-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Novel Escape Mutants Suggest an Extensive TRIM5α Binding Site
Spanning the Entire Outer Surface of the Murine Leukemia Virus Capsid
Protein
After entry into target cells, retroviruses encounter the host restriction
factors such as Fv1 and TRIM5α. While it is clear that these factors target
retrovirus capsid proteins (CA), recognition remains poorly defined in the
absence of structural information. To better understand the binding interaction
between TRIM5α and CA, we selected a panel of novel N-tropic murine
leukaemia virus (N-MLV) escape mutants by a serial passage of replication
competent N-MLV in rhesus macaque TRIM5α (rhTRIM5α)-positive cells using
a small percentage of unrestricted cells to allow multiple rounds of virus
replication. The newly identified mutations, many of which involve changes in
charge, are distributed over the outer ‘top’ surface of N-MLV CA,
including the N-terminal β-hairpin, and map up to 29 Ao apart.
Biological characterisation with a number of restriction factors revealed that
only one of the new mutations affects restriction by human TRIM5α,
indicating significant differences in the binding interaction between N-MLV and
the two TRIM5αs, whereas three of the mutations result in dual sensitivity
to Fv1n and Fv1b. Structural studies of two mutants show
that no major changes in the overall CA conformation are associated with escape
from restriction. We conclude that interactions involving much, if not all, of
the surface of CA are vital for TRIM5α binding.
Published in the journal: . PLoS Pathog 7(3): e32767. doi:10.1371/journal.ppat.1002011
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002011Summary
After entry into target cells, retroviruses encounter the host restriction
factors such as Fv1 and TRIM5α. While it is clear that these factors target
retrovirus capsid proteins (CA), recognition remains poorly defined in the
absence of structural information. To better understand the binding interaction
between TRIM5α and CA, we selected a panel of novel N-tropic murine
leukaemia virus (N-MLV) escape mutants by a serial passage of replication
competent N-MLV in rhesus macaque TRIM5α (rhTRIM5α)-positive cells using
a small percentage of unrestricted cells to allow multiple rounds of virus
replication. The newly identified mutations, many of which involve changes in
charge, are distributed over the outer ‘top’ surface of N-MLV CA,
including the N-terminal β-hairpin, and map up to 29 Ao apart.
Biological characterisation with a number of restriction factors revealed that
only one of the new mutations affects restriction by human TRIM5α,
indicating significant differences in the binding interaction between N-MLV and
the two TRIM5αs, whereas three of the mutations result in dual sensitivity
to Fv1n and Fv1b. Structural studies of two mutants show
that no major changes in the overall CA conformation are associated with escape
from restriction. We conclude that interactions involving much, if not all, of
the surface of CA are vital for TRIM5α binding.Introduction
Mammalian cells show different susceptibilities to retrovirus infection. Cells from mice exhibit different susceptibility to murine leukaemia virus (MLV) dependent on their genetic backgrounds; cell lines from different primates show defined patterns of lentiviral replication. In many cases, these variations are due to the presence of cellular proteins referred as restriction factors. The susceptibility to MLV infection in mouse cells is determined by a restriction factor called Fv1; different alleles of Fv1 restricting different strains of MLV. Fv1n restricts B-tropic MLV (B-MLV) but not N-tropic MLV (N-MLV) infections, whereas Fv1b does the reverse [1], [2], [3]. In primate cells, lentivirus and N-MLV are restricted by a second restriction factor, TRIM5α [4], [5], [6], [7], [8]. TRIM5 is a member of a large family of tri-partite motif proteins that contain RING, B-box and coiled-coil motifs, sometimes referred to collectively as an RBCC domain [9], [10]. The α isoform of TRIM5 (TRIM5α) also has an additional large B30.2 or PRYSPRY domain at its C-terminus [11], [12], important for recognizing the viral target [13], [14], [15], [16]. Both Fv1 [17], [18] and TRIM5α [5], [19] target the viral capsid protein (CA), blocking retrovirus replication after viral entry into the target cell. Restriction by TRIM5α, however, is clearly distinguished from that by Fv1 in that TRIM5α-mediated restriction normally occurs before or during reverse transcription [5], [20], whereas Fv1-mediated restriction occurs after the completion of reverse transcription but prior to viral integration [21], [22].
One of the most surprising features of TRIM5α restriction is the ability of TRIM5αs from different species to recognize and restrict multiple unrelated viruses often across different retroviral genera. For example, TRIM5α from the cotton-top tamarin can recognize at least one member from each of the lentivirus, gammaretrovirus, betaretrovirus and spumavirus genera and rhesus monkey (rh) TRIM5α will restrict both HIV-1 and N-MLV [23], [24], [25]. Although it is clear that Fv1 and TRIM5α target viral CA, in the absence of structural information the recognition process remains poorly defined. However, it appears likely that single-site binding is weak and that a productive interaction required for restriction is multivalent and dependent on the spacing of CA monomers/hexamers in the viral core [26], [27], [28].
One approach to investigating the problem of restriction specificity has been through genetic characterisation of naturally occurring or experimentally induced variants in CA that alter virus tropism [29]. In this way amino acid residues involved in the interaction with restriction factors have been identified. In the case of Fv1-mediated restriction of MLV, it is known that several surface-exposed amino acids in the region between α-helices 4 and 6 in CA, which include amino acids at positions 82, 92–94, 110, 114 and 117, can change the tropism of MLV [30]. Among these positions, the amino acid at position 110 is the main determinant, since substitution of amino acid at position 110 (Arg for N-tropic and Glu for B-tropic MLV) can switch the tropism of MLV [31]. Structural studies show that amino acids in positions 110, 114 and 117 are located in α-helix 6, residue 82 is in the loop between α-helices 4 and 5, and amino acids 92–94 are located in α-helix 5 [32]. Therefore, the region between α-helices 4 and 6 might be considered a ‘Fv1-binding pocket’ [27]. A number of amino acid substitution experiments have also suggested that this α4–α6 region may also contribute to TRIM5α-mediated restriction of N-MLV. These include an R110E mutation that confers resistance of N-MLV to restriction by human (hu) TRIM5α and an E110R mutation in B-MLV that results in susceptibility to restriction by huTRIM5α [19]. Other substitutions in CA at positions 82, 114 and 117 alter the TRIM5α species range able to restrict B - and N-MLV [8] and domain-swap experiments suggested that the equivalent region in HIV and other lentiviruses also contains the host-range determining segments [33], [34], [35], [36].
In this study, replication competent N-MLV was passaged in rhTRIM5α-positive cells to search for escape mutations in sites important for restriction and in the context of a natural infection. We isolated a number of N-MLV escape mutants, including substitutions at the previously identified positions 82 and 114. However, of particular interest is a new class of mutations including G8D, L10W and V116D that were discovered. They map outside the putative Fv1 binding pocket and display subtle structural changes. In these instances, escape from restriction by rhTRIM5α was conferred, but restriction by huTRIM5α was not affected. These observations have defined new regions of N-MLV CA that control virus growth in the presence of TRIM5α, revealed that the restriction factor-CA interface is larger than previously thought and shown that subtle changes in CA structure can have profound effects on TRIM5α susceptibility.
Results
In an initial attempt to isolate MLV variants resistant to TRIM5α-mediated restriction, Mus dunni tail fibroblasts (MDTF), MDTF expressing YFP and huTRIM5α (MDHu) or rhTRIM5α (MDRh) were inoculated with different dilutions of replication competent N-MLV (Stock A) and the infected cells were passed repeatedly. On each passage, the culture supernatant was harvested and reverse transcriptase (RT) activity in the filtered culture supernatant measured (Figure 1). After only three days high RT levels were measured in restriction factor-negative cells, even when the target cells were initially infected with low titre (1∶100 diluted virus) (Figure 1A). When restriction factor-negative cells were infected with the high titre undiluted virus, the RT activity decreased sharply at 6 days post infection (d.p.i.) but later recovered, due to virus-mediated cytopathic effects. In cells expressing huTRIM5α N-MLV replication was restricted completely, even when undiluted virus was inoculated (Figure 1B). By contrast, cells expressing rhTRIM5α did not prevent replication of high titre virus, and, although the time of RT release was delayed, maximal levels were similar to those seen with MDTF (compare Figure 1C and Figure 1A). No virus was recovered from MDRh infected with 1∶10 or 1∶100 diluted N-MLV (Figure 1C).
Taken together, these data imply (a) huTRIM5α restriction of N-MLV is more potent than that of rhTRIM5α and (b) rhTRIM5α restriction can be overcome by high levels of wild-type N-MLV in a rapid manner, presumably by saturation. However, under these conditions, an extended period of virus replication against a background of restriction does not seem to occur leaving little opportunity for escape mutants to arise. To maximize the chances of generating and recovering escape mutants we decided to focus on searching for escape mutants from rhTRIM5α using low input virus to avoid restriction factor saturation. To allow continued virus replication under selective conditions a mixture of MDTF and MDRh was infected.
Generation and selection of N-MLV escape mutants from rhesus TRIM5α
Low titres of N-MLV were used to infect a mixture of 90% TRIM5α-positive and 10% TRIM5α-negative cells. Virus spread was monitored by fluorescence staining using a monoclonal antibody directed against the viral p12 protein. As shown in Figure 2A, virus-infected cell populations were clearly distinguishable from virus-negative cells both in YFP-negative (TRIM5α-negative) and -positive (TRIM5α-positive) populations. Following infection of the first mixed culture, a small fraction of the YFP-positive cells appeared virus-positive as early as 3 d.p.i.; the virus spread into about 30% of the YFP-positive cell population within 13 d.p.i. and 60% by 18 d.p.i. (Figure 2B iv-vi). The culture supernatant was then collected, filtered, diluted tenfold and passed again in mixed cell culture for a further ten days (2nd culture). To select mutant viruses, the filtered and diluted culture supernatant from the second mixed cell culture was inoculated onto 100% MDRh cells (the 3rd and 4th cultures). In contrast to wild type N-MLV, which at 1∶10 dilution had shown no virus expansion during the first 18 days of MDRh culture (Figure 1C), the virus from the second culture took 7 days to grow in 100% MDRh cells in the third culture (Figure 2B). This was not a result of different levels of viral input because virus with RT activity of 20 mU was inoculated in the third culture compared to 514 mU RT activity in the first inoculum. The virus at the end of the third culture was stored and referred to as rhesus TRIM5α-escape mutant 1 (REM1).
Fig. 2. Selection of escape mutants from rhesus macaque TRIM5α. 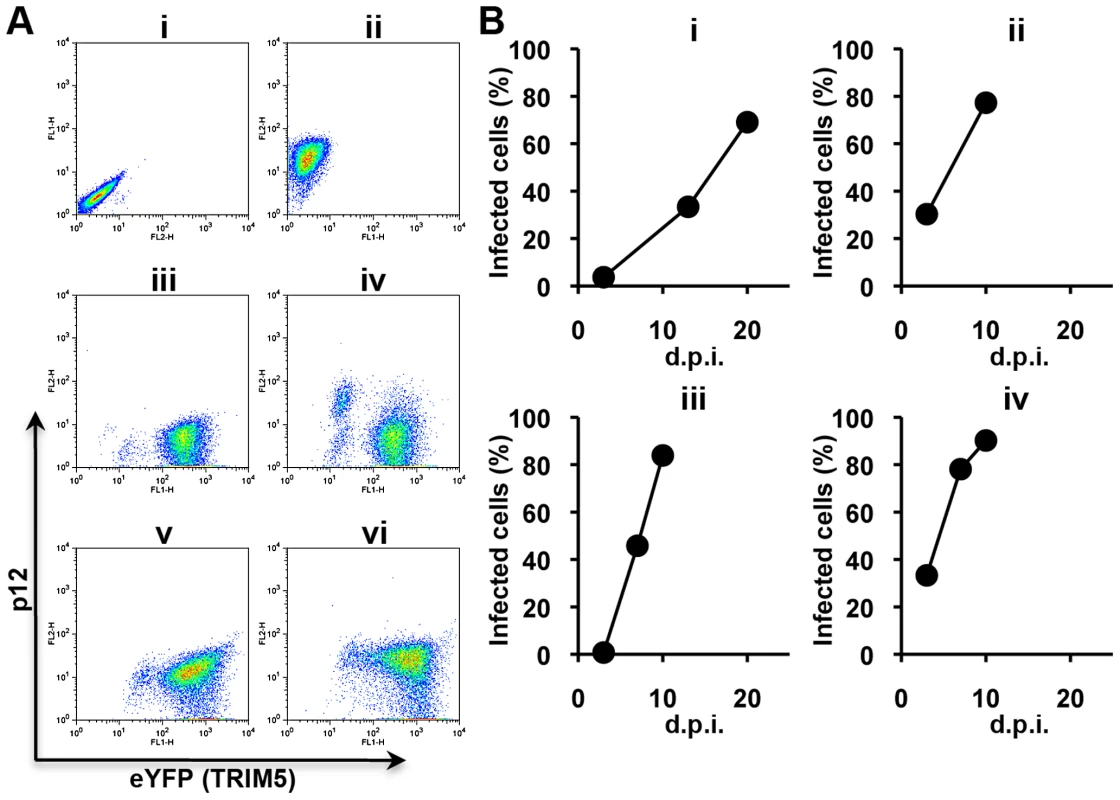
This procedure was repeated a further three times starting with N-MLV Stock A yielding REM2-4. In each experiment a similar time course was observed for isolation of a virus capable of unrestricted growth in the presence of rhTRIM5α. These results clearly demonstrate the utility of unrestricted refugia in the isolation of escape mutants from rhTRIM5α by allowing growth against a background of restriction.
The primary target for Fv1 and TRIM5α restriction is thought to be the viral CA protein. It was thus expected that escape mutations would map to the capsid so the nucleotide sequences of the capsid region of the viral gag gene was determined by direct sequencing of viral cDNA amplified from viral RNA in the culture supernatant. These data revealed that REM1 had acquired a single nucleotide substitution from T to G at nucleotide 1309 (AKV numbering) [37], which is predicted to produce a leucine to tryptophan substitution at amino acid position 10 in CA. The same L10W mutation in CA was seen in four independent experiments (isolates REM1-4) and no other mutations were detected in the capsid gene. This suggested that a small population of viruses carrying the L10W mutant may already exist in the virus stock prepared from the culture supernatant of chronically infected cells (Stock A) and the mutant was selected in the course of the virus passage experiments. As a first test of this hypothesis, the capsid gene of Stock A virus was amplified by RT-PCR and cloned. No sequence variation was observed in 50 independent clones (all 50 clones yielded sequences identical to N-MLV derived from pWN41) indicating that any minority species can only be present at very low levels.
We next derived a second stock of virus by transfection of 293T cells with self-ligated pWN41 provirus followed by three cell-free transfers to MDTF cells (Stock B). Two independent experiments with the new N-MLV stock following the same protocol used to isolate REM 1-4 resulted in the isolation of potential escape mutants REM5, 6. The only change in the capsid genes in these two viruses was a single T to A change at nucleotide 1627, giving rise to a V116D mutation. Four further experiments with freshly prepared Stock Bs gave rise to REM7-10; three carried single mutations resulting in L10W, H114N and H114R changes whereas REM10 carried three mutations causing L4S/A95D/S202G alterations.
A third set of experiments used Stock C, which was recovered directly following transfection of human 293T cells without passage through murine cells and should therefore have no mutations associated with reverse transcription (pWN41 encodes an ecotropic virus which will only infect murine cells), led to the isolation of REM11-15. Sequencing studies showed that these viruses carried mutations resulting in the following changes: G8D, N82D (twice), E92K and H114D.
To confirm the importance of these mutations in escape from rhTRIM5α they were introduced by Quikchange PCR into the N-MLV vector, pCIG3N and the resulting viruses tested for growth with different restriction factors. On the basis of these analyses (Figure 3 and Supplementary Figure S1), the escape mutants could be placed into two groups depending on the degree of residual sensitivity to rhTRIM5α. One group, typified by L10W and comprising L10W, H114D, H114N, H114R and E92K showed equivalent titration curves in the presence and absence of rhesus TRIM5α (though E92K grew poorly compared to wild type N-MLV). The second group, typified by V116D, contained in addition G8D and N82D. This group showed slightly reduced infectivity in the presence of rhTRIM5α. Two of the single amino acid changes present in the triple mutant, L4S and A95D, gave rise to viruses that would fall in the second group but together show complete resistance. The S202G change appeared to have no effect on restriction, as might be expected for a residue in the (interior) C terminal domain of CA. Perhaps surprisingly, only one of the mutations that result in escape from rhTRIM5α, E92K, affects restriction by huTRIM5α. Furthermore, three of the mutations that give rise to resistance to rhTRIM5α, G8D, H114D and V116D confer sensitivity to Fv1n while maintaining Fv1b susceptibility. These data are summarized in Table 1.
Fig. 3. Restriction of N-MLV vectors carrying L10W and V116D. 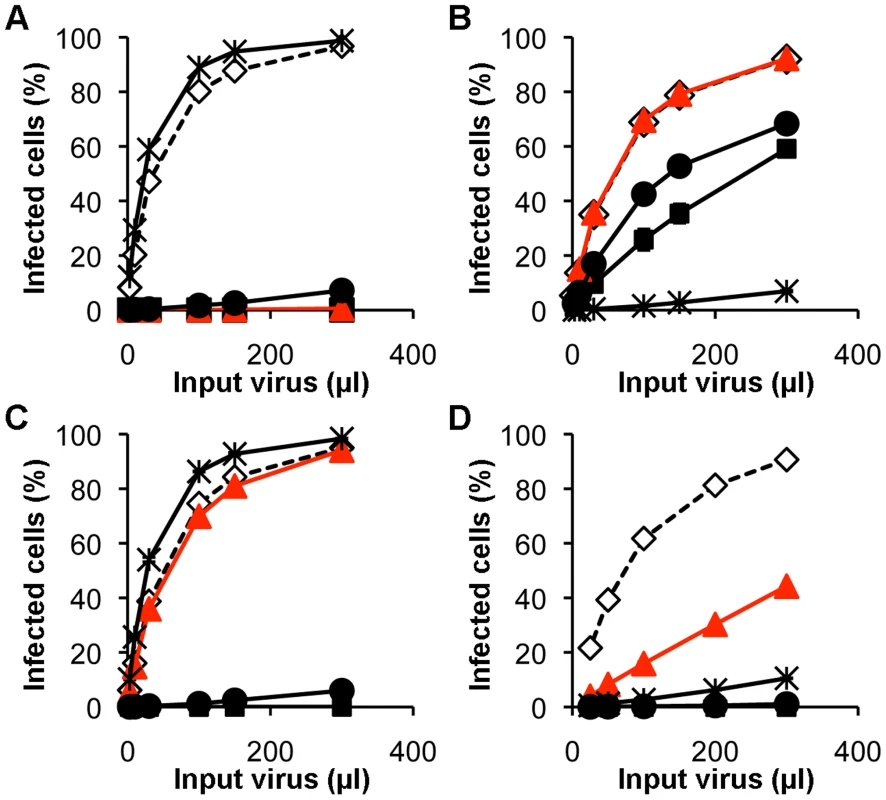
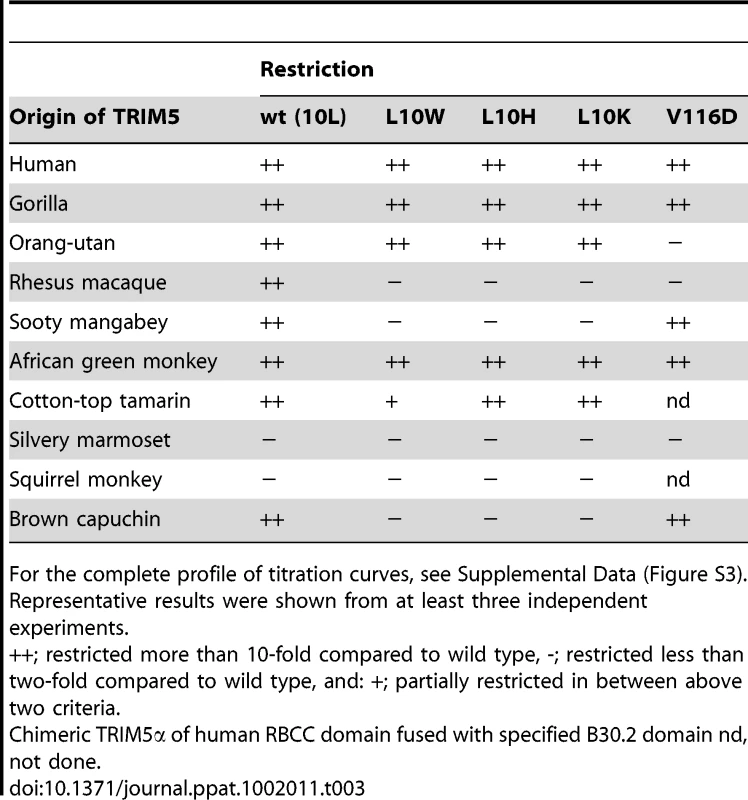
Tab. 1. Summary of the restriction profiles of the escape mutants. 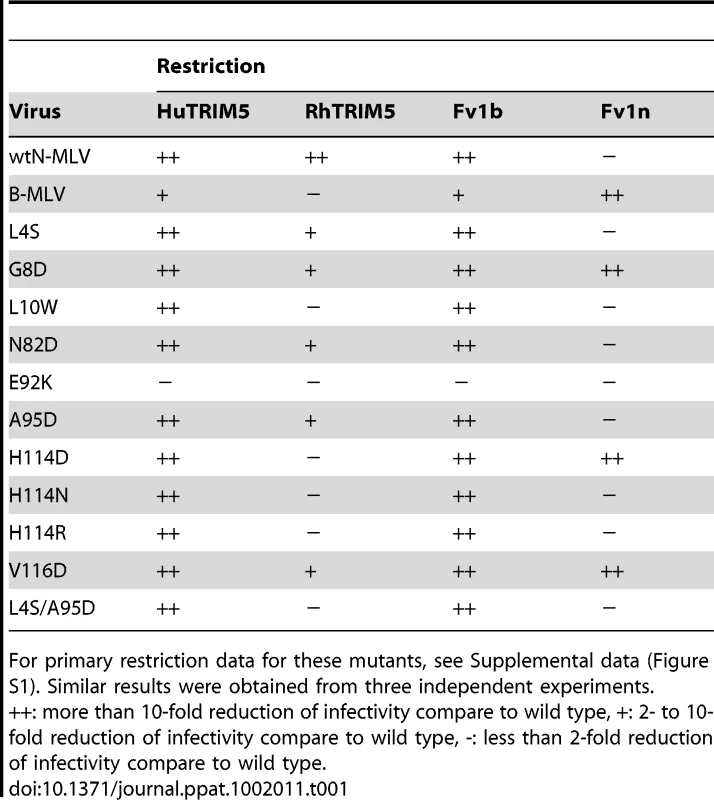
Mutant capsids are resistant to rhesus macaque TRIM5α mediated disassembly
Since mutations at positions 10 and 116 have not previously been reported to affect interactions with Fv1 or TRIM5α we next set out to explore the properties of the L10W and V116D mutations. To examine the interaction between viral cores carrying the L10W and V116D mutations and rhTRIM5α, we tested whether such cores would resist rhTRIM5α mediated disassembly using the previously described fate-of-capsid assay that follows the premature capsid disassembly thought to occur in an infected cell following virus encountering TRIM5α [38]. As expected, wt N-MLV cores were pelleted in TRIM5α-negative cell extract (Figure 4, lane 1) and B-MLV capsid cores, but not wt N-MLV, L10W and V116D mutant capsid cores, were pelleted when huTRIM5α-expressing cells were infected (Figure 4, lanes 2–5). This result was consistent with the observations that huTRIM5α restricted wt N-MLV, L10W and V116D mutant viruses, but not B-MLV. In contrast, in the presence of rhTRIM5α, B-MLV and the L10W and V116D N-MLVs were pelleted, but wild type N-MLV was not detected in the pellet fraction (Figure 4, lanes 6–9). Therefore, L10W and V116D mutant viruses escape disassembly of capsid core caused by rhTRIM5α, an observation consistent with the lack of restriction of these mutant viruses by rhTRIM5α and emphasizing the species-specific nature of escape by L10W and V116D mutants from TRIM5α.
Restriction profiles of L10 mutants and V116D by primate TRIM5αs
The selectivity difference of rhesus and human TRIM5αs prompted us to carry out a more detailed survey of the restriction profiles. Firstly, as only the L10W mutation was isolated through the in vivo passaging experiments we wondered what the effect of other residue types at position 10 in N-MLV CA might have on restriction factor sensitivity. To address this possibility, random amino acids were introduced at position 10 by PCR mutagenesis. Using this procedure fourteen of the possible nineteen substitutions and wt silent codon changes were recovered. The restriction data for rhTRIM5α are shown in Supplementary Figure S2 and summarized in Table 2. Out of the entire panel only two additional mutants, L10H and L10K, giving complete escape were identified. Two other substitutions (L10P and L10V) resulted in wt restriction phenotype; another gave non-infectious virus (L10R), whereas the remainder display intermediate phenotypes with restriction by TRIM5α at low virus titre but with only moderate restriction at higher virus titre. The variable phenotypes produced in this experiment highlight the importance of MLV residue 10 suggesting a direct involvement/interaction of this part of CA with rhTRIM5α in restriction of N-MLV but do not suggest a direct correlation between restriction activity and the size or charge of the amino acid residue at position 10.
Tab. 2. Restriction sensitivity of CA10 mutants. 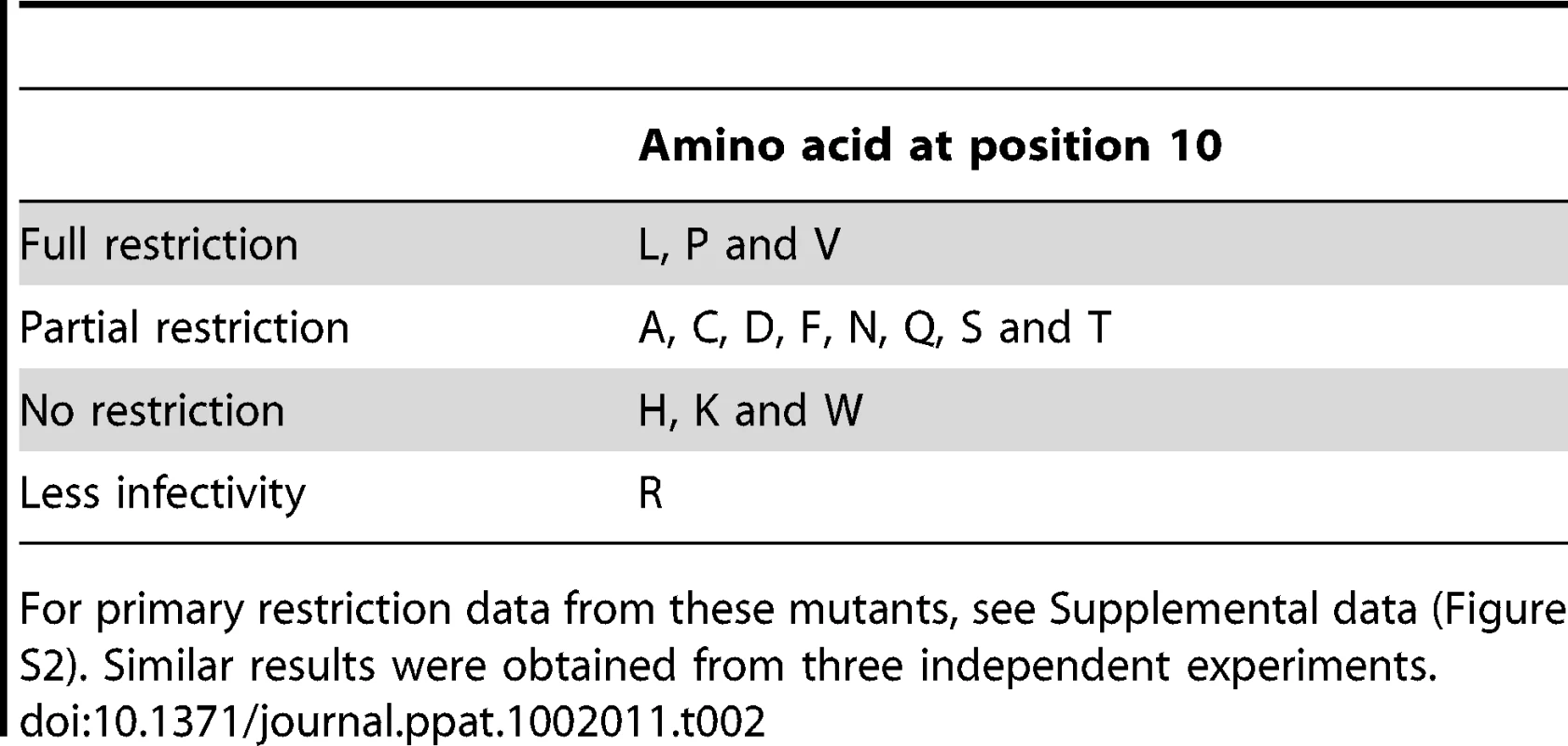
Next we tested the ability of a previously described panel of primate TRIM5αs to restrict the L10W, L10K, L10H and V116D mutant viruses [24]. Wild type N-MLV was restricted by all the ape and old world monkey TRIM5αs tested as well as some of the new world monkey TRIM5α including from Cotton-top tamarin and Brown capuchin (Table 3 and Supplementary Figure S3). All the L10 escape mutants were also restricted by ape TRIM5αs, which was perhaps not unexpected given the observation that huTRIM5α restricted the L10W mutant virus. However, among old world monkey TRIM5αs, only African green monkey TRIM5α restricted the L10 mutants and in each case these mutations also resulted in escape from restriction by the capuchin factor. On the other hand, the restriction profile of the V116D mutant virus was rather different. It was not restricted by orang-utan TRIM5α but was restricted by sooty mangabey and Brown capuchin TRIM5αs. These observations showed that whereas the viruses with mutations at position 10 showed a similar phenotype in terms of restriction by TRIM5α, the V116D mutant exhibited a different restriction profile by primate TRIM5α. These results imply that capsid recognition by TRIM5αs of different species may involve interactions with different amino acids on CA.
Involvement of B30.2 V1 and V2 regions in N-MLV CA recognition
These species differences in restriction profiles of the escape mutants allowed us to use a previously described series of chimeric human/macaque TRIM5αs [16], to ask which of the V1, V2 and V3 variable regions of the TRIM5α B30.2 domain are important for CA binding (Figure 5, Supplementary Figure S4). Each chimera was designed to contain a different combination of the variable regions of the B30.2 domain (Figure 5). Both human and rhesus TRIM5α restrict N-MLV and so not surprisingly MDTF cells stably expressing each of the eight chimeric TRIM5αs restricted wild type N-MLV. The position 10 mutants (L10W, L10K and L10H) show identical patterns of sensitivity to the chimeric constructs, being unrestricted by HR4, RH3 and RH4, but restricted by the other chimeric TRIM5α, suggesting that when either the V1 or V2 region is derived from huTRIM5α, the mutants are restricted, whereas when both V1 and V2 regions were derived from rhTRIM5α, the mutant N-MLV was not restricted. The V116D escape mutant also displays an identical profile to that of the position 10 mutants, supporting the idea that both V1 and V2 regions are involved in TRIM5α-mediated restriction of MLV.
The effect of β1-β2 mutations on HIV-1 susceptibility to Rhesus TRIM5α
The N-terminal domain of HIV-1 and MLV CAs share very similar structures [32], including the β-hairpin region. It therefore seemed possible that this region might affect sensitivity of HIV-1 to rhesus TRIM5α. To address this question, each amino acid between position 5 and 12 on HIV-1 capsid, which form the N-terminal β-hairpin loop, was substituted with a lysine residue. In addition, the methionine residue at position 10 of HIV-1 capsid was substituted randomly by mutagenesis PCR. However, although several of these mutations severely impaired Gag function, none of the viable HIV-1 mutants examined in this study, escaped rhTRIM5α (Supplementary Figure S5), implying that unlike MLV, the N-terminal β-hairpin loop of HIV-1 capsid does not seem to affect the restriction activity of TRIM5α. Based on these results, the ways by which rhTRIM5α recognizes viral capsid seems to be different between HIV and MLV.
Structure of N-MLV CA carrying the L10W mutation
On inspection of the structure of the MLV CA-NtD (Mortuza et al., 2004) it was apparent that although the side chains of both residues 10 and 116 were surface exposed on an assembled capsid structure, neither lay in the α4–α6 region, previously proposed as the Fv1 binding site (Mortuza et al., 2008). Residue 116 was located on the second turn of α6 on the face opposite the α4–α6 region while residue 10 was even more remote, located on the N-terminal β1–β2 hairpin. To further examine how the L10W mutation might effect rhTRIM5α restriction of MLV we determined the crystal structure of the CA-NtD of this variant at 2.0Å resolution. The structure has been refined to a final Rwork/Rfree of 17.4%/24.4% respectively and has excellent geometry with only 3 residues in the additionally allowed region of the Ramachandran plot (See Table 4 for details of crystal parameters, phasing and data refinement statistics).
Tab. 4. Statistics of data collection, phasing and refinement. 
The structure of L10W was typical of a retroviral CA-NtD consisting of an N-terminal β-hairpin and a core bundle of six α-helices. A comparison with the native MLV CA-NtD structure (Figure 6A) revealed an essentially identical backbone conformation. Pairwise comparisons of L10W with wt N-MLV CA-NtD gave an RMSD of 0.6Å across equivalent Cα atoms. However, minor deviations were seen at the top of helix-6 and in the loops and side chains on the surface of the molecule. In particular, the L10W mutation was clearly visible in the electron density (Supplementary Figure S6) and was located on the inward facing side of strand β2 adjacent to the α5-α6 loop and the top of helix α6.
Fig. 6. Structure of MLV CA-NtD (L10W). 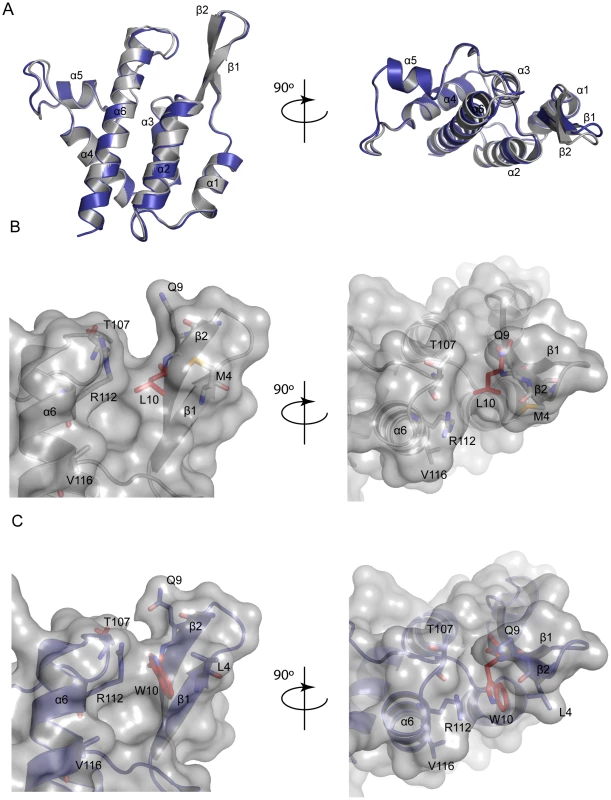
Examination of the wt N-MLV capsid surface in the proximity of L10 (Figure 6B) reveals the presence of a shallow channel between the β1–β2 hairpin and residues at the top of α6 and in the α5–α6 loop. The channel walls are formed by side-chains of M4 and Q9 from the β1–β2 hairpin together with R112 and T107 from the α5–6 loop. At the bottom of the channel the aliphatic L10 side-chain forms the base. However, in the L10W structure (Figure 6C) the much bulkier tryptophan side-chain protrudes into the channel along with R112 guanidinium moiety. Together they adopt a near planar arrangement reminiscent of a similar interaction seen in the structure of PSIV capsid [39]. Significantly, this side-chain configuration in L10W reduces the depth of the (β1–β2)-α6 channel by some 2.3Å, effectively closing the cavity completely. To test the importance of the W10 – R112 interaction for restriction, the R112 amino acid was mutated to an alanine in presence of both L10 and W10. Both viruses were susceptible to restriction by rhTRIM5α (Supplementary Figure S7). The observed structural change thus provided a simple potential explanation for the rhTRIM5α escape phenotype suggesting that in wt N-MLV the (β1–β2)-α6 channel constituted at least part of the rhTRIM5α binding site and that by blocking this channel in L10W the interaction with rhTRIM5α was abolished or sufficiently diminished to allow the virus to evade restriction.
Discussion
To better understand the interaction between TRIM5α and its viral target, we set out to isolate a panel of novel N-MLV escape mutants. Noting that direct inoculation of cells expressing a given restriction factor led, depending on the initial virus titre, either to rapid break-through of the block in replication or the absence of replication (Figure 1), we devised a procedure allowing replication against a background of restriction. We reasoned that the infection of a mixture of restricting and non-restricting cells would allow both multiple rounds of unrestricted replication as well as selection for viruses that were unrecognized by restriction factor. Experiments of the kind shown in Figure 2 illustrate the utility of such an approach and have resulted in the isolation of a number of MLV mutants that escape restriction by rhTRIM5α, apparently by escaping premature capsid disassembly (Figure 4). The lack of variation in mutants obtained with Stock A compared with that from Stocks B and C points to the possible accumulation of variant viruses in multi-passaged virus stocks even in the absence of overt selection or apparent sequence variation and emphasize the need for using freshly recovered virus in such studies.
β-hairpin mutants extend the MLV CA-restriction factor interface
The mutations identified by these passaging experiments together with in vitro mutagenesis studies all lie on the outer “top” surface of MLV CA (Figure 7) and can be divided into two groups. One group (Class-I) comprises N82D, E92K, A95D, and H114D/R/N, mutations that are within the previously defined Fv1 binding pocket in the loop region between helices 4 and 6 of CA [27], [30]. These mutations result in escape from rhesus TRIM5α mutations at these positions can affect susceptibility to Fv1. The other escape mutants, L4S, G8D, L10W, L10K, L10H and V116D are the founder members of a new group (Class-II) located on the opposing side of the central α6 helix and in the β1–β2 N-terminal β-hairpin. Residue 110 (R in N-MLV and E in B-MLV) is located on the top of α6 and is often referred to as the major specificity determinant of restriction as it was the first residue shown to determine susceptibility to Fv1 or TRIM5α, [8], [19], [31]. Our data now add further weight to the significance of residue 110 revealing that it is located at the centre of an extended restriction factor binding-site. This site encompasses the entire CA top surface from N82 across the region containing Class-I mutants to residues 8 and 10 in the β-hairpin. The G8D and mutations at L10 are of particular interest as they are located in a region of CA, the N-terminal β1–β2 hairpin, where mutations affecting the interaction of N-MLV with TRIM5α or Fv1 have never before been reported. The structure and properties of the L10W mutant (Figure 6) and the effect of the R112A change (Supplementary Figure S7) strongly suggest that the (β1–β2)-α6 channel present on CA forms part of the rhTRIM5α binding site. The observation that G8D and V116D cause susceptibility to Fv1n further suggests that the previous defined Fv1 binding pocket must be extended to include this region.
Fig. 7. Location of MLV restriction determinants. 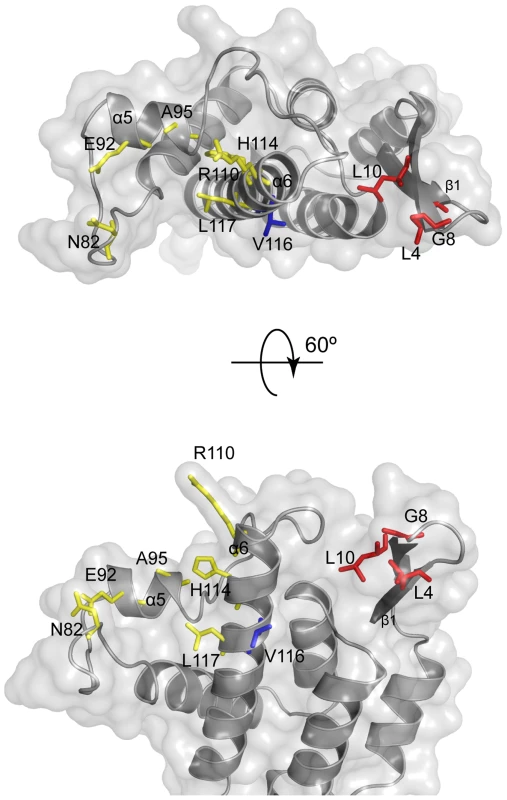
It is noteworthy that residues 10 and 82 are 29 Å (Cα-Cα) apart on either side of the exposed surface of CA monomers making up the core [32]. Moreover restriction sensitivity is affected by a number of amino acids (e.g. 92, 95, 110, 114, 116, 117) that are distributed between these two positions, covering much of the viral core that is exposed to cellular factors after entry into the cytoplasm of newly infected cells (Figure 7). Many of these residues posses poorly ordered side chains so their contribution to αrestriction factor binding seems most likely through side chain charge-charge or hydrophobic interactions at the molecular interface. Indeed many of the escape mutations we have isolated involve introduction or loss of charged amino-acids. Further, in cases of mutations affecting tropism for which we have detailed structural information, R110E [27] and L10W (this paper) there is no evidence for major change in the overall conformation of CA, indicating that mutations in the N terminal β-hairpin loop are not affecting the structure of other regions of CA for example the α4–α6 region. Taken together, these observations are consistent with the involvement of much, if not all, of the surface of N-MLV CA in recognition and binding by rhesus TRIM5α. Certainly the surfaces of the two proteins appear to lie in sufficiently close proximity that the introduction of charged residues in a variety of different positions prevents binding. An extensive recognition surface fits well with distance measurements of the B30.2 domain from TRIMs closely related to TRIM5 [40], [41] that reveal that the specificity determiningV1 and V3 regions are separated by about 30 Å, a number very similar to that between the two sides of CA residues (see above). However, attempts to identify regions of TRIM5α that bind specific CA residues using TRIM5α chimeras such as those illustrated in Figure 5 have not met with great success; rather it appears that well separated TRIM5α regions act in combinatorial fashion to determine restriction specificity.
Comparison of the restriction factor binding sites on different retroviruses
TRIM5α is capable of recognizing at least four genera of retroviruses despite extensive differences in primary sequences of CA [23], [24], [25]. However CA's show extensive structural conservation particularly in the core fold of helices 1–4 [42]. It thus appears likely that it is some shared feature of overall structure that represents the target for TRIM5α rather than direct sequence specific recognition alone. Loop exchange studies involving different lentiviruses suggest that the primary target for the restriction factor lies in the cyclophilin A binding loop, in a region analogous to the originally defined Fv1 binding site of MLV [33], [34]. The importance of this region is emphasized by the recent isolation of an HIV-1 escape mutant from rhTRIM5α, V86M, by methods similar to those used here [43] and the roles of HIV-2 P120 and G116 in resistance to cynomolgus macaque TRIM5α [35], [44]. HIV-1 V86 and MLV N82 both lie just C-terminal to helix 4 while HIV-1 G116 and HIV-2 P120 lie close to position 110 of MLV. Further, domain swap experiments also suggest a role for the β-hairpin region of SIV CA protein in the evasion from rhTRIM5α-mediated restriction [36].
Based on these results and our success of producing and mapping β-hairpin escape mutants of N-MLV that could evade restriction by rhTRIM5α, it seemed logical to extend these studies and test for commonality of binding by examining the restriction phenotypes of β-hairpin mutants in HIV-1. Moreover, a comparison of the CA-NtDs revealed that, although wider, the β1-hairpin-α6 cleft is also present in HIV-1 (Supplementary Figure S8). Therefore, it might be expected that the introduction of bulky or charged side chains into this channel would alter HIV susceptibility to TRIM5α in the same way as in N-MLV. To this end, the studies presented in supplementary Figure S5 were undertaken. However they showed that viable single amino acid mutations in the HIV-1 β-hairpin had no effect on restriction by rhTRIM5α. These data suggest that the β-hairpin-α6 cleft is not a shared target for all viruses, implying that an as yet unidentified binding region near the β-hairpin remains to be discovered. In any event, it seems most likely that lentiviruses have an extended TRIM5α binding site in analogous to that seen in MLV.
Human and rhesus TRIM5α restriction of MLV, the devil is in the detail
The results of the initial passaging studies (Figure 1) together with phenotypic analysis of our escape mutants (Figure 5) suggest that there are significant differences in the recognition of N-MLV by rhesus and human TRIM5α. From the outset, restriction of N-MLV by huTRIM5α was much more potent to the extent that we have been unable to overcome N-MLV restriction by the hu-TRIM5α. Moreover, it is also refractory to all but one of the changes isolated that allow escape from the rh-TRIM5α (Table 1). The one exception, E92K, was resistant to all restriction factors tested but showed a significant reduction in titre. These observations suggested that the interaction of huTRIM5α with N-MLV was either stronger and can elicit a more powerful host cell response or that the CA-TRIM5α interface is different and does not include the residues in the β-hairpin-α6 channel. These observations are somewhat perplexing given the degree of similarity of the TRIM5α B30.2 domains. However, it is notable that L10 and V116D mutations have differential effects on restriction by TRIM5αs derived from other species. For example V116D shows no restriction by orang-utan TRIM5α but is restricted by sooty mangeby whereas L10W shows the converse sensitivity (Table 1). Further, of the fourteen TRIM5αs we have previously examined only two, pigtailed macaque and sooty mangabey, show identical restriction profiles against a panel of ten different viruses [23], [24], [25].
Taken together, these variable determinants of restriction imply that TRIM5α recognizes its targets in a manner significantly different from many protein-protein interactions that involve a small number of highly conserved residues, exemplified by the interaction betweens HIV-1 CA and the cellular protein cyclophilin A [45]. Rather the interactions are predominately weak and spread over a large surface. Avidity could then be provided by multivalent binding to the regularly spaced CA molecules assembled on the viral core [26], [27], [28]. This notion is further supported by recent structural data suggesting that formation of a complementary TRIM5α lattice is actually a requirement for a productive interaction of the restriction factor with capsid αassemblies (Ganser-Pornillos et al, 2011). With the idea of lattice complementarity in mind it is also interesting to note that although differences in conformation around the top of Helix-6 between wt and L10W are only very small (∼1.2Å) this region is centrally located in the extended restriction factor binding-site. It is plausible that such small changes whilst not affecting hexamer or CA lattice formation when propagated throughout an entire capsid lattice could profoundly affect any lattice complementarity, altering restriction factor susceptibility and resulting in viral escape from some TRIM5αs. Whatever the case, retroviral pathogens comprise a wide range of sequence divergent but structurally related assembled repeated capsid moieties [42]. Perhaps weak interactions spread over a large binding surface are implicit in the design of a defence mechanism intended to recognize these diverse targets.
Materials and Methods
Cells
Tail fibroblast cells from Mus dunni (MDTF), MDTF cells that stably express restriction factors, and human 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum and antibiotics. MDTF cells expressing human and rhesus macaque TRIM5α (MDHu and MDRh) were established by an end-point dilution method. Stable, unselected expression of TRIM5α was observed in transduced cells for at least one month after cloning.
DNA
The cloning of human TRIM5α from TE671 and a chimeric human/rhesus macaque TRIM5α (human RBCC domain fused with rhesus macaque B30.2 domain) have been described previously [8], [24]. This chimeric human/rhesus macaque TRIM5α was used throughout this study to generate and select escape mutants of N-MLV, thereby allowing a direct comparison of interactions with the human and rhesus macaque B30.2 domains, avoiding confounding effects of the coiled coil region [16], [46], [47], and will be referred to as rhesus TRIM5α. Generation of transduction vectors expressing TRIM5α and Fv1 using the Gateway system was also described previously [24], [48]. Single nucleotide mutations were introduced into the capsid genes of Gag-Pol vector plasmids (pCIG3N and pCIG3B) and N-MLV provirus plasmid (pWN41 [17]) by the QuickChange mutagenesis PCR protocol. Random mutations were introduced at position 10 on MLV capsid by the QuickChange mutagenesis PCR using the following primer pair: forward 5′ - ggggggtaatggtcagNN(G/T)cagtactggccgttttc -3′ and reverse 5′ - gaaaacggccagtactg(C/A)NNctgaccattacccccc -3′, where N indicates any nucleotide and G/T and C/A indicate G or T and C or A, respectively. The sequences of the other primers used to prepare mutants are available upon request. All introduced mutations were verified by DNA sequencing.
Viruses
Single-cycle replication viruses were produced by transfection of VSV-G envelope (pcz VSV-G), retroviral gag-pol (pCIG3N and pCIG3B for N - and B-tropic viruses, respectively) and viral genomic plasmids (pczCFG2fEGFPf) into 293T cells by a conventional calcium phosphate method as described previously [48] or using Turbofect (Fermentas). Sixteen hours after transfection, cells were treated with 10 mM sodium butyrate for 6 hours, followed by replacement of the culture supernatant with fresh growth medium. Virus-containing supernatant was harvested 48 hours after transfection, filtered through a 0.45 µm pore filter disk and stored at −80°C until usage. Three stocks of replication competent N-MLV were used. One (Stock A) was prepared from the culture supernatant of MDTF cells transfected with N-MLV provirus (pWN41) followed by multiple (>20x) passage of the transfected cells. The second (Stock B) was generated by transfection of 293T cells with pWN41, transfer of the filtered supernatant to MDTF cells followed by three cell passages. The third (Stock C) was unpassaged supernatant of 293T cells transfected with pWN41. For transfection, pWN41 DNA was digested with Hind III to separate the permuted provirus from the vector, and purified viral DNA fragments were self-ligated with T4 DNA ligase at 16°C overnight. After purification, the ligated DNA was introduced into MDTF/293T cells using a commercial transfection reagent according to the manufacturer's instructions (TurboFect, Fermentas). Transfected cells were re-fed with fresh medium after 16 hours; culture supernatants were harvested after 48 hours.
Virus production in newly transfected/infected cells was monitored by protein blotting of pelleted culture supernatants with monoclonal antibodies specific to p12 and CA viral antigens (prepared from the culture supernatant of hybridomas 548 and R187 [ATCC]) or with a RT assay kit used according to the manufacturer's instructions (C-type RT assay kit, Cavidi Tech, Sweden).
Mutant virus selection
Target cells (MDTFs or rhTRIM5α expressing MDTFs or specified mixtures of the two cell types) were seeded on a 6-well plate at 2×105 cells per well one day before virus inoculation. Replication competent N-MLV was added to target cells at a titer equivalent to 514 mU of RT activity, followed by washing cells three times with the growth medium 16 hours after infection. The input virus was allowed to grow in the culture by passing infected cells and virus spread was assessed by fluorescent staining. Once the majority of restriction factor positive cells were infected with N-MLV, the culture supernatant was harvested, filtered, and then stored at −80°C until usage. The filtered supernatant was diluted 1∶10 with growth medium and inoculated onto fresh, restriction factor-expressing cells. This virus passage cycle was repeated several times until the virus spread rapidly in restriction factor positive cells.
FACS
Virus spread in infected cells was monitored by detection of an intracellular virus antigen, p12. Infected cells were harvested by trypsinization and washed with PBS, followed by fixation with 1.25% ice-cold paraformaldehyde in PBS. All of the following procedures were performed on ice. Fixed cells were washed once with permeabilization buffer (0.5 mg/ml digitonin and 1% bovine serum albumin [BSA] in PBS), followed by incubation with anti-p12 monoclonal antibody diluted in permeabilisation buffer. After washing three times with wash buffer (5% Tween-20 and 1% BSA in PBS), cells were then stained with anti-mouse IgG conjugated with AlexaFluor 594 (Invitrogen) diluted in permeabilisation buffer. After washing three times, cells were re-suspended in 1.25% paraformaldehyde in PBS and analysed by FACSCalibur within four hours.
Single-cycle replication experiments were performed as previously described [24], [48] using a delivery vector encoding restriction factor and eYFP and a tester virus encoding eGFP. Two colour FACS was carried out with a BD LSR II machine.
Sequencing of the capsid gene
Virus containing culture supernatant was harvested and filtered through a 0.45 µm-pore filter disk. The filtered supernatant was stored at −80°C until usage. The supernatant was treated with MgCl2 and RNase-free DNase I at 37°C for 2 hours to degrade contaminating DNA. DNase I-treated supernatant was subjected to viral RNA extraction using a commercial kit (Viral RNA mini kit, QIAGEN). A single strand cDNA spanning the capsid region was synthesized from viral RNA as a template with a primer designed in the protease gene (5′-gggttatcctgggttcaggggggggctcctgaccctg-3′) using a commercial kit (Transcriptor High Fidelity cDNA synthesis kit, Roche). The cDNA synthesized was used as a template to amplify viral capsid gene with a primer pair designed in viral matrix (5′-gtctcgcctgcggggcaaaagag-3′) and protease (the same as above) genes using PfuUltra high fidelity DNA polymerase (Stratagene). The PCR product was gel purified, and nucleotide sequence was determined by direct sequencing.
Fate-of-capsid assay
The fate-of-capsid assay was performed as previously described [38] with minor modifications. MDTF cells expressing TRIM5α (5×106 cells in a 80 cm2 tissue culture flask) were pre-plated one day before assay. Cells were inoculated with freshly prepared, chilled virus (14 ml per flask with 5 ug/ml polybrene) from the filtered culture supernatant of transiently transfected 293T cells. After incubation on ice for 30 minutes, cells were incubated at 37°C for 4 hours. Cells were then washed three times with ice-cold PBS and treated with 1 ml of 7 mg/ml pronase at room temperature for 5 minutes. Cells were re-suspended in ice-cold growth medium and washed three times with ice-cold PBS. Cells were re-suspended in 2.5 ml of hypotonic lysis buffer [10 mM Tris-HCl (pH 8.0), 10 mM KCl, 1 mM EDTA and protease inhibitor cocktail] and incubated on ice for 15 minutes. Swollen cells were lysed in a 7 ml-Dounce homogenizer with a ‘tight’ pestle (15 gentle strokes) and cell lysates cleared by centrifugation at 4°C and 1,850 xg for 3 minutes. Cleared cell extracts (2 ml) were layered over 50% sucrose cushions prepared in PBS and centrifuged at 4°C and 125,000 xg for 2 hours in a Beckman SW41 rotor and 100 µl of the cell extract was collected as a ‘input’ fraction. After the centrifugation, 100 µl of the topmost portion of the supernatant was collected as a ‘soluble’ fraction. The pellet was re-suspended in 100 µl 1×SDS sample loading buffer (‘pellet’ fraction). Samples were separated on acrylamide gels and blotted for CA as described above.
Protein crystallisation and structure determination
The CA N-terminal domain of N-MLV(L10W) was crystallised by sitting-drop vapour diffusion using a reservoir solution containing 24% PEG-8000, 0.1M phosphate citrate, pH 5.0 mixed with an equal volume of protein solution (14 mg/ml). Prior to data collection, crystals were transferred into a cryoprotectant of reservoir solution containing 20% glycerol and flash frozen in liquid nitrogen. X-ray diffraction data were collected at 100° K on a Rigaku Micromax-007HF with a Raxis-IV detector to a resolution of 2.0Å and processed using the HKL program package [49]. Crystals belonged to the space group C2 with the cell dimensions a = 71.9Å, b = 33.6Å, c = 59.3Å, β = 111.9°.
The structure of N-MLV(L10W) was determined by molecular replacement using a monomer of N-MLV CA-NtD (1U7K) as a search model in PHASER [50]. A single molecule was present in the asymmetric unit (Z-score 12.3, LLG 590). The model was rebuilt using Arp/Warp (Morris et al., 2003) followed by iterative rounds of refinement and model building in PHENIX [51] and COOT [52]. Nine TLS groups were included in final round of refinement as determined by TLSMD [53]. The structure was refined to a final Rwork/Rfree of 17.4%/24.4% respectively.
Coordinates
The coordinates and structure factors of N-MLV(L10W) have been deposited in the Protein Data Bank under accession number 2y4z.
Supporting Information
Zdroje
1. LillyF
1970
Fv-2: Identification and location of a second gene governing the
spleen focus response to Friend leukemia virus in mice.
J Natl Cancer Inst
45
163
169
2. PincusTHartleyJWRoweWP
1971
A major genetic locus affecting resistance to infection with
murine leukemia viruses. I. Tissue culture studies of naturally occurring
viruses.
J Exp Med
133
1219
1233
3. PincusTRoweWPLillyF
1971
A major genetic locus affecting resistance to infection with
murine leukemia viruses. II. Apparent identity to a major locus described
for resistance to Friend murine leukemia virus.
J Exp Med
133
1234
1241
4. HofmannWSchubertDLaBonteJMunsonLGibsonS
1999
Species-specific, postentry barriers to primate immunodeficiency
virus infection.
J Virol
73
10020
10028
5. StremlauMOwensCMPerronMJKiesslingMAutisslerP
2004
The cytoplasmic body component TRIM5α restricts HIV-1
infection in Old World monkeys.
Nature
427
848
853
6. HatziioannouTPerez-CaballeroDYangACowanSBieniaszPD
2004
Retrovirus resistance factors Ref1 and Lv1 are species-specific
variants of TRIM5α.
Proc Natl Acad Sci U S A
101
10774
10779
7. KeckesovaZYlinenLMJTowersGJ
2004
The human and African green monkey TRIM5α genes encode Ref1
and Lv1 retroviral restriction factor activities.
Proc Natl Acad Sci U S A
101
10780
10785
8. YapMWNisoleSLynchCStoyeJP
2004
Trim5α protein restricts both HIV-1 and murine leukemia
virus.
Proc Natl Acad Sci U S A
101
10786
10791
9. ReymondAMeroniGFantozziAMerlaGCairoS
2001
The tripartite motif family identifies cell
compartments.
EMBO J
20
2140
2151
10. NisoleSStoyeJPSaïbA
2005
Trim family proteins: retroviral restriction and antiviral
defence.
Nat Rev Microbiol
3
799
808
11. SardielloMCairoSFontanellaBBallabioAMeroniG
2008
Genomic analysis of the TRIM family reveals two groups of genes
with distinct evolutionary properties.
BMC Evol Biol
8
225
12. ShortKMCoxTC
2006
Sub-classification of the RBCC/TRIM superfamily reveals a novel
motif necessary for microtubule binding.
J Biol Chem
281
8970
8980
13. Perez-CaballeroDHatziioannouTYangACowanSBieniaszPD
2005
Human tripartite motif 5α domains responsible for retrovirus
restriction activity and specificity.
J Virol
79
8969
8978
14. SawyerSLWuLIEmermanMMalikHS
2005
Positive selection of primate TRIM5α identifies a critical
species-specific retroviral restriction domain.
Proc Natl Acad Sci U S A
102
In Press
15. SongBGoldBO'hUiginCJavanbakhtMLiX
2005
The B30.2(SPRY) domain of retroviral restriction factor
TRIM5α exhibits lineage-specific length and sequence variation in
primates.
J Virol
79
6111
6121
16. YapMWNisoleSStoyeJP
2005
A single amino acid change in the SPRY domain of human Trim5α
leads to HIV-1 restriction.
Curr Biol
15
73
78
17. BooneLRMyerFEYangDMOuC-YKohCK
1983
Reversal of Fv-1 host range by in vitro
restriction endonuclease fragment exchange between molecular clones of
N-tropic and B-tropic murine leukemia virus genomes.
J Virol
48
110
119
18. DesGroseillersLJolicoeurP
1983
Physical mapping of the Fv-1 tropism host range determinant of
BALB/c murine leukemia viruses.
J Virol
48
685
696
19. TowersGBockMMartinSTakeuchiYStoyeJP
2000
A conserved mechanism of retrovirus restriction in
mammals.
Proc Natl Acad Sci, USA
97
12295
12299
20. WuXAndersonJLCampbellEMJosephAMHopeTJ
2006
Proteasome inhibitors uncouple rhesus TRIM5α restriction of
HIV-1 reverse transcription and infection.
Proc Natl Acad Sci U S A
103
21. JolicoeurPBaltimoreD
1976
Effect of Fv-1 gene product on proviral DNA formation and
integration in cells infected with murine leukemia viruses.
Proc Natl Acad Sci, USA
73
2236
2240
22. PryciakPMVarmusHE
1992
Fv-1 restriction and its effects on murine
leukemia virus integration in vivo and in vitro.
J Virol
66
5959
5966
23. DiehlWEStansellEKaiserSMEmermanMHunterE
2008
Identification of Post-entry Restrictions to Mason-Pfizer Monkey
Virus Infection in New World Monkey Cells.
J Virol
82
11140
11151
24. OhkuraSYapMWSheldonTStoyeJP
2006
All three variable regions of the TRIM5α B30.2 domain can
contribute to the specificity of retrovirus restriction.
J Virol
80
8554
8565
25. YapMWLindemannDStankeNRehJWestphalD
2008
Restriction of foamy viruses by primate
Trim5alpha.
J Virol
82
5429
5439
26. Diaz-GrifferoFQinX-RHayashiFKigawaTFinziA
2009
A B-box 2 surface patch important for TRIM5α
self-association, capsid binding avidity and retrovirus
restriction.
J Virol
83
10737
10751
27. MortuzaGBDoddingMPGoldstoneDCHaireLFStoyeJP
2008
Structure of B-MLV capsid amino-terminal domain reveals key
features of viral tropism, gag assembly and core formation.
J Mol Biol
376
1493
1508
28. YapMWMortuzaGBTaylorIAStoyeJP
2007
The design of artificial retroviral restriction
factors.
Virology
365
302
314
29. FallerDVHopkinsN
1978
T1 oligonucleotide maps of N-, B-, and B->NB-tropic
murine leukemia viruses derived from BALB/c.
J Virol
26
143
152
30. StevensABockMEllisSLeTissierPBishopKN
2004
Retroviral capsid determinants of Fv1 NB - and
NR-tropism.
J Virol In Press
31. KozakCAChakrabortiA
1996
Single amino acid changes in the murine leukemia virus capsid
protein gene define the target for Fv1
resistance.
Virology
225
300
306
32. MortuzaGBHaireLFStevensASmerdonSJStoyeJP
2004
High-resolution structure of a retroviral capsid hexameric
amino-terminal domain.
Nature
431
481
485
33. KootstraNAMünkCTonnuNLandauNRVermaIM
2003
Abrogation of postentry restriction of HIV-1-based lentiviral
vector transduction in simian cells.
Proc Natl Acad Sci, USA
100
1298
1303
34. YlinenLMJKeckesovaZWilsonSJRanasingheSTowersGJ
2005
Differential restriction of human immunodeficiency virus type 2
and simian immunodeficiency virus SIVmac by TRIM5α
alleles.
J Virol
79
11580
11587
35. KuroishiABozekKShiodaTNakayamaEE
2010
A single amino acid substitution of the human immunodeficiency
virus type 1 capsid protein affects viral sensitivity to TRIM5
alpha.
Retrovirology
7
58
36. KonoKSongHYokoyamaMSatoHShiodaT
2010
Multiple sites in the N-terminal half of simian immunodeficiency
virus capsid protein contribute to evasion from rhesus monkey
TRIM5alpha-mediated restriction.
Retrovirology
7
72
37. HerrW
1984
Nucleotide sequence of AKV murine leukemia virus.
J Virol
49
471
478
38. StremlauMPerronMLeeMLiYSongB
2006
Specific recognition and accelerated uncoating of retroviral
capsids by the TRIM5α restriction factor.
Proc Natl Acad Sci U S A
103
5514
5519
39. GoldstoneDCYapMWRobertsonLEHaireLFTaylorWR
2010
Structural and functional analysis of prehistoric lentiviruses
uncovers an ancient molecular interface.
Cell Host Microbe
8
248
259
40. JamesLCKeebleAHKhanZRhodesDATrowsdaleJ
2007
Structural basis for PRYSPRY-mediated tripartite motif (TRIM)
protein function.
Proc Natl Acad Sci U S A
104
6200
6205
41. ParkEYKwonOBJeongBCYiJSLeeCS
2010
Crystal structure of PRY-SPRY domain of human
TRIM72.
Proteins
78
790
795
42. MortuzaGBGoldstoneDCPashleyCHaireLFPalmariniM
2009
Structure of the capsid amino-terminal domain from the
betaretrovirus, Jaagsiekte sheep retrovirus.
J Mol Biol
386
1179
1192
43. PachecoBFinziAStremlauMSodroskiJ
2010
Adaptation of HIV-1 to cells expressing rhesus monkey
TRIM5α.
Virology
408
204
212
44. SongHNakayamaEEYokoyamaMSatoHLevyJA
2007
A single amino acid of the human immunodeficiency virus Type 2
Capsid affects its replication in the presence of cynomolgus monkey and
human TRIM5αs.
J Virology
81
7280
7285
45. GambleTRVajdosFFYooSWorthylakeDKHouseweartM
1996
Crystal structure of human cyclophilin A bound to the
amino-terminal domain of HIV-1 capsid.
Cell
87
1285
1294
46. MaillardPVEccoGOrtizMTronoD
2010
The specificity of TRIM5α-mediated restriction is influenced
by its coiled-coil domain.
J Virol
84
5790
5801
47. NewmanRMHallLConnoleMChenGLSatoS
2006
Balancing selection and the evolution of functional polymorphism
in Old World monkey TRIM5alpha.
Proc Natl Acad Sci U S A
103
19134
19139
48. BockMBishopKNTowersGStoyeJP
2000
Use of a transient assay for studying the genetic determinants of
Fv1 restriction.
J Virol
74
7422
7430
49. OtwinowskiZMinorW
1997
Processing of X-ray diffraction data collected in oscillation
mode.
Meth Enzymol
276
307
326
50. McCoyAJGrosse-KunstleveRWAdamsPDWinnMDStoroniLC
2007
Phaser crystallographic software.
J Appl Crystallogr
40
658
674
51. ZwartPHAfoninePVGrosse-KunstleveRWHungLWIoergerTR
2008
Automated structure solution with the PHENIX
suite.
Methods Mol Biol
426
419
435
52. EmsleyPCowtanK
2004
Coot: model-building tools for molecular
graphics.
Acta Crystallogr D Biol Crystallogr
60
2126
2132
53. PainterJMerrittEA
2006
Optimal description of a protein structure in terms of multiple
groups undergoing TLS motion.
Acta Crystallogr D Biol Crystallogr
62
439
450
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in ChinaČlánek HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2Článek The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.Článek Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria ParasitesČlánek KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 MotifČlánek Viral EncephalomyelitisČlánek Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The Strain-Encoded Relationship between PrP Replication, Stability and Processing in Neurons is Predictive of the Incubation Period of Disease
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Human Macrophage Responses to Clinical Isolates from the Complex Discriminate between Ancient and Modern Lineages
- Dendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from
- Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China
- Rhesus TRIM5α Disrupts the HIV-1 Capsid at the InterHexamer Interfaces
- HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2
- Antigenic Variation in Malaria Involves a Highly Structured Switching Pattern
- The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Novel Escape Mutants Suggest an Extensive TRIM5α Binding Site Spanning the Entire Outer Surface of the Murine Leukemia Virus Capsid Protein
- Global Functional Analyses of Cellular Responses to Pore-Forming Toxins
- Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria Parasites
- Lung Adenocarcinoma Originates from Retrovirus Infection of Proliferating Type 2 Pneumocytes during Pulmonary Post-Natal Development or Tissue Repair
- Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors
- The Moving Junction Protein RON8 Facilitates Firm Attachment and Host Cell Invasion in
- KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 Motif
- The Coxsackievirus B 3C Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
- Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway
- Viral Encephalomyelitis
- Sheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice
- Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Dynamic Imaging of the Effector Immune Response to Infection
- The Lectin Receptor Kinase LecRK-I.9 Is a Novel Resistance Component and a Potential Host Target for a RXLR Effector
- Host Iron Withholding Demands Siderophore Utilization for to Survive Macrophage Killing
- The Danger Signal S100B Integrates Pathogen– and Danger–Sensing Pathways to Restrain Inflammation
- The RNome and Its Commitment to Virulence
- A Novel Nuclear Factor TgNF3 Is a Dynamic Chromatin-Associated Component, Modulator of Nucleolar Architecture and Parasite Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání