-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Lectin Receptor Kinase LecRK-I.9 Is a Novel Resistance Component and a Potential Host Target for a RXLR Effector
In plants, an active defense against biotrophic pathogens is dependent on a functional continuum between the cell wall (CW) and the plasma membrane (PM). It is thus anticipated that proteins maintaining this continuum also function in defense. The legume-like lectin receptor kinase LecRK-I.9 is a putative mediator of CW-PM adhesions in Arabidopsis and is known to bind in vitro to the Phytophthora infestans RXLR-dEER effector IPI-O via a RGD cell attachment motif present in IPI-O. Here we show that LecRK-I.9 is associated with the plasma membrane, and that two T-DNA insertions lines deficient in LecRK-I.9 (lecrk-I.9) have a ‘gain-of-susceptibility’ phenotype specifically towards the oomycete Phytophthora brassicae. Accordingly, overexpression of LecRK-I.9 leads to enhanced resistance to P. brassicae. A similar ‘gain-of-susceptibility’ phenotype was observed in transgenic Arabidopsis lines expressing ipiO (35S-ipiO1). This phenocopy behavior was also observed with respect to other defense-related functions; lecrk-I.9 and 35S-ipiO1 were both disturbed in pathogen - and MAMP-triggered callose deposition. By site-directed mutagenesis, we demonstrated that the RGD cell attachment motif in IPI-O is not only essential for disrupting the CW-PM adhesions, but also for disease suppression. These results suggest that destabilizing the CW-PM continuum is one of the tactics used by Phytophthora to promote infection. As countermeasure the host may want to strengthen CW-PM adhesions and the novel Phytophthora resistance component LecRK-I.9 seems to function in this process.
Published in the journal: . PLoS Pathog 7(3): e32767. doi:10.1371/journal.ppat.1001327
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001327Summary
In plants, an active defense against biotrophic pathogens is dependent on a functional continuum between the cell wall (CW) and the plasma membrane (PM). It is thus anticipated that proteins maintaining this continuum also function in defense. The legume-like lectin receptor kinase LecRK-I.9 is a putative mediator of CW-PM adhesions in Arabidopsis and is known to bind in vitro to the Phytophthora infestans RXLR-dEER effector IPI-O via a RGD cell attachment motif present in IPI-O. Here we show that LecRK-I.9 is associated with the plasma membrane, and that two T-DNA insertions lines deficient in LecRK-I.9 (lecrk-I.9) have a ‘gain-of-susceptibility’ phenotype specifically towards the oomycete Phytophthora brassicae. Accordingly, overexpression of LecRK-I.9 leads to enhanced resistance to P. brassicae. A similar ‘gain-of-susceptibility’ phenotype was observed in transgenic Arabidopsis lines expressing ipiO (35S-ipiO1). This phenocopy behavior was also observed with respect to other defense-related functions; lecrk-I.9 and 35S-ipiO1 were both disturbed in pathogen - and MAMP-triggered callose deposition. By site-directed mutagenesis, we demonstrated that the RGD cell attachment motif in IPI-O is not only essential for disrupting the CW-PM adhesions, but also for disease suppression. These results suggest that destabilizing the CW-PM continuum is one of the tactics used by Phytophthora to promote infection. As countermeasure the host may want to strengthen CW-PM adhesions and the novel Phytophthora resistance component LecRK-I.9 seems to function in this process.
Introduction
Plants deploy multiple strategies to defend themselves against pathogen attack. A key step is the perception of pathogen molecules in order to activate various defense responses. During infection pathogens produce microbe-associated molecular patterns (MAMPs) or elicit the production of host-derived damage-associated molecular patterns (DAMPs). These signals are recognized by the plant via so-called pattern recognition receptors (PRRs) and trigger cell wall-associated defenses, such as the production of antimicrobial compounds and cell wall strengthening [1], [2]. An active defense response is, however, dependent on a functional continuum between the cell wall (CW) and the plasma membrane (PM) [3]. When this continuum is disturbed the effectiveness of defense is lost. Destabilizing the CW-PM continuum to circumvent recognition could therefore be a strategy for a pathogen to promote infection.
In animals, cell adhesion is mediated by integrins, membrane-spanning receptors whose ligands are extracellular matrix (ECM) proteins carrying the cell-attachment motif Arg-Gly-Asp (RGD) [4]. Other proteins or peptides comprising the motif RGD can act as integrin antagonists and as such interfere with integrin-related functions, including cell adhesion and proliferation [5], [6]. Examples are certain viral, bacterial and fungal proteins [7]–[9], and various snake venom disintegrins [10].
Also plant pathogens produce proteins carrying a RGD motif. Well-studied is PtrToxA, a secreted proteinaceous toxin of the foliar wheat pathogen Pyrenophora trititci-repentis, which induces cell death in toxin-sensitive wheat mesophyll cells [11]. Internalization of PtrToxA is dependent on its RGD-containing solvent loop, which is largely identical to the integrin-binding RGD loop of the mammalian ECM glycoprotein vitronectin [12], [13]. PtrToxA proteins with mutations in the RGD-loop were found to be less toxic due to impaired internalization. Toxicity of PtrToxA was also reduced when RGD peptides were added as competitor and this supports the hypothesis of a RGD-dependent receptor-mediated endocytosis [13].
Several studies have shown that exogenously applied RGD peptides can have a strong disrupting effect on plant cells. For example, in cell suspensions of soybean and Arabidopsis, and in onion epidermal cells RGD peptides caused loss of CW-PM adhesions in a concentration dependent manner, whereas RGE or DGR peptides did not show this effect [14], [15]. Moreover, RGD peptides added to pea epicotyls reduced the production of the phytoalexin pisatin [16], and in shear-stressed Taxus cells these peptides negatively affected the alkalization response, as well as the accumulation of both, phenolics and reactive oxygen species (ROS) [17]. Accordingly, cowpea and pea cells treated with RGD peptides display a disturbed CW-PM continuum and decreased expression of cell wall-associated defense responses upon fungal penetration, whereas these effects were not seen when RGE peptides were added. Hence, the RGD-mediated reduction of defense response stimulated fungal penetration and intracellular hyphal growth [3].
IPI-O, an RXLR-dEER effector of the oomycete pathogen Phytophthora infestans, disrupts CW-PM adhesions in Arabidopsis through its RGD motif [18]. When the RGD motif in IPI-O is mutated to RGE or RGA the adhesions remain intact [18]. Plant plasma membranes possess high affinity RGD-binding sites, and binding was shown to be saturable, reversible and RGD-specific [15]. IPI-O interacts with these sites in a similar manner, and hence competes with other proteins possessing the RGD motif. A phage display, set up to find proteins interacting with the RGD motif of IPI-O, resulted in two peptides that act as RGD-binding antagonists and lead to the identification of an Arabidopsis legume-like lectin receptor kinase named LecRK-I.9 that binds IPI-O via its RGD motif [19]. Since LecRK-I.9 functions in CW-PM adhesions it is conceivable that LecRK-I.9 is involved in protein-protein interactions with RGD-containing proteins as potential ligands and plays a structural and signaling role at the plant cell surface. As yet, little is known about the function of LecRK-I.9 and other legume-like lectin receptor kinases [20] nor about their natural ligands.
Besides the RGD motif, IPI-O contains another characteristic motif called RXLR, that partially overlaps with RGD (i.e., RSLRGD). This motif is shared by numerous secreted oomycete effector proteins several of which are known to function as race-specific avirulence factor [21]. For two other RXLR effectors – i.e., P. infestans Avr3a and P. sojae Avr1b – it has demonstrated that the RXLR motif is crucial for transfer of the effector to the host cell [22]–[24]. The effector function of IPI-O is further supported by expression data; ipiO expression is not detectable in in vitro grown mycelium but induced in germ tubes invading host tissue. During infection of susceptible potato lines expression is highest in the periphery of the lesion where the hyphae ramify in the apoplast and in the sub-peripheral zone where plant cells are being invaded by haustoria, but is lacking in the necrotic center of the lesion and in the sporulating zone [25]. Recently we have shown that ipiO is the avirulence (Avr) gene Avr-blb1 that acts in a gene-for-gene manner with Rpi-blb1, a late blight resistance (R) gene from Solanum bulbocastanum which encodes a cytoplasmic NBS-LRR protein [26]. In potato plants carrying Rpi-blb1, colonization by P. infestans isolates that contain class I variants of IPI-O is blocked [27].
The observation of binding between a Phytophthora effector and an Arabidopsis legume-like lectin receptor kinase via the RGD cell attachment motif [19] raised questions about the role of LecRK-I.9 in the interaction with Phytophthora. Since, Arabidopsis is a non-host plant for P. infestans [28], we made use of another Phytophthora species, P. brassicae. For the interaction between P. brassicae and Arabidopsis various distinct incompatible and compatible isolate-accession combinations have been described, and this pathosystem can be regarded as an attractive model for the concurrent analysis of both host and pathogen [29]–[31]. In this study, we first analyzed expression of LecRK-I.9 under biotic and abiotic stress and determined the sub-cellular localization of LecRK-I.9. Subsequently, we analyzed the response of Arabidopsis LecRK-I.9 T-DNA insertion mutants (lecrk-I.9) to infection with P. brassicae and investigated the role of IPI-O in the infection process by making use of transgenic Arabidopsis lines expressing ipiO1. Interestingly, the lecrk-I.9 mutants and the ipiO1-expressing lines showed strikingly similar phenotypes. In both cases, we not only observed gain of susceptibility but also comparable patterns of pathogen - and MAMP-triggered callose deposition. Overall, our observations strongly suggest that LecRK-I.9 plays a role in disease resistance and point toward involvement of the RXLR effector IPI-O in infection processes.
Results
LecRK-I.9 expression is induced in incompatible interactions
Analysis of endogenous LecRK-I.9 expression in Arabidopsis Col-0 by RT-PCR revealed LecRK-I.9 transcripts in all tested tissues, i.e., in flowers, siliques, stems, rosette leaves and roots (data not shown). To analyze LecRK-I.9 expression in more detail we used a transgenic Arabidopsis Col-0 line carrying the reporter construct PLecRK-I.9-GUS consisting of the promoter region (−1486 to +9 bp) of LecRK-I.9 fused to the coding sequence of the β-glucuronidase (GUS) gene. Analysis of this line, selected because of a single copy insertion and a representative GUS staining pattern [32], revealed that LecRK-I.9 is differentially expressed during organ differentiation with very low or no expression in fully differentiated tissues.
We inoculated the PLecRK-I.9-GUS reporter line with two P. brassicae isolates; one that is compatible with Col-0 (isolate CBS686.95) and another one (isolate HH) that is incompatible with Col-0 but capable to infect other Arabidopsis accessions [29]. GUS staining upon inoculation with isolate CBS686.95 was comparable to the staining observed in un-inoculated or mock-treated leaves (Fig. 1A and 1B). In both cases we observed a blue color in the petiole, and in the primary veins of rosette leaves but not in leaf epidermal and mesophyll cells. In contrast, inoculation with HH resulted in a significant increase in GUS activity as early as 1 day post-inoculation (dpi). Fig. 1C shows the GUS activity at 6 dpi. A similar increase, also appearing within 1 dpi, was observed in the non-host interaction of Col-0 with P. infestans and upon inoculation with isolate IMI169558 of the necrotrophic grey mold fungus Botrytis cinerea, which can infect and colonize Col-0 (shown in Fig. 1D and 1E, respectively, and at 6 dpi). The prominent difference between an incompatible and compatible interaction was also observed by others (C. Balagué and D. Roby, personal communication). They found increased GUS activity within 6 hours after inoculation with the avirulent Pseudomonas syringae strain DC3000(avrRpm1), while in a compatible interaction with P. syringae DC3000 there was only a slight increase in GUS activity. The expression patterns that we observed in the PLecRK-I.9-GUS reporter line are consistent with expression data retrieved from publicly available repositories [20]. Taken together, these results reveal induction of expression of LecRK-I.9 upon infection with B. cinerea and in incompatible interactions with biotrophic Phytophthora and Pseudomonas pathogens.
Fig. 1. LecRK-I.9 expression is induced in an incompatible but not in a compatible interaction with P. brassicae. 
GUS activity in leaves of a transgenic Arabidopsis line carrying promoter-GUS construct PLecRK-I.9-GUS. (A) In mature non-inoculated leaves GUS activity is localized in the petiole (white arrows) and in veins (black arrows). GUS activity in leaves, 6 days post-inoculation (dpi) with the virulent P. brassicae isolate CBS686.95 (B), the avirulent P. brassicae isolate HH (C), P. infestans IPO-0 (D), 4 dpi with B. cinerea IMI169558 (E), and 2 days after wounding (F). LecRK-I.9 is localized at the plasma membrane
LecRK-I.9 contains a putative transmembrane domain [20], and hence hypothesized to be associated with a membrane. To determine the subcellular localization of LecRK-I.9 we constructed the binary vector pS-LecRK-I.9-GFP that is suitable for transient in planta expression of C-terminal GFP-tagged LecRK-I.9. Leaves of Nicotiana benthamiana were co-infiltrated with Agrobacterium strains carrying pS-LecRK-I.9-GFP and pBIN61-mCherry (free mCherry; [33]) and after 2 to 3 days examined by confocal laser scanning microscopy. GFP fluorescence was detected at the cell surface, whereas the red fluorescent signal of free mCherry was found to be distributed in the nucleus and the surrounding cytoplasm, and throughout the cytoplasm lining the cell surface (Fig. 2). Merging the GFP and mCherry images revealed distinct fluorescence of both GFP and mCherry at the cell surface with GFP more peripheral than mCherry (Fig. 2). Co-localization of GFP and mCherry was never observed within the nucleus or in the endoplasmic reticulum network that surrounds the nucleus, nor in cytoplasmic strands (data not shown). GFP fluorescence at the plasma membrane and distinct from free mCherry was reproducible in several independent experiments. This demonstrates that in these transient expression assays LecRK-I.9-GFP is targeted to the plasma membrane and points to association of LecRK-I.9 with the plasma membrane.
Fig. 2. LecRK-I.9 is localized at the plasma membrane. 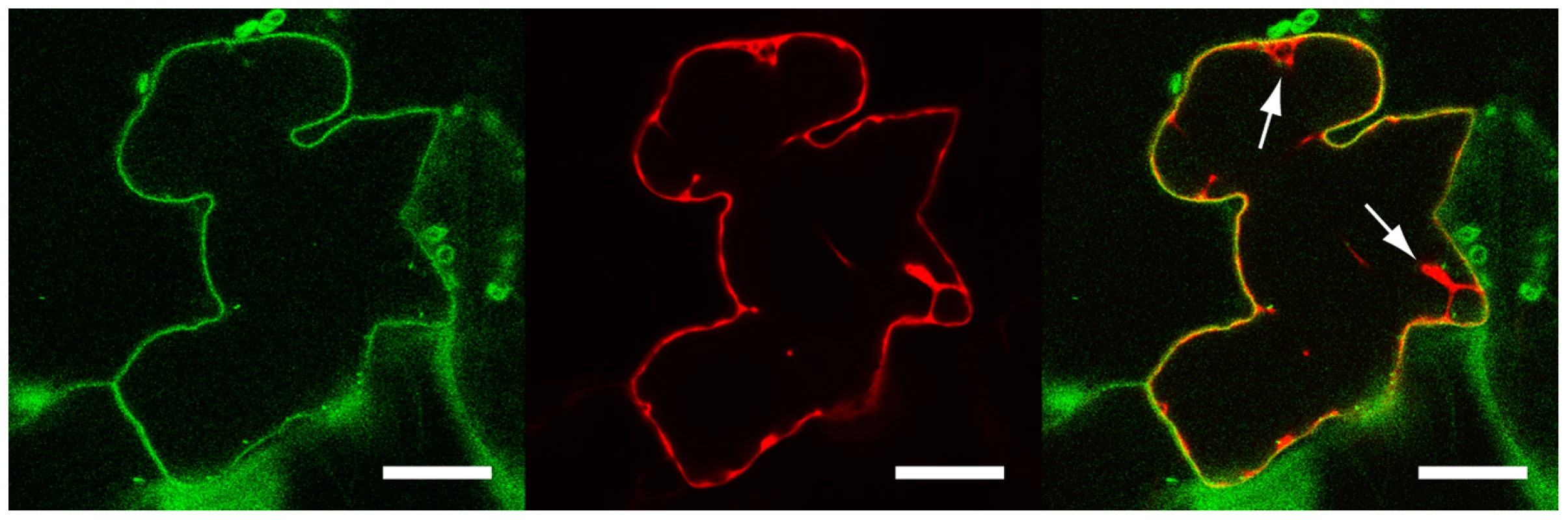
Confocal images of a N. benthamiana epidermal cell transiently expressing LecRK-I.9-GFP and mCherry. Single-channel (i.e., GFP left, mCherry middle) and merged fluorescence images (right). Arrows point towards cytoplasmic strands. Scale bars represent 20 µm. Arabidopsis mutants disrupted in LecRK-I.9 show gain of susceptibility towards P. brassicae
To be able to study the potential function of LecRK-I.9, we obtained two mutant lines with T-DNA insertions just downstream of the translation start site, i.e., lecrk-I.9-1 and lecrk-I.9-2 (Fig. 3A). RT-PCR using gene-specific primers confirmed that both lecrk-I.9 mutant lines lack LecRK-I.9 expression [32]. Growth behavior and morphology of the two homozygous lecrk-I.9 mutants and the recipient line Col-0 were comparable (data not shown). Also generation time, seed setting and seed germination were not affected in LecRK-I.9 deficient plants. We then compared the response of the lecrk-I.9 mutants and Col-0 to inoculation with P. brassicae. Isolate HH is incompatible with Col-0 and unable to colonize Col-0 plants. In contrast, plug-inoculation of lecrk-I.9 plants with isolate HH resulted in lesions that first appeared 2 days after inoculation and expanded further until complete leaf collapse after 7 days (Fig. 3B). Similar results were obtained when Col-0 and lecrk-I.9 plants were inoculated with zoospores of isolate HH (data not shown). This gain of disease susceptibility was observed in several assays on both lecrk-I.9-1 and lecrk-I.9-2. It is a stable phenotype and the infection efficiency (IE) of isolate HH on the two lecrk-I.9 lines is on average over 80 percent. Trypan blue staining of lecrk-I.9 leaves infected with HH revealed heavy tissue colonization and sporulation (Fig. 3C). This was further confirmed by UV fluorescence microscopy in lecrk-I.9 leaves infected with a GFP-tagged transformant of isolate HH (Fig. 3D) [34]. Comparable disease symptoms and IE were found when lecrk-I.9 plants were inoculated with P. brassicae isolate II, which is – like isolate HH – incompatible with Col-0 (Fig. S1).
Fig. 3. Arabidopsis mutants disrupted in LecRK-I.9 are impaired in resistance to P. brassicae. 
(A) Schematic representation of LecRK-I.9 and its gene product. The coding sequence is 2154 nt in length and has one continuous open reading frame. The two mutant lines lecrk-I.9-1 and lecrk-I.9-2 have a T-DNA insertion (black arrowheads) at position 176 and 566, respectively, relative to the translational start. The LecRK-I.9 protein contains an extracellular legume-like lectin domain (L-lectin), a transmembrane motif (TM), and an intracellular serine/threonine protein kinase domain (STK). (B) Col-0 and lecrk-I.9-1 plants 4 days post-inoculation with P. brassicae isolate HH. Arrows point at lesions. Colonization of lecrk-I.9-1 leaves by P. brassicae isolate HH visualized by trypan blue staining (C) and P. brassicae GFP transformant 155 m revealed by UV epifluorescence (D). Arrows in (C) point at sporangia. Scale bar in (C) represents 100 µm and in (D) 200 µm. To determine the specificity of this gain of disease susceptibility, we performed infection assays with other pathogens. Despite the fact that expression of LecRK-I.9 is induced in response to infection with B. cinerea (Fig. 1E), lecrk-I.9 did not show stronger disease symptoms compared to Col-0. Moreover, inoculation of lecrk-I.9 with the non-host pathogens P. infestans and Alternaria brassicicola, a necrotrophic fungus, did not result in lesion formation and no changes in disease susceptibility were found with respect to the hemibiotrophic fungus Colletotrichum destructivum, which forms a compatible interaction with Col-0.
Overexpression of LecRK-I.9 leads to developmental effects and enhanced resistance to P. brassicae
To further investigate the role of LecRK-I.9 in the defense response, transgenic Arabidopsis plants were generated that constitutively express LecRK-I.9. A construct containing the full-length coding sequence of LecRK-I.9 under the control of the constitutive cauliflower mosaic virus 35S (CaMV 35S) promoter was transferred to Arabidopsis accession Col-0 via flower-dip transformation. Multiple independent lines were obtained, two of which were selected for further analysis (i.e., C-0123 and C-0126). In comparison to the recipient line Col-0, both 35S-LecRK-I.9 lines had more compact rosettes with smaller and slightly wrinkled leaves (Fig. 4A), and were shorter in height (data not shown). Moreover, the transgenic lines displayed a substantially higher accumulation of anthocyanin and lignin, a phenomenon not observed in Col-0 or the lecrk-I.9 lines (Fig. 4B, and C). Anthocyanin pigmentation and lignin deposition was most intense in mature cotyledons, and along the petioles and midribs of young rosette leaves (Fig. 4B, and C).
Fig. 4. LecRK-I.9 overexpression in Arabidopsis leads to changes in morphology and accumulation of anthocyanin and lignin. 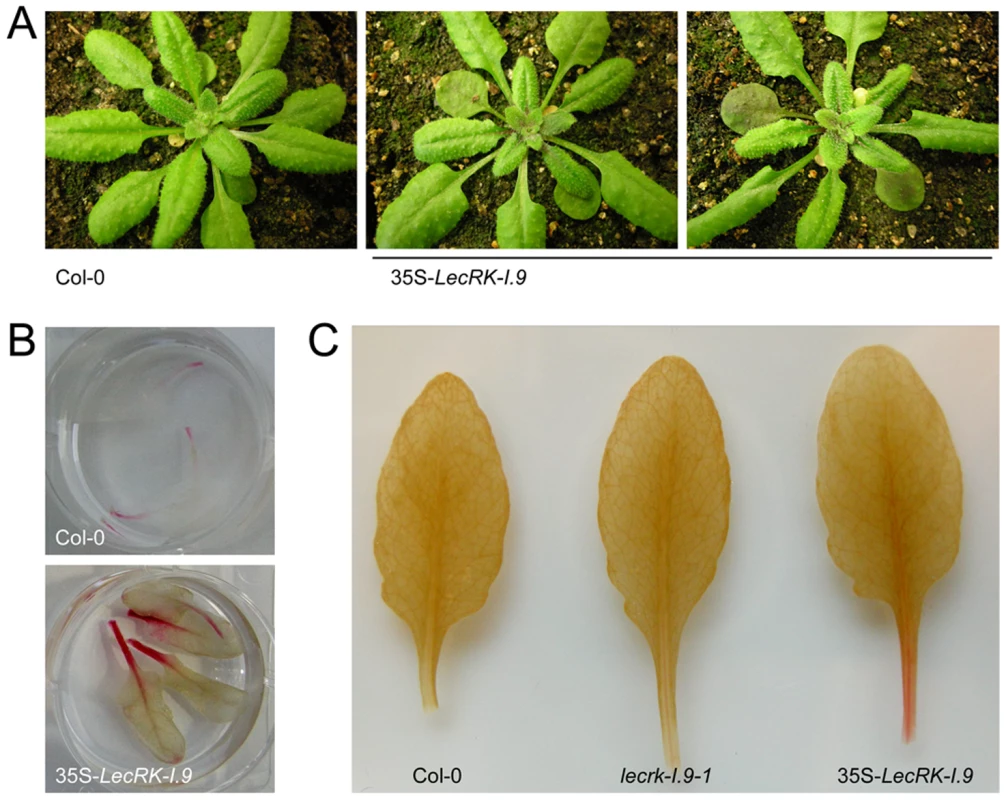
In comparison to the recipient line Col-0, 35S-LecRK-I.9 lines have more compact rosettes with smaller and slightly wrinkled leaves (A). Anthocyanin (B) and lignin (C) staining in rosette leaves of Col-0 and 35S-LecRK-I.9 lines. When we challenged the two 35S-LecRK-I.9 lines and the recipient line Col-0 with P. brassicae isolate HH we observed no differences. Col-0 is incompatible with HH and this incompatibility with HH is maintained in the 35S-LecRK-I.9 lines (data not shown). Col-0 is compatible though with P. brassicae isolate CBS686.95 and upon inoculation with this isolate, both with mycelium plugs and by drop inoculation with a zoospore suspension, Col-0 leaves became completely colonized. In contrast, such colonization was not observed in the transgenic 35S-LecRK-I.9 lines. These plants showed an elevated level of resistance towards this P. brassicae isolate (Fig. 5A, and B); there were no or only minor disease symptoms but instead there was a hypersensitive response (HR), including an increase in callose deposition, which was not found in Col-0 (Fig. 5C). The experiment was repeated several times with comparable results. No differences in disease progression were observed between Col-0 and the 35S-LecRK-I.9 lines upon inoculation with Botrytis cinerea (data not shown).
Fig. 5. LecRK-I.9 overexpression in Arabidopsis results in enhanced resistance to P. brassicae. 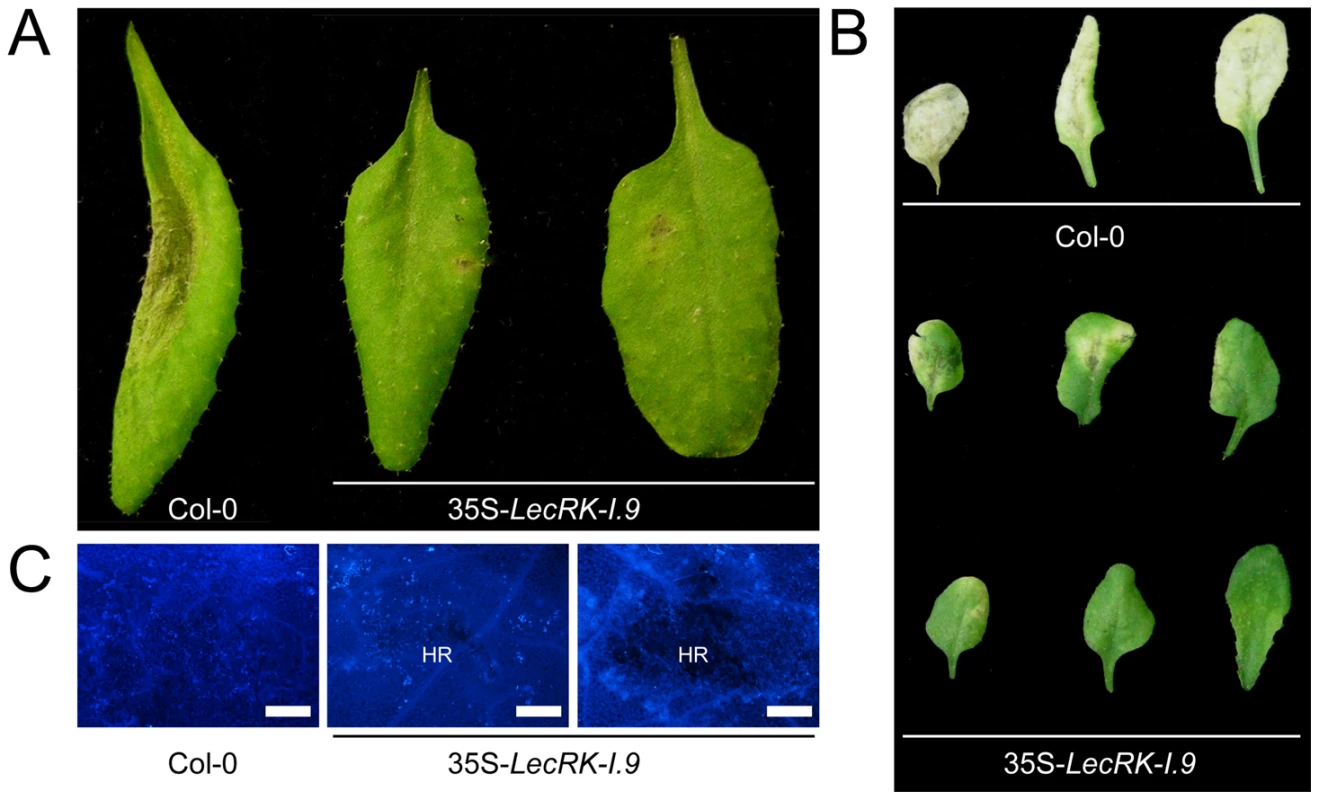
Arabidopsis leaves inoculated with plugs (A) or zoospores (B and C) of P. brassicae isolate CBS686.95. Pictures were taken 4 days post-inoculation (dpi) (A), 8 dpi (B), and 5 dpi (C). Lesion expansion was observed on Col-0 leaves, but not on leaves from two independent 35S-LecRK-I.9 lines. In (C) leaves were stained for callose (light blue fluorescence). HR; hypersensitive response. Scale bars in (C) represent 50 µm. lecrk-I.9 and 35S-ipiO1 lines are gain of susceptibility phenocopies
To examine whether IPI-O functions as an effector that manipulates host defense responses, we generated Arabidopsis transformants carrying a transgene composed of the coding sequence of the P. infestans ipiO1 gene fused to the constitutive CaMV 35S promoter in a Col-0 background. Transgene expression was confirmed by RT-PCR (data not shown). The transformants developed normal and, with respect to morphology and size, they could not be distinguished from Col-0 plants. Multiple independent transformants (35S-ipiO1) were tested for their response to P. brassicae. In contrast to Col-0, which, as expected, exhibited full resistance to P. brassicae isolate HH, the 35S-ipiO1 plants displayed clear disease symptoms 3 days after inoculation with HH (Fig. 6A). The foliar lesions developed gradually over time, and leaves were completely wilted after 7 days. This phenotype was observed in several independent experiments, and the IE on the 35S-ipiO1 plants was comparable (75%) to the IE on lecrk-I.9 lines. Trypan blue staining of infected leaf material revealed massively intercellular hyphal growth and sporulation similar to what was observed in the infected lecrk-I.9 lines (data not shown). Comparable disease symptoms and IE were found when the 35S-ipiO1 plants were inoculated with P. brassicae isolate II (Fig. S1). Also with respect to infection with the fungi A. brassicicola, C. destructivum and B. cinerea, and the bacterium P. syringae the 35S-ipiO1 plants responded similar as the two lecrk-I.9 lines (Table 1). These results show that in response to pathogens the lecrk-I.9 and 35S-ipiO1 lines behave as phenocopies.
Fig. 6. Arabidopsis lecrk-I.9 and 35S-ipiO1 lines are phenocopies. 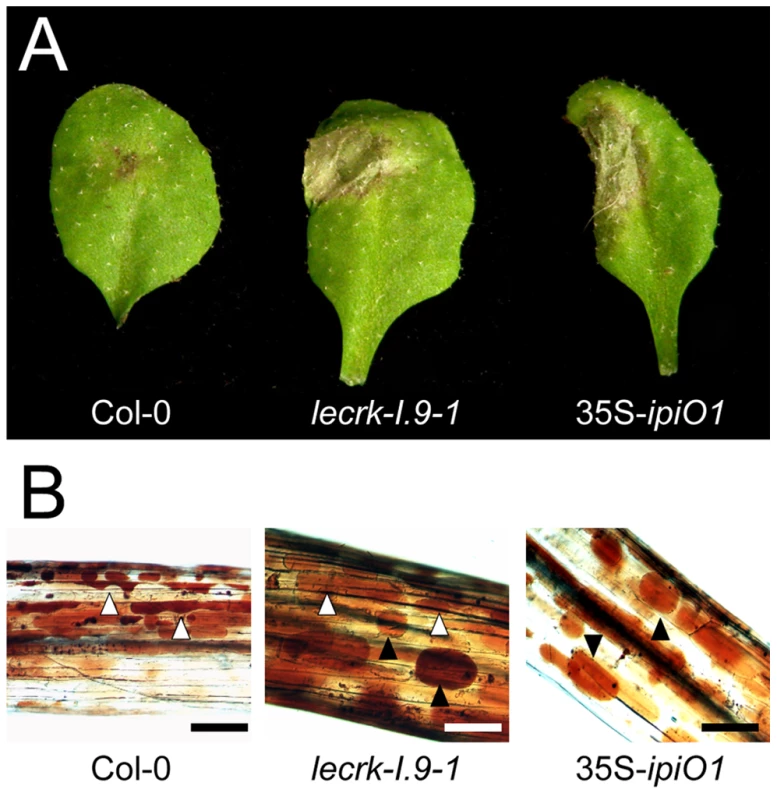
Both lines show (A) gain of susceptibility to P. brassicae, and (B) defects in cell wall integrity. (A) Leaves inoculated with P. brassicae isolate HH 3 days post-inoculation (dpi). (B) Elicitation of plasmolysis in etiolated hypocotyls by treatment with 0.4 M CaCl2. Shown are concave (white arrowheads) and convex (black arrowheads) forms of plasmolysis. In Col-0 only a few cells show convex plasmolysis, in lecrk-I.9 convex shapes occur more frequently whereas in 35S-ipiO1 the majority of cells is convex. Etiolated hypocotyls were stained with neutral red. Scale bars represent 100 µm. Tab. 1. Gain of susceptibility to Phytophthora brassicae in Arabidopsis Col-0, lecrk-I.9 and 35S-ipiO1 lines. 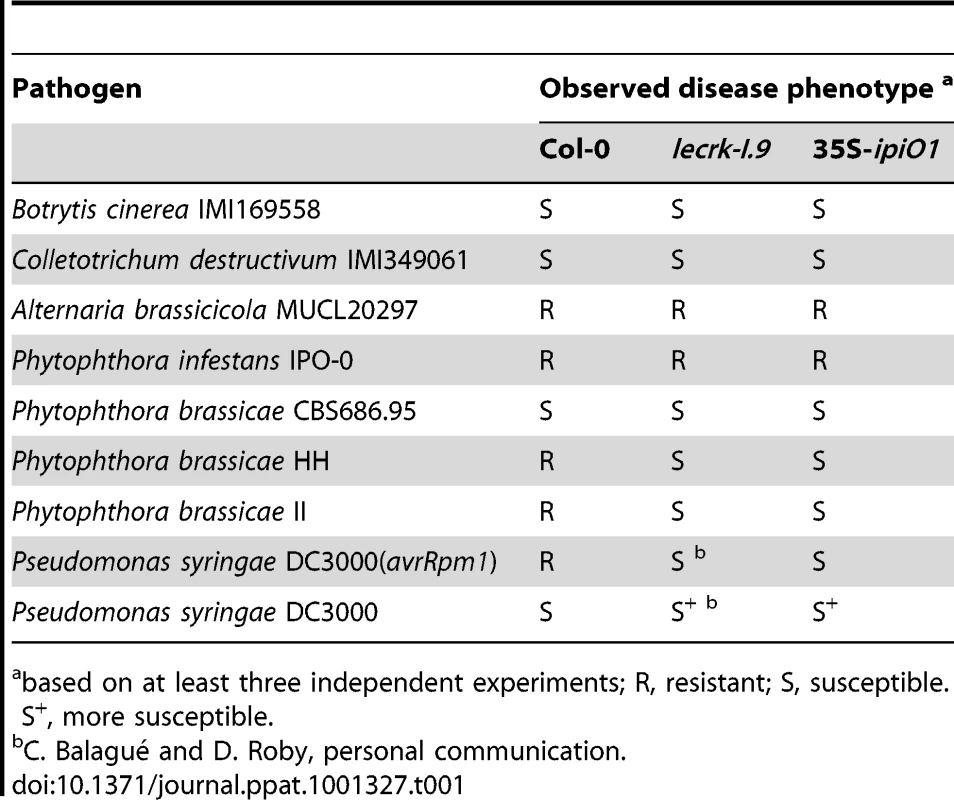
based on at least three independent experiments; R, resistant; S, susceptible. S+, more susceptible. lecrk-I.9 and 35S-ipiO1 lines show changes in the CW-PM continuum
Previously, it was shown that exposing Arabidopsis cells to IPI-O protein, obtained by heterologous ipiO expression in E. coli, results in disruption of the CW-PM continuum [18]. To investigate if ectopic expression of ipiO in Arabidopsis causes the same effect we examined the CW-PM continuum in 35S-ipiO1 plants. Plasmolysis was induced by soaking the hypocotyls in 0.4 M CaCl2. Upon staining with neutral red we observed that in Col-0 the PM readily separated from the CW, but at several points the adhesions between CW and PM were maintained, resulting in pockets that are concave with respect to cells. In contrast, convex forms of plasmolysis were observed in 35S-ipiO1 plants; the PM quickly separated from the CW reaching a near-spherical shape (Fig. 6C), similar to what was previously observed upon adding IPI-O1 protein to Arabidopsis cell suspension cultures [18]. To exclude that constitutive expression of ipiO leads to secondary effects, indirectly causing disruption of CW-PM continuum, we generated stable transformants in which ipiO1 expression is under control of the alcohol-inducible alcA promoter. Similar to the 35S-ipiO1 transformants, the alcA-ipiO1 transformants were morphological comparable to the parental line Col-0. After exposure of the alcA-ipiO1 plants to 0.01% (v/v) ethanol for 30 min, plasmolyzed hypocotyls displayed a phenotype identical that of 35S-ipiO1 plants: i.e., convex forms of plasmolysis (Table 2; Fig. S2). Hence, we can conclude that IPI-O causes the loss of CW-PM adhesions.
Tab. 2. The RGD motif in IPI-O is required to disrupt CW-PM adhesions in planta. 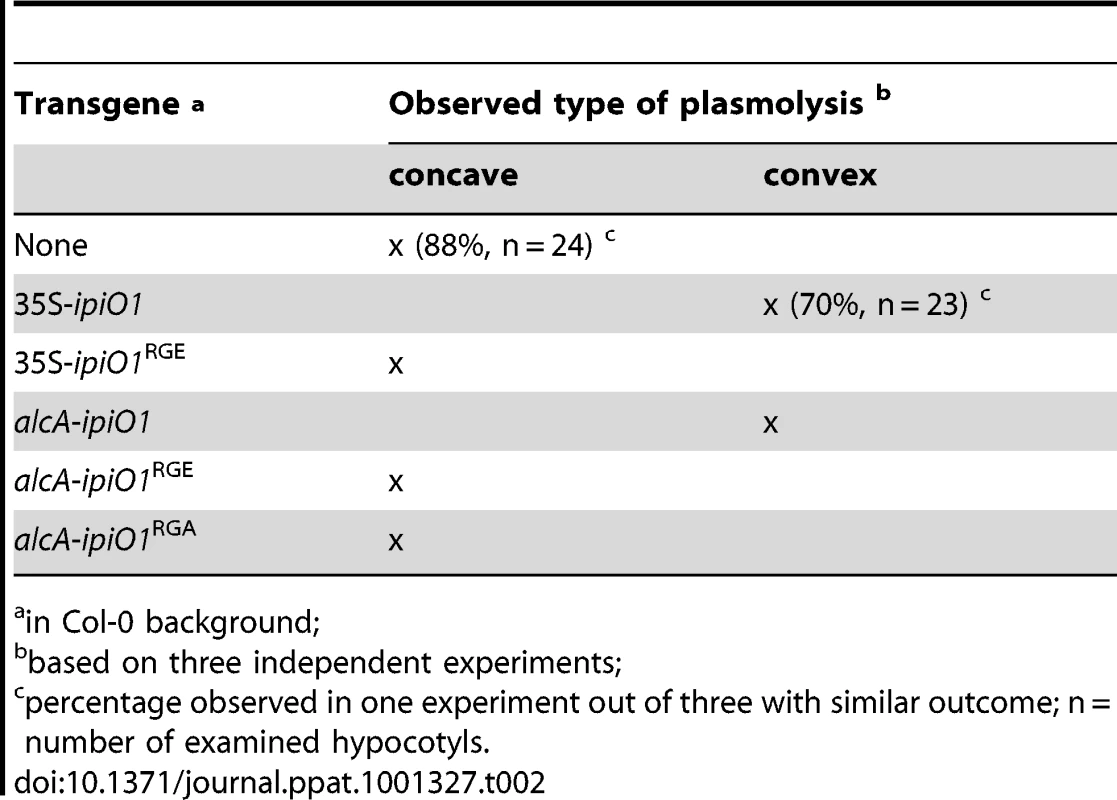
in Col-0 background; A logic next step was to examine the CW-PM adhesions in the phenocopy lecrk-I.9 lines. As shown in Fig. 6C, the convex forms of plasmolysis that were observed in 35S-ipiO1 and alcA-ipiO1 plants were also found in both knock-out lines, lecrk-I.9-1 and lecrk-I.9-2. It should be noted, however, that the relative number of hypocotyls with cells that showed a convex shape of plasmolysis was much less than in the 35S-ipiO1 lines. These observations imply that overexpression of LecRK-I.9 would lead to an increase in the strength of the CW-PM adhesions. Unfortunately, it was not possible to measure this. When we compared plasmolysed cells in hypocotyls from wild-type Col-0 and 35S-LecRK-I.9 lines no differences were observed and even at higher CaCl2 concentration (0.8 M) the shape in the plasmolysed Col-0 cells remained concave.
To determine whether CW-PM interactions are disturbed plant cells challenged with Phytophthora, we examined the CW-PM integrity in plasmolysed epidermal cells of N. benthamiana – which can be considered as a host plant [35] – shortly after infection with P. infestans (i.e., 5-7 hpi). To better visualize penetration, the leaves were inoculated with zoospores of a GFP-labeled P. infestans isolate (88069-GFP). Upon infection, we observed a strong detachment between CW and PM in epidermal cells penetrated by P. infestans. In contrast, this detachment was not observed in uninfected neighboring cells (Fig. S3).
lecrk-I.9 and 35S-ipiO1 lines are impaired in callose deposition
The gain of susceptibility and impairment of cell wall integrity suggests that cell wall associated defense response could be affected in the lecrk-I.9 and 35S-ipiO1 lines. To investigate this, we analyzed the level of callose deposition in the phenocopy lines after infiltration with P. syringae. Strain DC3000 produces effectors that suppress defense in Col-0 and, as a result, callose deposition is decreased [36]. The P. syringae hrcC mutant can no longer suppress defense resulting in callose accumulation. Microscopic analysis revealed that DC3000-infiltrated leaves did not (or hardly) display callose deposition in Col-0, and neither in lecrk-I.9 nor in 35S-ipiO1 lines (Fig. 7A, and B). As expected, Col-0 leaves infiltrated with the P. syringae hrcC mutant displayed a significant increase of callose deposition but in contrast, this increase was not observed in hrcC infiltrated leaves of lecrk-I.9 and 35S-ipiO1 plants (Fig. 7A, and B). Similarly, infiltration with the oligopeptide flg22 resulted in extensive callose deposition in Col-0, but hardly any callose deposition was found in lecrk-I.9 and 35S-ipiO1 lines (Fig. 7C, and D). This points at a defect in MAMP-triggered callose deposition in both, the lecrk-I.9 and 35S-ipiO1 lines.
Fig. 7. Pathogen- and MAMP-triggered callose deposition is reduced in lecrk-I.9 and 35S-ipiO1 lines. 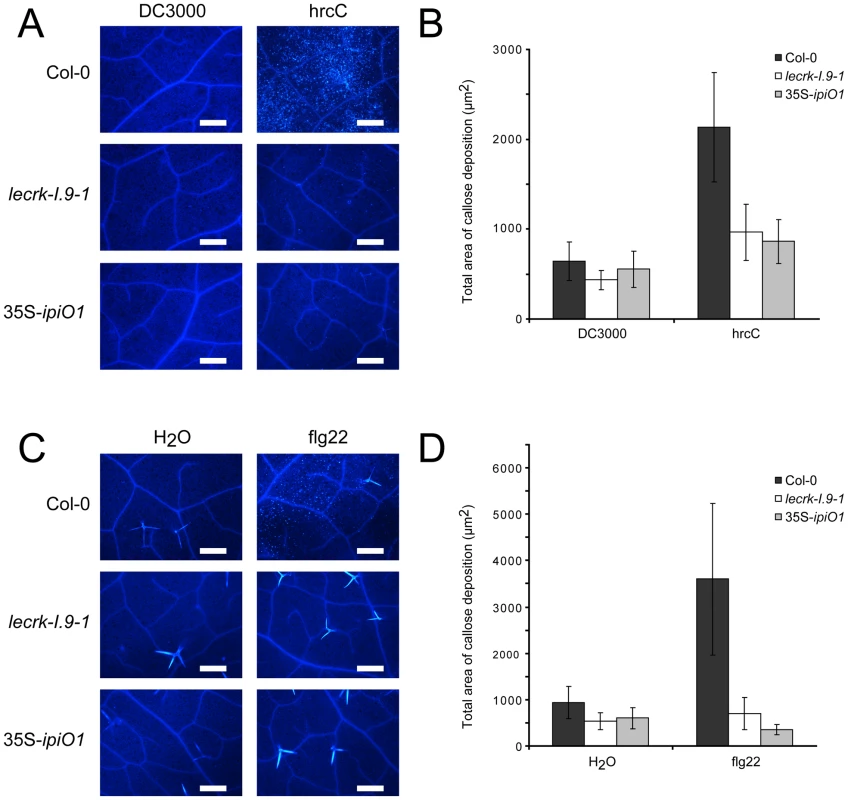
(A) Arabidopsis leaves stained with aniline blue after infiltration with Pseudomonas syringae DC3000 and hrcC. (B) Average totals of callose deposition (µm2) and associated 95% confidence intervals (CIs) after infiltration with DC3000 and hrcC (n = >16) (C). Callose deposition after infiltration with flg22. (D) Average totals of callose deposition after treatment with flg22 (µm2) +95% CIs (n = >10). Scale bars in (A) and (C) represent 50 µm. The RGD motif in IPI-O is a determinant of the phenotypic changes
We then addressed the role of the RGD motif in IPI-O and questioned if RGD is a determinant of the phenotypic changes observed in Arabidopsis upon ectopic ipiO1 expression. We therefore generated transgenic lines carrying ipiO1 constructs with site-directed mutations changing RGD to RGE or RGA. Two independent 35S-ipiO1RGE lines, one alcA-ipiO1RGE line and two alcA-ipiO1RGA lines were tested in a plasmolysis assay. In none of these lines a convex type of plasmolysis was observed, and as in Col-0 the CW-PM adhesions remained intact (Table 2). This is in agreement with the previous results that showed that in cell suspension cultures exogenously added mutant forms of IPI-O1 (IPI-O1RGE and IPI-O1RGA) had no disrupting effect [18]. Subsequently, we examined how a RGD-to-RGE mutation in IPI-O1 affects susceptibility to P. brassicae. As described above Col-0 exhibited full resistance to P. brassicae isolate HH whereas the leaves of 35S-ipiO1 plants were completely wilted leaves 5 days post-inoculation. On the two independent 35S-ipiO1RGE lines no lesions appeared and the phenotype was comparable to the incompatible interaction between Col-0 and HH (Fig. 8). Taken together, these results show that the RGD cell adhesion motif in IPI-O is crucial for disruption of the CW-PM continuum, as well as the gain of susceptibility to P. brassicae.
Fig. 8. The RGD motif in IPI-O is a determinant of the phenotypic changes. 
Arabidopsis transgenic lines expressing ipiO1 with a RGD-to-RGE targeted mutation show no gain of susceptibility to P. brassicae HH. Arabidopsis leaves inoculated with P. brassicae strain HH 5 days post-inoculation. Discussion
Membrane-spanning receptor proteins are supposed to play important roles in sensing alterations at the plant cell wall and, subsequently, to mediate response reactions [37]. Legume-like lectin receptor kinases (LecRKs) are regarded as candidates for monitoring cell wall integrity, and are likely functional in responses to various stresses. Up-till now only few reports have addressed the role of LecRKs in plant-pathogen interactions. Arabidopsis has a family of 45 LecRKs divided over nine clades and a few singletons [20]. This study revealed that one of these LecRKs, i.e., LecRK-I.9, is a novel membrane-associated Phytophthora resistance component. In incompatible interactions LecRK-I.9 plays a crucial role in arresting growth of the pathogen. In compatible interactions the pathogen exploits an effector carrying a RGD cell-attachment motif to disturb the CW-PM continuum, possibly by targeting the anchor protein LecRK-I.9.
LecRK-I.9 was initially identified in a phage display as a protein interacting with the P. infestans RXLR-dEER effector IPI-O via the RGD motif present in IPI-O [19]. In this study we first analyzed LecRK-I.9 gene expression and observed that under normal growth conditions LecRK-I.9 is expressed throughout the plant at a low level with higher levels of expression during organ differentiation. In compatible interactions with biotrophic pathogens LecRK-I.9 expression did not change. Infected and mock-treated leaves showed the same low basal level of LecRK-I.9 expression. In contrast, LecRK-I.9 expression was much higher when biotrophic pathogens encountered a strong hypersensitive response (HR) and were thus unable to colonize the leaves. LecRK-I.9 expression was also strongly increased in a non-host interaction with P. infestans, which shows an HR reminiscent of an incompatible interaction, and in lesions resulting from infection with the necrotrophic pathogen B. cinerea, in which cells are committed to programmed cell death [38]. Taken together, these expression patterns suggest that LecRK-I.9 functions in plant defense.
Strong evidence for a role for LecRK-I.9 in defense was obtained by making use of LecRK-I.9 T-DNA insertion mutants (lecrk-I.9) and LecRK-I.9 overexpressing lines (35S-LecRK-I.9). Their response to infection with various pathogens demonstrated that LecRK-I.9 is crucial for resistance of Arabidopsis to P. brassicae. Previously, it was reported that resistance in Arabidopsis to P. brassicae is not dependent on salicylic acid (SA), jasmonic acid (JA) and ethylene (ET) defense signaling pathways; i.e., disease resistance was maintained in mutants deficient in these pathways [29]. In contrast, mutants deficient in PAD2 lost resistance and the PAD2 gene is thus crucial for full-resistance to P. brassicae [29]. PAD2 encodes a γ-glutamylcysteine synthetase (GSH1), which is the first enzyme in the glutathione biosynthesis pathway [39]. Lower glutathione (GSH) levels in the pad2-1 mutant were found to be correlated with reduced accumulation of glucosinolates; toxic plant compounds which could have an negative effect on P. brassicae [40], [41]. So far, a link between GSH and lectin receptor kinases has not been reported. Intriguingly, in human cells a glutathione redox potential appears to regulate integrin-mediated cell adhesion [42] and hence, it is worth to investigate whether such a glutathione redox potential also influences cell adhesions and CW-PM adhesions in plants.
Apart from the change in disease phenotype both the insertion mutants and LecRK-I.9 overexpressing lines, showed certain developmental effects. In the 35S-LecRK-I.9 lines this was visible with the naked eye. In comparison to the parental Col-0 line, 35S-LecRK-I.9 plants were smaller in size, had wrinkled leaves and displayed accumulation of anthocyanins and lignin suggesting that these lines are some-how stressed. As yet, we have no clue if there is a correlation between the increase in anthocyanin and lignin and the enhanced resistance towards P. brassicae. Both anthocyanin and lignin are known to be induced after elicitation of basal defense. An increase in anthocyanin was shown to inhibit susceptibility of potato tubers to Pectobacterium carotovora ssp. carotovora [43], whereas Arabidopsis plants with an impaired monolignon synthesis exhibit enhanced susceptibility towards various bacterial and fungal pathogens [44]. The 35S-LecRK-I.9 plants also showed an increase in callose deposition upon inoculation with P. brassicae, whereas this increase was not found in Col-0. This suggests that LecRK-I.9 directly or indirectly triggers the enhancement of callose deposition, which is regarded as an early cellular marker of response upon cell wall damage, and recognition of MAMPs and pathogen elicitors.
The developmental effect that we observed in the lecrk-I.9 plants was more subtle. The morphology of the plants was not affected but at the cellular level we did find a phenotype that is in line with what we expected based on previous studies. Gouget et al. (2006) [19] who selected LecRK-I.9 in a phage display aimed at identifying proteins interacting with the RGD cell attachment motif in the P. infestans effector IPI-O, showed that the seven amino acid peptides resulting from the phage display disrupted the CW-PM adhesions in Arabidopsis hypocotyls as visualized by convex forms of plasmolysis. Since these peptides correspond to sequences in the extracellular domain of LecRK-I.9 we assumed that this disruption was due to competition with the natural ligands of LecRK-I.9 thereby disabling the function of endogenous LecRK-I.9. Also in lecrk-I.9 lines the function of LecRK-I.9 is disabled and indeed, the CW-PM adhesions seem to be slightly reduced. These observations support the hypothesis of Gouget et al. (2006) [19] that LecRK-I.9 functions in protein-protein interactions to mediate adhesions between the CW and PM.
LecRK-I.9 was identified as a protein potentially interacting with IPI-O [19]. The fact that, similar to LecRK-I.9 peptides, endogenously added IPI-O has the capacity to disrupt CW-PM adhesions [18] urged us to investigate the effect of ectopic expression of ipiO1 on CW-PM adhesions. As shown in Fig. 5C in planta expression of ipiO results in disturbance of the CW-PM continuum in an RGD dependent manner thereby demonstrating that the RGD cell attachment motif has to be intact to cause the disturbance. In the 35S-ipiO1 lines the convex form of plasmolysis was much stronger and more frequently observed than in the lecrk-I.9 lines. Expression analysis in lecrk-I.9 showed that in some tissues other clade I LecRK genes have increased expression levels compared to Col-0 suggesting that the lack of LecRK-I.9 is compensated by other LecRKs. This redundancy likely explains the difference between 35S-ipiO1 lines and lecrk-I.9 in the plasmolysis assays. Most interestingly, the 35S-ipiO1 lines were also found to display enhanced disease susceptibility to P. brassicae and thus behaved as phenocopies of lecrk-I.9. This phenocopy behaviour was also observed with respect to callose deposition: neither lecrk-I.9 nor 35S-ipiO1 lines accumulate callose upon pathogen - and MAMP treatment. Similarly, infiltration with P. syringae hrcC, resulted in callose deposition in Col-0 but not in the lecrk-I.9 and 35S-ipiO1 lines, and this is in agreement with the increase in severeness of disease symptoms caused by P. syringae in the phenocopy lines.
The effector gene ipiO1 was isolated from P. infestans, a pathogen that infects potato and tomato, but can not infect Arabidopsis. IPI-O belongs to the enormous reservoir of RXLR-dEER effectors present in all Phytophthora species analyzed so far [45]. The diversity among RXLR-dEER effectors is extremely high; not only between species but also within a species. For P. infestans and P. sojae many races are described that show differential interactions with cultivars of potato and soybean, respectively. We now know that the various resistance proteins present in those cultivars recognize different RXLR-dEER effectors from the pathogen [21]. Based on our current knowledge on the diversity in RXLR-dEER effectors it seems unlikely that P. brassicae, the species that infects Arabidopsis, contains an ipiO homologue. Nevertheless, P. brassicae may have a RGD-containing effector that targets LecRK-I.9 in Arabidopsis, but this should be revealed by genome sequencing of P. brassicae.
Evidence that pathogen effectors function by manipulating the host is accumulating. Suppression of defense is an effective mechanism that often requires entry of effectors into host cells. The RXLR-dEER motif that is shared by numerous oomycete effectors, including IPI-O, functions as a host cell targeting motif [22], [23], [24]. Indeed, for a few RXLR effectors it has been shown that the RXLR motif mediates transport into the plant cell and that they function inside the cell as suppressors of defense reactions. So far there is no experimental proof that IPI-O suppresses defense in its natural occurrence, i.e. the P. infestans-potato interaction. However, based on its activity as AVR factor [27] we have strong indirect evidence that, similar to other RXLR effectors, IPI-O ends up inside the host cell. The matching R protein is Rpi-blb1, an intracellular NBS-LRR protein that upon recognition of most IPI-O variants confers resistance to late blight in potato. This AVR activity of IPI-O, together with its activity unraveled in the current study, points to, at least, a dual function of IPI-O in the host-pathogen interaction. One function is executed when IPI-O resides extracellular and requires the RGD motif, whereas the other function operates intracellularly and is confined to the C-terminal domain of the effector protein. Host cell targeting, most likely mediated by the RXLR motif, can be considered as a third function.
In our model shown in Fig. 9A, we hypothesize that LecRK-I.9 is an RGD-binding protein that interacts with extracellular ligands. This interaction then results in activation or repression of kinase activity – mediated by the intracellular domain of LecRK-I.9 – leading to regulation of normal plant development. We further hypothesize that Phytophthora uses LecRK-I.9 as a gateway to establish infection and that one of the functions of the RGD-containing protein IPI-O is to mediate this early step in the infection process. The other anticipated functions of IPI-O are more downstream in the process and are not included in the model. The model is reminiscent of animal host-pathogen interactions. Also some animal pathogens make use of receptors present in the plasma membrane to gain entry and there are several examples where integrin receptors that bind RGD-containing proteins produced by the pathogen play a role in this process. Obviously, the role of these integrins is not to help pathogens to enter the host cell, but to regulate normal developmental processes that require ‘inside-out’ and ‘outside-in’ signaling over the plasma membrane and we anticipate that the same is true for LecRK-I.9. Based on our results we propose that in plants challenged by Phytophthora pathogens loosening of CW-PM adhesions is a ubiquitous step in the infection process. This is supported by the finding that these adhesions are disrupted in N. benthamiana leaf cells invaded by germ tubes of P. infestans but not in neighboring cells (Fig. S3). As shown previously [18], [19] and in the current study, IPI-O by itself can loosen the CW-PM adhesions and the presumed interaction of IPI-O with LecRK-I.9 via the RGD motif may further disrupt these adhesions. This model explains why lecrk-I.9 and 35S-ipiO1 lines show gain of susceptibility and behave as phenocopies, and also explains why overexpression of LecRK-I.9 enhances resistance (Fig. 9B).
Fig. 9. P. infestans effector IPI-O and its putative effector target LecRK-I.9. 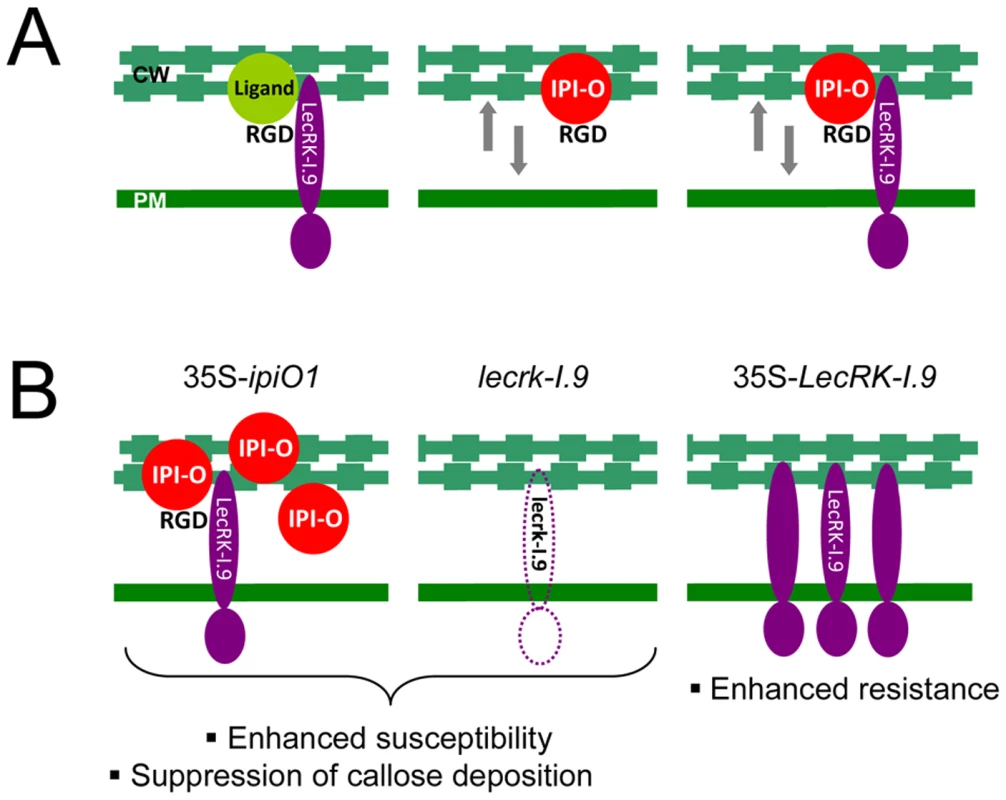
(A) Models depicting the membrane-associated LecRK-I.9 and the influence of IPI-O activity on adhesions between cell wall (CW) and plasma membrane (PM). LecRK-I.9 supposedly interacts with RGD-containing extracellular ligands (left panel). Addition of the RGD-containing effector IPI-O alters the CW-PM continuum (middle), and presumably binding of IPI-O to LecRK-I.9 further disrupts this continuum (right). The putative activity of IPI-O inside the plant cell is not included in these models. (B) Observed changes in the Arabidopsis phenocopy lines 35S-ipiO1 and lecrk-I.9 (left and middle panel, respectively), and the LecRK-I.9 overexpressing lines (right panel). Materials and Methods
Plant material and growth conditions
Arabidopsis plants were grown in soil or in vitro on solid MS medium (4.4 g/l Murashige and Skoog salts (Duchefa), 0.5% (w/v) sucrose and 1% (w/v) plant agar). Arabidopsis was grown in a conditioned growth chamber at 21–22°C with a 16 h photoperiod and at a relative humidity of 75–80%. Transgenic plants and T-DNA insertion lines were in Col-0 background. The Arabidopsis T-DNA insertion mutants lecrk-I.9-1 (SALK_042209), and lecrk-I.9-2 (SALK_024581) were obtained from the European Arabidopsis stock centre NASC (http://arabidopsis.info), and analyzed as described by Gouget (2005) [32]. Nicotiana benthamiana plants were grown under standard greenhouse conditions.
Pathogen growth and infection assays
Botrytis cinerea strain IMI169558 and Alternaria brassicicola strain MUCL20297 were grown and maintained at 22°C on malt agar and potato dextrose agar plates, respectively. Infection assays of Arabidopsis with B. cinerea and A. brassicola were performed as described by Van Esse et al. (2007) [46]. Colletotrichum destructivum strain IMI349061 was propagated at 22°C on Mathur's agar plates and inoculum was prepared as previously described [47]. Arabidopsis leaves were drop-inoculated with a conidial suspension (1*106 spores ml−1), and subsequently incubated at 22°C in trays covered with lids to maintain high humidity. Phytophthora infestans strain IPO-0, and the GFP-transformant 88069-GFP were grown on rye agar medium supplemented with 20 g l−1 sucrose at 18°C [48] and zoospores were isolated as described in Champouret et al., 2008 [27]. P. brassicae strains HH, II, CBS686.95 and the HH GFP-transformant 155 m [33] were grown at 18°C on 10% V8-juice agar plates [49], and zoospores were obtained as described in Bouwmeester and Govers, 2009 [31]. Inoculation was performed by placing plugs of young mycelium (Ø 5 mm) or 10 µl drops of a zoospore suspension (1*105 zoospores ml−1) on the abaxial leaf surface. Inoculated plants were kept in trays covered with lids to maintain a high humidity and placed in the dark, and placed in a growth chamber with a 16 h photoperiod at 18°C and a RH of 75%. The first day the trays were kept in the dark. After two days the mycelial plugs were removed from plants to stop the facilitation of nutrition from the agar medium. Pseudomonas syringae pv. tomato strains were grown on King B agar supplemented with the appropriate antibiotics at 28°C. Arabidopsis leaves were spray-inoculated with bacterial suspensions of 1*109 colony-forming units (cfu) ml−1, and incubated as earlier described [50]. Infection efficiencies (IEs) were calculated as percentages of successful infections relative to the total number of inoculations (n>72). All infection assays were performed at least 3 times.
Plasmid construction and plant transformation
For cloning ipiO1 (GenBank: L23939.1) in binary vectors ipiO1 fragments were PCR amplified from the plasmids pPIN18-c, pMBP-IPIO1D56A and pMBP-IPIO1D56E, respectively, using primers IPIOXHO-F and MAL-R (Table S1) [18]. PCR fragments were purified and digested with XhoI and PstI to release a 550 bp fragment containing the coding region of ipiO1, starting immediately after the signal peptide cleavage site, and ligated into the XhoI/PstI digested vector pRH80 [51]. Fragments containing the ipiO1 coding region fused to the TPI-II terminator sequence were released by digesting with XhoI and EcoRI, purified and ligated into XhoI/EcoRI-digested binary vector pRH90 [51] thereby fusing the ipiO1 coding sequence to the tobacco PR1a signal peptide sequence. The resulting binary plasmids were named pRW100, pRW101 and pRW102 with the latter two carrying RGD-tripeptide mutations E56A and D56E, respectively. Amplicons obtained by PCR amplification on pRW100, pRW101 and pRW102 with the primers PstPR1a and PR1 were digested by PstI and ligated into the PstI-digested vector pACN (Table S1) [52]. These plasmids were digested with HindIII, and the fragments were ligated into the HindIII-digested vector binSRNACatN [52]. In the resulting binary plasmids pRW110, pRW111 and pRW112, the ipiO1 coding sequence is fused to the ethanol-inducible promoter of the alcA gene.
For cloning the full-length coding sequence of LecRK-I.9 (GenBank: NM_125423.2) driven by the 35S CaMV promoter in a binary vector, a PCR was performed on BAC clone F15L12.17 with primers pK60300-s and pK60300-as or pK60300ms-as (Table S1). PCR fragments were cloned in pENTR/D-TOPO. LR recombination enabled cloning into binary vector pK2GW7 (http://www.psb.ugent.be/gateway) [53] and pSOL2095 resulting in plasmids pK-35S-LecRK-I.9 and pS-35S-LecRK-I.9-GFP, respectively. Cloning steps were verified by sequencing. Binary vectors were transformed to Agrobacterium tumefaciens strain GV3101 or AGL1 and cultured on medium containing the appropriate antibiotics. Arabidopsis thaliana accession Col-0 was transformed by the floral dip method [54]. Transformed plants were selected on MS agar with 50 mg/l kanamycin. Rooted seedlings were subsequently transferred to potting soil. Multiple independent lines were selected. Agroinfiltration assays were performed as previously described [27]. Leaves of 4–5 week old N. benthamiana plants were co-infiltrated in a 1∶1 ratio with A. tumefaciens suspensions of OD 0.5–1.0, and were analyzed by confocal laser scanning microscopy, 2–3 days after infiltration.
Staining techniques and microscopy
For GUS histochemical staining, Arabidopsis tissues were immersed and vacuum-infiltrated in X-gluc staining buffer [50 mM phosphate buffer pH 7.0, 0.1% (v/v) Triton X-100, 1 mM 5-bromo-4-chloro-3-indolyl-β-D-glucorunide (X-gluc, Biosynth, Staad, Switzerland), 1 mM K3Fe(CN)6*3H2O), 1 mM K4Fe(CN)6*3H2O and 10 mM EDTA] and incubated overnight at 37°C. Chlorophyll was removed by incubation in a 50–96% ethanol series. GUS stained samples were examined at low magnification under brightfield illumination. Pictures of GUS-stained tissues shown in this paper are representative results from at least three independent experiments. Neutral red staining and plasmolysis of etiolated Arabidopsis seedlings was performed as previously described with minor modifications [19]. Etiolated seedlings were incubated in a 0.05% neutral red solution for 30 min and rinsed afterwards in distilled water. Plasmolysis was induced by the addition of 0.4 M CaCl2. Pictures were taken after 5 min of plasmolysis. Trypan blue staining was performed as described earlier [55]. Lignification was visualized as earlier described by Mohr and Cahill (2007) [56]. In brief, leaves were cleared in ethanol and incubated overnight in a phloroglucinol-ethanol mixture, and subsequently placed for 5 min in 20% HCl and washed with water. To visualize anthocyanin pigmentation, leaves were incubated overnight in the dark at 4°C in 80% methanol containing 1% HCl. Brightfield and fluorescence microscopy was performed with a Nikon 90i epifluorescence microscope (Nikon, Amstelveen, The Netherlands). GFP and mCherry were visualized using a GFP-B (EX 460–500, DM 505, BA 510–560) and a TRITC (EX 540/25, DM 565, BA 606/55) filter cube. Confocol laser scanning microscopy was performed using a Zeiss Confocor 2–LSM 510 (Carl Zeiss, Jena, Germany). GFP and mCherry were excited with an Argon (488 nm line) and HeNe (543 nm line) laser, respectively. Fluorescence was captured through the band-pass emission filters BP505-530 (GFP) and 600–650 nm (mCherry). To visualize callose deposition, Arabidopsis leaves were harvested approximately 18 h after inoculation with bacteria or infiltration with 10 µM flg22, cleared overnight in an ethanol series (70–96%) and stained with 1% (w/v) aniline blue in 150 mM K2HPO4 (pH 9.5) for 1 h. Stained leaves were mounted in 50% glycerol, and fluorescent callose deposits were viewed using epifluorescence microscopy (DAPI filter; EX340-380, DM 400, BA 435-4850). Images of randomly selected fields were captured using a Nikon DS-5Mc digital camera and processed with ImageJ software (http://rsb.info.nih.gov/ij) to calculate the total area (µm2) of callose deposits. Values in Fig. 7B and D are the average of at least 16 and 10 microscopic fields, respectively. Error bars represent 95% confidence intervals (CIs).
RNA isolation and RT-PCR
Total RNA of Arabidopsis was isolated and purified using a NucleoSpin RNA Plant kit (Macherey-Nagel). RT-PCR was performed on a ABI7300 Real Time PCR system (Applied Biosystems) with use of a SYBR Green I qPCR kit (Eurogentec) and gene-specific primers (Table S1).
Accession numbers
IPI-O1; Q01918
LecRK-I.9; Q9LSR8/Q56XH0
Supporting Information
Zdroje
1. BollerT
FelixG
2009
A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors.
Annu Rev Plant Biol
60
379
407
2. HückelhovenR
2008
Cell wall-associated mechanisms of disease resistance and susceptibility.
Annu Rev Phytopathol
45
101
127
3. MellershDG
HeathMC
2001
Plasma membrane-cell wall adhesion is required for expression of plant defense responses during fungal penetration.
Plant Cell
13
413
424
4. RuoslahtiE
1996
RGD and other recognition sequences for integrins.
Annu Rev Cell Dev Biol
12
697
715
5. WatsonN
DuncanG
AnnanWS
van der WalleCF
2006
A tetravalent RGD ligand for integrin-mediated cell adhesion.
J Pharm Pharmacol
58
959
966
6. OlfaKZ
JoséL
SalmaD
AmineB
NajetSA
2005
Lebestatin, a disintegrin from Macrovipera venom, inhibits integrin-mediated cell adhesion, migration and angiogenesis.
Lab Invest
85
1507
1516
7. GarriguesHJ
RubinchikovaYE
DiPersioCM
RoseTM
2008
Integrin αVβ3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor.
J Virol
82
1570
1580
8. StockbauerKE
MagounL
LiuM
BurnsEHJr
GubbaS
1999
A natural variant of the cysteine protease virulence factor of group A Streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3.
Proc Natl Acad Sci U S A
96
242
247
9. HostetterMK
2000
RGD-mediated adhesion in fungal pathogens of humans, plants and insects.
Curr Opin Microbiol
3
344
348
10. CalveteJJ
MarcinkiewiczC
SanzL
2007
KTS and RTS-disintegrins: Anti-angiogenic viper venom peptides specifically targeting the α1β1 integrin.
Curr Pharm Des
13
2853
2859
11. SarmaGN
ManningVA
CiuffettiLM
KarplusPA
2005
Structure of Ptr ToxA: An RGD-containing host-selective toxin from Pyrenophora tritici-repentis.
Plant Cell
17
3190
3202
12. ManningVA
CiuffettiLM
2005
Localization of Ptr ToxA produced by Pyrenophora tritici-repentis reveals protein import into wheat mesophyll cells.
Plant Cell
17
3203
3212
13. ManningVA
HamiltonSM
KarplusPA
CiuffettiLM
2008
The Arg-Gly-Asp-containing, solvent-exposed loop of Ptr ToxA is required for internalization.
Mol Plant Microbe Interact
21
315
325
14. SchindlerM
MeinersS
ChereshDA
1989
RGD-dependent linkage between plant cell wall and plasma membrane: consequences for growth.
J Cell Biol
108
1955
65
15. CanutH
CarrascoA
GalaudJP
CassanC
BouyssouH
1998
High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall.
Plant J
16
63
71
16. KibaA
SugimotoM
ToyodaK
IchinoseY
YamadaT
1998
Interaction between cell wall and plasma membrane via RGD motif is implicated in plant defense responses.
Plant Cell Physiol
39
1245
1249
17. GaoH
GongYW
YuanYJ
2007
RGD-dependent mechanotransduction of suspension cultured Taxus cell in response to shear stress.
Biotechnol Prog
23
673
679
18. SenchouV
WeideR
CarrascoA
BouyssouH
Pont-LezicaR
2004
High affinity recognition of a Phytophthora protein by Arabidopsis via an RGD motif.
Cell Mol Life Sci
61
502
509
19. GougetA
SenchouV
GoversF
SansonA
BarreA
2006
Lectin receptor kinases participate in protein-protein interactions to mediate plasma membrane-cell wall adhesions in Arabidopsis.
Plant Physiol
140
81
90
20. BouwmeesterK
GoversF
2009
Arabidopsis L-type lectin receptor kinases: Phylogeny, classification, and expression profiles.
J Exp Bot
60
4383
4396
21. BouwmeesterK
van PoppelPMJA
GoversF
2009
Genome biology cracks enigmas of oomycete plant pathogens.
ParkerJE
Molecular aspects of plant disease resistance
Oxford, UK
Wiley-Blackwell
102
134
22. WhissonSC
BoevinkPC
MolelekiL
AvrovaAO
MoralesJG
2007
A translocation signal for delivery of oomycete effector proteins into host plant cells.
Nature
450
115
118
23. DouD
KaleSD
WangX
JiangRHY
BruceNA
2008
RXLR-mediated entry of Phytophthora sojae effector Avr1b into soybean cells does not require pathogen-encoded machinery.
Plant Cell
20
1930
1947
24. GoversF
BouwmeesterK
2008
Effector trafficking: RXLR-dEER as extra gear for delivery into plant cells.
Plant Cell
20
1728
1730
25. Van WestP
de JongAJ
JudelsonHS
EmonsAMC
GoversF
1998
The ipiO gene of Phytophthora infestans is highly expressed in invading hyphae during infection.
Fungal Genet Biol
23
126
138
26. VleeshouwersVGAA
RietmanH
KrenekP
ChampouretN
YoungC
2008
Effector genomics accelerates discovery and functional profiling of potato disease resistance and Phytophthora infestans avirulence genes.
PLoS ONE
3
e2875
27. ChampouretN
BouwmeesterK
RietmanH
van der LeeT
MaliepaardCA
2009
Phytophthora infestans isolates lacking class I ipiO variants are virulent on Rpi-blb1 potato.
Mol Plant Microbe Interact
22
1535
1545
28. VleeshouwersVGAA
Van DooijeweertW
GoversF
KamounS
ColonLT
2000
The hypersensitive response is associated with host and nonhost resistance to Phytophthora infestans.
Planta
210
853
64
29. RoetschiA
Si-AmmourA
BelbahriL
MauchF
Mauch-ManiB
2001
Characterization of an Arabidopsis-Phytophthora pathosystem: resistance requires a functional pad2 gene and is independent of salicylic acid, ethylene and jasmonic acid signalling.
Plant J
28
293
305
30. MauchF
TorcheS
SchlaeppiK
BranciardL
BelhajK
2009
Phytophthora brassicae as a pathogen of Arabidopsis.
LamourK
KamounS
Oomycete genetics and genomic: diversity, interactions and research tools
Hoboken, New Jersey
John Wiley & Sons
333
345
31. BouwmeesterK
GoversF
2009
A novel method for efficient and abundant production of Phytophthora brassicae zoospores on Brussels sprout leaf discs.
BMC Plant Biol
9
111
32. GougetA
2005
Etude fonctionnelle d'un récepteur lectine kinase (LecRK79), potentiel partenaire dans les contacts paroi-plasmalemme chez Arabidopsis thaliana.
[PhD thesis] Université Paul Sabatier-Toulouse
III
33. TamelingWIL
NooijenC
LudwigN
BoterM
SlootwegE
2010
RanGAP2 mediates nucleocytoplasmic partitioning of the NB-LRR immune receptor Rx in the Solanaceae, thereby dictating Rx function.
Plant Cell
22
4176
4194
34. Si-AmmourA
Mauch-ManiB
MauchF
2003
Quantification of induced resistance against Phytophthora species expressing GFP as a vital marker: β-aminobutyric acid but not BTH protects potato and Arabidopsis from infection.
Mol Plant Pathol
4
237
48
35. ShibataY
KawakitaK
TakemotoD
2010
Age-related resistance of Nicotiana benthamiana against hemibiotrophic pathogen Phytophthora infestans requires both ethylene - and salicylic acid-mediated signaling pathways.
Mol Plant Microbe Interact
23
1130
1142
36. HauckP
ThilmonyR
HeSY
2003
A Pseudomonas syringae type III effector suppresses cell wall-based extracellular defense in susceptible Arabidopsis plants.
Proc Natl Acad Sci U S A
100
8577
8582
37. HumphreyTV
BonettaDT
GoringDR
2007
Sentinels at the wall: Cell wall receptors and sensors.
New Phytol
176
7
21
38. Van KanJAL
2006
Licensed to kill: the lifestyle of a necrotrophic plant pathogen.
Trends Plant Sci
11
247
253
39. ParisyV
PoinssotB
OwsianowskiL
BuchalaA
GlazebrookJ
2007
Identification of PAD2 as a γ-glutamylcysteine synthetase highlights the importance of glutathione in disease resistance of Arabidopsis.
Plant J
49
159
172
40. SchlaeppiK
BodenhausenN
BuchalaA
MauchF
ReymondP
2008
The glutathione-deficient mutant pad2-1 accumulates lower amounts of glucosinolates and is more susceptible to the insect herbivore Spodoptera littoralis.
Plant J
55
774
786
41. SchlaeppiK
Abou-MansourE
BuchalaA
MauchF
2010
Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin.
Plant J
62
840
851
42. BallC
Vinod VijayanK
NguyenT
AnthonyK
BrayPF
2008
Glutathione regulates integrin αIIbβ3-mediated cell adhesion under flow conditions.
Thromb Haemost
100
857
863
43. Lorenc-KukułaK
JafraS
OszmiańskiJ
SzopaJ
2005
Ectopic expression of anthocyanin 5-O-glucosyltransferase in potato tuber causes increased resistance to bacteria.
J Agric Food Chem
53
272
281
44. QuentinM
AllasiaV
PegardA
AllaisF
DucrotPH
2009
Imbalanced lignin biosynthesis promotes the sexual reproduction of homothallic oomycete pathogens.
PLoS Pathog
5
e1000264
45. HaasBJ
KamounS
ZodyMC
JiangRHY
HandsakerRE
2009
Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans.
Nature
461
393
398
46. Van EsseHP
BoltonMD
StergiopoulosI
de WitPJGM
ThommaBPHJ
2007
The chitin-binding Cladosporium fulvum effector protein Avr4 is a virulence factor.
Mol Plant Microbe Interact
20
1092
1101
47. O'ConnellR
HerbertC
SreenivasaprasadS
KhatibM
Esquerré-TugayéMT
2004
A novel Arabidopsis-Colletotrichum pathosystem for the molecular dissection of plant-fungal interactions.
Mol Plant Microbe Interact
17
272
282
48. CatenCE
JinksJL
1968
Spontaneous variability of single isolates of Phytophthora infestans, I. Cultural variation.
Can J Bot
46
329
348
49. ErwinDC
RibeiroOK
1996
Phytophthora diseases worldwide.
St. Paul, Mn, USA
American Phytopathological Society
50. JambunathanN
McNellisTW
2003
Regulation of Arabidopsis COPINE 1 gene expression in response to pathogens and abiotic stimuli.
Plant Physiol
132
1370
1381
51. Van der HoornRAL
LaurentF
RothR
de WitPJGM
2000
Agroinfiltration is a versatile tool that facilitates comparative analyses of Avr9/Cf-9-induced and Avr4/Cf-4-induced necrosis.
Mol Plant Microbe Interact
13
439
446
52. CaddickMX
GreenlandAJ
JepsonL
KrauseKP
QuN
1998
An ethanol inducible gene switch for plants used to manipulate carbon metabolism.
Nat Biotechnol
16
177
180
53. KarimiM
InzeD
DepickerA
2002
GATEWAY vectors for Agrobacterium-mediated plant transformation.
Trends Plant Sci
7
193
195
54. CloughSJ
BentAF
1998
Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana.
Plant J
16
735
743
55. KeoghRC
DeberallBJ
McLeodS
1980
Comparison of histological and physiological responses to Phakospora pachyrhizi in resistant and susceptible soybean.
Trans Br Mycol Soc
74
329
333
56. MohrPG
CahillDM
2007
Suppression by ABA of salicylic acid and lignin accumulation and the expression of multiple genes, in Arabidopsis infected with Pseudomonas syringae pv. tomato.
Funct Integr Genomics
7
181
191
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in ChinaČlánek HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2Článek The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.Článek Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria ParasitesČlánek KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 MotifČlánek Viral EncephalomyelitisČlánek Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The Strain-Encoded Relationship between PrP Replication, Stability and Processing in Neurons is Predictive of the Incubation Period of Disease
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Human Macrophage Responses to Clinical Isolates from the Complex Discriminate between Ancient and Modern Lineages
- Dendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from
- Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China
- Rhesus TRIM5α Disrupts the HIV-1 Capsid at the InterHexamer Interfaces
- HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2
- Antigenic Variation in Malaria Involves a Highly Structured Switching Pattern
- The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Novel Escape Mutants Suggest an Extensive TRIM5α Binding Site Spanning the Entire Outer Surface of the Murine Leukemia Virus Capsid Protein
- Global Functional Analyses of Cellular Responses to Pore-Forming Toxins
- Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria Parasites
- Lung Adenocarcinoma Originates from Retrovirus Infection of Proliferating Type 2 Pneumocytes during Pulmonary Post-Natal Development or Tissue Repair
- Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors
- The Moving Junction Protein RON8 Facilitates Firm Attachment and Host Cell Invasion in
- KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 Motif
- The Coxsackievirus B 3C Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
- Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway
- Viral Encephalomyelitis
- Sheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice
- Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Dynamic Imaging of the Effector Immune Response to Infection
- The Lectin Receptor Kinase LecRK-I.9 Is a Novel Resistance Component and a Potential Host Target for a RXLR Effector
- Host Iron Withholding Demands Siderophore Utilization for to Survive Macrophage Killing
- The Danger Signal S100B Integrates Pathogen– and Danger–Sensing Pathways to Restrain Inflammation
- The RNome and Its Commitment to Virulence
- A Novel Nuclear Factor TgNF3 Is a Dynamic Chromatin-Associated Component, Modulator of Nucleolar Architecture and Parasite Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



