-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal Function in Flowers and Meristems
Arabidopsis TSO1 encodes a protein with conserved CXC domains known to bind DNA and is homologous to animal proteins that function in chromatin complexes. tso1 mutants fall into two classes due to their distinct phenotypes. Class I, represented by two different missense mutations in the CXC domain, leads to failure in floral organ development, sterility, and fasciated inflorescence meristems. Class II, represented by a nonsense mutation and a T-DNA insertion line, develops wild-type–like flowers and inflorescences but shows severely reduced fertility. The phenotypic variability of tso1 alleles presents challenges in determining the true function of TSO1. In this study, we use artificial microRNA, double mutant analysis, and bimolecular fluorescence complementation assay to investigate the molecular basis underlying these two distinct classes of phenotypes. We show that the class I mutants could be converted into class II by artificial microRNA knockdown of the tso1 mutant transcript, suggesting that class I alleles produce antimorphic mutant proteins that interfere with functionally redundant loci. We identified one such redundant factor coded by the closely related TSO1 homolog SOL2. We show that the class I phenotype can be mimicked by knocking out both TSO1 and its homolog SOL2 in double mutants. Such antimorphic alleles targeting redundant factors are likely prevalent in Arabidopsis and maybe common in organisms with many sets of paralogous genes such as human. Our data challenge the conventional view that recessive alleles are always hypomorphic or null and that antimorphic alleles are always dominant. This study shows that recessive alleles can also be antimorphic and can produce a phenotype more severe than null by interfering with the function of related loci. This finding adds a new paradigm to classical genetic concepts, with important implications for future genetic studies both in basic research as well as in agriculture and medicine.
Published in the journal: . PLoS Genet 7(11): e32767. doi:10.1371/journal.pgen.1002352
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002352Summary
Arabidopsis TSO1 encodes a protein with conserved CXC domains known to bind DNA and is homologous to animal proteins that function in chromatin complexes. tso1 mutants fall into two classes due to their distinct phenotypes. Class I, represented by two different missense mutations in the CXC domain, leads to failure in floral organ development, sterility, and fasciated inflorescence meristems. Class II, represented by a nonsense mutation and a T-DNA insertion line, develops wild-type–like flowers and inflorescences but shows severely reduced fertility. The phenotypic variability of tso1 alleles presents challenges in determining the true function of TSO1. In this study, we use artificial microRNA, double mutant analysis, and bimolecular fluorescence complementation assay to investigate the molecular basis underlying these two distinct classes of phenotypes. We show that the class I mutants could be converted into class II by artificial microRNA knockdown of the tso1 mutant transcript, suggesting that class I alleles produce antimorphic mutant proteins that interfere with functionally redundant loci. We identified one such redundant factor coded by the closely related TSO1 homolog SOL2. We show that the class I phenotype can be mimicked by knocking out both TSO1 and its homolog SOL2 in double mutants. Such antimorphic alleles targeting redundant factors are likely prevalent in Arabidopsis and maybe common in organisms with many sets of paralogous genes such as human. Our data challenge the conventional view that recessive alleles are always hypomorphic or null and that antimorphic alleles are always dominant. This study shows that recessive alleles can also be antimorphic and can produce a phenotype more severe than null by interfering with the function of related loci. This finding adds a new paradigm to classical genetic concepts, with important implications for future genetic studies both in basic research as well as in agriculture and medicine.
Introduction
During the transition from vegetative to reproductive phase all flowering plants develop flowers from stem cells at the shoot apex, called the inflorescence meristem (IM). In Arabidopsis thaliana, the IM gives rise to indeterminate number of floral meristems (FM). Each FM develops and subsequently differentiates into a flower with four distinct types of floral organs. Much has been learned about how the four floral organ types are specified by the four classes of floral homeotic genes [1], [2]. However, very little is known about how each floral organ grows and differentiates into its final shape, size, and morphology. This is partly owing to difficulties in identifying and analyzing mutants that fail to grow and differentiate, as their phenotypes may not be as distinct as floral homeotic mutants.
Arabidopsis tso1-1 appears to belong to this second class of flower mutants, as tso1-1 mutants fail to develop differentiated floral organs [3], [4]. Besides abnormal sepals, almost all other floral organs of tso1-1 flowers are missing and are replaced by a mass of callus-like undifferentiated tissues (Figure 1B). Rarely, tso1-1 flowers develop rudimentary floral organs, including petal-like structures (Figure1B) and unfused carpels. Since tso1-1 mutant plants do not develop normal reproductive organs, plants are completely sterile. In addition to the floral organ differentiation defects, inflorescence meristems of tso1-1 mutants are often enlarged and fasciated, splitting from one into several inflorescences (Figure 1B and [3], [4]). Although TSO1 mRNA is detected in all Arabidopsis tissues tso1-1 phenotypes are largely flower-specific.
Fig. 1. Phenotypic classes of tso1 alleles. 
(A) A wild type (WT) plant with fertile siliques indicated by an arrow (top panel) and a normal inflorescence (bottom). (B) A tso1-1 mutant inflorescence with an extremely fasciated meristem and undifferentiated flowers. Petal-like floral organ, occasionally seen in tso1-1 flowers, is indicated by an arrow. (C) A tso1-3 plant showing small siliques (arrows in top panel) but normal inflorescence and wild type-like flowers (bottom). (D) A tso1-5 mutant plant showing similar phenotypes as tso1-3 in (C). (E) A tso1-1/tso1-3 transheterozygous plant showing a fasciated inflorescence (bottom) and a lack of silique (an arrow, top panel). Bars in A and B: 500 µm. C, D, and E are at the same magnification as A. Using map-based cloning, we and others showed that the TSO1 gene (At3g22780) encodes a nuclear protein with two tandem cysteine-rich (CXC) repeats connected by a conserved intervening hinge region [3], [5]. Eight TSO1 homologs (CXC-Hinge-CXC or CHC genes) have been described in Arabidopsis and can be grouped into two different types [3], [4], [6]. TSO1 belongs to type I, together with two closely related homologs At3g22760 and At4g14770, which were also named as SOL1 and SOL2, respectively [5], and a fourth member At3g04850. Type II homologs, which are phylogenetically more distant from type I, include four genes, At4g29000, At2g20110, At5g25790, and At3g16160. Among the type I CHC proteins, TSO1 and SOL2 show highly similar expression patterns throughout the plant except in pollen and carpel tissues, where SOL2 is absent or expressed at a very low level [6]. On the other hand, SOL1 is predominantly expressed in all stages of pollen development. TSO1 transcript was also found in pollen development, but is limited to uninucleate microspores and bicellular pollen (not tricellular and mature pollen) [6]. The expression of the fourth member of the type I CHC proteins could not be detected and was suspected to encode a pseudogene.
The CHC proteins are absent in prokaryotes but present in all eukaryotes except fungi [6]. A CHC domain-containing protein was shown to bind DNA in soybean [7]. Also, CHC binds zinc ions and may define a novel zinc-finger domain [6]. The mammalian CHC protein, TESMIN, was originally identified in testes, but subsequently also detected in ovary development [8]–[10]. In Drosophila melanogaster, there are two CHC genes, Mip120 (myb-interacting protein 120) and Tombola, whose gene products function in two paralogous chromatin complexes [11]–[14]. The dREAM complex contains the Mip120 and was found to regulate cell cycle and cell differentiation [14]–[16]. The tMAC complex contains Tombola and regulates testis-specific programs [13]. The Caenorhabditis elegans CHC protein LIN-54, a component of the orthologous DRM complex, was recently shown to recognize and bind a hybrid E2F/DP and LIN-54 consensus motif and help recruit DRM to promoters of genes involved in cell cycle, development, and reproduction [17]. Blast searches identified plant homologs of almost all dREAM chromatin complex components, suggesting the possibility of a plant dREAM-like complex, whose activity may depend on TSO1.
Several different tso1 alleles have been previously described, all of which are recessive. The strongest allele is tso1-1 caused by a missense mutation in the second CXC repeat, replacing one of the highly conserved cysteines by a tyrosine [3]. tso1-2 allele, a result of replacing another conserved cysteine by a tyrosine in the first CXC repeat [3], caused a similar phenotype as tso1-1. In contrast, tso1-3 is a nonsense mutation that causes premature protein termination after the first CXC domain [3], [5]. However, tso1-3 phenotype is weak and differs significantly from tso1-1 and tso1-2. tso1-3 mutant plants develop normal flowers and do not exhibit meristem fasciation (compare Figure 1B with Figure 1C). The only defect is its severely reduced fertility as shown by the formation of very short siliques (seed pods) (Figure 1C and [5]). A fourth allele, tso1-5, was caused by a T-DNA insertion in the second CXC repeat, leading to undetectable levels of TSO1 transcripts [6]. tso1-5 is very similar to tso1-3 phenotypically with morphologically wild type flowers but small siliques (Figure 1D and [6]). Therefore, tso1 alleles can be grouped into two distinct classes. Class I includes tso1-1 and tso1-2 missense mutations that cause severe floral organ differentiation and meristem defects, and class II includes tso1-3 and tso1-5 loss-of-function mutations showing only reduced seed set.
1946 Nobel Prize winner H.J. Muller coined the terms amorph, hypomorph, hypermorph, antimorph and neomorph to indicate quantitative changes to the wild type characters based on his analyses of Drosophila mutants [18]. Today, “amorph” is often used interchangeably with “null”, hypomorph with “loss-of-function”, and antimorph with “dominant-negative”. Antimorphic (dominant-negative) mutant alleles, in a heterozygote state, antagonize the activity of corresponding wild type alleles to give a null-like phenotype and thus are thought to always act dominantly over wild type [18], [19].
The work reported here suggests that the tso1 class I alleles are antimorphic alleles, which however act recessively to their wild type allele. Specifically, experiments were conducted to answer questions why there is such a dramatic phenotypic difference between the missense class I alleles (tso1-1 and tso1-2) and the loss-of-function class II alleles (tso1-3 and tso1-5) and what is the nature of the tso1-1 and tso1-2 missense mutations. Using gene knockdown (artificial microRNA), T-DNA insertions, double mutant analyses, and Bimolecular Fluorescent Complementation (BiFC) assay, we obtained genetic and molecular data indicating that class I are recessive antimorphic alleles, which lost their normal function but interfered with the activity of a TSO1 homolog SOL2. Our work provides important mechanistic insights into recessive antimorphism and has broad implications both for basic science and for medicine and agriculture.
Results
Artificial MicroRNA Knockdown of TSO1 Suppressed tso1-1 Flower Phenotype
One obvious question is what the tso1 null allele is like. Since tso1-1 and tso1-2 (class I) exhibited stronger phenotypes, they could be null alleles. If the class I alleles were null, further reduction of tso1 mutant transcripts should not cause any change in their phenotypes. Alternatively, tso1-3 and tso1-5 (class II) could be null alleles as they cause protein truncation and undetectable RNA transcript, respectively [3], [5], . Consequently, the class I (tso1-1 and tso1-2) alleles, with a more severe mutant phenotype, are unlikely to be hypomorphic alleles. Instead, the class I alleles may act as recessive antimorphic alleles that not only lose TSO1 function but also interfere with functionally redundant TSO1 homologs, such as SOL1 and SOL2. This would explain why these class I alleles possess a more severe phenotype than the class II (null) alleles. If this second scenario were true, further reduction of tso1-1 transcripts in tso1-1 plants may remove the antimorphic (interfering) effect of tso1-1 and ameliorate the tso1-1 phenotype.
To test the above alternative hypotheses, an artificial microRNA was used to knock down tso1-1 mutant transcripts in tso1-1 mutants. This artificial microRNA construct, named 2044amiRTSO1, was designed to specifically target the 3′ end of the TSO1 gene (see Materials and Methods). Wild type plants were transformed with the construct to yield 63 first generation (T1) 2044amiRTSO1(WT) transgenic lines, none of which showed any phenotype (Figure 2G). Since tso1-1 homozygous plants are sterile, tso1-1/+ heterozygous plants were transformed with the 2044amiRTSO1 construct to yield 43 T1 transgenic lines. Four such lines were identified to be tso1-1/+; amiRTSO1 by genotyping, and all of them showed wild type phenotype (Figure 2F). On the other hand, five plants genotyped as tso1-1; amiRTSO1 exhibited inflorescence and flower phenotypes that were much milder than tso1-1 single mutants (compare Figure 2A with Figure 2B-2C, and Figure 1B with Figure 2D), indicative of a suppression of the tso1-1 phenotype by the amiRTSO1. To confirm that the observed phenotypic suppression in the tso1-1; amiRTSO1 plants was due to a reduction of tso1-1 transcripts, real-time RT-PCR was performed on two tso1-1; amiRTSO1 T1 transgenic lines, #1 and #7 (Figure 2H). The level of tso1-1 transcripts in both lines was reduced to about 15% of the untransformed tso1-1 level, suggesting that the reduction of tso1-1 mutant gene products in tso1-1; amiRTSO1 plants may underlie the phenotypic suppression.
Fig. 2. Artificial microRNA knockdown of tso1-1 transcripts suppresses tso1-1 phenotype. 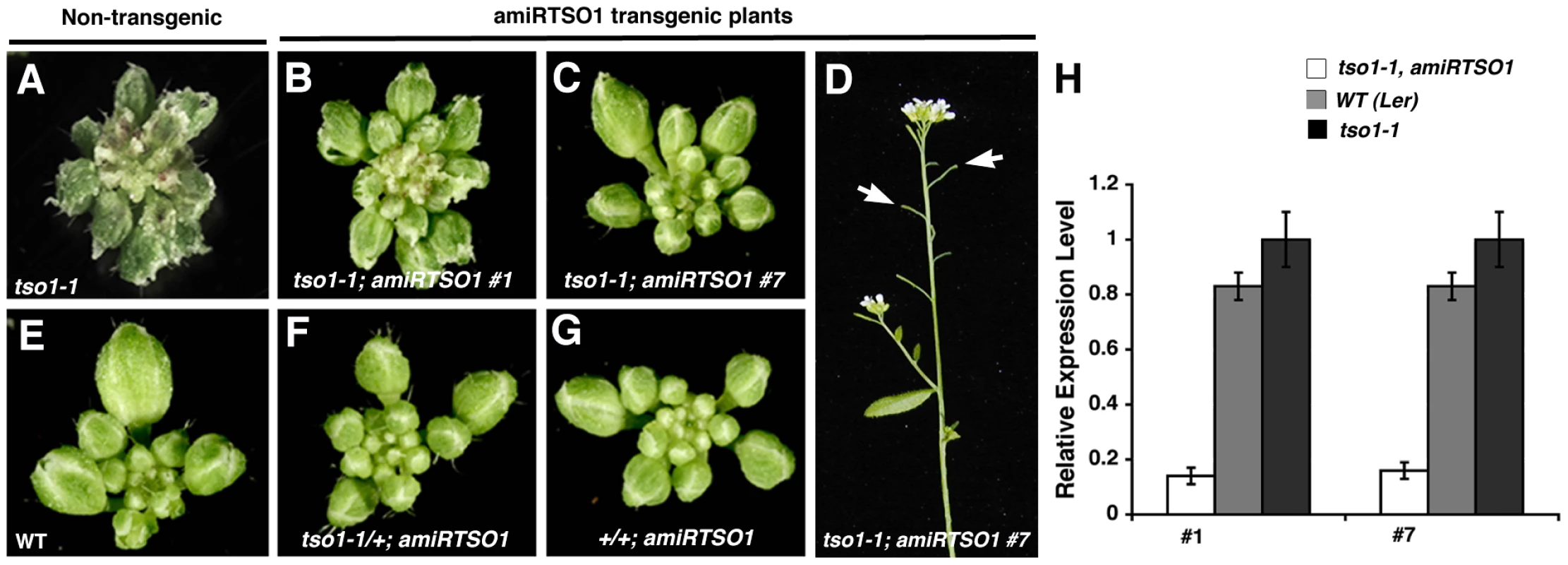
(A) A tso1-1 mutant inflorescence showing flower buds with jagged sepals. (B) An inflorescence of tso1-1; amiRTSO1 line #1 showing suppressed floral phenotype. (C) An inflorescence of tso1-1; amiRTSO1 line #7 with an even more suppressed floral phenotype. (D) Side view of a tso1-1; amiRTSO1 line #7 plant showing short and seedless siliques (arrows). (E) A wild type (Ler) inflorescence. (F) A tso1-1/+; amiRTSO1 inflorescence. (G) A +/+; amiRTSO1 inflorescence. (H) Real time RT-PCR analysis of tso1-1 transcript levels in tso1-1; amiRTSO1 (white bars) or tso1-1 (black bars) plants. The TSO1 transcript level in wild type (Ler, grey bars) is also shown. The relative level of tso1-1 transcripts in tso1-1 plants was designated as 1. Standard deviation was calculated based on three technical replicates. This result provides strong support for the second scenario that tso1-1, as well as other class I alleles, are likely recessive antimorphic alleles, while class II alleles (tso1-3 or tso1-5) are loss-of-function or near complete loss-of-function (near-null) alleles. The significant reduction of tso1-1 mutant gene products in tso1-1; amiRTSO1 plants removed the interfering effects of tso1-1 on potentially redundant factors.
Class II Alleles Cause Severe Fertility Defects
Although the tso1-1; amiRTSO1 plants described above were able to clear the antimorphic tso1-1, they still lack wild type TSO1 and thus resemble class II mutant plants. Specifically, tso1-1; amiRTSO1 plants formed extremely short siliques and were completely sterile despite their nearly normal floral and meristem development (Figure 2D, Figure 3H). Therefore, amiRTSO1 was able to convert a class I allele into a class II allele, and class II alleles represent severe loss-of-function or near-null alleles.
Fig. 3. tso1-3; sol2 or tso1-5; sol2 double mutants exhibit fertility defects similar to those of tso1-1 single mutants. 
(A) A silique (seed pod) of wild type (WT). (B) A silique of sol2-1. (C) A silique of sol2-2. (D) A tso1-3 silique. tso1-3 is in Ler background. (E) A tso1-3 silique in mixed Ler/Col background. (F) A gynoecium (a female reproductive organ) of tso1-5 that failed to develop into a silique. Note the abnormal gynoecium apical area (arrow). (G) A silique of tso1-5 (Col) formed later during plant development. (H) A silique of tso1-1; amiRTSO1. (I) A WT gynoecium consists of two fused carpels at the time of anthesis. (J–O) Absence of silique formation and the persistence of abnormal gynoecium in tso1-1 (J), tso1-1/tso1-3 (K), tso1-3; sol2-1 (L), tso1-3; sol2-2 (M), tso1-5; sol2-1 (N), and tso1-5; sol2-2 (O). Bars in H–J: 200 µm. All photos in A–O (except I) are at the same magnification. Photos of A–C were compressed vertically by 30% to fit the figure height. It is important to note that the severe fertility defects observed for both class I and class II alleles have rather distinct causes. The complete sterility of class I alleles are due to failure of proper reproductive floral organ formation. Figure 3J and 3K showed tso1-1 and tso1-1/tso1-3 mutant gynoecium (female reproductive organ) consisting of unfused and abnormally formed carpels that expose rudimentary ovules at the apex. In contrast, the gynoecia of class II alleles are wild type-like, shown by the two perfectly fused carpels (Figure 3D–3H). The reduced seed sets in class II mutants apparently result from defects in male and female gametes. The size of the silique (seed pod) positively correlates with the number of seeds inside. tso1-1; amiRTSO1 has the smallest silique (Figure 2D, Figure 3H) and is completely sterile. This is followed by tso1-3 with 0-1 viable seed per small silique (Figure 3D), and finally by tso1-5 with 1–5 viable seeds per silique (Figure 3F–3G). tso1-5 plants at first appeared to have severe fertility defects (Figure 3F). However, siliques that developed later from the same shoot were longer and had more seeds (Figure 3G).
The distinct phenotypes between class I and class II alleles are not caused by different ecotype backgrounds as the class I alleles, tso1-1 and tso1-2, and the class II allele tso1-3 are all in the Ler background. However within class II, tso1-3 (Ler) is less fertile than tso1-5 (Col) (Figure 3D and 3G), even though both alleles cause TSO1 protein truncation after the first CXC domain. By crossing tso1-3 into the Col background, the fertility of tso1-3 became similar to tso1-5 (Figure 3E and 3G). Therefore, the extent of infertility of class II mutants could be influenced by the ecotype.
The tso1-1 Antimorphic Allele Is Intrinsically Recessive to the TSO1 Wild-Type Allele
Antimorphic alleles, also termed “dominant-negative” alleles, usually interfere with the function of their wild type alleles and are defined as dominant alleles [18], [19]. tso1-1 appears to violate this rule as it is recessive to its wild type allele, but at the same time antimorphic in nature. One hypothesis is that tso1-1 may act in a dosage-dependent manner, being recessive when tso1-1 equals wild type in dosage. A higher tso1-1 dosage may overcome TSO1 wild type allele and cause a mutant phenotype. An alternative hypothesis is that tso1-1 is not antimorphic to its wild type allele (thus recessive to the wild type) but rather antimorphic to other TSO1 redundant factors. To test these hypotheses, 35S::tso1-1 (full-length tso1-1 cDNA driven by the strong 35S promoter) was introduced into wild type (Ler) plants. Out of 76 T1 transgenic lines, none showed any mutant phenotypes. In the T2 generation, where 25% of 35S::tso1-1 plants should become homozygous for the transgene, still none showed any mutant phenotype. The transcript level of tso1-1 from four independent T2 35S::tso1-1 transgenic lines was assayed by RT-PCR and shown to be at a higher level than the endogenous TSO1 (Figure S1). These results suggest that the over-expressed tso1-1 mutant gene product was unable to cause a mutant phenotype when wild type TSO1 is present and that tso1-1 is recessive to wild type TSO1 irrespective of its dosage.
The above conclusion is further supported by the reciprocal experiment, where TSO1 wild type cDNA was over-expressed in tso1-1 plants under the control of the 35S promoter. Specifically, 35S::TSO1-GFP transgene was introduced into tso1-1/+ plants. Through genotyping in T1 generation, four tso1-1; 35S::TSO1-GFP lines were identified. Three lines were completely rescued and are indistinguishable from wild type. The remaining one was not rescued probably due to positional effect of the transgene. Combined, our data suggest that tso1-1 readily succumbs to TSO1 wild type allele irrespective of its dosage to the wild type TSO1 and that tso1-1 only exerts its effect when wild type TSO1 is absent.
In tso1-1/tso1-3 (classI/classII) transheterozygotes, the amount of tso1-1 mutant protein is at 50% of the tso1-1/tso1-1 plants and the abnormal floral organ, fertility and meristem phenotypes are similar but milder than tso1-1 homozygotes (compare Figure 1B with 1E, and Figure 3J with 3K), suggesting that tso1-1 acts in a dosage-dependent fashion to interfere with some unknown factor(s) to yield the floral and meristem phenotype.
T-DNA Insertions in SOL1 and SOL2 Genes Caused No Phenotypic Defects
Possible redundant factors that tso1-1 interferes with could be the two most closely related TSO1 homologs, SOL1 and SOL2. SOL2 is the most likely candidate as it is expressed in a highly similar pattern to TSO1 with the only exception of pollen and carpel tissues, where SOL2 is absent [6]. It would explain why tso1 class II alleles never exhibit any defects in flowers and inflorescence meristems, as defects in these tissues are “masked” by the redundantly acting SOL2.
First, we characterized single mutants of SOL1 and SOL2. Two different T-DNA insertion lines for each gene were obtained from the ABRC stock center (Figure 4A). Real-time RT-PCR with gene specific primers demonstrated that sol2 alleles have a reduced SOL2 expression at about 20% of the wild type level (Figure 4B), and thus they may be loss-of-function alleles. On the other hand, two different sol1 alleles, sol1-1 and sol1-2, showed opposite effects on SOL1 expression (Figure 4C). In sol1-1, SOL1 expression was increased about three fold when compared to the wild type, most likely as a result of the T-DNA insertion in the SOL1 promoter (Figure 4A). In contrast, SOL1 expression was reduced to about 30% of the wild type level in sol1-2, and could represent a loss-of-function allele (Figure 4C). Although the expressions of SOL1 and SOL2 genes in corresponding T-DNA lines were dramatically changed, the mutant plants look indistinguishable from the wild type (Col) plant (Figure 5B–5E).
Fig. 4. Analyses of T-DNA insertion lines in TSO1, SOL1, and SOL2. 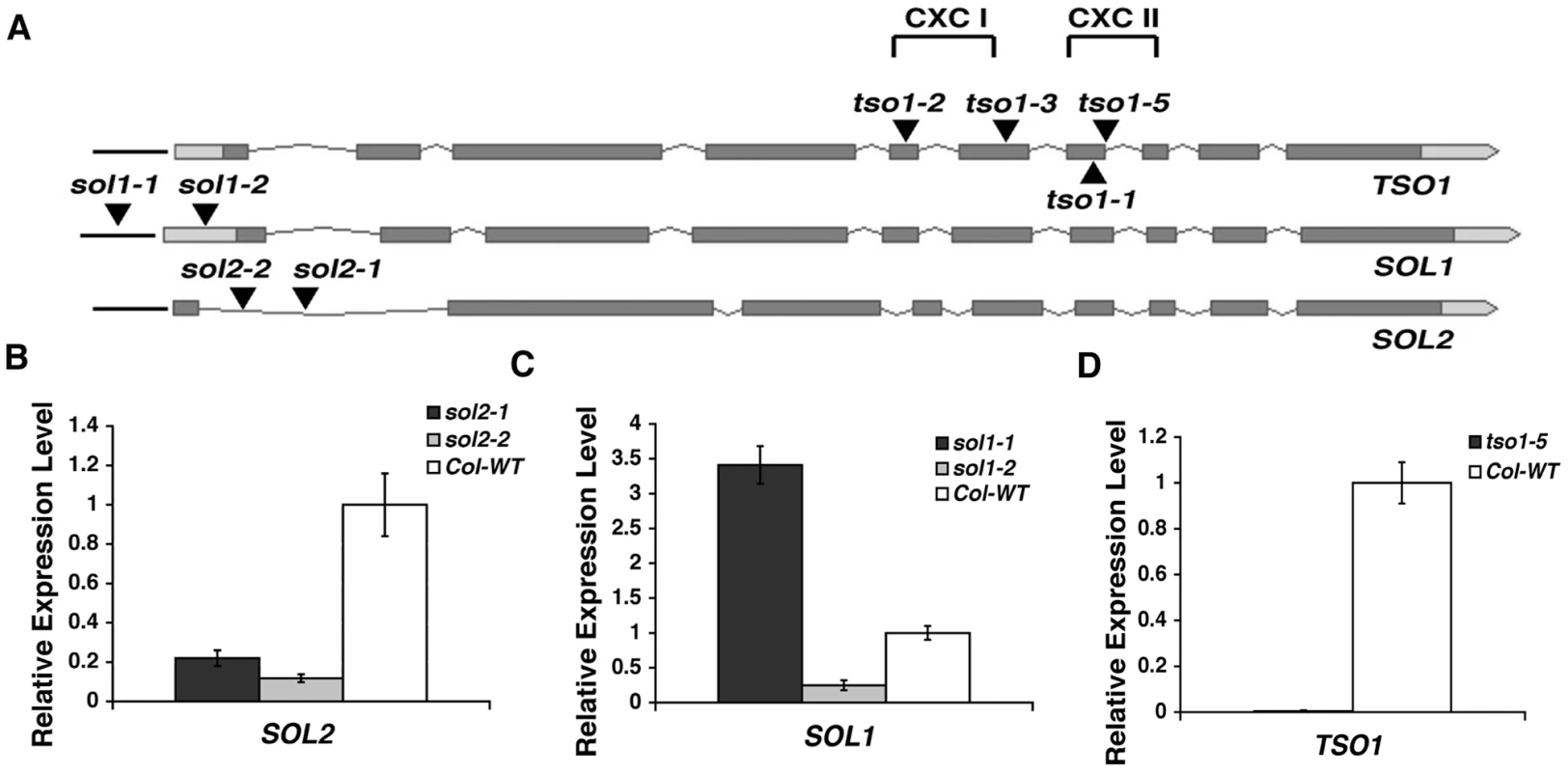
(A) Positions of mutations in TSO1, SOL1, and SOL2. Full-length genomic sequences, including promoters and 5′ and 3′ UTRs, are shown. Dark boxes represent exons. Light boxes indicate the UTRs. Grey thin lines represent introns. Black lines represent promoters. Black-filled triangles mark the position of T-DNA insertions or tso1 alleles. tso1-5 (Salk_102956) T-DNA is inserted in the seventh intron very close to the exon-intron boundary. sol1-1 (Salk_007957) is inserted in the SOL1 promoter. sol1-2 (Salk 013686) is inserted in the SOL1 5′UTR. sol2-1 (Sail 78_A12) and sol2-2 (Salk_021952) are both inserted in the first intron of SOL2. tso1-2 missense allele mutates a conserved cysteine in the first CXC domain. tso1-3 is a nonsense mutation in the hinge region between the two CXC domains. tso1-1 mutates a conserved cysteine located in the second CXC domain. (B–D) Real time RT-PCR analysis of SOL2, SOL1, and TSO1. Standard deviations were calculated based on two biological replicates, each with three technical replicates. The wild type level for each gene was assigned as value 1. Fig. 5. sol2, but not sol1, showed synergistic genetic interactions with tso1 class II alleles. 
Each panel consists of the top portion showing fertility phenotypes and bottom portion showing flower and inflorescence phenotypes. (A) tso1-1. (B–E) Fully fertile siliques and morphologically normal inflorescences developed in sol1-1 (B), so1-2 (C), sol2-1 (D), and sol2-2 (E) single mutants. (F) A sol1-2; amiRTSO1 inflorescence showing wild type phenotype. (G–J) tso1; sol2 double mutant plants showing an absence of silique and abnormal inflorescences of tso1-3; sol2-1 (G), tso1-3; sol2-2 (H), tso1-5; sol2-1 (I), and tso1-5; sol2-2 (J). We also characterized tso1-5, a T-DNA line inserted in the seventh intron of TSO1 gene near the exon-intron boundary (Figure 4A). Consistent with the previous report [6], real-time RT-PCR analysis showed undetectable levels of TSO1 mRNA in tso1-5 (Figure 4D).
sol2 But Not sol1 Showed Synergistic Genetic Interactions with tso1 Class II Alleles
To determine if SOL1, SOL2, or both encode the redundant factor(s), we aimed to construct double mutants between tso1 class II alleles and sol1 or sol2 loss-of-function alleles. Since SOL1 (At3g22760) and TSO1 (At3g22780) are closely linked on chromosome 3 (only 1545 bp apart and with one gene, At3g22770, in between), we couldn't construct double sol1 tso1 mutants. Instead, we knocked down TSO1 by crossing the amiRTSO1 into sol1-2, which has reduced SOL1 transcripts due to a T-DNA insertion at the 5′UTR (Figure 4A, 4C). The sol1-2; amiRTSO1 plants showed a wild type phenotype (Figure 5F) even though the amiRTSO1 caused a significant reduction of TSO1 (at about 11% of the wild type level) in the sol1-2; amiRTSO1 plant (Figure S2). This result suggests that SOL1 is unlikely a redundant factor of TSO1.
To test if SOL2 encodes a redundant factor for TSO1, we constructed sol2; tso1 double mutants by crossing sol2 loss-of-function alleles into the tso1 class II loss-of-function alleles, tso1-3 and tso1-5. If tso1-1 acts to interfere with the function of SOL2 then sol2; tso1-3 or sol2; tso1-5 double mutants should resemble tso1-1 mutants (Figure 5A). Genotyping identified tso1-3; sol2-1 and tso1-3; sol2-2 double mutants in F2, which showed severe morphological abnormalities in floral organs similar to those of tso1-1 (compare Figure 5A with Figure 5G–5H). The tso1-3; sol2 double mutants are completely sterile bearing no silique nor seeds. Instead, gynoecium with unfused carpels was formed (compare Figure 3B–3E, 3J–3K with Figure 3L–3M). Similar fertility and floral morphology defects were also observed in tso1-5; sol2-1 and tso1-5; sol2-2 double mutant plants (Figure 5I–5J, compare Figure 3B–3C, 3F–3G, 3J–3K with Figure 3N–3O), which were slightly weaker than those of tso1-3; sol2 double mutants.
Nevertheless, the tso1-3; sol2 and tso1-5; sol2 double mutants rarely showed meristem fasciation defects, which are typically seen in tso1 class I alleles. It is likely that additional redundant factor(s) may need to be knocked down in tso1-3; sol2 mutants to fully manifest the tso1-1 phenotype. Alternatively, the sol2 alleles used in the study may still retain some residual function, as 20% of the SOL2 transcript is still present in sol2 mutants (Figure 4B).
Biomolecular Fluorescence Complementation (BiFC) Assay Detected Direct Interaction between tso1-1 and SOL2
Our genetic data above strongly suggest that TSO1 and SOL2 act redundantly during flower and, less so, meristem development. Only when the function of both genes is compromised, either through antimorphic tso1-1 or by double knockdown, the class I phenotype can be revealed. To further investigate the molecular mechanisms underlying this genetic interaction, we tested direct physical interactions among TSO1, tso1-1, and SOL2 using BiFC. TSO1, tso1-1, and SOL2 cDNAs were fused in frame to the YFP N-terminal (YN) or the YFP C-terminal (YC) fragments. Pairs of YN and YC fusion constructs were co-infiltrated into the leaf epidermis of Nicotiana benthamiana. Direct interactions between the YN and YC fusion proteins can be detected by the YFP reconstitution and yellow fluorescence. YC-EER5 and YN-SAC3B are nuclear proteins, serving as negative controls in combination with test proteins. Wild type TSO1 was able to interact with itself in nuclei (compare Figure 6A–6B with Figure 6C) but was unable to interact with either SOL2 (Figure 6H) or tso1-1 (Figure 6D). This suggests that TSO1 may act independently of SOL2, for example, by acting in a different complex from SOL2. The absence of interaction between TSO1 and tso1-1 (Figure 6D) excludes the possibility of tso1-1 interfering with TSO1 through direct binding. Interestingly, strong nuclear YFP fluorescence was observed when YN-SOL2 and YC-tso1-1 were co-infiltrated (compare Figure 6E–6F with Figure 6G). This suggests a possible mechanism of tso1-1 sequestering or blocking SOL2 from performing SOL2 normal function in nucleus.
Fig. 6. BiFC analyses showing TSO1 to TSO1 as well as tso1-1 to SOL2 interactions. 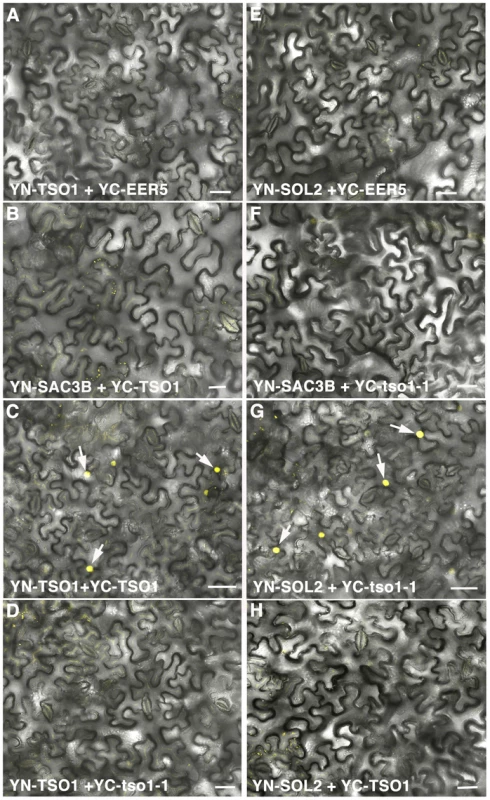
Interactions were detected by YFP reconstitution between the YN and YC fusion proteins, leading to yellow fluorescence shown by single confocal section images overlaid with Nomarsky differential interference contrast (DIC) images. YC-EER5 and YN-SAC3B nuclear proteins serve as negative controls as they function in unrelated processes from TSO1 or SOL2 [39]. Arrows point to nuclei expressing YFP fluorescence. Scale bars represent 50 µm. (A–B) Negative control combination of YN-TSO1 with YC-EER5 (A), or YN-SAC3B with YC-TSO1 (B). (C) Positive interaction between YN-TSO1 and YC-TSO1 indicated by fluorescent nuclei (arrows). (D) An absence of interaction between YN-TSO1 and YC-tso1-1 indicated by an absence of fluorescent signals. (E–F) Negative control combination of YN-SOL2 with YC-EER5 (E), or YN-SAC3B with YC-tso1-1 (F). (G) in vivo interaction between YN-SOL2 and YC-tso1-1 shown by fluorescent nuclei (arrows). (H) An absence of interaction between YN-SOL2 and YC-TSO1. Discussion
We observed and characterized two classes of tso1 alleles that exhibit dramatically different phenotypes. While the class I tso1 alleles, exemplified by the missense mutations of conserved cysteine residues in the CXC domain, develop abnormal floral organs and exhibit meristem fasciation, the class II tso1 alleles, represented by the tso1 nonsense allele and the T-DNA insertion allele, do not show any such defects in floral organ morphology or meristem fasciation, but rather they develop small siliques with reduced seed set. We showed that the class I phenotype can be suppressed and converted into the class II phenotype by artificial microRNA knockdown of the tso1 mutant transcript in class I mutants. This suggests that the class I alleles yielded antimorphic mutant products that were removed by the artificial microRNA and that the class II tso1 alleles are null or near-null alleles.
Antimorphic Alleles May Target Related Loci
Classical antimorphic alleles are only known to interfere with the wild type function at the same locus and are dominant over wild type [18], [19]. Here we show that an antimorphic allele can also interfere with the function of different loci with redundant functions. Such antimorphic alleles could serve as a powerful tool in the identification of gene function coded by functionally redundant gene families. We proposed that the antimorphic tso1-1 interferes with SOL2 and possibly other TSO1 family members. By removing functionally redundant factors, the tso1-1 antimorphic allele reveals a broader spectrum of TSO1 functions that are otherwise masked by the presence of redundant genes.
Our finding of SOL2 instead of SOL1 encoding the redundant factor is consistent with the highly similar tissue expression patterns between TSO1 and SOL2 throughout the plant except that SOL2 is not expressed or is expressed at a low level in pollen and ovule. Therefore, tso1 loss-of-function or null alleles only exhibit fertility defects due to an absence of SOL2 expression during the development of male and female gametes. On the other hand, SOL1 is predominantly expressed in all stages of pollen development, yet tso1 class II alleles still exhibit reduced male fertility supporting a non-redundant function between TSO1 and SOL1.
A Model on the Molecular Mechanism of tso1-1 Antimorphism
Currently, little is known about how TSO1 proteins function to regulate floral organ differentiation, meristem regulation, and gametophyte development. Based on the study of CHC-containing proteins in animal systems, TSO1 may function in a dREAM-like chromatin complex. Another important class of chromatin regulators, the Enhancer of zeste E(z) polycomb group proteins, contain two tandem CXC domains but lack the intervening hinge domain. Missense mutations of the conserved cysteine residue of CXC in the Drosophila E(z) proteins prevented the E(z)-containing complex (PRC2) from binding to polytene chromosomes [20], suggesting that one way tso1-1 could affect the chromatin complex is to impair its ability to bind DNA targets.
To gain insights into the molecular mechanism underlying class I tso1 antimorphism, we tested direct physical interaction among TSO1, tso1-1, and SOL2 using BiFC (Figure 6). While wild type TSO1 could interact with itself but not with SOL2, the antimorphic tso1-1 could no longer interact with wild type TSO1 but could interact strongly with SOL2. This suggests that tso1-1 may interfere with SOL2 function by direct binding and then disabling of SOL2. The model illustrated in Figure 7 provides one of several possible mechanisms, explaining different phenotypic outcomes in different tso1 genotypes. This model is proposed in the context of floral organ development where both TSO1 and SOL2 provide similar and redundant function in wild type (Figure 7A). In tso1-1/TSO1 heterozygotes (Figure 7B), a lack of physical interaction between tso1-1 and TSO1 excludes the possibility of tso1-1 interfering with TSO1 through direct binding. The presence of TSO1 wild type product is sufficient for the development of wild type flowers even when tso1-1 disables the SOL2. This is supported by the genetic dominance of wild type TSO1 over tso1-1 shown by the wild type phenotype of tso1-1/+, or 35S::tso1-1 (in wild type background), or 35S::TSO1-GFP (in tso1-1 background) plants. In tso1-1 (class I) plants (Figure 7C), both TSO1 and SOL2 complexes are nonfunctional due to an absence of wild type TSO1 and the inhibition of SOL2 by tso1-1. In tso1-3 (class II) mutants (Figure 7D), although wild type TSO1 is absent, the SOL2 complex provides sufficient function for normal flower development.
Fig. 7. A proposed model on the molecular mechanism of tso1-1 antimorphism in the context of flower development. 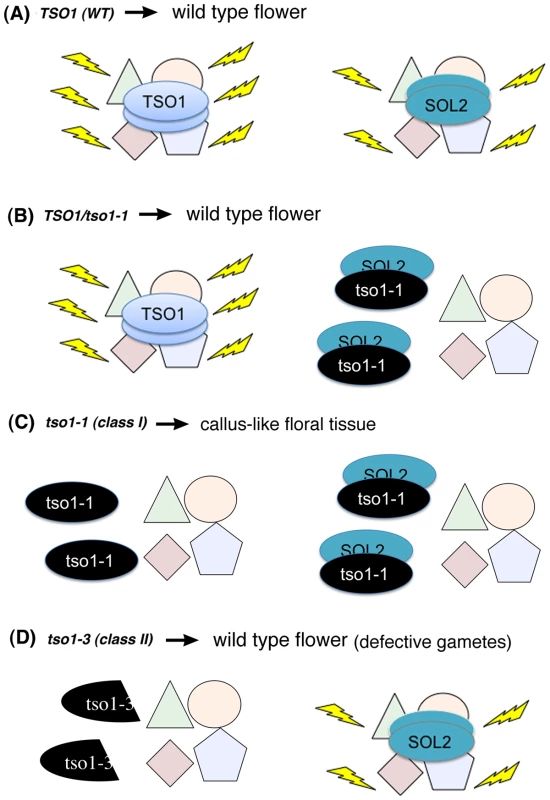
(A) In wild type (WT), TSO1 and SOL2 function as essential components of two independent, yet functionally redundant, chromatin complexes. Yellow flashes indicate functional complexes. (B) In tso1-1/TSO1 heterozygous plants, TSO1, at half of the wild type amount, is sufficient to confer wild type phenotype even in the presence of tso1-1, which completely or partially disables SOL2. (C) In tso1-1/tso1-1 (class I) mutants, both TSO1 and SOL2 are nonfunctional due to an absence of wild type TSO1 and the inhibition of SOL2 by tso1-1. (D) In tso1 class II mutants, such as tso1-3 or tso1-5, SOL2 is functional and compensates for a lack of TSO1, leading to the development of normal flowers. Recessive Antimorphic Alleles Are Likely Common in Arabidopsis
One might ask how common these recessive antimorphic alleles exist. Through our own work, as well as brief surveys of Arabidopsis literature, we found several cases similar to tso1-1. Two recessive missense alleles of BELLRINGER (BLR), blr-4 and blr-5, cause conserved amino acid change in the homeodomain and exhibit a phenotype of terminal carpelloid flowers [21] rarely observed in loss-of-function or null alleles [22]–[24]. blr-4 and blr-5 were proposed to interfere with other family members harboring redundant functions [21]. In a second case, three Arabidopsis genes encode the small subunit of the Ribonucleotide Reductase (RNR). tso2-1 (Ler) is a recessive missense mutation in one of these RNR genes, causing a strong flower and inflorescence phenotype [25]. In contrast, tso2-5, a T-DNA insertion at the N-terminal end of the TSO2 gene and thus a putative null, showed wild type phenotype (Wang and Liu, unpublished). In a third case, a recessive mutation of the Arabidopsis CORONA (CNA) gene, cna-1, located in a conserved domain of unknown function, showed a much stronger phenotype than a likely null allele, cna-2 [26]. In the above examples, the recessive missense alleles may interfere with the function of redundant factors to cause a different phenotype or exhibit a stronger phenotype than the corresponding null. In addition to recessive antimorphic alleles, there are many examples of semi-dominant or dominant missense alleles that act to interfere with the function of redundant factors. The clavatav1 (clv1) missense mutations bear striking parallels to the tso1 missense mutations [27]–[29]. The phenotypically medium to strong clv1 alleles were all missense alleles, while the weak clv1 alleles were all null or near-null. Co-suppression of clv1 missense alleles led to weakened phenotypes closely resembling the clv1 null [28]. The clv1 missense alleles were thought to interfere with a CLV1 homolog as well as with the wild type CLV1. In a second example, an unusual mutant allele of APETALA 2 (AP2), I28, exhibited a severe defect in shoot meristem development [30], which was observed in none of the previously characterized ap2 mutants. l28 causes a Glu to Lys change in the first AP2 domain that may be antimorphic by interfering with the function of a redundant factor, unmasking the function of AP2 in shoot meristems [30]. The Arabidopsis topless-1 (tpl-1) mutation transforms the shoot pole into a second root pole and tpl-1 is a dominant-negative mutation that interferes with the function of multiple TPL-related proteins in embryo development [31]. These examples illustrate the advantage of using antimorphic alleles, irrespective recessive or dominant, to unveil the role of functionally redundant gene family members.
We propose that the distinction between “recessive” and “dominant” antimorphic alleles resides in whether the antimorphic allele interferes with its wild type allele. If it does, dominant or semi-dominant effect results. If it does not, as shown for tso1-1, recessive effect results. Whether “recessive” or “dominant”, the antimorphysm is not limited to interfering with its own locus but also with related loci.
Broader Implications
Our findings have several important implications. First, null or near-null alleles, such as nonsense or T-DNA insertion alleles, are not necessarily always effective in revealing full gene functions when compared with missense mutations. Cautions should be exercised in making conclusions based on null alleles, especially those whose defective genes belong to gene families. While class I antimorphic alleles are able to reveal the range of TSO1 functions, the class II severe hypomorph or near-null alleles only reveal a subset of TSO1 function not complemented by the redundant factors. Second, antimorphic alleles can sometimes be recessive. In another word, not all antimorphic alleles are dominant or semi-dominant as defined in classical genetic analyses of Drosophila [18], [19]. Both dominant and recessive antimorphic alleles may interfere with the function of genes belonging to the same family. Third, our data challenge the conventional view that recessive alleles are always simple loss-of-function or null alleles. A different scenario illustrated in this study suggests that recessive alleles could also be antimorphic. Thus, alternative strategies aimed at eliminating rather than rescuing a genetic defect should be considered in ameliorating genetic abnormalities or diseases caused by recessive missense mutations.
Our work is potentially highly relevant to the study and interpretation of human genetic diseases. One example could be the wide spectrum of human diseases caused by mutations in the human A type lamin (LMNA) [32]. More than 10 different clinical syndromes including diseases of striated muscle, lipodystrophy syndromes, peripheral neuropathy, or accelerated aging are caused by various mutations in the LMNA gene. The striated muscle phenotype appears to be sensitive to reduced expression of LMNA and may represent hypomorphic alleles, while other symptoms might result from specific missense or splicing mutations that could lead to antimorphic LMNA proteins that interfere with LMNB or LMNC function. Our study reveals the strength of Arabidopsis as a genetic model whose gene number and genetic architecture are more appropriate for studying complex species like human, which is rich in low-copy repeats and paralogous segmental duplications (5%–10% of the human genome) [33]. Other models, such as Drosophila and C. elegans, have reduced gene sets, and thus reduced likelihood of discovering phenomena such as the recessive antimorphism discussed here.
Materials and Methods
Plant Growth, Mutant Strains, and Genetics
Arabidopsis thaliana plants were grown on Metromix soil (Griffin) under a 16 hour light-8 hour dark cycle at 20°C. tso1-1 and tso1-3 in Landsberg electra (Ler) background were previously described [3]–[5], [34]. tso1-1 and tso1-3 genotyping was done by standard PCR using primers listed in the Table S1. Since tso1-1 is 100% sterile, it is maintained as tso1-1+/+sup-5 (Ler/Ler) heterozygote that was transformed with all constructs described.
The following T-DNA insertion lines (in Columbia; Col background) were obtained from ABRC stock center: Salk_102956 (tso1-5, At3g22780), Salk_007957 (sol1-1, At3g 22760), Sail 742_H03 (sol1-2, At3g22760), Sail 78_A12 (sol2-1, At4g14770), and Salk_021952 (sol2-2, At4g14770). The genotyping of the T-DNA insertion lines was performed using standard PCR with primer pairs listed in Table S1. Conditions for standard PCR reaction were: 95°C for 3 minutes (min), followed by 35 cycles of 94°C for 30 seconds (s), 55°C for 30 s, 72°C for 75 s, and 72°C for 7 min.
Gene Expression Studies
Total RNA was isolated from inflorescences of wild type, tso1-1, sol1-1, sol1-2, sol2-1, sol2-2, transgenic 2044 amiRTSO1 (tso1-1), 2044 amiRTSO1(Ler); sol1-2, and 35S::tso1-1 (Ler) plants using RNeasy Plant Mini Kit (Qiagen Inc, Valencia CA, USA). First-strand cDNA was synthesized from 1 µg of total RNA using QuantiSure™ First-strand cDNA Kit (Accugen Biosciences, Rockville MD, USA). 1 µl of 10X diluted cDNA was used as a template in real-time and RT-PCR analysis. iQ™ SYBR® Green Supermix (Bio-Rad Laboratories, CA, USA) was used to set up real-time PCR reactions, which were run and analyzed on CFX96 Real-Time System (Bio-Rad Laboratories). Conditions for real-time PCR were as follows: 95°C for 3 min, followed by 40 cycles of 94°C for 15 s, 60°C for 15 s, 72°C for 30 s. Melting curve analysis was performed from 65°C to 95°C with increments of 0.5°C every 5 seconds. Gene specific primers and corresponding real-time PCR efficiencies for each primer pair are listed in Table S2. Primers used to test T-DNA lines were designed to detect transcripts 3′ of the insertion. The housekeeping gene GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE C SUBUNIT 1 (GAPC1, At3g04120) was used as a reference gene in all real-time PCR reactions. The Pfaffl formula 2−ΔΔCt [35] was used to calculate relative gene expression differences. ΔCt for every mutant equals CtMUTANT - CtGAPC1. Correspondingly, ΔCtWT = CtWT − CtGAPC1. ΔΔCt was calculated as ΔCtMUTANT - ΔCtWT.
For 35S::tso1-1 (Ler) RT-PCR analysis (Figure S1), endogenous TSO1 transcripts and the tso1-1 transcripts were assayed on four individual T2 lines using TSO1-specific and tso1-1 transgene-specific primers (Table S2). The PCR conditions were as follows: 95°C for 3 min, followed by 26 (or 28) cycles of 94°C for 30 s, 55°C (or 60°C for endogenous TSO1) for 30 s, 72°C for 60 s, and 72°C for 7 min.
Plasmid Constructions
Using Web MicroRNA Designer, version 2 (WMD 2, http://wmd2.weigelworld.org/cgi-bin/mirnatools.pl) [36], the microRNA sequence TAATGCTGGAATAGACCGTAC that targets 3′ end of TSO1 gene (at position 2044 bp of 2088 bp full length) was chosen to make 2044amiRTSO1. The primers used to construct 2044amiRTSO1 were:
-
mir-s: gaTAATGCTGGAATAGACCGTACtctctcttttgtattcc,
-
mir-a: gaGTACGGTCTATTCCAGCATTAtcaaagagaatcaatga,
-
mir*s: gaGTCCGGTCTATTCGAGCATTTtcacaggtcgtgatatg, and
-
mir*a: gaAAATGCTCGAATAGACCGGACtctacatatatattcct. pRS300 plasmid was used as a DNA template. Conditions for the PCR reaction were: 95°C for 3 min, followed by 30 cycles of 94°C for 30 s, 55°C for 30 s, 72°C for 75 s, and 72°C for 7 min.
The final PCR product was first cloned into pCR8/GW/TOPO using TA cloning kit (Invitrogen, Carsbad, CA, USA) and then introduced into the pEarleyGate100 plant transformation vector [37] using the Gateway technology (Invitrogen).
For constructing 35S::TSO1-GFP, pAVA120 containing GFP fused to the C-terminus of TSO1 [3] was cut with PstI. The released fragment was cloned into the PstI site in the pCGN1547 binary vector.
To construct 35S::tso1-1, total RNA was isolated from tso1-1 inflorescences and cDNA was produced as described above. 1 µl of 10x diluted cDNA was used as a template in PCR reaction. Phusion High-Fidelity PCR kit (New England Biolabs, USA) was used for tso1-1 cDNA amplification using gene-specific primers (Table S2). PCR conditions were as follows: 95°C for 3 min, followed by 25 cycles of 94°C for 30 s, 60°C for 30 s, 72°C for 90 s, and 72°C for 7 min. The resulting PCR fragment was cloned into pCR8/GW/TOPO (Invitrogen) and sequenced to verify the presence of the tso1-1 mutation. The tso1-1 cDNA was then introduced into the pEarleyGate100 binary vector [37].
For BiFC constructs, gene specific primers containing SpeI restriction sites (Table S2) were used to amplify TSO1, TSO1-1, and SOL2 cDNAs and then cloned into pCR8/GW/TOPO (Invitrogen). After verification by sequencing, inserts were released with SpeI and cloned into the SpeI site in the pCAMBIA2300 binary vector-based BiFC vectors, pSY736 and pSY735 [38]. Genes were fused in frame and downstream of the N-terminal (YN) or the C-terminal (YC) fragment of YFP, driven by the CaMV 35S promoter, and terminated by the NOS 3′ terminator. Two negative control plasmids YC-EER5 and YN-SAC3B were obtained from Jennifer Shemansky and Caren Chang. EER5 (also named AtTHP1) was previously shown to interact with SAC3B in nuclei via BiFC [39].
Biomolecular Fluorescence Complementation (BiFC) Assay
BiFC constructs were independently introduced into Agrobacterium tumefaciens strain C58C1 by electroporation. Agrobacterium cultures were spun down and resuspended at an OD600 of 0.4 in the tobacco infiltration media (10mM MgCl, 10mM MES, 100 µM Acetosyringone). Agrobacterium containing YN - or YC - fusion plasmids were mixed in equal parts and infiltrated into the leaves of 3–4 week old Nicotiana benthamiana based on a video (http://www.plantsci.cam.ac.uk/research/baulcombe/movies/agroInfil1.mpg), as well as published procedures [40]. The plants were returned to growth chamber at 25°C, 16 hr light/8 hr dark. After 48 hours, leaf sectors were placed on slides and examined under the Leica SP5X confocal laser scanning microscope with the 20x water immersion objective. YFP was visualized by excitation with an argon laser at 514 nm.
Plant Transformation and Analysis of Transgenic Plants
Constructs were introduced into Agrobacterium tumefaciens GV3101 by electroporation. The corresponding Agrobacterium was used to transform Arabidopsis thaliana wild-type (Ler) and tso1-1 heterozygous (tso1-1+/+sup-5) plants via floral dip. Primary transformants were selected on soil using 1∶3000 diluted BASTA herbicide (Liberty 200). 76 35S::tso1-1(WT) T1 plants were generated and analyzed for phenotypic changes. Four transgenic lines were further analyzed at the T2 generation.
For 2044 amiRTSO1, 43 T1 plants were obtained from transforming tso1-1+/+sup-5 plants. Detailed analysis was conducted on 14 T1 plants that were confirmed to contain the transgene. Among these 14 plants, five were homozygous for tso1-1, four were tso1-1 heterozygotes, and five were wild type for TSO1 and homozygous for sup-5. In addition, 63 T1 plants were obtained and analyzed from 2044 amiRTSO1 transformed into wild type (Ler) plants.
35S::TSO1-GFP was introduced into tso1-1+/+sup-5 plants and 29 T1 transformants were selected on kanamycin (50 µg/ml) plates and analyzed for the presence of GFP by standard PCR. Three out of four independent lines homozygous for tso1-1 and positive for the transgene were found to completely rescue the tso1-1phenotype.
Supporting Information
Zdroje
1. TheissenGSaedlerH 2001 Plant biology. Floral quartets. Nature 409 469 471
2. KrizekBAFletcherJC 2005 Molecular mechanisms of flower development: an armchair guide. Nat Rev Genet 6 688 698
3. SongJYLeungTEhlerLKWangCLiuZ 2000 Regulation of meristem organization and cell division by TSO1, an Arabidopsis gene with cysteine-rich repeats. Development 127 2207 2217
4. LiuZRunningMPMeyerowitzEM 1997 TSO1 functions in cell division during Arabidopsis flower development. Development 124 665 672
5. HauserBAHeJQParkSOGasserCS 2000 TSO1 is a novel protein that modulates cytokinesis and cell expansion in Arabidopsis. Development 127 2219 2226
6. AndersenSUAlgreen-PetersenRGHoedlMJurkiewiczACvitanichC 2007 The conserved cysteine-rich domain of a tesmin/TSO1-like protein binds zinc in vitro and TSO1 is required for both male and female fertility in Arabidopsis thaliana. J Exp Bot 58 3657 3670
7. CvitanichCPallisgaardNNielsenKAHansenACLarsenK 2000 CPP1, a DNA-binding protein involved in the expression of a soybean leghemoglobin c3 gene. Proc Natl Acad Sci U S A 97 8163 8168
8. SutouSMiwaKMatsuuraTKawasakiYOhinataY 2003 Native tesmin is a 60-kilodalton protein that undergoes dynamic changes in its localization during spermatogenesis in mice. Biol Reprod 68 1861 1869
9. SugiharaTWadhwaRKaulSCMitsuiY 1999 A novel testis-specific metallothionein-like protein, tesmin, is an early marker of male germ cell differentiation. Genomics 57 130 136
10. OlesenCMollerMByskovAG 2004 Tesmin transcription is regulated differently during male and female meiosis. Mol Reprod Dev 67 116 126
11. BeallELManakJRZhouSBellMLipsickJS 2002 Role for a Drosophila Myb-containing protein complex in site-specific DNA replication. Nature 420 833 837
12. JiangJBensonEBausekNDoggettKWhite-CooperH 2007 Tombola, a tesmin/TSO1-family protein, regulates transcriptional activation in the Drosophila male germline and physically interacts with always early. Development 134 1549 1559
13. BeallELLewisPWBellMRochaMJonesDL 2007 Discovery of tMAC: a Drosophila testis-specific meiotic arrest complex paralogous to Myb-Muv B. Genes Dev 21 904 919
14. LewisPWBeallELFleischerTCGeorletteDLinkAJ 2004 Identification of a Drosophila Myb-E2F2/RBF transcriptional repressor complex. Genes Dev 18 2929 2940
15. KorenjakMTaylor-HardingBBinneUKSatterleeJSStevauxO 2004 Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell 119 181 193
16. LitovchickLSadasivamSFlorensLZhuXSwansonSK 2007 Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell 26 539 551
17. TabuchiTMDeplanckeBOsatoNZhuLJBarrasaMI Chromosome-Biased Binding and Gene Regulation by the Caenorhabditis elegans DRM Complex. PLoS Genet 7 e1002074 doi:10.1371/journal.pgen.1002074
18. MullerHJ 1932 Further studies on the nature and causes of gene mutations. Proceedings of the 6th International Congress of Genetics 213 255
19. WilkieAO 1994 The molecular basis of genetic dominance. J Med Genet 31 89 98
20. CarringtonEAJonesRS 1996 The Drosophila Enhancer of zeste gene encodes a chromosomal protein: examination of wild-type and mutant protein distribution. Development 122 4073 4083
21. BaoXFranksRGLevinJZLiuZ 2004 Repression of AGAMOUS by BELLRINGER in floral and inflorescence meristems. Plant Cell 16 1478 1489
22. ByrneMEGrooverATFontanaJRMartienssenRA 2003 Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELLRINGER. Development 130 3941 3950
23. SmithHMHakeS 2003 The interaction of two homeobox genes, BREVIPEDICELLUS and PENNYWISE, regulates internode patterning in the Arabidopsis inflorescence. Plant Cell 15 1717 1727
24. RoederAHFerrandizCYanofskyMF 2003 The role of the REPLUMLESS homeodomain protein in patterning the Arabidopsis fruit. Curr Biol 13 1630 1635
25. WangCLiuZ 2006 Arabidopsis ribonucleotide reductases are critical for cell cycle progression, DNA damage repair, and plant development. Plant Cell 18 350 365
26. GreenKAPriggeMJKatzmanRBClarkSE 2005 CORONA, a member of the class III homeodomain leucine zipper gene family in Arabidopsis, regulates stem cell specification and organogenesis. Plant Cell 17 691 704
27. ClarkSEWilliamsRWMeyerowitzEM 1997 The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell 89 575 585
28. DievartADalalMTaxFELaceyADHuttlyA 2003 CLAVATA1 dominant-negative alleles reveal functional overlap between multiple receptor kinases that regulate meristem and organ development. Plant Cell 15 1198 1211
29. ClarkSERunningMPMeyerowitzEM 1993 CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397 418
30. WurschumTGross-HardtRLauxT 2006 APETALA2 regulates the stem cell niche in the Arabidopsis shoot meristem. Plant Cell 18 295 307
31. LongJAOhnoCSmithZRMeyerowitzEM 2006 TOPLESS regulates apical embryonic fate in Arabidopsis. Science 312 1520 1523
32. WormanHJBonneG 2007 “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 313 2121 2133
33. StankiewiczPLupskiJR 2002 Genome architecture, rearrangements and genomic disorders. Trends Genet 18 74 82
34. HauserBAVillanuevaJMGasserCS 1998 Arabidopsis TSO1 regulates directional processes in cells during floral organogenesis. Genetics 150 411 423
35. LivakKJSchmittgenTD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402 408
36. SchwabROssowskiSRiesterMWarthmannNWeigelD 2006 Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18 1121 1133
37. EarleyKWHaagJRPontesOOpperKJuehneT 2006 Gateway-compatible vectors for plant functional genomics and proteomics. Plant J 45 616 629
38. Bracha-DroriKShichrurKKatzAOlivaMAngeloviciRYalovskySOhadN 2004 Detection of protein-protein interactions in plants using bimolecular fluorescence complementation. Plant J 40 419 427
39. LuQTangXTianGWangFLiuKNguyenVKohalmiSEKellerWATsangEWHaradaJJRothsteinSJCuiY 2010 Arabidopsis homolog of the yeast TREX-2 mRNA export complex: components and anchoring nucleoporin. The Plant J 61 259 270
40. BrachaKLavyMYalovskyS 2002 The Arabidopsis AtSTE24 is a CAAX protease with broad substrate specificity. J Biol. Chem 277 29856 29864
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
-
Všechny články tohoto čísla
- De Novo Origins of Human Genes
- Duplication Hotspots Are Associated with Late-Replicating Regions of the Genome
- De Novo Origin of Human Protein-Coding Genes
- Cyclin D/CDK4 and Cyclin E/CDK2 Induce Distinct Cell Cycle Re-Entry Programs in Differentiated Muscle Cells
- Short Day–Mediated Cessation of Growth Requires the Downregulation of AINTEGUMENTALIKE1 Transcription Factor in Hybrid Aspen
- Physiological IRE-1-XBP-1 and PEK-1 Signaling in Larval Development and Immunity
- Role of Pirh2 in Mediating the Regulation of p53 and c-Myc
- Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution
- FOXO Regulates Organ-Specific Phenotypic Plasticity In
- Heritable Epigenetic Variation among Maize Inbreds
- Foxn1 Regulates Lineage Progression in Cortical and Medullary Thymic Epithelial Cells But Is Dispensable for Medullary Sublineage Divergence
- Attenuation of the Sensing Capabilities of PhoQ in Transition to Obligate Insect–Bacterial Association
- A Novel Protein LZTFL1 Regulates Ciliary Trafficking of the BBSome and Smoothened
- Activation of Bmp2-Smad1 Signal and Its Regulation by Coordinated Alteration of H3K27 Trimethylation in -Induced Senescence
- Histone H3K56 Acetylation, CAF1, and Rtt106 Coordinate Nucleosome Assembly and Stability of Advancing Replication Forks
- The SUN Protein Mps3 Is Required for Spindle Pole Body Insertion into the Nuclear Membrane and Nuclear Envelope Homeostasis
- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- Effect of Host Species on the Distribution of Mutational Fitness Effects for an RNA Virus
- Pch2 Acts through Xrs2 and Tel1/ATM to Modulate Interhomolog Bias and Checkpoint Function during Meiosis
- SOX9 Governs Differentiation Stage-Specific Gene Expression in Growth Plate Chondrocytes via Direct Concomitant Transactivation and Repression
- from the Aphid : A Missing Link from Facultative to Obligate Insect Endosymbiont
- Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal Function in Flowers and Meristems
- Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects
- Consequences of Eukaryotic Enhancer Architecture for Gene Expression Dynamics, Development, and Fitness
- Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- For Male , Sperm Activation Is a “Just-in-Time” Event
- PcG Complexes Set the Stage for Epigenetic Inheritance of Gene Silencing in Early S Phase before Replication
- The Gene Contains Hotspots for L1 Endonuclease-Dependent Insertion
- Relative Burden of Large CNVs on a Range of Neurodevelopmental Phenotypes
- Multiple Means to the Same End: The Genetic Basis of Acquired Stress Resistance in Yeast
- Genome-Wide Crossover Distribution in Meiosis Reveals Sex-Specific Patterns along Chromosomes
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
- Homologs of Retinoblastoma-Associated Protein 46/48 Associate with a Histone Deacetylase to Act Redundantly in Chromatin Silencing
- Genetic Interaction Maps in Reveal Functional Crosstalk among Cell Envelope Biogenesis Pathways
- The ERI-6/7 Helicase Acts at the First Stage of an siRNA Amplification Pathway That Targets Recent Gene Duplications
- PBX1 Genomic Pioneer Function Drives ERα Signaling Underlying Progression in Breast Cancer
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- De Novo Origins of Human Genes
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



