-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
Although it is generally believed that CD4+ T cells mediate anti-Leishmania immunity, some studies suggest that CD3+CD4−CD8 − (double negative, DN) T cells may play a more important role in regulating primary anti-Leishmania immunity. Here, we report that DN T cells extensively proliferate and produce effector cytokines in mice following primary and secondary L. major infections. Leishmania-reactive DN T cells utilize αβ T cell receptor (TCR) and are restricted by MHC class II molecules. Strikingly, DN T cells from healed mice display functional characteristics of protective anti-Leishmania memory-like cells: rapid and extensive proliferation, effector cytokine production in vitro and in vivo, and accelerated parasite control following secondary L. major challenge. These results directly identify DN T cells as important players in protective primary and secondary anti-L. major immunity in experimental cutaneous leishmaniasis.
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004396
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004396Summary
Although it is generally believed that CD4+ T cells mediate anti-Leishmania immunity, some studies suggest that CD3+CD4−CD8 − (double negative, DN) T cells may play a more important role in regulating primary anti-Leishmania immunity. Here, we report that DN T cells extensively proliferate and produce effector cytokines in mice following primary and secondary L. major infections. Leishmania-reactive DN T cells utilize αβ T cell receptor (TCR) and are restricted by MHC class II molecules. Strikingly, DN T cells from healed mice display functional characteristics of protective anti-Leishmania memory-like cells: rapid and extensive proliferation, effector cytokine production in vitro and in vivo, and accelerated parasite control following secondary L. major challenge. These results directly identify DN T cells as important players in protective primary and secondary anti-L. major immunity in experimental cutaneous leishmaniasis.
Introduction
The spectrum of disease collectively called Leishmaniasis is caused by several species of protozoan parasites belonging to the genus Leishmania. The disease is currently endemic in 88 countries, affecting an estimated 12 million people with over 1.5–2 million new cases and 70,000 deaths each year [1]. Because Leishmania parasites reside mainly within macrophages, a strong cell-mediated immunity is required to control intracellular parasite replication and disease progression [2], [3], [4], [5], [6]. Experimental L. major infection in mice closely mimics the human cutaneous disease and is an excellent model for understanding the factors that regulate cell-mediated immunity. Resistance to cutaneous leishmaniasis is associated with strong IFN-γ response, which activates infected macrophages leading to nitric oxide and reactive oxygen species production and destruction of the intracellular parasites [4], [7], [8], [9].
Although it is generally believed that CD4+ T cells play a primary role in mediating anti-Leishmania immunity, a study suggests that they may be dispensable and that MHC II-restricted CD3+CD4−CD8− (double negative, DN) T cells are critical for regulating primary anti-Leishmania immunity [10]. In addition, several studies have reported increased numbers of DN T cells in blood of Leishmania-infected patients [11], [12], dogs [13], and in spleens of Leishmania-infected mice [14]. These cells have been proposed to contribute to primary and vaccine-induced immunity against Leishmania. However, direct evidence implicating DN T cells in anti-Leishmania immunity has not yet been clearly documented. Here, we report for the first time, that infection with L. major leads to activation and proliferation of DN T cells in the draining lymph nodes (dLNs) and spleens of infected mice. These cells produce effector cytokines (IFN-γ and TNF), display functional characteristics of memory-like cells and contribute to optimal primary and secondary protection against L. major infection.
Results
DN T cells from healed mice proliferate and produce effector cytokines in response to L. major-infected BMDCs in vitro
Recovery from natural or experimental L. major infection is associated with strong T cell proliferation and IFN-γ production in spleens and dLNs. To investigate the contribution of CD4+ T cells in this process, we co-cultured CD8+ T cell-depleted splenocytes from healed mice with L. major-infected BMDCs in vitro. Surprisingly, we found in addition to CD4+ T cells, strong proliferative and IFN-γ responses by CD3+CD4−CD8− (DN) T cells (Fig. 1A and Fig. S1A and B). Proliferating DN T cells also produced TNF (Fig 1B), IL-17 (Fig. S2A) and little IL-2 (Fig. 1D), suggesting they are polyfunctional in cytokine production. Indeed, most of the IFN-γ-producing DN cells also co-produced TNF (Fig. 1C). Interestingly, although DN T cells proliferate significantly more than CD4+ T cells, their quantitative ability to produce IFN-γ and TNF was significantly lower than those of CD4+ T cells (Fig. S2B–D). In addition, DN T cells also produced GrzB (Fig. 1E), suggesting they may perform effector functions in L. major-infected mice. DN T cells from L. major-infected mice did not proliferate or produce IFN-γ following stimulation with OVA-loaded DCs (Fig. 1F), but were activated by DCs pulsed with SLA or freeze-thawed L. major (Fig. 1F). Collectively, these results suggest that the proliferation and cytokine production by DN T cells from healed mice are L. major specific.
Fig. 1. DN T cells from healed mice proliferate and produce effector cytokines following restimulation with L. major-infected BMDCs in vitro. 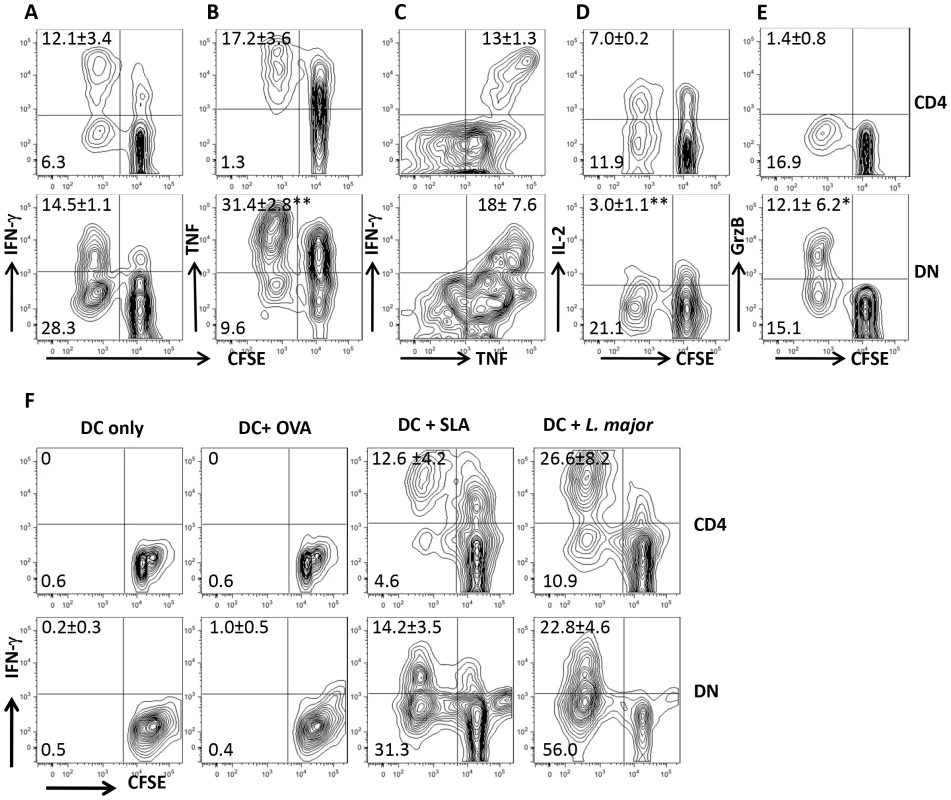
Highly purified CFSE-labeled T cells from spleens of L. major-infected and healed C57BL/6 mice (> 12 weeks) depleted of CD8+ T cells (by treatment with anti-CD8 mAb) were co-cultured with L. major-infected BMDCs for 5 days and proliferation (A and B) and IFN-γ (A) and TNF (B) production by CD4+ and DN T cells were analyzed by flow cytometry following gating on CD3+ T cells. In addition, the co-production of IFN-γ and TNF by CD4+ and DN cells was also assessed (C). DN T cells do not produce appreciable amounts of IL-2 (D) but are strong producers of granzyme B (E). CFSE-labeled T cells were co-cultured with BMDCs only or pulsed with OVA (50 µg/ml), SLA (50 µg/ml), or freeze-thawed L. major (107/ml) and proliferation and IFN-γ production by CD4+ and DN T cells were analyzed (F). Results are representative of 3 (A and B) and 2 (C–F) independent experiments (n = 3–4 mice) with similar results. *, p<0.05; **, p<0.01. DN T cells proliferate and produce IFN-γ in vivo after rechallenge with L. major and display memory markers
Our co-culture system showed that Leishmania-specific DN T cells are activated following in vitro recall response. To determine whether DN T cells are activated in vivo, we adoptively transferred CFSE-labeled T cells from healed Thy1.2 mice into naive Thy1.1 mice that were then challenged with L. major the next day. Both CD4+ and DN T cells from healed donor mice showed extensive proliferation and IFN-γ production compared to those from naive mice (Fig. 2A–D). The in vivo relevance of DN T cell response was further confirmed by BrdU incorporation (Fig. 2E and F). Interestingly and similar to CD4+ T cells, the percentage of proliferating and IFN-γ-producing DN T cells in healed mice were significantly higher than those in naïve mice following L. major challenge, suggesting that DN T cells display functional characteristics of memory T cells (rapid proliferation and cytokine production). Indeed, we found that the percentage of DN T cells in lymph nodes (Fig 3A) of healed mice that express CD62LhiCD44hi (central memory-like) was significantly higher (p<0.05) than those in naive mice (Fig. 3B). Following adoptive transfer of whole T cells from healed mice and subsequent L. major challenge, almost all the proliferating donor CD4+ T cells downregulated their CD62L expression (i.e. were CD62Llo). In contrast, the proliferating DN T cells contained an almost equal proportion of CD62Llo and CD62Lhi populations (Fig. 3C). In addition, more DN T cells were CD62LhiCD44hi compared to CD4+ T cells (Fig. 3D).
Fig. 2. DN T cells proliferate and produce IFN-γ in vivo. 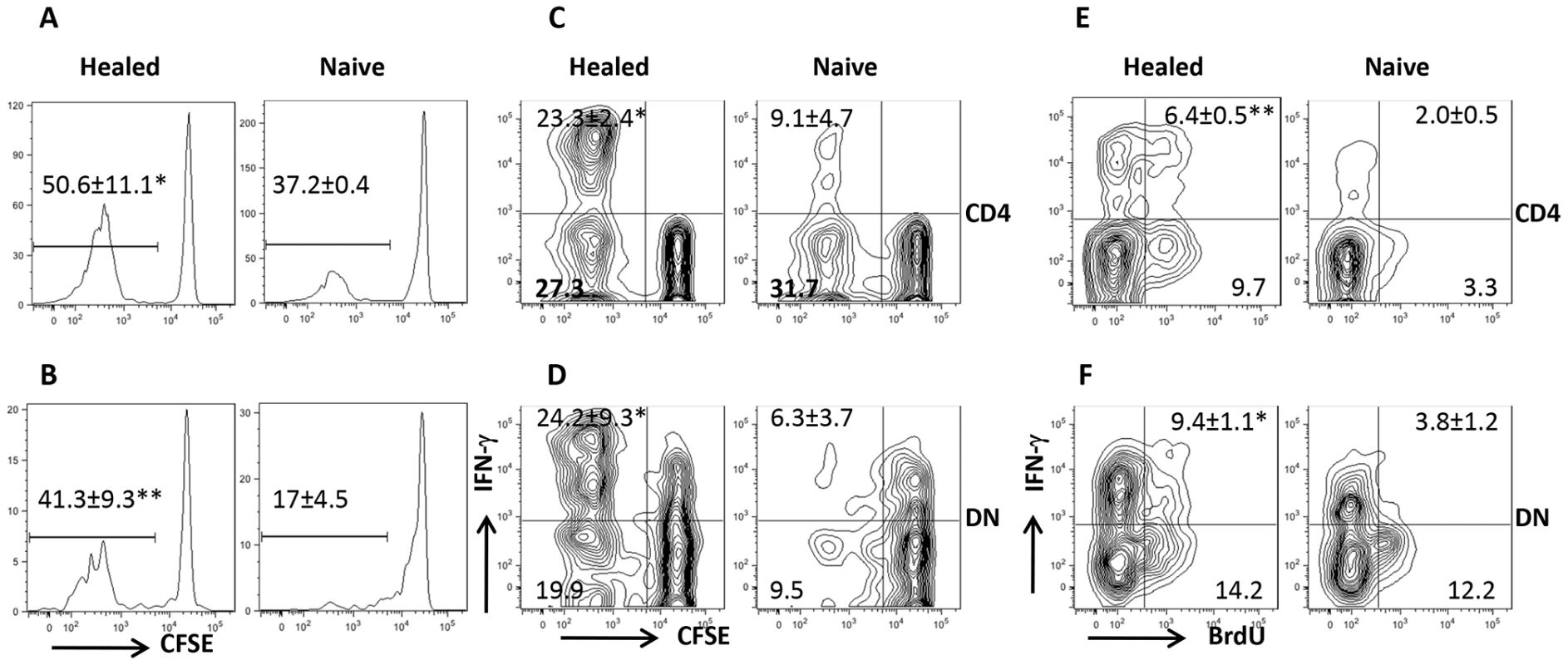
Highly enriched (> 98%) CFSE-labeled CD90.2+ T cells from naïve or healed Thy1.2 mice were adoptively transferred into naïve Thy1.1 recipients that were challenged with 5×106 L. major the next day. Seven days after challenge, mice were sacrificed and cell proliferation (A, B) and IFN-γ production (C, D) by CD4+ (A, C) and DN (B, D) T cells were analyzed directly ex vivo by gating on Thy1.2+CD3+ donor cell population. For BrdU incorporation assay, naïve or healed mice were challenged with L. major, treated daily with BrdU in drinking water and by i.p. injection, sacrificed after 7 days and BrdU incorporation and IFN-γ expression by CD4+ (E) and DN (F) T cells were analyzed. Results are presented as means +/− SE and representative of 2 independent experiments (n = 3–4 mice) with similar results. *, p<0.05; **, p<0.01 vs. naïve controls. Fig. 3. DN T cells from L. major-infected mice display phenotypic characteristic of memory cells. 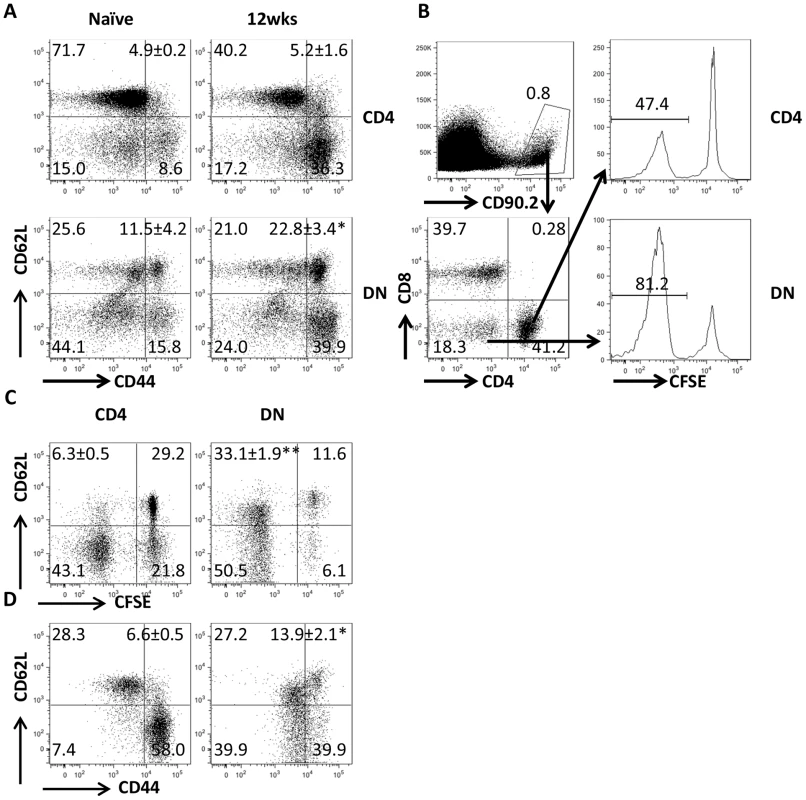
(A) Direct ex vivo expression of CD62L and CD44 on DN and CD4+ T cells from dLNs of naive and healed mice. (B–D) Phenotypic characteristics of Leishmania-reactive DN T cells in vivo. CFSE-labeled splenocytes from healed CD90.2 mice were transferred into naïve CD90.1 recipients that were then challenged with 5× 106 L. major. Challenged mice were sacrificed after 7 days and proliferation of donor CD4+ and DN T cells in dLNs was assessed directly ex vivo (B). In addition, the expression of CD62L within proliferating (C) and CD44hi (D) donor CD4+ and DN T cells was also assessed. Results are representative of 2 independent experiments with similar results using pooled cells from 3 mice with similar results. *, p<0.05; **, p<0.01; vs. CD4+ T cells. DN T cells predominately express αβ-TCR and do not have regulatory properties
In addition to αβ T cells, NKT and γδ T cells also do not express CD4 and CD8 molecules. To determine whether Leishmania-reactive DN T cells are NKT and γδ T cells, we assessed the expression of αβ, γδ and NK1.1 molecules on DN T cells by flow cytometry. As shown in Fig. 4A, DN T cells predominately (> 90%) expressed αβ TCR and not NK1.1 and γδ molecules, indicating that they are not NKT or γδ T cells. To further determine whether DN T cells are CD4+ or CD8+ T cells that have down-regulated their surface molecules following activation, we assessed highly enriched (> 99% purity, Fig. S3) DN, CD4+ and CD8+ T cells for CD4 and CD8 transcripts by RT-PCR. DN T cells did not express CD4 and CD8 mRNA (Fig. 2B), suggesting they are not CD4+ or CD8+ T cells that have down-regulated their surface molecules. In addition, highly purified CD4+, CD8+ and DN T cells maintained their respective phenotypes following in vitro restimulation for 5 days with L. major-infected BMDCs (Fig. 4C). To determine whether Leishmania-reactive DN T cells display regulatory properties as previously reported in other systems [12], [15], [16], we co-cultured CD4+ and DN T cells with L. major-infected BMDCs and assessed CD4+ T cell proliferation and IFN-γ production by flow cytometry. DN T cells did not affect CD4+ cell proliferation and IFN-γ production (Fig. 4D), suggesting that they do not exhibit regulatory/suppressive properties.
Fig. 4. DN T cells predominately express αβ-TCR and do not have regulatory properties. 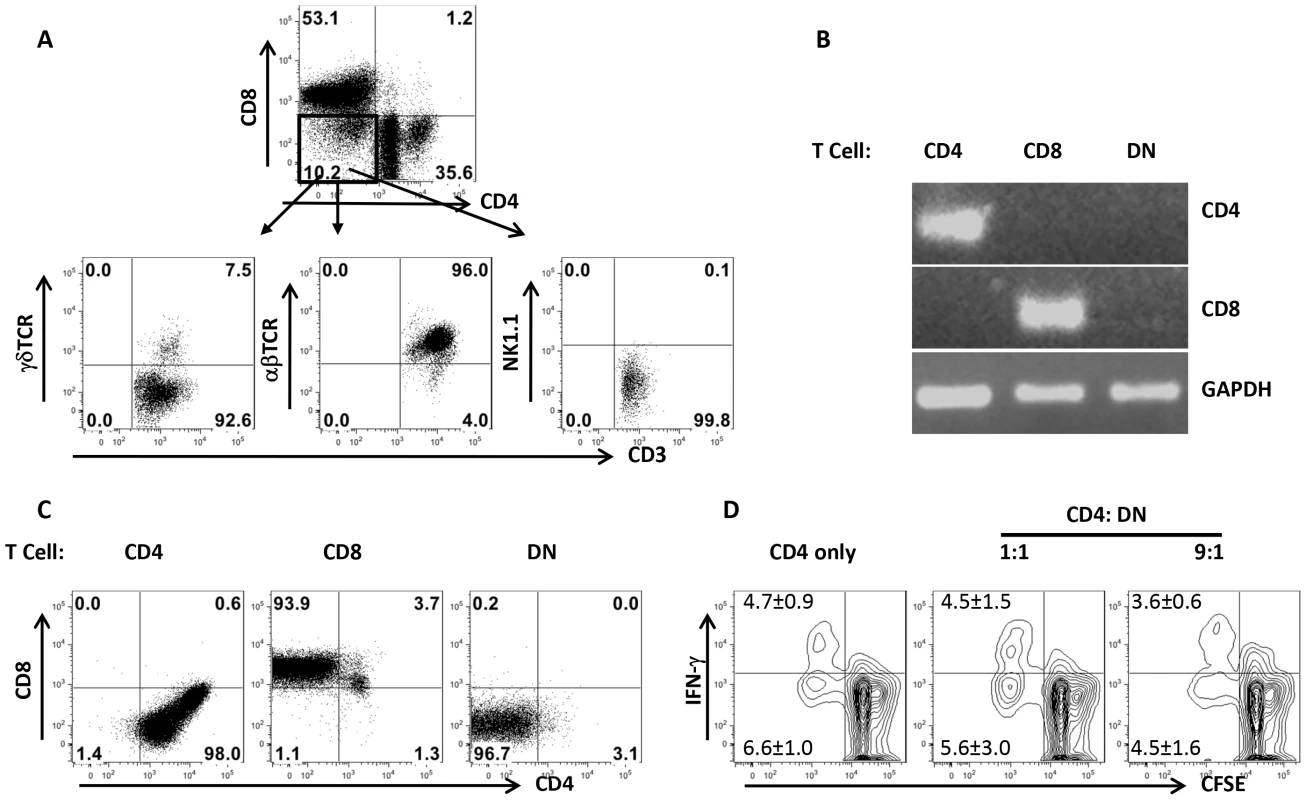
Highly purified T cells from healed mice were co-cultured with L. major-infected BMDCs for 5 days and the expression of αβ, γδ and NK1.1 molecules by DN T cells was analyzed by flow cytometry (A). In some experiments, CD4+, CD8+ and DN T cells were enriched by cell sorting and the expression of CD4 and CD8 gene transcripts in the enriched cells was assessed by RT-PCR (B). Highly purified CD4+, CD8+ and DN T cells were further stimulated with L. major-infected BMDCs for 5 days and CD4 and CD8 expression was analyzed by flow cytometry (C). Purified CFSE-labeled CD4+ cells from healed mice were cultured L. major infected BMDCs either alone or with DN T cells for 5 days and CD4+ T cell proliferation and IFN-γ production were analyzed by flow cytometry (D). Results are representative of 2–3 independent experiments with similar results. DN T cells are mostly restricted by MHC II
To determine whether Leishmania-reactive DN T cells are restricted by MHC II molecule, we co-cultured highly enriched T cells from healed mice with infected BMDCs in the presence or absence of anti-MHC II antibodies. Anti-MHC II antibodies blocked proliferation and IFN-γ production by both CD4+ and DN T cells in a dose-dependent manner (Fig. 5A). In addition, L. major-infected BMDCs from MHC II KO mice failed to induce proliferation and IFN-γ production by DN and CD4+ T cells (Fig. 5B). In contrast, proliferation and IFN-γ production by DN T cells were minimally affected following co-culture with infected BMDCs from CD1d KO mice (Fig. 5C), confirming that DN T cells are mostly restricted by MHC II molecules.
Fig. 5. DN T cells are restricted by MHC II molecules. 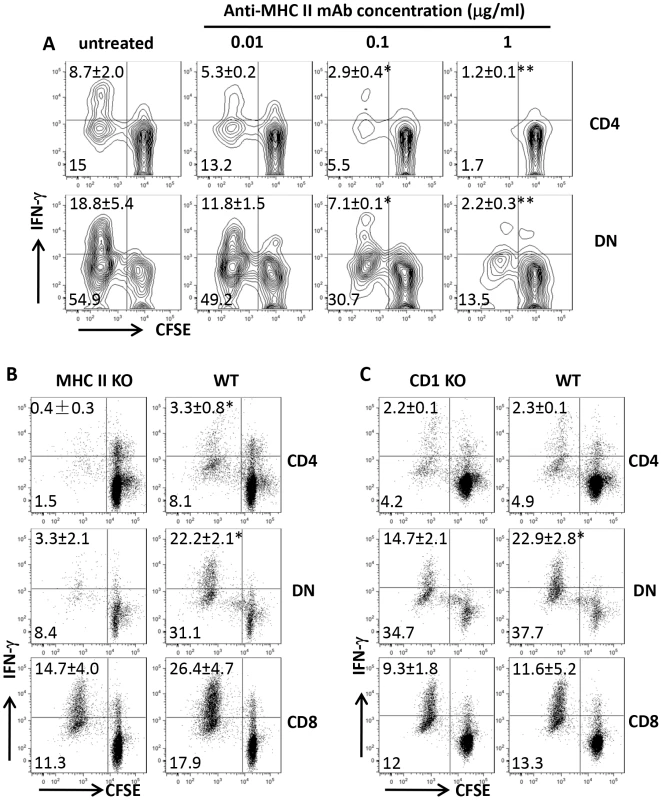
CFSE-labeled highly purified T cells from spleens of healed mice were co-cultured with L. major-infected BMDCs with or without varying concentrations of anti-MHC II mAb and proliferation and IFN-γ production by CD4+ and DN T cells were assessed after 5 days (A). In some experiments, the ability of infected BMDCs from MHC II KO (B) or CD1d KO (C) mice to induce proliferation and IFN-γ production by CD4+, CD8+ and DN T cells was compared with those from WT controls. Results are representative of 2–3 independent experiments with similar results. *, p<0.05; **, p<0.01; mAb treated vs. isotype controls or KO vs. WT groups. DN T cells are activated during primary L. major infection
We found that Leishmania-reactive DN T cells are recalled in healed mice following L. major challenge in vitro and in vivo suggesting that they may be induced following primary infection. To determine this, we assessed CD4+ and DN T cells response in the dLNs and spleens of infected mice C57BL/6 mice at different times after infection corresponding to early, peak and resolution of lesion progression (Fig. 6A). As expected, there was strong CD4+ T cell response (proliferation and IFN-γ production, Fig. 6B) at all times (3, 6 and 12 weeks) post-infection. Similarly, DN T cells from infected mice also strongly proliferated and produced IFN-γ following restimulation with infected BMDCs (Fig. 6C). In contrast, CD4+ and DN T cells from naïve mice did not proliferate or produce IFN-γ upon stimulation with L. major-infected BMDCs (Fig. 6B and C). Collectively, these results show that Leishmania-reactive DN T cells are induced during primary L. major infection and could contribute to anti-Leishmania immunity.
Fig. 6. DN T cells are activated during primary L. major infection. 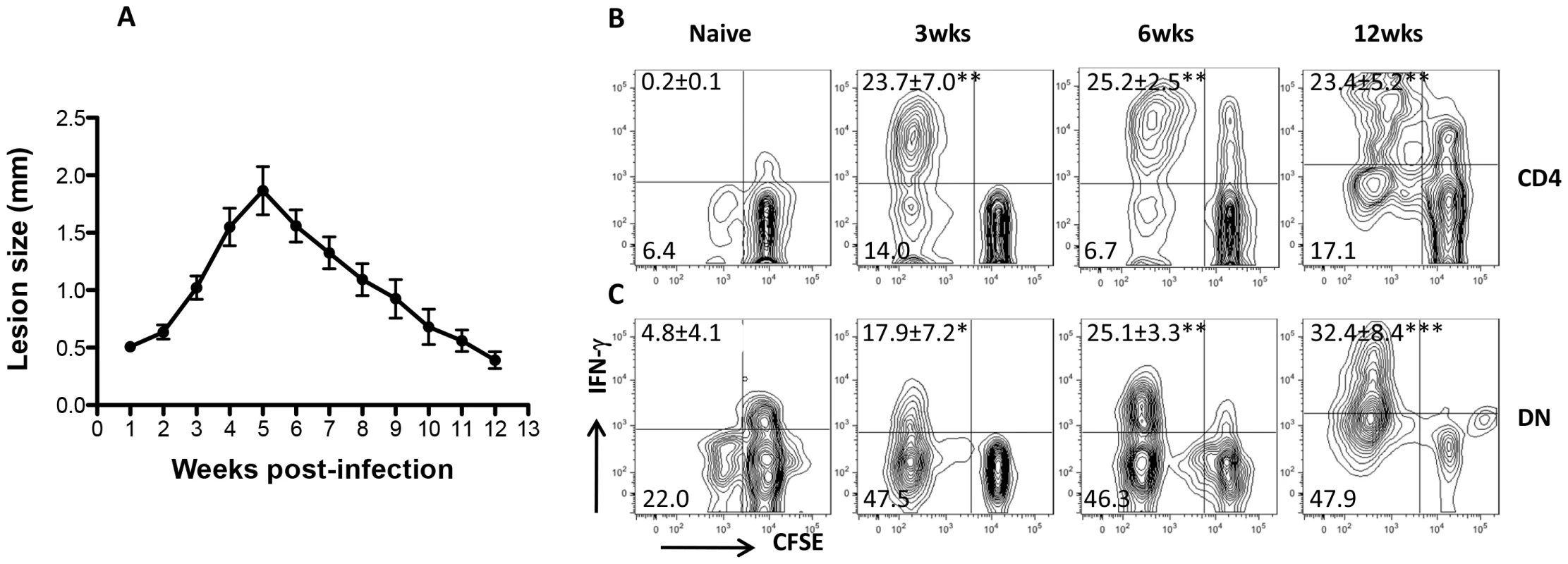
C57BL/6 mice were infected with 2×106 L. major and lesion progression was monitored weekly with calipers (A). At indicated times, infected mice were sacrificed and pooled splenic and dLN T cells were co-cultured with L. major-infected BMDCs for 5 days and cell proliferation and IFN-γ production by CD4+ (B) and DN (C) T cells were analyzed by flow cytometry. Results are representative of 2–3 independent experiments (n = 3–4 mice) with similar results. *, p<0.05; **, p<0.01; ***, p<0.001 infected vs. naïve (uninfected) controls. DN T cells contribute to optimal primary and secondary immunity against L. major
To determine if DN T cells contribute to primary immunity against L. major, we selectively depleted CD4+ and CD8+ or all T cells by treatment with anti-CD4/CD8 or anti-Thy1.2 mAbs, respectively, during the course of primary L. major infection (Fig. S4). Mice depleted of both CD4+ and CD8+ T cells still had some IFN-γ-producing CD3+ DN T cells (Fig. 7A and 7B) and harbor significantly (p<0.01) lower parasite burden (Fig. 7C) compared to those depleted of all T cells (by anti-Thy1.2 mAb treatment), indicating that DN T cells contribute to optimal control of parasite proliferation during primary L. major infection.
Fig. 7. DN T cells contribute to optimal primary and secondary immunity against L. major infection. 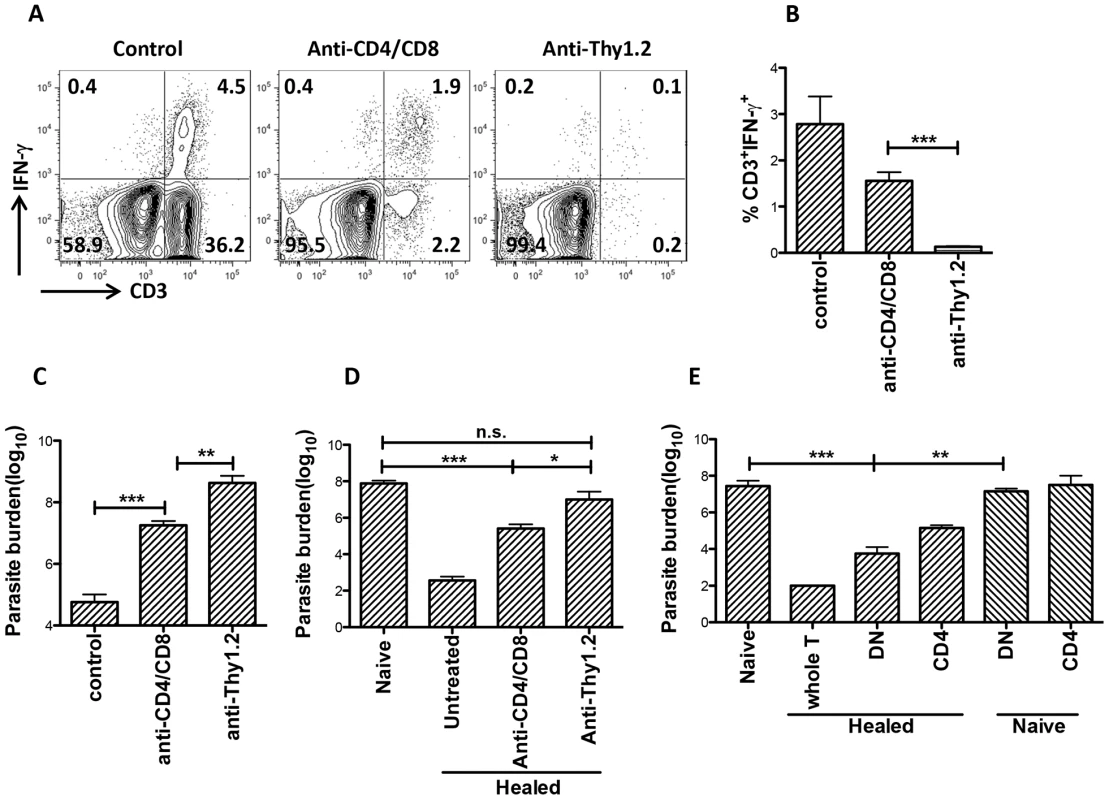
Naïve (A–C) or healed (> 12 weeks post infection, D) C57BL/6 mice were treated with anti-Thy1.2 or combined anti-CD4/anti-CD8 mAbs once weekly and challenged with 5×106 L. major 3 days after the first antibody treatment. At 3 weeks post-challenge, mice were sacrificed and dLN cells were stained directly ex vivo the percentage of CD3+IFN-γγ cells was determined by flow cytometry (A and B). Parasite burden in the challenged footpads was determined by limiting dilution (C and D). Highly enriched CD4+ and DN (> 99% purity) T cells from healed or naïve mice were transferred into naïve mice that were then challenged with 5×106 L. major. Challenged mice were sacrificed after 3 weeks to determine parasite burden (E). Results are representative of 2 independent experiments (n = 3–4 mice) with similar results. *, p<0.05; **, p<0.01; ***, p<0.001; anti-CD4/CD8 vs. anti-Thy1.2 mAb groups and healed DN vs. naïve DN recipient groups (E). Next, we used both in vitro and in vivo approaches to investigate whether DN T cells contribute to secondary anti-Leishmania immunity. Highly purified DN T cells from healed (but not naïve) mice significantly (p<0.05) inhibited parasite proliferation in infected BMDMs and this effect was comparable to those of CD4+ T cells (Fig. S5A and B). These results provide direct in vitro evidence that DN T cells could control parasite growth in L. major-infected BMDMs.
Next, we used two different experimental approaches to determine whether DN T cells contribute to secondary anti-Leishmania immunity in vivo. First, we selectively depleted CD4+ and CD8+ or all CD3+ T cells (as in Fig. 7A above) in healed mice and after 24 hr, rechallenged them with L. major. As shown in Fig. 7D, CD4+ and CD8+ T cells-depleted mice, which still had DN T cells, retained some level of infection-induced resistance as evidenced by significantly (p<0.01) lower parasite burden compared to naïve mice (primary infection). In contrast, depletion of all CD3+ T cells completely abrogated secondary immunity (Fig. 7D). Second, we assessed the ability of highly enriched (purity > 96%, Fig. S6) DN T cells from healed mice to protect naïve animals against virulent L. major challenge. Adoptively transferred DN T cells from healed mice protected naïve mice against virulent L. major challenge as evidenced by significantly lower parasite burden (Fig. 7E). Collectively, these in vitro and in vivo observations strongly implicate Leishmania-reactive DN T cells in contributing to optimal anti-Leishmania immunity in mice.
Increased transcriptional activity of innate genes in Leishmania-reactive DN T cells
Apart from lacking CD4 molecules, DN T cells display functional characteristics similar to CD4+ T cells (MHC-II restriction, proliferation, IFN-γ production and parasite control). To further investigate how Leishmania-reactive DN T cells differ from CD4+ T cells, we compared the transcriptional profile of proliferating DN and CD4+ T cells following restimulation with L. major-infected BMDCs. Although most of the 84 mouse innate and adaptive immune genes showed similar pattern and level of expression in both cell types, some genes were preferentially upregulated or downregulated in DN T cells compared to CD4+ T cells (Fig. 8A). The gene transcripts showing ≥ 2 folds difference in DN T cells were further analyzed and validated by quantitatively real-time PCR (Fig 8B and C). Interestingly, most of the upregulated transcripts in DN T cells were genes associated with innate immune responses, including C3, Mac-1 (CD11b), myeloperoxidase (Mpo), lysozyme, etc. In contrast, the downregulated transcripts (relative to CD4+ T cells) included genes associated with adaptive immunity, including CCR4, Foxp3, Gata-3, etc. Collectively, these results suggest that despite mediating anti-Leishmania immunity (akin to CD4+ T cells), Leishmania-reactive DN T cells are phenotypically distinct from conventional CD4+ T cells.
Fig. 8. Enhanced transcription of innate genes in Leishmania-reactive DN T cells. 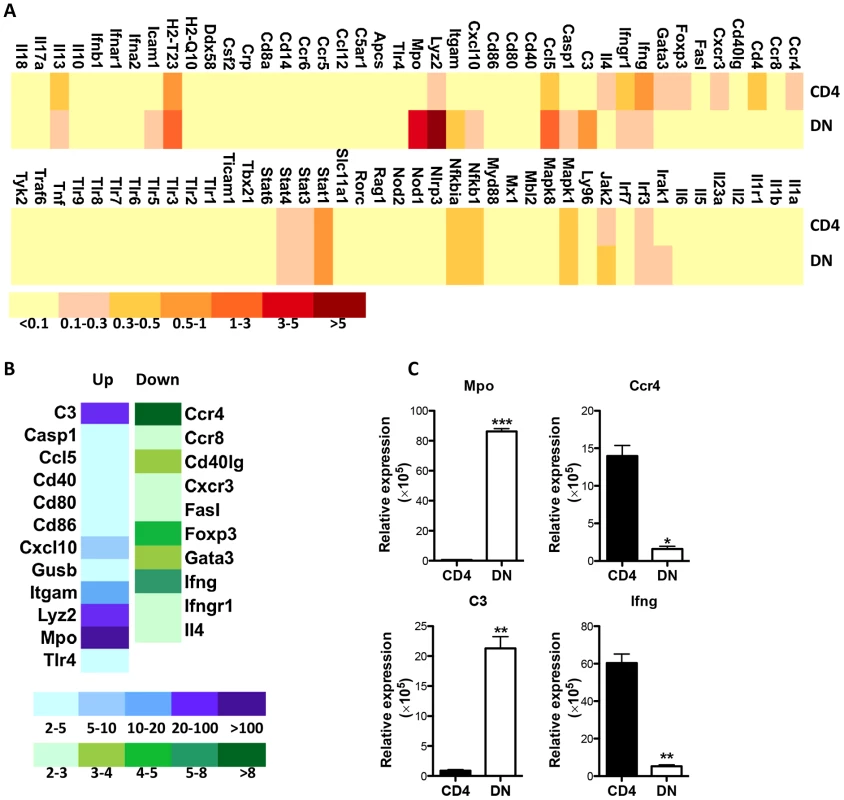
CFSE-labeled pooled splenocytes and dLN cells from healed mice were stimulated with L. major-infected BMDCs for 5 days and total mRNA was isolated from CFSElo (proliferating) CD4+ and DN T cells that were purified by cell sorting and used for PCR array. (A) Heat map showing gene expression profile by proliferating CD4+ and DN T cells. Relative expression levels were shown following normalization with internal controls. The genes expressing greater than two fold changes between CD4+ and DN T cells were further analyzed (B) and their expression levels were validated by qPCR following normalization with the housekeeping gene, 18S (C). *, p<0.05; **, p<0.01; ***, p<0.001. Discussion
We show here that DN T cells proliferate and produce effector cytokines in secondary lymphoid organs of mice following primary and secondary L. major challenges. DN T cells from healed mice display functional characteristics of anti-Leishmania memory-like cells: they rapidly proliferate and produce effector cytokines (TNF and IFN-γ) in response to L. major challenge in vitro and in vivo and mediate infection-induced immunity (rapid protection) following adoptive transfer in vivo. Leishmania-reactive DN T cells express predominantly αβ TCR, are restricted by MHC class II molecules, lack immunoregulatory properties and display transcriptional profile distinct from conventional CD4+ T cells. To the best of our knowledge, this is the first extensive characterization and demonstration of the protective ability of Leishmania-reactive DN T cells in vitro and in vivo.
It is generally believed that CD4+ T cells play a dominant role in anti-Leishmania immunity. However, the finding that CD4 deficient mice were resistant while MHC class II deficient mice were highly susceptible to L. major challenged this dogma [10] and suggests that MHC II-restricted CD4−CD8− T cells may be more important in regulating primary anti-Leishmania immunity. Indeed, several studies have reported the expansion of CD3+CD4−CD8− (DN) T cells in the blood of Leishmania-infected patients and dogs, and in spleens of Leishmania-infected mice [11], [12], [13], [14]. These cells have been proposed to contribute to primary and vaccine-induced immunity although a concrete evidence implicating them in immunity has not yet been demonstrated. Our studies directly show the importance of Leishmania-reactive DN T cells in mediating optimal primary and secondary anti-Leishmania immunity in mice.
The precise origin and development of peripheral DN T cells is not clearly understood and is controversial. Some reports suggest that DN T cells originate in the thymus by escaping negative selection [17], [18], [19]. In contrast, several reports suggest that DN T cells are generated in the periphery rather than in the thymus [19], [20], [21], [22]. These cells comprise about 1–5% of total T cells in non-transgenic mice and in humans [11], [23] making them difficult to isolate and subsequently study. TCR transgenic [24] or lpr (Fas mutation) mice [19], [25], which present increasing accumulation of DN T cells are widely used to investigate the function and developmental origin of DN T cells. DN T cells have been shown to influence long-term allograft survival [24], [26], [27], prevent the development of autoimmune disease [28], [29], [30], and contribute to control of intracellular pathogens [31], [32]. In addition, DN T cells have been shown to possess immunoregulatory and alloreactive properties, inhibit autoreactive CD4+ T cells and mediate MHC I-restricted killing of allogenic target cells [20], [24], [25]. Our studies show that Leishmania-reactive DN T cells are restricted by MHC class II and may not have immunoregulatory properties because they failed to suppress CD4+ T cell proliferation in vitro (Fig. 4D). Rather, a large percentage of proliferating DN T cells produced IFN-γ, TNF, IL-17 and GrzB, which is consistent with their effector functions as seen in other studies [11], [12], [29].
Previous studies that have reported the expansion and possible protective role of DN T cells in leishmaniasis focused mainly on primary Leishmania infection [11], [12], [13], [14]. We extend these studies during secondary immunity by showing rapid expansion and effector functions (cytokine production and parasite control) by DN T cells following challenge infection. Healed mice had more proliferating and IFN-γ-producing DN T cells compared with naive mice following L. major challenge (Fig. 2), and adoptive transfer of DN T cells from healed (but not naïve mice) rapidly protected naïve mice against virulent L. major change. Moreover, DN T cells from healed mice expressed high levels of CD44 and majority of them were CD62LhiCD44hi, which are characteristics markers expressed by central memory-like cells. Collectively, these results suggest that DN T cells display functional characteristics of memory cells and contribute to optimal secondary immunity against L. major.
How do DN T cells mediate their anti-Leishmania immunity? We speculate that this may be related in part to their ability to produce IFN-γ and TNF, key cytokines that activate infected macrophages leading to intracellular parasite killing. Indeed, we found that Leishmania-reactive DN T cells in the spleens and lymph nodes are highly proliferative and produce IFN-γ, TNF and granzyme B. Importantly, we also found that DN T cells from immune mice were recruited to and proliferate at the infected footpads (Fig. S7). In addition, our in vitro co-culture experiments with infected BMDMs and highly enriched DN T cells show that suppression of parasite proliferation was associated with increased nitric oxide production, a key effector molecule that mediate destruction of parasites in infected cells.
The findings that DN T cells mediate comparable (or even superior) protection against L. major in vitro and in vivo may challenge the dogma that CD4+ T cells are the major T cell subset that mediates anti-Leishmania immunity. Indeed, the proliferation of DN T cells was either comparable or sometimes higher than those of CD4+ T cells following in vitro or in vivo L. major challenge (see Figs. 1–3). Interestingly, although the percentage of IFN-γ-producing DN T cells was sometimes higher than those of CD4+ T cells, their MFI was significantly lower (Fig. 1C), an observation that explain the relatively lower IFN-γ transcripts in DN compared to CD4+ T cells (Fig. 8). In addition, the numbers of Leishmania-reactive CD4+ T cells were quantitatively (∼ 3–4 fold) higher than those of DN T cells. Thus, despite their superior proliferative response, DN T cells may still play a subordinate role to CD4+ T cells in vivo. Furthermore, it is conceivable that CD4+ T cells may be required for proper activation and effector functions of DN T cells. In line with this, we have observed that proliferation and IFN-γ production by highly enriched DN T cells is impaired in cultures devoid of immune CD4+ T cells in vitro and in vivo (Fig. S8). It is conceivable that DN T cells may assume increased roles in the absence of CD4+ T cells. For example, SIV infection in nonhuman primates does not result in immune dysfunction and progression to simian AIDS because DN T cells partially compensate for defective CD4+ T cell functions upon SIV-induced CD4+ T cell depletion in these animals [33], [34]. Similarly, a strong DN T cell-mediated HIV Gag-specific response has been associated with seronegativity in HIV-exposed individuals [35].
It is interesting that the expression of genes associated with innate immune responses including C3, were significantly higher in Leishmania-reactive DN T cells than in CD4+ T cells. While commonly associated with initiation of inflammation and critical molecule involved in first line of defense against pathogens, the complement proteins, particularly C3 and its degradation fragments are also known to prominently influence the adaptive immunity [36], [37]. Recent studies have been shown that some subset of T cells express C3 and that its intracellular activation is not only required for homeostatic T cell survival [38], but also in optimal Th1 induction and differentiation into effector cytokine (particularly IFN-γ) production [38], [39]. It is conceivable that C3-expressing DN T cells in L. major-infected mice might be involved in IFN-γ production leading to effective macrophage activation, nitric oxide production and parasite killing.
Collectively, our studies provide direct evidence for DN T cells in mediating anti-Leishmania immunity akin to CD4+ T cells. We propose that DN T cells complement CD4+ T cells to mediate efficient primary and secondary anti-Leishmania immunity in mice. In the absence of DN T cells, the induction of effective anti-Leishmania immunity may be either delayed or impaired. In a recent preliminary study, we observed impaired induction of DN T cells in spleens and draining lymph nodes of L. major-infected highly susceptible BALB/c mice. It would be interesting to determine whether the susceptibility of BALB/c mice to L. major infection is related in part to this impaired expansion of DN T cells. Collectively, our studies clearly identify DN T cells as important subset of T cells that contribute to optimal anti-Leishmania immunity.
Materials and Methods
Ethics statement
All mice were kept at the University of Manitoba Central Animal Care Services (CACS) facility in accordance to the Canadian Council for Animal Care guidelines. The University of Manitoba Animal Use Ethics Committee approved all studies involving animals, including infection, humane endpoints, euthanasia and collection of samples.
Mice
Six to 8 wk-old female C57BL/6 (Thy1.2) mice were obtained from Charles River, St Constante PQ, Canada. Thy1.1 and MHC class II deficient (MHC II KO) C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Female CD1d deficient C57BL/6 mice were kindly supplied by Dr. Xi Yang from a breeding colony maintained at the University of Manitoba Central Animal Care Services (CACS) Facility.
Infection protocol and parasite quantification
Leishmania major parasites (MHOM/80/Fredlin) were grown in M199 culture medium (Sigma, St. Louis, MO) supplemented with 20% heat inactivated FBS (HyClone, Logan, UT), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. For infection, mice were injected with 2×106 (primary infection) or 5×106 (secondary infection) stationary-phase promastigotes in 50 µl PBS suspension into the right (primary) or left (secondary) hind footpad. Lesion sizes were monitored weekly by measuring footpad swelling with calipers. Parasite burden in the infected footpads was determined by limiting dilution assay. Parasite titers were determined from the highest dilution at which growth was visible.
Bone marrow-derived macrophages (BMDMs) and dendritic cells (BMDCs) and in vitro infection
Bone marrow cells were isolated from the femur and tibia of naïve C57BL/c mice and differentiated into macrophages using complete medium supplemented with 30% L929 cell culture supernatant as previously described [40]. BMDCs were differentiated in petri dishes in the presence of rmGM-CSF (20 ng/ml; Peprotech, Rocky Hill, NJ). BMDMs and BMDCs were infected at a cell-to-parasite ratio of 1∶5 and after 6 hr, free parasites were washed away and infected BMDCs were used to stimulate purified CD3+, CD4+ or DN T cells from naïve or healed mice in vitro. To assess the ability of CD4+ or DN T cells to control parasite proliferation, infected BMDMs were co-cultured with CD4+ or DN T cells and parasite proliferation in infected BMDMs was determined at different times by counting Giemsa-stained cytospin preparations under light microscope at ×100 (oil immersion) objective.
In vitro recall responses
Infected mice were sacrificed and spleens and dLNs were collected and made into single-cell suspensions. Cells were labeled with CFSE dye (1.5 mM; Molecular Probes, Eugene, OR) and resuspended at a concentration of 2×106 cells per milliliter in RPMI 1640 supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 µg/ml streptomycin, and 5×10−5 M 2-mercaptoethanol (complete medium), plated with 100 µl per well in 96-well tissue culture plates, and stimulated with infected BMDCs (BMDC: T cell = 1∶100) or soluble anti-CD3/CD28 mAb (1 µg/ml; BioLegend, San Diego, CA). After 5 days, proliferation and cytokine production were determined by flow cytometry. In some experiments, CFSE-labeled T cells from spleens and dLNs of infected mice were co-cultured with L. major-infected WT, MHC II KO, or CD1d KO BMDCs for 5 days, stimulated with PMA, BFA and ionomycin for 4–6 hr and proliferation, IFN-γ, TNF, IL-2, IL-17 and GrzB expression by different T cell subsets were analyzed by flow cytometry. In some experiments, anti-MHC II antibodies were used to block MHC II-TCR interaction in vitro.
Purification of T cell subsets, cell labeling and adoptive transfer experiments
Healed (> 12 weeks post-infection) Thy1.2 C57BL/6 mice were sacrificed and single-cell suspensions from the dLNs and spleens were made. T cells (Thy1.2+) were enriched by positive selection using mouse CD90.2 (Thy1.2) selection kit according to the manufacturer's protocols (StemCell Technologies, Vancouver, BC). Enriched T cells (> 98% purity) were labeled with CFSE dye, and 107 cells were adoptively transferred into naive congenic (Thy1.1) mice by tail vein injection. After 24 hr, the recipient mice were challenged with 5×106 L. major, sacrificed after 7 days and cell proliferation and IFN-γ expression by donor (Thy1.2) cells in the dLNs and spleens were determined directly ex vivo.
Enrichment of DN T cells
For in vitro co-culture experiments, DN (CD3+CD4−CD8−) and CD4+ T cells were purified from pooled spleens and dLNs of healed or naïve mice by cell sorting (FACSAria III, BD Biosciences). For in vivo adoptive transfer studies, DN T cells were enriched using a combination of in vivo depletion and positive selection. Briefly, L. major-infected and healed mice (> 12 weeks post-infection) were first injected with 200 µl GK1.5 and TIB210 ascites (i.p) to deplete CD4+ and CD8+ cells. After 48 hr, DN T cells were purified using mouse CD90.2 selection kit (StemCell Technologies, Vancouver, BC). Enriched DN T cells were > 99% negative for CD4 and CD8 expression and > 95% positive for CD3 by flow cytometry.
Isolation of cells from infected footpads
To assess the numbers (percentages) and proliferation of DN T cells at the site of infection, CFSE-labeled whole T cells from L. major-infected Thy1.2 mice were adoptively transferred into naïve Thy1.1 mice that were then challenged with L. major. After 7 days, recipient mice were sacrificed and donor cells were recovered from the footpads as we previously described [41]. Briefly, the footpads were disinfected in 70% ethanol, the skins were peeled off and homogenized gently in PBS with tissue grinders. The crude homogenates were resuspended in 7 ml of cold PBS, carefully layered on top of 5 ml Ficoll and the infiltrating cells were separated by centrifugation according to the manufacturer's suggested protocols. The cells were collected, resuspended in 5 ml complete medium, counted, stained directly for expression of various cell surface markers and analyzed by flow cytometer by gating on Thy1.2+ donor cells.
RT-PCR, PCR array and qPCR
For reverse transcription-PCR (RT-PCR), cells from spleens of healed mice were stained with fluorescent-conjugated anti-CD3, anti-CD4 and anti-CD8 antibodies. CD4, CD8 and DN T cells were sorted to high purity by gating on CD3+ cells. CD4, CD8 and GAPDH gene expression in sorted cells were analyzed by RT-PCR. For PCR array, CFSE-labeled whole spleen cells from healed mice were stimulated with L. major-infected BMDCs for 5 days and proliferating (CFSElo) CD4+ and DN T cells were purified by cell sorting. Eighty-four innate and adaptive immune genes in CD4+ and DN T cells were analyzed with Mouse Innate & Adaptive Immune Responses PCR Array kit (Qiagen, Frederick, MD). PCR array was performed by a real-time cycler (Bio-Rad CFX96) and analyzed with web-based PCR Array Data Analysis Software (Qiagen, Frederick, MD). To quantify gene expression levels, equal amounts of cDNA were mixed with SYBR Green PCR master mix (Toyobo, Osaka, Japan) and primers specific for the gene of interest (Table S1). 18S rRNA was amplified as an internal control.
BrdU treatment
Naïve and healed mice were injected with 2 mg of BrdU i.p. per mouse and then challenged with 5×106 L. major in the next day. BrdU solution was prepared in sterile water, protected from light exposure, and changed daily. The night before the assay, mice were injected i.p. with 0.8 mg of BrdU in PBS. The next day, mice were sacrificed, spleens were harvested and BrdU staining was performed using BrdU Staining Kit according to the manufacturer's suggested protocol (BD PharMingen).
Antibody depletion in vivo
Healed mice were depleted of CD4 and/or CD8 T cells by injecting i.p. 200 µl ascites containing anti-CD4 (GK1.5) or anti-CD8 (TIB 210) mAb (or both) per mouse or depleted of total T cells by injecting i.p. 100 µg anti-Thy1.2 mAb (TIB 107) per mouse, once a week, and then challenged with 5×106 L. major.
Statistics
Data are presented as means and standard error of mean (SEM). Two-tailed Student's t-test or ANOVA were used to compare means and SEM between groups using GraphPad Prism software. Differences were considered significant at p<0.05.
Supporting Information
Zdroje
1. ReithingerR, DujardinJC, LouzirH, PirmezC, AlexanderB, et al. (2007) Cutaneous leishmaniasis. Lancet Infect Dis 7 : 581–596.
2. SacksD, Noben-TrauthN (2002) The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol 2 : 845–858.
3. PetersNC, EgenJG, SecundinoN, DebrabantA, KimblinN, et al. (2008) In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science 321 : 970–974.
4. ZaphC, UzonnaJ, BeverleySM, ScottP (2004) Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nature medicine 10 : 1104–1110.
5. LiuD, ZhangT, MarshallAJ, OkkenhaugK, VanhaesebroeckB, et al. (2009) The p110delta isoform of phosphatidylinositol 3-kinase controls susceptibility to Leishmania major by regulating expansion and tissue homing of regulatory T cells. Journal of immunology 183 : 1921–1933.
6. KayeP, ScottP (2011) Leishmaniasis: complexity at the host-pathogen interface. Nature reviews Microbiology 9 : 604–615.
7. SwihartK, FruthU, MessmerN, HugK, BehinR, et al. (1995) Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. The Journal of experimental medicine 181 : 961–971.
8. WangZE, ReinerSL, ZhengS, DaltonDK, LocksleyRM (1994) CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. The Journal of experimental medicine 179 : 1367–1371.
9. WeiXQ, CharlesIG, SmithA, UreJ, FengGJ, et al. (1995) Altered immune responses in mice lacking inducible nitric oxide synthase. Nature 375 : 408–411.
10. LocksleyRM, ReinerSL, HatamF, LittmanDR, KilleenN (1993) Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science 261 : 1448–1451.
11. AntonelliLR, DutraWO, OliveiraRR, TorresKC, GuimaraesLH, et al. (2006) Disparate immunoregulatory potentials for double-negative (CD4 - CD8-) alpha beta and gamma delta T cells from human patients with cutaneous leishmaniasis. Infect Immun 74 : 6317–6323.
12. GollobKJ, AntonelliLR, FariaDR, KeesenTS, DutraWO (2008) Immunoregulatory mechanisms and CD4-CD8 - (double negative) T cell subpopulations in human cutaneous leishmaniasis: a balancing act between protection and pathology. Int Immunopharmacol 8 : 1338–1343.
13. Alexandre-PiresG, de BritoMT, AlgueroC, MartinsC, RodriguesOR, et al. (2010) Canine leishmaniasis. Immunophenotypic profile of leukocytes in different compartments of symptomatic, asymptomatic and treated dogs. Veterinary immunology and immunopathology 137 : 275–283.
14. Lezama-DavilaCM, GallagherG (1995) CD4+, CD8+ and CD4 - CD8 - T cell-subsets can confer protection against Leishmania m. mexicana infection. Mem Inst Oswaldo Cruz 90 : 51–58.
15. HillhouseEE, BeauchampC, Chabot-RoyG, DugasV, LesageS (2010) Interleukin-10 limits the expansion of immunoregulatory CD4-CD8 - T cells in autoimmune-prone non-obese diabetic mice. Immunology and cell biology 88 : 771–780.
16. VoelklS, GaryR, MackensenA (2011) Characterization of the immunoregulatory function of human TCR-alphabeta+ CD4 - CD8 - double-negative T cells. European journal of immunology 41 : 739–748.
17. PriatelJJ, UttingO, TehHS (2001) TCR/self-antigen interactions drive double-negative T cell peripheral expansion and differentiation into suppressor cells. Journal of immunology 167 : 6188–6194.
18. WangR, Wang-ZhuY, GreyH (2002) Interactions between double positive thymocytes and high affinity ligands presented by cortical epithelial cells generate double negative thymocytes with T cell regulatory activity. Proceedings of the National Academy of Sciences of the United States of America 99 : 2181–2186.
19. D'AcquistoF, CromptonT (2011) CD3+CD4-CD8 - (double negative) T cells: saviours or villains of the immune response? Biochemical pharmacology 82 : 333–340.
20. PrinsRM, IncardonaF, LauR, LeeP, ClausS, et al. (2004) Characterization of defective CD4-CD8 - T cells in murine tumors generated independent of antigen specificity. J Immunol 172 : 1602–1611.
21. FordMS, ZhangZX, ChenW, ZhangL (2006) Double-negative T regulatory cells can develop outside the thymus and do not mature from CD8+ T cell precursors. Journal of immunology 177 : 2803–2809.
22. ZhangD, YangW, DegauqueN, TianY, MikitaA, et al. (2007) New differentiation pathway for double-negative regulatory T cells that regulates the magnitude of immune responses. Blood 109 : 4071–4079.
23. FischerK, VoelklS, HeymannJ, PrzybylskiGK, MondalK, et al. (2005) Isolation and characterization of human antigen-specific TCR alpha beta+ CD4(−)CD8 - double-negative regulatory T cells. Blood 105 : 2828–2835.
24. ZhangZX, YangL, YoungKJ, DuTempleB, ZhangL (2000) Identification of a previously unknown antigen-specific regulatory T cell and its mechanism of suppression. Nature medicine 6 : 782–789.
25. YoungKJ, KayLS, PhillipsMJ, ZhangL (2003) Antitumor activity mediated by double-negative T cells. Cancer Res 63 : 8014–8021.
26. YoungKJ, YangL, PhillipsMJ, ZhangL (2002) Donor-lymphocyte infusion induces transplantation tolerance by activating systemic and graft-infiltrating double-negative regulatory T cells. Blood 100 : 3408–3414.
27. LeeBP, MansfieldE, HsiehSC, Hernandez-BoussardT, ChenW, et al. (2005) Expression profiling of murine double-negative regulatory T cells suggest mechanisms for prolonged cardiac allograft survival. Journal of immunology 174 : 4535–4544.
28. FordMS, ChenW, WongS, LiC, VanamaR, et al. (2007) Peptide-activated double-negative T cells can prevent autoimmune type-1 diabetes development. European journal of immunology 37 : 2234–2241.
29. DuncanB, Nazarov-StoicaC, SurlsJ, KehlM, BonaC, et al. (2010) Double negative (CD3+ 4 - 8-) TCR alphabeta splenic cells from young NOD mice provide long-lasting protection against type 1 diabetes. PLoS One 5: e11427.
30. HillhouseEE, LesageS (2013) A comprehensive review of the phenotype and function of antigen-specific immunoregulatory double negative T cells. Journal of autoimmunity 40 : 58–65.
31. CowleySC, HamiltonE, FrelingerJA, SuJ, FormanJ, et al. (2005) CD4-CD8 - T cells control intracellular bacterial infections both in vitro and in vivo. The Journal of experimental medicine 202 : 309–319.
32. CowleySC, MeierovicsAI, FrelingerJA, IwakuraY, ElkinsKL (2010) Lung CD4-CD8 - double-negative T cells are prominent producers of IL-17A and IFN-gamma during primary respiratory murine infection with Francisella tularensis live vaccine strain. Journal of immunology 184 : 5791–5801.
33. MilushJM, MirKD, SundaravaradanV, GordonSN, EngramJ, et al. (2011) Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. The Journal of clinical investigation 121 : 1102–1110.
34. VintonC, KlattNR, HarrisLD, BriantJA, Sanders-BeerBE, et al. (2011) CD4-like immunological function by CD4 - T cells in multiple natural hosts of simian immunodeficiency virus. Journal of virology 85 : 8702–8708.
35. RestrepoC, RallonNI, del RomeroJ, RodriguezC, Sempere-OrtellsJM, et al. (2013) HIV Gag-specific immune response mediated by double negative (CD3(+)CD4(−)CD8(−)) T cells in HIV-exposed seronegative individuals. Journal of medical virology 85 : 200–209.
36. KemperC, AtkinsonJP (2007) T-cell regulation: with complements from innate immunity. Nature reviews Immunology 7 : 9–18.
37. BaudinoL, SardiniA, RusevaMM, Fossati-JimackL, CookHT, et al. (2014) C3 opsonization regulates endocytic handling of apoptotic cells resulting in enhanced T-cell responses to cargo-derived antigens. Proceedings of the National Academy of Sciences of the United States of America 111 : 1503–1508.
38. LiszewskiMK, KolevM, Le FriecG, LeungM, BertramPG, et al. (2013) Intracellular complement activation sustains T cell homeostasis and mediates effector differentiation. Immunity 39 : 1143–1157.
39. GhannamA, FauquertJL, ThomasC, KemperC, DrouetC (2014) Human complement C3 deficiency: Th1 induction requires T cell-derived complement C3a and CD46 activation. Molecular immunology 58 : 98–107.
40. MulemeHM, RegueraRM, BerardA, AzinwiR, JiaP, et al. (2009) Infection with arginase-deficient Leishmania major reveals a parasite number-dependent and cytokine-independent regulation of host cellular arginase activity and disease pathogenesis. Journal of immunology 183 : 8068–8076.
41. LiuD, UzonnaJE (2010) The p110 delta isoform of phosphatidylinositol 3-kinase controls the quality of secondary anti-Leishmania immunity by regulating expansion and effector function of memory T cell subsets. Journal of immunology 184 : 3098–3105.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



