-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
The emergence of viral respiratory pathogens with pandemic potential, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and influenza A H5N1, urges the need for deciphering their pathogenesis to develop new intervention strategies. SARS-CoV infection causes acute lung injury (ALI) that may develop into life-threatening acute respiratory distress syndrome (ARDS) with advanced age correlating positively with adverse disease outcome. The molecular pathways, however, that cause virus-induced ALI/ARDS in aged individuals are ill-defined. Here, we show that SARS-CoV-infected aged macaques develop more severe pathology than young adult animals, even though viral replication levels are similar. Comprehensive genomic analyses indicate that aged macaques have a stronger host response to virus infection than young adult macaques, with an increase in differential expression of genes associated with inflammation, with NF-κB as central player, whereas expression of type I interferon (IFN)-β is reduced. Therapeutic treatment of SARS-CoV-infected aged macaques with type I IFN reduces pathology and diminishes pro-inflammatory gene expression, including interleukin-8 (IL-8) levels, without affecting virus replication in the lungs. Thus, ALI in SARS-CoV-infected aged macaques developed as a result of an exacerbated innate host response. The anti-inflammatory action of type I IFN reveals a potential intervention strategy for virus-induced ALI.
Published in the journal: . PLoS Pathog 6(2): e32767. doi:10.1371/journal.ppat.1000756
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000756Summary
The emergence of viral respiratory pathogens with pandemic potential, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and influenza A H5N1, urges the need for deciphering their pathogenesis to develop new intervention strategies. SARS-CoV infection causes acute lung injury (ALI) that may develop into life-threatening acute respiratory distress syndrome (ARDS) with advanced age correlating positively with adverse disease outcome. The molecular pathways, however, that cause virus-induced ALI/ARDS in aged individuals are ill-defined. Here, we show that SARS-CoV-infected aged macaques develop more severe pathology than young adult animals, even though viral replication levels are similar. Comprehensive genomic analyses indicate that aged macaques have a stronger host response to virus infection than young adult macaques, with an increase in differential expression of genes associated with inflammation, with NF-κB as central player, whereas expression of type I interferon (IFN)-β is reduced. Therapeutic treatment of SARS-CoV-infected aged macaques with type I IFN reduces pathology and diminishes pro-inflammatory gene expression, including interleukin-8 (IL-8) levels, without affecting virus replication in the lungs. Thus, ALI in SARS-CoV-infected aged macaques developed as a result of an exacerbated innate host response. The anti-inflammatory action of type I IFN reveals a potential intervention strategy for virus-induced ALI.
Introduction
The zoonotic transmission of severe acute respiratory syndrome coronavirus (SARS-CoV) caused pneumonic disease in humans with an overall mortality rate of ∼10%. The exact reasons why some individuals succumbed to the infection while others remained relatively unaffected have not been clarified. Aging, an important risk factor in SARS-CoV-associated disease, is associated with changes in immunity [1],[2],[3]. Consequently, elderly individuals are at greater risk of contracting more severe and longer lasting infections with increased morbidity and mortality, exemplified by respiratory tract infections caused by influenza A virus and severe acute respiratory syndrome (SARS) coronavirus [4],[5],[6]. The clinical course of SARS-CoV-induced disease follows a triphasic pattern [5]. The first phase is characterized by fever, myalgia and other systemic symptoms that are likely caused by the increase in viral replication and cytolysis. The second phase of the disease is characterized by a decrease in viral replication that correlates with the onset of IgG conversion. Interestingly, it is also in this phase that severe clinical worsening is seen, which can not be explained by uncontrolled viral replication. It has been hypothesized that the diffuse alveolar lung damage in this phase is caused by an over exuberant host response [5],[7],[8]. The majority of patients recovers after 1–2 weeks, but up to one-third of the patients progress to the third phase and develop severe inflammation of the lung, characterized by acute respiratory distress syndrome (ARDS) [9]. The clinical course and outcome of SARS-CoV disease are more favorable in children younger than 12 years of age as compared to adolescents and adults [10],[11],[12]; elderly patients have a poor prognosis, with mortality rates of up to ∼50% [5],[6].
For SARS-CoV-associated disease in humans, it has been hypothesized that seemingly excessive pro-inflammatory responses, illustrated by elevated levels of inflammatory cytokines and chemokines, mediate immune-pathology resulting in acute lung injury (ALI) and ARDS [5],[13],[14],[15],[16]. Direct support for this concept, however, is scarce. ALI and ARDS are typified by inflammation, with increased permeability of the alveolar-capillary barrier, resulting in pulmonary edema, hypoxia, and accumulation of polymorphonuclear leukocytes and macrophages. Inflammatory cytokines, among which IL-1β and IL-8, play a major role in mediating and amplifying ALI/ARDS [9] and are elevated in SARS-CoV-infected patients as well [13],[14]. In vitro experiments confirm that SARS-CoV infection induces expression of cytokines/chemokines in a range of cell types [15],[17],[18]. Moreover, infection of cynomolgus macaques with SARS-CoV leads to a strong immune response, with expression of various cytokines/chemokines, resembling the host response seen in human SARS patients [19]. Nevertheless, the determinants that lead to severe virus-associated ALI/ARDS and that cause people to succumb to infection remain largely obscure, restraining development of appropriate treatments.
As advanced age is a predictor of adverse clinical outcome in both ARDS and SARS-CoV infections [5],[20], we used age as predisposing factor to study the pathogenesis of SARS-CoV in a macaque model. By performing comparative analyses of young adult and aged SARS-CoV-infected macaques regarding pathology, virus replication and host response, insight into the pathogenesis of SARS-CoV is obtained and a potential therapeutic intervention strategy for virus-induced ALI is revealed.
Results
SARS-CoV causes more severe pathology in aged than in young adult macaques
To obtain further insights in the pathogenesis of SARS-CoV, six aged (10–19 years old) and six young adult (3–5 years old) cynomolgus macaques were infected with SARS-CoV HKU39849 and euthanized four days after infection. Four young adult and four aged PBS-infected cynomolgus macaques were used as negative controls. During the 4-day experiment, some of the SARS-CoV-infected aged animals displayed decreased activity and mildly labored breathing. All aged infected macaques showed an increase in body temperature either during the night or during the day one to two days after infection (Figure 1A). The lungs of aged macaques showed large (multi)focal pulmonary consolidation that was severe (∼40–60% of affected lung tissue) in two macaques (Figure 1B and Figure S1). Microscopic examination revealed typical ALI-associated lesions, similar to what has been seen in SARS-CoV-infected humans that progress to ARDS [6]. Lesions involved the alveoli and terminal bronchioli, showing areas with acute or more advanced phases of diffuse alveolar damage (Figure 2). Lumina of alveoli were variably filled with protein rich edema fluid, cellular debris, alveolar macrophages and neutrophils, eosinophils, and lymphocytes (Figure 2 and Figure S2A–B). Moderately thickened alveolar walls were lined by cuboidal epithelial cells (type 2 pneumocyte hyperplasia; Figure 2 and Figure S2C). The epithelial origin of these enlarged type 2 pneumocytes with large vacuolated nuclei, prominent nucleoli and abundant vesicular cytoplasm was confirmed by keratin staining (Figure S2D). Hyaline membranes and multinucleated giant cells were occasionally observed in the alveoli (Figure S2E–F). In contrast, all young adult animals remained free of clinical symptoms and had no or less extensive pulmonary consolidation (Figure 1A–C). Hyaline membranes were not observed in SARS-CoV-infected young adult macaques. A multifocal mild chronic lymphoplasmacytic tracheo-bronchoadenitis, characterized by moderate numbers of lymphocytes, plasma cells, macrophages, less neutrophils and occasional eosinophils in the lamina propria of the bronchi, focally surrounding and infiltrating the submucosal glands, was observed in all young adult macaques, but not in aged macaques (Figure 2). Our data were confirmed by retrospective analysis of earlier experiments in which aged animals were used [19],[21],[22],[23]. Overall, aged macaques develop more severe SARS-CoV-associated ALI than young adults.
Fig. 1. Aged macaques are more prone to develop SARS-CoV-associated disease than young adults. 
(A) Fluctuations in body temperatures in four young adult and four aged SARS-CoV-infected macaques measured by transponders in the peritoneal cavity. Temperatures are shown from day six prior to infection until four days post infection. The arrow indicates day zero when animals were infected. Grey horizontal lines mark the average range of temperature fluctuations prior to infection. (B) Macroscopic appearance of (consolidated) lung tissue of young adult and aged SARS-CoV infected macaques at day 4 post infection. Lesions are arrowed. (C) Gross pathology scores of aged and young adult macaque groups were determined after necropsy and averaged (±standard error of the mean (s.e.m.)). Fig. 2. Histology of lungs from SARS-CoV-infected aged macaques. 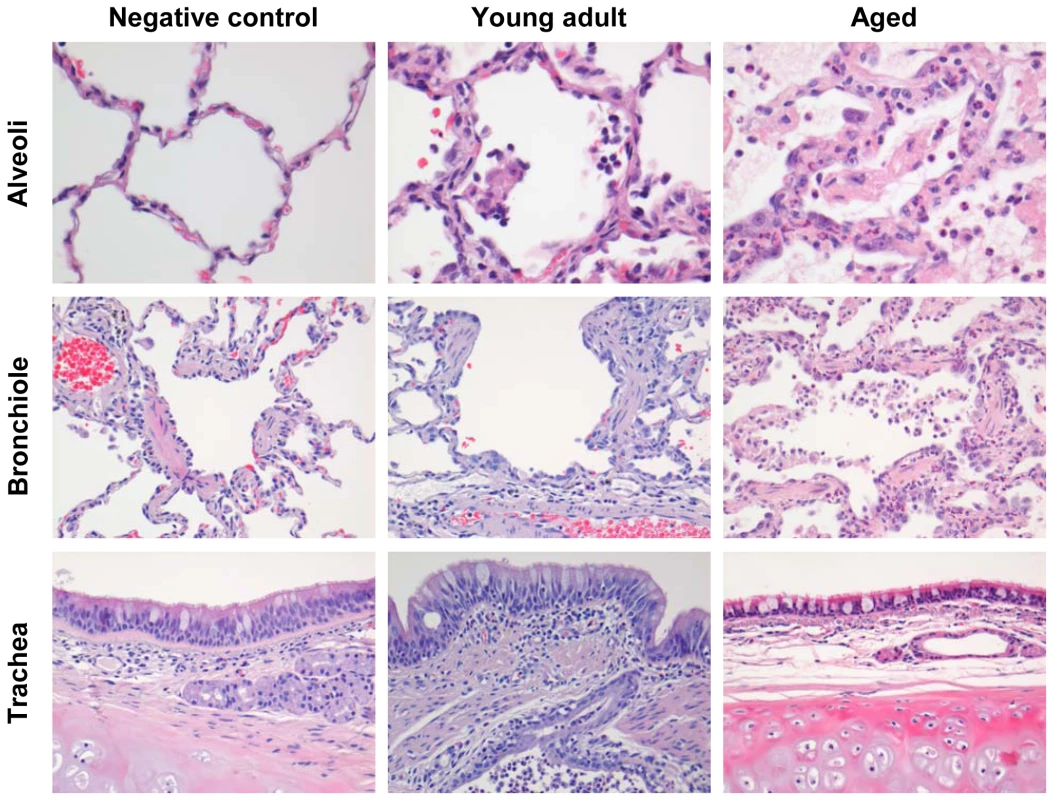
Lesions in lungs of PBS-infected (left panel) and SARS-CoV infected young adult (middle panel) and aged (right panel) macaques showing diffuse alveolar damage, characterized by disruption of alveolar walls causing edema and type II pneumocyte hyperplasia with influx of inflammatory cells in the alveoli and bronchioles. In the trachea, a multifocal mild chronic lymphoplasmacytic tracheobronchoadenitis was observed in young adult macaques. The level of viral replication in aged and young adult macaques is similar
Because viral replication is important for disease pathogenesis, we determined virus titers in aged and young adult animals. Virus excretion in the throat (Figure 3A) and nose (Figure 3B) of aged and young adult macaques at days 2 and 4 post infection was not significantly different. Moreover, no significant difference in quantity of SARS-CoV mRNA in the lungs of young adult and aged animals was observed (Figure 3C). Differences in the nature and percentage of SARS-CoV-infected cells in the lungs of aged and young adult macaques were not seen either (Figure 3D). Apparently, augmented pathology in aged macaques cannot be rationalized by increased viral replication.
Fig. 3. Viral replication levels in SARS-CoV-infected aged and young adult macaques are similar. 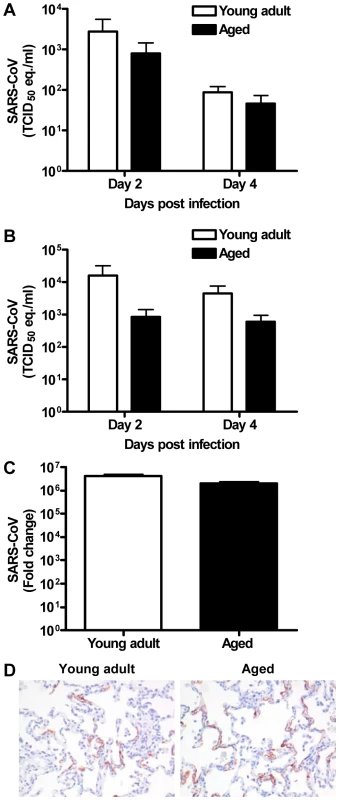
(A–B) SARS-CoV replication in the throat (A) and nose (B) of SARS-CoV infected aged (black bars) and young adult (white bars) macaques at day 2 and 4 post infection as determined by real-time RT-PCR. Viral RNA levels are displayed as TCID50 equivalents (eq.)/ml swab medium (±s.e.m.). (C) Average fold change in SARS-CoV mRNA levels (±s.e.m.) in the lungs of aged and young adult macaques compared to PBS-infected animals as determined by real-time RT-PCR and depicted on a log-scale. (D) Lung sections of SARS-CoV-infected aged and young adult macaques were stained with a mouse-anti-SARS-nucleocapsid IgG2a. Sections were counterstained with hematoxylin. Original magnifications are ×20. The host response to SARS-CoV infection is stronger in aged than in young adult macaques
To understand why SARS-CoV-infection in aged macaques results in more severe pathology than in young adult macaques, we determined global gene expression profiles by analyzing total RNA isolated from the lungs using microarray analysis. Hierarchical clustering methods were used to order gene transcripts and individual aged and young adult animals to identify groups of animals with similar expression patterns. These data were plotted as a heat map in which each entry represents a gene expression value (Figure 4). As expected in an animal experiment with outbred animals, the inter-animal variation was relatively high (Figure S3). There were two major roots to the hierarchical dendogram, with one root containing the PBS-infected control animals, and the second root containing the SARS-CoV-infected animals. The root of the PBS-infected control animals was divided in two minor roots, clustering young adults together and aged animals as a group. These data suggest that the baseline expression patterns are different in young adult and aged macaques. The root of the SARS-CoV-infected animals was also divided in two minor roots, largely clustering young adult animals together and grouping aged infected macaques. The hierarchical clustering heat map suggests that both age and SARS-CoV infection are key factors involved in determining transcription of cellular genes.
Fig. 4. Global gene expression profiles of individual young adult and aged animals. 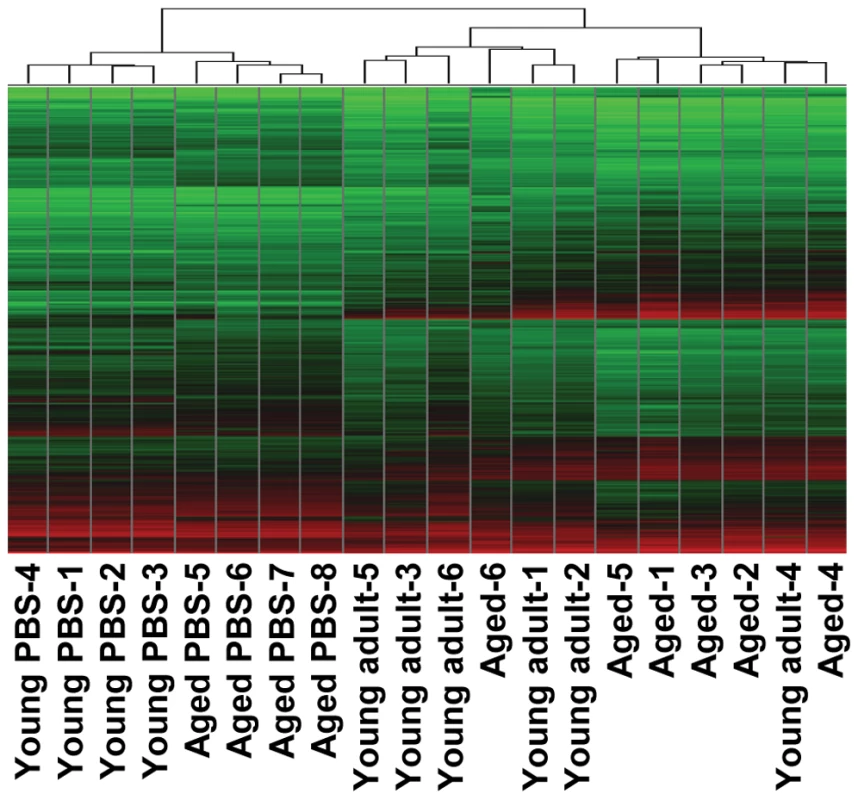
Global gene expression profiles of normalized log-2 based hybridization signals of individual young adult and aged macaques of a set of gene transcripts that were identified as being differentially regulated (fold change ≥2; FDR<0.05) in at least one of the comparisons of gene expression in the lungs of experimentally SARS-CoV-infected aged and young adult macaques versus gene expression in the lungs of PBS-infected macaques, and genes were included if they met the criteria of an absolute fold change of ≥2-fold (FDR<0.05) in at least one experiment. The data were plotted as a heat map, where each matrix entry represents a gene expression value. Normalized log-2 based hybridization signals ranged from 3 (green) to 14 (red). Dendograms (trees) of the heat map represent the degree of relatedness between the samples, with short branches denoting a high degree of similarity and long branches denoting a low degree of similarity. To determine whether aged and young adult animals respond differently to SARS-CoV-infection, their gene expression profiles were compared. In a direct comparison of aged (n = 6) versus young adult (n = 6) SARS-CoV-infected animals using an ANOVA-based analysis called LIMMA, 202 gene transcripts were differentially expressed (fold change ≥2; p<0.05; Table S1). Upon analysis of these gene transcripts within the context of biological processes and pathways using Ingenuity Pathways Knowledge Base, this subset of genes showed indications for an innate host response to viral infection. Among the top significantly differentially regulated (p<0.005) functional categories were immune response, inflammatory response and hematological system development and function, which included genes like F3, IL1RL1, IL1RN, IL6, IL8, S100A8, SERPINA1, SERPINA3, NP, ACPP, TFPI2, SPP1, IGF1, EDN3, DEFB1, and SOCS3 (Figure 5A) most of which were upregulated in SARS-CoV-infected aged animals compared to young adult infected animals. In addition, three of the most significantly regulated molecular/cellular functions (p<0.005) were associated with a pro-inflammatory response and included cell death, cell movement, and cell-to-cell signalling (Figure 5A). The top gene interaction network, showing the interplay between genes during the host response to viral infection, contained NF-κB as central node (Figure 5B). NF-κB is a redox-sensitive transcription factor implicated to play a major role in pro-inflammatory host responses and the development of ALI/ARDS [24],[25]. Several of the 202 differentially expressed gene transcripts, among which IL1RN, SERPINA1, IL8, F3 and TFPI2, are target genes for NF-κB. Thus, significant differences exist in the host response to SARS-CoV infection, corresponding with age.
Fig. 5. Direct comparison of gene expression profiles in the lungs of aged and young adult SARS-CoV-infected macaques. 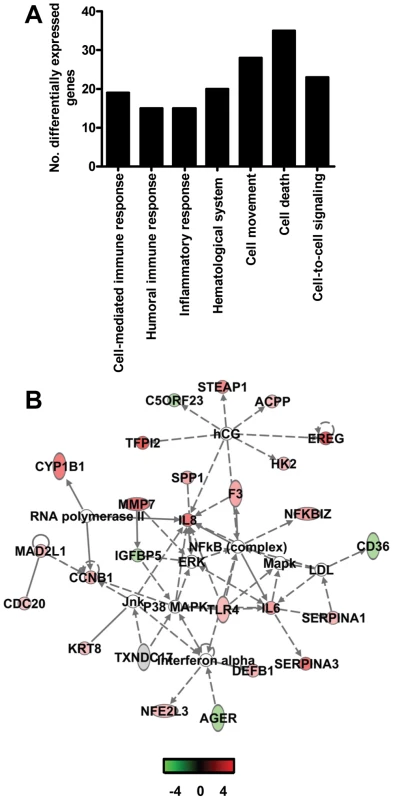
(A) Number of differentially expressed genes in the direct contrast of aged and young adult SARS-CoV-infected macaques with functions in immune response, inflammatory response, hematological system development and function, cell movement, cell death, or cell-to-cell signaling and interaction obtained from Ingenuity Pathways Knowledge Base. (B) This diagram shows a gene interaction network from Ingenuity Pathways Knowledge Base with genes that are differentially expressed in the contrast of aged and young adult SARS-CoV-infected animals. The central node is NF-κB, a key transcription factor in inflammation and ARDS. Genes depicted in green are downregulated and in red upregulated. To obtain a more in-depth view of the host response to infection, global gene expression profiles were determined in lungs of SARS-CoV-infected aged (n = 6) or young adult (n = 6) macaques in comparison to aged or young adult PBS-infected macaques (n = 4), respectively. Aged macaques differentially expressed 1577 gene transcripts (Figure 6A). Gene ontology analysis revealed that the majority of genes in the aged macaque group compared to aged PBS-infected animals were associated with a pro-inflammatory response and included cellular growth and proliferation, cell death, cell movement, and cell-to-cell signalling (Figure 6B). Although SARS-CoV-infected young adult macaques differentially expressed much less gene transcripts compared to young adult PBS-infected animals (Figure 6A), the most significantly regulated molecular/cellular functions also included cellular growth and proliferation, cell death, cell movement, and cell-to-cell signalling (Figure 6B). This suggested that the nature of the host response to infection in aged and young adult animals was strikingly similar, even though the severity was different.
Fig. 6. Aged macaques display a stronger host response to SARS-CoV infection than young adults. 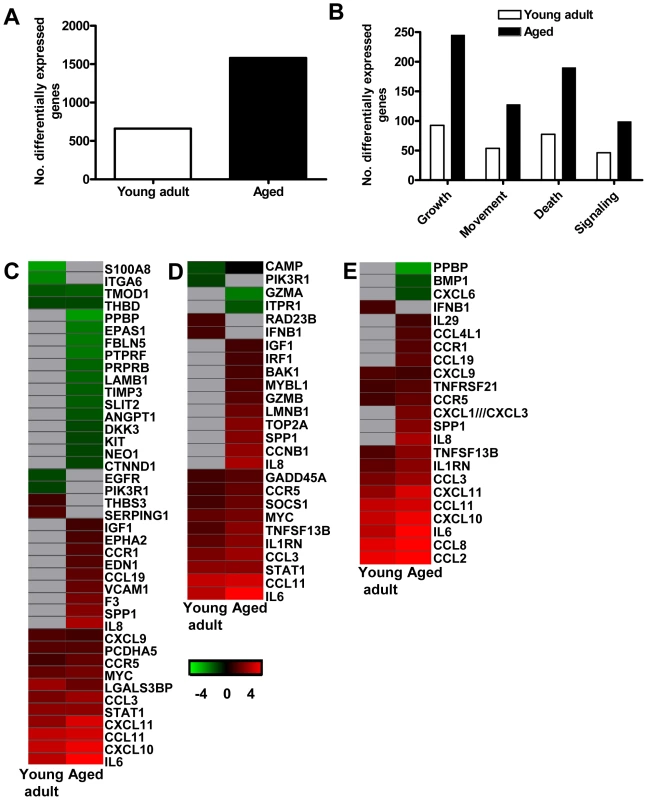
(A) Number of differentially expressed gene transcripts in aged and young adult SARS-CoV-infected macaques compared to aged and young adult PBS-infected animals, respectively (≥2-fold change, FDR<0.05, LIMMA analysis). (B) Number of differentially expressed genes in aged and young adult macaque groups compared to aged and young adult PBS-infected animals, respectively, with functions in cellular growth and proliferation, cell movement, cell death, or cell-to-cell signaling and interaction obtained from Ingenuity Pathways Knowledge Base. White bars for young adult and black bars for aged macaques. When SARS-CoV-infected aged and young adult macaques were compared directly, these cellular functions were significantly differentially expressed as well. (C–E) Gene expression profiles showing differentially expressed genes coding for proteins involved in cell adhesion (C), apopotosis (D), and cytokine/chemokine signalling (E) of aged and young adult macaques. Gene sets were obtained from Ingenuity Pathways Knowledge Base and changed ≥2-fold in at least one of the macaque groups as compared to PBS-infected controls. The data presented are error-weighted fold change averages for six young adult and aged animals. Genes shown in red were upregulated, in green downregulated, and in grey not significantly diferentially expressed in infected animals relative to PBS-infected animals (log (base 2) transformed expression values with minimum and maximum values of the color range being −4 and 4). Global test analysis of the direct contrast of SARS-CoV-infected aged versus young adult animals showed that these pathways were significantly differentially expressed (P<0.05). See Table S2 and S3 for full gene names and expression values. Because the above described gene ontology molecular/cellular functions are very broad, genes were further subdivided based on available annotations to gain insight in differences in the host response to infection in aged and young adult macaques compared to aged and young adult PBS-infected animals, respectively. Heat maps were generated for differentially regulated genes with pro-inflammatory functions such as cell adhesion (Figure 6C), apoptosis (Figure 6D), and cytokine/chemokine signalling (Figure 6E). The greater number of differentially expressed genes, as well as the brighter intensities (fold changes in transcripts) included in the heat map for aged macaques, suggested that aged macaques show a more zealous response to virus infection than young adult macaques. This assumption was corroborated using Goeman's global test [26] on the defined gene subsets cell adhesion, cytokine/chemokine signalling, and apoptosis. When macaques were grouped according to severity of pathology instead of age and compared to their respective PBS-infected controls, increased numbers of differentially expressed gene transcripts and increased fold changes for differentially expressed genes in inflammatory pathways correlated positively with gross pathology scores as well (Figure S4). Our data show that the innate host response to SARS-CoV infection changes during aging in macaques; age, pathology, and pro-inflammatory host response go hand-in-hand.
In order to understand the host responses in the context of senescence, we directly compared lung samples from PBS-infected aged (n = 4) and young adult (n = 4) macaques. LIMMA analysis revealed that 518 gene transcripts were differentially expressed (fold change ≥2; p<0.05), with categories such as immunological disease, haematological system and development, cell death, cell movement, and cellular growth and proliferation among the top significantly differentially regulated functions (p<0.005). Only 14 out of the 518 differentially expressed gene transcripts were also differentially expressed in the direct contrast of SARS-CoV-infected aged and young adult macaques. Our data indicate that significant differences exist in the basal gene expression levels of aged and young adult macaques, which may partly explain why differences in pathology were observed after SARS-CoV infection.
NF-κB signalling in SARS-CoV-infected macaques
As NF-κB target genes were differentially regulated in the direct comparison of SARS-CoV-infected aged and young adult macaques (Figure 5), we focussed on NF-κB in the indirect comparison of aged and young adult SARS-CoV-infected macaques compared to aged and young adult PBS-infected animals, respectively. A gene interaction network, showing the interplay between “immune response”-type genes with NF-κB as central node, revealed that aged SARS-CoV-infected macaques showed a much more robust regulation of these genes than young adult infected animals (Figure 7A) compared to their respective PBS-infected animals, which was corroborated by an analysis of differentially expressed target genes of NF-κB (Figure 7B). Several of these genes, among which VCAM1, F3, PTX3, and IL-8, have also been implicated in development of ARDS (Figure 7C) [9],[27],[28].
Fig. 7. NF-κB-signalling in aged and young adult macaques. 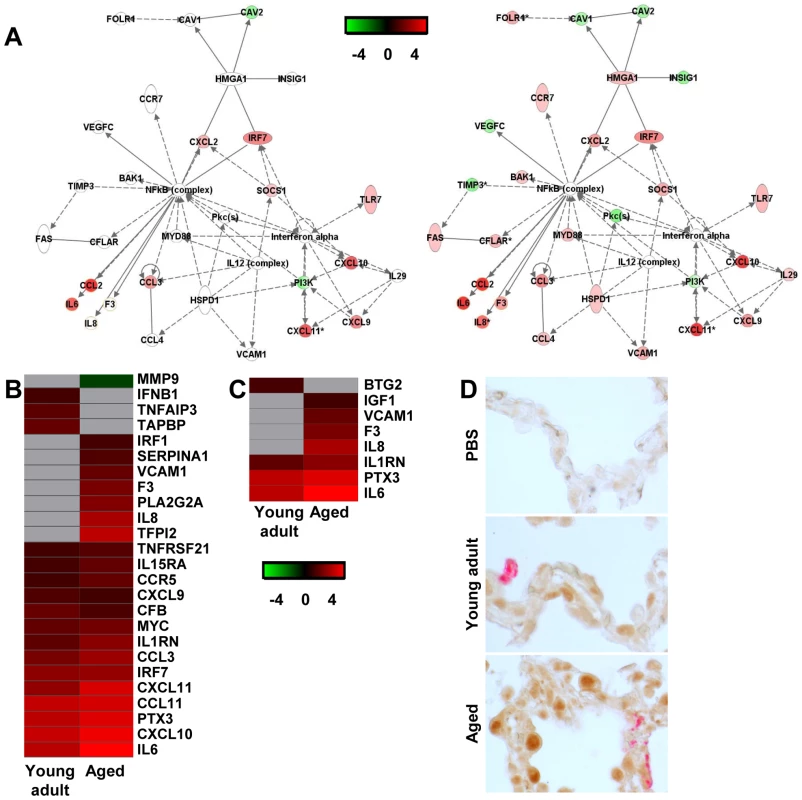
(A) These diagrams show a gene interaction network from Ingenuity Pathways Knowledge Base with genes that are differentially expressed in the contrast of aged SARS-CoV-infected animals versus aged PBS-infected macaques. The central node is NF-κB, a key factor in inflammation and development of ARDS. Genes depicted in green are downregulated and in red upregulated. As a reference, the same network is shown for young adult animals (left panel) and aged animals (right panel). (B–C) Gene expression profiles showing differentially expressed NF-κB target genes (B) and genes coding for proteins involved ARDS (C) of aged and young adult macaques. Gene sets were obtained from Ingenuity Pathways Knowledge Base or literature and changed ≥2-fold in at least one of the macaque groups as compared to PBS-infected controls. The data presented are error-weighted fold change averages for six young adult and aged animals. Genes shown in red were upregulated, in green downregulated, and in grey not significantly diferentially expressed in infected animals relative to PBS-infected animals (log (base 2) transformed expression values with minimum and maximum values of the color range being −4 and 4). See Table S2 and S3 for full gene names and expression values. (D) Lung sections of PBS and SARS-CoV-infected aged and young adult macaques were stained with an antibody against phosphorylated NF-κB (brown) and with a mouse-anti-SARS-nucleocapsid IgG2a (red). Sections were counterstained with hematoxylin. Original magnifications are ×40. In order to visualize NF-κB-signalling in the lungs of SARS-CoV-infected aged and young adult macaques, translocation of NF-κB was studied using immunohistochemistry with antibodies against phosphorylated NF-κB on day 4 after infection. As shown in Figure 7D, hardly any phosphorylated NF-κB could be detected in the nuclei of cells of PBS-infected macaques, while in the lungs of SARS-CoV-infected animals, cells with phosphorylated NF-κB in their nuclei were abundantly present. Phosphorylated NF-κB was detected primarily in the nuclei of non-infected cells (Figure 7D). No obvious differences in the translocation of NF-κB in the lungs of aged and young adult macaques were observed.
Type I interferon-β mRNA level is negatively correlated with gross pathology
Overall, our data indicate that SARS-CoV-infected aged macaques display a stronger pro-inflammatory host response to infection than young adult macaques. For example, mRNA levels for IL8, a key player in ALI/ARDS and a potent chemotactic factor essential in acute inflammation that is induced by a wide range of stimuli among which IL1β, viral products, and oxidative stress, were strongly upregulated in SARS-CoV-infected aged macaques as compared to young adult animals (Figure 5B, 7B, 8A). Despite the overall stronger activation of innate host gene responses in SARS-CoV-infected aged animals, microarray analyses revealed that IFN-β, well-known for its antiviral activities, was not differentially expressed in aged macaques compared to PBS-infected animals, in contrast to young adults (Figure 6E). RT-PCR analysis confirmed differential expression of IFN-β mRNA between young adult and aged macaques (Figure 8B). As shown in Figure 3, this difference in IFN-β levels in aged and young adult macaques did not affect viral replication efficiency. IFN-β mRNA levels, however, negatively correlated with gross pathology (Figure 8C).
Fig. 8. Quantitative RT-PCR confirmation of IFN-β mRNA levels. 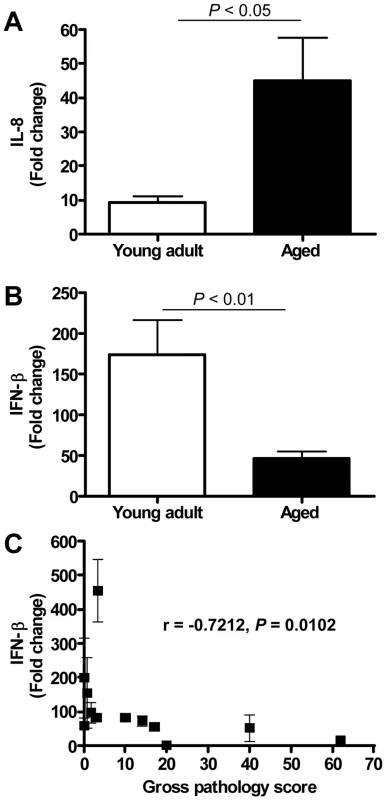
(A) Quantitative RT-PCR for IL-8 was performed on two-three separate lung samples per animal with substantial virus replication. The data presented are error-weighted (±s.e.m.) averages of the fold-change as compared to PBS-infected controls for young adult (n = 6) and aged (n = 6) animals. (B) Quantitative RT-PCR for IFN-β was performed on two-three separate lung samples per animal with substantial virus replication. The data presented are error-weighted (±s.e.m.) averages of the fold-change as compared to PBS-infected controls for young adult (n = 6) and aged (n = 6) animals. (C) The expression level of IFN-β (fold change) per animal was plotted against gross pathology score and the correlation coefficient was determined using Spearman's correlation test. Anti-inflammatory action of type I interferon mitigates pathology in SARS-CoV-infected aged macaques
The observation of a reverse correlation of IFN-β and IL-8 mRNA levels with age after SARS-CoV infection may reflect a physiological cross-regulation in which type I interferon and/or its respective signalling pathways modulate pro-inflammatory host responses [29],[30]. To corroborate this hypothesis, we treated uninfected human PBMC with IL-1β, which is known to rapidly activate NF-κB-signalling [31],[32], and observed the induction of pro-inflammatory cytokines in uninfected human PBMC, such as IL-1β and IL-8 (Figure 8A–B). An anti-inflammatory effect of pegylated IFN-α on IL-1β-induced responses was confirmed in vitro, as a dose-dependent inhibition of IFN-α on recombinant IL-1β-induced IL-1β and IL-8 mRNA levels in human PBMC was observed (Figure 9A–B).
Fig. 9. Anti-inflammatory type I IFN inhibits IL-1β-induced pro-inflammatory cytokine production in PBMCs. 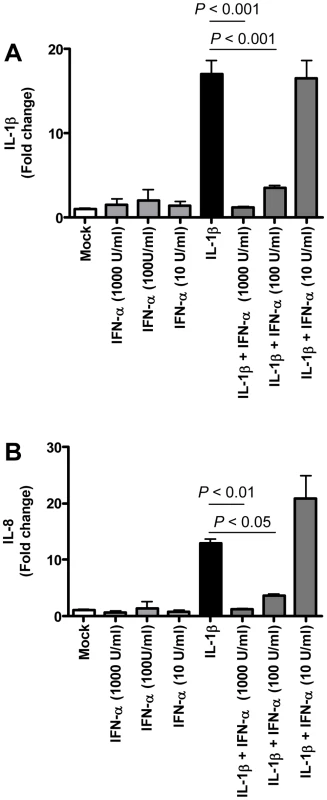
(A–B) Induction of IL-1β (A) and IL-8 (B) mRNAs after treatment of human PBMC with IL-1β (5 ng/ml), IFN-α (1000 U/ml, 100U/ml, or 10 U/ml) or both as determined by quantitative RT-PCR. The data presented are error-weighted (±s.e.m.) averages of the fold-change as compared to untreated (Mock) PBMC. Shown are representative data from one out of four donors. Because type I IFNs can inhibit pro-inflammatory signalling pathways, among which NF-κB signalling pathways [29],[30], we examined whether exogenous administration of type I IFN in SARS-CoV-infected aged macaques could influence SARS-CoV pathogenesis. Retrospective analyses of the lungs of SARS-CoV-infected aged animals treated therapeutically with type I IFN [22] showed that SARS-CoV-infected IFN-treated aged animals remained free of clinical symptoms and had no or less extensive pulmonary consolidation than untreated aged macaques (Figure 10A). Virus titers in the lungs, however, were similar between IFN-treated and untreated aged macaques (Figure 10B) and viral antigen expression in the lungs was not significantly different [22].
Fig. 10. Anti-inflammatory type I IFN inhibits virus-induced ALI in aged SARS-CoV-infected macaques. 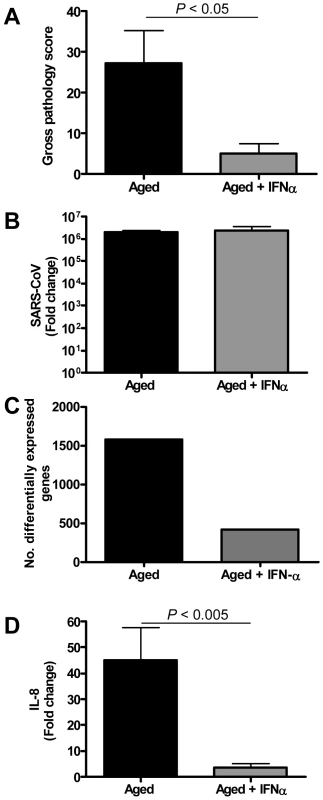
(A) Gross pathology scores of lungs from macaques were determined during necropsy and averaged (±s.e.m.). (B) Average fold change (±s.e.m.) in SARS-CoV mRNA levels in the lungs of pegylated IFN-α-treated (n = 3) and untreated aged (n = 6) macaques compared to aged PBS-infected (n = 4) animals as determined by real-time RT-PCR. (C) Number of differentially expressed gene transcripts compared to aged PBS-infected animals (≥2-fold change). (D) Quantitative RT-PCR for IL-8 was performed on two-three separate lung samples per animal with substantial virus replication. The data presented are error-weighted (±s.e.m.) averages of the fold-change as compared to PBS-infected controls for aged animals (n = 6) and aged animals treated with IFN-α (n = 3). In a direct comparison of the host response to infection in aged (n = 6) versus IFN-treated aged (n = 3) macaques using LIMMA, 961 gene transcripts were differentially expressed (fold change ≥2; p<0.05). Upon analysis of these gene transcripts within the context of genetic pathways, four of the most significantly regulated molecular/cellular functions (p<0.005) were associated with a pro-inflammatory response and included cellular growth and proliferation, cell death, cell movement, and cell-to-cell signalling, indicating that significant differences exist in the host response to SARS-CoV infection in animals treated with type I IFN compared to untreated aged macaques.
To obtain a broader view of the host response to infection, global gene expression profiles were determined in lungs of SARS-CoV-infected aged (n = 6) or IFN-treated aged macaques (n = 3) in comparison to aged PBS-infected macaques (n = 4). IFN-treated macaques differentially expressed (fold change ≥2; p<0.05) approximately four-fold less gene transcripts than untreated aged macaques (Figure 10C) as compared to PBS-infected animals. The most significantly regulated molecular/cellular functions in the IFN-treated macaque group compared to PBS-infected animals were associated with a pro-inflammatory response and included cellular growth and proliferation, cell death, cell movement, and cell-to-cell signalling, similar to what was observed for the aged macaque group, although less genes per function were differentially expressed (Figure S5A). These data suggested a common nature of the host response to infection in aged and IFN-treated aged animals, although the severity seemed different.
To gain more insight in differences in the host response to infection in aged and IFN-treated macaques compared to PBS-infected animals, heat maps were generated for differentially regulated genes involved in pro-inflammatory pathways apoptosis (Figure S5B) and cell adhesion (Figure S5C). Using Goeman's global test [26] on the defined gene subsets cell adhesion and apoptosis, significant differences between aged and IFN-treated animals in these pro-inflammatory pathways were obtained, providing statistical evidence for a difference in host response of aged and IFN-treated animals to SARS-CoV infection. Moreover, a decrease in differentially expressed target genes of NF-κB was observed (Figure S5D). Most notably, a dramatic decrease in the expression of cytokine/chemokine mRNA levels was observed, among which IL-8 (Figure 10D, Figure S5B–C). These data show that therapeutic treatment of SARS-CoV-infected aged macaques with type I IFN primarily results in downregulation of pro-inflammatory host responses.
Discussion
Age, pathology and pro-inflammatory host response go hand-in-hand
The present study aimed at gaining insight into the pathogenesis of SARS-CoV by studying the relationship between age, pathology, virus replication, and host response in a macaque model. In humans, SARS-CoV infection progresses from an atypical pneumonia to acute diffuse alveolar damage and ARDS [5]. The overall human fatality rate reached ∼10% and up to 50% in elderly [5],[6]. The acute lung injury observed after SARS-CoV infection in aged macaques is similar to what has been seen in humans that progress to ARDS [6]. This disease process includes an acute exudative phase, consisting of severe leukocyte infiltration, edema, the formation of hyaline membranes, and proliferation characterized by type II pneumocyte hyperplasia [33]. SARS-CoV-infected aged macaques develop more severe pathology than young adult animals, even though viral replication levels are similar. The chronic phase, which is characterized by persistent intra-alveolar and interstitial fibrosis and mortality was not observed because animals were sacrificed early after infection.
Comparative analyses of gene expression in aged and young adult SARS-CoV-infected macaques revealed that the host response to SARS-CoV infection is similar in nature, but differs significantly in severity in pro-inflammatory responses. Aged macaques had a stronger host response to virus infection than young adult macaques, with an increase in differential expression of genes associated with inflammation that center around the transcription factor NF-κB. Comparative analysis of PBS-infected aged and young adult macaques revealed significant differences in gene expression as a result of aging only. These observations are in line with earlier hypotheses that age-related accumulated oxidative damage and a weakened antioxidative defense system cause a disturbance in the redox balance, resulting in increased reactive oxygen species. Subsequently, the oxidative stress-induced redox imbalance activates redox-sensitive transcription factors, such as NF-κB, followed by the induction of pro-inflammatory genes including IL1β, IL6, TNFα and adhesion molecules, key players in the inflammatory process [34]. Oxidative stress may also potentiate the cellular responses to IL-1β [35], an early mediator of inflammation [36]. Thus, aging is associated not only with alterations in the adaptive immune responses, but also with a pro-inflammatory state in the host [34],[37],[38],[39]. Oxidative stress and toll-like receptor-4 signaling via NF-κB triggered by viral lung pathogens, such as SARS-CoV, may further amplify the host response ultimately resulting in ALI [25]. Taking the host gene expression profiles of PBS-infected aged and young adult macaques into account, we also observed a stronger activation of the pro-inflammatory pathways in SARS-CoV-infected aged macaques than in young adults. The finding that genes activated by NF-κB are significantly differentially upregulated in aged macaques infected with SARS-CoV is in line with the role of NF-κB as a redox-sensitive transcription factor in pro-inflammatory host responses and the development of ALI/ARDS [24],[25]. Given the fact that several SARS-CoV proteins block NF-κB signaling [40],[41],[42], we hypothesize that NF-κB-signaling in non-infected cells is largely responsible for the upregulated expression of NF-κB target genes, such as IL8, in aged compared to young adult macaques.
These observations are largely in line with transcriptome analyses in mice and SARS patients. In severe SARS patients, cytokines/chemokine involvement as the illness progresses may lead to widespread immune dysregulation and serious pathogenic events [13]. Aged mice show more pathology than young adult mice and the transcriptional profile in aged mice generally indicates a more robust pro-inflammatory response to virus infection than in young mice [43],[44].
Type I IFN signalling
Previously, we demonstrated IFN induction and signalling in SARS-CoV-infected macaques early after infection [19]. Based on the observation that plasmacytoid dendritic cells are able to produce type I IFNs after SARS-CoV infection in vitro [45], it was speculated that these cells are the IFN-producing cells in lungs of SARS-CoV-infected macaques. In addition, phosphorylated STAT-1 was observed in the nuclei of numerous cells in the lungs of SARS-CoV-infected macaques, indicating that these cells had been activated by IFNs or other agonists produced in the lung [19]. In SARS-CoV-infected cells, however, STAT-1 signalling was blocked [19], consistent with the fact that a range of SARS-CoV proteins can function as interferon antagonists that inhibit IFN production and signalling [41],[42]. Therefore, a large part of the genes activated downstream of STAT-1, observed in genomics analyses, is likely due to signalling in non-infected cells [19]. In the current study, we observed that aged macaques expressed significantly lower levels of IFN-β mRNA than young adult macaques and that IFN-β mRNA levels correlated negatively with severity of pathology. Interestingly, aged and young adult SARS-CoV-infected macaques showed opposite expression patterns for type I IFN-β and certain pro-inflammatory cytokines, such as IL-8. These data are corroborated by previous observations showing that higher amounts of pro-inflammatory cytokines, such as IL-1β and IL-8, are produced upon stimulation of leukocytes of the elderly, whereas induction of type I IFNs is decreased compared to young adults [46],[47],[48].
Cross-regulation between type I IFN and NF-κB signalling cascades
The observation of a reverse correlation of IFN-β and IL-8 mRNA levels with age after SARS-CoV infection may reflect a physiological cross-regulation between antiviral STAT-1 and proinflammatory NF-κB pathways. Evidence for such a cross-regulation between type I IFN/STAT-1 and pro-inflammatory/NF-κB signaling pathways exists. Type I interferons exert significant anti-inflammatory effects and provide at least partial protection from disease in collagen-induced arthritis, auto-immune encephalitis, and multiple sclerosis [49],[50],[51],[52],[53]. Not only inhibits IFN-beta expression of the IL8 gene at the transcriptional level [54], type I IFNs can also activate TAM receptor tyrosine kinases that inhibit toll-like receptor-induced cytokine-receptor cascades [55],[56] and induce the immunosuppresive cytokine IL-10 [57]. Direct NF-κB/STAT-1 protein-protein interactions [58] and modification of STAT-1 by acetylation, may be involved in this process [30]. A loss of type I IFN/STAT-1 signaling in aged macaques may negatively regulate interferon-induced gene expression and type I IFN signaling, which may lead to enhanced inflammatory responses. On the other hand, increased activation of NF-κB signaling pathways in aged macaques may negatively regulate interferon-induced gene expression and type I IFN signaling [29],[59],[60], which may enhance pro-inflammatory responses even further.
We have integrated our data and other findings on cross-regulation in a model (Figure 11). The model depicts the innate immune response to SARS-CoV infection as a coordinated series of signaling pathways aimed at clearing the virus while not harming host cells. Upon SARS-CoV infection, infected cells, depicted in the model as pneumocytes, produce inflammatory mediators that activate NF-κB, resulting in the production of pro-inflammatory cytokines and chemokines, such as IL-8. IL-1 is one of the cytokines highly upregulated on day 1 after infection upon SARS-CoV infection of macaques [19] and capable of activating NF-κB. At the same time, the virus is recognized by sentinel cells, such as pDCs, that produce type I IFNs to signal that a foreign invader has entered the host. The production of IFN induces neighboring non-infected cells to remodel the intracellular environment by producing a range of antiviral proteins, aiding in a block of viral replication. A cross-regulation between the “antiviral” and “pro-inflammatory” pathways occurs, which is a critical requirement to allow fine-tuning of the host response to infection and return to homeostasis. Disease outcome may be determined by the relative contribution of “antiviral” and “pro-inflammatory” pathways and apparently aging influences this intricate balance significantly.
Fig. 11. Model for cross-talk between “pro-inflammatory” and “antiviral” pathways during SARS-CoV infection. 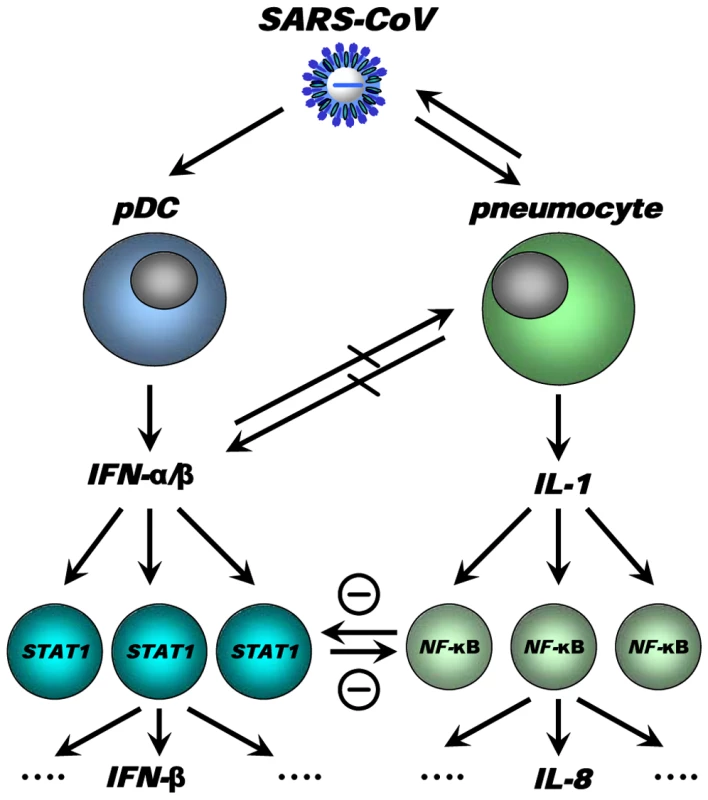
SARS-CoV infection results in activation of both “antiviral” and “pro-inflammatory” pathways. Subsets of uninfected cells, depicted by pDCs, start producing type I IFN (IFN-α), which results in STAT-1 activation in neighbouring cells, which in turn may produce other mediators (e.g. IFN-β). The SARS-CoV-infected cells produce inflammatory mediators, supposedly IL-1, which results in NF-κB activation in neighbouring uninfected cells and subsequent production of inflammatory mediators, such as IL-8. Cross-regulation between “antiviral” and “pro-inflammatory” pathways allows polarisation of antiviral or pro-inflammatory responses thereby modulating pathology. Modulation of transcription factors in the uninfected cells, e.g. by aging, may affect the overall outcome of the infection. Causal relationships between “antiviral” and “pro-inflammatory” pathways in macaques are difficult to prove and future studies in specific gene knock-out mice should therefore further clarify the complex interactions in the response to SARS-CoV. Our own in vitro experiments and the type I IFN intervention in SARS-CoV-infected aged macaques indicate that type I IFNs can play a role in mitigating pro-inflammatory host responses and severity of pathology. Therapeutic treatment of SARS-CoV-infected aged macaques with type I IFN reduces pathology and diminishes pro-inflammatory gene expression, including IL-8 levels, without affecting virus replication in the lungs. Antiviral effects of type I IFNs were not obvious, probably due to the fact that SARS-CoV infected cells inhibit STAT-1 signalling and viral replication peaks early after infection when treatment with pegylated IFN-α started. Given the fact that phosphorylated NF-κB was present mainly in the nuclei of non-infected cells in the lungs of SARS-CoV-infected macaques, these cells are potential targets for the action of IFN and subsequent STAT-1 signalling. It remains uncertain whether endogenously produced IFNs in young adult macaques are essential in the control of inflammatory responses or that enhanced activation of inflammatory pathways simply does not occur. Our data are in line with the observation that treatment of SARS-CoV-infected aged mice with type II IFN-γ, which like type I IFN also signals via STAT-1, protected against lethal respiratory illness, seemingly without an effect on viral replication [61]. Moreover, in humans with SARS, use of type I IFNs was associated with reduced disease-associated hypoxia and a more rapid resolution of radiographic lung abnormalities [62]. Whether the anti-inflammatory action of type I/II IFNs in macaques, mice and humans occurs via common pathways and is interchangeable between host species remains to be determined. Assuming that there is a conserved pathway in ALI/ARDS induced by multiple pathogens, including pandemic viruses that may emerge from avian influenza, modulation of the host response by type I IFNs provides a promising outlook for novel intervention strategies.
Materials and Methods
Macaque studies
Six young adult cynomolgus macaques (Macaca fascicularis), 3–5 years old, four of which carried active temperature transponders in the peritoneal cavity, and four aged cynomolgus macaques, 10–18 years old, which all carried active temperature transponders, were inoculated with SARS-CoV strain HKU39849, as described previously [19],[21],[22],[23]. Two additional aged animals (17 and 19 years old), previously infected with SARS-CoV strain HKU39849 [22], were enrolled in this study as well. Four young adult mock (PBS) infected animals from a previous study [19] and four aged macaques were taken as controls. Lung tissues stored in RNA-later from three cynomolgus macaques, 13 years old, previously inoculated with SARS-CoV strain HKU39849 and treated with pegylated IFN-α at a dose of 3 µg/kg intramuscularly on days 1 and 3 after infection, were taken along for molecular analyses [22]. All animals were infected with the same dose of virus, using the same inoculation procedure, by the same person to minimize inter-experiment variation. All animals were checked daily for clinical signs and anaesthetised with ketamine on days 0, 2 and 4 after infection to collect oral, nasal, and rectal swabs [22]. All animals were euthanized on day 4 post infection. Necropsies and sampling for histology/immunohistochemistry were performed as described [22]. The percentage of affected lung tissue from each lung lobe was determined at necropsy, recorded on a schematic diagram of the lung and the area of affected lung tissue was subsequently calculated (gross pathology score).
Ethics
Approval for animal experiments was obtained from the Institutional Animal Welfare Committee and performed according to Dutch guidelines for animal experimentation.
Immunohistochemistry
Serial 3 µm lung sections were stained using mouse-anti-SARS-nucleocapsid IgG2a (clone Ncap4; Imgenex) 1∶1600, mouse-anti-human neutrophil elastase (clone NP-57; DAKO) 1∶10, mouse-anti-human CD68 (clone KP1; DAKO) 1∶200, mouse-anti-human pankeratin (clone AE1/AE3; Neomarkers) 1∶100, rabbit anti p-NF-κB p65 (Santa Cruz) or rabbit control and isotype antibodies (clones 11711 and 20102; R&D), according to standard protocols [22],[23]. Quantitative assessment of SARS-CoV infection in the lungs was performed as described previously [22].
RNA-extraction and quantitative RT-PCR
RNA from 200 µl of swabs was isolated with the Magnapure LC total nucleic acid isolation kit (Roche) external lysis protocol and eluted in 100 µl. SARS-CoV RNA was quantified on the ABI prism 7700, with use of the Taqman Reverse Transcription Reagents and Taqman PCR Core Reagent kit (Applied Biosystems), using 20 µl isolated RNA, 1× Taqman buffer, 5.5 mM MgCl2, 1.2 mM dNTPs, 0.25 U Amplitaq gold DNA polymerase, 0.25 U Multiscribe reverse transcriptase, 0.4 U RNAse-inhibitor, 200 nM primers, and 100 nM probe [23]. Amplification parameters were 30 min at 48°C, 10 min at 95°C, and 40 cycles of 15 s at 95°C, and 1 min at 60°C. RNA dilutions isolated from a SARS-CoV stock were used as a standard. Average results (±s.e.m.) for young adult (n = 6) and aged macaque (n = 4) groups were expressed as SARS-CoV equivalents per ml swab medium.
Lung tissue samples (0.3–0.5 gram) were taken for RT-PCR and microarray analysis in RNA-later (Ambion, Inc.). RNA was isolated from homogenized post mortem tissue samples using Trizol Reagent (Invitrogen) and the RNeasy mini kit (Qiagen). cDNA synthesis was performed with 1 µg total RNA and Superscript III RT (Invitrogen) with oligo(dT), according to the manufacturer's instructions. Semi-quantitative RT-PCR was performed to detect SARS-CoV mRNA and to validate cellular gene expression changes as detected with microarrays [19]. Differences in gene expression are represented as the fold change in gene expression relative to a calibrator and normalized to a reference, using the 2−ΔΔCt method [63]. GAPDH (glyceraldehydes-3-phosphate dehydrogenase) was used as endogenous control to normalize quantification of the target gene. The samples from the young adult PBS-infected macaques were used as a calibrator. Average results (±s.e.m.) for young adult (n = 6), aged (n = 6), and IFN-α-treated aged (n = 3) macaque groups were expressed as fold change compared to young adult PBS-infected animals, respectively [63]. In addition, groups were based on severity of pathology: young adult macaques (n = 6), aged macaques with pathology (n = 4), and aged macaques with severe pathology with >40% of lungs affected (n = 2) (Supplementary Figure 4). As titration of lung homogenates gave inconsistent results in our hands and because the effects of endogenous and exogenous IFN may influence titration outcomes, we chose taqman and immunohistochemistry to determine viral replication levels in the lung.
Isolation and activation of PBMC
PBMC from healthy blood donors were isolated from heparinized venous blood using Lymphoprep (Axis-Shield). PBMC were resuspended at 2×106/ml in RPMI 1640 medium (Biowhittaker) supplemented with L-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml), and 10% fetal calf serum. Freshly isolated PBMC were incubated with IL-1β (5 ng/ml; eBioscience), IFN-α 2a (1000 U/ml, 100U/ml, or 10U/ml; Roferon-A; Roche) or both for 24 hours in duplo or triplo per donor. Total RNA from stimulated PBMC was isolated using Trizol Reagent (Invitrogen) and the RNeasy mini kit (Qiagen). cDNA synthesis was performed with 100 ng total RNA and Superscript III RT (Invitrogen) with oligo(dT), according to the manufacturer's instructions. Semi-quantitative RT-PCR was performed for IL-8 [19] and IL-1β (Taqman gene expression assays; Applied Biosystems) as described previously using the 2−ΔΔCt method [63]. Average results (±s.e.m.) were expressed as fold change compared to untreated (mock) cells [63].
Statistical analysis
Data (RT-PCR and gross pathology scores for SARS-CoV-infected young adult versus aged and aged versus aged animals treated with IFN) were compared using Student's t-test with Welch's correction. Differences were considered significant at P<0.05. One-way ANOVA and Bonferroni's multiple comparison test were used for the comparison of data in groups based on severity of pathology (low, medium, high) and in vitro IFN inhibition experiments. Correlation coefficients were determined using Spearman's correlation test.
RNA labeling, microarray hybridization, scanning and data preprocessing
Pooled total RNA (2.4 µg) from one-three separate lung pieces of all animals (including previously infected animals), with substantial SARS-CoV replication (>105 fold change), was labeled using the One-Cycle Target Labeling Assay (Affymetrix) and hybridized onto Affymetrix GeneChip Rhesus Macaque Genome Arrays (Affymetrix), according to the manufacturer's recommendations. Image analysis was performed using Gene Chip Operating Software (Affymetrix). Microarray Suite version 5.0 software (Affymetrix) was used to generate .dat and .cel files for each experiment. All data were normalized using a variance stabilization algorithm (VSN) [64]. Transformed probe values were summarized into one value per probe set by the median polish method [65]. Primary data is available at http://www.virgo.nl in accordance with proposed MIAME standards.
Microarray data analysis
Probe set (gene) wise comparisons between the experimental conditions (aged, young adult and IFN-treated animals versus young adult or aged PBS-infected animals and directly compared to each other) were performed by LIMMA (version 2.12.0) [66]. Correction for multiple testing was achieved by requiring a false discovery rate (FDR) of 0.05, calculated with the Benjamini-Hochberg procedure [67]. To understand the gene functions and the biological processes represented in the data and obtain differentially expressed molecular and cellular functions, Ingenuity Pathways Knowledge Base (http://www.ingenuity.com/) was used. Heat maps of pro-inflammatory pathways were produced using complete linkage and Euclidian distance in Spotfire DecisionSite for Functional Genomics version 9.1 (http://www.spotfire.com/) and Ingenuity Pathways Knowledge Base (http://www.ingenuity.com/), using log (base 2) transformed expression values with minimum and maximum values of the color range being −4 and 4, respectively. Differences between conditions in expression of specific pro-inflammatory pathways, e.g. direct comparison of defined gene sets (aged versus young adult and aged versus aged IFN-treated animals), were tested by Goeman's global test procedure [26]. Hierarchical clustering analysis of normalized log-2 based hybridization signals of individual young adult and aged macaques of a set of gene transcripts that were identified as being differentially regulated (fold change ≥2; FDR<0.05) in at least one of the comparisons of young adult versus young adult PBS or aged versus aged PBS animals were created using Spotfire DecisionSite for Functional Genomics version 9.1 (http://www.spotfire.com/) with complete linkage and Eucledian distance parameters.
Supporting Information
Zdroje
1. LicastroF
CandoreG
LioD
PorcelliniE
Colonna-RomanoG
2005 Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing 2 8
2. MeyerKC
2005 Aging. Proc Am Thorac Soc 2 433 439
3. PlackettTP
BoehmerED
FaunceDE
KovacsEJ
2004 Aging and innate immune cells. J Leukoc Biol 76 291 299
4. MeyerKC
2001 The role of immunity in susceptibility to respiratory infection in the aging lung. Respir Physiol 128 23 31
5. PeirisJS
ChuCM
ChengVC
ChanKS
HungIF
2003 Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 361 1767 1772
6. PeirisJS
GuanY
YuenKY
2004 Severe acute respiratory syndrome. Nat Med 10 S88 97
7. HuangKJ
SuIJ
TheronM
WuYC
LaiSK
2005 An interferon-gamma-related cytokine storm in SARS patients. J Med Virol 75 185 194
8. JiangY
XuJ
ZhouC
WuZ
ZhongS
2005 Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med 171 850 857
9. WareLB
MatthayMA
2000 The acute respiratory distress syndrome. N Engl J Med 342 1334 1349
10. HonKL
LeungCW
ChengWT
ChanPK
ChuWC
2003 Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet 361 1701 1703
11. LeungCW
ChiuWK
2004 Clinical picture, diagnosis, treatment and outcome of severe acute respiratory syndrome (SARS) in children. Paediatr Respir Rev 5 275 288
12. WongGW
LiAM
NgPC
FokTF
2003 Severe acute respiratory syndrome in children. Pediatr Pulmonol 36 261 266
13. CameronMJ
RanL
XuL
DaneshA
Bermejo-MartinJF
2007 Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J Virol 81 8692 8706
14. NagataN
IwataN
HasegawaH
FukushiS
YokoyamaM
2007 Participation of both host and virus factors in induction of severe acute respiratory syndrome (SARS) in F344 rats infected with SARS coronavirus. J Virol 81 1848 1857
15. TangNL
ChanPK
WongCK
ToKF
WuAK
2005 Early enhanced expression of interferon-inducible protein-10 (CXCL-10) and other chemokines predicts adverse outcome in severe acute respiratory syndrome. Clin Chem 51 2333 2340
16. WongCK
LamCW
WuAK
IpWK
LeeNL
2004 Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol 136 95 103
17. CheungCY
PoonLL
NgIH
LukW
SiaSF
2005 Cytokine responses in severe acute respiratory syndrome coronavirus-infected macrophages in vitro: possible relevance to pathogenesis. J Virol 79 7819 7826
18. LawHK
CheungCY
NgHY
SiaSF
ChanYO
2005 Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 106 2366 2374
19. de LangA
BaasT
TealT
LeijtenLM
RainB
2007 Functional genomics highlights differential induction of antiviral pathways in the lungs of SARS-CoV-infected macaques. PLoS Pathog 3 e112 doi:10.1371/journal.ppat.0030112
20. WareLB
2005 Prognostic determinants of acute respiratory distress syndrome in adults: impact on clinical trial design. Crit Care Med 33 S217 222
21. FouchierRA
KuikenT
SchuttenM
van AmerongenG
van DoornumGJ
2003 Aetiology: Koch's postulates fulfilled for SARS virus. Nature 423 240
22. HaagmansBL
KuikenT
MartinaBE
FouchierRA
RimmelzwaanGF
2004 Pegylated interferon-alpha protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat Med 10 290 293
23. KuikenT
FouchierRA
SchuttenM
RimmelzwaanGF
van AmerongenG
2003 Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet 362 263 270
24. FanJ
YeRD
MalikAB
2001 Transcriptional mechanisms of acute lung injury. Am J Physiol Lung Cell Mol Physiol 281 L1037 1050
25. ImaiY
KubaK
NeelyGG
Yaghubian-MalhamiR
PerkmannT
2008 Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133 235 249
26. GoemanJJ
van de GeerSA
de KortF
van HouwelingenHC
2004 A global test for groups of genes: testing association with a clinical outcome. Bioinformatics 20 93 99
27. FolkessonHG
MatthayMA
HebertCA
BroaddusVC
1995 Acid aspiration-induced lung injury in rabbits is mediated by interleukin-8-dependent mechanisms. J Clin Invest 96 107 116
28. ModelskaK
PittetJF
FolkessonHG
Courtney BroaddusV
MatthayMA
1999 Acid-induced lung injury. Protective effect of anti-interleukin-8 pretreatment on alveolar epithelial barrier function in rabbits. Am J Respir Crit Care Med 160 1450 1456
29. AmitI
GarberM
ChevrierN
LeiteAP
DonnerY
2009 Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 326 257 263
30. KramerOH
BausD
KnauerSK
SteinS
JagerE
2006 Acetylation of Stat1 modulates NF-kappaB activity. Genes Dev 20 473 485
31. AkiraS
UematsuS
TakeuchiO
2006 Pathogen recognition and innate immunity. Cell 124 783 801
32. AuronPE
1998 The interleukin 1 receptor: ligand interactions and signal transduction. Cytokine Growth Factor Rev 9 221 237
33. HaagmansBL
OsterhausADME
2006 Nonhuman primate models for SARS. PLoS Med 3 e194 doi:10.1371/journal.pmed.0030194
34. ChungHY
SungB
JungKJ
ZouY
YuBP
2006 The molecular inflammatory process in aging. Antioxid Redox Signal 8 572 581
35. RutkuteK
AsmisRH
Nikolova-KarakashianMN
2007 Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J Lipid Res 48 2443 2452
36. GasseP
MaryC
GuenonI
NoulinN
CharronS
2007 IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 117 3786 3799
37. AwD
SilvaAB
PalmerDB
2007 Immunosenescence: emerging challenges for an ageing population. Immunology 120 435 446
38. BruunsgaardH
PedersenM
PedersenBK
2001 Aging and proinflammatory cytokines. Curr Opin Hematol 8 131 136
39. FranceschiC
BonafeM
ValensinS
OlivieriF
De LucaM
2000 Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908 244 254
40. FangX
GaoJ
ZhengH
LiB
KongL
2007 The membrane protein of SARS-CoV suppresses NF-kappaB activation. J Med Virol 79 1431 1439
41. FriemanM
HeiseM
BaricR
2008 SARS coronavirus and innate immunity. Virus Res 133 101 112
42. Kopecky-BrombergSA
Martinez-SobridoL
FriemanM
BaricRA
PaleseP
2007 Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J Virol 81 548 557
43. BaasT
RobertsA
TealTH
VogelL
ChenJ
2008 Genomic Analysis Reveals Age Dependent Innate Immune Responses to SARS Coronavirus. J Virol 82 9465 9476
44. RockxB
BaasT
ZornetzerGA
HaagmansB
SheahanT
2009 Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol 83 7062 7074
45. Cervantes-BarraganL
ZustR
WeberF
SpiegelM
LangKS
2007 Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood 109 1131 1137
46. KongKF
DelrouxK
WangX
QianF
ArjonaA
2008 Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol 82 7613 7623
47. RinkL
CakmanI
KirchnerH
1998 Altered cytokine production in the elderly. Mech Ageing Dev 102 199 209
48. YoonP
KeylockKT
HartmanME
FreundGG
WoodsJA
2004 Macrophage hypo-responsiveness to interferon-gamma in aged mice is associated with impaired signaling through Jak-STAT. Mech Ageing Dev 125 137 143
49. PrinzM
SchmidtH
MildnerA
KnobelochKP
HanischUK
2008 Distinct and nonredundant in vivo functions of IFNAR on myeloid cells limit autoimmunity in the central nervous system. Immunity 28 675 686
50. AbreuSL
1982 Suppression of experimental allergic encephalomyelitis by interferon. Immunol Commun 11 1 7
51. BilliauA
2006 Anti-inflammatory properties of Type I interferons. Antiviral Res 71 108 116
52. GuoB
ChangEY
ChengG
2008 The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J Clin Invest 118 1680 1690
53. van HoltenJ
ReedquistK
Sattonet-RocheP
SmeetsTJ
Plater-ZyberkC
2004 Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res Ther 6 R239 249
54. NozellS
LaverT
PatelK
BenvenisteEN
2006 Mechanism of IFN-beta-mediated inhibition of IL-8 gene expression in astroglioma cells. J Immunol 177 822 830
55. O'NeillLA
2008 When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity 29 12 20
56. RothlinCV
GhoshS
ZunigaEI
OldstoneMB
LemkeG
2007 TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131 1124 1136
57. AmanMJ
TretterT
EisenbeisI
BugG
DeckerT
1996 Interferon-alpha stimulates production of interleukin-10 in activated CD4+ T cells and monocytes. Blood 87 4731 4736
58. GansterRW
GuoZ
ShaoL
GellerDA
2005 Differential effects of TNF-alpha and IFN-gamma on gene transcription mediated by NF-kappaB-Stat1 interactions. J Interferon Cytokine Res 25 707 719
59. PauliEK
SchmolkeM
WolffT
ViemannD
RothJ
2008 Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog 4 e1000196 doi:10.1371/journal.ppat.1000196
60. WeiL
SandbulteMR
ThomasPG
WebbyRJ
HomayouniR
2006 NFkappaB negatively regulates interferon-induced gene expression and anti-influenza activity. J Biol Chem 281 11678 11684
61. NagataN
IwataN
HasegawaH
FukushiS
HarashimaA
2008 Mouse-passaged severe acute respiratory syndrome-associated coronavirus leads to lethal pulmonary edema and diffuse alveolar damage in adult but not young mice. Am J Pathol 172 1625 1637
62. LoutfyMR
BlattLM
SiminovitchKA
WardS
WolffB
2003 Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. Jama 290 3222 3228
63. LivakKJ
SchmittgenTD
2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402 408
64. HuberW
von HeydebreckA
SultmannH
PoustkaA
VingronM
2002 Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 18 Suppl 1 S96 104
65. TukeyJW
1977 Some thoughts on clinical trials, especially problems of multiplicity. Science 198 679 684
66. SmythGK
2004 Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3 Article3
67. BenjaminiY
HochbergY
1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Soc B 57 289 300
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR AvidityČlánek Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 2- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Pathogen Entrapment by Transglutaminase—A Conserved Early Innate Immune Mechanism
- Broadly Protective Monoclonal Antibodies against H3 Influenza Viruses following Sequential Immunization with Different Hemagglutinins
- Neutrophil-Derived CCL3 Is Essential for the Rapid Recruitment of Dendritic Cells to the Site of Inoculation in Resistant Mice
- Differentiation, Distribution and γδ T Cell-Driven Regulation of IL-22-Producing T Cells in Tuberculosis
- IFN-α-Induced Upregulation of CCR5 Leads to Expanded HIV Tropism In Vivo
- An Extensive Circuitry for Cell Wall Regulation in
- TgMORN1 Is a Key Organizer for the Basal Complex of
- Direct Presentation Is Sufficient for an Efficient Anti-Viral CD8 T Cell Response
- Immunoelectron Microscopic Evidence for Tetherin/BST2 as the Physical Bridge between HIV-1 Virions and the Plasma Membrane
- A New Nuclear Function of the Glycolytic Enzyme Enolase: The Metabolic Regulation of Cytosine-5 Methyltransferase 2 (Dnmt2) Activity
- Genome-Wide mRNA Expression Correlates of Viral Control in CD4+ T-Cells from HIV-1-Infected Individuals
- Structural and Biochemical Characterization of SrcA, a Multi-Cargo Type III Secretion Chaperone in Required for Pathogenic Association with a Host
- A Major Role for the ApiAP2 Protein PfSIP2 in Chromosome End Biology
- HIV Controller CD4+ T Cells Respond to Minimal Amounts of Gag Antigen Due to High TCR Avidity
- Fis Is Essential for Capsule Production in and Regulates Expression of Other Important Virulence Factors
- Vaccinia Protein F12 Has Structural Similarity to Kinesin Light Chain and Contains a Motor Binding Motif Required for Virion Export
- A Novel Pseudopodial Component of the Dendritic Cell Anti-Fungal Response: The Fungipod
- Efficacy of the New Neuraminidase Inhibitor CS-8958 against H5N1 Influenza Viruses
- Long-Lived Antibody and B Cell Memory Responses to the Human Malaria Parasites, and
- IPS-1 Is Essential for the Control of West Nile Virus Infection and Immunity
- Transit through the Flea Vector Induces a Pretransmission Innate Immunity Resistance Phenotype in
- Ats-1 Is Imported into Host Cell Mitochondria and Interferes with Apoptosis Induction
- Six RNA Viruses and Forty-One Hosts: Viral Small RNAs and Modulation of Small RNA Repertoires in Vertebrate and Invertebrate Systems
- The Syk Kinase SmTK4 of Is Involved in the Regulation of Spermatogenesis and Oogenesis
- Optineurin Negatively Regulates the Induction of IFNβ in Response to RNA Virus Infection
- On the Diversity of Malaria Parasites in African Apes and the Origin of from Bonobos
- Five Questions about Viruses and MicroRNAs
- A Broad Distribution of the Alternative Oxidase in Microsporidian Parasites
- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Peptides Presented by HLA-E Molecules Are Targets for Human CD8 T-Cells with Cytotoxic as well as Regulatory Activity
- Interaction of Rim101 and Protein Kinase A Regulates Capsule
- Distinct External Signals Trigger Sequential Release of Apical Organelles during Erythrocyte Invasion by Malaria Parasites
- Exacerbated Innate Host Response to SARS-CoV in Aged Non-Human Primates
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
- Universal Features of Post-Transcriptional Gene Regulation Are Critical for Zygote Development
- Highly Differentiated, Resting Gn-Specific Memory CD8 T Cells Persist Years after Infection by Andes Hantavirus
- Arterivirus Nsp1 Modulates the Accumulation of Minus-Strand Templates to Control the Relative Abundance of Viral mRNAs
- Lethal Antibody Enhancement of Dengue Disease in Mice Is Prevented by Fc Modification
- Quantitative Comparison of HTLV-1 and HIV-1 Cell-to-Cell Infection with New Replication Dependent Vectors
- The Disulfide Bonds in Glycoprotein E2 of Hepatitis C Virus Reveal the Tertiary Organization of the Molecule
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Caspase-1 Activation via Rho GTPases: A Common Theme in Mucosal Infections?
- Kaposi's Sarcoma Associated Herpes Virus (KSHV) Induced COX-2: A Key Factor in Latency, Inflammation, Angiogenesis, Cell Survival and Invasion
- IL-1β Processing in Host Defense: Beyond the Inflammasomes
- Reverse Genetics in Predicts ARF Cycling Is Essential for Drug Resistance and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



