-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
Genome-wide association studies (GWAS) have successfully identified loci associated with quantitative traits, such as blood lipids. Deep resequencing studies are being utilized to catalogue the allelic spectrum at GWAS loci. The goal of these studies is to identify causative variants and missing heritability, including heritability due to low frequency and rare alleles with large phenotypic impact. Whereas rare variant efforts have primarily focused on nonsynonymous coding variants, we hypothesized that noncoding variants in these loci are also functionally important. Using the HDL-C gene LIPG as an example, we explored the effect of regulatory variants identified through resequencing of subjects at HDL-C extremes on gene expression, protein levels, and phenotype. Resequencing a portion of the LIPG promoter and 5′ UTR in human subjects with extreme HDL-C, we identified several rare variants in individuals from both extremes. Luciferase reporter assays were used to measure the effect of these rare variants on LIPG expression. Variants conferring opposing effects on gene expression were enriched in opposite extremes of the phenotypic distribution. Minor alleles of a common regulatory haplotype and noncoding GWAS SNPs were associated with reduced plasma levels of the LIPG gene product endothelial lipase (EL), consistent with its role in HDL-C catabolism. Additionally, we found that a common nonfunctional coding variant associated with HDL-C (rs2000813) is in linkage disequilibrium with a 5′ UTR variant (rs34474737) that decreases LIPG promoter activity. We attribute the gene regulatory role of rs34474737 to the observed association of the coding variant with plasma EL levels and HDL-C. Taken together, the findings show that both rare and common noncoding regulatory variants are important contributors to the allelic spectrum in complex trait loci.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002393
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002393Summary
Genome-wide association studies (GWAS) have successfully identified loci associated with quantitative traits, such as blood lipids. Deep resequencing studies are being utilized to catalogue the allelic spectrum at GWAS loci. The goal of these studies is to identify causative variants and missing heritability, including heritability due to low frequency and rare alleles with large phenotypic impact. Whereas rare variant efforts have primarily focused on nonsynonymous coding variants, we hypothesized that noncoding variants in these loci are also functionally important. Using the HDL-C gene LIPG as an example, we explored the effect of regulatory variants identified through resequencing of subjects at HDL-C extremes on gene expression, protein levels, and phenotype. Resequencing a portion of the LIPG promoter and 5′ UTR in human subjects with extreme HDL-C, we identified several rare variants in individuals from both extremes. Luciferase reporter assays were used to measure the effect of these rare variants on LIPG expression. Variants conferring opposing effects on gene expression were enriched in opposite extremes of the phenotypic distribution. Minor alleles of a common regulatory haplotype and noncoding GWAS SNPs were associated with reduced plasma levels of the LIPG gene product endothelial lipase (EL), consistent with its role in HDL-C catabolism. Additionally, we found that a common nonfunctional coding variant associated with HDL-C (rs2000813) is in linkage disequilibrium with a 5′ UTR variant (rs34474737) that decreases LIPG promoter activity. We attribute the gene regulatory role of rs34474737 to the observed association of the coding variant with plasma EL levels and HDL-C. Taken together, the findings show that both rare and common noncoding regulatory variants are important contributors to the allelic spectrum in complex trait loci.
Introduction
Numerous studies have associated low levels of high density lipoprotein cholesterol (HDL-C) with an increased risk of developing coronary heart disease (CHD) [1], [2], [3], [4], [5], [6], [7]. HDL-C levels are approximately 50% heritable [8]. Genome-wide association studies (GWAS) for lipid traits have identified many genes previously associated with HDL metabolism and numerous novel loci [9], [10], [11], [12], [13], [14]. However, the identification of the causal variants in these loci has proven difficult. Resequencing studies have not identified common coding variants that explain the associations. Such results may suggest that causal coding variants are rarer than anticipated [15] or lie in the gene regulatory regions. Furthermore, many of the variants identified by GWAS are embedded in gene deserts. Although a portion of these associated variants may tag less-common variants with strong phenotypic effects, some noncoding variants are likely to be causal themselves [16]. Nevertheless, combining the variation explained by all of the common variants identified to date leaves missing heritability [17] that may be explained, at least in part, by rare variants.
Several HDL-C candidate genes, including those with known physiological relevance to HDL-C metabolism, have been characterized though targeted gene-resequencing approaches [18]. Through these studies, the exons of HDL-C candidate genes (ABCAI, APOAI, LCAT) [19] and other mechanistically implicated genes (ANGPTL4, LIPG) [20], [21] have been sequenced in individuals at the extremes of the HDL-C phenotypic distribution. Rare coding loss-of-function variants were shown to segregate with the phenotype in a manner consistent with the known physiological role of the gene product in increasing or decreasing HDL-C levels. Causality of the identified variants was shown through a combination of in vitro functional studies and computational methods. Because the occurrence of each rare variant was too low to test its association in our sequencing cohorts, individual variants in each phenotypic extreme were grouped together (“collapsed”), and the total number of rare variants in the sequenced region was compared between cohorts. This method of rare variant association analysis, known as the cohort allelic sums test (CAST) [22], [23], has been instrumental in showing that rare loss-of-function variants modulate HDL-C levels in humans. However, few studies to date have utilized this approach to study rare regulatory variants, which do not always segregate with the phenotypic extremes of continuous traits as stringently as deleterious nonsynonymous variants. Additionally, the functional validation of identified variants in regulatory regions can be challenging, especially for unknown promoter or regulatory elements.
In the last decade, several HDL-C candidate genes have been identified, including many with large regulatory regions implicated in association studies. These findings, combined with the fact that HDL-C exists as a continuously distributed trait, make HDL-C candidate genes well-suited for understanding how rare regulatory variants influence complex traits. One HDL-C candidate gene associated in GWAS is LIPG [9], [10], [11], [12], [13], [24], [25], which encodes endothelial lipase (EL), a conserved plasma phospholipase expressed from endothelial cells [26], [27]. Compared to other plasma proteins, EL exhibits preferential HDL phospholipolysis activity in vitro [28]. Somatic overexpression of EL in mice causes a dose-dependent reduction in plasma HDL-C levels [29], whereas targeted deletion of LIPG [30] or inhibition of EL using polyclonal antibodies [31] raises HDL-C levels in vivo.
We recently identified rare loss-of-function coding variants in subjects with high HDL-C through a resequencing study of subjects at the extremes of the HDL-C phenotypic distribution [20]. Here, we expand our initial resequencing effort to include regulatory variations, thereby further characterizing the allelic spectrum of LIPG. Our findings show that both rare and common variations in regulatory regions of LIPG affect LIPG expression, plasma EL protein concentrations, and HDL-C levels.
Results
Identification and functional assessment of novel rare LIPG regulatory variants
We sequenced a portion of the promoter and the 5′ UTR (1755-bp immediately upstream of the transcription start site) in 388 unrelated individuals. Of the sequenced individuals, 195 individuals had extremely high HDL-C levels (≥95th percentile; HHDL Sequencing Cohort) and 193 had low HDL-C levels (≤25th percentile; LHDL Sequencing Cohort). A summary of the characteristics of the participants in the sequencing cohorts appears in Table 1. Through this study, we identified a total of 22 rare and common LIPG regulatory variants in the region sequenced (Figure 1).
Fig. 1. Rare and common LIPG regulatory variants studied. 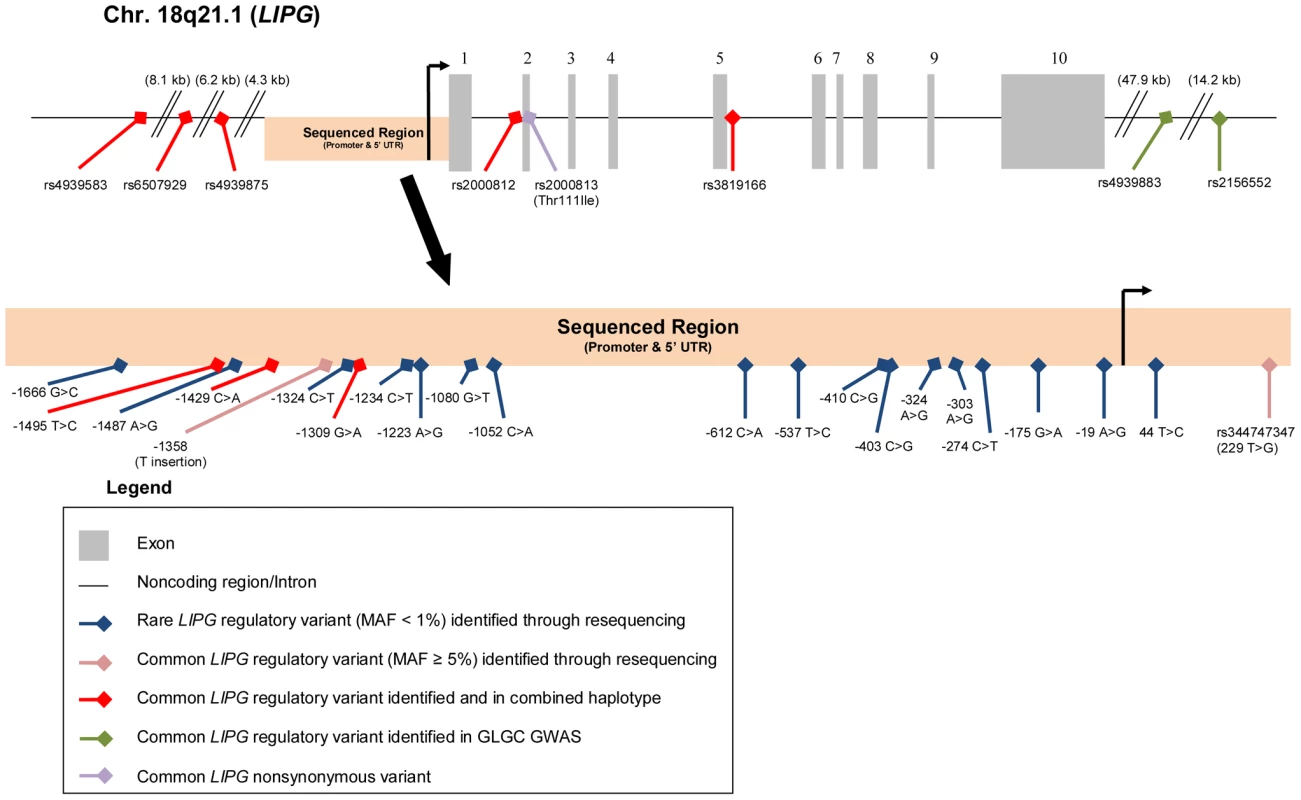
Diagram of Chr. 18q21.1 region containing LIPG with variants identified annotated. Tab. 1. Baseline characteristics of participants in LIPG promoter resequencing. 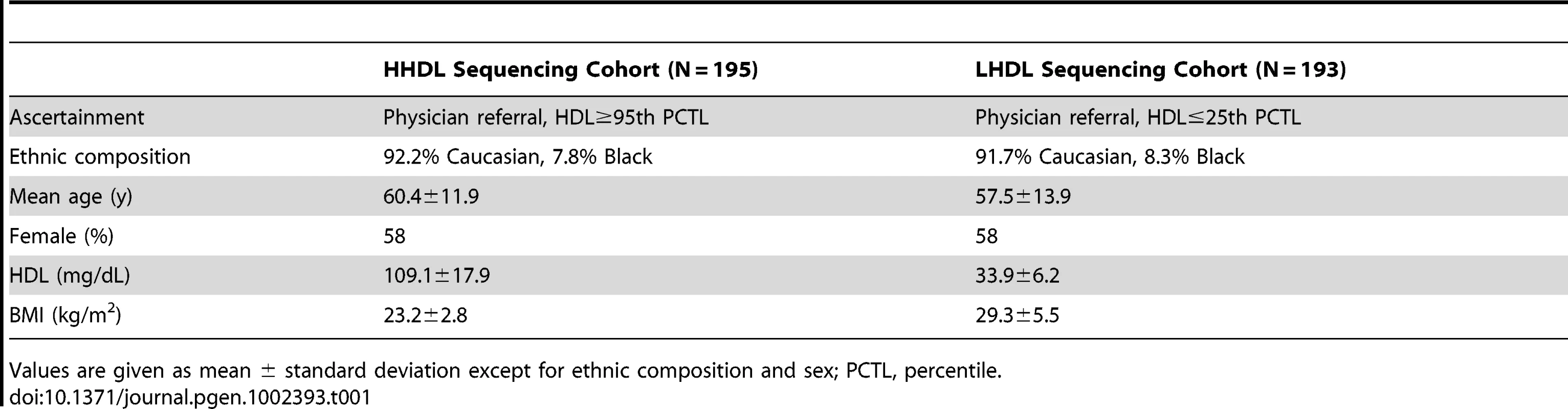
Values are given as mean ± standard deviation except for ethnic composition and sex; PCTL, percentile. 25 individuals from our sequencing cohorts harbored a rare variant (minor allele frequency [MAF]<1%) in the proximal promoter or 5′ UTR of LIPG. Of these 25 individuals, 16 were in the HHDL and 9 were in the LHDL Sequencing Cohort (Table 2). The main characteristics of each of these participants are summarized in Table S1. Of the 17 individual rare LIPG regulatory mutations we identified, 10 were found only in individuals with high HDL-C, 5 occurred only in individuals with low HDL-C, and the remaining 2 occurred in individuals from both cohorts. We did not find a disproportionate frequency of rare regulatory variants between the HHDL and LHDL cohorts (P = 0.2142, Table 3).
Tab. 2. Identified rarea LIPG regulatory variants. 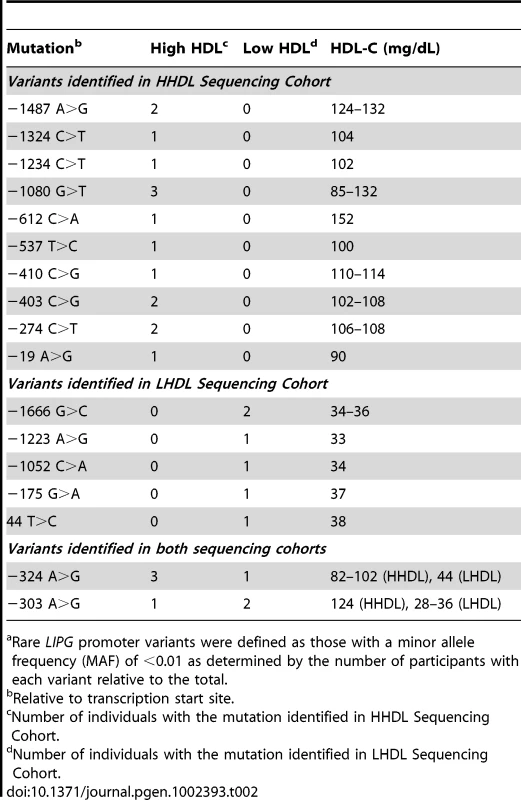
Rare LIPG promoter variants were defined as those with a minor allele frequency (MAF) of <0.01 as determined by the number of participants with each variant relative to the total. Tab. 3. Association of rare LIPG regulatory variants with HDL-C phenotypic extremes. 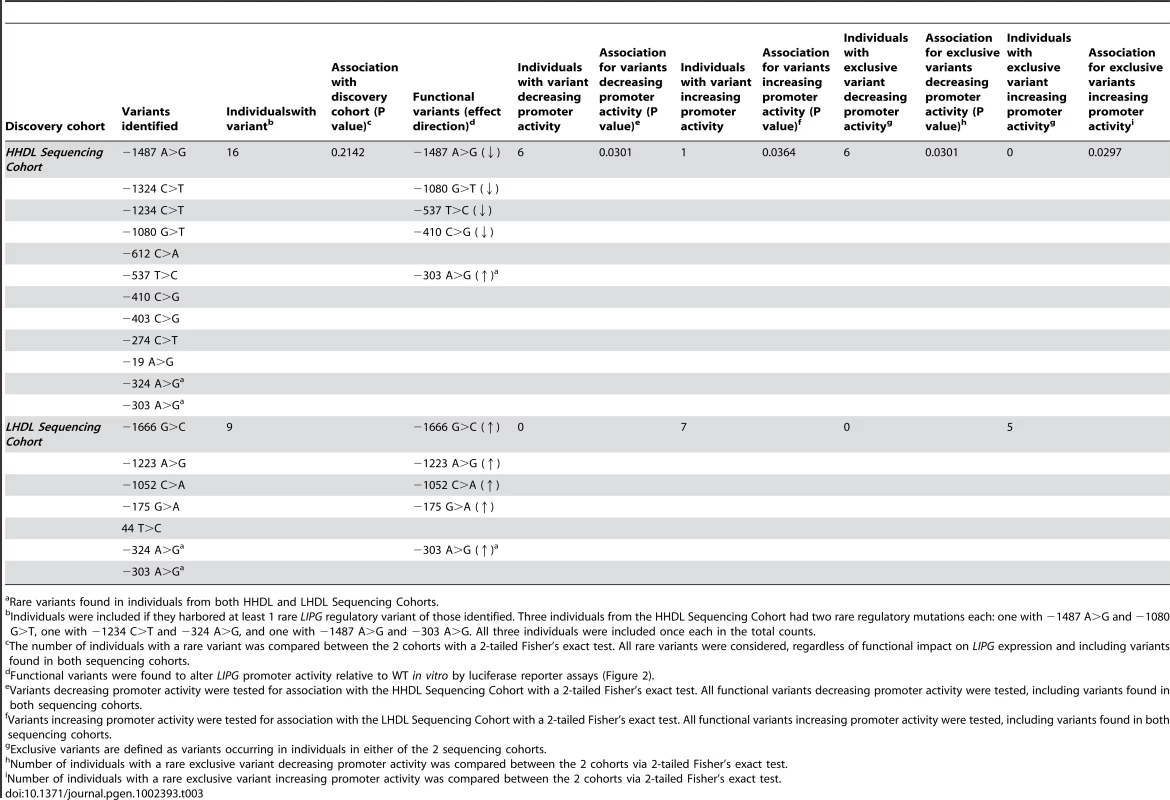
Rare variants found in individuals from both HHDL and LHDL Sequencing Cohorts. We also searched for these variants in the 1000 Genomes Project database (451 participants; [32]) and found that only the 2 variants present in both cohorts, −303 A>G and −324 A>G, occurred in individuals of the YRI ethnicity in this database (MAF = 0.014 for −303 A>G, MAF = 0.024 for −324 A>G). Neither of these variants was present in 1000 Genomes Project participants of other ethnicities, nor were any of the other 15 variants present in any population from this study.
To determine the functional significance of the identified variants in modulating LIPG promoter activity, variants were tested with a luciferase reporter assay in HUVECs, which endogenously express LIPG. A wild-type LIPG promoter construct corresponding to the sequenced portion of the LIPG promoter was constructed and tested against the promoter-less pGL3-basic construct. The WT LIPG promoter construct displayed approximately 31.9 times greater relative luciferase activity than the pGL3-basic construct (Figure S1).
We tested promoter constructs corresponding to the rare LIPG variants. Four of the 10 rare variants found only in high HDL-C individuals displayed decreased promoter activity relative to the WT promoter construct (Figure 2A). In contrast, 4 of the 5 rare variants found only in low HDL-C individuals displayed increased promoter activity (Figure 2B). The remaining 6 variants identified in only in high HDL-C individuals and 1 variant identified only in low HDL-C individuals did not alter promoter activity relative to WT (Figure S2A and S2B). One of the 2 rare regulatory variants found at both extremes (−303 A>G) caused increased promoter activity in vitro (Figure S2C). Six individuals from the HHDL Sequencing Cohort had a rare regulatory variant decreasing LIPG expression in vitro, compared to no individuals from the LHDL Sequencing Cohort (P = 0.0301, Fisher's exact test, Table 3). One individual from the HHDL Sequencing Cohort had a rare regulatory variant increasing promoter activity, compared with 7 individuals from the LHDL Sequencing Cohort (P = 0.0364, Table 3).
Fig. 2. Rare LIPG regulatory variants modulate transcriptional activity in vitro. 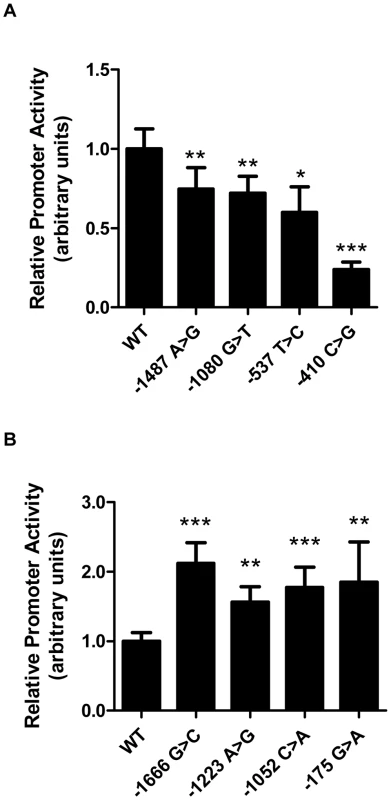
Relative promoter activity of rare variants (MAF<0.01) identified from resequencing of high HDL-C individuals (A) or low HDL-C individuals (B). Plasmid constructs expressing firefly luciferase under the control of wild-type (WT) or variant LIPG promoters were individually co-transfected with a Renilla luciferase reporter construct (pRL-SV40) in HUVECs. Firefly luciferase expression were measured and normalized to that of Renilla luciferase, and Renilla-normalized promoter activities for variant constructs were then compared to those of the WT construct to provide relative LIPG promoter activities of the variants. Assays were conducted with 6 replicates per experiment and data is given as mean ± standard deviation. *P-value<0.05, **P-value<0.01, ***P-value<0.0001, compared with WT. Next, we individually compared the number of individuals with functional rare regulatory variants identified in either sequencing cohort. We excluded the 2 regulatory mutations that were identified in individuals from both cohorts and reassessed the association of functional rare regulatory variants with the phenotypic extremes. Similar to the results obtained above, a significant excess of rare LIPG promoter variants causing decreased LIPG expression was found in individuals with high HDL-C (P = 0.0301, Table 3), and an excess of rare variants causing increased promoter activity was found in individuals with low HDL-C (P = 0.0297, Table 3). Notably, when we enriched for variants only present in either of the cohorts, no variants decreasing LIPG promoter activity in vitro were identified in individuals with low HDL-C. Likewise, no variants increasing promoter activity were present in individuals with high HDL-C.
Identification and association of common LIPG regulatory variants associated with HDL-C
In addition to discovering novel, rare LIPG regulatory variants, our sequencing effort identified 5 common variants (MAF≥5%), all of which were present in both high HDL-C and low HDL-C subjects (Figure 1 and Table 4). The minor alleles of 3 of the identified variants (rs9959847, −1495 T>C; rs4245232, −1429 C>A; rs3829632, −1309 A>G) are in complete LD with each other and constitute a common haplotype. According to the International HapMap Project dataset [33], this haplotype includes 3 additional SNPs upstream of the sequenced region (rs4939583, rs6507929, rs4939875) and 2 intronic SNPs (rs2000812, rs3819166) (Figure 1, Table S2, Figure S3). We assessed the association of 2 of the identified common variants, −1309 A>G (rs3829632) and −1358 (T insertion), with HDL-C and other HDL traits in the Framingham Heart Study Offspring cohort (FHS; 1089 subjects in this analysis, Table 5). The −1309 A>G variant was used as a tag SNP for the haplotype. Although the −1358 (T insertion) variant had a borderline association with decreased HDL3 subfraction, the −1309 A>G variant (and, thus, the entire haplotype) was strongly associated with decreased HDL-C by approximately 2 mg/dL (P<0.0002). This latter variant was also associated with decreases in HDL3, large HDL particles, apoA-I (the major protein component of all HDL), and HDL size. Consistent with these findings, a recent GWAS of >100,000 individuals by the Global Lipids Genetics Consortium (GLGC) found that the minor alleles of several variants of this haplotype were strongly associated with a reduction in HDL-C (Table S2) [13]. Neither the −1358 (T insertion) or −1309 A>G variants were associated with changes in any other lipid or lipoprotein measures in the FHS (data not shown).
Tab. 4. Identified commona LIPG regulatory variants. 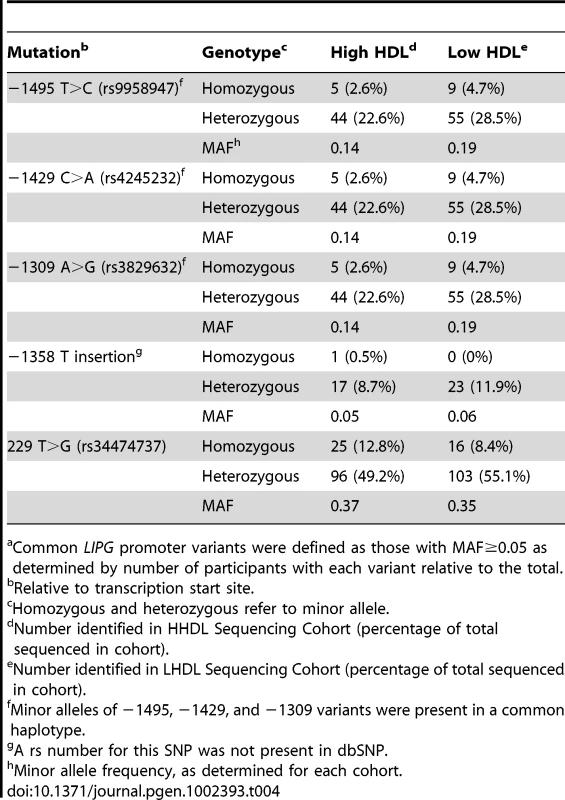
Common LIPG promoter variants were defined as those with MAF≥0.05 as determined by number of participants with each variant relative to the total. Tab. 5. Association of common variants with HDL in Framingham Heart Study. 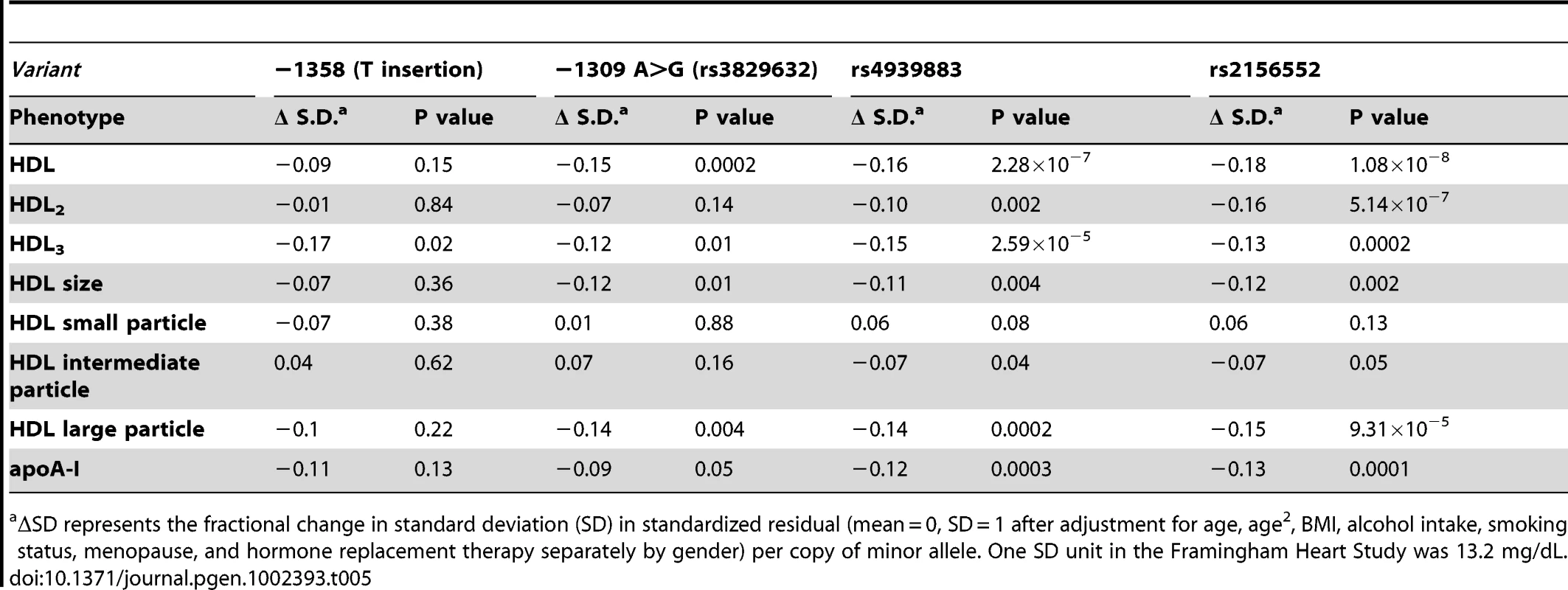
ΔSD represents the fractional change in standard deviation (SD) in standardized residual (mean = 0, SD = 1 after adjustment for age, age2, BMI, alcohol intake, smoking status, menopause, and hormone replacement therapy separately by gender) per copy of minor allele. One SD unit in the Framingham Heart Study was 13.2 mg/dL. Functional analysis of common LIPG regulatory variants
Reporter constructs corresponding to the common LIPG regulatory variant rs34474737 (229 T>G) and the −1358 T insertion variant, neither of which is known to be part of a haplotype extending beyond the LIPG promoter, were generated and used to test their impact on LIPG promoter activity in HUVECs (Figure 3). The rs34474737 variant caused a marked reduction in luciferase reporter activity (P<0.01 vs. WT), whereas the −1358 (T insertion) variant, which was not strongly associated with modulation of HDL-C in FHS, did not significantly alter LIPG promoter activity.
Fig. 3. Common LIPG regulatory variant rs34474737 affects LIPG promoter activity in vitro. 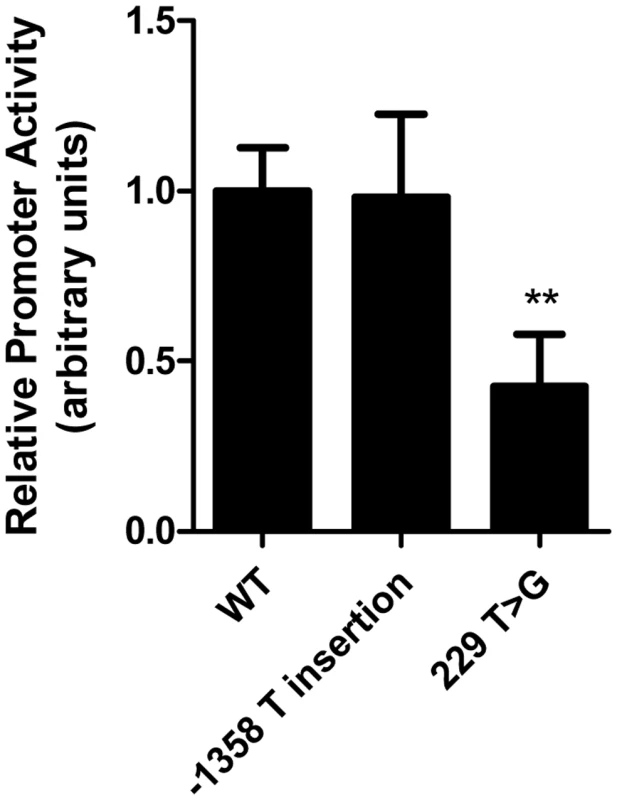
Relative LIPG promoter activity of common variants rs34474737 (229 T>G) and −1358 T insertion variant identified from resequencing of individuals with high and low HDL-C levels, measured as relative firefly luciferase expression of LIPG variant constructs in HUVECs. Assays were conducted with 6 replicates per experiment and data is given as mean ± standard deviation. **P-value<0.01, compared with WT. We hypothesized that the common LIPG regulatory variant rs34474737, which decreases promoter activity in vitro, would cause decreased plasma levels of EL in human subjects. If true, this finding would provide a mechanism through which the identified variants could increase HDL-C levels in humans. We also assessed the role of 2 recently associated noncoding variants (rs2156552 and rs4299883) and the common haplotype spanning the LIPG locus (rs3829632, −1309 A>G) in the regulation of LIPG expression, by testing the effects of these variants on plasma EL. The EL concentrations were measured in participants of the SIRCA study who were genotyped for variants rs34474737 (n = 761), rs2156552 (n = 570), rs4299883 (n = 755), and rs3829632 (n = 760) (Table 6).
Tab. 6. Association of common LIPG variants with plasma EL concentrations in SIRCA. 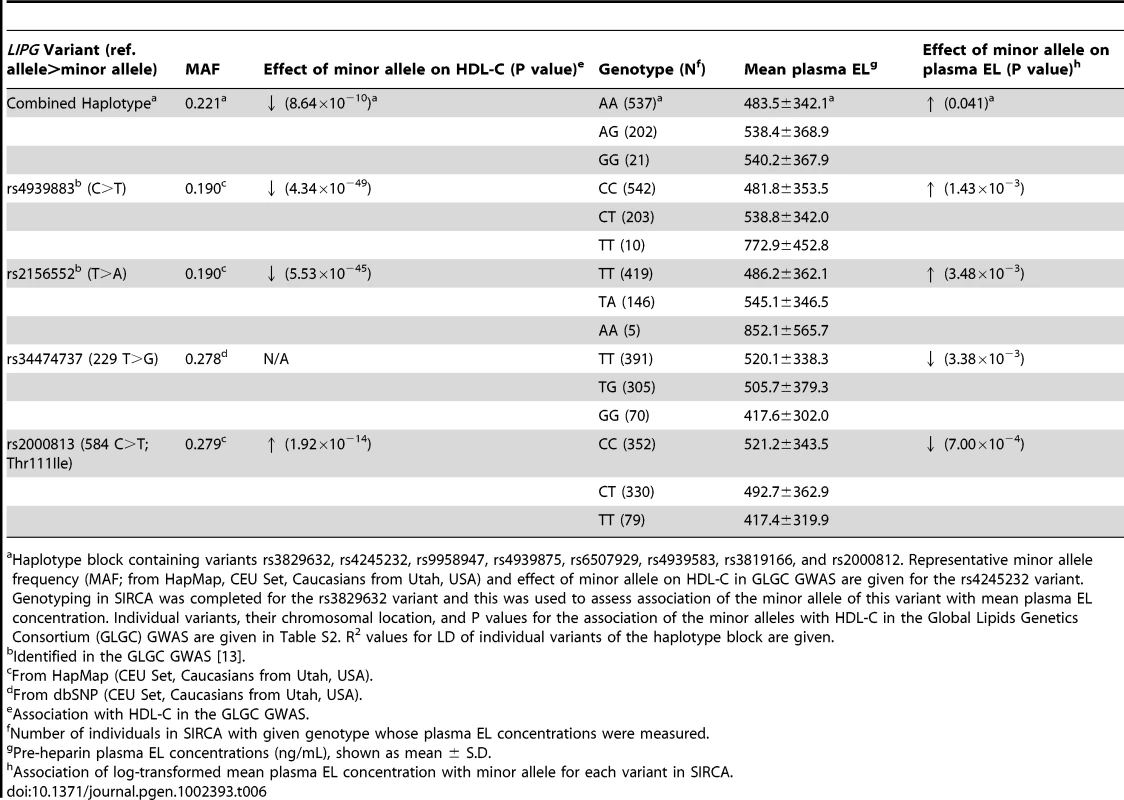
Haplotype block containing variants rs3829632, rs4245232, rs9958947, rs4939875, rs6507929, rs4939583, rs3819166, and rs2000812. Representative minor allele frequency (MAF; from HapMap, CEU Set, Caucasians from Utah, USA) and effect of minor allele on HDL-C in GLGC GWAS are given for the rs4245232 variant. Genotyping in SIRCA was completed for the rs3829632 variant and this was used to assess association of the minor allele of this variant with mean plasma EL concentration. Individual variants, their chromosomal location, and P values for the association of the minor alleles with HDL-C in the Global Lipids Genetics Consortium (GLGC) GWAS are given in Table S2. R2 values for LD of individual variants of the haplotype block are given. Minor alleles of the rs4299883 and rs2156552 variants were highly associated with decreased HDL-C in the GLGC GWAS (P<10−44 and P<10−48 respectively) [13]. We tested the association of these 2 variants with HDL-C and HDL subphenotypes in the FHS, and found that the minor alleles of these variants are associated with decreased HDL-C, HDL2, HDL3, and HDL particle sizes and apoA-I levels (Table 5). Consistent with these findings, the minor alleles of these variants were also associated with increased plasma EL (P<0.002 and P<0.004, respectively) (Table 6). The minor allele of the −1309 A>G variant was moderately associated with increased plasma EL (P<0.05), consistent with its role in decreasing plasma HDL-C, as suggested by the GLGC and FHS association studies.
The minor allele of the rs344747347 (229 T>G) variant was highly associated with decreased plasma EL (P<0.004), consistent with the luciferase reporter assay results. Plasma EL concentrations were measured for individuals in SIRCA genotyped for the rs2000813 variant (Thr111Ile; n = 761). This common nonsynonymous variant does not alter EL lipolytic activity in vitro or in vivo [20], but was associated with increased HDL-C in GLGC (P = 1.92×10−14). Plasma EL concentrations decreased with the minor allele of the Thr111Ile variant (P<0.0008, Table 6).
It may be that the Thr111Ile variant is in high LD with a regulatory variant that decreases EL expression, which would explain the decreased plasma EL of subjects with the Thr111Ile variant, as well as its association with HDL-C but normal lipolytic activity in GLGC. To test this possibility, using genotyping data for the common regulatory variants in SIRCA participants, we estimated their LD with Haploview software [34]. The rs34474737 (229 T>G) and rs2000813 (Thr111Ile) variants were in high LD (R2 = 0.8) (Figure 4).
Fig. 4. Linkage disequilibrium of rs34474737 (229 T>G) and rs2000813 (Thr111Ile) variants. 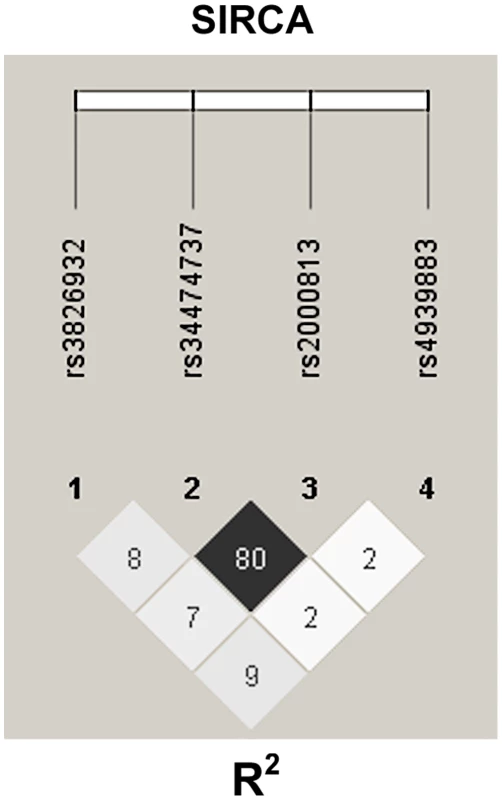
Genotyping of rs34474737 and rs2000813 variants was completed in SIRCA participants (761 in total). LD was estimated and plotted using this genotyping data using Haploview software. Values in the LD plot are estimated squared correlation coefficients (R2). Discussion
GWAS and candidate gene association techniques clearly contribute to the identification and validation of candidate genes for complex traits; however, they have fallen short in identifying causal variations. Although rare variants hold much promise for filling this void [35], the association of rare mutations with continuously distributed phenotypes has been hampered by the dual presence of functional and nonfunctional mutations. Moreover, studies have shown a lack of uniformity in incorporating the functional relevance of rare variants into their analyses. The direct influence of regulatory variants, for which functional significance is often ambiguous, also remains largely uncharacterized.
To address the phenotypic contributions of rare and common regulatory variants, we utilized the continuous trait HDL-C and candidate gene LIPG, which has significant genome-wide common and causal coding variations. By uncovering the allelic spectrum of LIPG regulatory regions through sequencing at the HDL-C extremes, rare and common regulatory mutations in LIPG were shown to contribute to observable variation in HDL-C levels. The findings also demonstrated that the functional impact of identified variants can help guide statistical analyses that assess their combined effect on a studied phenotype. To our knowledge, this study is one of the first applications of a rare variant association test to regulatory variants for a complex trait, as well as the first of such analyses to be informed by functional assays.
Association tests for rare variants of complex traits
Numerous methodologies have been described for statistically comparing the frequency differences of rare coding variants for a complex trait in cases and controls [22]. Some approaches assume that much of the heritability of complex traits arises from the combined presence of functionally important rare variants. These, which include CAST and combined multivariate and collapsing (CMC) method, collapse rare variants within a functional location (e.g., gene locus) and compare the frequencies of the aggregate variants between cases and controls [36], [37]. Other methods for evaluating rare, risk-conferring mutations include weighted sum methods that count both rare and common coding variants. These tests weight variants based on their frequency in controls [38] or are informed by computational prediction programs for assessing functionality [39]. Although these assessment methods demonstrate high statistical power, they are disadvantaged by their inclusion of both rare and common variants, as well as functional information that is largely inapplicable or unavailable for noncoding variants.
Although the effects of rare missense variants are frequently deleterious with regard to protein structure and function, the effects of rare regulatory variants are less readily interpretable [23], [36]. Such variants may cause increased or decreased gene expression, depending on their location; may act in a tissue-dependent manner, thereby weakening their association with complex traits; and may increase, decrease, or not affect transcription at all. Whereas nonfunctional coding variants can be predicted easily by synonymous or conservative amino acid substitutions, similar criteria cannot be applied to regulatory variants.
We first used CAST to investigate the contribution of rare regulatory variants to HDL-C without computationally predicting their effects. The results showed no significant excess of rare regulatory variants in LIPG in either cohort. However, the strength of rare variant aggregation methods increases when the functional validity of the variants is known [22], [23], [40], [41]. Therefore, we assessed the functional effects of each variant in a cell type that endogenously expresses LIPG (HUVECs). These putative functional effects were used to reassess the association of functional variants in the 2 cohorts. Using a modification of CAST, we separately tested the associations of variants that increase or decrease LIPG promoter activity. The results showed that variants segregated with the phenotypic extremes in a manner that was almost completely consistent with the contribution of the gene to the phenotype. For example, given that EL inversely affects HDL-C levels, variants that decrease EL should cause increased HDL-C and should occur at a higher frequency in high HDL-C individuals, and vice versa. Including functional information in the association analysis permitted the near-perfect demonstration of this distribution.
The only rare regulatory mutation inconsistent with the expected distribution was the −303 A>G variant, which increased LIPG promoter activity in vitro. This variant was found in 1 high and 2 low HDL-C individuals, which is the expected distribution, given its in vitro functionality. However, the high HDL-C individual with the −303 A>G variant also had another rare LIPG regulatory variant, −1487 A>G, which decreased promoter activity in vitro. Thus, the actual role of −303 A>G in contributing to high HDL-C levels must be considered in the context of the contribution from the additional rare variant in this individual.
Previously, Hegele et al. presented an elegant approach of refining association tests by using exclusively presenting coding variants [42]. In the present study, this approach was modified for application to noncoding variants. We examined the association of variant types with the phenotypic extremes after eliminating variants occurring at both extremes. The results showed that promoter-activating or -damaging rare LIPG variants occurred only in individuals with high or low HDL-C, respectively. Thus, our analysis method effectively enriched for functional variants with the greatest potential effect at either extreme. A limitation of this approach is that the exclusivity of any rare variant depends on the selection criteria and sizes of the cohorts. Nevertheless, even without this selectivity filter, the expected enrichment of opposing regulatory variant types occurred at the opposite phenotypic extremes.
The current literature contains additional rare variant association tests that evaluate the contribution of risk and protective rare variants to complex traits. One is a modified C-alpha score-test that measures the deviation of variance of each observed mutation from the expected variance with a binomial distribution. However, this method may not be valid for evaluating variants occurring only once in a test cohort, such as were identified in our study [43]. Another method, weighted sum test, calculates 2 one-sided statistics to quantify the association of variants in either phenotypic extreme. This test allows the incorporation of functional information of the identified variants and may be applicable to measuring the association of rare regulatory variants [44]. Yet neither of these methods is sufficiently robust to manage the large number of rare nonfunctional variants likely to be identified in resequencing studies of regulatory regions. In our study, nearly half of the rare variants identified in only one extreme failed to have any transcriptional effect. A recently reported modification of a previous methodology for studying common variants, the sequence kernel association test, may prove useful in studying the association of such rare variants without making any assumption of the functional direction or degree of effect of any individual variants [45].
Putative haplotype involving a causal regulatory and a nonfunctional coding variant of LIPG is associated with HDL-C levels
Exploration of the LIPG noncoding regions revealed the contributions of common regulatory variants. For example, the 229 T>G (rs34474737) variant in the 5′ UTR was found to decrease LIPG promoter activity in vitro and to raise plasma EL in humans. This variant was in LD with the common nonsynonymous variant Thr111Ile (rs2000813). Thr111Ile is a missense variant that does not damage EL function (according to the PolyPhen prediction program) and does not alter EL lipolytic activity in vitro or in vivo [20]. The association of Thr111lle with HDL is unclear, with some studies purporting a weak association with elevated HDL-C and others showing no association [46], [47], [48], [49], [50], [51], [52]. However, a recent GLGC GWAS metaanalysis of >100,000 individuals revealed significant association of this variant with increased plasma HDL-C (P = 1.92×10−14) [13], suggesting that Thr111Ile may be in LD with a regulatory variant.
Based on the high LD between 229 T>G and Thr111Ile, as well as the association of plasma EL with minor alleles of the 229 T>G and Thr111Ile variants in SIRCA participants, we propose that the 229 T>G variant may cause the association of Thr111Ile with HDL-C by decreasing plasma EL. To our knowledge, this finding represents the first identification of a putative haplotype involving a causal regulatory variant and a functionally benign coding variant. The result also highlights the potential misattribution that can occur when nonsynonymous coding variants are considered to be highly suggestive of causal mutations, and regulatory variants are ignored.
Interestingly, there are several reports of common nonsynonymous variants causing the association of noncoding variants in high LD with a phenotype. Kanda et al. reported that a common missense variant in high LD with a nearby promoter SNP at chromosome 10q26 independently explains the association of the locus with susceptibility to age-related macular degeneration [53]. A common functional missense variant in the B-cell scaffold protein BANK1 was shown to be in high LD with a common intronic variant that alters splicing, and both variants were strongly associated with systemic lupus erythematosus [54]. The functional heterogeneity of linked coding and noncoding SNPs highlights the complexity of haplotype structures, as well as the need to characterize the complete (i.e., coding and noncoding) variation in candidate loci for complex traits. Indeed, resequencing studies to identify haplotypes in candidate genes for inflammation, lipid metabolism, and blood pressure regulation are susceptible to missing partial or whole haplotype blocks when only coding variation is considered [55]. Common regulatory variation in observed haplotypes for several complex traits may have profound functional significance.
In our analysis of common regulatory variation in LIPG, we identified another haplotype with SNPs in the proximal promoter region. Three variants, −1495 T>C (rs9958947), −1429 C>A (rs4245232), and −1309 A>G (rs3829632), identified in the promoter region were in complete LD with each other. A study of HapMap data indicated that three SNPs upstream of the sequenced region (rs4839583, rs6507929, and rs4939875) and SNPs in the second and fifth introns of LIPG (rs2000812 and rs3819166, respectively) are also in high LD with these three promoter SNPs [33]. Because the region encompassed by this haplotype extends far upstream and within the LIPG gene (approximately 34.1 kb from the most 5′ to most 3′ of the variant constituents of the haplotype), it is not possible to assess its full functional impact with a reporter driven by part of the LIPG promoter. Characterization of the effects of single variants of this haplotype on LIPG expression in vitro could lead to erroneous implications about their functional significance, because their aggregate (and potentially synergistic) effects on transcription would be ignored. Therefore, we evaluated the contribution of the combined haplotype by measuring its effect on HDL-C levels and plasma EL concentrations from human subjects. Minor alleles of the haplotype variants were associated with decreased HDL-C in the FHS and GLGC GWAS studies, and the minor allele of 1 variant was associated with increased plasma EL. Together, these findings implicate this haplotype in the reduction of human HDL-C.
Association of minor alleles of the −1309 A>G, rs4939883, and rs2156552 variants with decreased HDL-C (P = 0.0002, 2.28×10−7, and 1.08×10−8, respectively) in the FHS was supported by a similar association with decreases in the HDL subphenotypes HDL2 and HDL3. A recent GWAS of 17 nonconventional, NMR-assessed lipoprotein measures also identified association of the rs4938993 variant with apoA-I and large HDL particles under both fasting and nonfasting conditions [56]. Together, these results demonstrate the reproducibility of such measurements in association studies. Future lipid genetic association studies using nonstandard measurements may provide additional insights beyond aggregate lipoprotein measures.
Finally, we evaluated 2 SNPs, rs2156552 and rs4299883, which were recently reported in the GLGC GWAS metaanalysis to be highly associated with HDL-C. Both variants are 40–65 kb downstream of the LIPG gene and are in high LD with each other [20], but not with Thr111Ile or Asn396Ser. In addition to being associated with decreased HDL-C, the minor alleles of these variants are associated with increased plasma EL in humans. We did not observe any LD with any of the common variants identified in our resequencing study. Further analysis of the regulatory region harboring these SNPs may help elucidate the mechanism by which these variants contribute to increased human LIPG expression.
The molecular regulators of LIPG expression are largely unknown. Investigations of induced EL secretion from human endothelial cells upon cytokine treatment have suggested that LIPG is regulated in an NFκB-dependent manner [57]. Subsequent studies utilizing electrophoretic mobility shift assays, chromatin immunoprecipitation (ChIP), and cotransfection experiments of luciferase reporter constructs determined that the LIPG promoter contains 2 NFκB binding sites, one of which (position −1250 relative to the transcription start site) exhibited strong NFκB binding in vitro [58]. In addition, ChIP combined with genome tiling arrays in HepG2 liver cell lines identified LIPG as a potential target of the SREBP1 transcription factor, a major regulator of cellular fatty acid synthesis and metabolism [59]. None of the promoter variants identified in this study disrupt the NFκB or SREBP1 binding sites. Further characterization of regulatory variants affecting LIPG expression may help elucidate key regulators of LIPG expression.
Conclusions
In this study, we demonstrate that regulatory variants, both common and rare, causally contribute to an associated phenotype. Given the complexities of interpreting the functionality of noncoding variants, direct experimental evaluation may be required to assess their impact accurately. By expanding on previous statistical association methods, this study provides an example of how such an evaluation may be done. As future whole-genome sequencing efforts will undoubtedly uncover myriad causal regulatory mutations for several polygenic traits, the findings in this study should encourage the development of methodologies to assess the contribution of rare noncoding variants.
Materials and Methods
Ethics statement
Written informed consent was obtained from all participants in the cohorts described. The UPenn Institutional Review Board (IRB) approved all study protocols.
Research participants for the sequencing cohorts
LIPG regulatory variants were identified in a discovery cohort of subjects selected from the extremes of the HDL-C phenotypic distribution in the following cohorts: University of Pennsylvania (UPenn) High HDL Cholesterol Study (HHDL), UPenn Catheterization cohort (PennCATH), Study of Inherited Risk of Coronary Atherosclerosis (SIRCA), and Philadelphia Area Metabolic Syndrome Network (PAMSyN).
HHDL is a cross-sectional study of genetic factors contributing to elevated HDL-C levels. Individuals with elevated HDL-C (>90th percentile for age and gender) were identified by physician referrals or through the Hospital of the UPenn clinical laboratory. PennCATH is composed of consecutive subjects undergoing coronary angiography at UPenn Health System hospitals and has been previously described [60]. SIRCA is a cross-sectional study of factors associated with coronary artery calcification in asymptomatic subjects recruited on the basis of a family history of premature coronary artery disease. Study design and initial findings have been previously published [61]. PAMSyN is a cross-sectional study of individuals with varying numbers of metabolic syndrome criteria, from none to all 5.
High HDL participants and low HDL participants were chosen from these cohorts. HHDL Sequencing Cohort participants are subjects with elevated HDL-C (≥95th percentile) for age and sex (females, range 87–174 mg/dL; males, range 85–166 mg/dL). LHDL Sequencing Cohort participants are subjects with low HDL-C (≤25th percentile), excluding individuals with HDL-C <20 mg/dL to eliminate participants with likely monogenic disorders of lipoprotein metabolism, leading to reduced HDL-C concentration (females, range 22–61 mg/dL; males, range 23–44 mg/dL). Approximately 92% of participants were Caucasian, while the remaining 8% were of African descent; 42% of the participants were males, which was representative of the overall demographics of the parent studies. In total, 195 high HDL participants and 193 low HDL participants were chosen for deep resequencing analysis of the LIPG promoter.
Research participants in Framingham Heart Study association
The Framingham Heart Study (FHS) Offspring Cohort, consisting of 5124 participants who were offspring of the original cohort recruited in 1948 and the spouses of the offspring, was initiated in 1971. Participants have been examined every 4 to 8 years. The examined genotypes were from a panel of 1778 unrelated individuals who provided blood samples for DNA extraction during the sixth examination cycle (1995–1998). HDL measurements were available at up to 7 time points for each individual. The HDL mean from the available measures for each individual was used. HDL2, HDL3, HDL size, HDL subfractions, and apoA-I, measured at exam 4, were determined as described previously [62], [63], [64]. The Institutional Review Board at Boston Medical Center approved the study, and all participants gave written informed consent.
Sequencing
A 1755-bp region of the promoter region (directly upstream of the transcription start site) of LIPG was amplified using a polymerase chain reaction (PCR)-based strategy. Genomic DNA was isolated from peripheral blood leukocytes using Nucleon extraction and purification protocols (Amersham). PCR reactions containing 200 ng of DNA template using Ready-to-Go PCR Beads (Amersham) were amplified in a final volume of 25 µL. The PCR program included denaturation at 95°C for 5 min, followed by 35 cycles (95°C for 1 min, 61.5°C for 30 s, and 72°C for 1 min), and extension at 72°C for 2 min. PCR products were purified with ExoSAP-IT (USB, Cleveland, OH). Purified PCR products were analyzed via Sanger sequencing on an ABI sequencer with Big Dye (Applied Biosystems) terminator chemistry. Sequences were aligned and chromatograms viewed with Sequencher Version 4.8 (Gene Codes) software. Allelic variations were verified by inspecting chromatograms. Putative variants identified in the HHDL and LHDL Sequencing Cohorts were searched for in the 1000 Genomes Project database. Rare variants were those with <1% MAF in our sequencing cohorts, and common variants were those with ≥5% MAF in our sequencing cohorts.
Genotyping
The −1309 A>G (rs3829632) and −1358 (T insertion) variants were genotyped in participants of the FHS for association analysis with HDL-C and other HDL traits by using Taqman custom genotyping assays (Applied Biosystems). For association of common variants with plasma EL concentration in SIRCA participants, genotyping was completed by using either Taqman custom genotyping assays (for −1309 A>G [rs3829632], 229 T>G [rs34474737], and rs4299883) or the ITMAT-Broad-CARe (IBC) cardiovascular gene genotyping array [65]. DNA was diluted to 50 ng/µL, and genotyping was performed at the Center for Applied Genomics (Children's Hospital of Pennsylvania) following manufacturer specifications for amplification and hybridization to the IBC array (HumanCVD beadchip, Illumina), as previously described [66].
Plasmid constructs and site-directed mutagenesis
A 2007-bp fragment consisting of the human LIPG promoter (1755-bp portion flanking the transcription start site) and the 5′ untranslated region (252-bp) was PCR-amplified from a human LIPG plasmid clone with PCR primers that introduced Kpn I and Xho I restriction sites at the 5′ and 3′ ends of the fragment, respectively. This amplified region was cloned into the pGL3-basic vector (Promega) with the Kpn I and Xho I restriction sites to generate a construct with wild-type LIPG promoter driving firefly luciferase expression and was confirmed by PCR.
Mutagenesis of the wild-type LIPG promoter (firefly luciferase) construct to generate mutant constructs for each of the identified regulatory variants was achieved by using QuikChange Site-Directed Mutagenesis Kit (Stratagene) according to the manufacturer's directions with primer sequences available in Table S3. Plasmids were sequenced after site-directed mutagenesis to confirm the changes and to rule out additional nonspecific changes.
Cell culture and dual-reporter luciferase assays
Clonetics human umbilical vein endothelial cells (HUVEC, Lonza) were cultured in Clonetics Endothelial Growth Medium (EGM-2, Lonza) at 37°C, 5% (v/v) CO2. In preparation for luciferase assays, HUVECs were passaged 3 times and plated (10,000 cells/well) overnight in 96-well tissue culture grade black-and-white microplates (Perkin-Elmer) in EGM-2. Cells were transfected by using 2 µg DNA/well (LIPG promoter construct and pRL-SV40 in a 50∶1 ratio) and Fugene HD transfection reagent (Roche) in a 1∶3 ratio of DNA to Fugene HD following the manufacturer's instructions. Cells were harvested at 36 h after transfection. Luciferase assays were performed with the Dual Luciferase Assay Kit (Promega) and a dual-injection microplate luminometer (Orion Microplate Luminometer, Berthold Detection Systems). Each well was normalized by Renilla luciferase luminescence values. Normalized values were compared to wild-type LIPG promoter constructs transfected on the same plate. Each construct was transfected with 6 replicate wells for each experiment. Each construct was evaluated at least three times.
Enzyme-linked immunosorbent assays
The preheparin mass of EL was measured from the plasma of SIRCA study participants genotyped for some of the identified common variants and 2 GWAS-identified noncoding variants. Detailed methods of the EL sandwich ELISA have been reported previously [67], [68]. Briefly, rabbit anti-human EL antibody was used to capture EL from diluted plasma samples, followed by incubation with biotin-conjugated rabbit anti-human EL antibody and streptavidin-horseradish peroxidase conjugate with O-phenylenediamine for detection.
Statistical analyses
Analysis and comparison of promoter activity between wild-type and variant LIPG promoter constructs from the luciferase assays were conducted by using unpaired Student's t-tests (P-values<0.05 were considered to be statistically significant). Numbers of individuals with a rare variant identified in each sequencing cohort were initially compared using 2-tailed Fisher's exact tests. Variants that did not alter promoter activity in vitro were discounted, and individuals harboring these variants were included in their respective sequencing cohort as individuals without a functionally altering variant. Numbers of individuals with variants decreasing promoter activity and with variants decreasing promoter activity in each sequencing cohort were then compared separately using 1-tailed Fisher's exact tests.
The FHS association analysis was completed by performing multiple linear regressions of the residuals of lipid phenotypes, separately by gender, after adjustment for means of age, age2, BMI, alcohol intake, and smoking status. In this analysis, for women, the proportion of exams that a woman was menopausal and on hormone replacement therapy was included as a covariate. For association of variant genotypes with effect on plasma EL in SIRCA, plasma EL concentrations were log-transformed to normalize the distribution and analyzed with linear regression. Linkage disequilibrium (LD) calculations and presentation were performed with Haploview software [34].
Supporting Information
Zdroje
1. KheraAVRaderDJ 2009 Discovery and validation of new molecular targets in treating dyslipidemia: the role of human genetics. Trends Cardiovasc Med 19 195 201
2. NatarajanPRayKKCannonCP High-density lipoprotein and coronary heart disease: current and future therapies J Am Coll Cardiol 55 1283 1299
3. AssmannGSchulteHvon EckardsteinAHuangY 1996 High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 124 Suppl S11 20
4. CastelliWPGarrisonRJWilsonPWAbbottRDKalousdianS 1986 Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA 256 2835 2838
5. CurbJDAbbottRDRodriguezBLMasakiKChenR 2004 A prospective study of HDL-C and cholesteryl ester transfer protein gene mutations and the risk of coronary heart disease in the elderly. J Lipid Res 45 948 953
6. SharrettARBallantyneCMCoadySAHeissGSorliePD 2001 Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 104 1108 1113
7. TurnerRCMillnsHNeilHAStrattonIMManleySE 1998 Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316 823 828
8. HellerDAde FaireUPedersenNLDahlenGMcClearnGE 1993 Genetic and environmental influences on serum lipid levels in twins. N Engl J Med 328 1150 1156
9. AulchenkoYSRipattiSLindqvistIBoomsmaDHeidIM 2009 Loci influencing lipid levels and coronary heart disease risk in 16 European population cohorts. Nat Genet 41 47 55
10. KathiresanSMelanderOGuiducciCSurtiABurttNP 2008 Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet 40 189 197
11. KathiresanSWillerCJPelosoGMDemissieSMusunuruK 2009 Common variants at 30 loci contribute to polygenic dyslipidemia. Nat Genet 41 56 65
12. SabattiCServiceSKHartikainenALPoutaARipattiS 2009 Genome-wide association analysis of metabolic traits in a birth cohort from a founder population. Nat Genet 41 35 46
13. TeslovichTMMusunuruKSmithAVEdmondsonACStylianouIM 2010 Biological, clinical and population relevance of 95 loci for blood lipids. Nature 466 707 713
14. WaterworthDMRickettsSLSongKChenLZhaoJH 2010 Genetic variants influencing circulating lipid levels and risk of coronary artery disease. Arterioscler Thromb Vasc Biol 30 2264 2276
15. CirulliETGoldsteinDB 2010 Uncovering the roles of rare variants in common disease through whole-genome sequencing. Nat Rev Genet 11 415 425
16. ViselARubinEMPennacchioLA 2009 Genomic views of distant-acting enhancers. Nature 461 199 205
17. MaherB 2008 Personal genomes: The case of the missing heritability. Nature 456 18 21
18. BauerRCStylianouIMRaderDJ Functional validation of new pathways in lipoprotein metabolism identified by human genetics. Curr Opin Lipidol 22 123 128
19. CohenJCKissRSPertsemlidisAMarcelYLMcPhersonR 2004 Multiple rare alleles contribute to low plasma levels of HDL cholesterol. Science 305 869 872
20. EdmondsonACBrownRJKathiresanSCupplesLADemissieS 2009 Loss-of-function variants in endothelial lipase are a cause of elevated HDL cholesterol in humans. J Clin Invest 119 1042 1050
21. RomeoSPennacchioLAFuYBoerwinkleETybjaerg-HansenA 2007 Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet 39 513 516
22. BansalVLibigerOTorkamaniASchorkNJ Statistical analysis strategies for association studies involving rare variants. Nat Rev Genet 11 773 785
23. MooneyS 2005 Bioinformatics approaches and resources for single nucleotide polymorphism functional analysis. Brief Bioinform 6 44 56
24. HeidIMBoesEMullerMKolleritsBLaminaC 2008 Genome-wide association analysis of high-density lipoprotein cholesterol in the population-based KORA study sheds new light on intergenic regions. Circ Cardiovasc Genet 1 10 20
25. WillerCJSpeliotesEKLoosRJLiSLindgrenCM 2009 Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet 41 25 34
26. HirataKDichekHLCioffiJAChoiSYLeeperNJ 1999 Cloning of a unique lipase from endothelial cells extends the lipase gene family. J Biol Chem 274 14170 14175
27. JayeMLynchKJKrawiecJMarchadierDMaugeaisC 1999 A novel endothelial-derived lipase that modulates HDL metabolism. Nat Genet 21 424 428
28. McCoyMGSunGSMarchadierDMaugeaisCGlickJM 2002 Characterization of the lipolytic activity of endothelial lipase. J Lipid Res 43 921 929
29. MaugeaisCTietgeUJBroedlUCMarchadierDCainW 2003 Dose-dependent acceleration of high-density lipoprotein catabolism by endothelial lipase. Circulation 108 2121 2126
30. IshidaTChoiSKunduRKHirataKRubinEM 2003 Endothelial lipase is a major determinant of HDL level. J Clin Invest 111 347 355
31. JinWMillarJSBroedlUGlickJMRaderDJ 2003 Inhibition of endothelial lipase causes increased HDL cholesterol levels in vivo. J Clin Invest 111 357 362
32. A map of human genome variation from population-scale sequencing. Nature 467 1061 1073
33. 2003 The International HapMap Project. Nature 426 789 796
34. BarrettJCFryBMallerJDalyMJ 2005 Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 263 265
35. FrazerKAMurraySSSchorkNJTopolEJ 2009 Human genetic variation and its contribution to complex traits. Nat Rev Genet 10 241 251
36. AltshulerDDalyMJLanderES 2008 Genetic mapping in human disease. Science 322 881 888
37. BodmerWBonillaC 2008 Common and rare variants in multifactorial susceptibility to common diseases. Nat Genet 40 695 701
38. MadsenBEBrowningSR 2009 A groupwise association test for rare mutations using a weighted sum statistic. PLoS Genet 5 e1000384 doi:10.1371/journal.pgen.1000384
39. PriceALKryukovGVde BakkerPIPurcellSMStaplesJ Pooled association tests for rare variants in exon-resequencing studies. Am J Hum Genet 86 832 838
40. KatsanisN 2009 From association to causality: the new frontier for complex traits. Genome Med 1 23
41. KryukovGVPennacchioLASunyaevSR 2007 Most rare missense alleles are deleterious in humans: implications for complex disease and association studies. Am J Hum Genet 80 727 739
42. JohansenCTWangJLanktreeMBCaoHMcIntyreAD Excess of rare variants in genes identified by genome-wide association study of hypertriglyceridemia. Nat Genet 42 684 687
43. NealeBMRivasMAVoightBFAltshulerDDevlinB Testing for an unusual distribution of rare variants. PLoS Genet 7 e1001322 doi:10.1371/journal.pgen.1001322
44. Ionita-LazaIBuxbaumJDLairdNMLangeC A new testing strategy to identify rare variants with either risk or protective effect on disease. PLoS Genet 7 e1001289 doi:10.1371/journal.pgen.1001289
45. WuMCLeeSCaiTLiYBoehnkeM Rare-variant association testing for sequencing data with the sequence kernel association test. Am J Hum Genet 89 82 93
46. HalverstadtAPharesDAFerrellREWilundKRGoldbergAP 2003 High-density lipoprotein-cholesterol, its subfractions, and responses to exercise training are dependent on endothelial lipase genotype. Metabolism 52 1505 1511
47. HutterCMAustinMAFarinFMViernesHMEdwardsKL 2006 Association of endothelial lipase gene (LIPG) haplotypes with high-density lipoprotein cholesterol subfractions and apolipoprotein AI plasma levels in Japanese Americans. Atherosclerosis 185 78 86
48. MaKCilingirogluMOtvosJDBallantyneCMMarianAJ 2003 Endothelial lipase is a major genetic determinant for high-density lipoprotein concentration, structure, and metabolism. Proc Natl Acad Sci U S A 100 2748 2753
49. Mank-SeymourARDurhamKLThompsonJFSeymourABMilosPM 2004 Association between single-nucleotide polymorphisms in the endothelial lipase (LIPG) gene and high-density lipoprotein cholesterol levels. Biochim Biophys Acta 1636 40 46
50. ParadisMECouturePBosseYDespresJPPerusseL 2003 The T111I mutation in the EL gene modulates the impact of dietary fat on the HDL profile in women. J Lipid Res 44 1902 1908
51. TangNPWangLSYangLZhouBGuHJ 2008 Protective effect of an endothelial lipase gene variant on coronary artery disease in a Chinese population. J Lipid Res 49 369 375
52. Yamakawa-KobayashiKYanagiHEndoKArinamiTHamaguchiH 2003 Relationship between serum HDL-C levels and common genetic variants of the endothelial lipase gene in Japanese school-aged children. Hum Genet 113 311 315
53. KandaAChenWOthmanMBranhamKEBrooksM 2007 A variant of mitochondrial protein LOC387715/ARMS2, not HTRA1, is strongly associated with age-related macular degeneration. Proc Natl Acad Sci U S A 104 16227 16232
54. KozyrevSVAbelsonAKWojcikJZaghloolALinga ReddyMV 2008 Functional variants in the B-cell gene BANK1 are associated with systemic lupus erythematosus. Nat Genet 40 211 216
55. CrawfordDCCarlsonCSRiederMJCarringtonDPYiQ 2004 Haplotype diversity across 100 candidate genes for inflammation, lipid metabolism, and blood pressure regulation in two populations. Am J Hum Genet 74 610 622
56. ChasmanDIPareGMoraSHopewellJCPelosoG 2009 Forty-three loci associated with plasma lipoprotein size, concentration, and cholesterol content in genome-wide analysis. PLoS Genet 5 e1000730 doi:10.1371/journal.pgen.1000730
57. JinWSunGSMarchadierDOcttavianiEGlickJM 2003 Endothelial cells secrete triglyceride lipase and phospholipase activities in response to cytokines as a result of endothelial lipase. Circ Res 92 644 650
58. KempeSKestlerHLasarAWirthT 2005 NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res 33 5308 5319
59. ReedBDCharosAESzekelyAMWeissmanSMSnyderM 2008 Genome-wide occupancy of SREBP1 and its partners NFY and SP1 reveals novel functional roles and combinatorial regulation of distinct classes of genes. PLoS Genet 4 e1000133 doi:10.1371/journal.pgen.1000133
60. LehrkeMMillingtonSCLefterovaMCumaranatungeRGSzaparyP 2007 CXCL16 is a marker of inflammation, atherosclerosis, and acute coronary syndromes in humans. J Am Coll Cardiol 49 442 449
61. ValdesAMWolfeMLTateHCGefterWRutA 2001 Association of traditional risk factors with coronary calcification in persons with a family history of premature coronary heart disease: the study of the inherited risk of coronary atherosclerosis. J Investig Med 49 353 361
62. ContoisJMcNamaraJRLammi-KeefeCWilsonPWMassovT 1996 Reference intervals for plasma apolipoprotein A-1 determined with a standardized commercial immunoturbidimetric assay: results from the Framingham Offspring Study. Clin Chem 42 507 514
63. FreedmanDSOtvosJDJeyarajahEJShalaurovaICupplesLA 2004 Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem 50 1189 1200
64. YangQLaiCQParnellLCupplesLAAdiconisX 2005 Genome-wide linkage analyses and candidate gene fine mapping for HDL3 cholesterol: the Framingham Study. J Lipid Res 46 1416 1425
65. KeatingBJTischfieldSMurraySSBhangaleTPriceTS 2008 Concept, design and implementation of a cardiovascular gene-centric 50 k SNP array for large-scale genomic association studies. PLoS ONE 3 e3583 doi:10.1371/journal.pone.0003583
66. EdmondsonACBraundPSStylianouIMKheraAVNelsonCP Dense Genotyping of Candidate Gene Loci Identifies Variants Associated With High-Density Lipoprotein Cholesterol. Circ Cardiovasc Genet 4 145 155
67. BadellinoKOWolfeMLReillyMPRaderDJ 2006 Endothelial lipase concentrations are increased in metabolic syndrome and associated with coronary atherosclerosis. PLoS Med 3 e22 doi:10.1371/journal.pmed.0030022
68. BadellinoKOWolfeMLReillyMPRaderDJ 2008 Endothelial lipase is increased in vivo by inflammation in humans. Circulation 117 678 685
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12- Růst a vývoj dětí narozených pomocí IVF
- Délka menstruačního cyklu jako marker ženské plodnosti
- Vztah užívání alkoholu a mužské fertility
- Intrauterinní inseminace a její úspěšnost
- Šanci na úspěšný průběh těhotenství snižují nevhodné hladiny progesteronu vznikající při umělém oplodnění
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



