-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice
A new variant of Creutzfeldt Jacob Disease (vCJD) was identified in humans and linked to the consumption of Bovine Spongiform Encephalopathy (BSE)-infected meat products. Recycling of ruminant tissue in meat and bone meal (MBM) has been proposed as origin of the BSE epidemic. During this epidemic, sheep and goats have been exposed to BSE-contaminated MBM. It is well known that sheep can be experimentally infected with BSE and two field BSE-like cases have been reported in goats. In this work we evaluated the human susceptibility to small ruminants-passaged BSE prions by inoculating two different transgenic mouse lines expressing the methionine (Met) allele of human PrP at codon 129 (tg650 and tg340) with several sheep and goat BSE isolates and compared their transmission characteristics with those of cattle BSE. While the molecular and neuropathological transmission features were undistinguishable and similar to those obtained after transmission of vCJD in both transgenic mouse lines, sheep and goat BSE isolates showed higher transmission efficiency on serial passaging compared to cattle BSE. We found that this higher transmission efficiency was strongly influenced by the ovine PrP sequence, rather than by other host species-specific factors. Although extrapolation of results from prion transmission studies by using transgenic mice has to be done very carefully, especially when human susceptibility to prions is analyzed, our results clearly indicate that Met129 homozygous individuals might be susceptible to a sheep or goat BSE agent at a higher degree than to cattle BSE, and that these agents might transmit with molecular and neuropathological properties indistinguishable from those of vCJD. Our results suggest that the possibility of a small ruminant BSE prion as vCJD causal agent could not be ruled out, and that the risk for humans of a potential goat and/or sheep BSE agent should not be underestimated.
Published in the journal: . PLoS Pathog 7(3): e32767. doi:10.1371/journal.ppat.1001319
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001319Summary
A new variant of Creutzfeldt Jacob Disease (vCJD) was identified in humans and linked to the consumption of Bovine Spongiform Encephalopathy (BSE)-infected meat products. Recycling of ruminant tissue in meat and bone meal (MBM) has been proposed as origin of the BSE epidemic. During this epidemic, sheep and goats have been exposed to BSE-contaminated MBM. It is well known that sheep can be experimentally infected with BSE and two field BSE-like cases have been reported in goats. In this work we evaluated the human susceptibility to small ruminants-passaged BSE prions by inoculating two different transgenic mouse lines expressing the methionine (Met) allele of human PrP at codon 129 (tg650 and tg340) with several sheep and goat BSE isolates and compared their transmission characteristics with those of cattle BSE. While the molecular and neuropathological transmission features were undistinguishable and similar to those obtained after transmission of vCJD in both transgenic mouse lines, sheep and goat BSE isolates showed higher transmission efficiency on serial passaging compared to cattle BSE. We found that this higher transmission efficiency was strongly influenced by the ovine PrP sequence, rather than by other host species-specific factors. Although extrapolation of results from prion transmission studies by using transgenic mice has to be done very carefully, especially when human susceptibility to prions is analyzed, our results clearly indicate that Met129 homozygous individuals might be susceptible to a sheep or goat BSE agent at a higher degree than to cattle BSE, and that these agents might transmit with molecular and neuropathological properties indistinguishable from those of vCJD. Our results suggest that the possibility of a small ruminant BSE prion as vCJD causal agent could not be ruled out, and that the risk for humans of a potential goat and/or sheep BSE agent should not be underestimated.
Introduction
Transmissible Spongiform Encephalopathies (TSEs) are fatal neurodegenerative diseases which include Scrapie in sheep and goats, Bovine Spongiform Encephalopathy (BSE) and Creutzfeldt-Jakob disease (CJD) in humans. Prions, the causal agents of these diseases are thought to be infectious protein particles essentially composed of a misfolded isoform (PrPSc) of the cellular prion protein (PrPC) [1], [2]. Scrapie has been detected more than two centuries ago, without epidemiological evidence of human transmission. BSE was diagnosed in cattle in the 80s [3] and subsequently acquired epidemic characteristics in several European countries. Ten years later, a variant form of CJD (vCJD) was identified in humans and linked to the consumption of BSE-infected products [4], [5]. During the BSE epidemic, sheep and goats have also been exposed to BSE-contaminated Meat and Bone Meal, so BSE transmission to these species may have occurred [6]. Sheep and goats are experimentally susceptible to BSE [7], [8], [9], [10], [11] and one confirmed [12] and one suspected [13] BSE-like case have been reported in goats in France and the United Kingdom (UK), respectively. While BSE infection is mostly restricted to the nervous system in cattle [14], [15], [16], [17], PrPSc is widely distributed in lymphoid tissues of experimentally BSE-infected sheep [18], [19], suggesting that infected sheep could provide a secondary and more dangerous source of BSE infection for humans.
Considering the protein-only hypothesis, one of the most difficult aspects to explain within prion diseases is the existence of prion strain diversity. Prion strains can be defined as isolates or sources of prion infectivity, that when transmitted to the same host, present distinct disease phenotypes, characterized by their incubation times, clinical signs, PrPSc biochemical properties, histopathological lesion profiles and PrPSc deposition patterns in the brain [20]. Intra-species prion transmission is characterized to be very efficient, maintaining these phenotypic traits on serial subpassaging. Although PrP primary sequence is highly conserved among mammals, inter-species prion propagation is limited by the so called transmission barrier, showing often at first passage lower attack rates and extended incubation times [21]. During years this transmission barrier has been called species barrier, suggesting to reside essentially in the degree of PrP amino acid sequence homology between donor and receiver [22], [23]. Nowadays there is strong evidence that also PrPSc conformation plays a critical role, not only in the cross-species transmission events, but also in the existence of different prion strains [24], [25], [26], [27], [28], [29].
In previous reports we demonstrated that BSE experimentally passaged in sheep homozygous for the A136R154Q171 allele of ovine PrP showed an enhanced virulence in transgenic mice expressing bovine and porcine PrP, compared to the original cattle BSE [29], [30]. The susceptibility of humans to a sheep or goat passaged BSE agent is still unknown. PrP polymorphism at codon 129, where either methionine or valine is encoded, has been described as a key factor influencing human prion susceptibility [31], [32], [33], [34] and seems to be particularly important in vCJD manifestation, as all but one clinical vCJD cases diagnosed so far are homozygous for methionine. In an attempt to evaluate the potential risk for humans of a sheep or goat BSE agent in this study we analyzed the susceptibility of transgenic mice expressing methionine 129 human PrP to sheep and goat BSE isolates.
Results
In two parallel studies, performed in two different laboratories, the susceptibility of 2 lines of human-PrP transgenic mice to BSE agent was assessed without or after an intermediate passage in either sheep or goat. Both human-PrP transgenic mouse lines overexpress human M129 PrP under the mouse Prnp promoter on a mouse PrP null background but they differ in the genetic construction, genetic background and human PrP protein expression levels: a PAC insert, a Sv129 background and a 6-fold expression levels for tg650 mice [35] and a MoPrP.Xho vector, a 129Ola/B6CBA background and a 4-fold expression levels for tg340 mice (generated as described in Material and Methods). Tg650 (at INRA, France) and tg340 (at CISA-INIA, Spain) were inoculated with several cattle, sheep and goat BSE isolates (see Table 1 for a description of the isolates used). The transmission efficiency was evaluated by the appearance of TSE clinical symptoms and by the presence of PrPres in the brain. The transmission data available to date, together with those obtained comparatively with vCJD or sCJD agents, are shown in Tables 2 and 3.
Tab. 1. Description of the different isolates used in this work. 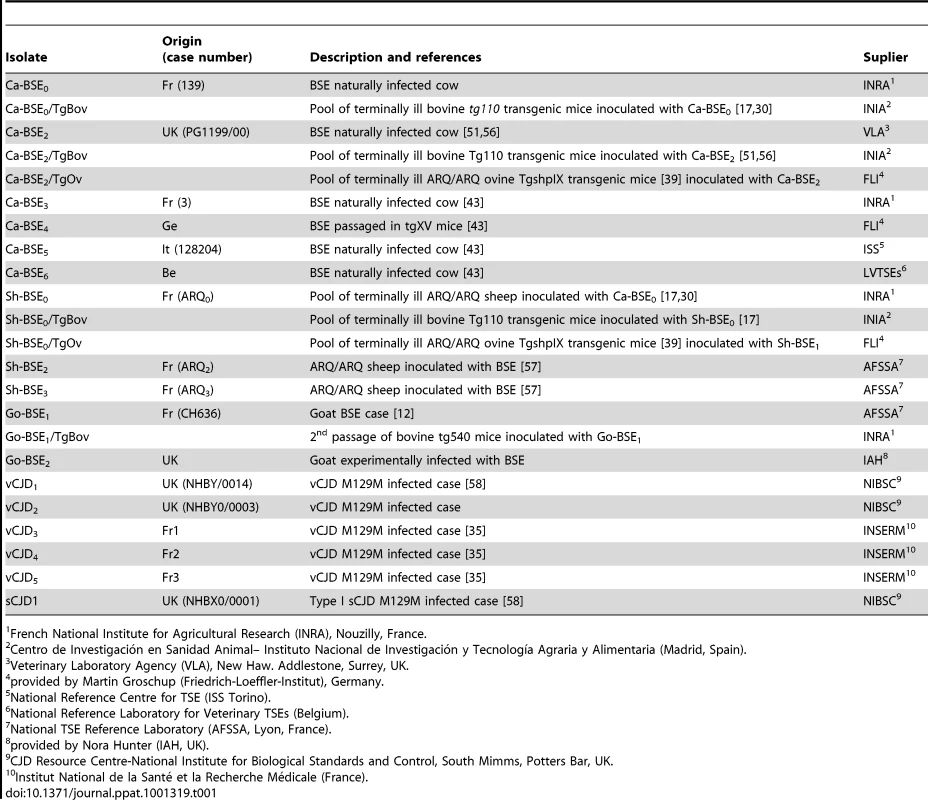
French National Institute for Agricultural Research (INRA), Nouzilly, France. Tab. 2. Transmission of bovine, ovine and goat-BSE isolates to tg650 (INRA). 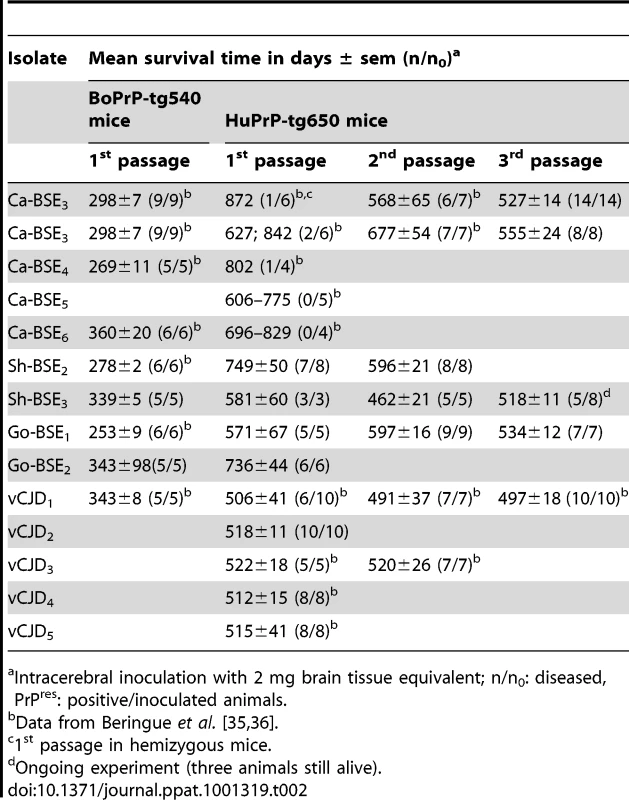
Intracerebral inoculation with 2 mg brain tissue equivalent; n/n0: diseased, PrPres: positive/inoculated animals. Tab. 3. Transmission of bovine, ovine and goat-BSE isolates to tg340 (CISA). 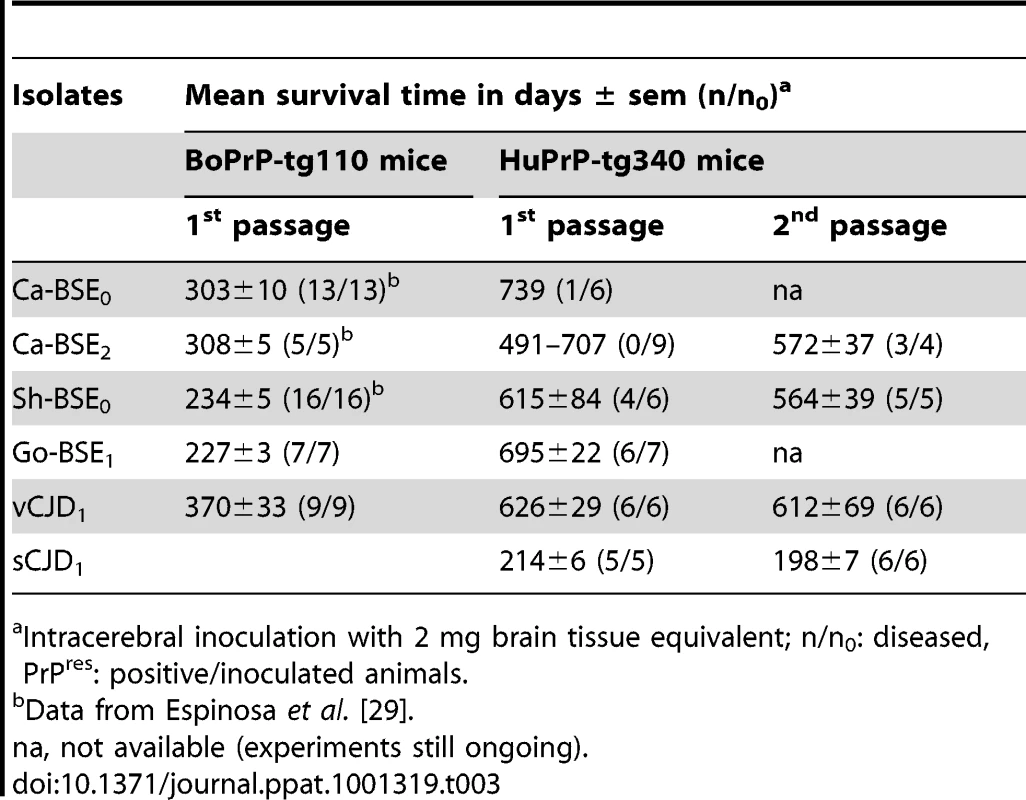
Intracerebral inoculation with 2 mg brain tissue equivalent; n/n0: diseased, PrPres: positive/inoculated animals. Low transmission efficiency of cattle-BSE prions in human-PrP mice
Both tg650 and tg340 lines were fairly susceptible to vCJD isolates with 100% clinical attack rates and mean survival times around 500 and 600 days post-infection (d.p.i.), respectively. These features were stable upon subpassaging, suggesting an absence of transmission barrier for this agent (Tables 2 and 3). Inoculation of cattle BSE isolates to tg340 mice produced markedly different results, as previously reported with tg650 mice [36]. At first passage, only one out of fifteen tg340 mice inoculated with BSE2 and BSE0 isolates was scored positive for brain PrPres and at a very late stage (739 d.p.i.) without clear clinical signs. The remaining inoculated mice failed to develop a clinical disease or to accumulate detectable levels of PrPres in the brain up to ∼700 days after inoculation. On second passage performed with brain homogenate from a PrPres-negative mouse (succumbed at 576 dpi) from the first passage (BSE2 isolate), 3 out of 4 inoculated tg340 mice tested positive for brain PrPres by western blot with a survival time of 572±37 d.p.i. It is important to note that all the cattle BSE isolates tested in this study were transmitted as efficiently as vCJD isolates or other BSE-related sources to bovine PrP transgenic mice (Table 2 and 3), thus suggesting that they may have a comparable infectivity in the absence of an apparent transmission barrier [36]. Overall, these results indicate that both human-PrP transgenic mouse lines exhibit a strong transmission barrier to cattle BSE, suggesting that human PrPC Met129 is a “bad substrate” for cattle BSE prions.
High transmission efficiency of sheep and goat BSE isolates to human-PrP mice
We next examined the transmission efficiency of sheep - and goat-passaged BSE prions. Several experimental or “natural” isolates of distinct origin were selected (Table 1). Upon primary transmission to both tg340 and tg650 mouse lines, sheep and goat BSE isolates showed significant higher transmission ability than cattle BSE isolates. Thus the attack rates approached 100% (clinical signs, PrPres detection in the brain) with almost all the sheep and goat BSE isolates used (Tables 2 and 3). Depending on the isolate used, the survival times varied between 571±67 and 749±50 d.p.i. in tg650 and 615±84 - 695±22 in tg340, thus much closer to the survival times observed for vCJD isolates inoculated in these mouse models (Tables 2 and 3). On second and third passage, 100% attack rates were obtained with all the sheep and goat BSE isolates tested. The incubation times were stable or for some isolates, slightly decreased and approached that of variant CJD. Overall, these data suggest a lower or an absence of apparent transmission barrier to sheep and goat BSE in human PrP transgenic mice.
Importantly, the comparatively higher attack rates seen with sheep and goat BSE isolates are not related to their initial PrPres content as dilution experiment results indicate that cattle BSE isolates (Ca-BSE2 and Ca-BSE3) contained higher PrPres levels in their brains than the sheep and goat BSE isolates (Figure 1).
Fig. 1. Comparative PrPres content of the cattle, sheep and goat isolates used for tgHu bioassays. 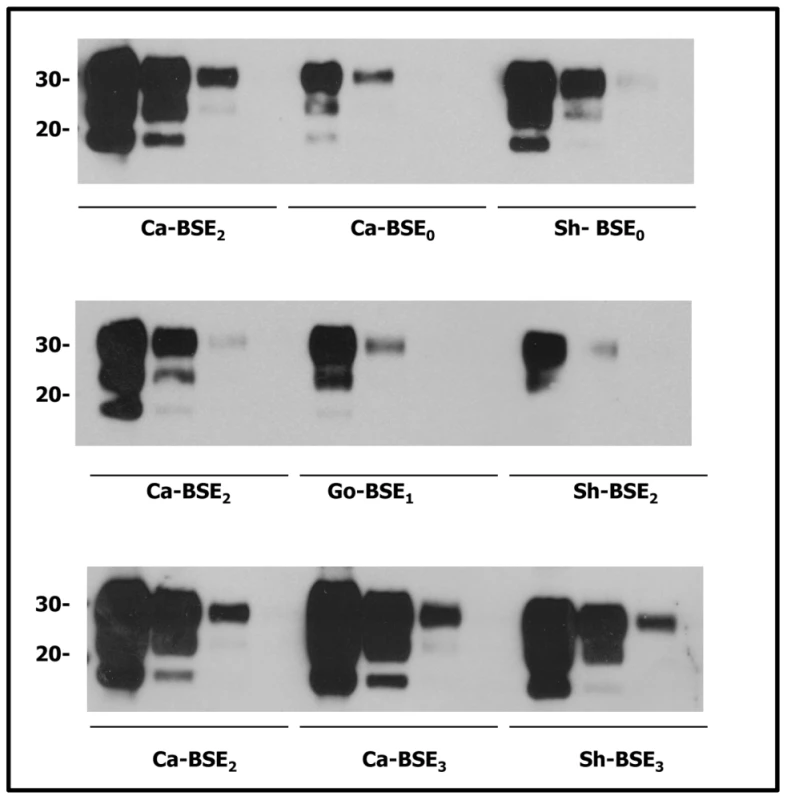
Immunoblots of brain PrPres detected with SHA 31 mAb. Direct sample and 1/5 dilutions were loaded on 12% Bis-Tris gels. Molecular weights (in kDa) are shown at the left side of the blot. Excluding the BSE0 isolate, all cattle BSE isolates presented higher PrPres contents than the sheep and goat BSE isolates. Cattle BSE2 and BSE3 isolates presented comparable PrPres contents while that in the BSE0 isolate was substantially lower. The sheep and goat BSE isolates used contained similar PrPres quantity. Human PrP transgenic mice accumulate vCJD-like PrPres following inoculation with cattle, sheep or goat BSE
Because distinct PrPSc conformations appear to encipher/encode distinct strains, brain PrPres electrophoretic mobility and glycoprofile characterization constitutes standard criteria to distinguish between strains. Brain PrPres of human PrP tg650 and tg340 transgenic mice inoculated with cattle, sheep and goat BSE isolates were analysed by western blot and the signature obtained was compared to that of variant CJD (Figures 2 and 3). A typical PrPvCJD banding pattern, characterized by low size fragments (∼19 kDa fragment for the aglycosyl band) and prominent diglycosylated species was consistently observed in the challenged, PrPres-positive mice. This signature clearly differed from that observed after inoculation of mice with sporadic CJD (Figure 3 and [35]). Similar results were obtained when the immunoblots were performed with the 12B2 anti-PrP antibody, whose epitope (89WGQGG93 according to the human PrP sequence) is known to be poorly protected from proteinase K digestion [29], [37] in vCJD and BSE-related isolates (Figure 4). The only exception was observed after second passage of one cattle BSE isolate (Ca-BSE2) in tg340 mice, one of the three positive mice presented a brain PrPres profile clearly distinct from PrPvCJD, that was comparable to that of typeI sCJD-inoculated tg340 mice with predominantly monoglycosylated and higher size fragments (∼21 kDa for the aglycosyl band) and preserved detection by 12B2 antibody (Figure 4).
Fig. 2. Western blots analysis of PrPres in the brains of tg650 mice infected with human, bovine, ovine and goat isolates. 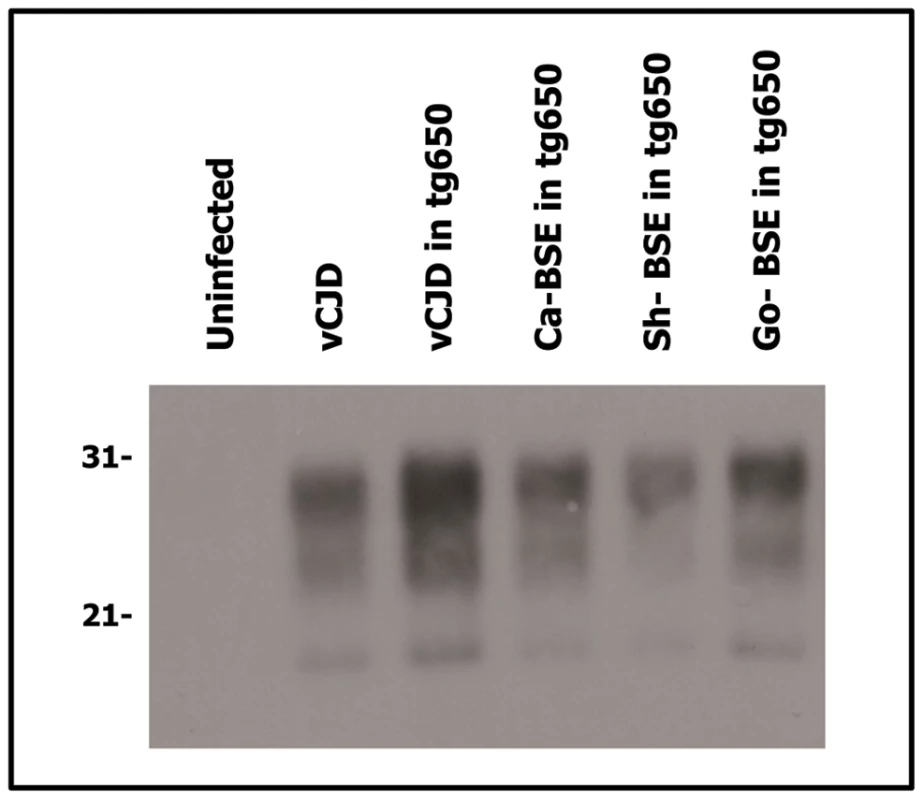
Immunoblots of brain PrPres detected with SHA 31 Mab. Similar amount of brain tissue equivalent (20 µg) was loaded for adequate comparison. vCJD isolate was loaded for comparison. Molecular weights (in kDa) are shown at the left side of the blot. Similar PrPres banding patterns were observed in the vCJD- and the cattle, sheep and goat BSE-challenged tg650 mice. Fig. 3. Western blots analysis of PrPres in the brains of tg340 mice infected with human, bovine, ovine and goat isolates. 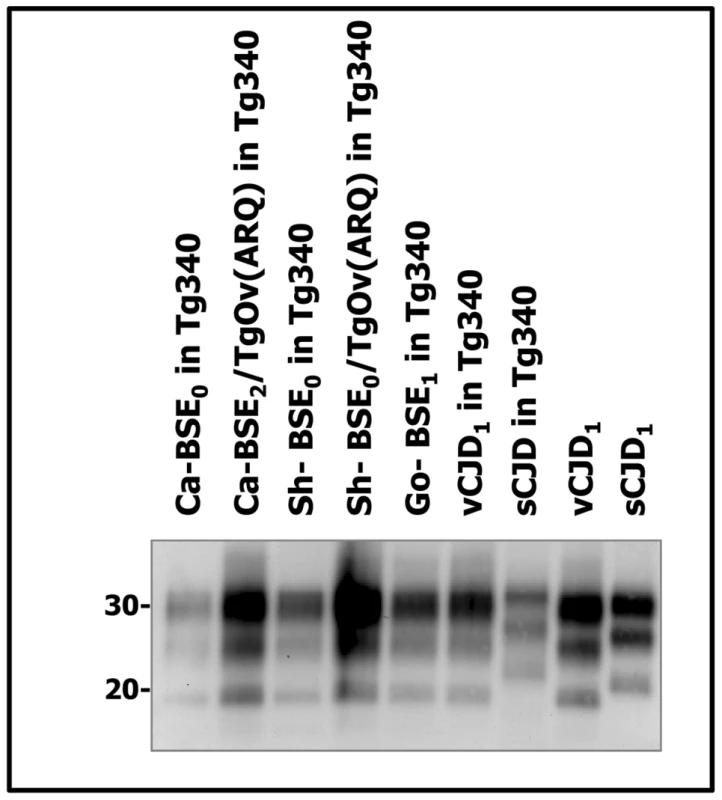
Immunoblots of brain PrPres detected with SHA 31 Mab. Similar quantities of PrPres were loaded for adequate comparison. sCJD and vCJD1 isolates were loaded for comparison. Molecular weights (in kDa) are shown at the left side of the blot. Note the typical PrPvCJD banding pattern, characterized by low size fragments and prominent diglycosylated species in the cattle, sheep and goat BSE-challenged mice. These banding patterns clearly differed from that observed after inoculation of mice with sporadic CJD. Fig. 4. Western blots analysis of PrPres in the brains of tg340 mice infected with human, bovine and ovine isolates. 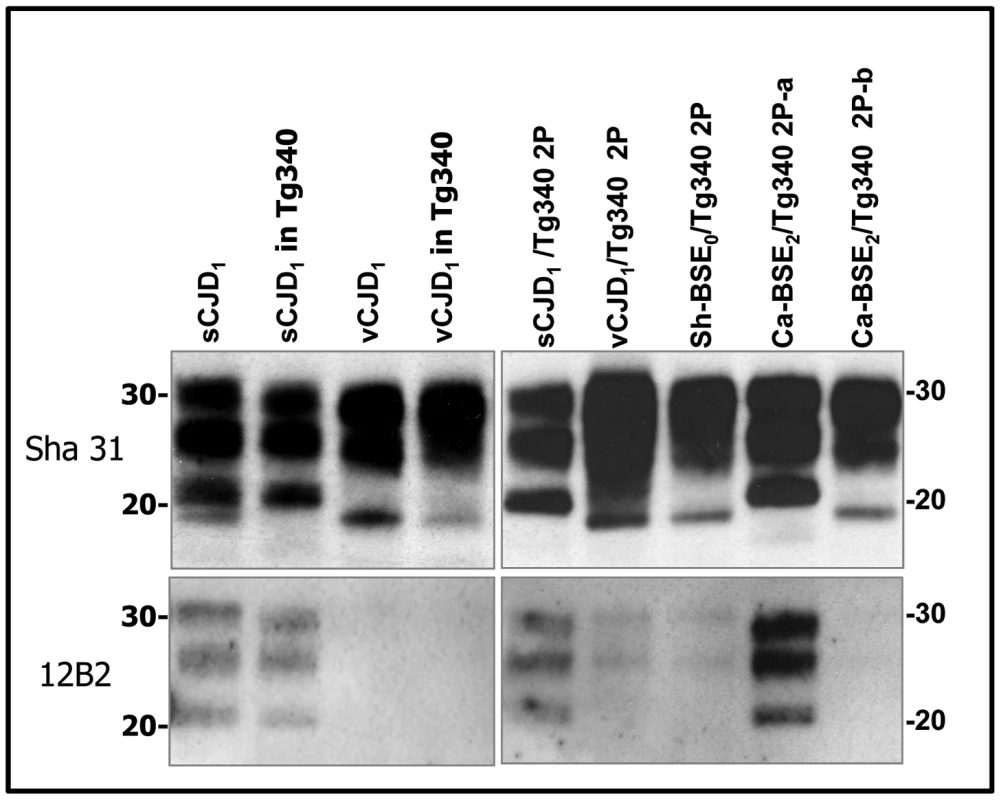
Immunoblots of brain PrPres detected with SHA 31 and 12B2 mAbs. Similar quantities of PrPres were loaded for adequate comparison. sCJD and vCJD1 isolates were loaded for comparison. Molecular weights (in kDa) are shown at the left side of the blot. Both vCJD- and type I sCJD-like PrPres were observed after second passage of Ca-BSE2 in tg340 mice (Ca-BSE2/Tg340 2P-b and Ca-BSE2/Tg340 2P-a respectively). Cattle, sheep and goat BSE isolates showed similar neuropathological features in human PrP mice
The regional distribution of PrPres and vacuolation in the brains are standard criteria to differentiate between strains/TSE agents [38]. We thus compared the neuropathological phenotypes of cattle, sheep and goat BSE agents by PrPres histoblotting and histopathological examination. The PrPres deposition pattern of cattle, sheep and goat BSE were clearly similar in both tg650 and tg340 mice on 2nd passage (not shown) and on 3rd passage in tg650. As illustrated in Figure 5, large plaque-like PrP deposits were detected throughout the brain, predominantly in the cerebral cortex, corpus callosum, thalamic nuclei, optic tract, brain stem and cerebellum, a distribution which is similar to that seen in the brains of vCJD-infected tg650 mice (Figure 5 and [35]).
Fig. 5. Regional distribution of PrPres in the brain of human PrP transgenic mice infected with BSE agent passaged through sheep, goat or human species. 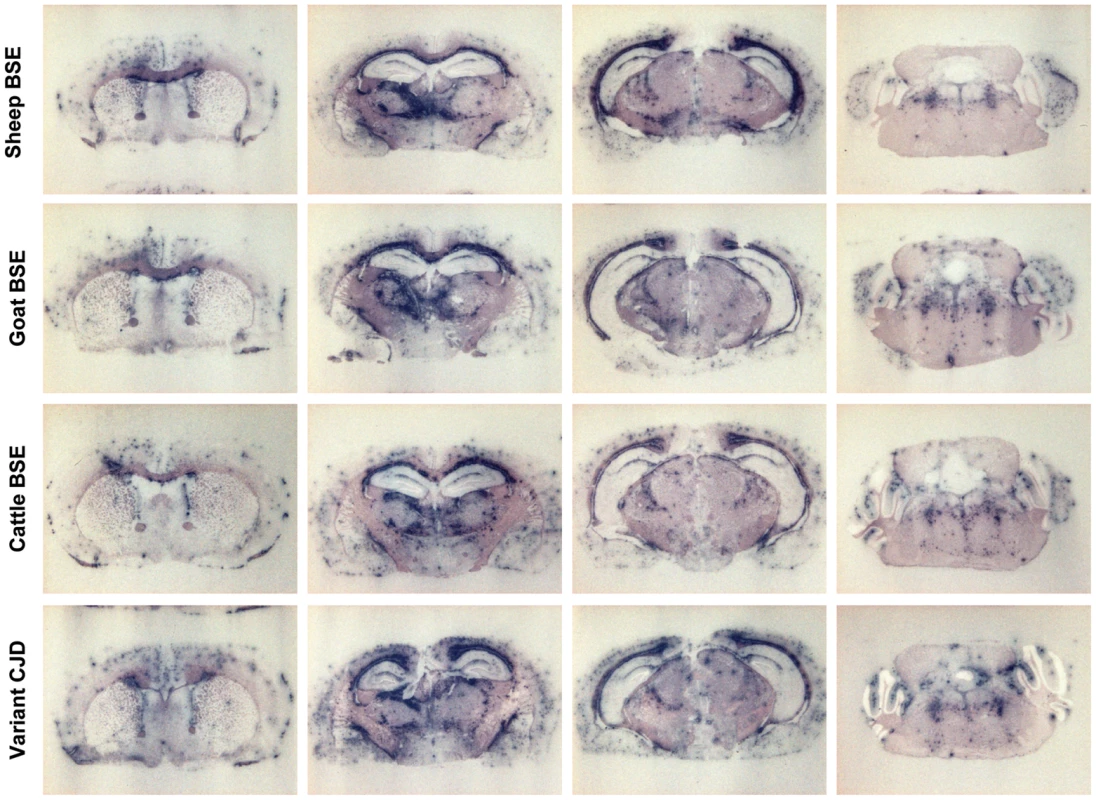
Histoblots of 4 representative anteroposterior mouse brain sections are shown. Comparison has been made at 3rd passage in tg650 mice. The patterns produced by sheep, goat and cattle BSE and variant CJD appear similar. At microscopic level, abundant amyloid-like plaques were present (Figure 6), as suggested by histoblotting. These plaques were associated with severe vacuolisation of the surrounding tissue (‘florid like’ aspect see Figure 6), precluding the establishment of a reliable standard lesion profile. However similar distribution of the vacuolar changes was observed in the brain of mice inoculated with the different BSE and the vCJD isolates. It mainly involved thalamic, hippocampal and cerebral cortex areas, while brainstem and cerebellar cortex remained poorly affected (Figure 6).
Fig. 6. Brain vacuolar changes and amyloid plaques in transgenic mice for the human PRNP gene (methionine 129 variant - tg650). 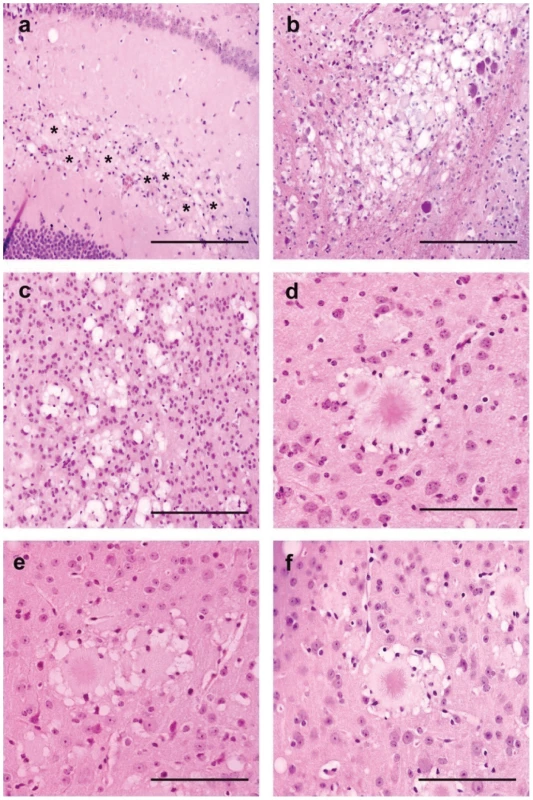
Hemalun-Eosin staining. Mice were inoculated with natural goat BSE (a – Bar 150 µm), cattle BSE (b: bar150 µm-e: bar 50 µm), vCJD (c: bar 150 µm-d: bar 50 µm) or experimental BSE in sheep (ARQ/ARQ genotype) (f: bar 50 µm). Vacuolar changes are associates to plaques deposits (a–b–c) (in a *: plaques deposits). Plaques deposits often harboured typical features of florid plaques: presence of crown of vacuoles and glial cell nuclei (d–e–f). Transmission efficiency of BSE prions into human-PrP mouse models is strongly influenced by the agent PrP primary sequence
Once known that the phenotypes of cattle, sheep and goat BSE appear indistinguishable in human PrP mice, we proceed to analyze in more detail the potential elements involved in the change on BSE transmission characteristics after passage into sheep or goat. One of the cattle BSE isolate studied (Ca-BSE2) was passaged into bovine (tg110) and ovine PrP transgenic mice (ARQ allele) [39] to propagate BSE agents with different PrP primary sequence (these isolates were termed Ca-BSE2/TgBov and Ca-BSE2/TgOv, Table 1). The Ca-BSE2/TgBov isolate did not induce a clinical disease nor PrPres accumulation in tg340 mice while an intermediate passage on the ovine PrPARQ sequence (Ca-BSE2/TgOv) restored the disease susceptibility, with survival times, biochemical and neuropathological features similar to those obtained with experimental sheep BSE isolates (Figure 7 and data not shown).
Fig. 7. Transmission to human PrP mice of different BSE isolates generated by intermediary passages in either ovine or bovine PrP transgenic mice. 
na: not available (experiments still ongoing). aIntracerebral inoculation with 2 mg brain tissue equivalent; n/n0: diseased, PrPres positive/inoculated animals. cTwo serial passages on tgBov mice. The opposite experiment was also performed. One sheep and one goat BSE isolate were passaged (twice for goat BSE) in bovine PrP transgenic mice (generating isolates Sh-BSE0/TgBov and Go-BSE1/TgBov, Table 1) before re-inoculation to human PrP transgenic mice (Figure 7). None of the mice inoculated with these isolates developed the disease nor accumulated PrPres, although one of them (Sh-BSE0/tgBov) produced the shortest disease in bovine PrP transgenic mice (Figure 7). This last result suggests that when sheep - or goat-BSE agents recovered their original bovine PrP sequence, the human transmission barrier was re-established. Moreover, the Sh-BSE0/TgOv isolate (which maintains its PrP ovine sequence) showed a full transmission rate in human transgenic mice with similar survival times as those of the original Sh-BSE0 isolate (Figure 7). Overall these data suggest that PrPSc primary sequence plays a critical role in the capacity of BSE prions to propagate on the human Met 129 PrP sequence.
Discussion
In this study, we compared the transmission features of cattle and sheep/goat BSE prions in two different models of transgenic mice expressing Met129 human PrP (tg650 and tg340 lines) in two different laboratories. In general, the transmission results obtained in both human-PrP transgenic mouse lines were very comparable. Some shortening in survival times was observed in tg650 mice (compared to the tg340 mice line), which was probably due to higher PrP expression levels in this line. Worryingly, our results support the view that an intermediate passage of BSE agent in small ruminants accelerates the appearance of a vCJD-like disease in human PrP mice or markedly increases its transmission efficiency. Because the apparent phenotype of cattle and sheep/goat BSE prions is conserved, these data also unravel an important role of PrPSc primary sequence in the cross-species transmission capacities of prion strains.
The transmission efficiency of cattle BSE isolates in both human-PrP transgenic mouse models was apparently low. With all BSE isolates, whose high infectivity has been demonstrated in bovine-PrP transgenic mice (Tables 2 and 3), very low attack rates were obtained on primary transmission to both tg650 and tg340 mice. Three passages were necessary to achieve a degree of fitness comparable to vCJD in the same mouse line. This low BSE transmission efficiency to human PrP transgenic mice -occasionally accompanied by a strain shift - has also been described by others [40], [41], [42], and suggests a strong although not absolute transmission barrier. Although the exact characteristics and further evolution of the vCJD epidemic still entail uncertainties owing to prolonged incubation times, this apparent high transmission barrier of humans to cattle BSE might be an explanation for the currently low vCJD incidence, considering the high exposure to BSE during the “mad cow” crisis.
Remarkably, a different picture emerged when the sheep and goat BSE isolates were inoculated to human PrP transgenic mouse models. Attack rates approaching 100% were observed from the primary passage onwards and mean incubation times were more consistent with those measured after transmission of vCJD. On further passaging, the neuropathological phenotype and PrPSc type of cattle and sheep/goat BSE agents appeared indistinguishable from the vCJD agent propagated in these mice, as previously demonstrated in bovine transgenic mice [29], thus strongly supporting the view that the same BSE prion strain has been propagated whatever the infecting species. Hence, these observations reproduced in two distinct human transgenic lines with different genetic background and PrP expression levels support the view that transmission efficiency of BSE prions is increased by an intermediate passage in sheep or goat. Although the electrophoretic pattern of sheep/goat and cattle BSE PrPres appeared similar in human-PrP transgenic mice, other assays are currently performed to further compare the biochemical or biophysical properties of the respective proteins are ongoing.
Importantly, the higher attack rates obtained after sheep and goat BSE transmissions compared to cattle BSE are not in accordance with the initial PrPres content of these isolates. In addition, the data from inoculation to BoPrP-Tg reporter mice suggest that cattle BSE and sheep and goat-BSE isolates could have similar transmission efficiency (Table 1 and 2) in the absence of apparent transmission barrier [36]. Furthermore, when the human PrP transgenic lines were inoculated with the BSE agent passaged into bovine and ovine transgenic mice, the transmission results were comparable to those of the cattle and sheep BSE isolates (Figure 7), further supporting the crucial role of the PrPSc primary sequence in the increase of transmission efficiency. Taken together all these considerations suggest that the higher transmission efficiency of sheep and goat BSE isolates in comparison to cattle BSE isolates cannot be linked to a higher infectious titer of the inoculum but must be the outcome of a modification in the pathogenicity of the agent.
Commonly, transmission barriers are determined considering attack rates and quantified by measuring the fall in the mean survival times between the first and second passage. Hence, if we consider PrPres detection as an indicator of successful transmission, our results imply that humans could be significantly more susceptible to a sheep or goat BSE agent than to a cattle BSE agent. On the other hand our results suggest that cattle BSE infection could produce very long latency in humans, with conversion efficiency far below the threshold of detectable PrPres, which is also very worrying since it suggests the possibility of silent carriers.
Our observations, made in two different mouse genetic backgrounds, suggest that the different transmission properties acquired by BSE after passage into either sheep or transgenic mice expressing ovine PrP are strongly related to the ovine PrP primary sequence, rather than to other host species-specific factors. Thus the transmission barrier observed with cattle BSE was fully restored when sheep/goat BSE experienced intermediate passaging into bovine transgenic mice before reinoculation to human PrP mice. In contrast, when the ovine sequence of sheep BSE was maintained, through passage into ARQ ovine PrP transgenic mice, the efficient transmission to human PrP mice was maintained. Apparently, an ovine/caprine PrPSc sequence appears to facilitate human PrP conversion by the BSE agent, compared to a bovine one.
The PrP primary sequence influence seems to depend strongly on the strain involved, since no PrPres was found in either first or second passages of sheep scrapie in tg340 mice (unpublished observations), suggesting no infection, in accordance with the lack of epidemiological evidence linking scrapie with human TSE. Moreover, the low transmission efficiency observed for the cattle BSE agent is not exclusively linked to the bovine PrP sequence since other uncommon BSE strains (BSE-L) are efficiently transmitted to human-PrP mice [41], [43]. Considering the conformational selection model [20], our results would suggest that M129 human PrPC prefers a BSE PrPSc with conformational characteristics templated by the ovine sequence, to a bovine BSE PrPSc. Because a similar increased transmission efficiency of sheep/goat BSE has been reported in wild type mice [44] and transgenic mice expressing elk [45], bovine [29] and porcine [30] PrP, the better structural compatibility conferred by sheep/goat primary PrPSc sequence may not be limited to human PrPC. One explanation might be an alteration in the quaternary structure (after passage into sheep/goat) generating PrPSc polymers less degraded or more rapidly/easily amplified favouring or enhancing the initial conversion. This question is currently being addressed by sedimentation velocity [46] and PMCA experiments. Another possibility, within the quasispecies concept [20], [47], might be that BSE prions confrontation with the sheep and goat primary PrP sequence increases the variety of BSE substrain components, with the following emergence of a markedly adapted component in response to the selection pressure imposed by the interspecies transmission events. On the other hand, this component would not be distinguishable from bovine-passaged BSE prions due to the current limits of the standard biological methods and/or the molecular tools employed here to characterize prion strains. Whatever the mechanism, the notion that a passage through an intermediate species can profoundly alter prion virulence for the human species has important public-health issues, regarding emerging and/or expanding TSEs, like atypical scrapie or CWD.
Although extrapolation of results from prion transmission studies by using transgenic mice has to be done very carefully, especially when human susceptibility to prions is analyzed, our results clearly indicate that Met129 homozygous individuals might be susceptible to a sheep or goat BSE agent at a higher degree than to cattle BSE, and that these agents might transmit with molecular and neuropathological properties indistinguishable from those of vCJD. Although no vCJD cases have been described in Val129 homozygous individuals so far it is relevant to analyze if similar results will be observed in this genotype. This issue is currently being addressed in transmission experiments using transgenic mice expressing Val129 human PrP.
Taken all together, our results suggest that the possibility of a small ruminant BSE prion as vCJD causal agent could not be ruled out, which has important implications on public and animal health policies. On one hand, although the exact magnitude and characteristic of the vCJD epidemic is still unclear, its link with cattle BSE is supported by strong epidemiological ground and several experimental data. On the other hand, the molecular typing performed in our studies, indicates that the biochemical characteristics of the PrPres detected in brains of our sheep and goat BSE-inoculated mice seem to be indistinguishable from that observed in vCJD. Considering the similarity in clinical manifestation of BSE - and scrapie-affected sheep [48], a masker effect of scrapie over BSE, as well as a potential adaptation of the BSE agent through subsequent passages, could not be ruled out. As BSE infected sheep PrPSc have been detected in many peripheral organs, small ruminant-passaged BSE prions might be a more widespread source of BSE infectivity compared to cattle [19], [49], [50]. This fact is even more worrying since our transmission studies suggest that apparently Met129 human PrP favours a BSE agent with ovine rather than a bovine sequence. Finally, it is evident that, although few natural cases have been described and so far we cannot draw any definitive conclusion about the origin of vCJD, we can not underestimate the risk of a potential goat and/or sheep BSE agent.
Materials and Methods
Ethics statement
Animal experiments were carried out in strict accordance with the recommendations in the guidelines of the Code for Methods and Welfare Considerations in Behavioural Research with Animals (Directive 86/609EC) and all efforts were made to minimize suffering. Experiments were approved by the Committee on the Ethics of Animal Experiments of the author's institutions (INRA and INIA); Permit Number: RTA06-091 and CT05-036353.
TSE isolates
The isolates used in this study are described in Table 1. For mouse inoculation, all isolated were prepared from brain tissues as 10% (w/v) homogenates in 5% glucose.
Mouse transmission studies
The tg650 transgenic mouse line over expresses human PrP M129 at a 6-fold level on a mouse PrP null background [35]. The tg340 mouse line expressing about 4-fold level of human PrP M129 on a mouse PrP null background has been generated following the same procedure previously described for the generation of other transgenic mouse line expressing different species PrP [51]. The details of this procedure are described below. Tg110 and tg540 mouse lines expresses bovine PrP at levels approximately 8-fold that in cattle brain [51], [52].
All inocula were prepared from brain tissues as 10% (w/v) homogenates. Individually identified 6–10 week-old mice were anesthetized and inoculated with 2 mg of brain homogenate in the right parietal lobe using a 25-gauge disposable hypodermic needle. Mice were observed daily and the neurological status was assessed weekly. When progression of a TSE disease was evident or at the end of lifespan, animals were euthanized because of ethical reasons. Once euthanized, necropsy was performed and brain was taken. A part of the brain was fixed by immersion in 10% formol to quantify spongiform degeneration by histopathology and PK resistant PrP accumulation (PrPres) by immunohistochemistry (IHQ) or histoblotting and the other was frozen at −20°C to determine presence of PrPres by Western blot (WB). In all cases, survival time and attack rate were calculated for each isolate. Survival time was expressed as the mean of the survival days post inoculation (d.p.i.) of all the mice scored positive for PrPres, with its correspondent standard error. Attack rate was determined as the proportion of mice scored positive for PrPres from all the mice inoculated. When all mice were scored negative for PrPres, the survival time range was shown. Brain homogenates from PrPres positive mice, when available, were used for further passaging. When all mice were scored negative for PrPres on primary passage, PrPres-negative brain homogenates were used for second passage.
Western blot
175±20 mg of frozen brain tissue were homogenized in 5% glucose in distilled water in grinding tubes (Bio-Rad) adjusted to 10% (w/v) using a TeSeE™ Precess 48™ homogenizer (Bio-Rad) following manufacturer instructions. Presence of PrPres in transgenic mice brains was determined by Western blot, following the procedure described below and using the reagents of the ELISA commercial test (TeSeE, Bio-Rad). 10–50 µl of a 10% (w/v) brain homogenate were diluted in a 10% (w/v) negative sheep brain homogenate, to obtain a 200 µl final volume. Homogenates were incubated for 10 min at 37°C with 200 µl of a 2% proteinase K solution (in buffer A). PrPres was recovered as a pellet after addition of 200 µl of buffer B and a centrifugation at 15,000× g for 7 min at 20°C. Supernatants were discarded and pellets were dried inverted over absorbent paper for 5 min. Pellets were solubilised in Laemmli buffer and samples were incubated for 5 min at room temperature, solubilised, and heated at 100°C for 5 min. Samples were centrifuged at 20,000× g for 15 min at 20°C and supernatants were recovered and loaded on a 12% Bis-Tris Gel (Criterion XT, BioRad or NuPage, Invitrogen). Proteins were electrophoretically transferred onto PVDF or nitrocellulose membranes (Millipore). Membranes were blocked O/N with 2% BSA blocking buffer. For immunoblotting, membranes were incubated with either Sha 31 [53] or 12B2 [37] monoclonal antibody (Mab). Immunocomplexes were detected incubating the membranes for 1 hour with horseradish peroxidase conjugated anti mouse IgG (Amersham Pharmacia Biotech). Immunoblots were developed with enhanced chemiluminescence ECL Plus (GE Healthcare Amersham Biosciences).
Histopathology
All procedures involving mice brains were performed as previously described [54]. Brain slices were realized, in order to allow lesion profiling according to the standard method described by Fraser and Dickinson [55]. Briefly, samples were fixed in neutral-1 buffered 10% formalin (4% 2 - formaldehyde) before being cut at determined levels and paraffin embedded. After deparaffinization, 2 µm-thick tissue sections were stained with haematoxylin and eosin.
Histoblots
Brains were rapidly removed from euthanised mice and frozen on dry ice. Thick 8–10 µm cryostat sections were cut, transferred onto Superfrost slides and kept at −20°C until use. Histoblot analyses were performed on 3 brains per infection at 2nd and 3rd passage, using the 3F4 anti-PrP antibody as previously described [35].
Generation of tg340 mouse line expressing human PrP
Tg340 mouse line expressing about 4-fold level of human PrP M129 on a mouse PrP null background has been generated following a similar procedure previously describe for the generation of other transgenic mouse line expressing different species PrP [51], [56]. Briefly, the open reading frame (ORF) of human PrP gene was isolated by PCR amplification from human genomic DNA encoding methionine at codon 129. The primers used created a XhoI restriction enzyme site adjacent to the translation start and stop sites of the human PrP ORF (5′ -CTCGAGATTATGGCGAACCTTGGCTGCTGG - 3′ and 5′ - CTCGAGTCATCCCACTATCAGGAAGATGAG - 3′, respectively). The PCR fragments obtained were sub cloned into a pGEM-T Easy Vector System (Promega) following manufacturer instructions, and inserts were sequenced to confirm no differences in the inferred amino acid sequence with respect to previously sequenced human PrP genes (GenBank accession number NM_183079) and to confirm the presence of the consequent codon 129 nucleotide variant (MetATG). The human PrP ORF was excised from the cloning vector using the restriction enzyme XhoI and inserted into the expression vector MoPrP.Xho [51], [56]. This vector contains the murine PrP promoter (including exon 1, intron 1, exon 2 and 3′ untranslated sequences) flanked by two XhoI restriction sites but could be distinguished from the wild type murine PrP gene because of the absence of intron 2. The vector was also digested with XhoI to excise the murine PrP ORF and the correspondent human PrP ORF were inserted by ligation, obtaining the plasmid pMo-huPrP129M.Xho.
The human transgene was excised from the plasmid vector using the restriction endonuclease Not I leading to DNA fragments of approximately 12 Kb. Finally, the DNAs were purified and dissolved in TE at a final concentration of 2 to 6 µg/ml and microinjected into pronuclear stage ova collected from super-ovulated B6CBAf1 females mated with 129/Ola males carrying a null mutation in endogenous PrP [51], [56].
DNA from founders' tails biopsies was extracted using a Extract-N-Amp Tissue PCR kit (Sigma-Aldrich) following manufacturer instructions. The presence of the human transgene in these founders was identified by PCR amplification using specific primers for the mouse PrP exon 2 and human PrP open reading frame. The absence of the murine PrP ORF in the transgenic mice generated was confirmed by PCR amplification using the primers: 5′ - TAGATGTCAAGGACCTTCAGCC - 3′ and 5′ - GTTCCACTGATTATGGGTACC -3′.
Eight different lines (founders) of human PrPC (huPrP) and murine PrPC (muPrP) heterozygous transgenic mice (PrP mu+/− hu+/−) were obtained. The expression of human PrPC in brain of these mouse lines was analyzed and compared with PrPC content in human brain homogenate by western blot using mAb 3F4 which recognizes the 109MKHM112 epitope (numbered according to the human PrP sequence). Human PrPC was detected in 100% of the tested lines (data not shown). From the initial 8 different mouse lines heterozygous for both murine and human PrP genes (PrP mu+/− hu+/−), the mouse line named as tg340 was selected for further experiments on the basis of the level of PrPC expression.
Homozygous Tg340 mouse line was established backcrossing these animals with homozygous null animals MuPrP−/− (Prnp−/−) to obtain a null murine PrP background (PrP mu−/− hu+/−). Interbreeding within these animals was performed to obtain homozygosis for the human PrP transgen within a murine PrP background (PrP mu−/− hu+/+). The absence of murine PrP gene was determined by PCR using specific primers. Human PrPC expression levels, determined more accurately in brain from homozygous tg340 animals was about 4-fold higher than PrPC levels in human brain homogenates as determined by dilution experiments in western blot (Figure 8).
Fig. 8. Brain PrPC expression in homozygous tg340 mouse line in comparison to both tg650 mice and human brain. 
A) Immunoblots of the brain PrPC expression in tg340 detected with Pri 308 Mab. Direct sample (10% brain homogenates) and ¼ dilutions were loaded on 12% Bis-Tris gels. The figure illustrates a representative set of three independent experiments. B) Brain PrPC expression in tg340 in comparison to tg650 detected with Pri 308 Mab. Inmunoblots illustrates a representative set of three independent experiments and the diagrams represent the mean densytometric values data from these experiments. Data from human brains (Human) were considered as 1 relative unit. Error bars represent the standard deviation of the mean values. Accession number
The GenBank accession number for the human Prnp gene used in this paper is NM_183079.
Zdroje
1. PrusinerSB
1982 Novel proteinaceous infectious particles cause scrapie. Science 216 136 144
2. GriffithJS
1967 Self-replication and scrapie. Nature 215 1043 1044
3. WellsGA
ScottAC
JohnsonCT
GunningRF
HancockRD
1987 A novel progressive spongiform encephalopathy in cattle. Vet Rec 121 419 420
4. BruceME
WillRG
IronsideJW
McConnellI
DrummondD
1997 Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature 389 498 501
5. HillAF
DesbruslaisM
JoinerS
SidleKC
GowlandI
1997 The same prion strain causes vCJD and BSE. Nature 389 448 450 526
6. BaylisM
HoustonF
KaoRR
McLeanAR
HunterN
2002 BSE - a wolf in sheep's clothing? Trends Microbiol 10 563 570
7. FosterJD
HopeJ
FraserH
1993 Transmission of bovine spongiform encephalopathy to sheep and goats. Vet Rec 133 339 341
8. BellworthySJ
DexterG
StackM
ChaplinM
HawkinsSA
2005 Natural transmission of BSE between sheep within an experimental flock. Vet Rec 157 206
9. BellworthySJ
DexterG
StackM
ChaplinM
HawkinsSA
2008 Oral transmission of BSE to VRQ/VRQ sheep in an experimental flock. Vet Rec 162 130 131
10. StackM
GonzalezL
JeffreyM
MartinS
MacaldowieC
2009 Three serial passages of bovine spongiform encephalopathy in sheep do not significantly affect discriminatory test results. J Gen Virol 90 764 768
11. HoustonF
GoldmannW
ChongA
JeffreyM
GonzalezL
2003 Prion diseases: BSE in sheep bred for resistance to infection. Nature 423 498
12. EloitM
AdjouK
CoulpierM
FontaineJJ
HamelR
2005 BSE agent signatures in a goat. Vet Rec 156 523 524
13. JeffreyM
MartinS
GonzalezL
FosterJ
LangeveldJP
2006 Immunohistochemical features of PrP(d) accumulation in natural and experimental goat transmissible spongiform encephalopathies. J Comp Pathol 134 171 181
14. WellsGA
HawkinsSA
GreenRB
AustinAR
DexterI
1998 Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet Rec 142 103 106
15. BuschmannA
GroschupMH
2005 Highly bovine spongiform encephalopathy-sensitive transgenic mice confirm the essential restriction of infectivity to the nervous system in clinically diseased cattle. J Infect Dis 192 934 942
16. WellsGA
SpiropoulosJ
HawkinsSA
RyderSJ
2005 Pathogenesis of experimental bovine spongiform encephalopathy: preclinical infectivity in tonsil and observations on the distribution of lingual tonsil in slaughtered cattle. Vet Rec 156 401 407
17. EspinosaJC
MoralesM
CastillaJ
RogersM
TorresJM
2007 Progression of prion infectivity in asymptomatic cattle after oral bovine spongiform encephalopathy challenge. J Gen Virol 88 1379 1383
18. FosterJD
BruceM
McConnellI
ChreeA
FraserH
1996 Detection of BSE infectivity in brain and spleen of experimentally infected sheep. Vet Rec 138 546 548
19. BellworthySJ
HawkinsSA
GreenRB
BlamireI
DexterG
2005 Tissue distribution of bovine spongiform encephalopathy infectivity in Romney sheep up to the onset of clinical disease after oral challenge. Vet Rec 156 197 202
20. CollingeJ
ClarkeAR
2007 A general model of prion strains and their pathogenicity. Science 318 930 936
21. PattisonIH
1966 The relative susceptibility of sheep, goats and mice to two types of the goat scrapie agent. Res Vet Sci 7 207 212
22. ScottM
FosterD
MirendaC
SerbanD
CoufalF
1989 Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell 59 847 857
23. PrusinerSB
ScottM
FosterD
PanKM
GrothD
1990 Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63 673 686
24. PeretzD
WilliamsonRA
LegnameG
MatsunagaY
VergaraJ
2002 A change in the conformation of prions accompanies the emergence of a new prion strain. Neuron 34 921 932
25. CollingeJ
SidleKC
MeadsJ
IronsideJ
HillAF
1996 Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383 685 690
26. ScottMR
WillR
IronsideJ
NguyenHO
TremblayP
1999 Compelling transgenetic evidence for transmission of bovine spongiform encephalopathy prions to humans. Proc Natl Acad Sci U S A 96 15137 15142
27. ScottMR
PeretzD
NguyenHO
DearmondSJ
PrusinerSB
2005 Transmission barriers for bovine, ovine, and human prions in transgenic mice. J Virol 79 5259 5271
28. NonnoR
Di BariMA
CardoneF
VaccariG
FazziP
2006 Efficient transmission and characterization of Creutzfeldt-Jakob disease strains in bank voles. PLoS Pathog 2 e12
29. EspinosaJC
AndreolettiO
CastillaJ
HervaME
MoralesM
2007 Sheep-passaged bovine spongiform encephalopathy agent exhibits altered pathobiological properties in bovine-PrP transgenic mice. J Virol 81 835 843
30. EspinosaJC
HervaME
AndreolettiO
PadillaD
LacrouxC
2009 Transgenic mice expressing porcine prion protein resistant to classical scrapie but susceptible to sheep bovine spongiform encephalopathy and atypical scrapie. Emerg Infect Dis 15 1214 1221
31. PalmerMS
DrydenAJ
HughesJT
CollingeJ
1991 Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature 352 340 342
32. WindlO
DempsterM
EstibeiroJP
LatheR
de SilvaR
1996 Genetic basis of Creutzfeldt-Jakob disease in the United Kingdom: a systematic analysis of predisposing mutations and allelic variation in the PRNP gene. Hum Genet 98 259 264
33. CollingeJ
1997 Human prion diseases and bovine spongiform encephalopathy (BSE). Hum Mol Genet 6 1699 1705
34. LeeHS
BrownP
CervenakovaL
GarrutoRM
AlpersMP
2001 Increased susceptibility to Kuru of carriers of the PRNP 129 methionine/methionine genotype. J Infect Dis 183 192 196
35. BeringueV
Le DurA
TixadorP
ReineF
LepourryL
2008 Prominent and persistent extraneural infection in human PrP transgenic mice infected with variant CJD. PLoS One 3 e1419
36. BeringueV
HerzogL
ReineF
Le DurA
CasaloneC
2008 Transmission of atypical bovine prions to mice transgenic for human prion protein. Emerg Infect Dis 14 1898 1901
37. YullHM
RitchieDL
LangeveldJP
van ZijderveldFG
BruceME
2006 Detection of type 1 prion protein in variant Creutzfeldt-Jakob disease. Am J Pathol 168 151 157
38. FraserH
DickinsonAG
1973 Scrapie in mice. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J Comp Pathol 83 29 40
39. KupferL
EidenM
BuschmannA
GroschupMH
2007 Amino acid sequence and prion strain specific effects on the in vitro and in vivo convertibility of ovine/murine and bovine/murine prion protein chimeras. Biochim Biophys Acta 1772 704 713
40. AsanteEA
LinehanJM
DesbruslaisM
JoinerS
GowlandI
2002 BSE prions propagate as either variant CJD-like or sporadic CJD-like prion strains in transgenic mice expressing human prion protein. Embo J 21 6358 6366
41. KongQ
ZhengM
CasaloneC
QingL
HuangS
2008 Evaluation of the human transmission risk of an atypical bovine spongiform encephalopathy prion strain. J Virol 82 3697 3701
42. BishopMT
HartP
AitchisonL
BaybuttHN
PlinstonC
2006 Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol 5 393 398
43. BeringueV
AndreolettiO
Le DurA
EssalmaniR
VilotteJL
2007 A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J Neurosci 27 6965 6971
44. GonzalezL
ChianiniF
MartinS
SisoS
GibbardL
2007 Comparative titration of experimental ovine BSE infectivity in sheep and mice. J Gen Virol 88 714 717
45. TamguneyG
MillerMW
GilesK
LemusA
GliddenDV
2009 Transmission of scrapie and sheep-passaged bovine spongiform encephalopathy prions to transgenic mice expressing elk prion protein. J Gen Virol 90 1035 1047
46. TixadorP
HerzogL
ReineF
JaumainE
ChapuisJ
2010 The physical relationship between infectivity and prion protein aggregates is strain-dependent. PLoS Pathog 6 e1000859
47. LiJ
BrowningS
MahalSP
OelschlegelAM
WeissmannC
2010 Darwinian evolution of prions in cell culture. Science 327 869 872
48. BaronTG
MadecJY
CalavasD
RichardY
BarilletF
2000 Comparison of French natural scrapie isolates with bovine spongiform encephalopathy and experimental scrapie infected sheep. Neurosci Lett 284 175 178
49. JeffreyM
RyderS
MartinS
HawkinsSA
TerryL
2001 Oral inoculation of sheep with the agent of bovine spongiform encephalopathy (BSE). 1. Onset and distribution of disease-specific PrP accumulation in brain and viscera. J Comp Pathol 124 280 289
50. FosterJD
ParnhamDW
HunterN
BruceM
2001 Distribution of the prion protein in sheep terminally affected with BSE following experimental oral transmission. J Gen Virol 82 2319 2326
51. CastillaJ
Gutierrez AdanA
BrunA
PintadoB
RamirezMA
2003 Early detection of PrPres in BSE-infected bovine PrP transgenic mice. Arch Virol 148 677 691
52. BeringueV
BencsikA
Le DurA
ReineF
LaiTL
2006 Isolation from cattle of a prion strain distinct from that causing bovine spongiform encephalopathy. PLoS Pathog 2 e112
53. FeraudetC
MorelN
SimonS
VollandH
FrobertY
2005 Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem 280 11247 11258
54. AndreolettiO
LacrouxC
ChabertA
MonnereauL
TabouretG
2002 PrP(Sc) accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol 83 2607 2616
55. FraserH
DickinsonAG
1968 The sequential development of the brain lesion of scrapie in three strains of mice. J Comp Pathol 78 301 311
56. CastillaJ
Gutierrez-AdanA
BrunA
DoyleD
PintadoB
2004 Subclinical bovine spongiform encephalopathy infection in transgenic mice expressing porcine prion protein. J Neurosci 24 5063 5069
57. BencsikA
BaronT
2007 Bovine spongiform encephalopathy agent in a prion protein (PrP)ARR/ARR genotype sheep after peripheral challenge: complete immunohistochemical analysis of disease-associated PrP and transmission studies to ovine-transgenic mice. J Infect Dis 195 989 996
58. CooperJK
LadhaniK
MinorPD
2007 Reference materials for the evaluation of pre-mortem variant Creutzfeldt-Jakob disease diagnostic assays. Vox Sang 92 302 310
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in ChinaČlánek HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2Článek The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.Článek Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria ParasitesČlánek KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 MotifČlánek Viral EncephalomyelitisČlánek Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- The Strain-Encoded Relationship between PrP Replication, Stability and Processing in Neurons is Predictive of the Incubation Period of Disease
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Human Macrophage Responses to Clinical Isolates from the Complex Discriminate between Ancient and Modern Lineages
- Dendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from
- Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China
- Rhesus TRIM5α Disrupts the HIV-1 Capsid at the InterHexamer Interfaces
- HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2
- Antigenic Variation in Malaria Involves a Highly Structured Switching Pattern
- The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Novel Escape Mutants Suggest an Extensive TRIM5α Binding Site Spanning the Entire Outer Surface of the Murine Leukemia Virus Capsid Protein
- Global Functional Analyses of Cellular Responses to Pore-Forming Toxins
- Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria Parasites
- Lung Adenocarcinoma Originates from Retrovirus Infection of Proliferating Type 2 Pneumocytes during Pulmonary Post-Natal Development or Tissue Repair
- Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors
- The Moving Junction Protein RON8 Facilitates Firm Attachment and Host Cell Invasion in
- KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 Motif
- The Coxsackievirus B 3C Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
- Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway
- Viral Encephalomyelitis
- Sheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice
- Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Dynamic Imaging of the Effector Immune Response to Infection
- The Lectin Receptor Kinase LecRK-I.9 Is a Novel Resistance Component and a Potential Host Target for a RXLR Effector
- Host Iron Withholding Demands Siderophore Utilization for to Survive Macrophage Killing
- The Danger Signal S100B Integrates Pathogen– and Danger–Sensing Pathways to Restrain Inflammation
- The RNome and Its Commitment to Virulence
- A Novel Nuclear Factor TgNF3 Is a Dynamic Chromatin-Associated Component, Modulator of Nucleolar Architecture and Parasite Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



