-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
Metabolic traits are molecular phenotypes that can drive clinical phenotypes and may predict disease progression. Here, we report results from a metabolome - and genome-wide association study on 1H-NMR urine metabolic profiles. The study was conducted within an untargeted approach, employing a novel method for compound identification. From our discovery cohort of 835 Caucasian individuals who participated in the CoLaus study, we identified 139 suggestively significant (P<5×10−8) and independent associations between single nucleotide polymorphisms (SNP) and metabolome features. Fifty-six of these associations replicated in the TasteSensomics cohort, comprising 601 individuals from São Paulo of vastly diverse ethnic background. They correspond to eleven gene-metabolite associations, six of which had been previously identified in the urine metabolome and three in the serum metabolome. Our key novel findings are the associations of two SNPs with NMR spectral signatures pointing to fucose (rs492602, P = 6.9×10−44) and lysine (rs8101881, P = 1.2×10−33), respectively. Fine-mapping of the first locus pinpointed the FUT2 gene, which encodes a fucosyltransferase enzyme and has previously been associated with Crohn's disease. This implicates fucose as a potential prognostic disease marker, for which there is already published evidence from a mouse model. The second SNP lies within the SLC7A9 gene, rare mutations of which have been linked to severe kidney damage. The replication of previous associations and our new discoveries demonstrate the potential of untargeted metabolomics GWAS to robustly identify molecular disease markers.
Published in the journal: . PLoS Genet 10(2): e32767. doi:10.1371/journal.pgen.1004132
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004132Summary
Metabolic traits are molecular phenotypes that can drive clinical phenotypes and may predict disease progression. Here, we report results from a metabolome - and genome-wide association study on 1H-NMR urine metabolic profiles. The study was conducted within an untargeted approach, employing a novel method for compound identification. From our discovery cohort of 835 Caucasian individuals who participated in the CoLaus study, we identified 139 suggestively significant (P<5×10−8) and independent associations between single nucleotide polymorphisms (SNP) and metabolome features. Fifty-six of these associations replicated in the TasteSensomics cohort, comprising 601 individuals from São Paulo of vastly diverse ethnic background. They correspond to eleven gene-metabolite associations, six of which had been previously identified in the urine metabolome and three in the serum metabolome. Our key novel findings are the associations of two SNPs with NMR spectral signatures pointing to fucose (rs492602, P = 6.9×10−44) and lysine (rs8101881, P = 1.2×10−33), respectively. Fine-mapping of the first locus pinpointed the FUT2 gene, which encodes a fucosyltransferase enzyme and has previously been associated with Crohn's disease. This implicates fucose as a potential prognostic disease marker, for which there is already published evidence from a mouse model. The second SNP lies within the SLC7A9 gene, rare mutations of which have been linked to severe kidney damage. The replication of previous associations and our new discoveries demonstrate the potential of untargeted metabolomics GWAS to robustly identify molecular disease markers.
Introduction
Genome-wide association studies (GWAS) search for associations between phenotypes and common variants within large collections of samples [1]. These studies usually focus on organismal phenotypes [2]–[6]. Recently however, molecular phenotypes, including gene-expression [7], [8] and metabotypes [9]–[14], have also been investigated. Studying the effects of genetic variations on molecular phenotypes is motivated by two characteristics common to the vast majority of GWAS on organismal phenotypes: first, the biological mechanisms underlying the associations are often unknown; and second, the significantly associated loci individually explain only a small fraction of variability of the organismal phenotype, and even cumulatively fall far from explaining the estimated heritability of the phenotype [15]. Molecular phenotypes can be considered as far less removed from the primary causal variants. In agreement with this, GWAS on these phenotypes uncover associations generally characterized by larger effect sizes and higher explained variances. For example, the study of gene expression data from different tissues revealed hundreds of SNPs explaining a significant portion (>5%) of the gene expression levels of (usually) neighboring genes. These expression quantitative trait loci (eQTL) overlaid with GWAS hits for organismal phenotypes reveal significant enrichment [16], hinting at the underlying causal biological mechanisms. Large effect sizes have also been observed for many metabolic quantitative trait loci (mQTL) (see [17] for a recent review). Indeed, several metabolite concentrations measured in urine or serum are genetically determined in a close-to-monogenic manner [10], [12], [18]. More recently, mQTLs have been studied in more depth in the context of organismal phenotypes in order to develop potential prognostic disease markers [11], [19].
The technologies used to measure the metabolome (generally mass spectrometry or NMR spectroscopy) produce high-dimensional raw data. Most GWAS for mQTLs employ estimates of metabolite concentrations that have been derived from these data after normalization. This data transformation is far from trivial, and is performed only for a subset of at most a few hundred metabolites of the much larger set of known human metabolites. The non-transformed data are ignored in the subsequent GWAS, so that this targeted approach to mQTL GWAS discards potentially valuable raw data captured by the analytical technique. In our study, we followed an untargeted approach, similar to the one previously used in the analysis of rodent [20], [21] and human metabolism [22]. In this approach, instead of seeking to transform normalized data into metabolite concentrations as target traits for GWAS, we use the normalized data themselves as phenotypes to be associated with the genotypes, thereby pinpointing metabolome features from these data that have a genetic association. The subsequent identification of metabolites is attempted only using these features, and thereby focused on compounds whose concentrations have a significant genetic determinant.
Results
Our study concerns metabolites in urine samples, measured by 1H-NMR spectroscopy (details on sample preparation and spectrum acquisition are provided in the Materials and Methods section). We binned the 1H-NMR spectra into approximately 2,000 uniform bins, and defined the average intensity of the NMR signal in a bin as a metabolome feature. In our untargeted approach, we used these features—which, combined, contain the full spectroscopic data—as molecular phenotypes. After quality filtering (Materials and Methods), we maintained 1,276 of these features for subsequent analysis. We then followed a two-stage GWAS design, wherein we tested all possible SNP-feature pairs for association in the Cohorte Lausannoise, or CoLaus (see figure 1A for the Manhattan plot corresponding to a single feature, figure S1 for a three-dimensional illustration of Manhattan plots for all features, and figure 1B for the P-value heat map summarizing only the significant associations). After pruning according to SNP linkage and feature correlation, pairs indicating suggestively significant association (P-value below 5×10−8) in CoLaus (N = 835) were tested for replication in the TasteSensomics cohort [23], [24] (N = 601). Out of 139 discovered independent associations, 56 replicated (see table S1 for detailed list).
Fig. 1. Genome- and metabolome-wide analysis results, first stage. 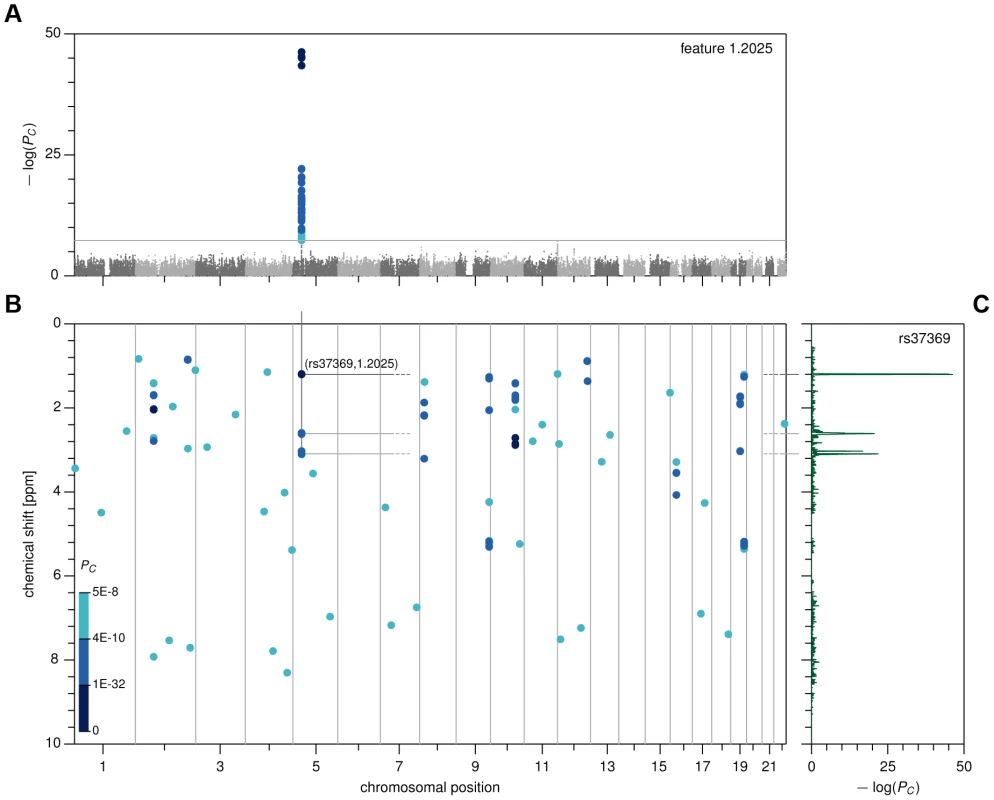
(A) Manhattan plot for feature 1.2025. (B) Genome- and metabolome-wide P-value heat map, showing associations with PC<5×10−8 in CoLaus. (C) Pseudo-spectrum for SNP rs37369, obtained by plotting the association P-values between rs37369 and all metabolic features. For this manageable set of reproducible associations, we then sought to identify the underlying metabolites. To this end, we devised a method that we call metabomatching. Our method makes use of the fact that the NMR spectrum of most metabolites comprises multiple peaks, so that the genetic effect of a SNP on a metabolite usually results in associations of that SNP with multiple metabolome features. This concept is best visualized by way of the pseudo-spectrum of a SNP (see figure 1C for an example), consisting of the set of significance values (−log(P-values)) of its associations with each of the 1,276 features. We observed that in cases where the genetic effect is sufficiently strong, the pseudo-spectrum tends to be similar to the NMR spectrum of the underlying metabolite, allowing its identification.
Specifically, for a given SNP, metabomatching assigns scores to all metabolites with known NMR spectrum. The scores are computed using the significance values of the features that correspond to peaks in the known spectra (see Materials and Methods for details). The metabolites are then ranked, based on these scores, to identify the candidate metabolites most likely to underlie the association. As an example, for SNP rs37369, the top-ranked candidate metabolite is 3-aminoisobutyrate, thereby replicating the association found in previous metabolomics studies [11], [12], [22]. Figure 2A shows how closely the NMR spectrum of 3-aminoisobutyrate (upper half) matches the pseudo-spectrum of rs37369 (lower half).
Fig. 2. Metabomatching. 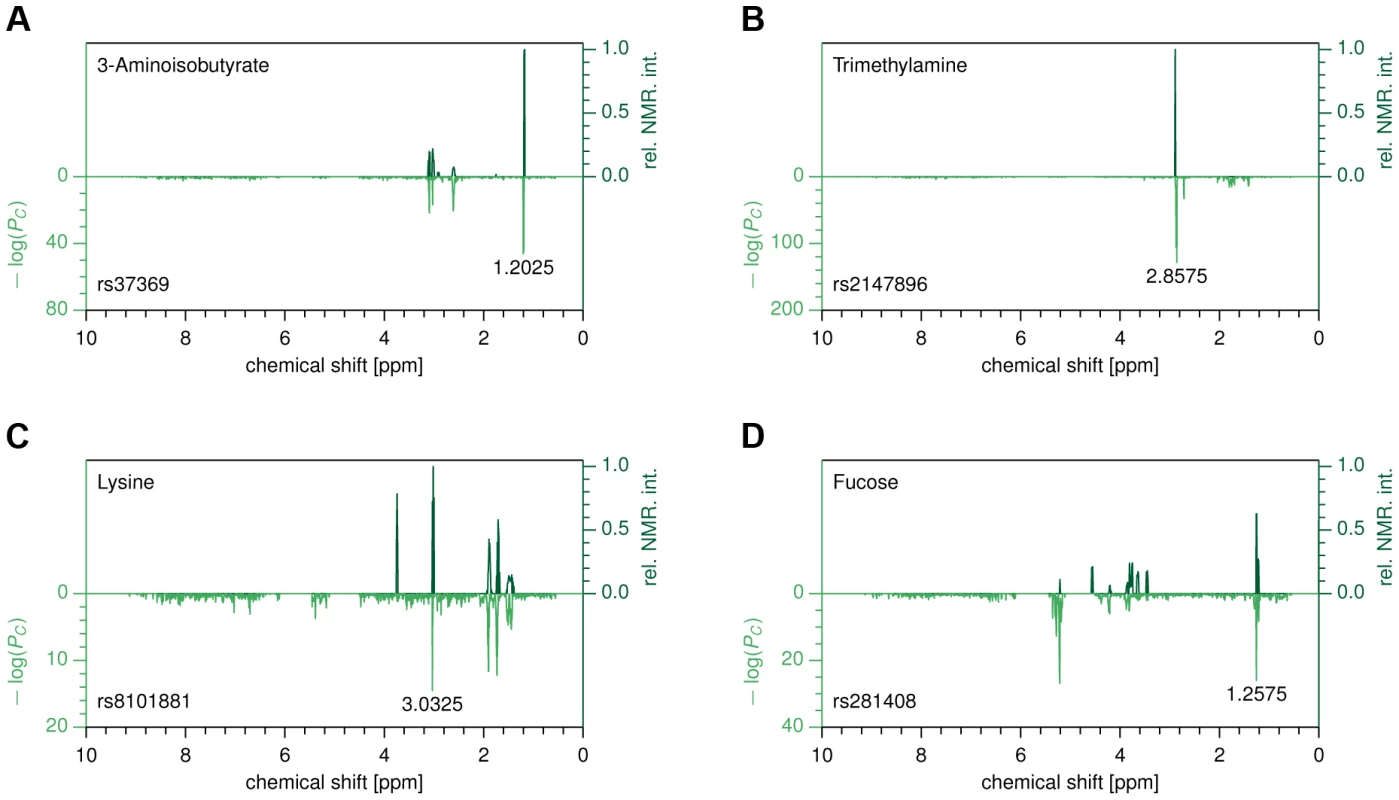
Each subfigure compares the CoLaus pseudo-spectrum (bottom half) with the NMR spectrum (top half) of the most likely candidate for the associated metabolite. (A) rs37369 vs. 3-aminoisobutyrate. (B) rs2147896 in PYROXD2 vs. trimethylamine (C) rs8101881 in SLC7A9 vs. lysine (D) rs281408 in FUT2 vs. fucose. In order to evaluate the robustness of the metabomatching method, we collected all known metabolites whose concentrations in urine had previously been found to be associated with SNPs by the two largest-to-date studies [12], [22]. Among these established SNP-metabolite pairs, we then considered only those for which our association P-values are below 10−6 and whose metabolites have a known NMR spectrum (see table S2). For these controls, metabomatching proved very efficient in selecting the reference compounds, which ranked within the top 1% for 5 out of 7 testable associations, and within the top 10% for the remaining two (see figure 2A–C and figure S2). Encouraged by these findings, we decided to use metabomatching to identify the metabolites (or metabolite families) underlying some of our associations.
Grouping features by metabolites and SNPs by genetic loci, we reduced our 56 SNP-feature associations to 11 locus-metabolite associations, listed in table 1. We replicated the previously published urine associations of ALMS1 with N-acetylated compounds (figure S2A), AGXT2 with 3-aminoisobutyrate (figure 2A), and PSMD9 with 2-hydroxyisobutyrate (figure S2D). For PYROXD2, we replicated the association with trimethylamine (figure 2B), but also found associations with several features not part of the spectrum of trimethylamine, suggesting that one or more additional metabolites could be implicated. Similarly, the published association of NAT2 is with the formate-succinate ratio [12], but neither of these compounds contains the features implicated by our association (Figure S2C). For the associations of SNPs in ACADL, ABO, and ACADS, linked SNPs have been found to associate with metabolite concentrations in serum. However, without conclusive identification of the metabolites underlying the associated features we could not determine whether our associations are the exact urine analogs of known serum associations, or whether they involve novel or related metabolites.
Tab. 1. Locus-metabolite associations. 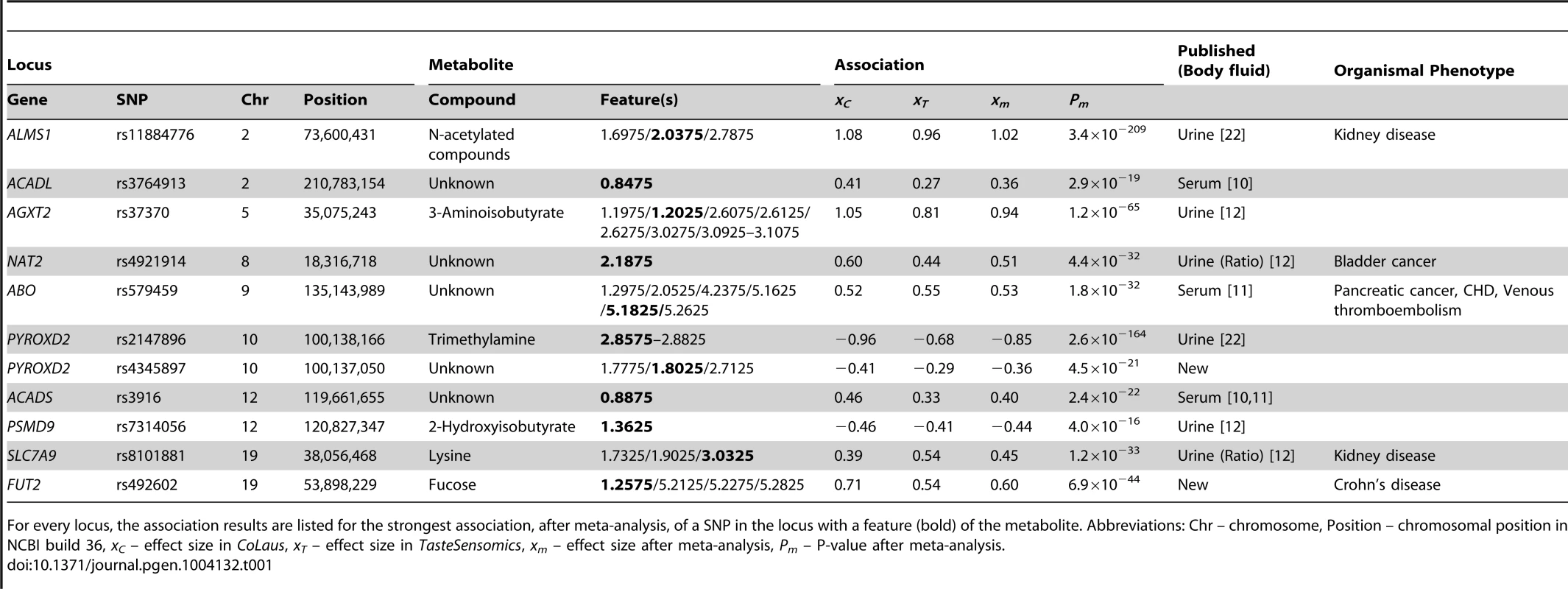
For every locus, the association results are listed for the strongest association, after meta-analysis, of a SNP in the locus with a feature (bold) of the metabolite. Abbreviations: Chr – chromosome, Position – chromosomal position in NCBI build 36, xC – effect size in CoLaus, xT – effect size in TasteSensomics, xm – effect size after meta-analysis, Pm – P-value after meta-analysis. In the traditionally applied SNP-pruning procedure, focus is given only to the most significant SNP and the phenomenon of (semi-)independent contribution of adjacent SNPs (termed as allelic heterogeneity) is ignored. To overcome this limitation, we tested for allelic heterogeneity for each of our 11 locus-feature pairs using multivariate association [25], [26]. We found evidence for secondary signals for four of these pairs in the CoLaus sample, and for two of them, both involving the AGXT2 locus, allelic heterogeneity was replicated in the TasteSensomics cohort (table 2). For these replicating cases, the variance explained by the multiple SNP association was up to 50% greater than that of the single SNP association, demonstrating the importance of allelic heterogeneity, still often overlooked in GWAS [26].
Tab. 2. Allelic heterogeneity at the AGXT2 locus. 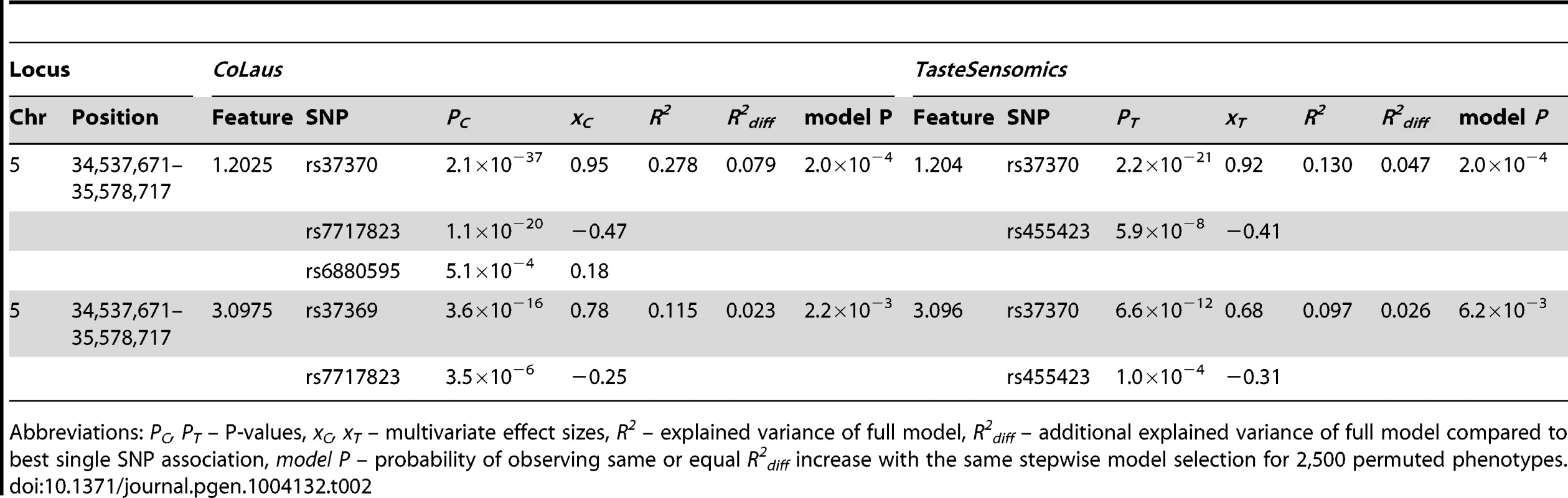
Abbreviations: PC, PT – P-values, xC, xT – multivariate effect sizes, R2 – explained variance of full model, R2diff – additional explained variance of full model compared to best single SNP association, model P – probability of observing same or equal R2diff increase with the same stepwise model selection for 2,500 permuted phenotypes. For our first novel association, metabomatching allowed the identification of the underlying metabolite. As illustrated in figure 2D, the pseudo-spectrum of rs281408 (lower half) closely resembles the NMR spectrum (upper half) of the top-ranked candidate, fucose. We confirmed this in-silico identification using NMR spectroscopy of fucose-spiked urine samples. In CoLaus, the SNPs associated with fucose fall within a large LD block on chromosome 19 encompassing the FUT2, RASIP1, and IZUMO1 genes. However, the TasteSensomics population has a different genetic structure within this region (figure S3), such that the combined association signal, led by rs492602 (r2 = 0.87 with rs281408), could be narrowed down to FUT2 specifically (Figure 3A). FUT2 encodes a fucosyltransferase enzyme that is essential for the secretion and display of ABO blood group antigens on mucosal surface cells. Mucosal ABO-antigens serve as attachment points for both beneficial gut bacteria and harmful viruses [27], [28], which is thought to have driven the complex evolution of FUT2 [29]. In addition, fucose, the substrate of the fucosyltransferase enzyme, was shown to impact human gut microbial composition [30], [31], and thereby gut health [32], [33]. The role of FUT2 in gut microbial ecology is further substantiated by the association of its SNP rs281379 (r2 = 0.76 with rs492602) with Crohn's disease (CD), as found in a sample of over 50 K individuals [34] (figure 4A). Several urinary metabolites (not including fucose) were shown to distinguish between inflammatory bowel disease patients (including those with CD) and healthy subjects [35]. Moreover, significantly elevated fucose levels in urine were found in mice with an interleukin-10 deficiency, the mouse model of CD [36], [37]. This FUT2-independent link between urinary fucose levels and CD may be indicating that the elevated urine fucose levels, also observed in human FUT2 non-secretors, do not simply result from the elimination of fucose that was not secreted into the mucosal layers. Instead this elevation may be a consequence of (and metabolic indicator for) early sub-symptomatic changes from a healthy gut flora towards the dysbiosis of CD. While its exact role is unclear, fucose is certainly an interesting candidate for further exploration of the metabolic causes and effects of CD, or inflammatory bowel disorders in general.
Fig. 3. Local Manhattan plots. 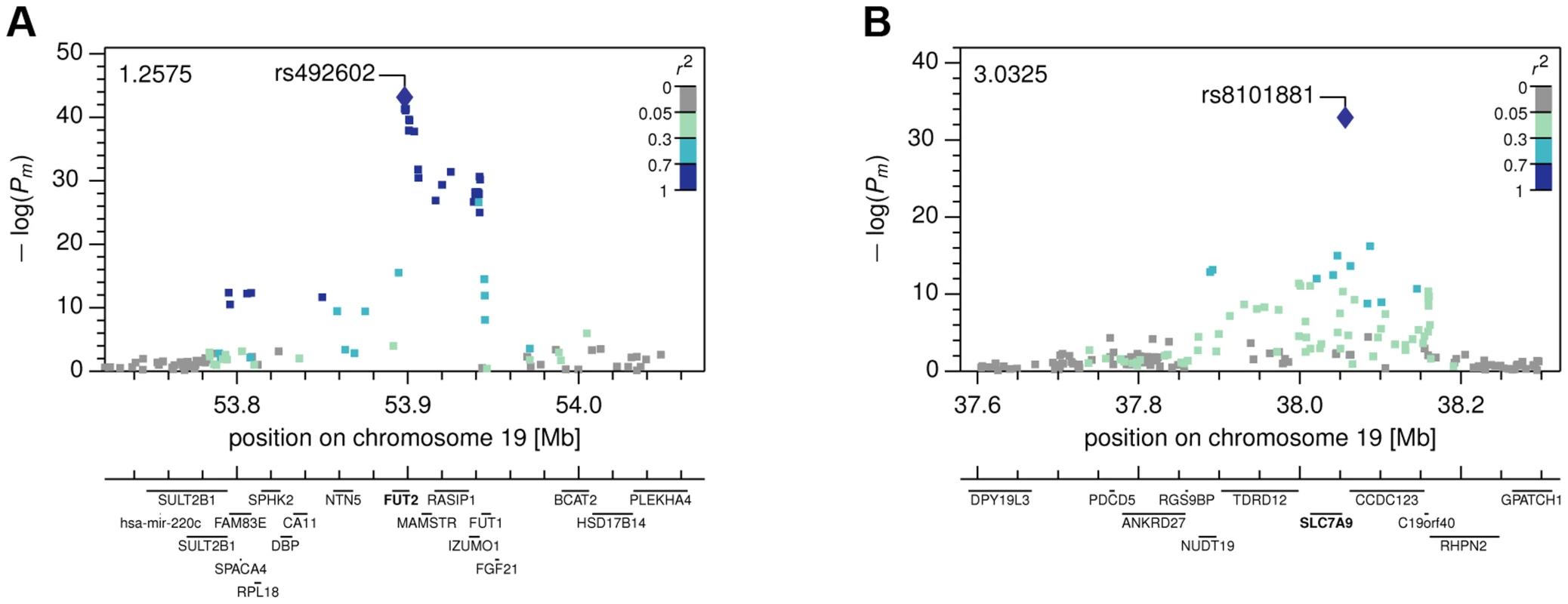
The Manhattan plots show combined −log(P-values) in the neighborhood of the most strongly associated SNP for (A) the FUT2 with fucose association, and (B) the SLC7A9 with lysine association. Fig. 4. Genotype-Metabotype-Phenotype associations. 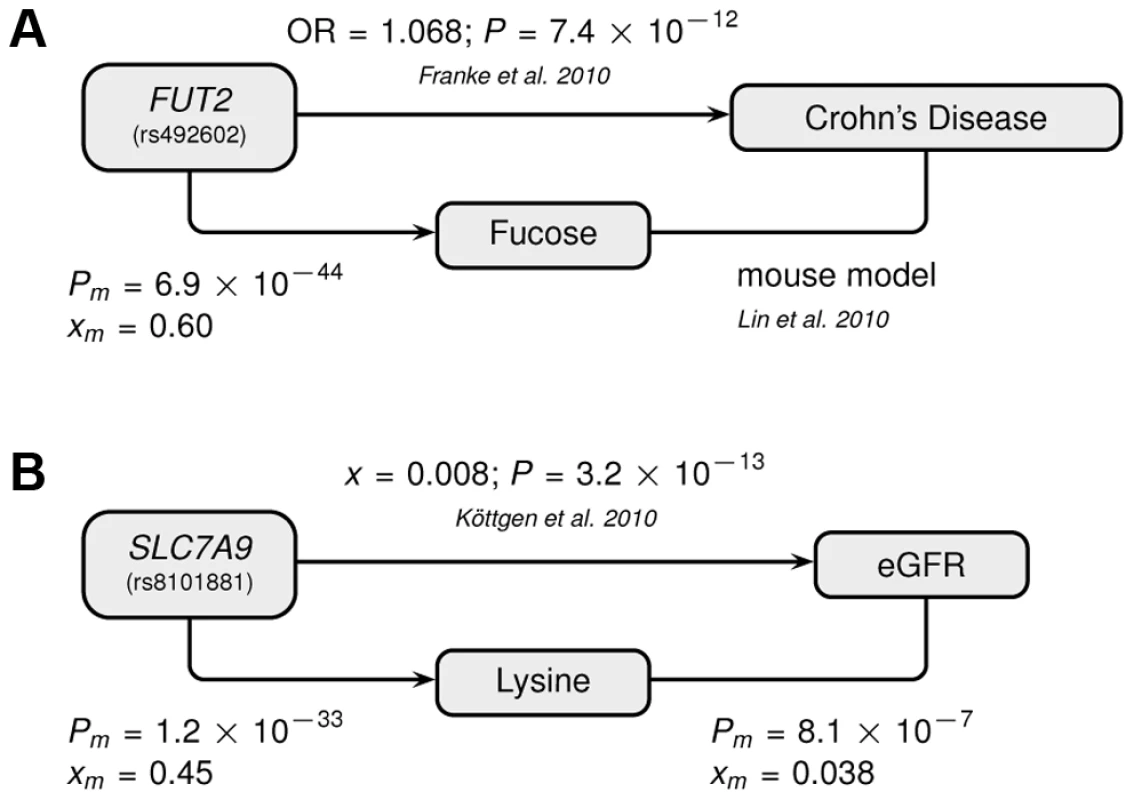
The two novel gene-metabolite associations of this study implicate SNPs that had previously been associated with disease-related phenotypes by the indicated publications: (A) Fucose–Crohn's disease–FUT2 (rs492602), (B) Lysine–eGFR–SLC7A9 (rs8101881). A link between the metabolite and the phenotype has been established for (A) based on a mouse model and for (B) by a direct correlation with the indicated significance and effect size. Abbreviations: OR refers to the odds ratio, x to the linear regression effect size, P to the corresponding P-value, and the m-index indicates values obtained in the combined CoLaus and TasteSensomics sample. Our second novel association links the SNP rs8101881 with a metabolite identified as lysine by our metabomatching method (figure 2C). This SNP falls within the SLC7A9 gene (in a different region of chromosome 19, see Figure 3B). SNPs at this locus have already been found to be significantly associated with the lysine/valine ratio [12], but not lysine alone. SLC7A9 is linked to kidney function: rare mutations in SLC7A9 cause severe kidney damage [38], and a common variant (rs12460876, linked to rs8101881 with r2 = 0.996) is associated with the estimated glomerular filtration rate (eGFR) [39], which is a key clinical measure of kidney health. Interestingly, lysine concentration shows a strong association with eGFR in the combined CoLaus and TasteSensomics sample (xm = 0.038, SE = 0.008, Pm = 8.1×10−7), regardless of the rs8101881 genotype. To further explore these links (figure 4B) we used Mendelian randomization (MR) [40], [41] in order to assess whether lysine levels may be causative for chronic kidney disease. We employed rs8101881 as instrument (F-statistic = 46.22) and the tests proposed by Glymour et al. [42] indicated no violation of the assumptions of MR. We then computed the two-stage least-squares (2SLS) estimate as done by Ehret et al. [2], where the rs8101881-lysine effect was calculated combining the results from the CoLaus and TasteSensomics cohorts, while the effect of rs8101881 on eGFR was estimated using CKDGen [39] summary statistics. Although the 2SLS estimate was consistent (overlapping in confidence interval) with the ordinary least-squares (OLS) estimate of lysine on eGFR (xm = 0.038), it was non-significant (x = 0.02, P = 0.54), hence we have no sufficient evidence to claim a causal effect of lysine levels on eGFR.
Discussion
We conducted a genome - and metabolome-wide association study of untargeted NMR data to reveal novel SNP-feature associations. Using both manual and automated annotation, we identified the metabolites underlying more than half of the discovered associations.
The high number of associations found to replicate (56 out of 139) is indicative of the robustness of mQTL GWAS in general, and our feature-based approach in particular. Our discovery and replication cohorts have different population origins —European for the Swiss cohort CoLaus, genetically admixed, from African, European, and Asian founders, for the Brazilian cohort TasteSensomics—indicating that the genetic effects on the metabotypes are likely to be both ethnicity-independent, and robust against potential variations of diet and other environmental factors.
The two metabolomic data sets we used for discovery and replication were collected independently, initially without the intention of combining them. As a result, the respective experimental conditions were not always well matched (see Materials and Methods for details). Since differences in the experimental setups can cause significant changes in the chemical shifts of specific metabolite absorption bands, one could have expected that this would cause a significant problem to our feature-based approach. Yet in practice, this did not appear to be a significant impediment, given the high rate of replication between our two studies. This indicates that the feature-based approach is rather robust against variations in experimental conditions. The reliability of the feature-based approach is further evidenced by the high overlap between our associations and previously described results [11], [12], [22].
In comparison to previous targeted approaches, where metabolite identification is applied before GWAS, the feature-based approach has two main advantages. The first, and most important one, is that by moving the identification of metabolite concentrations after the association phase, the complete metabolomic data captured by spectroscopy are analyzed. As a consequence, the feature-based approach can potentially provide additional association signals that would have been missed by a targeted approach.
The second advantage, which is of a more pragmatic nature, is that the burden associated with metabolic identification is considerably reduced. Indeed only the metabolites of interest, namely those found to have a genetic component, need identification. Even so, identification of all metabolites of interest can prove difficult, and cases may exist where identification will require further experimental work (like the collection of two-dimensional homo - and heteronuclear NMR spectra, for example). Such additional analysis was precluded in our study due to the destruction of samples after 1H-NMR analysis in accordance with study protocols and informed consent.
A key message of our study is that our metabomatching method may be useful for other cohort-based metabolomics projects when resources for compound identification in terms of material or expert time are limited. Essentially, the information inherent in the GWAS signals can complement (and sometimes even replace) traditional sample-based metabolite identification. As the information in databases of NMR spectra of individual metabolites increases, the method may become a powerful strategy for metabolite identification in GWAS involving untargeted metabolomics.
In summary, the replication of locus-metabolite associations with previous studies [9]–[13] and the unequivocal identification of two new gene-metabolite associations indicate that the feature-based approach, combined with pseudo-spectrum based identification, is a reliable approach for metabolome - and genome-wide association studies. In cases where newly identified association signals are of marginal strength, metabolite identification may be followed-up by model-based quantification of the metabolite [43], [44] to potentially improve the association signal, and provide a more accurate effect size estimate. While the assignment to metabolites of all associated features can require substantial follow-up work, this may not be necessary if the primary objective of a study was to elucidate novel genetic loci relevant for general metabolomic variability. Specifically, while associations with unidentified metabolites may lack a direct mechanistic interpretation, they can still prove to be valuable biomarkers of certain clinical phenotypes [45], [46]. Finally, the unidentified metabolite underlying an association may correspond to an unknown metabolite in the sense, used in Krumsiek et al. [47], of “a molecule which can reproducibly be detected and quantified […] but whose chemical identity has not been elucidated”, in which case the genetic association itself may provide identifying information.
Our GWAS revealed two new SNP-metabolite associations of potential clinical relevance. We found urine fucose concentration to be associated with variants in the FUT2 gene, which is linked to gut microbial ecology in general, and to Crohn's disease in particular. Furthermore, we found urine lysine concentration to be associated with SNPs in the SLC7A9 gene, which is linked to kidney function and to kidney failure specifically. We confirmed the link to kidney function with a significant lysine-eGFR association. Our Mendelian randomization was inconclusive for a causal link between urine lysine levels and eGFR (as a measure of kidney filtering capacity). Yet, we only had about 12% power and a sample size of at least 11,400 would be required for providing a conclusive answer (i.e. having over 80% power). Molecular trait association can not only help us to better understand the underlying biological processes, but also shed light on the interplay between genetic predisposition and environmental factors. In our case, figuring out how lysine levels are influenced by diet may thus help to develop nutritional intervention programs to counter kidney problems before they manifest themselves in a clinical phenotype. In summary, this study provided specific evidence that genetically influenced metabolite concentrations can play a crucial role in disease progression, and that these metabolites may provide an avenue for better diagnosis and prevention of diseases.
Materials and Methods
For the Cohorte Lausannoise (CoLaus) study, genotyping was performed using the Affymetrix GeneChip Human Mapping 500 K array set. Genotypes were called using BRLMM [48]. Duplicate individuals, and first and second degree relatives, were identified by computing genomic identity-by-descent coefficients, using PLINK [49]. The younger individual from each duplicate or relative pair was removed. Individuals with call rate below 90% were excluded from further analysis. The full set of unmeasured HapMap II SNPs (release 21) was imputed using 390,631 measured SNPs (with Hardy-Weinberg P-value above 10−7 and MAF above 1%). Imputation was performed using IMPUTE [50] version 0.2.0. Expected allele dosages were computed for 2,557,249 SNPs.
For the TasteSensomics study, genotyping was performed on the Illumina Human Omni-Quad1 platform. Genotype calling was performed with Beadstudio software (Illumina). Calls with a genotyping score below 0.2 were excluded from further analysis. SNPs with a call rate below 90% and individuals with a call rate below 95% were also excluded, leaving 989,972 available SNPs, with an overlap of 713,870 SNPs with the CoLaus cohort. No imputation was performed in this cohort, since none of the available HapMap panels were considered as sufficiently representative for the admixed population investigated in this study.
In the CoLaus cohort, 974 individuals each provided 1 urine sample for metabolic analysis. The CoLaus study was approved by the Institutional Ethics Committee of the University of Lausanne. All study participants gave written consent including for genetic studies. Prior to urinalysis, samples were stored at −80°C. Each sample was comprised of 400 µL urine and 200 µL of a 0.2M deuterated phosphate buffer solution (pH 7.4). Samples were centrifuged to remove precipitates, and to 500 µL aliquots of the resulting supernatant, 100 µL of a solution of 0.1% (w/v) sodium trimethylsilyl propionate (TSP) and 1% (w/v) sodium azide in D2O was added. The TSP provided a chemical shift reference (δ0.0), the sodium azide acted as a bactericide, and the D2O provided a deuterium field-frequency lock signal for the NMR spectrometer. 1H NMR spectra were acquired at 300 K on a Bruker Avance II 700 MHz spectrometer (Bruker Biospin, Rheinstetten, Germany) using a standard 1H detection pulse sequence with water suppression.
In the TasteSensomics cohort, 601 individuals donated 3 samples each over a period of 2 weeks. 3 mM sodium azide was added to the samples to prevent microbial growth. Samples were then frozen and stored at −80°C prior to urinalysis. Urine aliquots of 400 µL were adjusted to pH 6.8 using 200 µL of deuterated phosphate buffer solution (final concentration of 0.2M) containing 1 mM of sodium TSP. 1H NMR spectra were recorded at 300 K on a Bruker Avance II 600 MHz spectrometer, using a standard 1H detection pulse sequence with water suppression.
CoLaus 1H spectra were binned in chemical shift increments of 0.005 ppm, resulting in metabolic profiles of 2,200 metabolome features. Filtering out features then samples with more than 5% of missing values, a dataset composed of 1,276 features for 835 individuals was obtained. TasteSensomics 1H spectra were binned in increments of 0.0032 ppm, resulting in profiles of 2,400 features. More sophisticated binning procedures, such as adaptive binning [51], [52], could have been applied, but standard uniform binning has been shown to be successful by us [53], [54] and others [55], [56]. Bin intensities were log-averaged across replicate samples for each individual, and spectral qualities were such that all features and subjects were included in the analysis. For each individual, we applied a Z-score transformation in order to achieve zero mean and unit variance. This statistical normalization yields metabolic profiles similar to those resulting from common biological normalizations, such as normalization by total metabolite content (median correlation r = 0.92), or normalization by urinary creatinine measured before freezing and thawing (resulting in lower median correlation r = 0.45).
In addition to the standard confounding factors that are age, sex, post-menopausal status, and the principal components of the genotype, metabolic profiles are sensitive to lifestyle factors, dietary behavior, and creatinine levels. Among the 36 such factors available for the CoLaus sample, we select those which associated with at least 2% of the features, resulting in the 12 factor subset comprising age, sex, post-menopausal status, the 1st principal component of the genotype, the 2nd and 4th principal components of the dietary profile, smoking behavior, caffeine intake, alcohol intake, physical activity, urinary creatinine, and serum creatinine. For every feature, we use as covariates those factors which, in a stepwise method, significantly associate (P<0.05/12) with the feature. For the TasteSensomics feature, covariates were similarly selected (P<0.05/5) among the factors age, sex, BMI, and the first two principal components of the genotype.
We tested the 1,276 features for association in the CoLaus cohort with the 713,870 SNPs also measured in the TasteSensomics cohort. We pruned the suggestively significant (P<5×10−8) SNP-feature association pairs by considering two pairs equivalent if their SNPs were in LD (r2>0.3) and their features were correlated (r2>0.4). This procedure is an extension of the clumping method implemented in PLINK [49]. We then sought replication in the TasteSensomics cohort [23], [24]. Replication was declared if the discovery and replication effect directions were concordant, the replication P-value was below 0.05/#hits, and the combined association P-value below 5.7×10−10. The latter P-value threshold corresponds to the Bonferroni multiple testing correction for both features, where the effective number of tests was estimated [57] to be 125, and SNPs.
To use the admixed genetic background of the TasteSensomics cohort for narrowing down the genetic loci giving rise to the association signals, we grouped the replicating SNP-feature associations by genetic loci (1 Mb neighborhood), and ran associations between the implicated feature(s) with all available SNPs in both (discovery and replication) cohorts at the locus. We then meta-analyzed the local association summary statistics (see table S3). The combined results for the strongest association at each locus are reported in table 1.
Features do not directly correspond to the concentration of a single metabolite, so that feature ratios are difficult to interpret. Therefore, in contrast to previous metabolomics association studies, we do not include feature ratios in the first association phase, which substantially reduced the multiple testing burden.
The features involved in replicated associations were subjected to both manual and automated metabolite annotation. Manual annotation was performed using in-house libraries, reference spectra from public databases (HMDB http://www.hmdb.ca, BMRB http://www.bmrb.wisc.edu, Prime http://prime.psc.riken.jp), and the Chenomx NMR Suite software, version 7.1 (Chenomx Inc, Alberta, Canada). Automated annotation was performed by our metabomatching method (http://www.unil.ch/cbg), which compares the pseudo-spectrum (see main text) to the spectrum of all metabolites for which a reference spectrum is available in HMDB (to date around 850 metabolites). After pruning correlated spectral bins (to ensure independence) we quantified the similarity between the pseudo-spectrum and the spectrum of a given metabolite by summing up the squared association test statistics
corresponding to the k (independent) peaks present in the spectrum of the metabolite. The resulting test-statistic is χ2-distributed with k degrees of freedom. This allows for obtaining a P-value for having observed as good a match between the pseudo-spectrum and the NMR spectrum as by chance. The procedure is repeated for all metabolites in HMDB, which are then ranked according to their P-values.For each SNP with confirmed metabolite association, we examined the surrounding 1 Mb window searching for evidence of allelic heterogeneity or imperfect tagging. Within each 1 Mb region, we looked for the best multivariate model (in the sense of AIC) to explain the corresponding metabolic feature in the CoLaus sample. If this model provides a significantly better fit to the data than the lead SNP, we attempted to replicate in the TasteSensomics cohort. Note that due to the different LD structure in the CoLaus and TasteSensomics cohorts we did not attempt to replicate the exact same SNPs, but the locus. In case of successful replication we declare the locus to exhibit multiple independent signals. We also attempted fine-mapping of association signals in these regions, using 1000 Genomes imputed genotype association, but no stronger association was revealed.
Mendelian randomization (MR) was carried out by calculating two-stage least squares estimates and comparing them to the direct one stage effect size. We used an SLC7A9 SNP (rs8101881) as instrument to infer causality between lysine concentration and log transformed age - and sex-corrected eGFR. To verify the assumptions of MR, we noted that the instrument was strongly associated with lysine and, since it is a genotype, is very unlikely to have a common cause with eGFR. The final assumption of MR, namely that all causal effect of the SNP on eGFR is acting through lysine, was examined by verifying that our variables satisfied all of the tests of positive unmeasured confounding (leveraging prior casual assumptions) proposed by Glymour et al. [42]. The selected SNP was not found to be associated with any known confounding factors of eGFR. We used the Durbin-Hausman test [58] to compare the OLS and the 2SLS estimates.
Supporting Information
Zdroje
1. LaFramboiseT (2009) Single nucleotide polymorphism arrays: a decade of biological, computational and technological advances. Nucleic Acids Res 37 : 4181–4193.
2. EhretGB, MunroePB, RiceKM, BochudM, JohnsonAD, et al. (2011) Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature 478 : 103–109.
3. BarrettJC, ClaytonDG, ConcannonP, AkolkarB, CooperJD, et al. (2009) Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet 41 : 703–707.
4. SpeliotesEK, WillerCJ, BerndtSI, MondaKL, ThorleifssonG, et al. (2010) Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 42 : 937–948.
5. PatsopoulosNA, EspositoF, ReischlJ, LehrS, BauerD, et al. (2011) Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Ann Neurol 70 : 897–912.
6. EllinghausD, EllinghausE, NairRP, StuartPE, EskoT, et al. (2012) Combined analysis of genome-wide association studies for Crohn disease and psoriasis identifies seven shared susceptibility loci. Am J Hum Genet 90 : 636–647.
7. MontgomerySB, SammethM, Gutierrez-ArcelusM, LachRP, IngleC, et al. (2010) Transcriptome genetics using second generation sequencing in a Caucasian population. Nature 464 : 773–777.
8. StrangerBE, NicaAC, ForrestMS, DimasA, BirdCP, et al. (2007) Population genomics of human gene expression. Nat Genet 39 : 1217–1224.
9. GiegerC, GeistlingerL, AltmaierE, Hrabe de AngelisM, KronenbergF, et al. (2008) Genetics meets metabolomics: a genome-wide association study of metabolite profiles in human serum. PLoS Genet 4: e1000282.
10. IlligT, GiegerC, ZhaiG, Romisch-MarglW, Wang-SattlerR, et al. (2010) A genome-wide perspective of genetic variation in human metabolism. Nat Genet 42 : 137–141.
11. SuhreK, ShinSY, PetersenAK, MohneyRP, MeredithD, et al. (2011) Human metabolic individuality in biomedical and pharmaceutical research. Nature 477 : 54–60.
12. SuhreK, WallaschofskiH, RafflerJ, FriedrichN, HaringR, et al. (2011) A genome-wide association study of metabolic traits in human urine. Nat Genet 43 : 565–569.
13. MontoliuI, GenickU, LeddaM, CollinoS, MartinFP, et al. (2012) Current status on genome-metabolome-wide associations: an opportunity in nutrition research. Genes Nutr 8 (1) 19–27.
14. HomuthG, TeumerA, VolkerU, NauckM (2012) A description of large-scale metabolomics studies: increasing value by combining metabolomics with genome-wide SNP genotyping and transcriptional profiling. J Endocrinol 215 : 17–28.
15. ManolioTA, CollinsFS, CoxNJ, GoldsteinDB, HindorffLA, et al. (2009) Finding the missing heritability of complex diseases. Nature 461 : 747–753.
16. NicaAC, MontgomerySB, DimasAS, StrangerBE, BeazleyC, et al. (2010) Candidate causal regulatory effects by integration of expression QTLs with complex trait genetic associations. PLoS Genet 6: e1000895.
17. SuhreK, GiegerC (2012) Genetic variation in metabolic phenotypes: study designs and applications. Nat Rev Genet 13 : 759–769.
18. KettunenJ, TukiainenT, SarinAP, Ortega-AlonsoA, TikkanenE, et al. (2012) Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet 44 : 269–276.
19. Wang-SattlerR, YuZ, HerderC, MessiasAC, FloegelA, et al. (2012) Novel biomarkers for pre-diabetes identified by metabolomics. Mol Syst Biol 8 : 615.
20. DumasME, WilderSP, BihoreauMT, BartonRH, FearnsideJF, et al. (2007) Direct quantitative trait locus mapping of mammalian metabolic phenotypes in diabetic and normoglycemic rat models. Nat Genet 39 : 666–672.
21. RobinetteSL, HolmesE, NicholsonJK, DumasME (2012) Genetic determinants of metabolism in health and disease: from biochemical genetics to genome-wide associations. Genome Med 4 : 30.
22. NicholsonG, RantalainenM, LiJV, MaherAD, MalmodinD, et al. (2011) A genome-wide metabolic QTL analysis in Europeans implicates two loci shaped by recent positive selection. PLoS Genet 7: e1002270.
23. GenickUK, KutalikZ, LeddaM, DestitoMC, SouzaMM, et al. (2011) Sensitivity of genome-wide-association signals to phenotyping strategy: the PROP-TAS2R38 taste association as a benchmark. PLoS One 6: e27745.
24. LeddaM, KutalikZ, Souza DestitoMC, SouzaMM, CirilloCA, et al. (2014) GWAS of human bitter taste perception identifies new loci and reveals additional complexity of bitter taste genetics. Hum Mol Genet 23 : 259–267.
25. KutalikZ, BenyaminB, BergmannS, MooserV, WaeberG, et al. (2011) Genome-wide association study identifies two loci strongly affecting transferrin glycosylation. Hum Mol Genet 20 : 3710–3717.
26. EhretGB, LamparterD, HoggartCJ, WhittakerJC, BeckmannJS, et al. (2012) A multi-SNP locus-association method reveals a substantial fraction of the missing heritability. Am J Hum Genet 91 : 863–871.
27. McGovernDP, JonesMR, TaylorKD, MarcianteK, YanX, et al. (2010) Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum Mol Genet 19 : 3468–3476.
28. RauschP, RehmanA, KunzelS, HaslerR, OttSJ, et al. (2011) Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci U S A 108 : 19030–19035.
29. Ferrer-AdmetllaA, SikoraM, LaayouniH, EsteveA, RoubinetF, et al. (2009) A natural history of FUT2 polymorphism in humans. Mol Biol Evol 26 : 1993–2003.
30. PachecoAR, CurtisMM, RitchieJM, MuneraD, WaldorMK, et al. (2012) Fucose sensing regulates bacterial intestinal colonization. Nature 492 : 113–117.
31. CoyneMJ, ReinapB, LeeMM, ComstockLE (2005) Human symbionts use a host-like pathway for surface fucosylation. Science 307 : 1778–1781.
32. HooperLV, GordonJI (2001) Commensal host-bacterial relationships in the gut. Science 292 : 1115–1118.
33. MorganXC, TickleTL, SokolH, GeversD, DevaneyKL, et al. (2012) Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biology 13: R79.
34. FrankeA, McGovernDP, BarrettJC, WangK, Radford-SmithGL, et al. (2010) Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet 42 : 1118–1125.
35. StephensNS, SiffledeenJ, SuX, MurdochTB, FedorakRN, et al. (2012) Urinary NMR metabolomic profiles discriminate inflammatory bowel disease from healthy. J Crohns Colitis
36. LinHM, BarnettMP, RoyNC, JoyceNI, ZhuS, et al. (2010) Metabolomic analysis identifies inflammatory and noninflammatory metabolic effects of genetic modification in a mouse model of Crohn's disease. J Proteome Res 9 : 1965–1975.
37. LinHM, EdmundsSI, HelsbyNA, FergusonLR, RowanDD (2009) Nontargeted urinary metabolite profiling of a mouse model of Crohn's disease. J Proteome Res 8 : 2045–2057.
38. FeliubadaloL, FontM, PurroyJ, RousaudF, EstivillX, et al. (1999) Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat Genet 23 : 52–57.
39. KottgenA, PattaroC, BogerCA, FuchsbergerC, OldenM, et al. (2010) New loci associated with kidney function and chronic kidney disease. Nat Genet 42 : 376–384.
40. D. J. Balding MJB, Cannings C, editor (2007) Handbook of statistical genetics. Chichester: John Wiley & Sons.
41. SheehanNA, DidelezV, BurtonPR, TobinMD (2008) Mendelian randomisation and causal inference in observational epidemiology. PLoS Med 5: e177.
42. GlymourMM, TchetgenEJ, RobinsJM (2012) Credible Mendelian randomization studies: approaches for evaluating the instrumental variable assumptions. Am J Epidemiol 175 : 332–339.
43. Ala-KorpelaM, KangasAJ, SoininenP (2012) Quantitative high-throughput metabolomics: a new era in epidemiology and genetics. Genome Med 4 : 36.
44. WishartDS (2008) Quantitative metabolomics using NMR. Trends in Analytical Chemistry 27 : 228–237.
45. GallWE, BeebeK, LawtonKA, AdamKP, MitchellMW, et al. (2010) alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One 5: e10883.
46. FiehnO, GarveyWT, NewmanJW, LokKH, HoppelCL, et al. (2010) Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women. PLoS One 5: e15234.
47. KrumsiekJ, SuhreK, EvansAM, MitchellMW, MohneyRP, et al. (2012) Mining the unknown: a systems approach to metabolite identification combining genetic and metabolic information. PLoS Genet 8: e1003005.
48. Affymetrix (2006) BRLMM: an improved genotype calling method for the GeneChip© Human Mapping 500 K array set. pp. 1–18.
49. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81 : 559–575.
50. MarchiniJ, HowieB, MyersS, McVeanG, DonnellyP (2007) A new multipoint method for genome-wide association studies by imputation of genotypes. Nat Genet 39 : 906–913.
51. De MeyerT, SinnaeveD, Van GasseB, TsiporkovaE, RietzschelER, et al. (2008) NMR-based characterization of metabolic alterations in hypertension using an adaptive, intelligent binning algorithm. Anal Chem 80 : 3783–3790.
52. AndersonP, MahleD, DoomT, ReoN, DelRasoN, et al. (2010) Dynamic adaptive binning: an improved quantification technique. Metabolomics
53. CollinoS, MontoliuI, MartinFP, SchererM, MariD, et al. (2013) Metabolic signatures of extreme longevity in northern italian centenarians reveal a complex remodeling of lipids, amino acids, and gut microbiota metabolism. PLoS One 8: e56564.
54. ClausSP, ElleroSL, BergerB, KrauseL, BruttinA, et al. (2011) Colonization-induced host-gut microbial metabolic interaction. MBio 2: e00271–00210.
55. StaabJM, O'ConnellTM, GomezSM (2010) Enhancing metabolomic data analysis with Progressive Consensus Alignment of NMR Spectra (PCANS). BMC Bioinformatics 11 : 123.
56. KohlSM, KleinMS, HochreinJ, OefnerPJ, SpangR, et al. (2012) State-of-the art data normalization methods improve NMR-based metabolomic analysis. Metabolomics 8 : 146–160.
57. GaoX, StarmerJ, MartinER (2008) A multiple testing correction method for genetic association studies using correlated single nucleotide polymorphisms. Genet Epidemiol 32 : 361–369.
58. LawlorDA, HarbordRM, SterneJA, TimpsonN, Davey SmithG (2008) Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med 27 : 1133–1163.
Štítky
Genetika Reprodukční medicína
Článek Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the RatČlánek MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease PathogenesisČlánek Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 2- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- Fifteen Years Later: Hard and Soft Selection Sweeps Confirm a Large Population Number for HIV In Vivo
- The Same but Different: Worms Reveal the Pervasiveness of Developmental System Drift
- Serine Carboxypeptidase SCPEP1 and Cathepsin A Play Complementary Roles in Regulation of Vasoconstriction via Inactivation of Endothelin-1
- Coherent Functional Modules Improve Transcription Factor Target Identification, Cooperativity Prediction, and Disease Association
- A Long-Chain Flavodoxin Protects from Oxidative Stress and Host Bacterial Clearance
- Mammalian E-type Cyclins Control Chromosome Pairing, Telomere Stability and CDK2 Localization in Male Meiosis
- Influenza Virus Drug Resistance: A Time-Sampled Population Genetics Perspective
- Transcriptome-Wide Analyses of 5′-Ends in RNase J Mutants of a Gram-Positive Pathogen Reveal a Role in RNA Maturation, Regulation and Degradation
- Selective Disruption of Aurora C Kinase Reveals Distinct Functions from Aurora B Kinase during Meiosis in Mouse Oocytes
- X Chromosome Control of Meiotic Chromosome Synapsis in Mouse Inter-Subspecific Hybrids
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Extreme Population Differences in the Human Zinc Transporter ZIP4 (SLC39A4) Are Explained by Positive Selection in Sub-Saharan Africa
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Genomic Networks of Hybrid Sterility
- Natural Polymorphisms in Influence Negative Selection and CD4∶CD8 Lineage Commitment in the Rat
- Oxidative Stress Is Not a Major Contributor to Somatic Mitochondrial DNA Mutations
- Molecular Identification of Collagen 17a1 as a Major Genetic Modifier of Laminin Gamma 2 Mutation-Induced Junctional Epidermolysis Bullosa in Mice
- Uncoupling of Molecular Maturation from Peripheral Target Innervation in Nociceptors Expressing a Chimeric TrkA/TrkC Receptor
- MicroRNAs Located in the Hox Gene Clusters Are Implicated in Huntington's Disease Pathogenesis
- Loss of Trabid, a New Negative Regulator of the Immune-Deficiency Pathway at the Level of TAK1, Reduces Life Span
- Targeted Ablation of Nesprin 1 and Nesprin 2 from Murine Myocardium Results in Cardiomyopathy, Altered Nuclear Morphology and Inhibition of the Biomechanical Gene Response
- Identification of Novel Genetic Loci Associated with Thyroid Peroxidase Antibodies and Clinical Thyroid Disease
- CEP-1, the p53 Homolog, Mediates Opposing Longevity Outcomes in Mitochondrial Electron Transport Chain Mutants
- Transcriptomics and Functional Genomics of ROS-Induced Cell Death Regulation by
- Quantitative Genome-Wide Genetic Interaction Screens Reveal Global Epistatic Relationships of Protein Complexes in
- Cascades of Genetic Instability Resulting from Compromised Break-Induced Replication
- Serine- and Threonine/Valine-Dependent Activation of PDK and Tor Orthologs Converge on Sch9 to Promote Aging
- Zfp322a Regulates Mouse ES Cell Pluripotency and Enhances Reprogramming Efficiency
- Insertional Mutagenesis and Deep Profiling Reveals Gene Hierarchies and a -Dependent Bottleneck in Lymphomagenesis
- DAAM Is Required for Thin Filament Formation and Sarcomerogenesis during Muscle Development in Drosophila
- Plasma Cholesterol–Induced Lesion Networks Activated before Regression of Early, Mature, and Advanced Atherosclerosis
- High-Resolution Profiling of Stationary-Phase Survival Reveals Yeast Longevity Factors and Their Genetic Interactions
- Comparative RNAi Screens in and Reveal the Impact of Developmental System Drift on Gene Function
- Accurate and Robust Genomic Prediction of Celiac Disease Using Statistical Learning
- Sex-Specific Embryonic Gene Expression in Species with Newly Evolved Sex Chromosomes
- Chromosome X-Wide Association Study Identifies Loci for Fasting Insulin and Height and Evidence for Incomplete Dosage Compensation
- Negative Feedback and Transcriptional Overshooting in a Regulatory Network for Horizontal Gene Transfer
- DNA Sequence Explains Seemingly Disordered Methylation Levels in Partially Methylated Domains of Mammalian Genomes
- Insights into the Genomic Landscape: Comparative Genomics Reveals Variations in Ploidy and Nutrient Utilisation Potential amongst Wine Isolates
- Molecular Evidence for the Inverse Comorbidity between Central Nervous System Disorders and Cancers Detected by Transcriptomic Meta-analyses
- The Centriolar Satellite Protein AZI1 Interacts with BBS4 and Regulates Ciliary Trafficking of the BBSome
- Fine-Mapping the Region Detects Common Variants Tagging a Rare Coding Allele: Evidence for Synthetic Association in Prostate Cancer
- Transmission Distortion Affecting Human Noncrossover but Not Crossover Recombination: A Hidden Source of Meiotic Drive
- A Variant in the Neuropeptide Receptor is a Major Determinant of Growth and Physiology
- Mutation of SLC35D3 Causes Metabolic Syndrome by Impairing Dopamine Signaling in Striatal D1 Neurons
- NSUN4 Is a Dual Function Mitochondrial Protein Required for Both Methylation of 12S rRNA and Coordination of Mitoribosomal Assembly
- MicroRNA-133 Inhibits Behavioral Aggregation by Controlling Dopamine Synthesis in Locusts
- Convergence of Light and ABA Signaling on the Promoter
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
- Distinct Requirements for Cranial Ectoderm and Mesenchyme-Derived Wnts in Specification and Differentiation of Osteoblast and Dermal Progenitors
- Chk2 and P53 Regulate the Transmission of Healed Chromosomes in the Male Germline
- Ddc2 Mediates Mec1 Activation through a Ddc1- or Dpb11-Independent Mechanism
- Mapping the Fitness Landscape of Gene Expression Uncovers the Cause of Antagonism and Sign Epistasis between Adaptive Mutations
- Euchromatic Transposon Insertions Trigger Production of Novel Pi- and Endo-siRNAs at the Target Sites in the Germline
- miR-100 Induces Epithelial-Mesenchymal Transition but Suppresses Tumorigenesis, Migration and Invasion
- Canine Hereditary Ataxia in Old English Sheepdogs and Gordon Setters Is Associated with a Defect in the Autophagy Gene Encoding
- Within-Host Spatiotemporal Dynamics of Plant Virus Infection at the Cellular Level
- Analysis of Meiosis in SUN1 Deficient Mice Reveals a Distinct Role of SUN2 in Mammalian Meiotic LINC Complex Formation and Function
- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- Mechanistically Distinct Mouse Models for -Associated Retinopathy
- DAF-16/FoxO Directly Regulates an Atypical AMP-Activated Protein Kinase Gamma Isoform to Mediate the Effects of Insulin/IGF-1 Signaling on Aging in
- Chromosome I Controls Chromosome II Replication in
- Integrated Genomic Characterization Reveals Novel, Therapeutically Relevant Drug Targets in FGFR and EGFR Pathways in Sporadic Intrahepatic Cholangiocarcinoma
- The Iodotyrosine Deiodinase Ortholog SUP-18 Functions through a Conserved Channel SC-Box to Regulate the Muscle Two-Pore Domain Potassium Channel SUP-9
- The Genome of Highlights a Fish Pathogen Adapted to Fluctuating Environments
- Distinct DNA Binding Sites Contribute to the TCF Transcriptional Switch in and
- The Streamlined Genome of spp. Relative to Human Pathogenic Kinetoplastids Reveals a Parasite Tailored for Plants
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study of Metabolic Traits Reveals Novel Gene-Metabolite-Disease Links
- A Cohesin-Independent Role for NIPBL at Promoters Provides Insights in CdLS
- Classic Selective Sweeps Revealed by Massive Sequencing in Cattle
- Arf4 Is Required for Mammalian Development but Dispensable for Ciliary Assembly
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání




