-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
The invasion of erythrocytes by Plasmodium merozoites requires specific interactions between host receptors and parasite ligands. Parasite proteins that bind erythrocyte receptors during invasion are localized in apical organelles called micronemes and rhoptries. The regulated secretion of microneme and rhoptry proteins to the merozoite surface to enable receptor binding is a critical step in the invasion process. The sequence of these secretion events and the external signals that trigger release are not known. We have used time-lapse video microscopy to study changes in intracellular calcium levels in Plasmodium falciparum merozoites during erythrocyte invasion. In addition, we have developed flow cytometry based methods to measure relative levels of cytosolic calcium and study surface expression of apical organelle proteins in P. falciparum merozoites in response to different external signals. We demonstrate that exposure of P. falciparum merozoites to low potassium ion concentrations as found in blood plasma leads to a rise in cytosolic calcium levels through a phospholipase C mediated pathway. Rise in cytosolic calcium triggers secretion of microneme proteins such as the 175 kD erythrocyte binding antigen (EBA175) and apical membrane antigen-1 (AMA-1) to the merozoite surface. Subsequently, interaction of EBA175 with glycophorin A (glyA), its receptor on erythrocytes, restores basal cytosolic calcium levels and triggers release of rhoptry proteins. Our results identify for the first time the external signals responsible for the sequential release of microneme and rhoptry proteins during erythrocyte invasion and provide a starting point for the dissection of signal transduction pathways involved in regulated exocytosis of these key apical organelles. Signaling pathway components involved in apical organelle discharge may serve as novel targets for drug development since inhibition of microneme and rhoptry secretion can block invasion and limit blood-stage parasite growth.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000746
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000746Summary
The invasion of erythrocytes by Plasmodium merozoites requires specific interactions between host receptors and parasite ligands. Parasite proteins that bind erythrocyte receptors during invasion are localized in apical organelles called micronemes and rhoptries. The regulated secretion of microneme and rhoptry proteins to the merozoite surface to enable receptor binding is a critical step in the invasion process. The sequence of these secretion events and the external signals that trigger release are not known. We have used time-lapse video microscopy to study changes in intracellular calcium levels in Plasmodium falciparum merozoites during erythrocyte invasion. In addition, we have developed flow cytometry based methods to measure relative levels of cytosolic calcium and study surface expression of apical organelle proteins in P. falciparum merozoites in response to different external signals. We demonstrate that exposure of P. falciparum merozoites to low potassium ion concentrations as found in blood plasma leads to a rise in cytosolic calcium levels through a phospholipase C mediated pathway. Rise in cytosolic calcium triggers secretion of microneme proteins such as the 175 kD erythrocyte binding antigen (EBA175) and apical membrane antigen-1 (AMA-1) to the merozoite surface. Subsequently, interaction of EBA175 with glycophorin A (glyA), its receptor on erythrocytes, restores basal cytosolic calcium levels and triggers release of rhoptry proteins. Our results identify for the first time the external signals responsible for the sequential release of microneme and rhoptry proteins during erythrocyte invasion and provide a starting point for the dissection of signal transduction pathways involved in regulated exocytosis of these key apical organelles. Signaling pathway components involved in apical organelle discharge may serve as novel targets for drug development since inhibition of microneme and rhoptry secretion can block invasion and limit blood-stage parasite growth.
Introduction
Malaria continues to be a major public health problem in tropical regions of the world. It is responsible for significant morbidity and mortality with around 300 to 500 million malaria cases reported annually that result in about 2 million malaria-related deaths [1]. Of the Plasmodium species responsible for human malaria, Plasmodium falciparum is the most virulent and accounts for the vast majority of deaths attributed to malaria. Given the rapid spread of drug resistant malaria parasites, there is an urgent need to develop novel intervention strategies including new drugs and effective vaccines to combat malaria.
All the clinical symptoms of malaria are attributed to the blood stage of the parasite life cycle during which Plasmodium merozoites invade and multiply within host erythrocytes. Invasion of erythrocytes by Plasmodium merozoites is a complex multi-step process that is mediated by specific molecular interactions between host receptors and parasite ligands [2]. A clear understanding of the molecular mechanisms involved in erythrocyte invasion could lead to the development of novel approaches to inhibit invasion, limit blood-stage parasite growth and protect against malaria.
Plasmodium species belong to the phylum Apicomplexa and are characterized by the presence of apical membrane bound organelles called micronemes and rhoptries that play important roles in host cell invasion. A number of key parasite ligands that mediate critical interactions with host receptors during invasion are localized in these apical organelles [2]. Studies of the invasion process by light and electron microscopy suggest that the early steps of invasion include merozoite attachment to the erythrocyte surface, apical re-orientation, release of apical organelles including micronemes and rhoptries and formation of an irreversible ‘junction’ between the apical end of the invading merozoite and the target erythrocyte [3]–[6]. The precise timing and sequence of microneme and rhoptry secretion during invasion is not known. Moreover, the external signals and signaling pathways that trigger the coordinated release of microneme and rhoptry proteins remain to be identified.
In case of Plasmodium sporozoites cAMP as well as cytosolic Ca+2 have been shown to be involved in signaling mechanisms that trigger microneme secretion during hepatocyte invasion [7]. In P. falciparum merozoites rise in intracellular calcium has been implicated in phosphorylation of components of the motility machinery of merozoites, which is critical for invasion [8]. In the related Apicomplexan parasite Toxoplasma gondii, intracellular calcium regulates a number of critical processes including parasite motility, egress from host cells and secretion of microneme proteins [9]–[12]. Treatment of T. gondii tachyzoites with calcium modulating agents such as the ionophore A23187 or ethanol, which elevate cytosolic calcium levels, trigger release of microneme proteins such as MIC2 that play essential roles in host cell invasion [13]–[15]. Endogenous production of the phytohormone abscissic acid in T. gondii tachyzoites has been shown to trigger release of calcium from internal stores resulting in secretion of microneme proteins that mediate host cell invasion [16].
The rhoptries are bulbous membrane bound secretory organelles that are located at the apical end of Apicomplexan parasites. Both rhoptry neck proteins (RONs) as well as proteins localized in the rhoptry bulb are secreted during host cell invasion [17],[18]. T. gondii rhoptries contain a number of protein kinases, which play an important role as virulence factors and are secreted into the host cell during invasion [19],[20]. The external signals and signaling mechanisms responsible for secretion of rhoptry proteins are not known in any Apicomplexan parasite.
Here, we have investigated the role of intracellular calcium in the regulated secretion of apical organelles in P. falciparum merozoites during invasion. We describe the identification of physiologically relevant external signals that trigger the sequential release of microneme and rhoptry proteins. Importantly, we demonstrate that secretion of microneme and rhoptry proteins is triggered by distinct external signals. Exposure of merozoites to low potassium ion concentrations as found in blood plasma appears to provide the external signal that leads to a rise in cytosolic calcium, which triggers translocation of microneme proteins such as the 175 kD erythrocyte binding antigen (EBA175) [21]–[23] and apical membrane antigen-1 (AMA1) [24] to the merozoite surface. Following release to the merozoite surface, engagement of EBA175 with its receptor, glycophorin A (glyA), restores basal cytosolic calcium levels and triggers the release of rhoptry proteins such as CLAG3.1 [25] and PfRH2b [26]. These observations provide a starting point for the analysis of signaling pathways involved in regulated secretion of apical organelles during invasion. A clear understanding of these pathways may provide novel targets for intervention since inhibition of apical organelle release will block invasion and limit blood-stage parasite multiplication.
Results
Intracellular calcium levels in P. falciparum merozoites during erythrocyte invasion
Calcium is commonly used as a second messenger in signal transduction pathways that regulate key life processes in eukaryotic cells. Here, we have investigated whether calcium is involved in regulating processes during host cell invasion by P. falciparum merozoites. We have used time-lapse video microscopy to study changes in levels of free cytosolic calcium in P. falciparum merozoites during erythrocyte invasion. Mature P. falciparum schizonts were labeled with the calcium sensitive fluorescence indicator Fluo-4AM and incubated with erythrocytes to allow re-invasion. Free merozoites released from ruptured schizonts were fluorescent indicating presence of high levels of free intracellular calcium (Figure 1 and Videos S1, S2 and S3). Importantly, the distribution of the fluorescence signal observed in Fluo-4AM labeled free merozoites indicates that the calcium indicator disperses uniformly in the merozoite cytoplasm and does not accumulate in any internal organelles (Figure S1). Fluo-4AM can thus be used to report cytosolic calcium concentrations in merozoites. Following interaction with target erythrocytes, the fluorescence intensity in attached merozoites drops prior to invasion indicating a decrease in cytosolic calcium levels (Figures 1 and S2). In contrast, calcium levels remain high in free merozoites that do not attach to erythrocytes over comparable periods of time (Figure S2). These observations indicate that free merozoites released from ruptured schizonts in RPMI medium have high levels of free cytosolic calcium, which drop following attachment with target erythrocytes before invasion proceeds to completion.
Fig. 1. Cytosolic calcium levels in P. falciparum merozoites during erythrocyte invasion. 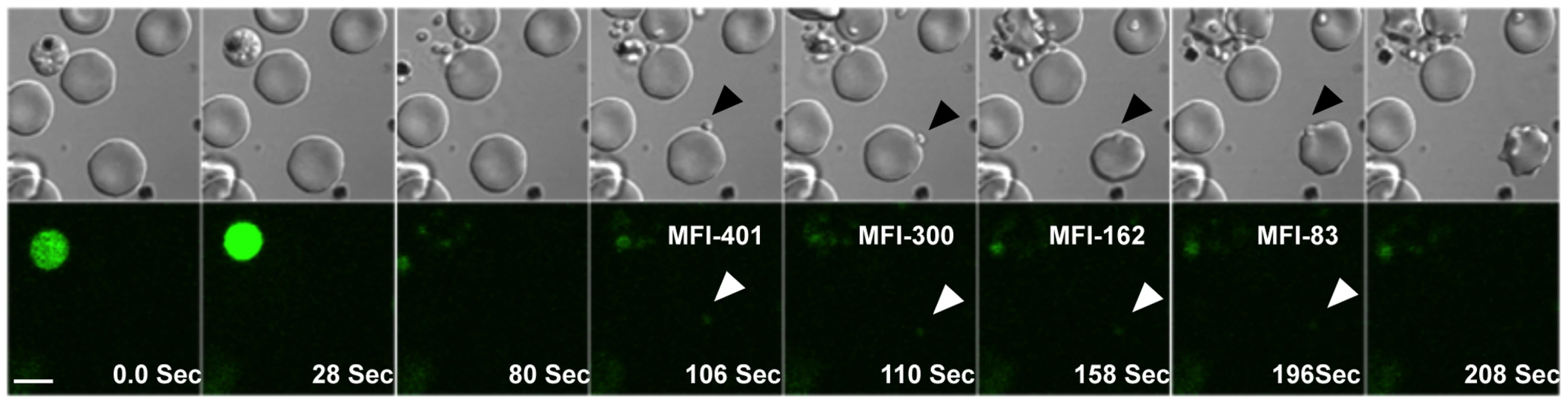
Late stage P. falciparum schizonts were labeled with Fluo-4AM and added to uninfected erythrocytes to allow re-invasion. Calcium levels were monitored in released merozoites during invasion by time-lapse video microscopy. Both DIC and fluorescence images were acquired at 2 second intervals. Selected pairs of DIC and fluorescence images with time elapsed between frames in seconds (Sec) are shown. The mean fluorescence intensity (MFI) after subtraction of background in an individual merozoite that successfully completed invasion (marked by arrows) is indicated. White bar indicates 5 µm. Also see Supplementary Information, Videos S1, S2 and S3. Elevation of intracellular calcium levels in P. falciparum merozoites triggers release of microneme proteins but not rhoptry proteins
We wondered whether the elevated cytosolic calcium levels observed in free P. falciparum merozoites released from schizonts could trigger the release of apical organelles. We developed methods to isolate viable P. falciparum merozoites and study translocation of microneme and rhoptry proteins to the merozoite surface in response to changes in intracellular calcium levels. P. falciparum cultures were synchronized by sorbitol treatment and late stage schizonts were allowed to mature and rupture naturally. Released merozoites were separated from unruptured schizonts and uninfected erythrocytes by differential centrifugation. To test their competence for invasion, ∼1×106 merozoites were incubated with ∼1×107 erythrocytes for 18–20 hours to allow invasion and newly invaded rings were scored by Giemsa staining. Based on the invasion rates measured, we estimate that 25.8±3.3% (n = 5) of merozoites invaded erythrocytes successfully to form rings. In parallel experiments, ∼1×105 late stage purified P. falciparum schizonts were incubated with ∼1×107 erythrocytes to allow schizont rupture and re-invasion. Newly invaded ring-infected erythrocytes were scored by Giemsa staining. Based on the invasion rates measured and assuming that each ruptured schizont releases ∼10 merozoites, we estimate that 17.4±4.1% of merozoites released naturally following rupture of schizonts invaded erythrocytes successfully. The invasion rates for merozoites isolated as described above and merozoites that invade target erythrocytes directly after release in culture following rupture of mature schizonts are therefore comparable. P. falciparum merozoites isolated for use in studies described here are thus viable and retain their competence for invasion.
We developed methods to label isolated P. falciparum merozoites with Fluo-4AM and measure cytosolic calcium levels by flow cytometry. Treatment of merozoites with the calcium ionophore A23187 results in an increase in cytosolic calcium (Figure 2A). The rise in free cytosolic calcium is blocked if merozoites are treated with the membrane permeable calcium chelator BAPTA-AM prior to addition of A23187 (Figure 2B). Merozoites isolated in RPMI and incubated with or without A23187 were fixed with p-formaldehyde and stained with specific antisera to detect changes in surface expression of microneme protein EBA175 [27], rhoptry protein encoded by cytoadherence-linked asexual gene 3.1 (CLAG3.1) [25] and merozoite surface protein 4 (MSP4) [28] by flow cytometry. Antiserum raised against a cytoplasmic protein, namely, nucleosome assembly protein-large (NAPL) [29], was used as a control to confirm that only proteins on the merozoite surface are detected by this method. Anti-NAPL serum does not yield any signal with p-formaldehyde-fixed merozoites either by flow cytometry (Figure S3) or by immunofluorescence assay (IFA) (Figure S4). NAPL is detected only if p-formaldehyde-fixed merozoites are permeabilized with saponin (Figure S3 and S4). The fixing method used thus does not permeabilize merozoites and only proteins on the merozoite surface are detected. Surface expression of EBA175 increases upon treatment of merozoites with A23187 (Figure 2C and Table S1). The increase in EBA175 on the merozoite surface is blocked if merozoites are treated with BAPTA-AM prior to treatment with A23187 (Figure 2C and Table S1). The increase in EBA175 levels on the merozoite surface following treatment with A23187 was confirmed by IFA (Figure 3). EBA175 secreted to the merozoite surface in response to the increase in cytosolic calcium is localized at the apical end (Figure 3). Treatment of merozoites with A23187 also triggers the release of microneme protein AMA1 to the merozoite surface (Figure S5 and Table S1). Levels of EBA175 and AMA1 detected in merozoites permeabilized with saponin remain unchanged before and after treatment with A23187 (Figures 2D and S5B). The increase in EBA175 and AMA1 on the merozoite surface following A23187 treatment can thus be attributed to translocation of these proteins to the surface from an intracellular location. Surface expression levels for MSP4, which is constitutively expressed on the merozoite surface, and CLAG3.1, which is localized in rhoptries, remain unchanged after treatment with A23187 (Figure 2E–F and Table S1). These observations indicate that rise in cytosolic calcium specifically triggers translocation of microneme proteins to the merozoite surface.
Fig. 2. Cytosolic calcium levels and expression of EBA175 on merozoite surface detected by flow cytometry following treatment with calcium ionophore A23187. 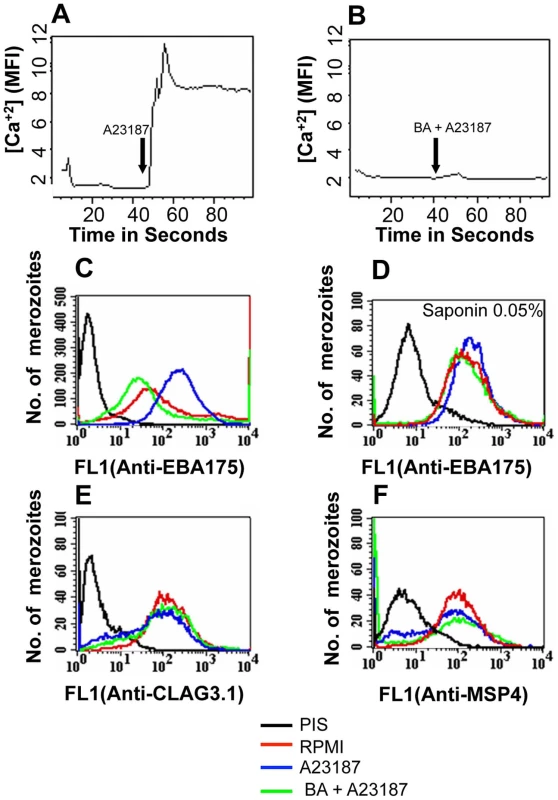
(A–B) Cytosolic calcium levels in merozoites upon treatment with ionophore A23187. Changes in cytosolic calcium levels were detected in P. falciparum merozoites labeled with Fluo-4AM by flow cytometry after treatment with either A23187 (A) or BAPTA-AM followed by A23187 (BA + A23187) (B). Mean fluorescence intensities (MFI), which reflect relative levels of free cytosolic calcium [Ca+2], measured by flow cytometry are shown as a function of time in seconds. Arrow indicates time-point at which A23187 was added. (C–F) Surface expression of microneme, rhoptry and merozoite surface proteins upon treatment with A23187. Expression of microneme protein EBA175 (C), rhoptry protein CLAG3.1 (E) and merozoite surface protein MSP4 (F) was detected on surface of P. falciparum merozoites isolated in RPMI1640 medium (RPMI, red) or following treatment with either A23187 (A23187, blue) or treatment with BAPTA-AM followed by A23187 (BA + A23187, green) using specific sera by flow cytometry. Microneme protein EBA175 was also detected in merozoites permeabilized with 0.05% saponin using specific sera (D). Untreated merozoites stained with pre-immune serum (PIS, black) were used as controls (C–F). Fig. 3. Expression of EBA175 on merozoite surface detected by immunofluorescence assay (IFA) following treatment with calcium ionophore A23187. 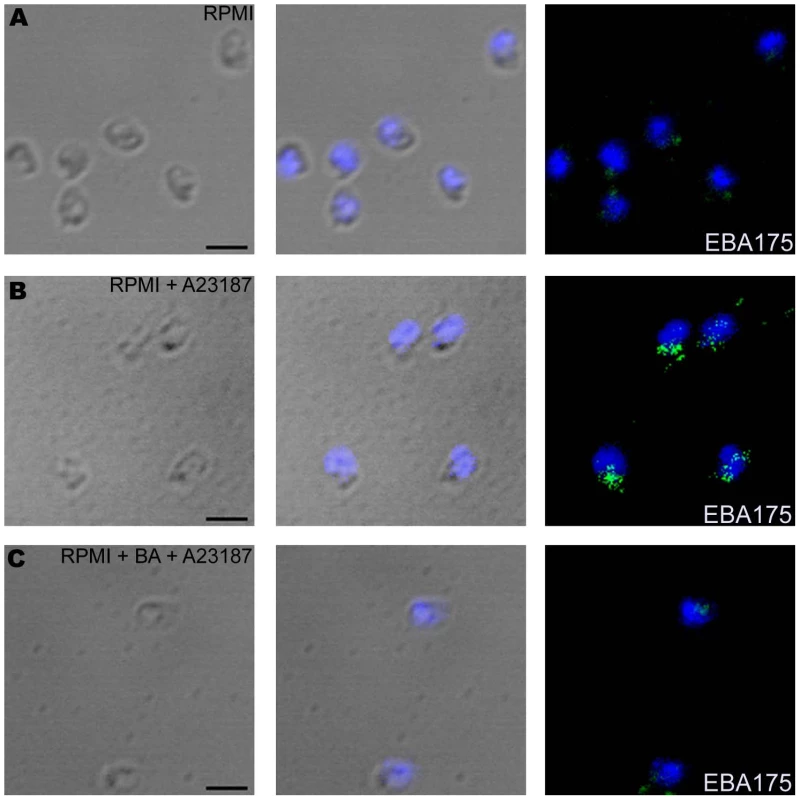
Expression of microneme protein EBA175 on surface of P. falciparum merozoites isolated in RPMI1640 medium (RPMI) (A) or following treatment with either A23187 (RPMI + A23187) (B) or BAPTA-AM followed by A23187 (RPMI + BA + A23187) (C) was detected by IFA using anti-EBA175 rabbit sera followed by FITC-conjugated anti-rabbit IgG goat sera (green). Nuclear DNA was counterstained with DAPI (blue). Bright field, bright field merged with DAPI staining and merged fluorescence images (DAPI and FITC) are shown. Black bar indicates 2 µm. Exposure of P. falciparum merozoites to low potassium ion concentrations elevates intracellular calcium levels and triggers microneme release
The physiologically relevant external signals that can induce a rise in intracellular calcium during invasion to trigger translocation of microneme proteins to the merozoite surface are not known. Given the drastic differences between the ionic environments in the erythrocyte cytosol and in blood plasma, we wondered whether exposure of merozoites to the ionic environment found in blood plasma could provide the signal for microneme release. The internal ionic environment in erythrocytes is characterized by low [Na+] (5 mM), high [K+] (140 mM) and low [Ca+2] (nanomolar) [30] whereas blood plasma has high [Na+] (140 mM), low [K+] (5 mM) and high [Ca+2] (1 mM).
We used Fluo-4AM to determine whether exposure of merozoites to ionic conditions found in blood plasma can trigger a rise in cytosolic calcium levels. Since Fluo-4AM is not a ratiometric dye, we first measured the in situ binding constants for Fluo-4AM in merozoites resuspended in buffers mimicking intracellular (IC buffer – 5 mM NaCl, 140 mM KCl, 1 mM EGTA) and extracellular (EC buffer - 140 mM NaCl, 5 mM KCl, 1 mM CaCl2) ionic conditions (Figure S6). The in situ binding constants for Fluo-4AM in IC (Kd = 1.13 µM) and EC buffers (Kd = 1.19 µM) are similar (Figure S6) confirming that Fluo-4AM can accurately report changes in cytosolic calcium concentrations in merozoites under these ionic conditions.
P. falciparum merozoites were isolated in IC buffer, labeled with Fluo-4AM, transferred from IC to EC buffer and observed for changes in cytosolic calcium levels by flow cytometry. Cytosolic calcium levels rise when merozoites are transferred from IC to EC buffer (Figure 4A). Next, we tested whether exposure of merozoites to extracellular ionic conditions can trigger the release of microneme proteins to the merozoite surface. Expression of EBA175 on the surface of merozoites increases upon transfer of merozoites from IC to EC buffer (Figure 4B and Table S2). However, treatment of merozoites with BAPTA-AM prior to transfer from IC to EC buffer blocks both the rise in cytosolic calcium and translocation of EBA175 to the merozoite surface (Figures 4A and 4B, Table S2). The translocation of EBA175 to the surface following transfer of merozoites from IC to EC buffer was confirmed by IFA (Figure 5). Microneme protein AMA1 is also secreted to the merozoite surface following transfer of merozoites from IC to EC buffer (Figure S7). EBA175 released to the merozoite surface in response to change in external ionic conditions appears to be primarily localized at the apical end of merozoites (Figure 5).
Fig. 4. Cytosolic calcium levels and expression of EBA175 on merozoite surface in response to changes in ionic conditions. 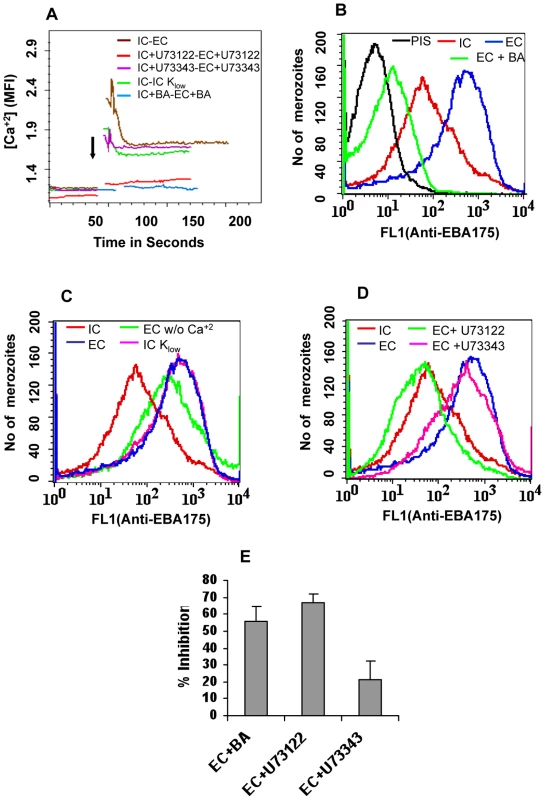
(A) Cytosolic calcium levels in merozoites under different ionic conditions. P. falciparum merozoites were isolated in buffer mimicking intracellular conditions (IC – 5 mM NaCl, 140 mM KCl, 1 mM EGTA). Merozoites were labeled with Fluo-4AM and cytosolic calcium levels [Ca+2] were measured by flow cytometry before and after transfer from IC buffer to buffer mimicking extracellular conditions (EC – 140 mM NaCl, 5 mM KCl, 1 mM CaCl2 (IC-EC, brown)) or IC Klow buffer (IC Klow - 5 mM NaCl, 5 mM KCl, 135 mM choline-Cl, 1 mM EGTA (IC-IC Klow, green)). Merozoites isolated in IC buffer were transferred to EC buffer after prior treatment with either BAPTA-AM (IC+BA-EC+BA, blue) or U73122 (IC+U73122-EC+U73122, red) or its inactive analog U73343 (IC+U73343-EC+U73343, purple). Mean fluorescence intensities (MFI), which reflect relative levels of free cytosolic calcium measured by flow cytometry, are shown as a function of time in seconds. Arrow indicates time-point at which merozoites were transferred from IC buffer. (B) Surface expression of EBA175 under different ionic conditions. Expression of EBA-175 was detected on surface of merozoites isolated in IC buffer (IC, red), following transfer from IC to EC buffer (EC, blue) or following transfer from IC to EC buffer after prior treatment with BAPTA-AM (EC + BA, green) using specific sera by flow cytometry. Merozoites isolated in IC buffer and stained with pre-immune serum (PIS, black) were used as controls. (C) Surface expression of EBA175 in response to change in [K+]. Expression of EBA-175 was detected on surface of merozoites isolated in IC buffer (IC, red), after transfer from IC to EC buffer (EC, blue), IC Klow buffer (IC Klow, pink) or EC w/o Ca+2 buffer (140 mM NaCl, 5 mM KCl, 1 mM EGTA (EC w/o Ca+2, green) using specific sera by flow cytometry. (D) Effect of PLC inhibitor U73122 on surface expression of EBA175. Expression of EBA175 was detected on surface of merozoites isolated in IC buffer (IC, red), following transfer from IC to EC buffer (EC, blue) or from IC to EC buffer following pre-treatment with either U73122 (EC+U73122, green) or its inactive analog U73343 (EC+U73343, pink) using specific sera by flow cytometry. (E) Invasion rates in presence of calcium signaling pathway inhibitors. Merozoites were allowed to invade erythrocytes in presence of calcium chelator BAPTA-AM (EC + BA), PLC inhibitor U73122 (EC+U73122) or its inactive analog U73343 (EC+U73343). Newly invaded rings were scored by Giemsa staining. Invasion rates in RPMI1640 medium in absence of any calcium modulating agents were used as controls to determine invasion inhibition rates in presence of BAPTA-AM, U73122 and U73343. Fig. 5. Expression of EBA175 on merozoite surface detected by immunofluorescence assay (IFA) in response to changes in ionic conditions. 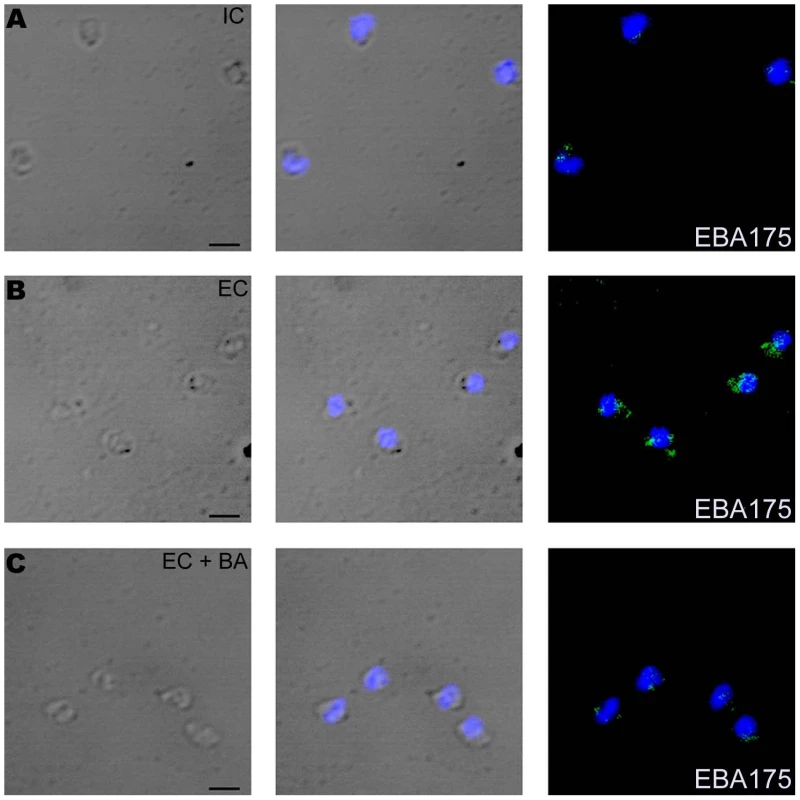
Expression of microneme protein EBA175 on surface of P. falciparum merozoites isolated in IC buffer (IC) (A) or following transfer to EC buffer (EC) (B) or following transfer to EC buffer after pre-treatment with BAPTA-AM (EC + BA) (C) was detected by IFA using anti-EBA175 rabbit sera followed by FITC-conjugated anti-rabbit IgG goat sera (green). Nuclear DNA was counterstained with DAPI (blue). Bright field, bright field merged with DAPI staining and merged fluorescence images (DAPI and FITC) are shown. Black bar indicates 2 µm. Following secretion to the merozoite surface, microneme proteins such as EBA175 and AMA1 are proteolytically cleaved and released in the supernatant. The presence of EBA175 (175kD) and AMA1 (44kD and 48kD fragments) in merozoite supernatants following incubation of merozoites in IC or EC buffers was detected by Western blotting. Levels of EBA175 and AMA1 were significantly higher in supernatants of merozoites incubated in EC buffer compared to IC buffer (Figure S8) confirming that extracellular ionic conditions that mimic those found in blood plasma triggers release of microneme proteins to the merozoite surface.
In order to identify the specific ion responsible for rise in cytosolic calcium and release of microneme proteins, we isolated merozoites in IC buffer, transferred them to IC-Klow buffer (5 mM NaCl, 5 mM KCl, 135 mM choline-Cl, 1 mM EGTA) and studied the levels of cytosolic calcium and EBA175 expression on the merozoite surface. Transfer of merozoites from IC to IC-Klow buffer, which exposes merozoites to a change in potassium ion concentration alone, triggers an increase in cytosolic calcium levels (Figure 4A) and translocation of EBA175 to the merozoite surface (Figure 4C and Table S2). Exposure of merozoites to low [K+] as found in blood plasma thus appears to be a key external signal that triggers a rise in cytosolic calcium and induces discharge of microneme proteins.
Transfer of merozoites from a high [K+] to low [K+] environment triggers a rise in intracellular calcium and microneme release even in the absence of any extracellular calcium (Figures 4A and 4C) implicating internal stores as the potential source of free calcium. Release of calcium from internal stores such as the endoplasmic reticulum (ER) usually involves phospholipase C (PLC) [31], which when activated cleaves phosphatidyl inositol bisphosphate (PIP2) to produce diacylglycerol (DAG) and inositol trisphosphate (IP3). Binding of IP3 to IP3 receptor on the ER can stimulate opening of calcium channels and release of free calcium from the ER to the cytoplasm [32]. Although IP3 receptor has not been identified in P. falciparum, pharmacological evidence suggests that it is likely to be present in Apicomplexan parasites [33]. The P. falciparum genome encodes a putative PLC and the PLC inhibitor U73122 has been shown to block P. falciparum blood-stage development [8],[34],[35]. We tested whether U73122 can block the release of calcium and translocation of microneme proteins when merozoites are transferred from IC to EC buffer. Merozoites isolated in IC buffer were treated either with U73122 [8],[34],[35] or its inactive analog U73343 [8],[34],[35] prior to transfer to EC buffer. Treatment with U73122 blocks the rise in cytosolic calcium and translocation of EBA175 to the merozoite surface upon transfer from IC to EC buffer (Figures 4A and 4D, Table S2). On the other hand, its inactive analog, U73343 [8],[34],[35], does not inhibit the rise in cytosolic calcium or translocation of EBA175 to the merozoite surface (Figures 4A and 4D, Table S2). Treatment of merozoites with U73122 also blocks erythrocyte invasion by P. falciparum merozoites in vitro (Figure 4E).
Interaction of EBA175 with its receptor, glycophorin A, restores basal intracellular calcium levels and triggers release of rhoptry proteins
While transfer of merozoites from IC to EC buffer triggers a rise in cytosolic calcium and release of microneme proteins, there is no increase in surface expression of rhoptry proteins such as CLAG3.1 (Figure 6A and Table S3) and PfRH2b (Figure S9) indicating that translocation of microneme and rhoptry proteins is likely to be triggered by distinct external signals. Given that microneme proteins such as EBA175, EBA140 and EBA181, bind erythrocyte receptors during invasion [2], we wondered whether receptor-engagement by these parasite ligands following translocation to the merozoite surface might provide the signal for secretion of rhoptry proteins. In order to test this hypothesis, we transferred P. falciparum merozoites from IC buffer to EC buffer containing glyA, the receptor for EBA175, and studied expression of CLAG3.1 on the merozoite surface. There is an increase in surface expression of CLAG3.1 when merozoites are transferred from IC buffer to EC buffer containing glyA (Figure 6A and Table S3). The translocation of CLAG3.1 to the merozoite surface following transfer of merozoites to EC buffer containing glyA was confirmed by IFA using anti-CLAG3.1 sera (Figure 7). CLAG3.1 is localized to the apical end following release to the merzoite surface (Figure 7). Similarly, transfer of merozoites from IC buffer to EC buffer containing glyA also triggers release of rhoptry proteins Rh2b to the merozoite surface (Figure S9). Importantly, cytosolic calcium levels drop when merozoites are transferred to EC buffer containing glyA (Figure 6B). The release of CLAG3.1 to the surface when merozoites are transferred from IC buffer to EC buffer containing glyA was confirmed by IFA (Figure 7). These observations suggest that glyA provides the external signal for restoration of basal intracellular calcium levels and release of rhoptry proteins presumably by interaction with its ligand EBA175. Indeed, when P. falciparum 3D7Δ175 merozoites, which have a deletion in the gene encoding EBA175 [36], are transferred from IC buffer to EC buffer containing glyA no increase in surface expression of CLAG3.1 is detected (Figure 6C and Table S3). P. falciparum 3D7Δ175 merozoites express EBA175 homologs such as EBA140 and EBA181. EBA140 binds glycophorin C (glyC) whereas EBA181 binds an as yet unidentified trypsin-resistant erythrocyte receptor [2]. EBA140 expression on the merozoite surface increases upon transfer of P. falciparum 3D7 and 3D7Δ175 merozoites from IC to EC buffer (Figure S10). Since purified glyC is not available, we tested whether red blood cell (RBC) ghosts, which contain glyC, can trigger rhoptry release in P. falciparum 3D7Δ175 merozoites. Transfer of P. falciparum 3D7Δ175 merozoites from IC buffer to EC buffer containing RBC ghosts triggers the release of rhoptry protein CLAG3.1 to the merozoite surface (Figure 6C and Table S3). Moreover, cytosolic calcium is restored to basal levels following transfer of P. falciparum 3D7Δ175 merozoites to EC buffer containing RBC ghosts (Figure 6D).
Fig. 6. Cytosolic calcium levels and translocation of rhoptry protein CLAG3.1 to merozoite surface in response to interaction with glyA. 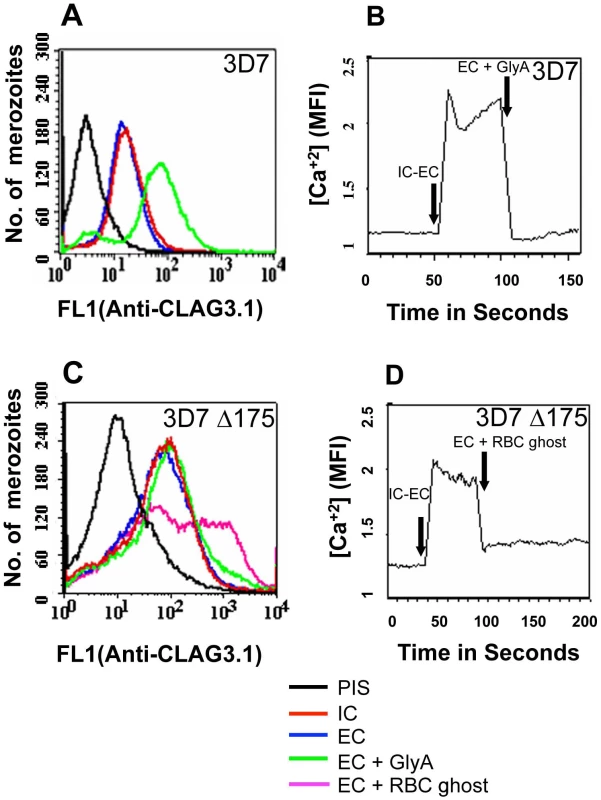
(A) Expression of CLAG3.1 on merozoite surface following interaction with glyA. Expression of CLAG3.1 was detected on surface of P. falciparum merozoites isolated in buffer mimicking intracellular conditions (IC – 5 mM NaCl, 140 mM KCl, 1 mM EGTA, red), or after transfer from IC buffer to buffer mimicking extracellular conditions (EC - 140 mM NaCl, 5 mM KCl, 1 mM CaCl2, blue) or after transfer from IC buffer to EC buffer containing glyA (EC + GlyA, green) using specific sera by flow cytometry. Merozoites isolated in IC buffer and stained with pre-immune sera (PIS, black) were used as controls. (B) Changes in cytosolic calcium levels in P. falciparum 3D7 merozoites following transfer from intracellular to extracellular ionic conditions and following engagement with glyA. P. falciparum 3D7 merozoites were isolated in IC buffer. Merozoites were labeled with Fluo-4AM and cytosolic calcium levels [Ca+2] were measured by flow cytometry before and after transfer from IC to EC buffer (IC-EC) and from EC buffer to EC buffer containing glyA (EC + GlyA). (C) Translocation of CLAG3.1 to surface of P. falciparum 3D7Δ175 merozoites following receptor engagement. Expression of CLAG3.1 was detected on surface of P. falciparum 3D7Δ175 merozoites isolated in IC buffer (IC, red), after transfer from IC buffer to EC buffer (EC, blue), after transfer from IC buffer to EC buffer containing glyA (EC + GlyA, green) or after transfer from IC buffer to EC buffer containing red blood cell (RBC) ghosts (EC + RBC ghosts, pink) using specific sera by flow cytometry. P. falciparum 3D7Δ175 merozoites in IC buffer stained with pre-immune sera (PIS, black) were used as control. (D) Changes in cytosolic calcium levels in P. falciparum 3D7Δ175 merozoites after transfer from intracellular to extracellular conditions and following binding to erythrocyte ghosts. P. falciparum 3D7Δ175 merozoites were isolated in IC buffer. Merozoites were labeled with Fluo-4AM and cytosolic calcium levels were measured by flow cytometry. Merozoites were transferred first from IC buffer to EC buffer (IC-EC) and from EC buffer to EC buffer containing red blood cell ghosts (EC + RBC ghosts). Mean fluorescence intensities (MFI), which reflect relative levels of free cytosolic calcium measured by flow cytometry are shown as a function of time in seconds (B, D). Arrows indicate time-points at which merozoites were transferred from IC buffer to EC buffer (IC-EC) (B, D) or from EC buffer to EC buffer containing gly A (EC + Gly A) (B) or from EC buffer to EC buffer containing RBC ghosts (EC + RBC ghosts) (D). Fig. 7. Expression of CLAG3.1 on merozoite surface detected by immunofluorescence assay (IFA) in response to interaction with glyA. 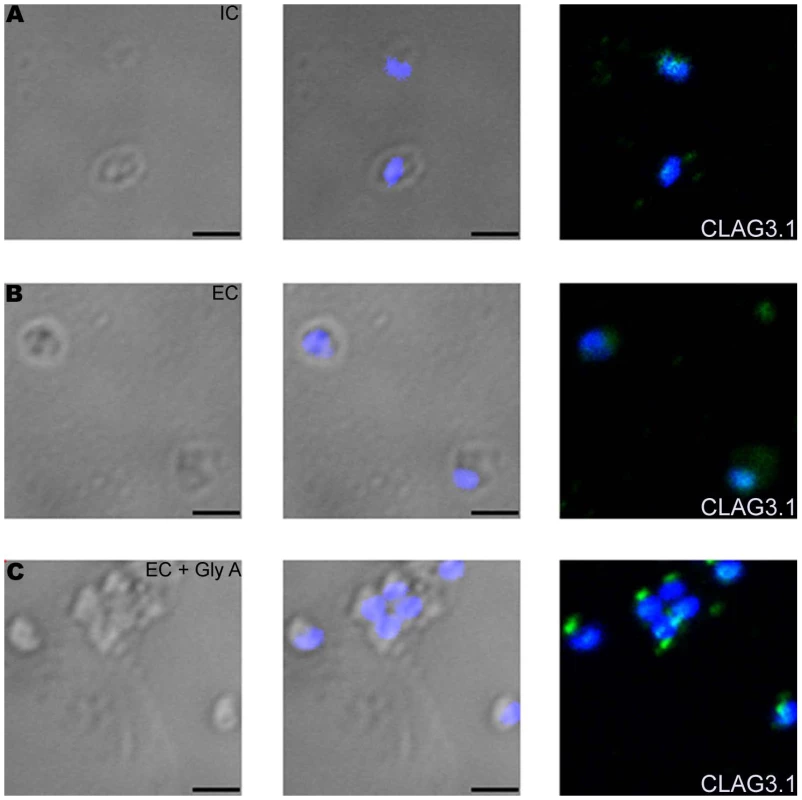
Expression of rhoptry protein CLAG3.1 on surface of P. falciparum merozoites isolated in IC buffer (IC) (A) or following transfer to EC buffer (EC) (B) or following transfer to EC buffer containing glyA (EC + GlyA) (C) detected by IFA using anti-CLAG3.1 rabbit sera followed by FITC-conjugated (green) anti-rabbit IgG goat sera. Nuclear DNA was counterstained with DAPI (blue). Bright field, bright field merged with DAPI staining and merged fluorescence images (DAPI and FITC) are shown. Black bar indicates 2 µm. Discussion
Parasites that belong to the phylum Apicomplexa are characterized by the presence of membrane bound organelles at the apical end of their invasive stages. These organelles, called micronemes and rhoptries, contain proteins that play essential roles in the process of host cell invasion. The regulated release of these apical organelles is critical for successful completion of host cell invasion. Here, we have identified the external signals that trigger the co-ordinated release of microneme and rhoptry proteins in P. falciparum merozoites during erythrocyte invasion.
We first investigated changes in levels of intracellular calcium in P. falciparum merozoites during erythrocyte invasion. Fluo-4AM labeled merozoites that are released in RPMI medium following rupture of mature schizonts in culture were fluorescent indicating that they had elevated levels of cytosolic calcium (Figures 1 and S2). The cytosolic calcium levels dropped following attachment of merozoites to target erythrocytes prior to invasion (Figures 1 and S2). Similar changes in calcium levels have been observed in T. gondii tachyzoites during host cell invasion [37].
Given the elevated calcium levels observed in P. falciparum merozoites prior to invasion we investigated whether calcium can regulate apical organelle secretion. We developed methods to isolate merozoites, measure levels of cytosolic calcium and study translocation of proteins to the merozoite surface. Treatment of merozoites with calcium ionophore A23187 resulted in elevation of cytosolic calcium (Figure 2A) and increase in expression of microneme proteins such as EBA175 (Figure 2C) on the merozoite surface. The increase in level of EBA175 on the merozoite surface in response to A23187 treatment was also detected by IFA (Figure 3) confirming results obtained by flow cytometry. Pre-treatment of merozoites with the intracellular calcium chelating agent BAPTA-AM blocked rise in cytosolic calcium (Figures 2B) as well as increase in EBA175 on the merozoite surface (Figures 2C and 3). Importantly, elevation of cytosolic calcium levels by A23187 treatment only triggered increased surface expression of microneme proteins. Surface expression of rhoptry proteins such as CLAG3.1 remained unchanged (Figure 2E) suggesting that the signaling pathways responsible for regulated secretion of microneme and rhoptry proteins are likely to be distinct.
Next, we identified a physiologically relevant natural signal that induces a rise in cytosolic calcium to trigger the release of microneme and rhoptry proteins in P. falciparum merozoites during invasion. We demonstrated that exposure of merozoites to low [K+] as found in blood plasma provided a key environmental signal that released calcium from intracellular stores through a PLC mediated pathway (Figure 3A). The signaling pathways by which exposure to low [K+] concentrations leads to a rise in cytosolic calcium remain to be defined. Importantly, transfer of merozoites to a low [K+] environment also triggers release of microneme proteins such as EBA175 to the merozoite surface as detected by flow cytometry and IFA (Figures 4 and 5). Moreover, higher levels of EBA175 and AMA1 are detected by Western blotting in supernatants of merozoites incubated in EC buffer compared to IC buffer (Figure S7) confirming that exposure of merozoites to extracellular ionic conditions as found in blood plasma triggers microneme protein release. Confirmation of flow cytometry results by two independent methods, namely, IFA and detection of secreted proteins by Western blotting, validates flow cytometry as a method to quantitatively detect expression of proteins on the merozoite surface.
Following translocation to the surface, binding of EBA175 and its homologues with their erythrocyte receptors triggers the release of rhoptry proteins such as CLAG3.1 (Figures 6 and 7) and PfRH2b (Figure S9). Receptor-engagement by EBA175 and its homologues also restores basal cytosolic calcium levels (Figures 6B and 6D), which may account for the drop observed in merozoites just prior to invasion by video microscopy (Figures 1 and S2). The drop in cytosolic calcium levels following receptor engagement may provide a feedback loop to switch off further secretion of microneme proteins to the merozoite surface.
The ionic environment within parasitized erythrocytes is influenced by the expression of parasite derived new permeation pathways following infection [38]. As a result, the Na+/K+ composition of the erythrocyte cytosol at late schizont stage is thought to be similar to that found in blood plasma [30],[39]. Moreover, the parasitophorous vacuolar membrane (PVM) is predicted to be freely permeable to ions in the schizont stage [39]. Merozoites developing within schizonts are thus likely to be exposed to a low [K+] environment prior to schizont rupture. Our model suggests that microneme proteins such as EBA175 and AMA1 may already be localized on the merozoite surface by the time of schizont rupture and merozoite egress. Indeed, AMA1 has been detected both in micronemes and on the surface of merozoites at the time of release from mature schizonts [40]. Following egress, as merozoites encounter uninfected erythrocytes, the interaction of parasite ligands such as EBA175 with their erythrocyte receptors such as glyA provides the signal for rhoptry release.
It has been previously reported that transgenic parasites that express truncated EBA175 with a deletion of the cytoplasmic domain can not use glyA as a receptor for invasion [41]. Phosphorylation of the cytoplasmic domain of EBA175 may be involved in signal transduction following receptor-engagement. Site-directed mutagenesis of two tyrosines present in the cytoplasmic tail, which are predicted to be phospohorylated, did not, however, result in any change in invasion efficiency [41]. Alternative phosphorylation sites in the cytoplasmic tail of EBA175 need to be investigated. Phosphorylation of the cytoplasmic domain of microneme protein AMA1 is functionally important for successful invasion of host cells [40]. In case of T. gondii, a conditional knock-out of AMA-1 results in a defect in rhoptry secretion [42]. In addition, deletion of the cytoplasmic domain of T. gondii microneme protein 8 (MIC8) also impairs secretion of rhoptry proteins during invasion [43]. These observations are in line with the induction of rhoptry secretion by EBA175 receptor-engagement reported here. The cytoplasmic domains of microneme proteins are likely to play important functional roles in signal transduction pathways that trigger rhoptry release during invasion.
This study opens the path for analysis of signal transduction pathways that mediate apical organelle release during invasion. Small molecules that target such pathways will block surface translocation of key parasite proteins needed for receptor-binding and may impair erythrocyte invasion. Indeed, we have demonstrated that treatment of P. falciparum merozoites with the PLC inhibitor U73122 blocks translocation of microneme proteins and inhibits erythrocyte invasion. Signaling pathway components that are involved in apical organelle release should therefore serve as attractive drug targets.
This study defines the sequence of apical organelle release during invasion. Our model suggests that surface translocation of microneme proteins precedes release of rhoptry proteins during invasion. Indeed, we have demonstrated that receptor engagement by microneme proteins such as EBA175 is required for release of rhoptry proteins. Targeting both microneme and rhoptry proteins with antibodies will thus block distinct steps in the invasion process and may provide a synergistic effect to inhibit erythrocyte invasion efficiently. Identification of key microneme and rhoptry proteins that play crucial roles in invasion should enable the development of efficacious multi-component blood-stage malaria vaccines that target distinct steps in the invasion process to block erythrocyte invasion efficiently and provide protection against malaria.
Materials and Methods
P. falciparum strain and in vitro culture
P. falciparum 3D7 was cultured in RPMI 1640 (Invitrogen, USA) supplemented with 27.2 mg/L hypoxanthine (Sigma, USA) and 0.5% Albumax I (Invitrogen, USA) (complete RPMI) using O+ RBCs in mixed gas environment (5% O2, 5% CO2 and 90% N2) as previously described [44].
Imaging calcium fluxes in P. falciparum merozoites during erythrocyte invasion by confocal microscopy
P. falciparum schizonts were purified from synchronized cultures using magnetic columns (Milteny Biotech, Germany), loaded with 2 µM Fluo-4AM (Molecular Probes, USA) for 30 mins at 37°C under mixed gas environment, washed with complete RPMI 1640 and added to uninfected erythrocytes in complete RPMI to allow re-invasion. The Fluo-4AM loaded P. falciparum schizont-uninfected erythrocyte suspension was injected into a Dvorak chamber [4] and observed on a Zeiss LSM510 confocal microscope equipped with a temperature controlled stage. Differential interference contrast (DIC) and fluorescence images (505–550 bandpass filter) were captured using a 63×, 1.4 NA lens. 12-bit time-lapse images of invasion events were captured and mean fluorescence intensity (MFI) within regions of interest containing merozoites in the focal plane were determined after background subtraction using Image Pro software.
Isolation of P. falciparum merozoites
P. falciparum 3D7 cultures were synchronized by treatment with sorbitol as described previously [45] in at least two successive cycles to obtain tight synchronization. Progress of synchronized P. falciparum schizonts was periodically monitored by light microscopy of Giemsa stained smears prepared from culture samples. When majority of infected erythrocytes reached the mature schizont stage with segmented merozoites, cultures were resuspended in complete RPMI or buffer mimicking intracellular ionic conditions (IC buffer – 5 mM NaCl, 140 mM KCl, 1 mM EGTA). Schizonts were allowed to rupture and release merozoites over a period of 1 hour. Cultures containing unruptured schizonts and released merozoites were centrifuged at 2000 rpm on a Sorvall RT7 centrifuge (500g) for 5 mins to separate released merozoites from unruptured schizonts and uninfected erythrocytes. Supernatants containing free merozoites were centrifuged at 5000 rpm on an Eppendorf 5810R centrifuge (3300 g) for 5 mins to collect merozoites. The merozoites were resuspended in RPMI 1640 medium (incomplete RPMI) or IC buffer for use in experiments. Merozoite preparations had less than 0.5% contaminating schizonts. Number of schizonts that ruptured was scored by Giemsa staining culture samples before and after collection period. Merozoite yields were scored by flow cytometry as described below. On average (5 experiments), 1 merozoite was retrieved per 4 ruptured schizonts.
Isolated merozoites were tested in erythrocyte invasion assays to estimate their viability. Isolated merozoites were mixed with liquid counting fluorescent beads (Becton Dickinson, USA) of known concentration and analyzed by flow cytometry on a FACSCalibur (Becton Dickinson, USA) to score merozoite density (number of merozoites per µl). Density of erythrocyte suspension (number of erythrocytes per µl) was scored using a hemocytometer. Approximately 1×106 merozoites were incubated with ∼1×107 erythrocytes in complete RPMI at 37°C for 18–20 hours under mixed gas environment to allow invasion. Invasion rates were determined by scoring the frequency of ring-infected erythrocytes by Giemsa (Sigma, USA) staining. In parallel, invasion assays were set up by incubating ∼1×105 late stage purified P. falciparum schizonts with ∼1×107 erythrocytes to allow schizont rupture and re-invasion. Newly invaded ring-infected erythrocytes were scored by Giemsa staining. Invasion rates from the two methods described were compared to determine whether viability of isolated merozoites is comparable to that of merozoites released from schizonts in culture.
Treatment of P. falciparum merozoites with calcium modulating agents and transfer to ionic conditions mimicking intracellular and extracellular conditions prior to analysis of cytosolic calcium levels or surface expression of merozoite proteins
P. falciparum merozoites isolated in RPMI 1640 were treated with 10 µM A23187 (Calbiochem, USA) for 15 mins at 37°C with or without pre-treatment with 50 µM BAPTA-AM for 15 mins at 37°C. Merozoites isolated in IC buffer were transferred to EC buffer, IC-Klow buffer (5 mM NaCl, 5 mM KCl, 135 mM choline-Cl, 1 mM EGTA), EC w/o Ca+2 buffer (140 mM NaCl, 5 mM KCl, 1 mM EGTA), EC buffer containing glyA (1 mg/ml; Sigma, USA ) or EC buffer containing red blood cell (RBC) ghosts with or without prior treatment with calcium modulators BAPTA-AM (50 µM; Calbiochem, USA), U73122 (10 µM; Calbiochem, USA) or its inactive analog U73343 (10 µM; Calbiochem, USA) for 15 mins at 37°C. RBC ghosts were prepared as follows. RBCs were lysed by resuspending RBC pellets in lysis buffer (cold, 10-fold diluted phosphate buffered saline (PBS)). Lysed RBCs were collected by centrifugation at 15,000 g for 5 mins and resuspended again in lysis buffer. This process was repeated 4–5 times to obtain RBC ghosts devoid of hemoglobin. RBC ghosts were finally resuspended in EC buffer and sonicated for 5 cycles with sonicator on or off for 0.5 seconds each before use. Protein content of RBC ghosts was measured by the bicinchoninic acid assay (BCA). RBC ghosts containing 250 µg of protein were added to 200 µl of merozoite suspension to stimulate rhoptry release.
Determination of free cytosolic calcium levels in P. falciparum merozoites by flow cytometry
P. falciparum merozoites isolated in complete RPMI or IC buffer as described above were loaded with 10 µM Fluo-4AM for 20 mins at 37°C, washed, resuspended in same buffers and used for experiments within 5 mins. Fluo-4AM loaded P. falciparum merozoites were treated with calcium modulating agents and/or transferred from ionic environments mimicking intracellular conditions to extracellular conditions as described above prior to analysis of fluorescence signal on FACSCalibur (Becton Dickinson, USA) using CellQuest software. The Fluo-4AM loaded merozoites were excited at 488 nm and fluorescence signal was detected with a 430/30 nm band pass filter for periods of 2–3 mins. Merozoites were gated on the basis of their forward scatter and side scatter. Histograms of mean fluorescence intensity (MFI), which reflects cytosolic calcium levels in merozoites, were plotted against time using FlowJo software.
Determination of in situ dissociation constants for Fluo-4AM in P. falciparum merozoites under intracellular and extracellular ionic conditions
In situ dissociation constants for Fluo-4AM were measured in P. falciparum merozoites under different ionic conditions using methods described earlier for mammalian cells [46]. Calcium Calibration Buffer Concentrate Kits (Molecular Probes, USA) containing 100 mM Ca-EGTA, pH 7.2 and 100 mM K2EGTA, pH 7.2 were used to generate IC and EC buffers containing a range of known concentrations of free calcium (17 nM to 39 µM) as described by the manufacturer. Merozoites were loaded with 10 µM Fluo-4AM for 20 mins at 37°C and washed with IC or EC buffers. Loaded merozoites were resuspended in IC and EC buffers containing range of calcium concentrations. Ionomycin (10 µM) (Calbiochem, USA) was added to the merozoite suspension to equilibrate intracellular and extracellular calcium concentrations. The fluorescence signal was measured at 485 nM using a Perkin Elmer Victor3 fluorimeter. Measured fluorescence intensity was used to plot Log[(F – Fmin)/Fmax – F)] against Log[Ca+2free] where F is the fluorescence intensity at different known free calcium concentrations, Fmin is the fluorescence intensity at zero free calcium concentration and Fmax is the fluorescence intensity at saturating free calcium concentration (39 µM free Ca+2). The x-intercept of the plot is Log Kd of Fluo-4AM. The Kd values determined represent affinity of Fluo-4 for calcium in the merozoite cytoplasm under ionic conditions that mimic intracellular and extracellular ionic environments.
Detection of proteins on P. falciparum merozoite surface by flow cytometry and fluorescence microscopy
P. falciparum merozoites isolated in complete RPMI or IC buffer were treated with calcium modulating agents and/or transferred from ionic environments mimicking intracellular conditions to extracellular conditions as described above prior to analysis of expression of microneme proteins (EBA175 and AMA1), rhoptry proteins (CLAG3.1 and RH2b) or merozoite surface protein (MSP4) on the merozoite surface. Merozoites were fixed with 0.15% chilled p-formaldehyde for 1 hour and used for detection of parasite proteins on the merozoite surface. Fixed merozoites were washed with PBS, incubated with sera raised against EBA175 (mouse sera at 1∶50), AMA1 (rabbit sera at 1∶50 dilution), CLAG3.1 (rabbit sera at 1∶50 dilution), PfRH2b (rabbit sera at 1∶50 dilution) and MSP4 (rabbit sera at 1∶50 dilution) for I hour on ice. Merozoites were washed two times with PBS, incubated with fluorescein isothiocyanate (FITC) conjugated anti-mouse IgG (Sigma, USA) or FITC conjugated anti-rabbit IgG (Sigma, USA) for I hour on ice, washed two times with PBS and used for analysis by flow cytometry using a Becton Dickinson FACS Callibur. Merozoites were excited at 488 nm and fluorescence signal was detected with a 430/30 nm band pass filter. Merozoites were gated on the basis of their forward scatter and side scatter. The gain of FL1 channel was set to ensure that fluorescence intensities of merozoite samples stained with pre-immune sera as well as immune sera against candidate proteins fall within the window of 100 to 104. In each experiment, data was acquired for 50,000 merozoites with the same FACS settings for each condition. FACS settings for independent experiments were not always identical. Data was analyzed using CellQuest software. Histograms showing logarithmic green fluorescence intensities (FL1) were plotted for each sample. Normalized relative MFI values from three independent experiments are reported for each condition to demonstrate reproducibility of the results. Merozoites were also labeled with mouse sera raised against the cytoplasmic protein NAPL at 1∶50 dilution as control. Merozoites were permeabilized by adding 0.05% saponin to primary and secondary sera to detect intracellular proteins. Expression of EBA175, CLAG3.1 and NAPL was also analyzed by IFA using a Nikon TE 300 fluorescence microscope following staining with primary and secondary sera as described above. Nuclear DNA was counterstained with 40 nM 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes, USA) in IFA.
Detection of proteins secreted from P. falciparum merozoites by Western blotting
P. falciparum merozoites isolated in IC buffer were incubated in either IC or EC buffer for 15 minutes at 37°C with or without prior treatment with BAPTA-AM for 20 mins at 37°C to evaluate the role of ionic conditions in secretion of microneme proteins. Merozoite supernatants were separated by centrifugation and used for detection of microneme proteins, EBA175 and AMA1, by Western blotting using specific rabbit antisera. Anti-EBA175 rabbit sera were used at a dilution of 1∶200, anti-AMA1 rabbit sera were used at 1∶2,000 dilution and horse radish peroxidase (HRP) conjugated anti-rabbit IgG goat sera (Sigma, USA) were used at a dilution of 1∶2,000 for Western blotting. Anti-NAPL rabbit sera were used at a dilution of 1∶2,000 as control for Western blotting with merozoite pellets as well as with merozoite supernatants for each condition. Densitometry analyses of Western blots of merozoite pellets and supernatants using anti-NAPL sera were used to normalize levels of EBA175 and AMA1 detected in merozoite supernatants under different conditions.
Measuring effect of calcium modulating agents on erythrocyte invasion by P. falciparum merozoites
Merozoites isolated from 2 ml of P. falcipaum 3D7 culture with 5% parasitemia were mock-treated with RPMI 1640 or treated with 1 mM BAPTA-AM (Calbiochem, USA), 10 µM U73122 (Calbiochem, USA) or 10 µM U73343 (Calbiochem, USA) for 15 mins at 37°C, washed with IC buffer, resuspended in EC buffer and incubated with 1×107 erythrocytes in EC buffer at 37°C under mixed gas environment for 2 hours to allow invasion. EC buffer was then replaced with complete RPMI. After 18–20 hours of incubation in complete RPMI under mixed gas environment to allow development of ring stages, the percentage of infected erythrocytes was scored by microscopy of Giemsa stained smears to determine invasion rates.
Supporting Information
Zdroje
1. SnowRW
GuerraCA
NoorAM
MyintHY
HaySI
2005 The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434 214 217
2. CowmanAF
CrabbBS
2006 Invasion of red blood cells by malaria parasites. Cell 124 755 766
3. AikawaM
MillerLH
JohnsonJ
RabbegeJ
1978 Erythrocyte entry by malarial parasites. A moving junction between erythrocyte and parasite. J Cell Biol 77 72 82
4. DvorakJA
MillerLH
WhitehouseWC
ShiroishiT
1975 Invasion of erythrocytes by malaria merozoites. Science 187 748 750
5. MillerLH
AikawaM
JohnsonJG
ShiroishiT
1979 Interaction between cytochalasin-treated malarial parasites and erythrocytes. J Exp Med 49 172 184
6. GilsonPR
CrabbBS
2009 Morphology and kinetics of the three distinct phases of red blood cell invasion by Plasmodium falciparum merozoites. Int J Parasitol 39 91 96
7. OnoT
Cabrita-SantosL
LeitaoR
BettiolE
PurcellLA
2008 Adenylyl cyclase alpha and cAMP signaling mediate Plasmodium sporozoite apical regulated exocytosis and hepatocyte infection. PLoS Pathog 4 e1000008 doi:10.1371/journal.ppat.1000008
8. VaidA
ThomasDC
SharmaP
2008 Role of Ca2+/CaM-PfPKB signaling pathway in erythrocyte invasion by Plasmodium falciparum. J Biol Chem 283 5589 5597
9. CarruthersVB
SibleyLD
1999 Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol 31 421 428
10. LovettJL
MarchesiniN
MorenoSN
SibleyLD
2002 Toxoplasma gondii microneme secretion involves intracellular Ca(2+) release from inositol 1,4,5-triphosphate (IP(3))/ryanodine-sensitive stores. J Biol Chem 277 25870 25876
11. MoudyR
ManningTJ
BeckersCJ
2001 The loss of cytoplasmic potassium upon host cell breakdown triggers egress of Toxoplasma gondii. J Biol Chem 276 41492 41501
12. WetzelDM
ChenLA
RuizFA
MorenoSN
SibleyLD
2004 Calcium-mediated protein secretion potentiates motility in Toxoplasma gondii. J Cell Sci 117 5739 5748
13. CarruthersVB
GiddingsOK
SibleyLD
1999 Secretion of micronemal proteins is associated with Toxoplasma invasion of host cells. Cell Microbiol 1 225 236
14. CarruthersVB
MorenoSNJ
SibleyLD
1999 Ethanol and acetaldehyde elevate intracellular [Ca2+] calcium and stimulate microneme discharge in Toxoplasma gondii. Biochem J 342 379 386
15. KawaseO
NishikawaY
BannaiH
ZhangH
ZhangG
2007 Proteomic analysis of calcium-dependent secretion in Toxoplasma gondii. Proteomics 7 3718 3725
16. NagamuneK
HicksLM
FuxB
BrossierF
ChiniEN
2008 Abscisic acid controls calcium-dependent egress and development in Toxoplasma gondii. Nature 451 207 210
17. HåkanssonS
CharronAJ
SibleyLD
2001 Toxoplasma evacuoles: a two-step process of secretion and fusion forms the parasitophorous vacuole. EMBO J 20 3132 3144
18. BesteiroS
MichelinA
PoncetJ
DubremetzJF
LebrunM
2009 Export of a Toxoplasma gondii rhoptry neck protein complex at the host cell membrane to form the moving junction during invasion. PLoS Pathog 5 e1000309 doi:10.1371/journal.ppat.1000309
19. SaeijJPJ
BoyleJP
CollerS
TaylorS
SibleyLD
2006 Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science 314 1780 1783
20. TaylorS
BarraganA
SuC
FuxB
FentressSJ
2006 A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science 314 1776 1780
21. CamusD
HadleyTJ
1985 Plasmodium falciparum antigen that binds to host erythrocytes and merozoites. Science 230 553 556
22. SimBK
OrlandiPA
HaynesJD
KlotzFW
CarterJM
1990 Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J Cell Biol 111 1877 1884
23. SimBK
ToyoshimaT
HaynesJD
AikawaM
1992 Localization of the 175-kilodalton erythrocyte binding antigen in micronemes of Plasmodium falciparum merozoites. Mol Biochem Parasitol 51 157 159
24. HealerJ
CrawfordS
RalphS
McFaddenG
CowmanAF
2002 Independent translocation of two micronemal proteins in developing Plasmodium falciparum merozoites. Infect Immun 70 5751 5758
25. KanekoO
Yim LimBY
IrikoH
LingIT
OtsukiH
2005 Apical expression of three RhopH1/Clag proteins as components of the Plasmodium falciparum RhopH complex. Mol Biochem Parasitol 143 20 28
26. TrigliaT
ThompsonJ
CaruanaSR
DelorenziM
SpeedT
CowmanAF
2001 Identification of proteins from Plasmodium falciparum that are homologous to reticulocyte binding proteins in Plasmodium vivax. Infect Immun 69 1084 1092
27. PandeyKC
SinghS
PattnaikP
PillaiCR
PillaiU
2002 Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol Biochem Parasitol 123 23 33
28. MarshallVM
SilvaA
FoleyM
CranmerS
WangL
1997 A second merozoite surface protein (MSP-4) of Plasmodium falciparum that contains an epidermal growth factor-like domain. Infect Immun 65 4460 4467
29. ChandraBR
OlivieriA
SilvestriniF
AlanoP
SharmaA
2005 Biochemical characterization of the two nucleosome assembly proteins from Plasmodium falciparum. Mol Biochem Parasitol 142 237 247
30. LeeP
YeZ
Van DykeK
KirkRG
1988 X-ray microanalysis of Plasmodium falciparum and infected red blood cells: effects of qinghaosu and chloroquine on potassium, sodium, and phosphorus composition. Am J Trop Med Hyg 39 157 165
31. BerridgeMJ
1993 Inositol trisphosphate and calcium signaling. Nature 361 315 325
32. RossCA
MeldolesiJ
MilnerTA
SatohT
SupattaponeS
1989 Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature 339 468 470
33. NagamuneK
MorenoSN
ChiniEN
SibleyLD
2008 Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem 47 70 81
34. BeraldoFH
GarciaCRS
2005 Products of tryptophan catabolism induce Ca2+ release and modulate the cell cycle of Plasmodium falciparum malaria parasites. J Pineal Res 39 224 230
35. VaidA
SharmaP
2006 PfPKB, a protein kinase B-like enzyme from Plasmodium falciparum: II. Identification of calcium/calmodulin as its upstream activator and dissection of a novel signaling pathway. J Biol Chem 281 27126 27133
36. DuraisinghMT
MaierAG
TrigliaT
CowmanAF
2003 Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc Natl Acad Sci U S A 100 4796 4801
37. LovettJL
SibleyLD
2003 Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. J Cell Sci 116 3009 3016
38. DesaiSA
KrogstadDJ
McCleskeyEW
1993 A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature 362 643 646
39. StainesHM
ElloryJC
KirkK
2001 Perturbation of the pump-leak balance for Na(+) and K(+) in malaria-infected erythrocytes. Am J Physiol Cell Physiol 280 1576 1587
40. TreeckM
ZacherlS
HerrmannS
CabreraA
KonoM
2009 Functional analysis of the leading malaria vaccine candidate AMA-1 reveals an essential role of the cytoplasmic domain in the invasion process. PLoS Pathog 5 e1000322
41. GilbergerTW
ThompsonJK
ReedMB
GoodRT
CowmanAF
2003 The cytoplasmic domain of the Plasmodium falciparum ligand EBA-175 is essential for invasion but not protein trafficking. J Cell Biol 162 317 327
42. MitalJ
MeissnerM
SoldatiD
WardGE
2005 Conditional expression of Toxoplasma gondii apical membrane antigen-1 (TgAMA1) demonstrates that TgAMA1 plays a critical role in host cell invasion. Mol Biol Cell 16 4341 4349
43. KesslerH
Herm-GötzA
HeggeS
RauchM
Soldati-FavreD
2008 Microneme protein 8–a new essential invasion factor in Toxoplasma gondii. J Cell Sci 121 947 956
44. TragerW
JensenJB
1976 Human malaria parasites in continuous culture. Science 193 673 675
45. LambrosC
VanderbergJP
1979 Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 65 418 420
46. ThomasD
ToveySC
CollinsTJ
BootmanMD
BerridgeMJ
2000 A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium 28 213 223
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



