-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Nucleolin Is Required for DNA Methylation State and the Expression of rRNA Gene Variants in
In eukaryotes, 45S rRNA genes are arranged in tandem arrays in copy numbers ranging from several hundred to several thousand in plants. Although it is clear that not all copies are transcribed under normal growth conditions, the molecular basis controlling the expression of specific sets of rRNA genes remains unclear. Here, we report four major rRNA gene variants in Arabidopsis thaliana. Interestingly, while transcription of one of these rRNA variants is induced, the others are either repressed or remain unaltered in A. thaliana plants with a disrupted nucleolin-like protein gene (Atnuc-L1). Remarkably, the most highly represented rRNA gene variant, which is inactive in WT plants, is reactivated in Atnuc-L1 mutants. We show that accumulated pre–rRNAs originate from RNA Pol I transcription and are processed accurately. Moreover, we show that disruption of the AtNUC-L1 gene induces loss of symmetrical DNA methylation without affecting histone epigenetic marks at rRNA genes. Collectively, these data reveal a novel mechanism for rRNA gene transcriptional regulation in which the nucleolin protein plays a major role in controlling active and repressed rRNA gene variants in Arabidopsis.
Published in the journal: . PLoS Genet 6(11): e32767. doi:10.1371/journal.pgen.1001225
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001225Summary
In eukaryotes, 45S rRNA genes are arranged in tandem arrays in copy numbers ranging from several hundred to several thousand in plants. Although it is clear that not all copies are transcribed under normal growth conditions, the molecular basis controlling the expression of specific sets of rRNA genes remains unclear. Here, we report four major rRNA gene variants in Arabidopsis thaliana. Interestingly, while transcription of one of these rRNA variants is induced, the others are either repressed or remain unaltered in A. thaliana plants with a disrupted nucleolin-like protein gene (Atnuc-L1). Remarkably, the most highly represented rRNA gene variant, which is inactive in WT plants, is reactivated in Atnuc-L1 mutants. We show that accumulated pre–rRNAs originate from RNA Pol I transcription and are processed accurately. Moreover, we show that disruption of the AtNUC-L1 gene induces loss of symmetrical DNA methylation without affecting histone epigenetic marks at rRNA genes. Collectively, these data reveal a novel mechanism for rRNA gene transcriptional regulation in which the nucleolin protein plays a major role in controlling active and repressed rRNA gene variants in Arabidopsis.
Introduction
In eukaryotic cells, ribosomal RNA genes (rRNA) are arranged in head-to-tail tandem arrays (depicted in Figure 1). The rRNA genes clustered at a single locus comprise nucleolar organizer regions (NORs), so named because the nucleolus, the site of ribosome synthesis, is organized around active rRNA genes during interphase [1]–[3]. Each rRNA gene transcription unit consists of sequences encoding a precursor transcript that includes the structural rRNAs (18S, 5.8S, 25S), the Internal Transcribed Spacers (ITS) and the External Transcribed Spacers (ETS). The rRNA gene units are separated from the adjacent gene in the array by an intergenic spacer (IGS) [4]. In plants, as in animals, coding sequences for the three structural rRNAs are highly conserved, even between distantly-related species, but the IGS and sequences which are removed during processing, including the ITS and ETS, are much less well conserved [5].
Fig. 1. Representation of rRNA gene repeats transcribed by RNA polymerase I. 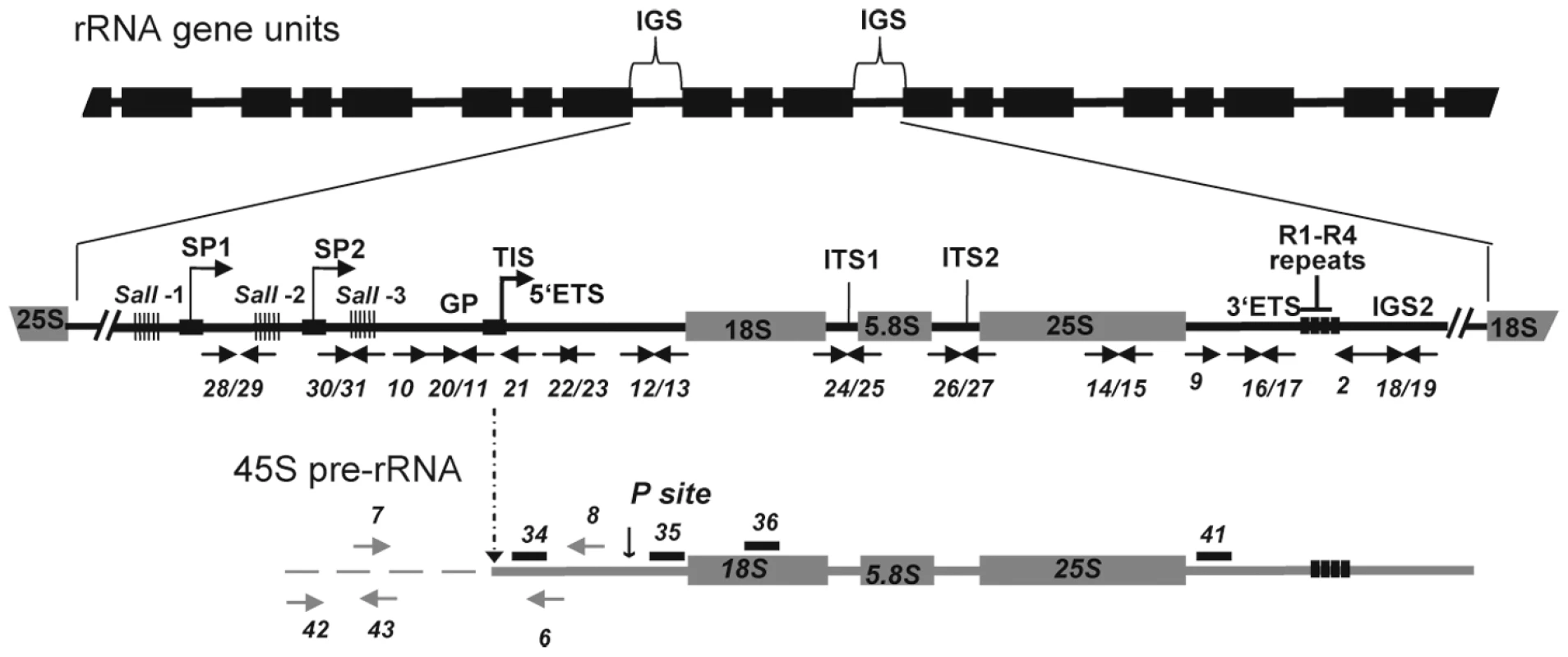
The top portion shows tandemly-arrayed 18S, 5.8S and 25S rRNA genes separated by intergenic sequences (IGS). In the middle, an enlarged rRNA unit is presented, with positions of the gene promoter (GP) and two spacer promoters (SP1 and SP2) located between the three repeat elements containing Sal I restriction site DNA repeat elements (SalI-1, -2 and -3 repeats). The arrow in GP indicates the Transcription Initiation Site (TIS). The lower scheme represents the primary 45S pre-rRNA, containing the external transcribed spacers (5′ETS and 3′ETS), and the structural rRNA sequences (18S, 5.8S and 25S rRNA in gray boxes) separated by internal transcribed spacers (ITS1 and ITS2). Four repeat sequences located in the 3′ETS are represented (R1-R4). The vertical arrow shows the primary cleavage site (P) in the 5′ETS. Positions of primers used to amplify or detect rRNA gene and/or pre-rRNA sequences are shown. Analysis of complete IGS sequences reveals considerable length and sequence heterogeneity in different plant species, including radish, wheat, A. thaliana, Brassica, Nicotiana and Solanum species [6]. However, all ribosomal IGS contain repeated sequences. IGS organization in plant rRNA genes resembles IGS organization in most higher eukaryotes, including at least one array of tandemly-repeated sequences located upstream from the transcription initiation site (TIS). In Xenopus [7] and mouse [8], repeated sequences in this location have been shown to possess enhancer activity, increasing the expression of the adjacent promoter after injection into frog oocytes or embryos or after transfection into cultured cells. Though Arabidopsis spacer repeats cloned next to a Xenopus rRNA gene promoter act as enhancers in frog oocytes [9], there is no evidence that they have analogous enhancer activity in plant cells [10]. In plants and animals not all rRNA genes are active and the mechanisms responsible for the selective activation and/or silencing of subsets of these genes have been sought for many years. Thus, questions persist as to whether or not IGS heterogeneity affects rRNA transcription; possibly playing a role in activation or silencing of rRNA genes or in stimulating rRNA transcription levels among active rRNA genes.
In the context of nucleolar dominance, activation or repression of specific rRNA genes is controlled mainly by rRNA chromatin modification [11]. In Arabidopsis suecica, an allotetraploid derived from A. thaliana and A. arenosa, the thaliana derived rRNA genes are repressed and those of arenosa are expressed [12]. The thaliana derived rRNA genes are hyper-methylated and are associated with histone H3 dimethylated on lysine 9 (H3dimethylK9), whereas the arenosa-derived genes are hypo-methylated and are associated with histone H3 trimethylated on lysine 4 (H3trimethylK4) [13]. Blocking de novo DNA methylation causes changes in histone modifications, and blocking histone deacetylation induces cytosine demethylation. Indeed, knockdown by RNAi of HDA6 and HDT1 (histone deacetylase and histone deacetylase –like protein encoding genes respectively) in A. suecica causes the derepression of thaliana-derived rRNA genes [13]–[14]. An RNAi knockdown screen in Arabidopsis identified DRM2, a de novo cytosine methyltransferase, and MBD6 and MBD10, two methyl cytosine binding domain proteins, as activities also required for nucleolar dominance [15]. More recently, it was demonstrated that HDA6 plays a role in activation of specific rRNA genes in A. thaliana [16].
Chromatin decondensation is generally correlated with transcriptional gene activation, in such a way that the amount of decondensed chromatin reflects gene expression activity, either real or potential [17]. AtHDA6 and AtNUC-L1 (a NUCleolin-Like protein), play a role in rRNA chromatin condensation in Arabidopsis [14], [18]. Decondensation of rRNA chromatin has no discernible effect on rRNA mature transcript levels in AtHDA6 mutants [14]. However, in plants with a disrupted AtNUC-L1 gene, the level of transcripts initiated from the gene promoter decreases and pre-rRNA cleaved at the P site accumulates [18], indicating that AtNUC-L1 plays a major role in controlling homeostatic rRNA gene expression.
In eukaryotic cells, nucleolin is one of the most abundant non-ribosomal proteins in the nucleolus [19]. Studies in vitro [20]–[22] or in animal cells with reduced amounts of nucleolin [23], [24], clearly show that nucleolin plays a role in different steps in ribosome biogenesis, including RNA pol I transcription and processing of pre-rRNA. However, a role for nucleolin in mechanisms responsible for controlling activation and/or silencing of specific rRNA genes has not been investigated.
Here we present evidence for a role of AtNUC-L1 in controlling activation and repression of a specific subset of rRNA genes located in distinctive NORs. Our findings indicate that AtNUC-L1 might play a central role in a mechanism responsible for switching expression of rRNA genes that involves IGS transcription and symmetric DNA methylation.
Results
Specific rRNA variants are expressed and/or repressed in Atnuc-L1 plants
We previously reported that disruption of a nucleolin like protein gene from A. thaliana (AtNUC-L1) affects nucleolar structure, rRNA chromatin condensation and accumulation of pre-rRNA [18]. To determine if AtNUC-L1 gene disruption affects transcription activation and/or repression of specific rRNA genes in Atnuc-L1 plants, we carried out a systematic cloning and sequencing strategy to characterize 3′ETS rRNA genes and pre-rRNA transcribed sequences from WT and two Atnuc-L1 mutant lines.
As shown in Figure 2A, PCR reactions with primers p1/p2 (shown in Figure 2C) to amplify 3′ETS rRNA gene sequences detect three major bands in genomic DNA prepared from WT (lane 1) and Atnuc-L1-1 and Atnuc-L1-2 plants (lanes 2 and 3). Cloning and sequencing analysis indicates that the genome of A. thaliana contains at least three major rRNA gene variants, VAR1, VAR2 and VAR 3. Sequencing analysis of ∼90 3′ETS rRNA gene clones showed that 48% of the sequences correspond to VAR1, while 30% and 22% correspond to VAR2 and VAR3 respectively. An additional rRNA gene variant (VAR4) was also identified by RT-PCR (see below) and confirmed by PCR amplification of genomic DNA using primers specific to this novel low copy number variant (data not shown). These variants can be distinguished from each other by the presence of a large insertion (VAR1) or deletions (VAR3) and/or additional short insertions or deletions (VAR2 and VAR4) located in the 3′ETS rRNA gene region (Figure 2C and Figure S3). Furthermore VAR2 and VAR3 can be separated into two sub variants, VAR2, VAR2a and VAR3, VAR3a, based on a short three base pair deletion (CAC and CGC respectively, double over lined in Figure S3). VAR1 contains four repeat sequences (R1-R4) located downstream from the 3′ETS cleavage site, in contrast to VAR2, VAR3 and VAR4 which contain only two complete repeats (R1 and R4). In contrast, PCR amplification of rRNA gene sequences (from −520 to +209) and sequencing analysis of ∼50 rRNA clones did not reveal major sequence variations in the promoter and/or in the 5′ETS region (data not shown).
Fig. 2. A. thaliana encodes and expresses specific rRNA gene variants. 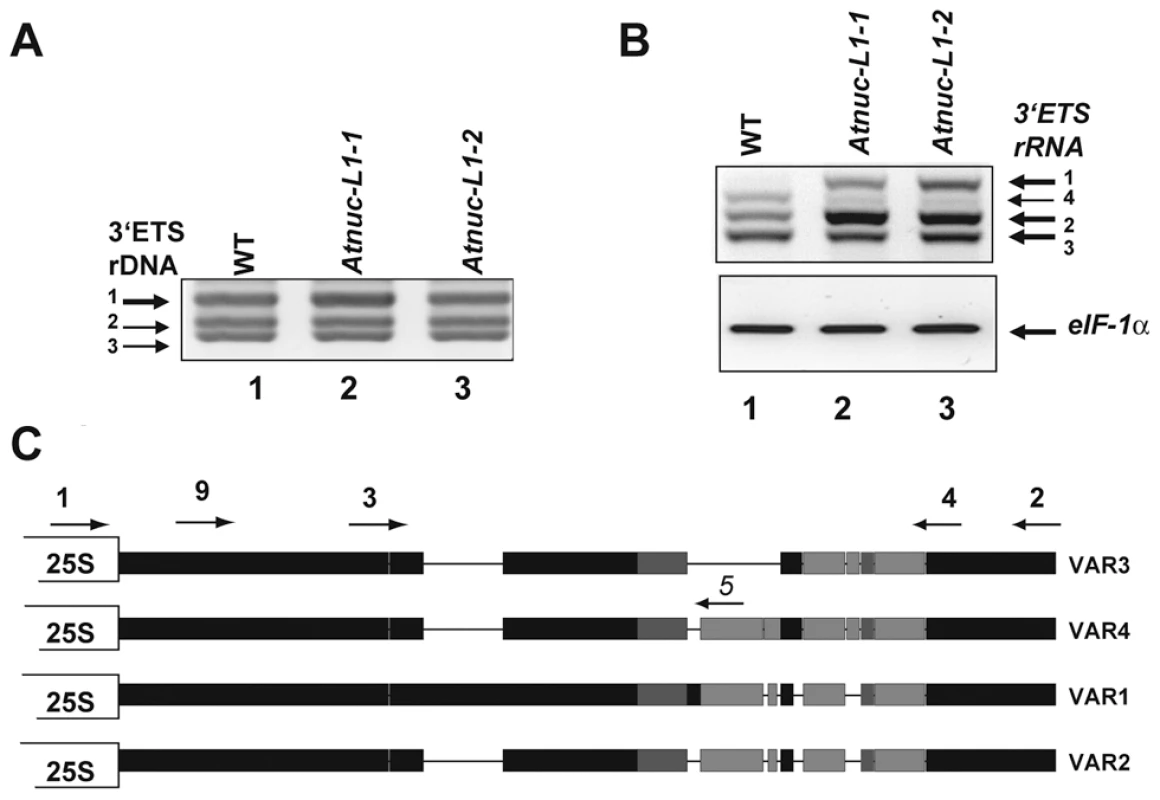
A) PCR amplification of 3′ETS rRNA gene sequences using primers p1/p2 and genomic DNA from WT (lane 1) and Atnuc-L1 mutant plants (lanes 2 and 3). Arrows show 3′ETS rRNA genes VAR1, VAR2 and VAR3 based on expected sizes and sequencing data (Figure S3). B) One Step RT-PCR analysis using primers p3/p4 to detect 3′ETS pre-rRNA sequence variants in WT (lane 1) and Atnuc-L1 mutant (lanes 2 and 3) plants. C) Representation of 3′ETS rRNA gene variants. Black, dark and/or light grey rectangles represent high, medium or low sequence identity respectively. Lines joining the rectangles indicate deletions in sequences of each rRNA gene variant. Positions of primers p1-p5 and p9are indicated. In order to determine if some or all of these rRNA gene variants are expressed, we performed RT-PCR analysis using primers p3/p4 that amplify shorter fragments than those used in Figure 2A, allowing better resolution between the four bands. As shown in Figure 2B, the amplification band corresponding to VAR1 (greater size) accumulates preferentially in Atnuc-L1 mutant plants (lane 2 and 3). No amplification band corresponding to VAR1 was detected in WT plants (lane 1). Amplification bands corresponding to VAR2 and VAR4 accumulate or decrease respectively in the Atnuc-L1 mutant compared to amplification signals in WT plants. In contrast, no significant differences in the amplification signal corresponding to VAR3 was observed between WT and Atnuc-L1 plants. The identity of each band was confirmed by sequencing. Similar results were obtained by RT-PCR reactions using the primers p1 and p2 used for amplification of rRNA genomic sequences (data not shown). Lower accumulation of rRNA VAR1 in Atnuc-L1-1 compared to Atnuc-L1-2 is not due to variations in the expression of this gene in individual plants. Indeed, RT-PCR analysis revealed similar increased amount of rRNA VAR1 in all individual Atnuc-L1-1 plants analyzed compared to wild-type (Figure S7).
To verify that activation of rRNA gene VAR1 is associated with the absence of AtNUC-L1 protein, we transformed Atnuc-L1 mutant plants with AtNUC-L1 genomic sequences under the control of its own promoter (Figure S2). Complementation of Atnuc-L1 mutants with AtNUC-L1 genomic sequences restore, at least partially, the amount of rRNA variants to those observed in WT plants (Figure S2B). Partial complementation is probably due to the fact that in these plants levels of AtNUC-L1 are significantly lower than those observed in WT (Figure S2C). This might suggest a dosage effect of AtNUC-L1 in rRNA gene variant expression. However, expression of transgenic AtNUC-L1 is sufficient to restore plant growth and developmental defects of Atnuc-L1 mutant plants (Figure S2A).
In conclusion, these analyses reveal the existence of several variants of rRNA genes, including an rRNA gene variant (VAR1), silent in wild-type plants, which is derepressed in A. thaliana plants lacking the AtNUC-L1 protein. Overall, they demonstrate a striking balance between active and inactive rRNA gene variants in WT and Atnuc-L1 plants.
Expression of rRNA gene variants in A. thaliana wild-type plants
Nucleolin like protein gene expression is up or down -regulated in meristematic tissues and at specific stages of plant development [18],[25]–[28]. In order to determine if the rRNA gene variants (VAR1-4) are differentially expressed in WT plants, we performed One Step RT-PCR using primers p3/p4 (shown in Figure 2C) to amplify 3′ETS pre-rRNA sequences in different organs and or plant conditions.
As observed in Figure 3A, the 3′ETS rRNA VAR2 and VAR3 are detected in roots, flowers, leaves and germinating seeds (lanes 3, 4 and 6, 7). rRNA VAR1 is detected essentially in seeds imbibed for 48 hrs (lane 7), though a very weak band is amplified in roots and flowers (lanes 3 and 4). In exponential or stationary cell culture (lanes 1 and 2), the rRNA VAR3 corresponds to the major amplification band. As shown previously, VAR1 is also detected in Atnuc-L1 mutant plants, used here as a control (lane 5). PCR reactions without reverse transcriptase did not reveal amplification products in any of the samples tested (data not shown). Amplification of eIF1α was performed to control RNA amounts in each sample (panel eIF1α).
Fig. 3. rRNA gene variants are differentially expressed in A. thaliana plants. 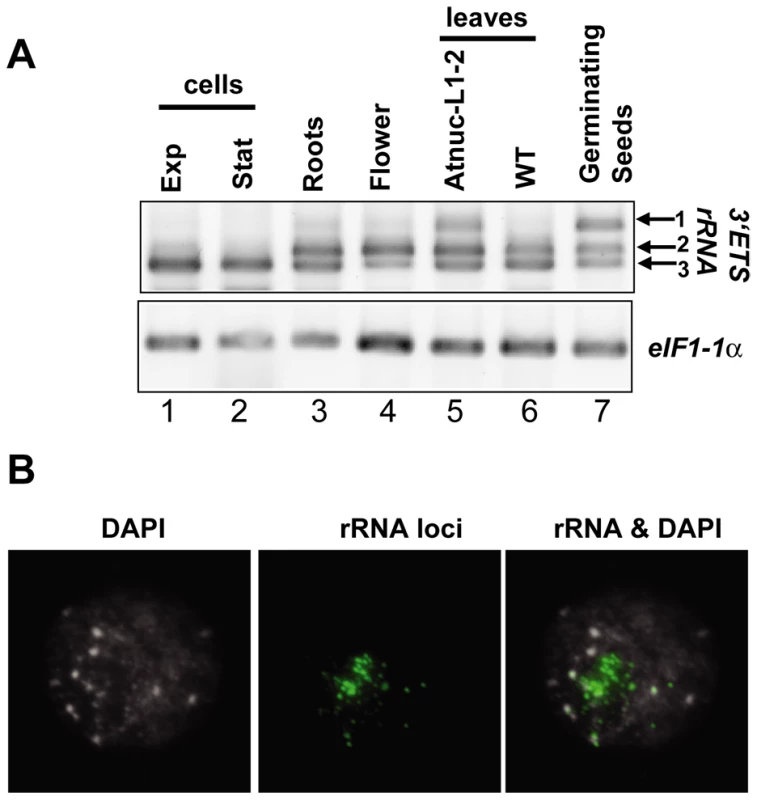
A) One Step RT-PCR analysis was using primers p3/p4 to detect 3′ETS pre-rRNA sequence variants in exponentially (lane 1) or stationary growing (lane 2) cells; in roots (lane 3), flower (lane 4), leaves from Atnuc-L1-2 mutant (lane 5) or WT (lane 6) plants or 2 days germinating seeds (lane 7). RNA from roots and leaves were prepared from 15 days old WT and Atnuc-L1 plant grown in MS medium as described previously [18]. Panel eIF1α; amplification of eIF1α mRNA was performed to control PCR reaction and total RNA in different samples. B) NOR condensation is affected in 2 day germinating seeds. Left, chromatin counterstained with DAPI, Middle FISH using 25S rRNA probe and Right, superposition of FISH and DAPI images. In a context of nucleolar dominance, at 2 days post germination in the allelotetraploid Arabidopsis suecica the thaliana -derived NORs are decondensed and the rRNA genes are expressed [29]. Here, to verify the chromatin state in the 2-days germinating A. thaliana seedlings expressing the rRNA VAR1 gene (refer to Figure 3A), we performed FISH analysis. As shown in Figure 3B, in the nucleus of 2 days germinating seedlings the rRNA chromatin is immature. In these nuclei several FISH signals are observed using a 25S probe, but these are smaller compared with those observed in 15 –day old plants (see Figure 6B). This is indicative of decondensed fibers of active rRNA genes, which can not be observed because are too thin, juxtaposed with condensed portions of heterochromatic rRNA signals (green labeling).
From these results, we conclude that rRNA variants are developmentally regulated in A. thaliana plants and chromatin state plays a major role in controlling the expression of rRNA VAR1.
45S pre–rRNA precursors are accurately processed in Atnuc-L1 plants
We previously reported that pre-rRNA cleaved at the primary cleavage site in the 5′ETS region (P site) accumulates in Atnuc-L1-1 mutant plants [18]. Here we examine accumulation of 45S pre-rRNA and processing in the 3′ETS region in Atnuc-L1-1 plants because 3′ETS cleavage is a co-transcriptional event that releases 45S pre-rRNA precursor [6]. To verify accumulation of these precursors in Atnuc-L1-1 plants we performed northern blot analysis using oligonucleotide probes p34 and p35 that specifically match 5′ETS sequences located either upstream and downstream from the P cleavage site, respectively, and oligonucleotide p41 to detects 3′ETS region (position shown in Figure 1 and Figure S4). As observed in Figure 4A, hybridization using p34, p35 and p41 primers generates stronger radioactive signals in the Atnuc-L1 mutant (lanes 1–2, 4–5 and 10–11) than in WT samples (lanes 3, 6 and 12). The larger radioactive signals detected using p41 (lanes 10–12), but also by p34 and p35 (lanes 1–6), correspond to the 45S pre-rRNA. The signals detected only by primer p35 correspond to an intermediate of 18S precursor forms (lanes 4–6). The smear detected with primer p34 might be due to exonucleolityc degradation of a 5′ETS cleave off product, while the signal indicated by an asterisk might correspond to a 5′ETS product produced by an alternative cleavage event upstream from the P site (lanes 1–3). Hybridization using primer p36 detects similar amounts of 18S rRNA in WT and Atnuc-L1 samples (lanes 7–9), Accumulation of pre-rRNA in Atnuc-L1-1 mutants was also observed by Northern blot using primers specific to ITS1, ITS2, 5.8S and 25S sequences. As for 18S we did not observe accumulation of either mature 5.8 or 25S rRNA (Figure S4).
Fig. 4. Processing of accumulated pre-rRNA in Atnuc-L1 mutant plants is accurate. 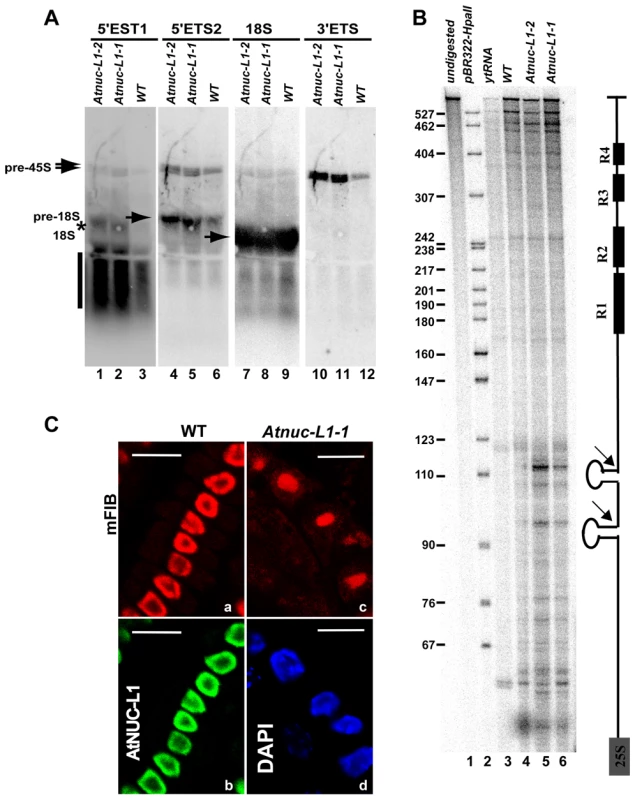
A) Northern blot analysis using total RNA isolated from WT and Atnuc-L1-1 mutant plants and [γ32P] 5′-end labeled primers p34, p35, p36 and p41 to detect 5′ETS1 (lanes 1–3), 5′ETS2 (lanes 4–6), 18S (lanes 7–9) and 3′ETS (lanes 10–12) pre-rRNA sequences respectively. The asterisk and vertical bar indicate expected 5′ETS cleave off and exonucleolityc products (See also Figure S4). B) RNAseA/T1 protection analysis was carried out with a radiolabelled probe complementary to the 3′ETS (right). The assay was performed with total RNA from WT (lane 4) and Atnuc-L1 (lanes 5 and 6) or with yeast tRNA as a control (lane 3). A control lane loaded with undigested riboprobe is shown (lane 1). Lane 2, pBR322 digested with HpaII and 5′end labeled with T4 PNK and [γ32P] ATP. C) Immunolocalization of fibrillarin in roots from WT and Atnuc-L1-1. Panel mFIB; Fibrillarin appears more abundant in the nucleolus of WT (a) than in the disorganized nucleolus of Atnuc-L1 plants (c) [18]. The nucleolar localization of fibrillarin practically overlaps the localization of AtNUC-L1 (b). Fibrillarin was detected with antibodies against mouse fibrillarin (mFIB 72B9) and Alexa-546 and AtNUC-L1 with antibodies against peptide AtNUC-L1 and Alexa-488. Chromatin in Atnuc-L1-1 is counterstained with DAPI (d). Bar, 10 µm. To determine if the 45S pre-rRNA is accurately processed at the 3′ETS cleavage site, we performed an RNase protection assay (Figure 4B). This analysis revealed two major radioactive signals of ∼95 and ∼110 nucleotides that accumulate to higher levels in Atnuc-L1 (lanes 5 and 6) versus WT (lane 4) plants, as expected. These signals map to cleavage sites in the stem base of two hairpin-loop structures that may be recognized by a RNase III implicated in co-transcriptional cleavage of pre-rRNA [30], [31]. The mapped cleavage sites are located upstream from four repeat sequence elements named R1 to R4 (Figure S3). Slight variations were observed upstream from the major cleavage site signals when the pattern of radioactive signal was compared between WT and Atnuc-L1 samples.
In Atnuc-L1 plants, the nucleolus is disorganized and this might affect the nucleolar localization of factors involved in the nucleolar step of rRNA synthesis [18]. Fibrillarin is a major nucleolar protein factor required for primary cleavage in the 5′ETS and methylation of pre-rRNA [32]. As shown in Figure 4C, after incubation of A. thaliana meristematic cells with murine monoclonal anti-fibrillarin autoantibody (mFIB, 72B9), the immunofluorescent labeling seems specifically concentrated in the nucleolus of both WT (a) and Atnuc-L1-1 plants (c). In WT plants the nucleolus is immunostained with antibodies against AtNUC-L1 (b) and in Atnuc-L1 mutants, DAPI is observed as a ring around the dark and unstained nucleolus (d).
Taken together, these results indicate that accumulated 45S pre-rRNA in Atnuc-L1 plants is accurately processed and suggest that pre-rRNA processing activity in the nucleolus is essentially not affected in the absence of the AtNUC-L1 protein.
RNA pol I transcription from the IGS in Atnuc-L1 mutant plants
Accumulation of pre-rRNA, which is higher in mutant compared to WT plants, can be controlled by increasing the number of rRNA genes being transcribed and/or by increasing the number of actively transcribing RNA Pol I enzymes [33], [34]. To obtain insight into the transcriptional origin of pre-rRNA transcripts accumulated in Atnuc-L1 plants, we used Chromatin Immuno Precipitation (ChIP) and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) experimental approaches.
To compare the level of RNA Pol I associated with rRNA genes in WT and Atnuc-L1 plants we performed ChIP using antibodies against the largest RNA pol I subunit and specific primers to amplify Gene Promoter (GP, p10/p11), 5′External Transcribed Spacer (5′ETS, p12/p13), 25S rRNA (25S, p14/p15), 3′External Transcribed Spacer (3′ETS, p16/p17) and Internal Gene Spacer 2 (IGS2, p18/p19) sequences (Figure 5A). Quantitative PCR analysis revealed higher GP, 5′ETS, 25S and 3′ETS amplification signals in samples from Atnuc-L1-1 plants (black bars) compared to signals in samples from WT plants (grey bars). In contrast, when the region located just downstream from the four repeat sequences (IGS2) was analyzed, the PCR amplification signals in samples from Atnuc-L1 and WT plants were similar.
Fig. 5. Accumulation of RNA polymerase I and rRNA transcripts from the IGS in Atnuc-L1 plants. 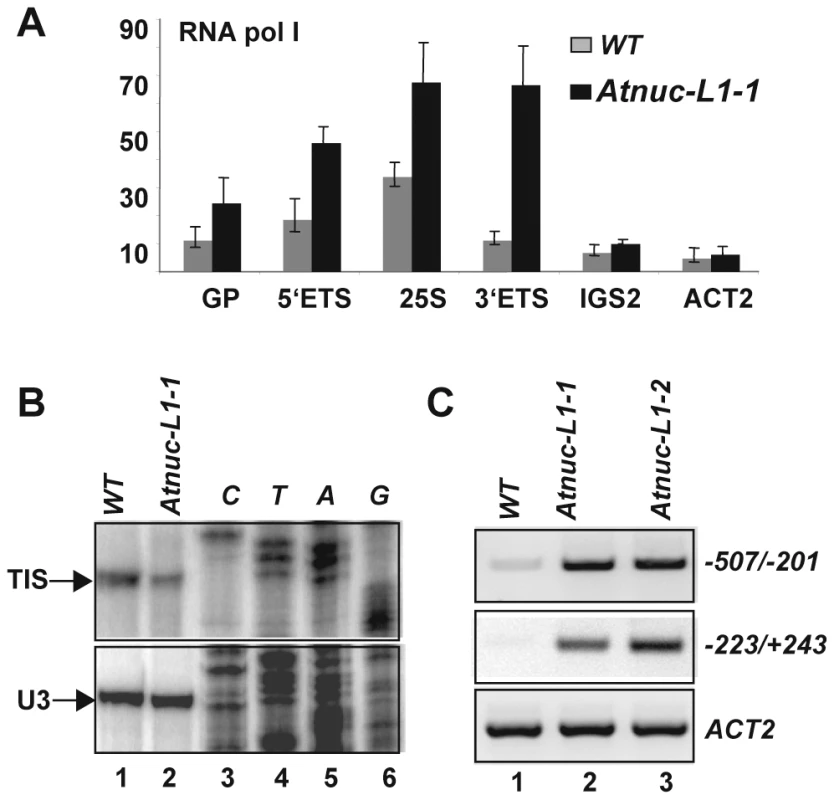
A) Chromatin samples prepared from WT (grey bars) and Atnuc-L1-1 mutant (black bars) plants were immunoprecipitated with antibodies against the largest RNA pol I subunit. ChIP samples were amplified with specific primers to GP (p10/p11), 5′ETS (p12/p13), 25S (p14/p15), 3′ETS (p16/p17) and IGS2 (p18/p19) rRNA sequences. Amplification of the ACT2 gene was performed to control ChiP and qRT-PCR reactions. B) Primer extension was performed using total RNA extracted from WT (lane 1) and AtnucL-1 (lane 2) plants and primer p6 for mapping transcription initiation site (TIS) from the gene promoter (GP). Primer extension using primer 3AtU3 that accurately maps the 5′ end of U3snoRNA was used to control similar amounts of total RNA in each reaction. Lanes 3–6 show DNA sequencing reactions used to map accurately the transcription initiation site (panel TIS) or verify the expected size for U3 snoRNA amplification. C) RT-PCR analysis using primers p42/p43 and p7/p8 to detect transcription from IGS(from −507 to −201 and from −223 to +243 respectively). One Step RT-PCR reaction of ACT2 transcripts was performed to control similar amount of total RNA in WT (lane 1) and Atnuc-L1 (lanes 2 and 3) samples. On the other hand, primer extension analysis shown in Figure 5B reveals that the level of transcripts initiated at the Transcription Initiation Site (TIS) in the gene promoter are lower in Atnuc-L1 than in WT plants. Accordingly, we would expect that accumulated pre-rRNA transcripts in Atnuc-L1 plants are generated from alternative transcription initiation sequences, possibly from spacer promoters (SP) located in the IGS (Figure 1). Indeed, it has been shown that RNA pol I is capable of initiating transcription from spacer promoter rRNA sequences in A. thaliana WT plants [9]. We performed RT-PCR reactions to verify transcription of pre-rRNA from the IGS in Atnuc-L1 mutant plants. As shown in Figure 5C, when PCR reactions were performed with oligonucleotides p42/p43 and p7/p8 to amplify sequences from −507 to −201 and from −223 to +243 respectively (shown in Figure 1) a specific band was amplified to a higher level in RNA samples from Atnuc-L1 mutants (lanes 2 and 3) compared with WT (lane 1) plants. RT-PCR reactions using primers that specifically amplify AtACT2 sequences were performed to verify similar amounts of RNA in each reaction (Panels ACT2, lanes 1–3). In contrast, RT-PCR reactions using primers located in the 3′ end of the 25S sequence and just downstream from SP1 to detect read through transcription from an upstream gene did not produce any amplification band (data not shown).
In conclusion, these results suggest that accumulation of pre-rRNA in Atnuc-L1 mutant plants is probably due to higher RNA pol I transcription from the IGS.
AtNUC-L1 binds specific and transcriptionally active rRNA genes in the NORs
We previously reported that the AtNUC-L1 protein specifically binds rRNA gene promoter sequences and directs rRNA transcription from the TIS [18]. Accordingly, we would expect that AtNUC-L1 also binds the IGS and other rRNA coding sequences to activate or repress specific rRNA genes located in any of the NOR associated loci on chromosome 2 (NOR2) and 4 (NOR4) [35], [36].
To investigate this hypothesis, we performed ChIP reactions with α-AtNUC-L1 antibody and purified chromatin samples from WT A. thaliana seedlings. As shown in Figure 6A, PCR reactions using increased amounts of ChIP fraction detect GP (p20/p21), 5′ETS (p22/p23), ITS1 (p24/p25), ITS2 (p26/p27), 3′ETS (p9/p2), Sal1-2 (p28/p29) and Sal1-3 (p30/p31) rRNA gene sequences. The amplification signals, GP, 5′ETS, ITS1, ITS2, 3′ETS and Sal1-2 were proportionally dependent on the amount of α-AtNUC-L1 antibodies added to the ChIP fraction (lanes 1 to 3). Note that amplification of 3′ETS rRNA gene sequences reveals two distinct bands. In contrast, PCR reactions to detect Sal1-3 rRNA sequences generated only a weak amplification signal that is not dependent on the amount of antibody added to the ChIP reaction. To control the specific ChIP reaction with α-AtNUC-L1, we performed PCR amplification reactions using ChIP fractions obtained from Atnuc-L1-1 plants (lanes 4–6). As expected, in plants lacking the AtNUC-L1 protein, amplification signals are similar to background signals detected in WT plants (lane 1, without α-AtNUC-L1) and are not dependent on the amount of α-AtNUC-L1 antibodies added to the ChIP sample. Only a weak amplification band corresponding to actin sequences was amplified in WT ChIP samples (ACT2 panel).
Fig. 6. AtNUC-L1 binds specific rRNA gene units. 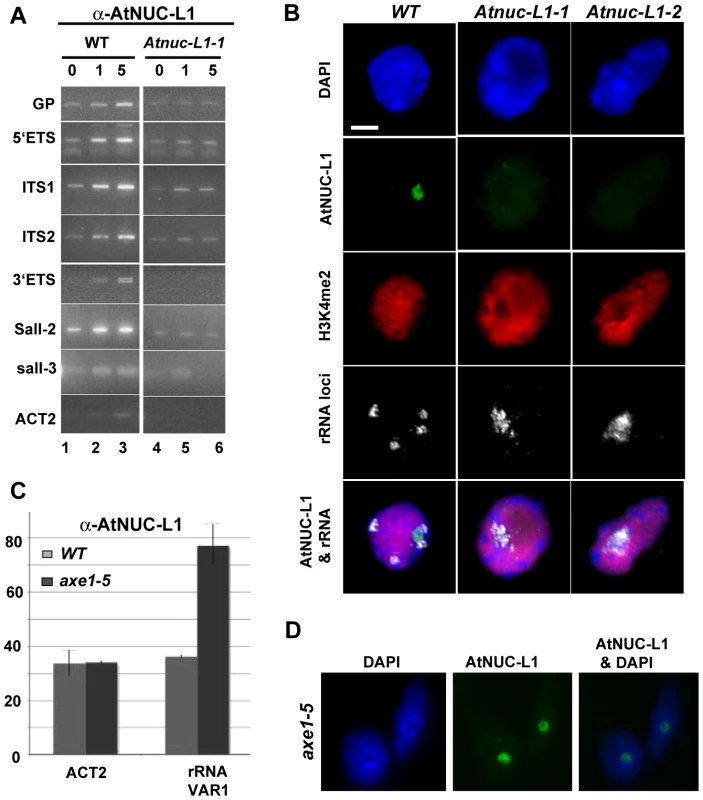
A) Chromatin isolated from WT plants was incubated either with 0, 1 or 5 µl of α-AtNUC-L1 antibody -conjugated to Protein-A (lanes 1 to 3). Immunoprecipitated DNA was analyzed by PCR using specific primers to detect coding 5′ETS (p22/p23), ITS1 (p24/p25), ITS2 (p26/p27) and 3′ETS (p9/p2) and non-coding GP (p20/p21), Sal1-2 (p28/p29) and Sal1-3 (p30/p31) rRNA sequences. Lanes 4–6 correspond to PCR amplification using chromatin isolated from Atnuc-L1-1 plants, which lack AtNUC-L1 protein, used as a control. Location of primers p2, p9 and p20-p31 on the rRNA gene is shown in Figure 1 and Figure 2. B) Co-localization of AtNUC-L1 and rRNA genes. Counterstaining with DAPI (blue); immunodetection of AtNUC-L1 (green); immunodetection of histone H3K4 dimethylation (H3K4me2; red), FISH with a 25S rRNA probe which reveals the 45S rRNA loci (white) and the merged image (AtNUC-L1 and rRNA) on nuclei from WT, Atnuc-L1-1 and Atnuc-L1-2 mutant plants. Bar = 5 µm. C) Chromatin samples prepared from WT (grey bars) and axe1.5 mutant (black bars) plants were immunoprecipitated with α-AtNUC-L1 antibody and amplified with specific primers (p32/p33) to rRNA gene VAR1. Amplification of the ACT2 gene was performed to control ChIP and PCR reaction. D) Nucleolar localization of AtNUC-L1 in axe5-1 mutant. Counterstaining with DAPI (blue); immunodetection of AtNUC-L1 (green) and the merged image (AtNUC-L1 & DAPI) is shown. Considering that VAR1 represents ∼50% of the rRNA genes identified in the genome of A. thaliana (Figure 2A), we wanted to determine if AtNUC-L1 binds either all or some of the NOR in the nucleus of A. thaliana plants. We performed immunofluoresence and in situ hybridization experiments to answer this question. As shown in Figure 6B, AtNUC-L1 protein is detected only in the nucleolus of WT plants as a green hybridization signal (Panel AtNUC-L1). On the other hand, four rRNA signals are detected in interphase nuclei of WT plants (Panel rRNA). These signals probably correspond to the two NOR2 and two NOR4 expected in a diploid thaliana cell. In a preceding paper, we showed that absence of nucleolin in Atnuc-L1 plants induces NOR decondensation [18]. Figure 6B of the present paper shows that in both Atnuc-L1-1 and Atnuc-L1-2 plants, the 45S rRNA chromatin is disorganized and invades the nucleolus. Superimposition of AtNUC-L1 and rRNA gene hybridization signals clearly shows that AtNUC-L1 co-localizes with only some (∼49%, see Table S1 for statistic analysis) of the rRNA loci (Panel AtNUC-L1 & rRNA). Additionally, AtNUC-L1 and rRNA gene signals co-localize only partially. Hybridization experiments using antibodies against H3K4me2, to examine global methylation status did not reveal major differences between WT and Atnuc-L1 plant samples (panel H3K4me2). DAPI staining was visualized around the nucleolus (panel DAPI).
To determine whether or not AtNUC-L1 interacts with rRNA gene VAR1 (inactive in WT plants), we performed ChIP using A. thaliana plants with a disrupted HDA6 gene [14]. In hda6 mutant plants (axe1-5) rRNA chromatin decondensation also induces expression of VAR1 [16]. Subsequently, to study interaction of AtNUC-L1 with either active or inactive rRNA gene VAR1, we performed ChIP reactions using α-AtNUC-L1 antibodies and purified chromatin samples from WT and axe1-5 mutant plants. As shown in Figure 6C, qPCR reactions using primers p32/p33 (shown in Figure S3) reveals specific interaction of AtNUC-L1 protein with VAR1 in axe-5 plants. Amplification of ACT2 in WT and in axe1-5 plants shows that interaction of AtNUC-L1 with VAR1 in WT is not specific. Indeed, amplification of ACT2 sequences is similar to signals of VAR1 rRNA gene in WT. We also demonstrated that expression of rRNA gene VAR1 is not due to a delocalization or repression of AtNUC-L1 protein expression in the axe1-5 mutant plants (Figure 6D). Inmunolocalization experiments show that AtNUC-L1 protein is detected in the nucleolus of hda6 mutant plants as a green hybridization signal (Panel AtNUC-L1). DAPI staining was visualized around the AtNUC-L1 signal in the nucleolus (panel AtNUC-L1 & DAPI).
We conclude that AtNUC-L1 interacts with transcriptionally active rRNA genes. These experiments also corroborate the role of AtNUC-L1 in chromatin condensation and suggest that the AtNUC-L1 protein binds active rRNA genes located in two specific NORs (either NOR2 or NOR4).
Symmetric methylation, but not post-translational histone modifications patterns, are altered in Atnuc-L1 mutants
In genetic hybrids, silencing of rRNA genes inherited from one parent requires DNA methylation and repressive histone modifications [37]. These modifications also involve specific small interfering RNAs (siRNAs) that direct specific DNA methylation [15]. In plants, DRM2 and CMT3 are responsible for methylation of CHH and CHG sites respectively, while MET1 maintains CG methylation [38].We tested if any of these features are involved in transcriptional activation and/or repression of the rRNA gene variants in Atnuc-L1 mutant plants.
To determine if specific siRNAs to 45S rRNAs sequences accumulate in Atnuc-L1 plants, we performed northern blot experiments using specific probes for promoter 45S rRNA sequences [39]. As shown in Figure 7A, the 45S siRNA probe hybridizes to a small RNA that accumulates ∼1.8 and ∼1.7 fold in Atnuc-L1-1 and Atnuc-L1-2 mutant plants respectively (lanes 3 and 1) compared with WT samples (lane 2). Higher levels of 45S siRNA were also observed in Northern blot experiments using total RNA extracted from WT and Atnuc-L1 flower buds (data not shown). Hybridization with primers corresponding to mir159 and AtSN1 siRNA did not detect major differences between WT and Atnuc-L1 samples (Panel miR159 and AtSN1). Hybridization with primers specific to small nuclear RNA U6 was used as an RNA loading control (panel snRNA U6).
Fig. 7. AtNUC-L1 gene disruption induces accumulation of siRNA 45S and rRNA gene hypomethylation on the 5′ETS. 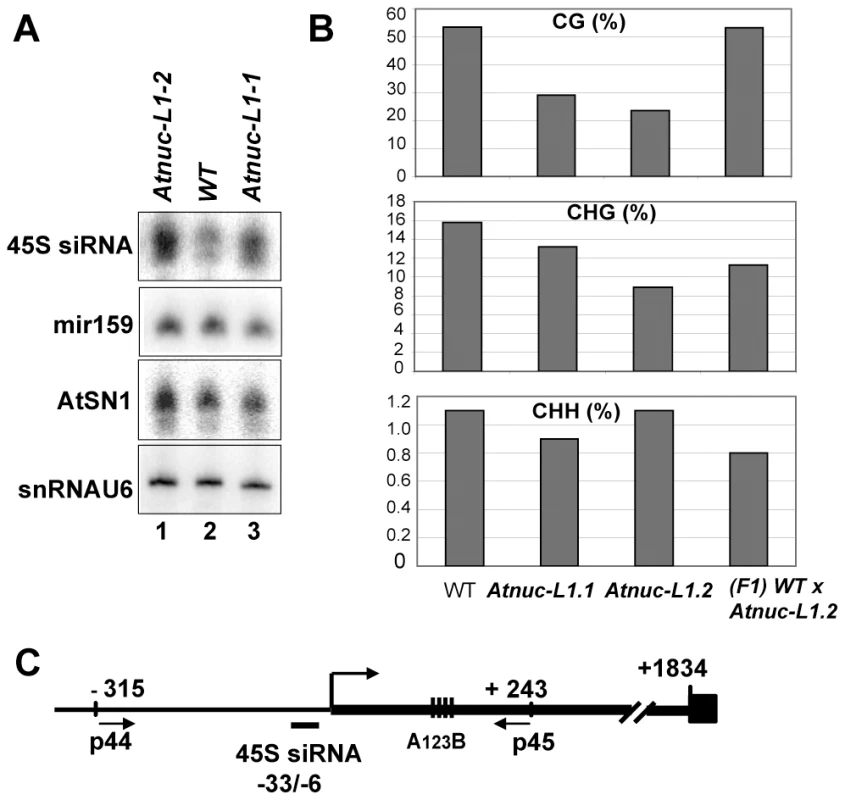
A) Total RNA from WT (lane 2) and Atnuc-L1 (lanes 1 and 3) plants were fractionated on PAGE and hybridized with [γ32P] 5′-end labeled primers to detect siRNA (45S, AtSN1) and miR159. Hybridization to detect snRNA U6 was performed to control RNA loading in each sample. B) Bisulfite sequencing analysis. The bar graphs show the representation (%) of methylated sites in the in 5′ETS rRNA gene sequences (from -+1 to +243) from WT, Atnuc-L1 mutant and F1 WT x Atnuc-L1-2 backcrossed plants in a CG (upper panel), CHG (middle panel) and CHH (lower panel) context. C) The schema shows the position of primers p44/p45 (located at −315 and at +243) and oligonucleotide 45S siRNA (from −33 to +6) used for sequencing bisulfite treated samples and for northern blot respectively. Conserved A123B motif in the 5′ETS is shown. To determine if siRNA accumulated in Atnuc-L1 mutant plants affected rRNA gene methylation, we performed bisulfite sequencing assays of both rRNA gene promoter (Figure S11) and 5′ETS (Figure 7B) sequences. In the 5′ETS (from +1 to +243) we observed lower CG methylation levels (panel CG) in both Atnuc-L1-1 (∼30%) and Atnuc-L1-2 (∼24%) mutant lines compared with WT plants (∼54%). A significant decrease of CHG methylation level (∼16% in WT) was also observed in Atnuc-L1-2 (∼9%) and to some extent in Atnuc-L1-1 (13%) mutant lines (panel CHG). Remarkably, decreased CG and CHG methylation was mainly observed in the 5′ETS rRNA sequences but not in sequences located upstream from the TIS (from −315 to +1, Figure S11). We did not observe significant changes in the CHH methylation level in Atnuc-L1 compared to WT plants (panel CHH), either in the promoter and/or in the 5′ETS regions (Figure 7B and Figure S11). Interestingly, we did not detect any major changes in the acetylation and methylation status of histone H3 in the rRNA genes from Atnuc-L1 plants (Figure S6), nor did we observe significant differences in the methylation status in the immunodetection experiment shown in Figure 6B (panel H3K4me2). When the Atnuc-L1-2 mutants were backcrossed with WT plants the CG methylation (53.2%) returned to WT levels (Figure 7B). In contrast, although the CHG methylation level increases (∼ 11.3%) in the F1 backcrossed plants, it remains lower compared with WT plants. Finally, in the F1 backcrossed plants the level of pre-rRNA return to those observed in WT plants (Figure S10).
We conclude that disruption of AtNUC-L1 affects accumulation of siRNA specific to 45S rRNA genes and symmetric methylation of 5′ETS rRNA sequences but does not affect the overall histone methylation status of 45S rRNA genes.
Discussion
The present results provide evidence that disruption of AtNUC-L1 gene expression affects RNA pol I transcription regulation at multiple levels. On one hand, by increasing RNA Pol I association with rRNA genes and, on the other, by switching the transcriptional state of specific rRNA gene variants.
The variations in the 45S rRNA genes detected in this work are in the 3′ETS sequences. We do not observe major variations in the promoter and/or in the 5′ETS rRNA gene sequences. Remarkably, among the four distinct 3′ETS rRNA gene variants present in the genome of Arabidopsis thaliana, the most highly represented (VAR1, ∼50%) is not expressed in WT but in Atnuc-L1 mutant plants (Figure 2 and data not shown). In this context it would be interesting to determine if rRNA VAR1 genes localize at NOR2 or NOR4 or at specific heterochromatic positions in the NORs.
We also observed that the rRNA variants are differentially expressed in A. thaliana WT plants. However, activation and/or repression of these rRNA variants are probably more dependent on the developmental stage and/or tissue rather than cell growth conditions (Figure 3). Remarkably, we detected rRNA VAR1 gene expression essentially in germinating seeds and Atnucl-L1 mutants dependent, i.e. when chromatin is decondensed (Figure 3B and Figure 6B). Developmental activation and repression of rRNA genes is also observed in genetic hybrids. For instance, in allotetraploid B. napus, the B. oleracea-derived rRNA is normally repressed and B. rapa-derived rRNA is active in vegetative tissue. However, rRNA transcripts from oleracea are found in all organs derived from floral meristems, suggesting that the oleracea genes silenced during vegetative growth are derepressed on flowering [40]. Similarly, developmental modulation of dominant and underdominant rRNA genes was also observed in Solanum allopolyploid species [41] and Arabidopsis hybrids [29].
Higher level of RNA pol I transcription of rRNA genes in the absence of AtNUC-L1 is supported by an increased amount of RNA pol I subunit associated with rRNA genes in Atnuc-L1 plants (Figure 5A). In agreement, in situ hybridization experiments confirm that accumulation of pre-rRNA is correlated with a higher concentration of RNA polymerase subunit in the nucleolus of Atnuc-L1 plants (Figure S5). Thus, it is possible that accumulation of pre-rRNA is due to a higher loading of RNA pol I subunits and/or an increased number of active rRNA genes. Interestingly, the level of preRNA transcripts from GP decreases in the Atnuc-L1 mutant (Figure 5B). Thus, accumulated pre-rRNAs are probably RNA pol I products transcribed from the IGS rather than the TIS in the rRNA gene promoter (Figure 5C). In agreement with these results, a previous report showed transcription from spacer promoter rRNA sequences in Arabidopsis thaliana [9]. Although the accumulation of pre-rRNA transcripts could also be generated by deficiency of the pre-rRNA machinery in Atnuc-L1 plants, our results indicate that this is not case. Indeed, fibrillarin remains localized in the disorganized nucleolus of Atnuc-L1 plants and pre-rRNA processing events are still accurate (Figure 4, Figure S4 and S5). However we do not discard the possibility that the rate of pre-rRNA processing activity of standard or longer pre-rRNA could be affected en absence of nucleolin.
RNA Pol I transcription from the IGS was also observed in mouse cell culture [42]. In this case, transcription from the IGS is dependent on the amount of TIP5, the large subunit of the chromatin remodeling complex NoRC [43]. As in Atnuc-L1, depletion of TIP5 decreases the level of promoter -initiated transcripts and leads to accumulation of pre-rRNA. However, in contrast to TIP5, disruption of AtNUC-L1 does not affect heterochromatic marks (Figure 6 and Figure S6). This suggests that AtNUC-L1 determines assembly or positioning of nucleosomes rather than chromatin epigenetic changes. Indeed, mammalian nucleolin binds histone H1 [44], [45] and play a role in histone chaperoning and assisting remodeling of nucleosomes [23]. A role in chromatin remodeling has also been demonstrated for nucleolar transcription factor UBF (Upstream Binding Factor) in animals. The UBF factor displaces histone H1 from nucleosomes [46], controls rRNA transcription [47] and was recently reported to determine the number of active genes by a methylation-independent mechanism [34]. Despite these similarities, AtNUC-L1 and UBF are structurally different proteins and consequently they might affect rRNA gene activation/repression through different molecular mechanism.
AtNUC-L1 co-localizes with only approximately half of the rRNA loci detected in the nucleus of WT plants (Figure 6 and Table S1). In Arabidopsis thaliana plants, rRNA genes are located at two NORs, one on chromosome 2 and the other one on chromosome 4 [35], [36]. We can not say if AtNUC-L1 binds NOR2 and/or NOR4. However, we predict that AtNUC-L1 only associates with potentially transcriptionally active rRNA genes located at one of the two NORs. This is based on the fact that nucleolin localizes in the nucleolus, which is a consequence of rRNA gene expression [1], [3], [48]. Moreover, while almost all the AtNUC-L1 signal is in the nucleolus, most of the rRNA signal is located in its periphery in the knob structures of condensed and inactive rRNA chromatin [49]. In addition, ChIP and inmunolocalization experiments using a plant mutant (axe1-5) in which the rRNA gene VAR1 is active (recently reported in [16]), also indicate that AtNUC-L1 binds transcriptionally active rRNA genes (Figure 6). Interaction of AtNUC-L1 with active rRNA genes is also supported by ChIP experiments that demonstrate that AtNUC-L1 co immunoprecipitates mainly with active A. arenosa –derived rRNA genes rather than repressed A. thaliana –derived rRNA genes in Arabidopsis suecica hybrid plants (Figure S9).
Although there is accumulation of 45S siRNA in the Atnuc-L1 plants (Figure 7), we did not observe major quantitative changes in the methylation and acetylation status of histone H3 in Atnuc-L1 mutant plants that might indicate higher or lower number of active or repressed rRNA genes in Atnuc-L1 mutant plants (Figure S6). In contrast we observed lower CpG methylation specifically in the 5′ETS region of the rRNA gene (Figure 7B) but not in the promoter region (Fig S11). Previous analyses of pericentromeric repeats and rRNA genes in Arabidopsis revealed that DNA methylation loss at CG dinucleotides persists into successive sexual generations [50]–[52]. Even when recessive mutations in genes required for CG methylation maintenance (e.g., DDM1 or MET1) are replaced by functional alleles, methylation within most repeats and transposons does not recover, or “reset” [53]. In contrast, our experiments with Atnuc-L1-2 documented a full recovery of CG methylation in rRNA genes after backcrossing this mutation with wild type Col0 (Figure 7B). We hypothesize that AtNUC-L1 deficiency impairs CG methylation maintenance without abolishing the underlying chromatin state required for DNA methylation to be reestablished. On the other hand, only a partial recovery of CHG methylation was observed in F1 backcrossed plants. A similar situation was also observed in hda6 mutants. Here, lost of CHG (and CG) methylation in the rRNA promoter region was also only partially recovered in the mutant plants rescued by the HDA6 transgene [16]. But in Atnuc-L1 mutant plants, the complete recovery of CG methylation is sufficient to restore wild-type expression of intergenic transcripts and rRNA gene variant expression (Figure S10). Interestingly, chromatin immunoprecipitation using antibodies that recognize dimethylated H3K9 reveal no significant change between wild-type Col-0 and Atnuc-L1 mutants (Figure S6). Taken together, H3K9 dimethylation is potentially the underlying chromatin state that allows CG methylation to be reestablished efficiently in Atnuc-L1 mutant heterozygote plants. This is not the case for pericentromeric repeats or rRNA genes after ddm1 or met1 back-crossing, but these mutants do show a loss of H3K9 dimethylation.
A few years ago we reported a plant nucleolin containing -U3snoRNP complex that specifically binds four conserved motifs in the 5′ETS (A123B, shown in Figure S8) and proposed a role for this complex in coupling transcription and processing of pre-rRNA [54], [55]. Here we demonstrate that AtNUC-L1 interacts directly with 5′ETS rRNA gene sequences but does not co-precipitate with RNA Pol I subunits (Figure S8). Thus, considering the nucleosome remodeling activity of nucleolin proteins [23], [56], it is rational to propose that binding of AtNUC-L1 to rRNA genes may be required to position nucleosomes in specific transcriptional frames that determine the ‘on’ or ‘off’ state of transcriptional active rRNA genes (Figure 8). Accordingly, demethylation of the 5′ETS rRNA gene sequences and activation of VAR1 suggest that AtNUC-L1 maintains gene methylation required for accurate nucleosome position for transcription (Figure 8). Indeed, several studies point to a major role of CpG methylation in nucleosome positioning and binding of multiples protein factors could be involve in repositioning of nucleosome [57], [58]. In this context, AtNUC-L1 activity might be required for rRNA gene methylation or demethylation to repress or activates RNA Pol I transcription. We do not know if AtNUC-L1 interacts with any DNA Methyl transferase (DNA-MT), demethylases (DME/ROS1) and/or Methyl cytocine –Binding protein (Mc-BP) (For references [59], [60]). However, nucleolin like proteins in plants interact in a large Ribo Nucleoprotein Complex (RNP) with the RNA methyltranferase fibrillarin (Figure S8) and other DNA/RNA modification activities, including RdRP and TSN proteins, which have been implicated in chromatin silencing [55].
Fig. 8. AtNUC-L1 controls symmetrical DNA methylation and nucleosome positioning to determine the “on” or “off” state of transcriptionally active rRNA genes. 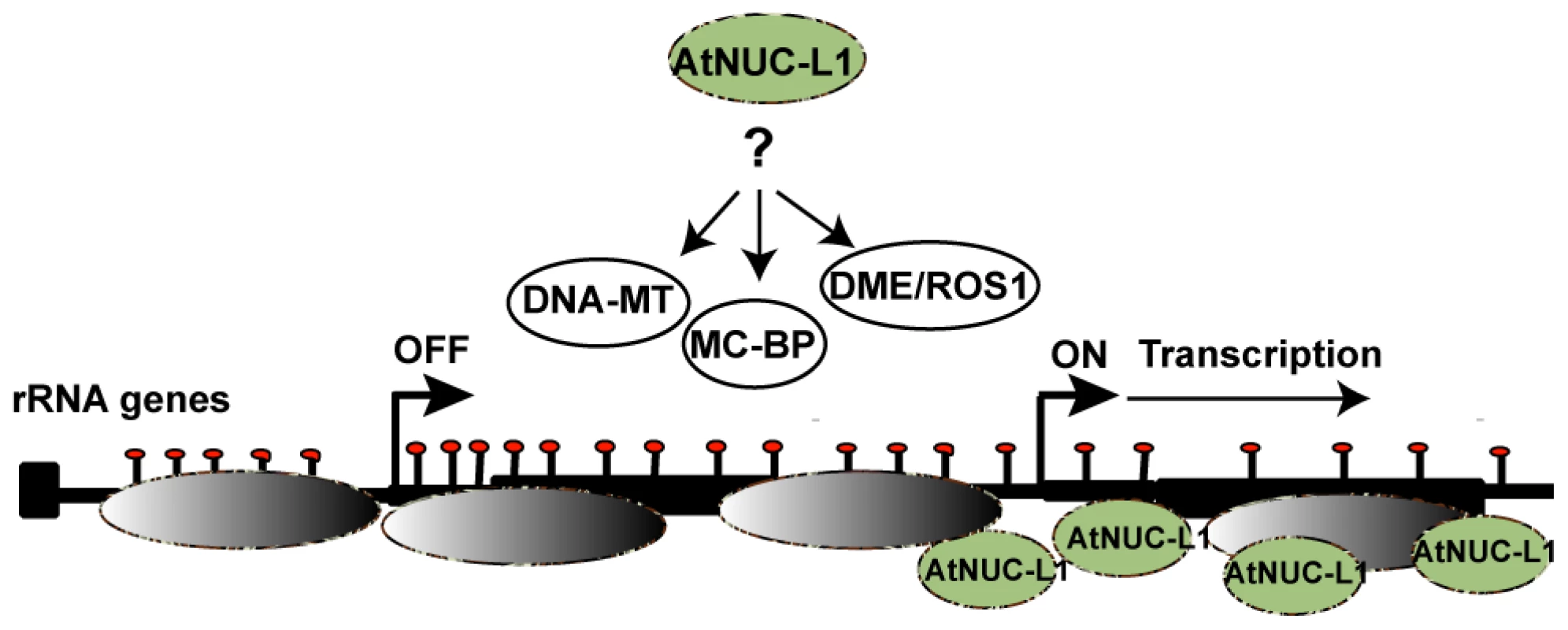
In WT plants AtNUC-L1 (in green) binds chromatin and 5′ETS rRNA sequences. AtNUC-L1 binding to the 5′ETS might maintain a suitable gene methylation pattern (red bullets) to set nucleosome (in grey) positioning and transcription from the GP. In this model, AtNUC-L1 might be required for nucleolar localization, assembly and/or activity of DNA-MT, MC-BP or DME/ROS1 protein complexes that repress or active rRNA transcription of specific loci in WT plants. Many questions remain, including mutual dependency and complex crosstalk among nucleolin-like proteins and other functionally related protein factors, such as HDA6 which has also been recently implicated in reactivation of rRNA gene VAR1 [16]. Finally, the AtNUC-L2 protein induced in Atnuc-L1 mutant plants ([18] and Figure S1) might also play a major role in controlling activation and/or repression of these rRNA gene variants. However, in WT plants we have not been able to find any correlation between AtNUC-L2 and rRNA VAR1 gene expression and consequently the physiological impact of a second nucleolin protein gene in plants remains an open question.
Materials and Methods
Plant growth conditions and mutant isolation
Seeds corresponding to Atnuc-L1-1 and Atnuc-L1-2 plants lines (SALK_053590 and SALK_002764 respectively) were obtained from the Nottingham Arabidopsis Stock Center (http://nasc.life.nott.ac.uk). Atnuc-L1-1 was reported previously in [18] and Atnuc-L1-2 corresponds to the parl1-2 allele described by [28]. The plant mutant axe1-5 was provided by C. Pikaard and described in [16]. To obtain backcrosses WT x Atnuc-L1-2 plants, pollen from Atnuc-L1-2 was used to fertilize WT Col0 plants. Then F1 heterozygous plants where selected by PCR as described previously in [18].
RNA extraction and blot analysis
Total RNA was isolated using TriZol reagent (GE Healthcare, Littler Chalfont, Bukimhamshire, UK) as described previously [18]. Total RNA (15 µg) was either fractionated on 0.8% formaldehyde agarose gels and transferred to nitrocellulose (Hybond+, GE Healthcare) or fractionated on 15% polyacrylamide gels and transferred to Hybond NX (GE Healthcare). For detection of pre-rRNA precursors and small RNA, 10 pmoles of oligonucleotide primers were 5′end labeled using 50 µCi of [γ32 P] ATP (6000 Ci/mmol) and T4 PNK (Promega). Oligonucleotides probes are listed in the supplementary material (Text S1). After overnight hybridization at 42°C (in 50% formamide, 0.5% SDS, 6X SSC, 5% Denhardt's solution buffer), filters were washed once 15 min. at 42°C with 2X SSC, 0.1% SDS and twice 15 min. at 65°C with 2X SSC, 0.1% SDS before exposure (over night for p34, p35, p37, p39 and p41 and 3 hrs for p36, p38 and p40) in a PhosphoImager (Molecular Dynamics).
Amplification and cloning of 3′ETS rRNA and pre–rRNA sequences
For cloning 3′ETS rRNA sequences, genomic DNA extracted from WT and Atnuc-L1 plants was amplified using GoTaq DNA polymerase (Promega). For cloning 3′ETS pre-rRNA, the RT-PCR reaction was performed using total DNA free - RNA (∼1 µg) and a OneStep RT_PCR Kit (QIAGEN). Corresponding pairs of specific primers used for amplification are indicated in each figure. The amplified rRNA and pre-rRNA sequences were then cloned in a pGemT easy vector (Promega). All clones were sequenced with a model 3130 DNA sequencer and an ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA).
Primer extension and RNase A/T1 protection assay
Primer extension analysis was performed using total RNA (15 µg) and specific 5′-end labeled primers as previously described [18]. Primers used were: p3 for detection of primary pre-rRNA precursor (+104 nucleotides from TIS) and 3AtU3 for detection of U3snoRNA used as loading control. Dideoxy sequencing reactions were performed using the fmol DNA Cycle Sequencing System (Promega) with a pGem-3Z plasmid vector containing the A. thaliana rRNA sequences from −520 to +1940 [61].
For RNase A/T1 mapping, the 3′ETS rRNA sequence that includes 16 nucleotides of the 3′ end of 25S rRNA followed by 664 bases of non coding sequence, was amplified from genomic DNA using primers p1 and p4 and cloned in the pGemT easy vector (Promega). The riboprobe was produced by in vitro transcription of the linearized antisense rRNA sequence by incorporating [α32P] CTP. The radiolabeled RNA probe was purified on 8% PAGE and the RNase protection assay performed as described previously [54].
Immunostaining and FISH
For the combination of immunostaining and FISH, immunodetection was performed prior to FISH. Young rosette leaves were squashed on slides in PBS 1X / 4% paraformaldehyde. Immunodetection of AtNUC-L1 was performed using α-AtNUC1 primary antibody followed by detection with the fluorochrome Alexa 488 - conjugated goat anti-rabbit IgG (Sigma). H3K4me2 immunodetection was performed using an anti-H3K4me2 primary antibody (Upstate) followed by detection with the fluorochrome Alexa 594 - conjugated goat anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO). FISH was subsequently performed as described previously [18]. Nuclei were stained with DAPI (4′, 6-diamidino-2-phenylindole) mounting medium (Vectashield, Vector Laboratories). A more detailed description of this immuno-FISH experiment is available in the supplementary information section (Text S1). Immunodetection of fibrillarin was performed using mouse fibrillarin (72B9) primary antibody prior to detection with the fluorochrome Alexa 488 - conjugated goat anti-rabbit IgG (Sigma). For image acquisition, an epifluorescence Imager Z1 microscope (Zeiss) with an Axiocam MRm camera (Zeiss) was used.
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed as described previously [18], [62]. To immunoprecipite rRNA chromatin associated with AtNUC-L1 protein, rabbit polyclonal antibodies against the C-terminal peptide sequence of AtNUC-L1 (Figure 4C) were custom made by NeoMPS (Strasbourg, France).
Real-time quantitative PCR
DNA was amplified using an Applied Biosystems model 7500 thermocycler with 0.5 units of Platinum Taq (Invitrogen), SYBR Green I (Invitrogen), and Internal Reference Dye (Sigma). Results were analyzed using the comparative CT method (Livak and Schmittgen, 2001) relative to input.
Bisulfite analysis
Genomic DNA from WT, Atnuc-L1 mutant and F1 Atnuc-L1-2 x WT plants was extracted using Illustra DNA extraction kit phytopure (GE Healthcare) following the manufacturer's instruction. 2 µg of DNA was digested overnight using 20 Units of BamHI restriction enzyme prior to bisulfite conversion. Bisulfite treatment was performed using Epitect Bisulfite kit (Qiagen). The primers p42 and p43 were used to amplify rRNA gene sequences (from −315 to +243) and resulting PCR products were clone in the pGemT easy vector (Qiagen). Between 31 and 41 clones per sample (WT, 41; Atnuc-L1-1, 35; Atnuc-L1-2, 31) were sequenced and analyzed using the CyMATE method [63].
Supporting Information
Zdroje
1. LamYW
Trinkle-MulcahyL
LamondAI
2005 The nucleolus. J Cell Sci 118 1335 1337
2. ShawP
DoonanJ
2005 The nucleolus. Playing by different rules? Cell Cycle 4 102 105. Epub 2005 Jan 2013
3. Saez-VasquezJ
MedinaFJ
2008 The plant nucleolus. Botanical Research: Incorporating Advances in Plant Pathology, Vol 47. San Diego Elsevier Academic Press Inc 1 46
4. GrummtI
2003 Life on a planet of its own: regulation of RNA polymerase I transcription in the nucleolus. Genes Dev 17 1691 1702
5. ReederRH
1992 Regulation of transcription by RNA polymerase I. Transcriptional Regulation: Cold Spring Harbor Laboratory Press
6. Saez-VasquezJ
EcheverriaM
2006 Polymerase I transcription.
GrasserKD
Regulation of transcription in plants Oxford, UK Blackwell 162 183
7. PikaardCS
ReederRH
1988 Sequence elements essential for function of the Xenopus laevis ribosomal DNA enhancers. Mol Cell Biol 8 4282 4288
8. PikaardCS
PapeLK
HendersonSL
RyanK
PaalmanMH
1990 Enhancers for RNA polymerase I in mouse ribosomal DNA. Mol Cell Biol 10 4816 4825
9. DoellingJH
GaudinoRJ
PikaardCS
1993 Functional analysis of Arabidopsis thaliana rRNA gene and spacer promoters in vivo and by transient expression. Proc Natl Acad Sci U S A 90 7528 7532
10. WanzenbockEM
SchoferC
SchweizerD
BachmairA
1997 Ribosomal transcription units integrated via T-DNA transformation associate with the nucleolus and do not require upstream repeat sequences for activity in Arabidopsis thaliana. Plant J 11 1007 1016
11. PreussS
PikaardCS
2007 rRNA gene silencing and nucleolar dominance: insights into a chromosome-scale epigenetic on/off switch. Biochim Biophys Acta 1769 383 392. Epub 2007 Mar 2012
12. ChenZJ
ComaiL
PikaardCS
1998 Gene dosage and stochastic effects determine the severity and direction of uniparental ribosomal RNA gene silencing (nucleolar dominance) in Arabidopsis allopolyploids. Proc Natl Acad Sci U S A 95 14891 14896
13. LawrenceRJ
EarleyK
PontesO
SilvaM
ChenZJ
2004 A concerted DNA methylation/histone methylation switch regulates rRNA gene dosage control and nucleolar dominance. Mol Cell 13 599 609
14. ProbstAV
FagardM
ProuxF
MourrainP
BoutetS
2004 Arabidopsis histone deacetylase HDA6 is required for maintenance of transcriptional gene silencing and determines nuclear organization of rDNA repeats. Plant Cell 16 1021 1034. Epub 2004 Mar 1022
15. PreussSB
Costa-NunesP
TuckerS
PontesO
LawrenceRJ
2008 Multimegabase silencing in nucleolar dominance involves siRNA-directed DNA methylation and specific methylcytosine-binding proteins. Mol Cell 32 673 684
16. EarleyKW
PontvianneF
WierzbickiAT
BlevinsT
TuckerS
2010 Mechanisms of HDA6-mediated rRNA gene silencing: suppression of intergenic Pol II transcription and differential effects on maintenance versus siRNA-directed cytosine methylation. Genes Dev 24 1119 1132
17. BenderJ
2004 Chromatin-based silencing mechanisms. Curr Opin Plant Biol 7 521 526
18. PontvianneF
MatiaI
DouetJ
TourmenteS
MedinaFJ
2007 Characterization of AtNUC-L1 reveals a central role of nucleolin in nucleolus organization and silencing of AtNUC-L2 gene in Arabidopsis. Mol Biol Cell 18 369 379
19. GinistyH
SicardH
RogerB
BouvetP
1999 Structure and functions of nucleolin. J Cell Sci 112 761 772
20. RogerB
MoisandA
AmalricF
BouvetP
2002 Repression of RNA polymerase I transcription by nucleolin is independent of the RNA sequence that is transcribed. J Biol Chem 277 10209 10219. Epub 12001 Dec 10231
21. RogerB
MoisandA
AmalricF
BouvetP
2003 Nucleolin provides a link between RNA polymerase I transcription and pre-ribosome assembly. Chromosoma 111 399 407. Epub 2003 Feb 2011
22. YanagidaM
ShimamotoA
NishikawaK
FuruichiY
IsobeT
2001 Isolation and proteomic characterization of the major proteins of the nucleolin-binding ribonucleoprotein complexes. Proteomics 1 1390 1404
23. AngelovD
BondarenkoVA
AlmagroS
MenoniH
MongelardF
2006 Nucleolin is a histone chaperone with FACT-like activity and assists remodeling of nucleosomes. Embo J 25 1669 1679. Epub 2006 Apr 1666
24. UgrinovaI
MonierK
IvaldiC
ThiryM
StorckS
2007 Inactivation of nucleolin leads to nucleolar disruption, cell cycle arrest and defects in centrosome duplication. BMC Mol Biol 8 66
25. BogreL
JonakC
MinkM
MeskieneI
TraasJ
1996 Developmental and cell cycle regulation of alfalfa nucMs1, a plant homolog of the yeast Nsr1 and mammalian nucleolin. Plant Cell 8 417 428
26. DidierDK
KleeHJ
1992 Identification of an Arabidopsis DNA-binding protein with homology to nucleolin. Plant Mol Biol 18 977 979
27. KojimaH
SuzukiT
KatoT
EnomotoKI
SatoS
2007 Sugar-inducible expression of the nucleolin-1 gene of Arabidopsis thaliana and its role in ribosome synthesis, growth and development. Plant J 6 6
28. PetrickaJJ
NelsonTM
2007 Arabidopsis nucleolin affects plant development and patterning. Plant Physiol 144 173 186. Epub 2007 Mar 2016
29. PontesO
LawrenceRJ
SilvaM
PreussS
Costa-NunesP
2007 Postembryonic establishment of megabase-scale gene silencing in nucleolar dominance. PLoS ONE 2 e1157 doi:10.1371/journal.pone.0001157
30. ComellaP
PontvianneF
LahmyS
VignolsF
BarbezierN
2008 Characterization of a ribonuclease III-like protein required for cleavage of the pre-rRNA in the 3′ETS in Arabidopsis. Nucleic Acids Res 36 1163 1175
31. KufelJ
DichtlB
TollerveyD
1999 Yeast Rnt1p is required for cleavage of the pre-ribosomal RNA in the 3′ ETS but not the 5′ ETS. Rna 5 909 917
32. BarnecheF
SteinmetzF
EcheverriaM
2000 Fibrillarin genes encode both a conserved nucleolar protein and a novel small nucleolar RNA involved in ribosomal RNA methylation in Arabidopsis thaliana. J Biol Chem 275 27212 27220
33. FrenchSL
OsheimYN
CiociF
NomuraM
BeyerAL
2003 In exponentially growing Saccharomyces cerevisiae cells, rRNA synthesis is determined by the summed RNA polymerase I loading rate rather than by the number of active genes. Mol Cell Biol 23 1558 1568
34. SanijE
PoortingaG
SharkeyK
HungS
HollowayTP
2008 UBF levels determine the number of active ribosomal RNA genes in mammals. J Cell Biol 183 1259 1274
35. CopenhaverGP
PikaardCS
1996 RFLP and physical mapping with an rDNA-specific endonuclease reveals that nucleolus organizer regions of Arabidopsis thaliana adjoin the telomeres on chromosomes 2 and 4. Plant J 9 259 272
36. CopenhaverGP
PikaardCS
1996 Two-dimensional RFLP analyses reveal megabase-sized clusters of rRNA gene variants in Arabidopsis thaliana, suggesting local spreading of variants as the mode for gene homogenization during concerted evolution. Plant J 9 273 282
37. LawrenceRJ
PikaardCS
2004 Chromatin turn ons and turn offs of ribosomal RNA genes. Cell Cycle 3 880 883. Epub 2004 Jul 2021
38. TariqM
PaszkowskiJ
2004 DNA and histone methylation in plants. Trends Genet 20 244 251
39. PontesO
LiCF
NunesPC
HaagJ
ReamT
2006 The Arabidopsis chromatin-modifying nuclear siRNA pathway involves a nucleolar RNA processing center. Cell 126 79 92
40. ChenZJ
PikaardCS
1997 Transcriptional analysis of nucleolar dominance in polyploid plants: biased expression/silencing of progenitor rRNA genes is developmentally regulated in Brassica. Proc Natl Acad Sci U S A 94 3442 3447
41. KomarovaNY
GrabeT
HuigenDJ
HemlebenV
VolkovRA
2004 Organization, differential expression and methylation of rDNA in artificial Solanum allopolyploids. Plant Mol Biol 56 439 463
42. MayerC
SchmitzKM
LiJ
GrummtI
SantoroR
2006 Intergenic transcripts regulate the epigenetic state of rRNA genes. Mol Cell 22 351 361
43. SantoroR
LiJ
GrummtI
2002 The nucleolar remodeling complex NoRC mediates heterochromatin formation and silencing of ribosomal gene transcription. Nat Genet 32 393 396. Epub 2002 Oct 2007
44. ErardMS
BelenguerP
Caizergues-FerrerM
PantaloniA
AmalricF
1988 A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur J Biochem 175 525 530
45. KharratA
DerancourtJ
DoreeM
AmalricF
ErardM
1991 Synergistic effect of histone H1 and nucleolin on chromatin condensation in mitosis: role of a phosphorylated heteromer. Biochemistry 30 10329 10336
46. KermekchievM
WorkmanJL
PikaardCS
1997 Nucleosome binding by the polymerase I transactivator upstream binding factor displaces linker histone H1. Mol Cell Biol 17 5833 5842
47. StefanovskyV
LangloisF
Gagnon-KuglerT
RothblumLI
MossT
2006 Growth factor signaling regulates elongation of RNA polymerase I transcription in mammals via UBF phosphorylation and r-chromatin remodeling. Mol Cell 21 629 639
48. GerbiSA
BorovjaginAV
LangeTS
2003 The nucleolus: a site of ribonucleoprotein maturation. Curr Opin Cell Biol 15 318 325
49. PontesO
LawrenceRJ
NevesN
SilvaM
LeeJH
2003 Natural variation in nucleolar dominance reveals the relationship between nucleolus organizer chromatin topology and rRNA gene transcription in Arabidopsis. Proc Natl Acad Sci U S A 100 11418 11423. Epub 12003 Sep 11422
50. KakutaniT
MunakataK
RichardsEJ
HirochikaH
1999 Meiotically and mitotically stable inheritance of DNA hypomethylation induced by ddm1 mutation of Arabidopsis thaliana. Genetics 151 831 838
51. KankelMW
RamseyDE
StokesTL
FlowersSK
HaagJR
2003 Arabidopsis MET1 cytosine methyltransferase mutants. Genetics 163 1109 1122
52. VongsA
KakutaniT
MartienssenRA
RichardsEJ
1993 Arabidopsis thaliana DNA methylation mutants. Science 260 1926 1928
53. LippmanZ
MayB
YordanC
SingerT
MartienssenR
2003 Distinct mechanisms determine transposon inheritance and methylation via small interfering RNA and histone modification. PLoS Biol 1 e67 doi:10.1371/journal.pbio.0000067
54. Saez-VasquezJ
Caparros-RuizD
BarnecheF
EcheverriaM
2004 A plant snoRNP complex containing snoRNAs, fibrillarin, and nucleolin-like proteins is competent for both rRNA gene binding and pre-rRNA processing in vitro. Mol Cell Biol 24 7284 7297
55. SamahaH
DelormeV
PontvianneF
CookeR
DelalandeF
2009 Identification of protein factors and U3 snoRNAs from a Brassica oleracea RNP complex involved in the processing of pre-rRNA. Plant J
56. MongelardF
BouvetP
2007 Nucleolin: a multiFACeTed protein. Trends Cell Biol 17 80 86
57. ChodavarapuRK
FengS
BernatavichuteYV
ChenPY
StroudH
2010 Relationship between nucleosome positioning and DNA methylation. Nature
58. SegalE
WidomJ
2009 What controls nucleosome positions? Trends Genet 25 335 343
59. MathieuO
ReindersJ
CaikovskiM
SmathajittC
PaszkowskiJ
2007 Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell 130 851 862
60. WooHR
DittmerTA
RichardsEJ
2008 Three SRA-domain methylcytosine-binding proteins cooperate to maintain global CpG methylation and epigenetic silencing in Arabidopsis. PLoS Genet 4 e1000156 doi:10.1371/journal.pgen.1000156
61. GruendlerP
UnfriedI
PointnerR
SchweizerD
1989 Nucleotide sequence of the 25S-18S ribosomal gene spacer from Arabidopsis thaliana. Nucleic Acids Res 17 6395 6396
62. WierzbickiAT
HaagJR
PikaardCS
2008 Noncoding transcription by RNA polymerase Pol IVb/Pol V mediates transcriptional silencing of overlapping and adjacent genes. Cell 135 635 648
63. HetzlJ
FoersterAM
RaidlG
Mittelsten ScheidO
2007 CyMATE: a new tool for methylation analysis of plant genomic DNA after bisulphite sequencing. Plant J 51 526 536
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Vliv melatoninu a cirkadiálního rytmu na ženskou reprodukci
- Délka menstruačního cyklu jako marker ženské plodnosti
- Intrauterinní inseminace a její úspěšnost
-
Všechny články tohoto čísla
- Genome-Wide Association Meta-Analysis of Cortical Bone Mineral Density Unravels Allelic Heterogeneity at the Locus and Potential Pleiotropic Effects on Bone
- Beyond QTL Cloning
- An Evolutionary Framework for Association Testing in Resequencing Studies
- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- The Functional Interplay between Protein Kinase CK2 and CCA1 Transcriptional Activity Is Essential for Clock Temperature Compensation in Arabidopsis
- Endogenous Viral Elements in Animal Genomes
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- DNA Methylation and Normal Chromosome Behavior in Neurospora Depend on Five Components of a Histone Methyltransferase Complex, DCDC
- Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes
- Genetic Basis of Growth Adaptation of after Deletion of , a Major Metabolic Gene
- Nomadic Enhancers: Tissue-Specific -Regulatory Elements of Have Divergent Genomic Positions among Species
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- CTCF-Dependent Chromatin Bias Constitutes Transient Epigenetic Memory of the Mother at the Imprinting Control Region in Prospermatogonia
- Systematic Dissection and Trajectory-Scanning Mutagenesis of the Molecular Interface That Ensures Specificity of Two-Component Signaling Pathways
- Nucleolin Is Required for DNA Methylation State and the Expression of rRNA Gene Variants in
- The Complex Genetic Architecture of the Metabolome
- ATM Limits Incorrect End Utilization during Non-Homologous End Joining of Multiple Chromosome Breaks
- Mutation Disrupts Synaptonemal Complex Formation, Recombination, and Chromosome Segregation in Mammalian Meiosis
- Mismatch Repair–Independent Increase in Spontaneous Mutagenesis in Yeast Lacking Non-Essential Subunits of DNA Polymerase ε
- The Kinesin-3 Motor UNC-104/KIF1A Is Degraded upon Loss of Specific Binding to Cargo
- Epigenetic Silencing of Spermatocyte-Specific and Neuronal Genes by SUMO Modification of the Transcription Factor Sp3
- A Coastal Cline in Sodium Accumulation in Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1
- Cyclin B3 Is Required for Multiple Mitotic Processes Including Alleviation of a Spindle Checkpoint–Dependent Block in Anaphase Chromosome Segregation
- Altered DNA Methylation in Leukocytes with Trisomy 21
- Human-Specific Evolution and Adaptation Led to Major Qualitative Differences in the Variable Receptors of Human and Chimpanzee Natural Killer Cells
- Leptotene/Zygotene Chromosome Movement Via the SUN/KASH Protein Bridge in
- RACK-1 Acts with Rac GTPase Signaling and UNC-115/abLIM in Axon Pathfinding and Cell Migration
- Genome-Wide Effects of Long-Term Divergent Selection
- Endless Forms Most Viral
- Conflict between Noise and Plasticity in Yeast
- Essential Functions of the Histone Demethylase Lid
- The Transcriptional Regulator Rok Binds A+T-Rich DNA and Is Involved in Repression of a Mobile Genetic Element in
- The Cellular Robustness by Genetic Redundancy in Budding Yeast
- Localization of a Guanylyl Cyclase to Chemosensory Cilia Requires the Novel Ciliary MYND Domain Protein DAF-25
- A Buoyancy-Based Screen of Drosophila Larvae for Fat-Storage Mutants Reveals a Role for in Coupling Fat Storage to Nutrient Availability
- A Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species (Green Peach Aphid)
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- Genome-Wide Effects of Long-Term Divergent Selection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



