-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
For Male , Sperm Activation Is a “Just-in-Time” Event
article has not abstract
Published in the journal: . PLoS Genet 7(11): e32767. doi:10.1371/journal.pgen.1002392
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002392Summary
article has not abstract
Sex-Specific Sperm Activation?
In the game of evolutionary fitness, males must maximize the chance that their sperm successfully fertilize oocytes. Species-specific strategies include making more, bigger, or faster sperm, or producing seminal fluid that does more than serve as a vehicle for sperm transfer [1]. Importantly, seminal fluid components not only modulate sperm function and promote their competitiveness and long-term viability, but also initiate various physiological changes within the female such as increasing her rate of ovulation and decreasing her receptivity to other males [2]–[4]. Adding to this complexity, ejaculate is often generated in a series of compositionally distinct spurts resulting from the sequential emptying of various sexual glands [2], [5]. Yet how most seminal proteins function, particularly in the context of sperm activation (Figure 1A), remains unclear.
Fig. 1. Sperm activation in C. elegans. 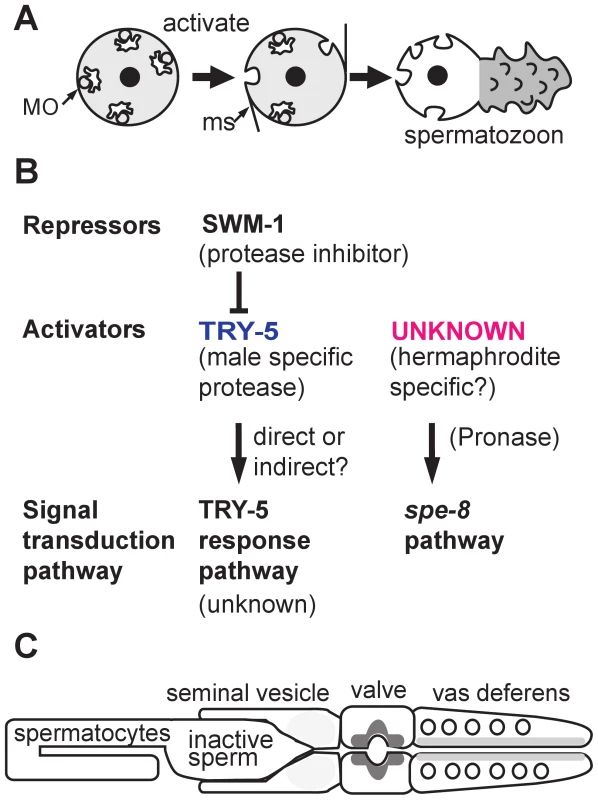
(A) Sperm activation involves the initial formation of microspikes, the fusion of membraneous organelles (MOs) with the plasma membrane, and the extension of motile pseudopods. (B) In males, SWM-1 prevents precocious activation of stored sperm by inhibiting the male-specific, extracellular factor TRY-5. Active TRY-5 triggers sperm activation through an unknown signal transduction pathway. In hermaphrodites, sperm are activated via an unknown activator that acts through the spe-8 signal transduction pathway. Both male and hermaphrodite sperm possess the two distinct signal transduction pathways (bottom row). The seminal fluid component TRY-5 can transactivate hermaphrodite sperm that have defects in the spe-8 group genes. In one model, the activator of the spe-8 signal transduction pathway is hermaphrodite-specific and activates try-5 male sperm within the uterus. In an alternative model, the activator of the spe-8 pathway is expressed in both hermaphrodites and, redundantly, in males. (C) Diagram of the C. elegans male gonad with the relative amount of TRY-5 within cells of the seminal vesicle, valve, and vas deferens indicated by different levels of gray shading. Even less is known about how reproductive fitness is maximized when both sexes (males and hermaphrodites) produce sperm. Certainly male and hermaphrodite Caenorhabditis elegans sperm face distinct challenges. In hermaphrodites, sperm are produced first, before their gonad switches over to exclusively producing oocytes [6]. Hermaphrodite sperm activate to form motile spermatozoa as they are physically pushed into the spermatheca by ovulating oocytes. Once there, they wait for fertilization opportunities as individual oocytes enter the spermatheca in an assembly-line fashion. Typically, every sperm gets an oocyte, and their only challenge is to remain in the spermatheca as the newly fertilized oocytes squeeze through the spermatheca on their way to the uterus [7].
In contrast, the larger male sperm are stored in a quiescent state within the seminal vesicle and only activate to form motile spermatozoa during the process of ejaculation. After insemination, male sperm must migrate from the vulva to the spermatheca and then out-compete the hermaphrodite's own sperm [8], [9].
Three lines of evidence suggest that C. elegans sperm activation is regulated in a sex-specific manner:
-
Molecular genetic studies identified components of a sperm-specific SPE-8 signal transduction cascade that function in both male and hermaphrodite sperm but are only essential for the activation of the hermaphrodite's own sperm (Figure 1B) [10]–[14]. Although mutant hermaphrodites are self-sterile, their sperm can be trans-activated by seminal fluid from either wild-type or spe-8 males. Conversely, mutant males are fertile, but their sperm activates abnormally in response to in vitro activation by the protease Pronase. Together, these results suggest that although the sperm activators are expressed in a sex-specific manner, both male and hermaphrodite sperm retain the capacity to respond to either activator.
-
Conversely, the somatically expressed protease inhibitor SWM-1 is specifically required for male, but not hermaphrodite, fertility [15]. SWM-1 specifically regulates the timing of sperm activation in males; in the absence of SWM-1, male sperm activate precociously within the seminal vesicle, precluding their efficient transfer to hermaphrodites.
-
Lastly, sperm activation was found to be a key factor in the evolution of hermaphroditism, as females from the male/female species Caenorhabditis remanei were experimentally transformed into hermaphrodites simply by lowering the activity of two genes: SWM-1 and the “female” promoting factor TRA-2 [16].
How do these findings fit together? In this issue of PLoS Genetics, Smith and Stanfield [17] provide new insights regarding two partially redundant signal transduction pathways that regulate C. elegans sperm activation.
TRY-5 Is a Sex-Specific Component of the Male Sperm Activation Pathway
Since male sperm activation can be triggered in vitro by proteases and blocked in vivo by the protease inhibitor SWM-1, the authors hypothesized that the male-specific sperm activator might be both a protease and a direct target of SWM-1. Taking advantage of both forward and reverse genetic approaches, they screened for mutations that suppress precocious sperm activation in swm-1 males and identified a single, secreted trypsin-class serine protease (TRY-5), whose depletion suppressed the precocious activation and transfer defects of swm-1 male sperm.
To further characterize this putative male sperm activator, the authors examined the effect of depleting TRY-5 alone, or in combination with previously characterized sperm activation mutants (Figure 1B). Since TRY-5 is expressed exclusively in males, it was not surprising that try-5 hermaphrodites were fertile. However, the discovery that try-5 males were also fertile suggested that their sperm were being activated via a second, TRY-5-independent pathway. Suspecting that this second pathway involved both the spe-8 signal transduction pathway and trans-activation by the hermaphrodite-specific activator, the authors demonstrated that mutant males lacking both TRY-5 and spe-8 class genes were infertile and that seminal fluid from try-5 males could not transactivate spe-8 hermaphrodite sperm. The authors concluded that, in C. elegans, sperm activation is controlled by two partially redundant pathways: an unknown signal transduction pathway triggered by the male-specific TRY-5 and the spe-8 signal transduction pathway triggered by an unknown hermaphrodite-specific activator.
TRY-5 Is a Component of Seminal Fluid
If TRY-5 functions directly as the sperm activator, it should be present in the seminal fluid, specifically during the process of ejaculation. To test whether TRY-5 is expressed at the correct time and in the correct place, the authors generated transgenic lines containing either a Ptry-5::GFP reporter or a Ptry-5::TRY-5::GFP fusion construct. Sure enough, try-5 was expressed specifically within the cells of the somatic male gonad that surround the exit path of the sperm (Figure 1C). Furthermore, prior to ejaculation, TRY-5 localized to secretory vesicles within these somatic cells. During ejaculation, TRY-5 was secreted as a component of the seminal fluid and was ejaculated in a reproducible pattern of spurts from distinct regions of the somatic gonad. The dynamics of TRY-5 secretion and transfer suggest that male sperm activation may occur within the uterus of the hermaphrodite rather than within the vas deferens of the male.
Answers and More Questions
The discovery and characterization of the protease TRY-5 as the putative male-specific sperm activator addresses both how and why male and hermaphrodite sperm are activated in a sex-specific manner. For male sperm, activation must be both robust and tightly coordinated with each ejaculation event. For hermaphrodite sperm, activation may be a one-time event and robustness may not be as critical. As TRY-5 is the first sex-specific component of the sperm-activation pathway to be identified, it will be critical both for elucidating the elements of the downstream signal transduction machinery and for addressing broader questions regarding the independent evolution of hermaphroditism.
At the same time, this study raises several new questions. Does TRY-5 activate sperm directly or does it function as part of a protease cascade? What are the sperm-expressed targets of the TRY-5 activation pathway? What is the hermaphrodite-expressed activator that functions upstream of the SPE-8 signal transduction pathway? Finally, are there really distinct male and hermaphrodite sperm activators, or do males normally express multiple activators while hermaphrodites express only the one that triggers the SPE-8 pathway (Figure 1B)?
Additionally, this is the first study to reveal C. elegans as a model for elucidating the temporal and physiological dynamics of seminal fluid production, as the TRY-5::GFP fusion protein can be directly observed through the transparent body of the worm. Future studies could use similar approaches to investigate the secretory dynamics of other seminal fluid proteins.
Zdroje
1. GomendioMRoldanER 2008 Implications of diversity in sperm size and function for sperm competition and fertility. Int J Dev Biol 52 439 447
2. PoianiA 2006 Complexity of seminal fluid: a review. Behav Ecol Sociobiol 60 289 310
3. WolfnerMF 2009 Battle and ballet: molecular interactions between the sexes in Drosophila. J Hered 100 399 410
4. den BoerSPBaerBBoomsmaJJ 2010 Seminal fluid mediates ejaculate competition in social insects. Science 19 1506 1509
5. Rodríguez-MartínezHKvistUErnerudhJSanzLCalveteJJ 2011 Seminal plasma proteins: what role do they play? Am J Reprod Immunol 66 Suppl 1 11 22
6. HubbardEJGreensteinD 2000 The Caenorhabditis elegans gonad: a test tube for cell and developmental biology. Dev Dyn 218 2 22
7. SingsonA 2001 Every sperm is sacred: fertilization in Caenorhabditis elegans. Dev Biol 230 101 109
8. WardSCarrelJS 1979 Fertilization and sperm competition in the nematode Caenorhabditis elegans. Dev Biol 73 304 321
9. LaMunyonCWWardS 1995 Sperm precedence in a hermaphroditic nematode (Caenorhabditis elegans) is due to competitive superiority of male sperm. Experientia 51 817 823
10. ShakesDCWardS 1989 Initiation of spermiogenesis in C. elegans: a pharmacological and genetic analysis. Dev Biol 134 189 200
11. MinnitiANSadlerCWardS 1996 Genetic and molecular analysis of spe-27, a gene required for spermiogenesis in Caenorhabditis elegans hermaphrodites. Genetics 143 213 223
12. NanceJDavisEBWardS 2000 spe-29 encodes a small predicted membrane protein required for the initiation of sperm activation in Caenorhabditis elegans. Genetics 156 1623 1633
13. GeldzilerBChatterjeeISingsonA 2005 The genetic and molecular analysis of spe-19, a gene required for sperm activation in Caenorhabditis elegans. Dev Biol 283 424 436
14. NishimuraHL'HernaultSW 2010 Spermatogenesis-defective (spe) mutants of the nematode Caenorhabditis elegans provide clues to solve the puzzle of male germline functions during reproduction. Dev Dyn 239 1502 1514
15. StanfieldGMVilleneuveAM 2006 Regulation of sperm activation by SWM-1 is required for reproductive success of C. elegans males. Curr Biol 16 252 263
16. BaldiCChoSEllisRE 2009 Mutations in two independent pathways are sufficient to create hermaphroditic nematodes. Science 326 1002 1005
17. SmithJRStanfieldGM 2011 TRY-5 is a sperm-activating protease in Caenorhabditis elegans seminal fluid. PLoS Genet 7 e1002375 doi:10.1371/journal.pgen.1002375
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 11- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- De Novo Origins of Human Genes
- Duplication Hotspots Are Associated with Late-Replicating Regions of the Genome
- De Novo Origin of Human Protein-Coding Genes
- Cyclin D/CDK4 and Cyclin E/CDK2 Induce Distinct Cell Cycle Re-Entry Programs in Differentiated Muscle Cells
- Short Day–Mediated Cessation of Growth Requires the Downregulation of AINTEGUMENTALIKE1 Transcription Factor in Hybrid Aspen
- Physiological IRE-1-XBP-1 and PEK-1 Signaling in Larval Development and Immunity
- Role of Pirh2 in Mediating the Regulation of p53 and c-Myc
- Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution
- FOXO Regulates Organ-Specific Phenotypic Plasticity In
- Heritable Epigenetic Variation among Maize Inbreds
- Foxn1 Regulates Lineage Progression in Cortical and Medullary Thymic Epithelial Cells But Is Dispensable for Medullary Sublineage Divergence
- Attenuation of the Sensing Capabilities of PhoQ in Transition to Obligate Insect–Bacterial Association
- A Novel Protein LZTFL1 Regulates Ciliary Trafficking of the BBSome and Smoothened
- Activation of Bmp2-Smad1 Signal and Its Regulation by Coordinated Alteration of H3K27 Trimethylation in -Induced Senescence
- Histone H3K56 Acetylation, CAF1, and Rtt106 Coordinate Nucleosome Assembly and Stability of Advancing Replication Forks
- The SUN Protein Mps3 Is Required for Spindle Pole Body Insertion into the Nuclear Membrane and Nuclear Envelope Homeostasis
- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- Effect of Host Species on the Distribution of Mutational Fitness Effects for an RNA Virus
- Pch2 Acts through Xrs2 and Tel1/ATM to Modulate Interhomolog Bias and Checkpoint Function during Meiosis
- SOX9 Governs Differentiation Stage-Specific Gene Expression in Growth Plate Chondrocytes via Direct Concomitant Transactivation and Repression
- from the Aphid : A Missing Link from Facultative to Obligate Insect Endosymbiont
- Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal Function in Flowers and Meristems
- Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects
- Consequences of Eukaryotic Enhancer Architecture for Gene Expression Dynamics, Development, and Fitness
- Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- For Male , Sperm Activation Is a “Just-in-Time” Event
- PcG Complexes Set the Stage for Epigenetic Inheritance of Gene Silencing in Early S Phase before Replication
- The Gene Contains Hotspots for L1 Endonuclease-Dependent Insertion
- Relative Burden of Large CNVs on a Range of Neurodevelopmental Phenotypes
- Multiple Means to the Same End: The Genetic Basis of Acquired Stress Resistance in Yeast
- Genome-Wide Crossover Distribution in Meiosis Reveals Sex-Specific Patterns along Chromosomes
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
- Homologs of Retinoblastoma-Associated Protein 46/48 Associate with a Histone Deacetylase to Act Redundantly in Chromatin Silencing
- Genetic Interaction Maps in Reveal Functional Crosstalk among Cell Envelope Biogenesis Pathways
- The ERI-6/7 Helicase Acts at the First Stage of an siRNA Amplification Pathway That Targets Recent Gene Duplications
- PBX1 Genomic Pioneer Function Drives ERα Signaling Underlying Progression in Breast Cancer
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- De Novo Origins of Human Genes
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



