-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe prevalence and characteristics of epilepsy in patients with relapsing-remitting multiple sclerosis treated with disease-modifying therapy
Prevalence a charakteristika epilepsie u pacientů s relabující-remitující formou roztroušené sklerózy léčených imunomodulační terapií
Cíl: Cílem bylo vyhledat pacienty s relabující-remitující formou RS (RRMS) léčené imunomodulační terapií, kteří mají v anamnéze neprovokovaný epileptický záchvat, či se léčí s epilepsií, a zhodnotit vliv epilepsie na průběh a prognózu RS.
Soubor a metodika: Do studie byli zařazení pacienti s RRMS léčení imunomodulační léčbou a docházející do centra RS fakultní nemocnice k 1. lednu 2019. Byly hodnoceny roční míra relapsů a Expanded Disability Status Scale (EDSS) před a po prvním záchvatu.
Výsledky: V kohortě pacientů s RRMS (n = 750) bylo nalezeno 17 pacientů (2,27 %) s historií neprovokovaných záchvatů nebo epilepsie. Aktivní epilepsie, definována užíváním antiepileptik či výskytem záchvatů v posledních 5 letech, byla zjištěna u 13 pacientů (73 %). Tři pacienti měli již diagnostikovanou epilepsii před prvními symptomy RS. Medián roční míry relapsů u pacientů s epilepsií byl 0,12 před prvním záchvatem a 0,5 po prvním záchvatu (p = 0,1). Nebyl nalezen signifikantní rozdíl mezi zhoršováním EDSS 2 roky před a 2 roky po prvním záchvatu (0,55 vs. 0,7; p = 0,38). Záchvat byl hodnocen jako fokální na základně klinické manifestace a/nebo EEG u 8 ze 17 pacientů (47 %).
Závěr: Frekvence neprovokovaného epileptického záchvatu či epilepsie u pacientů s RRMS byla v naší kohortě 2,27 %.
Klíčová slova:
roztroušená skleróza – epilepsie – záchvaty – prevalence
Authors: J. Kolčava; J. Kočica; P. Štourač; J. Bednařík
Authors place of work: Medicine, Masaryk University and University Hospital, Brno, Czech Repubic ; Department of Neurology, Faculty of
Published in the journal: Cesk Slov Neurol N 2020; 83/116(4): 424-427
Category: Krátké sdělení
doi: https://doi.org/10.14735/amcsnn2020424Summary
Aim: The aim was to identify and describe patients with relapsing-remitting MS (RRMS) treated with a disease-modifying therapy who had a history of unprovoked seizure or epilepsy and to investigate the impact of epilepsy on the course and prognosis of MS.
Materials and methods: Patients with RRMS treated with a disease-modifying therapy at a large MS centre of the university hospital by January 1, 2019 were included in this study. The annual relapse rate and Expanded Disability Status Scale (EDSS) before and after the first seizure were evaluated.
Results: 17 patients (2.27%) with a history of unprovoked seizure or epilepsy were identified within the cohort of RRMS patients (N = 750). Active epilepsy, defined as the use of antiepileptic drugs or incidence of seizures within the last 5 years, was disclosed in 13 (73%) of them. Three patients had developed epilepsy before the first signs of MS. The mean annual relapse rates in patients with epilepsy before and after the first seizure were 0.12 and 0.5 (P = 0.1), resp. No significant difference was found in terms of EDSS worsening at 2 years before and after the first seizure (0.55 vs. 0.7; P = 0.38). The attack semiology and/or electroencephalogram recordings indicated a focal onset of seizures in 8 out of 17 patients (47%).
Conclusion: The frequency of unprovoked seizure or epilepsy among RRMS patients in our cohort was 2.27%.
Keywords:
Multiple sclerosis – Epilepsy – prevalence – seizures
Introduction
Multiple sclerosis is a chronic inflammatory and degenerative disease of the CNS. It is the leading cause of neurologic disability (not arising out of trauma) in young adults and is characterized by focal white matter lesions; however, both deep and cortical grey matter are also involved [1,2]. Patients with MS and epilepsy had a higher risk of mortality compared with those without [3]. A recent study derived from the Swedish MS registry (N = 14,545) revealed a cumulative incidence of epilepsy of 3.5% in patients with MS and 1.4% in controls. Patients with relapsing-remitting MS (RRMS) had a cumulative incidence of epilepsy of 2.2%, whereas in patients with the progressive disease this reached 5.5% [4].
The frequency of active epilepsy among MS patients in Nordland (county in Norway) was 3.2%, approximately 4.5 times higher than in the general Norwegian population. The RRMS patients with active epilepsy had more likely converted to secondary progressive MS than the patients without active epilepsy [5].
The cause of such an increased occurrence of epilepsy among patients with MS is unknown, but it is reasonable to assume an epileptogenic role played by cortical lesions [6,7]. The aim of our retrospective cohort study was to identify and describe patients with RRMS treated with a disease--modifying therapy who had a history of unprovoked seizure or epilepsy and to assess the impact of epilepsy on the course of MS in terms of the annual relapse rate (ARR) and Expanded Disability Status Scale (EDSS) at 2 years before and after the first seizure.
Materials and Methods
A cohort of 750 patients (504 women and 246 men) currently suffering from RRMS according to the McDonald diagnostic criteria, 2017 [8], seen at the MS centre of the large university hospital in the region of 612,000 inhabitants in the period leading up to January 1, 2019 were screened. Their case histories based on the electronic patient ledger were scrutinized to identify seizures. Epilepsy was classified as focal or generalized, based on recorded seizure semiology and EEG findings. The age, sex and treatment for MS and epilepsy were noted. The values of ARR and EDSS were analysed over the periods lasting for 2 years – both before and after the first seizure, to establish whether the seizures were directly involved in the severity of MS. The ANOVA (Analysis of Variance) test was used for the correlation of ARRs and EDSS in our cohort. Patients presenting with seizures before MS diagnosis were excluded from the ARR or EDSS analysis.
Results
Among 750 patients with RRMS treated with a disease-modifying therapy, 17 patients (age 41.2 ± 10.4 years) with a history of seizure (prevalence 2.27%) were identified, 12 of them were women (age 43.6 ± 10.5 years) and 5 men (35.6 ± 10.5 years). Three patients had epilepsy before the first symptoms of MS (18%) (Fig. 1). The mean ARRs in patients with epilepsy at 2 years before and after the first seizure were 0.12 and 0.5 (P = 0.1), resp. The mean values of EDSS at 2 years before and after the first seizure were 2.9 and 4.15, resp. The EDSS at the first seizure was 3.45 in the cohort (N = 10). No significant difference was found in terms of EDSS worsening at 2 years before and after the first seizure (0.55 vs. 0.7; P = 0.38).
Fig. 1. Latency from the first symptom of MS to the first seizure.
Obr. 1. Latence od prvního symptomu RS k prvnímu záchvatu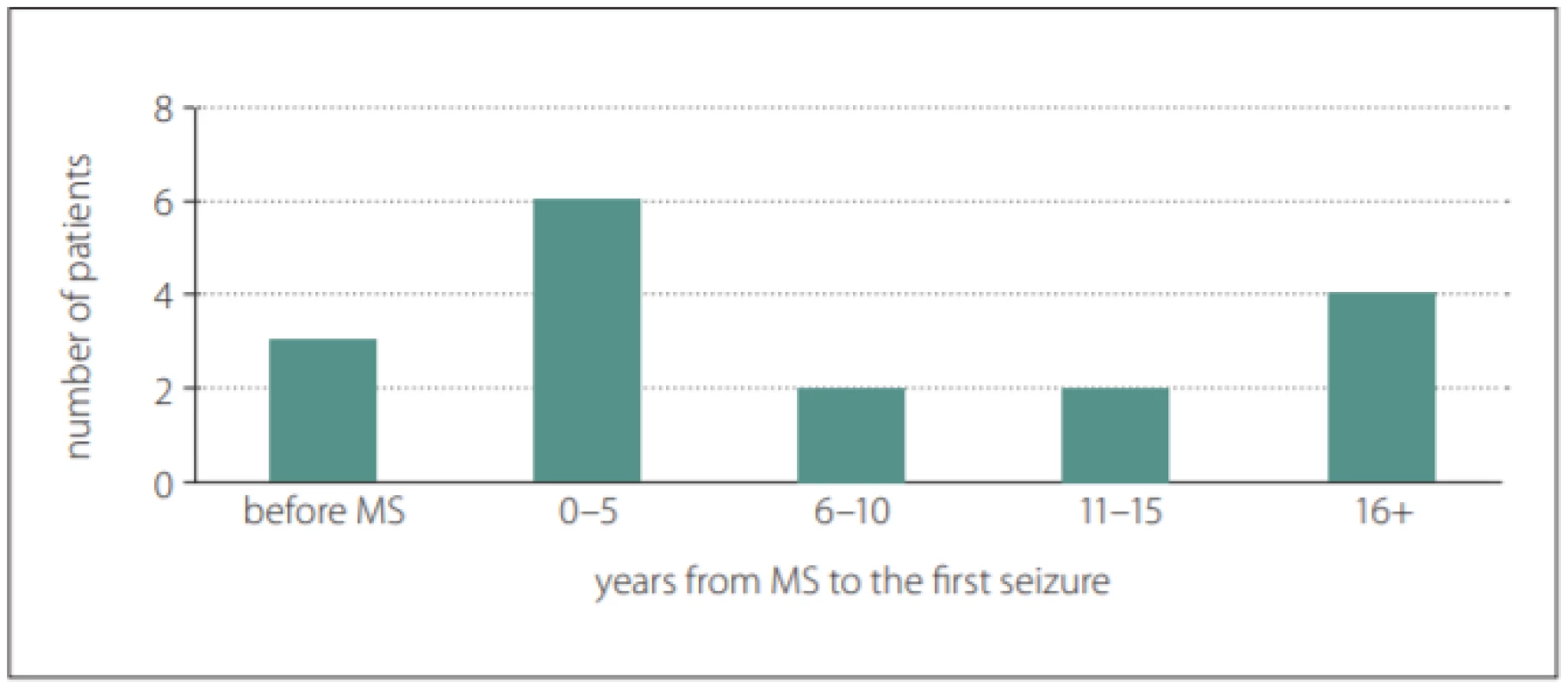
The mean age at which the first symptoms of MS appeared was 27.6 ± 10.3 years and the mean age at which the first seizure occurred was 35.1 ± 12.6 years. At least one EEG was recorded for all patients with MS and epilepsy. Epileptiform discharges appeared in six patients (35%). The demographic and clinical data of the cohort were analysed (Tab. 1).
Tab. 1. Demographic data of relapsing-remitting MS patients with epilepsy. 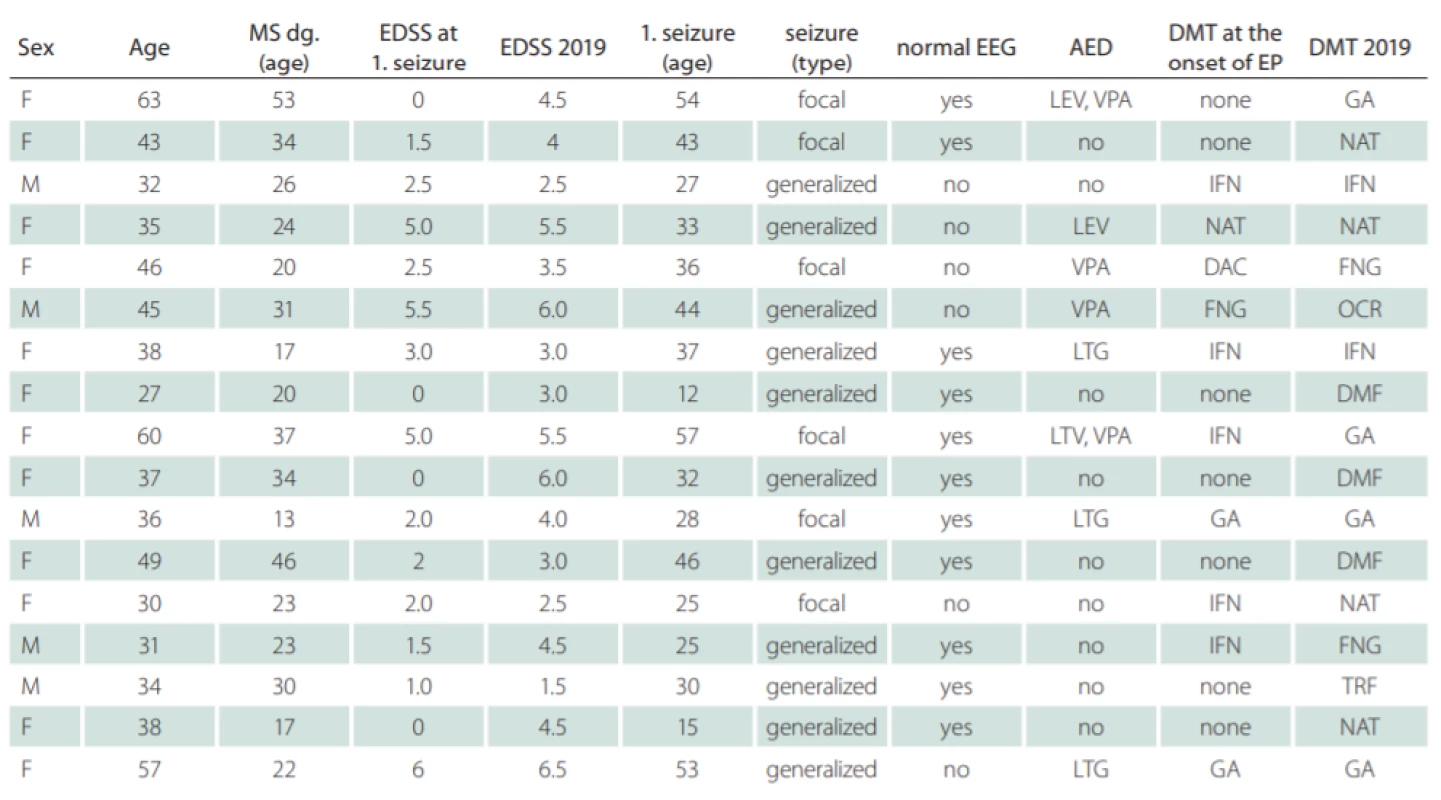
AED – antiepileptic drug; DAC – daclizumab; DMF – dimethyl fumarate; DMT – disease-modifying therapy; EDSS – Expanded Disability Status Scale; EEG – electroencephalogram; EP – epilepsy; F – female; FNG – fi ngolimod; GA – glatiramer acetate; IFN – interferon beta; LEV – levetiracetam; LTG – lamotrigine; M – male; NAT – natalizumab; OCR – ocrelizumab; TRF – terifl unomide; VPA – valporate Eight out of 14 patients (57%) exhibited new T2-lesions on brain MRI findings after the first seizure. However, detailed analysis of MRI finding was not possible because of the retrospectivity of this study. The determination of the number or extent of cortical lesions was not possible due to a change in the MRI protocol over the follow-up and inability to detect reliable cortical lesions in older findings.
Discussion
The frequency of unprovoked seizure or epilepsy among RRMS patients in this cohort was 2.27%, which is higher than in the general population [9]. The frequency of focal epilepsy was 47%. There remains a possibility that patients with generalized seizure and normal EEG had a focal onset of seizure with a focal-to-bilateral tonic-clonic seizure; the frequency of focal epilepsy may, therefore, be underestimated. These findings are in agreement with international reports. There was found a non-significant trend towards an increase in ARR in patients with epilepsy at 2 years before and after the first seizure. No significant difference in the mean EDSS was found 2 years before and after the first seizure.
The accuracy of the diagnosis of epilepsy may, however, give cause for concern, especially among MS patients in whom there may be a range of paroxysmal symptoms such as muscle cramps, dystonia and other involuntary movements.
Furthermore, the high frequency of focal epilepsy disclosed supports the proposition that localized MS pathology lies behind comorbid epilepsy. MS patients with epilepsy have a higher prevalence of cortical lesions than patients without seizures in their case histories [6,10].
Coincidental occurrence of epilepsy and MS could account for some of the cases. It is also possible that several of these patients suffer from idiopathic epilepsy, clinically triggered in a nonspecific way by the cerebral metabolic disturbance resulting from about MS. It has been argued that acute symptomatic seizures are caused by new MS lesions and that chronic epilepsy is unrelated to new attacks of the disease, but rather to the epileptogenic effect of chronic cortical plaques. Cortical lesions are also an independent predictor of the conversion from a clinically isolated syndrome to MS [11].
Neuroinflammation plays an important role in the pathogenesis of MS but is also commonly activated in epileptogenic brain regions in humans and is clearly involved in animal models of epilepsy [12]. Thus, neuroinflammation could serve as a possible link from MS to epilepsy and explain the development of seizures in a subset of MS patients.
While MS lesions in the white matter are readily visualized by MRI, conventional MRI techniques are of low sensitivity in the detection of gray-matter MS pathology, a drawback that hinders accurate assessment of the total lesion burden. Newer MRI sequences such as double inversion recovery and phase-sensitive inversion recovery are 1.5–5 times more sensitive than conventional MRI sequences in the detection of cortical lesions [13].
The findings could have important implications for clinicians since there exists a need to be aware of the risk of epilepsy in people with MS. The clinician may, in particular, wish to consider whether the patient displays any other risk factors for epilepsy, such as medication and/or alcohol abuse, which might potentially exacerbate the risk. Because of the possibility of new seizures and status epilepticus, attention has been drawn to the importance of early use of antiepileptic drugs in MS patients with epilepsy. Clinicians should also be aware of the hypothetical proconvulsive properties of interferon-beta arising out of metabolic interference with antiepileptic drugs or caused by direct neurotoxic effects [14].
Some progression on MRI was found after the first seizure in 57% patients. However, detailed analysis of MRI findings was not possible because of the retrospectivity of the study. Due to a change in the MRI protocol over the follow-up, determination of the number or extent of cortical lesions was not possible. The retrospective study has its limits, but it is a good starting point for planning a prospective multicentre study.
Limitations
This is a single centre cohort retrospective study. It was not possible to use the ILAE classification 2017 accurately due to the retrospectivity of the study. No recent information about the accurate prevalence of epilepsy in our region was found. A small cohort of patients exhibits epilepsy in our study. Detailed analysis of MRI findings was not possible. It was not possible to compare patients with and without epilepsy in the framework of/connection with demographic data.
Ethics approval
The research was approved by the local ethics committees (FN Brno; 04-170419/EK; 17.4.2019).
Acknowledgment
Supported by the Ministry of Health of the Czech Republic. Grant ref.: MH CZ – DRO, FNBr, 65269705. Tony Long (Svinosice) helped work up the English.
Conflict of interest
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Accepted for review: 8. 1. 2020
Accepted for print: 16. 6. 2020
MUDr. Jan Kočica
Department of Neurology Faculty of Medicine Masaryk University and University Hospital
Jihlavská 340/20
625 00 Brno
e-mail: kocica.jan@fnbrno.cz
Zdroje
1. Geurts JJ, Bö L, Pouwels PJ et al. Cortical lesions in multiple sclerosis: combined postmortem MR imaging and histopathology. AJNR Am J Neuroradiol 2005; 26 (3): 572–577.
2. Dagiasi I, Vall V, Kumlien E et al. Treatment of epilepsy in multiple sclerosis. Seizure 2018; 58 : 47–51. doi: 10.1016/j.seizure.2018.04.001.
3. Chou IJ, Kuo CF, Tanasescu R et al. Epilepsy and associated mortality in patients with multiple sclerosis. Eur J Neurol 2019; 26 (2): 342–e23. doi: 10.1111/ene.13821.
4. Burman J, Zelano J. Epilepsy in multiple sclerosis: a nationwide population-based register study. Neurology 2017; 89 (24): 2462–2468. doi: 10.1212/WNL.00000000 00004740.
5. Benjaminsen E, Myhr KM, Alstadhaug KB. The prevalence and characteristics of epilepsy in patients with multiple sclerosis in Nordland county, Norway. Seizure 2017; 52 : 131–135. doi: 10.1016/j.seizure.2017.09. 022.
6. Gasparini S, Ferlazzo E, Ascoli M et al. Epilepsy Study Group of the Italian Neurological Society: risk factors for unprovoked epileptic seizures in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci 2017; 38 (3): 399–406. doi: 10.1007/s10072-016-2803-7.
7. Martınez-Lapiscina EH, Ayuso T, Lacruz F et al. Cortico-juxtacortical involvement increases risk of epileptic seizures in multiple sclerosis. Acta Neurol Scand 2013; 128 : 24–31. doi: 10.1111/ane.12064.
8. Thompson A, Banwell B, Barkoff F. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald Criteria. Lancet Neurol 2018; 17 (2): 162–173. doi: 10.1016/S1474-4422 (17) 30470-2.
14. Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: a systematic review and meta--analysis of international studies. Neurology 2017; 88 (3): 296–303. doi: 10.1212/WNL.0000000000003 509.
10. Calabrese M, Grossi P, Favaretto A et al. Cortical pathology in multiple sclerosis patients with epilepsy: a 3-year longitudinal study. J Neurol Neurosurg Psychiatry 2012; 83 : 49–54. doi: 10.1136/jnnp-2011-300414.
11. Filippi M, Rocca MA, Calabrese M et al. Intracortical lesions: relevance for new MRI diagnostic criteria for multiple sclerosis. Neurology 2010; 75 : 1988–1994. doi: 10.1212/WNL.0b013e3181ff96f6.
12. Vezzani A, Balosso S, Ravizza T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 2019; 15 (8): 459–472. doi: 10.1038/s41582-019-0217-x.
13. Nelson F, Poonawalla AH, Hou P et al. Improved identification of intracortical lesions in multiple sclerosis with phase-sensitive inversion recovery in combination with fast double inversion recovery MR imaging. AJNR Am J Neuroradiol 2007; 28 : 1645–1649.
14. Walther EU, Hohlfeld R. Multiple sclerosis: side effects of interferon beta therapy and their management. Neurology 1999; 53 : 1622–1627.
Štítky
Dětská neurologie Neurochirurgie Neurologie
Článek EditorialČlánek Recenze knih
Článek vyšel v časopiseČeská a slovenská neurologie a neurochirurgie
Nejčtenější tento týden
2020 Číslo 4- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Magnosolv a jeho využití v neurologii
- Zolpidem může mít širší spektrum účinků, než jsme se doposud domnívali, a mnohdy i překvapivé
- Nejčastější nežádoucí účinky venlafaxinu během terapie odeznívají
-
Všechny články tohoto čísla
- Editorial
- Cytotoxic lesions of the corpus callosum (CLOCCs)
- Radial nerve injury associated with humeral shaft fracture
- It is evident when to make a surgery for lumbar disc herniation?
- Current diagnostics of secondary progressive form of multiple sclerosis and its treatment with siponimod
- Multiple sclerosis – behind the immunity curtains
- Airway clearance in patients with Parkinson‘s disease – overview and possibilities of physiotherapeutic intervention
- Clinical and social predictors of quality of life in children and young adults with autism spectrum disorder
- Safety of carotid endarterectomy in relation to the timing after ischemic stroke
- Glatirameracetate – the treatment of multiple sclerosis monitored in the ReMuS Registry
- Intensive computer-assisted cognitive rehabilitation in persons with multiple sclerosis – results of a 12-week randomized study
- Efficacy and safety of emergent microsurgical embolectomy in patients with acute ischemic stroke after the failure of intravenous thrombolysis and mechanical thrombectomy – a systematic review protocol
- Impact of the COVID-19 pandemic on sleep medicine in the Czech Republic and Slovakia
- The prevalence and characteristics of epilepsy in patients with relapsing-remitting multiple sclerosis treated with disease-modifying therapy
- Moyamoya syndrome associated with polycystic kidney disease – a rare case report and literature review
- Souběh dvou oportunních infekcí jako první projev HIV
- Dropped head syndrome in patient with progressive bulbar palsy
- Carotid body paraganglioma, a very rare pediatric tumor
- Využití kvantitativní MR venografie v indikaci stentingu stenózy žilního splavu
- Transvenous embolization of a ruptured brain arteriovenous malformation
- CGRP monoclonal antibodies in the treatment of migraine – indication criteria and therapeutic recommendations for the Czech Republic
- Recenze knih
- 2020 AAN Highlights Dlouhodobá data o účinnosti deplece CD20+ B-buněk v léčbě RS
- 2020 AAN Highlights Jak mění malá molekula průběh spinální svalové atrofie?
- Česká a slovenská neurologie a neurochirurgie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- It is evident when to make a surgery for lumbar disc herniation?
- CGRP monoclonal antibodies in the treatment of migraine – indication criteria and therapeutic recommendations for the Czech Republic
- Cytotoxic lesions of the corpus callosum (CLOCCs)
- Dropped head syndrome in patient with progressive bulbar palsy
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



