-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaConflict between Noise and Plasticity in Yeast
Gene expression responds to changes in conditions but also stochastically among individuals. In budding yeast, both expression responsiveness across conditions (“plasticity”) and cell-to-cell variation (“noise”) have been quantified for thousands of genes and found to correlate across genes. It has been argued therefore that noise and plasticity may be strongly coupled and mechanistically linked. This is consistent with some theoretical ideas, but a strong coupling between noise and plasticity also has the potential to introduce cost–benefit conflicts during evolution. For example, if high plasticity is beneficial (genes need to respond to the environment), but noise is detrimental (fluctuations are harmful), then strong coupling should be disfavored. Here, evidence is presented that cost–benefit conflicts do occur and that they constrain the evolution of gene expression and promoter usage. In contrast to recent assertions, coupling between noise and plasticity is not a general property, but one associated with particular mechanisms of transcription initiation. Further, promoter architectures associated with coupling are avoided when noise is most likely to be detrimental, and noise and plasticity are largely independent traits for core cellular components. In contrast, when genes are duplicated noise–plasticity coupling increases, consistent with reduced detrimental affects of expression variation. Noise–plasticity coupling is, therefore, an evolvable trait that may constrain the emergence of highly responsive gene expression and be selected against during evolution. Further, the global quantitative data in yeast suggest that one mechanism that relieves the constraints imposed by noise–plasticity coupling is gene duplication, providing an example of how duplication can facilitate escape from adaptive conflicts.
Published in the journal: . PLoS Genet 6(11): e32767. doi:10.1371/journal.pgen.1001185
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001185Summary
Gene expression responds to changes in conditions but also stochastically among individuals. In budding yeast, both expression responsiveness across conditions (“plasticity”) and cell-to-cell variation (“noise”) have been quantified for thousands of genes and found to correlate across genes. It has been argued therefore that noise and plasticity may be strongly coupled and mechanistically linked. This is consistent with some theoretical ideas, but a strong coupling between noise and plasticity also has the potential to introduce cost–benefit conflicts during evolution. For example, if high plasticity is beneficial (genes need to respond to the environment), but noise is detrimental (fluctuations are harmful), then strong coupling should be disfavored. Here, evidence is presented that cost–benefit conflicts do occur and that they constrain the evolution of gene expression and promoter usage. In contrast to recent assertions, coupling between noise and plasticity is not a general property, but one associated with particular mechanisms of transcription initiation. Further, promoter architectures associated with coupling are avoided when noise is most likely to be detrimental, and noise and plasticity are largely independent traits for core cellular components. In contrast, when genes are duplicated noise–plasticity coupling increases, consistent with reduced detrimental affects of expression variation. Noise–plasticity coupling is, therefore, an evolvable trait that may constrain the emergence of highly responsive gene expression and be selected against during evolution. Further, the global quantitative data in yeast suggest that one mechanism that relieves the constraints imposed by noise–plasticity coupling is gene duplication, providing an example of how duplication can facilitate escape from adaptive conflicts.
Introduction
For cellular adaptation gene expression must respond to changes in conditions. However, expression also varies stochastically among cells in a population. In the budding yeast Saccharomyces cerevisiae both variation across different conditions (‘expression plasticity’, [1]) and variation among individuals in a constant environment (‘expression noise’, [2]) have been quantified for thousands of genes. Comparisons across genes have shown that these two levels of expression correlate, suggesting that noise and plasticity may somehow be mechanistically coupled [3]–[7].
Correlations among different levels of expression variance are consistent with some theoretical proposals, which consider that stochastic, environmental, and mutational perturbations are likely to similarly affect biological systems [8]–[11]. The findings are also supported by mechanistic studies where mutations that increase noise have also increased plasticity [12], [13]. Further, several properties such as initiation from a TATA-box promoter [2], [4], [6] and high proximal promoter nucleosome occupancy [3], [14], [15] are enriched among genes with both high noise and high plasticity. It should be noted, however, that both properties are also associated with genes with a wide range of noise and plasticity levels [2]–[4], [6], [14], [15].
Theoretical work and intuition do, however, also suggest that noise and plasticity may not always by strongly coupled in this way. For example, by altering the size of transcriptional bursts, the interval time between bursts, or the number of decay steps in the degradation of a protein it should be possible to alter noise independently of plasticity [16]. Therefore it is important to ask whether coupling between expression noise and expression plasticity is, as reported [3], a general result. Or, rather, is coupling an evolvable trait that can vary among genes? Further, what is the mechanistic basis of coupling? Does coupling constrain expression evolution? How are such constraints relieved? And has coupling itself been subject to selection?
In some specific situations a strong coupling between noise and plasticity may be disfavored. For example, if high expression plasticity is beneficial, facilitating environmental adaptation, but high noise is detrimental, then strong coupling will cause a fitness cost–benefit conflict. This potential for an adaptive conflict predicts that for many genes strong coupling between noise and plasticity would reduce fitness. In short, when noise is detrimental, noise–plasticity coupling should be disfavored. It is not known if this is the case.
Here using global quantitative data from yeast it is shown that noise–plasticity coupling is not a general result, but rather a property of particular promoter architectures, and so is an evolvable trait. Promoter architectures that favor coupling are underrepresented among genes required for viability, and for these genes noise and plasticity are rather independent, consistent with selection against coupling. Following gene duplication, however, when the detrimental effects of expression variation in many cases will be reduced, the constraints on coupling and promoter architectures that favor coupling appear to be relaxed. Thus, noise–plasticity coupling is not a general trait, but one that likely is both constrained by selection and constrains the evolution of gene expression. Further, this constraint may be relieved following gene duplication, providing an example of escape from adaptive conflict [17], [18].
Results
Noise–plasticity coupling is related to promoter architecture
Considering all genes in yeast there is a reasonable correlation between levels of gene expression variation in a single condition (‘expression noise’ [2]) and the variability of expression across changing conditions (‘expression plasticity’ [1]) (Spearman's correlation coefficient, rho = 0.30, p<2.2E-16, n = 2049 [3]–[6]). However, considering different classes of genes shows that this is not a general result. In yeast only about 20% of genes are transcribed from promoters containing TATA box elements [19]. For these genes, noise and plasticity are strongly coupled (rho = 0.62, p<2.2E-16, n = 369, Figure 1A). In contrast, for non-TATA genes coupling is much weaker (rho = 0.16, p = 1.4E-10, n = 1680, Figure 1B). This indicates that the extent of coupling may relate to the mechanism of transcription initiation.
Fig. 1. Noise–plasticity coupling relates to promoter architecture. 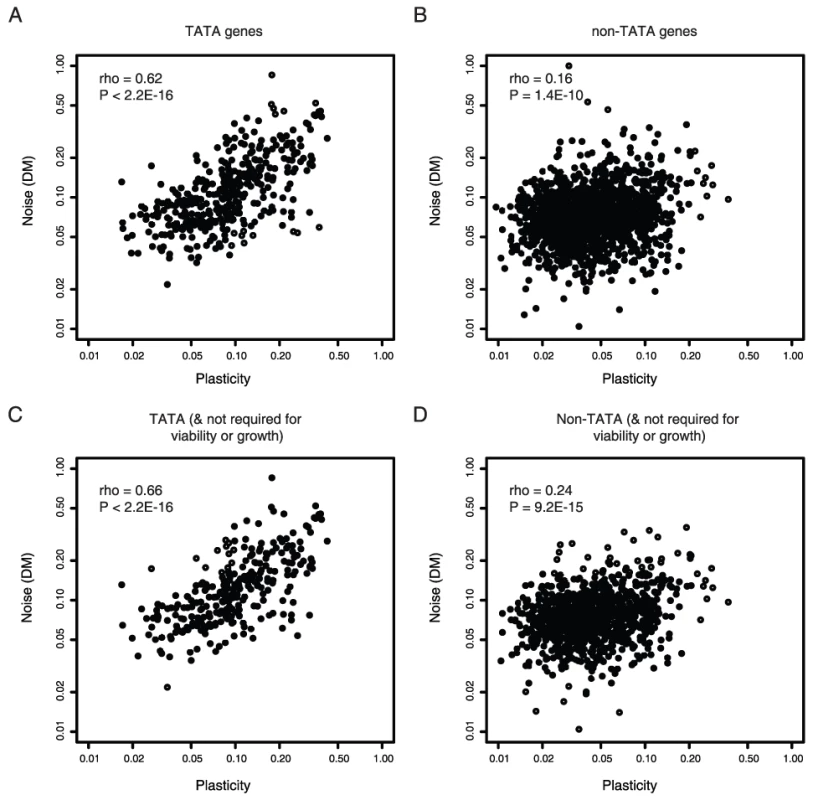
Noise–plasticity coupling for genes initiating from TATA-box promoters (A) and non-TATA promoters (B). Scaled noise and plasticity levels are shown for all genes with available data in yeast. In (C,D) the same comparison is made, but excluding all essential genes, haploinsufficient genes and genes required for growth (C–D). Correlation coefficients and P-values are shown inset. The stronger coupling of TATA genes is confirmed when controlling for possible confounding features such as the absolute level of plasticity (Table S1), the requirement of a gene for viability (Figure 1C and 1D), gene function (Table S3), expression level (Table S4), protein complex membership (Table S5), the number of upstream regulators (Table S6), the identity of upstream regulators (Table S7), histone exchange rates (Table S8), and nucleosome occupancy (Table 1). In summary, coupling between noise and plasticity in yeast appears related to the promoter architecture of a gene.
Tab. 1. Coupling between expression noise and expression plasticity for different gene classes in yeast. 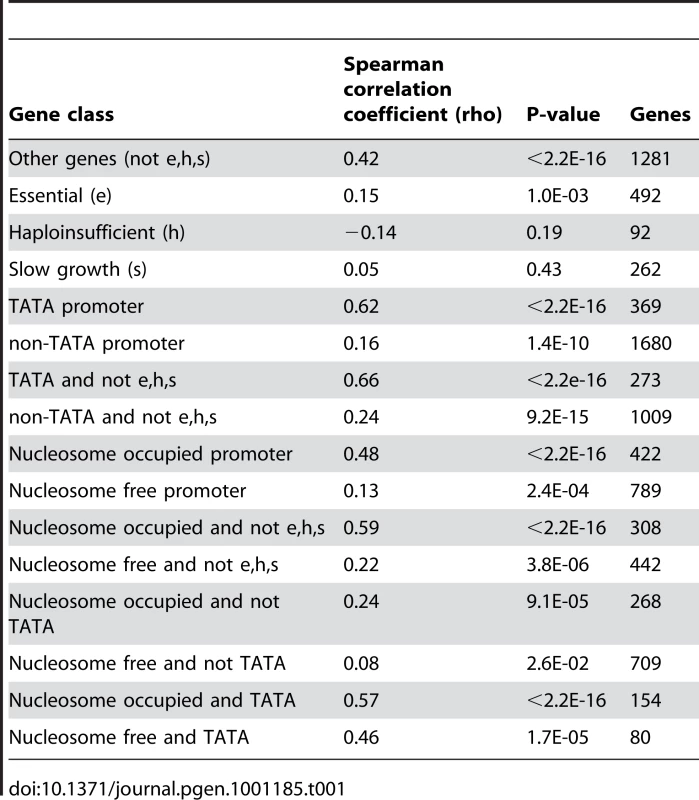
Noise–plasticity coupling and chromatin dynamics
In eukaryotes DNA is packaged into chromatin, and this chromatin structure varies across the promoters of different genes [14], [15], [20]. Chromatin remodeling is thought to be a major source of transcriptional noise [13], [21], [22]. Many genes in yeast contain a DNA-encoded region of low nucleosome occupancy in their proximal promoters, a feature often associated with low expression noise [14], [15]. Considering the nucleosome occupancy of promoters shows that high noise–plasticity coupling is also associated with high upstream nucleosome occupancy (Figure S1A and S1B, rho = 0.13, p = 2.4E-4, n = 789 for genes with upstream nucleosome free regions and rho = 0.48, p<2.2E-16, n = 273 for genes with high upstream nucleosome occupancy). This result is confirmed when controlling for gene importance (Figure 2A and 2B), the absolute levels of plasticity (Table S1), and also when only considering non-TATA promoters, even though these promoters show lower overall levels of coupling (Table 1, rho = 0.08 for non-TATA genes with upstream nucleosome free regions and rho = 0.24 for non-TATA genes with high upstream nucleosome occupancy).
Fig. 2. Noise–plasticity coupling is associated with promoter chromatin structure. 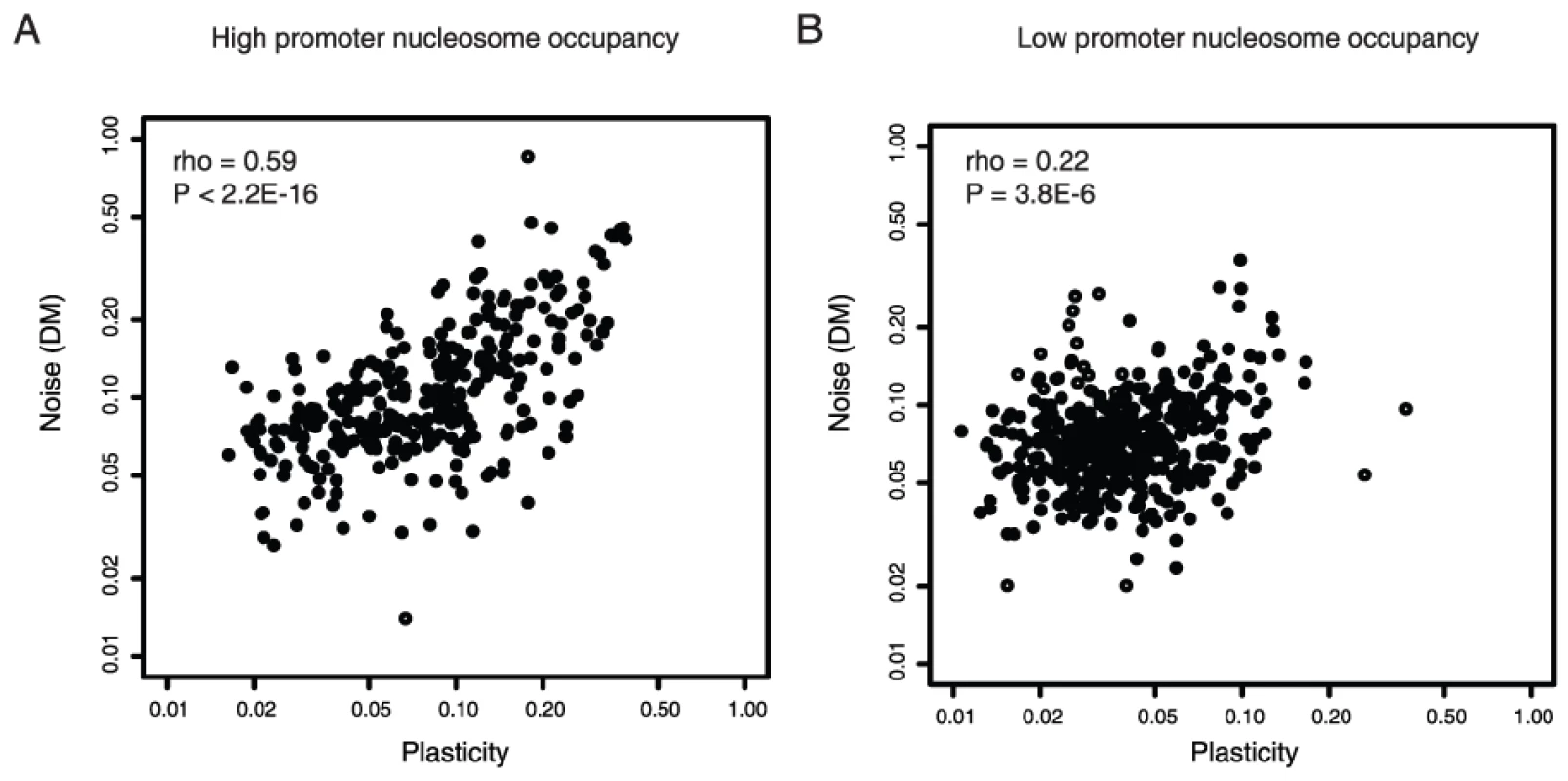
Noise–plasticity coupling for genes with high proximal promoter nucleosome occupancy [15] (A) and low proximal promoter nucleosome occupancy (B). Here only genes not required for growth or viability are considered. The comparison for all genes and for genes transcribed from non-TATA promoters is shown in Figure S1. Spearman correlation coefficients and P-values are shown inset. Promoters also differ in their nucleosome dynamics, and the exchange of core histones has been quantified across much of the genome [23]–[25]. High-noise plasticity coupling is also associated with high rates of histone exchange in promoter regions (Table S8). This is confirmed when controlling for TATA presence (Table S8), nucleosome occupancy (Table S9), or when only considering genes with low plasticity (Table S10). Thus, in addition to the link between noise–plasticity coupling and initiation from a TATA box, stronger coupling is also associated with higher and more dynamic promoter nucleosome occupancy. This strengthens the evidence that noise–plasticity coupling relates to the process of transcription initiation and indicates that coupling relates to chromatin remodeling. It also suggests that the extent of coupling is a trait that has the potential to change during evolution.
Noise–plasticity coupling is disfavored for essential genes
High expression variation can be detrimental if it results in insufficient protein production, and there is good evidence that this is the case in yeast because genes required for viability have low noise [2], [5], [26], [27]. This predicts that noise–plasticity coupling should also be disfavored for these genes: although genes required for viability still need to respond to external conditions (for example coupling growth rates to changes in the environment), excessive variation in their production would be detrimental.
Consistent with this, essential genes, haploinsufficient genes (genes that reduce fitness when their copy number is reduced by half) and genes required for growth all show little or no significant coupling between noise and plasticity (Figure 3, rho = 0.15 p = 0.001 n = 492 for essential genes, p = 0.19 for haploinsufficient genes, and p = 0.43 for genes required for growth). Similar results are seen when only considering genes with low absolute levels of plasticity (Table S1). Consistent with predictions that noise will be detrimental for protein complex subunits [27], coupling is also lower for these genes (Table S2). Thus when high noise is likely to be detrimental, noise and plasticity are largely unrelated traits in yeast.
Fig. 3. Low noise–plasticity coupling for core cellular components. 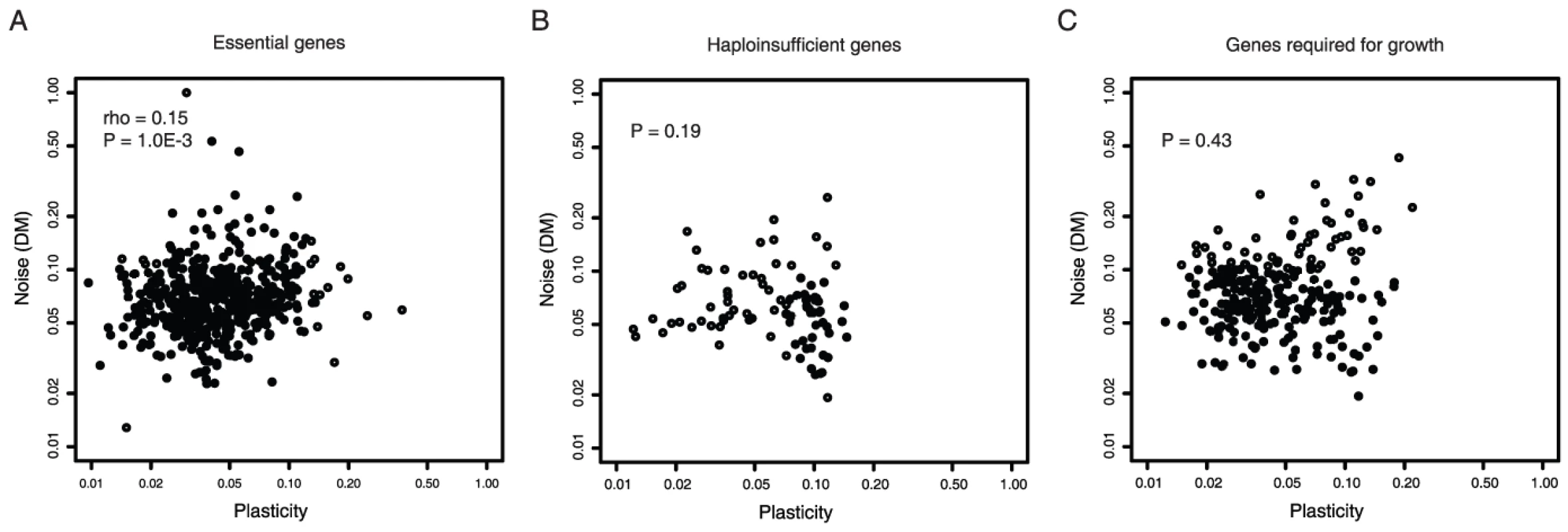
The correlation between gene expression noise and gene expression plasticity is shown for essential genes (A), haploinsufficient genes (B), and genes required for growth (C). Noise and plasticity data are scaled between 0 and 1. Spearman rank correlation coefficients (rho) and P-values are shown for each gene class. These findings are also consistent with differences in promoter architectures. Whereas 24% of non-essential genes use TATA promoters, only 3% of haploinsufficient genes, 9% of essential genes, and 11% of genes required for growth do (p<10E-14 in all cases, Fisher's exact test). Further, genes required for growth or viability usually have nucleosome free regions in their proximal promoters (74% compared to 57% for other genes, p<2.2E-16) and they have low levels of promoter histone exchange (mean 0.9 compared to 1.1 for other genes, p = 1.2E-11, Kolmogorov-Smirnov (KS) test). Thus, for genes encoding core cellular components, promoter architectures associated with high noise–plasticity coupling are avoided. This is consistent with a model in which selection disfavors coupling when it is detrimental.
Increased noise–plasticity coupling following gene duplication
In many cases, therefore, the evolution of highly responsive gene expression from TATA promoters may be constrained by the detrimental effects of high noise. How can genes escape this adaptive conflict and evolve highly plastic TATA-initiating expression? One event that has been proposed as a general mechanism to facilitate escape from adaptive conflicts is gene duplication [17], [18]. Here it is argued that one conflict that can be resolved by duplication is the conflict between gene expression noise and plasticity.
Following the duplication of a gene, variation in expression can be less detrimental if there is functional compensation between duplicates and a component of the expression variation of the duplicates is independent [28]–[30]. Thus, if the evolution of highly responsive, but noisy, expression is constrained, then this constraint may be relieved by duplication: promoter architectures that favor plasticity (but that also couple this plasticity to noise) should be less detrimental. Three sets of observations from yeast are consistent with this proposal.
First, duplicates in yeast have high levels of plasticity, and also high levels of noise (Figure 4A and 4B). Even duplicates known to redundantly perform a process required for viability or growth [31] have higher noise than single copy genes (p = 2.8E-7, KS test), showing that variation in their expression is not detrimental. Thus duplicates tolerate higher expression variation than other genes.
Fig. 4. Gene duplicates are enriched amongst genes with the highest expression noise and plasticity, and they tend to gain TATA promoters. 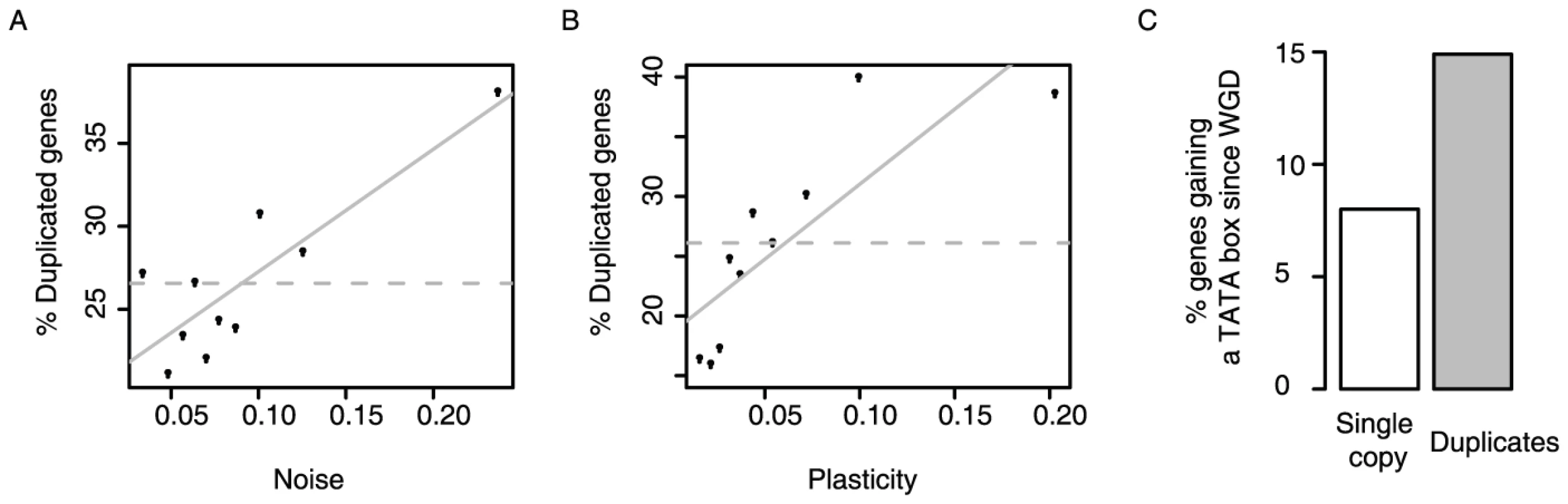
The proportion of duplicates is shown for genes with different expression noise (A) and plasticity (B). Proportions shown for equally populated bins of genes. (C) Genes retained as duplicates following the whole genome duplication in yeast are more likely to have gained a TATA box in their promoters since the duplication event than those reverting to a single copy (P = 1.24×10−5, Fischer's exact test). Here, only genes inferred to have ancestral TATA-less promoters are considered, N = 470 (retained as duplicates) and N = 1994 (not retained as duplicates). Second, TATA initiation, which facilitates plasticity but also couples noise and plasticity, is much more frequent among duplicates than among other genes: whereas 9% of genes required for viability or growth initiate from TATA promoters, this rises to 35% of gene duplicates redundantly required for growth or viability (p<2.2E-16, Fisher's exact test). TATA promoters are indeed strongly enriched among duplicates of all ages, and for duplicates arising from both small scale and whole genome duplication events (Table S11). Similar trends for the enrichment of TATA dependent transcription among duplicates are also seen in other species [32]. Thus, duplicates in general more frequently use promoters that couple noise to plasticity.
Third, evidence from the whole genome duplication (WGD) also supports this model. Following the WGD ∼100 million years ago, most genes reverted to a single copy but with a substantial number retained as duplicates [33]. This allows a direct comparison between genes retained as duplicates and those reverting to a single copy after a common ancestral event. Considering a set of genes inferred to have non-TATA promoters prior to the WGD (see Materials and Methods), those retained as duplicates are twice as likely to have gained a TATA promoter since the WGD than those reverting to a single copy (Figure 4C, P = 1.24×10−5, Fischer's exact test, N = 470 and N = 1994, respectively). This shows that not only are duplicates enriched for TATA promoters, but that they also tend to gain TATA promoters post-duplication. Thus, following duplication noise–plasticity coupling likely increased for many genes, which is consistent with this coupling being less detrimental.
In summary, the global data in yeast are consistent with duplication relieving a constraint on the evolution of highly plastic but variable gene expression. Thus one benefit of duplication may be that it allows escape from the adaptive conflict [17], [18] of noise–plasticity coupling.
Discussion
Noise–plasticity coupling is an evolvable trait
Previous studies have suggested that each promoter may have a “unique capacity to respond to external signals that can be environmental, genetic or even stochastic” [3]. Here it has been shown that this conclusion is not correct, but that the extent of noise–plasticity coupling relates to the mechanism of transcription initiation, and is confined to a subset of genes in yeast. This means that coupling can be an evolvable trait, as changes in promoter architecture associate with stronger or weaker coupling between expression plasticity and expression noise.
Cost–benefit conflicts constrain the evolution of promoters and gene expression
This study initiated from the hypothesis that the reported strong coupling between noise and plasticity could be detrimental because of the potential for fitness cost–benefit conflicts. The quantitative data from yeast support this idea, showing that both noise–plasticity coupling and promoter architectures that favor coupling are avoided when coupling is likely to be detrimental. Although only correlative in nature, the data are consistent with noise–plasticity coupling being not just an evolvable trait, but also one that has likely been constrained by selection.
High noise as a by-product of high plasticity for TATA genes
It has been shown here that TATA genes show a striking coupling between noise and plasticity. Thus, when high plasticity is adaptive, for TATA genes this will nearly always be accompanied by high noise. This means that although in some instances high noise may be beneficial [34]–[37], this should not be assumed as the case. Provided that it is not detrimental, high noise may be nothing more than a by-product of high plasticity.
Gene duplication facilitates escape from the adaptive conflict of noise–plasticity coupling
For many genes, however, there is good evidence that high noise would be detrimental [2], [5],[26],[27] and for these genes TATA-dependent initiation is disfavored and strong noise–plasticity coupling is not observed. How can genes escape a potential adaptive conflict between the benefits of plasticity and the costs of noise? One likely mechanism is gene duplication. Whereas prior to duplication the detrimental consequences of noise may limit the evolution of highly responsive expression, following duplication variation may be better tolerated due to functional compensation [28]–[30]. Constraints on the evolution of highly responsive, but noisy expression should therefore be relieved following duplication. The quantitative data from yeast are consistent with this model, showing that noise, plasticity, and the use of TATA promoters all increase among duplicates. Thus one general benefit of duplication may be that it facilitates escape from the adaptive conflict [17], [18] imposed by coupling between expression noise and expression plasticity, permitting the evolution of responsive and variable expression.
Materials and Methods
Gene expression plasticity
Expression plasticity is defined as the total responsiveness of each gene's expression to environmental change in a large compendium of over 1500 S. cerevisiae expression profiling experiments [1], as reported in [6]. Values shown in this manuscript, as for noise, are scaled between 0 and 1.
Gene expression noise
Expression noise is quantified from single cell-profiling measurements of fluorescently tagged proteins, using the ‘DM’ measure of Newman et al., which accounts for the influence of protein abundance on coefficient of variation measurements [2].
Nucleosome occupancy and histone turnover
Promoters were classified as ‘nucleosome occupied’ or ‘nucleosome free’ using in vivo nucleosome occupancy data [38] in 100 base pairs upstream of each gene as previously described [15]. A total of 1082 ‘nucleosome occupied’ proximal promoters (clusters 7 and 8 from Tirosh et al.) and 1940 ‘nucleosome free’ proximal promoters (clusters 2, 3, and 4) are considered. Histone H3 exchange data is from [25]. The average exchange in each promoter is used, with a promoter defined as 500 base pairs upstream of a gene's start site.
TATA box promoters
TATA containing promoters in S. cerevisiae were identified using the classification of Basehoar et al. [19]. Ancestral genes were considered as the set of genes with an ortholog present in each of the closely related pre-WGD species Zygosaccharomyces rouxii, Kluyveromyces lactis, Ashbya gossypii, Saccharomyces kluyveri, Kluyveromyces thermotolerans, and Kluyveromyces waltii [39]. TATA-boxes were identified in promoter regions of these species using the definition of Basehoar et al. by scanning the −70 to −310 region of each gene's promoter for the consensus site TATA(A/T)A(A/T)(A/G) [19]. In the analysis, ancestral non-TATA genes are those inferred from the absence of a consensus TATA-box in any pre-WGD species.
Gene duplicates
Duplicates were identified using the SYNERGY algorithm [40], which uses gene trees based on sequence similarity and shared gene order across 17 fungal genomes to resolve orthology and paralogy relationships [41].
Whole-genome duplicates
Whole-genome duplicates (WGD) and their orthologs in pre-WGD species were identified using the yeast genome order browser [42] version 3.0 [39]. Here conserved synteny and parsimony are used to identify ortholog groups.
Genetic redundancy
Genetically redundant genes were compiled from systematic studies and the literature, as described [31]. Here only redundant genes where the single gene deletions do not result in slow growth are considered. Considering all redundant genes or redundant genes arising in the WGD gave very similar results.
All statistical tests were performed using R (www.r-project.org).
Supporting Information
Zdroje
1. IhmelsJ
FriedlanderG
BergmannS
SarigO
ZivY
2002 Revealing modular organization in the yeast transcriptional network. Nat Genet 31 370 377
2. NewmanJR
GhaemmaghamiS
IhmelsJ
BreslowDK
NobleM
2006 Single-cell proteomic analysis of S. cerevisiae reveals the architecture of biological noise. Nature 441 840 846
3. ChoiJK
KimYJ
2009 Intrinsic variability of gene expression encoded in nucleosome positioning sequences. Nat Genet 41 498 503
4. LandryCR
LemosB
RifkinSA
DickinsonWJ
HartlDL
2007 Genetic properties influencing the evolvability of gene expression. Science 317 118 121
5. LehnerB
2008 Selection to minimise noise in living systems and its implications for the evolution of gene expression. Mol Syst Biol 4 170
6. TiroshI
WeinbergerA
CarmiM
BarkaiN
2006 A genetic signature of interspecies variations in gene expression. Nat Genet 38 830 834
7. TiroshI
BarkaiN
VerstrepenKJ
2009 Promoter architecture and the evolvability of gene expression. J Biol 8 95
8. LehnerB
2010 Genes Confer Similar Robustness to Environmental, Stochastic, and Genetic Perturbations in Yeast. PLoS ONE 5 e9035. doi:10.1371/ journal.pone.0009035
9. MeiklejohnCD
HartlDL
2002 A single mode of canalization. Trends Ecol Evoln 17 468 473
10. SatoK
ItoY
YomoT
KanekoK
2003 On the relation between fluctuation and response in biological systems. Proc Natl Acad Sci U S A 100 14086 14090
11. WaddingtonCH
1942 Canalization of Development and the Inheritance of Acquired Characters. Nature 150 563 565
12. BlakeWJ
BalazsiG
KohanskiMA
IsaacsFJ
MurphyKF
2006 Phenotypic consequences of promoter-mediated transcriptional noise. Mol Cell 24 853 865
13. RaserJM
O'SheaEK
2005 Noise in gene expression: origins, consequences, and control. Science 309 2010 2013
14. FieldY
KaplanN
Fondufe-MittendorfY
MooreIK
SharonE
2008 Distinct modes of regulation by chromatin encoded through nucleosome positioning signals. PLoS Comput Biol 4 e1000216 doi:10.1371/journal.pcbi.1000216
15. TiroshI
BarkaiN
2008 Two strategies for gene regulation by promoter nucleosomes. Genome Res 18 1084 1091
16. PedrazaJM
PaulssonJ
2008 Effects of molecular memory and bursting on fluctuations in gene expression. Science 319 339 343
17. HughesAL
1994 The evolution of functionally novel proteins after gene duplication. Proc Biol Sci 256 119 124
18. Des MaraisDL
RausherMD
2008 Escape from adaptive conflict after duplication in an anthocyanin pathway gene. Nature 454 762 765
19. BasehoarAD
ZantonSJ
PughBF
2004 Identification and distinct regulation of yeast TATA box-containing genes. Cell 116 699 709
20. TsankovAM
ThompsonDA
SochaA
RegevA
RandoOJ
2010 The role of nucleosome positioning in the evolution of gene regulation. PLoS Biol 8 e1000414 doi:10.1371/journal.pbio.1000414
21. KaernM
ElstonTC
BlakeWJ
CollinsJJ
2005 Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet 6 451 464
22. RajA
van OudenaardenA
2008 Nature, nurture, or chance: stochastic gene expression and its consequences. Cell 135 216 226
23. DionMF
KaplanT
KimM
BuratowskiS
FriedmanN
2007 Dynamics of replication-independent histone turnover in budding yeast. Science 315 1405 1408
24. JamaiA
ImoberdorfRM
StrubinM
2007 Continuous histone H2B and transcription-dependent histone H3 exchange in yeast cells outside of replication. Mol Cell 25 345 355
25. RufiangeA
JacquesPE
BhatW
RobertF
NouraniA
2007 Genome-wide replication-independent histone H3 exchange occurs predominantly at promoters and implicates H3 K56 acetylation and Asf1. Mol Cell 27 393 405
26. BatadaNN
HurstLD
2007 Evolution of chromosome organization driven by selection for reduced gene expression noise. Nat Genet 39 945 949
27. FraserHB
HirshAE
GiaeverG
KummJ
EisenMB
2004 Noise minimization in eukaryotic gene expression. PLoS Biol 2 e137 doi:10.1371/journal.pbio.0020137
28. CookDL
GerberAN
TapscottSJ
1998 Modeling stochastic gene expression: implications for haploinsufficiency. Proc Natl Acad Sci U S A 95 15641 15646
29. DeLunaA
SpringerM
KirschnerMW
KishonyR
Need-based up-regulation of protein levels in response to deletion of their duplicate genes. PLoS Biol 8 e1000347. doi:10.1371/journal.pbio.1000347
30. NowakMA
BoerlijstMC
CookeJ
SmithJM
1997 Evolution of genetic redundancy. Nature 388 167 171
31. VavouriT
SempleJI
LehnerB
2008 Widespread conservation of genetic redundancy during a billion years of eukaryotic evolution. Trends Genet 24 485 488
32. HaM
KimED
ChenZJ
2009 Duplicate genes increase expression diversity in closely related species and allopolyploids. Proc Natl Acad Sci U S A 106 2295 2300
33. WolfeKH
ShieldsDC
1997 Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387 708 713
34. AcarM
MettetalJT
van OudenaardenA
2008 Stochastic switching as a survival strategy in fluctuating environments. Nat Genet 40 471 475
35. BalabanNQ
MerrinJ
ChaitR
KowalikL
LeiblerS
2004 Bacterial persistence as a phenotypic switch. Science 305 1622 1625
36. ThattaiM
van OudenaardenA
2004 Stochastic gene expression in fluctuating environments. Genetics 167 523 530
37. ZhangZ
QianW
ZhangJ
2009 Positive selection for elevated gene expression noise in yeast. Mol Syst Biol 5 299
38. LeeW
TilloD
BrayN
MorseRH
DavisRW
2007 A high-resolution atlas of nucleosome occupancy in yeast. Nat Genet 39 1235 1244
39. GordonJL
ByrneKP
WolfeKH
2009 Additions, losses, and rearrangements on the evolutionary route from a reconstructed ancestor to the modern Saccharomyces cerevisiae genome. PLoS Genet 5 e1000485 doi:10.1371/journal.pgen.1000485
40. WapinskiI
PfefferA
FriedmanN
RegevA
2007 Automatic genome-wide reconstruction of phylogenetic gene trees. Bioinformatics 23 i549 558
41. WapinskiI
PfefferA
FriedmanN
RegevA
2007 Natural history and evolutionary principles of gene duplication in fungi. Nature 449 54 61
42. ByrneKP
WolfeKH
2005 The Yeast Gene Order Browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res 15 1456 1461
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 11- Akutní intermitentní porfyrie
- Růst a vývoj dětí narozených pomocí IVF
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Pilotní studie: stres a úzkost v průběhu IVF cyklu
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
-
Všechny články tohoto čísla
- Genome-Wide Association Meta-Analysis of Cortical Bone Mineral Density Unravels Allelic Heterogeneity at the Locus and Potential Pleiotropic Effects on Bone
- Beyond QTL Cloning
- An Evolutionary Framework for Association Testing in Resequencing Studies
- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- The Functional Interplay between Protein Kinase CK2 and CCA1 Transcriptional Activity Is Essential for Clock Temperature Compensation in Arabidopsis
- Endogenous Viral Elements in Animal Genomes
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- DNA Methylation and Normal Chromosome Behavior in Neurospora Depend on Five Components of a Histone Methyltransferase Complex, DCDC
- Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes
- Genetic Basis of Growth Adaptation of after Deletion of , a Major Metabolic Gene
- Nomadic Enhancers: Tissue-Specific -Regulatory Elements of Have Divergent Genomic Positions among Species
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- CTCF-Dependent Chromatin Bias Constitutes Transient Epigenetic Memory of the Mother at the Imprinting Control Region in Prospermatogonia
- Systematic Dissection and Trajectory-Scanning Mutagenesis of the Molecular Interface That Ensures Specificity of Two-Component Signaling Pathways
- Nucleolin Is Required for DNA Methylation State and the Expression of rRNA Gene Variants in
- The Complex Genetic Architecture of the Metabolome
- ATM Limits Incorrect End Utilization during Non-Homologous End Joining of Multiple Chromosome Breaks
- Mutation Disrupts Synaptonemal Complex Formation, Recombination, and Chromosome Segregation in Mammalian Meiosis
- Mismatch Repair–Independent Increase in Spontaneous Mutagenesis in Yeast Lacking Non-Essential Subunits of DNA Polymerase ε
- The Kinesin-3 Motor UNC-104/KIF1A Is Degraded upon Loss of Specific Binding to Cargo
- Epigenetic Silencing of Spermatocyte-Specific and Neuronal Genes by SUMO Modification of the Transcription Factor Sp3
- A Coastal Cline in Sodium Accumulation in Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1
- Cyclin B3 Is Required for Multiple Mitotic Processes Including Alleviation of a Spindle Checkpoint–Dependent Block in Anaphase Chromosome Segregation
- Altered DNA Methylation in Leukocytes with Trisomy 21
- Human-Specific Evolution and Adaptation Led to Major Qualitative Differences in the Variable Receptors of Human and Chimpanzee Natural Killer Cells
- Leptotene/Zygotene Chromosome Movement Via the SUN/KASH Protein Bridge in
- RACK-1 Acts with Rac GTPase Signaling and UNC-115/abLIM in Axon Pathfinding and Cell Migration
- Genome-Wide Effects of Long-Term Divergent Selection
- Endless Forms Most Viral
- Conflict between Noise and Plasticity in Yeast
- Essential Functions of the Histone Demethylase Lid
- The Transcriptional Regulator Rok Binds A+T-Rich DNA and Is Involved in Repression of a Mobile Genetic Element in
- The Cellular Robustness by Genetic Redundancy in Budding Yeast
- Localization of a Guanylyl Cyclase to Chemosensory Cilia Requires the Novel Ciliary MYND Domain Protein DAF-25
- A Buoyancy-Based Screen of Drosophila Larvae for Fat-Storage Mutants Reveals a Role for in Coupling Fat Storage to Nutrient Availability
- A Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species (Green Peach Aphid)
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- Genome-Wide Effects of Long-Term Divergent Selection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



