-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
Cells accumulate single-stranded DNA (ssDNA) when telomere capping, DNA replication, or DNA repair is impeded. This accumulation leads to cell cycle arrest through activating the DNA–damage checkpoints involved in cancer protection. Hence, ssDNA accumulation could be an anti-cancer mechanism. However, ssDNA has to accumulate above a certain threshold to activate checkpoints. What determines this checkpoint-activation threshold is an important, yet unanswered question. Here we identify Rif1 (Rap1-Interacting Factor 1) as a threshold-setter. Following telomere uncapping, we show that budding yeast Rif1 has unprecedented effects for a protein, inhibiting the recruitment of checkpoint proteins and RPA (Replication Protein A) to damaged chromosome regions, without significantly affecting the accumulation of ssDNA at those regions. Using chromatin immuno-precipitation, we provide evidence that Rif1 acts as a molecular “band-aid” for ssDNA lesions, associating with DNA damage independently of Rap1. In consequence, small or incipient lesions are protected from RPA and checkpoint proteins. When longer stretches of ssDNA are generated, they extend beyond the junction-proximal Rif1-protected regions. In consequence, the damage is detected and checkpoint signals are fired, resulting in cell cycle arrest. However, increased Rif1 expression raises the checkpoint-activation threshold to the point it simulates a checkpoint knockout and can also terminate a checkpoint arrest, despite persistent telomere deficiency. Our work has important implications for understanding the checkpoint and RPA–dependent DNA–damage responses in eukaryotic cells.
Published in the journal: . PLoS Genet 7(12): e32767. doi:10.1371/journal.pgen.1002417
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002417Summary
Cells accumulate single-stranded DNA (ssDNA) when telomere capping, DNA replication, or DNA repair is impeded. This accumulation leads to cell cycle arrest through activating the DNA–damage checkpoints involved in cancer protection. Hence, ssDNA accumulation could be an anti-cancer mechanism. However, ssDNA has to accumulate above a certain threshold to activate checkpoints. What determines this checkpoint-activation threshold is an important, yet unanswered question. Here we identify Rif1 (Rap1-Interacting Factor 1) as a threshold-setter. Following telomere uncapping, we show that budding yeast Rif1 has unprecedented effects for a protein, inhibiting the recruitment of checkpoint proteins and RPA (Replication Protein A) to damaged chromosome regions, without significantly affecting the accumulation of ssDNA at those regions. Using chromatin immuno-precipitation, we provide evidence that Rif1 acts as a molecular “band-aid” for ssDNA lesions, associating with DNA damage independently of Rap1. In consequence, small or incipient lesions are protected from RPA and checkpoint proteins. When longer stretches of ssDNA are generated, they extend beyond the junction-proximal Rif1-protected regions. In consequence, the damage is detected and checkpoint signals are fired, resulting in cell cycle arrest. However, increased Rif1 expression raises the checkpoint-activation threshold to the point it simulates a checkpoint knockout and can also terminate a checkpoint arrest, despite persistent telomere deficiency. Our work has important implications for understanding the checkpoint and RPA–dependent DNA–damage responses in eukaryotic cells.
Introduction
Telomeres protect chromosome ends from activating DNA damage responses that result in cell cycle arrest or inadvertent “repair.” Evidence that telomere dysfunction could be involved in carcinogenesis [3]–[5] suggests that some telomere-defective cells are able to avoid/escape arrest and generate genetically modified progenies. Checkpoint inactivation and checkpoint adaptation are potential routes to escape from arrest. Checkpoint adaptation is an intriguing process, in which checkpoint responses are terminated, despite persistent DNA damage and intact checkpoint pathways (reviewed by [6]). Several proteins involved in checkpoint adaptation have been identified. However, many if not all (Ku, Mre11, Rad50, Tid1, Srs2, Sae2, Cdc5Polo) also participate in processing the DNA damage [7]–[12]. Therefore, these proteins permit escape from arrest most likely indirectly, by affecting the substrate required for checkpoint activation.
Exciting discoveries in model organisms as diverse as Schizosaccharomyces pombe and Drosophila melanogaster suggest that eukaryotic cells are quite resourceful in their ways to prevent chromosome ends from being detected as DNA damage. For example, dysfunctional S. pombe telomeres do not recruit the checkpoint protein Crb253BP1, most likely because they lack a particular checkpoint substrate [13]. Drosophila uses transposons to protect and maintain chromosome ends, whereas S. pombe can use ribosomal DNA for the same purpose, but only when telomerase is inactivated [14]. In contrast, Saccharomyces cerevisiae can proliferate without transposons, telomeric or ribosomal DNA at chromosome ends when telomere maintenance pathways, e.g. telomerase and telomere recombination, are inactivated [15]. Such budding yeast strains, called PAL survivors, have no particular DNA sequences at chromosome ends, which shorten progressively, without triggering a cell cycle arrest. [15]. The existence of the PAL survivors suggests that eukaryotic cells can also prevent checkpoint responses to chromosome ends in a sequence-independent manner, perhaps with help from “anti-checkpoint” factors.
In this study, we identified arguably the first “anti-checkpoint” protein in Rif1 and demonstrate that checkpoint responses to damaged chromosome ends can be inhibited without significant modification of a major checkpoint substrate, the single stranded DNA. We propose that Rif1 has important physiological roles in preventing a cell cycle arrest to incipient or small single stranded DNA lesions occurring on chromosomes, particularly on chromosome ends. However, high levels of Rif1 may contribute to genomic instability by facilitating cell proliferation with even more DNA damage.
Results
Rif1 associates with DNA damage differently from Rap1
Telomeres successfully avoid stimulating the DNA damage checkpoint pathways, despite their resemblance to broken chromosome ends. Therefore, we hypothesized that proteins able to inhibit checkpoint sensors are among the telomere-associated proteins. To unmask potential checkpoint inhibitors, we tested how Rap1, Rif1 and other telomere-associated proteins respond to DNA damage. Rap1 is a major component of the telomeric chromatin [16], whereas Rif1 is a Rap1-interacting factor [17]. To induce DNA damage, we used the well-studied model system cdc13-1. Budding yeast cdc13-1 cells have a temperature-sensitive mutation in the telomere capping protein Cdc13Pot1. At restrictive temperatures, telomeres become uncapped and vulnerable to DNA processing factors. Hence, Sgs1 and other helicases unwind telomeres [18], whereas Exo1 and other nucleases resect the 5′-ended DNA strand. Together, they generate single stranded DNA (ssDNA), a potent checkpoint activator.
To determine whether the association of Rif1 and Rap1 with chromosomes was affected by the recruitment (and subsequently by the activities) of Sgs1 and Exo1, we induced telomere uncapping by shifting cdc13-1 cells from permissive (21°C) to restrictive (36°C) temperature. The dynamics of Sgs1 recruitment to uncapped chromosome ends is not known. Using chromatin immuno-precipitation, we found that Sgs1 did not significantly associate with sub-telomeres at 21°C (time 0 in Figure 1a). At the restrictive temperature 36°C however, Sgs1 progressively accumulated at 1, 8 and 15 kb from chromosome ends, associating with (sub)telomeres and single gene loci, whereas it did not associate with the centromere-proximal PAC2 locus (Figure 1a–1d). Exo1 accumulated at the same regions and with similar dynamics to Sgs1 (Figure 1e–1h), suggesting that: 1) DNA unwinding is closely followed by an Exo1-dependent resection and 2) with time, increasingly more sub-telomeres and single gene loci are processed by Exo1/Sgs1.
Fig. 1. The association of Sgs1, Exo1, Rap1, Rif1, and Rif1-CΔ with different chromosome regions during telomere dysfunction. 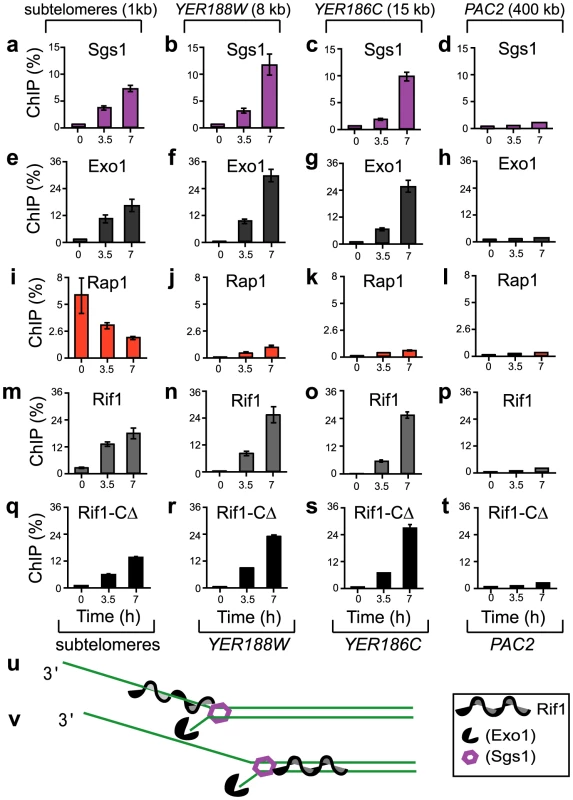
All strains were cdc13-1, grown overnight at 21°C, followed by 7 hours at 36°C to induce telomere uncapping. “ChIP (%)” was calculated for each sample (time-point) as the difference between the immuno-precipitated DNA and the background, divided by input DNA, and multiplied by 100. Error bars represent the standard deviation (SD) between three measurements. (a–d) The recruitment of Sgs1 to chromatin at the following distance from chromosome ends: (a) 1 kb (in Y′ sub-telomeric repeats) (b) 8 kb (in YER188W), (c) 15 kb (in YER186C) and (d) 400 kb (in PAC2). YER188W, YER186C and PAC2 are single gene loci on chromosome 5. Analyzed chromosome regions are indicated at the top of the figure. (e–h) As in a–d, except that Exo1 was analyzed; (i–l) Rap1 was analyzed; (m–p) Rif1 was analyzed; (q–t) Rif1-CΔ (1-1350 amino acids) was analyzed. Analyzed proteins are indicated above each graph. Exo1, Rif1 and Rif1-CΔ are Myc-tagged. Sgs1 and Rap1 are not tagged. (u–v) Preliminary models of interaction between Rif1 and resection forks: (u) Rif1 associates with ssDNA accumulating behind resection forks; (v) DNA unwinding pushes Rif1 ahead of the resection fork. Under these conditions, we also determined the dynamics of Rap1 and Rif1 onto DNA. We found that both Rap1 and Rif1 associated with (sub)telomeres at 21°C (time 0, Figure 1i, 1m), consistent with other studies [19], [20]. At the restrictive temperature 36°C however, Rap1 and Rif1 behaved differently from each other. Whereas Rap1 progressively dissociated from (sub)telomeres, Rif1 accumulated in (sub)telomeres (Figure 1i, 1m). Whereas only small levels of Rap1 (below 1%) were detected at single gene loci, 8 and 15 kb from the right end of chromosome 5 (Figure 1j–1k), high levels of Rif1 (up to 26%) were detected at these loci (Figure 1n–1o). In conclusion, Rif1 associated similarly to Sgs1 and Exo1; two different models of Rif1 association with DNA damage are presented in Figure 1u–1v. Moreover, Rif1 associated differently from Rap1. This is surprising, since it was thought that Rif1 associates with DNA through Rap1 [17].
To confirm that Rif1 does not require Rap1 to associate with DNA damage, we generated cdc13-1 strains lacking the C-terminus of Rif1 (Rif1-CΔ), required for association with Rap1 [17]. We found that Rif1-CΔ did not significantly associate with sub-telomeres at 21°C (Time 0 in Figure 1q), suggesting that Rif1 requires Rap1 to be recruited to normal (sub)telomeres, consistent with other studies [17]. Following telomere uncapping however, Rif1-CΔ progressively associated with sub-telomeres and single gene loci (Figure 1r–1t), similarly to Rif1 (and different from Rap1). These data indicate that Rif1 is recruited to DNA damage independently of Rap1. Interestingly, the behaviour of Rif1-CΔ bears a striking resemblance to that of mammalian Rif1 [2], [21], suggesting conserved functions. Similarly to Rif1-CΔ, mammalian Rif1 does not co-localize with Rap1 at normal telomeres, however it associates with dysfunctional telomeres and other damaged regions [2], [21].
Rif1 inhibits the association of Rad953BP1, Ddc1Rad9, Ddc2ATRIP, and RPA with DNA damage (DNA junctions)
At DNA damage, Rif1 may occupy the substrate for important DNA damage response (DDR) proteins. Ddc1Rad9 (part of the 9-1-1 checkpoint complex) has high affinity for 5′-junctions between single and double stranded DNA [22]. The RPA complex has high affinity for ssDNA, facilitating the recruitment of the Ddc2ATRIP checkpoint mediator [23], [24]. Rad953BP1 checkpoint protein associates with chromatin adjacent to DNA damage. Therefore, we determined whether Rif1 interfered with the association of any of these proteins with sub-telomeres and single gene loci. We used the same experimental design as in Figure 1, except that we incubated cdc13-1 cells at their mildest restrictive temperature (27°C). Interestingly, we found that several fold more checkpoint protein (Rad953BP1, Ddc1Rad9 and Ddc2ATRIP) and RPA associated with (sub)telomeres and YER188W in rif1D than in RIF1+ cells, during 7 h at 27°C (Figure 2a–2h), indicating that Rif1 strongly inhibits their recruitment to DNA damage.
Fig. 2. Rif1 inhibits the recruitment of checkpoint proteins and Rpa1 to sub-telomeres and single gene loci. 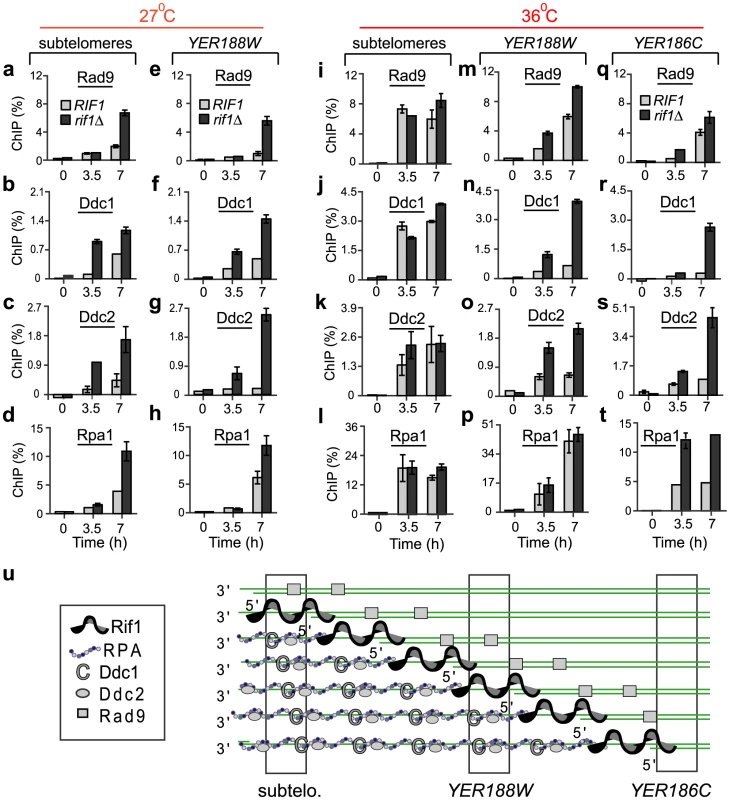
Cdc13-1 cells with a rif1Δ mutation (dark columns) or with wild-type RIF1 (light columns) were grown overnight at 21°C, followed by for 7 hours at 27°C or 36°C to induce telomere uncapping. ChIP (%) was calculated as in Figure 1. Analyzed chromosome regions are indicated at the top of the figure. (a–h) The dynamics of Rad9, Ddc1, Ddc2 and Rpa1 association with chromosome ends at 27°C. (i–t) The dynamics of Rad9, Ddc1, Ddc2 and Rpa1 association with chromosome ends at 36°C. Each analyzed protein is indicated above the respective graph. Ddc1 is HA-tagged; Rpa1 and Ddc2 are YFP-tagged; Rad9 is not tagged. Time 0 temperature and error bars are as in Figure 1. (u) A model of Rif1-association with excised chromosome ends in a population of cells. Vertical bars indicate regions analyzed in a–t. To inhibit checkpoint proteins and RPA, Rif1 may associate with ssDNA at random, similarly to RPA, or mainly with DNA-junctions and the adjacent (ss)DNA. To distinguish between these possibilities, we analyzed the effect of Rif1 at 36°C. At this temperature, resection is faster and affects many more chromosomes than at 27°C (see Figure 3). Consequently, DNA-junctions move faster from (sub)telomeres towards internal regions like YER188W and YER186C, leaving behind long ssDNA overhangs, bound by RPA and checkpoint proteins (Figure 2u). Therefore, we determined the location of Rif1 as follows: 1) If Rif1 associates with ssDNA at random, the Rif1-effect will be region-independent; 2) If Rif1 associates with DNA-junctions and the adjacent DNA, the Rif1-effect will appear stronger towards internal regions and weaker or absent towards (sub)telomeres. Importantly, we detected a strong Rif1-inhibitory effect on checkpoint/RPA proteins at YER188W and/or YER186C loci (Figure 2m–2t). Conversely, no such Rif1-effect was detected in sub-telomeres (Figure 2i–2l). These data strongly suggests that Rif1 associates with DNA damage at or around DNA-junctions (Figure 2u).
Fig. 3. Rif1 does not inhibit resection of sub-telomeres and single gene loci. 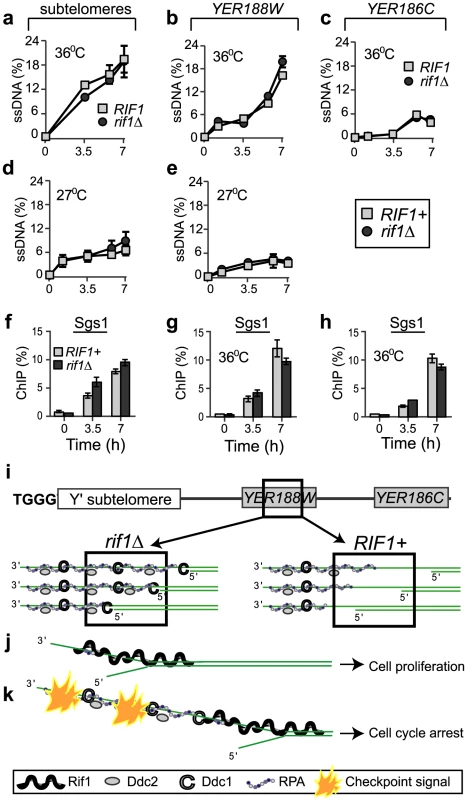
(a–c) Quantification of ssDNA generated in cdc13-1 cells with a rif1Δ mutation (dark circles) or with wild-type RIF1 (light squares) at 36°C at: (a) 1 kb, in sub-telomeres, (b) 8 kb, in YER188W and (c) 15 kb, in YER186C. (d–e) As in a–b, except that the temperature was 27°C. We used QAOS (the Quantitative Amplification Of ssDNA) to detect ssDNA as in [29], [51]. (f–h) The dynamics of Sgs1 association with chromosome ends following telomere uncapping at 36°C, in cdc13-1 cells with a rif1Δ mutation (dark columns) or with wild-type RIF1 (light columns) at: (f) 1 kb, in sub-telomeres, (g) 8 kb and (h) 15 kb. Time 0 temperature and error bars are as in Figure 1. (i) A model of RPA and checkpoint proteins association with YER188W in rif1Δ (left) versus RIF1+ cells (right). Squares indicate chromosomal regions detected by ChIP and QAOS. (j–k) The ssDNA tolerance threshold model: (j) When short ssDNA lesions are generated, Rif1 acts as a “band-aid” for these lesions, associating with DNA-junctions and the adjacent ssDNA and blocking access for RPA and checkpoint proteins. In consequence, cells avoid spending energy to arrest and to re-start the cell cycle. (k) When longer ssDNA lesions are generated, they extend beyond Rif1-protected regions. In consequence, the damage is detected, a checkpoint signal is fired and cells arrest the cell cycle. Rif1 does not affect resection of sub-telomeres or single gene loci
The ability of proteins like Cdc13 and KU to inhibit the recruitment of checkpoint proteins and/or RPA to DNA is indirect, by inhibiting DNA resection [25]. One possibility is that the Rif1-effect is also indirect. A recent study used in-gel hybridization assays to claim there is more ssDNA in cdc13-1 rif1Δ versus cdc13-1 cells at the very end of the chromosomes, the TG-telomeric sequences [26]. However, no other chromosomal regions were investigated. Because the in-gel hybridization assay is not sensitive enough for sub-telomeres or single gene loci, we used the ultra-sensitive qPCR-based method QAOS [27]–[29] to test whether a difference in ssDNA was responsible for the different accumulation of RPA and checkpoint proteins in RIF1+ versus rif1Δ cells. We found that this was clearly not the case. Unlike its strong inhibitory effect on RPA and checkpoint proteins, Rif1 did not significantly inhibit the accumulation of ssDNA in sub-telomeres or single gene loci, because similar amounts were detected in either rif1Δ or RIF1 cells at different time points during 7 h at 36°C or 27°C (Figure 3a–3e). Moreover, Rif1 did not affect the dynamics of the Sgs1 helicase on chromosome ends (Figure 3f–3h). Therefore, the inhibitory Rif1-effect on checkpoint proteins and RPA was not through inhibiting ssDNA formation.
In summary, several fold more RPA/checkpoint protein associated with sub-telomeres and single gene regions in rif1Δ versus RIF1 cells, despite the fact that very similar amounts of ssDNA accumulated at those regions in rif1Δ and RIF1 cells (Figure 3i). To our knowledge, this is the first report of a protein inhibiting the recruitment of RPA and checkpoint proteins to a chromosome region, without inhibiting resection or unwinding of that region. Rif1 most likely occupies the DNA junction and the adjacent DNA, thus blocking access for other proteins (e.g. checkpoint and RPA) to these important DDR structures. Through this effect, we propose that Rif1 sets the ssDNA tolerance threshold, facilitating cell proliferation with lower amounts of ssDNA (limited to regions protected by Rif1) or permitting arrest, if ssDNA is more extensive (Figure 3j, 3k). We tested this hypothesis by investigating the effect of Rif1 on cell proliferation and checkpoint responses, discussed next.
Rif1 inhibits the checkpoint responses to “below-threshold” ssDNA
To test whether Rif1 affects the ssDNA tolerance threshold, we investigated how much ssDNA could rif1Δ cells tolerate without arresting in G2/M, compared to RIF1+ cells. To generate low amounts of ssDNA, we incubated cdc13-1 cells (+/ - other relevant mutations) at the permissive temperature of 25°C. To generate more ssDNA, we incubated cdc13-1 cells at 27°C. Consistent with the idea that Rif1 facilitates cell proliferation with low amounts of ssDNA and also with some recent observations [26], [30], a rif1Δ mutation was incompatible with proliferation of cdc13-1 cells at 25°C (Figure 4a). In contrast, cdc13-1 cells with a C-terminal truncated version of Rif1 (Rif1-CΔ) proliferated as well as those with the wild type Rif1 (Figure 4a). This indicates that the interaction between Rif1 and Rap1 (disrupted in the truncated version) is not relevant for facilitating proliferation of telomere-uncapped cells, consistent with our observation that Rif1-CΔ continues to associate with DNA damage (Figure 1q–1t).
Fig. 4. Rif1 inhibits the checkpoint responses to telomere uncapping in cdc13-1 cells. 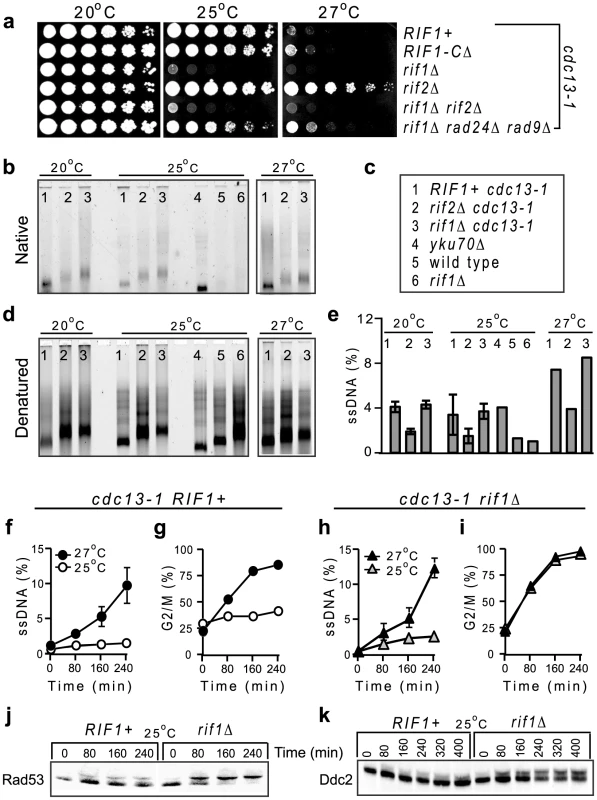
(a) Growth of serial dilution of cdc13-1 cells with or without additional mutations (indicated at the right of each row) at different temperatures, indicated above each plate. (b–e) Over-night cultures at 20°C were incubated with nocodazole for 2 h and then shifted to 25°C or to 27°C for 3 h. DNA was digested and hybridized with a CA-rich probe. (b) TG signals at native (non-denatured) telomeres. Numbers at the top of each lane correspond to different mutants, revealed in c. (c) Legend for b, d, and e indicating relevant mutations. (d) TG signals at denatured telomeres. (e) The percentage of ssDNA represents the fraction of native TG-ssDNA normalized to the total of TG sequences in each denatured sample. Error bars represent the standard deviation between measurements from two independent experiments. (f–g) The ssDNA was quantified by QAOS and the G2/M fraction counted by microscopy in cdc13-1 RIF1+ cells incubated for 240 min at 25°C (white circles) or 27°C (black circles). (h–i) As in f–g, except that cells were cdc13-1 rif1Δ at 25°C (white triangles) or 27°C (black triangles). (j) Rad53 activation detected by western blotting in cdc13-1 cells with wild type RIF1 (left) or with a rif1Δ mutation (right), incubated at 25°C, (except for the time 0 when the temperature was 21°C). (k) As in (j), except that the Ddc2 activation was detected. Since rif1Δ cdc13-1 cells had longer telomeres than cdc13-1 cells (about 0.6 kb versus 0.3 kb), we asked whether their enhanced temperature-sensitivity was somehow related to telomere length. Therefore, we tested a rif2Δ mutant, which has also longer telomeres [31]. In contrast to a rif1Δ mutation, a rif2Δ mutation permitted cdc13-1 cells to proliferate at 25°C and even at 27°C. Strikingly, deletion of RIF1 in cdc13-1 rif2Δ cells rendered them unable to proliferate at 25°C (Figure 4a), indicating a dominant effect for rif1Δ. Deletion of RAD24 and RAD9 checkpoint genes partially rescued the proliferation of cdc13-1 rif1Δ cells (Figure 4a). In conclusion, Rif1 is essential for proliferation of cdc13-1 and cdc13-1 rif2Δ cells at 25°C and 27°C respectively, through a mechanism independent of telomere length.
A recent report by Anbalagan et al attributed the increased temperature-sensitivity of cdc13-1 rif1Δ cells to increased ssDNA in the TG-rich telomeric repeats [26]. This interpretation is slightly at odds with our findings that Rif1 did not affect ssDNA accumulation at 0.6 kb distance from telomeres (in sub-telomeres) or at more internal regions, at 27°C and 36°C (Figure 3). It implies that Rif1 inhibits a very unusual resection activity that is restricted to TG-telomeres or to temperatures around 25°C. However, Anbalagan et al have not quantified and normalized the TG-signals to the total amount of TG sequences. It is known that rif1Δ cells have up to four times more TG sequences (longer telomeres) than RIF1+ cells. Therefore, normalizing the TG-signals is essential for determining whether stronger signals are caused by more ssDNA, or merely by more TG-sequences per ssDNA kilobase. To find out which one is true, we used similar techniques and experimental conditions as in Anbalagan et al., except that we quantified and normalized the original TG-ssDNA signals to the total amount of TG repeats, used a previously described AC-rich fluorescent probe [32], analyzed a cdc13-1 rif2Δ strain as control for signals generated by longer TG-telomeres and tested an additional temperature (27°C).
To determine whether Rif1 affects telomere resection at 25°C, we needed to compare ssDNA accumulation in cells expected to arrest proliferation in G2/M (cdc13-1 rif1Δ) versus cells expected to divide (cdc13-1 +/-rif2Δ). However, ssDNA lesions at cdc13-1 uncapped telomeres are generated almost exclusively in the G2/M phase [33]. Moreover, DNA replication factors would most likely repair (re-synthesize) some of the lesions during S-phase, if cells could proliferate. Therefore, nocodazole was used to arrest all strains in G2/M, thus permitting comparison between their resection rates based upon genetic differences, rather than cell cycle differences. Over-night cultures at 20°C were incubated with nocodazole for 2 h and then shifted to 25°C or to 27°C for 3 h. The signals given by TG-ssDNA (e.g. ssDNA hybridized with an AC-rich probe) at native and denatured chromosome ends are shown in Figure 4b and 4d, respectively. The TG-ssDNA signals normalized to the total amount of TG repeats are presented in Figure 4e.
In these experiments, the wild type and rif1Δ controls accumulated only about 1% TG-ssDNA, whereas strains with mutations in the telomere-capping proteins Cdc13 or Yku70 accumulated more ssDNA. However, we found similar fractions of ssDNA at telomeres of cdc13-1 and cdc13-1 rif1Δ cells: about 4% at 20–25°C and 7–8% at 27°C (Figure 4e). The error bars (the standard deviation between measurements from two different experiments) are also overlapping. This shows that Rif1 does not significantly affect resection of telomeres, consistent with similar results we obtained at other chromosome regions and temperatures using QAOS (Figure 3 and Figure 4). In contrast, ssDNA was two-fold lower in cdc13-1 rif2Δ versus cdc13-1 cells at any tested temperature (Figure 4e). Together, these data suggest that Rif1 and Rif2 have different effects: Rif1 does not affect resection, whereas Rif2 somehow facilitates resection at telomeres.
Interestingly, strains able (cdc13-1 and yku70Δ) or unable (cdc13-1 rif1Δ) to proliferate at 25°C had similar fractions of telomeric TG-ssDNA (about 4%) at this temperature (Figure 4e). Moreover, cdc13-1 rif1Δ cells had similar fractions of telomeric ssDNA at permissive (20°C) and restrictive (25°C) temperatures (Figure 4e). These data indicate that resection of TG-telomeres does not correlate with cell cycle arrest. Therefore, we tested whether progression of ssDNA to sub-telomeres correlated with G2/M arrest. We incubated cdc13-1 strains (+/ - other relevant mutations) at 25°C and 27°C, without nocodazole. We found that about 2% ssDNA accumulated in RIF1+ cells at 25°C (Figure 4f), however most cells continued to cycle (Figure 4g) and did not activate the Rad53Chk2 checkpoint kinase (Figure 4j) or the Ddc2ATRIP checkpoint protein (Figure 4k). At 27°C, about 6–10% ssDNA was detected in sub-telomeres when over 75% RIF1+ cells accumulated in G2/M (Figure 4f–4g). These data suggest that the majority of RIF1+ cells arrest proliferation in response to about 6–10% sub-telomeric ssDNA, whereas they tolerate 2% sub-telomeric ssDNA without activating a checkpoint response.
In contrast, rif1Δ cells responded to about 2% sub-telomeric ssDNA at 25°C by accumulating in G2/M as fast as they did in response to higher ssDNA levels at 27°C (Figure 4h–4i). A 2% sub-telomeric ssDNA corresponds roughly to one chromosome end per cell being resected as far as 1 kb. Another 1–2 telomeres are also likely to be single-stranded (since 4% ssDNA was detected in telomeres, Figure 4e), bringing the estimated total ssDNA to about 2 kb per cell. This is below the 10 kb ssDNA threshold predicted to activate checkpoints [10], [34]. These data indicate that Rif1 regulates the threshold for checkpoint activation in response to ssDNA, thus permitting proliferation with DNA damage.
High level of Rif1 expression mimics a checkpoint knockout
If Rif1 is a major ssDNA threshold-setter, then this threshold may increase with increased Rif1 expression. To test this hypothesis, we over-expressed RIF1 from the GAL1 promoter, induced by galactose. We found that Rif1 over-expression permitted cdc13-1 GAL-RIF1 cells to proliferate at 27°C and even at 29°C, similarly to the effect of a rad24Δ or a rad17Δ mutation (Figure 5a). Rad24Rad17 and Rad17Rad9 are checkpoint proteins, essential to arrest the cell cycle of telomere-damaged cdc13-1 cells [25]. Thus, Rif1 over-expression has the same effect as a checkpoint knockout, eliminating cell cycle arrest.
Fig. 5. Rif1 over-expression simulates a checkpoint knockout; Rif1-induction terminates a checkpoint response. 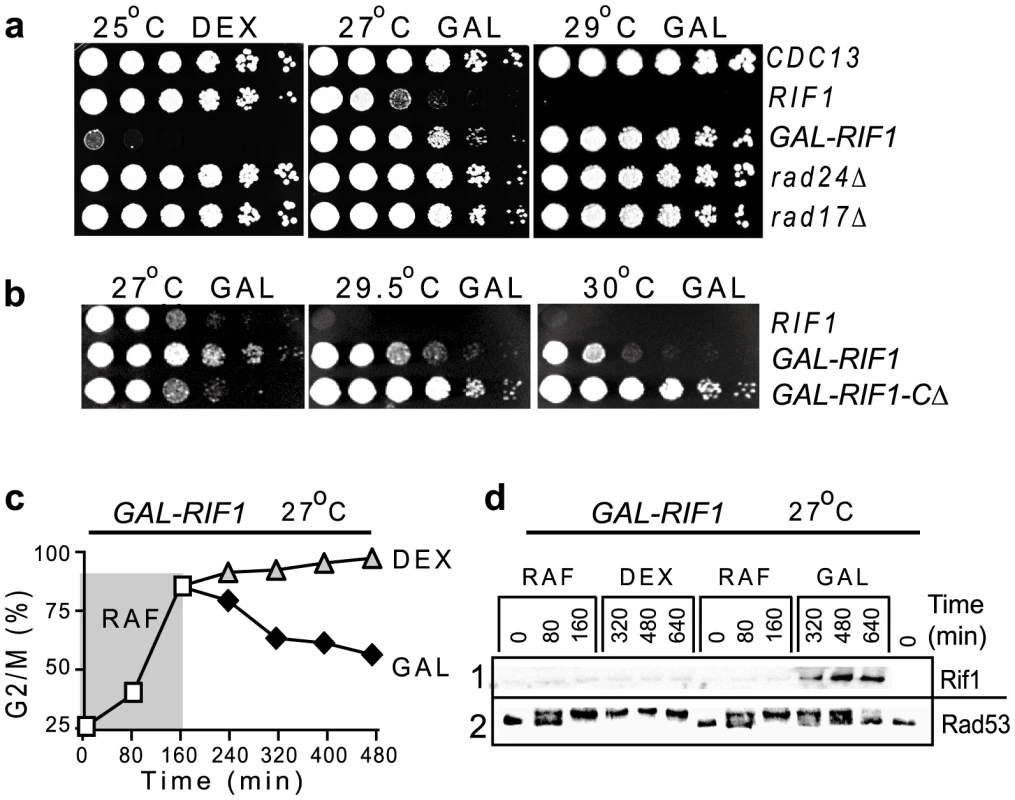
(a) Growth of serial dilution of wild type (first row, marked as CDC13) and cdc13-1 cells with RIF1 under its own promoter (second row) or under the GAL1 promoter (third row) or with additional mutations (indicated on the right of each row). (b) Growth of serial dilutions of cdc13-1 cells with RIF1 under its own promoter (first row); with RIF1 under the GAL1 promoter (second row); with the truncated RIF1-CΔ version under the GAL1 promoter. Temperatures and sugars in the medium are indicated above each plate: DEX stands for dextrose, GAL for galactose. (c) A culture of cdc13-1 GAL1-GFP-RIF1 cells was grown on raffinose (RAF) for 160 min at 27°C, then divided in two sub-cultures and either dextrose or galactose added; white squares represent the percentage of G2/M cells on RAF; triangles on DEX; black rombi on GAL. (d) Western blotting with anti-GFP to detect expression of GFP-Rif1 (lane 1) or with anti-Rad53 (lane 2) in samples collected during the experiment described in c. Interestingly, the over-expression of the C-terminal truncated variant RIF1-CΔ had additional effects on proliferation of cdc13-1 cells, increasing their temperature-resistance even further, to 30°C (Figure 5b). However, cdc13-1 GAL-RIF1-CΔ proliferated slower than cdc13-1 GAL-RIF1 on galactose (compare their growth at 27°C, Figure 5b). One hypothesis explaining these apparently paradoxical effects could be that an excess of RIF1-CΔ slows down DNA replication, which in turn allows more time for DNA replication factors to repair (re-synthesize) some of the ssDNA lesions at uncapped telomeres. Therefore, cells divide slower, but tolerate DNA damage better. Although we have not investigated the effects of over-expressing RIF1-CΔ any further, such mutants may be useful to determine whether Rif1 plays a role in other cellular processes like DNA replication.
Induction of Rif1 can terminate a checkpoint response
Another plausible consequence of the anti-checkpoint effect could be that Rif1 is able to terminate a checkpoint response when induced in cells that have already accumulated DNA damage. To test this hypothesis, we grew cdc13-1 GAL-RIF1 cells on raffinose at 27°C, to induce DNA damage and cell cycle arrest (Figure 5c). Then, we added either dextrose or galactose to the cultures. We found that with time, increasingly large fractions of GAL-RIF1 cells escaped from arrest in galactose, but not in dextrose (Figure 5c). Since galactose stimulates expression of Rif1, whereas dextrose inhibits it, this indicates that Rif1 over-expression abolishes a cell cycle arrest. Escape from arrest was confirmed by a progressive reduction in the active form of Rad53Chk2 (Figure 5d). In conclusion, (over-expressed) Rif1 was able to terminate a checkpoint-dependent arrest caused by a telomere uncapping.
The fact that over-expressed Rif1 inhibited the recruitment and activation of checkpoint proteins to the point it emulated a checkpoint knockout is consistent with the model presented in Figure 3j–3k, in which Rif1 out-competes the checkpoint proteins for their substrate. The fact that several hours after its induction, Rif1 was able to terminate a checkpoint arrest in a large fraction of cells, suggests that Rif1 took advantage of the turnover of RPA/checkpoint proteins on DNA damage to occupy their substrate of detection. Additionally, we suggest that Rif1 could be recruiting phosphatases to the DNA damage, to de-activate checkpoint proteins. Consistent with this hypothesis, Rif1 interacts physically with four different phosphatases: Glc7, Cdc14, Ptp1 and Psr2 [35]. Future studies will determine whether phosphatases are required for the Rif1-effect. In conclusion, Rif1 has an anti-checkpoint, anti-RPA effect at natural, but dysfunctional chromosome ends. This raises the question of whether Rif1 has similar effects at the ends of a double strand break (DSB).
Rif1 does not associate with an HO-DSB
A DSB poses an immediate threat to the viability of cells; in response to a DSB, cells arrest proliferation within one single cell cycle [36], [37]. Therefore, it is perhaps unlikely that checkpoint inhibitors would be acting at a DSB. To test this hypothesis, we induced the HO nuclease to cut a DSB (at a locus where it could not be repaired by homologous recombination) as previously described [7] and compared the recruitment of RPA and Ddc2ATRIP in RIF1+ versus rif1Δ strains. We found that Rif1 did not significantly affect the association of RPA or Ddc2ATRIP with chromosomal DNA at 0.2–2 kb distance from the DSB (Figure 6a–6d). We investigated the reasons behind this lack of effect and found that Rif1 was not significantly recruited to a DSB (Figure 6e–6f). This result reinforces the idea that Rif1 has to associate with DNA damage, in order to inhibit RPA/checkpoint proteins.
Fig. 6. Rif1 does not associate with an HO-induced DSB. Strains were grown overnight on raffinose; galactose was added to the medium at time 0 and samples collected every 2 hours. 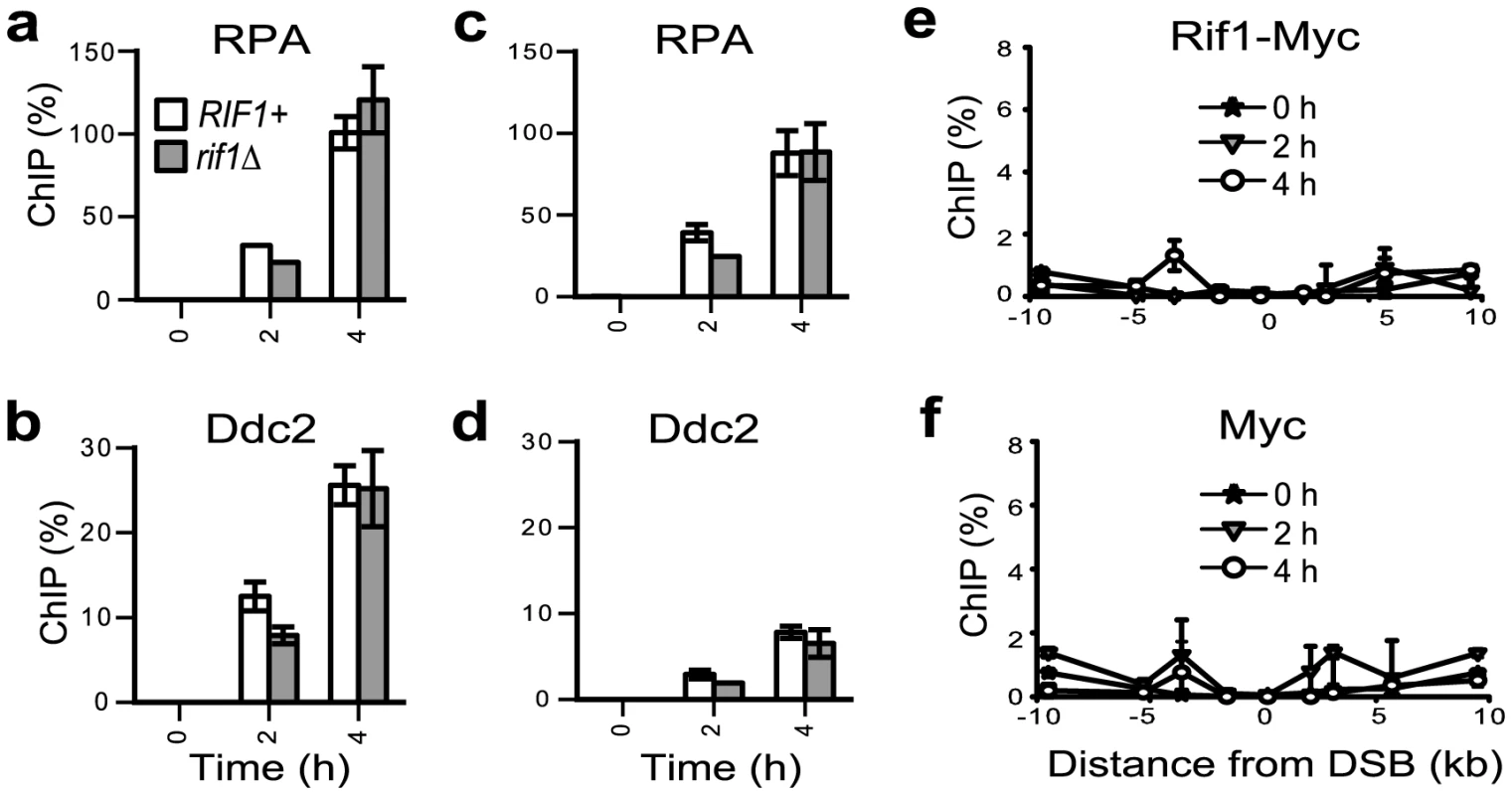
Galactose induces GAL-HO-nuclease to cut an unique DSB at the MATa locus [7]. Although a DSB is usually repaired by recombination, recombination was prevented in JKM139-derived strains by deletion of the donor locus [7]. (a–b) Chromatin immuno-precipitation experiments indicating the association of RPA and Ddc2 with chromatin at 0.2 kb right from the DSB, in RIF1+ (white columns) and rif1Δ (grey columns) JKM139-derived strains. (c–d) As in a–b, except that the association of RPA and Ddc2 with chromatin at 2 kb right from the DSB was analyzed. e) Chromatin immuno-precipitation with Myc antibody in the Rif1-Myc JKM139-derived strain. Legend indicates the time in galactose. The numbers on the X-axis indicate the location of the DSB (marked as 0) and the distance from the DSB (in kilobases). (f) As in (e), except that immuno-precipitation with Myc antibody was performed in the untagged JKM139 strain. Discussion
Our study indicates that Rif1 has a novel anti-RPA, anti-checkpoint effect. This effect manifested through less RPA and checkpoint protein recruited in RIF1+ versus rif1Δ cells to DNA damage (ssDNA) caused by telomere uncapping. Normal expression of Rif1 prevented a checkpoint-dependent cell cycle arrest in response to relatively low levels of ssDNA (estimated at 2–10 kb ssDNA per cell), whereas Rif1 over-expression prevented/abrogated a cell cycle arrest in cells with higher levels of ssDNA, similarly to the effect of a checkpoint knockout. Therefore, we proposed that Rif1 has a physiological role in regulating the checkpoint activation threshold in response to ssDNA; our model is presented in Figure 3i–3k.
We also investigated the mechanism(s) by which Rif1 inhibits checkpoint proteins/RPA and found that: 1) Rif1 did not significantly affect the amount of ssDNA at any chromosomal region (Figure 3, Figure 4); 2) Rif1 did not affect the amount of checkpoint protein in cells (Figure S1); 3) Rif1 inhibited the recruitment of checkpoint proteins/RPA when Rif1 associated with their DNA substrate (Figure 1, Figure 2, Figure 3). Conversely, Rif1 had no effect on checkpoint proteins/RPA, when Rif1 did not associate with their DNA substrate (Figure 6). These data strongly suggest that Rif1 inhibits the recruitment of checkpoint proteins/RPA to DNA damage through a competitive inhibition mechanism. Clues about the conditions required for Rif1 to out-compete RPA and checkpoint protein could be found in the different behavior of Rif1 at chromosome ends versus internal double strand breaks, discussed next.
Several hypotheses could explain the absence of Rif1 from an internal DSB: 1) Putative recruiters of Rif1 are missing from a DSB; 2) Rif1 is out-competed by other proteins. 3) Rif1 associates only with DNA damage initiated from telomeres. Although we have not pursued these hypotheses, our favourite is the second one. This is because a recent study showed that the majority of Rif1 is bound to the nuclear membrane [38], the place where telomeres are usually anchored. Therefore, we propose that Rif1 can efficiently out-compete other proteins for damages occurring near regions with abundant Rif1 (e.g. close to the nuclear periphery and/or to telomeric or sub-telomeric sequences). Conversely, Rif1 is usually out-competed at DNA damage occurring further from Rif1 anchor sites. Protein(s) preventing Rif1 from binding an internal DSB may also prevent checkpoint proteins from detecting lower amounts of ssDNA (caused by incipient resection/unwinding of the DSB), thus setting a Rif1-independent checkpoint-activation threshold. These do exclude the possibility that Rif1 may associate with internal DSBs under certain conditions, which remain to be investigated.
The association of Rif1 with chromatin within 15 kb from chromosome ends was demonstrated by chromatin immuno-precipitation in experiments presented in Figure 1. Interestingly, we found that Rif1 associated with DNA damage differently from Rap1 and that Rif1 also associated in the absence of its C-terminal Rap1-binding domain (Figure 1). If Rap1 was not required, perhaps other proteins facilitated the recruitment of Rif1 to DNA damage. Similarly to Rif1, a putative recruiter should associate with DNA-junctions and/or resection forks. Among candidates with these characteristics are the Rad24Rad17 checkpoint clamp loader and the Sgs1 helicase. Rad24Rad17 recruits Ddc1Rad9 (a protein investigated in Figure 2) as part of the 9-1-1 complex. If Rif1 has high affinity for Rad24Rad17, it will competitively inhibit Ddc1Rad9/9-1-1, thus providing an alternative explanation for some of the anti-checkpoint Rif1-effects. However, we found no evidence that Rad24Rad17 or Sgs1 are recruiting Rif1 (Figure S1, discussed in Text S1). The fact that Rif1 had a much stronger effect when over-expressed (without the need to over-express a potential recruiter) may suggest that Rif1 does not require a recruiter to DNA damage.
Previous studies suggested that Rif1 and Rif2 inhibit telomerase [31], [39], [40], an enzyme that requires a short ssDNA overhang and the activities of MRX and Tel1 to load onto DNA [41], [42]. Since Rif2 was found to inhibit an MRX-dependent DNA resection and the association of Tel1 with DNA damage [43], [44], this might explain how Rif2 inhibits telomerase. However, it cannot explain why Rif1 is a stronger telomerase-inhibitor than Rif2 (rif1Δ have longer telomeres than rif2Δ cells), since Rif1 has a much weaker effect on MRX/Tel1 compared to Rif2 [43], [44]. Our study shows that Rif1 associates with ssDNA overhangs at uncapped telomeres and protects them from RPA and checkpoint proteins. Similarly, Rif1 may associate with ssDNA overhangs generated during S-phase at normal telomeres and hide them from telomerase.
Although a molecular function for Rif1 is yet to be established in higher organisms, our study suggests conserved functions from yeast to man. We found that yeast Rif1 facilitates proliferation of cells with dysfunctional telomeres; other studies showed that mammalian Rif1 may facilitate the proliferation/viability of cells damaged by DNA polymerase inhibitors or ionizing radiation [2], [21], [45], [46]. We found that yeast Rif1 appears to move with resection forks driven by the Sgs1 helicase [18]; other studies showed that vertebrate Rif1 associates with the Sgs1-homologue, the BLM helicase, at replication forks [47]. Whether Rif1 and Sgs1 are acting in the same pathway is discussed in Text S1, Figure S1.
In conclusion, Rif1 has important and most likely conserved roles, inhibiting the checkpoint-dependent responses to DNA damage (ssDNA accumulation). Consequently, Rif1 permits cells to proliferate with DNA damage, which is a pre-requisite for chromosomal instability. Moreover, Rif1 is the first protein shown to inhibit the recruitment of RPA to ssDNA; through this effect, Rif1 could modulate important RPA-dependent processes, for example DNA replication. Further experiments will be required to understand all the consequences of the Rif1-effect in yeast and mammalian cells.
Methods
Yeast strains
All strains are derivates of W303 and are RAD5+. The cdc13-1 rif1Δ and cdc13-1 rif2Δ strains were generated by transformation of DLY1230 with PCR products using pFA6a-kanMX6 as a template [48]. RIF1-MYC strains were generated in the same way, using pFA6a-13Myc-KanMX6 as a template [48]. The last 1695 nt of the RIF1 gene were deleted to generate strains with a Rif1 C-terminus deletion (Rif1-CΔ). To over-express Rif1, we replaced the 0.5 kb genomic DNA upstream of the ATG of RIF1 with the GAL1 promoter, using pFA6a-kanMX6-PGAL1 and pFA6a-kanMX6-pGAL1-GFP as templates [48]. Other strains were obtained by genetic crosses between cdc13-1 rif1Δ and the following strains: HA2-DDC1 (YLL334 [49] and DDC2-YFP RFA1-CFP (W3924-11B [50]).
Cell culture, serial dilution, and cell cycle analysis
The YPD medium (Yeast extract, Peptone, and Dextrose) was supplemented with adenine at 50 mg/l. For experiments testing the maximum permissive temperature, cells grown overnight at 20°C were diluted to about 1.5×107 cells/ml, followed by 5-fold dilution series set up in 96-well plates. Small aliquots were transferred to YPD plates using metal prongs. Plates were incubated for 2.5 days at the indicated temperature. For Rif1 over-expression experiments, cells were grown in YPR medium (YP with 2% raffinose), followed by a 5-fold dilution series and transfer to YPD or to YPG plates (YP with 2% galactose). Cell cycle analysis was performed by fluorescent microscopy, after staining samples with DAPI and sonicating them, to separate individual cells. Following fractions were counted: cells without buds (in the G1 phase), with small buds (in the S-phase), with large anucleated buds (at the G2/M transition) or with nucleated buds (in anaphase/telophase). Wild type cells are equally distributed between these stages of the cell cycle, if growing exponentially.
Single-stranded DNA (ssDNA) measurements
Single-stranded DNA (ssDNA) measurements at sub-telomeric and single gene loci were performed by QAOS as previously described, except that we used asynchronous populations of cells cultivated at the indicated temperatures [29], [51]. Genomic DNA was extracted, purified and quantified at a centromere-proximal location (PAC2). SsDNA was quantified by QAOS within the Y′ sub-telomeric repeats (at about 1 kb from chromosome ends) and at the following single gene loci from the right arm of chromosome V: YER188W, YER186C and PAC2. Taqman primers and probes used for QAOS were previously described in [29], [51]. Single-stranded DNA measurements in the TG-telomeric repeats were performed using the fluorescent in-gel hybridization assay (FIGA) described in [32], with modifications (described next). Phenol-extracted DNA samples were diluted to about 800 ng/µl, digested with Xho1 and hybridized over night with a CA-rich fluorescent 5′ [CY5]CCCACCACACACACCCACACCC probe (Sigma). In the morning, a fraction of the digested and labelled DNA was denatured for 2 min at 100°C in a total volume of 30 µl and then chilled on ice for 1 h. This denaturing time is optimized for DNA up to 8 µg. DNA was exposed to gel electrophoresis to separate telomeric fragments and then scanned using a Typhoon Trio imager (GE Healthcare Fluorescent). Denatured and native DNA samples were scanned simultaneously, to ensure identical parameters/conditions of detection (leading to more realistic results following normalization of native to denatured DNA signals). The intensity of the signal in each sample was quantified on the original image generated by the Typhoon Trio imager, using the ImageJ (NIH) software. The percentage of ssDNA was calculated as the signal given by native telomeres (minus the immediately adjacent background) normalized to the signal given by denatured telomeres (minus the immediately adjacent background) and multiplied by 100. Additionally, DNA was stained with SYBR Safe for informative purposes.
Protein extraction and western blotting
Protein extracts were prepared by a trichloroacetic acid (TCA) method described in [52]. For western blotting, proteins were separated on SDS-PAGE and transferred to nitrocellulose membranes (GE healthcare). Membranes were blocked in 5% TBST, incubated with antibodies and analyzed by LAS-3000 (Fujifilm). We used the following antibodies: mouse monoclonal anti-Myc (sc-40, Santa Cruz), mouse monoclonal anti-GFP (11814460001, Roche), rat monoclonal anti-HA (11867423001, Roche) and goat polyclonal anti-Rad53 (sc-6749, Santa Cruz), anti-Sgs1 (sc-11993, Santa Cruz). Secondary antibodies included rabbit anti-mouse (ab6728, Abcam), donkey anti-goat (sc-2020, Santa Cruz) and rabbit anti-rat (ab6734, Abcam).
Chromatin immuno-precipitation (ChIP)
Chromatin immuno-precipitation (ChIP) was carried out by standard methods [53], [54].The association of Rif1-MYC, Ddc1-HA, Ddc2-YFP, Rpa1-CFP and Rif1-HA with chromatin was detected using antibodies (described above) directed against the respective tags; YFP and CFP were detected with anti-GFP antibodies. The association of Rad9 and Rap1 was detected with anti-Rap1 (sc-6663, Santa Cruz) and anti-Rad9 (sc-50442, Santa Cruz) antibodies, tested for their specificity by western blotting. RPA was also detected with specific antibodies PAB13584 (Abnova). Additionally, cells were treated with anti-goat antibodies (sc-2033, Santa Cruz) to assess the background cross-linking. For each time point, the background normalized to the input was subtracted from the immuno-precipitated DNA, also normalized to the input. Input, immuno-precipitated DNA and background were quantified by real-time PCR (StepOne Plus, Applied Biosystems) using genomic DNA standards.
Supporting Information
Zdroje
1. MichelsonRJRosensteinSWeinertT 2005 A telomeric repeat sequence adjacent to a DNA double-stranded break produces an anticheckpoint. Genes Dev 19 2546 59
2. SilvermanJTakaiHBuonomoSBEisenhaberFde LangeT 2004 Human Rif1, ortholog of a yeast telomeric protein, is regulated by ATM and 53BP1 and functions in the S-phase checkpoint. Genes Dev 18 2108 19
3. MurnaneJP 2010 Telomere loss as a mechanism for chromosome instability in human cancer. Cancer Res 70 4255 9
4. ReddelRR 2010 Senescence: an antiviral defense that is tumor suppressive? Carcinogenesis 31 19 26
5. ArtandiSEDePinhoRA 2010 Telomeres and telomerase in cancer. Carcinogenesis 31 9 18
6. ClemensonCMarsolier-KergoatMC 2009 DNA damage checkpoint inactivation: adaptation and recovery. DNA Repair (Amst) 8 1101 9
7. LeeSEMooreJKHolmesAUmezuKKolodnerRD 1998 Saccharomyces Ku70, mre11/rad50 and RPA proteins regulate adaptation to G2/M arrest after DNA damage. Cell 94 399 409
8. PellicioliALeeSELuccaCFoianiMHaberJE 2001 Regulation of Saccharomyces Rad53 checkpoint kinase during adaptation from DNA damage-induced G2/M arrest. Mol Cell 7 293 300
9. LeeSEPellicioliAMalkovaAFoianiMHaberJE 2001 The Saccharomyces recombination protein Tid1p is required for adaptation from G2/M arrest induced by a double-strand break. Curr Biol 11 1053 7
10. VazeMBPellicioliALeeSEIraGLiberiG 2002 Recovery from checkpoint-mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol Cell 10 373 85
11. ClericiMMantieroDLucchiniGLongheseMP 2006 The Saccharomyces cerevisiae Sae2 protein negatively regulates DNA damage checkpoint signalling. EMBO Rep 7 212 8
12. DonnianniRAFerrariMLazzaroFClericiMTamilselvan NachimuthuB PLoS Genet 6 e1000763 doi:10.1371/journal.pgen.1000763
13. CarneiroTKhairLReisCCBorgesVMoserBA 2010 Telomeres avoid end detection by severing the checkpoint signal transduction pathway. Nature 467 228 32
14. JainDHebdenAKNakamuraTMMillerKMCooperJP HAATI survivors replace canonical telomeres with blocks of generic heterochromatin. Nature 467 223 7
15. MaringeleLLydallD 2004 Telomerase - and recombination-independent immortalization of budding yeast. Genes Dev 18 2663 75
16. ShoreD 1994 RAP1: a protean regulator in yeast. Trends Genet 10 408 12
17. HardyCFSusselLShoreD 1992 A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev 6 801 14
18. NgoHPLydallD 2010 Survival and growth of yeast without telomere capping by Cdc13 in the absence of Sgs1, Exo1, and Rad9. PLoS Genet 6 e1001072 doi:10.1371/journal.pgen.1001072
19. BournsBDAlexanderMKSmithAMZakianVA 1998 Sir proteins, Rif proteins, and Cdc13p bind Saccharomyces telomeres in vivo, Mol Cell Biol 18 5600 8
20. SmithCDSmithDLDeRisiJLBlackburnEH 2003 Telomeric protein distributions and remodeling through the cell cycle in Saccharomyces cerevisiae. Mol Biol Cell 14 556 70
21. XuLBlackburnEH 2004 Human Rif1 protein binds aberrant telomeres and aligns along anaphase midzone microtubules. J Cell Biol 167 819 30
22. MajkaJBurgersPM 2003 Yeast Rad17/Mec3/Ddc1: a sliding clamp for the DNA damage checkpoint. Proc Natl Acad Sci U S A 100 2249 54
23. ZouLElledgeSJ 2003 Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science 300 1542 8
24. MajkaJNiedziela-MajkaABurgersPM 2006 The checkpoint clamp activates Mec1 kinase during initiation of the DNA damage checkpoint. Mol Cell 24 891 901
25. LydallD 2009 Taming the tiger by the tail: modulation of DNA damage responses by telomeres. Embo J 28 2174 2187
26. AnbalaganSBonettiDLucchiniGLongheseMP 2011 Rif1 supports the function of the CST complex in yeast telomere capping. PLoS Genet 7 e1002024 doi:10.1371/journal.pgen.1002024
27. MaringeleLLydallD 2002 EXO1-dependent single-stranded DNA at telomeres activates subsets of DNA damage and spindle checkpoint pathways in budding yeast yku70Delta mutants. Genes Dev 16 1919 33
28. ZubkoMKLydallD 2006 Linear chromosome maintenance in the absence of essential telomere-capping proteins. Nat Cell Biol 8 734 40
29. ZubkoMKMaringeleLFosterSSLydallD 2006 Detecting repair intermediates in vivo: effects of DNA damage response genes on single-stranded DNA accumulation at uncapped telomeres in budding yeast. Methods Enzymol 409 285 300
30. AddinallSGHolsteinEMLawlessCYuMChapmanK 2011 Quantitative fitness analysis shows that NMD proteins and many other protein complexes suppress or enhance distinct telomere cap defects. PLoS Genet 7 e1001362 doi:10.1371/journal.pgen.1001362
31. WottonDShoreD 1997 A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev 11 748 60
32. DewarJMLydallD Pif1 - and Exo1-dependent nucleases coordinate checkpoint activation following telomere uncapping. Embo J 29 4020 34
33. VodenicharovMDWellingerRJ 2006 DNA degradation at unprotected telomeres in yeast is regulated by the CDK1 (Cdc28/Clb) cell-cycle kinase. Mol Cell 24 127 37
34. ZubkoMKGuillardSLydallD 2004 Exo1 and Rad24 differentially regulate generation of ssDNA at telomeres of Saccharomyces cerevisiae cdc13-1 mutants. Genetics 168 103 15
35. BreitkreutzAChoiHSharomJRBoucherLNeduvaV A global protein kinase and phosphatase interaction network in yeast. Science 328 1043 6
36. HarrisonJCHaberJE 2006 Surviving the breakup: the DNA damage checkpoint. Annu Rev Genet 40 209 35
37. JacksonSPBartekJ 2009 The DNA-damage response in human biology and disease. Nature 461 1071 8
38. Park S, Patterson EE, Cobb J, Audhya A, Gartenberg MR, et al. Palmitoylation controls the dynamics of budding-yeast heterochromatin via the telomere-binding protein Rif1. Proc Natl Acad Sci U S A 108 14572 7
39. LevyDLBlackburnEH 2004 Counting of Rif1p and Rif2p on Saccharomyces cerevisiae telomeres regulates telomere length. Mol Cell Biol 24 10857 67
40. MillerKMFerreiraMGCooperJP 2005 Taz1, Rap1 and Rif1 act both interdependently and independently to maintain telomeres. Embo J 24 3128 35
41. GoudsouzianLKTuzonCTZakianVA 2006 S. cerevisiae Tel1p and Mre11p are required for normal levels of Est1p and Est2p telomere association. Mol Cell 24 603 10
42. SabourinMTuzonCTZakianVA 2007 Telomerase and Tel1p preferentially associate with short telomeres in S. cerevisiae. Mol Cell 27 550 61
43. BonettiDClericiMAnbalaganSMartinaMLucchini 2010 Shelterin-like proteins and Yku inhibit nucleolytic processing of Saccharomyces cerevisiae telomeres. PLoS Genet 6 e1000966 doi:10.1371/journal.pgen.1000966
44. HiranoYFukunagaKSugimotoK 2009 Rif1 and rif2 inhibit localization of tel1 to DNA ends. Mol Cell 33 312 22
45. WangHZhaoAChenLZhongXLiaoJ 2009 Human RIF1 encodes an anti-apoptotic factor required for DNA repair. Carcinogenesis 30 1314 9
46. BuonomoSBWuYFergusonDde LangeT 2009 Mammalian Rif1 contributes to replication stress survival and homology-directed repair. J Cell Biol 187 385 98
47. XuDMuniandyPLeoEYinJThangavelS 2010 Rif1 provides a new DNA-binding interface for the Bloom syndrome complex to maintain normal replication. Embo J 29 3140 55
48. LongtineMSMcKenzie A3rdDemariniDJShahNGWachA 1998 Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 953 61
49. LongheseMPPaciottiVFraschiniRZaccariniRPlevaniP 1997 The novel DNA damage checkpoint protein ddc1p is phosphorylated periodically during the cell cycle and in response to DNA damage in budding yeast. Embo J 16 5216 26
50. LisbyMBarlowJHBurgessRCRothsteinR 2004 Choreography of the DNA damage response: spatiotemporal relationships among checkpoint and repair proteins. Cell 118 699 713
51. BoothCGriffithEBradyGLydallD 2001 Quantitative amplification of single-stranded DNA (QAOS) demonstrates that cdc13-1 mutants generate ssDNA in a telomere to centromere direction. Nucleic Acids Res 29 4414 22
52. FoianiMMariniFGambaDLucchiniGPlevaniP 1994 The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol Cell Biol 14 923 33
53. BraunsteinMRoseABHolmesSGAllisCDBroachRJ 1993 Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev 7 592 604
54. DedonPCSoultsJAAllisCDGorovskyMA 1991 A simplified formaldehyde fixation and immunoprecipitation technique for studying protein-DNA interactions. Anal Biochem 197 83 90
Štítky
Genetika Reprodukční medicína
Článek A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the ChickenČlánek Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREXČlánek A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding SequencesČlánek Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor SystemČlánek Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 12- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- The Connection between Space and Thinking: An Interview with Rafael Viñoly
- An Assessment of the Individual and Collective Effects of Variants on Height Using Twins and a Developmentally Informative Study Design
- Widespread Cotranslational Formation of Protein Complexes
- Genomes Reveal Transition of Bacteria from Aquatic to Terrestrial Environments
- A Complex Genomic Rearrangement Involving the Locus Causes Dermal Hyperpigmentation in the Chicken
- Plasticity of BRCA2 Function in Homologous Recombination: Genetic Interactions of the PALB2 and DNA Binding Domains
- Transcription Is Required to Establish Maternal Imprinting at the Prader-Willi Syndrome and Angelman Syndrome Locus
- Substitutions in the Amino-Terminal Tail of Neurospora Histone H3 Have Varied Effects on DNA Methylation
- MAPK/ERK Signaling Regulates Insulin Sensitivity to Control Glucose Metabolism in
- A Comprehensive Analysis of Shared Loci between Systemic Lupus Erythematosus (SLE) and Sixteen Autoimmune Diseases Reveals Limited Genetic Overlap
- Genome Instability and Transcription Elongation Impairment in Human Cells Depleted of THO/TREX
- Genome-Wide Meta-Analysis of Five Asian Cohorts Identifies as a Susceptibility Locus for Corneal Astigmatism
- A Population Genetics-Phylogenetics Approach to Inferring Natural Selection in Coding Sequences
- HIF-1 Regulates Iron Homeostasis in by Activation and Inhibition of Genes Involved in Iron Uptake and Storage
- Ror2 Enhances Polarity and Directional Migration of Primordial Germ Cells
- DNA Methylation of the Gonadal Aromatase () Promoter Is Involved in Temperature-Dependent Sex Ratio Shifts in the European Sea Bass
- A Genetic Screening Strategy Identifies Novel Regulators of the Proteostasis Network
- Interspecific Sex in Grass Smuts and the Genetic Diversity of Their Pheromone-Receptor System
- The Synthetic Multivulva Genes Prevent Ras Pathway Activation by Tightly Repressing Global Ectopic Expression of EGF
- Mining the Allelic Spectrum Reveals the Contribution of Rare and Common Regulatory Variants to HDL Cholesterol
- Identification of a Genomic Reservoir for New Genes in Primate Genomes
- Genomic Distribution and Inter-Sample Variation of Non-CpG Methylation across Human Cell Types
- Identification of Evolutionarily Conserved Exons as Regulated Targets for the Splicing Activator Tra2β in Development
- Acute Multiple Organ Failure in Adult Mice Deleted for the Developmental Regulator Wt1
- Age-Related Neuronal Degeneration: Complementary Roles of Nucleotide Excision Repair and Transcription-Coupled Repair in Preventing Neuropathology
- Target Site Recognition by a Diversity-Generating Retroelement
- Ancestral Components of Admixed Genomes in a Mexican Cohort
- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- Autosomal Recessive Dilated Cardiomyopathy due to Mutations Results from Abnormal Dystroglycan O-Mannosylation
- SREBP Coordinates Iron and Ergosterol Homeostasis to Mediate Triazole Drug and Hypoxia Responses in the Human Fungal Pathogen
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- An Anti-Checkpoint Activity for Rif1
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Common Variants Show Predicted Polygenic Effects on Height in the Tails of the Distribution, Except in Extremely Short Individuals
- The Fission Yeast Stress-Responsive MAPK Pathway Promotes Meiosis via the Phosphorylation of Pol II CTD in Response to Environmental and Feedback Cues
- Integrating Genome-Wide Genetic Variations and Monocyte Expression Data Reveals -Regulated Gene Modules in Humans
- Repetitive Elements May Comprise Over Two-Thirds of the Human Genome
- A Novel Checkpoint and RPA Inhibitory Pathway Regulated by Rif1
- Hierarchical Generalized Linear Models for Multiple Groups of Rare and Common Variants: Jointly Estimating Group and Individual-Variant Effects
- The Major Roles of DNA Polymerases Epsilon and Delta at the Eukaryotic Replication Fork Are Evolutionarily Conserved
- A High-Resolution Whole-Genome Map of Key Chromatin Modifications in the Adult
- A Densely Interconnected Genome-Wide Network of MicroRNAs and Oncogenic Pathways Revealed Using Gene Expression Signatures
- A Functional Phylogenomic View of the Seed Plants
- Histone H3K9 Trimethylase Eggless Controls Germline Stem Cell Maintenance and Differentiation
- Ribosomal Protein Mutants Control Tissue Growth Non-Autonomously via Effects on the Prothoracic Gland and Ecdysone
- , , and Are Required to Activate or Delimit the Spread of the Transcriptional Response to Epidermal Wounds in
- Mechanisms Establishing TLR4-Responsive Activation States of Inflammatory Response Genes
- Candidate Gene Screen in the Red Flour Beetle Reveals as Ancient Regulator of Anterior Median Head and Central Complex Development
- Charcot-Marie-Tooth–Linked Mutant GARS Is Toxic to Peripheral Neurons Independent of Wild-Type GARS Levels
- The RNA–Methyltransferase Misu (NSun2) Poises Epidermal Stem Cells to Differentiate
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Targeted Proteolysis of Plectin Isoform 1a Accounts for Hemidesmosome Dysfunction in Mice Mimicking the Dominant Skin Blistering Disease EBS-Ogna
- The RNA Silencing Enzyme RNA Polymerase V Is Required for Plant Immunity
- The FGFR4-G388R Polymorphism Promotes Mitochondrial STAT3 Serine Phosphorylation to Facilitate Pituitary Growth Hormone Cell Tumorigenesis
- Target Site Recognition by a Diversity-Generating Retroelement
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



