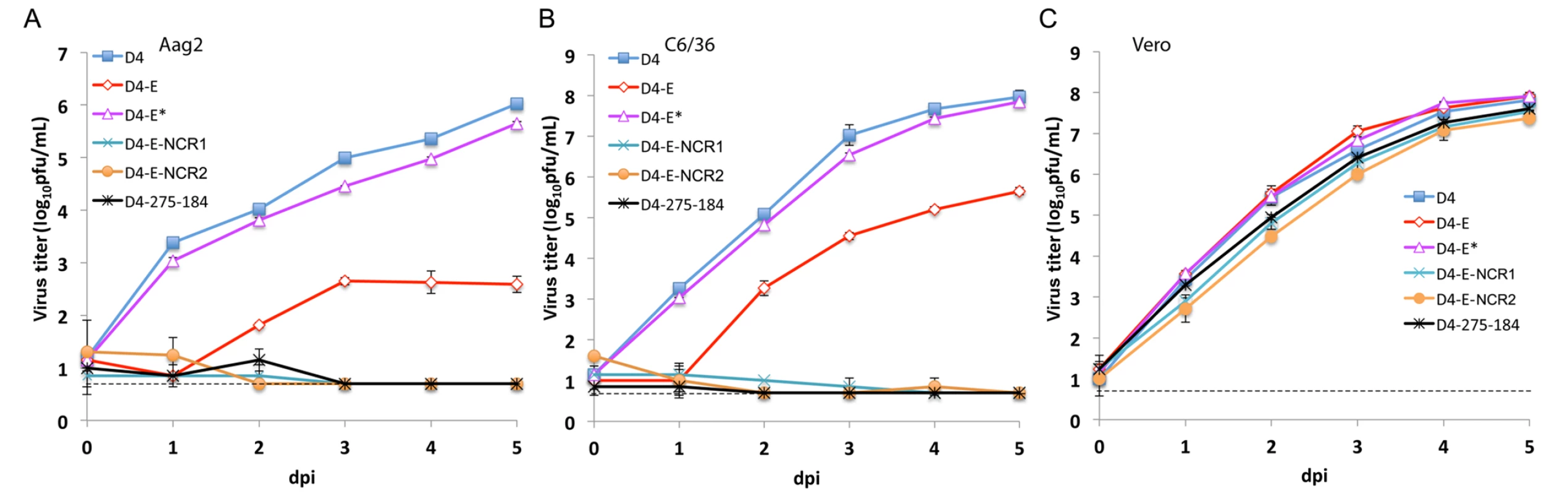
Confluent monolayers of Aag2 (A), C6/36 (B) and Vero (C) cells were infected in duplicate with either D4, D4-E, D4-E*, D4-275-184, D4-E-NCR1, and D4-E-NCR2 viruses at an MOI of 0.01. Each time point represents an average titer for two replicates ± standard deviation (shown as brackets). The dashed line indicates the limit of virus detection [0.7 log10 pfu/ml]. Fig. 6. Effect of mir-184 and mir-275 co-targeting of DEN4 genome in the ORF and 3’NCR on virus fitness in A. aegypti mosquitoes. 
A. aegypti were presented with blood meals containing approximately 7 log10 pfu/mL of indicated recombinant viruses and processed after 14 days. (A) Each point represents virus titer in an individual mosquito (n = 24 mosquitoes per group). Horizontal line represents the mean virus titer for all mosquitoes in the group. The dashed line indicates the limit of assay detection [0.2 log10 pfu/mosquito]. P-values used to compare mean titers of D4 and miRNA targeted viruses were calculated using unpaired one-tailed Student's t-test and adjusted using Bonferroni correction method to account for multiple comparisons. (B) Percentage of mosquitoes that became infected (blue) or developed a disseminated infection (orange) with each virus. Differences in infection and dissemination frequencies were compared between D4 and miRNA targeted viruses using one-tailed Fisher’s exact test, and P-values were adjusted using Bonferroni correction method to account for multiple comparisons. Even though a combined expression of mir-184 and mir-275 targets in the 3’NCR was sufficient to greatly restrict the D4-275-184 virus replication in mosquito cells and abolish infectivity in adult mosquitoes, an escape mutant lacking both authentic target sequences can theoretically emerge as a result of error prone flavivirus replication under miRNA-mediated selective pressure. To minimize the probability of such events, we generated a virus (D4-E-NCR1; Fig 1) expressing mir-184 and mir-275 target sequences in both the ORF and 3’NCR of DEN4 genome. As expected, the resulting virus was genetically stable and replicated well in Vero cells, but failed to initiate a productive infection in mosquito Aag-2 and C6/36 cells (Fig 5). Moreover, none of the A. aegypti mosquitoes that fed on a blood meal containing 6.8 log10 pfu/ml of D4-E-NCR1 became infected, or developed a disseminated infection into mosquito heads as tested at 14 dpi (Fig 6). These findings indicate that combined co-targeting of the DEN4 genome in the ORF and 3’NCR does not result in target interference during miRNA mediated flavivirus attenuation in mosquitos.
Silencing of DEN4 replication in mice and mosquitoes by multiple miRNA co-targeting of the viral genome
To explore if the miRNA targeting approach represents adequate means for simultaneous restriction of flavivirus replication in both the CNS of vertebrates and in mosquito vectors, we first evaluated neurovirulence of D4-E and D4-E-NCR1 viruses in a highly permissive animal model such as 3-day-old Swiss mice inoculated intracranially [34,36,42,44,48]. Both D4-E and D4-E-NCR1 viruses contained miRNA targets for mosquito-specific mir-184 and mir-275 and three copies of target sequences for vertebrate brain-specific mir-124 in the duplicated E/NS1 region (Fig 1). As a control virus for comparative assessment in the CNS of mice, we generated a D4-E** virus based on D4-E that contained synonymous mutations in the third base position of each codon of the CNS-specific mir-124 target sequences. The effect of mir-124 targeting in limiting neurotropism of flaviviruses has been extensively characterized in our laboratory previously [34–36]. Replication of D4-E and D4-E-NCR1 viruses in the mouse brain was strongly attenuated compared to that of D4 or D4-E** virus (Fig 7A, p<0.001 for each of control viruses; 2-way ANOVA). Moreover, none of the mice infected with D4-E or D4-E-NCR1 died, whereas the mortality rate for parental D4 was 100% (Fig 7B, p<0.001; log-rank test). This demonstrates that the miRNA targeting approach can be used for specific attenuation of flavivirus replication in more than one host or cell-type of interest. Interestingly, D4-E** virus with scrambled mir-124 target sequences in the ORF had a lower titer in the brain at each time point as compared with D4 virus (Fig 7A, p<0.001; 2-way ANOVA) and only 25% of mice infected with D4-E** virus died during a 21-days observation period (Fig 7B, p = 0.0014; log-rank test). This likely reflects that insertions of heterologous sequences (mir-275 and mir-184 targets) in the ORF can result in partial attenuation of DEN4 replication in the brain of mice.
Fig. 7. Effect of multiple mosquito- and brain-specific miRNA target insertions on DEN4 neurovirulence and replication in the brain of sucking mice. 
(A) Replication kinetics of D4, D4-E, D4-E**, D4-184, D4-E-NCR1, and D4-E-NCR2 viruses in the brains of mice. Three-day-old mice were inoculated IC with 3 log10 pfu of the indicated virus, and brains were collected from three mice in each group following the course of infection. Each time point represents an average titer for two replicates ± standard deviation (shown as brackets). The dashed line indicates the limit of virus detection [1.7 log10 pfu/ml]. Brain collection of mice infected with D4 and D4-184 was not performed at 14 dpi due to earlier paralysis of the animals. (B) Survival curves of sucking mice infected IC with 103 pfu of recombinant DEN4 viruses (litter of eight 3-day-old mice per virus). Mice were monitored daily for morbidity for 21 days. We selected a 3 log10 pfu dose of virus inoculation since parental DEN4 strain 814669 has an IC LD50 of 407 pfu as estimated previously for this age of mice [48]. Our previous studies [34,35] had demonstrated that increasing the number of miRNA targets in the 3’NCR of flavivirus genome significantly diminished virus replication in mouse brain and prevented virus escape from miRNA-mediated suppression. In order to minimize or reduce the emergence of DEN4 escape mutants during replication in the CNS, we developed an additional virus (D4-E-NCR2) containing a combination of brain and mosquito specific miRNA target sequences in the ORF and 3’NCR (Fig 1). As expected, the resulting D4-E-NCR2 virus efficiently replicated in Vero cells, but not in mosquito Aag2 or C6/36 cells (Fig 5; p<0.001; 2-way ANOVA). Moreover, no virus replication was detected in the brains of neonatal mice infected IC with D4-E-NCR2 (Fig 7A, p<0.001; 2-way ANOVA), and no death or neurological signs were observed (Fig 7B). In addition, the D4-E-NCR2 virus was unable to infect and generate disseminated infection in A. aegypti mosquitoes fed a blood meal containing 6.8 log10 pfu/ml of either virus (Fig 6B, p<0.001; Fisher’s exact test). We concluded that D4-E-NCR1 and D4-E-NCR2 viruses potentially could be used as a genetic background for development of chimeric live attenuated vaccine candidate against neurotropic flaviviruses, or a similar strategy could be applied for targeted attenuation of other flavivirus vaccine platforms such as yellow fever virus (YF) 17D or dengue viruses [13–19].
Discussion
In addition to the low level of viremia observed following use of most flavivirus LAV, several additional factors that limit the potential for mosquito transmission have been identified, most of which are associated with non-specific or incidental attenuation of the vaccine strains. Prolonged serial passage of the Asibi strain of YF in cell cultures prepared from embryonated eggs led to generation of the YF 17D virus. Even though YF 17D is capable of infecting the A. aegypti midgut, the virus cannot be transmitted to vertebrate hosts due to its inability to escape the midgut barrier, which has been found to be associated with an accumulation of selective mutations in E, NS2A, and NS4B genes [49–52]. Similarly, a deletion of 30 nts (Δ30 mutation) in the 3’NCR of the DEN4 LAV platform not only led to virus attenuation in vertebrate hosts, but also resulted in a significant decrease in the dissemination rate in Aedes mosquitoes [53]. Both YF17D and DEN4Δ 30 viruses were subsequently used as genetic platforms for development of LAV candidates against dengue, West Nile, Saint Louis encephalitis, Japanese encephalitis, and tick-borne encephalitis viruses [8–19,40,43,44]. Analysis of the resulting vaccine viruses in mosquitoes demonstrated that genome chimerization also leads to an additional decrease in transmission potential of chimeric viruses constructed based on either 17D [13–19] or DEN4Δ 30 [8–12] genetic backgrounds.
In this study, using mosquito-borne DEN4 flavivirus as a model virus, we demonstrated the effectiveness of the miRNA targeting approach for the simultaneous attenuation of a virus in two evolutionally distant host organisms: mice and mosquitoes. Since miRNA-mediated control of viral tropism in mammals has been achieved previously for both RNA and DNA viruses, including flaviviruses [23–25,34–36,54], as well as selective species-specific attenuation in mammals [30], then silencing of virus replication or complete blockade of virus infectivity in the insect vector alone or in both vertebrate and invertebrate hosts represents the unique focus of this study. We suggest that this approach can be adapted to support the development of environmentally safe, live attenuated virus vaccines restricted in their ability to be introduced into nature during feeding of competent mosquito vectors on viremic vaccinees, thereby limiting the possibility for a subsequent evolution of LAV-derived viruses which can result in unpredictable consequences [7]. The miRNA-mediated silencing of virus replication in mice and mosquitoes demonstrated in our studies can reinforce the safety of newly developed and existing vaccines for use in humans.
The precise mechanism involved in miRNA-mediated attenuation of viruses in mosquitoes and their cell lines is unknown and requires further investigation. A variety of factors might play a role and contribute to the effectiveness of cellular miRNA-mediated RNA transcriptional repression and can depend on the level of miRNA expression, the number of miRNA targets and their locations in mRNA or viral RNA genomes, and their accessibility and interactions with the RNA-induced silencing complex (RISC) [23,45,46,55,56].
As demonstrated in our studies, increasing the copy number of miRNA targets was generally associated with more efficient DEN4 attenuation in mosquito cells and in A. aegypti and A. albopictus mosquitoes, presumably due to a greater number of RISCs becoming engaged in interactions with the DEN4 RNA genome. Among three selected targets for mosquito-specific miRNAs that were inserted into the 3’NCR of DEN4 genome, the presence of a target for the highly expressed miRNAs in mosquito cells Aag2 or C710 (mir-184 and mir-275) reduced DEN4 replication to a greater extent than the inclusion of a target for the less expressed mir-1 miRNA (Figs 2 and S1) [37]. This indicates that inhibition of DEN4 replication occurs by a miRNA-mediated mechanism and is not a result of unspecific attenuation of viral replication due to insertion of heterologous sequences into the 3’NCR. This also suggests that miRNAs with the highest mosquito cell abundance should be used for effective viral genome targeting.
As described previously [57], insects have two functionally distinct RNase III enzymes (Dicers): Dicer-1 is primarily involved in biogenesis of cellular miRNAs, whereas Dicer-2 participates in the RNA interference (RNAi) response through production of small interfering RNAs from extended double stranded RNAs. It has been demonstrated that unlike Aag2, A. albopictus derived C6/36 cells exhibited impaired RNAi response due to aberrant Dicer-2 gene expression [58,59]. Since replication of miRNA-targeted D4-184 and D4-275 viruses in both Aag2 and C6/36 cell lines was attenuated compared to parental D4 virus, it can be assumed that inhibition of viral replication occurs primarily through a Dicer-1-dependent miRNA-mediated mechanism, but not through a Dicer-2-mediated RNAi mode (Fig 3).
Placement of miRNA targets in the 3’NCR has an overall stronger suppressive effect on the DEN4 replication in mosquito cells than target placement in the ORF region (Figs 3 and 5). Thus, replication of D4-275-184 containing targets for mir-275 and mir-184 in the 3’NCR was not detected in either Aag2 or C6/36 cells, whereas D4-E containing the same targets in the duplicated E-NS1 region replicated to titers of 2.5 and 5.5 log10 pfu/ml in these cells, respectively. Replication of D4-E in both cell lines was not due to loss of the miRNA targets and generation of escape mutants as was verified by sequence analyses (S3 Fig). In addition, combined expression of mir-275 and mir-184 targets in the 3’NCR was sufficient to completely block the D4-275-184 virus infectivity for A. aegypti and A. albopictus (Fig 4), whereas D4-E was detected in 5.6% (1/24) of A. aegypti mosquitoes (Fig 6). These findings are consistent with the notion that cellular mRNA translational repression occurs more frequently and efficiently by miRNA-targeting of mRNAs in the 3’NCR compared to that in the ORF or 5’NCR, presumably due to a lack of interference between the RISC and polysomes [45,46,60,61].
Surprisingly, infectivity of D4-275x2, containing two tandem targets for mir-275, in A. aegypti mosquitoes was significantly higher than that of single-copy targeted D4-275 virus (Fig 4). This phenomenon could be explained in part by assuming that introduction of a second mir-275 target results in the alteration in the 3’NCR secondary structure, which would make both mir-275 target sequences less accessible for miRNA-recognition and RISC binding. The m-fold RNA analysis predicts that two mir-275 targets could form a secondary structure with a 13 nt complementarity in the 3’NCR of the D4-275x2 genome (S4 Fig). The resulting structure could sequester both target sequences from interactions with RISC, as was previously shown for miRNA targets located in the regions of extensive secondary RNA structures [62]. Interestingly, this sequestration effect of the mir-275 tandem target was observed only for D4-275x2 infectivity in A. aegypti mosquitoes, but not in A. albopictus mosquitoes or in Aag2 or C6/36 cells (Figs 3 and 4). This suggests that structural folding of the 3’NCR of D4-275x2 genome as well as assembly, recognition, and binding with RISCs might vary between cell lines and mosquito species, which probably mirrors variation in specific cellular factors involved in these complex interactions.
The miRNA-based strategy of targeted host range restriction of flavivirus replication does not necessarily have to be confined to development of environmentally safe LAV against flaviviruses, but also could be applied for LAV against other arboviruses in Togaviridae and Bunyaviridae families. Moreover, similar to what was previously demonstrated for gain-of-function studies of influenza A virus [29,63–65], the miRNA based approach of host range restriction could potentially provide an additional level of biosafety for laboratories that use flaviviruses with altered pathogenicity or transmissibility characteristics. This should mitigate a risk for potential spread of novel pathogens in the nature after accidents/exposures in bio-containment laboratories.
Another significant observation of this study was the fact that targeting of the DEN4 genome with mosquito specific miRNA does not interfere with the capacity of CNS-enriched mir-124 miRNA to restrict replication of mir-124 targeted DEN4 viruses in the brain of newborn mice (Fig 7). This confirms the hypothesis that simultaneous targeting of RNA virus genome with sequences complementary to miRNAs overexpressed in different hosts can result in independent attenuation of virus replication in more than one host - or cell-type. This suggests that simultaneous multiple miRNA targeting can be applied to the construction of complex genetic systems with replication restricted to a very narrow range of target cells (organisms). This technique should find its use not only in LAV development but also in studies of virus pathogenesis using in vivo disease models [23,54].
Summary
Here, for the first time we demonstrate that a combined targeting of the mosquito-borne flavivirus genome can silence viral replication in two evolutionally distant species: mosquitoes and mice. We believe that the miRNA co-targeting approach can be adapted to support the design of environmentally safe, live attenuated virus vaccines by restricting their ability to be introduced into nature during feeding of competent vectors on viremic vaccinees, thereby limiting the possibility of subsequent viral evolution and unpredictable consequences. Thus, miRNA-mediated silencing of virus replication in mice and mosquitoes can reinforce the safety of newly developed and existing vaccines for use in humans. This engineered host range restriction in both insect vector and vertebrate host by miRNA-mediated mechanisms represents an alternative to non-specific strategies for the rational control of viral tissue tropism and pathogenesis in the vertebrate host and replicative efficacy in permissive vectors.
Materials and Methods
Plasmids
Recombinant cDNA clone of dengue type 4 virus strain 814669, referred to here as D4s (GenBank access # AY648301) has been described previously [40]. To generate a modified D4 version of D4s clone, DNA fragments of viral genome were amplified from D4s using Phusion DNA polymerase (New England Biolabs [NEB], Ipswich, MA) and cloned individually or in combinations into low copy number pACNR1181 plasmid vector [66] using conventional PCR-based methods [67]. Intron sequence [nt. 857–989 in HaloTag CMV-neo vector pHTC (GenBank access # JF920305)] was synthesized by GenScript Inc. (Piscataway, NJ) and was introduced at positions 3922 and 8836 of DEN4 genome at AGCT sites. cDNA of CMV promoter was amplified from pCMV-SPORT6 plasmid (Invitrogen, Carlsbad, CA) and was fused with 5' end of DEN4 genome using PCR-based techniques. The hepatitis delta ribozyme sequence was amplified from plasmid RBZ-17D/25C-GFP-FMDV2A-YFCO_full_prME (generous gift of Dr. I. Frolov, University of Alabama), and fused with polyA signal/translation terminator sequence that was amplified from pTriEx1.1 (Novagen, Germany). The resulting amplicon was fused with the 3’-end of DEN4 genome to assemble wild-type D4 cDNA clone. A Vero cell adaptive mutation L122→F [41–44] was introduced into NS4B protein gene to generate a D4 plasmid.
Target sequences for mosquito specific mir-1 (5’-CTCCATACTTCTTTACATTCCA-3’), mir-184 (5’-GCCCTTATCAGTTCTCCGTCCA-3’) and mir-275 (5’-GCGCTACTTCAGGTACCTGA-3’) or human brain-specific mir-124 (5’-GGCATTCACCGCGTGCCTTA-3’) were introduced into the 3’NCR of DEN4 genome between nts 10,277 and 10,278 (position 1, Fig 1) or 10,474 and 10,475 (position 2, Fig 1); these sites of target insertion are located 15 or 212 nts downstream of the TAA stop codon in the 3’NCR, respectively. To introduce miRNA targets in the ORF, we utilized the approach that has been described previously [34,47]. Specifically, the introduced sequence was inserted between nts 2451 and 2452 of DEN4 genome and contained five tandem targets for mir-124, mir-184 and mir-275 that were followed by a duplicated DEN4 E/NS1 region (nts from 2130 to 2451 of DEN4 genome) encoding 98 amino acids from the C-terminal end of the DEN4 E protein and 7 amino acids from the N-terminal end of the NS1 protein (Fig 1). Each codon (except for Met and Trp) within the duplicated E/NS1 region was mutated to a synonymous codon to minimize nucleotide sequence homology between repeated DEN4 genome segments (Fig 1). Each plasmid DNA was sequenced to verify its integrity. Detailed information for all plasmids is available from the authors upon request.
Cells and mosquitoes
Vero cells (African green monkey kidney) were cultured in serum free Opti-Pro medium (Invitrogen) as previously described [42]. Mosquito C6/36 (derived from A. albopictus; ATCC), C710 and Aag2 (derived from A. albopictus and A. aegypti, respectively; generous gift from Dr. A. Fallon, University of Minnesota) cells were maintained in Dulbecco minimal essential medium (DMEM) (Invitrogen) supplemented with 5% FBS (Lonza), 1x penicillin-streptomycin-glutamine (PSG) solution (Invitrogen), 1x MEM nonessential amino acids (Cellgro, Swedesboro, NJ), 1 × MEM vitamin solution (Invitrogen) and 5 μg/L of gentamicin (Invitrogen) at 32°C in an atmosphere of 5% CO2. The C6/36 cells were used in all but the initial experiments, because they exhibit superior viability in our experimental conditions compared to C710 cells.
Galveston colony of A. albopictus (generous gift of Dr. S. C. Weaver, UTMB) and NIH strain of A. aegypti mosquitoes have been described earlier [53,68]. Both mosquito species were maintained in carton containers supplemented with 10% sucrose on cotton balls at 28°C, 80% humidity and with a 16-hr daylight cycle.
Virus recovery
1) DNA transfection (D4 and its derivatives)
Twenty-four hours before transfection, 1.2x106 low-passage Vero cells were seeded into a 12.5 cm2 flask in 5 mL of DMEM media supplemented with 10% FBS and 1x PSG solution. Next day, Vero cells were transfected with 5 μg of plasmid DNA using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instructions. Cells were maintained in DMEM at 37°C, 5% CO2 for 5 days. Cell culture supernatant was harvested, and virus was titrated on Vero cell monolayers and was purified by one-step terminal dilution as described earlier [42]. Experimental virus stocks were prepared by two consecutive passages in Vero cells (passages 143–149) maintained in Opti-Pro (Invitrogen), followed by titration of viruses in Vero cells. Complete genomes of biologically-cloned viruses were sequenced to ensure genetic integrity.
2) RNA electroporation (D4s and its derivatives)
Five micrograms of plasmid DNA was linearized with AgeI restriction endonuclease (NEB, Ipswich, MA), followed by in vitro RNA transcription from the SP6 promoter using the mMESSAGE mMACHINE kit (Ambion, Austin, TX) according to manufacturer's instructions. Ten micrograms of RNA was electroporated into 5x107 Vero cells using a Gene Pulser Xcell electroporation system (Bio-Rad, Hercules, CA) with 4-mm cuvettes at the following conditions: 300V, pulse length 10μs, 5 pulses, with an interval between the pulses of 1s. Cells were transferred to a 75 cm2 flask and allowed to recover in 14 mL of Leibovitz’s L-15 medium (Invitrogen) supplemented with 10% FBS for 4 h at 37°C. The media was replaced with 14 mL of DMEM with 10% FBS, 1x PSG, and cells were incubated for 5 days at 37°C with 5% CO2, followed by harvest and titration in Vero cells. Experimental virus stocks were amplified by an additional passage in Vero cells maintained in Opti-Pro, and titrated on Vero cell monolayers. Presence of introduced miRNA targets in recovered viruses was verified by sequence analysis of viral cDNA genome.
To compare infectivity of in vitro transcribed RNAs, ten-fold dilutions of electroporated Vero cells were seeded in 1 mL of Opti-Pro media in 24-well plates containing 1x105 cells. Cells were incubated for 4h at 37°C, and culture media was replaced with Opti-MEM (Invitrogen) containing 1% methylcellulose (Invitrogen) and 2% FBS. After incubation for 5 days at 37°C, the cells were fixed with 100% methanol, and plaques were visualized by immunostaining with 4G2 monoclonal antibodies as described previously [42,43]. Infectious foci were counted and normalized to the number of cells used for electroporation.
Multicycle replication of miRNA targeted viruses in vertebrate and mosquito cells lines
To determine effect of miRNA targeting on DEN4 replication, miRNA targeted or control viruses were diluted in Opti-Pro medium supplemented with 2% FBS, 2 mM L-glutamine and were used to infect Vero, C6/36, Aag2 or C710 cells at an MOI of 0.01 in duplicate wells of a 6-well plate for 1 h at 37°C (Vero) or 32°C (mosquito cells). The cells were washed three times with Opti-Pro and 2.75 mL of fresh media was added. Aliquots of 0.25 mL were harvested daily and stored at −80°C until virus titration. Differences in virus replication kinetics were compared using 2-way ANOVA analyses implemented in Prism 6 software (La Jolla, CA).
Genetic stability of miRNA targeted viruses in simian Vero cells
Confluent monolayers of Vero cells in 25 cm2 flasks were infected at an MOI of 0.01 and cell culture supernatant was harvested at 3 dpi, diluted 1/10 with Opti-Pro medium, and 1 mL of inoculum was used to infect 25 cm2 flasks of fresh Vero cells. The cycle was repeated 5 times. At the end of the fifth passage, viral RNA was extracted from 0.14 mL of supernatant using the QIAamp Viral RNA Mini kit (Qiagen) according to manufacturer’s instructions. Genome regions flanking miRNA target sites were amplified using Titan One Tube RT-PCR kit (Roche, Indianapolis, IN) and sequenced.
Evaluation of viruses in mosquitoes
1) Intrathoracic infection (D4s and its derivatives)
Fifty 5-day-old female A. aegypti mosquitoes were immobilized on ice and inoculated intrathoracically with 1 μL of medium containing 5 log10 pfu/ml (100 pfu/mosquito) of DEN4 viruses. Mosquitoes were returned to carton containers, provided with 10% sucrose ad libitum, and held at 28°C and 80% humidity. After 7 days, live mosquitoes were collected with a hand aspirator, killed by chilling at -20°C for 5 min, and stored at -80°C.
2) Oral infections (D4 and its derivatives)
To prepare infectious blood meals, viruses were diluted with Opti-Pro media supplemented with 2% FBS, 2 mM L-glutamine, and 1x SPG (218 mM sucrose, 6 mM L-glutamic acid, 3.8 mM KH2PO4, 7.2 mM K2HPO4, pH 7.2) to titers equal to least concentrated virus used in analysis. Virus aliquot of 1 mL was mixed with 1 mL of defibrinated rabbit blood (Spring Valley Laboratories, Woodline, MD), and blood meals were offered to 4–5 day old female mosquitos in a 37°C preheated water-jacketed feeder or using the Hemotek membrane feeding system (Discovery Workshops, UK) covered in stretched Parafilm (American National Can, Chicago, IL). Blood meal samples were collected for virus titration. Feeding proceeded for 45 min, followed by sorting of engorged mosquitoes (stage 3+ [69]) on ice. Engorged females were returned to cages and incubated at 28°C, 80% humidity and 16/8-hr day/night cycle for 14 days. Live mosquitos were collected and stored as described above.
Virus infection was analyzed by plaque-forming assay of each whole mosquito body (intrathoracic infection) or in each decapitated mosquito (oral infection). To assess virus dissemination in orally infected mosquitoes, head and body homogenates were prepared separately. Mosquito bodies and heads were triturated in 0.25 mL of L-15 medium supplemented with 1x SPG, 0.05 mg/mL of Ciprofloxacin, 0.06 mg/mL of Clindamycin and 0.0025 mg/mL of Amphotericin B, and homogenates were clarified by centrifugation at 10,000 rpm for 3 min. Head homogenate aliquots of 0.1 mL were used to infect Vero cells in one well of 24-well plates. Body homogenate samples were serially 10-fold diluted and 0.1 mL aliquots were used to infect monolayers of Vero cells in 24-well plates, followed by plaque visualization as described above. Mosquitoes were considered infected if at least one virus plaque was detected in the lowest dilution of body homogenate after 5 days incubation. Virus was considered to develop a disseminated infection if at least one virus plaque was detected in head homogenates after 5 days incubation. Differences in viral titers in mosquitos were compared using one-tailed Student's t-test, and P-values were adjusted using Bonferroni correction method to account for multiple comparisons (P = P’xN where N is number of comparisons). Differences in infection and dissemination frequencies were compared using one-tailed Fisher’s exact test and P-values were adjusted using Bonferroni correction method.
Ethics statement
All animal experiments were done in compliance with the guidelines of the NIAID/NIH Institutional Animal Care and Use Committee. The NIAID DIR Animal Care and Use Program acknowledges and accepts responsibility for the care and use of animals involved in activities covered by the NIH IRP’s PHS Assurance #A4149-01, last issued 6/11/2011.
Evaluation of viruses in sucking mice
D4 and miRNA targeted viruses were diluted to 5 log10 pfu/ml with L-15 medium supplemented with 1x SPG. Three-day-old Swiss Webster mice (Taconic Farms) in groups of 10 were inoculated intracranially (IC) with 10 μL (3 log10 pfu) of virus and returned to the dam. For study of virus replication, the brains from three mice were harvested on 5, 8, 11, and 14 days post-infection (dpi). Each brain was weighted, and 10% homogenates were prepared using L-15 medium supplemented with 1x SPG, 0.05mg/mL of Ciprofloxacin, 0.06 mg/mL of Clindamycin and 0.0025 mg/mL of Amphotericin B. Virus titers in each brain suspension were determined by titration in Vero cells. Harvesting of brains from mice infected with D4 was not performed at 14 dpi due to earlier death of the animals. To study the effect of miRNA targeting on virus neurovirulence, 3-day-old Swiss Webster mice (Taconic Farms) were inoculated IC with 3 log10 pfu of parental D4 or its miRNA-targeted derivative and monitored for morbidity and mortality for 21 dpi. Mice that developed neurological signs (paralysis) were humanely euthanized. Differences in replication kinetics were compared using 2-way ANOVA and P-values were adjusted using Bonferroni correction method to account for multiple comparisons, and differences in survival were compared using Log-rank (Mantel-Cox) test implemented in Prism 6 software (La Jolla, CA).
Northern blot analysis of miRNA expression
The biotinylated probes complementary to mir-184 (5’Biotin-GCCCTTATCAGTTCTCCGTCCA-Biotin3’), mir-275 (5’Biotin-GCGCTACTTCAGGTACCTGA-Biotin3’), and mir-1 (5’Biotin-CTCCATACTTCTTTACATTCCA-Biotin3’) were synthesized by Bioresearch Technologies and used at 2–10 ng/mL. Ribo-oligonucleotides for artificial mir-184 (5’UGGACGGAGAACUGAUAAGGGC), mir-275 (5’UCAGGUACCUGAAGUAGCGC), and mir-1 (5’UGGAAUGUAAAGAAGUAUGGAG3’) were synthesized by Integrated DNA Technologies, and were used in northern blot as positive controls and molecular weight standards.
Total RNA was isolated with TRIzol SL reagent (Invitrogen) from 75-cm2 flasks containing confluent monolayers of Aag2, C6/36, C710, or Vero cells or from individual brains of 5-day old Swiss mice or from pools of 20 adult A. aegypti mosquitoes collected at 7 days post-emergence. miRNA detection was carried out by northern blot as described previously with minor modifications [70]. A 15 μg sample of total RNAs was mixed with 4 μL of 10X RNA Loading Solution (Quality Biologicals) and then mixed with an equal volume of formamide (Sigma-Aldrich). The RNA was denatured at 90°C for 5 min followed by rapid cooling on ice. A total of 14 μg of denatured RNA was separated at 150 V for 1 h in a 15% polyacrylamide tris-borate-EDTA gel supplemented with 7M urea (BioRad). The gels were washed and stained with 100 mL of 20 mM MOPS-NaOH and 0.5 μg/mL ethidium bromide buffer (pH7.0) for 15 min. miRNAs was electroblotted to Amersham Hybond-NX membrane (GE Healthcare) at 20 V for 2 h in 10mM MOPS-NaOH buffer (pH7.0), followed by cross-linking to the membrane using 12 mL of 0.13 M 1-methylimidazole (Sigma), 0.16 M 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (Sigma) solution (pH 8.0) at 60°C for 1h. Membranes were prehybridized in 25 mL of ULTRAhyb ultrasensitive hybridization buffer (Ambion) at 37°C for 1 h, followed by hybridization with biotinylated probes at 39°C overnight in ULTRAhyb Ultrasensitive Hybridization Buffer. Membranes were then washed twice with 1x Low Stringency Washing Solution #1 (Ambion) and miRNAs were detected using Chemiluminescent Nucleic Acid Detection Module kit (Thermo scientific) according to the manufacturer's instructions.
Supporting Information
Zdroje
1. Gubler DJ (1998) Resurgent vector-borne diseases as a global health problem. Emerg Infect Dis 4 : 442–450. 9716967
2. Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, et al. (2010) Dengue: a continuing global threat. Nat Rev Microbiol 8: S7–16. doi: 10.1038/nrmicro2460 21079655
3. Gubler DJ (2006) Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 277 : 3–16; discussion 16–22, 71–13, 251–253. 17319151
4. Ishikawa T, Yamanaka A, Konishi E (2014) A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine 32 : 1326–1337. doi: 10.1016/j.vaccine.2014.01.040 24486372
5. Schmitz J, Roehrig J, Barrett A, Hombach J (2011) Next generation dengue vaccines: a review of candidates in preclinical development. Vaccine 29 : 7276–7284. doi: 10.1016/j.vaccine.2011.07.017 21781998
6. Monath TP (2005) Yellow fever vaccine. Expert Rev Vaccines 4 : 553–574. 16117712
7. Seligman SJ, Gould EA (2004) Live flavivirus vaccines: reasons for caution. Lancet 363 : 2073–2075. 15207960
8. Hanley KA, Goddard LB, Gilmore LE, Scott TW, Speicher J, et al. (2005) Infectivity of West Nile/dengue chimeric viruses for West Nile and dengue mosquito vectors. Vector Borne Zoonotic Dis 5 : 1–10. 15815144
9. Blaney JE Jr., Sathe NS, Hanson CT, Firestone CY, Murphy BR, et al. (2007) Vaccine candidates for dengue virus type 1 (DEN1) generated by replacement of the structural genes of rDEN4 and rDEN4Delta30 with those of DEN1. Virol J 4 : 23. 17328799
10. Whitehead SS, Hanley KA, Blaney JE Jr., Gilmore LE, Elkins WR, et al. (2003) Substitution of the structural genes of dengue virus type 4 with those of type 2 results in chimeric vaccine candidates which are attenuated for mosquitoes, mice, and rhesus monkeys. Vaccine 21 : 4307–4316. 14505913
11. Blaney JE Jr., Hanson CT, Hanley KA, Murphy BR, Whitehead SS (2004) Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect Dis 4 : 39. 15461822
12. Engel AR, Mitzel DN, Hanson CT, Wolfinbarger JB, Bloom ME, et al. (2011) Chimeric tick-borne encephalitis/dengue virus is attenuated in Ixodes scapularis ticks and Aedes aegypti mosquitoes. Vector Borne Zoonotic Dis 11 : 665–674. doi: 10.1089/vbz.2010.0179 21142950
13. Reid M, Mackenzie D, Baron A, Lehmann N, Lowry K, et al. (2006) Experimental infection of Culex annulirostris, Culex gelidus, and Aedes vigilax with a yellow fever/Japanese encephalitis virus vaccine chimera (ChimeriVax-JE). Am J Trop Med Hyg 75 : 659–663. 17038690
14. Bhatt TR, Crabtree MB, Guirakhoo F, Monath TP, Miller BR (2000) Growth characteristics of the chimeric Japanese encephalitis virus vaccine candidate, ChimeriVax-JE (YF/JE SA14—14—2), in Culex tritaeniorhynchus, Aedes albopictus, and Aedes aegypti mosquitoes. Am J Trop Med Hyg 62 : 480–484. 11220763
15. Johnson BW, Chambers TV, Crabtree MB, Arroyo J, Monath TP, et al. (2003) Growth characteristics of the veterinary vaccine candidate ChimeriVax-West Nile (WN) virus in Aedes and Culex mosquitoes. Med Vet Entomol 17 : 235–243. 12941006
16. Johnson BW, Chambers TV, Crabtree MB, Bhatt TR, Guirakhoo F, et al. (2002) Growth characteristics of ChimeriVax-DEN2 vaccine virus in Aedes aegypti and Aedes albopictus mosquitoes. Am J Trop Med Hyg 67 : 260–265. 12408664
17. Johnson BW, Chambers TV, Crabtree MB, Guirakhoo F, Monath TP, et al. (2004) Analysis of the replication kinetics of the ChimeriVax-DEN 1, 2, 3, 4 tetravalent virus mixture in Aedes aegypti by real-time reverse transcriptase-polymerase chain reaction. Am J Trop Med Hyg 70 : 89–97. 14971704
18. Higgs S, Vanlandingham DL, Klingler KA, McElroy KL, McGee CE, et al. (2006) Growth characteristics of ChimeriVax-Den vaccine viruses in Aedes aegypti and Aedes albopictus from Thailand. Am J Trop Med Hyg 75 : 986–993. 17124001
19. McGee CE, Tsetsarkin K, Vanlandingham DL, McElroy KL, Lang J, et al. (2008) Substitution of wild-type yellow fever Asibi sequences for 17D vaccine sequences in ChimeriVax-dengue 4 does not enhance infection of Aedes aegypti mosquitoes. J Infect Dis 197 : 686–692. doi: 10.1086/527328 18266608
20. Pedersen CE Jr., Robinson DM, Cole FE Jr. (1972) Isolation of the vaccine strain of Venezuelan equine encephalomyelitis virus from mosquitoes in Louisiana. Am J Epidemiol 95 : 490–496. 4401801
21. Simmonds P, Becher P, Collett MS, Gould EA, Heinz FX, et al. (2011) Flaviviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ, editors. Virus Taxonomy: IXth Report of the International Committee on Taxonomy of Viruses. Oxford: Elsevier. pp. 1003–1020.
22. Kelly EJ, Russell SJ (2009) MicroRNAs and the regulation of vector tropism. Mol Ther 17 : 409–416. doi: 10.1038/mt.2008.288 19107117
23. tenOever BR (2013) RNA viruses and the host microRNA machinery. Nat Rev Microbiol 11 : 169–180. doi: 10.1038/nrmicro2971 23411862
24. Barnes D, Kunitomi M, Vignuzzi M, Saksela K, Andino R (2008) Harnessing endogenous miRNAs to control virus tissue tropism as a strategy for developing attenuated virus vaccines. Cell Host Microbe 4 : 239–248. doi: 10.1016/j.chom.2008.08.003 18779050
25. Kelly EJ, Hadac EM, Greiner S, Russell SJ (2008) Engineering microRNA responsiveness to decrease virus pathogenicity. Nat Med 14 : 1278–1283. doi: 10.1038/nm.1776 18953352
26. Cawood R, Chen HH, Carroll F, Bazan-Peregrino M, van Rooijen N, et al. (2009) Use of tissue-specific microRNA to control pathology of wild-type adenovirus without attenuation of its ability to kill cancer cells. PLoS Pathog 5: e1000440. doi: 10.1371/journal.ppat.1000440 19461878
27. Edge RE, Falls TJ, Brown CW, Lichty BD, Atkins H, et al. (2008) A let-7 MicroRNA-sensitive vesicular stomatitis virus demonstrates tumor-specific replication. Mol Ther 16 : 1437–1443. doi: 10.1038/mt.2008.130 18560417
28. Kelly EJ, Hadac EM, Cullen BR, Russell SJ (2010) MicroRNA antagonism of the picornaviral life cycle: alternative mechanisms of interference. PLoS Pathog 6: e1000820. doi: 10.1371/journal.ppat.1000820 20333250
29. Langlois RA, Albrecht RA, Kimble B, Sutton T, Shapiro JS, et al. (2013) MicroRNA-based strategy to mitigate the risk of gain-of-function influenza studies. Nat Biotechnol 31 : 844–847. doi: 10.1038/nbt.2666 23934176
30. Perez JT, Pham AM, Lorini MH, Chua MA, Steel J, et al. (2009) MicroRNA-mediated species-specific attenuation of influenza A virus. Nat Biotechnol 27 : 572–576. doi: 10.1038/nbt.1542 19483680
31. Ylosmaki E, Hakkarainen T, Hemminki A, Visakorpi T, Andino R, et al. (2008) Generation of a conditionally replicating adenovirus based on targeted destruction of E1A mRNA by a cell type-specific MicroRNA. J Virol 82 : 11009–11015. doi: 10.1128/JVI.01608-08 18799589
32. Ylosmaki E, Lavilla-Alonso S, Jaamaa S, Vaha-Koskela M, af Hallstrom T, et al. (2013) MicroRNA-mediated suppression of oncolytic adenovirus replication in human liver. PLoS One 8: e54506. doi: 10.1371/journal.pone.0054506 23349911
33. Ylosmaki E, Martikainen M, Hinkkanen A, Saksela K (2013) Attenuation of Semliki Forest virus neurovirulence by microRNA-mediated detargeting. J Virol 87 : 335–344. doi: 10.1128/JVI.01940-12 23077310
34. Teterina NL, Liu G, Maximova OA, Pletnev AG (2014) Silencing of neurotropic flavivirus replication in the central nervous system by combining multiple microRNA target insertions in two distinct viral genome regions. Virology 456–457 : 247–258.
35. Heiss BL, Maximova OA, Thach DC, Speicher JM, Pletnev AG (2012) MicroRNA targeting of neurotropic flavivirus: effective control of virus escape and reversion to neurovirulent phenotype. J Virol 86 : 5647–5659. doi: 10.1128/JVI.07125-11 22419812
36. Heiss BL, Maximova OA, Pletnev AG (2011) Insertion of microRNA targets into the flavivirus genome alters its highly neurovirulent phenotype. J Virol 85 : 1464–1472. doi: 10.1128/JVI.02091-10 21123372
37. Skalsky RL, Vanlandingham DL, Scholle F, Higgs S, Cullen BR (2010) Identification of microRNAs expressed in two mosquito vectors, Aedes albopictus and Culex quinquefasciatus. BMC Genomics 11 : 119. doi: 10.1186/1471-2164-11-119 20167119
38. Gu J, Hu W, Wu J, Zheng P, Chen M, et al. (2013) miRNA genes of an invasive vector mosquito, Aedes albopictus. PLoS One 8: e67638. doi: 10.1371/journal.pone.0067638 23840875
39. Bryant B, Macdonald W, Raikhel AS (2010) microRNA miR-275 is indispensable for blood digestion and egg development in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 107 : 22391–22398. doi: 10.1073/pnas.1016230107 21115818
40. Durbin AP, Karron RA, Sun W, Vaughn DW, Reynolds MJ, et al. (2001) Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3'-untranslated region. Am J Trop Med Hyg 65 : 405–413. 11716091
41. Blaney JE Jr., Manipon GG, Firestone CY, Johnson DH, Hanson CT, et al. (2003) Mutations which enhance the replication of dengue virus type 4 and an antigenic chimeric dengue virus type 2/4 vaccine candidate in Vero cells. Vaccine 21 : 4317–4327. 14505914
42. Engel AR, Rumyantsev AA, Maximova OA, Speicher JM, Heiss B, et al. (2010) The neurovirulence and neuroinvasiveness of chimeric tick-borne encephalitis/dengue virus can be attenuated by introducing defined mutations into the envelope and NS5 protein genes and the 3' non-coding region of the genome. Virology 405 : 243–252. doi: 10.1016/j.virol.2010.06.014 20594569
43. Rumyantsev AA, Chanock RM, Murphy BR, Pletnev AG (2006) Comparison of live and inactivated tick-borne encephalitis virus vaccines for safety, immunogenicity and efficacy in rhesus monkeys. Vaccine 24 : 133–143. 16115704
44. Blaney JE Jr., Speicher J, Hanson CT, Sathe NS, Whitehead SS, et al. (2008) Evaluation of St. Louis encephalitis virus/dengue virus type 4 antigenic chimeric viruses in mice and rhesus monkeys. Vaccine 26 : 4150–4159. doi: 10.1016/j.vaccine.2008.05.075 18586359
45. Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136 : 215–233. doi: 10.1016/j.cell.2009.01.002 19167326
46. Filipowicz W, Bhattacharyya SN, Sonenberg N (2008) Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 9 : 102–114. doi: 10.1038/nrg2290 18197166
47. Bonaldo MC, Mello SM, Trindade GF, Rangel AA, Duarte AS, et al. (2007) Construction and characterization of recombinant flaviviruses bearing insertions between E and NS1 genes. Virol J 4 : 115. 17971212
48. Pletnev AG, Putnak R, Speicher J, Wagar EJ, Vaughn DW (2002) West Nile virus/dengue type 4 virus chimeras that are reduced in neurovirulence and peripheral virulence without loss of immunogenicity or protective efficacy. Proc Natl Acad Sci U S A 99 : 3036–3041. 11880643
49. McElroy KL, Tsetsarkin KA, Vanlandingham DL, Higgs S (2006) Manipulation of the yellow fever virus non-structural genes 2A and 4B and the 3'non-coding region to evaluate genetic determinants of viral dissemination from the Aedes aegypti midgut. Am J Trop Med Hyg 75 : 1158–1164. 17172386
50. McElroy KL, Tsetsarkin KA, Vanlandingham DL, Higgs S (2006) Role of the yellow fever virus structural protein genes in viral dissemination from the Aedes aegypti mosquito midgut. J Gen Virol 87 : 2993–3001. 16963758
51. Jennings AD, Gibson CA, Miller BR, Mathews JH, Mitchell CJ, et al. (1994) Analysis of a yellow fever virus isolated from a fatal case of vaccine-associated human encephalitis. J Infect Dis 169 : 512–518. 7908925
52. L. W (1939) Failure of Aedes aegypti to transmit yellow fever cultured virus (17D). Am J Trop Med Hyg 19 : 19–26.
53. Troyer JM, Hanley KA, Whitehead SS, Strickman D, Karron RA, et al. (2001) A live attenuated recombinant dengue-4 virus vaccine candidate with restricted capacity for dissemination in mosquitoes and lack of transmission from vaccinees to mosquitoes. Am J Trop Med Hyg 65 : 414–419. 11716092
54. Pham AM, Langlois RA, TenOever BR (2012) Replication in cells of hematopoietic origin is necessary for Dengue virus dissemination. PLoS Pathog 8: e1002465. doi: 10.1371/journal.ppat.1002465 22241991
55. Pasquinelli AE (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13 : 271–282. doi: 10.1038/nrg3162 22411466
56. Saetrom P, Heale BS, Snove O Jr., Aagaard L, Alluin J, et al. (2007) Distance constraints between microRNA target sites dictate efficacy and cooperativity. Nucleic Acids Res 35 : 2333–2342. 17389647
57. Lee YS, Nakahara K, Pham JW, Kim K, He Z, et al. (2004) Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117 : 69–81. 15066283
58. Brackney DE, Scott JC, Sagawa F, Woodward JE, Miller NA, et al. (2010) C6/36 Aedes albopictus cells have a dysfunctional antiviral RNA interference response. PLoS Negl Trop Dis 4: e856. doi: 10.1371/journal.pntd.0000856 21049065
59. Scott JC, Brackney DE, Campbell CL, Bondu-Hawkins V, Hjelle B, et al. (2010) Comparison of dengue virus type 2-specific small RNAs from RNA interference-competent and-incompetent mosquito cells. PLoS Negl Trop Dis 4: e848. doi: 10.1371/journal.pntd.0000848 21049014
60. Gu S, Jin L, Zhang F, Sarnow P, Kay MA (2009) Biological basis for restriction of microRNA targets to the 3' untranslated region in mammalian mRNAs. Nat Struct Mol Biol 16 : 144–150. doi: 10.1038/nsmb.1552 19182800
61. Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, et al. (2007) MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27 : 91–105. 17612493
62. Ameres SL, Martinez J, Schroeder R (2007) Molecular basis for target RNA recognition and cleavage by human RISC. Cell 130 : 101–112. 17632058
63. Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, et al. (2012) Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336 : 1534–1541. doi: 10.1126/science.1213362 22723413
64. Imai M, Watanabe T, Hatta M, Das SC, Ozawa M, et al. (2012) Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486 : 420–428. doi: 10.1038/nature10831 22722205
65. Linster M, van Boheemen S, de Graaf M, Schrauwen EJ, Lexmond P, et al. (2014) Identification, characterization, and natural selection of mutations driving airborne transmission of A/H5N1 virus. Cell 157 : 329–339. doi: 10.1016/j.cell.2014.02.040 24725402
66. Bredenbeek PJ, Kooi EA, Lindenbach B, Huijkman N, Rice CM, et al. (2003) A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J Gen Virol 84 : 1261–1268. 12692292
67. Sambrook J, Fritsch E, Maniatis T (1989) Molecular Cloning: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
68. Vanlandingham DL, McGee CE, Klinger KA, Vessey N, Fredregillo C, et al. (2007) Relative susceptibilties of South Texas mosquitoes to infection with West Nile virus. Am J Trop Med Hyg 77 : 925–928. 17984355
69. Pilitt DR, Jones JC (1972) A qualitative method for estimating the degree of engorgement of Aedes aegypti adults. J Med Entomol 9 : 334–337. 5054499
70. Pall GS, Hamilton AJ (2008) Improved northern blot method for enhanced detection of small RNA. Nat Protoc 3 : 1077–1084. doi: 10.1038/nprot.2008.67 18536652
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human SkinČlánek Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?Článek The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious DiseasesČlánek Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting PolypeptideČlánek A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Pathogens as Biological Weapons of Invasive Species
- Selection and Spread of Artemisinin-Resistant Alleles in Thailand Prior to the Global Artemisinin Resistance Containment Campaign
- Endopeptidase-Mediated Beta Lactam Tolerance
- Prospective Large-Scale Field Study Generates Predictive Model Identifying Major Contributors to Colony Losses
- Relay of Herpes Simplex Virus between Langerhans Cells and Dermal Dendritic Cells in Human Skin
- Structural Determinants of Phenotypic Diversity and Replication Rate of Human Prions
- Sigma Factor SigB Is Crucial to Mediate Adaptation during Chronic Infections
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Heterologous Expression in Remodeled . : A Platform for Monoaminergic Agonist Identification and Anthelmintic Screening
- Novel Disease Susceptibility Factors for Fungal Necrotrophic Pathogens in Arabidopsis
- Interleukin 21 Signaling in B Cells Is Required for Efficient Establishment of Murine Gammaherpesvirus Latency
- Phosphorylation at the Homotypic Interface Regulates Nucleoprotein Oligomerization and Assembly of the Influenza Virus Replication Machinery
- Human Papillomaviruses Activate and Recruit SMC1 Cohesin Proteins for the Differentiation-Dependent Life Cycle through Association with CTCF Insulators
- Ubiquitous Promoter-Localization of Essential Virulence Regulators in
- TGF-β Suppression of HBV RNA through AID-Dependent Recruitment of an RNA Exosome Complex
- The Immune Adaptor ADAP Regulates Reciprocal TGF-β1-Integrin Crosstalk to Protect from Influenza Virus Infection
- Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable (NTHi) from Infected Lung
- The Epigenetic Regulator G9a Mediates Tolerance to RNA Virus Infection in
- Does the Arthropod Microbiota Impact the Establishment of Vector-Borne Diseases in Mammalian Hosts?
- Hantaan Virus Infection Induces Both Th1 and ThGranzyme B+ Cell Immune Responses That Associated with Viral Control and Clinical Outcome in Humans
- Viral Inhibition of the Transporter Associated with Antigen Processing (TAP): A Striking Example of Functional Convergent Evolution
- Plasma Membrane Profiling Defines an Expanded Class of Cell Surface Proteins Selectively Targeted for Degradation by HCMV US2 in Cooperation with UL141
- Optineurin Regulates the Interferon Response in a Cell Cycle-Dependent Manner
- IFIT1 Differentially Interferes with Translation and Replication of Alphavirus Genomes and Promotes Induction of Type I Interferon
- The EBNA3 Family of Epstein-Barr Virus Nuclear Proteins Associates with the USP46/USP12 Deubiquitination Complexes to Regulate Lymphoblastoid Cell Line Growth
- Hepatitis C Virus RNA Replication Depends on Specific and -Acting Activities of Viral Nonstructural Proteins
- A Neuron-Specific Antiviral Mechanism Prevents Lethal Flaviviral Infection of Mosquitoes
- The Aspartate-Less Receiver (ALR) Domains: Distribution, Structure and Function
- Global Genome and Transcriptome Analyses of Epidemic Isolate 98-06 Uncover Novel Effectors and Pathogenicity-Related Genes, Revealing Gene Gain and Lose Dynamics in Genome Evolution
- The Ebola Epidemic Crystallizes the Potential of Passive Antibody Therapy for Infectious Diseases
- Ebola Virus Entry: A Curious and Complex Series of Events
- Conserved Spirosomes Suggest a Single Type of Transformation Pilus in Competence
- Spatial Structure, Transmission Modes and the Evolution of Viral Exploitation Strategies
- Bacterial Cooperation Causes Systematic Errors in Pathogen Risk Assessment due to the Failure of the Independent Action Hypothesis
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Cerebrospinal Fluid Cytokine Profiles Predict Risk of Early Mortality and Immune Reconstitution Inflammatory Syndrome in HIV-Associated Cryptococcal Meningitis
- Utilize Host Actin for Efficient Maternal Transmission in
- Borna Disease Virus Phosphoprotein Impairs the Developmental Program Controlling Neurogenesis and Reduces Human GABAergic Neurogenesis
- An Effector Peptide Family Required for Toll-Mediated Immunity
- Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide
- A Redox Regulatory System Critical for Mycobacterial Survival in Macrophages and Biofilm Development
- Quadruple Quorum-Sensing Inputs Control Virulence and Maintain System Robustness
- Leukocyte-Derived IFN-α/β and Epithelial IFN-λ Constitute a Compartmentalized Mucosal Defense System that Restricts Enteric Virus Infections
- A Strategy for O-Glycoproteomics of Enveloped Viruses—the O-Glycoproteome of Herpes Simplex Virus Type 1
- Macrocyclic Lactones Differ in Interaction with Recombinant P-Glycoprotein 9 of the Parasitic Nematode and Ketoconazole in a Yeast Growth Assay
- Neofunctionalization of the α1,2fucosyltransferase Paralogue in Leporids Contributes to Glycan Polymorphism and Resistance to Rabbit Hemorrhagic Disease Virus
- The Extracytoplasmic Linker Peptide of the Sensor Protein SaeS Tunes the Kinase Activity Required for Staphylococcal Virulence in Response to Host Signals
- Murine CMV-Induced Hearing Loss Is Associated with Inner Ear Inflammation and Loss of Spiral Ganglia Neurons
- Dual miRNA Targeting Restricts Host Range and Attenuates Neurovirulence of Flaviviruses
- GATA-Dependent Glutaminolysis Drives Appressorium Formation in by Suppressing TOR Inhibition of cAMP/PKA Signaling
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- Genetic Analysis Using an Isogenic Mating Pair of Identifies Azole Resistance Genes and Lack of Locus’s Role in Virulence
- A Temporal Gate for Viral Enhancers to Co-opt Toll-Like-Receptor Transcriptional Activation Pathways upon Acute Infection
- Neutrophil Recruitment to Lymph Nodes Limits Local Humoral Response to
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Toxin-Induced Necroptosis Is a Major Mechanism of Lung Damage
- Transgenic Fatal Familial Insomnia Mice Indicate Prion Infectivity-Independent Mechanisms of Pathogenesis and Phenotypic Expression of Disease
- Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Infection
- EphrinA2 Receptor (EphA2) Is an Invasion and Intracellular Signaling Receptor for
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



