-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Candida albicans, the major fungal pathogen of humans, causes life-threatening infections in immunocompromised individuals. Due to limited available therapy options, this can frequently lead to therapy failure and emergence of drug resistance. To improve current treatment strategies, we have combined comprehensive chemical-genomic screening in Saccharomyces cerevisiae and validation in C. albicans with the goal of identifying compounds that can couple with the fungistatic drug fluconazole to make it fungicidal. Among the genes identified in the yeast screen, we found that only AGE3, which codes for an ADP-ribosylation factor GTPase activating effector protein, abrogates fluconazole tolerance in C. albicans. The age3 mutant was more sensitive to other sterols and cell wall inhibitors, including caspofungin. The deletion of AGE3 in drug resistant clinical isolates and in constitutively active calcineurin signaling mutants restored fluconazole sensitivity. We confirmed chemically the AGE3-dependent drug sensitivity by showing a potent fungicidal synergy between fluconazole and brefeldin A (an inhibitor of the guanine nucleotide exchange factor for ADP ribosylation factors) in wild type C. albicans as well as in drug resistant clinical isolates. Addition of calcineurin inhibitors to the fluconazole/brefeldin A combination only initially improved pathogen killing. Brefeldin A synergized with different drugs in non-albicans Candida species as well as Aspergillus fumigatus. Microarray studies showed that core transcriptional responses to two different drug classes are not significantly altered in age3 mutants. The therapeutic potential of inhibiting ARF activities was demonstrated by in vivo studies that showed age3 mutants are avirulent in wild type mice, attenuated in virulence in immunocompromised mice and that fluconazole treatment was significantly more efficacious when ARF signaling was genetically compromised. This work describes a new, widely conserved, broad-spectrum mechanism involved in fungal drug resistance and virulence and offers a potential route for single or improved combination therapies.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000753
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000753Summary
Candida albicans, the major fungal pathogen of humans, causes life-threatening infections in immunocompromised individuals. Due to limited available therapy options, this can frequently lead to therapy failure and emergence of drug resistance. To improve current treatment strategies, we have combined comprehensive chemical-genomic screening in Saccharomyces cerevisiae and validation in C. albicans with the goal of identifying compounds that can couple with the fungistatic drug fluconazole to make it fungicidal. Among the genes identified in the yeast screen, we found that only AGE3, which codes for an ADP-ribosylation factor GTPase activating effector protein, abrogates fluconazole tolerance in C. albicans. The age3 mutant was more sensitive to other sterols and cell wall inhibitors, including caspofungin. The deletion of AGE3 in drug resistant clinical isolates and in constitutively active calcineurin signaling mutants restored fluconazole sensitivity. We confirmed chemically the AGE3-dependent drug sensitivity by showing a potent fungicidal synergy between fluconazole and brefeldin A (an inhibitor of the guanine nucleotide exchange factor for ADP ribosylation factors) in wild type C. albicans as well as in drug resistant clinical isolates. Addition of calcineurin inhibitors to the fluconazole/brefeldin A combination only initially improved pathogen killing. Brefeldin A synergized with different drugs in non-albicans Candida species as well as Aspergillus fumigatus. Microarray studies showed that core transcriptional responses to two different drug classes are not significantly altered in age3 mutants. The therapeutic potential of inhibiting ARF activities was demonstrated by in vivo studies that showed age3 mutants are avirulent in wild type mice, attenuated in virulence in immunocompromised mice and that fluconazole treatment was significantly more efficacious when ARF signaling was genetically compromised. This work describes a new, widely conserved, broad-spectrum mechanism involved in fungal drug resistance and virulence and offers a potential route for single or improved combination therapies.
Introduction
Invasive fungal infections pose a serious health risk to hospitalized patients worldwide. Particularly affected are immunocompromised individuals with cancer or AIDS, people undergoing organ and hematopoietic stem cell transplantation (HSCT), and those receiving immunosuppressive therapy or implantable prosthetic devices [1],[2]. The growing population of these at-risk groups is reflected in an increase in invasive fungal infection over the last three decades [3]. Annual treatment costs for fungal therapies reach $2.6 billion in the US alone [4]. Despite available therapy options mortality rates approaching 30–50% (Candida species) and 30–80% (Aspergillus species) remain high [5],[6].
Candida and Aspergillus species together account for ∼70% of all invasive fungal infections, with Candida albicans and Aspergillus fumigatus predominating [7],[8],[9]. Currently, three classes of antifungal drugs are suitable for treatment of systemic infections caused by these fungi: polyenes (most notably amphotericin B) and azoles (e.g. fluconazole, FCZ) have been applied for decades, while the echinocandins (e.g. caspofungin, CF) represent a new class of antifungal that has entered treatment regimes over the past 10 years [10],[11]. While these therapy options can be effective, they also exhibit several shortcomings. First, current antifungals target a very limited number of biological processes. The majority of available drugs target ergosterol (polyenes) or inhibit lanosterol 14α-demethylase (azoles), resulting in the accumulation of toxic sterol intermediates that disrupt membrane integrity and lead to membrane stress. Because ergosterol, the major sterol in fungal cell membranes, is analogous to the mammalian lipid cholesterol, this strategy, particularly when amphotericin B is applied, can be problematic due to host toxicity [10]. Another complication of current antifungal strategies is that available drugs each possess a different spectrum of antifungal activities. For instance, azoles are typically fungistatic against pathogenic yeasts such as Candida species, but fungicidal against molds (Aspergillus species). CF, on the other hand, is fungicidal against yeasts and fungistatic against molds [12]. Finally, and most importantly, the small number of treatment options available has resulted in widespread drug resistance in pathogenic species. For each of the three major classes of antifungals (polyenes, azoles, echinocandins) isolation of drug-resistant clinical strains has been reported [11],[12],[13]; azole-resistant Candida, in particular, is now common among isolates from HIV-positive patients [14]. Developing new antifungal strategies, therefore, remains a pressing need.
One approach to satisfy this need is through combination antifungal therapy, where two (or more) agents combined are significantly more efficacious compared to either agent alone. This approach has recently been validated in a randomized, placebo-controlled trial, where approved antifungals were combined with immune regulatory agents [15]. Results from this study suggested that combining ergosterol inhibitors with a recombinant human monoclonal antibody against heat-shock 90 protein (HSP90) showed increased therapeutic benefits compared to monotherapy against Candida infection. Although the precise mechanisms involved remain elusive [16], extensive experiments have further established the benefits of such combinatorial approaches. For instance, a potent synergy resulted when inhibitors of HSP90 (geldanamycin, radicicol) or inhibitors of HSP90's key client protein, calcineurin (cyclosporin A (CsA), FK506) were combined either with azoles or echinocandins against C. albicans [10],[16],[17],[18],[19],[20],[21]. Similarly, pharmacological compromise of HSP90/calcineurin-signaling enhanced the efficaciousness of echinocandin treatment against A. fumigatus in vitro as well as in insect and mouse infection models [16],[17]. Although these examples clearly demonstrate the potential for combination antifungal therapy, human host toxicity associated with inhibition of HSP90 or suppression of the human immune system by CsA/FK506 currently precludes the use of such inhibitors in the clinic [16],[22]. While a non-immunosuppressive FK506 analogue (L-685, 818) has been identified, proprietary restrictions have currently prevented further testing [22]. Therefore, identification of new antifungal targets for optimal fungal killing remains a priority.
One of the challenges of finding new antifungal targets in C. albicans is the lack of sophisticated screening technologies often employed with, for example, Saccharomyces cerevisiae. Various large-scale chemical-genomic drug screening methods are now well established in S. cerevisiae, and have been effective for elucidating drug targets or revealing insights into the modes of action of bioactive compounds [23],[24],[25],[26],[27]. Similar approaches have only recently been applied directly to fungal pathogens [28]. Using S. cerevisiae as a model, we previously performed chemical-genomics to systematically analyze the genetic requirements to survive FCZ treatment [29]. In that work, we identified 22 genes that become essential for S. cerevisiae survival in the presence of FCZ.
Here, we expanded that work with the aim of identifying synergistic drug interactions that render FCZ fungicidal in C. albicans. To this end, we validated the S. cerevisiae FCZ-cidal gene set [29] in C. albicans. From 22 predicted genes, we found that only one gene, AGE3, mediated FCZ tolerance in the pathogen. We further show that both genetic and pharmacological compromise of ARF (ADP ribosylation factor) activities, a process that depends on Age3p, creates sensitivity to all three classes of antifungals used in clinics (polyenes, azoles, echinocandins), overrides clinical drug resistance and the calcineurin pathway, synergizes with fungistatic drugs against the two major pathogenic fungal species (C. albicans and A. fumigatus) and modifies fungal virulence in two established mouse models of candidiasis. Given that drug treatment in mice was significantly more efficacious when ARF activity was genetically compromised, this demonstrates that targeting ARF signaling has potential for antifungal therapies.
Results
Age3p mediates azole tolerance and sensitivity to cell wall inhibitors in C. albicans
To identify genes that become essential for survival in the presence of FCZ, we previously screened the non-essential S. cerevisiae knock-out collection (about 4900 strains) and identified 22 mutants that showed a robust FCZ-cidal phenotype [29]. BLAST searches identified C. albicans homologs for 21 of those S. cerevisiae genes. YDR532c appears to be the only gene that lacks a clear C. albicans homolog (Table S1). Recreating knockout or transposon insertion mutations of the 21 candidate genes in C. albicans, we found that four mutants (bem2, sac6, srb8 and ssn3) showed FCZ sensitivity comparable to WT (Table S2, Figure S1). Twelve C. albicans mutants (57%) showed increased FCZ sensitivity, but all of these mutants could still resume growth when incubated for extended time in the presence of FCZ. Four genes (GCN5, NGG1, ERG11 and NUP84) were linked to a slightly resistant FCZ phenotype. Only one mutant, age3 (ORF19.3683), showed the FCZ sensitive phenotype predicted from the yeast screen. We therefore focused further investigation on AGE3.
We validated the FCZ sensitivity of age3 cells by three different assays. When tested in a minimal inhibitory concentration (MIC) assay, the age3 mutant initially showed similar drug sensitivity as WT and revertant strains at 24 hours. Since FCZ on its own is fungistatic, however, WT cells demonstrated robust growth above the initial MIC point after prolonged incubation (72 hours), a feature referred to as tolerance [19]. In contrast, age3 cells did not resume growth above the 24 hours MIC point, indicating that age3 mutants lost tolerance to FCZ (Figure 1A, Table S2). These results were confirmed visually by growth on solid rich media in the presence of FCZ (Figure 1B). We further characterized the FCZ sensitivity of age3 cells by time-kill curves. Under FCZ treatment, the number of viable age3 cells slightly decreased over time, while growth of WT cells in the presence of FCZ continued (Figure 1C).
Fig. 1. Age3p plays a major role in azole tolerance in C. albicans. 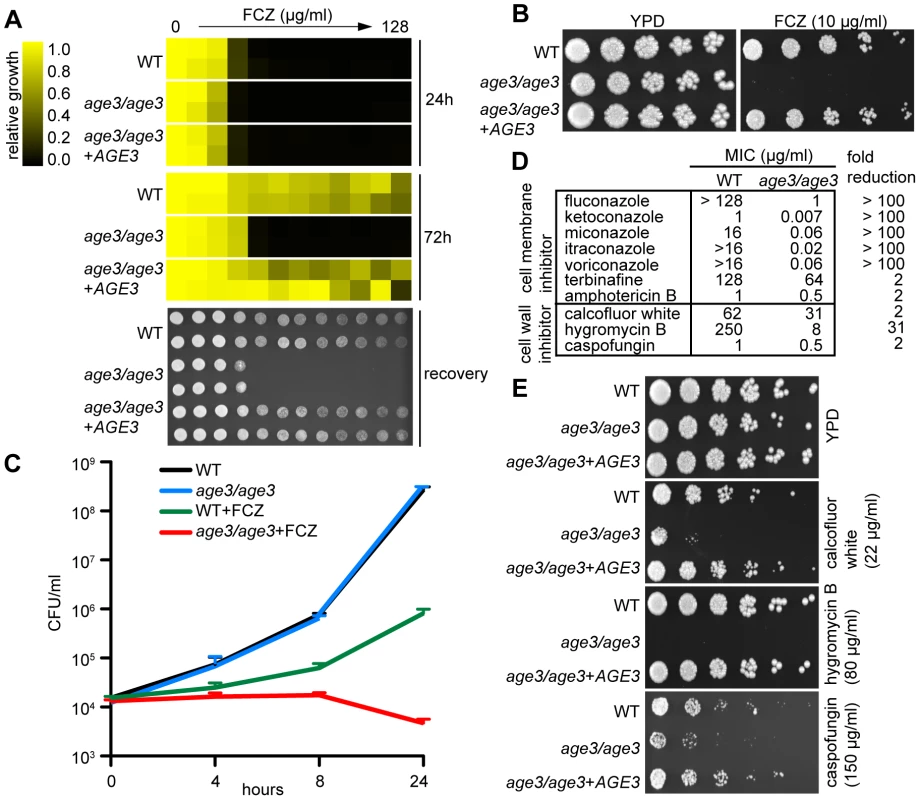
(A) Minimal Inhibitory Concentration (MIC) assay in rich YPD media showing that age3 cells are initially almost equally sensitive to FCZ compared to WT and revertant strains (24 hours reading, top), but fail to grow above this MIC threshold after prolonged incubation (72 hours reading, middle). MIC assays with two-fold serially diluted drug concentrations were done in duplicate and optical densities were normalized to drug-free control wells (see color bar). After 72 hours of incubation, 2 µl of each well of the MIC assay was spotted on fresh YPD media to assess the extent to which cells recover from the drug treatments (bottom). YPD recovery plates were incubated for 24 hours at 30°C. (B) FCZ sensitivity assayed on solid YPD media. No age3 colonies grew on YPD plates containing 24 hours-supra-MIC concentrations of FCZ. Overnight cultures were adjusted to OD600 of 0.1, and then serially diluted four-fold, before 2 µl were spotted on plates. Plates were incubated for 48 hours at 30°C. (C) Time-kill curves in YPD media confirming that knocking out AGE3 abrogates tolerance to FCZ. The number of viable age3 cells decreases slightly over time, while growth of WT cells in the presence of FCZ still occurs. FCZ was used at 10 µg/ml. Shown is the average of two independent experiments plus SD values. Note that age3 cells grow as efficiently as WT cells in the absence of drugs. (D) MIC assays in YPD media shows that age3 mutants are extremely sensitive to numerous azoles after 48 hours and mildly more sensitive to non-azole ergosterol inhibitors (terbinafine, amphotericin B) as well as cell wall inhibitors when compared to WT cells. Fold reduction represents the ratio of the MIC value for WT over the MIC value of the age3 mutant. (E) age3 cells show differential sensitivity to different cell wall inhibitors on YPD media plates. The assay was done as described in (B). We then tested the age3 mutant against a variety of antifungals to gauge the specificity of the mutation. We included various compounds, including second-generation azoles (voriconazole), non-azole ergosterol inhibitors (terbinafine) and other membrane-targeting drugs (amphotericin B). We found that age3 cells showed a generalized increased sensitivity to these compounds (Figure 1D). Among all cell-membrane drugs tested, the azoles caused by far the most significant enhancement in sensitivity in the age3 mutant.
In order to determine the effect of deleting AGE3 on the integrity of the cell wall, we tested the age3 mutant for sensitivity to a variety of cell wall perturbing agents and other agents whose effect have been linked to altered cell wall and glycosylation. The age3 mutant was slightly more sensitive to the β-1,3 glucan synthase inhibitor CF. Similarly, age3 cells were slightly more sensitive to calcofluor white, a phenotype that is usually associated with altered chitin structures along the cell wall [30]. More remarkably, age3 cells were extremely sensitive to hygromycin B, a phenotype usually seen in glycosylation mutants [31],[32] (Figure 1D, 1E). No change in sensitivity was observed in the presence of other agents such as caffeine, cycloheximide, menadione, nocodazole, rapamycin, 5-FC and wortmannin (data not shown). Together, these data suggest that while AGE3 plays a major role during membrane stress in C. albicans, its influence on the integrity of the cell wall remains somewhat less clear (see discussion).
Deleting AGE3 overrides clinical drug resistance and the calcineurin pathway
Among the most commonly encountered resistance mechanisms in drug treated clinical C. albicans isolates are over-expression of drug pumps or alterations in sterol biosynthesis [13],[33],[34]. To test whether such common mechanisms of drug resistance are still effective in the absence of AGE3, we deleted AGE3 in two FCZ-resistant clinical strains. The strain F5 carries a mutation in the transcription factor MRR1, which leads to constitutive over-expression of drug pumps. The strain S2 carries a mutation in the transcription factor UPC2, which causes up-regulation of ergosterol biosynthesis genes [35],[36]. Figure 2A shows that deleting AGE3 in strains F5 and S2 restored FCZ sensitivity even below WT levels, suggesting that loss of AGE3 abrogates FCZ-resistance in these clinical isolates.
Fig. 2. Deleting AGE3 overrides clinical drug resistance and the calcineurin pathway. 
(A) When AGE3 is knocked out in drug resistant clinical isolates F5 and S2, FCZ sensitivity is restored even below WT levels on solid YPD media. The assay was performed and analyzed as described in Figure 1B except plates were photographed after 24 hours. GOF = gain of function. (B) Calcineurin signaling stimulated either by extracellular CaCl2 or by a constitutively active mutation in strain DSY2146 leads to FCZ resistance. age3 mutants do not respond to extracellular CaCl2, while knocking out AGE3 in strain DSY2146 restored FCZ sensitivity. Disc diffusion assays were done by plating 2×105 cells on YPD plates followed by applying discs containing 50 mg of FCZ to the surface of agar. Plates were incubated for 24 hours at 30°C. Because age3 mutants lost tolerance to FCZ and because the calcineurin pathway is known to mediate FCZ-tolerance in WT as well as drug resistant clinical isolates [10],[19], we tested whether constitutive calcineurin signaling could reverse the AGE3-dependent FCZ sensitivity. The calcineurin pathway can be activated by addition of extracellular CaCl2 [19]. C. albicans WT became resistant to FCZ after only 24 hours of growth in the presence of extracellular CaCl2, while this rescue was not observed in the absence of AGE3 (Figure 2B). To further verify this observation, we deleted AGE3 in a constitutively active calcineurin signaling mutant (DSY2146) that is resistant to FCZ even in the absence of extracellular CaCl2 [19]. Deleting AGE3 in this constitutively active calcineurin mutant restored FCZ sensitivity both in the absence and in the presence of extracellular CaCl2 (Figure 2B). These results suggest that constitutive calcineurin signaling does not rescue the age3-dependent FCZ sensitivity. The results also support an argument that AGE3 and calcineurin-dependent processes could be linked (see discussion).
Pharmacological compromise of ARF cycling converts FCZ into a fungicidal drug in C. albicans
Given AGE3's role in sensitivity to various drugs and its implication in clinically relevant processes such as drug resistance, targeting either AGE3 or its biological process seemed a plausible avenue for combination therapies to render FCZ fungicidal. The S. cerevisiae homolog of C. albicans AGE3 is GCS1, which encodes an ARF GAP (ADP-Ribosylation Factor GTPase Activating Protein) [37]. ARFs are small G-proteins of the Ras GTPase superfamily that cycle between an active GTP-bound and an inactive GDP-bound state. ARF guanine nucleotide cycling, and hence function, is regulated by GAPs and GEFs (guanine nucleotide exchange factors) [38]. ARFs are involved in a variety of processes including vesicle trafficking (Golgi-to-ER retrograde vesicle trafficking, trans Golgi network-endosomal transport, transport from the Golgi to the membrane) and actin cytoskeleton organization [39],[40],[41],[42]. Brefeldin A (BFA), a metabolite from the fungus Penicillium decumbens, is a noncompetitive inhibitor of ARF activity. Protein crystal structures showed that BFA binds to a ternary complex of ARF-GDP-GEF, thus stabilizing this otherwise transient protein-protein interaction [43],[44],[45],[46],[47],[48]. Given that ARF cycling is a well-established target of BFA, we reasoned that BFA might be an ideal drug to synergize with FCZ in WT C. albicans by chemically mimicking the age3-dependent FCZ sensitivity. As illustrated by time-kill curves in Figure 3A, combining FCZ with BFA resulted in a potent fungicidal synergy in WT C. albicans, while either drug alone had only minor effects on cell growth. To compare the BFA/FCZ synergy to a well-established fungicidal synergy, we repeated the time-kill curves with CsA in combination with FCZ. CsA/FCZ co-treatment resulted in a similarly strong synthetic phenotype after 24 hours. This observation corroborates previous findings that calcineurin inhibition plus FCZ results in a potent fungicidal combination as assayed at 24 hours of drug treatment [19]. A triple drug combination of FCZ/BFA/CsA was significantly more efficacious than either FCZ/CsA or FCZ/BFA alone at 24 hours. However, when monitored for more than 24 hours, conditions that have not previously been reported [19], cells treated with FCZ/CsA could recover and resume growth, while cells treated with FCZ/BFA or FCZ/BFA/CsA could not resume growth above the detection limit (≈10 cells/ml). At 72 hours, drug combinations of either FCZ/BFA or FCZ/BFA/CsA appeared equally efficacious with no evidence of growth, while cells treated with FCZ/CsA continued to proliferate. Similar results were obtained when another calcineurin inhibitor, FK506, was combined with FCZ. These results suggest that while the combination of calcineurin inhibitors and an azole is initially efficient in pathogen killing, over prolonged drug treatment, combining ARF inhibitors with azoles is more efficacious.
Fig. 3. Pharmacological inhibition of ARF cycling results in a potent, fungicidal synergy in combination with FCZ in C. albicans. 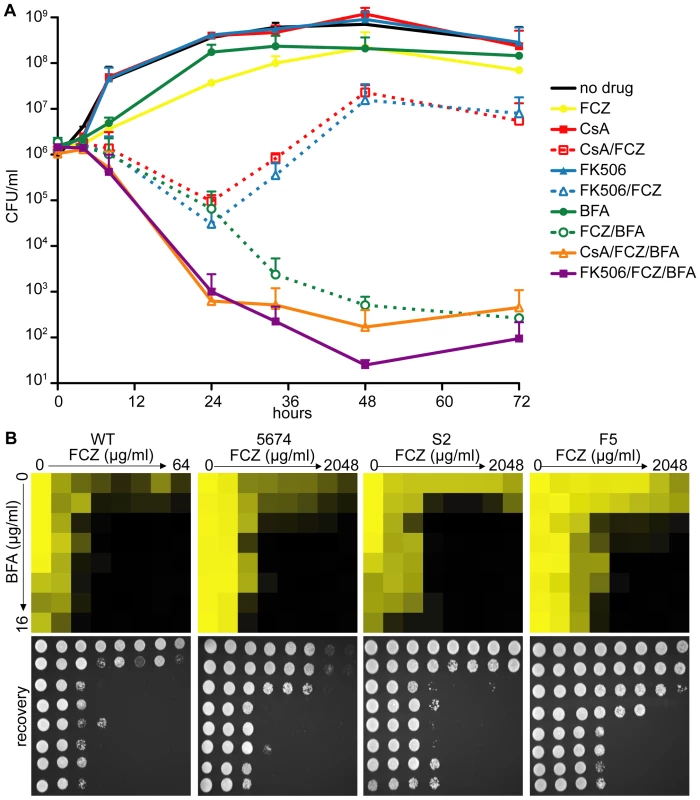
(A) Time-kill curves demonstrating that, while combining FCZ and BFA was initially equally efficacious in pathogen killing compared to combining FCZ and calcineurin inhibitors (FK506 or CsA), extended drug exposure only remained efficacious in pairwise BFA/FCZ combinations. Triple drug combinations of BFA/FCZ/calcineurin inhibitors were only initially (24 hours) more efficacious, but at 72 hours appeared equally efficacious compared to BFA/FCZ. The assay was done in YPD media. Drugs were used at 10 µg/ml for FCZ, 15 µg/ml for BFA, 1 µg/ml for CsA and 1 µg/ml for FK506. (B) Dose-matrix titration assay confirming the FCZ/BFA synergy in WT and drug resistant clinical isolates 5674, S2 and F5 in rich YPD media (top). Dose-matrix titration plates were incubated for 72 hours after which aliquots of each well were spotted on fresh YPD recovery plates (bottom). No-growth of recovery plates confirmed fungal cell death of the drug synergy. Recovery plates were incubated for 24 hours. Dose-matrix titration assays were analyzed as described for MIC assays in Figure 1A. A dose-matrix titration assay measuring growth of treated cells confirmed the synergy between FCZ/BFA (Figure 3B, Table S3). We also tested whether the FCZ/BFA synergy is still effective in C. albicans drug resistant clinical isolates and found that, although considerably higher concentrations of FCZ were needed, there was synergy in isolates F5 and S2 as well as in isolate 5674, which carries a gain-of-function mutation in TAC1, a transcriptional activator of CDR drug efflux pump genes [49]. Thus, genetic compromise of ARF cycling by deleting AGE3 abrogated FCZ tolerance, while pharmacological compromise of ARF cycling by adding BFA converted the fungistatic drug FCZ into a fungicidal agent in WT and FCZ-resistant C. albicans clinical isolates. Together, this suggests that the process of ARF cycling becomes essential during cell membrane stress in this pathogen.
Combining ARF inhibition with other drugs across fungal species
Importantly, genetic or pharmacological compromise of ARF cycling did not appear to significantly affect the cells' initial response to FCZ (Figure 1A, 3B, Table S2). Instead, ARF cycling inhibition seems to act on tolerance. To test whether the effect of BFA on tolerance is observed in combination with other azole drugs, we tested miconazole (MICO) and ketoconazole (KETO) in combination with BFA, because MICO and KETO alone did show a tolerance effect (Figure 4A). When tested in a dose-matrix titration format, BFA synergized with MICO and KETO against WT C. albicans, independently of which media were used (Figure 4A, Figure S2).
Fig. 4. Pharmacological compromise of ARF cycling synergizes with various azoles as well as the cell wall inhibitor CF. 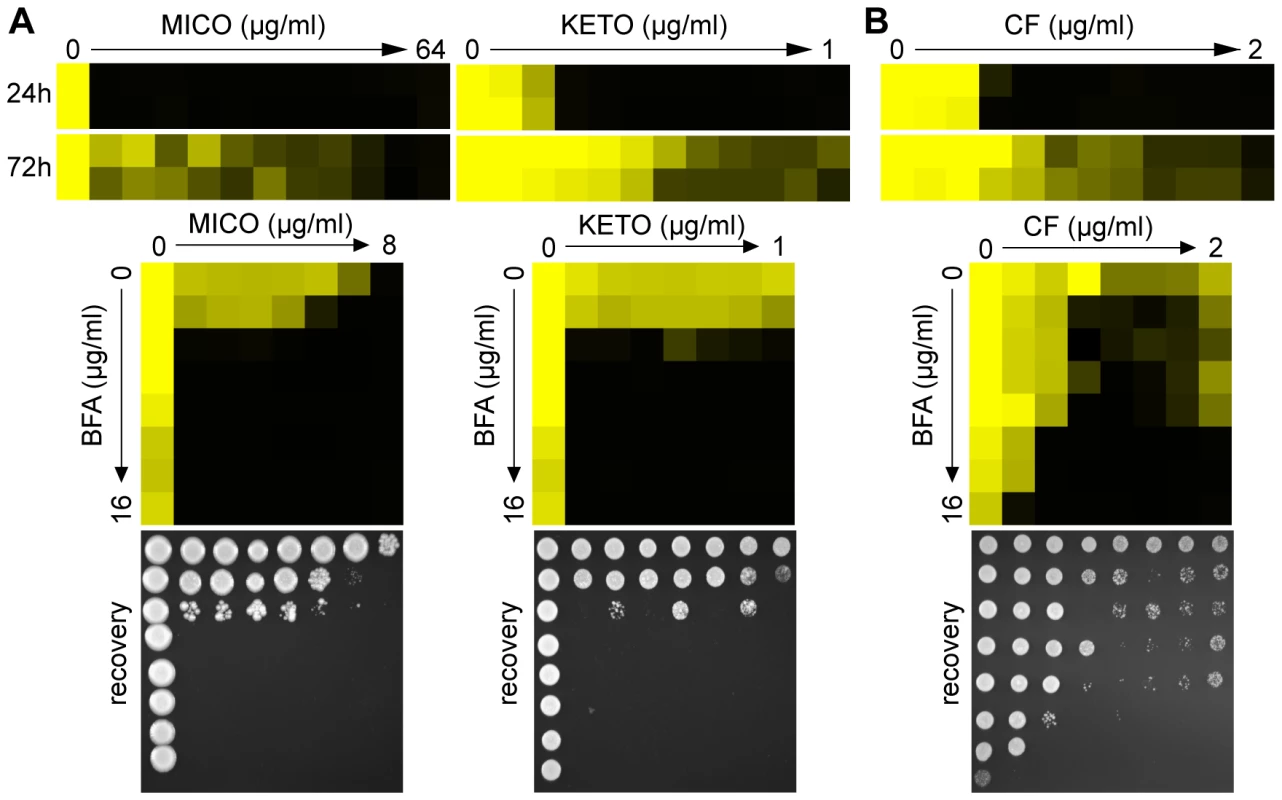
(A) MIC assays in YPD media demonstrated that WT C. albicans shows tolerance to MICO and KETO (top, compare 24 hours to 72 hours MIC readings). Combining BFA with either MICO or KETO resulted in a similarly potent fungicidal combination compared to BFA/FCZ (bottom). (B) CF showed trailing growth in rich YPD media (top) and synergized with BFA in a dose-matrix titration assay (bottom) against WT C. albicans. MIC and dose-matrix titration assays were performed and analyzed as described in Figure 1A and 3B, respectively. Although CF is generally considered fungicidal in C. albicans, we tested whether BFA would synergize with CF. Recent reports showed that C. albicans can start to grow at supra MIC concentrations of CF, an outcome referred to as the paradoxical or trailing growth effect [50],[51]. BFA did synergize with CF against WT C. albicans in a dose matrix titration assay at supra MIC concentrations of CF (Figure 4B). Together, these results indicate that genetic and pharmacological compromise of ARF cycling influences not only cell membrane stress, but also cell wall stress.
To examine whether BFA's inhibition of azole tolerance is conserved across other pathogenic Candida species, we tested BFA/drug interactions in C. tropicalis, C. parapsilosis, C. glabrata and C. krusei. Together, these species account for ∼30–40% of all Candida isolates causing invasive infections; furthermore, C. glabrata and C. krusei are notoriously difficult to treat with FCZ [7],[8],[12]. C. tropicalis, C. parapsilosis and C. glabrata showed tolerance in the presence of FCZ, MICO and KETO (Figure 5A); these azoles synergized with BFA in dose-matrix titration assays with these fungi. FCZ also synergized with BFA in the non-pathogenic yeast S. cerevisiae (data not shown). On the other hand, C. krusei did not show an obvious tolerance effect (i.e. >2 fold variations between 24 hours and 72 hours MIC readings), which could explain why BFA did not synergize with azoles in this pathogen (Figure 5B).
Fig. 5. BFA synergizes with different azoles in pathogenic non-albicans Candida species. 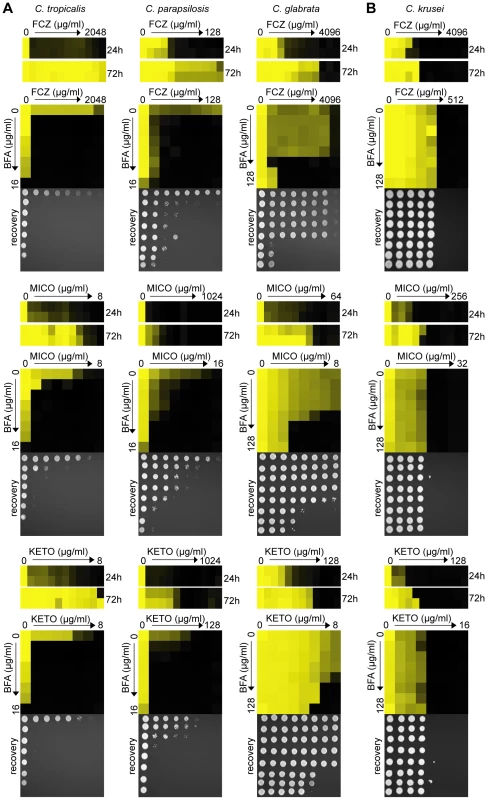
(A) When treated with FCZ, MICO or KETO, C. tropicalis, C. parapsilosis and C. glabrata isolates showed prominent growth above the initial MIC reading after extended incubation (24 hours vs. 72 hours). Dose-matrix titration assays confirming that BFA synergized with the three azoles in all three species. (B) No obvious tolerance effect was observed in C. krusei to any azoles tested and no synergy was observed when BFA was combined with these azoles. MIC and dose-matrix titration assays were performed and analyzed as described in Figure 1A and 3B. To further investigate ARF cycling inhibition as a mechanism of abrogating drug tolerance in non-Candida human fungal pathogens, we asked whether BFA would synergize with FCZ or CF against A. fumigatus. Consistent with the idea that ARF cycling inhibition acts primarily on tolerance, a disc diffusion assay in A. fumigatus demonstrated that BFA synergized with CF, a drug that is generally fungistatic in A. fumigatus (Figure 6). CF treatment created an inhibition zone against A. fumigatus where cells could still grow within that zone due to the fungistatic nature of CF. In contrast, when BFA was combined with CF, not only was the size of that inhibition zone increased, but growth within that zone was also remarkably reduced. A MIC assay in defined synthetic media independently confirmed that CF synergized with BFA against A. fumigatus (Table S3). On the other hand, we found that BFA did not synergize with FCZ, a drug that is considered fungicidal in A. fumigatus (data not shown) [12]. In general, pharmacological compromise of ARF cycling in A. fumigatus was not as efficacious as in Candida species, possibly because BFA on its own had a more pronounced impact on growth of A. fumigatus compared to Candida species (Figure 6, Table S3). Taken together, these results show that ARF cycling inhibition can couple with different fungistatic drugs in many pathogenic fungi to generate potentially fungicidal activity.
Fig. 6. BFA synergism with the fungistatic cell wall inhibitor CF in A. fumigatus. 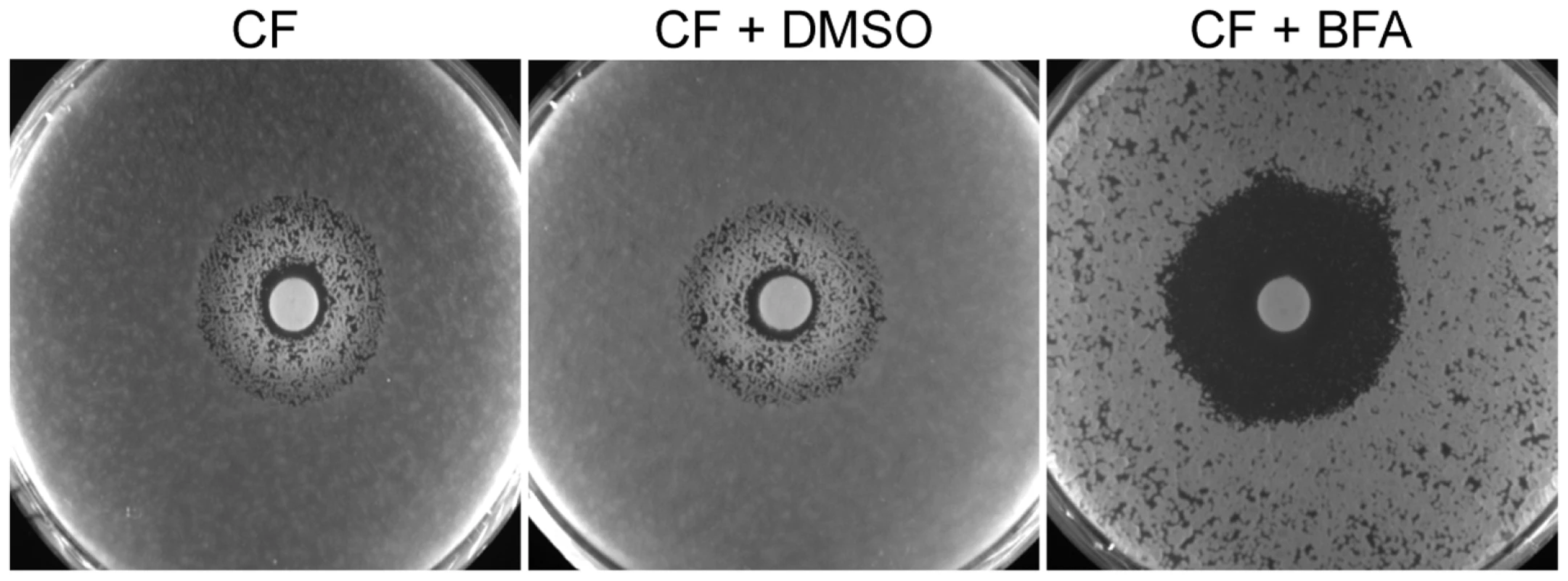
BFA/CF interaction in an A. fumigatus disk diffusion assay on half-strength YPD media. CF alone creates an inhibition zone that still allows fungal growth. Combining CF and BFA abrogated growth within that zone. Discs containing 160 µg CF were applied after 105 conidia were plated on plates containing water only, vehicle control (DMSO) or BFA (16 µg/ml), as indicated. Plates were incubated for 48 hours. Core transcriptional responses to FCZ and CF treatment are not significantly affected in the absence of AGE3
Analysis of transcriptional regulation has frequently been used to elucidate which cellular processes are linked to drug sensitivity or drug resistance in C. albicans [35],[52],[53]. We therefore performed microarray studies to test how transcriptional regulation is altered in age3 mutants. We first compared WT to age3 cells in the absence of drugs and found that 23 genes were differentially regulated when AGE3 was absent (Table S4). Among those 23 genes were five GPI-anchored cell wall proteins (ECM331, PGA13, CRH11, SAP9, PGA26) and two genes with phospholipase activity (FGR22 and PI-PLC). Because none of these genes have been linked to FCZ tolerance, it is currently unclear how they might influence the age3-dependent drug phenotypes.
We next analyzed the transcriptional response to FCZ. A typical transcriptional signature to FCZ is upregulation of ergosterol genes, presumably to compensate for depletion of these membrane lipids [54],[55]. This core response to FCZ was not changed in the absence of AGE3. All ergosterol genes that became significantly upregulated in FCZ-treated WT cells were similarly upregulated in FCZ-treated age3 cells (Table S5). Besides this core response to FCZ, clustering analysis of genes that were significantly regulated (>2 fold expression change and p-value <0.05) further confirmed that the overall transcriptional response to FCZ was very similar in WT and age3 cells (Figure S3A, Table S6).
A similar lack of AGE3-dependent transcriptional consequences could be observed in microarray experiments when the cell wall inhibitor CF was used. Clustering analysis revealed that CF-treated WT cells and CF-treated age3 cells showed an almost identical transcriptional response, with a significant overlap (p-value 4.8×10−194) of differentially regulated genes (Figure S3B, Tables S7, S8). This overlap of 168 genes showed further statistically significant similarity (p-value 1.8×10−7) when compared to core C. albicans CF-responsive genes previously identified in two independent studies [52],[56]. Therefore, the core transcriptional response to CF in C. albicans seems not to depend on AGE3. In summary, these microarray experiments suggest that deleting AGE3 does not cause major transcriptional changes in the presence of two different drug classes and further indicates that post-transcriptional processes might play a more dominant role in terms of ARF cycling-dependent drug phenotypes.
The age3 mutant is avirulent in WT mice
To evaluate whether AGE3 is a good drug target in vivo, we first injected age3 mutant cells into WT B6 mice, and found that age3 cells are avirulent in this mouse model. While 100% of mice infected with WT C. albicans became moribund within 11 days post-infection, none of the age3 mutant strain infected mice became moribund during the same time frame (Figure 7A). To test whether mice infected with age3 cells had cleared the infection, we sacrificed half of the mutant group on day 11 to analyze kidney fungal burden (Figure 7B). On average, kidney fungal load was significantly reduced (p-value<0.01, Mann Whitney test) in the age3 group compared to moribund WT-infected mice. We continued with the other half of age3 mutant-infected mice until the end of the experiment (day 21), but again found that none of the age3-infected mice became moribund. Comparing fungal load from these mice showed that three mice had cleared the infection, while two mice had a fungal load that was comparable to WT-infected mice. On average, however, there were significantly fewer age3 cells recovered from the host compared to moribund mice infected with WT C. albicans (p-value <0.02, Figure 7B).
Fig. 7. Genetic compromise of ARF cycling in C. albicans results in avirulence in a WT mouse model of disseminated disease. 
(A) C. albicans WT-infected mice become gradually moribund up to day 11, while mice infected with age3 mutants did not show any clinical signs until the end of the experiment on day 21. The dotted blue line indicates that half of the age3 mutant-infected mice were sacrificed to compare fungal load. Those mice were not moribund. (B) On average, fungal load of WT-infected mice, when moribund, is significantly higher compared to mutant fungal burden taken at indicated times. (C) Kidney section of WT-infected mice (left) showing fungal hyphal formation, which is also seen in mutant-infected kidneys (right). Kidneys were collected on day 11 for histological examination. Ten mice were used per experimental group and monitored according to approved standards. One key virulence factor in C. albicans pathogenicity is hyphal formation [57]. Because Arnold Bito and coworkers (Lettner T., Zeidler U., Gimona M., Breitenbach M., Bito A., manuscript submitted, personal communication from A. Bito) have observed some hyphal formation defects on solid media as well as defects in invasive growth in age3 mutants, we collected kidneys for histological examination on day 11 from both WT-infected and age3-infected mice. No obvious difference was found between kidney sections recovered from WT or from age3-infected mice (Figure 7C). In all kidney sections examined, age3 cells could be observed as elongated hyphal structures. Therefore, it remains unclear why age3-infected mice with a high fungal burden did not show any clinical signs, but might indicate that additional virulence factors are affected in age3 mutants.
Genetic inhibition of ARF cycling results in attenuated virulence in an immunocompromised mouse model of disseminated candidiasis and FCZ treatment is significantly more efficacious when ARF activity is genetically compromised
To help evaluate the therapeutic potential of FCZ treatment of age3 mutant-infected mice, we required a mouse model where age3 cells retain at least partial virulence. Cells of the C. albicans strains WT, age3 mutant and age3 revertant were therefore injected in an immunocompromised C5-deficient A/J mouse model [58]. In this very sensitive animal system, C. albicans WT and revertant-infected A/J mice became rapidly moribund after 20 to 24 hours, while age3 mutant-infected A/J mice survived significantly longer, with a median survival of two days (p-value<0.02, Log-rank test) (Figure 8A). However, comparing fungal burden indicated that mice infected with C. albicans age3 cells accumulated a significantly higher fungal load when moribund (p-value <0.02), with hyphal formation still observed in the age3 mutant-infected mice (Figure 8B, data not shown).
Fig. 8. age3 mutants are attenuated in virulence in A/J mice and FCZ treatment significantly extends survival of age3-infected A/J mice. 
(A) age3 mutant-infected mice survive significantly longer with a median survival of two days versus one day for WT and revertant-infected mice. Six mice were used per experimental group. (B) Fungal kidney burden was examined from moribund mice and was significantly higher in age3 mutant recovered cells compared to WT or revertant control groups. (C) A short FCZ therapy (4.5 mg/kg intraperitoneally once immediately after fungal infection, once on day one and once on day two post fungal infection) is significantly more efficacious when ARF cycling is genetically compromised as only the majority (83%) of mutant-infected mice survive until the end of the experiment (day 21). Six mice were used for WT and revertant groups and 12 mice for the age3 mutant group. To test whether genetic compromise of ARF activity holds therapeutic potential, we repeated this experiment and injected WT, age3 and the revertant strains in A/J mice to compare survival lengths after two days of FCZ therapy. FCZ administration extended survival of all three groups (p-value <0.001), but was significantly more efficacious in the mutant-infected mice, extending median survival time more than 10-fold compared to 7.5-fold median survival time extension for both the WT and revertant groups (p-value <0.01 for posthoc comparison between age3 mutant group with and without FCZ, versus p-value >0.05 posthoc comparison between WT with and without FCZ or p-value >0.05 for posthoc comparison between revertant with and without FCZ, Dunnett's Multiple Comparison) (Figure 8C). In summary, we conclude from these in vivo experiments that genetic compromise of ARF cycling results in avirulence in WT mice, significant attenuation in virulence in an immunocompromised mouse model and further suggests that FCZ treatment in A/J mice is more efficacious when ARF cycling is genetically compromised.
Discussion
Identifying new drug targets is an important step in the challenging task of developing new antifungal therapies, which are urgently needed due to the emergence of drug resistance to every class of antifungals currently in clinical use [11],[59]. Using a comprehensive reverse-genetics screen, we identified Age3p and the process of ARF cycling as potential new drug targets. We further established a widely conserved, potent fungicidal chemical synergy between the ARF cycling inhibitor BFA and several fungistatic drugs, and, through two murine infection models, validated the potential of ARF cycling as an antifungal target.
To render FCZ fungicidal through combination with other drugs, we applied a large-scale chemical-genomic approach in S. cerevisiae and found 22 FCZ-cidal genes (Table S1) [29]. The S. cerevisiae screen proved to have predictive power in that 12 of the 22 genes (57%) identified in S. cerevisiae were validated in C. albicans with an increased FCZ sensitivity (Figure S1, Table S2). Among those genes, only CDR1 and ADA2 have previously been linked to FCZ sensitivity in C. albicans [52],[60]. On the other hand, our screen linked four genes to FCZ resistance in the pathogen (NUP84, ERG11, GCN5 and NGG1). One of those genes, ERG11, has previously been linked to FCZ resistance in C. albicans [61]. Of note, Sanglard and coworkers have shown that the C. albicans erg11 mutant was sensitive to a variety of drugs, including BFA [61]. Two other C. albicans genes that have been linked to FCZ resistance in our screen (GCN5 and NGG1) are part of the SAGA, a conserved transcriptional co-activator [62]. Our data therefore expand the known events of transcriptional rewiring between S. cerevisiae and C. albicans in regards of drug resistance [63],[64], as another subunit of the SAGA complex, Ada2p, has been linked to increased drug sensitivity in our screen as well as in two previous studies [52],[60]. The observation that different subunits of the same transcriptional co-activator complex regulate the opposing phenotypes of drug sensitivity or drug resistance illustrates the extreme adaptability and flexibility of transcriptional regulatory networks in fungi.
Given that diverse essential processes exhibit significantly different regulation in S. cerevisiae and C. albicans [65],[66],[67], reorganization of transcriptional regulation might in general account for C. albicans mutants that showed different drug phenotypes compared to the yeast prediction. Despite these considerations, budding yeast genetics remains a convenient and powerful approach to predict phenotypes in pathogenic fungal species, at least until similar sophisticated screening techniques become available in the pathogen itself.
An alternative speculation why the success rate of our screen was not higher is that the gene encoding ERG11, the target of FCZ in both species, is essential only in S. cerevisiae [29],[61]. Thus, there may be redundant mechanisms for sterol biosynthesis in C. albicans, a suggestion that is further supported by the observation that another ergosterol biosynthesis gene, ERG24, is essential in S. cerevisiae, but not in C. albicans [68].
Chemical compromise of ARF cycling appeared far more potent than genetic compromise. When age3 mutants were treated with FCZ, the number of viable cells was reduced in time-kill curve experiments by less than one log value, whereas combining FCZ with BFA reduced the number of viable WT cells more than one log value over the same time (Figure 1C, Figure 3A). Similarly, chemical compromise appeared more potent than genetic compromise in terms of synergy with CF. The age3 mutant was only 2-fold more sensitive to CF than WT. On the other hand, combining BFA with CF resulted in a 32-fold reduction in sensitivity to CF in WT cells (Figure 1D and 1E, Figure 4B). These differences likely reflect that, while chemical interference with ARF function inhibits potentially all ARF GEFs, genetic inactivation was restricted to one ARF GAP (AGE3). Thus, it remains possible that some aspects of ARF signaling continue to function in the absence of AGE3 under drug conditions, a suggestion that is supported by work in yeast that demonstrated that several ARF GAPs provide redundant functions [39],[40].
The finding that both genetic and pharmacological blockage of ARF function result in increased azole sensitivity suggests that, among the multiple cellular roles described for the yeast homologue of AGE3, defects in proper ARF cycling and, therefore, defects in intracellular vesicle trafficking are responsible for drug phenotypes. One hypothesis to explain how incorrect vesicle trafficking could result in age3-dependent drug sensitivity is mislocalization of drug pumps. However, CDR1 and MDR1, two major drug pumps in C. albicans appear not be involved, as Arnold Bito and coworkers (Lettner T., Zeidler U., Gimona M., Breitenbach M., Bito A., manuscript submitted, personal communication from A. Bito) observed that CDR1 and MDR1 pumps are correctly localized to the plasma membrane in age3 mutants. They further established that CDR1 drug pump activity was not affected in the absence of AGE3.
Another plausible explanation that could account for the azole sensitivity of age3 mutants is defects in the biosynthesis of ergosterol or problems in transporting this membrane lipid to the cell membrane. Our microarray experiments provided evidence that the target pathway of azoles is not affected transcriptionally in the presence or the absence of FCZ (Figure S3A, Tables S5 and S6). We further found that the amount of ergosterol is similar in the plasma membrane of age3 mutants compared to WT (our unpublished data). These findings together with epistasis experiments showing that deletion of AGE3 restored azole sensitivity in different clinical isolates, suggest that the azole sensitivity of age3 cells is unlikely to depend on established mechanisms.
Finally, in support of the vesicle transport hypothesis is the observation that while age3 cells showed slightly increased sensitivity to different cell wall perturbing agents (CF and calcofluor white), the most impressive effect besides azoles was observed when age3 cells were treated with hygromycin B (Figure 1D). Hygromycin B sensitivity is usually linked to glycosylation defects [31],[32]. Therefore, it remains possible that some glycosylated proteins, including GPI-anchored proteins that normally reside in the cell wall, are not properly localized in age3 cells. How precisely defects in vesicle trafficking influence the observed drug phenotypes and whether drug sensitivity is caused by a general aspect of the secretory pathway or of a particular cell membrane or wall protein remains to be determined.
Drug resistance and virulence are two important biological aspects of pathogenic fungal species. While different fungal drug resistance mechanisms are now well understood [69],[70], various virulence-related attributes have been described that help Candida to cause infections [57],[59],[71]. Whereas genes critically involved either in drug resistance or virulence are attractive drug targets [59], an undeniably better option is targeting genes that are involved in both processes. The broad-range sensitivity to azoles and an echinocandin together with in vivo data showing that age3 mutants are avirulent in WT and exhibit significantly attenuated virulence even in an immunocompromised mouse model, indicates that Age3p and the process of ARF cycling is one such option. We further explored the therapeutic potential of ARF cycling inhibition by demonstrating that, in A/J mice, FCZ treatment was significantly more efficacious when ARF activity was genetically compromised.
One of our major problems was to reproduce the potent in vitro synergy of FCZ/BFA in animal models as we observed that a FCZ/BFA combination failed to rescue A/J mice infected with WT C. albicans (data not shown). One reason why the in vitro synergy failed to translate to in vivo conditions could be that BFA has low bioavailability characteristics [72] and efforts to chemically improve these unfavorable properties have not been successful so far [73],[74]. The ability of BFA to induce apoptosis in cancer cells has stimulated an interest for developing BFA as an anti-cancer therapeutic agent [75],[76],[77],[78],[79],[80]. Increasing evidence also shows that a variety of small G protein signaling pathways of the Ras superfamily, like RHO, RAS and ARF, have been linked to tumorigenesis [81],[82],[83]. Thus, despite being evolutionarily conserved, targeting ARF activities could be beneficial not only for antifungal, but also for cancer therapies. Clearly, one future challenge lies in finding fungal-specific ARF activity inhibitors that retain favorable bioavailability in vivo, a challenge that can now be approached cost - and time-effectively through virtual screening with both mammalian and yeast crystal structures of different ARF cycling proteins at hand [43],[44],[45],[46],[47],[48],[84],[85].
The age3 mutant lost tolerance to FCZ (Figure 1). This phenomenon of losing tolerance under membrane stress is not unique to ARF cycling. Previous work established that when HSP90 or calcineurin is genetically or pharmacologically inhibited in different fungal species, cells show a similar loss of tolerance and are not able to survive membrane stress [17],[18],[22],[86]. Additional phenotypes that calcineurin inhibition shares with ARF inhibition include synergism with azoles and echinocandins as well as reduced virulence in the bloodstream of the host [17],[19],[87]. These overlapping phenotypes, together with our epistasis experiments that showed that deleting AGE3 overrides constitutive calcineurin signaling, provide some arguments that ARF cycling and HSP90/calcineurin-dependent processes could be coupled. Although overlapping functions cannot be excluded, several lines of evidence support the current model that ARF and HSP90/calcineurin inhibition constitute two distinct mechanisms contributing to drug tolerance. First, a triple combination of azole/calcineurin/ARF inhibition is more efficacious than either pairwise combination at 24 hours. Second, consistent with previous observations that long term azole resistance evolves towards HSP90/calcineurin-independency [20], cells treated with calcineurin inhibitors plus FCZ recover after prolonged incubation, while BFA/FCZ treated cells do not. Finally, if ARF cycling is in fact coupled to HSP90/calcineurin signaling, then we might expect age3 mutants to copy other HSP90/calcineurin phenotypes besides the demonstrated reduced virulence and drug sensitivity. One explanation, for instance, why calcineurin mutants are avirulent is that they do not survive in the presence of FBS or Ca2+ ions present in host serum [19],[88]. We found, however, that age3 mutants can survive FBS and Ca2+ stresses (data not shown).
More work is therefore required to elucidate how ARF G protein signaling relates to HSP90/calcineurin and other signaling pathways that share ARF-related phenotypes. With four ARF proteins, six ARF GAPs and seven ARF GEFs identified in yeast so far [89], this provides a rich resource for further investigations into which aspects of vesicle transport and ARF G protein signaling are responsible for two important aspects of the biology of pathogenic fungal species.
Materials and Methods
Strains, plasmids, primers and culture conditions
All strains, primers and plasmids used in this study are described in supplementary Table S9, S10 and S11, respectively. C. albicans mutants were constructed either with the UAU1-transposon insertion strategy [90] or by deleting the coding sequence of genes (Table S9). For insertion and deletion mutant construction, at least two mutants independently derived from two distinct heterozygous mutants were analyzed in each case. With regards to the AEG3 gene (ORF19.3683), we propose to use AGE3 as standard name for the C. albicans homolog of S. cerevisiae's GCS1, because another gene (ORF19.5059) has already been named ‘GCS1’ in the Candida literature [91]. For the AGE3 deletion mutant, long 100-mer primers flanking up - and downstream sequences, respectively, of the coding sequence of AGE3 were used to amplify marker cassettes pFA-HIS1 and pFA-ARG4 [92]. Transformation was carried out according to standard protocols [93] and selected on synthetic media (2% dextrose, 6.7% yeast nitrogen base without amino acids, 2% agar) containing the necessary auxotrophic supplements. Correct marker integration was PCR-verified as described [94]. As genetic manipulation in C. albicans can frequently lead to aneuploidy [95],[96], we verified the absence of any chromosomal rearrangements by Comparative Genome Hybridization (CGH) and found that deletion mutants of AGE3 were aneuploidy free (Figure S4). For constructing the AGE3-revertant strain, the SAT1 flipper cassette was used [97]. Briefly, the AGE3 coding sequence including upstream and a short downstream flanking sequence was amplified with primers oEE242/oEE243, KpnI/XhoI-digested and cloned into the KpnI/XhoI-digested pSFS2A plasmid, resulting in plasmid pCaEE25, which was sequenced. A long downstream flanking sequence of AGE3 was amplified with primers oEE237/oEE238, NotI/SacII-digested and then cloned into the NotI/SacII-digested plasmid pCaEE25, resulting in plasmid pCaEE27. Following KpnI/SacII double digestion of plasmid pCaEE27, this digestion was transformed directly into the age3 deletion strain. Selection was done on YPD plates containing 200 µg/ml nourseothricin, as described [67] and PCR-verified. Counterselection of the nourseothricin marker was done as described [67].
The nourseothricin marker cassette [97] was also used to delete AGE3 in FCZ-resistant strains F5, S2 and DSY2146. Briefly, a long upstream coding sequence of AGE3 was first amplified from pEE27 with primers oEE235/oEE236, Kpn1/Xho1 digested and then cloned into the Kpn1/Xho1 digested plasmid pEE27, therefore replacing the coding sequence of AGE3. The resulting plasmid was designated pEE43.
Candida strains were routinely cultured at 30°C in either rich YPD media (1% yeast extract, 2% peptone, 2% dextrose, supplemented with 50 µg/ml uridine) or RPMI-MOPS media (RPMI-1640, SIGMA, supplemented with 0.3 g/l L-glutamine, 50 mg/ml uridine, 2% glucose, pH adjusted with MOPS buffer to 7.0). Media plates were supplemented with 2% agar. A. fumigatus was cultured in half-strength YPD media (not enriched with uridine) or RPMI-MOPS media (not enriched with uridine). Media plates were supplemented with 1.5% agar.
Antifungal susceptibility testing
Because drug susceptibility results did not differ significantly between WT C. albicans strains SC5314 (isolate) and SN95 (a standard laboratory auxotrophic mutant used to construct deletion mutants) [98] (Table S2), WT usually refers to SN95 unless indicated otherwise. Drug stock solutions were prepared using ethanol/10% Tween 20 as solvent for FK506 (5 mg/ml), cyclosporin A (25 mg/ml), DMSO for fluconazole (300 mg/ml), miconazole (100 mg/ml), ketoconazole (16.6 mg/ml), itraconazole (10 mg/ml), amphotericin B (20 mg/ml), calcofluor white (50 mg/ml), brefeldin A (20 mM), hygromycin B (50 mg/ml), and water for caspofungin (10 mg/ml). All drugs were obtained from Sigma, except caspofungin (Merck), fluconazole and itraconazole (both from SpectrumChemical, Mfg Corp, USA). Once in solution, drugs were stored at −20°C. Initial antifungal sensitivity testing with all C. albicans FCZ-cidal candidates was done using a modified version of the CLSI (formerly NCCLS) procedure [99]. Briefly, 50 µl of drugs at two-fold the final concentration was serially diluted in flat-bottom 96-well tissue culture plates (Corning Inc., NY, USA) and combined with 50 µl of overnight Candida cultures adjusted to 1×104 cells/ml. MIC plates were incubated at 30°C without shaking and optical densities were read at indicated time points with a Tecan Safire plate reader. The MIC was determined by the first well with a growth reduction of >95% in terms of OD600 values in the presence of a compound compared to untreated control cells. Before the 72 hours OD600 readings, plates were carefully shaken, so that a representative aliquot of 2 µl of each well could be spotted on fresh YPD recovery plates to assess the extent to which cells recover from the drug treatments. Recovery plates were incubated at 30°C between 24 hours and 48 hours before being photographed. Drug susceptibilities of robust hits that resulted from this initial MIC testing were then confirmed on solid media plates containing FCZ, disc diffusion or time-kill curve assays, as described [19].
Dose-matrix titration assays were used to evaluate drug synergies. Briefly, dose-matrix titration assays were done as described for the MIC assays, except that the final volume was 150 µl; 50 µl of three-fold the final drug concentration of drug A was dispensed in two-fold serial dilution steps across seven columns of the plate, 50 µl of three-fold the final drug concentration of drug B was dispensed in two-fold serial dilution steps down seven rows of the plate; 50 µl of overnight Candida cultures adjusted to 1.5×104 cells/ml was dispensed in all drug containing wells plus one control well containing no drugs. Synergy of a compound pair was quantified with respect to the Loewe additivity model [100] via the fractional inhibitory concentration index (FIC index) = (MICA in combo/MICA alone) + (MICB in combo/MICB alone), where “MICA in combo” is the MIC of drug A in combination, “MICA alone” is the MIC of drug A alone, “MICB in combo” is the MIC of drug B in combination and “MICB alone” is the MIC of drug B alone, respectively. Compound pairs were classified as synergistic if its FIC index is ≤0.5, the standard threshold [100],[101]. For calculation purposes of the FIC index, MIC values of >1, >2, >8, >16, >64, >128, >2048, >4096 were assumed to be 2, 4, 16, 32, 128, 256, 4096, 8192, respectively. MIC and dose-matrix titration results were visualized with TreeView version 1.6 (http://rana.lbl.gov/EisenSoftware.htm).
A. fumigatus disc diffusion assays were done as described [16], with the following modifications. Conidiation was induced on YPD plates incubated at 37°C for seven days. Conidia were then washed off the plates and suspended in PBS+0.1% Tween media before spreading 1×105 conidia on appropriate plates. Discs containing 6.4 µg caspofungin were applied and the plates were incubated at 35°C for 48 hours. A. fumigatus MIC assays were done exactly as described [102]. All MIC and dose-matrix titration assays with Candida and Aspergillus were independently performed on at least two different occasions.
Virulence studies
Virulence testing of C. albicans was done as previously described [58]. Briefly, 8 - to 12-week old C57BL/6J or A/J mice (Jackson Laboratories, Bar Harbor, ME) were inoculated via the tail vein with 200 µl of a suspension containing 3×105 C. albicans in PBS. Mice were closely monitored over a period of maximally 21 days for clinical signs of disease such as lethargy, ruffled fur, or hunched back. Mice showing extreme lethargy were considered moribund and were euthanized. All experimental procedures involving animals were approved by the Biotechnology Research Institute Animal Care Committee, which operates under the guidelines of the Canadian Council of Animal Care. Statistical analysis of survival curves as well as fungal load was done with GraphPad Prism version 5.0b. For kidney sections, extracted organs were fixed in 10% formaldehyde (Sigma) and processed at the Histology Core Facility at McGill (http://cancercentre.mcgill.ca/) by staining thin slices of tissue sections with Grocot–Gomori methenamine silver to visualize fungal cells.
Microarray and CGH studies
Comparative Genome Hybridization (CGH) analysis was done as previously described [49] with the following modifications. Genomic DNA was extracted from a C. albicans culture grown to saturation with the Qiagen Genomic DNA Extraction kit according to manufacturer's instructions. DNA hybridization was done with the Advalytix SlideBooster for 16 hours at 42°C according to manufacturer's instructions.
For the fluconazole microarray experiment, overnight cultures of C. albicans cells were diluted to OD600 of 0.05 in fresh YPD media, grown to early logarithmic phase (OD600 0.8) and split into two 50 ml cultures in 250 ml Erlenmayer flasks. One culture was fluconazole treated (600 mg/ml), while the other group received an equal amount of DMSO as control. Cultures were grown for one hour, spun down, the supernatant was removed and the cell pellet was nitrogen-flash frozen and stored at −80°C until further use. RNA was extracted according to the hot-phenol protocol [103]. For the caspofungin microarray experiment, logarithmically growing cells were treated with 125 ng/ml caspofungin for one hour, as previously described [52]. RNA was extracted with the RNase easy kit (Qiagen), as described [67]. Probe labeling, hybridization and slide washing was done as described [104], except that the SlideBooster was used for hybridization. At least three biological replicates including dye swaps were used for each condition on double spotted ORF microarrays (6,394 intragenic 70-mer oligonucleotide probes) [104]. Scanning was done with a ScanArray Lite microarray scanner (Perkin Elmer). QuantArray was used to quantify fluorescence intensities and data analysis was carried out using Genespring v.7.3 (Agilent Technologies). To compare overlap of different gene lists as well as analyzing Gene Ontology enrichment, p-values were calculated using the hypergeometric distribution as described in the GO Term Finder Tool web site (http://www.candidagenome.org/cgi-bin/GO/goTermFinder). Gene lists can be found in Tables S4, S5, S6, S7 and S8.
Supporting Information
Zdroje
1. NucciM
MarrKA
2005 Emerging fungal diseases. Clin Infect Dis 41 521 526
2. PappasPG
KauffmanCA
AndesD
BenjaminDKJr
CalandraTF
2009 Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48 503 535
3. PfallerMA
DiekemaDJ
2007 Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev 20 133 163
4. WilsonLS
ReyesCM
StolpmanM
SpeckmanJ
AllenK
2002 The direct cost and incidence of systemic fungal infections. Value Health 5 26 34
5. ShaoPL
HuangLM
HsuehPR
2007 Recent advances and challenges in the treatment of invasive fungal infections. Int J Antimicrob Agents 30 487 495
6. RichardsonM
Lass-FlorlC
2008 Changing epidemiology of systemic fungal infections. Clin Microbiol Infect 14 Suppl 4 5 24
7. Pfaller MichaelA
Pappas PeterG
Wingard JohnR
2006 Invasive Fungal Pathogens: Current Epidemiological Trends. Clinical Infectious Diseases 43 S3 S14
8. LeroyO
GangneuxJP
MontraversP
MiraJP
GouinF
2009 Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 37 1612 1618
9. PfallerMA
DiekemaDJ
2004 Rare and emerging opportunistic fungal pathogens: concern for resistance beyond Candida albicans and Aspergillus fumigatus. J Clin Microbiol 42 4419 4431
10. CowenL
2008 The evolution of fungal drug resistance: modulating the trajectory from genotype to phenotype. Nat Rev Micro 6 187 198
11. ChapmanSW
SullivanDC
ClearyJD
2008 In search of the holy grail of antifungal therapy. Trans Am Clin Climatol Assoc 119 197 215; discussion 215–196
12. CowenLE
SteinbachWJ
2008 Stress, drugs, and evolution: the role of cellular signaling in fungal drug resistance. Eukaryot Cell 7 747 764
13. CannonRD
LampingE
HolmesAR
NiimiK
BaretPV
2009 Efflux-mediated antifungal drug resistance. Clin Microbiol Rev 22 291 321, table of contents
14. TraederC
KowollS
ArastehK
2008 Candida infection in HIV positive patients 1985–2007. Mycoses 51 Suppl 2 58 61
15. PachlJ
SvobodaP
JacobsF
VandewoudeK
van der HovenB
2006 A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin Infect Dis 42 1404 1413
16. CowenLE
SinghSD
KohlerJR
CollinsC
ZaasAK
2009 Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A 106 2818 2823
17. SinghSD
RobbinsN
ZaasAK
SchellWA
PerfectJR
2009 Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 5 e1000532 doi:10.1371/journal.ppat.1000532
18. CruzMC
GoldsteinAL
BlankenshipJR
Del PoetaM
DavisD
2002 Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J 21 546 559
19. SanglardD
IscherF
MarchettiO
EntenzaJ
BilleJ
2003 Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol 48 959 976
20. CowenLE
LindquistS
2005 Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 309 2185 2189
21. MarchettiO
EntenzaJM
SanglardD
BilleJ
GlauserMP
2000 Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob Agents Chemother 44 2932 2938
22. SteinbachWJ
ReedyJL
CramerRAJr
PerfectJR
HeitmanJ
2007 Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Microbiol 5 418 430
23. LopezA
ParsonsAB
NislowC
GiaeverG
BooneC
2008 Chemical-genetic approaches for exploring the mode of action of natural products. Prog Drug Res 66 237, 239 271
24. HoonS
SmithAM
WallaceIM
SureshS
MirandaM
2008 An integrated platform of genomic assays reveals small-molecule bioactivities. Nat Chem Biol 4 498 506
25. HoCH
MagtanongL
BarkerSL
GreshamD
NishimuraS
2009 A molecular barcoded yeast ORF library enables mode-of-action analysis of bioactive compounds. Nat Biotechnol 27 369 377
26. ButcherRA
BhullarBS
PerlsteinEO
MarsischkyG
LaBaerJ
2006 Microarray-based method for monitoring yeast overexpression strains reveals small-molecule targets in TOR pathway. Nat Chem Biol 2 103 109
27. GiaeverG
FlahertyP
KummJ
ProctorM
NislowC
2004 Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc Natl Acad Sci U S A 101 793 798
28. XuD
JiangB
KetelaT
LemieuxS
VeilletteK
2007 Genome-wide fitness test and mechanism-of-action studies of inhibitory compounds in Candida albicans. PLoS Pathog 3 e92 doi:10.1371/journal.ppat.0030092
29. JansenG
LeeAY
EppE
FredetteA
SurprenantJ
2009 Chemogenomic profiling predicts antifungal synergies. Mol Syst Biol 5 338
30. UccellettiD
FarinaF
MorlupiA
PalleschiC
1999 Mutants of Kluyveromyces lactis with altered protein glycosylation are affected in cell wall morphogenesis. Res Microbiol 150 5 12
31. DeanN
1995 Yeast glycosylation mutants are sensitive to aminoglycosides. Proc Natl Acad Sci U S A 92 1287 1291
32. DeanN
PosterJB
1996 Molecular and phenotypic analysis of the S. cerevisiae MNN10 gene identifies a family of related glycosyltransferases. Glycobiology 6 73 81
33. MorschhauserJ
2002 The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta 1587 240 248
34. KanafaniZA
PerfectJR
2008 Antimicrobial resistance: resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis 46 120 128
35. MorschhauserJ
BarkerKS
LiuTT
BlaBWJ
HomayouniR
2007 The transcription factor Mrr1p controls expression of the MDR1 efflux pump and mediates multidrug resistance in Candida albicans. PLoS Pathog 3 e164 doi:10.1371/journal.ppat.0030164
36. DunkelN
LiuTT
BarkerKS
HomayouniR
MorschhauserJ
2008 A gain-of-function mutation in the transcription factor Upc2p causes upregulation of ergosterol biosynthesis genes and increased fluconazole resistance in a clinical Candida albicans isolate. Eukaryot Cell 7 1180 1190
37. PoonPP
WangX
RotmanM
HuberI
CukiermanE
1996 Saccharomyces cerevisiae Gcs1 is an ADP-ribosylation factor GTPase-activating protein. Proc Natl Acad Sci U S A 93 10074 10077
38. MossJ
VaughanM
1998 Molecules in the ARF orbit. J Biol Chem 273 21431 21434
39. PoonPP
CasselD
SpangA
RotmanM
PickE
1999 Retrograde transport from the yeast Golgi is mediated by two ARF GAP proteins with overlapping function. EMBO J 18 555 564
40. PoonPP
NothwehrSF
SingerRA
JohnstonGC
2001 The Gcs1 and Age2 ArfGAP proteins provide overlapping essential function for transport from the yeast trans-Golgi network. J Cell Biol 155 1239 1250
41. MyersKR
CasanovaJE
2008 Regulation of actin cytoskeleton dynamics by Arf-family GTPases. Trends Cell Biol 18 184 192
42. GillinghamAK
MunroS
2007 The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol 23 579 611
43. SingletonVL
BohonosN
UllstrupAJ
1958 Decumbin, a new compound from a species of Penicillium. Nature 181 1072 1073
44. RenaultL
GuibertB
CherfilsJ
2003 Structural snapshots of the mechanism and inhibition of a guanine nucleotide exchange factor. Nature 426 525 530
45. CherfilsJ
MenetreyJ
MathieuM
Le BrasG
RobineauS
1998 Structure of the Sec7 domain of the Arf exchange factor ARNO. Nature 392 101 105
46. GoldbergJ
1998 Structural basis for activation of ARF GTPase: mechanisms of guanine nucleotide exchange and GTP-myristoyl switching. Cell 95 237 248
47. SataM
MossJ
VaughanM
1999 Structural basis for the inhibitory effect of brefeldin A on guanine nucleotide-exchange proteins for ADP-ribosylation factors. Proc Natl Acad Sci U S A 96 2752 2757
48. MossessovaE
CorpinaRA
GoldbergJ
2003 Crystal structure of ARF1*Sec7 complexed with Brefeldin A and its implications for the guanine nucleotide exchange mechanism. Mol Cell 12 1403 1411
49. ZnaidiS
De DekenX
WeberS
RigbyT
NantelA
2007 The zinc cluster transcription factor Tac1p regulates PDR16 expression in Candida albicans. Mol Microbiol 66 440 452
50. StevensDA
EspirituM
ParmarR
2004 Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob Agents Chemother 48 3407 3411
51. FleischhackerM
RadeckeC
SchulzB
RuhnkeM
2008 Paradoxical growth effects of the echinocandins caspofungin and micafungin, but not of anidulafungin, on clinical isolates of Candida albicans and C. dubliniensis. Eur J Clin Microbiol Infect Dis 27 127 131
52. BrunoVM
KalachikovS
SubaranR
NobileCJ
KyratsousC
2006 Control of the C. albicans cell wall damage response by transcriptional regulator Cas5. PLoS Pathog 2 e21 doi:10.1371/journal.ppat.0020021
53. KarababaM
ValentinoE
PardiniG
CosteAT
BilleJ
2006 CRZ1, a target of the calcineurin pathway in Candida albicans. Molecular Microbiology 59 1429 1451
54. LepakA
NettJ
LincolnL
MarchilloK
AndesD
2006 Time course of microbiologic outcome and gene expression in Candida albicans during and following in vitro and in vivo exposure to fluconazole. Antimicrob Agents Chemother 50 1311 1319
55. SellamA
TebbjiF
NantelA
2009 Role of Ndt80p in sterol metabolism regulation and azole resistance in Candida albicans. Eukaryot Cell 8 1174 1183
56. LiuTT
LeeRE
BarkerKS
WeiL
HomayouniR
2005 Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob Agents Chemother 49 2226 2236
57. WhitewayM
BachewichC
2007 Morphogenesis in Candida albicans. Annu Rev Microbiol 61 529 553
58. MullickA
EliasM
PicardS
BourgetL
JovcevskiO
2004 Dysregulated inflammatory response to Candida albicans in a C5-deficient mouse strain. Infect Immun 72 5868 5876
59. GauwerkyK
BorelliC
KortingHC
2009 Targeting virulence: a new paradigm for antifungals. Drug Discov Today 14 214 222
60. SellamA
AskewC
EppE
LavoieH
WhitewayM
2009 Genome-wide mapping of the coactivator Ada2p yields insight into the functional roles of SAGA/ADA complex in Candida albicans. Mol Biol Cell 20 2389 2400
61. SanglardD
IscherF
ParkinsonT
FalconerD
BilleJ
2003 Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob Agents Chemother 47 2404 2412
62. TimmersHT
ToraL
2005 SAGA unveiled. Trends Biochem Sci 30 7 10
63. ZnaidiS
WeberS
Al-AbdinOZ
BommeP
SaidaneS
2008 Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot Cell 7 836 847
64. LiuTT
ZnaidiS
BarkerKS
XuL
HomayouniR
2007 Genome-wide expression and location analyses of the Candida albicans Tac1p regulon. Eukaryot Cell 6 2122 2138
65. TuchBB
LiH
JohnsonAD
2008 Evolution of eukaryotic transcription circuits. Science 319 1797 1799
66. HoguesH
LavoieH
SellamA
MangosM
RoemerT
2008 Transcription factor substitution during the evolution of fungal ribosome regulation. Mol Cell 29 552 562
67. AskewC
SellamA
EppE
HoguesH
MullickA
2009 Transcriptional Regulation of Carbohydrate Metabolism in the Human Pathogen Candida albicans. PLoS Pathog 5 e1000612 doi:10.1371/journal.ppat.1000612
68. JiaN
Arthington-SkaggsB
LeeW
PiersonCA
LeesND
2002 Candida albicans sterol C-14 reductase, encoded by the ERG24 gene, as a potential antifungal target site. Antimicrob Agents Chemother 46 947 957
69. SanglardD
CosteA
FerrariS
2009 Antifungal drug resistance mechanisms in fungal pathogens from the perspective of transcriptional gene regulation. FEMS Yeast Res 9 1029 1050
70. CowenL
SteinbachW
2008 Stress, Drugs, and Evolution: the Role of Cellular Signaling in Fungal Drug Resistance. Eukaryotic Cell 7 747 764
71. d'EnfertC
2009 Hidden killers: persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol 12 358 364
72. BruningA
IshikawaT
KneuselRE
MaternU
LottspeichF
1992 Brefeldin A binds to glutathione S-transferase and is secreted as glutathione and cysteine conjugates by Chinese hamster ovary cells. J Biol Chem 267 7726 7732
73. FoxBM
VromanJA
FanwickPE
CushmanM
2001 Preparation and evaluation of sulfide derivatives of the antibiotic brefeldin a as potential prodrug candidates with enhanced aqueous solubilities. J Med Chem 44 3915 3924
74. AnaduNO
DavissonVJ
CushmanM
2006 Synthesis and anticancer activity of brefeldin A ester derivatives. J Med Chem 49 3897 3905
75. ShaoRG
ShimizuT
PommierY
1996 Brefeldin A is a potent inducer of apoptosis in human cancer cells independently of p53. Exp Cell Res 227 190 196
76. ZhuJ-W
HoriH
NojiriH
TsukudaT
TairaZ
1997 Synthesis and activity of brefeldin a analogs as inducers of cancer cell differentiation and apoptosis. Bioorganic & Medicinal Chemistry Letters 7 139 144
77. ZhuJW
NagasawaH
NaguraF
MohamadSB
UtoY
2000 Elucidation of strict structural requirements of brefeldin A as an inducer of differentiation and apoptosis. Bioorg Med Chem 8 455 463
78. NojiriH
ManyaH
IsonoH
YamanaH
NojimaS
1999 Induction of terminal differentiation and apoptosis in human colonic carcinoma cells by brefeldin A, a drug affecting ganglioside biosynthesis. FEBS Lett 453 140 144
79. GuoH
TittleTV
AllenH
MaziarzRT
1998 Brefeldin A-mediated apoptosis requires the activation of caspases and is inhibited by Bcl-2. Exp Cell Res 245 57 68
80. HackiJ
EggerL
MonneyL
ConusS
RosseT
2000 Apoptotic crosstalk between the endoplasmic reticulum and mitochondria controlled by Bcl-2. Oncogene 19 2286 2295
81. SahaiE
MarshallCJ
2002 RHO-GTPases and cancer. Nat Rev Cancer 2 133 142
82. DownwardJ
2003 Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer 3 11 22
83. SabeH
OnoderaY
MazakiY
HashimotoS
2006 ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr Opin Cell Biol 18 558 564
84. CherfilsJ
ChardinP
1999 GEFs: structural basis for their activation of small GTP-binding proteins. Trends Biochem Sci 24 306 311
85. AmorJC
HortonJR
ZhuX
WangY
SullardsC
2001 Structures of yeast ARF2 and ARL1: distinct roles for the N terminus in the structure and function of ARF family GTPases. J Biol Chem 276 42477 42484
86. MarchettiO
MoreillonP
GlauserMP
BilleJ
SanglardD
2000 Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother 44 2373 2381
87. BaderT
BodendorferB
SchroppelK
MorschhauserJ
2003 Calcineurin is essential for virulence in Candida albicans. Infect Immun 71 5344 5354
88. BlankenshipJR
HeitmanJ
2005 Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect Immun 73 5767 5774
89. (SGD project. The Saccharomyces Genome Database, www.yeastgenome.org (as of October 2009))
90. DavisDA
BrunoVM
LozaL
FillerSG
MitchellAP
2002 Candida albicans Mds3p, a conserved regulator of pH responses and virulence identified through insertional mutagenesis. Genetics 162 1573 1581
91. ArnaudMB
CostanzoMC
SkrzypekMS
ShahP
BinkleyG
2007 Sequence resources at the Candida Genome Database. Nucleic Acids Res 35 D452 456
92. GolaS
MartinR
WaltherA
DünklerA
WendlandJ
2003 New modules for PCR-based gene targeting in Candida albicans: rapid and efficient gene targeting using 100 bp of flanking homology region. Yeast 20 1339 1347
93. ChenDC
YangBC
KuoTT
1992 One-step transformation of yeast in stationary phase. Curr Genet 21 83 84
94. WaltherA
WendlandJ
2008 PCR-based gene targeting in Candida albicans. Nat Protoc 3 1414 1421
95. BouchonvilleK
ForcheA
TangKE
SelmeckiA
BermanJ
2009 Aneuploid chromosomes are highly unstable during DNA transformation of Candida albicans. Eukaryot Cell 8 1554 1566
96. ArbourM
EppE
HoguesH
SellamA
LacroixC
2009 Widespread occurrence of chromosomal aneuploidy following the routine production of Candida albicans mutants. FEMS Yeast Res
97. ReussO
VikA
KolterR
MorschhäuserJ
2004 The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341 119 127
98. NobleSM
JohnsonAD
2005 Strains and Strategies for Large-Scale Gene Deletion Studies of the Diploid Human Fungal Pathogen Candida albicans. Eukaryotic Cell
99. NCCLS Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard-Second Edition. NCCLS document M27-A2 [ISBN 1-56238-469-4]. NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2002
100. LoeweS
1953 The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 3 285 290
101. BarchiesiF
Di FrancescoLF
CompagnucciP
ArzeniD
GiacomettiA
1998 In-vitro interaction of terbinafine with amphotericin B, fluconazole and itraconazole against clinical isolates of Candida albicans. J Antimicrob Chemother 41 59 65
102. NCCLS Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard. NCCLS document M38-A [ISBN 1-56238-470-8]. NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA, 2002
103. KohrerK
DomdeyH
1991 Preparation of high molecular weight RNA. Methods Enzymol 194 398 405
104. NantelA
RigbyT
HoguesH
WhitewayM
2006 Microarrays for studying pathogenicity in Candida albicans;
KavanaughKH
New Jersey Wiley Press
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



