-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaA Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species (Green Peach Aphid)
Aphids are amongst the most devastating sap-feeding insects of plants. Like most plant parasites, aphids require intimate associations with their host plants to gain access to nutrients. Aphid feeding induces responses such as clogging of phloem sieve elements and callose formation, which are suppressed by unknown molecules, probably proteins, in aphid saliva. Therefore, it is likely that aphids, like plant pathogens, deliver proteins (effectors) inside their hosts to modulate host cell processes, suppress plant defenses, and promote infestation. We exploited publicly available aphid salivary gland expressed sequence tags (ESTs) to apply a functional genomics approach for identification of candidate effectors from Myzus persicae (green peach aphid), based on common features of plant pathogen effectors. A total of 48 effector candidates were identified, cloned, and subjected to transient overexpression in Nicotiana benthamiana to assay for elicitation of a phenotype, suppression of the Pathogen-Associated Molecular Pattern (PAMP)–mediated oxidative burst, and effects on aphid reproductive performance. We identified one candidate effector, Mp10, which specifically induced chlorosis and local cell death in N. benthamiana and conferred avirulence to recombinant Potato virus X (PVX) expressing Mp10, PVX-Mp10, in N. tabacum, indicating that this protein may trigger plant defenses. The ubiquitin-ligase associated protein SGT1 was required for the Mp10-mediated chlorosis response in N. benthamiana. Mp10 also suppressed the oxidative burst induced by flg22, but not by chitin. Aphid fecundity assays revealed that in planta overexpression of Mp10 and Mp42 reduced aphid fecundity, whereas another effector candidate, MpC002, enhanced aphid fecundity. Thus, these results suggest that, although Mp10 suppresses flg22-triggered immunity, it triggers a defense response, resulting in an overall decrease in aphid performance in the fecundity assays. Overall, we identified aphid salivary proteins that share features with plant pathogen effectors and therefore may function as aphid effectors by perturbing host cellular processes.
Published in the journal: . PLoS Genet 6(11): e32767. doi:10.1371/journal.pgen.1001216
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001216Summary
Aphids are amongst the most devastating sap-feeding insects of plants. Like most plant parasites, aphids require intimate associations with their host plants to gain access to nutrients. Aphid feeding induces responses such as clogging of phloem sieve elements and callose formation, which are suppressed by unknown molecules, probably proteins, in aphid saliva. Therefore, it is likely that aphids, like plant pathogens, deliver proteins (effectors) inside their hosts to modulate host cell processes, suppress plant defenses, and promote infestation. We exploited publicly available aphid salivary gland expressed sequence tags (ESTs) to apply a functional genomics approach for identification of candidate effectors from Myzus persicae (green peach aphid), based on common features of plant pathogen effectors. A total of 48 effector candidates were identified, cloned, and subjected to transient overexpression in Nicotiana benthamiana to assay for elicitation of a phenotype, suppression of the Pathogen-Associated Molecular Pattern (PAMP)–mediated oxidative burst, and effects on aphid reproductive performance. We identified one candidate effector, Mp10, which specifically induced chlorosis and local cell death in N. benthamiana and conferred avirulence to recombinant Potato virus X (PVX) expressing Mp10, PVX-Mp10, in N. tabacum, indicating that this protein may trigger plant defenses. The ubiquitin-ligase associated protein SGT1 was required for the Mp10-mediated chlorosis response in N. benthamiana. Mp10 also suppressed the oxidative burst induced by flg22, but not by chitin. Aphid fecundity assays revealed that in planta overexpression of Mp10 and Mp42 reduced aphid fecundity, whereas another effector candidate, MpC002, enhanced aphid fecundity. Thus, these results suggest that, although Mp10 suppresses flg22-triggered immunity, it triggers a defense response, resulting in an overall decrease in aphid performance in the fecundity assays. Overall, we identified aphid salivary proteins that share features with plant pathogen effectors and therefore may function as aphid effectors by perturbing host cellular processes.
Introduction
Like most plant parasites, aphids require intimate associations with their host plants to gain access to nutrients. Aphids predominantly feed from the plant phloem sieve elements, and use their stylets to navigate between the cells of different layers of leaf tissue during which plant defenses may be triggered. Indeed, aphid feeding induces responses such as clogging of phloem sieve elements and callose formation, which are suppressed by the aphid in successful interactions with plant hosts [1]. In addition, some aphid species can alter host plant phenotypes, by for example inducing the formation of galls or causing leaf curling [2] indicating that there is an active interplay between host and aphid at the molecular level. During probing and feeding, aphids secrete two types of saliva: gelling saliva, which is thought to protect stylets during penetration, and watery saliva, which is secreted into various plant host cell types and the phloem [3]. The secretion of aphid saliva directly into the host-stylet interface [4], suggests that molecules present in the saliva may perturb plant cellular processes while aphids progress through different feeding stages. Interestingly, the knock-down of the C002 salivary gene in Acyrthosiphon pisum (pea aphid) negatively impacts survival rates of this aphid on plant hosts [5], [6]. Furthermore, proteomics studies based on artificial aphid diets showed the presence of secreted proteins, including C002, in aphid saliva indicating that these proteins are delivered inside the host plant during feeding [7], [8]. However, whether and how these aphid salivary proteins function in the plant host remains elusive.
Suppression of host defenses and altering host plant phenotypes is common in plant-pathogen interactions and involves secretion of molecules (effectors) that modulate host cell processes [9], [10]. Therefore it is likely that aphids, similar to plant pathogens, deliver effectors inside their hosts to manipulate host cell process enabling successful infestation of plants [9]. Effector-mediated suppression of plant defenses, such as Pathogen-Associated Molecular Pattern (PAMP)-triggered immunity (PTI), generally involves the targeting of a plant virulence target, or operative target [11]. However, plant pathogen effectors that are deployed to suppress host defenses are recognized by plant disease resistance (R) proteins in particular host genotypes, resulting in effector-triggered immunity (ETI) [12]. Interestingly, the R proteins that recognize plant pathogens and those that confer resistance to aphids, such as Mi-1.2 and Vat, share a similar structure, and contain a nucleotide binding site (NBS) domain and leucine rich repeat (LRR) regions [13]–[15]. The Mi-1.2 resistance gene confers resistance in tomato to certain clones of Macrosiphum euphorbiae (potato aphid), two whitefly biotypes, a psyllid, and three nematode species [16]–[19], indicating that there is significant overlap in plant pathogen and aphid recognition in plants. In addition, aphid resistance conferred by several resistance genes was shown to be race-specific [16], [20]. This suggests that depending on their genotype, certain aphid clones may be able to avoid and/or suppress plant defenses and fits with the gene-for-gene model in plant-pathogen interactions [21]. Therefore, it is likely that not only plant pathogens, but also aphids, secrete effectors that in addition to targeting host cell processes may trigger ETI depending on the host genotype.
Plant pathogen effectors generally share the common feature of modulating host cell processes [22]. Various assays have been developed to identify the functions of effectors from bacterial and eukaryotic filamentous plant pathogens [22]–[24]. One important and common function of plant pathogen effectors is the suppression of PTI. This activity is especially common among type III secretion system (T3SS) effectors. For example, the large majority of Pseudomonas syringae DC3000 effectors can suppress PTI responses, including the oxidative burst [25]. However, effectors from eukaryotic filamentous plant pathogens can also suppress PTI, as demonstrated for the AVR3a effector from Phytophthora infestans, which suppresses cell death induced by the PAMP-like elicitor INF1 [26], [27]. Another activity of plant pathogen effectors is the induction of phenotypes in plants. For example, several effectors, including CRN2 and INF1, from the oomycete plant pathogen P. infestans induce cell death upon overexpression in planta [28], [29], whereas other effectors, like AvrB from P. syringae DC3000 induce chlorosis [30]. Also, overexpression of effector proteins from plant pathogenic nematodes in host plants can affect plant phenotypes, as shown for the Heterodera glycines CLE protein Hg-SYV46 that alters host cell differentiation [31]. As effectors exhibit functions important for pathogenicity, their deletion can have detrimental effects on pathogen virulence. However, due to redundancy, the knock-down or deletion of single effectors does not always impact virulence. On the other hand, overexpression of plant pathogen effectors can enhance pathogen virulence, as shown for active AvrPtoB, which enhances virulence to P. syringae DC3000 in Arabidopsis [32], and for the H. schachtii effector 10A06 that, in addition to altering host plant morphology, increases nematode susceptibility in Arabidopsis [33].
We exploited publicly available aphid salivary gland sequences to develop a functional genomics approach for the identification of candidate aphid effector proteins from the aphid species Myzus persicae (green peach aphid) based on common features of plant pathogen effectors. Data mining of salivary gland expressed sequences tags (ESTs) identified 46 M. persicae predicted secreted proteins. Functional analyses showed that one of these proteins, Mp10, induced chlorosis and weak cell death in Nicotiana benthamiana, and suppressed the oxidative burst induced by the bacterial PAMP flg22. In addition, we developed a medium-throughput assay, based on transient overexpression in N. benthamiana, that allows screening for effects of aphid candidate effectors on aphid performance. Using this screen, we identified two candidate effectors, Mp10 and Mp42, that reduced aphid performance and one effector candidate, MpC002, that enhanced aphid performance. In summary, we found aphid secreted salivary proteins that share features with plant pathogen effectors and therefore may function as aphid effectors by perturbing host cellular processes.
Results
Description of functional genomics screen
We developed a functional genomics approach to identify candidate effectors from M. persicae using 3233 publicly available aphid salivary gland ESTs [34]. We hypothesized that aphid effectors are most likely secreted proteins that are delivered into the saliva through the classical eukaryotic endoplasmic reticulum (ER)-Golgi pathway of the salivary glands. A feature of proteins secreted through this pathway is the presence of an N-terminal signal peptide. Therefore, we used the SignalP v3.0 program [35] to predict the presence of signal peptides in the amino acid sequences encoded by the open reading frames (ORFs) found in salivary gland ESTs. Out of 5919 amino acid sequences corresponding to predicted ORFs, we identified 134 nonredundant sequences with signal peptide (Figure 1A). Out of these 134 proteins, 19 were predicted to contain a transmembrane domain in addition to the signal peptide, and are therefore likely to remain in the salivary gland membrane upon secretion. Hence, 115 predicted secreted proteins remained. In order to investigate the M. persicae candidate effector protein in functional assays, we started with the cloning of 46 candidates that corresponded to full-length sequences within the set of 115 candidates. Effectors are subject to diversifying selection because of the co-evolutionary arms race between host and pathogen proteins [36], [37]. Therefore, we used the presence of amino acid polymorphisms among alignments of deduced protein sequences of M. persicae and A. pisum ESTs as an additional criterion. Three candidates did not fulfill this criterion and were removed from our candidate set bringing the total to 43 candidates.
Fig. 1. Overview of functional genomics pipeline to identify candidate effectors from M. persicae. 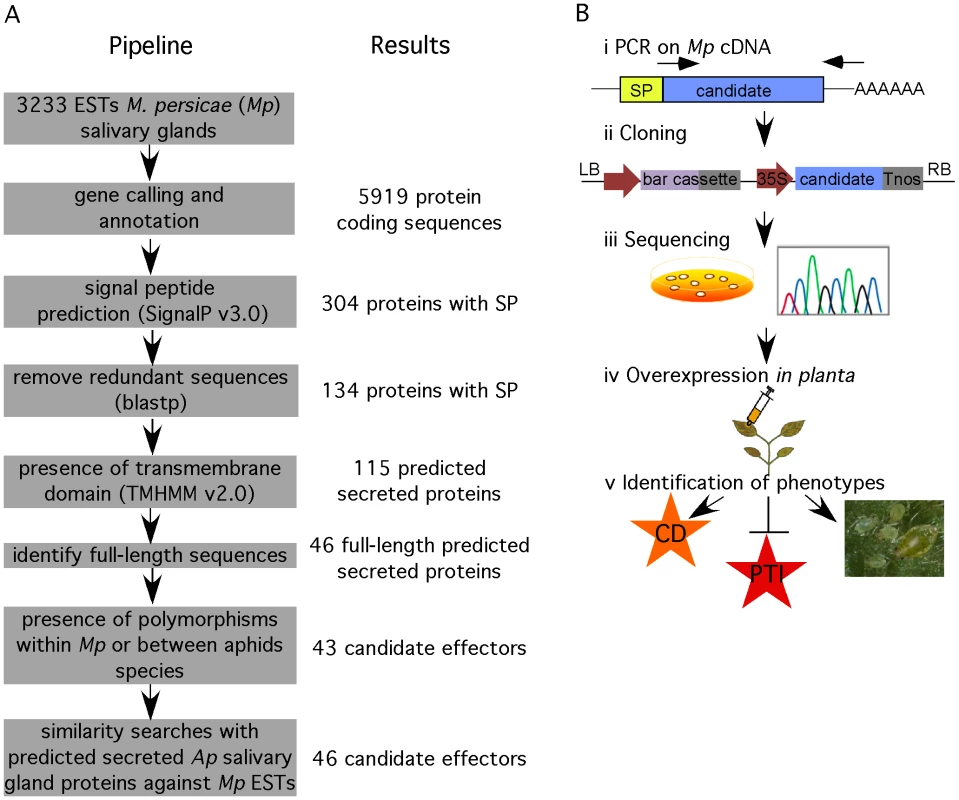
(A) Bioinformatics pipeline for data mining of M. persicae salivary gland expressed sequence tags (ESTs). (B) Cloning and functional analyses of candidates to identify effector activities. i) PCR amplification was performed on M. persicae cDNA. ii) Amplicons were cloned in the pCB302-3 vector under control of a 35S promoter and constructs were transformed into Agrobacterium tumefaciens. iii) Multiple clones were sequenced to identify polymorphic candidates. Clones were stored and cultured for subsequent functional assays. iv) Candidate effectors were overexpressed in Nicotiana benthamiana by agroinfiltration to determine whether they induce a phenotype in planta, such as cell death, suppress basal plant defences, PAMP-triggered immunity (PTI), and affect aphid performance. We applied a similar data mining approach as described above to 4517 publicly available salivary gland ESTs from A. pisum, thereby identifying 24 candidates (Table S1). In the A. pisum salivary gland ESTs we predicted only 1751 ORFs, explaining the relatively low number of A. pisum candidates. A total of three candidates were found in both M. persicae and A. pisum datasets (combinations Mp1/Ap1, Mp5/Ap7 and Mp16/Ap4). The remaining 21 non-overlapping A. pisum candidates were subjected to BLAST searches (E value<10−15) against all available M. persicae ESTs to identify putative M. persicae homologs. This led to the identification of three M. persicae sequences (Mp3, Mp54 and MpC002) that were added to the M. persicae candidate effector dataset bringing the total to 46 (Figure 1A, Table S2).
Interestingly, for two candidates, Mp39 and Mp49, no similar sequences were present in the publicly available aphid sequence datasets, including the A. pisum genome sequence (Table S2). Also, no homologs of these proteins were identified by BLAST searches against GenBank nucleotide and protein databases (E value<10−5). This suggests these proteins may be specific to M. persicae. A total of 11 candidates were shared between the independent salivary gland EST datasets from M. persicae and A. pisum but were not present in gut ESTs from M. persicae (Table S2) providing support that the corresponding proteins may share a similar function in both these aphid species. For four candidates matches were found in gut ESTs from M. persicae, suggesting these proteins may be derived from salivary gland contaminants in dissected gut tissues and not function uniquely in the salivary gland or saliva. Indeed, gene expression analysis of Mp51 in various aphid tissues dissected from aphids fed on N. benthamiana confirmed that this gene is specifically expressed in the aphid gut (Figure S1). In contrast, candidate effector genes Mp1, Mp2, Mp10, Mp30, Mp42, Mp47, Mp50 and MpCOO2, were expressed in aphid heads and salivary glands but not in aphid guts (Figure S1), suggesting that their corresponding proteins are indeed produced in the salivary glands. Furthermore, Mp1 and MpCOO2 were previously identified in saliva of M. persicae using a proteomics-based approach [7] confirming that these two proteins are secreted into aphid saliva.
To investigate the functions of the 46 effector candidates, we amplified the corresponding sequences encoding the mature proteins, without the signal peptide encoding sequences, from M. persicae cDNA for cloning (Figure 1B). To preserve the authentic sequence in the 3′ end of the ORF, we designed reverse primers in the 3′ untranslated regions (UTRs) based on EST sequences when possible. Amplicons were cloned in a 35S cassette and corresponding constructs were transformed directly into Agrobacterium tumefaciens followed by sequencing (Figure 1B). Two out of the 46 candidates, Mp7 and Mp38 could not be amplified from M. persicae cDNA. Of the remaining 44 candidates, four (Mp6, Mp17, Mp33 and Mp35) were represented by two polymorphic forms, with polymorphisms within the mature protein portion. Except for one of the polymorphic Mp6 sequences, all sequences were identical to those in the M. persicae EST databases. To rule out that the polymorphism in Mp6 was due to PCR errors, we repeated the Mp6 PCR and sequencing several times on individual aphids with similar results. Both forms of the four polymorphic candidates were cloned resulting in a total of 48 cloned M. persicae effector candidates. Functional assays were performed based on transient over-expression in N. benthamiana to assess whether the M. persicae candidate effectors 1) induce a phenotype in planta, 2) suppress PAMP-triggered immunity and 3) affect the ability of M. persicae aphids to reproduce (fecundity) (Figure 1B). We assessed fecundity of M. persicae lineage RRes (genotype O), which can utilize N. benthamiana as a host.
M. persicae candidate effector Mp10 induces chlorosis upon overexpression in N. benthamiana
Several plant pathogen effectors induce a phenotype upon overexpression in planta, which may reflect their virulence activity [22]. Hence, we performed transient overexpression of the effector candidates in N. benthamiana by agroinfiltration to screen for the induction of phenotypes. Out of the 48, one candidate effector, Mp10, induced chlorosis starting from 2 days post inoculation (dpi) (Figure 2A). In addition, we observed local cell death in a low number of infiltration sites (Figure S2A, S2B, S2C, S2D). The phenotype was not affected by co-expression with the silencing suppressor p19 (Figure S2E). To independently confirm the phenotype, we expressed Mp10 in N. benthamiana using a Potato virus X (PVX)-based vector (PVX-Mp10). Systemic PVX-based overexpression of Mp10 induced systemic chlorosis in N. benthamiana starting at 10 dpi (Figure 2B). This also suggests that the Mp10 response is not dependent on the presence of Agrobacterium. To determine whether the response to Mp10 was specific to N. benthamiana, we infected N. tabacum, Solanum lycopersicum (tomato) and N. benthamiana plants with PVX-Mp10 in parallel. Starting at around 10 dpi, systemic chlorosis was observed in N. benthamiana expressing PVX-Mp10, but not in control PVX-infected plants (Figure 2B). Whereas mosaic symptoms were observed in S. lycopersicum, indicative of PVX infection, no Mp10-induced chlorosis was observed (Figure 2C; Figure S3A, S3B). Mp10 expression was confirmed by semi-quantitative RT-PCR in systemically PVX-Mp10 infected leaves of S. lycopersicum suggesting that the lack of symptoms is not due to a loss of the Mp10 sequence from PVX-Mp10 (Figure 2E). In contrast, N. tabacum plants infected with PVX-Mp10 did not show mosaic symptoms indicative of virus infection, while N. tabacum inoculated with PVX alone did (Figure 2D; Figure S2B). No Mp10 expression could be detected in leaves of N. tabacum plants inoculated with PVX-Mp10, whereas expression of the viral coat protein was detected, indicating that PVX itself did systemically spread in N. tabacum (Figure 2E). In contrast, PVX-Mp42 did spread systemically in N. benthamiana, N. tabacum and S. lycopersicum, indicating that this aphid protein can be systemically expressed in these plant species using PVX (Figure S4). It is possible that PVX-Mp10 may evoke an avirulence response in N. tabacum causing the selection of PVX without the Mp10 insert. Loss of foreign gene fragments from the PVX genome has been reported previously and is most likely due to selection pressures forcing virus recombination [38]. The lack of mosaic symptoms in PVX-Mp10-inoculated N. tabacum plants is possibly due to the initially low abundance of recombined PVX-virus as compared to the vector control.
Fig. 2. The candidate effector Mp10 induces chlorosis specifically in N. benthamiana. 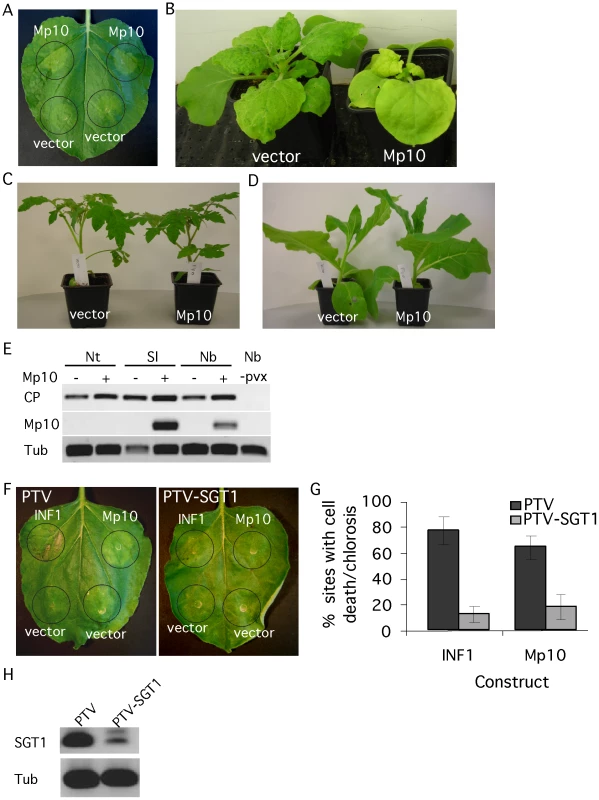
(A) Overexpression of 35S-Mp10 by agroinfiltration induces chlorosis in N. benthamiana. Symptoms of chlorosis started to appear from 2 days post infiltration (dpi). Photos were taken 4 dpi. (B) PVX-based expression of Mp10 in N. benthamiana. Symptoms of chlorosis started to appear from 10 days post wound-inoculation (dpwi). Photos were taken 14 dpwi. (C) PVX-based expression of Mp10 in Solanum lycopersicum (tomato). Photos were taken 14 dpwi. D) PVX-based expression of Mp10 in N. tabacum. Photos were taken 14 dpwi. (E) Semi-quantitative RT-PCR on RNA from N. benthamiana (Nb), N. tabacum (Nt) and S. lycopersicum (Sl) plants infected with PVX-Δgfp (vector) or PVX-Mp10 as well as non-infected N. benthamiana plants (Nb, -pvx). Primers were used to amplify sequences corresponding to the PVX virus coat protein (CP) and Mp10. The plant tubulin gene (Tub) was used as a control for equal RNA levels. Plant tissues were harvested 14 dpwi (F) Over-expression of 35S-INF1 and 35S-Mp10 in SGT1-silenced (TRV-SGT1) and control (TRV) N. benthamiana plants. Photos were taken 4 dpi. (G) Percentage of infiltration sites showing either INF1 cell death or Mp10 chlorosis 4 dpi on SGT1-silenced and control N. benthamiana plants. The graphs show the averages calculated from 3 replicated experiments, with 8–10 infiltration sites per individual replicate. Error bars indicate the standard error. H) Semi-quantitative RT-PCR on SGT1-silenced and control N. benthamiana plants with SGT1-specific primers. The plant tubulin gene (Tub) was used as a control for equal RNA amounts. The SGT1 protein, an ubiquitin-ligase associated protein, is required for plant cell death responses, including those involved in plant resistance [39]. To investigate whether SGT1 is required for the Mp10 chlorosis response, we generated SGT1-silenced N. benthamiana plants using Tobacco rattle virus (TRV)-based virus-induced gene silencing (VIGS). Silenced plants (treated with TRV-SGT1) and control plants (treated with TRV) (Figure 2H) were infiltrated with Agrobacterium strains expressing Mp10 or the positive control INF1, an elicitin from P. infestans that induces cell death in control plants, but not in SGT1-silenced plants [40]. Both the Mp10-induced chlorosis and the INF1-induced cell death were pronouncedly reduced in the SGT1-silenced plants, but not in the TRV-treated control plants (Figure 2F and 2G), indicating SGT1 is required for these chlorosis and cell death responses.
Candidate effector Mp10 suppresses the flg22 - but not the chitin-induced oxidative burst
Suppression of PTI induced by PAMPs like flg22 and chitin is a common feature of plant pathogen effectors. To determine whether aphid candidate effectors can suppress PTI, we assessed whether any of our 48 candidates suppressed the oxidative burst response induced by the bacterial PAMP flg22. We decided to screen for suppression of the oxidative burst induced by flg22 only, as this PAMP gives a strong and consistent oxidative burst response in N. benthamiana, which is convenient for use in large screens. N. benthamiana leaf discs overexpressing the effector candidate genes under control of the 35S promoter were challenged with the flg22 elicitor and the production of reactive oxygen species (ROS) was measured using a luminol-based assay [41]. The bacterial effector AvrPtoB, a suppressor of the flg22-mediated oxidative burst response [42], was included as a positive control. We found that Mp10 suppresses the flg22-induced oxidative burst in leaf discs harvested 2 days post agroinfiltration (three replicated experiments) (Figure 3A), whereas other candidate effectors did not (data not shown). Although the level of suppression by Mp10 was significant compared to that of the empty vector control, it was not as effective as AvrPtoB. We tested whether Mp10 also suppressed the oxidative burst induced by a fungal PAMP, chitin, and found that while Mp10 suppressed the flg22 response, no suppression of the chitin-induced oxidative burst was observed (Figure 3B). Thus, Mp10 specifically suppresses the oxidative burst induced by the PAMP flg22.
Fig. 3. Mp10 suppresses the oxidative burst induced by flg22, but not chitin, in N. benthamiana. 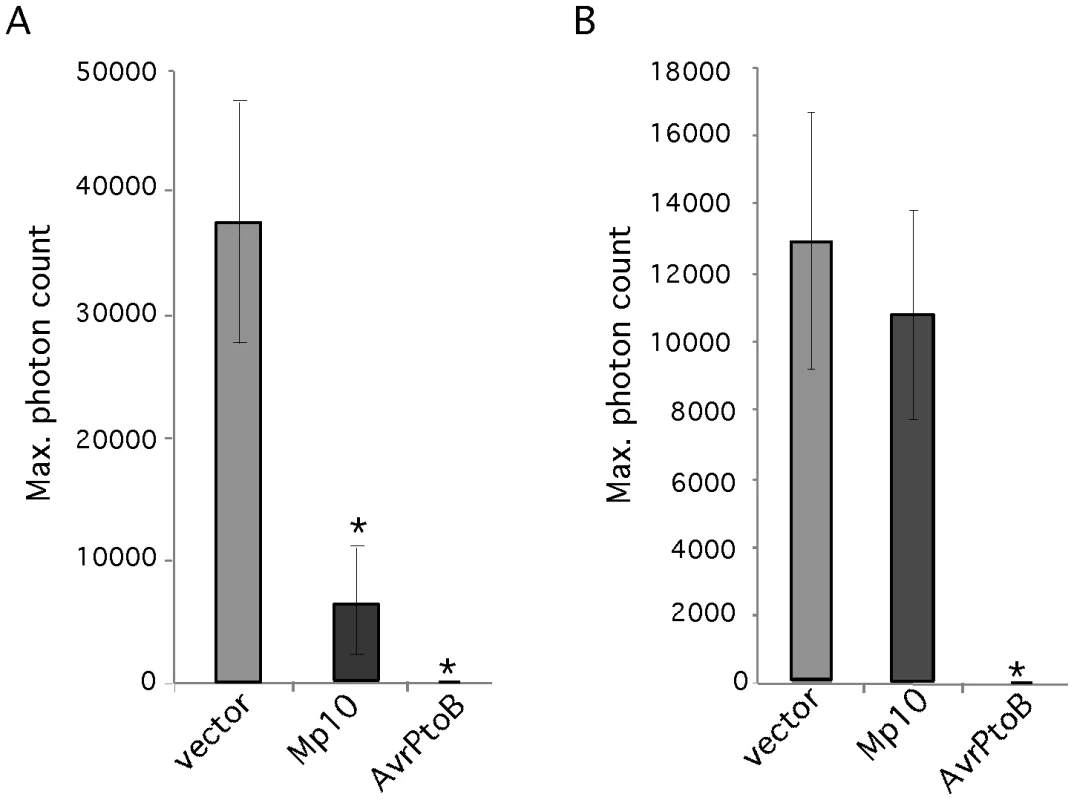
The induction of reactive oxygen species (ROS) induced by the flg22 and chitin was measured using a luminol-based assay. (A) The ROS response induced by flg22 in N. benthamiana leaf discs overexpressing Mp10, AvrPtoB (positive control) and the vector control upon agroinfiltration. The maximum photon count is based on the average of 8 leaf discs. The experiment was repeated 3 times with similar results. Error bars indicate standard error. Asterisks indicate statistical significance compared to the vector control (p≤0.043) (B) The ROS response induced by chitin in N. benthamiana leaf discs overexpressing Mp10, AvrPtoB (positive control) and the vector control upon agroinfiltration. The maximum photon count is based on the average of 8 leaf discs. The experiment was repeated 3 times with similar results. Error bars indicate standard error. Asterisks indicate statistical significance compared to the vector control (p≤0.028). Candidate effectors Mp10, Mp42, and MpC002 alter aphid fecundity on N. benthamiana
We developed a medium-throughput 24-well plate assay to assess M. persicae fecundity on N. benthamiana leaves transiently overexpressing the 48 candidate effectors (Figure 4A). Leaf discs were harvested from infiltrated leaves one day after agroinfiltration and placed upside down on water agar in 24-well plates. Four first-instar nymphs were placed on each leaf disc and the plate was incubated up-side-down under a light source. Aphids were moved every 6 days to plates with freshly infiltrated leaf discs, as expression levels of green fluorescent protein (GFP) in leaf discs were constant during 6 days and then tapered off (Figure S5). The aphids placed initially on the leaf discs generally started producing nymphs after about 10–11 days. Nymph production (fecundity) was assessed on day 12, 14 and 17 by counting and removing newly produced nymphs on each leaf disc. The total nymph production per adult was calculated and compared among the treatments and GFP and vector controls.
Fig. 4. A medium-throughput leaf disc-based assay identifies M. persicae effector candidates that affect aphid performance. 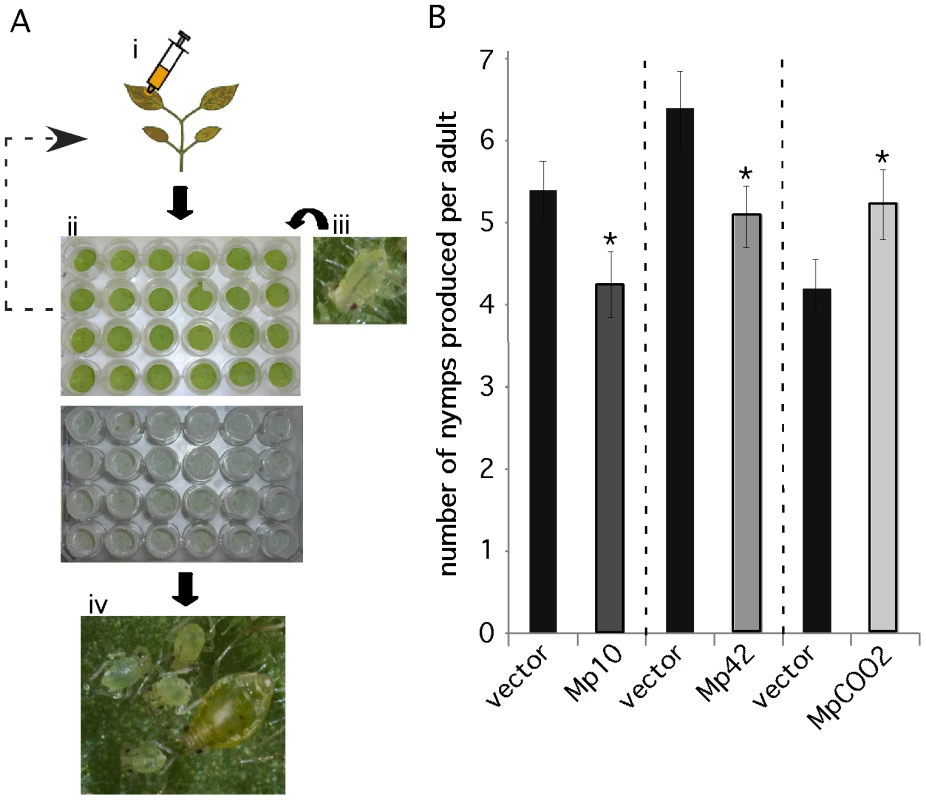
(A) A novel medium-throughput assay to determine whether in planta overexpresssion of effector candidates affects aphid performance. i) Effector candidates are overexpressed in Nicotiana benthamiana by agroinfiltration. ii) One day after agroinfiltration leaf discs are harvested from infiltration sites using a cork borer. Leaf discs are placed upside-down on water agar in a 24-wells plate. iii) Four first-instar nymphs are placed on each leaf disc and wells are covered with individual mesh caps. Every six days these four aphids are moved to fresh leaf discs overexpressing the effector candidates. iv) Nymph production is assessed up to 17 days after placing first-instar nymphs on the leaf discs on day 1. (B) Overexpression of Mp10 and Mp42 reduces aphid nymph production (fecundity) and overexpression of MpC002 increases aphid nymph production. For each effector candidate, agroinfiltrations and aphid assays were performed side-by-side with the vector control (vector). Graphs show the average number of nymphs produced per adult based on 3 replicated experiments, each consisting of 6 replicated leaf discs per candidate effector construct (n = 18). Error bars indicate the standard error. Asterisks indicate statistical significance compared to the vector control based on a one-way ANOVA (Mp10: p≤0.026, Mp42: p≤0.036 and MpCOO2: p≤0.038). In our initial screens, in which candidate effector constructs were infiltrated on different leaves and not always side-by-side with the vector control, we identified 14 candidates that either enhanced or reduced aphid fecundity by one time the standard error compared to the empty vector (EV) control (Figure S6). To confirm the effect on aphid fecundity of these 14 candidates, we conducted additional assays in which the candidates were infiltrated side-by-side with the vector control (EV) on the same leaves. Two candidates, Mp10 and Mp42, reduced aphid fecundity in three repeated confirmation assays compared to the vector control (Figure 4B). In addition, one candidate, MpC002, enhanced aphid fecundity in three repeated confirmation assays compared to the vector control (Figure 4B). Transient overexpression of Mp10 did not induce chlorosis in leaf discs (Figure S7) or leaves that were detached from the plant 24hrs after infiltration (data not shown). Thus, leaves need to be attached to the plant for chlorosis to occur and the chlorosis itself was therefore not likely responsible for the observed reduction in aphid performance. In summary, we have developed a novel assay to screen for effects of in planta expressed aphid salivary proteins on aphid performance and thereby identified three candidates that potentially function as effectors by eliciting plant defenses or promoting aphid infestation of host plants.
Homology searches of Mp10, Mp42, and MpC002
To determine whether the candidates that alter aphid fecundity, (i.e. Mp10, Mp42, and MpC002) share similarity to proteins of known or predicted function, we performed BLAST searches against the GenBank non-redundant (nr) protein database (E value<10−5). One of the three candidates, Mp10 showed homology to an insect protein of predicted function, the olfactory segment D2-like protein (OS-D2-like protein). The OS-D2-like protein is a member of a family of chemosensory proteins in aphids that contain the conserved cysteine pattern CX6CX18CX2C [43]. Mp10 also shows similarity to chemosensory proteins (CSPs) from other insects (E value<10−5), including the CSP5 protein from the mosquito Anopheles gambiae (Figure 5A). The four cysteines in Mp10 are conserved among different members of the CSP family [44], [45] (Figure 5A). Among the aphid sequences similar to Mp10, polymorphisms are predominantly present after the predicted signal peptide sequence, in the mature protein region. For Mp42 and MpC002, similar sequences were identified in the genome sequence of the aphid species A. pisum only, but these proteins have no similarities to proteins with known functions. Alignment of Mp42 to a putative A. pisum homolog shows strong sequence divergence especially in the mature protein regions (Figure 5B). Finally, alignment of MpC002 to A. pisum C002 shows sequence divergence consisting of both amino acid polymorphisms and a 45 amino acid gap in A. pisum C002 after the predicted signal peptide sequence (Figure 5C). The presence of polymorphisms mainly in the mature protein regions may reflect that the functional domains of these proteins have diversified due to distinct selective pressures.
Fig. 5. Amino acid alignments of M. persicae effector candidates that alter aphid fecundity. 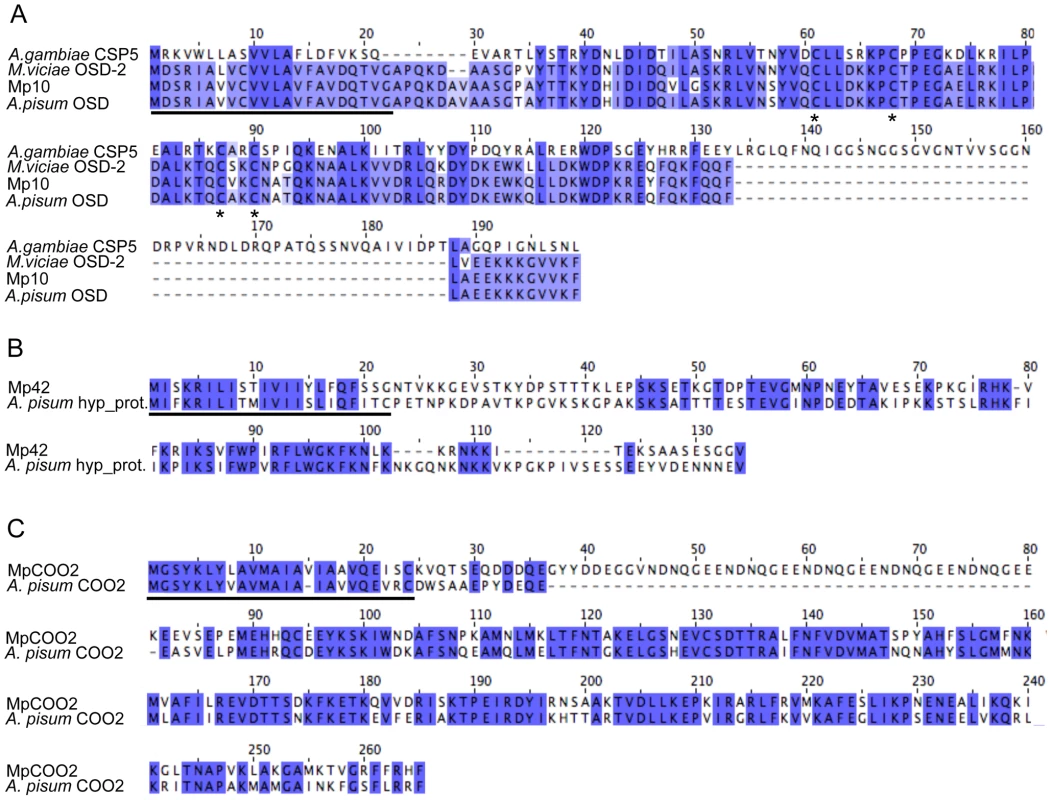
Black lines indicate the predicted signal peptide sequences. (A) Alignment of Mp10 with similar sequences from the aphid species Acyrthosiphon pisum (GenBank accession NP_001119652.1), Megoura viciae (GenBank accession CAG25435.1), and the mosquito species Anopheles gambiae (GenBank accession XP_317401.4). Asterisks indicate conserved cysteine residues. (B) Alignment of Mp42 with a similar sequence from A. pisum (GenBank accession XP_001948510). (C) Alignment of MpC002 with a similar sequence from A. pisum (GenBank accession XP_001948358.1). Discussion
Aphids, like other plant parasites, deliver repertoires of proteins inside their hosts that function as effectors to modulate host cell processes. These insects most likely secrete effectors into their saliva while progressing through the different plant cell layers during probing and feeding. The identification and characterization of these proteins will reveal new insights into the molecular basis of plant-insect interactions. Here, we have described a functional genomics pipeline to identify M. persicae effector candidates as well as various assays to determine whether the candidates share features with plant pathogen effectors. Using this approach, we identified three candidate effectors, Mp10 and Mp42, MpC002 that modulate host cell processes and affect aphid performance.
The induction of chlorosis and local cell death by Mp10 can reflect a genuine effector activity of this aphid salivary protein. Ectopic expression of bacterial TTSS as well as filamentous plant pathogen effectors can affect host immunity and induce a variety of phenotypes in plants, ranging from chlorosis to necrosis [22], [28]. Both the P. syringae type III effectors AvrB [30] and HOPQ-1 [46] induce chlorosis and for AvrB this activity is plant genotype specific [47]. No Mp10 induced chlorosis was observed in tomato despite expression levels of PVX-Mp10 that were comparable to N. benthamiana. This suggests that the Mp10 response was specific for N. benthamiana. Interestingly, PVX-Mp10 was unable to infect N. tabacum, suggesting this protein may induce an unknown defense mechanism that is effective against PVX-Mp10.
There are several possibilities that may explain the Mp10 phenotype in a biologically relevant context. The first possibility is that the artificially high expression of Mp10 could lead to the induction of the chlorosis/local cell death phenotype and therefore this response could be an artifact of the Agrobacterium-mediated overexpression assay. However, in this case we would expect that the induction of chlorosis and local cell death by Mp10 would be more widespread in various plant species, and would also be observed in N. benthamiana leaf discs or detached leaves. Another possibility is that the high expression of Mp10 could lead to excessive targeting of the operative target as well as other host proteins leading to an exaggeration of the true virulence activity [22]. Finally, the induction of chlorosis and local cell death could reflect avirulence activity of Mp10. Feeding of M. persicae is known to induce chlorosis and premature leaf senescence in plants, and this response is related to PAD4-mediated defense responses [48]. Therefore, Mp10 may exhibit an avirulence activity specifically in Nicotiana spp resulting in chlorosis and local cell death. The induction of chlorosis in N. benthamiana by P. syringae effector AvrB is thought to be due to weak activation of TAO1, an NBS-LRR protein, and requires the plant-signaling component Rar1 [49]. We found that chlorosis induction by Mp10 requires the co-chaperone SGT1, which is required for activation of NBS-LRR proteins and plant resistance responses [39]. Therefore, Mp10 may activate an NBS-LRR resistance protein resulting in ETI (further discussed below).
We also found that Mp10 suppressed the ROS response induced by flg22, suggesting that suppression of PTI may be a feature shared by plant pathogens and insects. Possibly, the flg22-induced signaling pathway may not be specific to bacteria as other (non-bacterial) PAMPs can induce this pathway. Also, plants may have a PTI pathway(s) that is induced by an unknown insect PAMP(s) and partially overlaps with the signaling pathway induced by flg22. To date the role of perception of PAMP-like molecules in plant-insect interactions remains elusive. However, chitin is a major structural component of the insect cuticle. Degradation of chitin by plant chitinases generates fragments that induce PTI [50]. Whether the chitin in the insect cuticle is degraded to induce plant defenses during interaction with host plants remains to be investigated. It has been hypothesized that sheath saliva protects the insect stylets, which mainly consist of chitin, from triggering plant defenses [51]–[53], potentially including PTI. Recent studies showed that insect saliva of both chewing insects [54] and aphids [55] contains elicitors that induce defense responses in host plants. The nature of these elicitors and their role in triggering PTI are unknown. Despite the lack of an understanding of the role in perception of PAMP-like molecules in plant-insect interactions, our data suggest that an aphid salivary protein, Mp10, can interfere with a specific PAMP response in a M. persicae host plant. It is therefore possible that Mp10 is a genuine suppressor of PTI. Alternatively, the overexpression of Mp10 may perturb a signaling component in the PTI pathway that is required for recognition of flg22. As Mp10 induces weak chlorosis starting from 2 dpi, it is possible that this response itself is responsible for loss of the oxidative burst response to PAMPs. However, the Mp10 chlorosis response does not interfere with the oxidative burst triggered by chitin. This suggests that the induction of chlorosis itself may not be sufficient to block the oxidative burst induced by flg22, but that Mp10 specifically interferes with the flg22-triggered signaling cascade.
Despite the suppression of the flg22-mediated oxidative burst by Mp10, its overexpression in N. benthamiana reduced aphid fecundity. A plausible explanation for this contradictory observation is that Mp10 may activate an NBS-LRR resistance protein resulting in ETI, thereby reducing aphid performance. Thus, the recognition of Mp10, potentially through ETI, in Nicotiana spp may mask the true virulence activity of this protein. If true, this recognition may be suppressed by other effectors during plant-aphid interactions so that Mp10 can exhibit its virulence function.
The leaf disc assay allowed us to generate vast amounts of functional data and directly implicated three effector candidates in plant-aphid interactions. The differences in aphid fecundity observed in our screens were quite variable, requiring replication of experiments. Despite the variation, Mp10, Mp42, and MpC002 showed consistent effects on aphid fecundity throughout the individual replicates (data not show). The fecundity was affected by Mp10, Mp42, and MpC002 by around 1–1.5 nymph produced per adult over a nymph production period of about 6 days. Although these differences may seem small, they are expected to have a large impact on the population size of aphids. Furthermore, M. persicae does not perform as well on N. benthamiana as it does on other hosts, such as Arabidopsis thaliana. Despite the low reproduction level on N. benthamiana, the fecundity differences found in our screens are similar to those observed over a 2-day period on A. thaliana in a study by Pegadaraju et al. [56] which shows that overexpression of PAD4 reduced aphid fecundity by about 1.5 nymphs per adult. The number of candidate effectors with an effect on aphid fecundity identified in this study may have been limited by our approach. For example, when the amount of an effector secreted by the aphid is sufficient to modulate host cell processes to promote feeding, in planta overexpression may not necessarily further enhance this effect. Also, there could be differences in plant responses to aphids in leaf discs versus whole plants as certain plant responses to aphids may require an intact plant transport system. Despite these limitations, the development of a novel leaf disc-based assay allowed us to identify three effector candidates from the aphid species M. persicae.
Out of the three candidates that affect aphid fecundity in the leaf-disc assays, only Mp10 shows homology to a protein of predicted function, namely OS-D2, a member of a family of predicted chemosensory proteins. Insect chemosensory proteins (CSP) are thought to be involved in olfaction and gustation. Indeed, several CSPs have been specifically found in chemosensory organs and are predicted to function in chemoperception [43], [57], [58]. However, for some members of this large protein family functions have been identified in insect development [59] and leg regeneration [60], suggesting that CSPs may have divergent functions. This is further supported by gene expression studies, which show that some CSPs are specifically expressed in antenna [61] or mouthparts [62], whereas others are expressed throughout the insect [63]. CSPs are thought to bind small molecules, such as fatty acids, and for some members of this protein family there is evidence that they bind to pheromones [64], [65]. In the aphid species Megoura viciae a Mp10 homolog was found to be expressed in aphid heads without antenna, indicating that it is not an antenna specific CSP [43]. Interestingly, in mosquitos, members of a family of odorant binding-related proteins, also with predicted functions in olfaction and gustation, are secreted into host cells to manipulate host physiology by for example scavenging host amines [66]. Counteracting host amines has evolved in various blood-feeding insects independently through adaptation of members of the lipocalin or odorant-binding protein families [66]. It is possible that also in plant feeding insects, proteins predicted to be involved in chemosensing are actually involved in early plant host recognition and plant host cell manipulation.
For Mp42 and MpC002 no homology was found to proteins of known or predicted function. This is not surprising as most plant pathogen effectors described to date do not show similarity to proteins of known function based on amino acid alignments. The reduction in aphid performance upon overexpression of Mp42 could reflect that Mp42 induces defense responses against aphids in the plant. In contrast, the enhancement of aphid fecundity by MpC002 suggests that this protein may exhibit an effector activity to promote aphid infestation. Indeed, the A. pisum homolog of MpC002, ApC002, has been implicated in aphid feeding [5]. Interestingly, ApC002 is secreted into plant tissues during aphid feeding and silencing of ApC002 gene expression reduces aphid survival on plants, but does not affect when aphids feed from diet [67]. However, whether A. pisum performs better upon overexpression of C002 in planta is not known. Our data suggest that the MpC002 homolog may exhibit a similar role in M. persicae, and that this protein is important during plant-aphid interactions. Future studies will be aimed at further characterizing these candidates to identify their plant targets and the molecular processes they perturb.
Methods
Sequence databases
We downloaded the following datasets in November 2008 from GenBank for bioinformatics analyses. A total of 3233 M. persicae salivary gland ESTs, 27868 M. persicae ESTs (all available ESTs), and 2558 M. persicae gut ESTs [34], as well as 4517 A. pisum salivary gland ESTs (GenBank accessions DV747494-DV752010). For similarity searches against the A. pisum genome sequence, we obtained the whole shotgun genome sequence scaffolds from GenBank (accessions EQ110773-EQ133570) in May 2010.
Bioinformatics analyses
The pipeline for the identification of M. persicae candidate effectors was developed as follows. The 3233 salivary gland ESTs from M. persicae were subjected to ORF calling. More specifically, we performed translations of all possible ORFs of 70+ amino acids, defined by an ATG to stop or an ATG to the end of a sequence, from both strands of the cDNA. We then applied the SignalP v 3.0 program [35] to predict the presence of signal peptides in the amino acid sequences with an HMM score cut-off value of >0.9 and a predicted cleavage site within the amino acid region 1–30. As some predicted secreted proteins were represented multiple times within the M. persicae salivary gland EST dataset, we used BLASTP searches to remove redundant sequences. Alignments were inspected manually and sequences that showed >95% identity throughout most of the alignment with an E value<10−10 were classified as being redundant. To remove sequences that in addition the signal peptide also contained a transmembrane domain we used TMHMM v.2.0. The remaining sequences were searched using TBLASTN (E value<10−5) against all M. persicae and A. pisum ESTs in our datasets as well as the A. pisum genome sequence to assess whether they encoded full-length proteins. Criteria for selecting full-length sequences were: 1) the presence of a conserved start and stop site in ESTs within the alignments; 2) the absence of a methionine within the alignments upstream of the methionine predicted to be the start of the ORF; 3) similarity to a predicted full-length A. pisum protein, when available. The remaining predicted secreted protein sequences were then assessed for the presence of polymorphisms within the alignments described above. Sequences not showing any sequence variation in alignments with M. persicae sequences and that contained up to one amino acid difference in alignments of the mature protein regions with A. pisum sequences were removed from the candidate list.
The 4517 salivary gland ESTs from A. pisum were analyzed with the same procedures except that no analyses was performed for the presence of polymorphisms. The amino acid sequences of the predicted secreted proteins (Table S1) were searched using BLASTP (E value of <10−5) against the amino acid sequences of the M. persicae candidates to identify overlap in the datasets. A. pisum candidates without a hit were then searched using TBLASTN against all available M. persicae ESTs (E value of <10−5) to identify M. persicae predicted secreted proteins with sequence similarity. The M. persicae candidates identified using our pipeline and subjected to cloning were designated MpC002, Mp1-12, Mp14-17, Mp19-24, Mp28-33, Mp35-37, Mp39-47, Mp49-51, Mp53-54, wherein Mp stands for M. persicae (Table S2).
Aphids
The M. persicae colony of lineage RRes (genotype O) was maintained in cages on N. tabacum plants. Cages were located in a contained growth room at 18°C under 16 hours of light.
Microbial strains and growth conditions
A. tumefaciens strain GV3101 was used in molecular cloning and agroinfiltration experiments and were routinely cultured at 28°C in Luria-Bertani (LB) media using appropriate antibiotics [68]. All bacterial DNA transformations were conducted by electroporation using standard protocols [68].
Cloning of Mp candidates
Primers were designed for amplification of sequences corresponding to the ORFs encoding the mature proteins (after the signal peptide encoding sequences) (Table S3). To confirm the 3′ end of the ORFs, we designed, where possible, the 3′-primer in the 3′UTR sequence. Sequences were amplified from M. persicae cDNA using Phusion polymerase (Finnzymes) and ligated into SpeI/BamHI, SpeI/BglII or BglII/BamHI digested pCB302-3 vector [69] to generate 35S-constructs. To assess whether sequences were polymorphic within the M. persicae clonal lineage used in our studies, we performed sequence analyses of 4 clones per construct. To generate constructs for PVX-based expression, we amplified sequences encoding mature ORFs and ligated these into ClaI/NotI digested pGR106 vector. The PTV vectors used in this study have been described previously [40].
Gene expression analyses by semi-quantitative RT-PCR
Aphids were dissected in PBS and tissues stored in RNA later. We collected 25 salivary glands, 10 guts, 5 heads and 5 whole aphids. RNA extractions were performed with the NucleoSpin RNA XS kit (Macherey-Nagel, Germany). cDNA was synthesized from 80 ng total RNA per sample using expand reverse transcriptase (Roche Diagnostics Ltd). RT-PCR was performed with gene specific primers for each effector candidate indicated in Table S3. MpActin primers were used as a control for equal cDNA template amounts.
For RT-PCR on plant tissues, 50 mg leaf tissue was ground in liquid nitrogen and RNA was extracted with the RNeasy Plant minikit (Qiagen). cDNA was synthesized from 500ng DNase treated RNA and subjected to PCR reactions with primer pairs Mp10-pvx-F/R and Mp42-pvx-F/R (Table S3) for amplification of Mp10 and Mp42 expressed in PVX, respectively. For amplification of the PVX coat protein we used primer pair PVX-CP-F/R and for amplification of plant tubulin we used the primer pair Tub-F/R (Table S3). Primers used for RT-PCR on RNA extracted from SGT - and HSP90-silenced plants were described elsewhere [26].
PVX agroinfection and agroinfiltration assays
Recombinant A. tumefaciens strains were grown as described elsewhere [70] except that the culturing steps were performed in LB media supplemented with 50 µg/mL of kanamycin. Agroinfiltration experiments were performed on 4–6 week-old N. benthamiana plants. Plants were grown and maintained throughout the experiments in a growth chamber with an ambient temperature of 22°–25°C and high light intensity.
For transient overexpression of candidate effectors by agroinfiltration, leaves of N. benthamiana were infiltrated with A. tumefaciens strain GV3101 carrying the respective constructs at a final OD600 of 0.3 in induction buffer (10mM MES, 10mM MgCl2, 150 µM acetosyringone, pH = 5.6).
For agroinfection assays, cotelydons of N. benthamiana, N. tabacum (cv Petite Gerard) or S. lycopersicum (MoneyMaker) were wound-inoculated with candidate effector clones using P200 pipette tips. Each strain was assayed on 2 replicated plants. As a control, plants were wound-inoculated with A. tumefaciens strains carrying pGR106-Δgfp [26]. Systemic PVX symptoms were scored 14 days post inoculation.
TRV-induced gene silencing
We performed gene silencing as described elsewhere [40]. A. tumefaciens suspensions expressing the binary TRV-RNA 1 construct, pBINTRA6, and the TRV-RNA2 vector, PTV00 or PTV-SGT1 were mixed in 1∶1 ratio (RNA1 - RNA2) in induction buffer (final OD600 is 0.6). Leaves were challenged with Agrobacterium strains carrying 35S-Mp10 and 35S-INF1 or the 35S vector.
Aphid fecundity assays in 24-well plates
We developed a medium-throughput 24-well assay to test whether overexpression in planta of effector candidates affects aphid nymph production rates. For this purpose, we overexpressed the candidates (35S-constructs) by agroinfiltration in N. benthamiana at a final OD600 of 0.3. One day after infiltration, leaf discs were collected using a cork borer (No. 7) from the infiltration sites and placed upside-down on top of 1ml water agar in 24-well plates. A total of 6 infiltration sites, from 6 different leaves, were used per construct and a total of 4 different constructs per 24-well plate. In initial screens, we infiltrated multiple sets of 4 candidate effectors at the same time, with one set including the vector and GFP controls (two candidate effectors plus the two controls). The 4 candidates within a set were infiltrated side-by-side on the same 6 leaves. Leaf discs from each set of candidates were placed in one 24-well plate (6 discs times 4 candidates). For the confirmation assays, we performed infiltrations of each candidate effector with the vector control side-by-side on the same 6 leaves, and leaf discs were placed in one 24-wells plate. On each leaf disc, we placed 4 M. persicae first-instar nymphs. The wells in the plate were individually sealed off using a cap of a 5ml BD Falcon round bottomed test tub with the top of the cap removed and covered with mesh. After 6 days, the nymphs were moved to a new 24-wells plate with fresh leaf discs infiltrated with the candidate effector constructs. Another 6 days later, the now adult aphids were again moved to a new 24-well plate with freshly infiltrated leaf discs. The numbers of adults (initially first-instar nymphs) were counted 6, 12, 14 and 17 days after setting up the first 24-wells plate and the number of newly produced nymphs were counted on day 12, 14 and 17. The newly produced nymphs were removed from the wells during counting. Wells wherein all 4 aphids that were initially placed on the discs died were taken out of the analyses. To calculate the production of nymphs per adult aphid, we calculated the average number of nymphs produced per adult by combining the average production rates throughout the experiment. These average production rates were obtained by dividing the number of nymphs on day 12 by the number of adults on day 6 (calculated per well), dividing the number of nymphs on day 14 by the number of adults on day 12, and dividing the number of nymphs on day 17 by the number of adults on day 14. To obtain the total average production rate, we calculated the sum of the average production rates for days 12, 14 and 17.
Measurements of reactive oxygen species
N. benthamiana leaf discs transiently overexpressing the effector candidates were subjected to a luminescence-based assay [41]. Leaf discs were floated overnight in 200ul water in a 96-well plate. The production of ROS was measured after replacing the water with a solution of luminol (20uM) and horseradish peroxidase (1ug) supplemented with either flg22 peptide (100nM) or chitin (100 µg/ml). Luminescence was measured using a Varioskan Flash plate reader. A total of 8 discs per construct, obtained from 4 different infiltration sites, were used per replicate. Assays with flg22 to screen the 48 candidates for suppression activity were repeated two times. The assays with chitin and flg22 were repeated three times.
Statistical analyses
All statistical analyses were conducted using Genstat 11. ROS assay was analysed using a two-sample t-test. Leaf discs fecundity assays were analysed using one-way ANOVA with “construct” as the treatment and “repeat” as the block. Data was checked for approximate normal distribution by visualising the residuals.
Supporting Information
Zdroje
1. WillT
TjallingiiWF
ThönnessenA
van BelAJ
2007 Molecular sabotage in plant defense by aphid saliva. Proc Natl Acad Sci U S A 104 10536 10541
2. BlackmanRL
EastopVF
2000 Aphids of the World's Crops - an identification and information guide England Wiley & Sons 466
3. PradoE
TjallingiiWF
2007 Behavioral evidence for local reduction of aphid-induced resistance. J Insect Sci 7 48
4. MartinB
CollarJL
TjallingiiWF
FereresA
1997 Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J Gen Virol 78 2701 2705
5. MuttiNS
LouisJ
PappanLK
PappanK
BegumK
2008 A protein from the salivary glands of the pea aphid, Acyrthosiphon pisum, is essential in feeding on a host plant. Proc Natl Acad Sci U S A 105 9965 9969
6. MuttiNS
ParkY
ReeseJC
ReeckGR
2006 RNAi knockdown of a salivary transcript leading to lethality in the pea aphid, Acyrthosiphon pisum. J Insect S 6 38
7. HarmelN
LétocartE
CherquiA
GiordanengoP
MazzucchelliG
2008 Identification of aphid salivary proteins: a proteomic investigation of Myzus persicae. Insect Mol Biol 17 165 174
8. CarolanJC
FitzroyCI
AshtonPD
DouglasAE
WilkinsonTL
2009 The secreted salivary proteome of the pea aphid Acyrthosiphon pisum characterised by mass spectrometry. Proteomics 9 2457 67
9. HogenhoutSA
Van der HoornRA
TerauchiR
KamounS
2009 Emerging concepts in effector biology of plant-associated organisms. Mol Plant Microbe Interact 22 115 122
10. KamounS
2007 Groovy times: filamentous pathogen effectors revealed. Curr Opin Plant Biol 10 358 365
11. Van der HoornRAL
KamounS
2008 From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell 20 2009 2017
12. JonesJD
DanglJL
2006 The plant immune system. Nature 444 323 329
13. KlinglerJ
CreasyR
GaoL
NairRM
CalixAS
2005 Aphid resistance in Medicago truncatula involves antixenosis and phloem-specific, inducible antibiosis, and maps to a single locus flanked by NBS-LRR resistance gene analogs. Plant Physiol 137 1445 1455
14. DogimontC
ChovelonV
TualS
BoissotN
RittenerV
2007 Molecular determinants of recognition specificity at the aphid and powdery mildew Vat/Pm-W resistance locus in melon. In XIII International Congress MPMI 2007, Sorrento (IT) 375
15. MilliganSB
BodeauJ
YaghoobiJ
KaloshianI
ZabelP
WilliamsonVM
1998 The root knot nematode resistance gene Mi from tomato is a member of the leucine zipper, nucleotide binding, leucine-rich repeat family of plant genes. Plant Cell 10 1307 1319
16. RossiM
GogginFL
MilliganSB
KaloshianI
UllmanDE
WilliamsonVM
1998 The nematode resistance gene Mi of tomato confers resistance against the potato aphid. Proc Natl Acad Sci U S A 95 9750 9754
17. CasteelCL
WallingLL
PaineTD
2006 Behavior and biology of the tomato psyllid, Bactericerca cockerelli, in response to the Mi 1.2 gene. Entomologia Experimentalis et Applicata 121 67 72
18. WallingLL
2008 Avoiding Effective Defenses: Strategies Employed by Phloem-Feeding Insects. Plant Phys 146 859 866
19. FrancisF
GuillonneauF
LeprinceP
De PauwE
HaubrugeE
2010 Tritrophic interactions among Macrosiphum euphorbiae aphids, their host plants and endosymbionts: investigation by a proteomic approach. J Insect Physiol 56 575 585
20. StewartSA
HodgeS
IsmailN
MansfieldJW
FeysBJ
2009 The RAP1 gene confers effective, race-specific resistance to the pea aphid in Medicago truncatula independent of the hypersensitive reaction. Mol Plant Microbe Interact 22 1645 1655
21. DanglJL
JonesJD
2001 Plant pathogens and integrated defence responses to infection. Nature 411 826 833
22. CunnacS
LindebergM
CollmerA
2009 Pseudomonas syringae type III secretion system effectors: repertoires in search of functions. Curr Opin Microbiol 12 53 60
23. HuitemaE
BosJI
TianM
WinJ
WaughME
KamounS
2004 Linking sequence to phenotype in Phytophthora-plant interactions. Trends Microbiol 12 193 200
24. SchornackS
HuitemaE
CanoLM
BozkurtTO
OlivaR
2009 Ten things to know about oomycete effectors. Mol Plant Pathol 10 795 803
25. GuoM
TianF
WamboldtY
AlfanoJR
2009 The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol Plant Microbe Interact 22 1069 1080
26. BosJIB
KannegantiTD
YoungC
CakirC
HuitemaE
2006 The C-terminal half of Phytophthora infestans RXLR effector AVR3a is sufficient to trigger R3a-mediated hypersensitivity and suppress INF1-induced cell death in Nicotiana benthamiana. Plant J 48 165 176
27. BosJI
ArmstrongMR
GilroyEM
BoevinkPC
HeinI
2010 Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc Natl Acad Sci U S A 107 9909 9914
28. TortoTA
LiS
StyerA
HuitemaE
TestaA
2003 EST mining and functional expression assays identify extracellular effector proteins from the plant pathogen Phytophthora. Genome Res 13 1675 1685
29. KamounS
van WestP
VleeshouwersVGAA
de GrootKE
GoversF
1998 Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell 10 1413 1425
30. ShangY
LiX
CuiH
HeP
ThilmonyR
2006 RAR1, a central player in plant immunity, is targeted by Pseudomonas syringae effector AvrB. Proc Natl Acad Sci U S A 103 19200 19205
31. WangX
MitchumMG
GaoB
LiC
DiabH
2005 A parasitism gene from a plant-parasitic nematode with function similar to CLAVATA3/ESR (CLE) of Arabidopsis thaliana. Mol Plant Pathol 6 187 191
32. Gimenez-IbanezS
HannDR
NtoukakisV
PetutschnigE
LipkaV
RathjenJP
2009 AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol 19 423 429
33. HeweziT
BaumTJ
2010 Sequence divergences between cyst nematode effector protein orthologs may contribute to host specificity. Plant Signal Behav 5 187 189
34. RamseyJS
WilsonAC
de VosM
SunQ
TamborindeguyC
2007 Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics 8 423
35. BendtsenJD
NielsenH
von HeijneG
BrunakS
2004 Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340 783 795
36. ChisholmST
CoakerG
DayB
StaskawiczBJ
2006 Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124 803 814
37. StahlEA
BishopJG
2000 Plant-pathogen arms races at the molecular level. Curr Opin Plant Biol 3 299 304
38. BarajasD
TenlladoF
Díaz-RuízJR
2006 Characterization of the recombinant forms arising from a Potato virus X chimeric virus infection under RNA silencing pressure. Mol Plant Microbe Interact 19 904 913
39. AzevedoC
BetsuyakuS
PeartJ
TakahashiA
NoëlL
2006 Role of SGT1 in resistance protein accumulation in plant immunity. EMBO J 25 2007 2016
40. KannegantiTD
HuitemaE
CakirC
KamounS
2006 Synergistic interactions of the plant cell death pathways induced by Phytophthora infestans Nepl-like protein PiNPP1.1 and INF1 elicitin. Mol Plant Microbe Interact 19 854 863
41. KepplerLD
BakerCJ
AtkinsonMM
1989 Active oxygen production during a bacteria-induced hypersensitive reaction in tobacco suspension cells. Phytopathology 79 974 978
42. HannDR
RathjenJP
2007 Early events in the pathogenicity of Pseudomonas syringae on Nicotiana benthamiana. Plant J 49 607 618
43. JacobsSP
LigginsAP
ZhouJJ
PickettJA
JinX
FieldLM
2005 OS-D-like genes and their expression in aphids (Hemiptera : Aphididae). Insect Mol Biol 14 423 432
44. AngeliS
CeronF
ScaloniA
MontiM
MontefortiG
1999 Purification, structural characterisation, cloning and immunocytochemical localisation of chemoreception proteins from Schistocerca gregaria. Eur J Biochem 292 745 754
45. TegoniM
CampanacciV
CambillauC
2004 Structural aspects of sexual attraction and chemical communication in insects. Trends Biochem Sci 29 257 264
46. WroblewskiT
CaldwellKS
PiskurewiczU
CavanaughKA
XuH
2009 Comparative large-scale analysis of interactions between several crop species and the effector repertoires from multiple pathovars of Pseudomonas and Ralstonia. Plant Physiol 150 1733 1749
47. AshfieldT
OngLE
NobutaK
SchneiderCM
InnesRW
2004 Convergent evolution of disease resistance gene specificity in two flowering plant families. Plant Cell 16 309 318
48. PegadarajuV
KnepperC
ReeseJ
ShahJ
2005 Premature leaf senescence modulated by the Arabidopsis PHYTOALEXIN DEFICIENT4 gene is associated with defense against the phloem-feeding green peach aphid. Plant Physiol 139 1927 1934
49. EitasTK
NimchukZL
DanglJL
2008 Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc Natl Acad Sci U S A 105 6475 6480
50. BollerT
1995 Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol 46 189 214
51. de VosM
KimJH
JanderG
2007 Biochemistry and molecular biology of Arabidopsis-aphid interactions. BioEssays 28 871 883
52. TjallingiiWF
2006 Salivary secretions by aphids interacting with proteins of phloem wound responses. J Exp Bot 57 739 745
53. MilesPW
1999 Aphid saliva. Biol Rev 74 41 85
54. DiezelC
von DahlCC
GaquerelE
BaldwinIT
2009 Different lepidopteran elicitors account for cross-talk in herbivory-induced phytohormone signaling. Plant Physiol 150 1576 1586
55. De VosM
JanderG
2009 Myzus persicae (green peach aphid) salivary components induce defence responses in Arabidopsis thaliana. Plant Cell Environ 32 1548 1560
56. PegadarajuV
LouisJ
SinghV
ReeseJC
BautorJ
2007 Phloem-based resistance to green peach aphid is controlled by Arabidopsis PHYTOALEXIN DEFICIENT4 without its signaling partner ENHANCED DISEASE SUSCEPTIBILITY1. Plant J 52 332 341
57. Nagnan-Le MeillourP
CainAH
Jacquin-JolyE
FrançoisMC
RamachandranS
2000 Chemosensory proteins from the proboscis of mamestra brassicae. Chem Senses 25 541 553
58. MontefortiG
AngeliS
PetacchiR
MinnocciA
2002 Ultrastructural characterization of antennal sensilla and immunocytochemical localization of a chemosensory protein in Carausius morosus Brünner (Phasmida: Phasmatidae). Arthropod Struct Dev 30 195 205
59. StathopoulosA
Van DrenthM
ErivesA
MarksteinM
LevineM
2002 Whole-genome analysis of dorsal-ventral patterning in the Drosophila embryo. Cell 111 687 701
60. NomuraA
KawasakiK
KuboT
NatoriS
1992 Purification and localization of p10, a novel protein that increases in nymphal regenerating legs of Periplaneta americana (American cockroach). Int J Dev Biol 36 391 398
61. CalvelloM
BrandazzaA
NavarriniA
DaniFR
TurillazziS
2005 Expression of odorant-binding proteins and chemosensory proteins in some Hymenoptera. Insect Biochem Mol Bio 35 297 307
62. MaleszkaR
StangeG
1997 Molecular cloning, by a novel approach, of a cDNA encoding a putative olfactory protein in the labial palps of the moth Cactoblastis cactorum. Gene 202 39 43
63. ZhouJJ
HuangW
ZhangGA
PickettJA
FieldLM
2004 “Plus-C” odorant-binding protein genes in two Drosophila species and the malaria mosquito Anopheles gambiae. Gene 327 117 129
64. Jacquin-JolyE
VogtRG
FrancoisMC
Nagnan-Le MeillourP
2001 Functional and expression pattern analysis of chemosensory proteins expressed in antennae and pheromonal gland of Mamestra brassicae. Chem Senses 26 833 844
65. BriandL
SwasdipanN
NespoulousC
BézirardV
BlonF
2002 Characterization of a chemosensory protein (ASP3c) from honeybee (Apis mellifera L.) as a brood pheromone carrier. Eur J Biochem 269 4586 4596
66. CalvoE
MansBJ
AndersenJF
RibeiroJM
2005 Function and evolution of a mosquito salivary protein family. J Biol Chem 281 1935 1942
67. MuttiNS
2006 Molecular studies of the salivary glands of the pea aphid, Acyrthosiphon pisum (Harris). PhD dissertation, Kansas State University, Kansas, USA
68. SambrookJ
RussellDW
2001 Molecular Cloning Cold Spring Harbor, New York, USA Cold Spring Harbor Laboratory Press
69. XiangC
HanP
LutzigerI
WangK
OliverDJ
1999 A mini binary vector series for plant transformation. Plant Mol Biol 40 711 717
70. Van der HoornRA
LaurentF
RothR
De WitPJ
2000 Agroinfiltration is a versatile tool that facilitates comparative analyses of Avr9/Cf-9-induced and Avr4/Cf-4-induced necrosis. Mol Plant-Microbe Interact 13 439 446
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 11- Akutní intermitentní porfyrie
- Farmakogenetické testování pomáhá předcházet nežádoucím efektům léčiv
- Růst a vývoj dětí narozených pomocí IVF
- IVF a rakovina prsu – zvyšují hormony riziko vzniku rakoviny?
- Délka menstruačního cyklu jako marker ženské plodnosti
-
Všechny články tohoto čísla
- Genome-Wide Association Meta-Analysis of Cortical Bone Mineral Density Unravels Allelic Heterogeneity at the Locus and Potential Pleiotropic Effects on Bone
- Beyond QTL Cloning
- An Evolutionary Framework for Association Testing in Resequencing Studies
- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- The Functional Interplay between Protein Kinase CK2 and CCA1 Transcriptional Activity Is Essential for Clock Temperature Compensation in Arabidopsis
- Endogenous Viral Elements in Animal Genomes
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- DNA Methylation and Normal Chromosome Behavior in Neurospora Depend on Five Components of a Histone Methyltransferase Complex, DCDC
- Sarcomere Formation Occurs by the Assembly of Multiple Latent Protein Complexes
- Genetic Basis of Growth Adaptation of after Deletion of , a Major Metabolic Gene
- Nomadic Enhancers: Tissue-Specific -Regulatory Elements of Have Divergent Genomic Positions among Species
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- CTCF-Dependent Chromatin Bias Constitutes Transient Epigenetic Memory of the Mother at the Imprinting Control Region in Prospermatogonia
- Systematic Dissection and Trajectory-Scanning Mutagenesis of the Molecular Interface That Ensures Specificity of Two-Component Signaling Pathways
- Nucleolin Is Required for DNA Methylation State and the Expression of rRNA Gene Variants in
- The Complex Genetic Architecture of the Metabolome
- ATM Limits Incorrect End Utilization during Non-Homologous End Joining of Multiple Chromosome Breaks
- Mutation Disrupts Synaptonemal Complex Formation, Recombination, and Chromosome Segregation in Mammalian Meiosis
- Mismatch Repair–Independent Increase in Spontaneous Mutagenesis in Yeast Lacking Non-Essential Subunits of DNA Polymerase ε
- The Kinesin-3 Motor UNC-104/KIF1A Is Degraded upon Loss of Specific Binding to Cargo
- Epigenetic Silencing of Spermatocyte-Specific and Neuronal Genes by SUMO Modification of the Transcription Factor Sp3
- A Coastal Cline in Sodium Accumulation in Is Driven by Natural Variation of the Sodium Transporter AtHKT1;1
- Cyclin B3 Is Required for Multiple Mitotic Processes Including Alleviation of a Spindle Checkpoint–Dependent Block in Anaphase Chromosome Segregation
- Altered DNA Methylation in Leukocytes with Trisomy 21
- Human-Specific Evolution and Adaptation Led to Major Qualitative Differences in the Variable Receptors of Human and Chimpanzee Natural Killer Cells
- Leptotene/Zygotene Chromosome Movement Via the SUN/KASH Protein Bridge in
- RACK-1 Acts with Rac GTPase Signaling and UNC-115/abLIM in Axon Pathfinding and Cell Migration
- Genome-Wide Effects of Long-Term Divergent Selection
- Endless Forms Most Viral
- Conflict between Noise and Plasticity in Yeast
- Essential Functions of the Histone Demethylase Lid
- The Transcriptional Regulator Rok Binds A+T-Rich DNA and Is Involved in Repression of a Mobile Genetic Element in
- The Cellular Robustness by Genetic Redundancy in Budding Yeast
- Localization of a Guanylyl Cyclase to Chemosensory Cilia Requires the Novel Ciliary MYND Domain Protein DAF-25
- A Buoyancy-Based Screen of Drosophila Larvae for Fat-Storage Mutants Reveals a Role for in Coupling Fat Storage to Nutrient Availability
- A Functional Genomics Approach Identifies Candidate Effectors from the Aphid Species (Green Peach Aphid)
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Two Novel Regions at 11p15.5-p13 and 1p31 with Major Impact on Acute-Phase Serum Amyloid A
- Analysis of the 10q11 Cancer Risk Locus Implicates and in Human Prostate Tumorigenesis
- The Parental Non-Equivalence of Imprinting Control Regions during Mammalian Development and Evolution
- Genome-Wide Effects of Long-Term Divergent Selection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



