-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
How do adapting populations navigate the tensions between the costs of gene expression and the benefits of gene products to optimize the levels of many genes at once? Here we combined independently-arising beneficial mutations that altered enzyme levels in the central metabolism of Methylobacterium extorquens to uncover the fitness landscape defined by gene expression levels. We found strong antagonism and sign epistasis between these beneficial mutations. Mutations with the largest individual benefit interacted the most antagonistically with other mutations, a trend we also uncovered through analyses of datasets from other model systems. However, these beneficial mutations interacted multiplicatively (i.e., no epistasis) at the level of enzyme expression. By generating a model that predicts fitness from enzyme levels we could explain the observed sign epistasis as a result of overshooting the optimum defined by a balance between enzyme catalysis benefits and fitness costs. Knowledge of the phenotypic landscape also illuminated that, although the fitness peak was phenotypically far from the ancestral state, it was not genetically distant. Single beneficial mutations jumped straight toward the global optimum rather than being constrained to change the expression phenotypes in the correlated fashion expected by the genetic architecture. Given that adaptation in nature often results from optimizing gene expression, these conclusions can be widely applicable to other organisms and selective conditions. Poor interactions between individually beneficial alleles affecting gene expression may thus compromise the benefit of sex during adaptation and promote genetic differentiation.
Published in the journal: . PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004149
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004149Summary
How do adapting populations navigate the tensions between the costs of gene expression and the benefits of gene products to optimize the levels of many genes at once? Here we combined independently-arising beneficial mutations that altered enzyme levels in the central metabolism of Methylobacterium extorquens to uncover the fitness landscape defined by gene expression levels. We found strong antagonism and sign epistasis between these beneficial mutations. Mutations with the largest individual benefit interacted the most antagonistically with other mutations, a trend we also uncovered through analyses of datasets from other model systems. However, these beneficial mutations interacted multiplicatively (i.e., no epistasis) at the level of enzyme expression. By generating a model that predicts fitness from enzyme levels we could explain the observed sign epistasis as a result of overshooting the optimum defined by a balance between enzyme catalysis benefits and fitness costs. Knowledge of the phenotypic landscape also illuminated that, although the fitness peak was phenotypically far from the ancestral state, it was not genetically distant. Single beneficial mutations jumped straight toward the global optimum rather than being constrained to change the expression phenotypes in the correlated fashion expected by the genetic architecture. Given that adaptation in nature often results from optimizing gene expression, these conclusions can be widely applicable to other organisms and selective conditions. Poor interactions between individually beneficial alleles affecting gene expression may thus compromise the benefit of sex during adaptation and promote genetic differentiation.
Introduction
The concept of a fitness landscape unites the three levels of evolutionary change – genotype, phenotype, and fitness – into a mathematical picture of the potential for, and constraints upon, adaptive evolution. By mapping genotypes to a measure of fitness, fitness landscapes guide our understanding of how epistasis – nonlinear interactions between the fitness effects of mutations – shapes evolution. Strong epistasis implies that landscapes are rugged, with many peaks, or locally optimally genotypes [1], [2]. The magnitude and form of epistasis is predicted to determine the number of evolutionary trajectories [3], [4], the rate and repeatability of adaptation [5]–[7], and the benefit of sex [8]. Recent experimental work with a wide variety of model organisms has revealed diminishing returns as a general trend of adaptation [9]–[13], with relatively few cases of synergy [11], [14] or sign epistasis [15] (i.e., the same mutation being beneficial or deleterious in different contexts [16]). Antagonism between adaptive mutations might imply that these populations are summiting peaks in their fitness landscapes with just a handful of genetic changes. This explanation might lead to further trends, such as a negative relationship between the initial selective coefficient of a mutation and its epistatic interactions that could prove to be a useful predictor of a saturating process of adaptation [17]. In order to definitely link diminishing returns to the ascent of local peaks, as well as to understand the existence of the peaks themselves, we must understand the phenotypes that link genotype and fitness in the adaptive landscape. Mathematically convenient formulations such as Fisher's geometric model for adaptation near a single peak [18] have been used to interpret the trend toward antagonism [19]. This approach assumes stabilizing selection a priori. What remains unclear is what types of physiological interactions give rise to fitness landscapes of varying shape and form, as well the constraints upon mutational changes to underlying phenotypes.
Models of metabolic pathways have been amongst the most successful in translating underlying biochemical phenotypes to fitness. The contribution of enzyme activities upon metabolic flux has been formalized via Metabolic Control Analysis (MCA) [20], [21]. The ability of this approach to predict the fitness consequences of changes in enzyme properties has been verified in experimental systems that vary from Escherichia coli in lactose-limited chemostats to the flight properties of butterflies (reviewed in [22]). Turning to multiple enzymes, MCA theory has suggested a general trend toward synergistic interactions between activity-increasing mutations in a metabolic pathway [23], [24]. A major limitation, however, has been that the costs of enzyme expression [25] have not been included in classical MCA. Whereas the dependence of flux through a metabolic pathway saturates with increasing levels of a given enzyme, the costs will continue to accumulate. The balance of these two selective factors will generate an intermediate optimum, and thus stabilizing selection. Inclusion of expression costs to MCA has enabled predictions of the optimum levels of a single enzyme [26], and was used to compare the differential utility of alternate, degenerate pathways [27]. An open question, however, is how the balance between catalytic benefits and expression costs plays out to optimize enzyme expression across many enzymes simultaneously.
In order to study how evolution would simultaneously optimize expression of multiple genes, we have developed a model system of an engineered Methylobacterium extorquens AM1 (EM) in which we altered its central metabolism to be dependent upon a foreign pathway (Figure 1A for details). M. extorquens grows on methanol by oxidizing it first to formaldehyde, and then through a series of steps to formate, which is either fully oxidized to CO2 or incorporated into biomass [28]–[32]. In the EM strain we removed the endogenous pathway for formaldehyde oxidation in wild-type (WT) [28] and replaced it with two genes encoding a foreign pathway that oxidizes formaldehyde via glutathione (GSH) derivatives [29]. Eight populations dependent upon this introduced metabolic pathway evolved in methanol-containing medium via serial transfers for 900 generations [10], [33].
Fig. 1. Adaptive mutations that optimized the expression of the GSH-linked pathway. 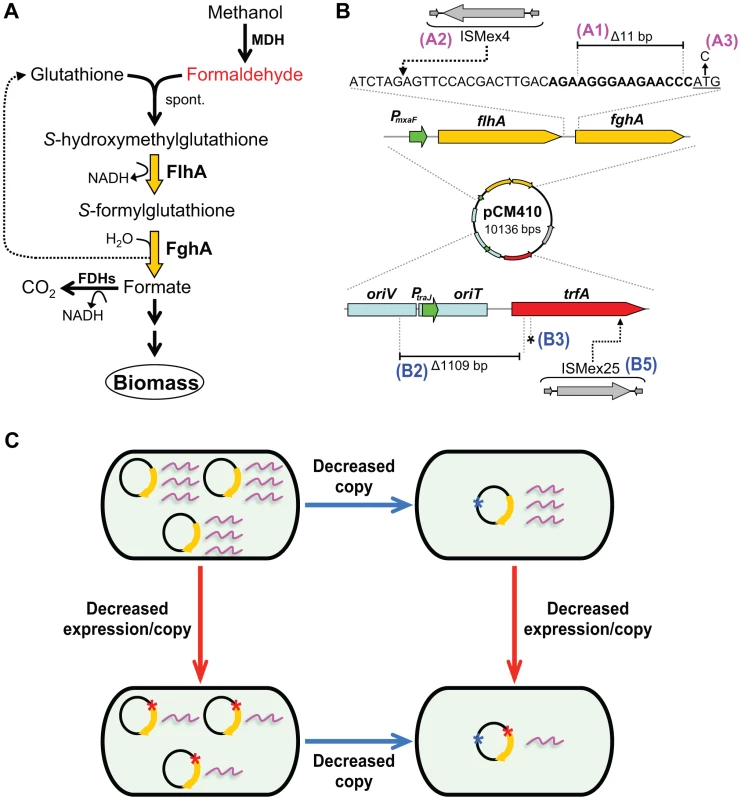
A) The GSH-linked formaldehyde oxidation pathway in the EM strain. The enzymes of the GSH-linked pathway are indicated with yellow arrows. MDH, methanol dehydrogenase; spont., spontaneous reaction of formaldehyde and GSH; FlhA, S-hydroxymethyl GSH dehydrogenase; FghA, S-formyl-GSH hydrolase; FDHs, formate dehydrogenases. B) Adaptive mutations identified on the introduced plasmid (pCM410) expressing the introduced formaldehyde pathway. Mutations occurring in the predicted ribosome-binding site (bold text) of fghA, or its upstream region are shown in magenta (Class A). Mutations occurring in regions that control plasmid replication are shown in blue (Class B). The fghA start codon is underlined. trfA, a gene encoding the TrfA protein essential to plasmid replication; PmxaF, promoter of the flhA-fghA gene cassette; oriV, origin of replication recognized by the TrfA protein; oriT, origin of transfer. ISMex4 and ISMex25, two insertion sequences native to M. extorquens AM1. C) Diagram of orthogonal mechanisms of Class A and B mutations on gene expression. Adaptation of the unfit EM strain to grow on methanol consistently involved beneficial mutations that altered expression of the foreign GSH pathway (Figure 1B). When the GSH pathway was introduced, the two enzymes were cloned together on a single mRNA transcript behind a strong native promoter present on a medium copy plasmid (∼9 cell−1) [10], [29], [34], [35]. As such, the costs of expression outweighed the catalytic benefits, and among the targets of adaptation we identified by resequencing strains evolved in separate populations, we universally obtained beneficial mutations that decreased expression of these enzymes [10], [34]. These mutations reduced expression of the GSH pathway through three classes of underlying mechanisms: Class A decreased expression per gene copy, Class B reduced gene dosage by lowering plasmid copy number, and Class C integrated the introduced pathway into the host genome, which also reduced plasmid copy number [33], [34] (Figure 1B). In terms of epistasis, mutations in multiple genes along a single adaptive trajectory – including one mutation (here ‘A1’) reducing expression of the GSH pathway – have been shown to exhibit a general trend of diminishing returns that was devoid of sign epistasis [10]. However, here we are interested in uncovering the trends and mechanisms underlying epistatic interactions between mutations that arose in separate adapting lineages and affect expression of the same metabolic pathway.
We combined independently-arising beneficial mutations affecting gene expression of this two-enzyme metabolic pathway and report strong antagonism and sign epistasis for fitness. These interactions were increasingly antagonistic for larger benefit mutations. Such strong antagonism did not stem from the effects of mutational combinations upon enzyme levels, but rather from the nonlinear mapping between enzyme expression and organismal fitness. By developing a quantitative model that relates expression cost and catalytic benefit to fitness, we characterized the overall shape of this fitness landscape and revealed that some of these single mutations can optimize multiple phenotypes simultaneously, leading to a big jump toward the single, global optimum.
Results
Interactions between mutations affecting expression of a two-enzyme metabolic pathway exhibit strong antagonism and sign epistasis
To explore the pattern of epistatic interactions between beneficial mutations affecting expression of the GSH-dependent pathway, we combined beneficial plasmid mutations that emerged during experimental evolution and affected distinct traits [34]. We focused upon Class A (decreased expression per copy) and B (reduced gene dosage) mutations because of their genetic tractability, and the prediction that these represent orthogonal mechanisms to achieve lower expression. We hypothesized that mutational combinations between these classes would result in enzyme levels that would be the product of the individual perturbations (Figure 1C). Three class A mutations, A1–A3, and one class B mutation, B5, occurred independently, whereas B2 and B3 were isolated together from the same plasmid. We generated 12 plasmids that paired each Class A mutation with each one from Class B, as well as with the B2–B3 pair, and measured their relative fitness via competitions with a fluorescently labeled ancestor [34] (Tables S1, S2). The observed fitness values for the mutational combinations were substantially less than expected based upon a simple multiplicative null model incorporating the single mutant effects (i.e., Wij = Wi×Wj; R2 = 0.53, adj-R2 = 0.32; Figure 2A).
Fig. 2. Epistasis at the level of expression phenotypes and fitness relative to independence or kinetic model. 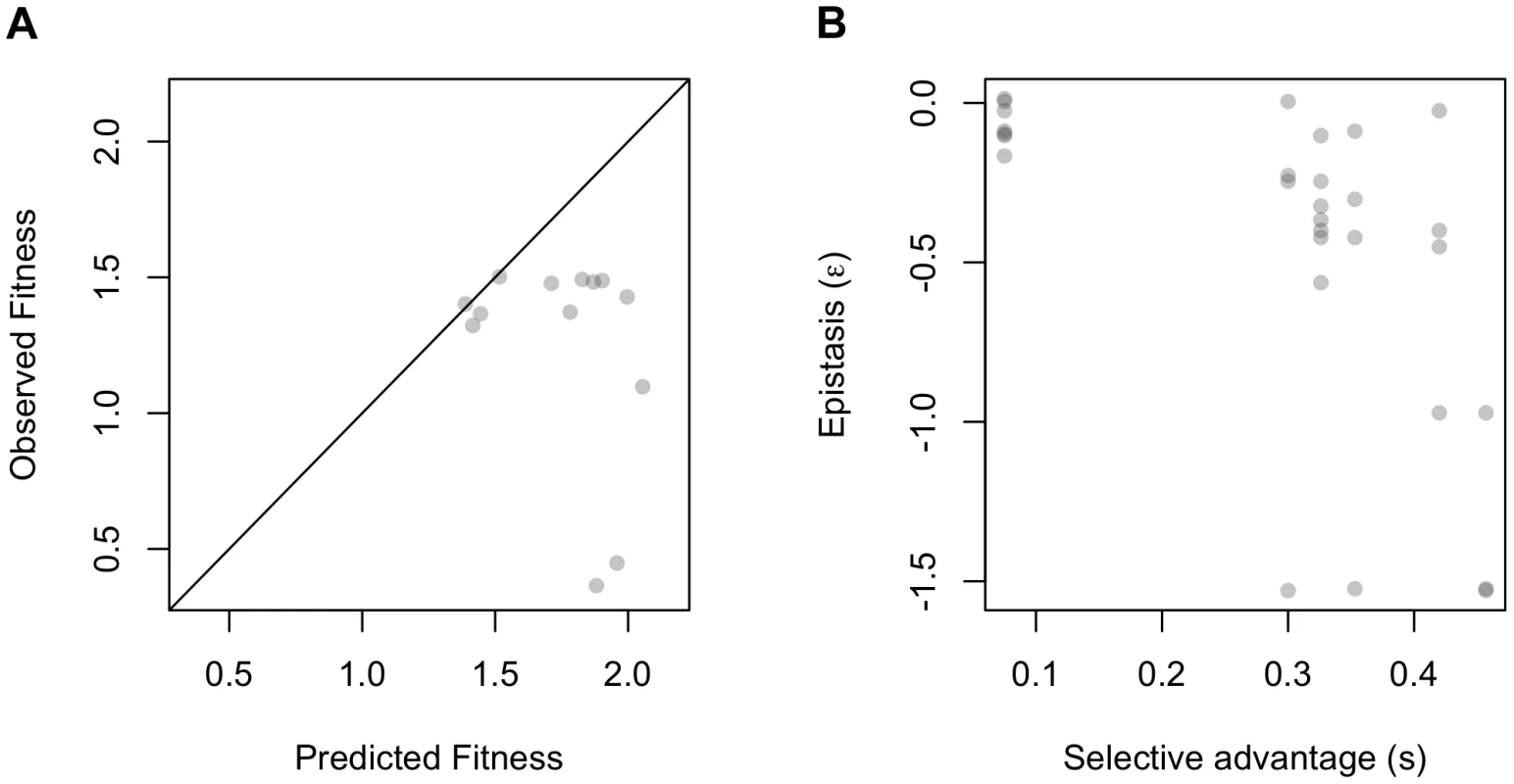
A) Fitness values of mutation combinations are consistently lower than expected by multiplicative independence. B) Mutations with larger with selective advantages (s) when tested alone in the ancestor have more negative ε values in combination with other mutations. The increasingly strong antagonism for higher expected fitness values suggested a potential negative relationship between the selective coefficients observed for each mutation and the average epistasis that mutation exhibited with other mutations. We observed that the individually most beneficial mutations (large s) engendered the greatest antagonism (ε<0) when combined with other mutations, including several examples of sign epistasis (Figure 2B).
The observed relationship between selective coefficient and average epistasis is observed for other biological systems
Several theoretical arguments suggest that the geometry of fitness landscapes might induce correlations between the size of a mutation and the strength and direction of epistasis. Epistasis has been observed to be coupled to the mean fitness effect of mutations [36], [37]. A single beneficial mutation of large effect may appreciably change both the mean fitness of subsequent mutations and the remaining distance to the optimum, potentially skewing its own epistatic coefficients.
Given the emerging empirical consensus and theoretical arguments for antagonistic epistatic interactions among beneficial mutations, we analyzed several other datasets to ask whether the strength and form of the relationship between selective effect and average epistatic effect held for intragenic and intergenic datasets. For this comparison we analyzed the relationship between s and ε for previous datasets from M. extorquens and E. coli where the beneficial mutations occurred consecutively in a variety of genes across the genome of a single adapting lineage [10], [11], combinations of mutations from two genes of the bacteriophage ID11 [12], and two datasets of within-protein interactions for β-lactamase [17], [38]. These datasets also displayed signs of a correlation between s and increasingly negative ε (as noted in [37]), with the exception of the intragenic data for β-lactamase (Figure S1).
Negative trends in the relationship between initial selective coefficient and epistasis may seem like obvious evidence for diminishing returns. However, recent theoretical work has shown that in models where mutations have random effects and no tendency to be either synergistic or antagonistic, a pattern of diminishing returns occurs between mutations if they are selected conditioned on being beneficial in the ancestral background [39]. In Supplementary Text S1 we show this behavior in a simple model of evolution on fitness landscapes with no mean epistatic tendency and show how it leads to a pattern of diminishing returns between beneficial mutations as a form of regression to the mean. This analysis suggests that genotype-fitness data alone, without knowledge of the phenotypic effects of mutations or the physiological causes for trade-offs, might be insufficient to infer the mechanism underlying a pattern of epistasis.
Pairs of orthogonal perturbations to gene expression act independently upon enzyme levels
What physiological factors underlie the strong antagonism observed between mutations affecting expression of the foreign GSH pathway? A first possibility is that mutational combinations lead to smaller changes in protein expression than expected from the single mutants and that such antagonistic behavior at the level of expression phenotypes merely propagated through as observed antagonism at the level of fitness. Because we used combinations that largely derived from pairing mutations that reduced expression per copy (Class A) with those that decreased plasmid copy number (Class B), our null hypothesis was that these mechanisms should act independently to alter expression, such that the expression level of an A+B mutant pair would simply be the product of these two values. Consistent with this prediction, enzyme levels were well described by the null model of multiplicative independence between paired perturbations (Figure 3A, Table S1). A simple linear model of log-transformed changes in enzyme levels as a function of the presence of the single mutations with no interaction terms explains much of the variation for both FlhA and FghA (adjusted-R2 = 0.85 (FlhA) and 0.86 (FghA)). This predictability can be seen in the high correlation between observed expression phenotypes for paired perturbations and those expected based upon the single changes.
Fig. 3. Mutations exert independent effects upon enzyme expression but a mechanistic model of benefits and costs of enzymes is required to predict combined effects on fitness. 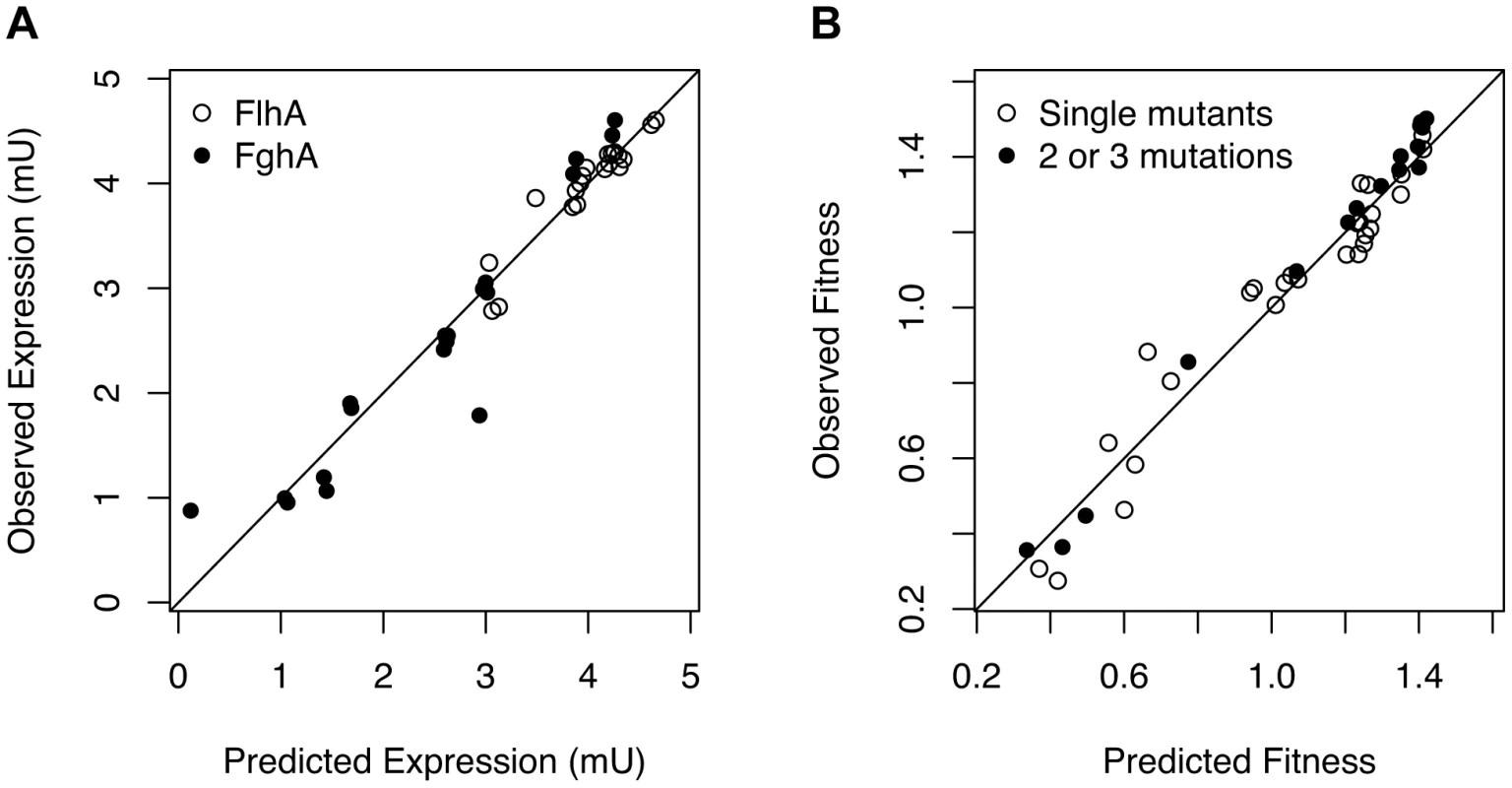
A) Expression levels (in mU) are well predicted by independent, multiplicative effects of each mutation on the FlhA and FghA enzymes. B) Using a kinetic model of catalysis and costs parameterized with data from the single mutants and inducible promoter constructs predicts the mutational combinations very well (R2 = 0.98). A fitness landscape model that incorporates benefits and costs predicts the fitness of mutational combinations
Since mutational combinations did not introduce epistatic interactions at the level of gene expression, we built a model of the fitness landscape based upon enzyme levels in order to ask how its shape would contribute to antagonism. Building upon earlier work on single enzymes [26] (Supplementary Text S2), we generated a model of the fitness landscape that calculates fitness as flux through the pathway above a threshold, minus the sum of two costs :
The hyperbolic expression for catalysis has been used before [40] to effectively describe the dependence of steady-state flux to the levels of a single enzyme and incorporates a “Vmax” term for the pathway, and an Eh half-maximal enzyme level term. We only model FlhA concentration as beneficial to fitness even though FghA is absolutely required for growth on methanol [34]. This is because, over the parameter range of our perturbations, fitness appeared to rise monotonically with decreasing levels of FghA. This suggests FghA is a typical enzyme that has a low metabolic “control coefficient” [20], [21] and that it only limits catalysis at exceptionally low levels. None of our perturbations pushed FghA levels below 2%, and for comparison β-galactosidase levels in lactose-limited chemostats only impacted fitness significantly if they decreased activity to ≤1% [41].The threshold flux term was added to the model to capture an unusual right-shift of the typical relationship between enzyme concentration and fitness observed with FlhA in these data, such that fitness approached zero even in the presence of measurable concentrations of functioning enzyme. We have observed similar behavior when manipulating levels of the analogous enzyme in the endogenous, tetrahydromethanopterin-dependent pathway for formaldehyde oxidation in WT (SM Carroll, CJM, unpublished). As both of these enzymes occur directly downstream of formaldehyde production, this threshold phenomenon may be explained by toxic effects of elevated steady-state formaldehyde concentrations at low enzyme levels. Finally, there are two cost terms for FlhA and FghA. The cost per molecule for each enzyme was treated as a linear function, consistent with prior work [26], [42].
The six parameters of this benefit - costs model were fit using the data from the EM ancestor, single mutants, as well as strains with inducible promoter plasmids (27 data points; Table S3). The inducible promoter plasmids contained a cumate-responsive repressor to modulate the levels of flhA-fghA from ancestral levels to lower values (Table S1). These data were critical for capturing the steep decline of the fitness landscape at low values of FlhA. The resulting benefit - costs model captured the curvature of the fitness landscape (Figure 4) and, unlike the simple multiplicative model, it was able to predict the 17 combinations of mutations that were not used for model fitting with high precision (R2 = 0.98) (Figure 3B). From the perspective of the ancestral genotype, in the model fitness rises gently with decreased expression of either enzyme, but then declines rapidly upon reaching catalytically-limiting levels of FlhA. A similar cliff exists for low values of FghA [34], but at enzyme levels beyond the range of our dataset and below the detection threshold of our enzyme assay method (see Methods).
Fig. 4. Fitness landscape of the GSH-linked pathway. 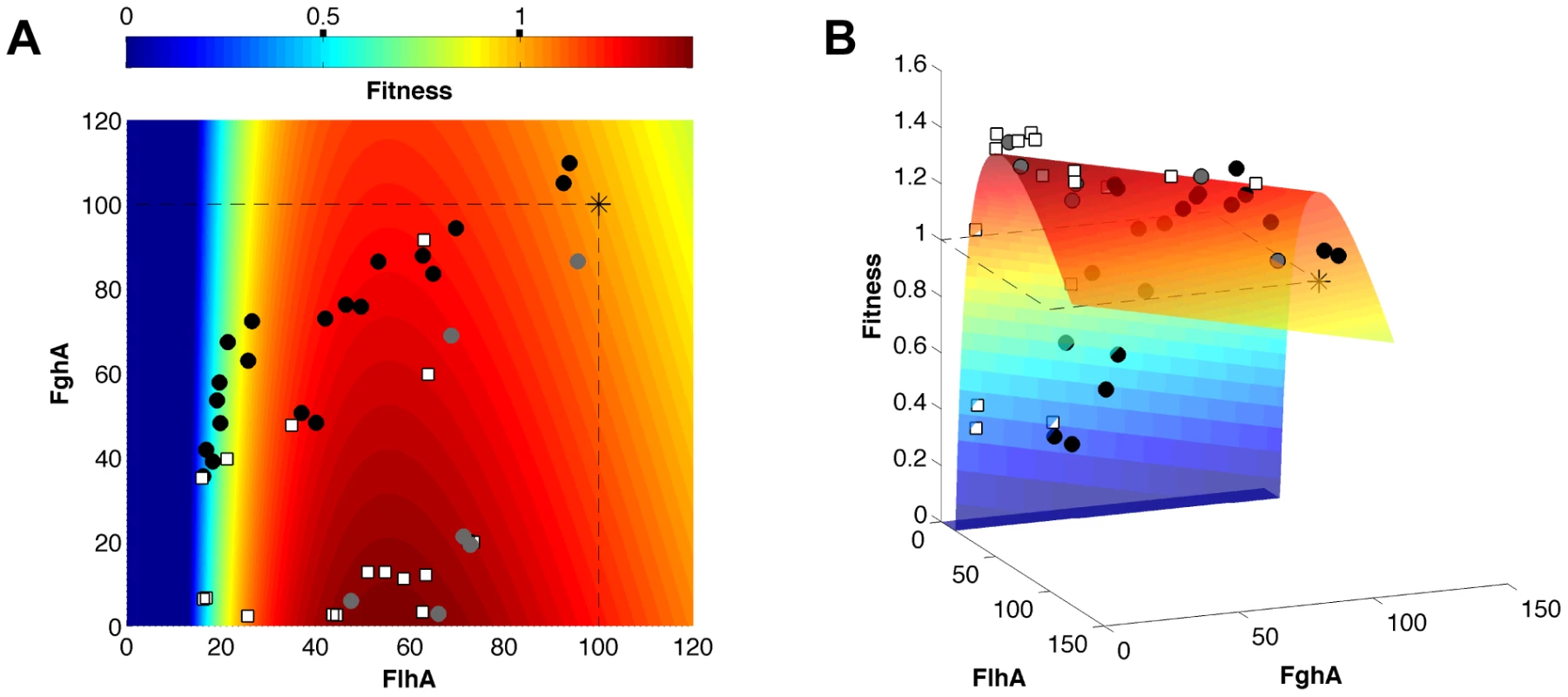
A) A two-dimensional heatmap and B) three-dimensional surface showing the shape of the fitness landscape predicted by the model. The x and y axes are FlhA and FghA levels relative to ancestor (set at 100). Though the plasmid cost is included in the model, this fourth dimension was corrected for to allow a three-dimensional visualization (see Methods). Experimental data points indicate the ancestor (asterisk), single mutants (grey circles), mutational combinations (white squares), and inducible expression vectors (black circles). Beneficial mutations moved directly toward the global expression optimum rather than in the locally steepest direction on the fitness landscape
Our fitness landscape model that precisely maps phenotypes to fitness allowed us to explore how much local topography may have influenced the direction of phenotypic change during evolution by de novo mutations (Figure 5). We compared the changes in enzyme expression caused by single beneficial mutations relative to three factors: 1) the local gradient in the fitness landscape for the ancestor (greater decreases of FlhA versus FghA because the former is more costly), 2) the direct vector pointing to the global fitness optimum and 3) equal proportional changes between the enzymes which might be expected due to the physical constraint of their being expression from a single transcript. All mutations moved toward the global optimum rather than ascend in the phenotypically steepest direction on the local fitness landscape. Mutations B2 and B5 affected copy number but, through mechanisms we do not currently understand, led to greater decreases in FghA than FlhA. In contrast, B3 was directly along the line of equivalent change in both enzymes. This mutation was identified along with B2 as a plasmid haplotype, and this B2–B3 combination allowed this lineage to accomplish a similar phenotypic (and fitness) change as the other mutants.
Fig. 5. Adaptive mutations tended to move phenotypes toward the global optimum. 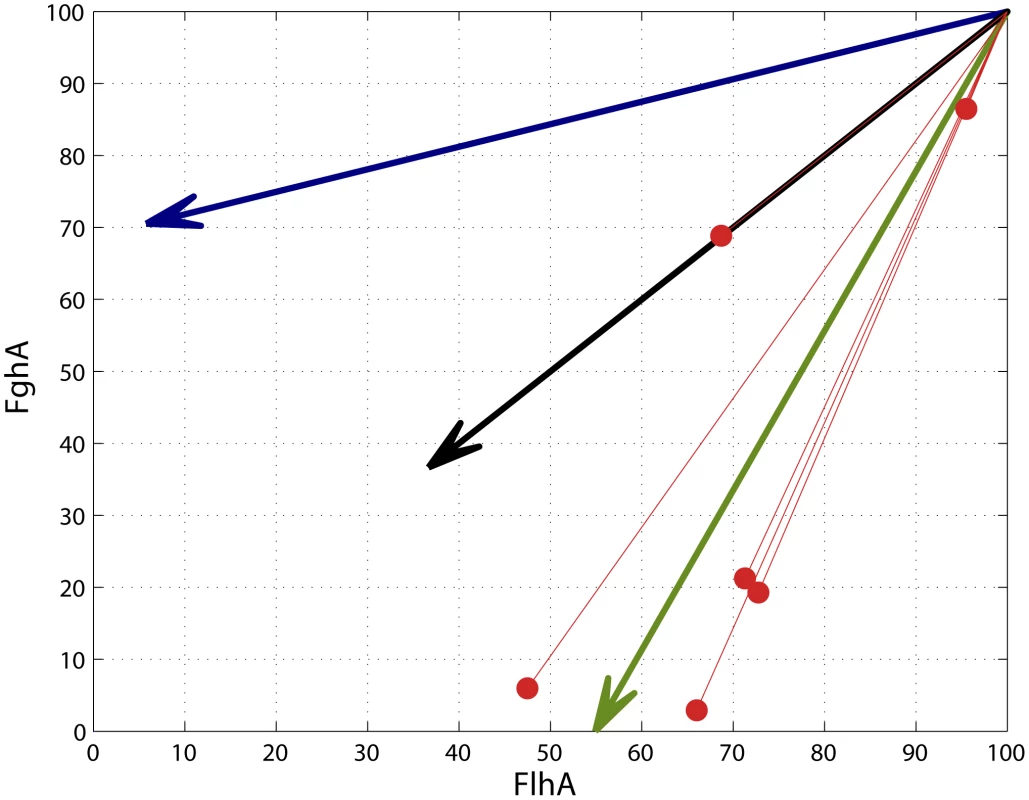
The direction of phenotypic movements for strains with single mutations (red lines; enzyme levels of ancestor set to 100) are compared to the vectors indicating of the locally steepest fitness gradient for the ancestor (blue), complete 1∶1 correlation between phenotypes (black), and the global optimum (green). The direction of phenotypic movement for all single mutations was closer to the vector for the global optimum relative to the local gradient around the ancestor. Discussion
We found that combining adaptive mutations that optimize expression of a two-enzyme pathway exhibited strong antagonistic interactions and sign epistasis. Fitness values of mutational combinations were generally less than expected relative to a null model of independent, multiplicative effects upon fitness. We further observed a negative relationship between s and ε for individual mutations. Other datasets of intragenic epistasis revealed similar trends between s and ε; however, this overall trend of epistasis (e.g., antagonism) does not imply a specific connection with properties of the individual mutations, such as s. For example, this trend will also arise as a consequence of regression to the mean when the beneficial mutations assayed are conditioned to be beneficial in the ancestral background and the effect of a mutation has a component that is independently distributed on each possible genetic background. Therefore, to extract biological insight from the quantitative relationship between s and ε , we must interrogate the mechanisms that lead to antagonistic epistasis.
The first possible explanation for antagonism in our data would be non-linearities in the way mutations combined to affect enzyme expression. However, as expected from having chosen combinations that combined class A mutations with those from class B, these orthogonal mechanistic effects resulted in independent effects on enzyme expression that were jointly well predicted with a simple, multiplicative model. As in this system, many ecologically relevant genes are encoded on plasmids whose regulation and gene dosage may both be effected by separate sets of mutations. More broadly, mutations that influence different traits that make joint contribution to a higher phenotype such as fitness are common. At the level of individual genes, for example, catalytic improvement of an enzyme often results from the joint contribution of mutations that improve protein stability and those that enhance kinetic parameters [43]–[45].
The second factor that could generate antagonism is the curvature of the underlying fitness landscape for gene expression. Recent theory has shown that almost any formulation of fitness based upon multiple underlying phenotypes will generate epistasis at the level of fitness, even when the mutations – as we observed here – do not interact epistatically on the underlying trait phenotypes [46]. Previous models have formulated fitness as a function of gene expression correctly predicted the evolution of optimal levels of gene expression [26]. Here we extended this model framework to multiple enzymes and used it for the first time to interpret beneficial mutational effects from phenotype to fitness, which we characterized both individually and in combination. Our fitness landscape model was able to predict the fitness values of the mutation combinations with high precision (R2 = 0.98). The asymmetry in the curvature of this fitness landscape results from the relatively gentle effects of expression costs relative to the sharp transition in fitness effects due to rate limitation upon catalysis [41]. This observed selection to maintain an intermediate optimum of enzyme levels is distinct from the selective neutrality on a catalytic plateau that was predicted by classical MCA analyses that did not incorporate expression costs [40].
Knowledge of the underlying fitness landscape allows us to understand aspects of the epistatic interactions not evident from fitness values alone. For example, we observed that the A3 and B5 mutations had fairly comparable individual fitness values (1.420±0.032 vs. 1.457±0.033; mean and 95% CI), but the former had three-fold more antagonistic epistasis than the latter (average = −0.46 vs. −1.34; t-test, p = 0.025; Figure 6). The modeled fitness landscape illuminates the underlying reason for this difference. Both mutations rest near the peak value of enzyme expression, but on opposite sides (B5 has 70% the level of FghA as A3). This poises B5 such that it is much more sensitive to further reductions in expression than A3. Thus, although these two mutations are essentially equivalent if one only considers their fitness values, their locations in phenotypic space change their likelihood for antagonism and sign epistasis.
Fig. 6. Phenotype and not just fitness value determines epistatic interactions. 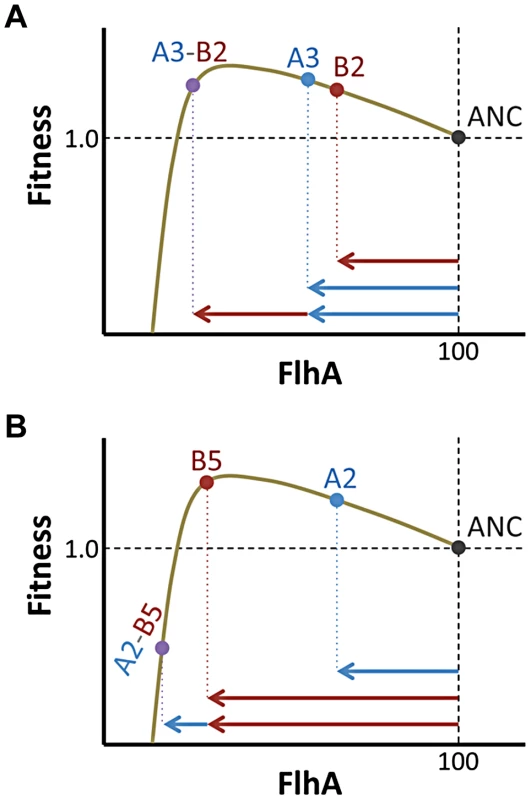
Mutations (A) A3 and (B) B5 had similar fitness values, but resided on opposite flanks of the optimal phenotype (shown here for simplicity as FlhA enzyme levels). In interactions with secondary beneficial mutations (B2 and A2, respectively), B5 fares worse due to being closer to the fitness cliff that occurs when FlhA catalysis becomes limiting. One consequence of sign epistasis between mutations affecting the phenotypes like gene expression is a reduction of the benefit caused by recombination bringing beneficial mutations together into the same genome (i.e., Fisher-Muller model). This tradeoff between benefits and costs is inherent to gene expression, and thus results in stabilizing selection. These patterns of epistasis are likely very common, given the apparent ubiquity of stabilizing selection upon gene expression from microbes to primates [47]–[50].
With a complete fitness landscape defined by biochemical phenotypes, we can now interpret the genetic landscape in terms of what was accessible to individual mutations. In contrast to how selection acts upon standing genetic variation, the de novo mutations fixed in experimental populations ignored the phenotypically-local best direction of change and “jumped” towards the global optimum. This highlights that the classic quantitative genetics intuition of climbing in the direction of the steepest selection gradient [51], which is appropriate for small populations containing standing genetic variation, fails to capture the phenotypic potential of a sizeable pool of de novo mutations arising from large populations. In our case, it was not the large magnitude of expression change that was surprising, per se, but change in the ratio of their expression. Given that flhA and fghA are encoded on the same transcript, it was notable that all but one of the single mutations down-regulated FghA to a large extent while only cutting FlhA levels in half. Whereas this system allowed individual mutations to reach near-optimality, a multi-step trajectory was required for the directed evolution of the LacI repressor to reverse its regulatory logic [52]. In that system, the first round mutation simply broke the old logic to become constitutive, which in combination with two latter mutations allowed the “anti-LacI” phenotype to emerge and locate the fitness peak they predicted from a computational model. Our results suggest that relatively large moves in multi-phenotype space can emerge as winners, provided the genetic architecture at least allows rare mutations to achieve this possibility.
Finally, the near optimal expression levels of these beneficial mutations becomes even more remarkable when considering that this optimization did not happen in isolation, but in adapting populations that contained many beneficial mutations simultaneously [33], [34], [53]. In varying environments, such diversity in large microbial populations may lead to genetically complex adaptation such as stable polymorphisms [54]. In a stable environment, this diversity leads to ‘clonal interference’ [55], a type of serial fixation that effectively sorts for mutations of the greatest effect amongst what was possible. This would have impacted the mutations that affected GSH pathway expression in two ways. Firstly, there were many different genetic solutions to reducing expression of these enzymes [34]. One type in particular - the Class C mutations that resulted from integration of the introduced plasmid into the host chromosome – occur at very high rates and emerged to detectable levels repeatedly, up to 17 times per population [33]. These mutations confer ∼⅔ the benefit of the Class A and B mutations [34], however, and were only found to rise to fixation in three of eight populations despite more than 100 observed occurrences [33]. In this regard, clonal interference aids finding optimal solutions by allowing only the best individual mutations to fix. Recently, however, it has been shown that fixation probability of contending mutations is only partly dependent upon their individual effect because they commonly hitchhike with other beneficial mutations present [56]–[58]. This leads to a second effect of clonal interference, which is competition between lineages carrying beneficial mutations affecting distinct phenotypic processes. Indeed, the ancestral genotype faced a variety of phenotypic challenges besides just optimizing expression of the GSH pathway [14], [59], [60]. Some of these mutations in other loci had beneficial effects up to 3× larger than those described here [10] and were segregating at the same time as mutations affecting expression of the GSH pathway [33], [53]. Even with so much turmoil in the populations, the eventual winners discovered nearly optimal solutions to this local, two-enzyme expression optimization in order to win the battle for fixation. Population size thus contributed to the fixation of optimal solutions by both increasing the number of mutations occurring and escaping drift in the first place, and by facilitating competition between multiple potential solutions. These factors conspired to allow selection to reward – when mutationally possible – lineages that made long-range, lucky jumps to distant peaks on the phenotypic landscape.
Materials and Methods
Experimental evolution and growth conditions
The EM strain was generated previously by deleting the mptG gene of M. extorquens AM1 in the white strain WT CM502 [61] lacking carotenoid pigments due to an unmarked mutation in crtI (encoding phytoene desaturase) [62], followed by introduction of pCM410 [10]. Eight replicate populations seeded by the EM strain were grown in 9.6 ml methanol (15 mM) minimal media incubated in a 30°C shaking incubator at 225 rpm. Populations were transferred to fresh media at a 1/64 dilution rate (thus six generations per growth cycle, Nfinal≈109) and propagated for 600 generations. One liter of minimal media consists of 100 ml of phosphate buffer (25.3 g of K2HPO4 and 22.5 g of NaH2PO4 in 1 liter of deionized water), 100 ml of sulfate solution (5 g of (NH4)2SO4 and 0.98 g of MgSO4 in 1 liter of deionized water), 799 ml of deionized water, and 1 ml of trace metal solution. One liter of the trace metal solution consists of 100 ml of 179.5 mM FeSO4, 800 ml of premixed metal mix (12.738 g of EDTA disodium salt dihydrate, 4.4 g of ZnSO4·7H2O, 1.466 g of CaCl2·2H2O, 1.012 g of MnCl2·4H2O, 0.22 g of (NH4)6Mo7O24·4H2O, 0.314 g of CuSO4·5H2O, and 0.322 g of CoCl2·6H2O in 1 liter of deionized water, pH 5), and 100 ml of deionized water [14].
Plasmid and strain construction
All strains and plasmids used are indicated in Table S2. All plasmids constructed in this study were maintained in E. coli 10-beta strain (New England Biolabs) and were transferred to M. extorquens via electroporation [63] or tri-parental mating with the helper strain pRK2073 [64]. Plasmid DNA in E. coli was extracted using the QIAprep Spin MiniPrep Kit (Qiagen). The PmxaF expression vector pCM160 [65], its variant pCM410 in the EM strain expressing the flhA-fghA cassette [10], and the cumate-inducible vector pHC112 expressing the flhA-fghA cassette [34] have been described previously. In order to combine Class A and B mutations which accumulated on separate pCM410 derivatives during experimental evolution of the EM strain (Figure 1B, Table S1), Class B mutations (from pCM410B2B3, pCM410B2, pCM410B3, and pCM410B5) were moved to pCM410 derivatives bearing Class A mutations (pCM410A1, pCM410A2, and pCM410A3) through the procedures delineated below. Fragments containing B2–B3, B2, and B3 mutations were first obtained by digesting pCM410B2B3, pCM410B2, or pCM410B3 with SfiI and NheI. These were then ligated into the plasmid backbone of pCM410A1, pCM410A2, or pCM410A3 cut with the same enzymes. A fragment containing the B5 mutation was obtained by digesting of pCM410B5 with SfiI and SexAI and then ligated into the plasmid backbone pCM410A1, pCM410A2, or pCM410A3 cut by the same enzymes. The above procedures were also applied to introduce B2–B3, B2, B3, and B5 mutations into pHC112 in order to generate pHC112 derivatives that vary in their plasmid copy number.
Fitness assays
Fitness assays were performed by a previously described procedure [34]. Strains were first physiologically acclimated through one 4-day growth cycle in 9.6 ml of minimal media supplemented with 15 mM methanol. In addition, for strains bearing cumate-inducible promoter plasmids (pHC112 derivatives), different concentrations of cumate (Table S1) were added to growth media to modulate the expression of FlhA and FghA enzymes. After this acclimation phase, each of these strains was mixed with a fluorescent variant (CM1232) of the EM ancestor [10] by a 1∶1 volume ratio, diluted 1/64 into 9.6 ml of fresh growth media, and incubated in a 30°C shaking incubator at 225 rpm. The ratios of the two populations before (R0) and after (R1) competitive growth were quantified by a LSR II flow cytometer (BD Biosciences) for at least 50000 cell counts per sample. The forward scatter threshold of LSRII was adjusted to 300 to ensure unbiased detection of the test and reference strains despite their potential differences in cell size. Fitness values (W) relative to the reference strain were calculated by a previously described equation assuming an average of 64-fold size expansion of mixed populations during competitive growth [35]:
In order to convert to absolute differences in growth rate, the EM ancestor grows under these conditions with a growth rate of 0.0654+/−0.0016 h−1 [10].Enzyme assays
The activities of FlhA [66] and FghA [67] were assayed in three replicates as described using cells harvested from mid-exponential phase cultures. Cells were collected through centrifugation at 10,000× g for 10 min, frozen at −80°C, and used for enzyme assays within a week. Right before assays frozen cell pellets were suspended in 50 mM Tris-HCl buffer (pH 7.5) and physically disrupted in tubes containing Lysing Matrix B and shaken at speed 6.0 m/s on a FastPrep®-24 bead beater (MP Biomedicals) for 40 seconds. Insoluble debris in the cell lysate was removed by centrifugation at 13,000× g, 4°C for 15 min. The total protein concentration of the cell lysate was quantified using the Bradford method [68]. Kinetic analysis of FlhA and FghA activities over 10 min at 30°C was performed in 200 µl reaction mixtures using a SpectraMax M5 Plate Reader (Molecular Devices).
Quantification of plasmid copy numbers
The copy number of pCM410 derivatives in M. extorquens was quantified by a real-time PCR approach described previously [34]. Briefly genomic DNA of M. extorquens from mid-exponential phase cultures was extracted by an alkaline lysis method [69]. Detection of plasmid DNA was targeted at the kan gene using primers HC410p18 (5′-GAAAACTCACCGAGGCAGTTCCATAG-3′) and HC410p19 (5′-TCAGTCGTCACTCATGGTGATTTCTCA-3′). Detection of chromosomal DNA was targeted at the rpsB gene (encoding the 30S ribosomal protein S2) in the chromosome META1 using primers HCAM111 (5′-TGACCAACTGGAAGACCATCTCC-3′) and HCAM113 (5′-TTGGTGTCGATCACGAACAGCAG-3′). Real-time PCR experiments were performed in three replicates with the PerfeCTa SYBR Green SuperMix (Quanta Biosciences) on a DNA Engine Opticon2 (MJ Research), and the average threshold cycle (Ct) of each PCR reaction was determined using the Opticon Monitor v. 2.02 software (MJ Research). Each real-time PCR reaction contained 25 ng of genomic DNA extracted from various strains and kan - or rpsB-specific primers. To establish a standard curve (SC) of plasmid copy numbers, 1, 0.1, 0.01, and 0.001 ng of pCM410 (equivalent to 9.09×107, 9.09×106, 9.09×105, and 9.09×104 plasmid molecules, respectively) were mixed with 25 ng of genomic DNA (equivalent to 3.03×106 genome copies) of the plasmid-less, white WT M. extorquens (CM502) [61]. The standard curve is a plot of ΔCt (i.e. Ctkan–CtrpsB) versus plasmid molecules on a log2 scale. For each strain, by interpolating its ΔCt value against the SC the absolute quantity of plasmid DNA can be estimated using the following equation:
Model fitting and comparison
Multiplicative models predicting fitness or gene expression were fit and assessed as standard linear models following a log transformation of the response variable. The model for the fitness landscape was fit using a non-linear routine in Matlab. The raw data as well as commented code in Matlab and R that completely recreates the analysis and figures has been deposited at www.datadryad.org (doi:10.5061/dryad.8hb23).
Supporting Information
Zdroje
1. WrightS (1932) The roles of mutation, inbreeding, crossbreeding, and selection in evolution. Proc VIth Int Congress of Genetics 1 : 356–366.
2. PhillipsPC (2008) Epistasis – the essential role of gene interactions in the structure and evolution of genetic systems. Nat Rev Genet 9 : 855–867.
3. FrankeJ, KlözerA, de VisserJAGM, KrugJ (2011) Evolutionary accessibility of mutational pathways. PLoS Comput Biol 7: e1002134.
4. TenaillonO, Rodríguez-VerdugoA, GautRL, McDonaldP, BennettAF, et al. (2012) The molecular diversity of adaptive convergence. Science 335 : 457–461 doi:10.1126/science.1212986
5. KryazhimskiyS, TkacikG, PlotkinJB (2009) The dynamics of adaptation on correlated fitness landscapes. Proc Natl Acad Sci USA 106 : 18638–18643 doi:10.1073/pnas.0905497106
6. DraghiJA, ParsonsTL, PlotkinJB (2011) Epistasis increases the rate of conditionally neutral substitution in an adapting population. Genetics 187 : 1139–1152 doi:10.1534/genetics.110.125997
7. SzendroIG, FrankeJ, de VisserJAGM, KrugJ (2013) Predictability of evolution depends nonmonotonically on population size. Proc Natl Acad Sci USA 110 : 571–576 doi:10.1073/pnas.1213613110
8. deVisserJAGM, ParkSC, KrugJ (2009) Exploring the effect of sex on empirical fitness landscapes. Am Nat 174: S15–30 doi:10.1086/599081
9. SanjuánR, MoyaA, ElenaSF (2004) The contribution of epistasis to the architecture of fitness in an RNA virus. Proc Natl Acad Sci USA 101 : 15376–15379.
10. ChouH-H, ChiuH-C, DelaneyNF, SegrèD, MarxCJ (2011) Diminishing returns epistasis among beneficial mutations decelerates adaptation. Science 332 : 1190–1192 doi:10.1126/science.1203799
11. KhanAI, DinhDM, SchneiderD, LenskiRE, CooperTF (2011) Negative epistasis between beneficial mutations in an evolving bacterial population. Science 332 : 1193–1196.
12. RokytaDR, JoyceP, CaudleSB, MillerC, BeiselCJ, et al. (2011) Epistasis between beneficial mutations and the phenotype-to-fitness map for a ssDNA virus. PLoS Genet 7: e1002075 doi:10.1371/journal.pgen.1002075
13. MaharjanRP, FerenciT (2013) Epistatic interactions determine the mutational pathways and coexistence of lineages in clonal Escherichia coli populations. Evolution 67 : 2762–2768 doi:10.1111/evo.12137
14. ChouH-H, BerthetJ, MarxCJ (2009) Fast growth increases the selective advantage of a mutation arising recurrently during evolution under metal limitation. PLoS Genet 5: e1000652 doi:10.1371/journal.pgen.1000652
15. KvitekDJ, SherlockG (2011) Reciprocal sign epistasis between frequently experimentally evolved adaptive mutations causes a rugged fitness landscape. PLoS Genet 7: e1002056 doi:10.1371/journal.pgen.1002056
16. WeinreichDM, WatsonRA, ChaoL (2005) Perspective: Sign epistasis and genetic constraint on evolutionary trajectories. Evolution 59 : 1165–1174.
17. SchenkMF, SzendroIG, SalverdaML, KrugJ, de VisserJAGM (2013) Patterns of epistasis between beneficial mutations in an antibiotic resistance gene. Mol Biol Evol 30 : 1779–1787 doi:10.1093/molbev/mst096
18. Fisher RA (1930) The genetical theory of natural selection. Clarendon Press, Oxford, UK.
19. MartinG, ElenaSF, LenormandT (2007) Distributions of epistasis in microbes fit predictions from a fitness landscape model. 39 : 555–560.
20. KascerH, BurnsJA (1973) The control of flux. Symp Soc Exp Biol 27 : 65–104.
21. HeinrichR, RapoportTA (1974) A linear steady-state treatment of enzymatic chains. General properties, control and effector strength 42 : 89–95.
22. WattWB, DeanAM (2000) Molecular-functional studies of adaptive genetic variation in prokaryotes and eukaryotes. Annu Rev Genet 34 : 593–622.
23. SzathmaryE (1993) Do deleterious mutations act synergistically? Metabolic control theory provides a partial answer. Genetics 133 : 127–132.
24. KeightleyPD (1996) Metabolic models of selection response. J Theor Biol 182 : 311–316.
25. StoebelDM, DeanAM, DykhuizenDE (2008) The cost of expression of Escherichia coli lac operon proteins in in the process, not in the products. Genetics 178 : 1653–1660 doi:10.1534/genetics.107.085399
26. DekelE, AlonU (2005) Optimality and evolutionary tuning of the expression level of a protein. Nature 436 : 588–592 doi:10.1038/nature03842
27. FlamholzA, NoorE, Bar-EvenA, LiebermeisterW, MiloR (2013) Glycolytic strategy as a tradeoff between energy yield and protein cost. Proc Natl Acad Sci USA 110 : 10039–10044 doi:10.1073/pnas.1215283110
28. ChistoserdovaL, VorholtJA, ThauerRK, LidstromME (1998) C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic Archaea. Science 281 : 99–102.
29. MarxCJ, ChistoserdovaL, LidstromME (2003) Formaldehyde-detoxifying role of the tetrahydromethanopterin-linked pathway in Methylobacterium extorquens AM1. J Bacteriol 185 : 7160–7168.
30. MarxCJ, LaukelM, VorholtJA, LidstromME (2003) Purification of the formate-tetrahydrofolate ligase from Methylobacterium extorquens AM1 and demonstration of its requirement for methylotrophic growth. J Bacteriol 185 : 7169–7175.
31. MarxCJ, Van DienSJ, LidstromME (2005) Flux analysis uncovers the key role of functional redundancy in formaldehyde metabolism. PLoS Biol 3: e16.
32. CrowtherGJ, KosályG, LidstromME (2008) Formate as the main branch point for methylotrophic metabolism in Methylobacterium extorquens AM1. J Bacteriol 190 : 5057–5062 doi:10.1128/JB.oo228-08
33. LeeM-C, MarxCJ (2013) Synchronous waves of failed soft sweeps in the laboratory: remarkably rampant clonal interference of alleles at a single locus. Genetics 193 : 943–952 doi:1534/genetics.112.148502
34. ChouH-H, MarxCJ (2012) Optimization of gene expression through divergent mutational paths. Cell Rep 1 : 133–140 doi:10.1016/j.celrep.2011.12.003
35. LeeM-C, ChouH-H, MarxCJ (2009) Asymmetric, bimodal trade-offs during adaptation of Methylobacterium to distinct growth substrates. Evolution 63 : 2816–2830 doi:10.1111/j.1558-5646.2009.00757.x
36. WilkeCO, AdamiC (2001) Interaction between directional epistasis and average mutational effects. Proc Biol Sci 268 : 1469–1474.
37. GrosPA, Le NagardH, TenaillonO (2009) The evolution of epistasis and its links with genetic robustness, complexity and drift in a phenotypic model of adaptation. Genetics 182 : 277–293 doi:10.1534/genetics.108.099127
38. WeinreichDM, DelaneyNF, DepristoMA, HartlDL (2006) Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312 : 111–114.
39. DraghiJA, PlotkinJ (2013) Selection biases the prevalence and type of epistasis among beneficial substitutions. Evolution 67 : 3120–3131 doi:10.1111/evo.12192
40. HartlDL, DykhuizenDE, DeanAM (1985) Limits of adaptation: the evolution of selective neutrality. Genetics 111 : 655–674.
41. DykhuizenDE, DeanAM, HartlDL (1987) Metabolic flux and fitness. Genetics 115 : 25–31.
42. DongH, NilssonL, KurlandCG (1995) Gratuitous overexpression of genes in Escherichia coli leads to growth inhibition and ribosome destruction. J Bacteriol 177 : 1497–1504.
43. LunzerM, MillerSP, FelsheimR, DeanAM (2005) The biochemical architecture of an ancient adaptive landscape. Science 310 : 499–501.
44. GiverL, GershensonA, FreskgardP-O, ArnoldFH (1998) Directed evolution of a thermostable esterase. Proc Natl Acad Sci USA 95 : 12809–12813 doi:10.1073/pnas.95.22.12809
45. DePristoMA, WeinreichDM, HartlDL (2005) Missense meanderings in sequence space: a biophysical view of protein evolution. Nat Rev Genet 6 : 678–687.
46. ChiuH-C, MarxCJ, SegrèD (2012) Epistasis from functional dependence of fitness on underlying traits. Proc Biol Sci 279 : 4156–4164 doi:10.1098/rspb.2012.1449
47. DenverDR, MorrisK, StreelmanJT, KimSK, LynchM, et al. (2005) The transcriptional consequences of mutation and natural selection in Caenorhabditis elegans. Nat Genet 37 : 544–548.
48. LemosB, MeiklejohnCD, CáceresM, HartlDL (2005) Rates of divergence in gene expression profiles of primates, mice, and flies: stabilizing selection and variability among functional categories. Evolution 59 : 126–137.
49. WagnerA (2005) Energy constraints on the evolution of gene expression. Mol Biol Evol 22 : 1365–1374.
50. BedfordT, HartlDL (2009) Optimization of gene expression by natural selection. Proc Natl Acad Sci USA 106 : 1133–1138 doi:10.1073/pnas.0812009106
51. LandeR, ArnoldSJ (1983) The measurement of selection on correlated characters. Evolution 37 : 1210–1226.
52. PoelwijkFJ, de VosMG, TansSJ (2011) Tradeoffs and optimality in the evolution of gene regulation. Cell 146 : 462–470 doi:10.1016/j.cell.2011.06.035
53. ChubizLM, LeeM-C, DelaneyNF, MarxCJ (2012) FREQ-Seq: a rapid, cost-effective, sequencing-based method to determine allele frequencies directly from mixed populations. PLoS One 7: e47959 doi:10.1371/journal.pone.0047959
54. YiX, DeanAM (2013) Bounded population sizes, fluctuating selection and the tempo and mode of coexistence. Proc Natl Acad Sci USA 110 : 16945–16950 doi:10.1073/pnas.1309830110
55. GerrishPJ, LenskiRE (1998) The fate of competing beneficial mutations in an asexual population. Genetica 102–103 : 127–144.
56. WoodsRJ, BarrickJE, CooperTF, ShresthaU, KauthMR, et al. (2011) Second-order selection for evolvability in a large Escherichia coli population. Science 331 : 1433–1436 doi:10.1126/science.1198914
57. LangGI, BotsteinD, DesaiMM (2011) Genetic variation and the fate of beneficial mutations in asexual populations. Genetics 188 : 647–661 doi:10.1534/genetics.111.128942
58. LangGI, RiceDP, HickmanMJ, SodergrenE, WeinstockGM, et al. (2013) Pervasive genetic hitchhiking and clonal interference in forty evolving yeast populations. Nature 500 (7464) 571–4 doi:10.1038/nature12344
59. LeeM-C, MarxCJ (2012) Repeated, selection-driven genome reduction of accessory genes in experimental populations. PLoS Genet 8: e1002651 doi:10.1371/journal.pgen.1002651
60. CarrollSM, MarxCJ (2013) Evolution after introduction of a novel metabolic pathway consistently leads to restoration of wild-type physiology. PLoS Genet 9: e1003427 doi:10.1371/journal.pgen.1003427
61. MarxCJ (2008) Development of a broad-host-range sacB-based vector for unmarked allelic exchange. BMC Res Notes 1 : 1 doi:10.1186/1756-0500-1-1
62. Van DienSJ, MarxCJ, O'BrienBN, LidstromME (2003) Genetic characterization of the carotenoid biosynthetic pathway in Methylobacterium extorquens AM1 and isolation of a colorless mutant. Appl Environ Microbiol 69 : 7563–7566.
63. MarxCJ, LidstromME (2004) Development of an insertional expression vector system for Methylobacterium extorquens AM1 and generation of null mutants lacking mtdA and/or fch. Microbiol 150 : 9–19.
64. ChistoserdovAY, ChistoserdovaLV, McIntireWS, LidstromME (1994) Genetic organization of the mau gene cluster in Methylobacterium extorquens AM1: complete nucleotide sequence and generation and characteristics of mau mutants. J Bacteriol 176 : 4052–4065.
65. MarxCJ, LidstromME (2001) Development of improved versatile broad-host-range vectors for use in methylotrophs and other Gram-negative bacteria. Microbiol 147 : 2065–2075.
66. RasJ, Van OphemPW, ReijndersWN, Van SpanningRJ, DuineJA, et al. (1995) Isolation, sequencing, and mutagenesis of the gene encoding NAD - and glutathione-dependent formaldehyde dehydrogenase (GD-FALDH) from Paracoccus denitrificans, in which GD-FALDH is essential for methylotrophic growth. J Bacteriol 177 : 247–251.
67. HarmsN, RasJ, ReijndersWN, van SpanningRJ, StouthamerAH (1996) S-formylglutathione hydrolase of Paracoccus denitrificans is homologous to human esterase D: a universal pathway for formaldehyde detoxification? J Bacteriol 178 : 6296–6299.
68. BradfordMM (1976) Rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 : 248–254 doi:10.1016/0003-2697(76)90527-3
69. LeeCL, OwDS, OhSK (2006) Quantitative real-time polymerase chain reaction for determination of plasmid copy number in bacteria. J Microbiol Methods 65 : 258–267.
Štítky
Genetika Reprodukční medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene FunctionČlánek Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 2- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání






