-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Obesity is one of the largest health problems facing the world today. Although twin and family studies suggest about two-thirds of obesity is caused by genetic factors, only a small fraction of this variance has been unraveled. There are still large numbers of genes to be identified that cause variations in body fatness and the associated diseases encompassed in the metabolic syndrome (MetS). A locus near a sequence tagged site (STS) marker D6S1009 has been linked to obesity or body mass index (BMI). However, its genetic entity is unknown. D6S1009 is located in the intergenic region between SLC35D3 and NHEG1. Here we report that the ros mutant mice harboring a recessive mutation in the Slc35d3 gene show obesity and MetS and reduced membrane dopamine receptor D1 (D1R) with impaired dopamine signaling in striatal neurons. SLC35D3 is localized to both endoplasmic reticulum (ER) and early endosomes and interacts with D1R. In ros striatal D1 neurons, lack of SLC35D3 causes the accumulation of D1R on the ER to impair its ER exit. The MetS phenotype is reversible by the administration of D1R agonist to the ros mutant. In addition, we identified two mutations in the SLC35D3 gene in patients with MetS, which alter the subcellular localization of SLC35D3. Our results suggest that the SLC35D3 gene, close to the D6S1009 locus, is a candidate gene for MetS, which is involved in metabolic control in the central nervous system by regulating dopamine signaling.
Published in the journal: . PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004124
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004124Summary
Obesity is one of the largest health problems facing the world today. Although twin and family studies suggest about two-thirds of obesity is caused by genetic factors, only a small fraction of this variance has been unraveled. There are still large numbers of genes to be identified that cause variations in body fatness and the associated diseases encompassed in the metabolic syndrome (MetS). A locus near a sequence tagged site (STS) marker D6S1009 has been linked to obesity or body mass index (BMI). However, its genetic entity is unknown. D6S1009 is located in the intergenic region between SLC35D3 and NHEG1. Here we report that the ros mutant mice harboring a recessive mutation in the Slc35d3 gene show obesity and MetS and reduced membrane dopamine receptor D1 (D1R) with impaired dopamine signaling in striatal neurons. SLC35D3 is localized to both endoplasmic reticulum (ER) and early endosomes and interacts with D1R. In ros striatal D1 neurons, lack of SLC35D3 causes the accumulation of D1R on the ER to impair its ER exit. The MetS phenotype is reversible by the administration of D1R agonist to the ros mutant. In addition, we identified two mutations in the SLC35D3 gene in patients with MetS, which alter the subcellular localization of SLC35D3. Our results suggest that the SLC35D3 gene, close to the D6S1009 locus, is a candidate gene for MetS, which is involved in metabolic control in the central nervous system by regulating dopamine signaling.
Introduction
The worldwide prevalence of obesity (OMIM 601665, http://www.ncbi.nlm.nih.gov/omim/) is increasing (data from the International Obesity Taskforce (IOTF) website, http://www.iaso.org/iotf/obesity/). This has resulted in a significant increase in morbidity and mortality associated with the metabolic syndrome (MetS, OMIM 605552). Obesity and associated MetS or body mass index (BMI, OMIM 606641) are regarded as complex traits influenced by both additive genetic effects and environmental factors [1]. It has been estimated that genetic factors explain 67% of the variance in human obesity [2]. Currently, more than 150 loci have been implicated in the development of monogenic obesity, syndromic obesity and polygenic obesity. However, only about 2% of the variance in this trait has been explained [3], [4]. About 200 cases of severe obesity have been reported to be associated with a single gene mutations in a cohort of 11 genes [5]. Studies on extremely obese children have been successful in the characterization of the causative genes for monogenic obesity. However, progress with this approach has been very slow, and is expected to be faster in the era of whole exome sequencing. On the other hand, the identification of the FTO gene as an obesity gene is an example of loci uncovered by genome-wide association or linkage studies [6]. It remains a challenge to uncover genes responsible for mild or moderate obese phenotypes, especially those which develop in adulthood.
Genome-wide linkage analyses have revealed that a locus on chromosome 6q23-25 is linked to obesity in the Framingham Heart Study, with a major locus near the sequence tagged site (STS) marker D6S1009 [7]–[9]. D6S1009 is located within the intergenic region between SLC35D3 (55,419 bp apart at the centromere side) and NHEG1 (867 bp apart at the telomere side) in the NCBI Map Viewer (http://www.ncbi.nlm.nih.gov/mapview/). NHEG1 (neuroblastoma highly expressed 1) is a predicted gene with unknown function. No association with obesity of this gene has been documented. SLC35D3 (solute carrier family 35, member D3) is predicted as an orphan nucleotide sugar transporter or a fringe connection-like protein with 10 transmembrane domains. Previous studies have characterized the recessively inherited ros-/− mutant mouse (ros hereafter), which has a spontaneous intracisternal A particle (IAP) insertion at the first exon of the Slc35d3 gene to disrupt its function [10]. Platelet dense granules are absent in the ros mutant, suggesting that SLC35D3 is involved in the biogenesis of platelet dense granules [10], [11]. This function seems not to do with the solute carrier, and requires further investigation. Mouse Slc35d3 is specifically expressed in the brain as determined by multiple tissue Northern blots [10], suggesting it has specific roles in the central nervous system. In addition, Slc35d3 expression was restricted to the striatonigral medium spiny neurons (MSNs) expressing dopamine receptor D1 (D1R) rather than the striatopallidal MSNs expressing dopamine receptor D2 (D2R) [12]. Interestingly, during the breeding of this mouse, we observed that the adult ros mice gained weight progressively. Here we have characterized the ros mutant as a mouse model of MetS and obesity. In addition, we found two MetS patients with mutations of the SLC35D3 gene. Our results suggest that SLC35D3 is a candidate gene for obesity-related MetS, which is involved in metabolic control in the central nervous system by regulating dopamine signaling.
Results
Ros mutant exhibits metabolic syndrome and lowered energy expenditure
During the breeding of the ros mutant mice, we observed that adult ros mice became obese compared to sex and age-matched wild-type (WT) mice (Fig. 1A). Growth curves showed progressive and significant weight gain of ros mice relative to WT controls, starting at 8 weeks in males, which is similar to the features of late-onset obesity in humans. However, the expression levels of SLC35D3 in the striatum did not change at different postnatal stages within 6 months (Fig. S1). Based on this observation, we chose mice at 12 weeks of age for behavior and molecular tests, mice at 24 weeks of age for phenotypic analyses of MetS. At 24 weeks of age, ros males were 31.5% heavier than age-matched WT males (Fig. 1B), while the naso-to-anal body length was increased by 6.2% in ros mice relative to WT controls (Fig. 1C).
Fig. 1. Ros mice exhibit features of metabolic syndrome. 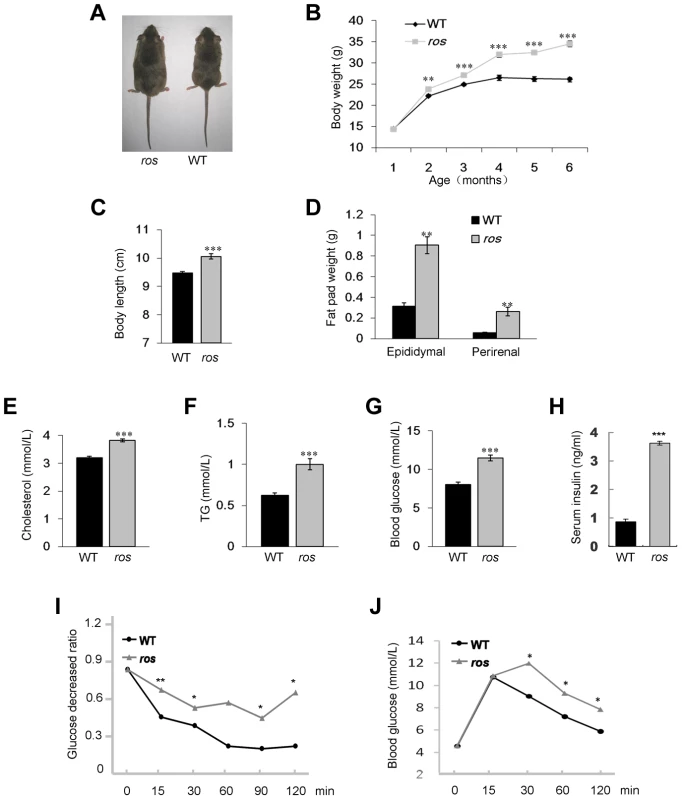
(A) Comparison of ros mouse and wild-type (WT) C3H/HeSnJ mouse at 24 weeks of age. (B) Growth curves of male WT and ros mice. Body weights of ros mice are significantly higher than those of WT starting at 8 weeks. (C) ros mice show significantly increased naso-to-anal body length (10.06±0.09 cm, n = 10) compared with the WT controls (9.47±0.06 cm, n = 10) at 24 weeks of age. (D) Perirenal and epididymal fat pad weight in ros mice (n = 10) are significantly higher than those of the WT (n = 10) respectively at 24 weeks of age. (E–G) Serum levels of cholesterol (Chol), triglycerides (TG) and blood glucose (Gluc) in fasted ros mice (n = 14) are significantly higher than those in WT controls (n = 14) at 24 weeks of age. In ros, mean values of Chol, TG, Gluc are 3.82, 1.0, 11.47 mmol/L, respectively; In WT, mean values of Chol, TG, Gluc are 3.21, 0.62, 8.03 mmol/L, respectively. (H) Plasma insulin levels were increased in non-fasted ros mice (3.62±0.20 ng/ml, n = 3) than in WT (0.87±0.08 ng/ml, n = 3) at 24 weeks of age. (I) Insulin tolerance tests (ITT) showed a tendency of impaired tolerance in ros mice compared with WT mice at 24 weeks of age (n = 3). (J) Glucose tolerance tests (ITT) showed a tendency of impaired tolerance in ros mice compared with WT mice at 24 weeks of age (n = 3). *P<0.05, **P<0.01, ***P<0.001. To determine whether the increased weight of ros mice reflects body composition changes, we dissected and weighed two distinct fat pads, epididymal and perirenal white adipose tissue (WAT). Both epididymal and perirenal fat mass were enormously increased in ros mice (Fig. 1D). Serum cholesterol and triglycerides levels were increased about 19% and 61% respectively compared with the WT controls (Figs. 1E and 1F). Blood glucose levels in ros mice were increased about 43% (Fig. 1G). In addition, serum insulin was increased about 4.2-fold in ros mice (Fig. 1H), while the insulin tolerance test (ITT) and glucose tolerance test (GTT) showed ros mice were insulin resistant and glucose intolerant (Figs. 1I and 1J). Taken together, the ros mice exhibited multiple features of the MetS with late-onset obesity, hyperlipidemia, hyperglycemia and hyperinsulinemia.
The development of obese ros mice may result from elevated energy intake and/or decreased energy expenditure. To assess whether ros mice were hyperphagic, daily food intake was monitored in animals fed a standard chow diet ad libitum for 7 consecutive days at the age of 24 weeks when ros mice were already obese. There was no significant difference in daily food intake between WT and ros mice (Fig. 2A). This suggests that energy intake is unaffected in ros mice. On the other hand, ros mice had significantly decreased physical activity including decreased movement distance, average velocity and movement duration (Figs. 2B–D).
Fig. 2. Food intake, spontaneous locomotor activity and total and resting energy expenditure. 
Mice at 12 weeks of age were used in this study. (A) There was no significant difference in daily food intake between wild-type (WT) (n = 14) and ros mice (n = 15). P = 0.066. (B) The distance traveled during 30 min for ros mice (n = 25) was lower than that for the WT (n = 20). (C) The average movement velocity during 30 min for ros mice (n = 25) was shorter than the WT (n = 20). (D) The total movement duration during 30 min for ros mice (n = 25) was shorter than the WT (n = 20). (E–G) Total energy expenditure (Watts) (E), resting energy expenditure (Watts) by Method 1 (F) and resting energy expenditure (Watts) by Method 2 (G) for WT and ros mice measured at age 8 to 12 weeks (n = 8 per group). WT mice are black symbols and ros mice are white symbols. Fitted linear regressions for each data set are shown. There was a significant effect of body weight and genotype but no interaction in total energy expenditure (ANCOVA: Body weight effect, F1,13 = 44.85, p<0.001); genotype effect, F1,13 = 10.47, p = 0.007; interaction, not significant (ns).). There was a significant effect of body weight but no genotype or interaction effect in resting energy expenditure by Method 1 or 2 (Method 1 ANCOVA: Body weight effect, F1,13 = 8.78, p = .011; genotype effect, F1,13 = 0.35, p = 0.566; interaction: ns. Method 2 ANCOVA: Body weight effect, F1,13 = 22.29, p<0.001; genotype effect, F1,13 = 0.03, p = 0.873; interaction, ns.). *P<0.05, **P<0.01. The decreased physical activity in ros mice prompted us to measure daily energy expenditure over 5 day periods using an Oxymax system (details in the Materials and Method and Fig. S2). Total energy expenditure (resting plus activity metabolism) in the ros mice at age 2–3 months, prior to development of obesity, was significantly lower than in the WT mice using analysis of covariance (ANCOVA) [13] (Fig. 2E). However, there was no difference between the genotypes in their resting metabolic rates, independent of how the resting metabolism was evaluated (Figs. 2F and 2G). This indicated that the difference in energy expenditure between the genotypes was contributed to only by the differences in physical activity expenditure. When we included distance traveled in the respirometry chambers as a covariate in the analysis of total metabolic rate, this only marginally reduced the effect of genotype (F1,12 = 11.48, P = 0.05), suggesting the impact of the mutation is on the total amount of activity as well as the energy costs of locomotion.
Plasma membrane D1R is reduced in ros striatonigral neurons which impairs dopamine signaling
The brain-specific expression pattern in the multiple Northern blots [10] indicated a restricted expression and specific function of SLC35D3 in the brain. We detected no mutant SLC35D3 protein in striatum, substantia nigra and olfactory bulb of the ros mutant with an antibody to mouse SLC35D3 (Fig. 3A), although transcription was upregulated in mutant tissues [10]. SLC35D3 protein was readily detectable in these tissues of WT mice (Fig. 3A). Although the expression of SLC35D3 in the Allen Brain Atlas (http://www.brain-map.org/) shows a wider distribution, in either WT or mutant mice, we did not detect the SLC35D3 protein in other brain sub-regions and especially in the obesity-related brain tissues such as thalamus and hypothalamus (Fig. 3A), as well as in several organs involved in energy homeostasis such as adipose tissue, pancreas, liver and skeletal muscle (Fig. 3B). In addition, no apparent morphological changes or fat accumulation was observed in these organs (Figs. 3C–3F). Considering the enlargement of WAT fat pads (Fig. 1D), the adipocytes in adult ros mice exhibited hyperplasia (increase in number), rather than hypertrophy (increase in size), to contribute mainly to the weight gain. The presence of SLC35D3 in non-neuronal tissues is only known so far in platelets and it plays a role in the biogenesis of platelet dense granules [10], [11]. These results suggest that SLC35D3 is selectively expressed in certain types of neurons with particular enrichment in the basal ganglia, and that ros mice allow us to investigate the phenotypes related to its dysfunction in these neurons.
Fig. 3. SLC35D3 expression in brain sub-regions and non-neuronal tissues. 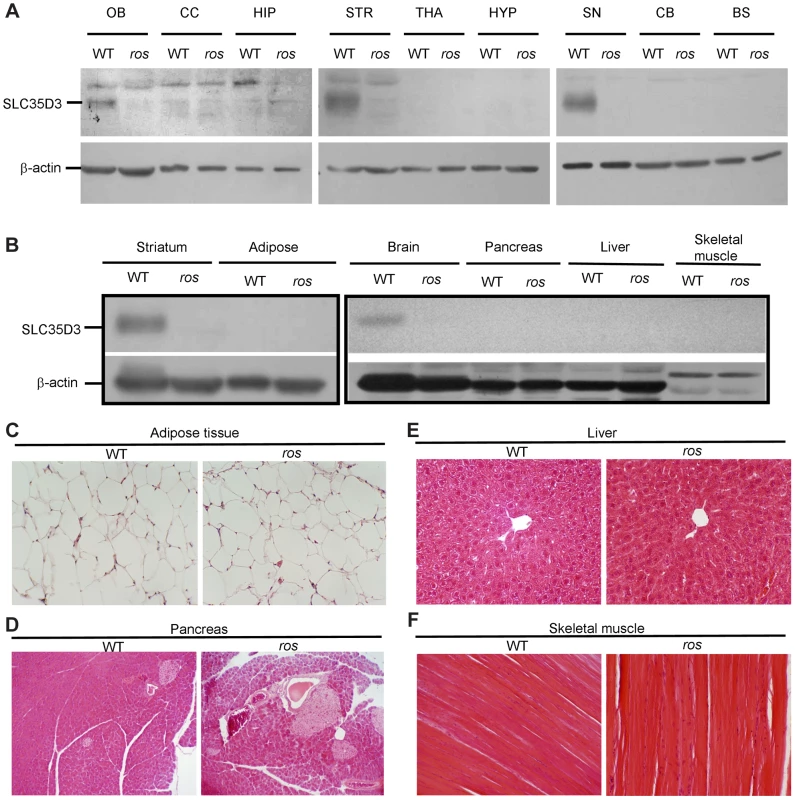
Mice at 24 weeks of age were used in this study. (A) Immunoblotting of SLC35D3 protein in nine brain sub-regions of wild-type (WT) and ros mice. OB: olfactory bulb, CC: cerebral cortex, HIP: hippocampus, STR: striatum, THA: thalamus, HYP: hypothalamus, SN: substantia nigra, CB: cerebellum, BS: brain stem. (B) Immunoblotting assay showed no expression of SLC35D3 in adipose tissue, pancreas, liver, or skeletal muscle compared with striatum or brain in WT mice. There was no expression of SLC35D3 in any of these tissues in ros mice. β-actin serves as a loading control. (C) The size of adipose from epididymal fat pad of ros mice was normal. (D–F) No apparent fat accumulation was observed in pancreas, liver, or skeletal muscle in ros mice. 200× magnification was applied in the sections with H–E staining. It has been reported that Slc35d3 is specifically expressed in the striatonigral MSNs expressing D1R rather than the striatopallidal MSNs expressing D2R [12], therefore, we investigated whether SLC35D3 regulates the function of D1 neurons. Immunohistochemical analysis using an antibody to D1R showed that numerous intensely immunoreactive cell bodies were present in ros striatum (Fig. 4A), which is similar to the intracellular accumulation or internalization of D1R after D1R agonist treatment [14]. To confirm this, in immuno-electronic microscopic (IEM) pictures labeled by anti-D1R, we quantified the gold-labeled particles located on the plasma membrane (PM) and endomembrane structures (EnM) (Fig. 4B). The proportion of D1R in EnM was significantly higher (66%) in ros striatum than that (48.3%) in wild-type (Fig. 4C).
Fig. 4. Plasma membrane D1R and its signaling are reduced in ros striatonigral neurons. 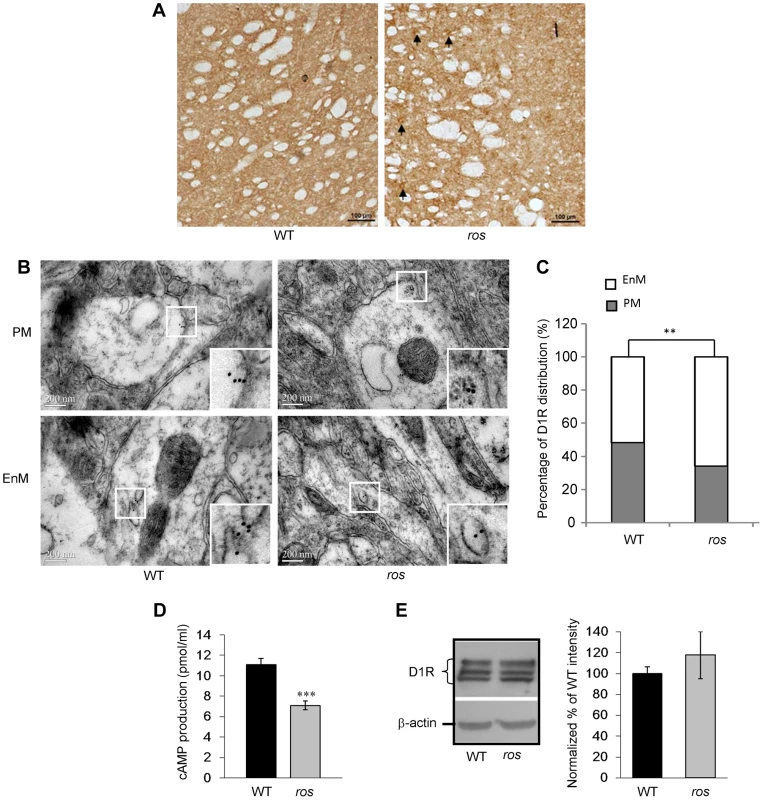
Mice at 12 weeks of age were used in this study. (A) Immunohistochemical detection of D1R on the coronal brain sections of the striatum. In ros mice, visible immunoreactive cell bodies are present (arrows). Scale bar: 100 µm. (B, C) Representative immuno-EM pictures of D1R-labeled particles on plasma membrane (PM, upper panels) and endomembrane structures (EnM, lower panels) in wild-type (WT) and ros striatal neurons are shown without much difference (B). However, the quantification test revealed that the particles on PM in ros mice (34.0%, n = 103) is significantly lower than that in WT (51.7%, n = 87), **P<0.01. Scale bar: 200 nm. (D) SKF82958–induced cAMP accumulation in striatal membranes prepared from WT and ros mice. As compared with the WT (11.09±0.61 pmol/ml, n = 6), cAMP activity in ros membranes (7.11±0.45 pmol/ml, n = 6) was reduced about 36%. *P<0.001. (E) Immunoblot analysis of D1 receptor. left panel, Striatum tissues (20 µg) from WT and ros mice were probed with the monoclonal D1R antibody. β-actin was used as a loading control. The immunoblots shown are representative of three independent experiments. right panel, Normalized percentages of the band intensities shown in left panel are means ± SEM (n = 3). There is no significant difference of total D1R levels between WT and ros mice (P>0.05). We then tested cyclic AMP (cAMP) production to detect functional D1R activation at the cell surface. Stimulation of D1R by the specific agonist SKF82958 (10 µM) produced an accumulation of cAMP in both groups. Consistently, cAMP production in ros striatum was reduced about 36% compared with the WT controls (Fig. 4D), which may be attributable to the reduction of plasma membrane D1R. Western blotting of striatum lysates showed that total D1 receptor expression levels were similar between wild-type and ros samples (Fig. 4E), indicating the total number of striatal D1 neurons in ros mice is not changed. In comparison, total D2R and the fraction of D2R on the plasma membrane were unchanged in the striatum of ros mutant mice (Fig. S3), consistent with the observation that SLC35D3 is not expressed in the D2 neurons [12]. This indicates that SLC35D3 plays a role in D1R trafficking in the striatal D1 neurons, but does not affect D2R trafficking in the striatal D2 neurons. Taken together, our results suggest that loss of SLC35D3 in ros striatum causes intracellular accumulation of D1R and reduces D1 receptors on the plasma membrane.
SLC35D3 is localized to the ER and early endosomes and it interacts with D1R
To ascertain the underlying mechanism of the accumulation of D1R within the ros neurons, we first examined the subcellular localization of mouse SLC35D3. Consistent with a recent report [11], the EGFP-SLC35D3 protein was selectively localized to the ER and early endosomes, but not to the Golgi apparatus or late endosomes/lysosomes (Figs. 5A–5D).
Fig. 5. Subcellular localization of SLC35D3 protein and the effect of SLC35D3 on trafficking of D1R. 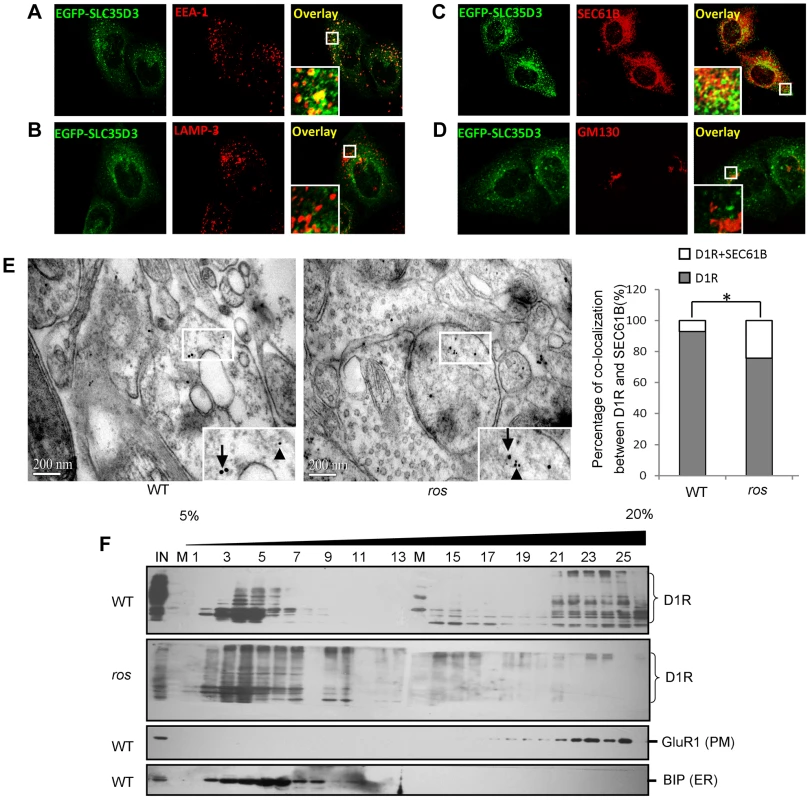
(A–D) In HEK293T cells, EGFP-SLC35D3 co-localized with the ER marker SEC61B and the endosome marker EEA1, but not with the Golgi marker GM130 or the lysosome/late endosome marker LAMP3. (E) Representative pictures to show that the smaller gold-labeled D1R particles (10 nm, arrowheads) and the larger gold-labeled SEC61B particles (ER marker) (15 nm, arrows) are colocalized in ros striatum compared with the particles on different structures in wild-type (WT) under immuno-EM at 12 weeks of age. Scale bar: 200 nm. The percentage of D1R colocalized with SEC61B in ros mutant (19.6%, n = 97) is significantly higher than that in wild-type (WT) (6.8%, n = 74), *P<0.05. (F) Immunoblotting of Optiprep (Biocomp, USA) gradient (5–20%) fractions. One microgram tissue lysates of striatum at 12 weeks of age were separated into 26 fractions. D1R was shifted from plasma membrane (PM: GluR1 as a marker) in fractions 16–25 to intracellular fractions 2–10 mainly in endoplasmic reticulum (ER: BIP as a marker) in ros mice compared with WT mice. IN: lysate input; M: molecular marker. We then investigated the intracellular location of accumulated D1R in ros striatal neurons. We performed immuno-EM by double-labeling with anti-D1R and anti-SEC61B (an ER marker) and found that the D1R particles co-residing with SEC61B in ros striatal neurons (19.6%) were significantly higher than that in wild-type (6.8%) (Fig. 5E). This indicates that the increased proportion of ER-retained D1R (12.8%, Fig. 5E) may mostly account for the reduction of plasma membrane D1R (17.7%, Fig. 4B) in ros mice. Our OptiPrep gradient assays further confirmed the shift of D1R from plasma membrane (fractions 16–25) to intracellular fractions 2–10 mainly corresponding to ER in ros striatum compared with the wild-type (Fig. 5F).
We then tested whether there is a physical interaction between SLC35D3 and D1R by co-immunoprecipitation. Indeed, we observed that Myc-SLC35D3 co-precipitated with Flag-D1R (Fig. 6A). Reciprocally, Myc-D1R co-precipitated with Flag-SLC35D3 (Fig. 6B). In addition, we found that the N-terminal portion of SLC35D3 (1–241aa) interacted with the C-terminal region of D1R (217 - 446aa) (Figs. 6A, 6B).
Fig. 6. SLC35D3 interacts with D1R and mutations of SLC35D3 alter its subcellular distribution. 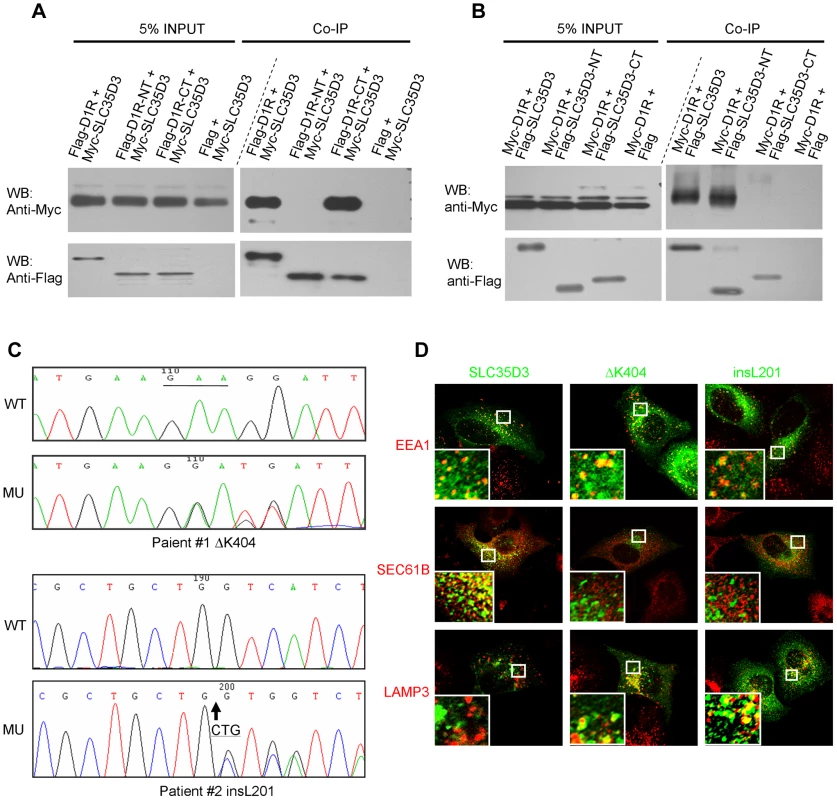
(A) C-terminal D1R interacted with SLC35D3. Cell lysates with the co-expression of Myc-SLC35D3 and Flag-D1R (or Flag-D1R-NT (1–216aa), or Flag-D1R-CT (217–446aa)) were immunoprecipitated. Both the full-length D1R and the D1R-CT bound with Myc-SLC35D3. (B) N-terminal SLC35D3 interacted with D1R. Cell lysates with the co-expression of Myc-D1R and Flag-SLC35D3 (or Flag-SLC35D3-NT (1–301aa), or Flag-SLC35D3-CT (242–422aa)) were immunoprecipitated. Both the full-length SLC35D3 and the SLC35D3-NT bound with Myc-D1R. (C) Two heterozygous mutations were identified in two patients with MetS. WT: wild-type SLC35D3; MU: mutant SLC35D3. In patient #1, a 3-bp deletion (c.1209_1211delGAA) as underlined led to an in-frame deletion of K404. In patient #2, a 3-bp insertion (c.601_602insCTG) as the arrow indicated led to an in-frame insertion of L201. (D) In HEK293T cells, wild-type SLC35D3 was partially colocalized with EEA1 (early endosome) and SEC61B (ER), without colocalization with LAMP3 (late endosome/lysosome). The ΔK404 and insL201 mutations were mislocalized mainly on late endosomes/lysosomes. Taken together, our results indicate that SLC35D3 is likely involved in the membrane trafficking of D1R on its ER exit, and that loss of SLC35D3 leads to the intracellular D1R retention mainly on ER, thus reducing the amount of plasma membrane D1R receptors and their signaling.
Mutational screen of human SLC35D3 in patients with metabolic syndrome
The above findings in ros mice prompted us to investigate whether there are mutations in the orthologous human SLC35D3 gene in patients with MetS. We screened 363 Chinese Han patients with MetS and 217 unaffected individuals by sequencing the two exons and adjacent exon/intron boundaries together with 1 kb untranslated sequence upstream of the start codon of the SLC35D3 gene.
Two variants of SLC35D3 leading to the frame-shift of the coding sequence were found in two unrelated patients. These variants were absent in the control group or the NCBI SNP database for the SLC35D3 gene (Locus ID 340146, http://www.ncbi.nlm.nih.gov/SNP/). In patient #1 (Male, Age: 55, BMI: 26.1, waist circumference: 109 cm, blood pressure: 135/85 mmHg, TG: 4.23 mmol/L, Chol: 5.28 mmol/L, Gluc: 4.4 mmol/L), a heterozygous ΔK404 was identified (Fig. 6C). The mutated SLC35D3 showed the miscolocalization to LAMP3-positive late endosomes/lysosomes in transfected cells compared with WT protein (Fig. 6D), suggesting its subcellular localization has been altered. In patient #2 (Male, Age: 51, BMI: 27.1, waist circumference: 100 cm, blood pressure: 120/80 mmHg, TG: 2.52 mmol/L, Chol: 5.94 mmol/L, Gluc: 5.2 mmol/L), a heterozygous insL201 was identified (Fig. 6C). Similarly, the mutant insL201 colocalized with LAMP3, but not EEA1 or SEC61B (Fig. 6D), also suggesting that these mutations alter the subcellular localization of SLC35D3. The residues around L201 are conserved in human, chimpanzee, dog, mouse and rat. However, the residues around K404 are less conserved in these species. The mislocalization of these two variants (insL201 and ΔK404) implicates localization or sorting signals may lie on these mutational sites.
We did not find a second mutation in the SLC35D3 gene in these two patients after excluding possible large deletions, suggesting that both patients are likely affected in the heterozygous state. Both patients were diagnosed as having MetS with central obesity according to the guidelines of International Diabetes Federation (IDF) [15] and central obesity in China [16]. Similarly, we observed moderate weight gain in heterozygous ros+/− mice at 5 months of age compared with WT littermates (WT: 29.4±0.38, n = 7; ros+/−: 30.9±0.24, n = 8; P<0.01). The more severe weight gain in homozygous ros−/ − mice at the same age is suggestive that the SLC35D3 mutation may have a gene dosage effect on D1R trafficking. It is unknown whether patients with homozygous or compound mutations may have more severe phenotypes. Unfortunately, we were not able to get access to the blood samples of the family members of these two patients, which precluded us to explore the penetrance of the mutations. This study suggests that mutant human SLC35D3 does not function properly in the ER exit of D1R, thus likely impairing the membrane trafficking of D1R and D1 signaling in the patients in a similar mechanism as revealed in ros mice.
D1R agonist reduces body weight and reverses hyperlipidemia in ros mice
To test whether the pathological phenotype in obese ros mice is reversible by the treatment with a D1R agonist, adult male mice received a daily intraperitoneal injection of D1 receptor agonist SKF38393. Following the 12-day treatment period, we observed that body weight loss of ros mice (13%) was significantly higher than that of the wild-type (7%). In contrast, body weight changes of saline-treated wild-type mice or ros mice were not significant (Fig. 7A). Treatment with SKF38393 did not change the levels of serum lipids and glucose compared with saline-treatment in wild-type mice. Strikingly, serum cholesterol and triglycerides levels were significantly decreased for SKF38393-treated ros mice to levels that were similar to those of wild-type mice. Blood glucose levels in ros mice were significantly reduced after the treatment of SKF38393 (Figs. 7B–7D). Thus, administration of SKF38393 caused body weight loss and rescued the hyperlipidemia in ros mice. In addition, physical activity was increased significantly after SKF38393 treatment in ros mice compared with WT (Fig. 7E). These results suggest that impaired D1R signaling could be reversible by D1R agonists in ros mice and likely in patients with MetS who carry SLC35D3 mutations.
Fig. 7. Effect of SKF38393 treatment on body weight loss, serum lipids and glucose, and activity of wild-type or ros mice. 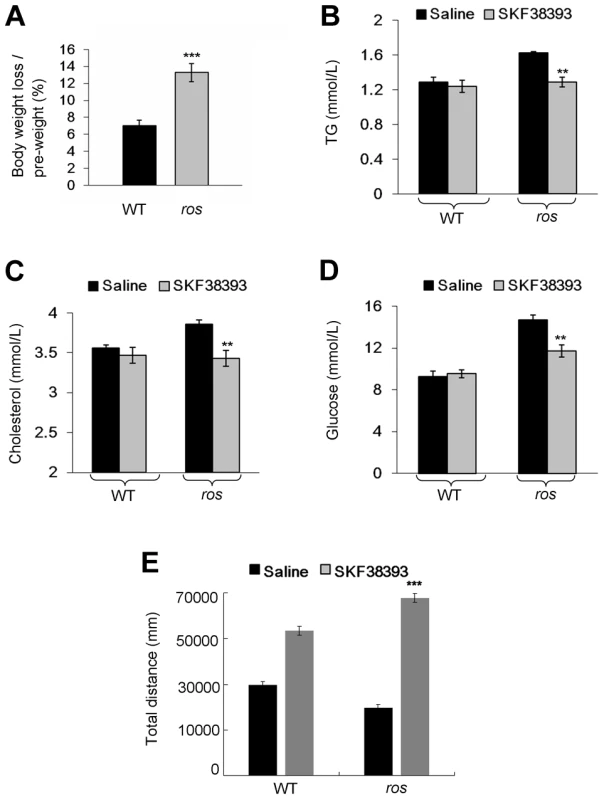
Mice at 24 weeks of age were used in this study. (A) Wild-type (WT) or ros mice (n = 9 per group) received a daily intraperitoneal injection of 20 mg/kg SKF38393 for 12 days and weighed on day 14. Body weights of saline-treated WT or ros mice had no significant changes, whereas body weights of SKF38393-treated WT mice were reduced approximately 7% (from 29.61±0.76 g to 27.5±0.6 g), and body weights of SKF38393-treated ros mice were reduced approximately 13% (from 38.56±0.79 g to 33.39±0.58 g). ***P<0.001. (B–D) Compared with saline-treated WT mice (n = 8), SKF38393-treated WT mice (n = 9) exhibit no significant changes of serum levels of triglycerides (TG), cholesterol (Chol) and blood glucose (Gluc). In SKF38393-treated ros mice, serum levels of Chol, TG and Gluc were significantly reduced compared to saline-treated ros littermates. SKF38393 vs. saline, Chol: 3.43±0.08 mmol/L (n = 9) vs. 3.86±0.05 mmol/L (n = 7); TG: 1.29 ± 0.06 mmol/L (n = 8) vs. 1.63±0.01 mmol/L (n = 7); Gluc: 11.7±0.57 mmol/L (n = 9) vs. 14.67±0.46 mmol/L (n = 7). **P<0.01. (E) Total distances traveled during the period of 30 min to 60 min after SKF38393 treatment were increased in both WT and ros mice. However, the distance traveled in ros mice was greater than that in WT (***P<0.001). Discussion
Obesity is caused by perturbations of the balance between energy intake and energy expenditure, which in turn is regulated by a complex physiological system that requires the coordination of several peripheral and central signals in the brain [17]–[19]. Dopaminergic signaling pathways are involved in the regulation of food intake and energy expenditure, including the mesolimbic pathway in food reward, the mesohypothalamic pathway in satiety and the nigrostriatal pathway in energy expenditure [20]–[22]. Both the D1 and D2 dopamine (DA) receptors act synergistically in the regulation of the basal ganglia function in the striatal MSNs [23], [24]. Dysregulation of DA signaling has been previously implicated in the development of obesity [25]. However, the precise mechanism by which DA receptors regulate energy balance is still unclear [26]. Positron emission tomography revealed that striatal D2 receptor availability is lower in obese humans compared to lean individuals [25], [27], but to date no human imaging studies have assessed the involvement of D1 receptors in obesity.
Reduction of DA receptors on the cell surface could result from 1) increased internalization, 2) reduced reinsertion to the plasma membrane due to increased degradation, and 3) reduced trafficking or expression ab initio. Previous extensive studies have focused on understanding the internalization of DA receptors following agonist occupancy, including agonist-elicited receptor desensitization, endocytosis, and resensitization or degradation [28]–[31]. Unlike D2R, which is generally trafficked to the lysosomes for degradation [32], endocytosed D1R is recycled back to the plasma membrane [14], [33]. However, mechanistic studies of the trafficking of D1R to the cell surface are limited [34], and its relevance of this trafficking to metabolic disorders has not been reported.
Transit out of the ER has been shown to be a critical control point and rate-limiting step in the expression of D1 receptors at the cell surface [35], [36]. A number of DA receptor-interacting proteins have been identified [37]. One ER protein, DRiP78, acts as a chaperon for D1 receptor trafficking [35]. Similarly, our data have shown that SLC35D3 is localized to the ER and endosomes, where it interacts with D1R. Loss of SLC35D3 in ros mice blocks the ER exit of D1R, thus leading to retention in the ER and reduced D1R distribution on the cell surface, thereby impairing D1R signaling. We have not completely excluded the possibility that D1R trafficking from early endosomes to plasma membrane is also blocked. Given that the ER-retained fraction of D1R accounts for the greatest proportion of reduced plasma membrane D1R (Figs. 4B and 5E), we speculate that SLC35D3 plays a major role in the ER exit of D1R. Likewise, in human patients, the mutant SLC35D3 (insL201 or Δ404K) is mistargeted to late endosomes/lysosomes and therefore is likely unable to function properly in the ER exit of D1R. Therefore, SLC35D3 is identified as a novel regulator of D1R membrane trafficking from ER.
The restricted expression of SLC35D3 in the brain and the absence of expression in other peripheral organs (except for the platelets) laid the foundation of our hypothesis that the MetS phenotype in ros mice is attributable to lesions in the central nervous system. Since D2R distribution (Fig. S2) is unaffected, and SLC35D3 was not expressed in D2 neurons [12], the impaired D1R signaling perturbs the D1R/D2R balance, which likely led to the reduced movement and energy expenditure due to the dysfunction of the basal ganglion DA loop.
No apparent obesity phenotypes have been documented in several D1r-knockout (KO) mouse lines as listed in the MGI database (MGI:99578, http://www.informatics.jax.org/). However, reduced spontaneous locomotor activity was reported in a line of D1r-KO mutants [38]. Although the number of plasma membrane D1R in ros mice is about 65% (34%/51.7%) of the WT mice (Fig. 4C), the ros mutant does not mimic the D1r+/ − mice as the total number of D1R is unchanged but redistributed mainly from the plasma membrane to ER. The other significant difference is that SLC35D3 is selectively expressed in striatal D1R-expressing neurons which may manifest specific effects related to D1R reduction. The D1r-KO mice in contrast may have additional defects given the wider expression of D1R in both neuronal and non-neuronal tissues. In fact the D1r-KO mice showed postnatal growth retardation [39]. Thus, complex multiple interacting effects may preclude the development of obesity in the D1r+/ − or D1r−/ − mice. In other words, the ros mutant mouse mimics a D1-neuron specific knockdown of plasma membrane D1R, rather than mimicking the conventional D1r−/ − or D1r+/ − mouse.
In contrast to the ob/ob mice which develop obesity from the age of weaning [40], the ros mice exhibited progressive weight gain starting from 2 months of age. The delayed weight gain in ros mice with late-onset obesity is still a mystery given that the indicated protein is present in early stages after birth (Fig. S1). In addition, the two patients with SLC35D3 mutations were diagnosed with adult central obesity, which also suggests a late-onset obesity phenotype in humans.
Our studies have elucidated the underlying genetic entity of a long-standing unresolved linked locus near the marker D6S1009. Both mouse and human mutations of the SLC35D3 gene are associated with MetS, suggesting that SLC35D3 is a novel candidate gene for MetS. Considering that obesity affects 10∼25% of the European population and nearly one third of the US population [41], a mutational screen of SLC35D3 in the obese population would be cost-effective as a precursor to potential D1R agonist treatment.
Obese children receiving D1R agonist treatment reverse weight gain [42]. Similarly, administration of D1R agonist reversed most of the phenotype of MetS in ros mice. This effect may be caused by the stimulation of the residual D1R on the plasma membrane of ros striatum, or redirection of the ER-retained D1R to plasma membrane for its signaling. In addition, the reversible phenotypes upon D1R agonist treatment suggest that the reduced D1R numbers on the plasma membrane could be the primary cause of MetS in the ros mutant mice, although we have not excluded the effects on the substantia nigra and olfactory bulb where SLC35D3 are also expressed (Fig. 3A). SLC35D3 deficiency caused obesity primarily via effects on physical activity levels, supporting that genetic factors could be a component of low physical activity [43]. As reduced physical activity is the primary consequence of impaired D1R signaling, encouraging elevations in physical activity in these patients might be an alternative way to prevent or alleviate their symptoms of obesity [44].
Materials and Methods
Mice
The ros mutant (ros−/−) [10] and control C3H/HeSnJ mice (wild-type, WT) were originally obtained from Dr. Richard T. Swank's laboratory and bred in the animal facility of the Institute of Genetics and Developmental Biology (IGDB), Chinese Academy of Sciences. To ensure the genotypes of ros−/− and wild-type, we developed a PCR method of genotyping based on the nature of the insertional mutation in the Slc35d3 gene [10]. For the locomotion tests, only male mice of each genotype at 12 weeks old were selected to control for potentially confounding hormonal effects during the estrous cycle in females. Mice were housed in a room with a 12-hr light/dark cycle (lights on at 7 : 30 a.m. and off at 7 : 30 p.m.) with access to food and water ad libitum. For other phenotypic analyses, males at 24 weeks of age were used except for those specified in figure legends or materials and methods.
Study subjects
We recruited 363 unrelated Chinese Han patients with MetS from The Affiliated Hospital of Qingdao University Medical College and The Affiliated Children's Hospital of Nanjing Medical University, and 217 unaffected individuals from Beijing Tongren Hospital of Capital Medical University. The patients were diagnosed as MetS according to the guidelines of International Diabetes Federation (IDF) [15] and central obesity in China [16]. In brief, MetS is diagnosed as abdominal obesity (or central obesity) with any two of the following parameters, 1) TG>1.7 mmol/L, 2) HDL<1.03 mmol/L (male) or <1.29 mmol/L (female), 3) blood pressure >130/85 mmHg, 4) fasting plasma glucose >5.6 mmol/L. For the diagnosis of abdominal obesity in Chinese population, we choose either 1) BMI >28 as general obesity or 2) BMI between 24 to 28 as overweight, and waist circumference >90 cm (male) or >85 cm (female). Eight mililiter peripheral blood samples were collected from all subjects participating in this study. We designed primers for amplifying the two exons and about 1 kb upstream of the human SLC35D3 gene. Amplified PCR products were subjected to direct sequencing by an ABI PRISM 3700 automated sequencer (Applied Biosystems, Foster City, CA).
Measurements of obesity features and metabolites in mice
Growth curves for males were obtained by measuring body weight once a month from 4 to 24 weeks of age. For determination of body length, mice were anesthetized and fully extended to measure the naso-anal distance. Epididymal and perirenal fat pads were harvested from male mice and weighed. Blood was collected by cardiac puncture after an overnight fast for measuring blood glucose, serum cholesterol and triglycerides by colorimetric kit assays (Leadman, Beijing, China) and analyzed using an automatic biochemical analyzer (Hitachi, Tokyo, Japan). Insulin was measured by a rat/mouse insulin enzyme-linked immunoassay (ELISA) Kit on non-fasted mice (Millipore, Bedford, MA, USA). For the insulin tolerance test (ITT) and glucose tolerance test (GTT), fasting plasma glucose levels were measured (16 hours fast, blood taken from the tail vein) using a glucosimeter (Teromo, Japan). Then insulin (Roche Diagnostics, Switzerland) was injected intraperitoneally (1 U/kg) and blood glucose was measured again at 15, 30, 60, 90 and 120 min post injection. Alternatively, D-glucose (Sigma-Aldrich, St. Louis, Missouri, USA) was injected intraperitoneally (2 g/kg body weight) and blood glucose was measured again at 15, 30, 60 and 120 min post injection.
Spontaneous locomotor activity
Mice were pre-exposed to the chamber before testing to allow environmental habituation, and activity was monitored under indirect dim light and sound-attenuated conditions. A single mouse was placed in a chamber (40 cm length×40 cm width×45 cm height) for 30 min. Total distance traveled, average velocity and total movement duration measured spontaneous activity. All these parameters were measured by JLBehv software (JLGY, Shanghai, China). Behavioral testing was performed between 8 : 00 and 12 : 00 a.m.
Food intake
Before measurement of daily food intake, mice fed ad libitum were individually housed for 3 days to allow environmental habituation. Food was measured at 3 : 00 p.m. each day for 7 consecutive days.
VO2 and VCO2 measurements
Mice aged 8 to 12 weeks (prior to development of obesity) were measured using an indirect calorimetry system (Oxymax, Ohio, USA). Oxygen consumption, CO2 production and physical activity (beam breaks) were recorded at 30-min intervals for 5 consecutive days (48 times a day). Volume of oxygen consumption (VO2) and carbon dioxide production (VCO2) were measured using electrochemical and spectrophotometric sensors respectively. Oxygen consumption data were converted to energy expenditure (Watts) using the measured RQ values using procedures outlined in Arch et al [45]. To report the total energy expenditure we averaged the 48 measurements collected each day across days 2 to 5, allowing the animals to acclimate during the first 24 h in the chambers [13]. We recorded simultaneously the physical activity levels of the mice. Typical temporal patterns of oxygen consumption and physical activity are shown in Figs. S2A and S2B respectively. To establish the resting metabolic rate we used two different strategies. First we summarized all the half hourly oxygen consumption data in a histogram and then calculated the mean of the lowest 5% of values. Typical histogram for the data shown in Fig. S2A is shown in Fig. S2C. A second method was to use the regression approach outlined by Nonogaki et al [46]. This involved plotting the time matched data for VO2 and physical activity levels (Fig. S2D) and then evaluating the resting metabolic rate from the intercept of a fitted linear regression model. The relationship between total metabolic rate and body weight for each genotype is shown in Fig. 2E, and that for the two resting metabolic rate approaches in Figs. 2F and 2G respectively. Following derivation of the total and resting rates of metabolism we corrected for the potentially confounding effects of body weight (Figs. 2E–2G) as recommended in Arch et al [45] and Tschop et al [13].
Treatment of D1 receptor agonists on mice
Twenty-four to 25-week-old male mice of each genotype received a daily intraperitoneal injection of dopamine D1 receptor SKF38393 (20 mg/kg) (Sigma-Aldrich) for a period of 12 days. Saline-treated mice served as controls. Body weight in all mice was measured on the fourteenth day. Blood glucose, serum cholesterol and triglycerides, and locomotor activities were measured as described above.
cAMP ELISA measurement
The dorsal striatum of mice was dissected as above and homogenized in Buffer A (10 mM Tris pH 7.4, 1 mM EDTA, 30 µM leupeptin, 1 µM pepstatin A) with 10% sucrose. Membranes were isolated by centrifugation (65 min at 100,000 g) onto a cushion of Buffer A with 44.5% (w/v) sucrose. The membranes at the interface were transferred to a new tube and washed twice with Buffer A and collected by centrifugation (30 min at 100,000 g). Protein concentrations were determined with Protein Assay (Bio-Rad, Hercules, CA, USA). Adenylyl cyclase activity was determined by incubating membrane protein (20 µg) at 30°C for 10 min in 0.1 ml of buffer containing 10 mM imidazole (pH 7.4), 0.2 mM EGTA, 0.5 mM MgCl2, 0.5 mM DTT, 0.1 mM ATP, 0.5 mM IBMX, and 10 µM D1 receptor agonists SKF82958 (Sigma-Aldrich). Reactions were terminated by placing the tubes into boiling water for 2 min. The cAMP concentrations were measured using the Direct cAMP Enzyme-linked Immunoassay Kit (Sigma-Aldrich) following the manufacturer's instructions. Optical density was measured at 405 nm by a microplate reader (Bio-Rad).
Antibodies
The polyclonal rabbit anti-mouse SLC35D3 antiserum (1∶1000) for immunoblotting (WB) was prepared using the purified C-terminal 322–422aa peptide as an antigen. The monoclonal mouse anti-GFP (1∶1000) and polyclonal rabbit anti-Myc (1∶1000) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Monoclonal mouse anti-Flag antibody (Sigma-Aldrich) was used for WB (1∶7000) and immunocytochemical (ICC) analysis (1∶3000). Mouse monoclonal antibody against calnexin (1∶200) for ICC was purchased from Abcam (Cambridge Science Park, Cambridge, UK). Mouse monoclonal antibody against D1 receptor (Chemicon, Temecula, CA, USA) was used for WB (1∶600) and ICC (1∶300). Polyclonal rabbit anti-D2 receptor (Millipore) was used for WB (1∶2000). Mouse polyclonal antibody against GM130 for ICC (1∶500) was a kind gift from Dr. S. Bao (IGDB, CAS, China). Polyclonal rabbit anti-LAMP3 (Chemicon) was used for ICC (1∶200). Rabbit polyclonal anti-SEC61B antibody was purchased from Millipore and used for ICC (1∶600). Mouse monoclonal anti-EEA1 was from BD Biosciences (Franklin Lakes, NJ, USA) for ICC (1∶500). Rabbit polyclonal anto-GluR1 was from Millipore for WB (1∶1000). Goat polyclonal anti-BIP was from Santa Cruz Biotechnology for WB (1∶400). Mouse monoclonal anti-β-actin (Sigma-Aldrich) was used for WB (1∶10000). Alexa Fluor 594-conjugated donkey anti-mouse and donkey anti-rabbit IgG (H+L) were purchased from Molecular Probes (Invitrogen, Carlsbad, CA, USA).
Western blotting
Cell lysates, immunoprecipitates or tissue lysates were combined with loading buffer and subjected to 8–12% SDS polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were blotted onto polyvinylidene difluoride membranes in phosphate buffer with 0.1% Tween-20 (PBST), blocked for 1 h in 5% non-fat dry milk/PBST and probed for 2 h with primary antibodies at room temperature. The membranes were rinsed three times (10 min each) with PBST prior to incubation with appropriate peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology) and developed with enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ, USA).
Constructs and co-immunoprecipitation
We prepared constructs for co-immunoprecipitation and immunofluorescence experiments. Mouse entire coding regions of wild-type Slc35d3 (RefSeq, NM_029529, http://www.ncbi.nlm.nih.gov/refseq/), D1r (RefSeq, NM_010076), D2r (RefSeq, NM_010077), and human SLC35D3 (RefSeq, NM_001008783) were subcloned into the pEGFP-C2 or -N2 vector (with GFP-tag), pCMV-tag2B vector (with Flag-tag) and pCMV-tag3B vector (with Myc-tag) as specified in the figures. The fragments of mouse SLC35D3 and mouse D1R specified in figure legends were generated by subcloning. The mutant human SLC35D3 constructs (ΔK404 and insL201) were generated by site-directed mutagenesis (Takara, Japan) using human wild-type SLC35D3 construct.
Transfected HEK-293T cells grew to confluency on 6-well plates. Cells were harvested and lysed in 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and protease inhibitors. Cell lysates were centrifuged at 18,000 g for 10 min, and the supernatant was collected and recentrifuged. The supernatant was incubated overnight with 3 µg mouse monoclonal anti-FLAG M2 antibody (Sigma-Aldrich) and washed 6 times with ice-cold wash buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl). The samples were eluted with elution buffer (5 µg/µl 3× FLAG peptide) and subjected to SDS-PAGE and Western blotting with anti-Myc or anti-Flag antibody as described above.
Histological and immunohistochemical staining
Mice were perfused through the heart with 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) under deep pentobarbital anesthesia. The brains were removed, and 20 µm frozen sections in the coronal plane were prepared for hematoxylin and eosin (H–E) or immunohistochemical staining (IHC). The H–E staining followed routine procedures. A standard H–E staining protocol was applied to sections of adipose tissue, liver, pancreas and skeletal muscle. For IHC, the endogenous peroxidase activity was blocked by treatment with 0.3% hydrogen peroxide in methanol, sections were blocked with 0.01 M PBS containing 10% goat serum and were then incubated overnight at 4°C with the mouse monoclonal antibody against D1R (1∶400). Following 0.01 M PBS rinses, sections were incubated in a biotinylated secondary antibody (Zhongshan Goldenbridge, Beijing, China) for an hour at room temperature, treated for another hour at room temperature with peroxidase-ligated streptavidin. The results were captured by a TS100 microscope (Nikon, Tokyo, Japan).
Immunofluorescence confocal imaging
HEK293T cells transfected with EGFP-SLC35D3 (wild-type or mutant or other constructs as specified in the results or figure legends) were grown on glass cover slips in 24-well plates until 30–50% confluence. 18–20 hrs after transfection, they were fixed with freshly prepared 4% paraformaldehyde for 10 min. Cells were washed 3 times with 0.01 M phosphate buffer (pH 7.4). The permeabilization of cells was carried out in the presence of 0.3% Triton X-100 in PBS for 10 min. After blocking in 0.01 M PBS containing 1% BSA for 1 hr at 37°C, fixed cells were incubated with various antibodies as indicated in the results overnight at 4°C. Cells were then washed 3 times in 0.01 M PBS containing 0.1% Triton X-100 before incubating with Alexa Fluor 594-conjugated secondary antibody at 1∶2000 dilution for 1 h at 37°C. Cells were then washed 3 times before glass cover slips were mounted. Images were acquired with an ×100 lens on a D-ECLIPSE-si confocal microscope (Nikon).
Immuno-electronic microscopy examination
Mice were anesthetized with pentobarbital (0.1 g/kg, Sigma-Aldrich). Brains were separated and cut by a vibratome (DSK, model DTK-1000, Japan). The striatum was fixed with 2% paraformaldehyde, 2.5% glutaraldehyde and 0.1% tannic acid in 0.1 M natrium cacodylicum. Then sections were rinsed and postfixed with 1% osmium tetroxide for 30 min. After washing, the sections were dehydrated in an ascending series of dilution of acetone and impregnated in Epon 60°C, 24 hours. Ultrathin (70 nm) sections were collected on nickel grids, rinsed and incubated with mouse anti-D1R (1∶10) or rabbit anti-SEC61B (1∶50) in 1% BSA buffer overnight at 4°C, washed and incubated with 10 nm gold-anti-mouse IgG or 15 nm anti-rabbit IgG (1∶50). Sections were observed in JEM 2000 electron microscope (Japan). All the reagents were purchased from Electronic Microscope Science (EMS, Hatfield, PA, USA).
OptiPrep gradient assay
The dissected striatum was immediately homogenized with 1 ml HB lysis buffer (250 mM sucrose, 20 mM Tris-HCl, pH 7.4, 1 mM EDTA). The sample was placed onto the top of an 11 ml continuous 5%–20% Optiprep (Axis-Shield, Norway) gradient in HB buffer. The gradient was centrifuged at 200,000 g (34100 rpm) for 14 hours in a Beckman SW41 rotor. Twenty-six fractions (400 µl each) were collected from the top using auto-collector (BioComp, USA). Equal aliquots from each fraction were analyzed for immunoblotting.
For the fractionation assay, the dissected striatum was immediately homogenized with 200 µl HB lysis buffer. 800 µl 20% Optiprep and 800 µl 5% Optiprep in HB buffer were placed into the tube constitutively. The tissue lysate was placed onto the top of the gradient. The sample was centrifuged at 28,000 rpm (TLS-55, Beckman, USA) for 14 hours. Eighteen fractions (100 µl each) were collected from the top. Based on the pilot Western assay, the fractions were combined as the 1–10th tube (mostly cytoplasm fraction) and the 11–18th tube (mostly plasma membrane fraction) respectively. Equal aliquots from each fraction were analyzed for immunoblotting.
Data collection and analyses
cAMP ELISA was performed in duplicate and was repeated three or four times. The standard curves were generated using non-linear regression curve fitting. The specific protein bands on Western blots were scanned and analyzed using the software program NIH Image J. All data were obtained from at least three independent experiments. Data were expressed as mean ± SEM and statistical significance was tested by Student's t-test. Data from the calorimetry system was tested by ANCOVA. Distribution of gold-labeled particles in immuno-EM pictures were counted and statistical significance was tested by Chi-square test. Intensities of immunofluorescence in cultured cells were analyzed using NIH Image J.
Ethics statements
All mouse procedures were approved by the Institutional Animal Care and Use Committee of IGDB (mouse protocol KYD2006-002). The study of human subjects was approved by the Bioethic Committee of IGDB, Chinese Academy of Sciences (IRB approval number, IGDB-2011-IRB-002). The study was conducted according to the Declaration of Helsinki Principles. Written informed consents were obtained from all subjects.
Supporting Information
Zdroje
1. KopelmanPG (2000) Obesity as a medical problem. Nature 404 : 635–643.
2. MaesHH, NealeMC, EavesLJ (1997) Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 27 : 325–351.
3. DrongAW, LindgrenCM, McCarthyMI (2012) The genetic and epigenetic basis of type 2 diabetes and obesity. Clin Pharmacol Ther 92 : 707–715.
4. MutchDM, ClementK (2006) Unraveling the genetics of human obesity. PLoS Genet 2: e188.
5. RankinenT, ZuberiA, ChagnonYC, WeisnagelSJ, ArgyropoulosG, et al. (2006) The human obesity gene map: the 2005 update. Obesity (Silver Spring) 14 : 529–644.
6. FischerJ, KochL, EmmerlingC, VierkottenJ, PetersT, et al. (2009) Inactivation of the Fto gene protects from obesity. Nature 458 : 894–898.
7. FoxCS, Heard-CostaNL, WilsonPW, LevyD, D'AgostinoRBSr, et al. (2004) Genome-wide linkage to chromosome 6 for waist circumference in the Framingham Heart Study. Diabetes 53 : 1399–1402.
8. AryaR, LehmanD, HuntKJ, SchneiderJ, AlmasyL, et al. (2003) Evidence for bivariate linkage of obesity and HDL-C levels in the Framingham Heart Study. BMC Genet 4(Suppl 1): S52.
9. AtwoodLD, Heard-CostaNL, CupplesLA, JaquishCE, WilsonPW, et al. (2002) Genomewide linkage analysis of body mass index across 28 years of the Framingham Heart Study. Am J Hum Genet 71 : 1044–1050.
10. ChintalaS, TanJ, GautamR, RusiniakME, GuoX, et al. (2007) The Slc35d3 gene, encoding an orphan nucleotide sugar transporter, regulates platelet-dense granules. Blood 109 : 1533–1540.
11. MengR, WangY, YaoY, ZhangZ, HarperDC, et al. (2012) SLC35D3 delivery from megakaryocyte early endosomes is required for platelet dense granule biogenesis and is differentially defective in Hermansky-Pudlak syndrome models. Blood 120 : 404–414.
12. LoboMK, KarstenSL, GrayM, GeschwindDH, YangXW (2006) FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci 9 : 443–452.
13. TschopMH, SpeakmanJR, ArchJR, AuwerxJ, BruningJC, et al. (2011) A guide to analysis of mouse energy metabolism. Nat Methods 9 : 57–63.
14. DumartinB, CailleI, GononF, BlochB (1998) Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J Neurosci 18 : 1650–1661.
15. AlbertiKG, ZimmetP, ShawJ (2006) Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23 : 469–480.
16. BaoY, LuJ, WangC, YangM, LiH, et al. (2008) Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis 201 : 378–384.
17. WoodsSC, SeeleyRJ, PorteDJr, SchwartzMW (1998) Signals that regulate food intake and energy homeostasis. Science 280 : 1378–1383.
18. SchwartzMW, WoodsSC, PorteDJr, SeeleyRJ, BaskinDG (2000) Central nervous system control of food intake. Nature 404 : 661–671.
19. GuyenetSJ, SchwartzMW (2012) Clinical review: Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab 97 : 745–755.
20. RamosEJ, MeguidMM, CamposAC, CoelhoJC (2005) Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition 21 : 269–279.
21. MartelP, FantinoM (1996) Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav 53 : 221–226.
22. MeguidMM, FetissovSO, VarmaM, SatoT, ZhangL, et al. (2000) Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition 16 : 843–857.
23. SurmeierDJ, DingJ, DayM, WangZ, ShenW (2007) D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci 30 : 228–235.
24. LoboMK (2009) Molecular profiling of striatonigral and striatopallidal medium spiny neurons past, present, and future. Int Rev Neurobiol 89 : 1–35.
25. WangGJ, VolkowND, LoganJ, PappasNR, WongCT, et al. (2001) Brain dopamine and obesity. Lancet 357 : 354–357.
26. WangGJ, VolkowND, ThanosPK, FowlerJS (2009) Imaging of brain dopamine pathways: implications for understanding obesity. J Addict Med 3 : 8–18.
27. VolkowND, WangGJ, TelangF, FowlerJS, ThanosPK, et al. (2008) Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42 : 1537–1543.
28. KimOJ, GardnerBR, WilliamsDB, MarinecPS, CabreraDM, et al. (2004) The role of phosphorylation in D1 dopamine receptor desensitization: evidence for a novel mechanism of arrestin association. J Biol Chem 279 : 7999–8010.
29. CorvolJC, StudlerJM, SchonnJS, GiraultJA, HerveD (2001) Galpha(olf) is necessary for coupling D1 and A2a receptors to adenylyl cyclase in the striatum. J Neurochem 76 : 1585–1588.
30. IwamotoT, OkumuraS, IwatsuboK, KawabeJ, OhtsuK, et al. (2003) Motor dysfunction in type 5 adenylyl cyclase-null mice. J Biol Chem 278 : 16936–16940.
31. MarcheseA, ChenC, KimYM, BenovicJL (2003) The ins and outs of G protein-coupled receptor trafficking. Trends Biochem Sci 28 : 369–376.
32. JiY, YangF, PapaleoF, WangHX, GaoWJ, et al. (2009) Role of dysbindin in dopamine receptor trafficking and cortical GABA function. Proc Natl Acad Sci U S A 106 : 19593–19598.
33. VargasGA, Von ZastrowM (2004) Identification of a novel endocytic recycling signal in the D1 dopamine receptor. J Biol Chem 279 : 37461–37469.
34. TanCM, BradyAE, NickolsHH, WangQ, LimbirdLE (2004) Membrane trafficking of G protein-coupled receptors. Annu Rev Pharmacol Toxicol 44 : 559–609.
35. BermakJC, LiM, BullockC, ZhouQY (2001) Regulation of transport of the dopamine D1 receptor by a new membrane-associated ER protein. Nat Cell Biol 3 : 492–498.
36. Petaja-RepoUE, HogueM, LaperriereA, WalkerP, BouvierM (2000) Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem 275 : 13727–13736.
37. WangM, LeeFJ, LiuF (2008) Dopamine receptor interacting proteins (DRIPs) of dopamine D1-like receptors in the central nervous system. Mol Cells 25 : 149–157.
38. TranAH, TamuraR, UwanoT, KobayashiT, KatsukiM, et al. (2005) Dopamine D1 receptors involved in locomotor activity and accumbens neural responses to prediction of reward associated with place. Proc Natl Acad Sci U S A 102 : 2117–2122.
39. KobayashiM, IaccarinoC, SaiardiA, HeidtV, BozziY, et al. (2004) Simultaneous absence of dopamine D1 and D2 receptor-mediated signaling is lethal in mice. Proc Natl Acad Sci U S A 101 : 11465–11470.
40. IngallsAM, DickieMM, SnellGD (1950) Obese, a new mutation in the house mouse. J Hered 41 : 317–318.
41. Pi-SunyerFX (2002) The obesity epidemic: pathophysiology and consequences of obesity. Obes Res 10(Suppl 2): 97S–104S.
42. GalluzziF, SaltiR, StagiS, La CauzaF, ChiarelliF (2005) Reversible weight gain and prolactin levels–long-term follow-up in childhood. J Pediatr Endocrinol Metab 18 : 921–924.
43. de Vilhena e SantosDM, KatzmarzykPT, SeabraAF, MaiaJA (2012) Genetics of physical activity and physical inactivity in humans. Behav Genet 42 : 559–578.
44. CasazzaK, FontaineKR, AstrupA, BirchLL, BrownAW, et al. (2013) Myths, presumptions, and facts about obesity. N Engl J Med 368 : 446–454.
45. ArchJR, HislopD, WangSJ, SpeakmanJR (2006) Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 30 : 1322–1331.
46. NonogakiK, AbdallahL, GouldingEH, BonaseraSJ, TecottLH (2003) Hyperactivity and reduced energy cost of physical activity in serotonin 5-HT(2C) receptor mutant mice. Diabetes 52 : 315–320.
Štítky
Genetika Reprodukční medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 2- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



