-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
Eukaryotic genomes are partitioned into active and inactive domains called euchromatin and heterochromatin, respectively. In Neurospora crassa, heterochromatin formation requires methylation of histone H3 at lysine 9 (H3K9) by the SET domain protein DIM-5. Heterochromatin protein 1 (HP1) reads this mark and directly recruits the DNA methyltransferase, DIM-2. An ectopic H3 gene carrying a substitution at K9 (hH3K9L or hH3K9R) causes global loss of DNA methylation in the presence of wild-type hH3 (hH3WT). We investigated whether other residues in the N-terminal tail of H3 are important for methylation of DNA and of H3K9. Mutations in the N-terminal tail of H3 were generated and tested for effects in vitro and in vivo, in the presence or absence of the wild-type allele. Substitutions at K4, K9, T11, G12, G13, K14, K27, S28, and K36 were lethal in the absence of a wild-type allele. In contrast, mutants bearing substitutions of R2, A7, R8, S10, A15, P16, R17, K18, and K23 were viable. The effect of substitutions on DNA methylation were variable; some were recessive and others caused a semi-dominant loss of DNA methylation. Substitutions of R2, A7, R8, S10, T11, G12, G13, K14, and P16 caused partial or complete loss of DNA methylation in vivo. Only residues R8-G12 were required for DIM-5 activity in vitro. DIM-5 activity was inhibited by dimethylation of H3K4 and by phosphorylation of H3S10, but not by acetylation of H3K14. We conclude that the H3 tail acts as an integrating platform for signals that influence DNA methylation, in part through methylation of H3K9.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002423
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002423Summary
Eukaryotic genomes are partitioned into active and inactive domains called euchromatin and heterochromatin, respectively. In Neurospora crassa, heterochromatin formation requires methylation of histone H3 at lysine 9 (H3K9) by the SET domain protein DIM-5. Heterochromatin protein 1 (HP1) reads this mark and directly recruits the DNA methyltransferase, DIM-2. An ectopic H3 gene carrying a substitution at K9 (hH3K9L or hH3K9R) causes global loss of DNA methylation in the presence of wild-type hH3 (hH3WT). We investigated whether other residues in the N-terminal tail of H3 are important for methylation of DNA and of H3K9. Mutations in the N-terminal tail of H3 were generated and tested for effects in vitro and in vivo, in the presence or absence of the wild-type allele. Substitutions at K4, K9, T11, G12, G13, K14, K27, S28, and K36 were lethal in the absence of a wild-type allele. In contrast, mutants bearing substitutions of R2, A7, R8, S10, A15, P16, R17, K18, and K23 were viable. The effect of substitutions on DNA methylation were variable; some were recessive and others caused a semi-dominant loss of DNA methylation. Substitutions of R2, A7, R8, S10, T11, G12, G13, K14, and P16 caused partial or complete loss of DNA methylation in vivo. Only residues R8-G12 were required for DIM-5 activity in vitro. DIM-5 activity was inhibited by dimethylation of H3K4 and by phosphorylation of H3S10, but not by acetylation of H3K14. We conclude that the H3 tail acts as an integrating platform for signals that influence DNA methylation, in part through methylation of H3K9.
Introduction
The primary structures of histones, the small basic proteins that are complexed with DNA to form chromatin in eukaryotes, are highly conserved but not invariant [1], [2]. For example, comparisons between a sea urchin and the filamentous fungus Neurospora crassa reveal that the two most highly conserved histones, H3 and H4, have 16/135 and 9/102 amino acid differences, respectively [3], [4]. Pioneering studies with the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe revealed that mutation, or even deletion, of some histone residues is not lethal, allowing for genetic studies of structure/function relationships of these prominent proteins [5]–[9].
Histones are subject to a variety of posttranslational modifications including methylation, acetylation, phosphorylation, ubiquitylation, sumoylation, as well as ADP-ribosylation, deimination and proline isomerization [1], [10]. An explosion of findings has implicated these modifications in various fundamental cellular processes, including transcription, alternative splicing, replication, chromosome condensation, recombination, DNA repair and DNA methylation [1], [10], [11]. One approach to investigate potential involvement of modifications of a particular histone residue is to test for effects of substitutions that prevent the modification or mimic the modified state. For example, because acetylation of lysines reduces the positive charge of this residue, it can be informative to test the effect of substituting lysines with non-modifyable residues that are either neutral (e.g. glutamine) or positively charged (e.g. arginine) [12]. To gain insight into residues involved in DNA methylation and other cellular processes, we carried out a systematic analysis of the heavily modified N-terminus of N. crassa histone H3 [13].
Neurospora is an excellent organism to study effects of histone H3 mutations in part because the wild-type genome contains a single H3 gene, hH3 [3], [4]. A direct link between H3 modifications and DNA methylation was originally demonstrated in Neurospora when DNA methylation was found to depend on trimethylation of histone H3 lysine 9 (H3K9me3) [14], [15]. In this organism, DNA methylation is primarily found at sequences that are products of a genome defense mechanism called RIP (Repeat Induced Point mutation), which is triggered by sequence repeats [16], [17]. Duplicated sequences are efficiently detected and mutated during the sexual phase of the Neurospora life cycle in the period between fertilization and nuclear fusion. Both copies of duplicated sequences are peppered with numerous C:G to T:A mutations, rendering the resulting sequences AT-rich, and the remaining cytosines are typically methylated [18], [19]. A single DNA methyltransferase (DIM-2), guided by Heterochromatin Protein 1 (HP1) [20], [21], is responsible for all known DNA methylation in Neurospora [22]. HP1 binds to H3K9me2 and even more tightly to H3K9me3, the predominant form found in Neurospora [23], [24]. Mutation of the dim-5 gene, which encodes an H3K9 methyltransferase responsible for most if not all H3K9me3 in Neurospora, results in complete loss of DNA methylation [14]. DIM-5 is part of the DCDC (DIM-5/-7/-9/Cul4/DDB1 Complex), and depends on DIM-7 for recruitment to future heterochromatin domains [25]–[27]. All components of the DCDC are required for normal H3K9 methylation [26]. It is also known that methylation of some regions requires dephosphorylation of H3S10 by PP1 [28]. The current study was carried out to decipher which residues of H3 play a role in DNA methylation. We expected to identify interactions with DIM-5 as well as interactions with other components of the methylation machinery, such as HP1. In plants [29]–[31] and animals [32], H3K9 methylation also directs some DNA methylation, raising the possibility that our findings will provide insights into shared mechanisms operating in a variety of organisms.
Initial experiments demonstrated that introduction of an ectopic H3 gene bearing substitutions at lysine 9 (e.g. hH3K9R) can cause dominant loss of DNA methylation and can reactivate a methylation-silenced transgene, hphme [14]. Structural studies demonstrated intimate contacts between the H3 tail (residues A7-G12) and the DIM-5 backbone [33], suggesting that additional residues may be important for DIM-5 activity and DNA methylation. We wished to test both for possible dominant effects, as initially detected for K9 substitutions [14], and for possible recessive effects on DNA methylation, H3K9me and DIM-5 activity. We therefore first transformed a strain harboring the methylation-silenced transgene (hphme) to screen for substitutions that cause a dominant loss of DNA methylation. We next constructed substitution strains to reveal recessive mutations. Our current results suggest that multiple H3 tail residues, or their modifications, are involved in DNA methylation. We found both recessive and dominant effects.
Results
Amino-acid substitutions in the N-terminal tail of H3 reactivate a transgene and reduce DNA methylation
Roughly half of the approximately 40 amino acid residues in the highly conserved N-terminal tail of histone H3 are subject to covalent modifications (Figure S1) [1], [2], [10]. To improve our understanding of the role of H3 in DNA methylation, we carried out mutational analyses of these and other residues of potential interest, such as those around lysine 9 (Figure 1A). Acetylatable lysines were changed to arginines (K→R) or to glutamines (K→Q) to simulate the hypoacetylated or acetylated states, respectively. Lysines and arginines that might be methylated were changed to leucines (K/R→L), and potentially phosphorylated serines and threonines were changed to alanines (S/T→A) to prevent modification. We also tested for a constraint on the spacing of residues by inserting an extra glycine at GG12,13 (+G13). We took advantage of a simple genetic test for effects on DNA methylation. Mutant alleles were introduced into a hygromycin-sensitive reporter strain, N644, in which the hygromycin phosphotransferase gene, hph, was reversibly silenced by DNA methylation [34]. In our initial experiments, we co-transformed N644 with the mutant constructs along with a dominant selectable marker, BenR, which confirms resistance to benomyl [14]. Loss of hph methylation caused by dominant or semi-dominant effects of the hH3mutant alleles would render the strain resistant to hygromycin (Figure 1B). Although co-tranformation is extremely efficient in Neurospora (typically 50–90% of transformants incorporate the non-selected DNA), representative transformants were tested to verify that the H3 construct had been integrated in a fraction of the tranformants that did not produce hygromycin resistant strains (data not shown).
Fig. 1. Reactivation of a methylation-silenced transgene by amino acid substitutions in the N-terminal tail of histone H3. 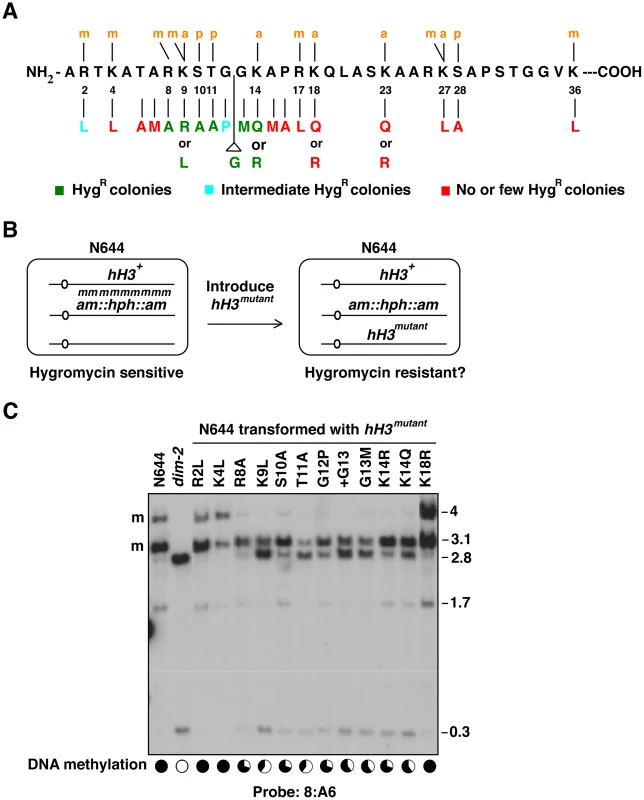
(A) Sequence of N. crassa H3 amino-terminal tail and summary of results after transformation of strain N644 with hH3 alleles bearing indicated mutations. (See also Figure S1). (B) Scheme to test the possible role of H3 residues in DNA methylation. Strain N644 harbors a methylated, silenced hph transgene, which confers resistance to hygromycin when it is expressed. Transformation of this strain with mutant forms of hH3 normally result in a mixture of wild-type and mutant histone in the cell. If the tested amino acid residue is critical for DNA methylation this may cause a semi-dominant loss of DNA methylation and thus expression of hph. (C) Partial loss of DNA methylation in transformants with native hH3 plus ectopic mutant hH3 alleles. DNA from selected hygromycin-resistant transformants [hH3R2L (N3079); hH3K4L (N3081); hH3R8A (N3082); hH3K9L (N3084); hH3S10A (N3085); hH3T11A (N3087); hH3G12P (N3088); hH3+G13 (N3090); hH3G13M (N3092); hH3K14R (N3094); hH3K14Q (N3096); hH3K18R (N3098)] was digested with 5-methylcytosine-sensitive restriction enzymes (BamHI and EcoRI) and analyzed by Southern hybridization. The blots were probed for the 8:A6 region [18], which is normally methylated (m), giving rise to 4.0 and 3.1 kb fragments. Complete loss of methylation, as in the dim-2 mutant, gives rise to 2.8 and 0.3 kb fragments. The levels of DNA methylation in various strains, determined by the relative intensities of the 3.1 and 2.8 kb bands using ImageJ [79] are indicated in the pie-charts (bottom). To convert the images into the approximate methylation levels conveyed in the pie graphs, we used densitometry to compare the intensity of bands representing methylated and unmethylated sites and normalized the results to that in wild-type (defined as 100%). Similar results were observed for other methylated regions including ψ63, 2:B3 (see also Figure S2, Table S4), 5:B8, 8:G3, 1d21 and 8:F10 (data not shown). We found that substitutions of any residue between positions 8 to 14 caused hygromycin resistance (Figure 1A, Table 1 and Table S1). Similarly, insertion of an extra glycine at GG12-13 (+G13) caused hygromycin resistance. Transformation experiments were repeated 4–8 times with similar results (Table 1). Some substitutions, notably G12P and R2L, gave variable or weaker hygromycin resistance; transformations with constructs bearing these substitutions only gave rise to hygromycin resistant colonies in ∼50% of the transformations. Overall, phenotypic analyses of transformants suggested that silencing depends on R2 and residues around K9 (R8 to K14) and that the spacing between residues around GG12-13 is important.
Tab. 1. DNA methylation analyses of HygR colonies obtained in cotransformations of N644 with hH3 alleles. 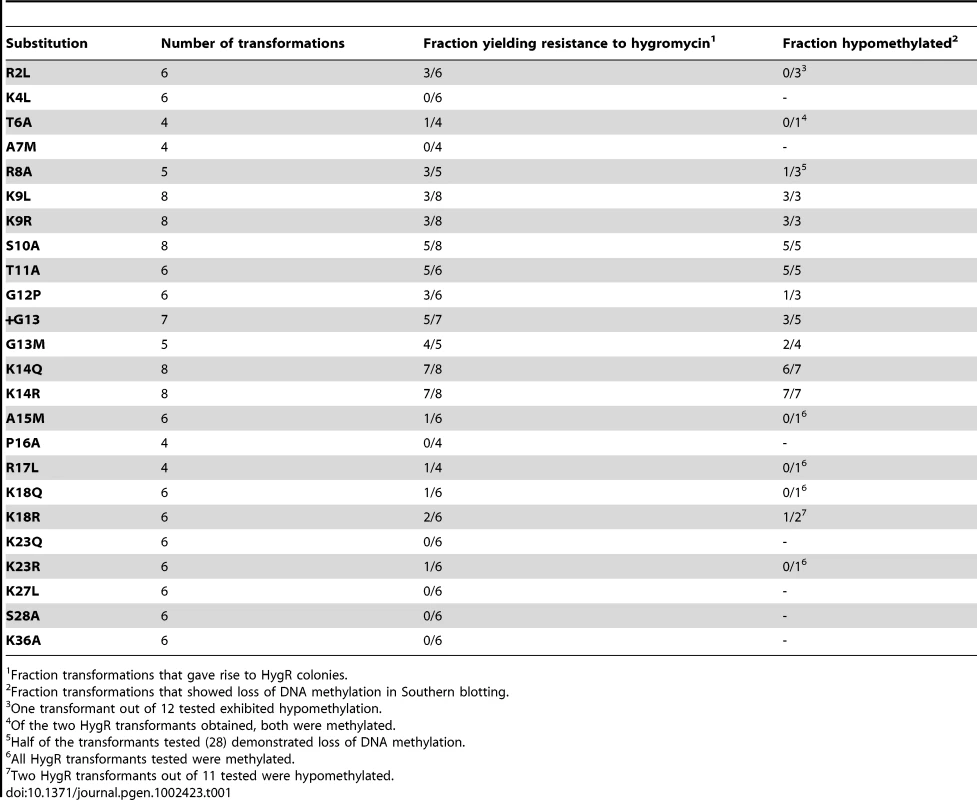
Fraction transformations that gave rise to HygR colonies. The methylated hph allele is somewhat unstable; its reversion frequency is ∼10−5 [25], [34]. To confirm that H3 mutations caused loss of DNA methylation, we directly examined the methylation status of genomic DNA by Southern hybridization using 5-methylcytosine-sensitive restriction endonucleases. Blots were probed for several genomic regions that are typically methylated [18]. DNA from the parental strain (N644) and from a DNA methyltransferase mutant (dim-2) served as positive and negative controls, respectively, and DNA from several independent transformants were analyzed for each hH3 construct. Consistent reductions in DNA methylation were observed for all transformants with S10A, T11A, G12P, +G13, G13M, K14R, K14Q, K9L and K9R mutations (Figure 1C, Figure S2A and S2B, Table 1 and Table S1). Curiously, about half of the hygromycin-resistant colonies that came from the R8A transformations exhibited loss of DNA methylation. None of the few hygromycin-resistant colonies that were obtained with the T6A, A15M, R17L, K18R, K18Q and K23R constructs showed changes in DNA methylation. In the case of R2L, although hygromycin-resistant colonies were obtained in half of the trials, only one of twelve mutant strains analyzed showed a significant reduction in DNA methylation. This variability might result from differences in copy number and/or chromosomal location of the integrated mutant alleles. Indeed, we found that some hygromycin-resistant transformants had single ectopic integrations of hH3mutant genes while others had multiple integrations (Figure S2C). Multiple integrations might result in a dose-dependent loss of DNA methylation. Of course, hygromycin resistance may also result from unknown genetic or epigenetic effects. To address these possibilities, we developed a system to construct strains with single copies of mutant alleles inserted at a particular location in the genome, as described below.
Amino acid residues surrounding K9 are required for DIM-5 activity in vitro
H3 substitutions may cause loss of DNA methylation through effects on site recognition or catalysis by the histone methyltransferase, DIM-5. Alternatively, H3 substitutions might affect the DNA methylation pathway downstream of H3K9me. In one approach to distinguish between these possibilities, we tested the in vitro activity of DIM-5 on various H3 substrates. We constructed modified forms of a GST-H3 (residues 1–57) fusion corresponding to the amino acid replacements that we tested in vivo. The fusion proteins were incubated with DIM-5 and radio-labeled methyl-group donor (S-adenosyl methionine), fractionated by SDS-polyacrylamide gel electrophoresis and tested for incorporation of 3H-methyl groups by fluorography (Figure 2A). As expected from our previous finding that H3K9 is the only target for DIM-5 [14], the K9L substitution abolished DIM-5 activity. The R8A and G12P mutations also completely inhibited DIM-5 activity. The S10A and T11A mutations greatly reduced, but did not abolish, DIM-5 activity. Slightly reduced activity was observed with the G13M protein in most assays performed. In contrast, K4L, A7M, +G13, K14R, A15M and P16A mutations had no significant effect on DIM-5 activity. The K14R mutation was not inhibitory in vitro while this change caused striking hygromycin resistance and loss of methylation in vivo (Figure 1). We conclude that the effects of mutations in H3 residues 8–12 are likely due to direct effects on DIM-5/H3 interactions while the inhibitory effects on DNA methylation of K14R is presumably due to some unknown downstream effects (e.g. on HP1 or DIM-2 action).
Fig. 2. DIM-5 activity on histone H3 peptides. 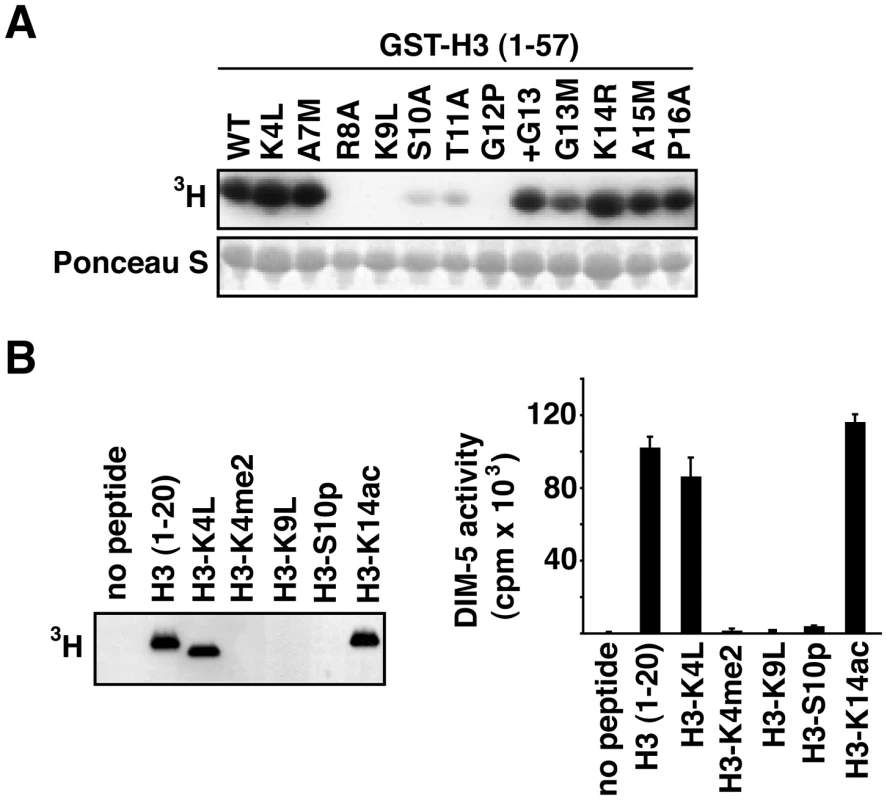
(A) Residues surrounding H3K9 are critical for DIM-5 activity. Histone methyltransferase assays were performed with GST-H3 (1–57) bound to glutathione-agarose and S-adenosyl [methyl-3H]-L-methionine as the methyl group donor and analyzed by gel electrophoresis and fluorography. The membrane was stained with Ponceau S to confirm amount of protein loaded (bottom panel). (B) DIM-5 is sensitive to modifications of the H3 tail. In vitro assays were performed with unmodified H3 peptide (residues 1–20) or with peptides bearing covalent modifications (K4me2, S10ph and K14ac) or a substitution (K4L). Incorporation of the methyl group was analyzed by fluorography (left panel) or by liquid scintillation counting (right panel, each bar represents an average of three reactions). Methylation of H3K4 and phosphorylation of H3S10, but not acetylation of K14, inhibit methylation of H3K9 by DIM-5
The H3 tail is subject to a variety of post-translational modifications (Figure S1) that could potentially play a role in controlling DIM-5 action. Indeed, there is precedence for effects of modifications on the activity of histone methyltransferases related to DIM-5. In particular, SUV39H1, SETDB1 and Clr4 have been shown to be sensitive to phosphorylation of H3S10 (H3S10ph) [35]–[37]. We were most interested in the possibility that H3K4me, a mark common in euchromatic regions, might interfere with DNA methylation in Neurospora. In addition, we wished to test the effect of acetylation of H3K14 (H3K14ac) because this modification is apparently countered by histone deacetylase 1, which is important for DNA methylation in Neurospora [38], and because substitution of K14 inhibited DNA methylation in vivo but did not noticeably affect DIM-5 activity in vitro (Figure 1A, 1C and Figure 2A). We therefore tested the effect of methylation of H3K4, acetylation of H3K14 and phosphorylation of H3S10 on in vitro DIM-5 activity with peptides covering residues 1–20 of H3 (Figure 2B). As expected, a peptide that had a substitution at the target residue, H3K9, completely abolished DIM-5 activity. Robust activity was found with a H3K14ac peptide. This contrasts results obtained with the H3K9 methyltransferase SETDB1 [37] but is consistent with findings with the SUV39H1 and Clr4 H3K9 methyltransferases [35], [36]. Essentially no DIM-5 activity was detected with H3S10ph or H3K4me2 peptides, whereas a peptide bearing the K4L substitution showed full activity. H3K4me2 did not affect H3K9 methylation by SETDB1 or Clr4 [36], [37]. Our results support the idea that H3K4me2 and H3S10ph impact DNA methylation by preventing DIM-5 activity. In contrast, the importance of H3K14 presumably results from an effect downstream of H3K9 methylation by DIM-5.
Recessive effects of hH3 mutations
In theory, Neurospora transformants of strain N644 may have resulted either from ectopic insertion of hH3mutant genes or from gene replacement events. In practice, only ectopic insertions were obtained, and most transformants had single integrations (Figure S2C), which is unusual for transformation of Neurospora. This raised the possibility that transformants bearing exclusively, or even predominantly, altered H3 alleles may not be viable. To test this possibility, and to investigate if some H3 substitutions might be recessive and thus show defects that were masked by the simultaneous presence of wild-type H3, we developed a system to test hH3 constructs as single copies at a defined genomic location, in the presence or absence of a wild-type allele. The system takes advantage of a non-functional hH3 allele at the endogenous locus (hH3RIP1) that we generated by RIP when we crossed two strains homozygous for an hH3S10E allele at the his-3 locus [28]. The resulting hH3RIP1 strain with hH3S10E at his-3 was crossed with transformants bearing other hH3 substitution alleles integrated at his-3 to test whether progeny with substitution alleles at his-3 would be viable in the absence of a functional allele (Figure 3A; Text S1). For most of our substitutions, including those at R2, A7, R8, S10, A15, P16, R17, K18 and K23, we were able to build such (hH3RIP1; hH3mutant) strains. Thus the entire cellular pool of H3 carries the relevant substitution in these strains. Although viable, all these strains showed defects in vegetative development (Figure 3B, Figure S3) and were female sterile (data not shown). For substitutions of other residues (K9, G12, G13, K14, K27, S28 and K36), we were only able to obtain strains with the substitution allele at his-3 when the wild-type allele was present at the native locus (hH3WT; hH3mutant), suggesting that these mutations are lethal in the absence of wild-type H3. We also found evidence that substitutions of K4 and T11 are not tolerated in the absence of a wild-type allele (data not shown). Although it was conceivable that some or all of these mutations simply affected protein stability, their semi-dominant effects argued against this.
Fig. 3. Substitutions in the H3 tail can cause recessive or semi-dominant loss of DNA methylation. 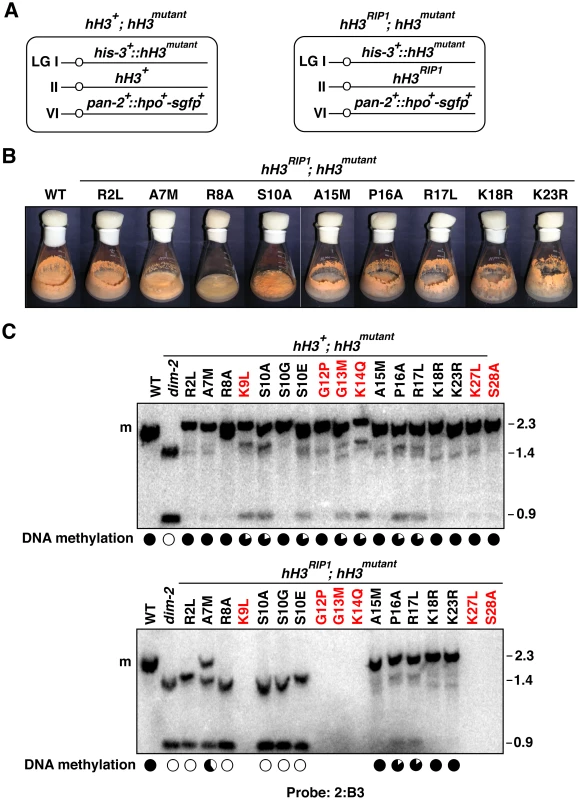
(A) Generalized genotypes of strains harboring two copies of hH3. Various substitution alleles (hH3mutant) were integrated at an ectopic location (his-3) and combined with either the wild-type allele (hH3+; left) or the null allele (hH3RIP1; right) at the native hH3 locus. A gene encoding HP1-GFP was integrated at the pan-2 locus to visualize heterochromatin by microscopy (Figure 4B). (B) Defective asexual development in stains containing mutant H3. The H3 mutants showed reduced growth relative to wild-type (N150) and reduced production of asexual spores. The strains tested carried the following mutations: hH3R2L (N3520); hH3A7M (N3537); hH3R8A (N3542); hH3S10A (N3474); hH3A15M (N3553); hH3P16A (N3556); hH3R17L (N3560); hH3K18R (N3565); hH3K23R (N3568). All strains were grown on solidified minimal medium with 1.5% sucrose for 7 days at 32°C. (See also Figure S3). (C) Loss of DNA methylation caused by H3 substitutions. Southern blotting with DNA isolated from strains that contain either a mixture of wild-type and altered H3 [top panel: hH3R2L (N3524); hH3A7M (N3540); hH3R8A (N3543); hH3K9L (N3544); hH3S10A (N3494); hH3S10G (N3475); hH3S10E (N3476); hH3G12P (N3546); hH3G13M (N3548); hH3K14Q (N3550); hH3A15M (N3554); hH3P16A (N3558); hH3R17L (N3562); hH3K18R (N3566); hH3K23R (N3570); hH3K27L (N3572); hH3S28A (N3573)] or just altered H3 [bottom panel: hH3R2L (N3520); hH3A7M (N3537); hH3R8A (N3542); hH3S10A (N3474); hH3S10G (N3477); hH3S10E (N3478); hH3A15M (N3553); hH3P16A (N3556); hH3R17L (N3560); hH3K18R (N3565); hH3K23R (N3568)]. DNA was digested with the 5mC-sensitive restriction enzyme AvaII and the blots were probed for the methylated region 2:B3 [18]. Levels of DNA methylation are summarized as in Figure 1 based on the relative intensitites of 2.3 and 0.9 kb bands. Similar results were observed for other methylated regions including 2:G12 and 8:A6 (see also Figures S4 and S5, Table S4). Analyses of DNA methylation in the targeted H3 mutant strains was informative (Figure 3). Where comparisons could be made with results obtained with the strains bearing one or more copies of mutant constructs at undefined sites, the strains with precisely one wild-type and one mutant H3 gene gave similar results (Figure 3C and Figure S4; Table S4). For example, mutation of K14 gave equivalent results with both experimental schemes. [Incidentally, it is noteworthy that the extent of loss of DNA methylation caused by substitutions in K14 was similar to that caused by substitutions in K9 even though the K14 mutations, unlike the K9 mutations, did not substantially interfere with methylation by DIM-5 in vitro (Figure 2) or in vivo (data not shown)]. We did observe some variability between experiments and among methylated regions tested (Table S4). R8A, which caused a partial (∼25%) reduction in DNA methylation in cotransformation experiments (Figure 1 and Figure S2), showed comparable reduced methylation at the 2:G12 region when the mutant allele was at the his-3 locus but did not show significant loss of methylation at 8:A6 or 2:B3. The K9L mutation, which resulted in clear loss of methylation in cotransformants at all regions tested also showed obvious reduced methylation at 8:A6 and 2:B3 but not at 2:G12 when targeted to his-3. Substitutions of S10 with A or E resulted in partial loss of DNA methylation at all sites examined but S10G did not cause loss of methylation at any region tested, when there was a wild-type H3 gene in the same strain. Mutations of R2, A7, A15, K18, K23, K27 and S28, which did not reveal loss of silencing in the cotransformation experiments also showed no loss when the mutant allele was targeted to his-3 in the presence of the native H3 gene. Mutations of P16 and R17, which also showed no effect in the cotransformation experiments, caused modest reduction of DNA methylation, but only in the 2:B3 region. In contrast, substitution of residues G12 showed significant loss of methylation in the contransformation experiments but not in the targeted transformants and mutation of G13 showed a modest reduction of methylation in the latter experiments and a greater reduction in the contransformants.
The results summarized above unequivically indicated that a number of mutations of the H3 gene are semi-dominant. Interestingly, we also found several mutant constructs that showed dramatic effects on DNA methylation only when they provided all of the H3, i.e. they were recessive. The most striking results were obtained for the R2L mutation. Although this change showed little, if any, effect in the presence of normal H3 (Figure 1 and Figure 3), the mutant allele, when alone, caused a nearly complete loss of DNA methylation (Figure 3C, Figures S4 and S5) Interestingly, methylation of H3 K9 and HP1 binding appeared normal (Figure 4), suggesting that R2 plays a role downstream of these events.
Fig. 4. Reduced H3K9 methylation and HP1 binding in selected hH3 substitution strains. 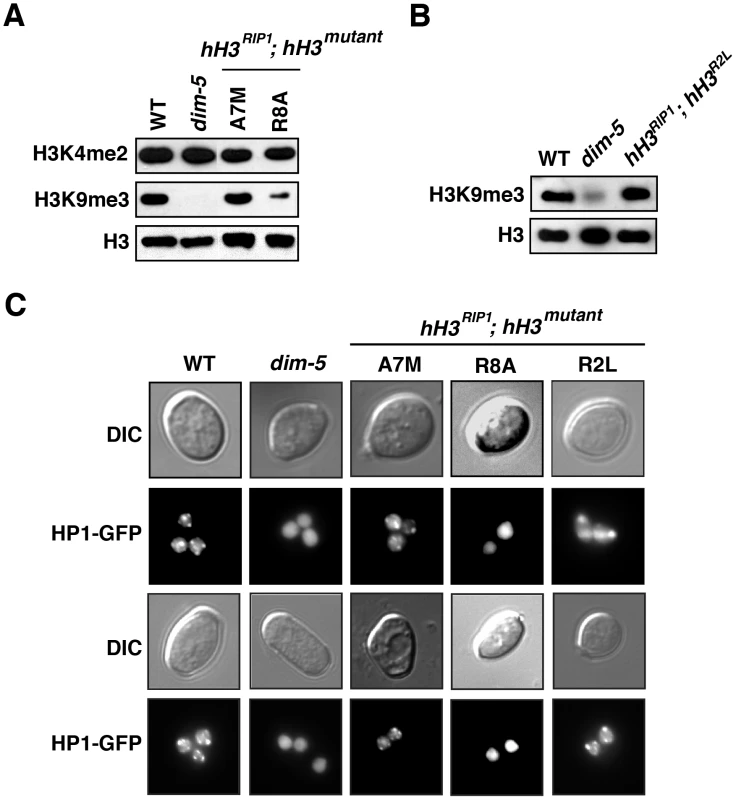
(A) Reduction in H3K9 methylation in the A7M and R8A substitution strains. Western blots of nuclear extracts from wild-type (N150), dim-5 (N2264), hH3A7M (N3537) and hH3R8A (N3542) strains. An antibody recognizing the C-terminus of H3 served as a loading control. (B) Apparently normal H3K9 methylation and HP1 binding in the hH3R2L mutant. Western blots of nuclear extracts from wild-type (N150), dim-5 (N2264) and hH3R2L (N3520) strains. An antibody recognizing the C-terminus of H3 served as a loading control. (C) Localization of HP1-GFP in H3 substitution strains. Duplicate sets of images generated by light (DIC) and fluorescence (HP1-GFP) microscopy on germinating conidia of wild type (N2534), dim-5 (N2542), hH3A7M (N3537), hH3R8A (N3542) and hH3R2L (N3520) strains are shown. The A7M and R8A substitution alleles also showed marked reductions in DNA methylation alone, but little or no reduction when present along with the wild-type allele (Figure 3C). Of these two mutants, R8A gave the stronger effect, consistent with its marked inhibition of H3K9 methylation in vitro (Figure 2). We confirmed that the R8A substitution strain, but not the A7M strain, also shows greatly reduced H3K9me3 and mislocalization of HP1 in vivo (Figure 4).
Discussion
We took advantage of the single-copy status of the histone H3 gene in Neurospora to explore the possible involvement of particular amino acid residues of H3 in DNA methylation. The study was facilitated by a methylated hph allele that allowed detection of partial loss of DNA methylation. Previous work revealed that transformation of the reporter strain with alleles of hH3 bearing substitutions in H3K9 caused almost complete loss of DNA methylation, while retaining a wild-type allele of hH3, i.e. the mutations were semi-dominant, as if the altered H3 “poisoned” the methylation machinery [14]. This may reflect a requirement for the HP1 dimer to bind pairs of methylated H3K9 residues within one nucleosome or between adjacent nucleosomes [39]. Failure to isolate transformants bearing multiple copies of the mutant allele further suggested that a preponderance of H3 with this substitution might not be tolerated by Neurospora [14].
Here we explored the generality of these observations by extending our analysis to include all residues in the N-terminal tail that are thought to be subject to post-translational modification [1], [10]. In addition to testing various residues with our system pioneered for K9, we also developed a scheme to test directly for viability of strains bearing mutant alleles in the absence of a wild-type copy of hH3 [28]. This approach identified a number of residues that are essential for viability and revealed nonlethal mutations that resulted in recessive DNA methylation defects (Table 2). The most striking recessive defects were due to R2L, A7M, R8A and S10G substitutions. S10A and S10E showed semi-dominant defects that became much more pronounced in the absence of the wild-type allele while some other changes, including P16A and R17L, showed modest defects either in the presence or absence of the wild-type allele. Most, but not all, residues identified as important for DNA methylation in vivo were also important for DIM-5 histone methyltransferase activity in vitro. In particular, R8, K9, S10, T11, G12 and G13 were required for H3K9me3 by DIM-5 in vitro, while substitutions in every residue from A7 through R17 caused loss of DNA methylation in vivo. We also found that the spacing between residues can be important since introduction of an extra glycine at GG12-13 caused a loss of DNA methylation. Finally, we found that DIM-5 is sensitive to certain H3 modifications (H3K4me2 and H3S10ph) but not to others (H3K14ac).
Tab. 2. Summary of H3 mutagenesis results. 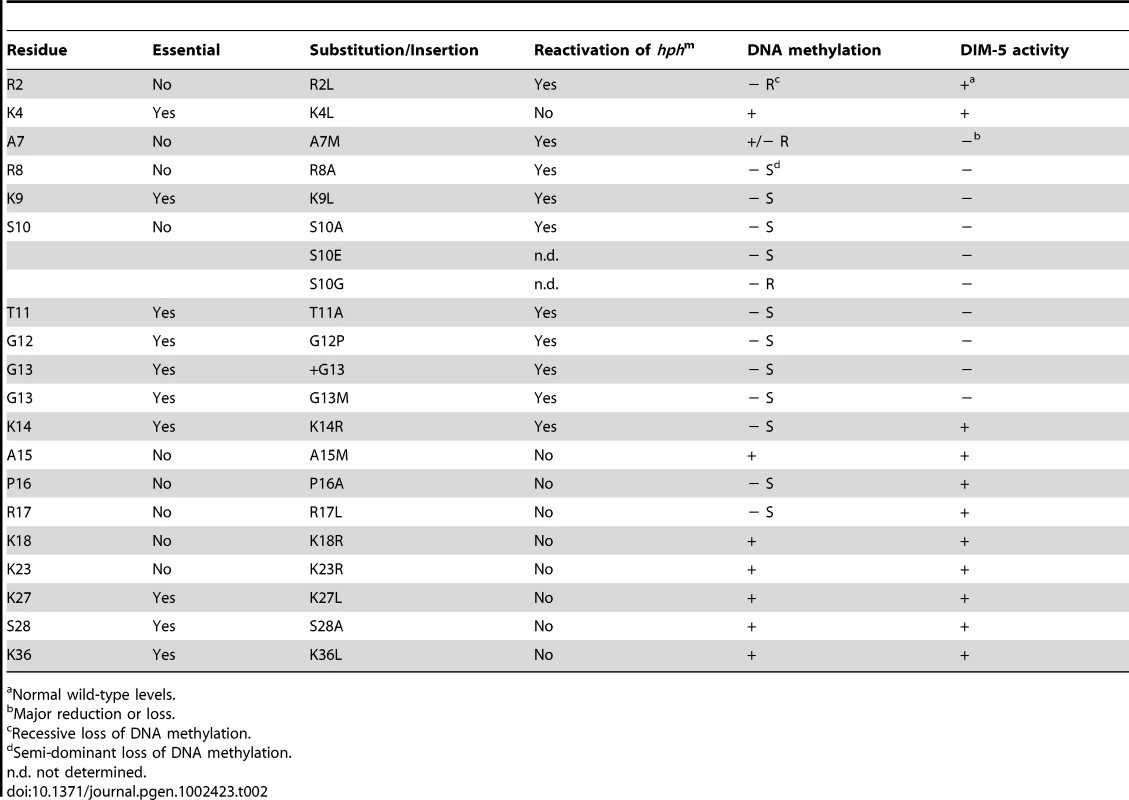
Normal wild-type levels. Our results provide clues to the role of histone H3 in the control of DNA methylation. No comparable study has been carried out in a system with DNA methylation. Extensive mutational analyses of histones have been carried out in the budding yeast S. cerevisiae [6]–[8], however, and more limited studies have been carried out in the fission yeast S. pombe [9], which unlike S. cerevisiae, sports H3K9 methylation. We found that point mutations in K4, K9, T11, G12, G13, K14, K27 and S28 could not be tolerated by Neurospora in the absence of a normal allele. Interestingly, unlike yeasts that have been examined, K27 is subject to methylation in Neurospora, but this modification is not essential (K. Jamieson and E. Selker, unpublished observation). Substitutions in K4, K9, S10 and K14 are tolerated in fission yeast [9], [40] although they do interfere with heterochromatin function, as in Neurospora. A curious result from studies in budding yeast is that effects of histone mutations appear to depend to some extent on strain background [7]. Our observation that a mutant allele containing an extra residue (+G13) is tolerated only in presence of a sheltering wild-type allele implies that the proper spacing between H3 N-terminal tail residues is critical for viability. This provides a possible explanation for the observed lethality of a deletion of residues 4 through 10 in budding yeast, even though substitutions in individual residues from 1 through 39 were tolerated [7].
Phosphorylation of T3, T6, S10, T11 and S28 in the H3 tail has been found to be required for chromatin-dependent processes including gene silencing in several systems [28], [41]–[47]. Based on the crystal structure of DIM-5, the presence of a phosphate on H3S10 should prevent the interactions between the hydroxyl group of S10 and residues in DIM-5 that are essential for optimal catalytic activity (Y283 and D209) [33]. The expected importance of the dephosphorylated state for H3K9 methylation by DIM-5 was supported by findings with strains deficient in the H3S10 phosphatase PP1; an increase in global levels of H3S10ph caused reduced levels H3K9me3 in regions of the genome that loose DNA methylation [28]. This was supported by the observed complete loss of H3K9me and DNA methylation in hH3S10A, hH3S10G, hH3S10E substitution strains (Figure 3C, Figures S4 and S5 and [28]). Information from other systems suggests that phosphorylation of the neighboring residue, T11, may be also important. H3T11ph is associated with chromosome condensation during mitosis and meiosis in plants [48]. In mammals, phosphorylation of H3T11 by PRK1 in response to androgen-dependent stimulation accelerates demethylation of H3K9me3 by the JMJD2C demethylase and activates transcription of target genes [43]. Protein kinase Chk1 also phosphorylates H3T11 and its dissociation from chromatin in response to DNA damage causes a decrease in H3T11ph and release of the H3K9 acetyltransferase GCN5, leading to transcriptional repression [42]. We found that replacement of this residue with alanine caused a semi-dominant loss of DNA methylation (Figure 1C and Figure S2). The main chain carbonyl group of H3T11 forms a hydrogen bond with the DIM-5 backbone (Q285) and the alanine substitution would disrupt this interaction, presumably resulting in the observed loss of DIM-5 activity in vitro (Figure 2A and [33]). H3T11ph, like H3S10ph, may impact H3K9 methylation and DNA methylation
It is interesting that substitutions of H3K14 caused a semi-dominant loss of silencing and DNA methylation, especially considering that neither mutation nor acetylation of this residue appreciably affected DIM-5 activity in vitro (Figure 2; [49]). Similarly, mammalian SUV39H1 and fission yeast Clr4 are not affected by H3K14ac [35], [36]. One possibility is that H3K14 is important for an activity downstream of methylation of H3K9 by DIM-5. In Neurospora, H3K9me3 is recognized by HP1, which then directly recruits the DNA methyltransferase DIM-2 to the heterochromatin domains [20], [21]. The fact that the H3K14 mutations can only be studied while effectively heterozygous confounded attempts to determine whether HP1 binding is reduced but it is interesting that we found little if any change in H3K9 methylation and HP1 binding in the K14Q mutant (Figure S6). It is noteworthy, that acetylation of this K14 does not affect binding of the S. pombe HP1 homologue, Swi6 [50], in vitro while mutation of H3K14 in S. pombe causes mislocalization of Swi6 [9]. Alhough H3K14ac does not affect H3K9 methyltransferase activity in vitro, it remains possible that it interferes with the establishment of H3K9 methylation in vivo. In both S. pombe and N. crassa, the H3K9 methyltransferases are components of multi-protein complexes, the CLRC and DCDC, respectively [25], [26], [51], [52]. Although there are important mechanistic differences between these two complexes, K14 mutations may influence H3 interaction with proteins that guide the H3K9 methyltransferases. The possible importance of H3K14ac in regulation of heterochromatin formation is consistent with the observed requirement of histone deacetylases (HDACs) for DNA methylation in N. crassa and for heterochromatin formation in S. pombe [38], [50], [53]–[55].
An important new finding from our study is that mutation of H3R2 disrupted DNA methylation. Information from other organisms suggests that this arginine is one of several in H3 that are subject to methylation, namely R2, R8, R17 and R26 [56]. Thus it will be of interest to determine if methylation of one or more of the arginines in the H3 N-terminal tail (Figure S1) impact methylation of H3K9 and DNA. Substitutions of both R8 and R17 (hH3R8A and hH3R17L) caused semi-dominant loss of DNA methylation, whereas substitution of R2 (hH3R2L) caused recessive loss of DNA methylation (Figure 3C, Figures S4 and S5). Presumably this reflects different roles of these residues in heterochromatin formation. The structure of DIM-5 complexed with an H3 peptide shows intimate contacts between R8 and the DIM-5 backbone [33]. Methylation of R8 is expected to prevent these interactions, consistent with the lack of DIM-5 activity in vitro on a substrate with the R8A substitution (Figure 2A and [49]) and with the observations on strains bearing this substitution, namely reduced H3K9me3, mislocalization of HP1 and loss of DNA methylation (Figure 4A and 4B). In contrast to the situation with R8, R2 lies outside of the region bound by DIM-5 (A7-G12) [33] and substitutions in R2 have little effect on DIM-5 activity in vitro [49]. Conceivably, the loss of DNA methylation observed in the R2L mutant is mediated by H3K4 methylation. It is known that H3R2me2 indirectly blocks H3K4me3 in both budding yeast and mammals [57]–[59]. Though H3K4me2 inhibits DIM-5 activity in vitro (Figure 2B and 2C), we observed wild-type levels of H3K9 methylation in the R2L mutant in vivo and apparently normal localization of HP1 (Figure 4). These observations suggests that the recessive loss of DNA methylation caused by R2L substitution results from disruption of a step downstream of H3K9 methylation and HP1 binding.
Our finding that DIM-5 activity is inhibited by methylation of H3K4 (Figure 2B) raises the possibility that effects on DNA methylation may reflect effects on methylation of H3K4. Indeed, methylated forms of H3K4 and H3K9 appear mutually exclusive in the Neurospora genome [19]. The mammalian H3K9 methyltransferase G9a is similarly sensitive to methylated forms of K4 [60] but, surprisingly, other H3K9 HMTases including S. pombe Clr4, mammalian SETDB1 and dSU(VAR)3-9, have been reported to methylate H3 peptides bearing K4me2 [35], [36], [61]. The establishment of H3K9me in these organisms apparently requires the concerted activity of various H3K4 demethylases, specifically SU(VAR)3-3 in Drosophila and Lid2 in fission yeast [62], [63]. In mammals, the DNA methyltransferase regulator DNMT3L directly interacts with the H3 tail and this interaction is abolished by methylation of H3K4me [64]. Additionally, Cfp1, a component of the H3K4 methyltransferase complex Setd1, binds unmethylated CpGs and helps establish H3K4me3 domains free of DNA methylation [65], [66].
In summary, our observations in Neurospora suggest that H3 amino-terminal tail residues and their covalent modifications regulate methylation of H3K9, binding of HP1, and one or more downstream events required for the establishment, maintenance and propagation of DNA methylation. Strains generated in this study should be useful to elucidate the role of H3 in other processes in Neurospora including the gene silencing mechanisms, RIP, meiotic silencing and quelling, as well as in other chromatin-based processes including transcription, DNA repair, recombination and the genomic arrangement of histone variants.
Materials and Methods
Neurospora strains and methods
Strains used in this study are listed in Table S2. Standard procedures were followed to grow strains and to perform crosses [67]–[69]. Primers used in this study are listed in Table S3.
Isolation of genomic DNA and Southern analyses
Genomic DNA was prepared as described previously [70], [71] from strains grown with shaking in Vogel's medium N with appropriate supplements at 32°C for 3 days. For Southern blotting, approximately 1 µg DNA was digested overnight with 3–5 units of the desired restriction enzyme, fractionated on 0.8 or 1.0% agarose gels and transferred to nylon membrane [72]. Hybridizations were performed with probes prepared by random hexamer priming [73], as previously described [74].
Histone H3 methyltransferase assay
DIM-5 assays were carried out in a volume of 20 µl at 10°C for 1 h with 5 µg of the appropriate recombinant GST-H3 variant (H3 residues 1–57) and 2.75 µCi of S-adenosyl [methyl-3H]-L-methionine (NEN), as previously described [14]. The reaction products were fractionated on SDS-PAGE gel (16%, acrylamide/bis, 29∶1) and incorporation of the radioactive methyl group was analyzed by fluorgraphy using ENTENSIFY (DuPoint). To test the effect of covalent H3 modifications or amino acid substitutions on DIM-5 activity, similar assays were performed with 0.5 µg peptide substrates (modified or unmodified H3 residues 1–20) [15]. The reaction products were analyzed by fluorography or precipitation with 20% TCA, filtration (Millipore GF/F filter), washing and liquid scintillation counting [75].
Western blotting
Nuclei were isolated as previously described [76], but with minor modifications. The following enzyme inhibitors were added to all buffers: 1 mM sodium butyrate, 1 µM Trichostatin A, 1 µM PMSF, 3 mM DTT, 10 mM sodium fluoride, 1 mM sodium vanadate, 0.1% phosphatase inhibitor cocktail (Sigma, P2850) and 1 µM each of leupepsin, pepstatin and E-64. Western blotting was performed with ∼100 µg of nuclear protein as described previously [15]. The following antibodies were used: α-H3K4me2 (Upstate, 07-030), α-H3K9me3 [15] and α-H3 C-terminal (Active Motif/LP Bio, AR-0144-200). Modifications on histones are represented according to the nomenclature proposed by Turner [77]. All antibodies were used at a dilution of 1∶5000. Horseradish peroxidase (HRP)-conjugated goat antibody against rabbit IgG was used to detect antibody-peptide complexes by chemiluminescene (Thermo Fischer Scientific Inc, USA).
Microscopy
Dilute suspensions of vegetative spores (conidia) were germinated on solidified Vogel's N medium with 1.5% sucrose and the required supplements for 2 hrs at 30°C. Square pieces of agar with the germinating conidia were cut out from plates and placed on glass slides. Agar pieces were flooded with a few drops of liquid Vogel's N medium and then overlayed with coverslips [78]. Bright-field and fluorescence images were collected on a Zeiss Axioplan 2 Imaging System with a EBQ 100 isolated light source, Endow GFP (S65T) filter (excitation 470, emission 525) and Plan-APOCHROMAT 100×/1.46 N.A. objective. Images were processed with Axiovision (version 4.6.3) and Adobe Photoshop CS (version 8) software.
Supporting Information
Zdroje
1. CamposEReinbergDanny 2009 Histones: Annotating Chromatin. Annu Rev Genet 43
2. MalikHSHenikoffS 2003 Phylogenomics of the nucleosome. Nat Struct Biol 10 882 891
3. WoudtLPPastinkAKempers-VeenstraAEJansenAEMagerWH 1983 The genes coding for histone H3 and H4 in Neurospora crassa are unique and contain intervening sequences. Nucleic Acids Res 11 5347 5360
4. HaysSMSwansonJSelkerEU 2002 Identification and characterization of the genes encoding the core histones and histone variants of Neurospora crassa. Genetics 160 961 973
5. SmithMMSantistebanMS 1998 Genetic dissection of histone function. Methods 15 269 281
6. NakanishiSSandersonBWDelventhalKMBradfordWDStaehling-HamptonK 2008 A comprehensive library of histone mutants identifies nucleosomal residues required for H3K4 methylation. Nat Struct Mol Biol 15 881 888
7. DaiJHylandEMYuanDSHuangHBaderJS 2008 Probing nucleosome function: a highly versatile library of synthetic histone H3 and H4 mutants. Cell 134 1066 1078
8. MatsubaraKSanoNUmeharaTHorikoshiM 2007 Global analysis of functional surfaces of core histones with comprehensive point mutants. Genes Cells 12 13 33
9. MelloneBGBallLSukaNGrunsteinMRPartridgeJF 2003 Centromere silencing and function in fission yeast is governed by the amino terminus of histone H3. Curr Biol 13 1748 1757
10. KouzaridesT 2007 Chromatin modifications and their function. Cell 128 693 705
11. LucoRFPanQTominagaKBlencoweBJPereira-SmithOM 2010 Regulation of alternative splicing by histone modifications. Science 327 996 1000
12. MegeePCMorganBAMittmanBASmithMM 1990 Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science 247 841 845
13. XiongLAdhvaryuKKSelkerEUWangY 2010 Mapping of lysine methylation and acetylation in core histones of Neurospora crassa. Biochemistry 49 5236 5243
14. TamaruHSelkerEU 2001 A histone H3 methyltransferase controls DNA methylation in Neurospora crassa. Nature 414 277 283
15. TamaruHZhangXMcMillenDSinghPBNakayamaJ 2003 Trimethylated lysine 9 of histone H3 is a mark for DNA methylation in Neurospora crassa. Nat Genet 34 75 79
16. SelkerEU 1990 Premeiotic instability of repeated sequences in Neurospora crassa. Annu Rev Genet 24 579 613
17. GalaganJESelkerEU 2004 RIP: the evolutionary cost of genome defense. Trends Genet 20 417 423
18. SelkerEUTountasNACrossSHMargolinBSMurphyJG 2003 The methylated component of the Neurospora crassa genome. Nature 422 893 897
19. LewisZAHondaSKhlafallahTKJeffressJKFreitagM 2009 Relics of repeat-induced point mutation direct heterochromatin formation in Neurospora crassa. Genome Res 19 427 437
20. FreitagMHickeyPCKhlafallahTKReadNDSelkerEU 2004 HP1 is essential for DNA methylation in Neurospora. Mol Cell 13 427 434
21. HondaSSelkerEU 2008 Direct interaction between DNA methyltransferase DIM-2 and HP1 is required for DNA methylation in Neurospora. Mol Cell Biol 28 6044 6055
22. KouzminovaEASelkerEU 2001 Dim-2 encodes a DNA-methyltransferase responsible for all known cytosine methylation in Neurospora. EMBO Journal 20 4309 4323
23. LachnerMO'CarrollDReaSMechtlerKJenuweinT 2001 Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature 410 116 120
24. JacobsSATavernaSDZhangYBriggsSDLiJ 2001 Specificity of the HP1 chromo domain for the methylated N-terminus of histone H3. Embo J 20 5232 5241
25. LewisZAAdhvaryuKKHondaSShiverALSelkerEU 2010 Identification of DIM-7, a protein required to target the DIM-5 H3 methyltransferase to chromatin. Proc Natl Acad Sci U S A
26. LewisZAAdhvaryuKKHondaSShiverALKnipM 2010 DNA Methylation and Normal Chromosome Behavior in Neurospora Depend on Five Components of a Histone Methyltransferase Complex, DCDC. PLoS Genet 6 e1001196 doi:10.1371/journal.pgen.1001196
27. JacobsSAKhorasanizadehS 2002 Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 295 2080 2083
28. AdhvaryuKKSelkerEU 2008 Protein phosphatase PP1 is required for normal DNA methylation in Neurospora. Genes Dev 22 3391 3396
29. EbbsMLBenderJ 2006 Locus-specific control of DNA methylation by the Arabidopsis SUVH5 histone methyltransferase. Plant Cell 18 1166 1176
30. JacksonJPLindrothAMCaoXJacobsenSE 2002 Control of CpNpG DNA methylation by the KRYPTONITE histone H3 methyltransferase. Nature 416 556 560
31. JacksonJPJohnsonLJasencakovaZZhangXPerezBurgosL 2004 Dimethylation of histone H3 lysine 9 is a critical mark for DNA methylation and gene silencing in Arabidopsis thaliana. Chromosoma 112 308 315
32. LehnertzBUedaYDerijckAABraunschweigUPerez-BurgosL 2003 Suv39h-mediated histone H3 lysine 9 methylation directs DNA methylation to major satellite repeats at pericentric heterochromatin. Curr Biol 13 1192 1200
33. ZhangXYangZKhanSIHortonJRTamaruH 2003 Structural basis for the product specificity of histone lysine methyltransferases. Mol Cell 12 177 185
34. IrelanJTSelkerEU 1997 Cytosine methylation associated with repeat-induced point mutation causes epigenetic gene silencing in Neurospora crassa. Genetics 146 509 523
35. ReaSEisenhaberFO'CarrollDStrahlBDSunZW 2000 Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature 406 593 599
36. NakayamaJRiceJCStrahlBDAllisCDGrewalSI 2001 Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 292 110 113
37. SchultzDCAyyanathanKNegorevDMaulGGRauscherFJ3rd 2002 SETDB1: a novel KAP-1-associated histone H3, lysine 9-specific methyltransferase that contributes to HP1-mediated silencing of euchromatic genes by KRAB zinc-finger proteins. Genes Dev 16 919 932
38. SmithKMDobosyJRReifsnyderJERountreeMRAndersonDC 2010 H2B - and H3-specific Histone Deacetylases are Required for DNA Methylation in Neurospora crassa. Genetics
39. CanzioDChangEYShankarSKuchenbeckerKMSimonMD 2011 Chromodomain-mediated oligomerization of HP1 suggests a nucleosome-bridging mechanism for heterochromatin assembly. Mol Cell 41 67 81
40. XhemalceBKouzaridesT 2010 A chromodomain switch mediated by histone H3 Lys 4 acetylation regulates heterochromatin assembly. Genes Dev 24 647 652
41. Casas-MollanoJAJeongBRXuJMoriyamaHCeruttiH 2008 The MUT9p kinase phosphorylates histone H3 threonine 3 and is necessary for heritable epigenetic silencing in Chlamydomonas. Proc Natl Acad Sci U S A 105 6486 6491
42. ShimadaMNiidaHZineldeenDHTagamiHTanakaM 2008 Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell 132 221 232
43. MetzgerEYinNWissmannMKunowskaNFischerK 2008 Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol 10 53 60
44. DunnKLDavieJR 2005 Stimulation of the Ras-MAPK pathway leads to independent phosphorylation of histone H3 on serine 10 and 28. Oncogene 24 3492 3502
45. MetzgerEImhofAPatelDKahlPHoffmeyerK 2010 Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature 464 792 796
46. KellyAEGhenoiuCXueJZZierhutCKimuraH 2010 Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 330 235 239
47. WangFDaiJDaumJRNiedzialkowskaEBanerjeeB 2010 Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 330 231 235
48. HoubenADemidovDRuttenTScheidtmannKH 2005 Novel phosphorylation of histone H3 at threonine 11 that temporally correlates with condensation of mitotic and meiotic chromosomes in plant cells. Cytogenet Genome Res 109 148 155
49. RathertPZhangXFreundCChengXJeltschA 2008 Analysis of the substrate specificity of the Dim-5 histone lysine methyltransferase using peptide arrays. Chem Biol 15 5 11
50. YamadaTFischleWSugiyamaTAllisCDGrewalSI 2005 The nucleation and maintenance of heterochromatin by a histone deacetylase in fission yeast. Mol Cell 20 173 185
51. ChenESZhangKNicolasECamHPZofallM 2008 Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 451 734 737
52. ZhangKMoschKFischleWGrewalSI 2008 Roles of the Clr4 methyltransferase complex in nucleation, spreading and maintenance of heterochromatin. Nat Struct Mol Biol 15 381 388
53. NicolasEYamadaTCamHPFitzgeraldPCKobayashiR 2007 Distinct roles of HDAC complexes in promoter silencing, antisense suppression and DNA damage protection. Nat Struct Mol Biol 14 372 380
54. MotamediMRHongEJLiXGerberSDenisonC 2008 HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms. Mol Cell 32 778 790
55. SugiyamaTCamHPSugiyamaRNomaKZofallM 2007 SHREC, an effector complex for heterochromatic transcriptional silencing. Cell 128 491 504
56. BedfordMTClarkeSG 2009 Protein arginine methylation in mammals: who, what, and why. Mol Cell 33 1 13
57. KirmizisASantos-RosaHPenkettCJSingerMAVermeulenM 2007 Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature 449 928 932
58. GuccioneEBassiCCasadioFMartinatoFCesaroniM 2007 Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature 449 933 937
59. KirmizisASantos-RosaHPenkettCJSingerMAGreenRD 2009 Distinct transcriptional outputs associated with mono - and dimethylated histone H3 arginine 2. Nat Struct Mol Biol 16 449 451
60. ChinHGPradhanMEstevePOPatnaikDEvansTCJr 2005 Sequence specificity and role of proximal amino acids of the histone H3 tail on catalysis of murine G9A lysine 9 histone H3 methyltransferase. Biochemistry 44 12998 13006
61. EskelandRCzerminBBoekeJBonaldiTRegulaJT 2004 The N-terminus of Drosophila SU(VAR)3-9 mediates dimerization and regulates its methyltransferase activity. Biochemistry 43 3740 3749
62. RudolphTYonezawaMLeinSHeidrichKKubicekS 2007 Heterochromatin formation in Drosophila is initiated through active removal of H3K4 methylation by the LSD1 homolog SU(VAR)3-3. Mol Cell 26 103 115
63. LiFHuarteMZaratieguiMVaughnMWShiY 2008 Lid2 is required for coordinating H3K4 and H3K9 methylation of heterochromatin and euchromatin. Cell 135 272 283
64. OoiSKTQiuCBernsteinELiKJiaD 2007 DNMT3L connects unmethylated lysine 4 of histone H3 to de novo methylation of DNA. Nature 448 714 717
65. ButlerJSLeeJHSkalnikDG 2008 CFP1 interacts with DNMT1 independently of association with the Setd1 Histone H3K4 methyltransferase complexes. DNA Cell Biol 27 533 543
66. ThomsonJPSkenePJSelfridgeJClouaireTGuyJ 2010 CpG islands influence chromatin structure via the CpG-binding protein Cfp1. Nature 464 1082 1086
67. DavisRHDe SerresFJ 1970 Genetic and microbiological research techniques for Neurospora crassa. Meth Enzymol 17A 47 143
68. PerkinsDD 1986 Hints and precautions for the care, feeding and breeding of Neurospora. Fungal Genetics Newsl 33 35 41
69. DavisRH 2000 Neurospora: Contributions of a Model Organism Oxford University Press 333
70. IrelanJMiaoVSelkerEU 1993 Small scale DNA preps for Neurospora crassa. Fungal Genetics Newsletter 40 24
71. OakleyBRRinehartJEMitchellBLOakleyCECarmonaC 1987 Cloning, mapping and molecular analysis of the pyrG (orotidine - 5′-phosphate decarboxylase) gene of Aspergillus nidulans. Gene 61 385 399
72. SouthernEM 1975 Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol 98 503 517
73. FeinbergAPVogelsteinB 1983 A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity (I). Anal Biochem 132 6 13
74. SelkerEUStevensJN 1987 Signal for DNA methylation associated with tandem duplication in Neurospora crassa. Mol Cell Biol 7 1032 1038
75. ZhangXTamaruHKhanSHortonJKeefeL 2002 Structure of the Neurospora SET Domain Protein DIM-5, a Histone H3 Lysine Methyltransferase. Cell 111 117 127
76. BaumJAGilesNH 1986 DNase I hypersensitive sites within the inducible qa gene cluster of Neurospora crassa. Proc Natl Acad Sci U S A 83 6533 6537
77. TurnerBM 2005 Reading signals on the nucleosome with a new nomenclature for modified histones. Nat Struct Mol Biol 12 110 112
78. HickeyPCJacobsonDReadNDLouise GlassNL 2002 Live-cell imaging of vegetative hyphal fusion in Neurospora crassa. Fungal Genet Biol 37 109 119
79. AbramoffMDMagelhaesPJRamSJ 2004 Image Processing with ImageJ. Biophotonics International 11 36 42
80. NelsonCJSantos-RosaHKouzaridesT 2006 Proline isomerization of histone H3 regulates lysine methylation and gene expression. Cell 126 905 916
81. DuncanEMMuratore-SchroederTLCookRGGarciaBAShabanowitzJ 2008 Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell 135 284 294
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



