-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Cyclin D/CDK4 and Cyclin E/CDK2 Induce Distinct Cell Cycle Re-Entry Programs in Differentiated Muscle Cells
Cell proliferation and differentiation are regulated in a highly coordinated and inverse manner during development and tissue homeostasis. Terminal differentiation usually coincides with cell cycle exit and is thought to engage stable transcriptional repression of cell cycle genes. Here, we examine the robustness of the post-mitotic state, using Caenorhabditis elegans muscle cells as a model. We found that expression of a G1 Cyclin and CDK initiates cell cycle re-entry in muscle cells without interfering with the differentiated state. Cyclin D/CDK4 (CYD-1/CDK-4) expression was sufficient to induce DNA synthesis in muscle cells, in contrast to Cyclin E/CDK2 (CYE-1/CDK-2), which triggered mitotic events. Tissue-specific gene-expression profiling and single molecule FISH experiments revealed that Cyclin D and E kinases activate an extensive and overlapping set of cell cycle genes in muscle, yet failed to induce some key activators of G1/S progression. Surprisingly, CYD-1/CDK-4 also induced an additional set of genes primarily associated with growth and metabolism, which were not activated by CYE-1/CDK-2. Moreover, CYD-1/CDK-4 expression also down-regulated a large number of genes enriched for catabolic functions. These results highlight distinct functions for the two G1 Cyclin/CDK complexes and reveal a previously unknown activity of Cyclin D/CDK-4 in regulating metabolic gene expression. Furthermore, our data demonstrate that many cell cycle genes can still be transcriptionally induced in post-mitotic muscle cells, while maintenance of the post-mitotic state might depend on stable repression of a limited number of critical cell cycle regulators.
Published in the journal: . PLoS Genet 7(11): e32767. doi:10.1371/journal.pgen.1002362
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002362Summary
Cell proliferation and differentiation are regulated in a highly coordinated and inverse manner during development and tissue homeostasis. Terminal differentiation usually coincides with cell cycle exit and is thought to engage stable transcriptional repression of cell cycle genes. Here, we examine the robustness of the post-mitotic state, using Caenorhabditis elegans muscle cells as a model. We found that expression of a G1 Cyclin and CDK initiates cell cycle re-entry in muscle cells without interfering with the differentiated state. Cyclin D/CDK4 (CYD-1/CDK-4) expression was sufficient to induce DNA synthesis in muscle cells, in contrast to Cyclin E/CDK2 (CYE-1/CDK-2), which triggered mitotic events. Tissue-specific gene-expression profiling and single molecule FISH experiments revealed that Cyclin D and E kinases activate an extensive and overlapping set of cell cycle genes in muscle, yet failed to induce some key activators of G1/S progression. Surprisingly, CYD-1/CDK-4 also induced an additional set of genes primarily associated with growth and metabolism, which were not activated by CYE-1/CDK-2. Moreover, CYD-1/CDK-4 expression also down-regulated a large number of genes enriched for catabolic functions. These results highlight distinct functions for the two G1 Cyclin/CDK complexes and reveal a previously unknown activity of Cyclin D/CDK-4 in regulating metabolic gene expression. Furthermore, our data demonstrate that many cell cycle genes can still be transcriptionally induced in post-mitotic muscle cells, while maintenance of the post-mitotic state might depend on stable repression of a limited number of critical cell cycle regulators.
Introduction
During the terminal stages of differentiation, cells usually arrest proliferation and permanently exit the division cycle. Insight in how this post-mitotic state is established and maintained is of both fundamental and clinical importance. Entry into the cell cycle requires activation of Cyclin Dependent Kinases (CDKs) in the G1 phase of the cell cycle. These kinases promote expression of cell cycle genes that are controlled by E2F/DP (together named “E2F”) transcription factors (for review: [1]). CDK inhibitors (CKIs), such as p21Cip1 and p27Kip1, counteract cell cycle entry through association with Cyclin/CDK complexes and inhibition of their activity. In addition, members of the retinoblastoma protein (pRb) family inhibit cell cycle entry through repression of E2F-regulated cell cycle genes [2]. When activated in the G1 phase, Cyclin D/CDK4(6) and Cyclin E/CDK2 kinases phosphorylate pRb, which prevents its association with E2F and allows activating E2F transcription factors to induce S phase gene expression. Differentiation signals are thought to induce cell cycle arrest through activation of negative regulators of G1 progression, probably in parallel with chromatin remodeling and modification complexes that induce stable repression of cell cycle genes [3]–[5].
Only a few examples have been reported of post-mitotic cells that re-enter the cell cycle while maintaining the differentiated state. In Drosophila, expression of E2F together with the Cdc25c phosphatase String or Cyclin E/CDK2 induces continued division of differentiated cells during eye and wing development [6]. In mammals, loss of the pRb tumor suppressor allows proliferation of certain terminally differentiated cells, such as the post-mitotic hair cells of the mouse inner ear [7]. In addition, inactivation of pRb family members can result in the development of retinoblastoma or related tumors from fully differentiated neurons [8], [9]. Thus, at least some terminally differentiated cells that are normally arrested can be induced to initiate cell proliferation with no apparent de-differentiation.
In this study, we use the nematode C. elegans to examine the cell cycle arrest associated with terminal differentiation. C. elegans shows a tight inverse relationship between proliferation and differentiation, and a highly reproducible pattern of terminal differentiation [10], [11]. Except for 55 ‘blast’ cells, all cells differentiate and become post-mitotic before embryonic development completes. The single Cyclin D (cyd-1) and CDK4/6 (cdk-4) genes are essential for G1/S progression in postembryonic development [12], [13], while the single Rb-family member, lin-35, and the cki-1 and cki-2 Cip/Kip inhibitors act as negative regulators of cell cycle entry [13]–[18]. Notably, lin-35 Rb inactivation combined with cki-1/cki-2 inhibition causes only limited over-proliferation in blast-cell lineages ([13], JK and SvdH, unpublished observations). Thus, in C. elegans, cell cycle exit can occur without a functional pRb family protein, as was recently also observed in mice [19].
Here, we examine to what extent terminally differentiated C. elegans body-wall muscle cells respond to, or resist, cell-cycle inducing signals. Expression of G1 Cyclin/CDK combinations triggered expression of S phase genes and partial cell cycle re-entry in differentiated muscle cells, while not interfering with the differentiation status. Tissue-specific transcriptional profiling and single molecule FISH experiments revealed that CYD-1/CDK-4 and CYE-1/CDK-2 induce a substantial and overlapping set of genes that are strongly associated with cell cycle functions. However, CYD-1/CDK-4 also triggered up-regulation or down-regulation of large numbers of genes with metabolism-associated functions, and induced DNA replication, in contrast to CYE-1/CDK-2. Notably, several key G1/S regulators, such as cye-1, cdc-25.1, and cdk-2, were not induced by CYD-1/CDK-4. Thus, differentiated muscle cells remain remarkably flexible for cell cycle induction, while at the same time, robust repression of a few key regulators appears to maintain a stable post-mitotic state.
Results
G1 Cyclin/CDK induction overcomes cell cycle quiescence
We set out to examine if cell cycle entry can be induced by transcriptional induction of single G1 Cyclins (CYD-1 Cyclin D or CYE-1 Cyclin E), CDKs (CDK-4, CDK-2, or CDK-2AF, a mutated form of CDK-2 that lacks the negative Tyr14 and Thr15 phosphorylation sites), or combined expression of CYD-1/CDK-4, CYE-1/CDK-2 or CYE-1/CDK-2AF. We first examined the effects of G1 Cyclin/CDK transgene expression in cells that are temporarily arrested (quiescent) but not terminally differentiated. In wild-type larvae that hatch from the egg, precursor cells of the post-embryonic lineages remain quiescent under starvation conditions. When food is added, development resumes and postembryonic blast cells initiate proliferation [14]. Expression of any of the three Cyclin/CDK combinations from the intestine-specific elt-2 promoter prevented the normal cell cycle arrest in the intestine of late embryonic and starved L1 animals (see Figure S1). Based on BrdU incorporation, DNA replication continued in starvation-arrested larvae with intestinal Cyclin/CDK expression (Figure S1D). The number of intestinal nuclei in these starved L1 animals regularly exceeded the maximal number of 34 nuclei in normal adults (Figure S1B and S1F). Expression of either CYD-1 or CYE-1 alone was sufficient to trigger nuclear division and DNA replication, whereas CDK expression alone did not induce an apparent cell cycle response (Figure S1E and S1F; and data not shown). This probably indicates that the temporally arrested cells contain residual CDK proteins, but not G1 Cyclins. We conclude that transcriptional induction of a G1 Cyclin is sufficient to prevent cell cycle quiescence of intestinal cells.
Cyclin/CDK expression in muscle leads to cell cycle re-entry during larval development
Next, we examined if G1 Cyclin/CDK expression could trigger cell cycle re-entry in terminally differentiated body-wall muscle. The C. elegans larva is born with 81 fully differentiated body-wall muscle cells [10]. We chose the myo-3 promoter (Pmyo-3) to drive expression of Cyclins and CDKs in muscle, as the muscle myosin gene myo-3 is turned on in post-mitotic embryonic body-wall muscle [20], [21]. When expressed from this promoter, CYE-1 and CDK-2AF showed muscle-specific expression in immunostaining, and complex formation in immunoprecipitation/western blotting experiments (Figure S2). Next, we introduced the different Cyclin/CDK combinations together with a reporter construct, Pmyo-3::GFP::H2B, to facilitate the detection of muscle nuclei, and generated strains with integrated arrays to avoid mosaic expression (Figure 1A).
Fig. 1. G1 Cyclin/CDK expression induces mitotic events in body-wall muscle cells. 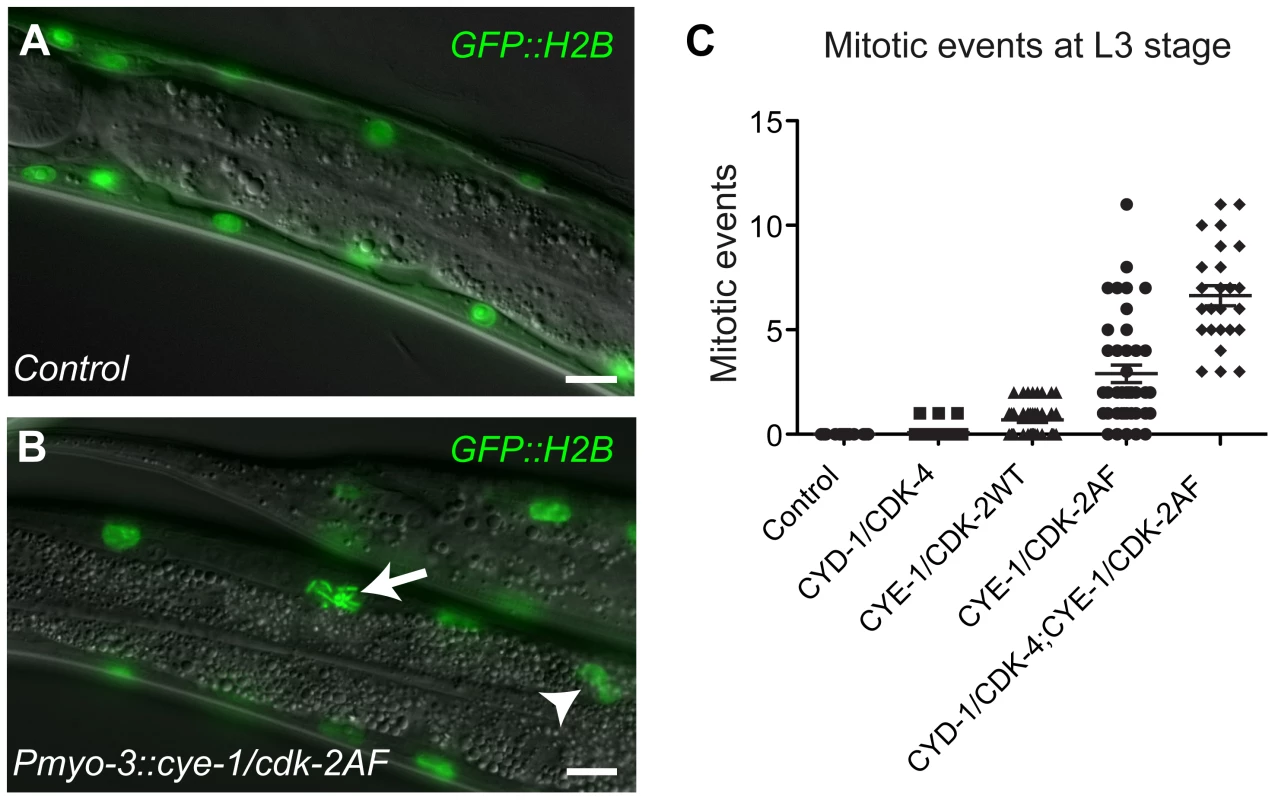
(A) Control animal expressing the GFP::H2B fusion protein in body-wall muscle. (B) Animals that express CYE-1, CDK-2AF and GFP::H2B together from the myo-3 promoter show chromosome condensation (arrow) and nuclear division (arrowhead) in differentiated body-wall muscle. (C) Quantification of mitotic events for each Cyclin/CDK combination at the L3 stage. Each dot represents the number of mitotic muscle nuclei (with condensed DNA, metaphase or anaphase figures or nuclear division) in a single animal. In each animal, 58 muscle nuclei anterior of the prospective vulva were counted, to exclude muscle cells formed during post-embryonic development in the Mesoblast lineage. The highest mitotic index was 12% (7/58) in the strain with both Cyclin/CDKs. Error bars represent S.E.M. In contrast to our findings in the intestine, animals expressing any of the three Cyclin/CDK combinations in muscle hatched with a normal complement of muscle nuclei (Figure S3A and S3B). Thus, Pmyo-3-driven Cyclin/CDK expression does not lead to extra muscle cell division during embryogenesis. However, from the L2 stage onwards, some body-wall muscle cells started to show signs of mitosis, including chromosome condensation, chromosome congression, anaphase and nuclear division, sometimes even resulting in clusters of small nuclei (Figure 1B; Figure S3C-S3E). In contrast to quiescent intestinal cells, expression of CYD-1 or CYE-1 alone did not induce mitotic events in differentiated muscle cells. CYE-1/CDK-2AF was much more efficient in inducing mitotic events than CYD-1/CDK-4, while CYE-1/CDK-2WT expression gave an intermediate effect (Figure 1C). Western blotting experiments indicated that the extent of mitotic induction did not correspond to the protein expression level, but rather the type of CDK (and Cyclin) expressed (Figure S4). The combination of CYD-1/CDK-4 and CYE-1/CDK-2AF caused the strongest mitotic induction (Figure 1C).
We looked for additional indications of cell cycle re-entry in body-wall muscle cells. Staining for the mitosis specific phospho-histone H3S10 epitope readily visualized mitotic nuclei in the body-wall muscle of CYE-1/CDK-2AF animals (Figure 2A and 2B). Moreover, we observed expression of S-phase reporters in muscle cells. One of these reporters uses the rnr-1 ribonucleotide reductase promoter to express tdTomato in frame with a destruction box containing N-terminal CYB-1 Cyclin B fragment [22]. In addition, we used a transgenic strain with a single-copy translational fusion of C. elegans MCM-4::mCherry under its native promoter. Both reporters were completely silent in body-wall muscle of normal larvae. In contrast, MCM-4::mCherry was detectable as early as the L1 stage in all body-wall muscle cells of CYD-1/CDK-4 and CYE-1/CDK-2AF-expressing animals (Figure 2C and 2D, and data not shown). Expression of rnr-1::CYB-1desBox::tdTomato became detectable from the L2 stage onward, and even remained detectable in the muscle of adult animals.
Fig. 2. G1 Cyclin/CDK expression induces S and M phase markers in larval muscle. 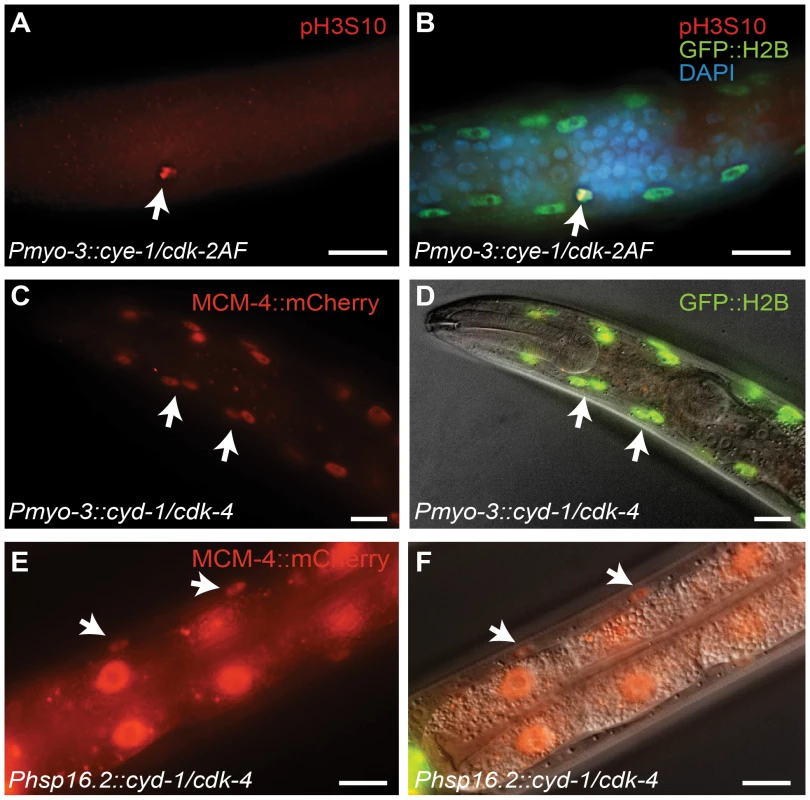
(A,B) A body-wall muscle cell expressing CYE-1/CDK-2AF stains positive for the mitosis-specific phospho-histone H3S10 epitope (arrow). (C,D) Expression of the S-phase marker Pmcm-4::MCM-4::mCherry (MCM4) in all body-wall muscle cells of an adult animal expressing CYD-1/CDK-4 from the myo-3 promoter. (D) merged image of MCM-4::mCherry, GFP::H2B and DIC. (E,F) Expression of the Pmcm-4::MCM-4::mCherry S-phase marker in an L4 animal after heat-shock induced expression of CYD-1/CDK-4 at the L2/L3 stage. (F) merged image of MCM-4::mCherry and DIC. Arrows point to muscle nuclei, scale bars indicate 10 μm. Importantly, heat-shock promoter driven expression of CYD-1 and CDK-4 during larval development also induced S-phase reporter gene expression. After heat shock, we observed MCM-4::mCherry expression in the body-wall muscle of hsp-16.2::CYD-1/hsp-16.2::CDK-4 transgenic strains (Figure 2E and 2F; in 3 of 3 strains), but never in control heat-shock treated animals without the Cyclin/CDK transgenes (n = 50 animals examined). Even adult animals showed MCM-4 expression after heat-shock induction of CYD-1 and CDK-4, further illustrating that the S phase reporter could be turned on after terminal differentiation. Together, these results indicate that at least some cell cycle genes are not irreversibly silenced in post-mitotic muscle cells.
C. elegans Cyclin D/CDK-4, but not Cyclin E/CDK-2, induces S phase in muscle cells
Next, we tested if G1 Cyclin/CDK expression is sufficient for induction of DNA replication in differentiated muscle cells. We used two independent methods to detect DNA synthesis. First, we examined incorporation of EdU, a thymidine analogue that can be used to visualize DNA replication in combination with antibody staining of muscle nuclei [22], [23]. We stained L4 animals for both EdU and GFP and analyzed muscle cells anterior of the vulva, to exclude muscles formed in the postembryonic Mesoblast lineage. To our surprise, only in 2 out of 35 CYE-1/CDK-2AF expressing animals EdU incorporation was detectable in a few muscle cells, while all other muscle nuclei were completely EdU negative (Figure 3C and 3D, circles). In contrast, the anterior of more than half (19/35) of the CYD-1/CDK-4 animals contained clearly EdU positive body-wall muscle cells (Figure 3A and 3B, arrowheads).
Fig. 3. C. elegans Cyclin D/CDK-4 induces DNA replication in muscle. 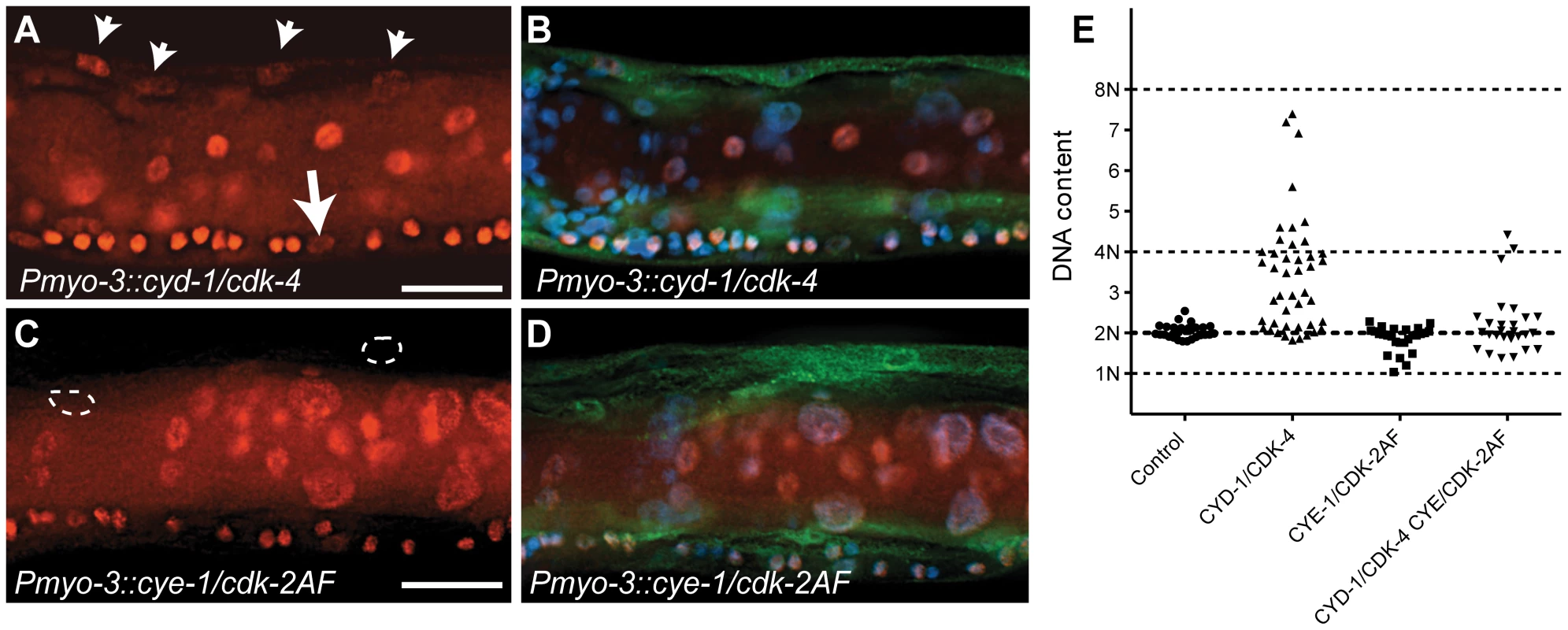
(A-D) Detection of EdU incorporation in body-wall muscle nuclei. (A,C) EdU staining, (B,D) merged image of EdU, GFP and DAPI staining. EdU-positive nuclei are readily detectable in CYD-1/CDK-4 expressing body-wall muscle (A, arrowheads), but not in CYE-1/CDK-2AF expressing body-wall muscle (C, circles). For comparison, the arrow in (A) indicates a Pn.p cell, which completed one round of DNA replication in the presence of EdU. (E) Quantitative determination of DNA content reveals DNA replication in CYD-1/CDK-4 expressing muscle cells. Each dot indicates the DNA content, based on propidium iodide staining, of a single body-wall muscle nucleus. Pn.p nuclei in the ventral cord were used as 2n controls. L3/L4 stage larvae were stained in A-D, GFP staining reveals muscle expressed GFP::H2B and CDK-2/4::Venus. Quantitative determination of DNA content based on propidium iodide (PI) intercalation confirmed and expanded these results (Figure 3E). While body-wall muscle in the Pmyo-3::H2B::GFP control strain showed a G1 DNA content (2n), muscle cells with CYD-1/CDK-4 expression often contained a larger amount of DNA, which corresponded to a partly or completely duplicated genome (4n). CYE-1/CDK-2 expressing muscle cells did not contain more than 2n DNA, and very few muscle cells with combined expression of CYD-1/CDK-4 and CYE-1/CDK-2 contained 4n DNA (Figure 3E). Thus, CYD-1/CDK-4 promotes DNA replication, while CYE-1/CDK-2 even appears to inhibit induction of DNA synthesis by CYD-1/CDK-4. Together, CYE-1/CDK-2AF expression in differentiated muscle triggers S-phase gene expression and mitosis, but not DNA replication, while the CYD-1/CDK-4 combination induces a more normal cell cycle that includes DNA replication in S phase, but usually arrests prior to M phase.
Activation of a cell-cycle transcriptional program without loss of muscle differentiation
Our combined data indicate that differentiated body-wall muscle cells can re-enter the cell cycle post-embryonically in response to G1 Cyclin/CDK expression. Next, we wanted to examine if the observed cell cycle re-entry coincides with loss of muscle differentiation. Animals expressing G1 Cyclin/CDK complexes in the body-wall muscle appear phenotypically normal and show apparently normal sinusoidal movement (Figure 4C). To examine muscle structure, we stained animals for UNC-15/Paramyosin, a component of thick muscle filaments in C. elegans [24]. Muscle cells in L4 and adult CYE-1/CDK-2AF animals displayed a normal pattern of thick muscle filaments, even when nuclei with clear mitotic figures were present (Figure 4A and 4B). These data support the idea that CYE-1/CDK-2AF expression does not change muscle structure and function.
Fig. 4. Animals with mitotic body-wall muscle retain normal motility and muscle structure. 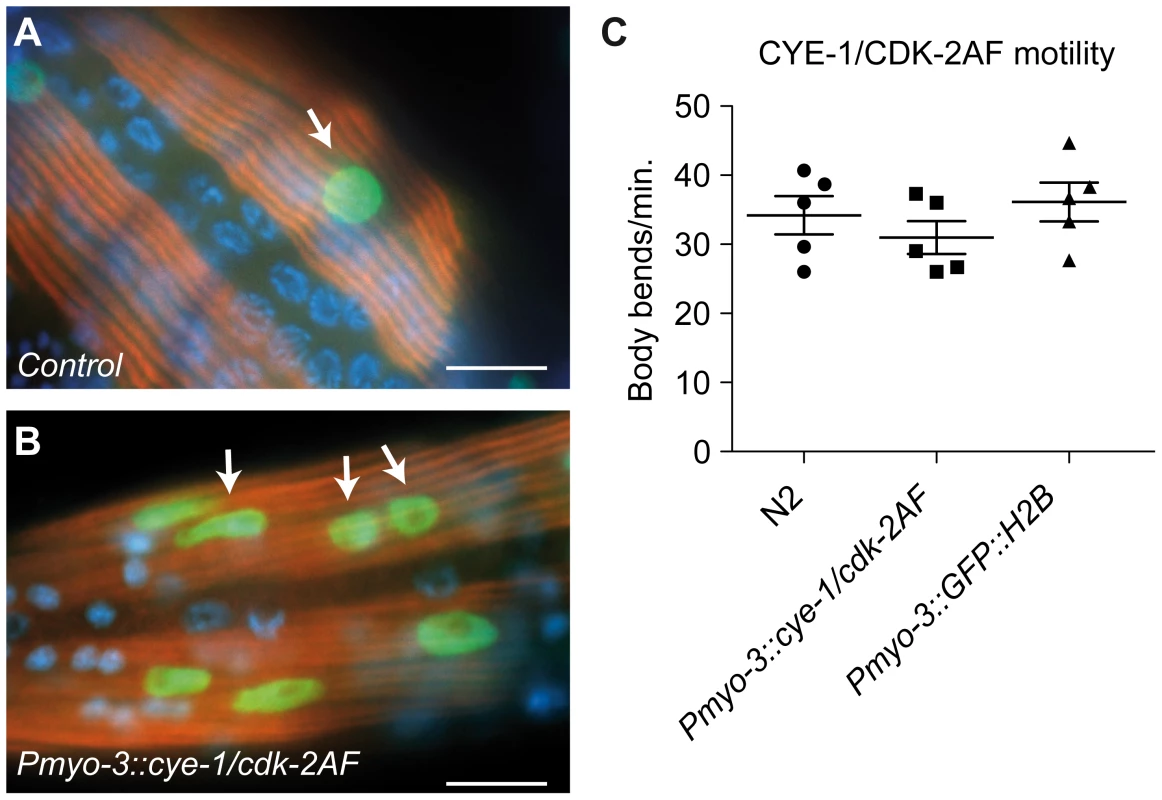
(A,B) UNC-15/Paramyosin staining (red) of a control (Pmyo-3::GFP::H2B) line and a line with muscle expression of CYE-1 and CDK-2AF. Arrows mark GFP (green) expressing nuclei of body-wall muscle cells. (B) Body-wall muscle in both animals show thick filament structures, despite the signs of mitosis (arrows). (C) Motility assay of L4 larvae: N2 wild type, SV858 (Pmyo-3::CYE-1/CDK-2AF) and SV859 (Pmyo-3::GFP::H2B control). Each dot represents a single animal. Error bars represent S.E.M., scale bars indicate 10 μm. To obtain a more comprehensive picture of the changes induced by CYD-1/CDK-4 and CYE-1/CDK-2AF expression, we performed tissue-specific mRNA profiling of body-wall muscles. Key to this approach is muscle-specific expression of a FLAG-tagged PAB-1 poly(A)-binding protein [25]. As a validation of the method, immunopurification of PAB-1-crosslinked mRNA from Pmyo-3::FLAG::PAB-1 transgenic animals yielded mRNAs that were highly enriched for muscle-expressed genes (Table S1). We next compared the mRNA profiles of control L1 animals (carrying the Pmyo-3::GFP::H2B and Pmyo-3::FLAG::PAB-1 transgenes), and L1 animals that, additionally, expressed CYE-1/CDK-2AF or CYD-1/CDK-4 in muscle cells. Most genes in CYD-1/CDK-4 or CYE-1/CDK-2AF expressing muscle cells were not significantly up - or down - regulated (Figure S5A and S5B, Table S2). This includes nearly all muscle-specific genes, confirming maintenance of the muscle-specific fate (see below).
A set of 219 genes was significantly upregulated (fold-change ≥ 2, p<0.05) in CYE-1/CDK-2AF animals compared to the control strain. Manual annotation of these induced genes and GO-term enrichment analysis using Funcassociate [26] revealed a large overrepresentation of genes involved in various aspects of the cell cycle, including G1/S regulation, DNA replication, DNA damage response, mitosis, cytokinesis and checkpoint control (Figure 5B and 5E, Table S2). Similar to CYE-1/CDK-2AF, CYD-1/CDK-4 expression in the body-wall muscle induced a set of 395 genes with a strong cell cycle signature (Figure 5A, Table S2).
Fig. 5. Microarray analysis reveals induction of cell-cycle gene transcripts in differentiated muscle cells. 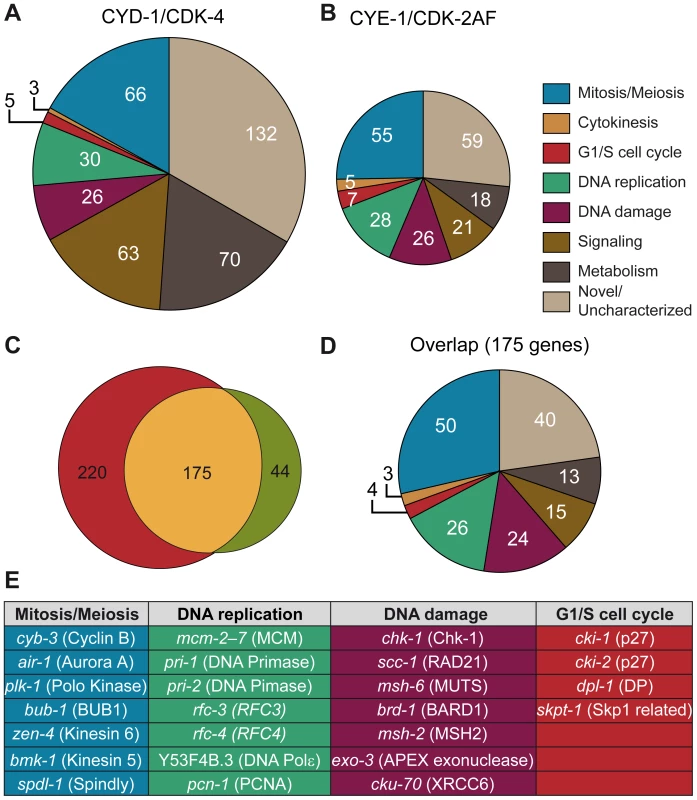
(A,B) Functional annotation of genes significantly increased more than 2-fold in Pmyo-3::CYD-1/CDK-4 and Pmyo-3::CYE-1/CDK-2AF muscle cells. A large overrepresentation of genes involved in various stages of the cell cycle is seen in both data sets, as well as a distinct increase in the proportion of metabolism and signaling genes in CYD-1/CDK-4 animals. (C) Overlap in more than 2-fold upregulated genes between CYE-1/CDK-2AF and CYD-1/CDK-4 body-wall muscle cells. The majority of the genes induced by CYE-1/CDK-2AF are also induced in CYD-1/CDK-4, but the latter also contains a substantial number of non-cell cycle genes. (D) Functional annotation of the genes present in the overlap between CYD-1/CDK-4 and CYE-1/CDK-2AF reveals a strong (60%) representation of known cell cycle genes. (E) Table of representative cell cycle genes and their orthologs that are induced by both CYD-1/CDK-4 and CYE-1/CDK-2AF (>2-fold upregulated). The majority of genes upregulated in CYE-1/CDK-2AF were also upregulated in CYD-1/CDK-4 muscle (Figure 5C and 5D). The overlapping gene set has a particularly strong cell cycle signature, with over 60% of the genes with functional annotations in a cell cycle category (Figure 5D and 5E). Interestingly, CYD-1/CDK-4 also induced a set of 143 genes (> 2x, p<0.05) that are not significantly upregulated by CYE-1/CDK-2AF expression (p>0.05) (Table S2, Table S3). These genes were enriched for GO terms related to biosynthesis, though at adjusted P values slightly above 0.05 (0.055 to 0.092). Manual annotation confirmed that many of these genes (45 of 87 genes with recognizable homologues) have ascribed cellular growth and metabolism functions (Figure 5A, Table S2). This suggests that CYD-1/CDK-4 does not only activate a cell-cycle transcriptional program, but also functions in G1 to stimulate cellular growth and metabolism associated gene expression.
A major function of G1/S Cyclin/CDK complexes is the activation of E2F transcription factors through inhibitory phosphorylation of pRb proteins [1]. We therefore analyzed the promoter sequences of genes upregulated in CYD-1/CDK-4 or CYE-1/CDK-2AF for enrichment in consensus transcription factor binding sites (Materials and Methods), including a C. elegans specific profile for the EFL-1 (E2F) transcription factor [27]. We analyzed the promoter regions of 439 genes upregulated ≥ 2 fold in CYD-1/CDK-4 or CYE-1/CDK-2AF expressing muscle. In both sets of promoters the highest scoring transcription factor site motif was that of E2F1, while the C. elegans specific EFL-1 site ranked fifth and third respectively (Table S4). These results are consistent with the activation of a set of E2F target genes by both CYD-1/CDK-4 and CYE-1/CDK-2. We next analyzed the 143 genes upregulated in CYD-1/CDK-4 animals but not in CYE-1/CDK-2 animals. This set of genes was hardly enriched for E2F1 and EFL-1 sites (Table S4). Together with the functional annotations, these results indicate that CYD-1/CDK-4 and CYE-1/CDK-2 induce a core set of E2F regulated cell cycle genes, while CYD-1/CDK-4 also activates a set of genes with a broader range of functions.
CYE-1/CDK-2 expression in muscle caused down-regulation of only 50 genes with no functional enrichment. In contrast, CYD-1/CDK-4 expression also resulted in down-regulation (≥ 2x, p<0.05) of a substantial set of 555 transcripts (Table S5). This group shows a remarkable enrichment with GO terms related to catabolic processes, including peptidase, lipase, esterase and hydrolase activities. While muscle cells remain functional and morphologically normal, 60 of the down-regulated genes are normally highly expressed in muscle (Table S4). Thus, CYD-1/CDK-4 causes upregulation of cell-cycle and biosynthesis-associated genes, as well as down-regulation of genes primarily associated with biodegradation and energy production.
Key G1/S regulators are not induced by G1 Cyclin/CDK expression in muscle
Despite the robust induction of many E2F targets, some key activators of G1/S progression were not induced. For instance, in CYD-1/CDK-4 expressing muscle, the induction factor (log2, mean of 4 experiments) was 0.11 for cye-1 Cyclin E, 0.04 for cdk-2, and -1.3 for cdc-25.1. These genes are well-established E2F targets in mammals, Drosophila, and, at least cye-1 and cdc-25.1, also in C. elegans [27], [28]. These observations suggest that in contrast to many other E2F targets, the actual regulators that promote cell cycle entry may be more tightly repressed.
We used single molecule FISH analysis to verify the gene expression analysis. This method makes use of approximately 48 individually labeled short oligonucleotide probes, which together allow detection of individual mRNA molecules [29]. As a first test, we examined myo-3 mRNA abundance. The control strain, expressing myo-3::H2B::GFP in muscle, and four different strains with muscle-induced G1 Cyclin/CDK expression, all showed readily detectable fluorescent myo-3 mRNA spots in the cytoplasm of muscle cells (Figure 6A–6C, 6G). Very similar numbers were obtained in different strains and experiments; illustrating that single molecule FISH gives reproducible results (Figure 6G, and data not shown). Next, we used this technique to examine mRNA levels of the E2F-target mcm-6. All muscle cells with G1 Cyclin/CDK expression showed substantial mcm-6 mRNA induction as early as 3 hrs of postembryonic L1 development, in agreement with the microarray data (Figure 6H). Induction of mcm-6 was observed in CYE-1/CDK-2AF and CYE-1/CDK-2 expressing strains, and in two independent CYD-1/CDK-4 strains, including one with low levels of CYD-1/CDK-4 expression in muscle (CYD-1/CDK-4 #2; Figure 6H, Figure S4).
Fig. 6. Single molecule FISH shows gene expression in individual muscle cells and limited cye-1 induction by CYD-1/CDK-4. 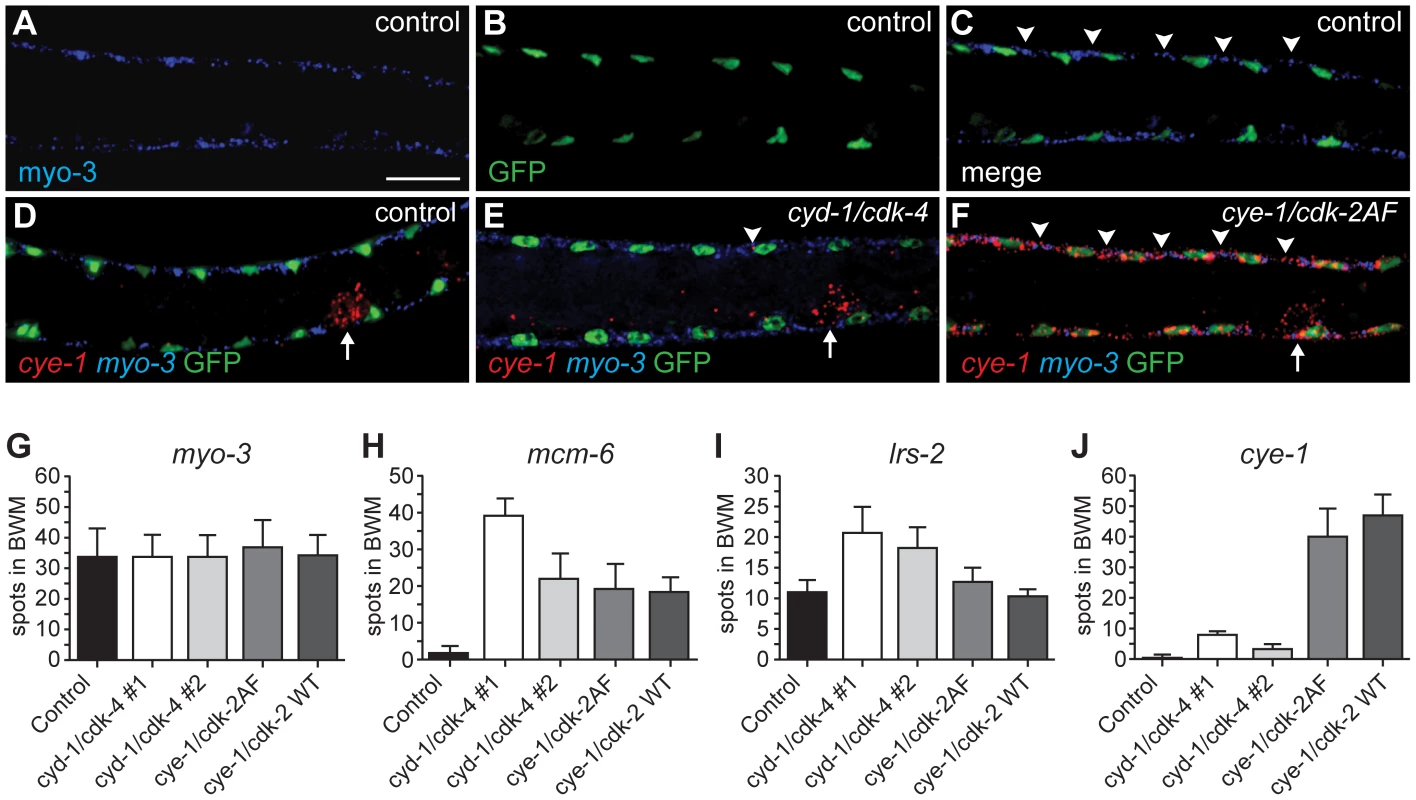
(A-F) Single mRNA molecules labeled with probes against myo-3 (blue) or cye-1 (red) mRNA. Muscle nuclei are marked in green by myo-3::H2B::GFP. Analyzed are strains expressing only Pmyo-3::H2B::GFP (control, SV859: A-D), and Pmyo-3::H2B::GFP together with Pmyo-3::CYD-1/CDK-4 (SV857: E) or Pmyo-3::CYE-1/CDK-2AF (SV858: F). Arrowheads indicate areas of myo-3 mRNA (C) or cye-1 mRNA (E, F) expression in dorsal body-wall muscle cells. Arrows in (D-F) indicate cye-1 expression in the gonad. Scale bar indicates 10 µm, all panels show the same magnification. (G-J) Quantification of the number of fluorescent spots in seven body-wall muscle cells located anterior of the gonad on the dorsal side of animals. All animals were hybridized with myo-3 probes to identify body-wall muscle cells. Signal corresponding to mRNA molecules of myo-3 (G), mcm-6 (H), lrs-2 (I), or cye-1 (J) were counted. The strains analyzed are SV859 (control), SV857 (cyd-1/cdk-4 #1), SV860 (cyd-1/cdk-4 #2), SV858 (cye-1/cdk-2AF) and SV861 (cye-1/cdk-2WT). Note that overlap between spots greatly reduced the counted number of myo-3 and mcm-6 mRNAs. In contrast, the few and small cye-1 labeled spots are likely all single mRNA molecules. The microarray data showed weak upregulation of the Leucyl amino-acyl tRNA synthetase gene lrs-2 in muscle with CYD-1/CDK-4 (induction factor 1.38) but not in CYE-1/CDK-2AF expressing muscle (induction 0.04). FISH experiments showed the same trend: strain 2 with only low CYD-1/CDK-4 expression showed lrs-2 upregulation, while even the high CYE-1/CDK-2 expressing strain did not differ from the control (Figure 6I). In contrast, cye-1 mRNA was nearly absent in CYD-1/CDK-4 expressing muscle cells, with occasionally a single or at most two small dots in muscle cells (Figure 6E, 6J). Thus, the single molecule FISH experiments support our conclusions from the microarray data, and show with single cell resolution that cye-1 mRNA expression is not even induced in a subset of muscle cells.
Discussion
In this study, we demonstrate that post-mitotic C. elegans muscle cells remain remarkably competent to express cell cycle genes, and that cell cycle re-entry can occur coincident with the differentiated state. In addition, we demonstrate differential activity of CYD-1/CDK-4 versus CYE-1/CDK-2 in the regulation of gene expression. These results challenge prevailing views on the irreversible commitment to cell cycle exit upon terminal differentiation and the linear pathway of CDK activities.
To our knowledge, our data show for the first time that the CYD-1/CDK-4 complex controls expression of metabolic genes, in addition to cell cycle genes. These transcriptome data fit well with observations that Cyclin D is directly controlled by mitogenic signals as the most upstream regulator in cell cycle entry (reviewed in [30]), and that Drosophila Cyclin D and Cdk4 predominantly act to promote growth rather than cell cycle progression [31], [32]. While many studies implicate Cyclin D and Cyclin E CDKs as regulators of the Rb/E2F module, our data provide strong support for CYD-1/CDK-4 control of additional transcription factors. Interestingly, Cyclin D has been observed to interact with several transcriptional regulators, to localize to promoters, and to affect transcription independently of CDK4/6 (e.g., [33]). It will be of great interest to find if these results relate to our observations, and what molecular mechanisms underlie the regulation of metabolic genes by Cyclin D/CDK4. As a potential substrate, several metabolic genes that are specifically up-regulated (e.g., acs-2) or down-regulated (e.g., ech-9/F01G10.3) by CYD-1/CDK-4 are transcriptional targets of NHR-49, a member of the Hepatocyte Nuclear Factor 4 (HNF4) family of nuclear hormone receptors [34].
CYD-1/CDK-4 also induced DNA replication in muscle, while CYE-1/CDK-2, in contrast, appeared to antagonize DNA synthesis. As a possible explanation, DNA synthesis might depend on a growth-related signal that is uniquely induced by CYD-1/CDK-4. Alternatively, or in addition, the different effects of the two Cyclin/CDK combinations may result from different roles in DNA replication origin licensing. CYE-1/CDK-2 antagonizes the formation of DNA pre-replication complexes (pre-RCs), at least in some cell types [35]-[37]. This activity helps to restrict DNA replication to only once per cell cycle. The observed induction of DNA replication in muscle cells indicates that CYD-1/CDK-4 does not share this CYE-1/CDK-2 function, but allows the formation and function of pre-RCs. In arrested intestinal cells, CYE-1 Cyclin E was sufficient to induce DNA replication, which points to an important difference in the arrested state of quiescent versus post-mitotic cells.
Progression from temporary arrest to irreversible cell cycle exit is thought to depend on epigenetic silencing of cell cycle promoters (e.g., [3]). Our transcriptional profiling indicates that such a repressive chromatin state can be reversed at many cell cycle promoters. As Cyclin/CDK expression does not appear to induce full cell division of body-wall muscle, additional safeguards probably inhibit proliferation. Our expression studies indicate that these safeguards focus on a limited number of critical regulators. Interestingly, expression of E2F in differentiating Drosophila eye and wing cells, and differentiation of mouse Rb-family triple knockout cells have also revealed additional levels of control that impinge on Cyclin E during terminal differentiation [19], [38].
Coincident differentiation and proliferation has been observed in some situations, such as proliferating horizontal interneurons in mouse retinoblastoma, proliferating pRb(-/-) hair cells of the mouse inner ear, and Drosophila wing hairs and eye cells expressing E2F and Cyclin E [6]-[8]. Our preliminary results indicate that CYD-1/CDK-4 expression also triggers S phase gene expression in differentiated C. elegans neurons (data not shown). Together, the different examples indicate potential for finding a common set of gene alterations that can induce cell proliferation in terminally differentiated cells. Despite the success of induced pluripotent stem cells, this could ultimately provide an attractive regeneration strategy, in particular if limited proliferation could be achieved without genome-wide remodeling of the chromatin and loss of the differentiated state.
Materials and Methods
Strains, molecular cloning, and transgenes
All strains and culture conditions are listed in Text S1. Expression constructs for cye-1, cyd-1, cdk-4, cdk-2, cdk-2AF, and GFP::H2B were created using a 2.4 Kb myo-3 promoter (PCGS1 (Pmyo-3), body-wall muscle expression) or a 5 kb elt-2 promoter (intestinal expression [39]). Coding sequences of Venus YFP with C. elegans-optimized codons (a kind gift of Yuichi Iino) were inserted before the translational stop in all CDK constructs. The CDK-2AF mutant was created by mutating two conserved Wee1 phosphorylation sites (Thr25-to-Ala and Tyr26-to-Phe) by site-directed mutagenesis. Pmcm-4::MCM-4::mCherry::mcm-4 3′UTR was recently described [22] and integrated as a single copy using the MosSCI technique [40]. The Prnr::CYB-1DesBox::TdTomato marker expresses an N-terminal 100 amino acid part of C. elegans CYB-1 (N-CYB-1), which harbors a KEN destruction box sequence for recognition by the APC/C coupled to the TdTomato fluorophore [22]. Multiple transgenic lines were analyzed for each Cyclin/CDK combination. Representative lines with 40-70% F2 transmission were selected for γ-irradiation. Integrated lines were backcrossed with N2 a minimum of 4 times before analysis.
Immunostaining and detection of DNA replication
For immunostaining of larval stages, animals were fixed in methanol (5 min. at −20°C) and acetone (20 minutes at −20°C) according to [22]. BrdU and EdU staining were performed as described [22], [41]. Primary antibodies used: rabbit anti-phospho-H3S10 (1∶200, Abcam), mouse anti-GFP (1∶100, Sigma), mouse anti-CYE-1 (E. Kipreos, 1∶200), rabbit anti-GFP (1∶100, Molecular Probes), mouse monoclonal antibody 5-23 to UNC-15 (1∶3, tissue supernatant, Developmental Studies Hybridoma Bank). Secondary antibodies used: Donkey anti-mouse FITC or TexasRed and Donkey anti-rabbit FITC or TexasRed (1∶200, Jackson Immunolaboratories).
Quantitative DNA measurements were performed as previously described [41]. In short, series of Z-sections were taken of propidium iodide-stained animals with a confocal scanning laser microscope, and pixel intensities of all sections were added and recalculated to DNA content, using Pn.p ventral cord precursor nuclei as a 2n DNA standard.
Tissue-specific microarray analysis
Tissue-specific mRNA was isolated from synchronized L1 larvae fed for 3 hours by purification of the poly-A binding protein FLAG::PAB-1 specifically expressed in body-wall muscle cells of L1 larvae [25]. Poly(A)+ RNA was isolated essentially as described [25]. Four independently grown biological samples were used for each different line: SV912 (control), SV911 (CYE-1/CDK-2AF), and SV985 (CYD-1/CDK-4). Array data is submitted to ArrayExpress accession no.: E-TABM-886. Procedures used in the hybridizations, data analysis and transcription factor analysis are detailed in Text S1.
Functional analysis
Manual annotation of genes upregulated >2-fold was done using WormBase (release WS207). GO-term enrichment was determined using Funcassociate [26].
Single-molecule Fluorescence In Situ Hybridization
Single molecule Fluorescence In Situ Hybridization was performed as described by [29]. Starved L1 animals were fed for three hours on OP50, followed by fixation in 4% formaldehyde and hybridization with sets of the 48 labeled oligonucleotide probes. Images were taken with a DeltaVision Core wide-field microscope, quantification of the number of mRNAs was performed using the 3D imaging software Volocity version 5 (Perkin Elmer). For quantification, a region of interest was drawn around seven body-wall muscle nuclei, located anteriorly from the gonad on the dorsal side of the animal, to exclude cells in the ventral nerve cord and mesoblast lineage. Further details on the procedure, microscopy and data analysis are provided in Text S1.
Supporting Information
Zdroje
1. van den HeuvelSDysonNJ 2008 Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 9 713 724
2. CobrinikD 2005 Pocket proteins and cell cycle control. Oncogene 24 2796 2809
3. JacobsJJKieboomKMarinoSDePinhoRAvan LohuizenM 1999 The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397 164 168
4. BlaisAvan OevelenCJMargueronRAcosta-AlvearDDynlachtBD 2007 Retinoblastoma tumor suppressor protein-dependent methylation of histone H3 lysine 27 is associated with irreversible cell cycle exit. J Cell Biol 179 1399 1412
5. ButtittaLAEdgarBA 2007 Mechanisms controlling cell cycle exit upon terminal differentiation. Curr Opin Cell Biol 19 697 704
6. ButtittaLAKatzaroffAJPerezCLde la CruzAEdgarBA 2007 A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev Cell 12 631 643
7. SageCHuangMKarimiKGutierrezGVollrathMA 2005 Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 307 1114 1118
8. AjiokaIMartinsRABayazitovITDonovanSJohnsonDA 2007 Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell 131 378 390
9. XuXLFangYLeeTCForrestDGregory-EvansC 2009 Retinoblastoma has properties of a cone precursor tumor and depends upon cone-specific MDM2 signaling. Cell 137 1018 1031
10. SulstonJEHorvitzHR 1977 Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol 56 110 156
11. SulstonJESchierenbergEWhiteJGThomsonJN 1983 The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol 100 64 119
12. ParkMKrauseMW 1999 Regulation of postembryonic G(1) cell cycle progression in Caenorhabditis elegans by a cyclin D/CDK-like complex. Development 126 4849 4860
13. BoxemMvan den HeuvelS 2001 lin-35 Rb and cki-1 Cip/Kip cooperate in developmental regulation of G1 progression in C. elegans. Development 128 4349 4359
14. HongYRoyRAmbrosV 1998 Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125 3585 3597
15. LuXHorvitzHR 1998 lin-35 and lin-53, two genes that antagonize a C. elegans Ras pathway, encode proteins similar to Rb and its binding protein RbAp48. Cell 95 981 991
16. BoxemMvan den HeuvelS 2002 C. elegans class B synthetic multivulva genes act in G(1) regulation. Curr Biol 12 906 911
17. SaitoRMPerreaultAPeachBSatterleeJSvan den HeuvelS 2004 The CDC-14 phosphatase controls developmental cell-cycle arrest in C. elegans. Nat Cell Biol 6 777 783
18. BuckSHChiuDSaitoRM 2009 The cyclin-dependent kinase inhibitors, cki-1 and cki-2, act in overlapping but distinct pathways to control cell cycle quiescence during C. elegans development. Cell Cycle 8
19. WirtSEAdlerASGebalaVWeimannJMSchafferBE 2010 G1 arrest and differentiation can occur independently of Rb family function. J Cell Biol 191 809 825
20. FireAWaterstonRH 1989 Proper expression of myosin genes in transgenic nematodes. EMBO J 8 3419 3428
21. FoxRMWatsonJDVon StetinaSEMcDermottJBrodiganTM 2007 The embryonic muscle transcriptome of Caenorhabditis elegans. Genome Biol 8 R188
22. KorzeliusJTheIRuijtenbergSPortegijsVXuH 2011 C. elegans MCM-4 is a general DNA replication and checkpoint component with an epidermis-specific requirement for growth and viability. Dev Biol 350 358 369
23. SalicAMitchisonTJ 2008 A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A 105 2415 2420
24. MoermanDGWilliamsBD 2006 Sarcomere assembly in C. elegans muscle. WormBook 1 16
25. RoyPJStuartJMLundJKimSK 2002 Chromosomal clustering of muscle-expressed genes in Caenorhabditis elegans. Nature 418 975 979
26. BerrizGFKingODBryantBSanderCRothFP 2003 Characterizing gene sets with FuncAssociate. Bioinformatics 19 2502 2504
27. KirienkoNVFayDS 2007 Transcriptome profiling of the C. elegans Rb ortholog reveals diverse developmental roles. Dev Biol 305 674 684
28. DuronioRJO'FarrellPH 1995 Developmental control of the G1 to S transition in Drosophila: cyclin E is a limiting downstream target of E2F. Genes Dev 9 1456 1468
29. RajAvan den BogaardPRifkinSAvan OudenaardenATyagiS 2008 Imaging individual mRNA molecules using multiple singly labeled probes. Nat Methods 5 877 879
30. SherrCJ 2004 Principles of tumor suppression. Cell 116 235 246
31. DatarSAJacobsHWde la CruzAFLehnerCFEdgarBA 2000 The Drosophila cyclin D-Cdk4 complex promotes cellular growth. EMBO J 19 4543 4554
32. MeyerCAJacobsHWDatarSADuWEdgarBA 2000 Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J 19 4533 4542
33. BienvenuFJirawatnotaiSEliasJEMeyerCAMizerackaK 2010 Transcriptional role of cyclin D1 in development revealed by a genetic-proteomic screen. Nature 463 374 378
34. Van GilstMRHadjivassiliouHJollyAYamamotoKR 2005 Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol 3 e53 doi:10.1371/journal.pbio.0030053
35. MailandNDiffleyJF 2005 CDKs promote DNA replication origin licensing in human cells by protecting Cdc6 from APC/C-dependent proteolysis. Cell 122 915 926
36. KimJFengHKipreosET 2007 C. elegans CUL-4 prevents rereplication by promoting the nuclear export of CDC-6 via a CKI-1-dependent pathway. Curr Biol 17 966 972
37. KorzeliusJvan den HeuvelS 2007 Replication licensing: oops!... I did it again. Curr Biol 17 R630 632
38. ButtittaLAKatzaroffAJEdgarBA 2010 A robust cell cycle control mechanism limits E2F-induced proliferation of terminally differentiated cells in vivo. J Cell Biol 189 981 996
39. FukushigeTHawkinsMGMcGheeJD 1998 The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev Biol 198 286 302
40. Frokjaer-JensenCDavisMWHollopeterGTaylorJHarrisTW Targeted gene deletions in C. elegans using transposon excision. Nat Methods 7 451 453
41. BoxemMSrinivasanDGvan den HeuvelS 1999 The Caenorhabditis elegans gene ncc-1 encodes a cdc2-related kinase required for M phase in meiotic and mitotic cell divisions, but not for S phase. Development 126 2227 2239
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 11- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
-
Všechny články tohoto čísla
- De Novo Origins of Human Genes
- Duplication Hotspots Are Associated with Late-Replicating Regions of the Genome
- De Novo Origin of Human Protein-Coding Genes
- Cyclin D/CDK4 and Cyclin E/CDK2 Induce Distinct Cell Cycle Re-Entry Programs in Differentiated Muscle Cells
- Short Day–Mediated Cessation of Growth Requires the Downregulation of AINTEGUMENTALIKE1 Transcription Factor in Hybrid Aspen
- Physiological IRE-1-XBP-1 and PEK-1 Signaling in Larval Development and Immunity
- Role of Pirh2 in Mediating the Regulation of p53 and c-Myc
- Signatures of Environmental Genetic Adaptation Pinpoint Pathogens as the Main Selective Pressure through Human Evolution
- FOXO Regulates Organ-Specific Phenotypic Plasticity In
- Heritable Epigenetic Variation among Maize Inbreds
- Foxn1 Regulates Lineage Progression in Cortical and Medullary Thymic Epithelial Cells But Is Dispensable for Medullary Sublineage Divergence
- Attenuation of the Sensing Capabilities of PhoQ in Transition to Obligate Insect–Bacterial Association
- A Novel Protein LZTFL1 Regulates Ciliary Trafficking of the BBSome and Smoothened
- Activation of Bmp2-Smad1 Signal and Its Regulation by Coordinated Alteration of H3K27 Trimethylation in -Induced Senescence
- Histone H3K56 Acetylation, CAF1, and Rtt106 Coordinate Nucleosome Assembly and Stability of Advancing Replication Forks
- The SUN Protein Mps3 Is Required for Spindle Pole Body Insertion into the Nuclear Membrane and Nuclear Envelope Homeostasis
- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- Effect of Host Species on the Distribution of Mutational Fitness Effects for an RNA Virus
- Pch2 Acts through Xrs2 and Tel1/ATM to Modulate Interhomolog Bias and Checkpoint Function during Meiosis
- SOX9 Governs Differentiation Stage-Specific Gene Expression in Growth Plate Chondrocytes via Direct Concomitant Transactivation and Repression
- from the Aphid : A Missing Link from Facultative to Obligate Insect Endosymbiont
- Recessive Antimorphic Alleles Overcome Functionally Redundant Loci to Reveal Function in Flowers and Meristems
- Over-Expression of DSCAM and COL6A2 Cooperatively Generates Congenital Heart Defects
- Consequences of Eukaryotic Enhancer Architecture for Gene Expression Dynamics, Development, and Fitness
- Distinct Genetic Architectures for Male and Female Inflorescence Traits of Maize
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- For Male , Sperm Activation Is a “Just-in-Time” Event
- PcG Complexes Set the Stage for Epigenetic Inheritance of Gene Silencing in Early S Phase before Replication
- The Gene Contains Hotspots for L1 Endonuclease-Dependent Insertion
- Relative Burden of Large CNVs on a Range of Neurodevelopmental Phenotypes
- Multiple Means to the Same End: The Genetic Basis of Acquired Stress Resistance in Yeast
- Genome-Wide Crossover Distribution in Meiosis Reveals Sex-Specific Patterns along Chromosomes
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
- Homologs of Retinoblastoma-Associated Protein 46/48 Associate with a Histone Deacetylase to Act Redundantly in Chromatin Silencing
- Genetic Interaction Maps in Reveal Functional Crosstalk among Cell Envelope Biogenesis Pathways
- The ERI-6/7 Helicase Acts at the First Stage of an siRNA Amplification Pathway That Targets Recent Gene Duplications
- PBX1 Genomic Pioneer Function Drives ERα Signaling Underlying Progression in Breast Cancer
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Evidence-Based Annotation of Gene Function in MR-1 Using Genome-Wide Fitness Profiling across 121 Conditions
- De Novo Origins of Human Genes
- Capture of MicroRNA–Bound mRNAs Identifies the Tumor Suppressor miR-34a as a Regulator of Growth Factor Signaling
- TRY-5 Is a Sperm-Activating Protease in Seminal Fluid
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



