-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
, , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
The epidermis is the largest organ of the body for most animals, and the first line of defense against invading pathogens. A breach in the epidermal cell layer triggers a variety of localized responses that in favorable circumstances result in the repair of the wound. Many cellular and genetic responses must be limited to epidermal cells that are close to wounds, but how this is regulated is still poorly understood. The order and hierarchy of epidermal wound signaling factors are also still obscure. The Drosophila embryonic epidermis provides an excellent system to study genes that regulate wound healing processes. We have developed a variety of fluorescent reporters that provide a visible readout of wound-dependent transcriptional activation near epidermal wound sites. A large screen for mutants that alter the activity of these wound reporters has identified seven new genes required to activate or delimit wound-induced transcriptional responses to a narrow zone of cells surrounding wound sites. Among the genes required to delimit the spread of wound responses are Drosophila Flotillin-2 and Src42A, both of which are transcriptionally activated around wound sites. Flotillin-2 and constitutively active Src42A are also sufficient, when overexpressed at high levels, to inhibit wound-induced transcription in epidermal cells. One gene required to activate epidermal wound reporters encodes Dual oxidase, an enzyme that produces hydrogen peroxide. We also find that four biochemical treatments (a serine protease, a Src kinase inhibitor, methyl-ß-cyclodextrin, and hydrogen peroxide) are sufficient to globally activate epidermal wound response genes in Drosophila embryos. We explore the epistatic relationships among the factors that induce or delimit the spread of epidermal wound signals. Our results define new genetic functions that interact to instruct only a limited number of cells around puncture wounds to mount a transcriptional response, mediating local repair and regeneration.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002424
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002424Summary
The epidermis is the largest organ of the body for most animals, and the first line of defense against invading pathogens. A breach in the epidermal cell layer triggers a variety of localized responses that in favorable circumstances result in the repair of the wound. Many cellular and genetic responses must be limited to epidermal cells that are close to wounds, but how this is regulated is still poorly understood. The order and hierarchy of epidermal wound signaling factors are also still obscure. The Drosophila embryonic epidermis provides an excellent system to study genes that regulate wound healing processes. We have developed a variety of fluorescent reporters that provide a visible readout of wound-dependent transcriptional activation near epidermal wound sites. A large screen for mutants that alter the activity of these wound reporters has identified seven new genes required to activate or delimit wound-induced transcriptional responses to a narrow zone of cells surrounding wound sites. Among the genes required to delimit the spread of wound responses are Drosophila Flotillin-2 and Src42A, both of which are transcriptionally activated around wound sites. Flotillin-2 and constitutively active Src42A are also sufficient, when overexpressed at high levels, to inhibit wound-induced transcription in epidermal cells. One gene required to activate epidermal wound reporters encodes Dual oxidase, an enzyme that produces hydrogen peroxide. We also find that four biochemical treatments (a serine protease, a Src kinase inhibitor, methyl-ß-cyclodextrin, and hydrogen peroxide) are sufficient to globally activate epidermal wound response genes in Drosophila embryos. We explore the epistatic relationships among the factors that induce or delimit the spread of epidermal wound signals. Our results define new genetic functions that interact to instruct only a limited number of cells around puncture wounds to mount a transcriptional response, mediating local repair and regeneration.
Introduction
The development of a specialized epidermal barrier layer represents a key step during the evolution of multi-cellular organisms. This outer integument provides protection from the environment and helps maintain cellular homeostasis. Epidermal barriers consist of epithelial cells that are tightly joined by adherens and other types of junctional complexes, as well an apical extracellular matrix layer that is highly variable. The mammalian epidermal barrier is constructed from a constantly renewing multicellular layer, in which cells follow a complex process of cell division and differentiation to form the stratum corneum [1]. In arthropods like Drosophila melanogaster, a single epidermal cell layer secretes a multilayered matrix of cross-linked lipid, protein, and chitin to generate a largely impermeable cuticle barrier [2], [3]. Despite the great differences between the components and physical makeup of their epidermal barriers, both mammals and arthropods make use of conserved cellular mechanisms, transcriptional regulators, and signaling pathways during the generation of epidermal barriers as well as during their regeneration after wounding [4], [5], [6], [7]. There are many complex processes that contribute to epidermal wound healing; these include clot formation, reepithelialization, cellular proliferation, inflammation, and barrier replacement [8]. Drosophila is a genetically tractable system for discovering evolutionarily conserved genes involved in such epidermal wound healing processes, as it has been for discovering genes that regulate animal septic wound responses [9].
One useful system for elucidating cellular mechanisms involved in wound healing has been Drosophila dorsal closure—where sheets of embryonic epidermal cells migrate to join at the dorsal midline—which uses some of the same cellular processes that are used to heal wounds [10], [11]. For example, both dorsal closure and wound healing involve the recruitment of an actin-cytoskeleton “purse-string” to help close the edge of the wound or the edge of a gap in a migrating dorsal epidermal sheet [12]. Several evolutionarily conserved transcriptional regulatory pathways have been linked to developmental control of barrier formation as well as wound healing [13]. For example, Grainy head (Grh) transcription factors are required in a variety of animals for the development of impermeable epidermal barriers as well as normal wound repair [5], [6], [14], [15], [16], [17]. In Drosophila, Grh accomplishes these functions in part through regulation of the Dopa Decarboxylase (Ddc) and Tyrosine hydroxylase (ple) genes, which encode enzymes that produce cuticle protein cross-linkers [5], [18].
Other transcription factors with conserved roles in wound repair are those in the JUN family, which are required for wound reepithelialization in both mammals and Drosophila [7], [19]. Upstream of JUN, the JUN amino-terminal kinase (JNK) signaling pathway [20] is required in the Drosophila epidermis for dorsal closure and wound reepithelialization [4], [21], [22]. misshapen (msn), which encodes a Drosophila JNK-kinase-kinase-kinase, is distinctive because it is transcriptionally activated around embryonic, larval and adult epidermal wounds [4], [21], [22], [23], [24], [25]. Recent reports have shown that JNK signaling is also required during Drosophila wing imaginal disc regeneration [26], [27]. Another signaling pathway involving the gene stitcher (stit), which encodes a receptor tyrosine kinase, is also activated around epidermal wound sites in Drosophila embryos, and is required for normal wound reepithelialization, and activation of some epidermal barrier repair genes [28].
There have been a few focused genetic screens for Drosophila mutants required for normal epidermal wound repair. One was a screen of 665 P-element insertional mutants for abnormal wound reepithelialization phenotypes after laser wounding during embryogenesis. The mutations with the most severe defects were in the genes for the JUN transcription factor and ßHeavy Spectrin [7]. Interestingly, wound closure defects were not observed in several genes that are required for dorsal closure and epithelial migration during Drosophila development, indicating that wound closure and dorsal closure are, to some extent, under the control of distinct genetic systems [7]. Another Drosophila genetic screen used a combination of dominant negative and RNAi-mediated knockdowns to test about 180 genes, focusing on Receptor Tyrosine Kinases (RTKs), JNK signaling components, and cytoskeletal components after pinch or puncture wounding of the larval epidermis [22], [29]. The knockdown or knockout of function in about 20 genes showed defective reepithelialization after larval wounding. Genes required for normal larval reepithelialization include those encoding components of the JNK signaling pathway like the transcription factors JUN (Drosophila Jra) and FOS (Drosophila kay), as well as the Drosophila PDGF/VEGF-like receptor (Pvr), and some proteins that regulate or remodel the actin cytoskeleton [22], [29]. A few of the tested genes (encoding JUN, JNK, and JUNKK, respectively) were also required for the transcriptional activation of a wound response reporter gene (misshapen-lacZ) in larval epidermal cells surrounding sterile wound sites [22].
We have initiated a large, unbiased, genetic screen to identify mutations that are required for localized activation of epidermal genes around clean puncture wounds in Drosophila embryos. At this point, there are cis-regulatory wound enhancers identified from the Ddc, ple, msn, kkv, and stit genes [25], [28]. These enhancers, when attached to fluorescent reporter genes (hereafter called wound reporters) provide a visible readout of wound-induced gene activation after epidermal wounding, and can be used to identify mutations that are required to activate or localize (delimit) this response. A few hours after wounding late stage embryos, fluorescent signal from these epidermal wound reporters can be observed in a zone that extends ∼5–10 cells from puncture sites. Some particularly interesting regulatory genes that we discuss in this paper are those required to delimit or localize the activity of wound reporters to a zone within a few cell diameters from wound sites. Mutations in such genes result in a global activation of wound reporters in most or all epidermal cells after wounding. One of the wound localization genes we identified is reggie-1/Flotillin-2 (referred to as flotillin-2 or Flo-2 in Flybase), which was originally isolated as a gene that is activated in wounded, regenerating goldfish neurons [30], and as a protein enriched in lipid rafts [31]. At the cellular level, Flo-2 appears to be involved in a variety of cell signaling and adhesion functions, at least in part via its role in clathrin-independent vesicular trafficking [32], [33], [34], [35], [36]. Analogous to wounded fish neurons, the Flo2 gene in Drosophila embryos is transcriptionally activated in cells surrounding epidermal wounds. We find that Flo-2 interacts in a pathway involving Drosophila Src42A to delimit epidermal wound responses, and that overexpression of either Flo-2 or activated Src42A can inhibit wound reporter activation, whether it is triggered locally by epidermal puncture, or globally by injection of trypsin or hydrogen peroxide.
Results
Monitoring the activity of epidermal wound reporters in late stage Drosophila embryos [25] provides an in vivo assay that can be used to screen for mutations that are required to activate or localize the expression of genes that respond to epidermal wounding. We began such a screen using a collection of well-defined small chromosomal deletions of the Drosophila melanogaster genome [37], searching for regions containing zygotic functions required for normal activation of the wound reporter ple-WE1 [25]. One advantage of this screen is that most Drosophila zygotic mutants survive to late stages of embryogenesis, differentiate their epidermis (which can be assayed by the activation of an anal pad specific enhancer that exists alongside the wound enhancer within the ple-WE1 sequence), and can still be assayed for wound reporter activity. At this point, we have screened 300 deletions that include approximately 4,600 genes on the X and 2nd chromosomes (Figure 1A). Sixteen of these deletions had abnormal ple-WE1 expression, and therefore contained putative epidermal wound regulatory genes. Analysis of the genes within the collection of deletions indicates that the zygotic functions of many signaling pathways, transcription factors, and other regulators of cellular properties have no effect on the activation of the ple-WE1 epidermal wound reporter (Table 1). For example, deletion mutants of patched (Hedgehog pathway), shaggy/GSK3 or disheveled (Wingless pathway), Notch (Notch signaling pathway), Pvr (PDGF/VEGF signaling pathway), domeless (JAK/STAT pathway), and wengen (TNF pathway) all showed normal ple-WE1 expression after wounding. It is possible that the maternal contribution of some of these genes is sufficient to rescue potential effects on wound reporter activation in zygotic mutants.
Fig. 1. Summary of epidermal wound reporter mutants and their phenotypes. 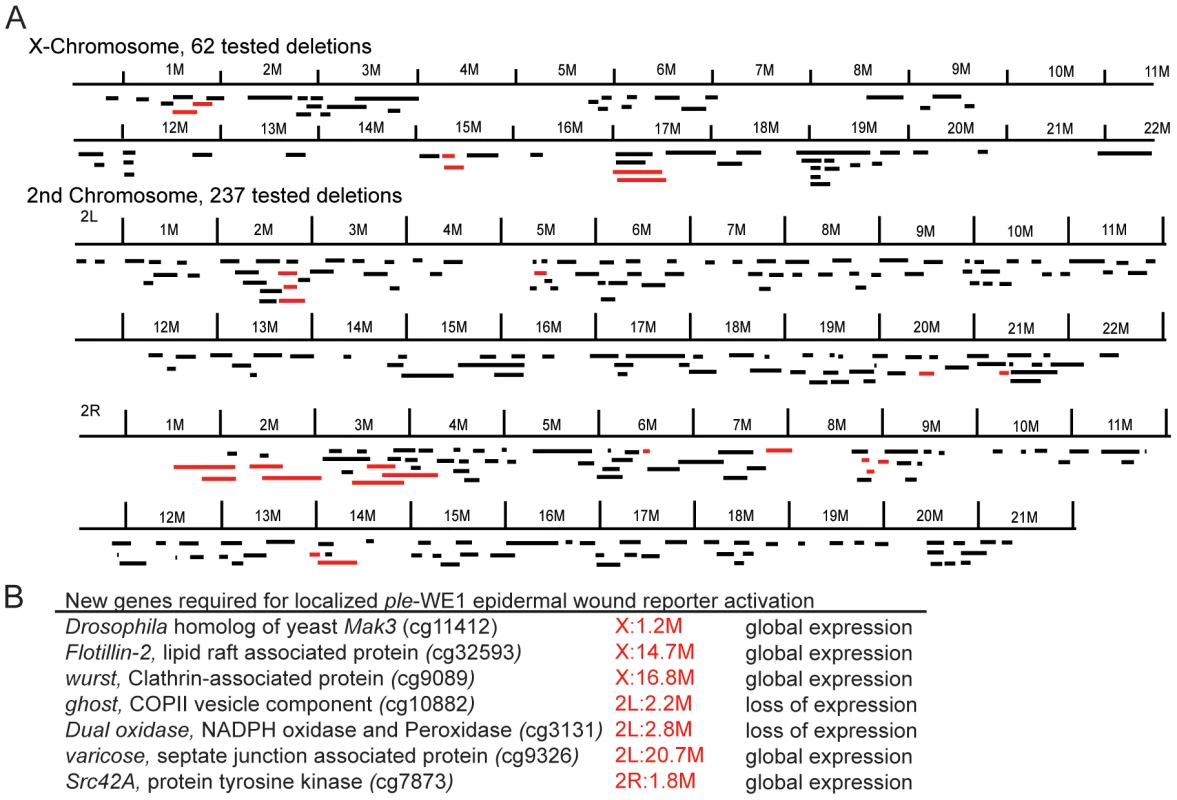
(A) Maps of X and 2nd chromosomes. Black bars represent deleted regions that have a normal epidermal wound reporter phenotype. Red bars represent deleted regions that have an altered epidermal wound reporter express pattern. (B) A list of new genes identified that affect the localization of the epidermal wound reporter. X:1.2M, for example, denotes 1.2 Megabases on the Drosophila genome map of the X chromosome [93]. Tab. 1. Zygotic mutants in these genes show <i>ple</i>-WE1 wound reporter expression in a normal zone in the epidermis around wounds. 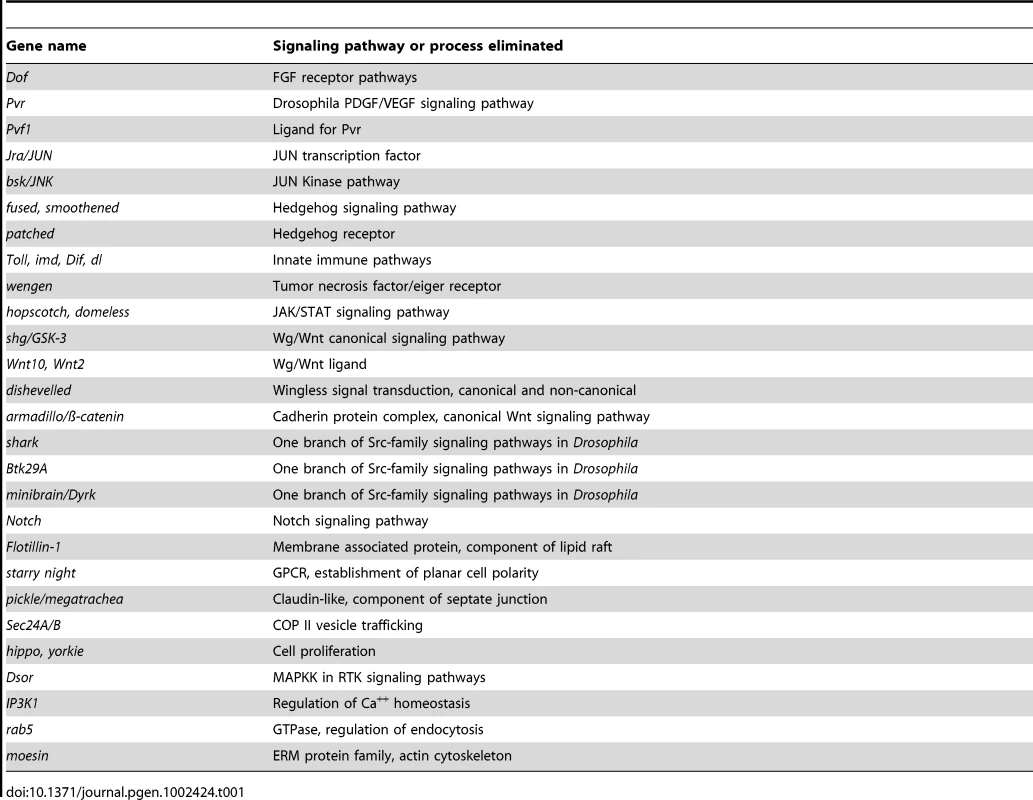
Within the 16 regions required for normal epidermal wound reporter expression, we have currently defined 7 single gene mutations with new functions in wound reporter activation or localization (Figure 1B). The functions of two of these genes (ghost/stenosis, and Duox) are required for wound gene activation, and five of the genes (flotillin-2, wurst, varicose, Src42A, and the Drosophila homolog of yeast MAK3) are required to localize wound reporter activity to the immediate vicinity of wounds. One focus of this paper is on flotillin-2 (Flo-2). The Flo-2 protein has been well characterized at the cellular and biochemical levels, but the genetic interactions of Flo-2 are still enigmatic in the diverse cellular processes in which it participates [38]. The Drosophila genome encodes only one Flo-2 ortholog [39]. Null mutants that eliminate Drosophila Flo-2 protein also accumulate little or none of the related Flo-1 protein, since it is apparently destabilized in the absence of Flo-2 [40]. Flo-2 mutant animals have normal morphology and are viable and fertile [40]. Despite having normal adult morphology, Flo-2 mutants show a reduced spread of Wnt and Hedgehog signals in wing imaginal discs [40], [41]. Flo-1 mouse mutants are viable and fertile under standard lab conditions and the mutants have somewhat reduced Flo-2 protein levels [42]. We tested a Drosophila deletion mutant that eliminated the Flo-1 gene, but ple-WE1 expression was normal after wounding (Table 1).
Normal epidermal activation of our fluorescent protein wound reporters can be easily detected 4 hours after wounding wild type stage 16 embryos (Figure 2A, 2B), although transcripts from the same endogenous wound-activated genes in a narrower zone of cells can be detected in fixed embryos within 30 minutes after wounding [5], [25]. The fluorescent protein reporters have the great advantage of being easily detectable in living embryos or larvae, whereas nucleic acid or antiserum probe permeability into late stage embryos or larvae with partially or fully differentiated cuticle is labor intensive at best. However, the fluorescent protein reporters typically represent a delayed version (by our estimate, a few hour delay) of the transcriptional response in cells surrounding wounds. This is due to the requirement of enhanced-GFP or enhanced-dsRed proteins to oxidatively mature to fluorescence. We estimate that the fluorescent reporter proteins we used [5], [25], [43]. have a half-time to maturation of about an hour in fly embryos after the reporter gene RNAs are translated. Additionally, time is required to accumulate detectable levels of fluorescent protein, and this is dependent on the strength of the epidermal wound enhancer being tested. For example, the Ddc .47 wound enhancer appears to be slightly stronger than the ple-WE1 enhancer [5], [25].
Fig. 2. Flo-2 functions to inhibit widespread activation of epidermal wound reporters. 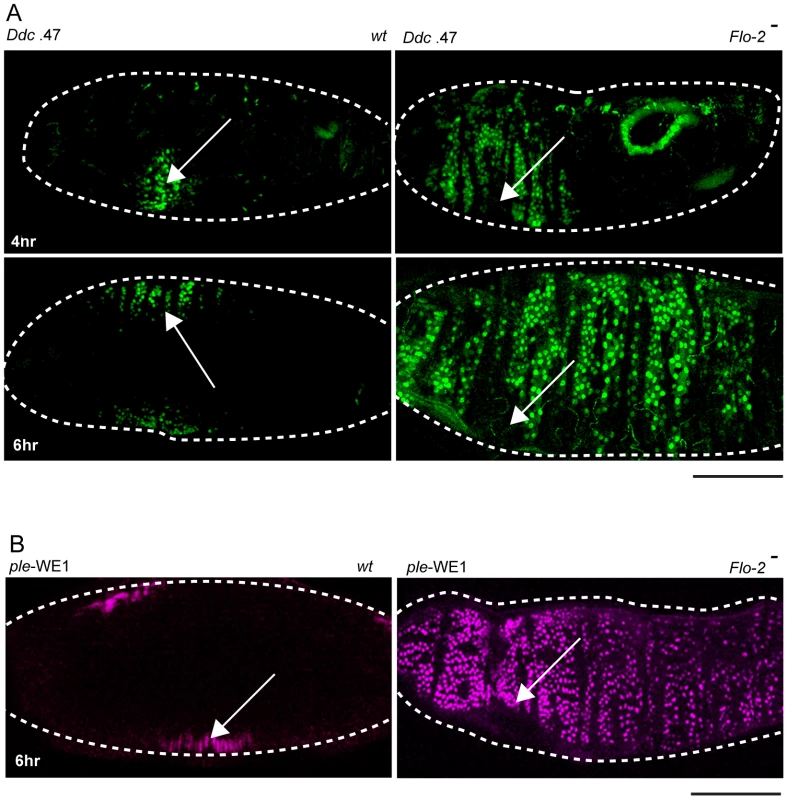
(A) Confocal images of Ddc .47-GFP epidermal wound reporter activity (4–6 hours after puncture wounding). Wild type (wt) embryos show the reporter in cells around the wound site. Flo-2 mutant embryos show expansion of Ddc .47 reporter expression to most epidermal cells. (B) ple-WE1-dsRed epidermal wound reporter expression expands to most epidermal cells in Flo-2 mutant embryos 6 hours after wounding. Arrows mark the site of the wound. Dashed lines in the data panels mark the outlines of embryos. Scale bars = 50 µM. In Flo-2 mutants, fluorescent wound reporter proteins driven by the ple-WE1 and Ddc .47 wound enhancers are detected at 4 hours after wounding, but reporter expression is significantly expanded compared to wild type embryos (Figure 2A, 2B). By 6 hours post-wounding reporter expression in Flo-2 mutants spreads to include most epidermal cells in late embryos (Figure 2A, 2B). Using in situ hybridization or protein immunodetection, we also tested the effect of Flo-2 mutants on the activation pattern of several endogenous wound response genes (ple, Ddc, msn, stit, and Src42A), and found comparable expanded expression domains after wound induction (Figure S1, and data not shown). Nearly all (∼90%) of wounded Flo-2 mutant embryos survive, hatch to become larvae, and progress to adulthood, which was similar to that observed in wounded wild type embryos (data not shown). We did not detect any characteristics of abnormal epidermal wound healing [26], or ectopic melanization in the punctured Flo-2 mutants, although we did not carefully examine the kinetics of healing in the mutants. Activation of wound reporters in Flo-2 mutants was wound-dependent; in no instances was constitutive expression of reporters detected in mutant embryos.
Flo-2 transcripts normally accumulate in all cells of the embryo, including the epidermis (Figure 3A), with higher apparent levels in the central nervous system [40], [41]. The insertional mutant into Flo-2 (Flo-2[KG00210]) used in this study fails to accumulate transcripts (Figure S1A; [40]). A 500 bp region within the largest of the Flo-2 introns contains predicted high affinity binding sites for the Grh, AP-1, and ETS transcription factors. Such sites are found in clusters in previously characterized epidermal wound response enhancers from the ple, Ddc, msn, and stit genes [25], [28]. To test whether Flo-2 is transcriptionally activated around epidermal wounds, we carried out in situ hybridizations on wounded embryos using a Flo-2 probe. As seen in Figure 3B, Flo-2 transcripts are expressed at higher levels in cells surrounding epidermal wound sites, overlapping with the wound induced activation of the endogenous Ddc gene. Previous studies showed the activation of some epidermal wound response genes depends on the function of the Grh transcription factor [25], [28]. As seen in Figure 3C, Flo-2 transcriptional activation around epidermal wound sites is dramatically reduced in grh mutant embryos compared to wild type siblings (Figure 3B), consistent with Flo-2 being activated in a Grh-dependent manner.
Fig. 3. Flo-2 transcriptional activation at wound site depends on grainy head function. 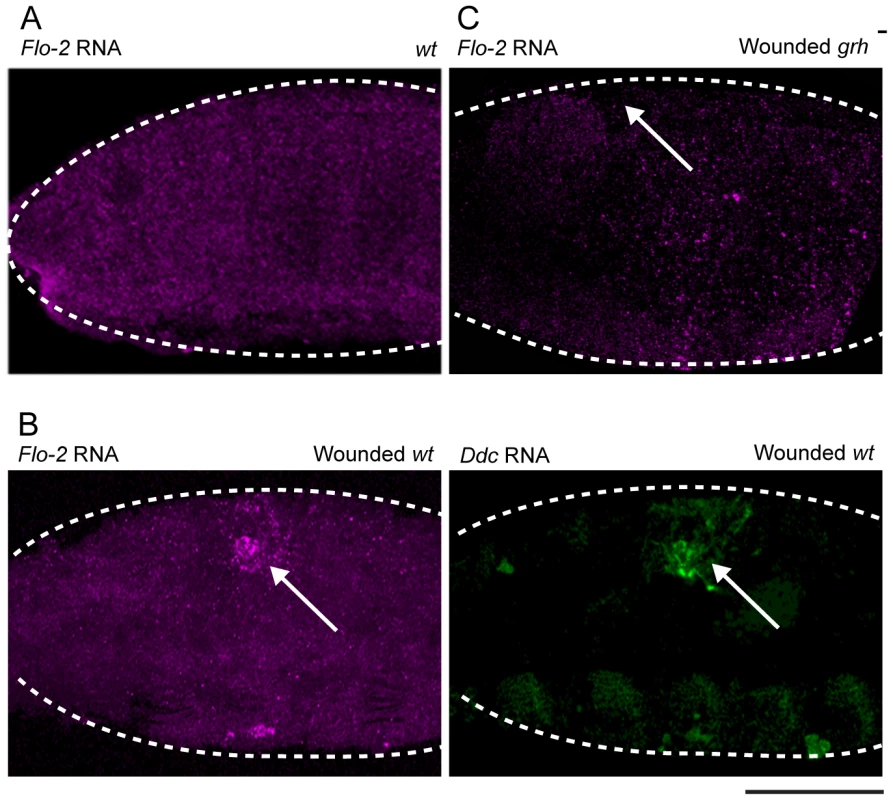
Confocal images of in situ hybridizations with fluorescently labeled probes made from Flo-2 and Ddc cDNA clones. (A) Flo-2 transcripts accumulate in all epidermal cells of late stage embryos. (B) 30-minutes after puncture wounding, Flo-2 transcripts show increased accumulation in cells around wound sites, in a similar pattern as wound-induced Ddc transcript activation in the epidermis. (C) 30-minutes after puncture wounding, grainy head (grh) mutant embryos fail to show increased accumulation of Flo-2 transcripts around epidermal wound sites. Arrows mark the wound site. Dashed lines in the data panels mark the outlines of embryos. Scale bar = 50 µM. With Flo-2 loss of expression resulting in widespread activation of epidermal wound genes, we wished to test whether overexpression of Flo-2 would have an effect on wound gene activation. This was accomplished using a fly line in which the Flo-2 cDNA was fused to a UAS promoter, in combination with an arm-GAL4 driver [44]. armadillo (arm), the Drosophila homolog of ß-catenin, is expressed ubiquitously in embryos, and the arm-GAL4 driver can induce high levels of UAS-Flo-2 expression beginning at stage 10 of embryonic development (Figure S2A, [45]). Ubiquitous high levels of Flo-2 inhibit the activation of the Ddc .47 and ple-WE1 epidermal wound reporters around wound sites (Figure 4A, Figure S3A). To test whether the inhibition caused by overexpression of Flo-2 was cell autonomous, we used en-GAL4 to drive high levels of Flo-2 in the engrailed (en), posterior compartment of each embryonic segment (Figure S2B, [46]). Overexpression of Flo-2 using en-GAL4 is sufficient to silence the activation of the Ddc .47 and ple-WE1 epidermal wound reporters in all cells near a wound, even in those that do not produce Flo-2 at higher levels (Figure 4C, data not shown). The lack of any activation of the epidermal wound reporter in the en>Flo-2 overexpression experiments suggests that Flo-2 can act cell non-autonomously, at least at short range, to inhibit the ability of cells to respond to wound signals. Higher levels of Flo-2 do not appear to be toxic, as overexpression of Flo-2 with either the arm-GAL4 or the en-GAL4 drivers does not obviously alter embryonic development, and animals so treated survive to produce viable and fertile adults (data not shown).
Fig. 4. Overexpression of Flo-2 inhibits activation of an epidermal wound reporter. 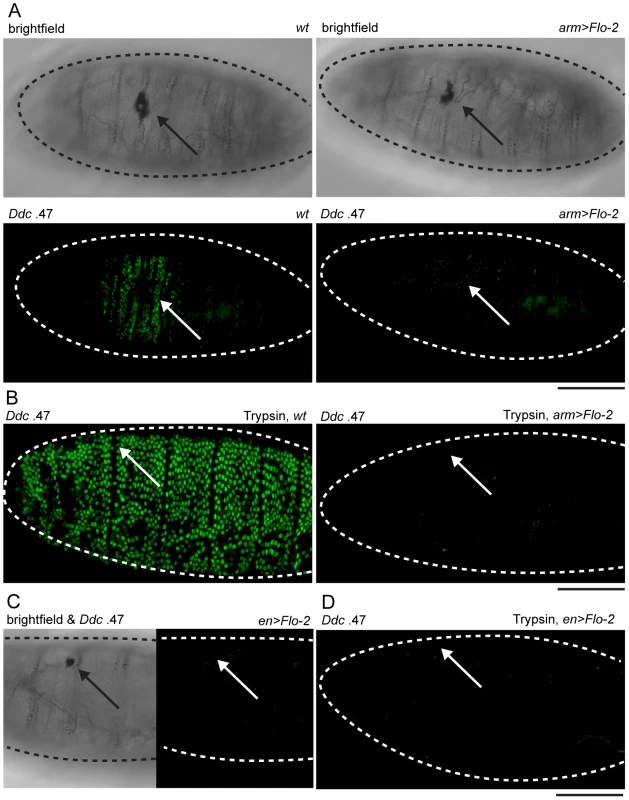
(A) Brightfield and fluorescent confocal images of late stage embryos with Ddc .47-GFP epidermal wound reporter genes. Wild type (wt) embryos, or embryos with overexpressed Flo-2 (arm>Flo-2) have similar melanized clots in the cuticle at puncture wound sites. Ddc .47 reporter expression accumulates around punctures to high levels at 4–6 hours post-wounding. In arm>Flo-2 embryos, Ddc .47 wound reporter expression is not detected around punctures at any time post-wounding. (B) After wounding with needles loaded with trypsin, wild type (wt) late stage embryos dramatically activate Ddc .47-GFP reporter expression throughout most or all epidermal cells at 6 hours post-wounding. In late stage embryos overexpressing Flo-2 (arm>Flo-2), the trypsin-induced Ddc .47 wound reporter activation is completely repressed. (C,D) Embryos with the Ddc .47 epidermal wound reporter that overexpress Flo-2 in stripes using the en-GAL4 driver (en>Flo-2) show inhibition of both wound-induced (C) and trypsin-induced (D) activation of the Ddc .47 wound reporter in all epidermal cells, not just the epidermal cells that overexpress Flo-2 (see Figure S2B). Arrows show wound sites. Dashed lines in the data panels mark the outlines of embryos. Scale bar = 50 µM. Although we do not know the signaling mechanisms that allow cells 5–10 cell diameters from a wound site to sense the presence of an epidermal break and activate wound gene transcription, one system that might be involved is an activation of serine protease cascades. Serine proteases regulate the production of some localized developmental signals [47], infectious innate immune signals [9], and activate localized melanization around wounds [48]. We tested whether a serine protease, trypsin, would be sufficient to induce widespread activation of epidermal wound reporter genes when injected into late stage embryos. Injection of trypsin into the body cavity (or into the perivitelline space) of stage 16 embryos results in a global activation of Ddc .47 and ple-WE1 epidermal wound reporters (Figure 4B, Figure S3B). This trypsin treatment does not appear to result in widespread epidermal cell death, nor is the epidermal paracellular barrier—which prevents diffusion of all but very small molecules through epithelia—breached when trypsin is injected into the perivitelline space of stage 16 embryos (R.P., unpublished results). Although trypsin is sufficient to activate the ple and Ddc wound reporter genes, as yet we have no current evidence that a specific endogenous serine protease is required to activate epidermal wound-induced transcriptional responses. Strikingly, overexpression of Flo-2 under the control of either arm-GAL4 or en-GAL4 is sufficient to inhibit trypsin-induced activation of Ddc .47 or ple-WE1 wound reporters throughout the entire embryonic epidermis (Figure 4B, 4D; Figure S3C, S3D). This finding suggests that puncture-induced and trypsin-induced activation of wound genes might act through a common pathway that can be inhibited by overexpression of Flo-2. The inhibition of protease-induced wound reporter gene activation observed with en>Flo-2 overexpression is consistent with the idea that Flo-2 can act cell non-autonomously.
Similar to Flo-2 mutants, mutants in Drosophila Src42A show more widespread activation of the ple wound reporter or Ddc transcription in epidermal cells after localized punctures (Figure 5A, 5B). Since previous research has uncovered functional and biochemical interactions between Flotillins and Src family tyrosine kinases [33], [49], we decided next to focus on the relationships between Flo-2 and Src42A in the regulation of epidermal wound response genes. Src42A is the Drosophila homolog of vertebrate c-Src [50]. Src family kinases were found to play important roles in several signaling pathways [51]. Like Flo-2, Src42A is itself a wound response gene; Scr42A transcripts accumulate to high levels in cells surrounding wound sites in wild type embryos (Figure 5C). Src42A transcription is also globally activated in all epidermal cells in wounded Flo-2 mutant embryos, although the converse is not true, as Flo-2 transcript levels are unchanged in Scr42A mutants (data not shown). In this sense at least, Flo-2 wound-dependent transcriptional activation is not behaving as other wound-induced genes like Ddc and ple, which show widespread wound-induced transcription in Src42A mutants.
Fig. 5. Src42A functions to inhibit widespread epidermal wound reporter activity after wounding. 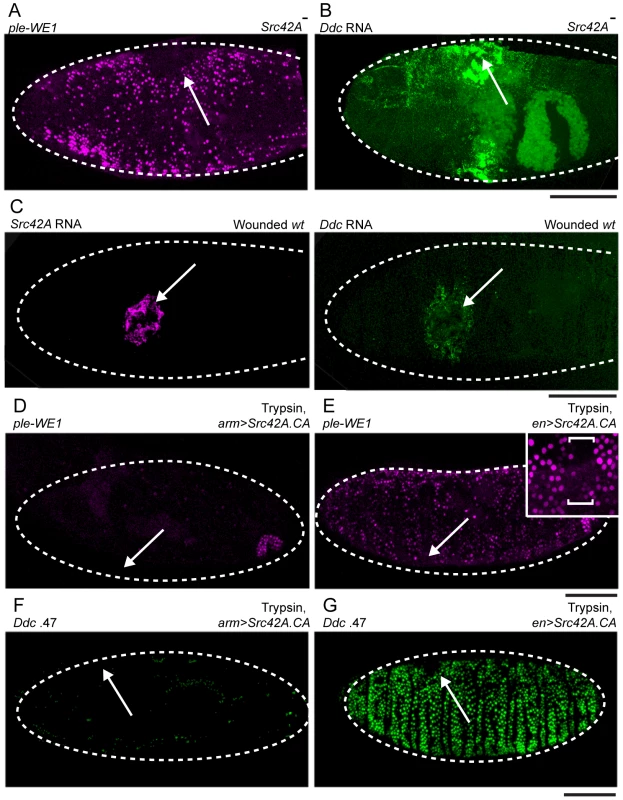
(A) Fluorescent confocal image of ple-WE1-dsRed epidermal wound reporter activity in a late stage Src42A mutant embryo. At 6 hours after wounding, the Src42A mutants show activation of the ple-WE1 wound reporter in a very broad area of the embryonic epidermis surrounding the wound site (compare with Figure 1B). (B,C) Confocal images of fluorescent in situ hybridization experiments. (B) 30-minutes after wounding, Src42A mutant embryos show accumulation of Ddc transcripts in a widespread zone of epidermal cells around the wound site (compare with Ddc expression in wounded wild type (wt) in panel C- right side). (C) Using double in situ hybridization with probes labeled with different fluorophores [9] (both images in C are taken from the same wild type (wt) embryo 30 minutes after wounding), we observed that Src42A transcripts are activated to high levels around wound sites (left panel), and in a slightly smaller zone than Ddc transcripts at the same stage. (D,E) Fluorescent confocal images of ple-WE1-dsRed epidermal wound reporter activity in late stage embryos (6 hours post-wounding) after body cavity injection of trypsin. (D) Ubiquitous expression with Src42A.CA inhibits the trypsin-induced ple-WE1 reporter in late stage embryos (compare with Figure S3B). (E) Overexpression of Src42A.CA in stripes with the en-GAL4 driver only inhibits ple-WE1 reporter expression in the cells where en-GAL4 is activating high levels of Src42A.CA protein expression. The insert provides higher magnification, and the bracket shows the stripe of cells where Src42A.CA is overexpressed by the en-GAL4 driver. (F,G) Confocal images of Ddc .47 epidermal wound reporter activity in late stage embryos (6 hours post-wounding) after body cavity injection of trypsin. (F) Ubiquitous expression with Src42A.CA inhibits the trypsin-induced Ddc .47 wound reporter in late stage embryos. (G) Overexpression of Src42A.CA in stripes with the en-GAL4 driver only inhibits Ddc .47 reporter expression in the cellular stripes where en-GAL4 is activating high levels of Src42A.CA protein expression. Arrows mark sites of wounds. Dashed lines in the data panels mark the outlines of embryos. Scale bar = 50 µM. Similar to Flo-2, overexpression of Src42A.CA (a constitutively activated form, [52]) with arm-GAL4 inhibits both the local puncture, as well as trypsin-induced, activation of the Ddc .47 and ple-WE1 epidermal wound reporters (Figure 5D, 5F; data not shown). In contrast to Flo-2, overexpression of Src42A.CA in stripes using en-GAL4 inhibits the trypsin-induced activation of the ple-WE1 and Ddc .47 epidermal wound reporters only in the cells where en>Src42A.CA is over-expressed (Figure 5E, 5G). Thus, overexpressed Src42A.CA acts cell autonomously to inhibit epidermal wound reporter activity. Embryos with deletion mutations that eliminate other Src family tyrosine kinases, e.g. Btk29A (Tec homolog), shark (Syk homolog), hopscotch (JAK homolog), and minibrain (DYRK homolog) all had normal, localized ple-WE1 wound reporter activity (Table 1). This suggests that the Src42A inhibition of epidermal wound reporter activity is specific, and not a general property of Src family tyrosine kinases.
To test whether chemical inhibition of Src function would result in widespread wound reporter activation, we simultaneously wounded and injected one such chemical inhibitor, SU6656 [53], into the body cavity. This treatment induced widespread, patchy expression of the Ddc .47 and ple-WE1 wound reporters throughout the embryonic epidermis (Figure S4A, S4B). This widespread activation of wound reporter genes after inhibition of Src kinase function was not suppressed by overexpression of Flo-2 using the arm-GAL4 driver (Figure S4A, S4B).
Flo-2 is associated with, and may stabilize the Flotillin-dependent fraction of lipid rafts in membranes [33]. Therefore, we were prompted to test whether chemicals that disrupt lipid rafts might influence the spread of wound reporter activation in the epidermis. One chemical that inhibits lipid raft formation is methyl-ß-cyclodextrin (MßCD), which depletes cholesterol (and other similar lipids) from cell membranes, and can influence many intracellular signaling pathways [54], [55], [56]. Simultaneous wounding and injection of MßCD into the body cavity of Drosophila embryos is sufficient to activate the Ddc .47 and ple-WE1 wound reporters throughout the entire embryonic epidermis (Figure 6A and data not shown). This result suggests that the effect of reducing functional Flo-2 on the wound response can be mimicked by more severe disruptions in lipid raft organization and membrane composition.
Fig. 6. Chemical activation of the epidermal wound reporters by H2O2 and methyl-ß-cyclodextrin (MßCD) is consistent with the affects of Duox and Flo-2 mutations on wound reporter expression. 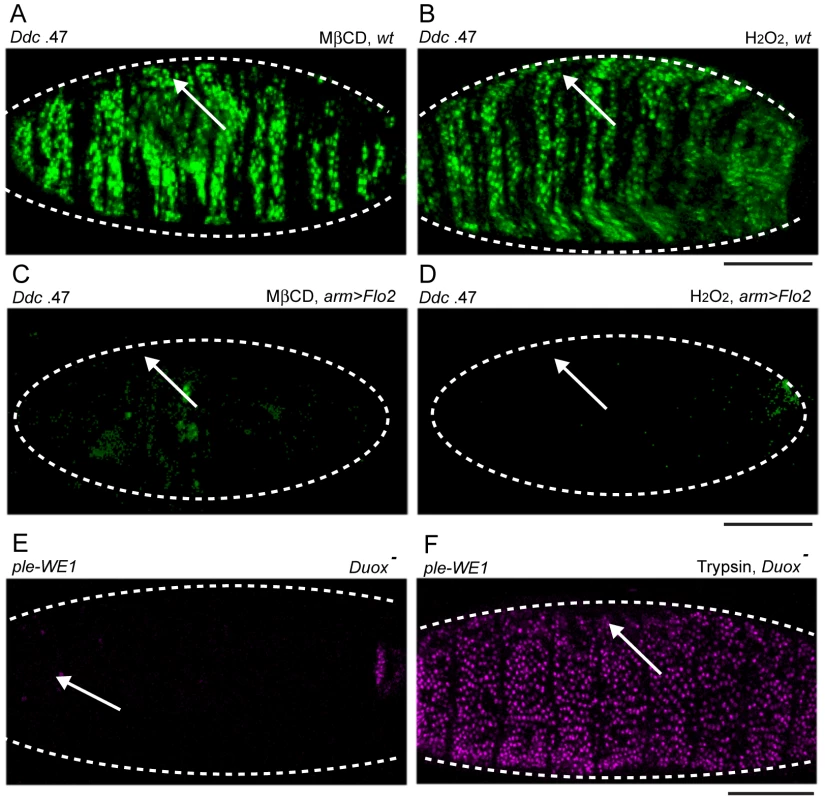
(A–F) Fluorescent confocal images of Ddc .47 or ple-WE1 epidermal wound reporters (all embryos shown are 6 hours post-wounding). Body cavity injection of MßCD (A) and H2O2 (B) into wild type (wt) embryos activates the Ddc .47 wound reporter throughout the entire epidermis. Overexpression of Flo-2 with an arm-GAL4 driver (arm>Flo-2) can inhibit both MßCD (C) and H2O2-induced (D) activation of the Ddc .47 wound reporter (or the ple-WE1 wound reporter, not shown). (E) In Duox mutant embryos, wound-induced ple-WE1 reporter expression is not detected (F) However, in Duox mutant embryos treated with trypsin ple-WE1 reporter expression is detected in all epidermal cells. Scale bar = 50 µM. One wound-induced signal that can function to attract blood cells to the site of clean wounds in zebrafish and Drosophila embryos is Hydrogen Peroxide (H2O2) [57], [58], [59]. We wished to test whether injection of H2O2 into the body cavity of Drosophila embryos was sufficient to activate the Ddc .47 and ple-WE1 epidermal wound reporters in embryos, and found that a wide range of concentrations of H2O2 could activate the Ddc .47 and ple-WE1 wound reporters in most or all epidermal cells (Figure 6B, and data not shown). Interestingly, Flo-2 or Src42A.CA overexpression is sufficient to inhibit the both MßCD and H2O2 activation of the Ddc .47 and ple-WE1 epidermal wound reporters (Figure 6C, 6D; Figure S4C, S4D, and data not shown). These results suggest that high levels of Flo-2 or Src42A.CA are potent inhibitors of chemically-induced transcriptional activation of these epidermal wound reporters. To determine whether H2O2 production was required for the induction of epidermal wound reporters, we tested a Drosophila mutant in the gene for the Dual oxidase protein (Duox), the enzyme responsible for the production of H2O2, [57]. We found that Duox mutant embryos show a dramatically decreased activation of the ple-WE1 epidermal wound reporter (Figure 6E). However, in the Duox mutant background, trypsin injection is still sufficient to activate the ple-WE1 reporter throughout the epidermis (Figure 6F), suggesting H2O2-induced wound signaling might be upstream of, or in parallel to, a serine protease-dependent activation of epidermal wound reporters.
Discussion
Drosophila melanogaster wound healing is an example of a regenerative process, which requires localized epidermal cytoskeletal changes, and localized wound-induced changes in epidermal transcriptional activity [60]. Our genetic screen with wound-dependent reporters has allowed us to identify novel components that regulate the localized transcriptional response to wounding in epidermal cells. This research identifies seven genes that are required to either activate (Duox and ghost/stenosis) or localize (Flo-2, Src42A, wurst, varicose, and Drosophila homolog of yeast Mak3) the expression patterns of epidermal wound reporters. The number of new functions involved in the delimitation of epidermal wound response near wound sites was unexpected, but indicates that considerable genetic effort is devoted to localizing the activity of transcriptional wound responses during regeneration.
One of the genes that limits the spread of epidermal wound reporters after clean epidermal punctures is Flo-2, as mutants of this gene show a broad expansion of epidermal wound gene activation. Drosophila Flo-2 is itself transcriptionally activated around epidermal wound sites, consistent with an evolutionarily conserved role in regeneration after wounding. In vertebrates, reggie-1/Flo-2 gene expression is activated in wounded fish optic neurons [30], and reggie-1/Flo-2 and reggie-2/Flo-1 morpholino knockdowns in wounded zebrafish retinal explants reduced axon outgrowth compared to controls [61]. Flo-2 transcriptional activation around Drosophila epidermal wound sites is dependent on the grh genetic function, which is required to activate at least a few other epidermal wound response genes [5], [25], [28]. Flo-2 thus appears to act in the same pathway as grh, although it may act both downstream and upstream of grh, since overexpression of Flo-2 can inhibit the activation of other grh-dependent wound response genes. In this respect, Flo-2 resembles the stit receptor tyrosine kinase gene, which is both transcriptionally activated by Grh, as well as required for grh-dependent activation of other downstream wound genes [28]. Amazingly, overexpression of Flo-2 can even inhibit the global activation of the Ddc .47 and ple-WE1 wound reporters that are induced by the serine protease trypsin, or by hydrogen peroxide. The inhibitory function of overexpression of Flo-2 on wound induced transcription is cell non-autonomous, at least over the range of a few cell diameters, as shown by the ability of striped overexpression of Flo-2 to silence puncture or trypsin-induced gene activation throughout the epidermis.
The only animal where Flo-2 null mutants have so far been characterized is Drosophila, where Flo-2 has been shown to regulate the spread of Wingless (Wg) and Hedgehog (Hh) signals in the wing imaginal discs [40], [41]. In the wing discs, both the secretion rate and the diffusion rate of these two lipid-modified morphogens were increased when Flo-2 was overexpressed, and decreased when Flo-2 and Flo-1 proteins were not expressed [41]. Despite the reduced spread of Wg and Hh morphogen proteins in Flo-2 mutant imaginal discs, adult morphology of mutants was normal, presumably because of compensatory mechanisms that occur later in development. Whereas a reduced range of activation of wg and hh long range transcription target genes was observed in Flo-2 mutant imaginal discs, we observe a greatly increased range of wound-induced gene activation in Flo-2 mutant embryos. This apparent discrepancy could be explained if one invokes of a long-range wound-induced inhibitory signal that in wild type embryos diffuses faster and farther than a wound activating signal, and thereby functions to limit the wound response to nearby epidermal cells [62], and that in Flo-2 mutants this potential inhibitory signal has reduced secretion, concentration, and/or diffusion range. This notion is consistent with the cell non-autonomous effect of overexpressed Flo-2 on inhibiting wound - or trypsin-induced gene activation. A similar scheme of controlling signal spreading has been seen in the way that Mmp2 acts cell non-autonomously to limit FGF signaling during Drosophila tracheal development and branch morphogenesis [63]. It's also possible that Flo-2 normally is required to set a global threshold that wound-induced signals must overcome in order to activate wound transcription, for example via Flo-2-dependent endocytosis/degradation of a diffusible wound signal and its receptor (perhaps the Stit RTK [28]), and that signal strength normally surpasses the Flo-2 threshold only in the vicinity of a wound. In this model, loss of Flo-2 would result in all epidermal cells being able to exceed the wound signal threshold, and overexpression of Flo-2 would prevent any cells from exceeding the wound signal threshold. The cell non-autonomous effects of Flo-2 overexpression under this model might be explained by an increase in Flo-2-dependent endocytosis/degradation that rapidly depletes an activating signal from the extracellular space.
Many previous studies have documented biochemical, molecular biological, and cell biological interactions between Src family kinases and Flotillins [33], [34], [49], [64]. In Drosophila, lack of Src42A and Flo-2 leads to expanded spread of wound gene activation, and overexpression of Flo-2 or activated Src42A can inhibit wound gene activation, which is consistent with an interaction between the two functions during the process of wound gene regulation. In cultured mammalian cells, Flo-2 can be phosphorylated by Src family kinases in an extracellular signal-dependent fashion. This phosphorylation is associated with changes in the normal intracellular trafficking of Flotillin-containing membrane microdomains and vesicles [33], [35], [49]. Since overexpressed Flo-2 in Drosophila can act in a cell non-autonomous fashion to inhibit wound gene activation, and overexpressed Src42A acts in a cell autonomous fashion to inhibit wound gene activation, one interpretation is that Flo-2 lies genetically upstream of Src42A in the epidermal wound response. This hypothesis appears to be inconsistent with the vertebrate biochemical data indicating that Src kinases phosphorylate Flotillins to activate their diverse functions. However, an observation that is consistent with Src42A activating Flo-2 protein function, is that even when Flo-2 is overexpressed, addition of chemical inhibitors of Src family kinases to wounded embryos, results in widespread Ddc .47 or ple-WE1 wound reporter activation. One interpretation of this results Flo-2 protein, no matter the level of expression, is inactive in the absence of Src42A function. Complex feedback loops involving signaling proteins being regulated by a transcription factor, while the activity of the same transcription factors is regulated by the same signaling pathway, have been observed in the control of Drosophila epidermal wound gene expression and reepithelialization [22], [28], so there may be similar dynamic cross-regulatory interactions between Flo-2 and Src42A in the localization of the epidermal wound response, interactions not easily captured in linear genetic pathway diagrams [65].
The inhibitory effect of Src42A on wound gene activation suggests that it might antagonize a signaling cascade that leads to the epidermal wound response. A good candidate for such a signaling cascade is the RTK pathway involving the Stit kinase. Stit is a RET-family RTK that is required for robust activation of the Ddc and stit wound reporter genes in wounded embryos [28]. Other evidence consistent with RTK pathway importance in wound gene activation is that phosphotyrosine accumulates persistently around wound sites [5], [28], and that ERK kinase function is required for robust activation of the Ddc wound reporter gene [5]. Interestingly, Src42A has been shown to act as an inhibitor of some Drosophila RTK proteins (those encoded by the torso, Egfr, and sevenless genes) in a few different tissues during Drosophila development [66]. The Flo-2 and Src42A functions in epidermal wound localization after clean wounding are reminiscent of the role of Drosophila WntD during infectious wounding. WntD mutants show higher levels of some antimicrobial peptide genes after septic injury of adults [67].
Previous evidence suggested that H2O2 and Duox could provide wound-induced inflammatory signals and antimicrobial activities [58], [59], [68], [69], [70], [71]. Our studies show that Duox is required to activate wound reporter genes after epidermal wounding, and that injected exogenous H2O2 is sufficient to activate widespread epidermal wound gene expression. Overexpression of either Flo-2 or Src42A.CA can inhibit the H2O2 -dependent wound reporter expression, suggesting that all of these components are in a common pathway controlling the activation of epidermal wound reporters. However, the ability of trypsin injection to activate the Ddc .47 and ple-WE1 wound reporters in Duox mutants suggests that a serine protease might act downstream of, or in parallel to, H2O2-dependent wound signals. A recent report showed that in cultured mammalian cells, a Src kinase phosphorylates and inhibits a Flo-2-associated enzyme, peroxiredoxin-1, which results in increased stability of H2O2 [72]. This is consistent with our results placing Flo-2, Src42A, and H2O2 in a common wound signaling pathway.
Like H2O2, the injection of methyl-ß-cyclodextrin (MßCD) into wounded embryos triggers a global wound response in the epidermis. MßCD strongly depletes cholesterol and other sterols from membranes and disrupt lipid rafts [73], [74], but was also shown to remove sphingolipid-associated proteins such as Src-Family Kinases [54]. The effects of MßCD, in combination with the effects of loss of Flo-2, suggests that the integrity of lipid rafts and associated proteins are required to inhibit epidermal wound signals. In cultured cells, MßCD treatments trigger a release of EGF receptors from membrane microdomains, which increases EGFR, and perhaps other RTK, signaling in a ligand-independent manner [75]. Interestingly, in cultured keratinocytes, MßCD treatment can induce the expression of involucrin [76], which encodes a protein, analogous to Drosophila Ple/tyrosine hydroxylase, which is required for the formation of an epidermal barrier. Similarly, MßCD injections into Drosophila embryos might also cause an increase the levels of a wound signal produced or released from cells adjacent to the wound site, allowing more widespread transcriptional activation of wound reporter genes. Our observations that overexpression of Src42A or Flo-2 can inhibit the MßCD -triggered activation of epidermal wound reporter genes suggest that high levels of these proteins might overcome lipid raft-inhibitory effects on wound signaling pathways.
Other genes (wurst and varicose) identified in the screen have phenotypes similar to Flo-2 and Src42A mutants (Figure 1). wurst encodes an evolutionarily conserved trans-membrane protein, containing a heat shock cognate protein 70 binding domain and a clathrin binding motif [77]. wurst is ubiquitously expressed in embryonic epithelial cells, strongly up-regulated during endocytosis-dependent luminal clearance, and mislocalized in mutants with endocytosis defects [77]. wurst mutant embryos have tortuous tracheal tubes, due to a failure to properly endocytose matrix material from the tracheal lumen [77]. varicose encodes an evolutionarily-conserved septate junction scaffolding protein, in the Membrane Associated GUanylate Kinase (MAGUK) family [78], [79], [80]. varicose is expressed in epidermally-derived cells (including the hindgut and trachea) and co-localizes with the septate junction proteins, Coracle and Neurexin4 [80]. varicose mutant embryos develop permeable tracheal tubes and paracellular barrier defects in epithelia [79], [80]. Like wurst mutants, varicose mutants also have abnormal matrix composition in the tracheal lumen, and may also have abnormal extracellular matrix composition produced by other epidermal cells.
Another gene (ghost, also known as stenosis) identified in this screen is required for wound reporter activation like Duox or grh (Figure 1). ghost encodes the Drosophila Sec24CD homolog, a coat protein of COPII vesicles in the ER/Golgi trafficking pathway [81], [82]. Transport of cargo from the ER to the Golgi via COPII vesicles is required to achieve normal amounts of secretion of extracellular matrix proteins into the developing Drosophila tracheae and normal apical-basal localization of membrane proteins [81], [82], [83]. Presumably, similar secretion and membrane localization defects occur in non-tracheal epidermal cells, which account for the severe cuticle deposition defects in ghost (Sec24CD) mutants. It is fascinating to note that our finding that ghost (Sec24CD) is required for transcriptional activation of epidermal wound reporter genes is consistent with the finding that RNAi knockdowns of Sec24C in a planaria (Schmidtea mediterranea) interfered with normal regeneration after amputation wounds [84]. It is possible that the ghost mutants do not secrete enough wound signals, or the protein matrix necessary for the propagation of a wound signal.
Another gene required for the activation of wound reporters is shroud (sro). Based on a previous paper by Giesen et al. (2003) [85], we believed sro to be an allele in the Drosophila Fos-D isoform [25], and hypothesized that one of the Drosophila kayak/Fos transcription factors was required for the activation of some epidermal wound gene reporters [25]. However, as Niwa et al. (2010) [86] recently discovered, sro[1] and other sro point mutant alleles do not map in the kayak/Fos gene, but in an immediately adjacent transcription unit (Nm-g/sro) that encodes an enzyme in the sterol metabolic pathway that is necessary for production of ecdysone hormone. At first glance, the requirement of sro to activate some wound reporters suggested that these reporters rely on ecdysone signaling. This is possible, although we have tested deletions that eliminate zygotic functions of the ecdysone receptor gene, as well as of the phantom gene (which encodes another enzyme in the ecdysone synthesis pathway), and embryos that are zygotic mutants in either gene show normal activation of the ple-WE1 wound reporter after puncture wounding.
In summary, from our large unbiased screen, we have identified several genes that add to our understanding of the complex pathways that control the signals that activate wound response transcription near puncture wounds. At the cellular level, there appears to be a correlation between genetic functions required to localize wound-induced gene activation, and cellular functions required for endocytosis and/or apical-basal polarity. For example, one function of Flo-2 is in signal-dependent endocytosis, although Flo-2 also plays other roles in vesicular trafficking [32], [33], [34], [35], [36]. There have been many studies showing that endocytosis can regulate extracellular signaling strength and duration [87]. For example, one study found that tagged-FGF8 showed increased accumulation, spread, and target gene activation when Rab-5-mediated endocytosis was reduced in zebrafish embryos [88]. We believe that further studies on wound response signaling may provide new insights into how membrane microdomains, endocytosis of membrane receptors, and the composition and organization of the extracellular matrix, regulates the transmission of wound signals.
Materials and Methods
Drosophila Stocks
Fluorescent Balancers, Deficiencies, and Mutant alleles were obtained from the Bloomington Drosophila Stock Center: FKG = FM7c, P{GAL4-Kr.C}DC1, P{UAS-GFP.S65T}DC5, CKG = CyO, P{GAL4-Kr.C}DC3, P{UAS-GFP.S65T}DC7, Flo-2{KG00210}, Src42A-E1, UAS-Src.CA, arm-GAL4, en-GAL4, Duox{KG07745}, wurst{G814}, varicose{03953b}, and ghost{KG029061}. UAS-Flo-2 was provided by Vladimir Katanaev. Ddc .47 and ple-WE1 were previously described [25].
Wounding Procedure
Embryos were collected on apple juice agar plates and aged to 15–17 h at 25°C. Embryos were washed into mesh baskets, dechorionated in bleach for 1 min, then washed copiously with water. Embryos were then transferred to a clean slab of apple juice agar and aligned for 30–60 min at 18°C, transferred to slides with double-sided tape, then covered in a 1∶1 ratio of 700∶27 weight halocarbon oil. Embryos were then wounded bilaterally with fresh microinjection needles made from an automated puller mounted on a micromanipulator, allowed to recover for 3–8 h at room temperature, and visualized under fluorescent light in a compound microscope to determine wound reporter activity. At least 3 independent experiments with at least 50 successfully wounded embryos were performed. Assays involving homozygous deletion or mutant embryos were performed in parallel to heterozygous-balancer embryos. A Kr-GFP fluorescent marker on the balancer chromosome [89], was used to determine the genotype of the embryos. Assays involving UAS-GAL4 overexpression were performed in parallel to UAS-non-GAL4 controls. All embryos were impaled using a micromanipulator so that the needle protruded 1 embryo-width from the exit wound. Wound reporter responses were rated on a scale of “no activity, localized activity, or global activity.” Images were obtained by wounding embryos with microinjection needles and imaged on a Leica SP2 confocal microscope, selecting representative embryos to image. Images were resized while constraining proportions to maintain resolution. Adobe Photoshop adjustment functions were used equally on images to enhance clarity, but not to obscure, eliminate, or misrepresent any information. Original images are available on request.
Body Cavity Injection
Individual embryos were simultaneously wounded and injected by using a syringe to expel the various solutions into the body cavity of the embryo. A Pipetman was used to load the solutions to be injected into the pulled capillary microinjection needles. Needles were broken on the side of a glass cover slip on a glass slide. Serine Protease-Trypsin from bovine pancreas was solubilized in 1 mM HCl pH 3.0 to 2 mg/mL (Sigma). Src Inhibitor-SU6656 was solubilized in 50% DMSO to 100 µM (Calbiochem). Methyl-ß-cyclodextrin (MßCD) was solubilized in 1 mM NaOH to 3 mM (Sigma). Hydrogen Peroxide-H2O2 was diluted in H2O to 0.6 M (Fisher). Chemical-wounded embryos were simultaneously wounded and injected with a 1∶4 ratio of 1% toluidine blue dye and solubilized compounds. Toluidine blue dye allowed for visual confirmation of solubilized compounds being injected into the body cavity. Control embryos were wounded with a broken needle containing 1∶4 ratios of 1% toluidine blue dye and solute without chemical. A wide range of chemical concentrations was tested to obtain optimal activation of the epidermal wound reporter and maintain high levels of embryo survival after body cavity injection.
Multiplex Fluorescent In Situ Hybridization
Probes were generated from partial or full cDNA clones from the Drosophila Gene Collection [90], [91]. anti-Stitcher antibody was provided by Christos Samakovilis. Probe labeling and hybridization protocol was as described in Dave Kosman's multiplex FISH protocol [92].
Supporting Information
Zdroje
1. SegreJ 2003 Complex redundancy to build a simple epidermal permeability barrier. Curr Opin Cell Biol 15 776 782
2. LockeM 2001 The Wigglesworth Lecture: Insects for studying fundamental problems in biology. J Insect Physiol 47 495 507
3. MoussianB 2010 Recent advances in understanding mechanisms of insect cuticle differentiation. Insect Biochem Mol Biol 40 363 375
4. MartinPParkhurstSM 2004 Parallels between tissue repair and embryo morphogenesis. Development 131 3021 3034
5. MaceKAPearsonJCMcGinnisW 2005 An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science 308 381 385
6. TingSBCaddyJHislopNWilanowskiTAudenA 2005 A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science 308 411 413
7. CamposIGeigerJASantosACCarlosVJacintoA 2010 Genetic Screen in Drosophila melanogaster Uncovers a Novel Set of Genes Required for Embryonic Epithelial Repair. Genetics 184 129 140
8. GurtnerGCWernerSBarrandonYLongakerMT 2008 Wound repair and regeneration. Nature 453 314 321
9. LemaitreBHoffmannJ 2007 The host defense of Drosophila melanogaster. Annu Rev Immunol 25 697 743
10. JacintoAWoodWBalayoTTurmaineMMartinez-AriasA 2000 Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol 10 1420 1426
11. JacintoAWoodWWoolnerSHileyCTurnerL 2002 Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr Biol 12 1245 1250
12. WoodWJacintoAGroseRWoolnerSGaleJ 2002 Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol 4 907 912
13. SchaferMWernerS 2007 Transcriptional control of wound repair. Annu Rev Cell Dev Biol 23 69 92
14. BraySJKafatosFC 1991 Developmental function of Elf-1: an essential transcription factor during embryogenesis in Drosophila. Genes Dev 5 1672 1683
15. YuZLinKKBhandariASpencerJAXuX 2006 The Grainyhead-like epithelial transactivator Get-1/Grhl3 regulates epidermal terminal differentiation and interacts functionally with LMO4. Dev Biol 299 122 136
16. ChalmersADLachaniKShinYSherwoodVChoKW 2006 Grainyhead-like 3, a transcription factor identified in a microarray screen, promotes the specification of the superficial layer of the embryonic epidermis. Mech Dev 123 702 718
17. KimMMcGinnisW 2011 Phosphorylation of Grainy head by ERK is essential for wound-dependent regeneration but not for development of an epidermal barrier. Proc Natl Acad Sci U S A 108 650 655
18. AndersenSOHojrupPRoepstorffP 1995 Insect cuticular proteins. Insect Biochem Mol Biol 25 153 176
19. LiGGustafson-BrownCHanksSKNasonKArbeitJM 2003 c-Jun is essential for organization of the epidermal leading edge. Dev Cell 4 865 877
20. WestonCRDavisRJ 2007 The JNK signal transduction pathway. Curr Opin Cell Biol 19 142 149
21. GalkoMJKrasnowMA 2004 Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol 2 e239 doi:10.1371/journal.pbio.0020239
22. LeschCJoJWuYFishGSGalkoMJ 2010 A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics 186 943 957
23. SuYCTreismanJESkolnikEY 1998 The Drosophila Ste20-related kinase misshapen is required for embryonic dorsal closure and acts through a JNK MAPK module on an evolutionarily conserved signaling pathway. Genes Dev 12 2371 2380
24. RametMLanotRZacharyDManfruelliP 2002 JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol 241 145 156
25. PearsonJCJuarezMTKimMDrivenesOMcGinnisW 2009 Multiple transcription factor codes activate epidermal wound-response genes in Drosophila. Proc Natl Acad Sci U S A 106 2224 2229
26. BoschMSerrasFMartin-BlancoEBagunaJ 2005 JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol 280 73 86
27. BergantinosCVilanaXCorominasMSerrasF 2010 Imaginal discs: Renaissance of a model for regenerative biology. Bioessays 32 207 217
28. WangSTsarouhasVXylourgidisNSabriNTiklovaK 2009 The tyrosine kinase Stitcher activates Grainy head and epidermal wound healing in Drosophila. Nat Cell Biol 11 890 895
29. WuYBrockARWangYFujitaniKUedaR 2009 A Blood-Borne PDGF/VEGF-like Ligand Initiates Wound-Induced Epidermal Cell Migration in Drosophila Larvae. Curr Biol 19 1473 1477
30. SchulteTPaschkeKALaessingULottspeichFStuermerCA 1997 Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development 124 577 587
31. BickelPESchererPESchnitzerJEOhPLisantiMP 1997 Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem 272 13793 13802
32. FrickMBrightNARientoKBrayAMerrifiedC 2007 Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol 17 1151 1156
33. RientoKFrickMSchaferINicholsBJ 2009 Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J Cell Sci 122 912 918
34. BabukeTTikkanenR 2007 Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol 86 525 532
35. LanghorstMFReuterAJaegerFAWippichFMLuxenhoferG 2008 Trafficking of the microdomain scaffolding protein reggie-1/flotillin-2. Eur J Cell Biol 87 211 226
36. StuermerCA 2010 The reggie/flotillin connection to growth. Trends Cell Biol
37. ParksALCookKRBelvinMDompeNAFawcettR 2004 Systematic generation of high-resolution deletion coverage of the Drosophila melanogaster genome. Nat Genet 36 288 292
38. StuermerCA 2011 Reggie/flotillin and the targeted delivery of cargo. J Neurochem 116 708 713
39. GalbiatiFVolonteDGoltzJSSteeleZSenJ 1998 Identification, sequence and developmental expression of invertebrate flotillins from Drosophila melanogaster. Gene 210 229 237
40. HoehneMde CouetHGStuermerCAFischbachKF 2005 Loss - and gain-of-function analysis of the lipid raft proteins Reggie/Flotillin in Drosophila: they are posttranslationally regulated, and misexpression interferes with wing and eye development. Mol Cell Neurosci 30 326 338
41. KatanaevVLSolisGPHausmannGBuestorfSKatanayevaN 2008 Reggie-1/flotillin-2 promotes secretion of the long-range signalling forms of Wingless and Hedgehog in Drosophila. Embo J 27 509 521
42. LudwigAOttoGPRientoKHamsEFallonPG 2010 Flotillin microdomains interact with the cortical cytoskeleton to control uropod formation and neutrophil recruitment. J Cell Biol 191 771 781
43. BaroloSCastroBPosakonyJW 2004 New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP
44. BrandAHPerrimonN 1993 Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401 415
45. SansonBWhitePVincentJP 1996 Uncoupling cadherin-based adhesion from wingless signalling in Drosophila. Nature 383 627 630
46. PhelpsCBBrandAH 1998 Ectopic gene expression in Drosophila using GAL4 system. Methods 14 367 379
47. ChoYSStevensLMSteinD 2010 Pipe-dependent ventral processing of Easter by Snake is the defining step in Drosophila embryo DV axis formation. Curr Biol 20 1133 1137
48. TangHKambrisZLemaitreBHashimotoC 2006 Two proteases defining a melanization cascade in the immune system of Drosophila. J Biol Chem 281 28097 28104
49. Neumann-GiesenCFernowIAmaddiiMTikkanenR 2007 Role of EGF-induced tyrosine phosphorylation of reggie-1/flotillin-2 in cell spreading and signaling to the actin cytoskeleton. J Cell Sci 120 395 406
50. TakahashiFEndoSKojimaTSaigoK 1996 Regulation of cell-cell contacts in developing Drosophila eyes by Dsrc41, a new, close relative of vertebrate c-src. Genes Dev 10 1645 1656
51. ParsonsSJParsonsJT 2004 Src family kinases, key regulators of signal transduction. Oncogene 23 7906 7909
52. TatenoMNishidaYAdachi-YamadaT 2000 Regulation of JNK by Src during Drosophila development. Science 287 324 327
53. BlakeRABroomeMALiuXWuJGishizkyM 2000 SU6656, a selective src family kinase inhibitor, used to probe growth factor signaling. Mol Cell Biol 20 9018 9027
54. IlangumaranSHoessliDC 1998 Effects of cholesterol depletion by cyclodextrin on the sphingolipid microdomains of the plasma membrane. Biochem J 335 Pt 2 433 440
55. LambertSVind-KezunovicDKarvinenSGniadeckiR 2006 Ligand-independent activation of the EGFR by lipid raft disruption. J Invest Dermatol 126 954 962
56. ZidovetzkiRLevitanI 2007 Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta 1768 1311 1324
57. MathiasJRPerrinBJLiuTXKankiJLookAT 2006 Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J Leukoc Biol 80 1281 1288
58. NiethammerPGrabherCLookATMitchisonTJ 2009 A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459 996 999
59. MoreiraSStramerBEvansIWoodWMartinP 2010 Prioritization of competing damage and developmental signals by migrating macrophages in the Drosophila embryo. Curr Biol 20 464 470
60. StramerBMartinP 2005 Cell biology: master regulators of sealing and healing. Curr Biol 15 R425 427
61. MunderlohCSolisGPBodrikovVJaegerFAWiechersM 2009 Reggies/flotillins regulate retinal axon regeneration in the zebrafish optic nerve and differentiation of hippocampal and N2a neurons. J Neurosci 29 6607 6615
62. GiererAMeinhardtH 1972 A theory of biological pattern formation. Kybernetik 12 30 39
63. WangQUhlirovaMBohmannD 2010 Spatial restriction of FGF signaling by a matrix metalloprotease controls branching morphogenesis. Dev Cell 18 157 164
64. StuermerCALangDMKirschFWiechersMDeiningerSO 2001 Glycosylphosphatidyl inositol-anchored proteins and fyn kinase assemble in noncaveolar plasma membrane microdomains defined by reggie-1 and -2. Mol Biol Cell 12 3031 3045
65. AveryLWassermanS 1992 Ordering gene function: the interpretation of epistasis in regulatory hierarchies. Trends Genet 8 312 316
66. LuXLiY 1999 Drosophila Src42A is a negative regulator of RTK signaling. Dev Biol 208 233 243
67. GordonMDDionneMSSchneiderDSNusseR 2005 WntD is a feedback inhibitor of Dorsal/NF-kappaB in Drosophila development and immunity. Nature 437 746 749
68. HaEMOhCTBaeYSLeeWJ 2005 A direct role for dual oxidase in Drosophila gut immunity. Science 310 847 850
69. BaeYSChoiMKLeeWJ 2010 Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol 31 278 287
70. BuchonNPoidevinMKwonHMGuillouASottasV 2009 A single modular serine protease integrates signals from pattern-recognition receptors upstream of the Drosophila Toll pathway. Proc Natl Acad Sci U S A 106 12442 12447
71. WesleyUVBovePFHristovaMMcCarthySvan der VlietA 2007 Airway epithelial cell migration and wound repair by ATP-mediated activation of dual oxidase 1. J Biol Chem 282 3213 3220
72. WooHAYimSHShinDHKangDYuDY 2010 Inactivation of peroxiredoxin I by phosphorylation allows localized H(2)O(2) accumulation for cell signaling. Cell 140 517 528
73. HancockJF 2006 Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7 456 462
74. LingwoodDSimonsK 2010 Lipid rafts as a membrane-organizing principle. Science 327 46 50
75. PikeLJ 2005 Growth factor receptors, lipid rafts and caveolae: an evolving story. Biochim Biophys Acta 1746 260 273
76. JansRAtanasovaGJadotMPoumayY 2004 Cholesterol depletion upregulates involucrin expression in epidermal keratinocytes through activation of p38. J Invest Dermatol 123 564 573
77. BehrMWingenCWolfCSchuhRHochM 2007 Wurst is essential for airway clearance and respiratory-tube size control. Nat Cell Biol 9 847 853
78. BachmannADragaMGraweFKnustE 2008 On the role of the MAGUK proteins encoded by Drosophila varicose during embryonic and postembryonic development. BMC Dev Biol 8 55
79. MoyerKEJacobsJR 2008 Varicose: a MAGUK required for the maturation and function of Drosophila septate junctions. BMC Dev Biol 8 99
80. WuVMYuMHPaikRBanerjeeSLiangZ 2007 Drosophila Varicose, a member of a new subgroup of basolateral MAGUKs, is required for septate junctions and tracheal morphogenesis. Development 134 999 1009
81. ForsterDArmbrusterKLuschnigS 2010 Sec24-dependent secretion drives cell-autonomous expansion of tracheal tubes in Drosophila. Curr Biol 20 62 68
82. NorumMTangEChavoshiTSchwarzHLinkeD 2010 Trafficking through COPII stabilises cell polarity and drives secretion during Drosophila epidermal differentiation. PLoS ONE 5 e10802 doi:10.1371/journal.pone.0010802
83. TsarouhasVSentiKAJayaramSATiklovaKHemphalaJ 2007 Sequential pulses of apical epithelial secretion and endocytosis drive airway maturation in Drosophila. Dev Cell 13 214 225
84. ReddienPWBermangeALMurfittKJJenningsJRSanchez AlvaradoA 2005 Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev Cell 8 635 649
85. GiesenKLammelULangehansDKrukkertKBunseI 2003 Regulation of glial cell number and differentiation by ecdysone and Fos signaling. Mech Dev 120 401 413
86. NiwaRNamikiTItoKShimada-NiwaYKiuchiM 2010 Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box’ of the ecdysteroid biosynthesis pathway. Development 137 1991 1999
87. WileyHSBurkePM 2001 Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic 2 12 18
88. ScholppSBrandM 2004 Endocytosis controls spreading and effective signaling range of Fgf8 protein. Curr Biol 14 1834 1841
89. CassoDRamirez-WeberFKornbergTB 2000 GFP-tagged balancer chromosomes for Drosophila melanogaster. Mech Dev 91 451 454
90. StapletonMCarlsonJBroksteinPYuCChampeM 2002 A Drosophila full-length cDNA resource. Genome Biol 3 RESEARCH0080
91. StapletonMLiaoGBroksteinPHongLCarninciP 2002 The Drosophila gene collection: identification of putative full-length cDNAs for 70% of D. melanogaster genes. Genome Res 12 1294 1300
92. KosmanDMizutaniCMLemonsDCoxWGMcGinnisW 2004 Multiplex detection of RNA expression in Drosophila embryos. Science 305 846
93. TweedieSAshburnerMFallsKLeylandPMcQuiltonP 2009 FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Research 37 D555 D559
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



