-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
Hosts are equipped with sophisticated machineries for detecting and eliminating invading viruses before they cause significant physiological damage. Unlike mammals which have both innate and adaptive immune systems, insects rely solely on the innate immune system to limit viral infection. Mosquitoes are natural vectors for many human pathogenic viruses, such as dengue virus (DENV) and yellow fever virus. Despite lacking an immunoglobulin-based humoral response, mosquitoes arm themselves with a functional complement-like system to ward off invading pathogens. Herein, we show that a system composed of complement-related factors recognizes and limits flaviviruses by inducing antimicrobial peptides expression. Understanding antiviral mechanisms in arthropods may provide novel strategies for limiting arboviral transmission in nature.
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1004027
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004027Summary
Hosts are equipped with sophisticated machineries for detecting and eliminating invading viruses before they cause significant physiological damage. Unlike mammals which have both innate and adaptive immune systems, insects rely solely on the innate immune system to limit viral infection. Mosquitoes are natural vectors for many human pathogenic viruses, such as dengue virus (DENV) and yellow fever virus. Despite lacking an immunoglobulin-based humoral response, mosquitoes arm themselves with a functional complement-like system to ward off invading pathogens. Herein, we show that a system composed of complement-related factors recognizes and limits flaviviruses by inducing antimicrobial peptides expression. Understanding antiviral mechanisms in arthropods may provide novel strategies for limiting arboviral transmission in nature.
Introduction
Animals have evolved multiple immune systems to eradicate pathogenic viruses. The innate immune response plays a crucial antiviral role in the early stage of infection. Immune responses are initiated based on the recognition of viral components, including viral surface glycoproteins and genetic material, by a group of recognition receptors. This recognition activates the complement system and intracellular antiviral signalling cascades, leading to the elimination of viruses and infected cells. Adaptive immunity is then evoked to produce virus-specific immunoglobulins for viral eradication. The antiviral machineries of blood-sucking arthropods, such as mosquitoes, ticks, and sand flies, are quite different from those of mammals [1]. Arthropods lack an immunoglobulin-based adaptive immune response [2]. Thus, the innate immune system of arthropods plays a central role in the antiviral processes of these organisms.
The complement system functions during the early phase of infection and directly mediates pathogen elimination [3]. The recent identification of complement-like factors in arthropods indicates that this system shares common ancestry as an immune defense mechanism in both vertebrates and invertebrates [4], [5], [6], [7]. Thioester (TE)-containing proteins (TEPs) share high similarity with mammalian C3 [8]. TEPs are generally divided into 3 distinct families, including alpha-2-macroglobulins (A2Ms), C3/C4/C5 complement factors, and insect TEPs (iTEPs) [9]. An Anopheles TEP induced by Plasmodium berghei can bind and kill parasitic ookinetes [5]. Multiple iTEPs in Anopheles (AgTEP1, AgTEP3 and AgTEP4) [10] play a role in clearing bacteria via phagocytosis. Drosophila TEPs (DmTEP2 and DmTEP3) are required for efficient phagocytosis of Gram-positive or Gram-negative bacteria in S2 cells [11]. However, TEPs mutation in Drosophila did not significantly influence survival rate after different bacterial infections, suggested Drosophila TEPs act redundantly or that their absence can be compensated by other components of the immune response [12]. Macroglobulin complement-related factors (MCRs), which are members of the iTEP family, are highly conserved in metazoans and form an independent branch separate from other iTEP subfamilies in the phylogenetic tree. RNA interference screening has identified a Drosophila MCR (known as DmTEP6) that acts as a key factor in the efficient phagocytosis of Candida albicans [11]. Overall, the TEP-based complement-like system functions as an evolutionarily conserved anti-microbial mechanism in arthropods.
Scavenger receptors (SR) are cell surface glycoproteins defined by their ability to bind chemically modified low-density lipoproteins. These receptors are categorized into 6 classes, without conserved domains, suggesting that different primary amino acid (aa) sequences can encode similar 3-dimensional structures [13]. Several classes of SRs are located on the surface of immune cells and function as pathogen recognition receptors. SR-C, a membrane receptor identified in Drosophila that contains 2 complement control protein (CCP) domains (also designated Sushi repeat domains) in its extracellular region, is capable of recognizing both Gram-positive and Gram-negative bacteria and is thought to act as a pattern recognition receptor for phagocytosis [14]. The 2 CCP domains of Drosophila SR-C are thought to be responsible for microbial interaction.
The CCP domain is a signature module in many mammalian complement proteins. Each CCP consists of a domain of ∼60 aa residues, including a consensus pattern of 4 invariant cysteine residues and some additional conserved residues [15]. CCP has been shown to mediate the protein-protein interactions of complement components and to interact with pathogenic microorganisms. For example, complement receptor 2, containing 16 CCP repeats, acts as a receptor for the cleaved products of C3. Complement receptor 2 is also a well-known cellular entry receptor for Epstein-Barr virus in humans, and its CCP-1 and -2 domains are required for Epstein-Barr virus binding [16]. A membrane cofactor protein (MCP) with 4 CCP repeats has been demonstrated to function as a cellular receptor for measles virus [17]. Structural analysis has shown that the CCP-1 and -2 domains of MCP are responsible for measles virus recognition [15]. Another complement regulator with 20 CCP repeats, factor H, reportedly binds the human immunodeficiency virus surface glycoproteins gp41 and gp120 [18], [19].
Many mosquito-transmitted flaviviruses, such as West Nile virus, Japanese encephalitis virus, dengue virus (DENV) and yellow fever virus (YFV), are etiologic agents of severe human diseases, including hemorrhagic fever, biphasic fever, encephalitis, and meningitis. DENV is maintained in a transmission cycle between humans and Aedes mosquitoes [20], [21]. Four serotypes of DENV, sequentially designated DENV1-4, exist in nature [22]. Aedes aegypti, a member of the Culicinae subfamily, shows a close association with human populations and is a primary vector for DENVs [23]. Because A. aegypti is easy to cultivate in the laboratory and its genome has been well characterized [24], [25], it has become an ideal model for the investigation of flaviviral pathogenesis and innate immune responses in invertebrates [7], [26], [27], [28]. Herein, we report that an A. aegypti MCR (AaMCR) is a crucial effector in opposing the flaviviral invasion of mosquitoes. Furthermore, we identified an SR-C with 2 CCP domains in A. aegypti that efficiently recognizes DENV and recruits AaMCR to stimulate the expression of antimicrobial peptides (AMPs). Our findings suggest a new pattern recognition receptor pathway that senses flaviviruses and initiates an antiviral cytokine-like response in A. aegypti.
Results
Phylogenetic analysis of MCR factors in A. aegypti
The proteins of the TEP superfamily are key constituents of the immune systems of both vertebrates and invertebrates. TEPs are generally divided into 3 distinct subfamilies: complement C3/C4/C5, A2Ms, and iTEPs. Previous studies have suggested that several iTEP genes found in Anopheles and Drosophila play a role in microbial elimination [10], [11]. Furthermore, silencing an A. aegypti TEP homologue (AAEL012267, known previously as AeTEP1) significantly enhances flaviviral infections [7], indicating a potential role of iTEPs in combating the viral infection of insects. Given the complicated immune function of iTEPs, we analyzed the phylogenetic relationships of iTEP proteins among Anopheles gambiae, A. aegypti, and Drosophila melanogaster. MCRs, characterized as a subfamily of iTEPs, cluster along a single branch (Figure 1A). AAEL012267 is grouped into the MCR family, indicating that it is an A. aegypti MCR homologue (see Figure 1A).The AAEL012267-encoded protein is predicted to contain domains that are identical to those of Drosophila MCR (DmMCR), suggesting possible conserved functions of these proteins (Figure 1B). We have therefore re-designated AAEL012267 as AaMCR throughout this study. The thioester domain of iTEPs binds the surface of microbes via a covalent bond and triggers the phagocytosis and opsonization of microbes. However, not all TEPs contain the TE module (Figure 1C). The lack of this domain in MCRs suggests a distinct mechanism of action.
Fig. 1. Comparison of the functional domains and phylogenetic analysis of insect thioester-containing proteins (iTEPs). 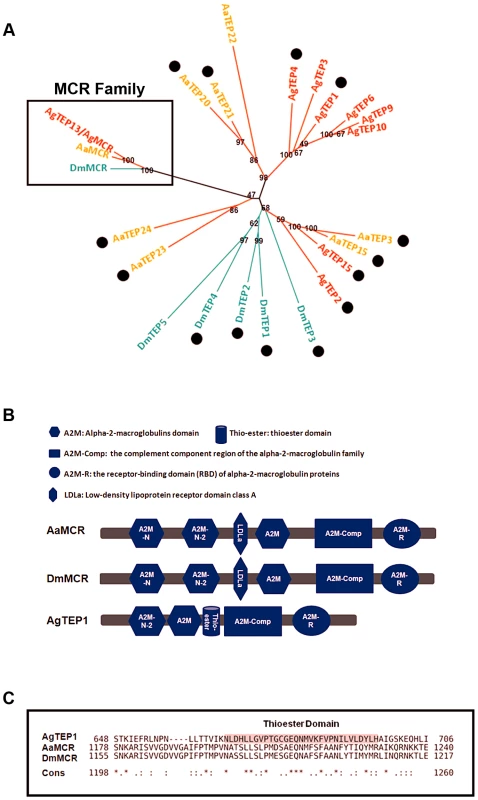
(A) Unrooted phylogenetic tree of iTEPs. The tree was constructed using the neighbour-joining (NJ) method based on the alignment of 23 iTEP protein sequences. The bootstrap values of 500 replicates (%) are indicated on the branch nodes. Anopheles gambiea (Ag), Aedes Aegypti (Aa) and Drosophila melanogaster (Dm) are indicated with red, yellow and green, respectively. Period represents the iTEPs with thioester domain. (B) Schematic representation of AaMCR, DmMCR and AgTEP1. The functional modules of MCRs and TEPs were predicted using the SMART (http://smart.embl-heidelberg.de/smart/set_mode.cgi?GENOMIC=1) and Pfam websites (http://pfam.sanger.ac.uk/). (C) Alignment of the sequence of thioester domain using CLUSTAL-X. The shadowed sequence is the predicted thoiester domain. Asterisk indicates the identical residues in all sequences of the alignment; colon indicates the conserved substitutions; period indicates the semi-conserved substitutions. AaMCR resists the flaviviral infections of A. aegypti
To examine the physiological role of AaMCR in flaviviral infection, we knocked down AaMCR using double-stranded RNA (dsRNA) via thoracic inoculation. The expression of AaMCR was significantly decreased at both the mRNA (Figure 2A) and protein (Figure 2B) levels following dsRNA treatment. Three days after gene silencing, 4 DENV serotypes and YFV were microinjected into the mosquitoes, and their viral loads were assessed 6 days post-infection via quantitative polymerase chain reaction (qPCR) and plaque assay. Consistent with our previous results [7], AaMCR knockdown significantly increased the levels of DENV 1–4 and YFV transcripts (Figure 2C–2G) and the number of virions (Figure S1A–S1E), confirming an important antiviral function of AaMCR in mosquitoes.
Fig. 2. AaMCR restricts flaviviral infections. 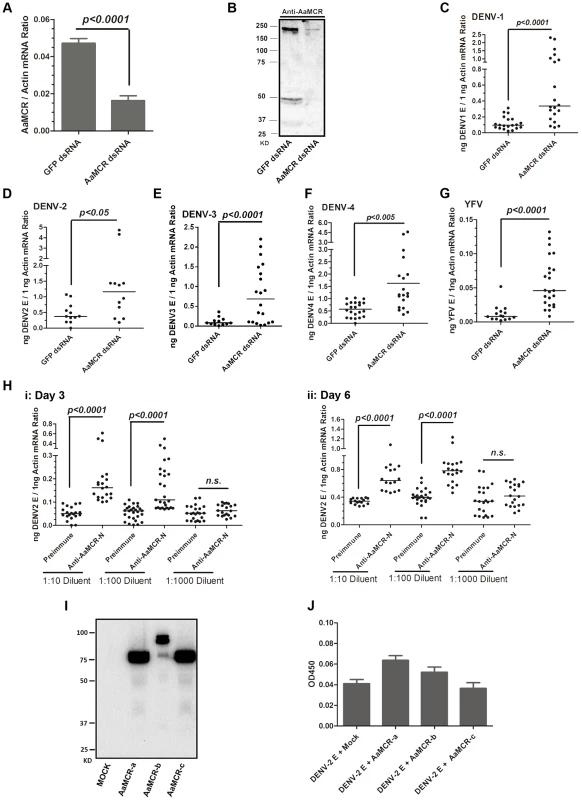
(A-B) Knockdown of the AaMCR gene. The mosquitoes were microinjected with AaMCR or GFP dsRNA. The inoculated mosquitoes were sacrificed, and AaMCR abundance was assessed via qPCR (A) and immuno-blotting with an AaMCR antibody (B) at 6 days post microinjection. 50 μg of protein from mosquito lysates was loaded into each lane (B). (C-G) Silencing of AaMCR enhanced DENVs and YFV (17D) infections in A. aegypti. 10 MID50 DENVs or YFV was inoculated at 3 days post-AaMCR silencing. The viral load was assessed at 6 days post-infection through qPCR and normalized against A. aegypti actin (AAEL011197). The primers and probes used for qPCR are described in Table S2. The experiment was repeated three times with similar results. One dot represents 1 mosquito and the horizontal line represents the median of the results. The data were analyzed statistically by the non-parametric Mann-Whitney test. (H) Immuno-blockade of AaMCR enhanced the DENV-2 infection of A. aegypti. The AaMCR-N antibody, in 10-fold serial dilutions, was premixed with 10 MID50 DENV-2 for thoracic co-microinjection. The treated mosquitoes were sacrificed to examine the viral load at 3 (i) and 6 (ii) days post-infection via qPCR and normalized against A. aegypti actin. The results were pooled from 3 independent experiments. One dot represents 1 mosquito and the horizontal line represents the median of the results. The data were analyzed statistically using the non-parametric Mann-Whitney test. (I) The expression of AaMCR fragment peptides in Drosophila S2 cells. Three fragments of the AaMCR (a, 30–601 aa; b, 590–1,240 aa; c, 1,200–1,793 aa) with a C-terminal HA tag were cloned into the pMT/BiP/V5-His A vector and expressed in the S2 cell supernatant. The supernatant from empty vector-transfected S2 cells was used as a mock. The recombinant peptides were detected with an anti-HA antibody via western blotting. (J) The fragments of AaMCR do not directly interact with DENV-2 E protein in ELISA. The cell supernatant, including the AaMCR fragments were collected at 48 hrs after transfection. The DENV-2 E protein was expressed and purified from a Drosophila expression system. Each plate well was coated with 2 ug of DENV-2 E. The interaction was determined using an anti-HA antibody. The empty DNA vector-transfected S2 cell supernatant was used as a negative control. The data are expressed as the mean ± standard error from 3 independent experiments. To understand the role of AaMCR in viral infections more thoroughly, we expressed and purified an N-terminal peptide of from AaMCR (AaMCR-N, 30–601 aa) from Escherichia coli (Figure S1F) and generated mouse polyclonal antiserum against this fragment. The antibody recognized a full-length AaMCR of ∼200 kDa and a smaller band of ∼50 kDa in mosquito lysates (Figure S1G) and hemolymph (Figure S1H). To validate the above results, we generated rabbit polyclonal antiserum using 2 synthetic peptides from the N-terminus of AaMCR. The same bands of ∼200 kDa and ∼50 kDa were detected in a mosquito lysates (Figure S1I). These results suggest that AaMCR is likely constitutively and rapidly processed into small active fragments and its cleaved peptides function in soluble form in the hemolymph of A. aegypti. To rule any off-target effects dsRNA-mediated RNA interference and validate the function of AaMCR, we premixed serial dilutions of the AaMCR-N antibody with the DENV-2 virus and thoracically microinjected the mixture into A. aegypti. Blocking the function of AaMCR using this specific antibody dramatically enhanced DENV-2 infectivity in mosquitoes at both 3 days (Figure 2H, i) and 6 days (Figure 2H, ii) post-inoculation.
Previous reports have demonstrated a direct interaction between iTEPs and pathogens via a TE bond. Anopheles TEP1 kills P. berghei ookinetes by binding to their surface proteins [5]. We therefore assessed the interaction between AaMCR and DENV-2. AaMCR is a 1,793-aa protein comprised of multiple complement-like domains but lacks an internal TE domain (Figure 1B). According to the predicted domains, we divided AaMCR into 3 fragments (AaMCR-a, -b, -c; Figure S1J) to be expressed in Drosophila S2 cells (Figure 2I). None of the 3 segments of AaMCR interacted directly with the DENV-2 envelope (E) protein in enzyme-linked immunosorbent assays (ELISAs) (Figure 2J) or co-immunoprecipitation (co-IP) assays (data not shown). We also assessed AaMCR mRNA expression in various mosquito tissues. We noted that the abundance of AaMCR mRNA varied among the tissues, with the highest abundance being found in the salivary glands, followed by the hemocytes and midgut. However, DENV-2 infection did not induce AaMCR mRNA expression in the investigated tissues at serial time points (Figure S1K).
Proteins containing CCP domains restrict the flaviviral infections of A. aegypti
The CCP domain is an evolutionarily conserved module in the complement system that is essential for reactions in the complement cascade. AaMCR shares high homology with mammalian C3/C4/C5 complement factors. The cleaved fragments of C3 and C4 are recognized by the CCP domains of the complement receptors [29]. However, the CCP domains of several membrane proteins can be manipulated by microbial pathogens to be used as receptors for cellular entry [16], [17]. Our results show that AaMCR does not bind directly to DENV-2, indicating that adaptor molecules may exist to facilitate DENV recognition by AaMCR. We therefore speculated that proteins with CCP domains found in A. aegypti may play a role in the AaMCR-mediated antiviral response.
To test this hypothesis, we identified a family of 10 proteins with CCP domains in A. aegypti (Table S1). These 10 CCP genes were knocked down individually via thoracic microinjection of dsRNA. DENV-2 was then inoculated into the mosquitoes, and the resultant viral loads were quantified through qPCR 6 days after infection. Individual knockdown of 7 CCP genes in mosquitoes significantly enhanced the DENV-2 burden compared with that found in green fluorescent protein (GFP) dsRNA-treated mosquitoes (Figure 3A). Moreover, the silencing of 6 CCP genes increased mosquito susceptibility to YFV (17D vaccine strain) infection (Figure 3B), indicating a general antiviral role for the CCP genes of A. aegypti. Among all of the identified family members, AAEL006361 exhibited the most significant effect on DENV-2 infection. Viral loads were enhanced by ∼4 folds in AAEL006361 dsRNA-treated mosquitoes compared with the GFP dsRNA group (see Figure 3A and 3B). AAEL006361 was effectively silenced at both the mRNA (Figure S2A) and protein (Figure S2B) levels, indicating that the enhancement of DENV infection was correlated with dsRNA inoculation. Similar results were observed for other DENV serotypes (Figure S2C and S2D).
Fig. 3. The role of CCP domain-containing proteins in flaviviral infection. 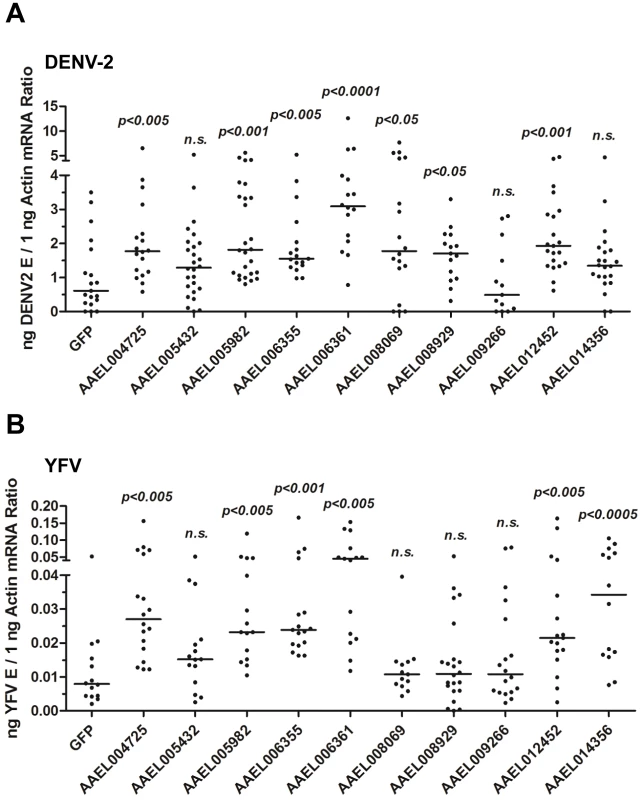
Each CCP gene was silenced through thoracic microinjection of dsRNA and the effect on the viral burden was assessed on 6 days post-DENV-2 (A) or YFV (B) infection. GFP dsRNA-inoculated mosquitoes served as mock controls. The viral loads were measured via qPCR, and normalized against A. aegypti actin. One dot represents 1 mosquito and the horizontal line represents the median. The data were analyzed statistically using the non-parametric Mann-Whitney test. The results were combined from 2 independent experiments. AAEL006361 is an SR that recognizes flaviviruses in vitro and in vivo
AAEL006361 encodes a trans-membrane protein with a signal peptide that contains 2 CCP domains in its extracellular region. Sequence analysis that AAEL006361 is a homologue of Drosophila SR-C (DmSR-C; Figure 4A), and the protein encoded by AAEL006361 presents the same highly conserved domains as DmSR-C (Figure 4B). We therefore designated AAEL006361 as A. aegypti SR-C (AaSR-C) in this study. To detect the endogenous expression of AaSR-C, we generated a rabbit polyclonal anti-AaSR-C antibody against the extracellular region. A ∼50-kDa protein band was detected in mosquito lysates (Figure S3A), and its size corresponded to the predicted full length of AaSR-C. We therefore cloned and expressed the extracellular region of AaSR-C (AaSR-C-Ex, 23–400 aa) in a Drosophila expression system. The recombinant peptide was then purified and detected through immunoblotting (Figure 4C, left panel) and Coomassie Blue staining (Figure 4C, right panel).
Fig. 4. A Scavenger receptor-C with CCP domains recognizes DENV-2 in vitro and in vivo. 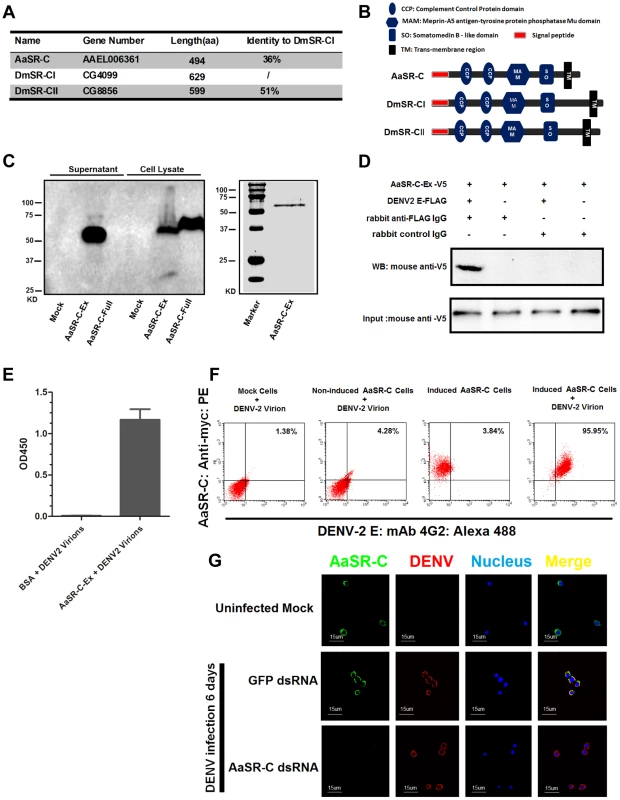
(A) Percentage of amino acid identity of insect SR-Cs. (B) Schematic representation of SR-Cs in A. aegypti and D. melanogaster. The functional modules were predicted in SMART (http://smart.embl-heidelberg.de/smart/set_mode.cgi?GENOMIC=1) and Pfam websites (http://pfam.sanger.ac.uk/). (C) Expression and purification of AaSR-C from Drosophila S2 cells. The extracellular region (AaSR-C-Ex) or full length (AaSR-C-Full) AaSR-C was cloned into the pMT/BiP/V5-His-A expression vector. The recombinant plasmids were transfected into Drosophila S2 cells, and their expression was probed using an anti-V5 mAb. The supernatant or lysates from mock-transfected S2 cells was used as the mock control (Left panel). Recombinant AaSR-C-Ex, produced in Drosophila cells, was purified using an Ni-His column (Right panel). (D) AaSR-C-Ex interacted with DENV-2 E proteins in co-immunoprecipitation assay. Purified AaSR-C-Ex (V5) and DENV-2 E (FLAG) were used to investigate the interaction of the proteins. Control rabbit IgG was used as a mock control to exclude non-specific interactions. The protein complex was pulled down with a rabbit anti-FLAG antibody and detected using a mouse anti-V5 antibody. We reproduced the experiment 3 times. (E) AaSR-C-Ex captured DENV-2 virions in an ELISA. Binding was probed using the flavivirus E mAb 4G2. The data are presented as the mean ± standard error. The experiment was reproduced 3 times. (F) AaSR-C bound DENV-2 virions on the cell surface. A Cu2+-inducible stable S2 cell line was generated to express the full-length AaSR-C. DENV-2 virions were incubated with AaSR-C expressing cells at 4°C for 1 hr. Non-induced stable cells and empty vector-transfected S2 cells containing virions served as the mock control groups. The interaction between AaSR-C and DENV was measured through flow cytometry. The DENV virions were stained using the flaviviral E mAb 4G2 and anti-mouse IgG Alexa-488; AaSR-C was probed using a Myc mAb and anti-rabbit IgG Phycoerythrin (PE). The data was analyzed using FlowJo software. The presented data was representative of 3 independent experiments with similar results. (G) The in vivo association between AaSR-C and DENV-2 in A.aegypti hemocytes. Hemolymph was collected from uninfected mosquitoes, AaSR-C silenced infected mosquitoes and GFP dsRNA treated infected mosquitoes to undergo immunofluorescence staining. AaSR-C was stained with anti-rabbit IgG Alexa-488 (Green), and the DENV-2 E protein was identified using anti-mouse IgG Alexa-546 (Red). Nuclei were stained blue with To-Pro-3 iodide (Blue). Images were examined using a Zeiss LSM 780 meta confocal 63×objective lens. To understand the antiviral mechanism of AaSR-C, we next assessed whether AaSR-C binds directly to DENV-2. The purified AaSR-C-Ex peptide interacted strongly with the DENV-2 E protein in a co-IP assay (Figure 4D). Purified AaSR-C-Ex also captured DENV-2 virions efficiently (Figure 4E). AaSR-C was predicted to be a membrane protein (Figure 4B). To verify that DENV virions bind AaSR-C on the cell surface, we cloned and expressed full-length AaSR-C with its trans-membrane region (AaSR-C-Full, 23–494 aa; see Figure 4C, left panel) and constructed a Cu2+-inducible AaSR-C-expressing stable cell line. AaSR-C-expressing cells were incubated with DENV-2 virions, and their association with the cell surface was examined using flow cytometry. Non-induced stable cells and empty vector-transfected S2 cells were included as controls. More than 90% of the AaSR-C-expressing cells were bound by DENV-2 virions and showed double-positive staining compared with a negligible number of double-stained cells that were observed in the controls (Figure 4F). We next performed immunofluorescence staining to explore the location of native AaSR-C and DENV-2 virions. AaSR-C was highly expressed on the surface of hemocytes (Figure 4G, the 1st row). Following AaSR-C silencing, the polyclonal antibody against AaSR-C did not detect endogenous AaSR-C expression on hemocytes (Figure 4G, the 3rd row), indicating the high specificity of the antibody. Overlapping localization of AaSR-C and DENV was observed on mosquito hemocytes (Figure 4G, the 2nd row), suggesting that both AaSR-C and DENV locate to function on the cell surface. In agreement with the immunofluorescence staining results, AaSR-C transcripts were found to be more highly expressed in hemocytes compared with salivary glands and the midgut (Figure S3B). We also noted that AaSR-C mRNA expression remained unchanged after DENV-2 infection (Figure S3B).
AaSR-C acts in conjunction with AaMCR in a pathway that opposes DENV infection
In the mammalian complement system, several complement proteins have CCP-mediated interaction with C3 and C4 cleaved fragments. In the present study, we assumed that AaSR-C may also interact with AaMCR in the process of viral elimination. Three fragments (AaMCR-a to AaMCR-c) arranged from the N - to C-terminus were expressed in Drosophila S2 cells (see Figure 2I). The binding of AaMCR segments to AaSR-C was then determined through co-IP assays. AaSR-C interacted strongly with the N-terminal of AaMCR-a (30-601 aa). However, no interaction was observed between AaSR-C and AaMCR-b (590–1,240 aa) or AaMCR-c (1,200–1,793 aa) (Figure 5A). We next expressed and purified AaMCR-a in a Drosophila expression system (Figure 5B, left panel). The purification of the protein was confirmed through western blotting using an anti-HA tag monoclonal antibody (see Figure 5B, right panel). The purified AaMCR-a interacted with AaSR-C-Ex in an ELISA (Figure S4A) and a co-IP assay (Figure S4B).
Fig. 5. AaMCR and AaSR-C function in a pathway that opposes DENV-2 infection. 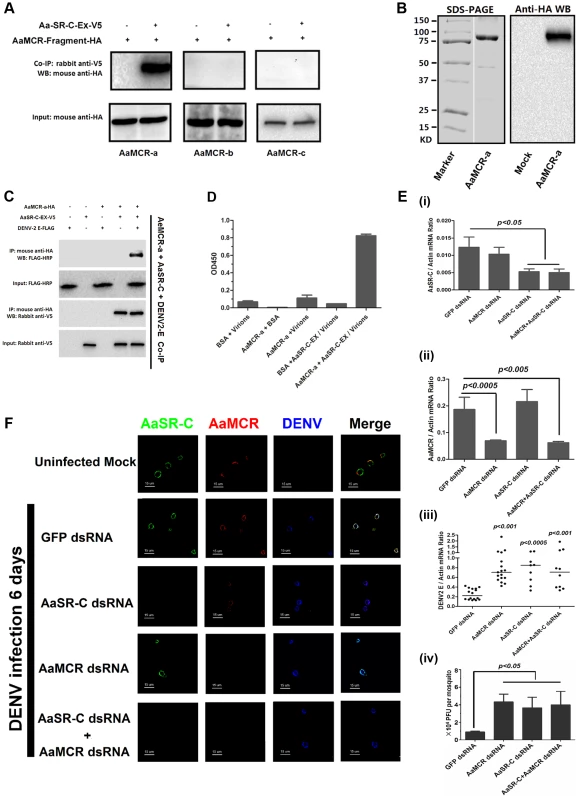
(A) The interaction between 3 AaMCR fragments and AaSR-C in co-IP assays. Three AaMCR gene fragments were cloned into the pMT/BiP/V5-His A vector. The recombinant plasmids were transiently transfected into S2 cells. The cell supernatant was used for investigation of the AaMCR/AaSR-C interaction. The protein complex was pulled down with a rabbit anti-V5 antibody and probed using a mouse anti-HA antibody. The experiment was reproduced 3 times. (B) Expression and purification of AaMCR-a in Drosophila S2 cells. The purified AaMCR-a was separated through SDS-PAGE (Left Panel) and detected with an anti-HA antibody via western blotting (Right Panel). The supernatant from empty vector-transfected S2 cells was used as the mock control. (C) AaSR-C-Ex acted as an adaptor in the interaction between the AaMCR-a and DENV-2 E proteins. The purified AaSR-C-Ex, AaMCR-a and DENV-2 E proteins were mixed and pulled down with a mouse anti-HA antibody (AaMCR-a) and detected using a rabbit anti-V5 antibody (AaSR-C) and anti-FLAG-HRP antibody (DENV-2 E). The experiment was repeated 3 times with similar results. (D) AaSR-C-Ex connected AaMCR-a to DENV-2 virions. Purified AaMCR-a or BSA was pre-coated into the ELISA plate wells. DENV-2 virions either mixed with AaSR-C-Ex or without AaSR-C-Ex were added to the protein-coated wells. The signal was detected using the flavivirus E mAb 4G2. The data are expressed as the mean ± standard error. The experiment was reproduced by 3 times with similar results. (E) Double knockdown of AaMCR and AaSR-C showed similar effects on DENV-2 infection to individual knockdown. Both AaSR-C (i) and AaMCR (ii) were knocked down using a dsRNA mixture in the AaSR-C/AaMCR co-silenced group. DENV-2 replication and the numbers of infectious DENV-2 virions in the mosquitoes were measured via qPCR (iii) and plaque assays (iv). Statistical analysis was performed using the non-parametric Mann-Whitney test. The data on gene silencing (i, ii) and from plaque assays (iv) are expressed as the mean ± standard error. The horizontal line depicts the median (iii). Each dot represents an individual mosquito. The result was representative of 3 independent experiments. (F) Immunostaining of AaMCR, AaSR-C and DENV-2 in A. aegypti hemocytes. The hemocytes were dissected from uninfected mosquitoes, AaMCR and/or AaSR-C silenced infected mosquitoes and GFP dsRNA treated infected mosquitoes at 6 days post-infection. AaSR-C was stained with anti-rabbit IgG Alexa-488 (Green); AaMCR was probed using anti-mouse IgG Alexa-546 (Red); the DENV-2 E protein was probed with DENV-2 human antiserum (purified IgG) and anti-human IgG Alexa-633 (Blue). Images were examined using a Zeiss LSM 780 meta confocal 63×objective lens. We have demonstrated crucial roles for both AaSR-C and AaMCR in controlling the DENV-2 infection of A. aegypti in addition to a direct interaction of AaSR-C with DENV-2 or the N-terminus of AaMCR. We next examined whether AaSR-C and AaMCR cooperate in a pathway that opposes DENV infection. As shown in Figure 5C, purified DENV-2 E, AaSR-C-Ex, and AaMCR-a formed a complex in co-IP assays, whereas AaMCR-a did not bind directly to the DENV-2 E protein (Figure 5C), suggesting that AaSR-C-Ex functions as a linker to bring the other 2 proteins together. Likewise, AaSR-C-Ex was shown to be necessary for AaMCR-a binding to DENV-2 virions in an ELISA (Figure 5D). To determine the relationship between AaSR-C and AaMCR in DENV infection, we co-silenced AaSR-C (Figure 5E, i) and AaMCR (see Figure 5E, ii) in mosquitoes using dsRNA. Double knockdown had the same effect on DENV replication (Figure 5E, iii) and the number of DENV-2 virions (Figure 5E, iv) as single knockdown, suggesting that AaSR-C and AaMCR function in the same pathway opposing the DENV infection of A. aegypti.
In the above experiments, the mosquitoes were infected through intra-thoracic inoculation. To further evaluate the dissemination of DENV in various mosquito tissues following the infection by oral feeding, we silenced AaMCR and AaSR-C, both individually and together, via intra-thoracic microinjection of dsRNA. Three days after the dsRNA treatment, the mosquitoes were fed with Vero cells-generated DENV-2 and fresh human blood and the specific tissues were dissected at serial time points to evaluate the kinetics of viral dissemination. In accordance with our previous results obtained through intra-thoracic infection (Figure 5E, iii), in the oral feeding experiment, silencing AaMCR and/or AaSR-C significantly enhanced DENV-2 burden in whole mosquitoes and various tissues, indicating that the complement-like system works as a general mechanism to limit viral infection in mosquitoes (Figure S5A-S5C). Moreover, the silencing of these complement components increased the number of infectious DENV-2 virions in salivary glands extracts (SGE) as determined in plaque assay (Figure S5D), suggesting that antiviral immunity in mosquitoes may influence their viral transmission capacity.
To understand the association between AaSR-C, AaMCR, and DENV more thoroughly in vivo, we determined their localization in hemocytes using immunofluorescence microscopy. Under an uninfected status, AaMCR showed very little co-localization with AaSR-C on the hemocyte surface (Figure 5F, the 1st row). After infection, AaSR-C co-localized well with AaMCR and DENV on the hemocyte surface (Figure 5F, the 2nd row). Moreover, knockdown of AaMCR eliminated AaMCR staining in hemocytes, demonstrating the specificity of the AaMCR antibody (Figure 5F, the 4th row). Because AaSR-C but not AaMCR binds DENV directly, knockdown of AaMCR did not influence DENV localization to the hemocyte membrane (Figure 5F, the 4th row). Interestingly, AaSR-C silencing reduced, but did not fully abolish the fluorescence staining of AaMCR, indicating that additional proteins may recruit AaMCR to hemocytes. Furthermore, we still observed DENV staining after AaSR-C/AaMCR silencing (Figure 5F). This is because the DENV can still bind to its cellular entry receptors [27] or some other membrane proteins on hemocytes. Without AaSR-C and AaMCR, DENV binding to hemocyte surface does not produce AMPs, but still leads to cellular entry via its receptors, resulting in DENV replication.
AaSR-C/AaMCR induces AMPs expression to oppose DENV infection
The induction of AMPs as a response to pathogen infection is a crucial mechanism of innate immunity in insects [30]. Previous studies have revealed that AMP expression is induced by DENV infection in mosquitoes, and AMPs exert antiviral activities against many types of viral infections [31], [32], [33]. We therefore examined whether the AaSR-C/AaMCR axis regulates AMP expression in response to DENV infection. Seventeen AMPs belonging to the defensin (DEF), cecropin (CEC), gambicin, and attacin families were identified through sequence comparison. We measured the abundance of AMP mRNA in DENV-2-infected and uninfected mosquitoes using qPCR after 6 h of infection. The mRNA expression of 7 AMPs was significantly enhanced by DENV-2 infection (more than 2 fold; Table 1). To investigate the influence of the AaSR-C/AaMCR pathway on AMPs regulation, we silenced AaSR-C and AaMCR individually or simultaneously through dsRNA treatment. Three days later, the gene-silenced mosquitoes were infected with DENV-2, and AMPs expression was assessed via qPCR at 6 h post-infection. Among the 7 DENV-induced AMPs, the mRNA abundance of 3 DEFs and 2 CECs was decreased in the AaSR-C - and/or AaMCR-silenced mosquitoes compared with GFP dsRNA mock-treated mosquitoes, suggesting that AaSR-C and AaMCR play vital roles in DENV-mediated AMP induction (Figure 6A). Furthermore, double knockdown of AaSR-C and AaMCR had the same effect on AMP abundance as single knockdown (see Figure 6A), further indicating that AaSR-C and AaMCR function in an immune signaling pathway related to AMPs regulation. To elucidate the antiviral roles of these AMPs, we silenced these 5 AMP genes in A. aegypti and assessed the effect on viral burden 6 days after DENV-2 infection. The expression of these AMPs was significantly decreased by dsRNA treatment (Figure S6). Compared with GFP dsRNA-inoculated mosquitoes, mosquitoes in which DEF C, DEF D, and CEC N were silenced showed 2 - to 3-fold enhancement of the viral burden (Figure 6B). However, knockdown of DEF A and CEC E did not significantly enhance the DENV-2 burden (see Figure 6B). Our data convincingly demonstrate that AaSR-C/AaMCR signaling induces AMPs as effectors involved in DENV limitation in A. aegypti.
Fig. 6. The AaMCR/AaSR-C pathway induces antimicrobial peptides production to control dengue infection. 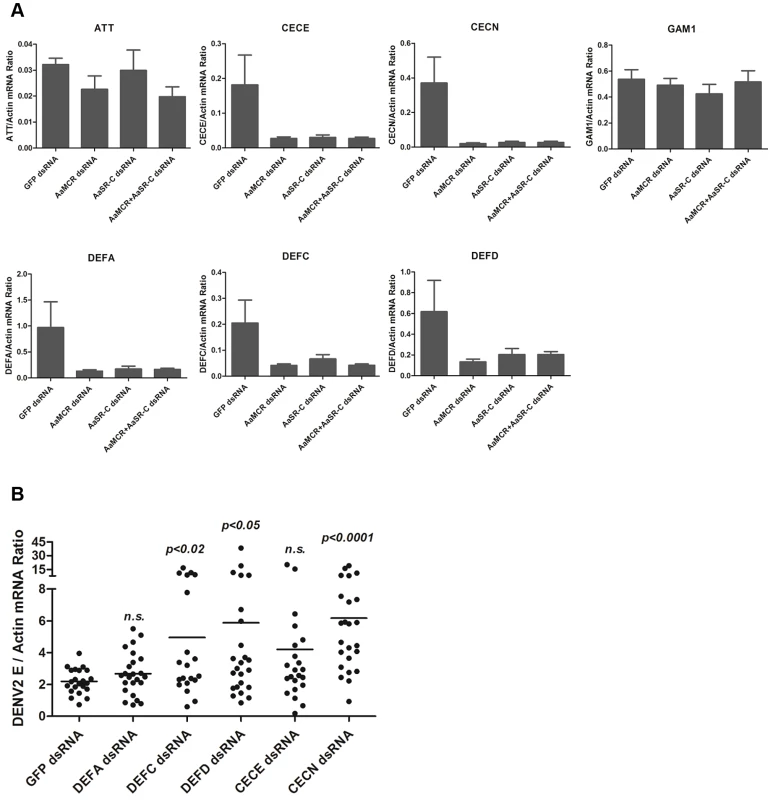
(A) Effects of AaMCR and AaSC-R knockdown on the expression of dengue-induced antimicrobial peptides. AaSR-C and AaMCR were knocked down individually or simultaneously through thoracic microinjection of dsRNA. Three days later, the gene-silenced mosquitoes were infected with (1,000 MID50) of DENV-2, and the AMP mRNA expression was assessed at 6 h post-infection via qPCR. The qPCR primers are provided in Table S2. The data is expressed as the mean± standard error of the results. The experiment was repeated 3 times. (B) Silencing of AMPs enhanced DENV-2 infection in A. aegypti. The 5 AaMCR/AaSR-C-regulated AMPs were knocked down through thoracic microinjection of dsRNA. DENV-2 (10 MID50) was inoculated at 3 days post AMP silencing. The viral load was assessed at 6 days post-infection via qPCR and normalized against A. aegypti actin (AAEL011197). The primers and probes used for qPCR are described in Table S2. The result was pooled from 3 independent experiments. One dot represents 1 mosquito and the horizontal line represents the mean value of the results. The data was analyzed statistically using the non-parametric Mann-Whitney test. Tab. 1. Regulation of antimicrobial peptides abundance in DENV-2 infection of <i>A. aegypti</i>. 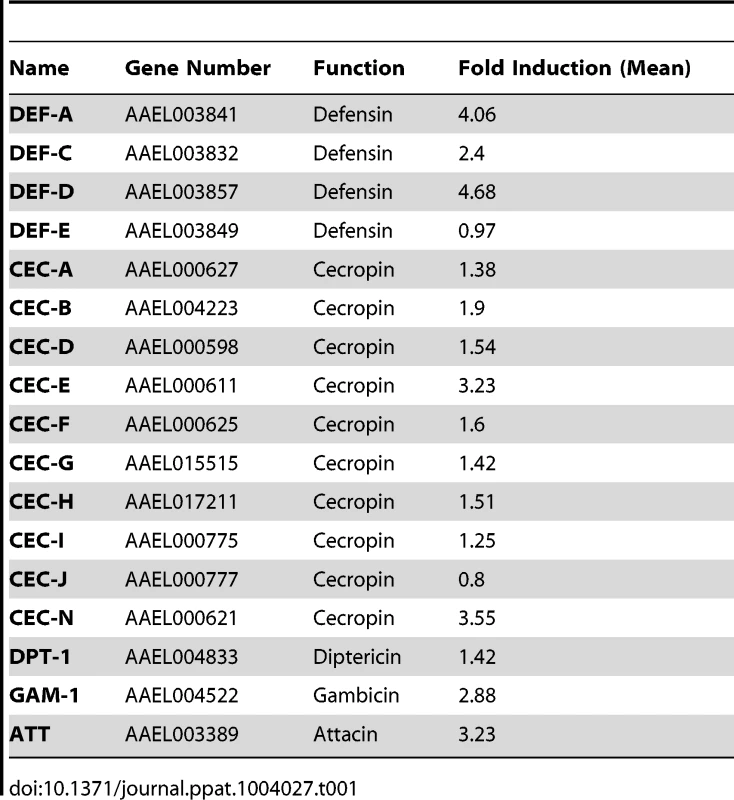
Discussion
Hosts are equipped with sophisticated machineries to detect and eliminate invading pathogens before they cause significant physiological damage. Unlike mammals, which have both innate and adaptive immune systems, insects rely solely on RNA interference and potentially on cytokine-like responses to limit viral infection [34], [35]. Despite lacking an immunoglobulin-based humoral response, insects have a functional complement-like system for warding off invading pathogens. For instance, Anopheles TEP1 eradicates Plasmodium [5], and other Anopheles TEPs recognize E. coli and Staphylococcus aureus to target them for phagocytosis [10]. DmMCR, which shares multiple common domains with insect TEPs and mammalian complement C3/C4/C5, has been observed to opsonize and phagocytize C. albicans [11]. However, whether the TEP-based complement-like system plays a role in mosquito defense against viral agents currently remains unclear.
The human complement system consists of more than 30 secreted and membrane-bound proteins. Invading pathogens are recognize by pattern receptors, including C1q, ficolin, and mannose-binding C-type lectin (MBL), subsequently triggering a complement cascade. However, the mechanism of pathogen recognition in insects may be quite different from that in mammals. Comparative genomic analyses have shown that no homologues of human C1q or ficolin exists in A. aegypti (Cheng G, Xiao XP, unpublished data). Moreover, the extracellular C-type lectins found in mosquitoes, which are the putative homologues of human MBL, have recently been shown to act as cellular receptors facilitating West Nile virus [26] and DENV [27] infection, rather than as antiviral pattern recognition receptors. Herein, we demonstrate that a homologue of macroglobulin complement-related factors (AaMCR) is crucial for restricting flaviviral infections. Silencing of AaMCR or immuno-blockade of AaMCR impaired the antiviral ability of mosquitoes. However, AaMCR does not directly interact with DENV surface proteins, suggesting that the antiviral effect of AaMCR is mediated by a certain particular adaptor. To further explore the essential players in this MCR-based antiviral response, we identified 10 proteins containing CCP domains and investigated their role in flaviviral infection in A. aegypti. Knockdown of AAEL006361 enhanced flaviviral infections in the mosquitoes most significantly. Sequence analyses suggested that the protein encoded by AAEL006361 contains 2 CCP domains at its N-terminus and is a homologue of Drosophila SR-C, which is a well-known pattern recognition receptor of bacterial surface components that initiates an antibacterial phagocytic response [14], [36]. In accordance with these findings, our results show that AaSR-C is capable of binding DENV surface proteins. The virion-bound AaSR-C then recruits the complement-like factor AaMCR, which induces antiviral immune factors such as AMPs to control viral infection. Indeed, in mammals, most CCP-containing complement components function as regulators of the complement cascades. For example, factor H act as a negative regulator of the alternative complement pathway [37], and C4-binding protein (C4BP) functions as an inhibitor of complement C4 [38]. This observation indicates the common distant complement system mechanism between mammals and arthropods.
TEPs generally need to be cleaved into active products following microbial attack. In human complement proteins, the alpha chain of C3 is primarily cleaved into the C3a and C3b active forms after microbial infection. C3a is involved in the inflammatory immune response, whereas C3b is further degraded into C3c and C3d. C3d plays a role in B-cell activation through binding to complement receptor 2. C3c is deposited on the pathogen surface and triggers the phagocytosis and opsonization of pathogens [29], [39]. Anopheles TEP1 is cleaved into 2 fragments of ∼75 kDa and ∼100 kDa in response to septic wounds and microbial infection [40]. However, emerging evidence indicates that not all iTEPs are necessarily proteolytically processed to become active. DmMCR is not cleaved in the Drosophila S2 supernatant, and the full-length protein takes its active form without any proteolytic processing [11]. Ixodes ricinus MCR lacks the lysine-arginine cleavage motif that is a typical feature of C3-complement molecules [6]. In the present study using 2 polyclonal antibodies against the N-terminus of AaMCR, we detected a ∼50-kDa band below the predicted 206-kDa full-length protein in adult female A. aegypti lysates through both reducing and non-reducing sodium dodecyl sulphate polyacrylamide gel electrophoresis (data not shown), indicating that AaMCR is likely constitutively cleaved into its active form for function.
Mammalian complement proteins C3 and C4, which contain TE and multiple A2M domains, are the central players in the complement system opposing pathogen invasion [41], [42]. The TE modules of C3 and C4 bind to the surface of microbes via a covalent bond, resulting in the phagocytosis and opsonization of microbes. Activated complement proteins C3 and C4 are deposited on the viral surface and subsequently eliminate virions, independent of phagocytes [41]. Anopheles TEP1, which has been reported to be a key factor in bacterial [10] and parasitic [5] elimination, also interacts with pathogens through its TE bond. However, not all of the TEP-pathogen interactions are apparently mediated by covalent TE bonds. Anopheles TEP3 (AGAP010816), which is an insect TEP without a TE domain [6], [43], recognizes Gram-negative bacteria for phagocytosis [10]. DmMCR, which also lacks a TE domain, interacts with C. albicans for pathogen eradication [11]. These observations imply an unknown alternative mechanism for the recognition of pathogens by iTEPs. Indeed, as demonstrated by our data, AaMCR, a mosquito TEP without a TE domain, recognizes DENV via AaSR-C to enhance AMP production. Given the established interaction between CCP domains and the cleaved C3 fragment or the surface components of pathogens, we speculate that the CCP domains of AaSR-C may play central role in the formation of the AaMCR/AaSR-C/virus complex.
AMPs are evolutionarily conserved components of the innate immune response. Mammalian AMPs have been demonstrated to efficiently eliminate bacteria, enveloped viruses, and fungi [44]. Given their conserved sequences, insect AMPs may play a crucial role in antiviral defense. In fact, a CEC-like peptide was recently found to be induced by DENV infection and to limit the virus in the salivary gland of A. aegypti [32]. Several AMPs belonging to the CEC and DEF families have been shown to present anti-DENV activity in A. aegypti [30]. In agreement with these findings, our results revealed that 7 mosquito AMPs are upregulated by DENV-2 infection, among which 3 DEFs and 2 CECs are regulated by the AaMCR-AaSR-C signalling pathway. Multiple AMPs exert antiviral activity against DENV infection. Considering the data obtained in the present study, we propose the following antiviral immune signalling pathway in A. aegypti: (1) viral recognition: AaSR-C recognizes the flaviviral surface protein and subsequently recruits AaMCR to form a protein-virus complex; (2) AMP induction: the conjugation of AaSR-C and AaMCR with viruses triggers an immune signalling pathway to stimulate AMPs expression; and (3) viral inactivation: the positive charges of AMPs can electrostatically or hydrophobically associate with the components of the viral surface [45], subsequently resulting in the flaviviral inactivation.
Similar to Drosophila, mosquitoes equip at least three immune signalling cascades: the Toll, Immune Deficiency (Imd), and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathways to fight pathogenic invasion [31], [46], [47], [48]. Compared with the Imd pathway, the Toll and JAK-STAT pathways are more specific for response to viruses [31], [48]. Toll pathway is activated in response to DENV infection in A. aegypti, which triggers robust expression of several Toll pathway marker genes, such as attacin, defensins, and cecropins, to control DENV replication in mosquito tissues [49]. The JAK-STAT pathway has also been implicated in anti-viral defenses [48]. Moreover, Dong et al. showed that both the Toll and JAK-STAT pathways are activated by Beauveria bassiana infection, leading to resistance of dengue infection in A. aegypti [49]. Previous studies implied that both the Toll and JAK-STAT pathways may be regulated at least in part by the same signal transduction cascade. Consistent with this hypothesis, the JAK-STAT pathway can be indirectly activated by the Toll cascade in D. melanogaster [50]. Here, our results revealed that several mosquito AMPs, regulated by the AaMCR/AaSR-C system, are also modulated by Toll/JAK-STAT responses [49]. Given the partial overlap between Toll/JAK-STAT and AaMCR/AaSR-C regulated AMPs, we speculate that complement-like factors may directly or indirectly function together with the Toll/JAK-STAT pathways. The relationship between complement-like factors and the Toll/JAK-STAT signaling pathways remains to be elucidated in the future.
The antiviral systems of mosquitoes limit viral infection and prevent invading pathogens from causing significant physiological damage in tissues. Our results presented above show that several complement-like factors play a vital role in resistance against dengue infection in A. aegypti. These factors are unable to eliminate viruses from mosquitoes but can limit the viral burden to a tolerable level that does not elicit significant tissue damages in mosquitoes and that allows successful viral transmission. Without these antiviral mechanisms, viral replication could cause damages to mosquito physiology and thus decrease the mosquito life span. Previous studies have demonstrated a negative correlation between viral titers and mosquito longevity [51], [52]. We examined the life span of individual - or co-AaMCR/AaSR-C dsRNA-treated, DENV-infected mosquitoes and noted that the life span of the AaMCR/AaSR-C silenced mosquitoes was shorter (though not statistically significantly) than that of GFP dsRNA controls following DENV infection (Cheng G, Xiao XP, Unpublished data). Many studies suggest that arboviral infection leads to apoptosis in mosquito tissues. The midgut epithelial cells of Culex pipiens undergo apoptosis after West Nile virus infection [53]. Apoptosis of the salivary glands and midgut of Aedes albopictus was observed following Sindbis virus infection [54]. Moreover, the level of apoptosis is correlated with virus replication in infected cells [55]. We assessed apoptosis in mosquito tissues by TUNEL staining, and noted that the number of apoptotic cells in AaMCR/AaSR-C silenced mosquitoes was greater than in GFP dsRNA controls (Cheng G, Xiao XP, Unpublished data). It is plausible based on the above observations that the antiviral complement mechanism favors DENV transmission and maintenance in nature by extending the life span of DENV infected mosquitoes.
In summary, we have elucidated the antiviral activity of a system composed of the factors, involving multiple complement-related proteins in A. aegypti. A similar antiviral mechanism may be present in other arthropods such as Drosophila and Ixodes, which also exhibit MCR and SR-C homologues. Understanding the antiviral mechanisms of arthropods may provide novel strategies for limiting arboviral transmission in nature.
Materials and Methods
Ethics statement
Collection of human blood samples was conducted with approval of the local ethics committee at Tsinghua University. Human blood taken from healthy donors, who provided written informed consent, was used for mosquito blood feeding.
Mosquitoes, cells, and viruses
Aedes aegypti (the Rockefeller strain) was maintained in a low-temperature illuminated incubator, model 818 (Thermo Electron Corporation, Waltham, MA) at 26°C and 80% humidity according to standard rearing procedures [31]. Aedes albopictus C6/36 cells were grown at 30°C in Dulbecco's modified Eagle's medium for DENVs and YFV production. Drosophila melanogaster S2 cells were cultured in Schneider's medium. All media were supplemented with 10% heat-inactivated foetal bovine serum, 1% L-glutamine, and 100 U/mL each of penicillin and streptomycin. Flaviviruses, including DENV-1 (Hawaii strain), DENV-2 (New Guinea C strain), DENV-3 (Guangdong strain), DENV-4 (H241 strain), and YFV (17D vaccine strain) were passaged in A. albopictus C6/36 cells and stocked in a −80°C ultra-freezer. The viruses for in vivo experiments were titrated to 50% mosquito infective dose (MID50). The MID50 measurement procedure has been described previously [26].
Antibodies
A flavivirus E protein 4G2 monoclonal antibody was generated from a hybridoma cell line (ATCC, D1-4G2-4-15) [56]. The antibodies for tags were all purchased from Medical and Biological Laboratories Co., LTD. (Nagoya, Japan). Anti-mouse immunoglobulin G (IgG)-Alexa 488, anti-rabbit IgG-Alexa 546, and anti-human IgG-Alexa 633 were obtained from Invitrogen (Carlsbad, CA) and anti-rabbit IgG-phycoerythrin was purchased from eBioscience (San Diego, CA). The polyclonal antibodies for AaSR-C were produced in rabbits. Owing to inefficient expression of AaSR-C in E. coli (data not shown), we synthesised 2 peptides in the AaSR-C extracellular region (CSSGDASRRARKTGP and CNSNGTSEEPVTESD) for immunization. The polyclonal antibody was generated with 3 boosts. Murine anti-AaMCR-N polyclonal antibody was produced by the N-terminal peptide of AaMCR (AaMCR-N, 30–601 aa). AaMCR-N was amplified and cloned into pET28 vector for expression in E. coli. The cloning primers are shown in Table S2. An abundant protein expressed in inclusion bodies was washed and dissolved in 8 M urea solution and the protein was purified with a 6×His affinity column. The purified AaMCR-N protein in 2 M urea solution was inoculated into mice for polyclonal antibody production. The other AaMCR antibody (AaMCR-SP antibody), immunised with 2 synthesised peptides of the N-terminus (YNPLDPFNRQRYNP and RPRTFNPQSNRNVF), was generated in rabbits with 3 boosts.
Bioinformatics
The sequences of TEP genes in A. gambiae (Ag), A. Aegypti (Aa), and D. melanogaster (Dm) were obtained from VectorBase (https://www.vectorbase.org/) and Flybase (http://flybase.org). The unrooted phylogenetic tree was built with the neighbour-joining method [57] using MEGA 5.2.2 software based on the alignment of the sequences determined using MUSCLE [58]. The bootstrap consensus tree was inferred from 500 replicates. The functional modules of MCRs, TEPs, and SR-Cs were predicted using the SMART (http://smart.embl-heidelberg.de/smart/set_mode.cgi?GENOMIC=1) and Pfam websites (http://pfam.sanger.ac.uk/). The sequence accession numbers of iTEP genes are AgTEP1, AGAP010815; AgTEP2, AGAP008366; AgTEP3, AGAP010816; AgTEP4, AGAP010812; AgTEP6, AGAP010814; AgTEP9, AGAP010830; AgTEP10, AGAP010819; AgTEP13/AgMCR, AGAP008407; AgTEP15, AGAP008364; AaMCR, AAEL012267; AaTEP2, AAEL008607; AaTEP15, AAEL014755; AaTEP20, AAEL001794; AaTEP21, AAEL001802; AaTEP22, AAEL000087; AaTEP23, AAEL001163; AaTEP24, AAEL017023; DmTEP1, CG18096; DmTEP2, CG7052; DmTEP3, CG7068; DmTEP4, CG10363; DmTEP5, CG13079; DmMCR, CG7586. AaMCR and DmMCR were named as AaTEP13 and DmTEP6 in the previous study [43].
Gene silencing and viral infection in A. aegypti
Genes were silenced in vivo via dsRNA thoracic microinjection. dsRNA synthesis has been described previously [26], [59]. The primers for dsRNA synthesis are shown in Table S2. The quality of dsRNA was checked after annealing via gel electrophoresis. We have described elsewhere the detailed procedures for gene silencing and viral challenge in A. aegypti [26]. Briefly, female A. aegypti mosquitoes were cold-anaesthetised on a cold tray, and 1 μg/300 nL of dsRNA was injected into their thoraxes. The injected mosquitoes were allowed to recover 3 days under standard rearing conditions for viral infection. The mosquitoes were thoracically microinjected with 10 MID50/300 nL (for functional investigation) or 1,000 MID50/300 nL viruses (for the detection of gene expression) and then were maintained in double containers for additional investigations.
To investigate the role of AaMCR using an immuno-blocking approach, we premixed the serial diluents of AaMCR-N polyclonal antibodies with 10 MID50 DENV-2 and microinjected the combination into the thoraxes of mosquitoes. The treated mosquitoes were reared in double containers under standard condition. At 3 and 6 days post-infection, the inoculated mosquitoes were killed and the total RNA from the whole body was isolated to assess DENV burden with qPCR. The primers for DENV-2 detection are shown in Table S2.
Sample preparation for immuno-blotting
Hemolymph was collected into denaturing protein loading buffer (Thermo Scientific, 26149) through proboscis clipping [60]. For the preparation of whole mosquito extracts, three mosquitoes were collected in a single tube and homogenized in 200 μl protein lysis buffer/protease inhibitor cocktail (Thermo Scientific, 1862209) using a pestle grinder system (FisherSci, 03-392-106). The lysates was centrifuged to remove debris and lipids. Hemolymph and whole mosquito extracts were denatured in protein loading buffer at 65°C for 5 min for SDS-PAGE and immuno-blotting [40], [60].
Detection of viral burden in mosquitoes by qPCR
The whole body of infected mosquitoes was homogenised in Buffer I of RNA isolation kit (Qiagen, 74106) with a Pestle Grinder System (FisherSci, 03-392-106). The detailed procedure of total RNA isolation is described in the kit manual. Complementary DNA (cDNA) was randomly reverse-transcribed using a cDNA Synthesis Kit (Bio-Rad, 1708891). The viral burden was then quantified with qPCR. The primers and probes for DENV 1-4 E and YFV E genes are shown in Table S2. The amount of virus was normalised with A. aegypti actin (AAEL011197).
Detection of the AMP abundance in A. aegypti
We measured AMP abundance in DENV-2 infected or mock mosquitoes using qPCR after 6 h of infection. DENV-2 virus (1,000 MID50) was thoracically inoculated into mosquitoes. The mock mosquitoes were microinjected with phosphate-buffered saline (PBS). Six hours post-viral inoculation, the whole bodies of mosquitoes were homogenised for total RNA isolation. cDNA was randomly reverse-transcribed with a cDNA Synthesis Kit. The abundance of AMP genes was quantified with qPCR. The primers for AMPs detection are shown in Table S2. The number of genes was normalized with A. aegypti actin.
Protein generation in a Drosophila expression system
The genes of AaMCR and AaSR-C-Ex were amplified from a cDNA library of adult female A. aegypti. The PCR fragments were inserted into the pMT/BiP/V5-His A vector (Invitrogen, V4130-20), and the recombinant plasmids were transfected into Drosophila S2 cells in combination with the hygromycin selection vector pCo-Hygro for stable cell construction. The primers for PCR and gene cloning are shown in Table S2. The transfected cells were selected using 300 μg/mL hygromycin-B (Invitrogen, 10687-010) for 4 weeks. The resistant cells were grown in spinner flasks, switched to serum-free medium (GIBCO, Invitrogen, 10486025) for 3 days, and induced with copper sulphate at a final concentration of 500 μM for 4 days. The culture medium was cleared with centrifugation at 1,000 × g for 5 min and collected for protein purification with a metal affinity resin (Clontech, Mountain View, CA, PT1320-1). The protein was eluted with 150 mM imidazole, extensively dialysed against PBS (pH 7.8), and concentrated via centrifugal filtration through a 5-kDa filter (Millipore Corp., Bedford, MA, pLCC07610) [26]. The protein concentration was measured using Protein Assay Dye (Bio-Rad, 500-0006) and a Nanodrop 2000c spectrophotometer. The protein purity was checked with sodium dodecyl sulphate polyacrylamide gel electrophoresis, and the specificity of purification was confirmed with immunoblotting using mouse mAbs (Medical and Biological Laboratories Co., LTD.).
Co-immunoprecipitation
To perform the co-IP assays with purified proteins (see Figures 4D, 5C, and S4B), DENV-2 E, AaMCR-a, or AaSR-C was expressed and purified using a Drosophila expression system. Two micrograms each of the purified proteins was incubated at 4°C for 2 hr. Subsequently, 1 μg of a baited antibody was added, and a 2 hr incubation was performed to pull down the protein complex. For experiments involving cell supernatants (see Figure 5A), recombinant plasmids encoding AaMCR fragments were transiently transfected into Drosophila S2 cells using a transfection kit (Qiagen, 301425). Following copper induction, the cell supernatant was collected 48 hr after transfection, and AaMCR expression was detected via western blotting using an anti-HA antibody. The supernatant was incubated with 5 μg of purified AaSR-C-Ex protein for the co-IP assay. The experimental details of the co-IP assays are described in the Classic IP kit manual (Thermo Scientific, 26146).
ELISA
A microtiter test plate (Nunc, Roskilde, Denmark) was coated with 2 μg of bait protein overnight at 4°C. After 5 washes with PBS containing 0.05% Tween 20 (PBST), 5 μg of purified protein or cell lysate was added to each well, followed by incubation at room temperature (RT) for 2 hr. The wells were then washed 5 times with PBST. after which the primary antibody was added, and incubation was continued at RT for 2 hr. The wells were washed again, and 100 μL of a secondary IgG-horseradish peroxidase antibody was added. Following incubation at RT for 1 hr, a commercial peroxidase substrate system was applied (Kirkegaard & Perry Laboratories, Inc., MA, 50-76-11), and the optical density at 450 nm was measured with an ELISA reader.
The interaction between the proteins and DENV-2 virions was also measured via ELISA. In this procedure, the microtiter test plate was coated with 2 μg of recombinant protein at 4°C overnight. After 5 washes with PBST, 2 μg of purified inactivated DENV-2 virions (MicroBix, Canada, EL-22-02-001) in PBS was added to each well, followed by incubation for 2 hr at 4°C. After washing with PBST, a flavivirus E protein 4G2 mAb was added, followed by incubation for an additional 2 hr at 4°C. The analysis followed the procedure outlined above.
Flow cytometry
The full-length AaSR-C was cloned into pMT/BiP/V5-His A for the stable cell construction. An Myc tag was introduced into the N-terminal of AaSR-C protein. AaSR-C expression on the cell surface was induced with copper sulphate at a final concentration of 500 μM. Inactivated DENV-2 virions were incubated with AaSR-C-expressing cells at 4°C for 2 hr, and the non-induced cells or mock cells with DENV-2 virions served as control groups. Cells were then fixed with 4% paraformaldehyde (USB Corporation, Cleveland, OH) and stained with primary and second antibodies. The treated cells were then examined using a FACS Calibur flow cytometer (BD Biosciences, San Diego, CA). Dead cells were excluded on the basis of forward and side light scatter. Data was analysed using FlowJo software.
Immunofluorescence microscopy
Hemocytes were isolated and placed on sialylated slides (PGC Scientific, Gaithersburg, MD), washed in PBS, and fixed in 4% paraformaldehyde at 4°C for 2 h. Detailed mosquito dissection procedures have been described previously [61]. Hemolymph was dried on the sialylated slides. Samples were blocked in PBS with 1% bovine serum albumin and 0.1% Triton X-100 at RT for 2 hr before antibody incubation. After being incubated with primary and secondary antibodies, the slides were imaged with the multitrack mode of Zeiss LSM 780 meta confocal microscope.
Plaque assay
The numbers of DENV and YFV virions were measured in plaque assay. Briefly, mosquitoes were sacrificed on serial days post-infection, and individual mosquitoes were then homogenized in 200 μl PBS using a pestle grinder system (FisherSci, 03-392-106). The samples were centrifuged for 10 min at 4°C and the supernatant were collected for virological assays. Vero cells were seeded into flat-bottomed 12-well plates and grown overnight. The mosquito lysates were serially diluted 10 times (10−1∼10−6) with DMEM and each dilution being included in 3 parallel wells. After 2 hrs at 37°C, the fresh medium was replaced in each well, and the cells were covered with melting Agarose (Lonza, 50302) to be culture for additional days. The cells were stained with 1% crystal violet. The viral titration was calculated based on the number of plaque-forming units.
Supporting Information
Zdroje
1. WeaverSC, BarrettAD (2004) Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol 2 : 789–801.
2. GanesanS, AggarwalK, PaquetteN, SilvermanN (2011) NF-kappaB/Rel proteins and the humoral immune responses of Drosophila melanogaster. Curr Top Microbiol Immunol 349 : 25–60.
3. ShishidoSN, VarahanS, YuanK, LiX, FlemingSD (2012) Humoral innate immune response and disease. Clin Immunol 144 : 142–158.
4. LevashinaEA, MoitaLF, BlandinS, VriendG, LagueuxM, et al. (2001) Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell 104 : 709–718.
5. BlandinS, ShiaoSH, MoitaLF, JanseCJ, WatersAP, et al. (2004) Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116 : 661–670.
6. BuresovaV, HajdusekO, FrantaZ, LoosovaG, GrunclovaL, et al. (2011) Functional genomics of tick thioester-containing proteins reveal the ancient origin of the complement system. J Innate Immun 3 : 623–630.
7. ChengG, LiuL, WangP, ZhangY, ZhaoYO, et al. (2011) An in vivo transfection approach elucidates a role for Aedes aegypti thioester-containing proteins in flaviviral infection. PLoS ONE 6: e22786.
8. DoddsAW, LawSK (1998) The phylogeny and evolution of the thioester bond-containing proteins C3, C4 and α2-macroglobulin. Immunol Rev 166 : 15–26.
9. BlandinS, LevashinaEA (2004) Thioester-containing proteins and insect immunity. Mol Immunol 40 : 903–908.
10. MoitaLF, Wang-SattlerR, MichelK, ZimmermannT, BlandinS, et al. (2005) In vivo identification of novel regulators and conserved pathways of phagocytosis in Anopheles gambiae. Immunity 23 : 65–73.
11. Stroschein-StevensonSL, FoleyE, O’FarrellPH, JohnsonAD (2006) Identification of Drosophila gene products required for phagocytosis of Candida albicans. PLoS Biol 4 : 87–99.
12. Bou AounR, HetruC, TroxlerL, DoucetD, FerrandonD, MattN (2011) Analysis of thioester-containing proteins during the innate immune response of Drosophila melanogaster. J Innate Immun 3 : 52–64.
13. GoughPJ, GordonS (2000) The role of scavenger receptors in the innate immune system. Microbes Infect 2 : 305–311.
14. RämetM, PearsonA, ManfruelliP, LiX, KozielH, et al. (2001) Drosophila scavenger receptor CI is a pattern recognition receptor for bacteria. Immunity 15 : 1027–1038.
15. CasasnovasJM, LarvieM, StehleT (1999) Crystal structure of two CD46 domains reveals an extended measles virus-binding surface. EMBO J 18 : 2911–2922.
16. MolinaH, BrennerC, JacobiS, GorkaJ, CarelJC, et al. (1991) Analysis of Epstein-Barr virus-binding sites on complement receptor 2 (CR2/CD21) using human-mouse chimeras and peptides. At least two distinct sites are necessary for ligand-receptor interaction. J Biol Chem 266 : 12173–12179.
17. DörigRE, MarcilA, ChopraA, RichardsonCD (1993) The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75 : 295–305.
18. StoiberH, ClivioA, DierichMP (1997) Role of complement in HIV infection. Annu Rev Immunol 15 : 649–674.
19. PangburnMK (2000) Host recognition and target differentiation by factor H, a regulator of the alternative pathway of complement. Immunopharmacology 49 : 149–157.
20. Rigau-PérezJG, ClarkGG, GublerDJ, ReiterP, SandersEJ, et al. (1998) Dengue and dengue haemorrhagic fever. Lancet 352 : 971–977.
21. GouldEA, SolomonT (2008) Pathogenic flaviviruses. Lancet 371 : 500–509.
22. RanjitS, KissoonN (2011) Dengue hemorrhagic fever and shock syndromes. Pediatr Crit Care Med 12 : 90–100.
23. HalsteadSB (2008) Dengue virus-mosquito interactions. Annu Rev Entomol 53 : 273–291.
24. GublerDJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11 : 480–496.
25. NeneV, WortmanJR, LawsonD, HaasB, KodiraC, et al. (2007) Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316 : 1718–1723.
26. ChengG, CoxJ, WangP, KrishnanMN, DaiJ, et al. (2010) A C-type lectin collaborates with a CD45 phosphatase homolog to facilitate West Nile virus infection of mosquitoes. Cell 142 : 714–725.
27. LiuY, ZhangFC, LiuJY, XiaoXP, ZhangSY, et al. (2014) Transmission-blocking antibodies against mosquito C-type lectins for dengue prevention. PLoS Pathog 10: e1003931.
28. ColpittsTM, CoxJ, VanlandinghamDL, FeitosaFM, ChengG, et al. (2011) Alterations in the Aedes aegypti transcriptome during infection with West Nile, dengue and yellow fever viruses. PLoS Pathog 7: e1002189.
29. DempseyPW, AllisonME, AkkarajuS, GoodnowCC, FearonDT (1996) C3d of complement as a molecular adjuvant, bridging innate and acquired immunity. Science 271 : 348–350.
30. PanX, ZhouG, WuJ, BianG, LuP, et al. (2011) Wolbachia induces reactive oxygen species (ROS)-dependent activation of the Toll pathway to control dengue virus in the mosquito Aedes aegypti. Proc Natl Acad Sci USA 109: E23–31.
31. XiZ, RamirezJL, DimopoulosG (2008) The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4: e1000098.
32. LuplertlopN, SurasombatpattanaP, PatramoolS, DumasE, WasinpiyamongkolL, et al. (2011) Induction of a peptide with activity against a broad spectrum of pathogens in the Aedes aegypti salivary gland, following infection with dengue virus. PLoS Pathog 7: e1001252.
33. RamirezJL, Souza-NetoJ, Torres CosmeR, RoviraJ, OrtizA, et al. (2012) Reciprocal tripartite interactions between the Aedes aegypti midgut microbiota, innate immune system and dengue virus influences vector competence. PLoS Negl Trop Dis 6: e1561.
34. WangXH, AliyariR, LiWX, LiHW, KimK, et al. (2006) RNA interference directs innate immunity against viruses in adult Drosophila. Science 312 : 452–454.
35. ArjonaA, WangPH, MontgomeryRR, FikrigE (2011) Innate immune control of West Nile virus infection. Cell Microbiol 13 : 1648–1658.
36. PearsonA, LuxA, KriegerM (1995) Expression cloning of dSR-CI, a class C macrophage-specific scavenger receptor from Drosophila melanogaster. Proc Natl Acad Sci USA 92 : 4056–4060.
37. SchmidtCQ, HerbertAP, HockingHG, UhrínD, BarlowPN (2008) Translational mini-review series on complement factor H, structural and functional correlations for factor H. Clin Exp Immunol. 151 : 14–24.
38. LeungE, BlomAM, ClemenzaL, IsenmanDE (2006) The complement regulator C4b-binding protein (C4BP) interacts with both the C4c and C4dg subfragments of the parent C4b ligand, evidence for synergy in C4BP subsite binding. Biochemistry 45 : 8378–8392.
39. MillerEC, ChaseNM, DensenP, HintermeyerMK, CasperJT, et al. (2012) Autoantibody stabilization of the classical pathway C3 convertase leading to C3 deficiency and Neisserial sepsis, C4 nephritic factor revisited. Clin Immunol 145 : 241–250.
40. FraitureM, BaxterRH, SteinertS, ChelliahY, FroletC, et al. (2009) Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5 : 273–284.
41. FuchsA, LinTY, BeasleyDW, StoverCM, SchwaebleWJ, et al. (2010) Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin. Cell Host Microbe 8 : 186–195.
42. AvirutnanP, HauhartRE, MarovichMA, GarredP, AtkinsonJP, et al. (2011) Complement-mediated neutralization of dengue virus requires mannose-binding lectin. MBio 2: e00276–e002711.
43. WaterhouseRM, KriventsevaEV, MeisterS, XiZ, AlvarezKS, et al. (2007) Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316 : 1738–1734.
44. HancockRE, SahlHG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24 : 1551–1557.
45. HancockRE, RobertEW, RozekA (2002) Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett 206 : 143–149.
46. DongY, AguilarR, XiZ, WarrE, MonginE, et al. (2006) Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog 2: e52.
47. GarverLS, DongY, DimopoulosG (2009) Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog 5: e1000335.
48. Souza-NetoJA, SimS, DimopoulosG (2009) An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA 106 : 17841–17846.
49. DongY, MortonJCJr, RamirezJL, Souza-NetoJA, DimopoulosG (2012) The entomopathogenic fungus Beauveria bassiana activate toll and JAK-STAT pathway-controlled effector genes and anti-dengue activity in Aedes aegypti. Insect Biochem Mol Biol 42 : 126–132.
50. LagueuxM, PerrodouE, LevashinaEA, CapovillaM, HoffmannJA (2000) Constitutive expression of a complement-like protein in toll and JAK gain-of-function mutants of Drosophila. Proc Natl Acad Sci USA 97 : 11427–11432.
51. Maciel-de-FreitasR, KoellaJC, Lourenço-de-OliveiraR (2011) Lower survival rate, longevity and fecundity of Aedes aegypti (Diptera: Culicidae) females orally challenged with dengue virus serotype 2. Trans R Soc Trop Med Hyg 105 : 452–458.
52. SylvestreG, GandiniM, Maciel-de-FreitasR (2013) Age-dependent effects of oral infection with dengue virus on Aedes aegypti (Diptera: Culicidae) feeding behavior, survival, oviposition success and fecundity. PLoS ONE 8: e59933.
53. VaidyanathanR, ScottTW (2006) Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis 11 : 1643–1651.
54. KellyEM, MoonDC, BowersDF (2012) Apoptosis in mosquito salivary glands: Sindbis virus-associated and tissue homeostasis. J Gen Virol 93 : 2419–2424.
55. YenYT, ChenHC, LinYD, ShiehCC, Wu-HsiehBA (2008) Enhancement by tumor necrosis factor alpha of dengue virus-induced endothelial cell production of reactive nitrogen and oxygen species is key to hemorrhage development. J Virol 82 : 12312–12324.
56. HenchalEA, GentryMK, McCownJM, BrandtWE (1982) Dengue virus-specific and flavivirus group determinants identified with monoclonal antibodies by indirect immunofluorescence. Am J Trop Med Hyg 31 : 830–836.
57. SaitouN, NeiM (1987) The neighbor-joining method, a new method for reconstructing phylogenetic trees. Mol Biol Evol 4 : 406–425.
58. Edgar RChttp://nar.oxfordjournals.org/content/32/5/1792.short - corresp-1 (2004) MUSCLE, multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32 : 1792–1797.
59. BrackneyDE, FoyBD, OlsonKE (2008) The effects of midgut serine proteases on dengue virus type 2 infectivity of Aedes aegypti. Am J. Trop Med Hyg 79 : 267–274.
60. PovelonesM, WaterhouseRM, KafatosFC, ChristophidesGK (2009) Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324 : 258–261.
61. HanYS, ChunJ, SchwartzA, NelsonS, PaskewitzSM (1999) Induction of mosquito hemolymph proteins in response to immune challenge and wounding. Dev Comp Immunol 23 : 553–562.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 ActivationČlánek Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



