-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Ins and Outs of Rust Haustoria
article has not abstract
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004329
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004329Summary
article has not abstract
Rust diseases caused by fungi of the order Pucciniales afflict a wide range of plants, including cereals, legumes, ornamentals, and fruit trees, and pose a serious threat to cropping systems and global food security. The obligate parasitic lifestyle of these fungi and their complex life cycles, often involving alternate hosts for the sexual and asexual stages, also make this group of pathogens of great biological interest. One of the most remarkable adaptations of rust fungi is the specialized infection structure that underpins the sustained biotrophic association with hosts; the haustorium (Figure 1A and C). This organ forms after penetration of the wall of a live host cell, expanding on the inner side of the cell wall while invaginating the surrounding host plasma membrane (Figure 1C). Through haustoria, the pathogen derives nutrients from the host and secretes virulence proteins called effectors, which are believed to be the key players that manipulate the physiological and immune responses of host cells [1]–[4]. Analogous terminal feeding structures have independently evolved in other organisms such as the haustorium in powdery mildews (ascomycetes) and downy mildews (oomycetes, not true fungi), and the arbuscules in arbuscular mycorrhizae, suggesting that such architecture represents a successful adaptation of these organisms to interact with their respective host plants [5], [6].
Fig. 1. Schematic representation of the developmental phases of rust infection on wheat and example of macroscopic symptoms. 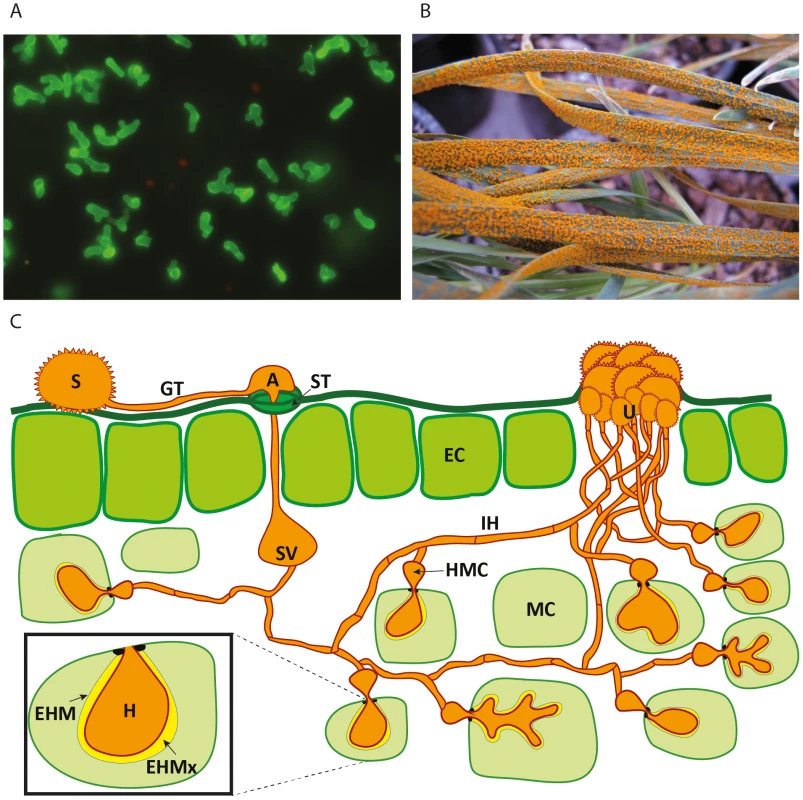
A) Confocal microscopy of isolated haustoria from Pst-wheat infected tissue [34] stained with the lectin Concanavalin A (which has affinity for α-D-mannosyl and α-D-glucosyl groups present in various sugars, glycoproteins, and glycolipids), conjugated to Alexa Fluor 488. B) Massive uredospore production at advanced stages of the stripe rust disease on wheat seedlings. C) Representation of the asexual cycle of Puccinia spp. on wheat. The dikaryotic uredospore (S) lands on the leaf surface and produces a germination tube (GT) within 6 hours. Subsequently, it produces an appresorium (A) over the stomatal aperture and enters to the leaf interior through the stoma (ST), where it differentiates into a substomatal vesicle (SV). Primary infection hyphae (IH) propagate through the leaf, and once in contact with mesophyll cells, haustorial mother cells (HMC) differentiate. These penetrate the host mesophyll cell (MC) wall to form the haustorium (H). The haustorium remains separated from the host cell cytoplasm by the extrahaustorial matrix (EHMx) and the host-derived extrahaustorial membrane (EHM). After the establishment of the first haustorium, secondary hyphae develop, colonize the intercellular spaces, and give rise to more HMCs and haustoria. The cycle is completed within 10–11 days, when the invasive hyphae form sporogenous basal cells in the uredia (U) and thousands of new infective uredospores erupt through the leaf epidermis. Rust Haustoria Possess a Specialised Metabolism
The primary disease-causing stage of the rust life cycle is the asexual stage. Dikaryotic uredospores germinate on the leaf surface and then colonize the leaf tissue to establish the biotrophic interaction, which can be very long-lasting (Figure 1C). Ultimately, the infection gives rise to sporulating pustules that release vast numbers of new spores that can repeat the infection cycle through the growing season (Figure 1B, C). Early ultrastructural studies of dikaryotic rust infection processes showed that haustorium formation begins when a haustorial mother cell (HMC) (Figure 1C) differentiates from intercellular hyphae by laying down a septum near the hyphal tip [7]. During haustorium formation, the cytoplasmic contents of the HMC, including the two haploid nuclei, migrate into the haustorium through the neck structure, leaving the HMC enucleate and highly vacuolated. The HMC septum undergoes complex changes during host wall penetration and haustorial maturation, including occlusion of the central pore, thereby preventing continuity of the cytoplasmic contents throughout the hyphae [7]. Thus, the HMC and haustorium are separated from the hyphae, which may aid the development of independent transcriptional and metabolic programs in these cells.
The ability to purify rust fungi haustoria from infected plant tissue (Figure 1A) enabled the first analysis of haustorial gene expression, conducted in the bean rust fungus Uromyces fabae (Uf) [8]. This work identified several genes apparently involved in nutrient acquisition, including genes encoding a hexose transporter, HXT1 [2], and three amino acid transporters, AAT1, AAT2, and AAT3 [1], [9], [10]. Immunolocalization studies showed the exclusive localization of HXT1 and AAT2 at the haustorial plasma membrane [1], [2], while biochemical studies revealed that AAT1 and AAT3 function as proton-dependent transporters with preference for histidine/lysine and leucine/methionine/cysteine respectively [9], [10]. These studies provided the first compelling evidence for involvement of haustoria in nutrient uptake.
Since then, the emergence of high-throughput DNA and mRNA sequencing has greatly increased our understanding of the metabolic function of the haustorium. For instance, the transcriptomic analysis of isolated haustoria from wheat stripe rust Puccinia striiformis f. sp tritici (Pst), indicated that they are active in uptake of sugar, amino acids, nitrogen, and other nutrients through high expression of transmembrane transporters, and also in incorporation of these nutrients into biosynthetic and energy-manufacturing pathways for their utilization in fungal development [11]. This is in contrast to germinating stripe rust spores, which show expression patterns consistent with acquisition of energy from stored compounds. The haustorial transcriptomes from other rusts, such as the common bean and soybean rust pathogens Uromyces appendiculatus and Phakopsora pachyrhizi [12], and the wheat stem rust Puccinia graminis f. sp tritici (Pgt) [13], [14], show similar expression patterns, suggesting that rust haustoria share similar mechanisms to exploit host-derived resources. The four sequenced rust genomes, including the Puccinia species, Pgt and Pst [13], [15], [16], and the two Melampsora species, Melampsora larici-populina and Melampsora lini [13], [17], lack genes encoding key assimilatory enzymes for inorganic nitrate and sulphur, suggesting that rust pathogens obtain the reduced versions of these nutrients from plant cells.
Haustoria Produce and Deliver Effectors to the Host Cytoplasm
Seminal studies on the bean and flax rust pathogens provide support for the idea that haustoria of rust fungi are responsible for the production and secretion of effectors, with a number of these proteins targeted to the host cytoplasm where they are thought to promote the infection. Rust transferred protein 1 (RTP1) from Uf and its homologue from Uromyces striatus were the first such proteins proven to be expressed specifically in the haustorium and transferred to the host cytoplasm during a compatible biotrophic interaction [8], [18]. RTP1 shares similarity with cysteine protease inhibitors and can inhibit proteolytic activity in yeast culture supernatants, so may act to inhibit host defence-associated proteases [19]. It can also form aggregates and filamentous-like structures inside the extrahaustorial matrix and the host cytoplasm, which may have a structural role in stabilizing the host cell allowing accommodation of the haustorium [20]. Emerging transcriptomic and genomic data from a range of rust fungi have identified RTP1 homologues in at least 13 rust species, suggesting that this protein could play an important role in the biotrophic lifestyle [19].
Four avirulence (Avr) genes, which encode effectors that are recognised by immune receptors encoded by host resistance (R) genes, have been identified in the flax rust M. lini [3], [21], [22]. All encode small secreted proteins that are expressed in haustoria and are recognised in the host cytoplasm, implying that these proteins are delivered into the host cell during infection. This was confirmed by direct visualisation of the effector AvrM inside infected flax cells [23]. This study also found evidence that at least some effectors can be taken up into the host cytosol independently of a specialized pathogen delivery system, since secreted AvrM and AvrL567 expressed by tobacco cells accumulated in the cytosol despite being targeted efficiently to the plant secretory system. Structural and functional studies of AvrM revealed a dimeric protein with intrinsic membrane-binding properties, which possesses a conserved hydrophobic surface patch required for pathogen-independent internalization [23], [24]. Although AvrM can bind negatively-charged phospholipids, this is not essential for its transport across the plant plasma membrane [24]. Overall, the mechanisms of effector delivery from rusts and other filamentous pathogens remain unknown and are the subject of much debate [5], [6]
Rust Haustoria Express Many Effector Candidates
The characterisation of RTP1 and Avr proteins implied the existence of a class of rust effectors delivered into host cells from haustoria, some of which could become targets of recognition by immune receptors. Over 30 Avr specificities have been described in flax rust, and around 50 in each of Pgt, Pst, and Puccinia triticina, suggesting large families of such effectors. Indeed, genomic and transcriptomic studies on rust fungi have revealed large sets (500 to 1,500) of potential effector genes. In contrast to effectors in some other filamentous plant pathogens, such as the RxLR and crinkler class effectors of oomycetes [5], no conserved amino acid motifs are widely present in these proteins [16], [25]. In the absence of defined and conserved hallmarks in the sequences of effector genes, their prediction has been based on three main criteria: (1) presence of a secretion signal, (2) lack of transmembrane domains, and (3) expression in haustoria or infected tissue. For example, in the genomes of Pgt, Pst, M. larici-populina, and M. lini [13], [15], [17], about 8% of their predicted proteomes corresponds to candidate effectors that fulfil these criteria. Infection tissue-specific transcriptomes of these pathogens [11], [13], [26] and other rusts, including Uf [27], have identified large numbers of predicted effectors expressed in planta. More recently, haustoria-specific transcriptomic data detected expression of 70% of the predicted in planta effector complement in the haustorium of Pst (Jackson, et al. unpublished) and 58% in Pgt [14], lending additional support to the idea that the haustorium is the main source of effector proteins. Sperschneider, et al. (2014) [28] used an alternative, unbiased approach for effector prediction based on the comparison of 174 fungal genomes and the classification of genes into families associated with pathogenicity. This study revealed a cluster of proteins enriched in secretion signals, small amino acids and cysteine residues, confirming that these are useful criteria for effector prediction. The generation of lists of candidate effectors is an important first step that precedes functional assays to uncover their contributions to pathogenicity.
Evolutionarily Diverged Effector Candidates May Control Host Specificity
Avirulence genes often exhibit high levels of polymorphism and display signatures of diversifying selection [3], [22], [29] as a result of antagonistic co-evolution with plant defences. For instance, positively selected polymorphic residues in AvrL567 are exposed on the protein surface and are responsible for differences in recognition specificity by host immune receptors [30], explaining the underlying molecular basis driving diversifying selection of this gene family to escape recognition. Likewise, AvrM is recognised by direct interaction with the corresponding M resistance protein, and differences in recognition are governed by surface-exposed polymorphic residues [24], [31]. Effectors are probably also under selection to adapt to alterations in host proteins targeted by their virulence functions or to acquire new virulence targets. Comparison of effector complements from multiple rust species [17], [25] reveals some families that are widely conserved and are enriched for proteins with signatures of enzyme activity that may play general roles in virulence, e.g., as cell wall–degrading enzymes. In contrast, many candidate effectors are not conserved across genus or species boundaries [16], [17] and can be highly variable between isolates of the same species [14], [32]. This class includes known Avr proteins from flax rust and is likely to be enriched for such determinants of host specificity.
Conclusion
The use of modern technologies to study the highly specialised dikaryotic haustorium of rust fungi has provided convincing support of the early idea that it comprises a feeding apparatus that allows the pathogen to parasitise the host. The intimate and long-lasting relationship between pathogen and plant also demands that the host immune system is dampened or disabled. Both of these functions are likely to be dependent upon the secretion of effector proteins that condition the host to accommodate the infection. Although the availability of genomes and transcriptomes of rust fungi have helped to uncover their effector coding potential, precise roles for effectors during infection is an unexplored frontier with great potential to define fascinating new aspects of biology. Thus, the development of systems to screen candidate effectors for their role in disease [33] will expand our understanding of these important proteins and increase the options to control rust pathogenic fungi.
Zdroje
1. MendgenK, StruckC, VoegeleRT, HahnM (2000) Biotrophy and rust haustoria. Physiol Mol Plant Pathol 56 : 141–145.
2. VoegeleRT, StruckC, HahnM, MendgenK (2001) The role of haustoria in sugar supply during infection of broad bean by the rust fungus Uromyces fabae. Proc Natl Acad Sci U S A 98 : 8133–8138.
3. CatanzaritiAM, DoddsPN, LawrenceGJ, AyliffeMA, EllisJG (2006) Haustorially expressed secreted proteins from flax rust are highly enriched for avirulence elicitors. Plant Cell 18 : 243–256.
4. VoegeleRT, MendgenK (2003) Rust haustoria: nutrient uptake and beyond. New Phytol 159 : 93–100.
5. BozkurtTO, SchornackS, BanfieldMJ, KamounS (2012) Oomycetes, effectors, and all that jazz. Curr Opin Plant Biol 15 : 483–492.
6. RafiqiM, EllisJG, LudowiciVA, HardhamAR, DoddsPN (2012) Challenges and progress towards understanding the role of effectors in plant-fungal interactions. Curr Opin Plant Biol 15 : 477–482.
7. Littlefield LJ, Heath MC (1979) Ultrastructure of rust fungi. New York: Academic Press.
8. HahnM, MendgenK (1997) Characterization of in planta induced rust genes isolated from a haustorium-specific cDNA library. Mol Plant Microbe Interact 10 : 427–437.
9. StruckC, ErnstM, HahnM (2002) Characterization of a developmentally regulated amino acid transporter (AAT1p) of the rust fungus Uromyces fabae. Mol Plant Pathol 3 : 23–30.
10. StruckC, MuellerE, MartinH, LohausG (2004) The Uromyces fabae UfAAT3 gene encodes a general amino acid permease that prefers uptake of in planta scarce amino acids. Mol Plant Pathol 5 : 183–189.
11. GarnicaDP, UpadhyayaNM, DoddsPN, RathjenJP (2013) Strategies for Wheat Stripe Rust Pathogenicity Identified by Transcriptome Sequencing. Plos One 8: e67150.
12. LinkTI, LangP, SchefflerBE, DukeMV, GrahamMA, et al. (2013) The haustorial transcriptomes of Uromyces appendiculatus and Phakopsora pachyrhizi and their candidate effector families. Mol Plant Pathol 15 : 379–393.
13. DuplessisS, CuomoCA, LinYC, AertsA, TisserantE, et al. (2011) Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc Natl Acad Sci U S A 108 : 9166–9171.
14. UpadhyayaNM, GarnicaDP, KaraogluH, NemriA, SperschneiderJ, et al. (2014) Comparative genomics of Australian stem rust (Puccinia graminis f. sp. tritici) isolates reveals extensive polymorphism in candidate effector genes. Front Plant Sci In press.
15. CantuD, GovindarajuluM, KozikA, WangM, ChenX, et al. (2011) Next generation sequencing provides rapid access to the genome of Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust. PLoS ONE 6: e24230.
16. ZhengW, HuangL, HuangJ, WangX, ChenX, et al. (2013) High genome heterozygosity and endemic genetic recombination in the wheat stripe rust fungus. Nat Commun 4 : 2673.
17. NemriA, SaundersDGO, AndersonC, UpadhyayaNM, WinJ, et al. (2014) The genome sequence and effector complement of the flax rust pathogen Melampsora lini. Front Plant Sci 5 : 98.
18. KemenE, KemenAC, RafiqiM, HempelU, MendgenK, et al. (2005) Identification of a protein from rust fungi transferred from haustoria into infected plant cells. Mol Plant Microbe Interact 18 : 1130–1139.
19. PretschK, KemenA, KemenE, GeigerM, MendgenK, et al. (2013) The rust transferred proteins-a new family of effector proteins exhibiting protease inhibitor function. Mol Plant Pathol 14 : 96–107.
20. KemenE, KemenA, EhlersA, VoegeleR, MendgenK (2013) A novel structural effector from rust fungi is capable of fibril formation. Plant J 75 : 767–780.
21. DoddsPN, LawrenceGJ, CatanzaritiAM, AyliffeMA, EllisJG (2004) The Melampsora lini AvrL567 avirulence genes are expressed in haustoria and their products are recognized inside plant cells. Plant Cell 16 : 755–768.
22. BarrettLG, ThrallPH, DoddsPN, van der MerweM, LindeCC, et al. (2009) Diversity and evolution of effector loci in natural populations of the plant pathogen Melampsora lini. Mol Biol Evol 26 : 2499–2513.
23. RafiqiM, GanP, RavensdaleM, LawrenceG, EllisJ, et al. (2010) Internalization of flax rust avirulence proteins into flax and tobacco cells can occur in the absence of the pathogen. Plant Cell 22 : 2017–2032.
24. VeT, WilliamsSJ, CatanzaritiAM, RafiqiM, RahmanM, et al. (2013) Structures of the flax-rust effector AvrM reveal insights into the molecular basis of plant-cell entry and effector-triggered immunity. Proc Nat Acad Sci U S A 110 : 17594–17599.
25. SaundersDGO, WinJ, CanoLM, SzaboLJ, KamounS, et al. (2012) Using Hierarchical Clustering of Secreted Protein Families to Classify and Rank Candidate Effectors of Rust Fungi. PLoS ONE 7: e29847.
26. CantuD, SegoviaV, MacleanD, BaylesR, ChenX, et al. (2013) Genome analyses of the wheat yellow (stripe) rust pathogen Puccinia striiformis f. sp. tritici reveal polymorphic and haustorial expressed secreted proteins as candidate effectors. BMC Genomics 14 : 270.
27. LinkTI, VoegeleRT (2008) Secreted proteins of Uromyces fabae: similarities and stage specificity. Mol Plant Pathol 9 : 59–66.
28. SperschneiderJ, YingE, DoddsPN, UpadhyayaNM, GardinerDM, et al. (2014) Adaptative Evolution in Expanded Pathogen-Associated, Effector-like Gene Families in the Stem Rust Fungus. Front Plant Sci 5 : 372.
29. DoddsPN, LawrenceGJ, CatanzaritiAM, TehT, WangCIA, et al. (2006) Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc Nat Acad Sci U S A 103 : 8888–8893.
30. WangCIA, GuncarG, ForwoodJK, TehT, CatanzaritiAM, et al. (2007) Crystal structures of flax rust avirulence proteins AvrL567-A and -D reveal details of the structural basis for flax disease resistance specificity. Plant Cell 19 : 2898–2912.
31. CatanzaritiAM, DoddsPN, VeT, KobeB, EllisJG, et al. (2010) The AvrM Effector from Flax Rust Has a Structured C-Terminal Domain and Interacts Directly with the M Resistance Protein. Mol Plant Microbe Interact 23 : 49–57.
32. BruceM, NeugebauerKA, JolyDL, MigeonP, CuomoCA, et al. (2014) Using transcription of six Puccinia triticina races to identify the effective secretome during infection of wheat. Front Plant Sci 4 : 520.
33. UpadhyayaNM, MagoR, StaskawiczBJ, AyliffeMA, EllisJG, et al. (2014) A Bacterial Type III Secretion Assay for Delivery of Fungal Effector Proteins into Wheat. Mol Plant Microbe Interact 27 : 255–264.
34. GarnicaDP, RathjenJP (2014) Purification of fungal haustoria from infected plant tissue by flow cytometry. Methods Mol Biol 1127 : 103–110.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



