-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
Malaria, caused by a protozoa parasite, Plasmodium, affects more than 200 million people per year. The infection triggers an acute febrile illness, the paroxysms, occurring every 48 or 72 hours depending on the species. Plasmodium vivax, in most cases, does not cause severe malaria, but it is the most geographically widespread parasite responsible for human disease and causes substantial costs to individuals and governments. Once the parasite reaches the blood stream, they infect reticulocytes that can be destroyed by phagocytes. Our goal was to assess the importance of monocyte subsets during malaria. We found that P. vivax infection causes an increase in frequency of circulating monocytes, which were defined as classical, inflammatory, and patrolling, based on the expression of membrane molecules. Classical and inflammatory monocytes produced higher levels of pro-inflammatory cytokines and were distinguished from patrolling monocytes by displaying larger and more active mitochondria. Importantly, inflammatory monocytes were more efficient phagocytes; produced high levels of intracellular reactive oxygen species and TNF and consequently control better Plasmodium vivax infection. Hence, our results support the hypothesis that CD14+CD16+ monocytes display effector functions involved in parasite control during malaria.
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004393
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004393Summary
Malaria, caused by a protozoa parasite, Plasmodium, affects more than 200 million people per year. The infection triggers an acute febrile illness, the paroxysms, occurring every 48 or 72 hours depending on the species. Plasmodium vivax, in most cases, does not cause severe malaria, but it is the most geographically widespread parasite responsible for human disease and causes substantial costs to individuals and governments. Once the parasite reaches the blood stream, they infect reticulocytes that can be destroyed by phagocytes. Our goal was to assess the importance of monocyte subsets during malaria. We found that P. vivax infection causes an increase in frequency of circulating monocytes, which were defined as classical, inflammatory, and patrolling, based on the expression of membrane molecules. Classical and inflammatory monocytes produced higher levels of pro-inflammatory cytokines and were distinguished from patrolling monocytes by displaying larger and more active mitochondria. Importantly, inflammatory monocytes were more efficient phagocytes; produced high levels of intracellular reactive oxygen species and TNF and consequently control better Plasmodium vivax infection. Hence, our results support the hypothesis that CD14+CD16+ monocytes display effector functions involved in parasite control during malaria.
Introduction
Plasmodium vivax is the most widely distributed malaria parasite and responsible for approximately 70–80 million cases, annually. In addition, P. vivax is responsible for the majority of malaria cases and represents a significant impediment to social and economic development in Latin America and Asia [1]. Both innate and acquired immunity are thought to play critical role in host resistance to infection and pathogenesis of malaria [2], [3]. However, the mechanisms by which the innate immune response mediate resistance to Plasmodium infection or promote a deleterious systemic inflammation associated with malaria sepsis are poorly understood [2]. This is particularly true in the case of P. vivax malaria [4].
The blood is the main tissue affected during P. vivax malaria since sequestration is not a central event in this infection. When parasitized reticulocytes rupture in the blood stream, parasite components are sensed by the innate immune receptors and activate monocytes [5]. The innate immune system recognizes Plasmodium sp. through different pattern-recognition receptors expressed by monocytes and initiates a broad spectrum of defense mechanisms [6], [7], [8], [9], [10]. Importantly, the same immune mediators involved in host resistance, such as pro-inflammatory cytokines are also thought to mediate pathology during acute malaria episodes [8], [11], [12]. However, the full spectrum of monocyte subsets and the specific functions of each monocyte population during malaria have not been defined.
Besides supplying peripheral tissues with macrophage and dendritic cell (DC) precursors, monocytes contribute directly to immune defense against microbial pathogens [13], [14], [15]. Monocytes were initially identified by their expression of large amounts of CD14 [16], [17]. However, recent studies have revealed that monocytes in human peripheral blood are heterogeneous and can be divided into three distinct subsets described based on their expression of phenotypic markers. These cells are referred to as, classical (CD14+CD16− cells), inflammatory or intermediate (CD14+CD16+) and patrolling (CD14loCD16+) monocytes [18], [19]. Given the importance of monocytes as a major source of pro-inflammatory cytokines and potential effector cells during malaria, in this study, we have attempted to define the role of the various monocyte subsets during P. vivax infection. To address this question, we phenotypically and functionally characterized the classical, inflammatory, and patrolling monocytes present in the peripheral blood from patients experiencing acute malaria episodes.
We demonstrate that the frequency of circulating monocytes is elevated during acute infection with P. vivax and that the classical and inflammatory monocyte subsets are the primary source of pro-inflammatory cytokines. Importantly, we found that CD16+ cells, and in particular the CD14+CD16+ LFA-1highICAM-1highPECAM-1high monocytes display augmented effector functions such as phagocytosis and intracellular reactive oxygen species production and are thus likely to be an important cell subset controlling parasitemia, and mediating host resistance to P. vivax infection.
Methods
Patients
A total of 35 P. vivax-infected patients with uncomplicated malaria were enrolled in this study at Centro de Pesquisa de Medicina Tropical de Rondônia (CEPEM) in Porto Velho, Rondônia, a malaria endemic area in the Amazon region of Brazil. According to the World Health Organization, uncomplicated malaria is a symptomatic infection with malaria parasitemia without signs of severity and/or evidence of vital organ dysfunction. Up to 100 ml of peripheral blood was collected immediately after confirmation of P. vivax infection by thick blood smear film and again 30–45 days after chemotherapy (n = 35, ranging from 18 to 66 years old [35±9.5]) (Table S1). Additional 36 P. vivax-infected patients were enrolled for reticulocyte analysis: percentage: mean: 1.383, SD: 0.655; median: 1.150, IQR: 0.925–1.650; absolute numbers: mean: 51,779, SD: 18,390; median: 48,100, IQR: 38,970–59,520. Patients were treated for 3 days with chloroquine followed by 7 days with primaquine according to the Brazilian Ministry of Health. P. vivax infection and parasitological cure were confirmed by thick blood smear film and polymerase chain reaction (PCR) [20]. Identification of the three species of human malaria parasites was done by nested PCR that targets variant sequences in the small subunit rRNA gene. The clinical manifestations of acute malaria were fever, myalgia, chills, arthralgia, nausea, vomiting or diarrhea. Peripheral blood was also collected from 15 healthy donors (HD) ranging from 21 to 56 years old [32±8] living in Porto Velho and negative for P. vivax infection.
Ethics statement
These studies were performed under protocols reviewed and approved by the Ethical Committees on Human Experimentation from Centro de Pesquisa em Medicina Tropical de Rondônia (CEP-CEPEM 095/2009) and Centro de Pesquisas René Rachou, Fundação Oswaldo Cruz (CEP-CPqRR 2004), the National Ethical Committee (CONEP 15652) from Ministry of Health, Brazil, as well as by the Institutional Review Board from the University of Massachusetts Medical school. Only adults, 18 years old, were enrolled in the study. All patients enrolled in this study provided written informed consent.
Cellular immunophenotyping
Peripheral blood mononuclear cells (PBMC) were prepared from heparinized venous blood of adult volunteers by Ficoll-Hypaque density gradient centrifugation (GE Healthcare Life Sciences). Cells were stained for surface molecules for 15 minutes at room temperature. Subsequently, PBMC were washed with FACS buffer (PBS and 2%FBS), and fixed and permeabilized according to the manufacture's instruction (Cytofix/Cytoperm, BD Biosciences). Cells were then stained for the intracellular antigens. Cells were finally suspended and maintained in 200 µl of PBS 2% paraformaldehyde (Sigma Aldrich). At least 100,000-gated events were acquired for analysis using FACSCan upgraded with a second laser (5 colors) with Cellquest Pro and Rainbow from Cytek or LSR II with Diva (both from BD Biosciences). The antibody panels included the following: anti-CD31 (clone WM59)-FITC, anti-CD11a (LFA-1) (clone HI111)-FITC, anti-HLA-DR (clone LN3)-PE, anti-CD14 (clone 61D3)-APC, anti-CD62L (clone DREG56)-APC, anti-HLA-DR (clone LN3)-efluor 780, anti-CX3CR1 (clone 2A9-1)-biotin, anti-CD106 (VCAM-1) (clone STA)-biotin and streptavidin-PE, all purchased from eBioscience; anti-CD54 (ICAM-1) (clone HA58)-PE, anti-TNF-α (clone MAb11)-PE, anti-IL-6 (clone MQ2-13A5)-PE, anti-CD197 (CCR7) (clone 3D12)-PECy7 and anti-CD16 (clone 3G8)-PercPCy5.5, purchased from BD Biosciences; anti-CCR2 (clone 48607)-PE, purchased from R&D Systems. Data were analyzed using FlowJo Version 9.3.2 (TreeStar). A forward scatter height (FSC-H) and a side scatter height (SSC-H) gate were used to initially remove debris and to capture leucocytes. CD14 versus SSC-H gate was then used to select monocytes. An additional HLA-DR versus CD16 gate was performed to exclude possible contamination by neutrophils (CD16+HLA-DR−). A more detailed analysis of monocyte subpopulations was done based on CD14 and CD16 expression and here designated as: classical monocytes (CD14+CD16−), inflammatory monocytes (CD14+CD16+), and patrolling monocytes (CD14l°CD16+). The activation/cell presentation molecule, HLA-DR; the cellular adhesion molecules, VCAM-1, ICAM-1, PECAM-1, and LFA-1; the chemokine receptors, CCR2, CX3CR1 and CCR7; and the cytokine TNF-α was analyzed within CD14+ cells and also within each monocyte subpopulation. Data is shown in frequencies or mean fluorescence intensity (MFI). In the latter, when graphs are overlaid, the y-axis is left on automatic scaling and the axis represents % of Maximum. This normalization is used because different numbers of events is collected for the monocyte subsets analyzed and allow us to focus on the important and relevant variations between the levels of expression of different markers on the x-axis.
Cytokine measurements
IL-6, IL-8 and IL-10 were measured in cryopreserved plasma using the Cytometric Bead Array kit (CBA, BD Biosciences Pharmingen) as recommended by the manufacturer. The concentration of cytokines in each sample was calculated using the BD FCAP Array Software v 1.0.1 (BD Biosciences).
P. vivax-parasitized reticulocytes enrichment and staining
The red blood cells pellet from the Ficoll-Hypaque (GE Healthcare Life Sciences) density gradient centrifugation was harvested and washed three times and then resuspended in RPMI 1640 medium (Sigma Aldrich) to a final hematocrit of 10%. Five milliliters of this suspension was overlaid on a 5 mL 45% Percoll solution in a 15 mL tube. After centrifugation, floating mature Pv-reticulocytes (Pv-Ret) were collected and resuspended in RPMI 1640 [21], [22]. The enrichment of Pv-Ret was assessed by optic microscopy and a purity of 95% was obtained. Where indicated, enriched Pv-Ret were stained with 1 µM carboxyfluorescein succinimidyl ester (CFSE) at 1×106 cells/mL for 8 minutes at room temperature before phagocytosis assays (Molecular Probes-Invitrogen). After stained Pv-Ret were washed three times in RPMI and 10%FBS.
Expression of NADPH subunits by monocyte subsets
P47phox and p67phox expression was assessed by incubating PBMC in suspension at 37°C in complete RPMI 1640 supplemented with 2 µM glutamine, 10 mM HEPES and 50 µM 2-ME for 3 hours in medium alone and with P. vivax-Ret (0.5 Pv-Ret/PBMC) in the presence of 10% immune serum. PBMC were then washed with FACS buffer, stained for surface molecules for 15 minutes at room temperature, fixed and permeabilized according to the manufacture's instruction (Cytofix/Cytoperm, BD Biosciences).. Cells were then stained for the intracellular antigens (p47phox and p67phox) for 20 minutes, following additional 20 minutes incubation with anti-IgG1. After staining, plates were kept on ice for 15 minutes and cells were harvested with ice-cold PBS, 2.5 mM EDTA and maintained in 200 µl of PBS 2% paraformaldehyde. At least 100,000-gated events were acquired for analysis using a LSRFortessa (BD Biosciences). The antibody panels included the following: anti-CD16 (clone 3G8)-Alexa Fluor 700, anti-HLA-DR (Tu39)-FITC, anti-CD14 (clone M5E2)-APC, purified anti-phox47 (1/p47Phox) and anti-phox67 (D-6), and anti-IgG1 (A851)-PE. The MFI of each monocyte subpopulation expressing the NADPH subunits was determined by flow cytometry. Data were analyzed using FlowJo Version X 10.0.7.
Phagocytosis
Phagocytosis was assessed by incubating PBMC in suspension using 96-wells polystyrene plates at 37°C in complete RPMI 1640 supplemented with 10% heat-inactivated FCS, 2 µM glutamine, 10 mM HEPES, and 50 µM 2-ME for 1, 4 and 12 hours with P. vivax-Ret previously stained with CFSE (0.5 Pv-Ret/PBMC) in the absence of immune serum and in the presence of serum or inactivated serum. In some experiments monoclonal anti-CD11a (1 mg/mL) (clone G43-25B, BD), anti-CD31 (0.5 mg/mL) (clone WM59), anti-CD36 (0.5 mg/mL) (clone CB38) and anti-CD54 (1 mg/mL) (clone HA58) blocking antibodies were added in the cultures 30 minutes before the addition of P. vivax-Ret. After staining, plates were kept on ice for 15 minutes and cells were harvested with ice-cold PBS containing 2.5 mM EDTA. The frequencies of total monocytes and each monocyte subpopulation positive for CFSE were determined by flow cytometry and data were analyzed using FlowJo Version 9.3.2 (TreeStar).
ROS detection
To detect ROS production at the single cell level, the Image-iT LIVE Green Reactive Oxygen Species Detection kit (Invitrogen) was used following the manufacturer's instructions. Briefly, PBMC were washed twice with PBS and incubated in medium alone or with Pv-Ret (0.5 Pv-Ret/PBMC) or phorbol 12-myristate 13-acetate (PMA, 10 ng/mL) and ionomycin (500 ng/mL). Pre-warmed 25 µM 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) was added to the cells for 3 hours at 37°C. Mitochondrial ROS was assessed by MitoSox red mitochondrial superoxide indicator (Invitrogen) following the manufacturer's instructions. Briefly, PBMC were washed twice with HBSS and incubated in medium alone or with Pv-Ret (0.5 Pv-Ret/PBMC) or PMA (10 ng/mL) and ionomycin (500 ng/mL) and 10 µm MitoSox. After 30 minutes incubation, monoclonal antibodies against CD14 and CD16 were added to cell cultures to allow monocyte subpopulations analysis. Cell suspension was washed after additional 30 minutes incubation with HBSS at 37°C. Plates were kept on ice for 15 minutes and cells were harvested with ice-cold PBS containing 2.5 mM EDTA. Cells were acquired by flow cytometry and data were analyzed using FlowJo Version 9.3.2.
Purification of monocyte subpopulations
After PBMC preparation CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes from HD and P. vivax-infected patients were sorted with a FACSAria II cell sorter (BD Biosciences), using the combination of antibodies described above. CD14+CD16−, CD14+CD16+ and CD14l°CD16+ monocytes were then collected and fixed with 2.5% buffered glutaraldehyde solution, 0.1 M, for electron microscopy or with RLT buffer (QIAGEN) supplemented with β-mercaptoethanol for mRNA detection and nanostring analysis as described below.
Electron microscopy
After FACS-sorting, cells were prepared as previously described [23], [24]. Briefly, cells were fixed in 2.5% buffered glutaraldehyde solution, 0.1 M, pH 7.2, 6 h, 8°C. Cells were then washed with the same buffer. The pellets were included in phosphate buffer, 4% agarose and left overnight at 4°C. Next, the cells were fixed in a mixture of 1% osmium tetroxide and 1.5% (w/v) potassium ferrocyanide, dehydrated in a graded series of ethanol solutions, infiltrated, and embedded in Araldite 502 (Electron Microscopy Sciences, Hatfield, PA, USA). After polymerization, thin sections were obtained using a diamond knife on a Sorvall MT-2B ultramicrotome (Dupont, Wilmington, DE, USA) and mounted on uncoated 200-mesh copper grids (Ted Pella, Inc., Redding, CA, USA). Sections were stained with 2% uranyl acetate and Reynolds lead citrate and then analyzed using transmission electron microscopy (EM 10A Zeiss).
mRNA detection
mRNA was assessed by nanostring analysis [25]. nCounter CodeSets were constructed for detecting selected human-specific genes. A total of 1×104 cells of each subset were lysed in RLT buffer (QIAGEN) supplemented with β-mercaptoethanol. This lysate was mixed with capture and reporter probes, hybridized to the Codeset for 16 hr and loaded onto the nCounter prep station, and then quantified with the nCounter Digital Analyzer. For side-by-side comparisons of nCounter experiments, data were normalized in two ways described previously [26]. Briefly, the first normalization was for small variations utilizing the internal positive controls that are present in each CodeSet. Then the samples were normalized with 7 housekeeping genes that were included in the CodeSet. The data was analyzed with n Solver software. The heatmap was constructed using log2 transformed data and the Tiger Multi Experiment Viewer software.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software, version 5.0. The results were analyzed using two-tailed paired t-test. Wilcoxon testing was used when data did not fit a Gaussian distribution. The results were analyzed using unpaired t-test when two groups were compared. Mann-Whitney (MW) test was used when a normality test failed. Analyses were also done between HD (represented in the graphs as dashed line) and patients after cure and no significant differences were found. The correlation analyses were performed using the Spearman's rank. Differences were considered to be statistically significant, when p≤0.05.
Results
P. vivax-infected patients display higher levels of plasma cytokines accompanied by increased frequencies of circulating monocytes
High levels of the pro-inflammatory cytokines, IL-6 and IL-8, and regulatory cytokine IL-10, were found in the circulation of P. vivax-infected patients before treatment initiation, when compared to the same patients after anti-malarial therapy (Figure 1A). While the cytokinemia of P. vivax-infected patients was significantly higher than individuals after cure, the absolute numbers of total leukocytes decreased with infection (Figure 1B). The frequencies of lymphocytes were lower, whereas the proportions of polymorphonuclear cells and monocytes were higher in symptomatic malaria patients. Monocyte frequencies were also assessed within PBMC by flow cytometry. The frequencies of CD14+ monocytes were significantly higher in P. vivax-infected patients than in the same individuals after treatment (Figure 1C).
Fig. 1. P. vivax-infected patients display higher levels of cytokines accompanied by increased frequencies of circulating monocytes. 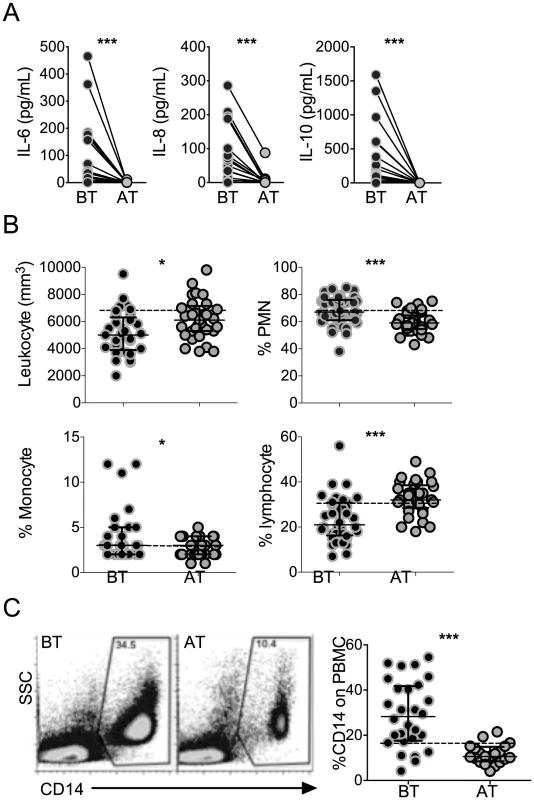
(A) IL-6, IL-8 and IL-10 were measured in plasma of P. vivax-infected individuals before (BT, black circles) and 30–45 days after (AT, grey circles) treatment (n = 20). Levels of cytokine were measured by Cytometric Bead Array. (B) Leukocyte counts and frequencies from P. vivax-infected individuals before (BT, black circles) and after (AT, grey circles) treatment were assessed at a clinical laboratory (n = 33). C) Representative density plots of CD14+ monocytes (left panel) and frequencies of CD14+ monocytes (right panel) within PBMCs from P. vivax-infected individuals (BT, n = 28 and AT, n = 20). Circles indicate individual patients and lines represent median values and interquartile ranges. Dotted lines represent medians of a given measurements from healthy donors. *p<0.05, ***p<0.01. P. vivax infection alters the expression of HLA-DR, adhesion molecules and chemokine receptors on circulating monocytes
The expression of the activation marker, HLA-DR, cell adhesion molecules, CD54, CD106, CD31, and chemokine receptors, CXCR3, CCR7, was analyzed on circulating monocytes (Figure 2). Significantly lower levels of HLA-DR were found on monocytes from P. vivax-infected patients when compared to the same patients after treatment (Figure 2). Monocytes from acute malaria patients also displayed significantly lower levels of the adhesion molecule CD31 and the chemokine receptor CCR7 (Figure 2). In contrast, significantly higher expression of the adhesion molecules CD106 (VCAM-1), CD54 (ICAM-1), and the chemokine receptor CX3CR1, were observed on monocytes from acute malaria patients before treatment initiation (Figure 2). The expression of all these molecules on monocytes from malaria patients reached the levels found in healthy donors when analyzed 30 days after treatment. Thus, monocytes from P. vivax-infected patients exhibit a distinct activation state during acute infection.
Fig. 2. P. vivax infection alters the expression of activation markers, adhesion molecules and chemokine receptors on circulating monocytes. 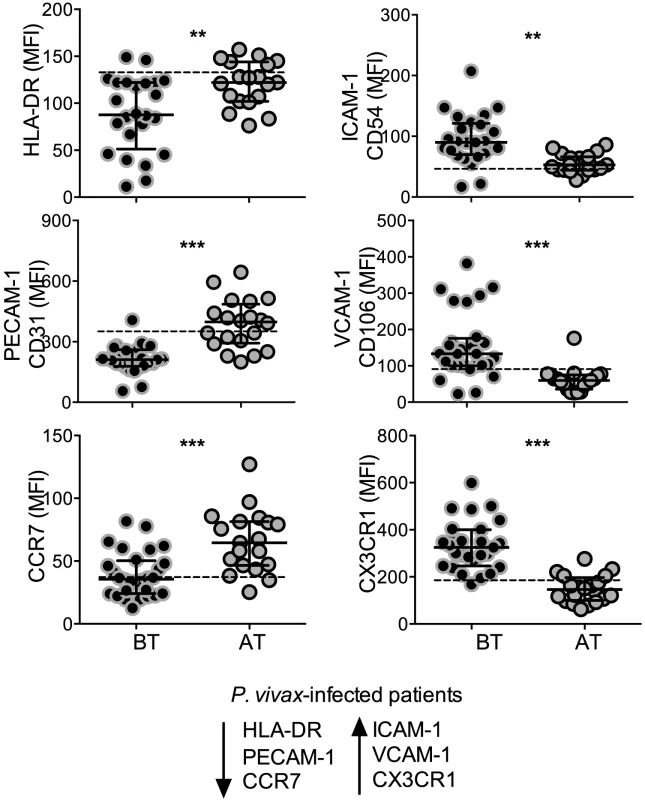
Mean fluorescence intensity (MFI) of HLA-DR (BT, n = 24 and AT, n = 19), CD31 (BT, n = 25 and AT, n = 20), CCR7 (BT, n = 28 and AT, n = 19) (left panels, from the top to the bottom), CD54 (BT, n = 25 and AT, n = 19), CD106 (BT, n = 28 and AT, n = 19), CX3CR1 (BT, n = 28 and AT, n = 20) (right panels, from the top to the bottom) was evaluated on monocytes from P. vivax-infected subjects, before (BT, black circles) and 30–45 days after treatment (AT, grey circles). Circles indicate individual patients and lines represent median values and interquartile ranges. Dotted lines represent medians of a given measurements from healthy donors. Levels of the molecules above were measured by flow cytometry. **0.05>p>0.01, *** p<0.01. Monocyte subpopulations from P. vivax-infected patients display distinct activated phenotypes
As noted above, human monocyte subsets can be distinguished by flow cytometry based on the expression of CD14 and CD16 [18]. The majority of monocytes express CD14 but not CD16, and those expressing CD16 can be subdivided into two subpopulations, CD14+CD16+ and CD14loCD16+ cells (Figure 3A). They are also slightly different in granularity as previously described and shown in Figure S1 [18]. To exclude any contaminating neutrophils, only HLA-DR+ cells were included in the analysis and CD16+ cells were included only if they also expressed HLA-DR. Higher frequencies of CD14+CD16− monocytes were found in P. vivax-infected patients when compared with patients after treatment (Figure 3B). The frequencies of CD14+CD16+ and CD14loCD16+ monocytes did not differ significantly between malaria patients before and after treatment.
Fig. 3. Characterization of monocyte subsets in malaria patients. 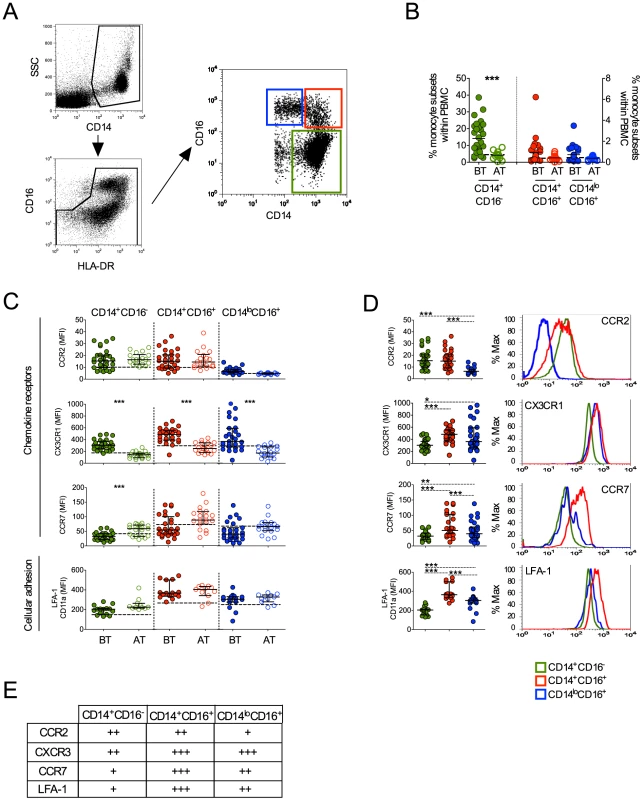
(A) Representative dot plots showing the gate strategy for the identification of CD14+CD16− (green gate), CD14+CD16+ (red gate), CD14loCD16+ (blue gate) monocyte subsets. CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes are represented by green, red and blue symbols. (B) Frequencies of CD14+CD16−, CD14+CD16+ and CD14lo monocytes within PBMC from P. vivax-infected patients before (BT, filled symbols) and 30–45 days after treatment (AT, open symbols) (n = 28). (C) Mean fluorescence intensity (MFI) of CCR2 (BT, n = 28 and AT, n = 18), CX3CR1 (BT, n = 26 and AT, n = 19), CCR7 (BT, n = 28 and AT, n = 19) and LFA-1 (BT, n = 15 and AT, n = 11) (from the top to the bottom) was evaluated on monocyte subsets (CD14+CD16− (left panel), CD14+CD16+ (middle panel), CD14loCD16+ (right panel)) from P. vivax-infected subjects, before and 30–45 days after treatment. Dotted lines represent medians of a given measurements from healthy donors. (D) Scattered dot plots (left panels) and representative histograms (right panels) showing MFI of the molecules described above on CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes from P. vivax-infected patients before treatment (open histograms). Levels of the molecules above were measured by flow cytometry. Circles indicate individual patients and lines represent median values and interquartile ranges. (E) Levels of molecules expressed by the monocyte subsets analyzed according to D. * p<0.05, **0.05>p>0.01, ***p<0.01. Chemokine receptors and the adhesion molecule, LFA-1, have been reported to be differently expressed on monocyte subsets. Previous studies described that CCR2 is expressed by both CD14+CD16− and CD14+CD16+ but not by CD14loCD16+ monocytes [18], [27]. Similarly in P. vivax-infected patients, low levels of CCR2 were found in CD14+CD16− and CD14+CD16+ monocytes and, as expected, CCR2 was barely expressed by CD14loCD16+ cells (Figure 3C and 3D). In addition, no changes in LFA-1 expression in the different monocyte subsets were observed in P. vivax patients as a result of treatment (Figure 3C). Higher LFA-1 levels were observed in CD14+CD16+, followed by CD14loCD16+ and CD14+CD16− monocytes (Figure 3D). The expression of CX3CR1 along with LFA-1 has been implicated in the ability of CD14loCD16+ monocytes to crawl on the inner surface endothelium of blood vessels [18], [28]. Importantly, P. vivax infection triggered a significantly increased expression of CX3CR1 on CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes (Figure 3C). CX3CR1 was expressed at lower levels by CD14+CD16− monocytes, when compared to the CD16+ populations (Figure 3D). Lastly, changes in CCR7 expression were observed only on CD14+CD16− monocytes, with those from P. vivax infected patients expressing lower levels of CCR7 compared to monocytes from treated individuals (Figure 3C). Thus, all the monocyte subpopulations from P. vivax-infected display a distinct phenotypic profile in patients undergoing acute malaria compared with the same individuals after treatment.
P. vivax induces a variety of inflammatory genes during malaria infection
We next FACS-sorted the CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocyte subpopulations from healthy donors and P. vivax-infected patients (Figure 4A). The purity of each cell population is shown in Figure 4A. Since the numbers of each circulating monocyte subset obtained from the FACS-sort was limited, we chose to assess the expression of several genes involved in inflammatory responses by nanostring [25]. The expression of 72 selected genes involved in innate immune response, cell adhesion, migration and phagocytosis was evaluated, and we found that 41 genes had their expression significantly altered in at least one of the monocyte subpopulations upon P. vivax infection (Figure 4B). Differences greater than 4-fold cannot be appreciated in the heatmap, once the range from −4.0 to +4.0 fold was selected to better reveal differences in the majority of the genes induced by malarial infection in monocyte subsets. Once changes in gene expression were detected upon P. vivax infection, the expression of each of these genes was compared among the monocytes subset from P. vivax-infected patients (Figure 4C). In general, the CD14+ subpopulations, i.e., the classical and inflammatory monocytes, expressed higher levels of RNAs encoded by pro-inflammatory genes. The chemokines CCL2 and CXCL2 were highly expressed by CD14+CD16− and CD14+CD16+ subpopulations, but were expressed at lower levels by CD14loCD16+ monocytes (Figure 4C). The same pattern of expression was observed for TNFR1/TNFRSF1A and ICAM-1 with CD14+CD16− and CD14+CD16+ expressing higher levels than CD14loCD16+ monocytes (Figure 4C). The classical monocytes, CD14+CD16− expressed higher counts of mRNA for the receptor for IFN-gamma and for the IL-1 receptor agonist IL-1RA (Figure 4C). The expression of cytokine genes also varied among monocyte subsets. Both IL6 and IL10 had higher expression in CD14+CD16+ compared to CD14+CD16− cells. CD14loCD16+ monocytes expressed higher levels of mRNA for TNF and lower levels of IL8 than the CD14+CD16− subset. CD14+CD16− as well as CD14+CD16+ monocytes expressed higher amounts of NLRP3 and CASPASE1 than patrolling monocytes. The same pattern of expression was observed for NFKB1 and NFKB1A, involved respectively with induction and regulation of cytokine expression, with CD14+CD16− and CD14+CD16+ expressing higher levels than CD14loCD16+ monocytes. REL that encodes a protein that is a member of the Rel/NFKB family was more highly expressed on CD14+CD16+ monocytes than on the other monocyte subpopulations. The expression of the costimulatory molecule CD80 was higher in CD14+CD16+ than classical and patrolling monocytes (Figure 4C). Together these data indicate that CD14+CD16− and CD14+CD16+ monocytes have a more activated and inflammatory profile than patrolling monocytes during malaria.
Fig. 4. Monocyte subsets display distinct gene expression. 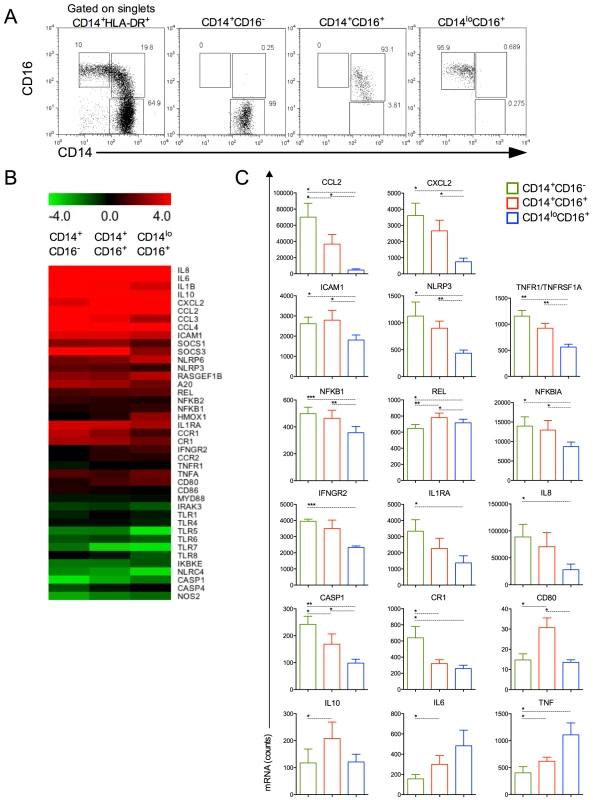
CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes from healthy donors and P. vivax-infected patients were isolated by FACS. (A) Representative dot plots showing monocyte subpopulations before (left panel) and after FACS-sorting (right panels). (B) A nanostring analysis of monocyte subsets from 5 patients infected with P. vivax and 5 healthy donors. Heatmap representation of 41 differentially regulated genes upon malaria infection compared to healthy donors is depicted. (C) CCL2, CXCL2, ICAM1, NLRP3, TNFR1/TNFRSF1A, NFKB1, REL, NFKB1A, IFNGR2, IL1RA, IL8, CASP1, CR1, CD80, IL10, IL6 and TNF were differentially induced among CD14+CD16− (green bars), CD14+CD16+ (red bars) and CD14loCD16+ (blue bars) monocytes from P. vivax-infected patients. *p<0.05, **0.05>p>0.01, ***p<0.01. Monocytes from P. vivax infected patients exhibit morphologic changes
Patrolling monocytes can be distinguished from the classical and inflammatory subsets based on size and granularity [18], but no ultrastructural analysis had been previously performed on these cell subpopulations. Electronic microscopy was performed attempting to reveal morphological changes suggestive of functional alterations. FACS-sorted CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes from healthy donors (n = 5) and P. vivax-infected patients (n = 6) (Figure 4A) were fixed and processed for ultrastructural analysis by electron microscopy (Figure 5A). CD14+CD16− and CD14+CD16+ monocytes from HD (Figure 5A, upper panel) and P. vivax-infected patients (Figure 5A, lower panel) had a larger and a higher number of mitochondria (white arrows) when compared to CD14loCD16+ monocytes. All monocyte subsets from P. vivax-infected patients displayed morphological features compatible with activation (Figure 5A). Moreover, mitochondria from CD14+CD16+ cells from P. vivax-infected patients were significantly larger than those in the two other monocyte subsets, when mitochondria area was measured using the software ImageJ 1.47K (NIH) (Figure 5B). Mitochondria area was assessed in at least six cells of each monocyte subpopulation per patient.
Fig. 5. Circulating monocyte subpopulations display distinct morphology, mitochondrial and NADPH subunit content. 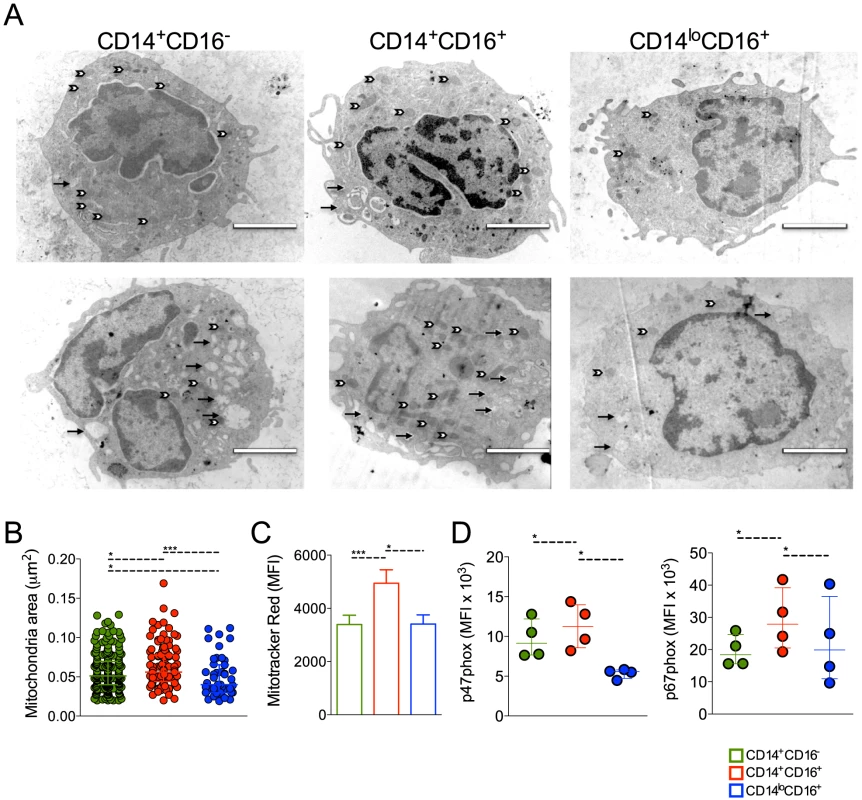
FACS-sorted CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes from healthy donors and P. vivax-infected patients were fixed and prepared for electron microscopy. Monocyte subsets from a single healthy donor (A, upper panel) and a single patient (A, lower panel) shown are representative of the analysis of at least six cells of each monocyte subpopulation per patient and of the analysis of 5 controls and 6 patients. Mitochondria (white arrows) and vesicles (black arrows). Scale bar, 2 µm. (B) Mitochondria area from CD14+CD16− (green circles), CD14+CD16+ (red circles) and CD14loCD16+ (blue circles) monocytes was assessed using ImageJ. Circles indicate individual mitochondria. (C) Mitochondria content in CD14+CD16− (green bars), CD14+CD16+ (red bars) and CD14loCD16+ (blue bars) monocytes from P. vivax-infected patients was measured based on Mitotracker reactivity (n = 11). (D) Mean fluorescence intensity (MFI) of p47phox and p67phox within CD14+CD16−, CD14+CD16+, CD14loCD16+ monocytes from P. vivax-infected patients was measured by flow cytometry 3 hours after culture in medium alone or P. vivax-infected reticulocytes (n = 4). Results are representative of 2 independent experiments. Symbols represent individual subject. *p<0.05, ***p<0.01. ROS are generated in multiple compartments and by multiple enzymes in the cell and important contributions include proteins within the plasma membrane, e.g., NADPH oxidases, and mitochondria [29], [30]. Since mitochondria are at least in part responsible for the generation of ROS [31], [32], we further assessed the content of mitochondria in the monocyte subsets from P. vivax-infected patients. MitoTracker Red CMX-Ros was used for this propose. The MFI of MitoTracker Red, probe sensitive to membrane potential, was assessed in CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocyte subsets by flow cytometry (Figure 5C). CD14+CD16− and CD14loCD16+ monocytes similarly react with Mitotracker Red while significantly higher reactivity was found in CD14+CD16+ monocytes (Figure 5C). Our data show that CD14+CD16+ monocytes have larger and more active mitochondria suggesting differential metabolic activity during P. vivax infection. The expression of p47phox and p67phox, cytosolic components of the NADPH oxidase, was also measured in monocyte subsets from malaria patients after a short-term culture with P. vivax-infected reticulocytes. Higher expression of p47phox and p67phox were found in CD14+CD16+ monocytes when comparing to their other counterparts (Figure 5D).
Monocyte subpopulations from malaria patients display distinct levels of molecules involved in cell activation and migration
Upon activation monocytes undergo several changes, including expression of molecules involved with antigen presentation, cell adhesion and migration [33], [34], [35]. CD14+CD16− monocytes from patients undergoing P. vivax infection expressed lower levels of HLA-DR (Figure 6A). In contrast, the expression of HLA-DR did not differ in CD14+CD16+ and CD14loCD16+ monocytes when cells from malaria patients were compared before and after treatment (Figure 6A). Higher levels of HLA-DR were found in CD14+CD16+ monocytes compared to their other counterparts during P. vivax infection (Figure 6A, B, C).
Fig. 6. Monocyte subsets from P. vivax-infected patients display a highly activated phenotype. 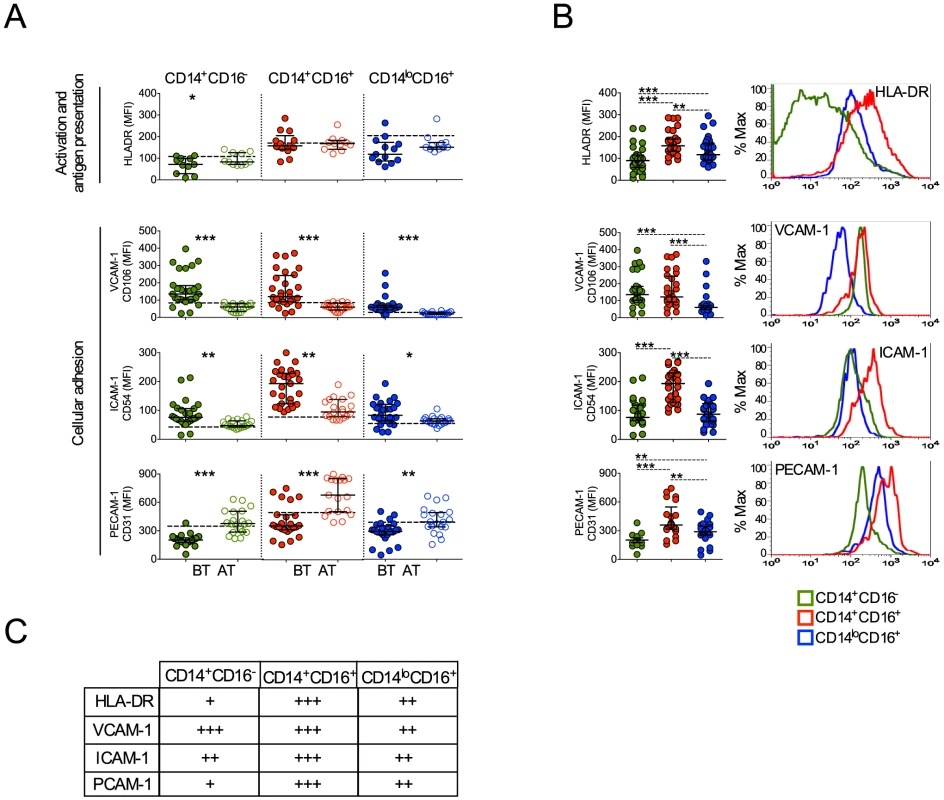
CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes are represented by green, red and blue symbols. (A) Mean fluorescence intensity (MFI) of HLA-DR (BT, n = 11 and AT, n = 11), CD106 (BT, n = 26 and AT, n = 19), CD54 (BT, n = 28 and AT, n = 19) and CD31 (BT, n = 25 and AT, n = 20) (from the top to the bottom) was evaluated on monocyte subsets (CD14+CD16− (left panel), CD14+CD16+ (middle panel), CD14loCD16+ (right panel)) from P. vivax-infected subjects, before (filled symbols) and 30–45 days after treatment (open symbols). Dotted lines represent medians of given measurements from healthy donors. (B) Scattered dot plots (left panels) and representative histograms (right panels) showing MFI of the molecules described above on CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes from P. vivax-infected patients before treatment. Levels of the molecules above were measured by flow cytometry. Circles indicate individual patients and lines represent median values and interquartile ranges. (C) Levels of molecules expressed by the monocyte subsets analyzed according to B. *p<0.05, **0.05>p>0.01, ***p<0.01. We also observed that P. vivax infection triggered the expression of VCAM-1 and ICAM-1 in all three monocyte subpopulations (Figure 6). In contrast, a decreased expression of PECAM-1 was observed in patients experiencing malaria when compared to the same patients after treatment (Figure 6A). Importantly, CD14+CD16+ monocytes from P. vivax-infected patients expressed the highest levels of ICAM-1 and PECAM-1, when compared to CD14+CD16− and CD14loCD16+ monocytes (Figure 6B, C). Taken together these results further corroborate that CD14+ monocytes, especially the CD14+CD16+ subset, are highly activated during P. vivax malaria.
CD16+CD14+ monocytes display elevated phagocytosis
We used CFSE labeled P. vivax-infected reticulocytes (Pv-Ret) to quantify phagocytosis by different monocyte subpopulations from malaria patients before treatment initiation. CD14+CD16+ cells displayed significantly higher levels of phagocytosis of Pv-Ret than the other monocyte subsets (Figure 7A). The phagocytic ability of CD14+CD16+ cells was followed by the CD14loCD16+ patrolling monocytes, which was significantly better than the CD14+CD16− monocytes (Figure 7A). It is important to note that significant differences are found in the phagocytic ability of monocyte subsets when uninfected reticulocytes are purified from healthy donors and co-cultured with PBMC from patients and healthy donors. Much lower frequencies of reticulocytes containing monocytes are detected when cultures are performed with uninfected reticulocytes compared to P. vivax-infected reticulocytes (CD14+CD16−: 3.54%±0.52% vs. 71.14%±11.11%, CD14+CD16+: 23.20%±2.18% vs. 98.33%±1.40%, CD14loCD16+: 23.18%±4.22% vs. 87.13%±8.10%). The same phenomenon was shown for P. falciparum [36].
Fig. 7. CD16+CD14+ monocytes display pronounced phagocytic ability than other subsets. 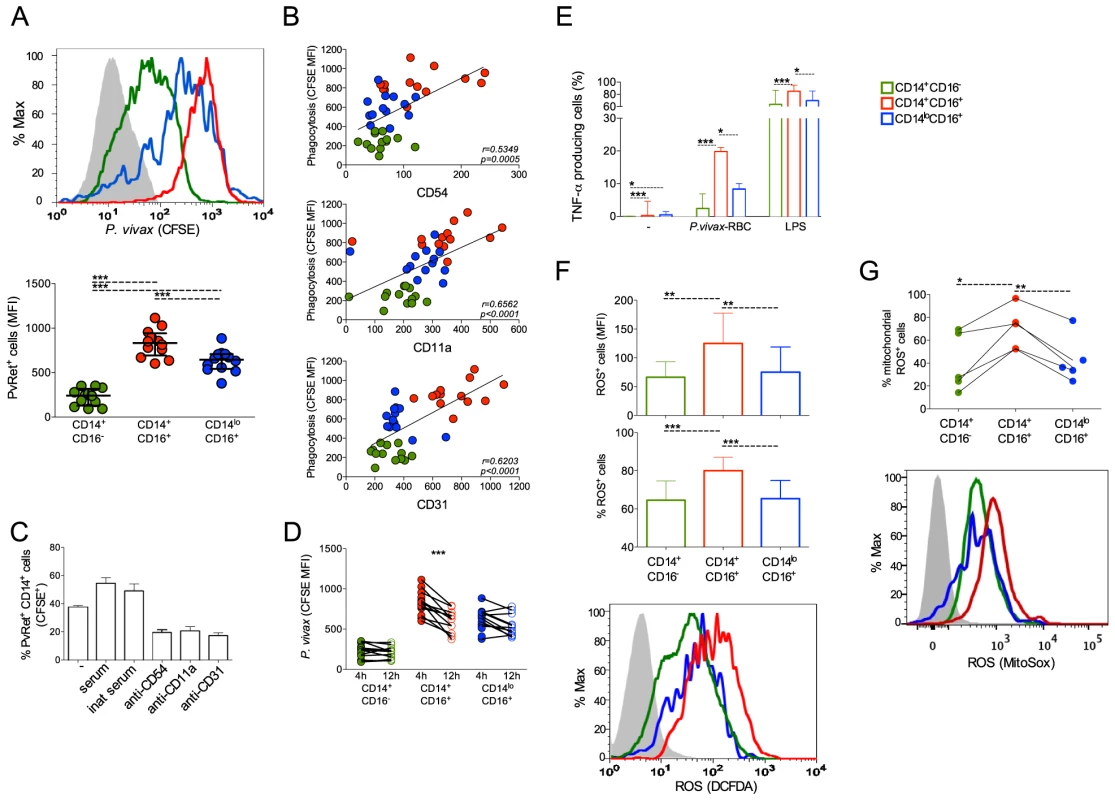
CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes are represented by green, red, and blue symbols, respectively. P. vivax-infected reticulocytes (Pv-Ret) were purified, CFSE labeled (A, B, C) or not (D, E) and cultured with PBMC from acutely P. vivax-infected subjects. (A) Mean fluorescence intensity (MFI) of CFSE within CD14+CD16−, CD14+CD16+, CD14loCD16+ monocytes exposed to P. vivax was measured by flow cytometry after 4 hours of culture. Each circle represents a single patient (n = 12) and lines represent median values and interquartile ranges (B) Correlation between P. vivax phagocytosis (CFSE MFI) and CD54, CD11a or CD31 expression by CD14+CD16−, CD14+CD16+, CD14loCD16+ monocytes. Circles indicate individual patients (n = 13). (C) Phagocytosis of Pv-Ret by CD14+ cells was measured in the absence of serum, presence of inactivated serum and presence of serum, and in the presence of anti-CD54, CD11a and CD31 blocking antibodies (lower graph). Bars represent median values and interquartile ranges (n = 6). (D) MFI of CFSE within CD14+CD16−, CD14+CD16+, CD14loCD16+ monocytes exposed to P. vivax was measured by flow cytometry after 4 and 12 hours of culture. Connecting circles represent values of CFSE MFI of a single patient after 4 and 12 hours of culture, (n = 12) (E) Frequencies of TNF-α producing monocyte subsets were measured after culture with medium alone, Pv-Ret or LPS. Bars represent median values and interquartile ranges (n = 6). (F) ROS production was detected by measuring MFI (top panel) and the proportions (middle panel) of carboxy-H2DCDA+ CD14+CD16−, CD14+CD16+ and CD14loCD16+ monocytes after 3 hours incubation with P. vivax-infected reticulocytes. Bars represent median values and interquartile ranges (n = 9). Representative histograms showing MFI of carboxy-H2DCFDA expressing CD14+CD16−, CD14+CD16+, CD14loCD16+ monocytes (bottom panel). Grey histogram represents CD14+ cells cultured in the absence of Pv-Ret. (G) Mitochondrial ROS was assessed in monocyte subsets from P. vivax-infected patients measuring MitoSox by flow cytomery after culture with P. vivax-infected reticulocytes (n = 4). Connecting circles represent frequencies of different monocyte subsets producing mitochondrial ROS. Symbols represent individual subject. Representative histograms showing MFI of MitoSox expressing CD14+CD16−, CD14+CD16+, CD14loCD16+ monocytes (bottom panel). Grey histogram represents MitoSox- cells. *p<0.05, **0.05>p>0.01, ***p<0.01. Interestingly, higher levels of phagocytosis of Pv-Ret correlated with higher expression of the adhesion molecules ICAM-1 (CD54), LFA-1 (CD11a) and PECAM-1 (CD31) by monocytes (Figure 7B). As adhesion molecules, such as CD54, expressed by cell lines interact with P. vivax-infected reticulocytes [22], we used blocking antibodies to assess whether phagocytosis of P. vivax-infected erythrocytes was dependent on those interactions. Indeed, the phagocytosis of Pv-Ret by CD14+ cells was partially blocked by either anti-CD54 or -CD11a or -CD31, as observed by the significantly decreased frequencies of monocytes containing Pv-Ret (Figure 7C). To assess the ability of each monocyte subpopulation to kill Pv-Ret, PBMC were left in cultures for 12 h in the presence of CFSE-labeled-Pv-Ret, and the mean fluorescence intensity of CFSE were measured in each monocyte subset. The MFI of CFSE was only decreased in CD14+CD16+ monocytes when analyzed 12 h and compared to 4 h of culture (Figure 7D). Both CD14+CD16− and CD14l°CD16+ displayed similar levels of Pv-Ret containing monocytes when the MFI was compare between 4 and 12 h of culture. Important to mention that no significant increase in apoptosis or changes in the proportions of monocyte subsets were detected when PBMC were cultured for 4 and 12 hours in medium or Pv-Ret (Figure S2) (Text S1).
Higher levels of intracellular TNF and ROS are found in CD14+CD16+ monocytes compared to their other counterparts
To examine the mechanism of killing, we evaluated the ability of the different monocytes to produce TNF-α and ROS, which are key effector molecules made by activated monocyte/macrophages. Significantly higher frequencies of TNF-α producing cells were found among CD14+CD16+ monocytes when PBMC were cultured with Pv-Ret (or LPS) (Figure 7E).
ROS production by PBMC and monocyte subsets from P. vivax-infected patients was measured by luminescence (RLU) of luminol or fluorescence (RFU) of H2DCFDA (Figure S4). PBMC from P. vivax-infected patients produced detectable amounts of total ROS spontaneously or in response to PMA, when measured by luminol (Figure S4A) (Text S1). PBMC from acutely infected patients produce higher levels of ROS than the same patients after treatment. In addition, total ROS production was measured in purified monocyte subsets, but no significant differences were found among them (Figure S4B). Intracellular ROS production by each monocyte subpopulation was also measured in the single-cell level by flow cytometry. Consistent with the phagocytosis and TNF-α results, CD14+CD16+ monocytes exposed to Pv-Ret generated significantly higher levels of ROS than the other monocyte subpopulations (Figure 7F).
We then performed experiments using rotenone and DPI (diphenylene iodonium) to block respectively the mitochondria complex I and NADPH oxidase plus nitric oxide synthases [37], [38], [39]. The inhibition of ROS production was assessed in PBMC by luminescence using luminol and in monocyte subsets using H2DCFDA by flow cytomety (Figure S4C and S4D). Rotenone was able to partially inhibit ROS production by PBMC (Figure S4C) and by CD14+CD16+ cells cultured with parasitized reticulocytes (Figure S4D). When DPI was added in the culture, it was able to abrogated ROS production by PBMC (Figure S4C). Partial inhibition was observed when H2DCFDA+CD14+CD16+ cells from malaria patients were cultured with Pv-Ret and assessed by flow cytometry (Figure S4D). To more specifically analyze whether mitochondrial ROS were differentially produced by monocyte subsets during malaria, MitoSox staining was performed. Corroborating with total intracellular ROS production assessed with H2DCFDA, CD14+CD16+ cells were the monocyte subset that most reacted with MitoSox (Figure 7G).
Taken together, our data indicate that CD14+CD16+ inflammatory monocytes play important effector activity during P. vivax infection.
Discussion
Although pro-inflammatory cytokines play an important role in host resistance to Plasmodium infection, various studies reported that they may contribute to deleterious effects during malaria [40], [41], [42]. Thus, the pathways involved on induction of these mediators during malaria, represent checkpoints for immunological intervention to prevent poor outcome of disease. Monocytes have been described as a major source of cytokines during P. vivax infection [5]. Although infection with Plasmodium spp is known to dramatically alter monocyte differentiation [43], the role of monocyte subsets in host resistance to infection and pathogenesis of malaria remains poorly understood. The findings reported here clearly demonstrate that both the classical (CD14+CD16−) and intermediate or inflammatory (CD14+CD16+) monocytes are important sources of cytokines during acute P. vivax infection. Intriguingly, the CD14+CD16+ cells displayed the highest mitochondria content and activity, being an important source of ROS and were the most efficient phagocytes of P. vivax infected reticulocytes.
Both in mice and human, different monocyte subsets seem to reflect developmental stages with distinct physiological roles, such as recruitment to inflammatory lesions or entry to normal tissues [17]. Consistent with our results assessing the monocyte subsets in P. vivax-infected patients, the majority of monocytes found in steady state, known as classical monocytes, express CD14 but not CD16, and the remaining monocytes express both CD14 and CD16: CD14+CD16+ (inflammatory) and CD14loCD16+ (patrolling) monocytes [18]. This classification still gives rise to discussion. Ziegler-Heitbrock and coworkers has defined CD14loCD16+ as non-classical and CD14+CD16+ as intermediate monocytes [44]. Moreover, some studies have been analyzed monocytes based on the expression of molecules related to differentiation/activation found in macrophages [45], [46]. Despite several of these molecules were assessed in this study many others, such as CD68, CD163 and CD206 have been strongly correlated with different monocyte subsets [45].
As described here, both classical and inflammatory monocytes expressed the chemokine receptor CCR2. It was previously shown in the P. chabaudi rodent model of malaria that inflammatory monocytes migrate to spleens, in CCR2 dependent manner, where they are important effector cells implicated in the control of parasite burden, likely through their phagocytic activity and release of ROS [47]. CCR2, the chemokine receptor for CCL2 (also known as monocyte chemotactic protein-1) is a marker for inflammatory monocytes. These monocyte subsets were also shown to express higher levels of mRNA encoding CCL2 when compared to the patrolling monocytes. Similarly, a previous study reported that patients infected with either P. vivax or P. falciparum have high levels of circulating CCL2 [48]. Classical and inflammatory monocytes from P. vivax-infected patients also expressed high levels of inflammatory mediators, including CXCL2 and the receptors for TNF-α, IFN-γ and IL-1. Despite the similarities described above, CD14+CD16+ monocytes displayed the highest frequencies of TNF-α producing cells when exposed to Pv-Ret. Interestingly, CD14+CD16+ monocytes also expressed higher mRNA for IL-10 than the other monocyte subsets. It has been described that highly activated effector cells can acquire regulatory features. During Leishmania [49], [50], T. cruzi [51] and T. gondii [52] infection polarized Th1 cells produce IL-10 along to IFN-γ, in attempt to control immunopathology. The same has been described for monocytes. A recent article shows in the murine model of toxoplasmosis that Ly6Chi monocytes entering the gastrointestinal tract responded to commensal ligands by adopting a regulatory phenotype. For instance inflammatory monocytes became capable to control parasite burden while limiting collateral damage to tissue [53]. Indeed, plasma levels of IL-10 are lower with increased disease severity during P. vivax infection [42]. Thus, the expression of this counter regulatory cytokine may also represent an important role of CD14+CD16+ monocytes in preventing immunopathology during P. vivax malaria.
Different adhesion molecules, including CD36, ICAM-1, VCAM-1 and PECAM-1 have been described as important receptors that bind P. falciparum infected red blood cells and influence the outcome of disease [54], [55]. P. falciparum-infected erythrocytes are able to tether and roll on CD36, ICAM-1, P-selectin, and VCAM-1 in a shear-dependent fashion. In addition, CD36 has an important role in phagocytosis of P. falciparum infected cells [56], [57], [58]. On the other hand, ICAM-1, but not CD36, was implicated in the cythoadhesion of P. vivax to endothelium cells [22]. We found that CD14+CD16+CCR2+ inflammatory monocytes from P. vivax malaria patients express the highest levels of ICAM-1, PECAM-1, and LFA-1. Although these adhesion molecules were originally identified as endothelium receptors for parasitized red blood cells, their expression on monocytes may favor biding and uptake of P. vivax infected reticulocytes. Indeed, our results indicate that the phagocytic activity of different monocyte subsets positively correlated with the expression of ICAM-1, PECAM-1 and LFA-1 and blockade of each of these adhesion molecules efficiently inhibited phagocytosis of Pv-Ret.
It is noteworthy that CD14+CD16+ monocytes have also been reported to expand in a group of P. falciparum-infected patients, and total monocytes from these patients were able to better control parasite growth in vitro, through antibody dependent cellular inhibition [59], [60]. CD16, a Fcγ receptor, has a high affinity for IgG1 and IgG3 [61] and therefore may be involved in phagocytosis of Plasmodium infected red blood cells. In contrast, experiments performed with Staphylococcus aureus or Echerichia coli showed that CD14+ monocytes have higher phagocytic activity than CD14lo monocytes. However no differences were observed between CD14+CD16+ and CD14+CD16− monocytes [62]. In our system, the expression of CD16 was not the only molecule involved in phagocytosis since the ability of Pv-Ret internalization was different between CD14+ and CD14lo, both expressing CD16. The expression of adhesion molecules ICAM-1, PECAM-1 and LFA-1, though, appeared to play an important role in parasite internalization by inflammatory monocytes. In vitro studies have shown that hemozoin interferes in the upregulation of MHC class II and CD54 on monocytes after IFN-γ stimulation [63], altering also their differentiation and maturation in dendritic cells [64] and antigen presentation [65]. Despite hemozoin is known to impair the ability of monocytes to repeat phagocytosis [66], no pigment was found when PBMC were evaluated in our study. Moreover, impairment in phagocytosis was not observed and the monocytes still produced ROS in response to PMA, in oppose to monocytes previously exposed to parasitized red blood cells [63]. We believe that phagocytosis of P. vivax-infected reticulocytes by monocytes are taking place in the spleen, where bona fide undifferentiated monocytes reside in equivalent numbers in circulation [67].
Besides their activated phenotype, CD14+ monocytes displayed an activated morphology with larger mitochondrias. Interestingly, the CD14+CD16+ subset showed higher mitochondrial activity than the other monocyte subpopulations. Malaria infection triggers production of high levels of total ROS. Despite similar levels of total ROS are produced by different monocyte subsets, higher frequencies and levels of intracellular ROS, and higher expression of p47phox and p67phox were found in CD14+CD16+ cells compared to CD14+CD16− and CD14loCD16+ monocytes. In addition, staining with MitoSox reveals that CD14+CD16+ monocytes produce higher levels of mitochondrial ROS. ROS are important effector free radicals involved in Plasmodium killing [47], [68]. Indeed, either blockade of mitochondrial complex I or blockage of NADPH oxidases, both responsible for ROS generation, efficiently reduces ROS levels in PBMC and CD14+CD16+ monocytes from malaria patients. Despite mitochondrial ROS have been regarded as byproducts of oxidative respiration, studies have indicated that mitochondria are recruited to vacuoles containing pathogens through an active process mediated by immune signaling [69], [70], [71]. Moreover, West and coworkers showed that the reduction of macrophage mitochondrial ROS results in defective bacterial killing [71].
Similarly to ROS production, higher frequencies of cells producing TNF-α, a cytokine known to trigger the respiratory burst, were found among CD14+CD16+ monocytes exposed to Pv-Ret. In a different context, patients experiencing severe malarial anemia triggered by P. falciparum display increased numbers of circulating monocytes, with significant augment in the numbers of TNF-α-producing CD14+CD16+ monocytes [72]. It is still unclear what is the cause of anemia during malaria. Despite recent reports have shown evidence demonstrating that P. vivax malaria may be associated with higher frequency and more severe anemia than P. falciparum, most of these studies were performed with children already suffering from malnutrition and hospitalized subject [73], [74], [75]. Only a small proportion of the P. vivax-infected patients analyzed in this study presented mild anemia (Table S1) and their reticulocyte counts are according to the reference values [76]. In fact, P. vivax infection is responsible for very low frequencies of infected red blood cells. We believe that during uncomplicated vivax malaria, phagocytosis of reticulocytes by monocytes and the production of inflammatory mediators will preferentially help in the parasite control.
It is important to mention that P. vivax-infected patients display elevated levels of hepatic biomarkers such as aspartate (AST), alanine (ALT) aminotransferase and bilirubin. And despite no correlation was found between monocyte activation markers and most of the laboratory parameters, such as hematocrit and platelet counts, higher levels of AST correlated with higher expression of CCR2 and VCAM-1 on both CD14+CD16− and CD14+CD16+ monocytes and ICAM-1 on CD14+CD16− monocytes (Figure S3). These data indicate that in the attempt of controlling parasitemia, monocytes might cause inflammatory damage.
Finally, we observed that CD14loCD16+ patrolling monocytes from P. vivax malaria patients did not express CCR2 but expressed high levels of LFA-1 and CX3CR1. The latter two receptors appear to be responsible for the ability of monocytes to patrol blood vessels in vivo [18]. Consistent with this hypothesis, CD16+ monocytes display enhanced capacity to adhere to endothelial cells in vitro. This ability is partially dependent on fractalkine, the CX3CR1 ligand and CD11a, the α chain of LFA-1 [77]. Importantly, patrolling monocytes have been shown to express high levels of TLR8 and TLR9 [18], and may also play an important role on cytokine production in response to parasite RNA and DNA. Thus, patrolling monocytes may also contribute to the early inflammatory response observed during P. vivax infection.
In conclusion, our findings support the concept that highly activated monocytes are characteristic of acute malaria. P. vivax-infection leads to cytokine production by classical and inflammatory monocytes, and this response is likely to be largely responsible for many of the signs and symptoms observed in malaria sepsis. Importantly, we demonstrate for the first time that CD14+CD16+ monocytes in malaria patients exhibited greater phagocytic activity and produced higher levels of intracellular TNF-α and reactive oxygen species, indicating their important role in parasite control and host resistance to infection. Further delineation of the differential roles of monocyte subsets in P. vivax malaria could lead to identification of specific targets for therapeutic intervention in this extremely important but highly neglected parasitic disease.
Supporting Information
Zdroje
1. GethingPW, ElyazarIR, MoyesCL, SmithDL, BattleKE, et al. (2012) A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl Trop Dis 6: e1814.
2. LanghorneJ, NdunguFM, SponaasAM, MarshK (2008) Immunity to malaria: more questions than answers. Nat Immunol 9 : 725–732.
3. SpencePJ, LanghorneJ (2012) T cell control of malaria pathogenesis. Curr Opin Immunol 24 : 444–448.
4. MuellerI, GalinskiMR, BairdJK, CarltonJM, KocharDK, et al. (2009) Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect Dis 9 : 555–566.
5. LeorattiFM, TrevelinSC, CunhaFQ, RochaBC, CostaPA, et al. (2012) Neutrophil paralysis in Plasmodium vivax malaria. PLoS Negl Trop Dis 6: e1710.
6. GazzinelliRT, DenkersEY (2006) Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nat Rev Immunol 6 : 895–906.
7. TakeuchiO, AkiraS (2010) Pattern recognition receptors and inflammation. Cell 140 : 805–820.
8. FranklinBS, ParrocheP, AtaideMA, LauwF, RopertC, et al. (2009) Malaria primes the innate immune response due to interferon-gamma induced enhancement of toll-like receptor expression and function. Proc Natl Acad Sci U S A 106 : 5789–5794.
9. ParrocheP, LauwFN, GoutagnyN, LatzE, MonksBG, et al. (2007) Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 104 : 1919–1924.
10. SharmaS, DeOliveiraRB, KalantariP, ParrocheP, GoutagnyN, et al. (2011) Innate immune recognition of an AT-rich stem-loop DNA motif in the Plasmodium falciparum genome. Immunity 35 : 194–207.
11. KwiatkowskiD, HillAV, SambouI, TwumasiP, CastracaneJ, et al. (1990) TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet 336 : 1201–1204.
12. McCallMB, NeteaMG, HermsenCC, JansenT, JacobsL, et al. (2007) Plasmodium falciparum infection causes proinflammatory priming of human TLR responses. J Immunol 179 : 162–171.
13. AntonelliLR, Gigliotti RothfuchsA, GoncalvesR, RoffeE, CheeverAW, et al. (2010) Intranasal Poly-IC treatment exacerbates tuberculosis in mice through the pulmonary recruitment of a pathogen-permissive monocyte/macrophage population. J Clin Invest 120 : 1674–1682.
14. GoncalvesR, ZhangX, CohenH, DebrabantA, MosserDM (2011) Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J Exp Med 208 : 1253–1265.
15. SerbinaNV, ChernyM, ShiC, BleauSA, CollinsNH, et al. (2009) Distinct responses of human monocyte subsets to Aspergillus fumigatus conidia. J Immunol 183 : 2678–2687.
16. PasslickB, FliegerD, Ziegler-HeitbrockHW (1989) Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 74 : 2527–2534.
17. GordonS, TaylorPR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5 : 953–964.
18. CrosJ, CagnardN, WoollardK, PateyN, ZhangSY, et al. (2010) Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33 : 375–386.
19. ZawadaAM, RogacevKS, RotterB, WinterP, MarellRR, et al. (2011) SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood 118: e50–61.
20. KanekoO, KimuraM, KawamotoF, FerreiraMU, TanabeK (1997) Plasmodium falciparum: allelic variation in the merozoite surface protein 1 gene in wild isolates from southern Vietnam. Exp Parasitol 86 : 45–57.
21. IhalamullaRL, MendisKN (1987) Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg 81 : 25–28.
22. CarvalhoBO, LopesSC, NogueiraPA, OrlandiPP, BargieriDY, et al. (2010) On the cytoadhesion of Plasmodium vivax-infected erythrocytes. J Infect Dis 202 : 638–647.
23. Chiarini-GarciaH, HornickJR, GriswoldMD, RussellLD (2001) Distribution of type A spermatogonia in the mouse is not random. Biol Reprod 65 : 1179–1185.
24. Chiarini-GarciaH, RussellLD (2002) Characterization of mouse spermatogonia by transmission electron microscopy. Reproduction 123 : 567–577.
25. GeissGK, BumgarnerRE, BirdittB, DahlT, DowidarN, et al. (2008) Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol 26 : 317–325.
26. DixitE, BoulantS, ZhangY, LeeAS, OdendallC, et al. (2010) Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141 : 668–681.
27. GripO, BredbergA, LindgrenS, HenrikssonG (2007) Increased subpopulations of CD16(+) and CD56(+) blood monocytes in patients with active Crohn's disease. Inflamm Bowel Dis 13 : 566–572.
28. AuffrayC, FoggD, GarfaM, ElainG, Join-LambertO, et al. (2007) Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317 : 666–670.
29. LambethJD (2004) Nox enzymes and the biology of reactive oxygen. Nature Reviews Immunology 4 : 181–189.
30. BalabanRS, NemotoS, FinkelT (2005) Mitochondria, oxidants, and aging. Cell 120 : 483–495.
31. ChanceB, SiesH, BoverisA (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59 : 527–605.
32. BalabanRS, NemotoS, FinkelT (2005) Mitochondria, oxidants, and aging. Cell 120 : 483–495.
33. HightonJ, CarlisleB, PalmerDG (1995) Changes in the phenotype of monocytes/macrophages and expression of cytokine mRNA in peripheral blood and synovial fluid of patients with rheumatoid arthritis. Clin Exp Immunol 102 : 541–546.
34. PhillipsRJ, LutzM, PremackB (2005) Differential signaling mechanisms regulate expression of CC chemokine receptor-2 during monocyte maturation. J Inflamm (Lond) 2 : 14.
35. TrialJ, BirdsallHH, HallumJA, CraneML, Rodriguez-BarradasMC, et al. (1995) Phenotypic and functional changes in peripheral blood monocytes during progression of human immunodeficiency virus infection. Effects of soluble immune complexes, cytokines, subcellular particulates from apoptotic cells, and HIV-1-encoded proteins on monocytes phagocytic function, oxidative burst, transendothelial migration, and cell surface phenotype. J Clin Invest 95 : 1690–1701.
36. TurriniF, GinsburgH, BussolinoF, PescarmonaGP, SerraMV, et al. (1992) Phagocytosis of Plasmodium-Falciparum-Infected Human Red-Blood-Cells by Human Monocytes - Involvement of Immune and Nonimmune Determinants and Dependence on Parasite Developmental Stage. Blood 80 : 801–808.
37. BuluaAC, SimonA, MaddipatiR, PelletierM, ParkH, et al. (2011) Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). Journal of Experimental Medicine 208 : 519–533.
38. HancockJT, JonesOTG (1987) The Inhibition by Diphenyleneiodonium and Its Analogs of Superoxide Generation by Macrophages. Biochemical Journal 242 : 103–107.
39. StuehrDJ, FasehunOA, KwonNS, GrossSS, GonzalezJA, et al. (1991) Inhibition of Macrophage and Endothelial-Cell Nitric-Oxide Synthase by Diphenyleneiodonium and Its Analogs. Faseb Journal 5 : 98–103.
40. OthoroC, LalAA, NahlenB, KoechD, OragoAS, et al. (1999) A low interleukin-10 tumor necrosis factor-alpha ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. J Infect Dis 179 : 279–282.
41. KurtzhalsJA, AdabayeriV, GokaBQ, AkanmoriBD, Oliver-CommeyJO, et al. (1998) Low plasma concentrations of interleukin 10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. Lancet 351 : 1768–1772.
42. AndradeBB, Reis-FilhoA, Souza-NetoSM, ClarencioJ, CamargoLM, et al. (2010) Severe Plasmodium vivax malaria exhibits marked inflammatory imbalance. Malar J 9 : 13.
43. SkorokhodOA, AlessioM, MordmullerB, AreseP, SchwarzerE (2004) Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect. J Immunol 173 : 4066–4074.
44. Ziegler-HeitbrockL, AncutaP, CroweS, DalodM, GrauV, et al. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116: e74–80.
45. FadiniGP, CappellariR, MazzucatoM, AgostiniC, Vigili de KreutzenbergS, et al. (2013) Monocyte-macrophage polarization balance in pre-diabetic individuals. Acta Diabetol 50 : 977–982.
46. MantovaniA, GarlandaC, LocatiM (2009) Macrophage diversity and polarization in atherosclerosis: a question of balance. Arterioscler Thromb Vasc Biol 29 : 1419–1423.
47. SponaasAM, Freitas do RosarioAP, VoisineC, MastelicB, ThompsonJ, et al. (2009) Migrating monocytes recruited to the spleen play an important role in control of blood stage malaria. Blood 114 : 5522–5531.
48. FernandesAA, CarvalhoLJ, ZaniniGM, VenturaAM, SouzaJM, et al. (2008) Similar cytokine responses and degrees of anemia in patients with Plasmodium falciparum and Plasmodium vivax infections in the Brazilian Amazon region. Clin Vaccine Immunol 15 : 650–658.
49. NylenS, MauryaR, EidsmoL, Das ManandharK, SundarS, et al. (2007) Splenic accumulation of IL-10 mRNA in T cells distinct from CD4(+) CD25(+) (Foxp3) regulatory T cells in human visceral leishmaniasis. Journal of Experimental Medicine 204 : 805–817.
50. AndersonCF, OukkaM, KuchrooVJ, SacksD (2007) CD4(+)CD25(−)Foxp3(−) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. Journal of Experimental Medicine 204 : 285–297.
51. RoffeE, RothfuchsAG, SantiagoHC, MarinoAPMP, Ribeiro-GomesFL, et al. (2012) IL-10 Limits Parasite Burden and Protects against Fatal Myocarditis in a Mouse Model of Trypanosoma cruzi Infection. Journal of Immunology 188 : 649–660.
52. JankovicD, KullbergMC, FengCG, GoldszmidRS, CollazoCM, et al. (2007) Conventional T-bet(+)Foxp3(−) Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. Journal of Experimental Medicine 204 : 273–283.
53. GraingerJR, WohlfertEA, FussIJ, BouladouxN, AskenaseMH, et al. (2013) Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nature Medicine 19 : 713–+.
54. NewboldC, WarnP, BlackG, BerendtA, CraigA, et al. (1997) Receptor-specific adhesion and clinical disease in Plasmodium falciparum. Am J Trop Med Hyg 57 : 389–398.
55. RoweJA, ClaessensA, CorriganRA, ArmanM (2009) Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med 11: e16.
56. UdomsangpetchR, ReinhardtPH, SchollaardtT, ElliottJF, KubesP, et al. (1997) Promiscuity of clinical Plasmodium falciparum isolates for multiple adhesion molecules under flow conditions. J Immunol 158 : 4358–4364.
57. AyiK, PatelSN, SerghidesL, SmithTG, KainKC (2005) Nonopsonic phagocytosis of erythrocytes infected with ring-stage Plasmodium falciparum. Infect Immun 73 : 2559–2563.
58. McGilvrayID, SerghidesL, KapusA, RotsteinOD, KainKC (2000) Nonopsonic monocyte/macrophage phagocytosis of Plasmodium falciparum-parasitized erythrocytes: a role for CD36 in malarial clearance. Blood 96 : 3231–3240.
59. ChimmaP, RoussilhonC, SratongnoP, RuangveerayuthR, PattanapanyasatK, et al. (2009) A distinct peripheral blood monocyte phenotype is associated with parasite inhibitory activity in acute uncomplicated Plasmodium falciparum malaria. PLoS Pathog 5: e1000631.
60. JaworowskiA, KamwendoDD, ElleryP, SonzaS, MwapasaV, et al. (2007) CD16+ monocyte subset preferentially harbors HIV-1 and is expanded in pregnant Malawian women with Plasmodium falciparum malaria and HIV-1 infection. J Infect Dis 196 : 38–42.
61. NimmerjahnF, RavetchJV (2011) FcgammaRs in health and disease. Curr Top Microbiol Immunol 350 : 105–125.
62. Skrzeczynska-MoncznikJ, BzowskaM, LosekeS, Grage-GriebenowE, ZembalaM, et al. (2008) Peripheral blood CD14high CD16+ monocytes are main producers of IL-10. Scand J Immunol 67 : 152–159.
63. SchwarzerE, TurriniF, UlliersD, GiribaldiG, GinsburgH, et al. (1992) Impairment of macrophage functions after ingestion of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. Journal of Experimental Medicine 176 : 1033–1041.
64. SkorokhodOA, AlessioM, MordmullerB, AreseP, SchwarzerE (2004) Hemozoin (malarial pigment) inhibits differentiation and maturation of human monocyte-derived dendritic cells: a peroxisome proliferator-activated receptor-gamma-mediated effect. Journal of Immunology 173 : 4066–4074.
65. ScorzaT, MagezS, BrysL, De BaetselierP (1999) Hemozoin is a key factor in the induction of malaria-associated immunosuppression. Parasite Immunol 21 : 545–554.
66. SchwarzerE, AlessioM, UlliersD, AreseP (1998) Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect Immun 66 : 1601–1606.
67. SwirskiFK, NahrendorfM, EtzrodtM, WildgruberM, Cortez-RetamozoV, et al. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325 : 612–616.
68. KumarS, ChristophidesGK, CanteraR, CharlesB, HanYS, et al. (2003) The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci U S A 100 : 14139–14144.
69. MatsumotoA, BesshoH, UehiraK, SudaT (1991) Morphological studies of the association of mitochondria with chlamydial inclusions and the fusion of chlamydial inclusions. J Electron Microsc (Tokyo) 40 : 356–363.
70. SinaiAP, WebsterP, JoinerKA (1997) Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci 110 (Pt 17): 2117–2128.
71. WestAP, BrodskyIE, RahnerC, WooDK, Erdjument-BromageH, et al. (2011) TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472 : 476–480.
72. OgondaLA, OragoAS, OtienoMF, AdhiamboC, OtienoW, et al. (2010) The levels of CD16/Fc gamma receptor IIIA on CD14+ CD16+ monocytes are higher in children with severe Plasmodium falciparum anemia than in children with cerebral or uncomplicated malaria. Infect Immun 78 : 2173–2181.
73. AlexandreMA, FerreiraCO, SiqueiraAM, MagalhaesBL, MouraoMP, et al. (2010) Severe Plasmodium vivax malaria, Brazilian Amazon. Emerg Infect Dis 16 : 1611–1614.
74. DouglasNM, AnsteyNM, BuffetPA, PoespoprodjoJR, YeoTW, et al. (2012) The anaemia of Plasmodium vivax malaria. Malar J 11 : 135.
75. Rodriguez-MoralesAJ, SanchezE, VargasM, PiccoloC, ColinaR, et al. (2006) Anemia and thrombocytopenia in children with Plasmodium vivax malaria. J Trop Pediatr 52 : 49–51.
76. Lewis SM, Bain BJ, Bates I (2006) Dacie and Lewis Practical Haematology. Philadelphia: Churchill Livingstone Elsevier. 736 p.
77. AncutaP, RaoR, MosesA, MehleA, ShawSK, et al. (2003) Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. J Exp Med 197 : 1701–1707.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



