-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
A Coastal Cline in Sodium Accumulation in Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1
The genetic model plant Arabidopsis thaliana, like many plant species, experiences a range of edaphic conditions across its natural habitat. Such heterogeneity may drive local adaptation, though the molecular genetic basis remains elusive. Here, we describe a study in which we used genome-wide association mapping, genetic complementation, and gene expression studies to identify cis-regulatory expression level polymorphisms at the AtHKT1;1 locus, encoding a known sodium (Na+) transporter, as being a major factor controlling natural variation in leaf Na+ accumulation capacity across the global A. thaliana population. A weak allele of AtHKT1;1 that drives elevated leaf Na+ in this population has been previously linked to elevated salinity tolerance. Inspection of the geographical distribution of this allele revealed its significant enrichment in populations associated with the coast and saline soils in Europe. The fixation of this weak AtHKT1;1 allele in these populations is genetic evidence supporting local adaptation to these potentially saline impacted environments.
Published in the journal: . PLoS Genet 6(11): e32767. doi:10.1371/journal.pgen.1001193
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001193Summary
The genetic model plant Arabidopsis thaliana, like many plant species, experiences a range of edaphic conditions across its natural habitat. Such heterogeneity may drive local adaptation, though the molecular genetic basis remains elusive. Here, we describe a study in which we used genome-wide association mapping, genetic complementation, and gene expression studies to identify cis-regulatory expression level polymorphisms at the AtHKT1;1 locus, encoding a known sodium (Na+) transporter, as being a major factor controlling natural variation in leaf Na+ accumulation capacity across the global A. thaliana population. A weak allele of AtHKT1;1 that drives elevated leaf Na+ in this population has been previously linked to elevated salinity tolerance. Inspection of the geographical distribution of this allele revealed its significant enrichment in populations associated with the coast and saline soils in Europe. The fixation of this weak AtHKT1;1 allele in these populations is genetic evidence supporting local adaptation to these potentially saline impacted environments.
Introduction
Uncovering the genetic polymorphisms that underlie adaptation to environmental gradients is a critical goal in evolutionary biology, and will lead to a better understanding of both the types of genetic changes and the gene functions involved. Such understanding will not only provide insight into how organisms may respond to future global climate change, but will also provide tools for the development of agricultural systems and ecological services that are more resilient to such changes.
Patterns of phenotypic diversity across environmental gradients can be indicative of adaptive responses to selection, and evaluation of these patterns has the potential to lead to the identification of the genetic polymorphisms underlying these adaptive responses. Numerous studies in animals and plants have identified phenotypic clines in various life history traits, but only a few have determined the genetic changes driving such traits. In Arabidopsis thaliana, plasticity in seasonally regulated flowering appears to be modulated by a network of gene interactions responsive to both vernalization and photoperiod signals [1]. Adaptive clines in resistance to oxidative stress and chilling [2], and wing size [3] in Drosophila melanogaster are modulated by the Insulin-like Receptor (InR) and Drosophila cold acclimation (Dca) genes, respectively. While adaptation to high altitude in Peromyscus maniculatus (Deer mice) is associated with enhanced pulmonary O2 loading driven by alterations in α-globin and β-globin genes [4]. These genetic changes are all associated with adaptation to variation in environmental factors that vary with latitude or altitude. Such systematic variation has greatly facilitated the discovery of these loci and their adaptive significance. Clines in various life history traits have also been identified in plants growing on serpentine [5], saline [6], [7], and mine impacted soils [8]. Progress has been made in outlining the genetic architecture of these adaptive traits [5], [8]–[10], though a molecular genetic understanding is still needed.
A. thaliana is broadly distributed in its native Europe and central Asia, where it experiences a wide range of altitudinal, climatic, and edaphic conditions, leading to a range of selective pressures [11]. Whether the wide variety of natural phenotypic and genetic variation observed in A. thaliana [12] contributes to its local adaptation is an important unresolved question that is currently attracting a significant amount of attention [13].
Because of its relevance to crop production, salinity tolerance in plants has been studied intensively [14], and natural plant populations adapted to such conditions have provided an excellent system for studying the evolutionary mechanisms of adaptation and speciation in coastal [6], [10] and salt marsh [7], [9], [15]–[18] environments. The primary effects of excess Na+ on plants are water deficit resulting from a water potential gradient between the soil solution and plant cells, and cytotoxicity due of intracellular Na+ accumulation [14]. To overcome these effects plants must both accumulate solutes for osmotic regulation, and detoxify intracellular Na+ either by limiting its accumulation, or by compartmentalizing Na+ into the vacuole. In addition, Na+ compartmentalization facilitates vacuolar osmotic adjustment that is necessary to compensate for the osmotic effects of salinity by maintaining turgor pressure for cell expansion and growth. Plants therefore need to strike a balance between the accumulation of Na+ to maintain turgor, and the need to avoid Na+ chemical toxicity, and this balance will depend in part on soil salinity levels. Given the critical role Na+ accumulation plays in salinity tolerance, we used this life history trait to probe the global A. thaliana population for signals of adaptive selection for growth in saline impacted environments.
Results/Discussion
We grew 349 accessions of A. thaliana in a controlled common garden in non-saline soil, and analyzed leaf Na+ accumulation. We observed a wide range of leaf Na+ accumulation across the accessions (330–4,848 mg kg−1 dry weight). If this natural variation in leaf Na+ accumulation capacity is related to adaptation to growth in saline soils we would expect to find evidence of an adaptive cline, or a gradient of leaf Na+ accumulation that correlates with the geographical distribution of variation in soil salinity. Salinity impacted soils are expected to occur in coastal regions due to air born deposition of sea spray which can occur many tens of km inland [19]–[22], but can also occur in areas distant from the coast through high Na+ in the soil or ground water. Elevated soil salinity can also be caused by inappropriate irrigation practices such as irrigation with saline water or poor drainage.
To test for the existence of an adaptive cline in leaf Na+ accumulation capacity and soil salinity we related leaf Na+ accumulation capacity to the distance of the collection site for each accession to the coast, or to the nearest known saline soil, whichever is the shortest. We focused on European accessions since a good soil salinity map exists for this region [23], which left 300 accessions. Regressing the distance to the coast, or nearest known saline soil, on leaf Na+ for all 300 accessions revealed a significant relationship (p-value<2e-12), establishing that accessions with elevated leaf Na+ are more likely to grow in potentially saline impacted soils (Figure 1A and 1B).
Fig. 1. A coastal/saline soil cline in leaf Na+ accumulation in A. thaliana driven by natural variation at the AtHKT1;1 locus. 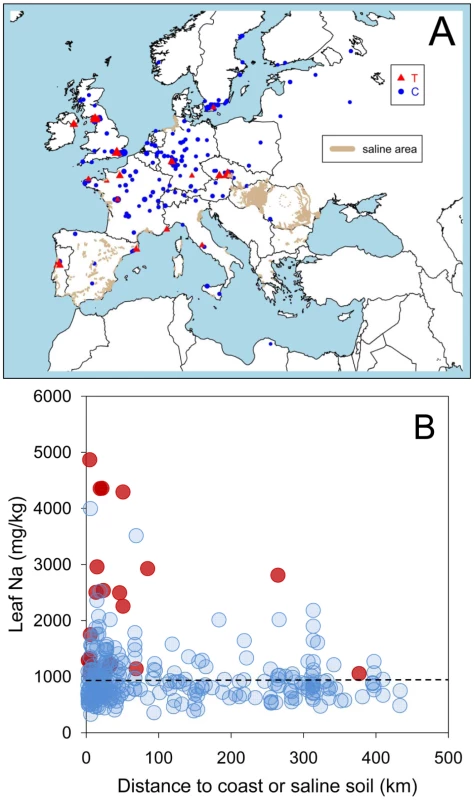
A. Map showing the geographical position for the collection site of 300 accessions of A. thaliana. The genotype at Chr4:6392276 of each accession is represented by the type of symbol (red triangle Chr4:6392276 = T, blue circle Chr4:6392276 = C). Leaf Na+ accumulation of each accession, measured in a common garden experiment, is represented by the size of the symbols. Known areas of saline soils were obtained from Tóth et al., [23] and are represented here in green. B. Leaf Na+ accumulation in 300 accessions of A. thaliana, measured in a common garden experiment, and its relationship with the distance to the collection site of each accession to the coast or known saline soils (whichever is the smallest). Red symbols genotype at Chr4:6392276 = T, blue symbols genotype at Chr4:6392276 = C. Dashed black line represents the average leaf Na+ for the Col-0 accession. To investigate the genetic architecture underlying this cline in leaf Na+ accumulation capacity we performed a genome-wide association (GWA) study (previously described for a smaller data set [24]) to identify regions of the genome at which genetic variation is associated with leaf Na+ accumulation capacity. The 337 A. thaliana accessions used in our GWA study, which are a subset of the 349 accessions phenotyped for leaf Na+, were genotyped using the Affymetrix SNP-tilling array Atsnptile1a which can interrogate 248,584 SNPs. To assess evidence of association between SNPs and leaf Na+ accumulation we used a mixed-model approach [25] to correct for population structure, as previously described [24]. In the current analysis we identified a single strong peak of SNPs associated with leaf Na+, with the peak centered on AtHKT1;1 (Figure 2), a gene known to encode a Na+-transporter [26]. Accessions with a thymine (T) at the SNP most significantly associated with leaf Na+ at position 6392276 bp on chromosome 4 (Chr4 : 6392276) have significantly higher leaf Na+ than accessions with a cytosine (C) at this same position (2,325 vs. 955 mg Na+ kg−1 dry weight, p-value<2e-16). This SNP explains 32% (without accounting for population structure) of the total variation in leaf Na+ accumulation observed.
Fig. 2. Genome-wide association analysis of leaf Na+ accumulation in 349 A. thaliana accessions grown in a common garden. 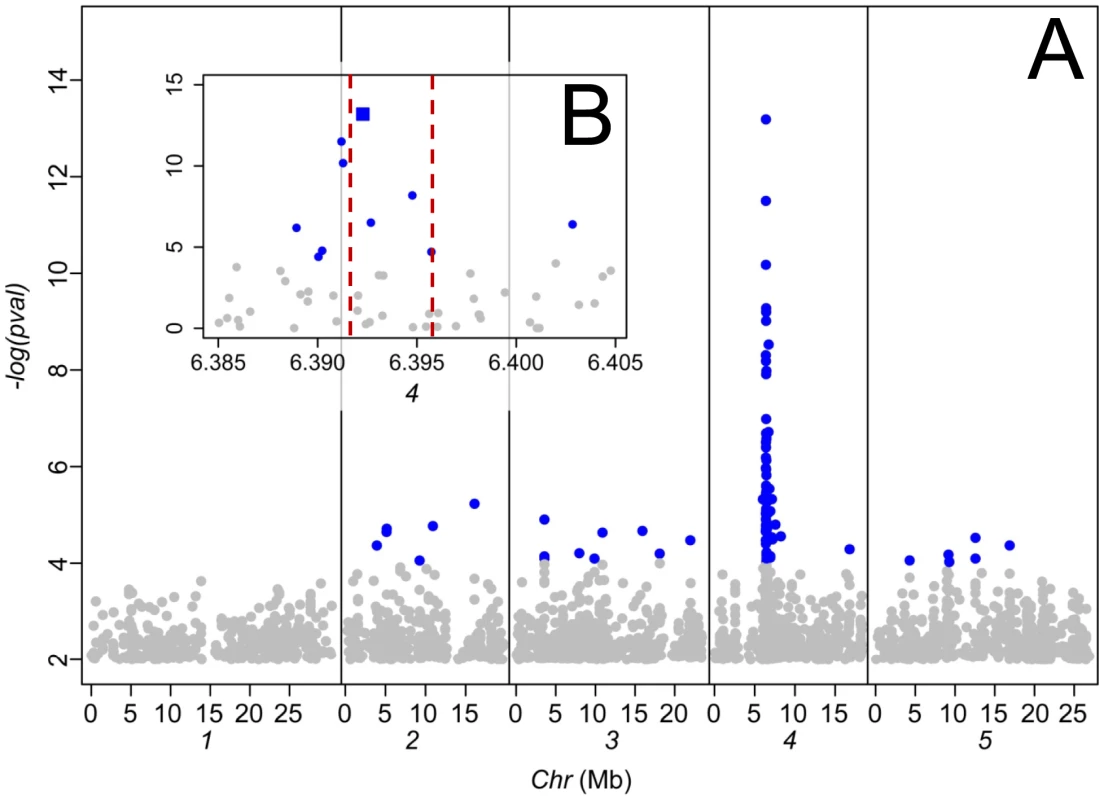
A. Genome-wide p-values from a mixed model analysis implemented in EMMA [24]. Associations with a p-value>0.001 are shaded in grey. B. Magnification of the genomic region surrounding AtHKT1;1, the position and extent of which is indicated by vertical lines (locus At4g10310 Chr4:6391984–6395877). The SNP most significantly associated with leaf Na+ accumulation (Chr4:6392276) is represented by a square symbol, all other SNPs represented by circles. Mb, megabase. Previously, in independent test crosses between the high leaf Na+ accessions Ts-1 and Tsu-1 (both containing a T at Chr4 : 6392276) and the low leaf Na+ accession Col-0 (containing a C at Chr4 : 6392276) QTLs for leaf Na+ centered on AtHKT1;1 were identified in both F2 populations [27]. Such genetic evidence provides independent support that the peak of SNPs associated with leaf Na+ observed in our GWA analysis, centered at AtHKT1;1 (Figure 2), represents a true positive association and not a false positive driven by the high degree of population structure known to exist in A. thaliana [24]. Reduced expression of AtHKT1;1 in Ts-1 and Tsu-1 was concluded to drive the elevated leaf Na+ observed in these two accessions [27]. Here, we expand on this observation by establishing the strength of the AtHKT1;1 alleles in four further high Na+ accumulating accessions (Bur-1, Duk, PHW-20 and UKNW06-386) that all contain a T at Chr4 : 6392276, along with a low leaf Na+ accession (Nd-1) with a C at Chr4 : 6392276. By examining the leaf Na+ accumulation in F1 plants from crosses of each of these accessions to Col-0hkt1-1 and Col-0HKT1, we were able to establish a significant correlation between leaf Na+ accumulation and the strength of the AtHKT1;1 alleles (Figure 3A). These crosses confirmed that all accessions tested with elevated leaf Na+, and that contain a T at Chr4 : 6392276, have hypofunctional alleles of AtHKT1;1 relative to the Col-0 allele. Furthermore, analysis of the expression of AtHKT1;1 in the same set of accessions revealed that allelic variation in AtHKT1;1 strength is modulated at the level of gene expression (Figure 3B), consistent with what was previously observed for Ts-1 and Tsu-1 [27]. Though the SNP most significantly associated with leaf Na+ (Chr4 : 6392276) is unlikely to be causal for these AtHKT1;1 expression level polymorphisms, this SNP can be used as a linked genetic marker to determine the type of AtHKT1;1 allele present, with a T at this SNP being associated with weak AtHKT1;1 alleles.
Fig. 3. AtHKT1;1 allele strength in various A. thaliana accessions assessed by genetic complementation and expression level. 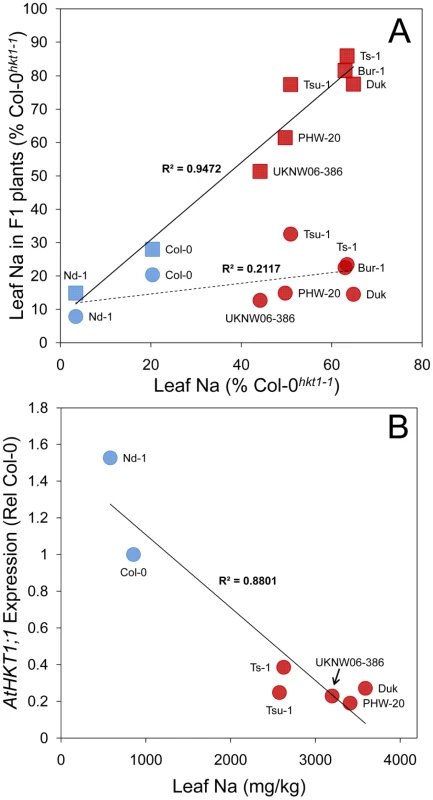
A. Leaf Na+ accumulation of F1 plants generated from crosses of various accessions to either Col-0hkt1-1 (squares) or Col-0HKT1 (circles) compared to leaf Na+ accumulation of the non-Col parent of the cross, and represented as a percentage of leaf Na+ accumulation of Col-0hkt1-1. Solid line represents linear regression of data represented by square symbols, dashed line represents linear regression of data represented by circular symbols. B. Root expression level of AtHKT1;1 in various accessions as determined by qRT-PCR, represented as relative to AtHKT1;1 expression in Col-0, and compared to leaf Na+ accumulation. Red symbols genotype at Chr4:6392276 = T, blue symbols genotype at Chr4:6392276 = C. Using the SNP at Chr4 : 6392276 as a genetic marker for the type of AtHKT1;1 allele (strong or weak) allowed us to test the hypothesis that the leaf Na+ soil salinity cline we observe in European populations of A. thaliana (Figure 1A and 1B) is associated with weak alleles of AtHKT1;1. By comparing the means of distances to the coast, or known saline soil, for the collection site of all 300 accessions with and without a T at Chr4 : 6392276, we determined that a significant association (parametric test p-value = 0.0001; non-parametric Wilcoxon rank-sum test p-value = 0.0062) exists between A. thaliana growing on potentially saline impacted soils and the presence of a weak allele of AtHKT1;1 (Figure 1A and 1B). Such a strong correlation between the presence of allelic variation at AtHKT1;1 known to drive elevated leaf Na+, and the observed cline in leaf Na+ and saline soils, is evidence for the involvement of AtHKT1;1 in determining this geographical distribution. Furthermore, using 13 SNPs within a 20kb region centered on HKT1;1 to define the HKT1;1 haplotype, we identify 7 haplotypes (6 if you combine haplotypes with only 1 SNP different) in accessions with high leaf Na+ (>2,500 ppm), suggesting that weak alleles of HKT1;1 have arisen independently multiple times.
However, to be credible it is also important to provide evidence that selection for growth on saline soils could be acting on the phenotype driven by allelic variation at AtHKT1;1; in this case elevated leaf Na+. Such evidence is provided by the previous observation that the weak allele of AtHKT1;1 in the coastal Tsu-1 A. thaliana accession not only causes elevated leaf Na+ but is also genetically linked to the elevated salinity tolerance of this accession [27].
In A. thaliana AtHKT1;1 functions to unload Na+ from xylem vessels in the root, controlling translocation and accumulation of Na+ in the shoots [26], [28]. Therefore, modulation of its function would allow the balancing of Na+ accumulation in the shoot with soil salinity. We note here that the hkt1-1 null mutation in the Col-0 background causes plants to exhibit dramatic leaf Na+ hyperaccumulation and increased NaCl sensitivity [29], [30]. We interpret this to mean that expression of AtHKT1;1 in the hkt1-1 null mutant is reduced to such an extent that leaf Na+ accumulation saturates the capacity for cellular detoxification of Na+ by vacuolar compartmentalization. We propose that the naturally occurring weak alleles of AtHKT1;1, that we show are associated with populations growing in potentially saline impacted environments, allow sufficient Na+ to accumulate in leaves for osmotic adjustment, conferring elevated Na+ tolerance. However, these weak, but not complete loss-of-function AtHKT1;1 alleles, do not saturate the mechanism whereby the accessions avoid Na+ cytotoxicity. The basis of this Na+ detoxification mechanism remains to be determined, though an active leaf vacuolar Na+ compartmentalization mechanism driven by AtNHX1 is one likely candidate.
In conclusion, here we provide evidence supporting the involvement of specific cis-regulatory polymorphisms at AtHKT1;1 in the potentially adaptive cline in leaf Na+ accumulation capacity we observe in A. thaliana populations to saline impacted environments. We have identified a strong association between the AtHKT1;1 allele frequency in A. thaliana populations and their growth on potentially saline impacted soils (Figure 1A and 1B). Further, we have confirmed by GWA mapping, experimental complementation crosses, and gene expression studies, that this allelic variation directly causes changes in the clinally varying leaf Na+ accumulation phenotype via cis-regulatory polymorphisms (Figure 2 and Figure 3). And, finally, we have previously established that the weak AtHKT1;1 alleles we show to be associated with potentially saline soils, are also linked to elevated salinity tolerance [27], providing a plausible mechanistic link between selection for growth on saline soils and variation in AtHKT1;1 allele frequency. Such discoveries provide tantalizing evidence that points to selection acting at AtHKT1;1 in natural populations of A. thaliana in adaptation to growth in saline environments.
Materials and Methods
Plant Material and Plant Growth Conditions
Plants were grown in a controlled environment with 10 h light/14 h dark (90 µmol m−2s−1 photosynthetically active light) and 19 to 22°C, as previously described [31]. Briefly, seeds were sown onto moist soil (Promix; Premier Horticulture) in 10.5″×21″ 20 row trays with various elements added to the soil at subtoxic concentrations (As, Cd, Co, Li, Ni, Rb, and Se [31]) and the tray placed at 4°C for 3 days to stratify the seeds and help synchronize germination. Each tray contained 108 plants, six plants each from 18 accessions, with three plants of each accession planted in two different parts of the tray. Each tray contained four common accessions (Col-0, Cvi-0, Fab-2 and Ts-1) used as controls, and 14 test accessions. Trays were bottom-watered twice per week with 0.25-strength Hoagland solution in which Fe was replaced with 10 µM Fe-HBED[N,N′-di(2-hydroxybenzyl)ethylenediamine-N,N′-diacetic acid monohydrochloride hydrate; Strem Chemicals, Inc.). After 5 weeks plants were non-destructively sampled by removing one or two leaves and the elemental composition of the tissue analyzed by Inductively Couple Plasma Mass Spectroscopy (ICP-MS). The plant material was rinsed with 18 MΩ water and placed into Pyrex digestion tubes. For complementation experiments plants were crossed to Col-0 or Col-0hkt1-1 and approximately 12 F1 plants were grown in the conditions described above.
Mapping Population
A set of 360 A. thaliana accessions were selected from 5,810 worldwide accessions to minimizing redundancy and close family relatedness, based on the genotypes at 149 SNPs developed in a previous study [32]. Figure S1 and Table S1 show the genetic variation in the core set of 360 accessions vs. a random set of 360 accessions chosen from the genotyped 5,810 accessions. From the selected core set of 360 accessions a subset of 349 were phenotyped using ICP-MS, and of these 337 were genotyped using the Affymetrix SNP-tilling array Atsnptile1 which contains probe sets for 248,584 SNPs. Details of the SNP-tilling array and methods for array hybridization and SNP-calling are the same as previously described [24]. In brief, approximately 250 ng of genomic DNA was labeled using the BioPrime DNA labeling system (Invitrogen) and 16 µg of the labelled product hybridized to each array. SNPs were called using the Oligo package after slight modifications. Quality control (QC) of the genotypes, and imputation of the missing SNPs were performed following the procedure previously described [24], except that a 15% mismatch rate was used to filter out low quality arrays. After QC and imputation, the 337 accessions had genotypes for at least 213,497 SNPs. The core set of 360 accessions selected are all available from the Arabidopsis Biological Resource Center (http://abrc.osu.edu/), and the SNP genotypes for the 337 accessions used for the GWA study are available from http://borevitzlab.uchicago.edu/resources/genetic/hapmap/BaxterCore/.
Tissue Elemental Analysis
Samples were analyzed as described by Lahner et al. [31]. Tissue samples were dried at 92°C for 20 h in Pyrex tubes (16×100 mm) to yield approximately 2–4 mg of tissue for elemental analysis. After cooling, seven of the 108 samples from each sample set were weighed. All samples were digested with 0.7 ml of concentrated nitric acid (OmniTrace; VWR Scientific Products), and diluted to 6.0 ml with 18 MΩ water. Elemental analysis was performed with an ICP-MS (Elan DRCe; PerkinElmer) for Li, B, Na, Mg, P, S, K, Ca, Mn, Fe, Co, Ni, Cu, Zn, As, Se, Rb, Mo, and Cd. A liquid reference material composed of pooled samples of A. thaliana leaves was run every 9th sample to correct for ICP-MS run to run variation and within-run drift. All samples were normalized to the calculated weights, as determined with an iterative algorithm using the best-measured elements, the weights of the seven weighed samples, and the solution concentrations, implemented in the Purdue Ionomics Information Management System (PiiMS) [33] (for a full description see www.ionomicshub.org). Data for all elements is available for viewing and download at www.ionomicshub.org in trays 1478–1504.
Quantification of AtHKT1;1 mRNA
To quantify the levels of AtHKT1;1 mRNA in roots of the various accessions studied, we used a protocol similar to that of Rus et al. [27]. Roots from plants grown under identical conditions to those used for ICP-MS analysis were separated from the shoots and rinsed thoroughly with deionized water to remove any soil contamination. The samples were frozen in liquid nitrogen and stored at −80°C until extraction. Total RNA was extracted, and DNase digestion was performed during the extraction, using the Invitrogen PureLink RNA Mini Kit. Two micrograms of total RNA were used as a template to synthesize first-strand cDNA with random hexamers, using SuperScript II Reverse Transcriptase (Invitrogen Life Technologies). Quantitative real-time PCR (qRT-PCR) was performed with first strand cDNA as a template on four technical replicates from three independent biological samples for each accession, using a sequence detector system (StepOne Plus, Applied Biosystems). For normalization across samples within a qRT-PCR run the expression of the Actin 1 gene (At2g37620) was used with the following primers: CPRD66, 5′-TGG AAC TGG AAT GGT TAA GGC TG-3′ and CPRD67, 5′-TCT CCA GAG TCG AGC ACA ATA C-3′. For quantification of AtHKT1;1 the following primers were used: HKT-RTF, 5′-TGG GAT CTT ATA ATT CGG ACA GTT C-3′ and HKT-RTR, 5′-GAT AAG ACC CTC GCG ATA ATC AGT-3′. The fold induction relative to AtHKT1;1 expression in Col-0 roots was calculated following the method of Livak and Schmittgen [34]. CT values were determined based on efficiency of amplification. The mean CT values were normalized against the corresponding Actin 1 gene and ΔCT values calculated as CTAtHKT1;1–CTActin 1. The expression of AtHKT1;1 was calculated using the 2∧(ΔCT) method [34]. To normalize between samples analyzed in separate qRT-PCR runs, we divided the ΔCT for each line by the ΔCT of Col-0 roots in that run.
Data Analysis
ICP-MS measurements below zero and extreme outliers (those values that were greater than the 90th percentile + percentile) within each tray were removed. To account for variation in the growth environment, the four control accessions included in each tray were used to create a tray specific normalization factor. Briefly, for each element, each control accession in a given tray was compared to the overall average for that accession across all trays to obtain an element×line×tray specific normalization factor. The four element×line×tray factors in a give tray were then averaged to create a tray×element normalization factor for the tray. Every value for the element in the tray was then multiplied by the normalization factor. See Figure S2 for data of control accessions before and after the normalization. The mean of each accession was then used for all subsequent analysis. Normalized Na+ values and their frequency distribution can be found in Dataset S1 and Figure S3.
Genotype calls for all 349 accessions were obtained using the methods previously described [24]. GWA analysis was done with correction for confounding using a mixed-model that uses a genetic random effect with a fixed covariance structure to account for population structure [25] implemented in the program EMMA [24]. The contribution of the best performing SNP (C or T at Chr4 : 6392276 = isT) was checked using un-normalized Na+ data and the linear model:(1)using the lm and anova functions from R v2.9.1. The control accessions were excluded from this analysis. The output of the statistical model can be found in Text S1. Although the samples were nested in trays, Figure S4 indicates that the best performing SNP is essentially evenly distributed across all trays.
The geographical location of each accession was obtained from TAIR (www.arabidopsis.org). When processing the original data, we found an inconsistency for one of the high-Na accessions, CS28373 (also known as Jm-1). The listed latitude and longitude (49, 15) of the accession do not match the location name “Jamolice” from where this accession was collected. The town Jamolice is located at 49.0721283 latitude and 16.2532139 longitude (http://www.gpsvisualizer.com/geocode). In the interests of consistency, we used the original coordinates, although altering the location did not materially change the analysis. The distance to the coast or saline/sodic areas was calculated by obtaining the longitudes and latitudes of the shoreline/coast from the National Oceanic and Atmospheric Administration's National Geophysical Data Center (NOAAs NGDC http://www.ngdc.noaa.gov/ngdc.html) and the saline and sodic soils data from the European Soils database [23]. The pointDistance function in R 2.10.0 and the package raster were used to calculate the Great-circle distance to the shoreline or saline/sodic areas. We created a variable (toSeaSal) representing the shortest distance from the target accession to the shoreline/coast or saline/sodic area. The accession coordinates, distance to sea, distance to saline environment and SNP genotype at Chr4 : 6392276 can all be found in Dataset S1.
The method used to collect accessions and assemble the population might introduce unintended confounding effects that violate the assumption of independent locations used by our models. To determine whether the locations of the accessions were spatially dependent we performed a Mantel test [35] on the distances from the 300 accessions to the coast or known saline/sodic areas. The simulated p-values of 50 permutations tests with 999 repeatedly simulated samples are 0.996, indicating that an assumption of independency for the response variable toSeaSal is acceptable.
To test for associations between leaf Na+ (Na), genotype at the highest scoring SNP (C or T at Chr4 : 6392276 = isT), and the distance to the nearest coast or saline/sodic area (toSeaSal), we used the package lm in R 2.10.0 to fit linear models, with the weights determined by the following approach. First, to quantify the strength of the relationship between toSeaSal and the leaf sodium Na, we fit the data into a linear model and regressed toSeaSal on Na.(2)Second, we applied a regression approach to single-factor analysis [36] between toSeaSal and isT and tested if the average distance to coast or saline/sodic areas of samples having the high Na T allele is significantly different from the average of samples having the C allele.(3)Finally, we regressed toSeaSal on the interaction between Na and isT to inspect how the two predictors jointly affect the distance to sea or saline/sodic.(4)To perform the significance tests on the linear coefficients, Na should be centered at the mean [36]. The extent of variation of distances to saline environments changes with both leaf Na+ concentrations and genotypes (Figure S5). Therefore, all three models account for this heterogeneity of variation, and parameters of the models are fitted using weighted least squares. The variances of the error terms in equation 2, 3, and 4 are not constant, and are related to the predictors according to the diagnosis on the model residuals. The models were fit using iterative weighted least squares [36]. In addition to the parametric test (model 3), we performed a non-parametric test (Wilcoxon rank-sum test or Wilcoxon-Mann-Whitney test [37]) using the wilcox.test function in R package stats, to assess whether toSeaSal is higher in the lines with the T allele than those with the C allele at Chr4 : 6392276. The p-value of the Wilcoxon rank-sum test is 0.006224 indicating that both the parametric and non-parametric approaches reach the same conclusion. The statistical output of all models can be found in Text S1.
Supporting Information
Zdroje
1. WilczekAM
RoeJL
KnappMC
Lopez-GallegoC
MartinLJ
2009 Effects of genetic perturbation on seasonal life history plasticity. Science 323 930 934
2. PaabyAB
BlacketMJ
HoffmannAA
SchmidtPS
2010 Identification of a candidate adaptive polymorphism for Drosophila life history by parallel independent clines on two continents. Mol Ecol 19 760 774
3. McKechnieSW
BlacketMJ
SongSV
RakoL
CarrollX
2010 A clinally varying promoter polymorphism associated with adaptive variation in wing size in Drosophila. Mol Ecol 19 775 784
4. StorzJF
RunckAM
SabatinoSJ
KellyJK
FerrandN
2009 Evolutionary and functional insights into the mechanism underlying high-altitude adaptation of deer mouse hemoglobin. Proc Natl Acad Sci U S A 106 14450 14455
5. BradyK
KruckebergA
BradshawHJ
2005 Evolutionary ecology of plant adaptation to serpentine soils. Annual Review of Ecology, Evolution, and Systematics 36 243 266
6. LowryDB
RockwoodRC
WillisJH
2008 Ecological reproductive isolation of coast and inland races of Mimulus guttatus. Evolution 62 2196 2214
7. WelchME
RiesebergL
2002 Habitat divergence between a homoploid hybrid sunflower species, Helianthus paradoxus (Asteraceae), and its progenitors. Am J Bot 89 472
8. MacnairMR
1993 The genetics of metal tolerance in vascular plants. New Phytol 124 541 559
9. LexerC
WelchME
DurphyJL
RiesebergLH
2003 Natural selection for salt tolerance quantitative trait loci (QTLs) in wild sunflower hybrids: implications for the origin of Helianthus paradoxus, a diploid hybrid species. Mol Ecol 12 1225 1235
10. LowryDB
HallMC
SaltDE
WillisJH
2009 Genetic and physiological basis of adaptive salt tolerance divergence between coastal and inland Mimulus guttatus. New Phytol 183 776 788
11. HoffmannMH
2002 Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae). J Biogrogr 29 125 134
12. Alonso-BlancoC
AartsMG
BentsinkL
KeurentjesJJ
ReymondM
2009 What has natural variation taught us about plant development, physiology, and adaptation? Plant Cell 21 1877 1896
13. Mitchell-OldsT
SchmittJ
2006 Genetic mechanisms and evolutionary significance of natural variation in Arabidopsis. Nature 441 947 952
14. MunnsR
TesterM
2008 Mechanisms of salinity tolerance. Annu Rev Plant Biol 59 651 681
15. EdelistC
LexerC
DillmannC
SicardD
RiesebergLH
2006 Microsatellite signature of ecological selection for salt tolerance in a wild sunflower hybrid species, Helianthus paradoxus. Mol Ecol 15 4623 4634
16. KarrenbergS
EdelistC
LexerC
RiesebergL
2006 Response to salinity in the homoploid hybrid species Helianthus paradoxus and its progenitors H. annuus and H. petiolaris. New Phytol 170 615 629
17. LexerC
LaiZ
RiesebergLH
2004 Candidate gene polymorphisms associated with salt tolerance in wild sunflower hybrids: implications for the origin of Helianthus paradoxus, a diploid hybrid species. New Phytol 161 225 233
18. LexerC
WelchME
RaymondO
RiesebergLH
2003 The origin of ecological divergence in Helianthus paradoxus (Asteraceae): selection on transgressive characters in a novel hybrid habitat. Evolution 57 1989 2000
19. FranzénLG
1990 Transport, deposition and distribution of marine aerosols over southern Sweden during dry westerly storms. Ambio 19 180 188
20. GouldingKWT
PoultonPR
ThomasVH
WilliamsRJB
1986 Atmospheric deposition at Rothamsted, Saxmundham, and Woburn experimental stations, England, 1969–1984. Water, Air, & Soil Pollution 29 1573 2932
21. GustafssonMER
FranzénLG
2000 Inland transport of marine aerosols in Southern Sweden. Atmospheric Environment 34 313 325
22. SuzukiT
IizukaY
MatsuokaK
FurukawaT
KamiyamaK
2002 Distribution of sea salt components in snow cover along the traverse route from the coast to Dome Fuji station 1000 km inland at east Dronning Maud Land, Antarctica. Tellus 54B 407 411
23. TóthG
MontanarellaL
RuscoE
2008 61 74 Threats to soil quality in Europe EUR 23438 - Scientific and Technical Research series Luxembourg: Office for Official Publications of the European Communities
24. AtwellS
HuangYS
VilhjalmssonBJ
WillemsG
HortonM
2010 Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465 627 631
25. YuJ
PressoirG
BriggsWH
VrohBL
YamasakiM
2006 A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet 38 203 208
26. HorieT
HauserF
SchroederJI
2009 HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 14 660 668
27. RusA
BaxterI
MuthukumarB
GustinJ
LahnerB
2006 Natural variants of AtHKT1 enhance Na+ accumulation in two wild populations of Arabidopsis. PLoS Genet 2 e210 doi:10.1371/journal.pgen.0020210
28. MollerIS
GillihamM
JhaD
MayoGM
RoySJ
2009 Shoot Na+ exclusion and increased salinity tolerance engineered by cell type-specific alteration of Na+ transport in Arabidopsis. Plant Cell 21 2163 2178
29. BerthomieuP
ConejeroG
NublatA
BrackenburyWJ
LambertC
2003 Functional analysis of AtHKT1 in Arabidopsis shows that Na(+) recirculation by the phloem is crucial for salt tolerance. EMBO J 22 2004 2014
30. MaserP
EckelmanB
VaidyanathanR
HorieT
FairbairnDJ
2002 Altered shoot/root Na+ distribution and bifurcating salt sensitivity in Arabidopsis by genetic disruption of the Na+ transporter AtHKT1. FEBS Lett 531 157 161
31. LahnerB
GongJ
MahmoudianM
SmithEL
AbidKB
2003 Genomic scale profiling of nutrient and trace elements in Arabidopsis thaliana. Nat Biotechnol 21 1215 1221
32. PlattA
HortonM
HuangYS
LiY
AnastasioAE
2010 The scale of population structure in Arabidopsis thaliana. PLoS Genet 6 e1000843 doi:10.1371/journal.pgen.1000843
33. BaxterI
OuzzaniM
OrcunS
KennedyB
JandhyalaSS
2007 Purdue ionomics information management system. An integrated functional genomics platform. Plant Physiol 143 600 611
34. LivakKJ
SchmittgenTD
2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 25 402 408
35. MantelN
1967 The detection of disease clustering and a generalized regression approach. Cancer Research 27 209
36. KutnerMH
NachtsheimCJ
LiW
NeterJ
2005 in Building the Regression Model III: Remedial Measures Ch. 11, 16, 22, (McGraw Hill)
37. MannHB
WhitneyDR
2947 On a test of whether one of two random variables is stochastically larger than the other. The Annals of Mathematical Statistics 18 50 60
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Genome-Wide Association Meta-Analysis of Cortical Bone Mineral Density Unravels Allelic Heterogeneity at the Locus and Potential Pleiotropic Effects on Bone
- Beyond QTL Cloning
- An Evolutionary Framework for Association Testing in Resequencing Studies
- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- The Functional Interplay between Protein Kinase CK2 and CCA1 Transcriptional Activity Is Essential for Clock Temperature Compensation in Arabidopsis
- Endogenous Viral Elements in Animal Genomes
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- DNA Methylation and Normal Chromosome Behavior in Neurospora Depend on Five Components of a Histone Methyltransferase Complex, DCDC
- Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes
- Genetic Basis of Growth Adaptation of after Deletion of , a Major Metabolic Gene
- Nomadic Enhancers: Tissue-Specific -Regulatory Elements of Have Divergent Genomic Positions among Species
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- CTCF-Dependent Chromatin Bias Constitutes Transient Epigenetic Memory of the Mother at the Imprinting Control Region in Prospermatogonia
- Systematic Dissection and Trajectory-Scanning Mutagenesis of the Molecular Interface That Ensures Specificity of Two-Component Signaling Pathways
- Nucleolin Is Required for DNA Methylation State and the Expression of rRNA Gene Variants in
- The Complex Genetic Architecture of the Metabolome
- ATM Limits Incorrect End Utilization during Non-Homologous End Joining of Multiple Chromosome Breaks
- Mutation Disrupts Synaptonemal Complex Formation, Recombination, and Chromosome Segregation in Mammalian Meiosis
- Mismatch Repair–Independent Increase in Spontaneous Mutagenesis in Yeast Lacking Non-Essential Subunits of DNA Polymerase ε
- The Kinesin-3 Motor UNC-104/KIF1A Is Degraded upon Loss of Specific Binding to Cargo
- Epigenetic Silencing of Spermatocyte-Specific and Neuronal Genes by SUMO Modification of the Transcription Factor Sp3
- A Coastal Cline in Sodium Accumulation in Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1
- Cyclin B3 Is Required for Multiple Mitotic Processes Including Alleviation of a Spindle Checkpoint–Dependent Block in Anaphase Chromosome Segregation
- Altered DNA Methylation in Leukocytes with Trisomy 21
- Human-Specific Evolution and Adaptation Led to Major Qualitative Differences in the Variable Receptors of Human and Chimpanzee Natural Killer Cells
- Leptotene/Zygotene Chromosome Movement Via the SUN/KASH Protein Bridge in
- RACK-1 Acts with Rac GTPase Signaling and UNC-115/abLIM in Axon Pathfinding and Cell Migration
- Genome-Wide Effects of Long-Term Divergent Selection
- Endless Forms Most Viral
- Conflict between Noise and Plasticity in Yeast
- Essential Functions of the Histone Demethylase Lid
- The Transcriptional Regulator Rok Binds A+T-Rich DNA and Is Involved in Repression of a Mobile Genetic Element in
- The Cellular Robustness by Genetic Redundancy in Budding Yeast
- Localization of a Guanylyl Cyclase to Chemosensory Cilia Requires the Novel Ciliary MYND Domain Protein DAF-25
- A Buoyancy-Based Screen of Drosophila Larvae for Fat-Storage Mutants Reveals a Role for in Coupling Fat Storage to Nutrient Availability
- A Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species (Green Peach Aphid)
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- Genome-Wide Effects of Long-Term Divergent Selection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



