-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaExposure-Dependent Control of Malaria-Induced Inflammation in Children
Malaria remains a major cause of disease and death worldwide. When mosquitoes infect people with malaria parasites for the first time, the parasite rapidly multiplies in the blood and the body responds by producing molecules that cause inflammation and fever, and sometimes the infection progresses to life-threatening disease. However, in regions where people are repeatedly infected with malaria parasites, most infections do not cause fever and parasites often do not multiply uncontrollably. For example, in Mali where this study was conducted, children are infected with malaria parasites ≥100 times/year but only get malaria fever ∼2 times/year and often manage to control parasite numbers in the blood. To understand these observations we collected immune cells from the blood of healthy children before the malaria season and 7 days after malaria fever. We simulated malaria infection at these time points by exposing the immune cells to malaria parasites in a test-tube. We found that re-exposing immune cells to parasites after malaria fever results in reduced expression of molecules that cause fever and enhanced expression of molecules involved in parasite killing. These findings help explain how the immune system prevents fever and controls malaria parasite growth in children who are repeatedly infected with malaria parasites.
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1004079
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004079Summary
Malaria remains a major cause of disease and death worldwide. When mosquitoes infect people with malaria parasites for the first time, the parasite rapidly multiplies in the blood and the body responds by producing molecules that cause inflammation and fever, and sometimes the infection progresses to life-threatening disease. However, in regions where people are repeatedly infected with malaria parasites, most infections do not cause fever and parasites often do not multiply uncontrollably. For example, in Mali where this study was conducted, children are infected with malaria parasites ≥100 times/year but only get malaria fever ∼2 times/year and often manage to control parasite numbers in the blood. To understand these observations we collected immune cells from the blood of healthy children before the malaria season and 7 days after malaria fever. We simulated malaria infection at these time points by exposing the immune cells to malaria parasites in a test-tube. We found that re-exposing immune cells to parasites after malaria fever results in reduced expression of molecules that cause fever and enhanced expression of molecules involved in parasite killing. These findings help explain how the immune system prevents fever and controls malaria parasite growth in children who are repeatedly infected with malaria parasites.
Introduction
In previously unexposed individuals, blood-stage Plasmodium falciparum parasites rapidly replicate and almost invariably induce fever and other symptoms of malaria [1] through the production of pro-inflammatory cytokines and chemokines [2]–[4]. Although the initial systemic inflammatory response is crucial for setting in motion the innate and adaptive immune effector mechanisms that control blood-stage parasites [5], [6], dysregulated inflammation has been linked to severe malaria [7], [8] which only occurs in a minority of individuals with infrequent or no prior malaria exposure [9]. Conversely, in malaria endemic areas where individuals are repeatedly exposed, P. falciparum infections more commonly cause a mild febrile illness or no symptoms at all, and parasite numbers in the blood are generally kept in check, even in young children [10]–[12] who have yet to acquire a fully protective antibody repertoire [13]. The nature of the immune response that enables most children to restrain P. falciparum-induced inflammation while maintaining control of parasite replication remains elusive [6], [14].
The notion of malaria ‘tolerance’ has long been invoked to explain the common finding of low-level, asymptomatic blood-stage infection in endemic areas [15], particularly among children, as antibodies that reliably protect against febrile malaria are only acquired after many years of exposure to genetically diverse and clonally variant P. falciparum antigens [13]. Several mechanisms have been proposed to explain malaria tolerance or ‘anti-disease’ immunity [14], [16] including antibody-mediated neutralization of P. falciparum pathogen-associated molecular pattern (PAMP) molecules such as GPI anchors [14], [17], [18]; desensitization of pattern-recognition receptor (PRR)-mediated signaling as a result of repeated stimulation [16]; and the production of anti-inflammatory mediators such as IL-10 [2], [19]–[21] and TGF-β [21]–[23] that suppress inflammation-driven anti-parasite effector mechanisms once parasite replication has been controlled [14].
Interestingly, it has long been speculated that parallels exist between malarial tolerance and bacterial endotoxin tolerance (reviewed in [16]). This speculation is based in part on early studies in humans that showed that malaria induces cross-tolerance to the febrile response normally induced by bacterial endotoxin [24], [25]. The traditional view of endotoxin tolerance holds that immune cells that are exposed to endotoxin have an altered response when re-challenged with endotoxin, such that the production of pro-inflammatory cytokines and chemokines is attenuated relative to the response induced at homeostasis [26]. Whether P. falciparum infection in humans ‘tolerizes’ immune cells in an analogous manner is unknown. More specifically, there is no direct evidence that febrile malaria in humans induces regulatory responses that limit the production of pro-inflammatory cytokines and chemokines upon re-exposure to P. falciparum parasites relative to responses induced at homeostasis before malaria in the same individual. Also, it is unclear how current views of malaria-induced tolerance/regulatory responses account for the observation that most children in endemic areas are able to restrain P. falciparum-induced inflammation and simultaneously control parasite replication, since generalized suppression of P. falciparum-triggered immune responses predicts that parasite replication would proceed unhindered and cause severe disease.
A potential solution to this problem is that febrile malaria temporarily alters immune cells such that the host responds to P. falciparum re-exposure by downregulating the production of acute phase pro-inflammatory mediators that contribute to fever and other malaria symptoms while enhancing anti-parasite effector mechanisms that control parasite replication, such as phagocytosis-mediated clearance of blood-stage parasites. This hypothesis is supported by a recent study by Foster et al. who used an in vitro model of lipopolysaccharide (LPS) tolerance in murine macrophages to show that the regulation of LPS-triggered inflammation is component-specific, such that pro-inflammatory mediators are transiently silenced or ‘tolerized’ while antimicrobial effectors are primed or enhanced upon re-challenge with LPS [27]. A similar phenotype was observed in a human model of LPS tolerance [28], but whether these observations reflect events induced by natural infections in humans is unknown.
Here we tested the hypothesis that febrile malaria alters children's immune cells such that pro-inflammatory mediators are downregulated and anti-parasite effector responses are upregulated upon re-exposure to P. falciparum parasites compared to that induced at homeostasis before malaria in the same children. In addition, given the pivotal role of IL-10 in regulating Plasmodium-induced inflammation in murine models [19], [29], we sought to elucidate the identity, function and kinetics of P. falciparum-specific IL-10-producing cells. We further asked if P. falciparum-inducible regulatory responses that limit inflammation are maintained in untreated children who harbor chronic asymptomatic infections, and whether these responses can be recalled in children who have not been exposed to P. falciparum for extended periods of time. To address these questions we applied a systems biology approach [30] to a longitudinal analysis of peripheral blood mononuclear cells (PBMCs) sampled from Malian children over a 12-month period—starting at their healthy baseline before the six-month malaria season; seven days after treatment of their first febrile malaria episode of the ensuing malaria season (when malaria symptoms had resolved); and after the subsequent six-month dry season, a period of little to no P. falciparum transmission.
We found that febrile malaria induces a marked shift in the response to P. falciparum re-exposure with cells producing lower levels of pro-inflammatory cytokines and chemokines and higher levels of anti-inflammatory cytokines compared to responses induced at homeostasis before malaria. Re-exposure was also associated with enhanced expression of pathways involved in phagocytosis and activation of adaptive immunity. This shift was accompanied by a marked increase in P. falciparum-specific CD4+Foxp3− T cells that co-produce IL-10, IFN-γ and TNF. IL-10 remained partially inducible in untreated children with chronic asymptomatic infections whereas IL-10 was no longer inducible in children whose infections had been cleared by treatment.
Results
P. falciparum-inducible inflammation is downregulated and anti-parasitic effectors are upregulated after febrile malaria relative to responses induced at the healthy baseline
To obtain a global view of transcriptional changes that persist in children's PBMCs after the clinical resolution of febrile malaria, compared to each child's own healthy baseline, we profiled RNA expression of PBMCs collected from 34 healthy Malian children before the six-month malaria season, when blood smears were negative for P. falciparum parasites, and 7 days after treatment of their first febrile malaria episode of the ensuing malaria season, when malaria symptoms had resolved. The average age of these children was 8.5 years and 32% were female. At their first febrile malaria episode of the season, children had an axillary temperature of >37.5°C (or their parents reported fever within 24 hours), were infected with P. falciparum (geometric mean density 17,817 asexual parasites/µl of blood) and had no other cause of fever discernible on physical examination. The average incidence of febrile malaria during the six-month malaria season was similar for the 34 children in this study compared to children in this age group in the larger cohort (1.6 and 1.5 episodes, respectively) [31]. By definition, these malaria-susceptible children had yet to acquire P. falciparum-specific antibodies that reliably protect from febrile malaria. Individual demographic and clinical data are shown in Table S1. Malaria was effectively treated in all subjects with a standard 3-day course of artemether/lumefantrine.
PBMCs were first analyzed directly ex vivo (not re-stimulated). Principal components analysis of the microarray data showed segregation of transcription profiles based on time-point (healthy baseline vs. day 7 after malaria), but not age, gender or batch effects (Figure S1A). Within-subject gene-expression changes were computed and resulted in 1497 differentially expressed genes (DEGs)—1,351 increased and 146 decreased after the resolution of febrile malaria relative to baseline (Table S2). Ingenuity Pathway Analysis (IPA) identified “Infectious Disease”, “Immunological Disease” and “Inflammatory Disease” among the top five functional categories enriched with DEGs. Notably, all differentially expressed genes encoding cytokines and chemokines that have been associated with P. falciparum-induced fever and inflammation [2]–[4], [32], including the canonical pyrogenic cytokines IL1B and TNF as well as the pro-inflammatory chemokines IL8, CCL3 (MIP-1α) and CXCL2 (MIP-2α), were suppressed after the resolution of malaria to lower levels than observed at baseline (Figure 1A). Conversely, genes expressing molecules directly involved in microbial killing and activation of adaptive immunity were significantly upregulated after the resolution of febrile malaria relative to the healthy baseline (Figure 1A). Specifically, IPA identified the following canonical pathways as significantly upregulated: “Toll-like receptor signaling” (P = 2.15e-6), “Fcγ receptor-mediated phagocytosis in macrophages and monocytes” (P = 1.8e-4), “Production of nitric oxide and reactive species in macrophages” (P = 1.09e-7), “Antigen presentation pathway” (P = 0.0463), “T cell receptor signaling” (P = 1.19e-5) and “Interferon signaling” (P = 5.08e-6) (Figure S1B). The expression of several PRRs including Toll-like receptor (TLR)2 and TLR4 was increased after malaria relative to baseline (Figure 1A), consistent with recent in vivo exposure to P. falciparum PAMPs such as GPI anchors [33], hemozoin [34], CpG-containing DNA motifs bound to hemozoin [35] and AT-rich DNA motifs [36], but of note, NLRP3, a putative receptor for P. falciparum hemozoin-induced IL-1β production [37], was the only PRR to be downregulated after malaria relative to homeostasis (Figure 1A).
Fig. 1. A molecular pattern of restrained inflammation and enhanced anti-parasite effector function upon P. falciparum re-exposure. 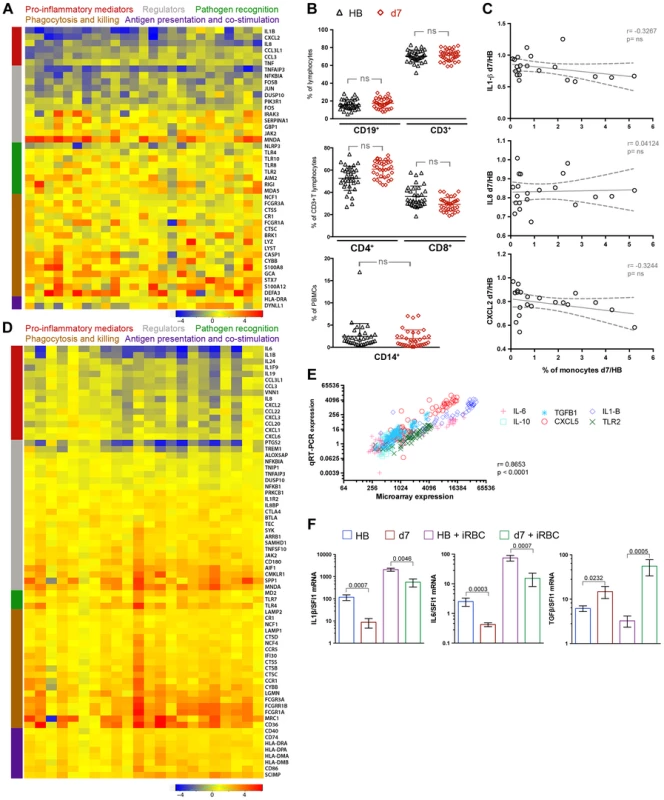
(A) PBMCs were collected from 34 healthy children with blood smears negative for P. falciparum infection before the malaria season (HB) and 7 days after treatment of their first febrile malaria episode of the ensuing malaria season when malaria symptoms had resolved (d7). RNA was extracted from PBMCs immediately after thawing and hybridized onto Affymetrix GeneChip Human 1.0 ST arrays. RNA from all 68 PBMC samples was of sufficient quantity and quality for microarray analysis. Nine of 68 samples did not pass the microarray quality assessment and were removed from further analysis (see Supplemental Figure 1A) such that 25 children with paired RNA samples at the healthy baseline and 7 days after malaria were analyzed. The heat map shows ex vivo RMA-normalized log2 ratios (d7/HB) of differentially expressed genes (rows) for each child (columns). Genes are grouped and color-coded by function as indicated. (B) PBMCs analyzed by FACS for B cells (CD19+), T cells (CD3+), CD3+CD4+ T cells, CD3+CD8+ T cells, and monocytes (CD14+) at the healthy baseline and after malaria. (n = 34 children; except CD14+ monocytes, n = 30). (C) Ratio of monocyte percentage (d7/HB) versus the ratio of the expression level of monocyte-derived pro-inflammatory cytokines and chemokines (d7/HB). Each point represents an individual subject (n = 21 children with paired samples). (D) RNA was extracted from PBMCs of the same 34 children after 18 h of in vitro stimulation with P. falciparum-infected red blood cell (iRBC) lysate. After stimulation with iRBC lysate, 22 of the 34 children had RNA samples from both time points of sufficient quantity and quality for microarray analysis and also passed the microarray quality assessment. The heat map shows RMA-normalized log2 ratios (d7/HB) of differentially expressed genes (rows) for each child (columns) in response to in vitro iRBC lysate stimulation. Genes are grouped and color-coded by function as indicated. (E) q-RT-PCR confirmation of the microarray data. The data represent the results of one experiment with 6 genes (IL1B, IL6, IL10, TGFB1, TLR2, CXCL5) from 17 subjects at two time points (d7 and HB) from both the ex vivo unstimulated and in vitro iRBC-stimulated datasets. Each symbol represents a single gene at a given time point. PCR expression computed as antilog2 –dCT. n = 497 XY pairs. (F) q-RT-PCR expression of genes encoding the pro-inflammatory cytokines IL1-β and IL-6 and the anti-inflammatory cytokine TGF-β in PBMCs of children (n = 17) collected at the healthy baseline (HB) and after resolution of febrile malaria (d7), either directly ex vivo (unstimulated) or after in vitro stimulation with iRBCs for 18 h. ns, not significant (P≥0.05), P values determined by the paired ttest (B), Pearson's (C), Spearman's (E) or paired Wilcoxon rank sum test (F). Data are shown as the means ± s.d. (B) or means ± s.e.m. (F). Because changes in mRNA levels in PBMCs can reflect an altered cell composition of the PBMC compartment or changes in gene expression in discrete cell populations, we analyzed the PBMCs used for gene-expression profiling by FACS and found no significant differences in the percentage of CD4+ or CD8+ T cells, B cells or monocytes after the resolution of febrile malaria relative to the healthy baseline (Figure 1B). Moreover, at the individual subject level we found that genes encoding myeloid-expressed pro-inflammatory mediators were downregulated after resolution of malaria relative to baseline irrespective of changes in the percentage of monocytes (Figure 1C and Figure S1C), and even as mRNA levels of other genes identified as myeloid-specific [38] were unchanged or increased after malaria relative to baseline (Figure S1D). Taken together, these data indicate that the changes in mRNA levels that persist after the resolution of febrile malaria relative to the healthy baseline reflect differential regulation of gene expression rather than gross alterations in the composition of the PBMC compartment.
These data indicate that febrile malaria induces transcriptional changes in PBMCs that persist after the resolution of febrile malaria; namely, we observed that the expression of genes encoding pro-inflammatory mediators is suppressed while the expression of molecules involved in microbial killing and activation of adaptive immunity is enhanced after malaria relative to baseline. On the basis of these findings we hypothesized that re-exposure to P. falciparum parasites soon after the resolution of febrile malaria would induce a qualitatively different immune response relative to that induced at the healthy baseline. To test this hypothesis and to investigate the molecular and cellular basis of regulatory responses induced upon P. falciparum re-exposure, we analyzed the same PBMCs from the same 34 children (collected at the healthy baseline before the malaria season and seven days after treatment of the first febrile malaria episode) following in vitro stimulation with P. falciparum-infected red-blood cell (iRBC) lysate. iRBC-inducible gene expression as well as secreted and intracellular cytokine production were examined with each child serving as his or her own healthy baseline control.
Within-subject gene expression changes induced by iRBC stimulation at both time points were computed and resulted in 456 DEGs—148 decreased and 308 increased after the resolution of febrile malaria relative to that induced at the healthy baseline (Table S3). IPA identified “Inflammatory Response” as the functional category with the highest enrichment score (P = 3.36e-34). Within this functional category, all differentially expressed pro-inflammatory cytokines and chemokines (IL1B, IL6, IL8, IL19, IL24, CCL3, CCL3L1, CCL19, CCL20, CCL22, CXCL1, CXCL2, CXCL3, and CXCL6) were downregulated in response to iRBC stimulation after the resolution of malaria relative to the iRBC-induced response at baseline (Figure 1D). In line with this result, IPA identified the following canonical pathways as significantly downregulated: “Acute phase response signaling” (P = 5.94e-4), “Role of hypercytokinemia/hyperchemokinemia in pathogenesis of Influenza (P = 1.19e-3), “IL-6 signaling” (P = 1.18e-3), “Agranulocyte adhesion and diapedesis” (P = 8.82e-6), “Granulocyte adhesion and diapedesis” (P = 6.55e-9) and “Role of cytokines in mediating communication between immune cells” (P = 0.017) (Figure S1E). Consistent with the downregulation of pro-inflammatory responses, NFKB1 and TREM-1, a positive regulator of inflammation [39], were downregulated, while several negative regulators of inflammation were upregulated including IL18BP, IL1R2, BTLA and SAMHD1 (Figure 1D).
In contrast to the downregulation of pro-inflammatory cytokine and chemokine responses, genes encoding molecules involved in microbial killing and activation of adaptive immunity were upregulated by iRBC stimulation after malaria relative to responses induced by iRBC stimulation at baseline. These included molecules involved in opsonic and non-opsonic phagocytosis, phagolysosome maturation, antigen presentation and co-stimulation (Figure 1D). IPA identified the following canonical pathways as significantly upregulated: “iNOS signaling” (P = 0.0012), “Antigen presentation pathway” (P = 6.87e-5), “T helper cell differentiation” (P = 1.88e-3) and “T cell receptor signaling” (P = 0.035) (Figure S1E). Together these data suggest that re-exposure to P. falciparum parasites after a recent episode of febrile malaria induces the differential expression of functionally distinct components of the immune response, whereby acute phase pro-inflammatory cytokines and chemokines that drive the initial systemic inflammatory response are restrained, while pathways involved in microbial killing and activation of adaptive immunity are upregulated.
To validate the expression of selected immune-related genes, we used quantitative real time (qRT)-PCR to analyze PBMCs from the 17 children who had microarray data from the ex vivo and iRBC-stimulated experiments at both time points (before and after malaria). We found a positive correlation (r = 0.8653; P<0.0001) for gene expression as detected by microarray and qRT-PCR (Figure 1E and Table S4). The qRT-PCR data confirmed decreased expression of the canonical fever-inducing cytokines IL1B and IL6 and increased expression of the anti-inflammatory cytokine TGFB after resolution of febrile malaria relative to the healthy pre-malaria baseline, in both the unstimulated and iRBC-stimulated experiments (Figure 1F). Therefore, the qRT-PCR data confirmed a molecular pattern of restrained P. falciparum-inducible inflammation in children who had recently recovered from febrile malaria.
P. falciparum-inducible IL-10 is upregulated after the resolution of febrile malaria and partially maintained in children with persistent asymptomatic infection
IL-10 plays a critical role in controlling and resolving inflammation by limiting the production of pro-inflammatory cytokines and chemokines [40], yet we did not observe differential expression of IL10 in the microarray data. Given the known temporal dissociation between IL-10 transcription and translation [41], we assayed supernatants of iRBC-stimulated PBMCs for secreted IL-10 using a multiplex assay that also measured IL-1β, IL-6, IL-8 and TNF. We observed that P. falciparum-inducible IL-10 production was higher after the resolution of febrile malaria relative to the healthy baseline of the same children before the malaria season (P<0.0001; Figure 2A), while P. falciparum-inducible production of the pro-inflammatory chemokine IL-8 was lower after the resolution of febrile malaria relative to baseline (P<0.0001; Figure 2A), in agreement with the microarray data. P. falciparum-inducible production of IL-1β and IL-6 trended toward lower levels after the resolution of febrile malaria relative to baseline, but the decrease was not statistically significant (Figure 2A). Because IL-1β and IL-6 are primarily produced by monocytes/macrophages, we isolated monocytes/macrophages (Figure S2A) from 9 additional children who had PBMCs available at their healthy baseline before the malaria season and 14 days after their first febrile malaria episode of the ensuing malaria season (Table S1). We stimulated these monocytes/macrophages with iRBCs for 6 hours and measured IL-1β and IL-6 in the supernatants. We found that P. falciparum-inducible production of IL-1β and IL-6 by monocytes/macrophages was lower after the resolution of febrile malaria relative to that induced at baseline (P = 0.0066 and P = 0.0003 for IL-1β and IL-6 respectively; Figure 2B), consistent with a reduced risk of fever in children who are exposed to ongoing P. falciparum transmission during the malaria season.
Fig. 2. P. falciparum-inducible IL-10 production is upregulated upon re-exposure and partially maintained by persistent asymptomatic infection. 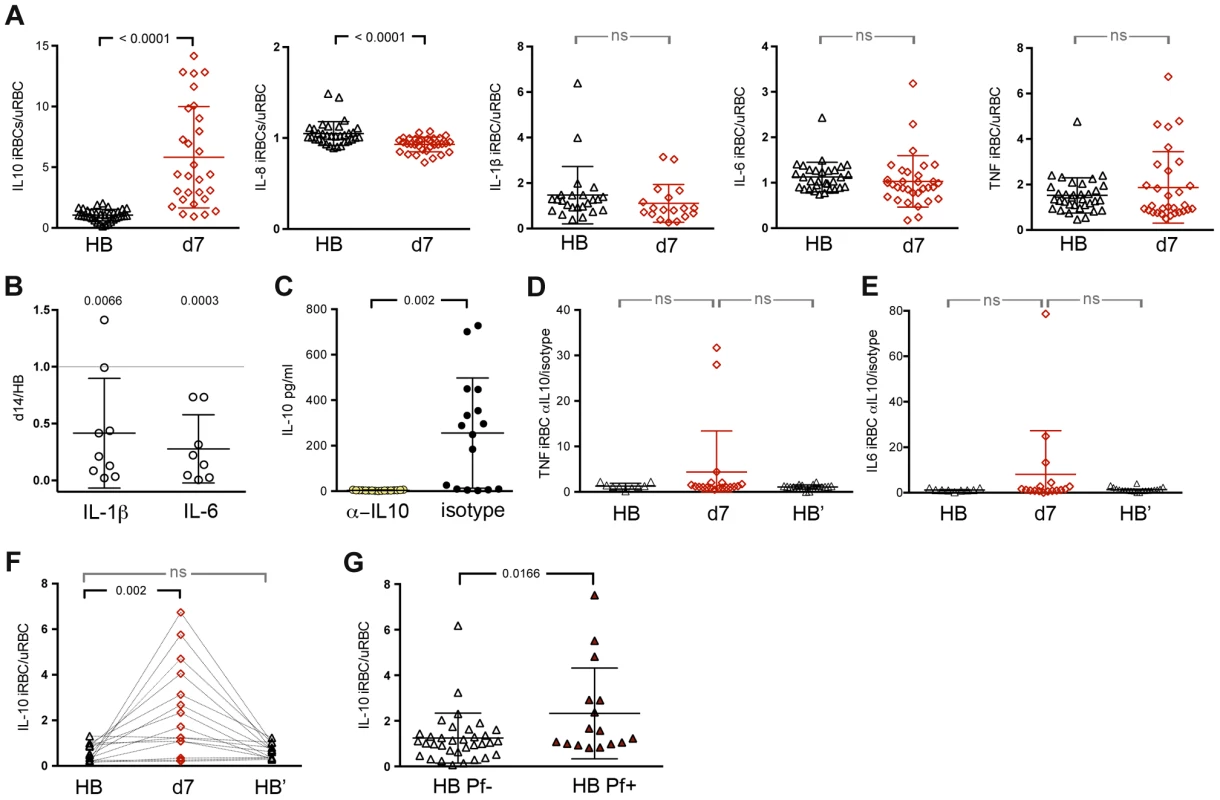
(A) Production of IL-10, IL-8, IL1-β, IL-6 and TNF by PBMCs in response to in vitro stimulation with iRBC lysate at the healthy baseline before the malaria season (HB) and 7 days after malaria (d7) (n = 28 children with paired samples). (B) Production of IL-1β and IL-6 by isolated monocytes/macrophages after 6 h of in vitro stimulation with iRBC lysate. Results are shown as the ratio of cytokines produced 14 days after malaria (d14) versus the healthy baseline before the malaria season (HB) (n = 9 children with paired samples) (P = 0.0066 and P = 0.0003 for IL-1β and IL-6 respectively). (C) A positive control showing IL-10 production by PBMCs in response to in vitro stimulation with iRBC lysate in the presence of blocking antibodies specific for IL-10 and the IL-10 receptor or the isotype control (n = 17). (D,E) TNF and IL-6 production by PBMCs in response to in vitro stimulation with iRBC lysate in the presence of blocking antibodies specific for IL-10 and the IL-10 receptor at the healthy baseline (HB), 7 days after malaria (d7) and at the healthy baseline after the subsequent 6-month dry season (HB′) (n = 20 children, 9 paired in the 3 conditions). (F) IL-10 production by PBMCs in response to in vitro stimulation with iRBC lysate at the healthy baseline before the malaria season (HB), 7 days after malaria (d7) and at the healthy baseline after the subsequent 6-month dry season (HB′), a period of little to no P. falciparum transmission (n = 15 children). (G) IL-10 production by PBMCs in response to in vitro stimulation with iRBC lysate among children with asymptomatic P. falciparum infection at the end of the dry season (HB Pf+, n = 16) versus aged-matched, healthy uninfected children at the same time point (HB Pf−, n = 34). Data are presented as fold change relative to PBMCs stimulated with uninfected RBC (uRBC) lysate (A, F, G). ns, not significant (P≥0.05), P values were determined by a paired ttest (A, C), one-sample Student's T-tests comparing the mean ratio against a 1∶1 ratio (B), paired ttest followed by Bonferroni's test (D–F) or unpaired ttest (G). Data are shown as the means ± s.d. In an independent experiment, we sought to determine if the upregulation of P. falciparum-inducible IL-10 production after malaria influences the production of pro-inflammatory cytokines. We observed that blocking IL-10 activity with antibodies specific for IL-10 and the IL-10 receptor (Figure 2C) enhanced iRBC-inducible TNF and IL-6 production in some but not all children after the resolution of febrile malaria compared to baseline (Figures 2D and E).
We next asked if P. falciparum-inducible IL-10 responses could be recalled in children who had not been exposed to P. falciparum transmission for an extended period of time. We performed iRBC stimulation of PBMCs collected from 18 additional children (Table S1) at their healthy baseline before the malaria season, 7 days after treatment of their first malaria episode of the ensuing 6-month malaria season, and after the following 6-month dry season, a period of little to no P. falciparum transmission. This independent experiment confirmed that P. falciparum-inducible IL-10 is upregulated after the resolution of febrile malaria relative to baseline (P = 0.0082; Figure 2F). However, in the absence of ongoing malaria exposure, children reverted to an apparent homeostatic baseline in which IL-10 production was no longer inducible (Figure 2F), suggesting that ongoing malaria exposure is required to maintain P. falciparum-inducible IL-10 production capacity. To test this hypothesis we identified 16 untreated children (Table S1) whose asymptomatic P. falciparum infections persisted through the six-month dry season, and compared their P. falciparum-inducible IL-10 response to age-matched children who were uninfected at the same time point at the end of the dry season. We observed that P. falciparum-inducible IL-10 responses of persistently infected asymptomatic children were higher than responses of age-matched uninfected children (P = 0.0166; Figure 2G), suggesting that P. falciparum-inducible IL-10 upregulation is partially maintained by ongoing P. falciparum exposure and that IL-10 upregulation may contribute to protection from febrile malaria in the context of ongoing P. falciparum exposure.
P. falciparum-inducible IL-10 is produced by CD4+CD25+Foxp3− T cells that co-produce IFN-γ and TNF
Despite evidence that IL-10 plays a critical role in regulating Plasmodium-induced inflammation in murine models, the cellular sources of IL-10 and the functionality and kinetics of IL-10-producing cells in the context of human malaria remain unclear [42]. To identify the predominant cellular source of P. falciparum-inducible IL-10 and to investigate longitudinally the functionality and kinetics of IL-10-producing cells in children exposed to intense seasonal malaria, we analyzed PBMCs by FACS with intracellular staining for IL-10, IFN-γ and TNF after in vitro iRBC stimulation at the healthy baseline before the malaria season, 7 days after malaria treatment (when symptoms had resolved), and after the 6-month dry season. Consistent with the kinetics of P. falciparum-inducible secreted IL-10 production described above (Figure 2F), we found that P. falciparum-inducible IL-10 production by CD4+ T cells increased significantly after the resolution of febrile malaria relative to the healthy baseline (P = 0.0095; Figure 3A), and then reverted to a state in which IL-10 production was no longer inducible by the end of the following six-month dry season (Figure 3A). Interestingly, the majority of P. falciparum-inducible IL-10-producing PBMCs following febrile malaria were CD3+CD4+ T cells (Figure 3B; mean 53.1%, 95%CI: 44.8–61.5) and most of these were CD25+FOXP3− (Figure 3B; mean 78.8%, 95%CI: 72.2–85.5), while FOXP3+CD4+ T cells (regulatory T cells) represented only a small percentage of IL-10-producing T cells. P. falciparum-inducible IFN-γ - and TNF-producing CD4+ T cells also increased after the resolution of malaria compared to baseline, and like IL-10, reverted to homeostasis after the dry season (Figure 3A). At the single-cell level the majority of IL-10-producing P. falciparum-inducible CD4+ T cells also produced IFN-γ, or IFN-γ plus TNF (Figure 3C–E), thus identifying these cells as ‘self-regulating’ Th1 effector cells [43]. From these results and the microarray data emerges a consistent theme whereby re-exposure to P. falciparum parasites after recent febrile malaria induces exposure-dependent regulatory mechanisms that limit the production of pro-inflammatory mediators that drive systemic inflammation while enhancing effector mechanisms that control parasite replication.
Fig. 3. P. falciparum-inducible IL-10 is mainly produced by CD4+CD25+Foxp3− T cells that co-produce IFNγ and TNF. 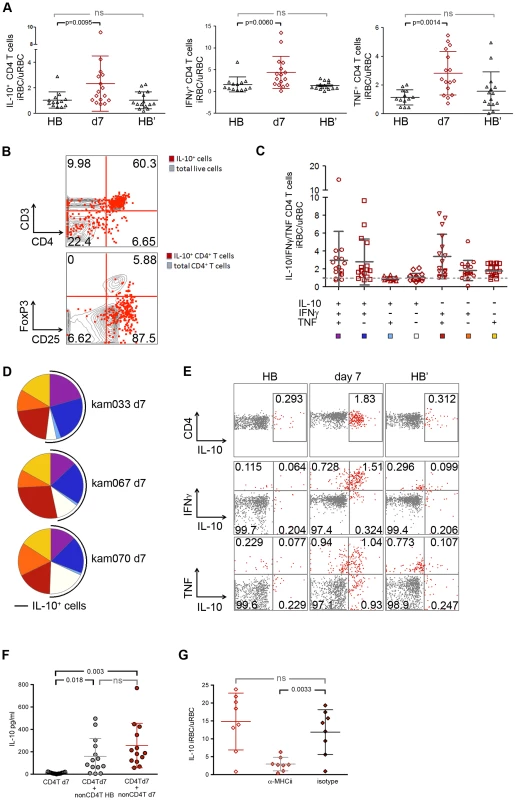
(A) PBMCs from the healthy baseline (HB), 7 days after malaria (d7), and at the healthy baseline at the end of the subsequent dry-season (HB′) were stimulated for 18 h with iRBC lysate and assayed for the production of IL-10, IFNγ and TNF by intra-cellular FACS. Results are shown as the ratio of live CD3+ CD4+ antigen-experienced cells (CD45RO+ CD27+, CD45RO+ CD27−, and CD45RO− CD27−) producing IL-10, IFN-γ or TNF in response to stimulation with iRBC lysate vs. uninfected RBC (uRBC) lysate (n = 16, 13 paired samples). (B) Overlay of IL-10-producing cells (red) among all live cells (gray) in a CD3 vs. CD4 dot plot (top) (n = 14), and IL-10-producing CD4+ T cells (red) with all CD4+ T cells (gray) in CD25 vs. FoxP3 dot plot (bottom) (n = 9; representative subject shown). (C) Using SPICE analysis, cytokine-producing CD4+ T cells were divided into 7 distinct subpopulations producing any combination of IL-10, IFNγ and TNF (n = 16). (D) Pie chart representation of the combination of cytokines produced by CD4+ T cells after iRBC stimulation for 3 representative donors 7 days after malaria (d7). The black arcs indicate the IL-10-producing CD4+ T cells. (E) Representative FACS plots of live CD3+ CD4+ antigen-experienced cells producing IL-10, IFNγ and TNF after iRBC stimulation of PBMCs collected at the healthy baseline (HB), 7 days after malaria (d7) and at the healthy baseline at the end of the subsequent dry-season (HB′). (F) CD4+ T cells were isolated from PBMCs which had been collected from children 7 days after malaria and were then stimulated for 18 h with iRBC or uRBC lysate in the absence (CD4+T d7) or presence of non-CD4+T cells isolated from PBMCs of the same individuals collected at either the healthy baseline (CD4+T d7 + nonCD4+T HB) or 7 days after malaria (CD4+T d7 + nonCD4+T d7) (n = 8 paired samples). (G) PBMCs collected from children 7 days after malaria were stimulated for 18 h with iRBC lysate and assayed for the production of IL-10 in the presence (αMHC-II) or absence (isotype) of antibodies specific for HLA-DR, -DQ and -DP (n = 8). ns, not significant (P≥0.05), P values determined by a linear mixed model for repeated measures ANOVA with Tukey HSD post hoc tests (A) and permutation re-sampling tests (F, G). Data are shown as the means ± s.d. Because whole microbe stimulation with P. falciparum iRBCs involves a complex mixture of antigens and stimuli for innate receptors, we asked whether P. falciparum-inducible IL-10 production by CD4+ T cells requires antigen presenting cells (APCs) and T cell receptor engagement. We magnetically isolated CD4+ T cells that had been collected after the resolution of febrile malaria and found that they failed to produce IL-10 in response to iRBC stimulation in the absence of antigen-presenting cells (Figure 3F). Moreover, iRBC-induced IL-10 production by CD4+ T cells was abrogated in PBMC cultures in the presence of antibodies that block major histocompatibility complex (MHC) class II molecules (Figure 3G). Together these data demonstrate that P. falciparum-inducible IL-10 production by CD4+ T cells is T cell receptor-dependent.
Having established that iRBC-inducible IL-10 production by CD4+ T cells requires APCs and T cell receptor engagement, we sought to understand the role that in vivo conditioning of APCs plays in modulating IL-10 production by P. falciparum-specific CD4+ T cells. We magnetically isolated CD4+ T cells collected after the resolution of febrile malaria and cultured these cells with autologous APCs collected at the healthy baseline before the malaria season or after the resolution of febrile malaria. Under both conditions iRBC-inducible IL-10 production by CD4+ T cells was restored to similar levels (Figures 3F), suggesting that the in vivo conditions during acute febrile malaria shape the functional response of CD4+ T cells in a manner that is independent of the in vivo conditioning of APCs.
Discussion
In our previous investigations at this study site we observed that the risk of febrile malaria slowly decreases over years as individuals are exposed to intense seasonal P. falciparum transmission such that adults rarely experience febrile malaria when infected with blood-stage parasites [44]. The gradual acquisition of blood-stage immunity that reliably protects from the onset of febrile malaria likely reflects the need for repeated infections over years to achieve levels of broadly reactive antibodies that exceed a protective threshold [13], [45]. However, even malaria-susceptible children at this study site (who by definition have yet to acquire reliably protective antibodies) experience only 1 to 2 febrile malaria episodes per six-month malaria season despite ≥100 infective mosquito bites per person each season, and generally these children manage to keep parasite numbers in the blood in check [44]. These observations prompted us to investigate immune mechanisms beyond antibody responses that might contribute to protection from febrile malaria and parasite replication in children who are exposed to repeated P. falciparum infections, and also to investigate how children become susceptible again to febrile malaria after a period of decreased P. falciparum exposure.
We found that acute febrile malaria alters children's PBMCs such that P. falciparum re-exposure results in downregulation of acute phase pro-inflammatory cytokines that drive fever and systemic inflammation (e.g. IL-1β and IL-6 from monocytes/macrophages), and upregulation of immune mechanisms involved in control of inflammation (e.g. IL-10-producing CD4+ T cells) and parasite clearance (e.g. IFN-γ-producing CD4+ T cells, phagocytosis and phagolysosome maturation) (Figure 4). The maintenance of this regulatory state appears to depend on recent or ongoing P. falciparum exposure as children revert to a homeostatic baseline in the absence of ongoing P. falciparum exposure during the six-month dry season. The short-lived, exposure-dependent nature of this response mirrors the kinetics of P. falciparum-specific antibody responses in children [44], [45], suggesting that these responses work in concert to protect children as long as P. falciparum exposure is ongoing. These data offer mechanistic insights into how children who are repeatedly infected with P. falciparum commonly manage to remain afebrile and control parasite replication, and how they become susceptible again to febrile malaria after a period of reduced P. falciparum exposure. The possibility that treatment with artemether/lumefantrine contributed to these findings cannot be excluded.
Fig. 4. Proposed model by which children remain asymptomatic and control parasitemia upon P. falciparum re-exposure. 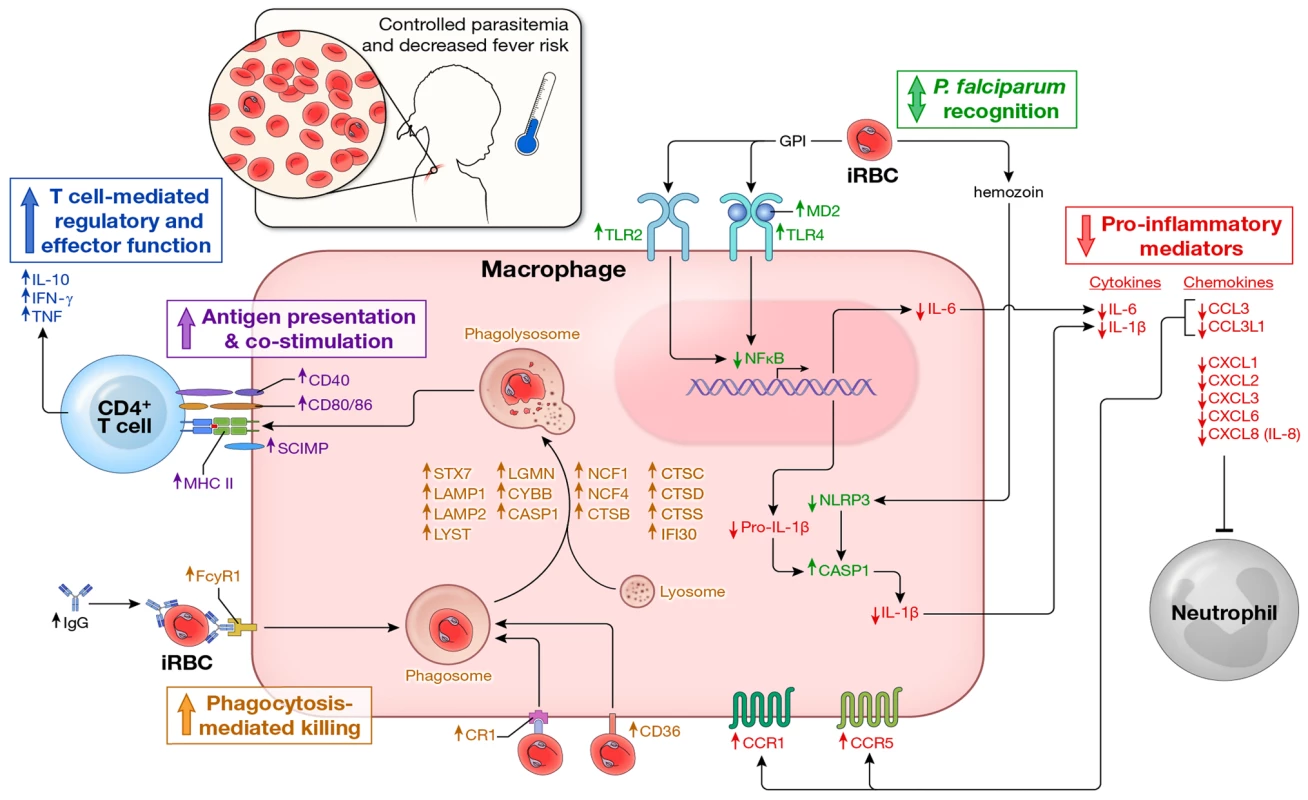
In children without prior or recent malaria exposure, P. falciparum infection induces a robust pro-inflammatory cytokine and chemokine response (e.g. IL-1β, IL-6, IL-8) whereas effector mechanisms that mediate parasite clearance (phagocytosis, phagolysosome activation, antigen presentation, T cell co-stimulation and IFN-γ production by CD4+ T cells) are not readily inducible, leaving children susceptible to fever and other systemic symptoms of malaria as well as poorly controlled parasite replication. In contrast, febrile malaria induces an exposure-dependent regulatory state (shown here) whereby re-exposure to P. falciparum results in reduced production of pro-inflammatory cytokines and chemokines and enhanced expression of regulatory cytokines (e.g. IL-10 production by CD4+ T cells) and pathways involved in phagocytosis-mediated clearance of infected red blood cells and activation of adaptive immunity, thus enabling children to remain asymptomatic and control parasite replication in the face of ongoing P. falciparum exposure. In addition, P. falciparum-specific IgG levels are low in children who have not been recently exposed to malaria, but transiently increase in response to P. falciparum infection [44], [45], further enhancing exposure-dependent parasite clearance through opsonization and phagocytosis of infected erythrocytes. Arrows indicate the direction of expression observed in this study of molecules at the mRNA and/or protein levels induced by P. falciparum re-exposure after febrile malaria relative to responses induced by P. falciparum exposure at the healthy baseline. Molecules are color-coded by biological function. These data shed light on the long-standing and enigmatic clinical notion of ‘premunition’—a partially effective, exposure-dependent immune response that protects against illness and high numbers of parasites in the blood without completely eliminating the infection [12], [46], [47]. Although premunition is often viewed as a state of immune dysregulation or suppression [12], [46], [47], we speculate that it evolved as an appropriate immune response in the face of unrelenting exposure to genetically and antigenically diverse parasites such that young children are at least partially protected from potentially life-threatening inflammation and unchecked parasite replication before they acquire durable, broadly reactive antibodies that reliably protect against the onset of malaria symptoms. Although we did not study severe malaria per se—an overlapping set of syndromes [48] which have been linked to excessive inflammation [49]—it is conceivable that the ability to rapidly downregulate P. falciparum-inducible inflammation in early life contributes to the rapid acquisition of strain-transcendent immunity to severe malaria which may occur after only one or two symptomatic infections [50], and conversely, that the small percentage of children who develop severe malaria are those whose genetic background, environment (e.g. co-infection history, microbiota, nutritional status) or specific interaction with parasite virulence factors [51]–[54] tips them toward dysregulated pathologic inflammatory responses.
The prospective design of this study, in which each subject served as their own healthy control, provides a rare view of the regulation and functional plasticity of innate and adaptive immune cells in response to a natural infection in humans. In general, innate immune cells such as monocytes/macrophages first detect pathogens through PRRs such as TLRs and NOD-like receptors (NLRs) which recognize highly conserved PAMPs [55]. Through these initial host-pathogen interactions, innate immune cells provide the first line of defense against pathogen invasion and also direct the quality of antigen-specific B and T cell responses. To date, only a handful of P. falciparum PAMPs and their respective PRRs have been identified. These include GPI anchors (TLR2>TLR4) [33], hemozoin (NLRP3) [34], CpG-containing DNA motifs bound to hemozoin (TLR9) [35] and AT-rich DNA motifs (unknown cytosolic receptor) [36]. Studies in vitro and in animal models show that these PAMPs drive monocytes/macrophages to produce pro-inflammatory cytokines and chemokines such as IL-1β, IL-6, IL-8 and TNF [33]–[35]. These observations are consistent with studies in humans that show these cytokines and chemokines rise and fall in the serum of individuals treated for febrile malaria [2]–[4]. However, prior to this study, the nature of the inflammatory response induced by P. falciparum re-exposure relative to that induced at the healthy baseline of the same individuals was unknown. Here we show that the capacity of monocytes/macrophages to produce the canonical pyrogenic cytokines IL-1β and IL-6 is reduced upon re-exposure to P. falciparum parasites relative to that induced at the healthy baseline of the same individuals—a finding we observed at the mRNA level by microarray and qRT-PCR in PBMCs, and at the protein level in isolated monocytes/macrophages.
Although the precise molecular mechanisms that regulate P. falciparum-inducible inflammation remain to be fully elucidated, this study offers insight into the multiple levels at which this regulation might occur. For example, we observed that NFKB1 expression was downregulated after malaria relative to baseline. NFKB1 encodes the p50 component of the canonical p65/p50 NF-κB heterodimeric transcription factor that stimulates the expression of pro-inflammatory cytokines such as IL-1β and IL-6 [56]. We also observed decreased expression of NLRP3 after malaria relative to baseline. NLRP3 encodes a component of the NALP3 inflammasome which is expressed in myeloid cells and activates caspase-1, thereby promoting the maturation and secretion of IL-1β [57]. Other differentially expressed PRRs such as TLR2 and TRL4 were upregulated after malaria relative to baseline, suggesting that the regulation of P. falciparum-inducible inflammation does not occur at the level of TLR expression. Interestingly, TLR expression is also upregulated in the context of tolerance induced by gram positive bacteria [58], whereas tolerance induced by gram negative bacteria is associated with reduced expression of TLR2 and TLR4 [59], underscoring the microbe-specific nature of immune-regulation.
After the resolution of febrile malaria we also observed increased expression of genes encoding proteins that limit the inflammatory response including IL18BP, IL1R2, CTLA4, BTLA, SAMHD1 and TNFSF10, as well as decreased expression of genes encoding proteins that promote inflammation including PTGS2 and TREM1. Further studies are needed to more clearly elucidate the signaling networks involved in regulating the immune response to P. falciparum infection and to fully understand the relationships between perturbations to these networks and the variability in malaria clinical outcomes.
The gene expression microarray data also shed light on the regulation of chemotactic responses [60] in malaria. Relative to the response induced at the healthy baseline, re-exposure to P. falciparum parasites was associated with downregulated expression of chemokines that recruit macrophages (CCL3, CCL3L1; Figures 1A, 1D and 4) and neutrophils (CXCL1, CXCL2, CXCL3, CXCL6; Figures 1A, 1D and 4), but upregulated expression of monocyte/macrophage-specific chemokine receptors (CCR1, CCR5; Figure 4). We postulate that this pattern reflects the fine-tuning of chemotactic responses in the face of ongoing or repeated P. falciparum exposure, whereby systemic chemokine release is restrained to decrease the potential for tissue damage caused by aberrant trafficking and accumulation of effector cells such as neutrophils, whereas the reciprocal regulation of monocyte/macrophage-specific chemokines (repressed) and chemokine receptors (increased) enhances the sensitivity of monocyte/macrophages to detect decreased concentrations of chemokines.
Murine models clearly demonstrate that IL-10 and TGF-β play critical roles in regulating Plasmodium-induced inflammation [19], [22]. In humans, IL-10 and TGF-β levels increase in serum during acute febrile malaria and then fall after treatment [2], [20], consistent with a role for these cytokines in restraining and resolving P. falciparum-induced inflammation during a single malaria episode. However, whether febrile malaria conditions the immune system to modify the production of IL-10 and TGF-β upon subsequent exposure to P. falciparum within the same individual remained an open question. Here we show that P. falciparum-inducible IL-10 and TGF-β production/expression is upregulated after the resolution of febrile malaria relative to that which is inducible at the healthy baseline of the same individuals. In addition, we observed that IL-10 blockade in vitro enhanced IL-6 and TNF production in some but not all children, consistent with a role for IL-10 in controlling inflammation in the setting of P. falciparum re-exposure, but also highlighting the complexity of regulatory responses that restrain P. falciparum-induced inflammation.
Given the role of IL-10 in regulating Plasmodium-induced inflammation, we sought to illuminate the identity, function and kinetics of P. falciparum-specific IL-10-producing cells. Previous studies in humans have shown that total FOXP3+ T regulatory cells (Tregs) increase in response to experimental [23] and natural [61] P. falciparum infection (reviewed in [42]), which suggested that Treg-generated anti-inflammatory cytokines play an important role in controlling P. falciparum-inducible inflammation. However, subsequent cross-sectional studies failed to conclusively show significant differences in Treg responses between individuals with mild and severe malaria [62], [63]. Here we show that CD4+CD25+Foxp3− T cells are the predominant source of P. falciparum-inducible IL-10, whereas Tregs contributed minimally to the overall IL-10 response. We demonstrate that IL-10 producing CD4+CD25+Foxp3− T cells are P. falciparum-specific, in that they require APCs and T cell receptor engagement to produce IL-10. We also found that P. falciparum-inducible IL-10 production by CD4+ T cells isolated after malaria did not change significantly when these cells were co-cultured with homologous APCs collected before malaria, although there was a trend toward enhanced IL-10 production by these CD4+ T cells when cultured with APCs collected after malaria.
Interestingly, we observed that a significant proportion of P. falciparum-specific IL-10 producing CD4+CD25+Foxp3− T cells co-produced the Th1 cytokines IFN-γ and/or TNF. Similar ‘self-regulating’ Th1 cells that co-produce IL-10 and IFN-γ were first identified in the lungs of patients with active pulmonary tuberculosis [64] and have since been observed in mice infected with Toxoplasma gondii [65] and Leishmania major [66] as well as in humans with visceral leishmaniasis [67]. Intriguingly, we observed that P. falciparum-specific IL-10 was only inducible in activated Th1 cells after recent febrile malaria, and that after the dry season IL-10 and IFN-γ were no longer inducible through P. falciparum stimulation. This is consistent with a recent study of Ugandan children by Jagannathan et al. in which the frequencies of P. falciparum-specific CD4+ T cells co-producing IFN-γ and IL-10 were inversely associated with days since last malaria episode [68]. Together these data support the hypothesis that IL-10 production by antigen-specific Th1 cells represents a normal phase of their differentiation program which is reached after full activation in order to restrain the inflammatory response while still allowing an efficacious immune response [43], [65], namely, IFN-γ production that promotes phagocytosis-mediated clearance of blood-stage parasites.
Importantly, we show that P. falciparum-specific IL-10 production remains inducible in some but not all untreated children whose low-level asymptomatic P. falciparum infections persisted through the six-month dry season, suggesting that the production of IL-10 and IFN-γ is finely tuned such that parasitemia is controlled without inducing clinically overt inflammation—potentially explaining the long-standing clinical observation that most individuals, if left untreated after their initial bout of febrile malaria, become afebrile and maintain control of parasitemia for months before the infection is finally cleared. The exposure-dependent inducibility of IL-10 production by Th1 cells may also explain our previous observation at the same study site that children with asymptomatic P. falciparum infection at the end of the dry season are at lower risk of febrile malaria during the ensuing malaria season [31], whereas uninfected children at the end of the dry season are at increased risk—corresponding temporally with their return to a homeostatic baseline in which P. falciparum exposure induces a pro-inflammatory phenotype. The exposure-dependent inducibility of IL-10 is also consistent with anecdotal reports of rapidly waning clinical immunity to febrile malaria in those who emigrate from malaria endemic areas [69]. Taken together these data point toward a protective effect of P. falciparum-specific IL-10 producing Th1 cells in malaria, a hypothesis supported by a cross-sectional study in The Gambia which showed a higher frequency of total IL-10 producing Th1 cells in children with mild versus severe malaria [62]. In contrast, a study in Uganda recently reported that frequencies of CD4+ T cells co-producing IFN-γ and IL-10 were not associated with protection from future malaria, although imprecise measures of malaria exposure may have led to spurious associations with protection [68]. More studies are needed to define the potential role of these cells in protection from malaria and to elucidate the molecular basis of their remarkable functional plasticity [70]—information that could define ways in which these cells could be safely induced and maintained through vaccination. Further studies are also needed to disentangle the relative contributions of IL-10 upregulation and antibodies to protection from malaria.
Malaria-induced regulatory responses that control inflammation are often viewed as globally immunosuppressive, which predicts that parasites would grow unimpeded in individuals residing in areas of ongoing P. falciparum transmission. However, this model is at odds with the common finding of low-level, asymptomatic infection among children in endemic areas. Therefore a key finding of this study is that despite the downregulation of P. falciparum-inducible inflammation after the resolution of malaria, pathways involved in clearance of blood-stage parasites and activation of adaptive immunity were upregulated. Specifically, we observed P. falciparum-inducible upregulation of genes encoding proteins that mediate opsonic (e.g. FCGR1) and non-opsonic (e.g. CR1, CD36) phagocytosis, phagolysosome maturation, antigen processing and presentation, and T cell co-stimulation (Figure 4). The limited blood volume available from children enrolled in this study precluded concomitant functional confirmation of these observations; however, the observed gene expression pattern of enhanced anti-microbial activity after the resolution of febrile malaria is consistent with the results of a study in which malaria-naïve adults, who were experimentally infected with P. falciparum, showed enhanced macrophage phagocytic activity after treatment relative to baseline [71]. Non-opsonic phagocytosis of iRBCs is considered to be an important first line of defense in non-immune or partially immune hosts who have yet to acquire P. falciparum-specific opsonizing antibodies [72]. Indeed, others have shown that the scavenger receptor CD36 mediates phagocytosis of non-opsonized iRBCs [73], and interestingly, does so without inducing pro-inflammatory cytokines [73], [74].
This study reveals several intriguing parallels between the regulation of P. falciparum-triggered inflammation and endotoxin tolerance [75], a link that is particularly germane in light of earlier studies in humans that showed that malaria induces cross-tolerance to the febrile response normally induced by bacterial endotoxin [24], [25], suggesting at least partial overlap of regulatory pathways induced by Plasmodium and gram negative bacteria. Indeed, similar to what has been described in an in vitro model of LPS tolerance in murine macrophages [27], we observed that the regulation of P. falciparum-triggered responses is component-specific, such that acute phase pro-inflammatory mediators such as IL-1β and IL-6 are transiently downregulated or ‘tolerized’, while anti-parasitic effector pathways are primed or enhanced upon re-challenge with P. falciparum parasites. Further reductionist studies are needed to define the molecular mechanisms by which this regulation occurs including the potential role of chromatin modification [76] and microRNAs [77]. It will be of interest to understand how malaria-induced epigenetic reprogramming of innate immune cells—or “trained immunity”—differs from that induced by other pathogens [78], [79].
To our knowledge, no human study has evaluated the genome-wide transcriptional response to a natural infection in which each subject serves as his or her own healthy control. Nearly all individuals at the study site become infected with P. falciparum within a predictable window of time each year [80], which enabled us to compare intra-individual changes in PBMC gene expression at the healthy baseline before the malaria season and after the resolution of febrile malaria—both directly ex vivo and after re-exposing PBMCs to P. falciparum parasites in vitro. An important limitation of blood transcriptome analysis is that changes in mRNA levels can be driven by de novo transcriptional regulation or changes in the composition of PBMCs in peripheral blood [81]. Three lines of evidence indicate that the observed changes in mRNA levels in this study are driven by de novo transcriptional regulation. First, by flow cytometry we did not observe gross changes in the composition of the study subjects' PBMCs from before to after malaria. This is consistent with the observation that immune cells traffic out of the peripheral circulation during acute malaria but then return to the peripheral circulation after the infection has resolved [82]. Second, at the individual subject level we found that genes encoding myeloid-expressed pro-inflammatory mediators were downregulated after malaria relative to baseline, irrespective of changes in the percentage of monocytes, even as mRNA levels of other genes identified as myeloid-specific [38] were unchanged or increased relative to baseline. And finally, we observed that in vitro stimulation of fixed populations of cells (PBMCs and isolated monocytes/macrophages) induces de novo expression of immune-related genes.
In summary, this longitudinal study of Malian children shows that febrile malaria induces exposure-dependent P. falciparum-specific regulatory responses that limit pathogenic inflammation and enhance anti-parasite effector responses upon P. falciparum re-exposure. These findings offer mechanistic insights into several long-standing clinical observations in malaria including the high incidence of asymptomatic P. falciparum infection in endemic areas [69], reduced fever with repeated experimental Plasmodium infections in humans [83], the rapid acquisition of immunity to severe malaria [50], the rapid loss of clinical immunity to febrile malaria in the absence of ongoing P. falciparum exposure [6] and Plasmodium-induced hetero-tolerance to endotoxin challenge [25]. Longitudinal studies of symptomatic and asymptomatic individuals who are repeatedly exposed to P. falciparum will refine our understanding of the mechanisms underlying the regulation and dysregulation of Plasmodium-induced inflammation and may help define the potential for interventions that safely prevent or mitigate Plasmodium-induced immunopathology without compromising control of parasite replication.
Materials and Methods
Ethics statement
The Ethics Committee of the Faculty of Medicine, Pharmacy, and Dentistry at the University of Sciences, Techniques, and Technologies of Bamako, and the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health approved this study. Written informed consent was obtained from the parents or guardians of participating children.
Study subjects
Study subjects were enrolled in an observational cohort study conducted in Kambila, Mali, a rural village of ∼1500 inhabitants where intense seasonal P. falciparum transmission occurs from July through December. The cohort is an age-stratified random sample of the entire village population. A detailed description of the study site and design of the cohort study has been published elsewhere [31]. The present study focused on children aged 5–13 years who had PBMCs collected at their healthy baseline before the malaria season, and 7 or 14 days after treatment of their first malaria episode of the ensuing malaria season, as well as a subset of children who also had PBMCs collected after the following six-month dry season, a period of little to no P. falciparum transmission. Individual demographic and clinical data are given in Table S1. Febrile malaria episodes were detected prospectively by self-referral to the study clinic, which was staffed by a physician 24 hours/day. Malaria episodes were treated with a standard 3-day course of artemether/lumefantrine.
Detection of P. falciparum infection
Thick blood smears were stained with Giemsa and counted against 300 leukocytes, and P. falciparum densities were recorded as the number of asexual parasites/µl of whole blood based on an average leukocyte count of 7500/µl. Each smear was evaluated separately by at least two expert microscopists. P. falciparum was detected by PCR from dried blood spots preserved on 903 Protein Saver filter paper (Whatman) as previously described [80].
Processing of PBMCs
Blood samples (8 ml) were drawn by venipuncture into sodium citrate-containing cell preparation tubes (BD, Vacutainer CPT Tubes) and transported 20 km to the laboratory where PBMCs were isolated and frozen within three hours according to the manufacturer's instructions. PBMCs were frozen in fetal bovine serum (FBS) (Gibco, Grand Island, NY) containing 7.5% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO), kept at −80°C for 24 hours, and then stored at −196°C in liquid nitrogen. For each individual, PBMCs from all time points were thawed and assayed at the same time. The trypan blue dye exclusion assay consistently demonstrated >80% viability of PBMCs after thawing.
Microarray chip processing and data analysis
RNA extraction, cDNA amplification, synthesis and labeling was performed as previously described [84]. Hybridization, fluidics and scanning were performed according to standard Affymetrix protocols. GeneChip Operating Software GCOS v1.4 was used to convert the image files to cell intensity data (cel files). All cel files, representing individual samples were normalized using the Robust Multiarray Average (RMA) method from the affy package library in the R project for Statistical Computing (R Core Team 2013). Nine outlier chips were identified among the unstimulated samples using quality control plots from Partek Genomics Suite software (Partek, inc. St. Louis, Mo., v6.5 6.11.310) and principal components analyses (PCA) computed using R. An empirical Bayes moderated paired T-test was computed using the limma package library in R to obtain false discovery rate (FDR) adjusted p-values and fold changes. Probes were considered statistically significant if their FDR-adjusted P values were <0.05 and their absolute fold change was >1.25. Heatmaps were generated with the gplots package library in R. Log fold change ratios, p-values and false discovery rates from the empirical Bayes T-tests were imported into Ingenuity Pathways Analysis to examine enrichment of pathways and functional groups.
Quantitative real-time PCR
Human yeast Sfi1 homolog spindle assembly associated gene (Sfi1) was selected as a reference gene based on its low coefficient of variation (CV) across DNA microarray analysis. Seven mRNAs were analyzed by q-RT-PCR to validate DNA microarray findings: chemokine (C-X-C motif) ligand 5 (CXCL5), interleukin-1 beta (IL1B), interleukin-6 (IL6), interleukin-10 (IL10), toll-like receptor 2 (TLR2), and transforming growth factor, beta 1 (TGFB1). All six probe and primer sets were designed using Primer Express version 3.0 (ThermoFisher Scientific, Waltham, MA) and are listed inTable S5. Seventeen out of 34 patients were selected for q-RT-PCR validation. Four RNAs were analyzed from each patient representing the two time points HB and d7 and two experimental conditions (‘ex vivo unstimulated’ and ‘in vitro stimulated with iRBC’). Template preparation and q-RT-PCR analysis was performed as described previously [84].
Preparing P. falciparum-infected red blood cell lysate for in vitro stimulation of PBMCs
3D7 P. falciparum parasites were maintained in fresh human ORh+erythrocytes at 3% hematocrit in RPMI 1640 medium (KD Medical) supplemented with 10% heat-inactivated ORh+ human serum (Interstate Blood Bank, Memphis, Tennessee), 7.4% Sodium Bicarbonate (GIBCO, Invitrogen) and 25 µg/ml of gentamycin (GIBCO, invitrogen), at 37°C in the presence of a gas mixture containing 5% O2, 5% CO2 and 90% N2. Parasite cultures were shown to be free of mycoplasma and acholeplasma using an ELISA-based Mycoplasma Detection Kit (Roche) which contains polyclonal antibodies specific for M. arginini, M. hyorhinis, A. laidlawii and M. orale. P. falciparum schizont iRBCs were isolated in RPMI 1640 medium supplemented with 0.25% Albumax (GIBCO, Invitrogen) and 7.4% Sodium Bicarbonate (GIBCO, Invitrogen) using magnetic columns (LD MACS Separation Columns, Miltenyi Biotec). Control preparations of uninfected red blood cells (uRBC) from the same blood donor were obtained and tested in all experiments. Lysates of P. falciparum-infected and uninfected RBCs were obtained by three freeze-thaw cycles in liquid nitrogen and 37°C water bath.
In vitro stimulation of PBMCs with P. falciparum-infected red blood cell lysate
PBMCs were cultured in complete RPMI (RPMI 1640 plus 10% fetal calf serum, 1% penicillin/streptomycin, 2-mercaptoethanol) in flat-bottom 96 well plates, at 37°C in a 5% CO2 atmosphere. 500,000 PBMCs were stimulated with lysate of infected red blood cells (iRBCs) or uninfected RBCs (uRBCs) in a ratio of 3 RBCs per PBMC for 18 h, with or without 1.25 µg/ml Brefeldin A (BFA) (Sigma-Aldrich) for the last 15 h of stimulation. A 3∶1 ratio of RBC to PBMC was used on the basis of titration experiments (from 5∶1 to 1∶1) and is consistent with previous reports [85]. PBMCs stimulated with 1.18 µg/ml Staphylococcal enterotoxin B from Staphylococcus aureus (SEB) (Sigma-Aldrich) was used as a positive control for cytokine production in supernatants and within cells. Following stimulation, cells were centrifuged and supernatants were recovered and frozen at −80°C for cytokine analysis. Cells stimulated in the presence of BFA were centrifuged, washed and recovered for intracellular staining and flow cytometry analysis.
Isolation of monocytes/macrophages and in vitro stimulation with P. falciparum-infected red blood cell lysate
Monocyte/macrophages were isolated from PBMCs of Malian children by negative selection using the MACS Pan Monocyte Cell Negative Isolation kit II (Miltenyi Biotec), an indirect magnetic labeling system for the isolation of untouched monocytes/macrophages. Non-monocyte/macrophage cells were directly depleted by using a cocktail of biotin-conjugated antibodies followed by magnetic removal of labeled cells. Monocyte/macrophage purity was verified by flow cytometry using fluorescently labeled antibodies specific for CD3 PE (UCHT1), CD4 APC (RPA-T4), CD8 APC-Cy7 (SK1), CD14 FITC (M5E), CD16 Pacific blue (3G8) (BD Biosciences), CD19 PerCP-Cy5.5 (SJ25C1) (eBioscience), and 7-Aminoactinomycin D (7-AAD) viability staining (BD Biosciences). FACS analysis was performed on a BD LSR II Table flow cytometer (BD Bioscience) and analyzed using FlowJo software (Tree Star). Purified monocytes/macrophages were then stimulated in a ratio of 30 RBCs per monocyte for 6 h with lysate of P. falciparum-infected RBCs and cytokines were measured in supernatants.
Flow cytometry
PBMCs were washed in PBS with 4% heat-inactivated FCS and cells were incubated for 30 min at 4°C with fluorescently labeled antibodies specific for CD3 PE (UCHT1), CD4 APC (RPA-T4), CD8 APC-Cy7 (SK1), CD14 FITC (M5E) and CD16 Pacific blue (3G8) purchased from BD Biosciences; and CD19 PerCP-Cy5.5 (SJ25C1) purchased from eBioscience. All phenotypic analyses were performed using mouse mAbs specific for human markers conjugated to fluorophores. FACS analyses were performed on a BD LSR II Table flow cytometer (BD Biosciences) and analyzed using FlowJo software (Tree Star, Inc).
Measurement of cytokines in supernatants of stimulated PBMCs
Supernatants were thawed and immediately analyzed with Bio-plex human cytokine assays (Bio-Rad Laboratories, Inc.) as recommended by the manufacturer. The following cytokines were measured: IL-1β, IL-6, IL-8, IL-10 and TNF. Briefly, 25 µL of supernatant was diluted 1∶2 in medium and incubated with anti-cytokine antibody-coupled magnetic beads for 30 min at room temperature shaking at 300 RPM in the dark. Between each step the complexes were washed three times in wash buffer, using a vacuum manifold. The beads were then incubated with a biotinylated detector antibody for 30 min before incubation with streptavidin-phycoerythrin for 30 minutes. Finally, the complexes were resuspended in 125 µL of detection buffer and 100 beads were counted with a Luminex 200 device (Bio-Rad Laboratories, Inc.). Final concentrations were calculated from the mean fluorescence intensity and expressed in pg/mL using standard curves with known concentrations of each cytokine.
IL-10 blocking
PBMCs were cultured in complete RPMI in flat-bottom 96 well plates, at 37°C in a 5% CO2 atmosphere. 500,000 PBMCs were stimulated for 18 h with lysate of infected (iRBCs) or uninfected RBCs (uRBCs) in a ratio of 3 RBCs per PBMC in the presence of anti-IL-10 (BD Pharmigen, USA) and anti-IL-10R (R&D Systems, Inc.) or in the presence of the respective isotype controls. Following stimulation cells were centrifuged and supernatants were recovered for cytokine analysis.
Intracellular cytokine staining
After stimulation a total of 1×106 PBMCs were sequentially stained for surface and intracellular markers in round-bottom 96-well plates at room temperature. To exclude dead cells, PBMCs were stained for 30 min using the LIVE/DEAD Fixable Violet Dead Cell Stain Kit (Invitrogen) followed by a surface staining with PerCP-Cy5.5 anti-human CD27 (M-T271) and APC-H7 anti-human CD45RO (UCHL1) for 20 min. After fixing and permeabilizing the cells according to the manufacturer's protocol using the FoxP3 Staining Buffer Set (eBioscience), the cells were stained with BD Horizon V500 anti-human CD3 (UCHT1), PerCP anti-human CD4 (SK3), Alexa Fluor 700 anti-human IFNγ (B27), FITC anti-human TNF (MAb11), APC anti-human IL-10 (JES3-19F1), PE-Cy7 anti-human CD25 (BC96) and PE anti-human FoxP3 (236A/E7) for 30 min. Fluorescently labeled antibodies against TNF, CD25 and FoxP3 were purchased from eBioscience, the remaining antibodies were purchased form BD Biosciences. Cells were acquired using a BD LSR II Table flow cytometer (BD) and analyzed using FlowJo software (Tree Star) and SPICE software [86].
Isolation of CD4+ T cells and in vitro stimulation with P. falciparum infected red blood cell lysate
CD4+ T cells were isolated from PBMCs of Malian children by negative selection using the MACS CD4+ T Cell Negative Isolation kit II (Miltenyi Biotec), an indirect magnetic labeling system for the isolation of untouched CD4+ T helper cells. Non-CD4+ T cells were directly depleted by using a cocktail of biotin-conjugated antibodies against CD8, CD14, CD16, CD19, CD36, CD56, CD123, TCR g/d and Glycophorin A and anti-Biotin microbeads, followed by magnetic removal of labeled cells. CD4+ T cells purity was verified by flow cytometry, using fluorescently labeled antibodies specific for CD3 (PE) and CD4 (APC) (BD Bioscience) and a BD LSR II Table flow cytometer (BD Bioscience, USA) and analyzed using FlowJo software (Tree Star, Inc). Purified CD4+ T cells were then incubated either in the presence or absence of non-CD4+ cells and stimulated for 18 h with lysate of infected (iRBCs) or uninfected RBCs (uRBCs), for cytokine analysis of supernatants.
MHC II blocking
PBMCs were cultured in complete RPMI in flat-bottom 96 well plates, at 37°C in a 5% CO2 atmosphere. 500,000 PBMCs were stimulated for 18 h with lysate of infected (iRBCs) or uninfected RBCs (uRBCs) in a ratio of 3 RBCs per PBMC, in the presence of anti-human leukocyte antigen HLA-DQ (SPVL-3; Beckmann Coulter, USA), anti-HLA-DP (B7/21; abcam, USA) and anti-HLA-DR (L243; Biolegend, USA), or in the presence of respective isotype controls. Following stimulation, cells were centrifuged and supernatants were recovered for cytokine analysis.
Statistical analysis
Continuous data were compared using the paired or unpaired Student's T-test, paired Wilcoxon rank sum test or permutation tests of mean paired differences as appropriate. Bonferroni adjustments were applied to correct for multiple comparisons when appropriate. A linear mixed model for repeated measures ANOVA with Tukey HSD post hoc tests was also used to compare continuous variables. Pearson correlation coefficients and linear regressions with 95% confidence bands were used to examine the correlation between continuous variables. Fisher's exact test was used for contingency table analyses. The statistical test used is specified in the figure legends. Statistical significance was defined as a 2-tailed P value of ≤.05. Statistical tests were computed using R version 2.13.2 (http://www.R-project.org), GraphPad Prism version 5.0d (http://www.graphpad.com/scientific-software/prism/) or JMP 10.0 (www.jmp.com).
Supporting Information
Zdroje
1. CollinsWE, JefferyGM (1999) A retrospective examination of sporozoite - and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity during primary infection. Am J Trop Med Hyg 61 : 4–19.
2. DayNP, HienTT, SchollaardtT, LocPP, ChuongLV, et al. (1999) The prognostic and pathophysiologic role of pro - and antiinflammatory cytokines in severe malaria. J Infect Dis 180 : 1288–1297.
3. LykeKE, BurgesR, CissokoY, SangareL, DaoM, et al. (2004) Serum levels of the proinflammatory cytokines interleukin-1 beta (IL-1beta), IL-6, IL-8, IL-10, tumor necrosis factor alpha, and IL-12(p70) in Malian children with severe Plasmodium falciparum malaria and matched uncomplicated malaria or healthy controls. Infect Immun 72 : 5630–5637.
4. WaltherM, WoodruffJ, EdeleF, JeffriesD, TongrenJE, et al. (2006) Innate immune responses to human malaria: heterogeneous cytokine responses to blood-stage Plasmodium falciparum correlate with parasitological and clinical outcomes. J Immunol 177 : 5736–5745.
5. UrbanBC, IngR, StevensonMM (2005) Early interactions between blood-stage plasmodium parasites and the immune system. Curr Top Microbiol Immunol 297 : 25–70.
6. LanghorneJ, NdunguFM, SponaasAM, MarshK (2008) Immunity to malaria: more questions than answers. Nat Immunol 9 : 725–732.
7. KwiatkowskiD, HillAV, SambouI, TwumasiP, CastracaneJ, et al. (1990) TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336 : 1201–1204.
8. GrauGE, TaylorTE, MolyneuxME, WirimaJJ, VassalliP, et al. (1989) Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med 320 : 1586–1591.
9. Molineaux L (1985) The impact of parasitic diseases and their control on mortality, with emphasis on malaria and Africa. In: Vallin J, Lopez AD, editors. Health Policy, Social Policy and Mortality Prospects. Liege: Ordina. pp. 13–44.
10. BaliraineFN, AfraneYA, AmenyaDA, BonizzoniM, MengeDM, et al. (2009) High prevalence of asymptomatic plasmodium falciparum infections in a highland area of western Kenya: a cohort study. J Infect Dis 200 : 66–74.
11. BottiusE, GuanzirolliA, TrapeJF, RogierC, KonateL, et al. (1996) Malaria: even more chronic in nature than previously thought; evidence for subpatent parasitaemia detectable by the polymerase chain reaction. Trans R Soc Trop Med Hyg 90 : 15–19.
12. McGregorIA, GillesHM, WaltersJH, DaviesAH, PearsonFA (1956) Effects of heavy and repeated malarial infections on Gambian infants and children; effects of erythrocytic parasitization. Br Med J 2 : 686–692.
13. PortugalS, PierceSK, CromptonPD (2013) Young lives lost as B cells falter: what we are learning about antibody responses in malaria. J Immunol 190 : 3039–3046.
14. RileyEM, WahlS, PerkinsDJ, SchofieldL (2006) Regulating immunity to malaria. Parasite Immunol 28 : 35–49.
15. SintonJA (1938) Immunity or Tolerance in Malarial Infections: (Section of Comparative Medicine). Proc R Soc Med 31 : 1298–1302.
16. BoutlisCS, YeoTW, AnsteyNM (2006) Malaria tolerance–for whom the cell tolls? Trends Parasitol 22 : 371–377.
17. SchofieldL, HewittMC, EvansK, SiomosMA, SeebergerPH (2002) Synthetic GPI as a candidate anti-toxic vaccine in a model of malaria. Nature 418 : 785–789.
18. BoutlisCS, RileyEM, AnsteyNM, de SouzaJB (2005) Glycosylphosphatidylinositols in malaria pathogenesis and immunity: potential for therapeutic inhibition and vaccination. Curr Top Microbiol Immunol 297 : 145–185.
19. LiC, CorralizaI, LanghorneJ (1999) A defect in interleukin-10 leads to enhanced malarial disease in Plasmodium chabaudi chabaudi infection in mice. Infect Immun 67 : 4435–4442.
20. WenischC, ParschalkB, NarztE, LooareesuwanS, GraningerW (1995) Elevated serum levels of IL-10 and IFN-gamma in patients with acute Plasmodium falciparum malaria. Clin Immunol Immunopathol 74 : 115–117.
21. DodooD, OmerFM, ToddJ, AkanmoriBD, KoramKA, et al. (2002) Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 185 : 971–979.
22. OmerFM, RileyEM (1998) Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med 188 : 39–48.
23. WaltherM, TongrenJE, AndrewsL, KorbelD, KingE, et al. (2005) Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23 : 287–296.
24. HeymanA, BeesonPB (1949) Influence of various disease states upon the febrile response to intravenous injection of typhoid bacterial pyrogen; with particular reference to malaria and cirrhosis of the liver. J Lab Clin Med 34 : 1400–1403.
25. RubensteinM, MulhollandJH, JefferyGM, WolffSM (1965) Malaria Induced Endotoxin Tolerance. Proc Soc Exp Biol Med 118 : 283–287.
26. WestMA, HeagyW (2002) Endotoxin tolerance: a review. Crit Care Med 30: S64–73.
27. FosterSL, HargreavesDC, MedzhitovR (2007) Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447 : 972–978.
28. del FresnoC, Garcia-RioF, Gomez-PinaV, Soares-SchanoskiA, Fernandez-RuizI, et al. (2009) Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol 182 : 6494–6507.
29. SpencePJ, LanghorneJ (2012) T cell control of malaria pathogenesis. Curr Opin Immunol 24 : 444–448.
30. TranTM, SamalB, KirknessE, CromptonPD (2012) Systems immunology of human malaria. Trends Parasitol 28 : 248–257.
31. CromptonPD, TraoreB, KayentaoK, DoumboS, OngoibaA, et al. (2008) Sickle Cell Trait Is Associated with a Delayed Onset of Malaria: Implications for Time-to-Event Analysis in Clinical Studies of Malaria. J Infect Dis 198 : 1265–1275.
32. OchielDO, AwandareGA, KellerCC, HittnerJB, KremsnerPG, et al. (2005) Differential regulation of beta-chemokines in children with Plasmodium falciparum malaria. Infect Immun 73 : 4190–4197.
33. KrishnegowdaG, HajjarAM, ZhuJ, DouglassEJ, UematsuS, et al. (2005) Induction of proinflammatory responses in macrophages by the glycosylphosphatidylinositols of Plasmodium falciparum: cell signaling receptors, glycosylphosphatidylinositol (GPI) structural requirement, and regulation of GPI activity. J Biol Chem 280 : 8606–8616.
34. ShioMT, EisenbarthSC, SavariaM, VinetAF, BellemareMJ, et al. (2009) Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog 5: e1000559.
35. ParrocheP, LauwFN, GoutagnyN, LatzE, MonksBG, et al. (2007) Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 104 : 1919–1924.
36. SharmaS, DeOliveiraRB, KalantariP, ParrocheP, GoutagnyN, et al. (2011) Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 35 : 194–207.
37. GriffithJW, SunT, McIntoshMT, BucalaR (2009) Pure Hemozoin is inflammatory in vivo and activates the NALP3 inflammasome via release of uric acid. J Immunol 183 : 5208–5220.
38. ChaussabelD, QuinnC, ShenJ, PatelP, GlaserC, et al. (2008) A modular analysis framework for blood genomics studies: application to systemic lupus erythematosus. Immunity 29 : 150–164.
39. SharifO, KnappS (2008) From expression to signaling: roles of TREM-1 and TREM-2 in innate immunity and bacterial infection. Immunobiology 213 : 701–713.
40. CouperKN, BlountDG, RileyEM (2008) IL-10: the master regulator of immunity to infection. J Immunol 180 : 5771–5777.
41. PrabhakarU, ConwayTM, MurdockP, MooneyJL, ClarkS, et al. (2005) Correlation of protein and gene expression profiles of inflammatory proteins after endotoxin challenge in human subjects. DNA Cell Biol 24 : 410–431.
42. FinneyOC, RileyEM, WaltherM (2010) Regulatory T cells in malaria–friend or foe? Trends Immunol 31 : 63–70.
43. O'GarraA, VieiraP (2007) T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol 7 : 425–428.
44. WeissGE, TraoreB, KayentaoK, OngoibaA, DoumboS, et al. (2010) The Plasmodium falciparum-specific human memory B cell compartment expands gradually with repeated malaria infections. PLoS Pathog 6: e1000912.
45. CromptonPD, KayalaMA, TraoreB, KayentaoK, OngoibaA, et al. (2010) A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A 107 : 6958–6963.
46. SmithT, FelgerI, TannerM, BeckHP (1999) Premunition in Plasmodium falciparum infection: insights from the epidemiology of multiple infections. Trans R Soc Trop Med Hyg 93(Suppl 1): 59–64.
47. HansenDS, SchofieldL (2010) Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog 6: e1000771.
48. MarshK, ForsterD, WaruiruC, MwangiI, WinstanleyM, et al. (1995) Indicators of life-threatening malaria in African children. N Engl J Med 332 : 1399–1404.
49. SchofieldL, GrauGE (2005) Immunological processes in malaria pathogenesis. Nat Rev Immunol 5 : 722–735.
50. GuptaS, SnowRW, DonnellyCA, MarshK, NewboldC (1999) Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5 : 340–343.
51. AvrilM, TripathiAK, BrazierAJ, AndisiC, JanesJH, et al. (2012) A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc Natl Acad Sci U S A 109: E1782–1790.
52. ClaessensA, AdamsY, GhumraA, LindergardG, BuchanCC, et al. (2012) A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci U S A 109: E1772–1781.
53. LavstsenT, TurnerL, SagutiF, MagistradoP, RaskTS, et al. (2012) Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci U S A 109: E1791–1800.
54. TurnerL, LavstsenT, BergerSS, WangCW, PetersenJE, et al. (2013) Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature 498 : 502–505.
55. TakeuchiO, AkiraS (2010) Pattern recognition receptors and inflammation. Cell 140 : 805–820.
56. HaydenMS, GhoshS (2008) Shared principles in NF-kappaB signaling. Cell 132 : 344–362.
57. DavisBK, WenH, TingJP (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29 : 707–735.
58. BuckleyJM, WangJH, RedmondHP (2006) Cellular reprogramming by gram-positive bacterial components: a review. J Leukoc Biol 80 : 731–741.
59. ZhongB, MaHY, YangQ, GuFR, YinGQ, et al. (2008) Decrease in toll-like receptors 2 and 4 in the spleen of mouse with endotoxic tolerance. Inflamm Res 57 : 252–259.
60. MantovaniA, BonecchiR, LocatiM (2006) Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol 6 : 907–918.
61. FinneyOC, NwakanmaD, ConwayDJ, WaltherM, RileyEM (2009) Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur J Immunol 39 : 1288–1300.
62. WaltherM, JeffriesD, FinneyOC, NjieM, EbonyiA, et al. (2009) Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 5: e1000364.
63. MinigoG, WoodberryT, PieraKA, SalwatiE, TjitraE, et al. (2009) Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 5: e1000402.
64. GerosaF, NisiiC, RighettiS, MiccioloR, MarchesiniM, et al. (1999) CD4(+) T cell clones producing both interferon-gamma and interleukin-10 predominate in bronchoalveolar lavages of active pulmonary tuberculosis patients. Clin Immunol 92 : 224–234.
65. JankovicD, KullbergMC, FengCG, GoldszmidRS, CollazoCM, et al. (2007) Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. J Exp Med 204 : 273–283.
66. AndersonCF, OukkaM, KuchrooVJ, SacksD (2007) CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med 204 : 285–297.
67. NylenS, MauryaR, EidsmoL, ManandharKD, SundarS, et al. (2007) Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med 204 : 805–817.
68. JagannathanP, Eccles-JamesI, BowenK, NankyaF, AumaA, et al. (2014) IFNgamma/IL-10 Co-producing Cells Dominate the CD4 Response to Malaria in Highly Exposed Children. PLoS Pathog 10: e1003864.
69. MarshK, KinyanjuiS (2006) Immune effector mechanisms in malaria. Parasite Immunol 28 : 51–60.
70. O'SheaJJ, PaulWE (2010) Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 327 : 1098–1102.
71. LeitnerWW, KrzychU (1997) Plasmodium falciparum malaria blood stage parasites preferentially inhibit macrophages with high phagocytic activity. Parasite Immunol 19 : 103–110.
72. UrbanBC, RobertsDJ (2002) Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr Opin Immunol 14 : 458–465.
73. McGilvrayID, SerghidesL, KapusA, RotsteinOD, KainKC (2000) Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96 : 3231–3240.
74. SerghidesL, KainKC (2002) Mechanism of protection induced by vitamin A in falciparum malaria. Lancet 359 : 1404–1406.
75. BiswasSK, Lopez-CollazoE (2009) Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol 30 : 475–487.
76. FosterSL, MedzhitovR (2009) Gene-specific control of the TLR-induced inflammatory response. Clin Immunol 130 : 7–15.
77. QuinnEM, WangJ, RedmondHP (2012) The emerging role of microRNA in regulation of endotoxin tolerance. J Leukoc Biol 91 : 721–727.
78. KleinnijenhuisJ, QuintinJ, PreijersF, JoostenLA, IfrimDC, et al. (2012) Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 109 : 17537–17542.
79. QuintinJ, SaeedS, MartensJH, Giamarellos-BourboulisEJ, IfrimDC, et al. (2012) Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe 12 : 223–232.
80. TranTM, LiS, DoumboS, DoumtabeD, HuangCY, et al. (2013) An intensive longitudinal cohort study of Malian children and adults reveals no evidence of acquired immunity to Plasmodium falciparum infection. Clin Infect Dis 57 : 40–47.
81. ChaussabelD, PascualV, BanchereauJ (2010) Assessing the human immune system through blood transcriptomics. BMC Biol 8 : 84.
82. HviidL, TheanderTG, AbdulhadiNH, Abu-ZeidYA, BayoumiRA, et al. (1991) Transient depletion of T cells with high LFA-1 expression from peripheral circulation during acute Plasmodium falciparum malaria. Eur J Immunol 21 : 1249–1253.
83. CollinsWE, JefferyGM (1999) A retrospective examination of secondary sporozoite - and trophozoite-induced infections with Plasmodium falciparum: development of parasitologic and clinical immunity following secondary infection. Am J Trop Med Hyg 61 : 20–35.
84. Mackey-LawrenceNM, GuoX, SturdevantDE, VirtanevaK, HernandezMM, et al. (2013) Effect of the Leptin Receptor Q223R Polymorphism on the Host Transcriptome following Infection with Entamoeba histolytica. Infect Immun 81 : 1460–1470.
85. Artavanis-TsakonasK, RileyEM (2002) Innate immune response to malaria: rapid induction of IFN-gamma from human NK cells by live Plasmodium falciparum-infected erythrocytes. J Immunol 169 : 2956–2963.
86. RoedererM, NozziJL, NasonMX (2011) SPICE: Exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 79 : 167–174.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial PeptidesČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 ActivationČlánek Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



