-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors
Botulinum neurotoxins (BoNTs) include seven bacterial toxins (BoNT/A-G) that target presynaptic terminals and act as proteases cleaving proteins required for synaptic vesicle exocytosis. Here we identified synaptic vesicle protein SV2 as the protein receptor for BoNT/D. BoNT/D enters cultured hippocampal neurons via synaptic vesicle recycling and can bind SV2 in brain detergent extracts. BoNT/D failed to bind and enter neurons lacking SV2, which can be rescued by expressing one of the three SV2 isoforms (SV2A/B/C). Localization of SV2 on plasma membranes mediated BoNT/D binding in both neurons and HEK293 cells. Furthermore, chimeric receptors containing the binding sites for BoNT/A and E, two other BoNTs that use SV2 as receptors, failed to mediate the entry of BoNT/D suggesting that BoNT/D binds SV2 via a mechanism distinct from BoNT/A and E. Finally, we demonstrated that gangliosides are essential for the binding and entry of BoNT/D into neurons and for its toxicity in vivo, supporting a double-receptor model for this toxin.
Published in the journal: . PLoS Pathog 7(3): e32767. doi:10.1371/journal.ppat.1002008
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002008Summary
Botulinum neurotoxins (BoNTs) include seven bacterial toxins (BoNT/A-G) that target presynaptic terminals and act as proteases cleaving proteins required for synaptic vesicle exocytosis. Here we identified synaptic vesicle protein SV2 as the protein receptor for BoNT/D. BoNT/D enters cultured hippocampal neurons via synaptic vesicle recycling and can bind SV2 in brain detergent extracts. BoNT/D failed to bind and enter neurons lacking SV2, which can be rescued by expressing one of the three SV2 isoforms (SV2A/B/C). Localization of SV2 on plasma membranes mediated BoNT/D binding in both neurons and HEK293 cells. Furthermore, chimeric receptors containing the binding sites for BoNT/A and E, two other BoNTs that use SV2 as receptors, failed to mediate the entry of BoNT/D suggesting that BoNT/D binds SV2 via a mechanism distinct from BoNT/A and E. Finally, we demonstrated that gangliosides are essential for the binding and entry of BoNT/D into neurons and for its toxicity in vivo, supporting a double-receptor model for this toxin.
Introduction
Botulinum neurotoxins (BoNTs), one of the six Category A potential bioterrorism agents, are a family of bacterial toxins that cause the fatal disease botulism in humans and animals [1], [2], [3]. These toxins target and enter presynaptic nerve terminals by receptor-mediated endocytosis. Once inside neurons, they act as proteases to cleave host proteins essential for synaptic vesicle exocytosis. Blocking vesicle exocytosis abolishes the release of neurotransmitters from nerve terminals, thus paralyzing muscles, and may cause death due to respiratory failure [1], [4]. The ability of BoNTs to block synaptic vesicle release also provides the basis for their medical applications: local injections of minute amounts of toxin can attenuate neuronal activity in the targeted region which can be beneficial in many medical conditions such as dystonia [5], [6], [7], [8].
BoNTs are classified into seven serotypes (BoNT/A-G) based on their antigenic properties [1], [9]. They share a similar overall domain structure composed of a heavy chain (∼100 kDa) and a light chain (∼50 kDa) connected via a disulfide bond [1], [4], [10]. The heavy chain contains two functional domains: the C-terminal receptor binding domain (HCR, ∼50 kDa) and the N-terminal domain (HN) that mediates the translocation of the toxin light chain (LC) across endosomal membranes. The LCs act as zinc-dependent proteases. The specificity of each toxin LC for host proteins has been well-established: BoNT/B, D, F, and G cleave synaptic vesicle protein synaptobrevin II (Syb, also known as VAMP II) [11], [12], [13], [14], [15], [16]. BoNT/A, E, and C cleave peripheral membrane protein synaptosomal-associated protein of 25 kDa (SNAP-25) [12], [17], [18], [19], [20], [21]. In addition, BoNT/C can also cleave the plasma membrane protein syntaxin (Syx) [22], [23]. These three proteins are known as soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNARE) that form the basic machinery mediating the fusion of synaptic vesicle membranes to plasma membranes [24], [25], [26], [27], [28].
There are two key functional variations among BoNTs: the particular SNARE proteins that their LCs cleave, and the cellular receptors that they use to enter cells. Understanding these key determinants for each BoNT is critical for developing effective strategies to counteract their toxicity and for utilizing them for scientific and therapeutic applications. Thus, it has been a major focus to identify the receptors for each BoNT [29], [30].
The first binding components identified for BoNTs are widely expressed complex forms of gangliosides (polysialiogangliosides, PSG), a family of glycosphingolipids [31], [32], [33], [34], [35], [36]. PSG are abundantly expressed in neurons and their direct interactions with all seven BoNTs have been characterized. Ganglioside-binding sites within each toxin have been proposed and further supported by mutagenesis and crystal structural studies [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. On the functional level, it has been shown that blocking ganglioside synthesis using chemical inhibitors reduced the binding and entry of BoNT/A and B in cells [47], [48], [49]. Recently, knockout (KO) mice lacking the ability to synthesize PSG have been created. It has been shown that lacking PSG in these KO mice reduced the toxicity of all seven BoNTs at motor nerve terminals using an ex vivo phrenic nerve hemi-diaphragm preparation [42], [43], [46], [50]. Furthermore, BoNT/A, B, E, and G failed to bind and enter hippocampal neurons cultured from PSG deficient mice and this defect can be restored using exogenous gangliosides [49], [51]. Finally, mice lacking PSG showed decreased sensitivities to BoNT/A, B, C, and G in vivo [49], [52], [53].
In addition to gangliosides, accumulating evidence suggests that there are specific protein receptors for BoNTs and a double-receptor model has been proposed [29], [54]. Previous studies have established two isoforms of synaptic vesicle membrane protein synaptotagmin (Syt) I and II, in conjunction with PSG, as the receptors for BoNT/B and G [35], [46], [49], [55], [56], [57], [58]. Co-crystal structure of BoNT/B bound to Syt II revealed that the toxin binds the membrane adjacent region of Syt [48], [59]. This binding mechanism is shared by BoNT/G, which has the highest sequence similarity to BoNT/B among the seven BoNTs [40], [41], [46], [58].
The protein receptor for BoNT/A and E was subsequently identified as the synaptic vesicle protein SV2 [51], [60], [61]. SV2 contains twelve transmembrane domains and one major luminal domain (the fourth luminal domain, L4) [62], [63], [64], [65]. In contrast to our detailed understanding of BoNT/B-Syt interactions, how BoNT/A and E recognize SV2 at the molecular level remains to be characterized. What have been shown are: 1) Binding of BoNT/A and E are mediated by SV2-L4; 2) BoNT/A can bind SV2-L4, while there is no detectable binding of BoNT/E to recombinant SV2-L4; 3) all three mammalian isoforms of SV2 (SV2A, B, and C) can function as the receptor for BoNT/A, while BoNT/E likely only utilizes SV2A and SV2B; 4) Mutating a conserved N-linked glycosylation site within SV2-L4 (N573Q in SV2A) blocked the entry of BoNT/E and also reduced the entry of BoNT/A into neurons. In addition, it was suggested that BoNT/F, which has the highest sequence similarity to BoNT/E within the seven BoNT-HCRs, also uses SV2 as its receptor [43], [45]. However, functional evidence is still lacking for the role of SV2 in mediating the binding and entry of BoNT/F into neurons.
The remaining serotypes, BoNT/C and BoNT/D, share the highest sequence similarity to each other among the seven BoNTs [9], [66]. Whether these two toxins share the same mode of receptor-recognition with the other five BoNTs remains unsolved. It has been suggested that BoNT/C and BoNT/D do not need protein receptors since treating rat brain synaptosomes with proteases and heating did not diminish toxin binding [53]. It was further suggested that BoNT/D binds phosphatidylethanolamine but not gangliosides, and lacking PSG did not reduce the toxicity of BoNT/D in mice [53]. On the other hand, recent studies have demonstrated that BoNT/D can bind gangliosides and the toxicity of BoNT/D is reduced at phrenic nerve hemi-diaphragm preparations from PSG deficient mice [42].
Here we established that BoNT/D uses SV2 as its protein receptor via a binding-mechanism distinct from BoNT/A and E. We further determined that gangliosides are essential for the binding and entry of BoNT/D into neurons and for its toxicity in vivo, thus extending the “double-receptor” model to this toxin and revealing how members of BoNTs converge onto a central theme yet also have their own individual receptor recognition strategies.
Results
BoNT/D enters neurons via recycling of synaptic vesicles
Exocytosis of synaptic vesicles and subsequent endocytosis of vesicle components is a major membrane recycling event at presynaptic terminals – the target site for BoNTs [67], [68]. Using the cleavage of Syb by BoNT/D as a functional readout for toxin entry, we found that stimulating vesicle exocytosis in cultured rat hippocampal neurons with high levels of potassium solution (high K+ buffer) increased Syb cleavage as compared to resting conditions (Figure 1A). We next constructed the receptor binding domain of BoNT/D (BoNT/D-HCR) fused with a HA tag in order to directly assay the binding of toxins to cell surfaces, since there were no suitable antibodies available for BoNT/D detection. This recombinant BoNT/D-HCR is capable of competing with BoNT/D for binding receptors as it reduced the cleavage of Syb by BoNT/D (Figure 1B). We found that high K+ buffer increased the binding of BoNT/D-HCR to neurons (Figure 1C). Binding occurs mainly at presynaptic terminals as shown by high degrees of co-localization between BoNT/D-HCR and the presynaptic marker synapsin (Figure 1C, overlay). Moreover, treating neurons with tetanus neurotoxin (TeNT), which cleaves Syb and blocks synaptic vesicle exocytosis [11], blocked the binding of BoNT/D-HCR to neurons (Figure 1D). Together, these data suggest that BoNT/D enters neurons through recycling of synaptic vesicles.
Fig. 1. Stimulation of synaptic vesicle recycling increases the binding and entry of BoNT/D into neurons. 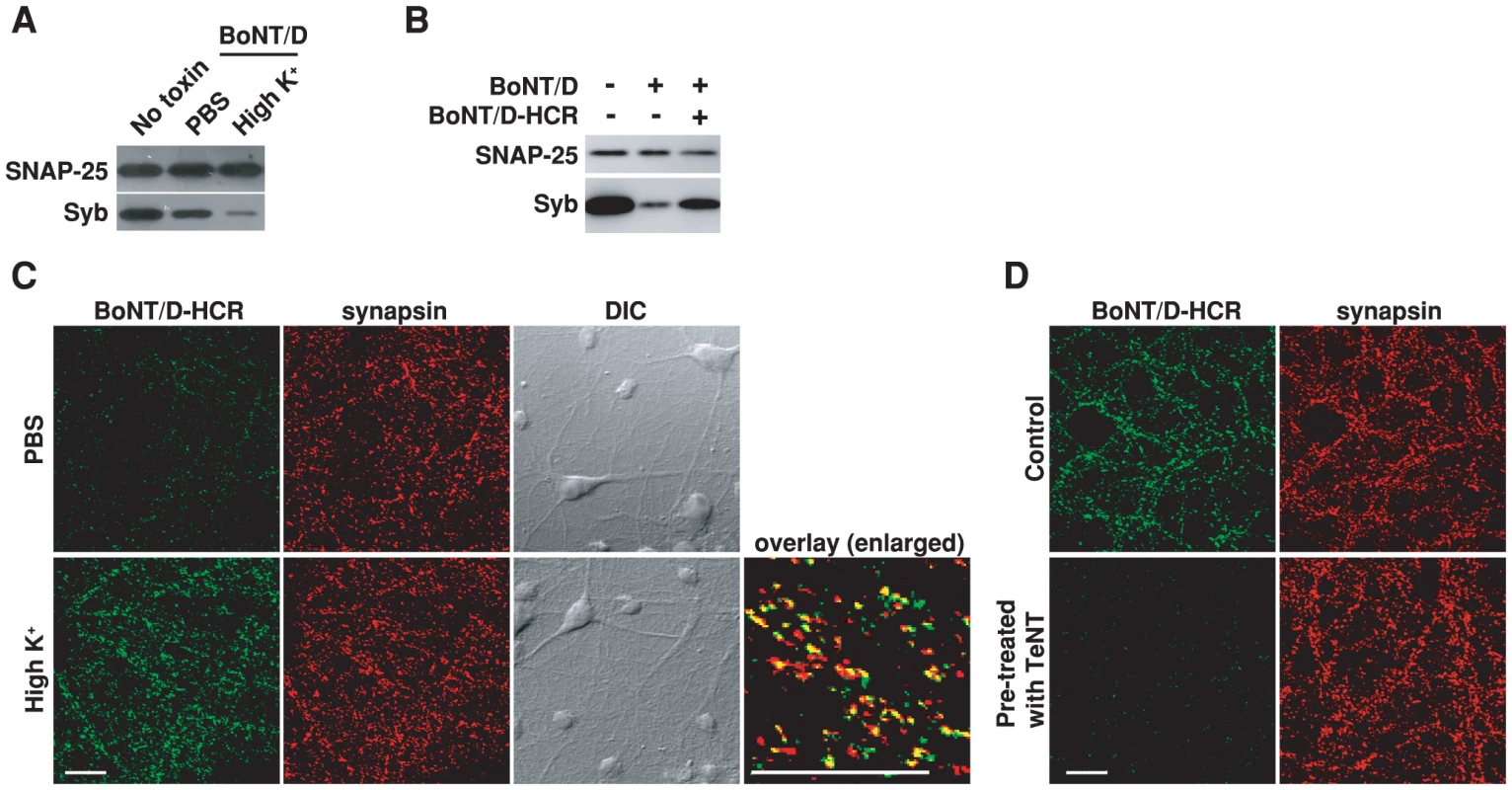
A) Cultured rat hippocampal neurons were exposed to BoNT/D (100 pM, 5 min) in either resting condition (PBS) or stimulated conditions (high K+ buffer: PBS with 56 mM KCl and 1 mM Ca2+). Cells were washed and further incubated in toxin-free media for 6 hrs. Cell lysates were subjected to immunoblot analysis. Cells that were not exposed to toxins served as the control (No toxin). SNAP-25 was detected as an internal control for loading of cell lysates. The cleavage of Syb by BoNT/D resulted in loss of Syb immunoblot signals. Stimulation of synaptic vesicle recycling with high K+ increased the cleavage of Syb. In all the following experiments, high K+ buffer was used to load toxins into neurons using the same procedures described here and cell lysates were subjected to immunoblot analysis, unless otherwise indicated in the Figure Legends. B) Neurons were exposed to BoNT/D (100 pM) with (+) or without (−) the presence of 1 µM BoNT/D-HCR. The presence of BoNT/D-HCR reduced the cleavage of Syb by BoNT/D. C) Neurons were exposed to BoNT/D-HCR (80 nM, 5 min) in either PBS or high K+ buffer. Cells were washed, fixed, permeabilized, and subjected to immunostaining analysis. Binding of BoNT/D-HCR was detected using an anti-HA antibody. Synapsin was labeled as a marker for presynaptic terminals. High K+ buffer increased the binding of BoNT/D-HCR to presynaptic terminals. The “Overlay” panel is enlarged from the center region of the “high K+” sample, showing high degrees of co-localization between BoNT/D-HCR (green) and synapsin (red). The scale bars represent 20 µm in all figures. D) Neurons were pre-treated with TeNT (1 nM, 12 hrs in media) and then tested for the binding of BoNT/D-HCR as described in panel C. Control cells were not exposed to TeNT. Pre-treatment with TeNT prevented the binding of BoNT/D-HCR to neurons. SV2 is a synaptic vesicle protein binding to BoNT/D-HCR
We next purified BoNT/D-HCR as a glutathione S-transferase (GST) fusion protein and used it to pull-down interacting proteins from rat brain detergent (Triton X-100) extracts. Bound materials were subjected to immunoblot analysis using antibodies for major synaptic vesicle membrane proteins [68], [69]. The HCRs of BoNT/A, E, B, as well as GST protein alone, were assayed in parallel as controls. BoNT/D-HCR pulled-down significant amounts of vesicle protein SV2 and low levels of Syt I, but not other vesicle proteins such as synaptophysin (Syp) or synaptogyrin I (Syg), in a similar manner to the HCRs of BoNT/A and E (Figure 2A). BoNT/A and E are known to use SV2 as their receptor and the low levels of bound Syt I might be due to the association of Syt I with SV2 as previously characterized [70], [71], [72], [73], [74]. Consistently, the HCR of BoNT/B, which uses Syt I/II as its receptors, pulled-down Syt I but not SV2 (Figure 2A). These data suggest that BoNT/D-HCR can bind SV2.
Fig. 2. BoNT/D-HCR can pull-down and co-immunoprecipitate SV2 from rat brain detergent extracts. 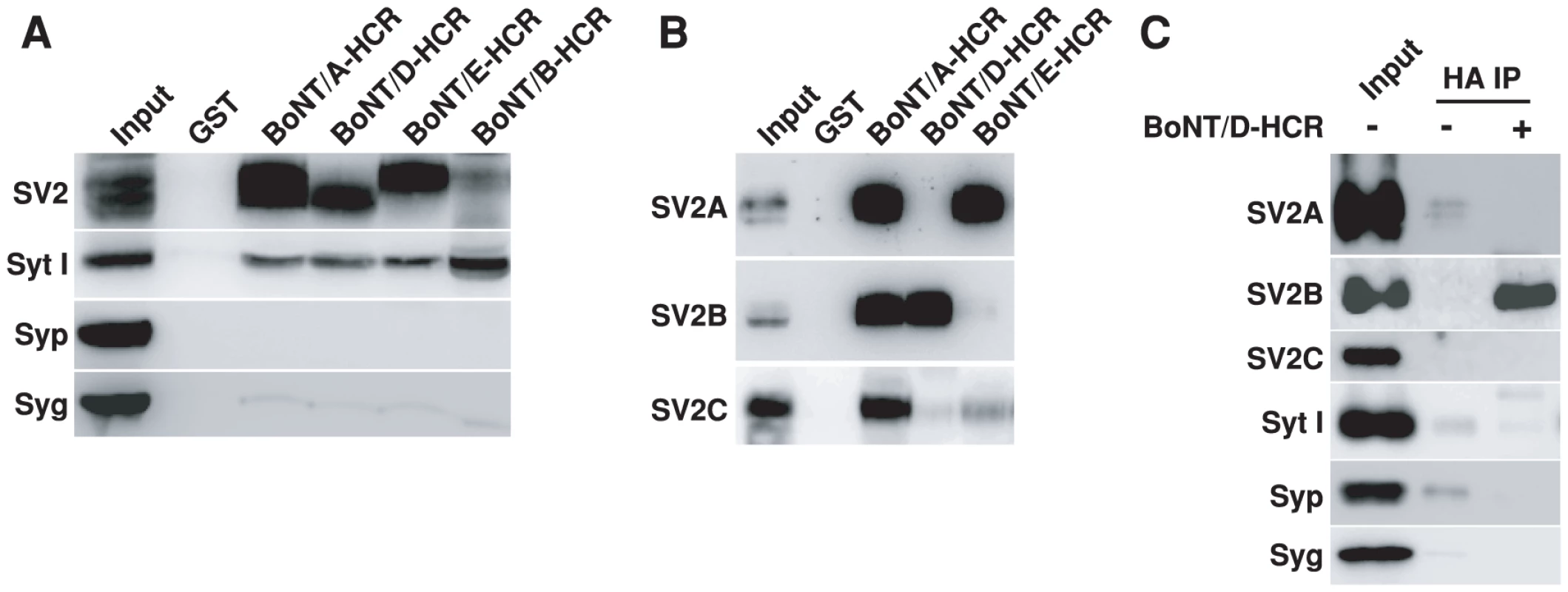
A) The HCRs of BoNT/A, D, E, and B were purified as GST-fusion proteins, immobilized on beads (500 µg), and incubated with 1.5 ml rat brain detergent (Triton X-100) extracts (2 mg/ml, 1 hr at 4°C). GST protein was assayed in parallel as a control. Bound materials were analyzed by immunoblot analysis. BoNT/A, D and E pulled-down SV2 and low levels of Syt I, while BoNT/B pulled-down Syt I. B) Bound materials from HCRs of BoNT/A, D, and E as described in panel A were analyzed using polyclonal antibodies that recognize SV2A, B, or C, respectively. BoNT/A-HCR pulled-down all three isoforms. BoNT/D-HCR pulled-down SV2B, while BoNT/E-HCR pulled-down SV2A. C) HA-tagged BoNT/D-HCR (100 nM) was incubated with brain detergent extracts (0.5 ml, 1 hr at 4°C). Co-immunoprecipitation assays were carried out using a monoclonal anti-HA antibody (HA IP). BoNT/D-HCR co-immunoprecipitated SV2B, but not other vesicle proteins. SV2 shown in Figure 2A was detected using an antibody that recognizes all three mammalian SV2 isoforms. We noticed that the molecular weight of SV2 pulled-down by BoNT/D-HCR and BoNT/E-HCR appears to be different (Figure 2A). Therefore, we further analyzed the bound materials using antibodies specific for each SV2 isoform (Figure 2B). While BoNT/A-HCR pulled-down all three SV2 isoforms, BoNT/D-HCR and BoNT/E-HCR showed clear preferences: BoNT/D-HCR for SV2B (which is of lower molecular weight than SV2A and C), and BoNT/E-HCR for SV2A, respectively. We note that although BoNT/E-HCR did not pull-down detectable levels of SV2B, we have previously demonstrated that SV2B can function as the receptor to mediate the binding and entry of BoNT/E in neurons [51]. The likely explanation for these apparently contradictory results is that the pull-down assay using detergent-solubilized materials may only preserve the strongest binding interactions, while the neuronal surface may provide an optimal environment for SV2-BoNT/E interactions. Similarly, the preference of BoNT/D-HCR for SV2B does not exclude other SV2 isoforms as its receptors on neuronal surfaces, but suggests that BoNT/D-HCR may have the highest binding affinity to SV2B under our assay conditions.
In addition, we also carried out co-immunoprecipitation assays using a HA antibody to immunoprecipitate soluble BoNT/D-HCR incubated with brain detergent extracts (Figure 2C). Immunoprecipitated materials were analyzed using antibodies against different synaptic vesicle proteins. SV2B is the only one co-immunoprecipitated with BoNT/D-HCR at significant levels (Figure 2C); further confirming BoNT/D-SV2 interactions.
SV2 is required for the binding and entry of BoNT/D into neurons
To determine whether SV2 plays a role for the binding and entry of BoNT/D, we used hippocampal neurons cultured from SV2A/B double KO mice as a cell model. We have previously found that hippocampal neurons mainly express two of the three SV2 isoforms – SV2A and SV2B, thus SV2A/B KO neurons can serve as a loss-of-function model [51], [60]. We found that BoNT/D failed to enter SV2A/B KO neurons, as demonstrated by the lack of Syb cleavage (Figure 3A). Furthermore, expressing SV2A, B or C in SV2A/B KO neurons via lentiviral infection restored the entry of BoNT/D and resulted in the cleavage of Syb (Figure 3A). These results demonstrated that SV2 is essential for the functional entry of BoNT/D into neurons, and all three isoforms of SV2 can mediate the entry of BoNT/D.
Fig. 3. SV2 is essential for the binding and entry of BoNT/D into neurons. 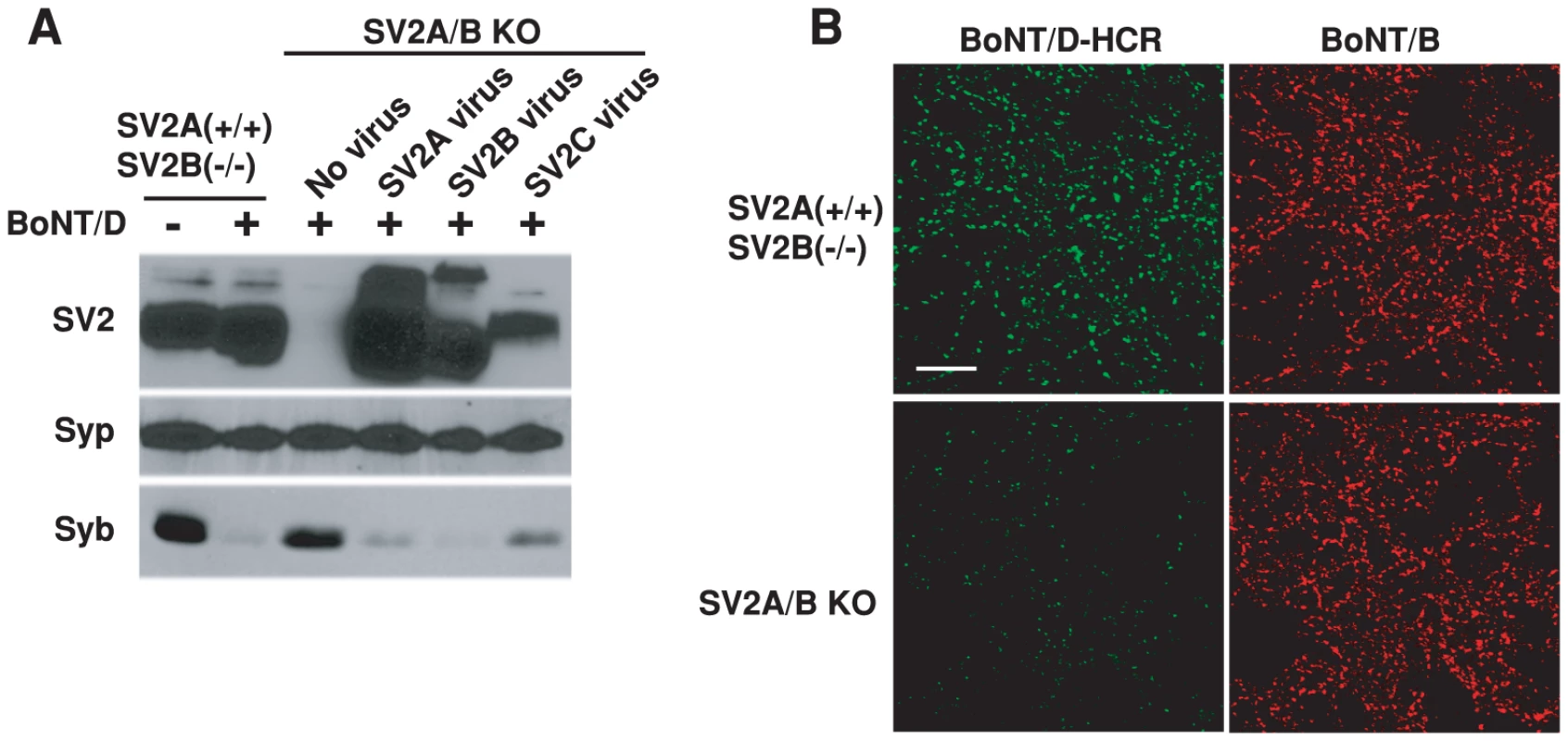
A) SV2A/B double KO mice were generated by breeding mice with the genotype of SV2A(+/−)SV2B(−/−). Thus, SV2A(+/+)SV2B(−/−) mice from the same litter as SV2A/B double KO mice served as the control. Hippocampal neurons cultured from these mice were exposed to BoNT/D (100 pM). Cell lysates were subjected to immunoblot analysis using antibodies for SV2, Syp (as a loading control), and Syb. SV2 A/B KO neurons infected with lentiviruses expressing SV2A, B or C were also assayed in parallel. BoNT/D failed to enter SV2 A/B KO neurons as evidenced by the lack of Syb cleavage. Expression of SV2A, B or C restored the entry of BoNT/D. B) SV2A(+/+)SV2B(−/−) and SV2A/B KO neurons were exposed to BoNT/D-HCR (80 nM) and BoNT/B (20 nM) in high K+ buffer for 5 min. Cells were subjected to immunostaining analysis. BoNT/B can bind both types of neurons. BoNT/D-HCR failed to bind SV2A/B KO neurons. We next examined whether SV2 is required for the binding of BoNT/D to neurons. As shown in Figure 3B, BoNT/D-HCR failed to bind SV2A/B KO neurons. BoNT/B served as an internal control, which bound to both control and SV2A/B KO neurons, demonstrating that loss of BoNT/D-HCR binding is not due to any potential defects in the vesicle recycling process in SV2A/B KO neurons. Furthermore, binding of BoNT/D-HCR was also observed for a subpopulation of SV2A/B KO neurons that still express SV2C and bound BoNT/D-HCR largely co-localizes with endogenous SV2C (Figure S1). Together, these results indicate that SV2 likely mediates the binding of BoNT/D to neurons.
The binding mechanism of BoNT/D to SV2 is distinct from BoNT/A and BoNT/E
SV2 has only one major extracellular domain (luminal domain) with significant length (SV2-L4, ∼130 amino acids, Figure 4A). We previously demonstrated that BoNT/A can bind recombinant SV2-L4 fragments directly [60], while BoNT/E requires glycosylation at a particular site within the SV2-L4 domain [51]. We next carried out a series of studies to determine whether BoNT/D shares SV2-binding mechanisms with either BoNT/A or BoNT/E.
Fig. 4. BoNT/D utilizes a SV2-binding mechanism distinct from BoNT/A and E. 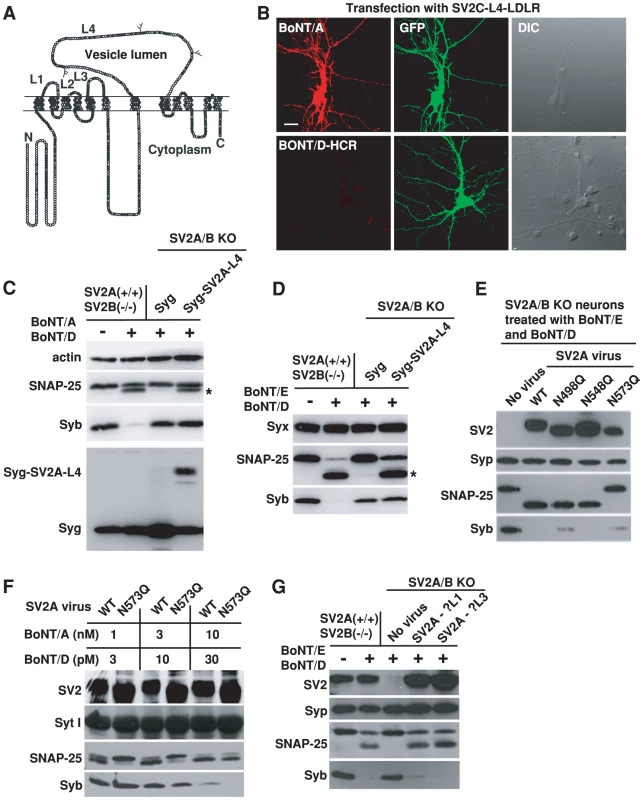
A) Schematic drawing of SV2. Filled circles indicate conserved residues in all SV2 isoforms; gray circles are residues conserved in two SV2 isoforms; and open circles represent non-conserved residues. Three glycosylation sites within the L4 domain are also indicated. B) SV2A/B KO neurons were transfected with a chimeric receptor containing the SV2C-L4 at the N-terminus of the transmembrane and cytosolic domain of LDL receptor (SV2C-L4-LDLR, [51]). Cells were exposed to either BoNT/A (20 nM) or BoNT/D-HCR (80 nM) for 20 min in culture media, washed and fixed for immunostaining analysis. Transfected cells were marked by co-expressed GFP. SV2C-L4-LDLR mediated the binding of BoNT/A, but failed to mediate the binding of BoNT/D-HCR. C) Chimeric protein Syg-SV2A-L4 was constructed by inserting SV2A-L4 into the second luminal domain of Syg. Syg and Syg-SV2A-L4 were expressed in SV2A/B KO neurons via lentiviral infection. Cells were exposed to BoNT/A (10 nM) and BoNT/D (100 pM). Actin serves as a loading control. Expression of Syg and Syg-SV2A-L4 was confirmed using a polyclonal anti-Syg antibody. Cleavage of SNAP-25 by BoNT/A yielded a smaller fragment that is indicated by an asterisk. Syg-SV2A-L4 mediated the entry of BoNT/A as indicated by the cleavage of SNAP-25, but failed to mediate the entry of BoNT/D as shown by the lack of Syb cleavage. D) Experiments were carried out as described in panel C, except that neurons were exposed to BoNT/E (200 pM) and BoNT/D (100 pM). Cleavage of SNAP-25 by BoNT/E yielded a smaller fragment that is indicated by an asterisk. Syx was used as a loading control. Syg-SV2A-L4 mediated the functional entry of BoNT/E, but not BoNT/D. E) Three glycosylation sites within the SV2A-L4 domain were abolished by site-directed mutagenesis, respectively. These mutants were expressed in SV2 A/B KO neurons via lentiviral infection. Neurons were exposed to BoNT/D (100 pM) and BoNT/E (200 pM). Syp serves as a loading control. Mutation N573Q abolished the entry of BoNT/E. Entry of BoNT/D was not affected by abolishing any one of the three glycosylation sites. F) SV2A/B KO neurons were infected with lentiviruses that express either WT SV2A or SV2A N573Q mutant. Cells were exposed to BoNT/A and BoNT/D at indicated concentrations (5 min in high K+ buffer, 12 hrs incubation in media afterwards). Syt I serves as a loading control. Mutation N573Q reduced the cleavage of SNAP-25 by BoNT/A at low toxin concentrations (1 and 3 nM). Cleavage of Syb by BoNT/D is similar at all toxin concentrations between neurons expressing WT SV2A and N573Q mutant. G) SV2A mutants harboring disrupted luminal domain 1 (SV2A-ΔL1) or L3 (SV2A-ΔL3) were expressed in SV2A/B KO neurons via lentiviral infection. Neurons were exposed to BoNT/E (100 pM) and BoNT/D (100 pM). Disrupting L1 or L3 domain did not reduce the functional entry of either BoNT/E or BoNT/D. In previous studies, we have constructed chimeric receptors containing SV2-L4 plus the transmembrane and cytoplasmic domains of low density lipoprotein receptor (LDLR) [51]. Once expressed in SV2A/B KO neurons, these chimeric receptors (SV2-L4-LDLR) were able to mediate the binding of BoNT/A (Figure 4B, Figure S2, [51]) and BoNT/E [51], but failed to mediate the binding of BoNT/D-HCR (Figure 4B, Figure S2).
To address the concern that SV2-L4-LDLR does not localize to synaptic vesicles, we next inserted SV2A-L4 into the luminal domain of another multiple membrane spanning synaptic vesicle protein Syg. Once expressed in SV2A/B KO neurons, this chimeric protein (Syg-SV2A-L4) mediated the entry of BoNT/A (Figure 4C) and BoNT/E (Figure 4D) into neurons at a comparable efficiency to endogenous SV2A expressed in control neurons, as indicated by the similar levels of SNAP-25 cleavage. In contrast, Syg-SV2A-L4 failed to mediate the entry of BoNT/D as shown by the lack of Syb cleavage (Figures 4C–D). These data suggest that the SV2-L4 domain, expressed in chimeric receptors, can provide a binding site for BoNT/A and E, but it is not sufficient for BoNT/D.
Because glycosylation at the third site within the SV2-L4 (N573 in SV2A) has been shown to be essential for the entry of BoNT/E and also can enhance the entry of BoNT/A at low toxin concentrations [51], we next examined whether BoNT/D shares this requirement. Three mutant forms of SV2A that harbor point mutations at each N-linked glycosylation consensus sequence (N498Q, N548Q, N573Q), respectively, were expressed in SV2A/B KO neurons. As we previous reported, N573Q mutation completely blocked the entry of BoNT/E and protected SNAP-25 (Figure 4E). In contrast, none of the mutants blocked the entry of BoNT/D (Figure 4E). Furthermore, N573Q did not affect the entry of BoNT/D when we reduce BoNT/D concentrations from 100 pM (Figure 4E) to 30 and 10 pM (Figure 4F). As a control, we confirmed our previous finding that N573Q mutation reduced BoNT/A entry at low toxin concentrations (3 and 1 nM, Figure 4F). These data again suggest that BoNT/D has a SV2-binding mechanism distinct from BoNT/A and BoNT/E. In addition, these studies also demonstrated that the SV2A(N573Q) mutant is able to mediate the entry of toxins, providing strong evidence that mutating the third glycosylation site in SV2A does not affect the function and localization of SV2, but rather specifically abolishes the receptor function for BoNT/E and reduces the binding affinity for BoNT/A.
Besides L4, SV2 has two other short luminal domains (L1: ∼15 amino acids; L3: ∼20 amino acids, Figure 4A). To test whether these two minor domains play any roles for BoNT/D, we constructed two mutant forms of SV2A by deleting the middle portions of L1 and L3 (residue 196-200 of L1 and 321-331 of L3), respectively. Both mutants, when expressed in SV2 A/B KO neurons, were able to restore the entry of BoNT/D and BoNT/E (Figure 4G), indicating these two short luminal domains are unlikely participants in providing the binding site for BoNT/D or BoNT/E.
Localization of SV2C on plasma membranes reconstituted the binding site for BoNT/D-HCR on the surface of neurons and non-neuronal cells
Whether SV2 can provide the binding site for BoNT/D on cell surfaces is a key question in establishing it as a receptor for BoNT/D. SV2 luminal domains are the only regions that can be transiently exposed to the outside of cells during vesicle recycling. The finding that LDLR - or Syg-based chimeric receptors failed to mediate the entry of BoNT/D did not exclude the L4 domain as the toxin binding site, especially considering that the L4 domain in SV2 is anchored to membranes through both N - and C-terminal transmembrane domains (Figure 4A), yet these membrane adjacent regions are disrupted in chimeric receptors. Unfortunately, our attempts to include the transmembrane domains of SV2 in different chimeric receptors, as well as various mutations within the L4 domains and the L4 domain deletion all resulted in mis-folded proteins that are not expressed/trafficked in cells, suggesting a rigid requirement for a specific conformation within the L4 domain. In order to examine whether SV2 provided the binding site for BoNT/D, we have to find a way to present SV2 luminal domains onto cell surfaces in their native conformation. The solution comes from a new observation we made when examining the binding of BoNT/D-HCR to SV2A/B KO neurons transfected with different SV2 isoforms (Figure 5A).
Fig. 5. Localization of SV2C to plasma membranes mediated binding of BoNT/D-HCR to the cell surface in both neurons and HEK293FT cells. 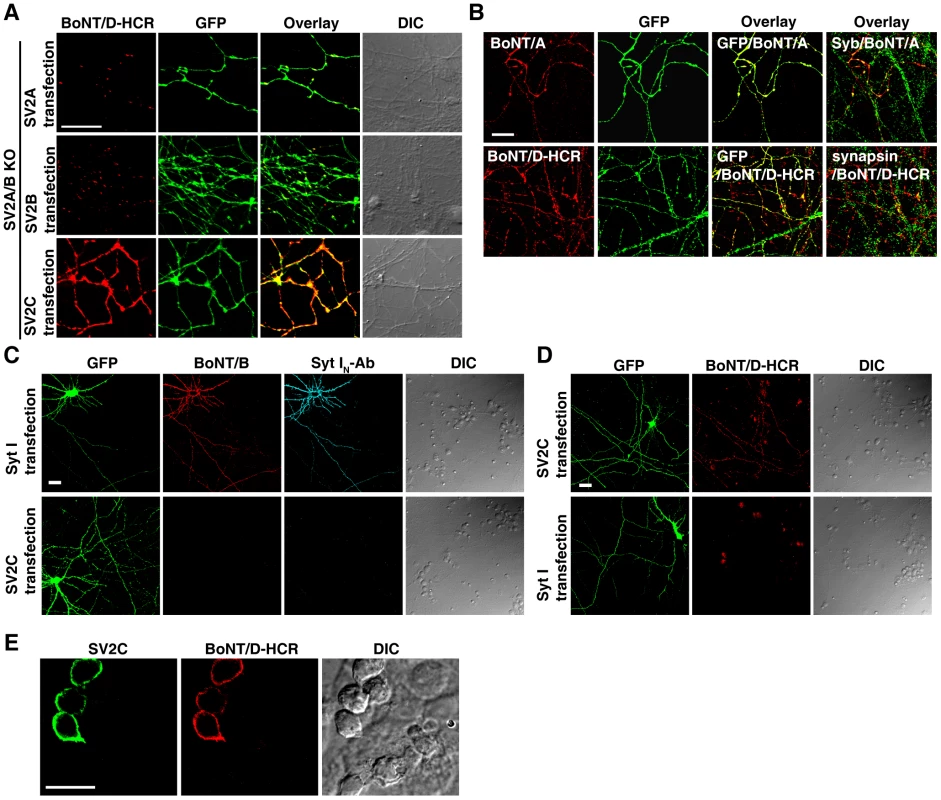
A) SV2A/B KO neurons were transfected with SV2A, B, or C. Transfected cells were marked by co-expressed GFP. Cells were exposed to BoNT/D-HCR (80 nM, 5 min in high K+ buffer), washed, fixed, and permeabilized for immunostaining. Binding of BoNT/D-HCR was observed as puncta along processes in neurons transfected with SV2A (upper panel) or SV2B (middle panel). Binding of BoNT/D-HCR to SV2C-transfected neurons appears to be continuous along neuronal processes (lower panel). B) SV2A/B KO neurons were transfected with SV2C. Cells were exposed to BoNT/A (20 nM, upper panel) or BoNT/D-HCR (80 nM, lower panel) for 10 min in cold media (incubation on ice). Cells were then washed in cold media and fixed. Immunostaining was first performed without permeabilizing cells to detect the surface binding of BoNT/A or BoNT/D-HCR. Cells were subsequently permeabilized and immunostaining was further carried out to label neuronal processes using an anti-GFP antibody and presynaptic terminals using antibodies for Syb (upper panel) or synapsin (lower panel). Transfection of SV2C resulted in the binding of BoNT/A (upper panel) and BoNT/D-HCR (lower panel) to neuronal surface at both presynaptic terminals (co-localized with Syb (green) or synapsin (green)) as well as regions outside the synapse. C) Cultured rat hippocampal neurons were transfected with either Syt I (upper panel) or SV2C (lower panel). Neurons were exposed to BoNT/B (20 nM) and an antibody that recognizes the luminal domain of Syt I (Syt IN-Ab, 1∶200) for 10 min in cold media, washed, and fixed. Immunostaining was first performed without permeabilizing cells to detect the surface binding of BoNT/B and Syt IN-Ab. Cells were subsequently permeabilized and immunostaining was carried out using an anti-GFP antibody. Transfection of Syt I, but not SV2C, resulted in the binding of both BoNT/B and Syt IN-Ab to neuronal surfaces. D) Experiments were carried out as described in panel C, except that neurons were exposed to BoNT/D-HCR (80 nM). Expression of SV2C, but not Syt I, mediated the binding of BoNT/D-HCR to neuronal surfaces. E) HEK293FT cells were transfected with SV2C and exposed to BoNT/D-HCR (80 nM, 30 min in media at 37°C). Immunostaining was first carried out without permeabilizing cells to detect BoNT/D-HCR. Cells were subsequently permeabilized and immunostaining was carried out using a polyclonal anti-SV2C antibody. Expression of SV2C mediated the binding of BoNT/D-HCR to the surface of HEK293FT cells. SV2 normally resides on synaptic vesicles. As expected, transfecting SV2A or SV2B restored the binding of BoNT/D-HCR to SV2A/B KO neurons in a puncta pattern (Figure 5A, upper and middle panels), suggesting that binding occurs at presynaptic terminals. To our surprise, binding of BoNT/D-HCR to SV2C-transfected neurons showed a continuous binding pattern along neuronal processes (Figure 5A, lower panel). The likely explanation is that a significant portion of SV2C localizes onto plasma membranes and mediates the binding of BoNT/D-HCR to regions outside of synapses.
To confirm the localization of SV2C to plasma membranes, we tested the binding of BoNT/A to SV2C-transfected SV2A/B KO neurons under a low temperature condition, which stops membrane trafficking and only allows the binding to occur at cell surfaces. Furthermore, surface-bound toxins were detected via immunostaining without permeabilizing cells. Under these assay conditions, we detected the binding of BoNT/A to SV2C-transfected neurons in a continuous pattern along neuronal processes (Figure 5B, upper panel). BoNT/A is known to bind SV2C-L4 [60], [61], thus this result demonstrates that SV2C is located on cell surfaces with its luminal domains exposed to the outside of cells. In addition, we subsequently permeabilized these cells and detected synaptic marker Syb (Figure 5B, overlay Syb/BoNT/A). We found that Syb distributed along BoNT/A-bound neuronal processes in a puncta pattern, indicating that these processes are axons harboring presynaptic terminals and also demonstrating that BoNT/A binding occurs at both presynaptic terminals and regions outside of synapses. Under the same assay conditions, we observed robust binding of BoNT/D-HCR to SV2C-transfected neurons (Figure 5B, lower panel) at both presynaptic terminals (labeled by synapsin) and regions outside of synapses, demonstrating that SV2C mediates the binding of BoNT/D-HCR to cell surfaces.
This low temperature surface-binding assay allows us to examine toxin binding even in WT neurons. Using this assay, we found that the receptor for BoNT/B, Syt I, also has a significant portion localized on plasma membranes when over-expressed in rat neurons, as demonstrated by the surface binding of BoNT/B and the binding of Syt IN Ab that recognizes the N-terminus of Syt I luminal domain (Figure 5C, upper panel). Under the same assay conditions, we did not detect the binding of BoNT/B or Syt IN Ab to SV2C-transfected neurons (Figure 5C, lower panel). Consistently, BoNT/D-HCR binds to SV2C-transfected rat neurons (Figure 5D, upper panel), but not to Syt I-transfected neurons (Figure 5D, lower panel), demonstrating the specificity of BoNT/B and BoNT/D-HCR in recognizing their respective receptors under our assay conditions.
Finally, we expressed SV2C in non-neuronal HEK293FT cells. Transfected cells were exposed to BoNT/D-HCR and immunostaining was first carried out without permeabilizing cells to detect the surface binding of BoNT/D-HCR. Cells were subsequently permeabilized to confirm the expression of SV2C using a polyclonal SV2C antibody. As shown in Figure 5E, expression of SV2C mediated the binding of BoNT/D-HCR to the surfaces of HEK293FT cells. Furthermore, the polyclonal SV2C antibody, which recognizes the N-terminal cytoplasmic domain of SV2C, failed to stain SV2C in transfected cells without permeabilizing cells (Figure S3), suggesting that SV2C maintains the correct membrane topology on the surface of HEK293FT cells. Together, the experiments described in this section demonstrate that SV2 functions as a receptor providing the binding site for BoNT/D on cell surfaces.
PSG are required for the binding and entry of BoNT/D into neurons
We next determined whether BoNT/D requires gangliosides as co-receptors. We note that a previous study concluded that PSG are not required for BoNT/D binding and entry based on a KO mouse line lacking GM3 synthase, which only depletes a - and b - series, but not o-series PSG. O-series PSG are not abundant in WT neurons; however, it has been shown that their levels are significantly elevated in GM3 KO neurons [75]. Thus, we assessed the role of PSG using a different KO mouse line lacking the gene encoding GM2/GD2 synthase (GS KO), an enzyme required for synthesis of all major PSG [76]. Using a well-established rapid-time-to-death assay, we examined the sensitivity of KO mice versus their wild type (WT) littermates to BoNT/D. The assay was conducted by injecting a large amount of toxins (105–106 mean lethal doses, LD50) into mice that resulted in death within 30 min to 1 hour. Within this range of toxin concentrations, the effective toxicity in vivo can be estimated based on how long the mice survive using a standard curve [57], [77]. When injected with the same amount of BoNT/D, the KO mice survived significantly longer than WT mice (Figure 6A). The effective toxicity in KO mice was reduced to only 10% of the level in WT mice (Figure 6A), demonstrating that PSG are essential for the toxicity of BoNT/D in vivo.
Fig. 6. PSG are essential for the binding and entry of BoNT/D into neurons. 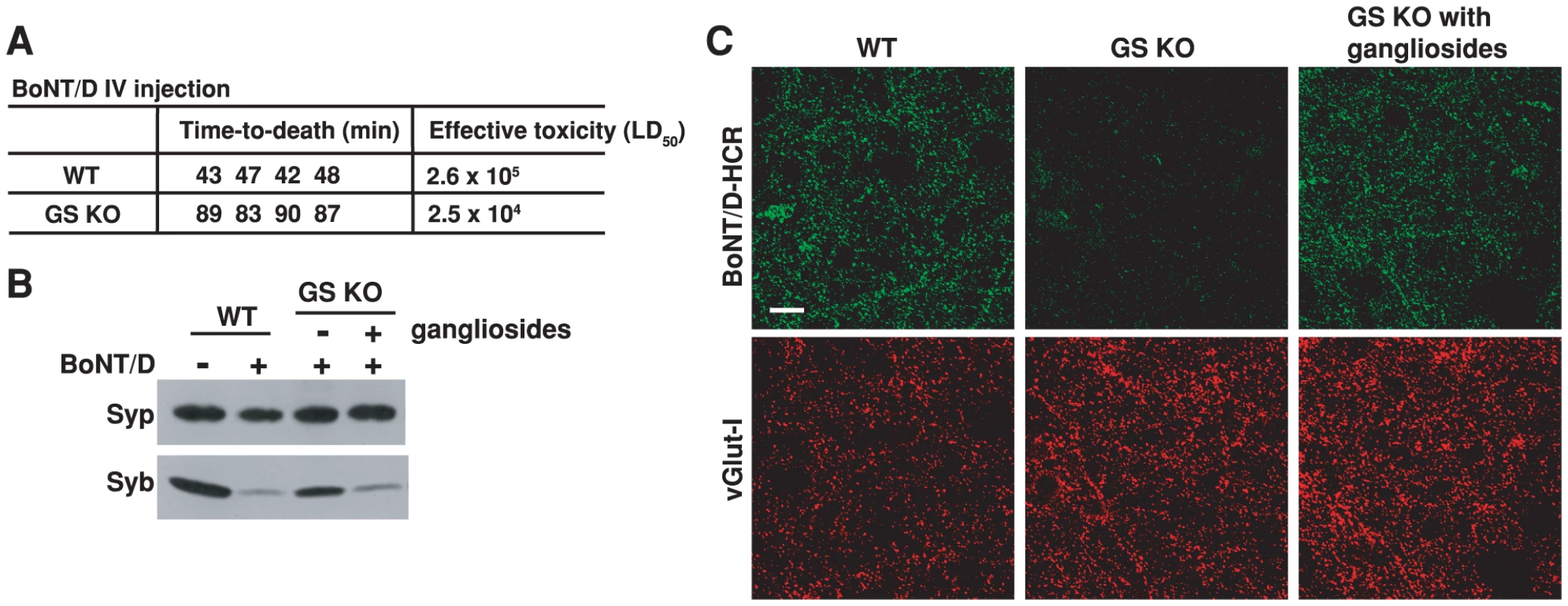
A) The sensitivity of four pairs of WT and GS KO mice to BoNT/D was assayed using a rapid-time-to-death assay [57]. When injected with the same amount of toxins, all four GS KO mice survived longer than WT mice, corresponding to a 10-fold reduction of effective toxicity in vivo. B) Neurons cultured from GS KO mice, with (+) or without (−) incubation with exogenous gangliosides (250 µg/ml ganglioside mixture, 12 hrs in media), were exposed to BoNT/D (100 pM). WT neurons from the same littermates were assayed in parallel. BoNT/D failed to enter GS KO neurons as evidenced by the lack of cleavage of Syb; the entry was restored by adding exogenous gangliosides. C) Neurons cultured from GS KO, with (right panel) or without (middle panel) pre-incubation with exogenous gangliosides, were exposed to BoNT/D-HCR (80 nM) for 5 min in high K+ buffer. WT neurons were assayed in parallel (left panel). Cells were washed, fixed, and subject to immunostaining analysis. Vesicular glutamate transporter I (vGlut-I) was labeled as a marker for presynaptic terminals. BoNT/D-HCR failed to bind GS KO neurons and the binding was restored by loading exogenous gangliosides to cell membranes. We next assayed whether reduced toxicity of BoNT/D in GS KO is due to the decrease of BoNT/D entry into neurons. Using hippocampal neurons cultured from GS KO mice as a cell model, we found that lacking gangliosides reduced the entry of BoNT/D into neurons as evidenced by the reduction of Syb cleavage (Figure 6B). Furthermore, the functional entry of BoNT/D was restored by loading exogenous gangliosides into the cell membrane (Figure 6B).
The next question is whether PSG are required for the neuronal binding step of the toxin action. To directly examine this, we tested the binding of BoNT/D-HCR to hippocampal neurons cultured from GS KO mice and their WT littermates. Binding of BoNT/D-HCR was abolished in GS KO neurons (Figure 6C, middle panel), and it was restored by loading exogenous gangliosides onto the cell membrane (Figure 6C, right panel), demonstrating that gangliosides are required for the binding of BoNT/D to neurons. Together, these studies provided critical evidence at both animal and cellular levels for the conclusion that PSG are essential co-receptors for BoNT/D.
Entry of BoNT/C and BoNT/F into hippocampal neurons requires PSG but not SV2
Among the seven BoNTs, BoNT/C has the highest sequence similarity to BoNT/D, while BoNT/F has the highest sequence similarity to BoNT/E within the seven BoNT-HCRs [9]. We next assessed whether BoNT/C and BoNT/F share the same requirement for receptor-recognition with BoNT/D or BoNT/E, taking advantage of available PSG and SV2 KO mouse lines.
We found that lacking PSG abolished the functional entry of BoNT/C, as shown by the lack of cleavage of SNAP-25 (Figure 7A). Entry was restored by adding exogenous gangliosides to cell membranes (Figure 7A). Similarly, lacking PSG reduced the entry of BoNT/F, as evidenced by the decreased cleavage of Syb (Figure 7B). Loading gangliosides into cell membranes restored the entry of BoNT/F (Figure 7B). These results are consistent with previous studies demonstrating that gangliosides can bind BoNT/C and F, and are essential for their toxicity in mice and in phrenic nerve hemi-diaphragm preparations [43], [45], [53]. Our studies provided further cellular evidence to establish gangliosides as a shared co-receptor for all seven BoNTs.
Fig. 7. Entry of BoNT/C and BoNT/F into hippocampal neurons requires PSG but not SV2. 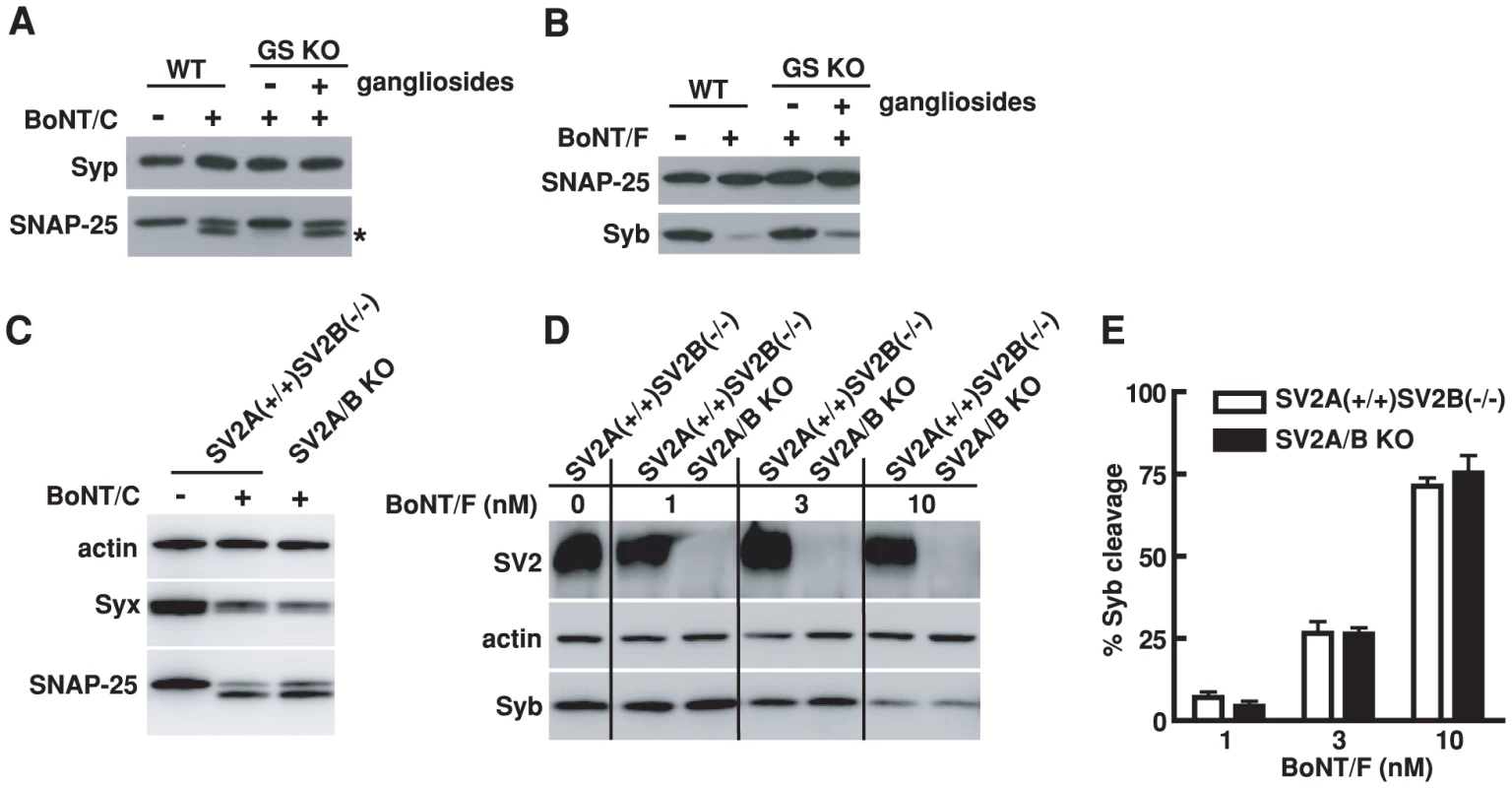
A) Experiments were carried out as described in Figure 6B, except that neurons were exposed to BoNT/C (10 nM). Cleavage of SNAP-25 by BoNT/C yielded a smaller fragment that is indicated by an asterisk. BoNT/C failed to enter GS KO neurons as shown by the lack of cleavage of SNAP-25, and exogenous gangliosides restored the entry of BoNT/C. Syp serves as a loading control. B) Experiments were carried out as described in panel A, except that neurons were exposed to BoNT/F (10 nM). BoNT/F failed to enter GS KO neurons as evidenced by the lack of cleavage of Syb; the entry was restored by adding exogenous gangliosides. SNAP-25 serves as a loading control. C) Hippocampal neurons cultured from SV2A/B KO mice and their SV2A(+/+)SV2B(−/−) littermates were exposed to BoNT/C (10 nM). In addition to SNAP-25, the second target protein of BoNT/C, Syx, was also detected. Actin serves as a loading control. The cleavage of Syx by BoNT/C resulted in loss of Syx immunoblot signals. Similar degrees of Syx and SNAP-25 cleavage by BoNT/C were observed in SV2A/B KO neurons as compared to control neurons. D) Hippocampal neurons cultured from SV2A/B KO mice and their SV2A(+/+)SV2B(−/−) littermates were exposed to a titration of BoNT/F at indicated concentrations. Lacking SV2 in hippocampal neurons did not significantly reduce the entry of BoNT/F into neurons, as shown by similar levels of Syb cleavage at each toxin concentration between SV2A/B KO neurons and control SV2A(+/+)SV2B(−/−) neurons. E) Degrees of Syb cleavage by BoNT/F assayed in panel D were quantified by densitometry and normalized to the levels of actin. Error bars represent standard deviation (N = 4). Lacking SV2, on the other hand, did not significantly reduce the cleavage of SNAP-25 and Syx by BoNT/C in cultured hippocampal neurons as compared to the control neurons that still express SV2A (Figure 7C), suggesting that BoNT/C does not share the same protein receptor requirement as BoNT/D in hippocampal neurons. We also found that the absence of SV2 did not reduce the sensitivity of hippocampal neurons to BoNT/F compared to control neurons that still express SV2A, as evidenced by the similar levels of Syb cleavage at all toxin concentrations examined (Figure 7D,E). Although many questions remain to be determined such as whether other SV2 isoforms play a role in BoNT/F entry, whether SV2 mediates BoNT/F entry into other types of neurons and whether other proteins can compensate the loss of SV2 for BoNT/F, it is clear that BoNT/F does not share the same receptor-binding mechanism with BoNT/E in hippocampal neurons.
Discussion
To identify the receptor for BoNT/D, we first determined that BoNT/D uses synaptic vesicle recycling to enter neurons. Using BoNT/D-HCR as bait, we identified synaptic vesicle protein SV2 as the toxin binding protein. Utilizing hippocampal neurons cultured from SV2A/B KO mice, we demonstrated that SV2 is essential for the binding and entry of BoNT/D into neurons and all three isoforms of SV2 can mediate the binding and entry of BoNT/D. Our key finding is that localization of over-expressed SV2C onto plasma membranes mediated the binding of BoNT/D to cell surfaces in both neurons and HEK293 cells, suggesting that the luminal domains of SV2 provide the binding site for BoNT/D and demonstrating that other synaptic vesicle proteins are not required. Together, these data establish SV2 as the protein receptor for BoNT/D.
Interestingly, BoNT/D appears to have a SV2-recognition strategy distinct from BoNT/A and BoNT/E. First, SV2-L4 domain expressed in LDLR-based or Syg-based chimeric proteins can function as the receptor for BoNT/A and E, but failed to mediate the entry of BoNT/D. Second, N573Q mutation that abolished a glycosylation site within the SV2A-L4 domain blocked the entry of BoNT/E and also reduced the entry of BoNT/A, but has no significant effect on the entry of BoNT/D. These data suggest that BoNT/D has a distinct SV2-binding mechanism that has yet to be understood. In fact, we still do not understand whether BoNT/A and BoNT/E share similar mechanisms recognizing SV2 at the molecular level. The finding that BoNT/D also uses SV2 as a receptor, together with recent progress solving the crystal structures of BoNTs and BoNT-HCRs [39], [42], [44], [78], [79], provided an opportunity for comparative studies in order to understand the molecular and structural basis for seemingly diverse SV2-binding mechanisms utilized by different BoNTs.
Among the seven BoNTs, BoNT/B and G display the highest similarity and they share Syt I/II as their receptors [9], [58]. BoNT/A and E are fairly close to each other and they share SV2 as the receptor [9]. The finding that BoNT/D also uses SV2 as the receptor, on the other hand, may not be explained by sequence similarity especially considering that the binding mechanism appears to be different from BoNT/A and E. This surprising convergence may suggest that SV2 possesses certain characteristics/functions that make it an attractive receptor candidate. One possibility is that the complex glycan structure in SV2 may facilitate the binding of toxins. It is also interesting to note that Syt is known to function as the Ca2+ sensor for triggering vesicle release and also plays an important role for maintaining the rate of synaptic vesicle endocytosis [80], [81], [82], [83], [84]. Although the function of SV2 has not been established, recent studies showed that lacking SV2 results in elevated Ca2+ levels in the presynaptic terminals and also reduces the rate of compensatory membrane retrieval after synaptic vesicle release [85]. Moreover, it has been shown that SV2 associates with Syt and may regulate the endocytosis of Syt [70], [74], [82]. The roles of Syt and SV2 in Ca2+ signaling and compensatory endocytosis, two critical functions in a vesicle cycle, may provide strategic reasons for toxins to exploit them as receptors to target recycling synaptic vesicles.
Using BoNT/A as a specific SV2 luminal domain probe, we showed that significant portions of SV2C localize onto plasma membranes when over-expressed in neurons. This is likely due to over-expression of exogenous proteins since it was not observed for endogenous SV2C (Figure S1). This phenomenon was also seen for Syt I. It has been proposed that the plasma membrane is a default destination for Syt I and its sorting to synaptic vesicles requires endocytotic sorting adaptors that could be overwhelmed by over-expressed Syt I [86]. It remains to be seen whether a similar mechanism causes the localization of over-expressed SV2C on plasma membranes.
In addition to protein receptors, we also examined the role of PSG for the binding and entry of BoNT/D in neurons utilizing GS KO mice. We showed that these ganglioside deficient mice are less sensitive to BoNT/D in vivo. We further showed that BoNT/D cannot bind and enter neurons cultured from GS KO mice; binding and entry can be restored by loading exogenous gangliosides to cell membranes. We extended these studies to BoNT/C and BoNT/F, and demonstrated that PSG are required for the functional entry of both toxins in cultured neurons. Together, these studies contribute to the growing body of evidence that PSG are a shared binding platform for all seven BoNTs.
Finally, we note that a natural chimeric toxin composed of the light chain of BoNT/C and the receptor binding domain of BoNT/D has been tested in patients [87], [88]. This toxin is designated as BoNT/C-D, but has also been marketed as a subtype of BoNT/C [9], [89]. Its receptor binding domain is identical to BoNT/D. Our studies indicate that this toxin targets neurons by recognizing SV2 and gangliosides.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Standing Committee on Animals of Harvard Medical School (Permit Number: 04619). All efforts were made to minimize suffering of animals.
Antibodies, mouse lines, toxins and other materials
Mouse monoclonal antibodies for Syb (Cl 69.1), Syt I (Syt IN Ab: Cl 604.4; Syt I cytoplasmic domain Cl 41.1), SV2 (pan-SV2), synaptophysin (Cl 7.2), syntaxin (HPC-1) and SNAP-25 (Cl 71.2) were generously provided by E. Chapman (Madison, WI). Rabbit polyclonal antibodies for BoNT/A and BoNT/B were described previously [57], [60]. Rabbit polyclonal antibodies for SV2A, B, C, and Syg were generously provided by R. Janz (Houston, TX) and were described previously [65], [90], [91]. The following antibodies were purchased from indicated vendors: mouse monoclonal anti-HA (16B12, Covance); rabbit polyclonal anti-synapsin and guinea pig anti-vesicular glutamate transporter I (vGlut-I, Millipore); chicken polyclonal anti-GFP and mouse monoclonal anti-actin (Abcam).
GM2/GD2 synthase KO mice have been previously described [76] and were obtained from the Consortium for Functional Glycomics (Grant number: GM62116). The SV2A/B knockout mice were described previously [90] and were generously provided by R. Janz.
Bovine brain gangliosides were purchased from Matreya LLC (PA). TeNT was purchased from List Biological Lab (CA). BoNT/A (Hall-A), BoNT/B (Okra), BoNT/C (Brazil), BoNT/D (D1873), BoNT/E (Alaska) and BoNT/F (Langeland) were purified in E. Johnson's lab from indicated strains.
cDNA, constructs and protein purification
Rat SV2A/B/C and Syg cDNAs were described previously [62], [63], [64], [65] and were generously provided by R. Janz. Rat Syt I cDNA were generously provided by T.C. Sudhof (Palo Alto, CA).
Lox-Syn-Syn lentivirus vector [92] was used for all constructs expressing exogenous proteins in neurons. This vector contains two separate neuronal-specific promoters (synapsin promoter). One promoter controls expression of indicated proteins and the other controls expression of EGFP.
LDLR-based chimeric receptors were generated by fusing the L4 domains of each SV2 isoform (residues 468–595 in SV2A, 410–539 in SV2B, 453–580 in SV2C) to the N-terminus of a fragment encoding the transmembrane and cytosolic domain of human LDLR-2 (residues 788-860) as described previously [51]. Syg-based chimeric receptor was constructed by inserting the SV2A-L4 domain between residue 140 (L) and 141 (N) within the second luminal domain of Syg. Deletion mutants SV2A-ΔL1 and SV2A-ΔL3 were generated by replacing residues 196-200 of L1 and 321–331 of L3 with a peptide sequence derived from the first eleven amino acids of rat Syt I [93]. This sequence can be recognized by Syt IN-Ab, which we found only recognizes rat but not mouse Syt I [51], thus serving as a tag. Point mutations at N-glycosylation sites of SV2A have been described previously [51].
The cDNA encoding the HCRs of BoNT/A and BoNT/B were generously provided by J. Barbieri (Milwaukee, WI) and were previously described [73]. The cDNA encoding the HCRs of BoNT/D (residues 859–1276, GenBank: CAA38175.1) and BoNT/E (residues 820–1252 GenBank: X62683.1) were synthesized by Geneart Inc. (Germany) with codon optimized for E. Coli expression. They were subcloned into pGEX4T vector for expression as GST fusion proteins. In addition, BoNT/D-HCR was also subcloned into pET-28 vector, with a HA-tag (YPYDVPDYA) fused to its N-terminus. This HA-tagged BoNT/D-HCR was purified as N-terminal tagged His6-fusion proteins. Both GST-fusion and His6-fusion proteins were purified as previously described [94], [95], except that the induction conditions were changed to 16°C overnight with 0.25 mM IPTG.
Brain detergent extracts, GST pull-down assay and co-immunoprecipitation
Rat brain detergent extracts were prepared by homogenizing one fresh dissected adult rat brain in 15 ml 320 mM sucrose buffer, followed by a centrifugation at 5000 rpm for 2 min at 4°C in a Sorvall SS-34 rotor. Supernatants were collected and centrifuged at 11,000 rpm for 12 min using the same rotor. The pellet was collected and solubilized for 30 min in 15 ml Tris-buffered saline (TBS: 20 mM Tris, 150 mM NaCl) plus 2% of Triton X-100 and a cocktail of protease inhibitors (Roche, CA). Samples were subsequently centrifuged at 17,000 rpm for 20 min in a Sorvall SS-34 rotor to remove the insoluble materials. The final brain detergent extracts yielded ∼2 mg/ml proteins.
GST pull-down assays were carried out using 500 µg GST fusion proteins or GST protein immobilized on glutathione-Sepharose beads, mixed with 1.5 ml rat brain detergent extracts for 1 hr at 4°C. Beads were washed three times with the washing buffer (TBS plus 0.5% Triton X-100). Ten percent of bound proteins were subjected to SDS-PAGE and immunoblot analysis following standard western blot procedures using the enhanced chemiluminescence (ECL) method (Pierce). ‘Input’ corresponds to 0.5% of total brain extracts incubated with each HCR protein.
Co-immunoprecipitation experiments were carried out by first incubating 100 nM HA-tagged BoNT/D-HCR with 0.5 ml rat brain detergent extracts for 1 hr at 4°C, and then after the addition of monoclonal anti-HA antibody (4 µl) incubating for a further 1 hr. Protein G Fast Flow beads (50 µl, GE Bioscience) were then added and incubated for additional 1 hr. The beads were washed three times in the washing buffer (TBS plus 0.5% Triton X-100). Bound proteins were analyzed by immunoblot analysis.
Neuron cultures, transfection and lentiviral infection
Rat hippocampal neurons were prepared from E18-19 embryos. Mouse hippocampal neurons were prepared from P1 mice. Dissected hippocampi were dissociated with papain following manufacture instructions (Worthington Biochemical, NJ). Cells were plated on poly-D-lysine coated glass coverslips and cultured in Neurobasal medium supplemented with B-27 (2%) and Glutamax (Invitrogen). Experiments were carried out generally using DIV (days in vitro) 12–18 neurons. Transfection of neurons was carried out using Lipofectamine 2000 (Invitrogen) at DIV5. Lentiviral particles were produced by HEK293FT (Invitrogen) cells co-transfected with the virus packaging vectors (VSV-G and Δ8.9) as described previously [92]. Viruses were added to neurons at DIV5.
Binding and entry of toxins into neurons, surface binding assays and toxin binding to HEK293FT cells
The control buffer (PBS) used in Figure 1A contains (mM: NaCl 140, KCl 3, KH2PO4 1.5, Na2HPO4 8, MgCl2 0.5). High K+ buffer is the same as the control buffer but adjusted to 56 mM KCl and 87 mM NaCl plus 1 mM CaCl2. In general, the binding of BoNT/D-HCR to neurons was assayed by incubating neurons with 80 nM BoNT/D-HCR for 5 min in high K+ buffers at 37°C. Cells were washed three times. Immunostaining was carried out by fixing cells with 4% paraformaldehyde, permeabilized with 0.3% Triton in PBS solution, and incubated with indicated primary antibodies for 1 hr at room temperature, followed by the incubation with secondary antibodies conjugated with Alexa dyes (Invitrogen, CA) for 1 hr at room temperature. Images were collected using a Leica TCS SP confocal microscope using a 40x oil objective.
Surface binding assays described in Figure 5B–D were carried out by first incubating neurons in cold media (4°C, 5 min), and then exposing them to indicated reagents in cold media on ice for 10 min. Cells were washed and fixed. Immunostaining was first carried out without the permeabilization step to detect the surface binding of BoNT/D-HCR, BoNT/A, BoNT/B, or Syt IN-Ab. Cells were subsequently permeabilized and immunostaining was carried out for the additional indicated intracellular proteins.
In Figure 5E, HEK293FT cells were growing on poly-D-lysine coated coverslips and were transfected with full-length SV2C in pCMV5 vector using Lipofectamine 2000 when cells reached 70–80% confluence. Cells were exposed to BoNT/D-HCR (80 nM) for 30 min at 37°C 48 hrs after the transfection. Cells were washed and fixed. Immunostaining was first carried out without permeabilization to detect BoNT/D-HCR. Subsequently, cells were permeabilized to detect SV2C using a polyclonal SV2C antibody. We note that the binding of BoNT/D-HCR was observed mostly in cells with round cell shapes – a morphology that becomes more prominent when cells reach complete confluence.
Functional entry of BoNTs into neurons was assayed by examining the cleavage of their substrate proteins in neurons. In general, neurons were exposed to indicated toxins for 5 min in high K+ buffers at 37°C. Neurons were washed, further incubated in toxin-free media for an additional 6 hrs, and lysed in the cell lysate buffer (PBS with 1% Triton X-100, 0.05% SDS and protease inhibitor cocktail (Roche, CA), 100 µl per one well of 24-well plates). Lysates were centrifuged for 10 min using a microcentrifuge at 4°C, and the supernatants were assayed by immunoblot analysis.
Rapid-time-to-death assay and ganglioside loading
A rapid-time-to-death assay was utilized to assess the toxicity of BoNTs in GS KO mice, following a well-established protocol as previously described [57], [77]. Briefly, the WT and KO littermates were injected with the same amount of toxins intravenously (lateral tail vein), and their time-to-death was recorded. The apparent intraperitoneal LD50/ml of toxins in each mouse (effective toxicity) was determined using a standard curve as previously described [57], [77].
The stock of gangliosides was prepared by dissolving mixed bovine brain gangliosides in Chloroform:Methanol (2 : 1) solution. They were dried in glass tubes using nitrogen gas, resuspended in Neurobasal media at 1 mg/ml, and added to culture media at 250 µg/ml concentrations for 12 hrs to load into cell membranes.
Supporting Information
Zdroje
1. SchiavoGMatteoliMMontecuccoC 2000 Neurotoxins affecting neuroexocytosis. Physiol Rev 80 717 766
2. ArnonSSSchechterRInglesbyTVHendersonDABartlettJG 2001 Botulinum toxin as a biological weapon: medical and public health management. JAMA 285 1059 1070
3. SimpsonLL 1986 Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol 26 427 453
4. MontalM 2010 Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem 79 591 617
5. SchantzEJJohnsonEA 1992 Properties and use of botulinum toxin and other microbial neurotoxins in medicine. Microbiol Rev 56 80 99
6. JohnsonEA 1999 Clostridial toxins as therapeutic agents: benefits of nature's most toxic proteins. Annu Rev Microbiol 53 551 575
7. MontecuccoCMolgoJ 2005 Botulinal neurotoxins: revival of an old killer. Curr Opin Pharmacol 5 274 279
8. DollyJOLawrenceGWMengJWangJ 2009 Neuro-exocytosis: botulinum toxins as inhibitory probes and versatile therapeutics. Curr Opin Pharmacol 9 326 335
9. HillKKSmithTJHelmaCHTicknorLOFoleyBT 2007 Genetic diversity among Botulinum Neurotoxin-producing clostridial strains. J Bacteriol 189 818 832
10. SimpsonLL 2004 Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol 44 167 193
11. SchiavoGBenfenatiFPoulainBRossettoOPolverino de LauretoP 1992 Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature 359 832 835
12. SchiavoGRossettoOCatsicasSPolverino de LauretoPDasGuptaBR 1993 Identification of the nerve terminal targets of botulinum neurotoxin serotypes A, D, and E. J Biol Chem 268 23784 23787
13. SchiavoGShoneCCRossettoOAlexanderFCMontecuccoC 1993 Botulinum neurotoxin serotype F is a zinc endopeptidase specific for VAMP/synaptobrevin. J Biol Chem 268 11516 11519
14. SchiavoGMalizioCTrimbleWSPolverino de LauretoPMilanG 1994 Botulinum G neurotoxin cleaves VAMP/synaptobrevin at a single Ala-Ala peptide bond. J Biol Chem 269 20213 20216
15. YamasakiSBinzTHayashiTSzaboEYamasakiN 1994 Botulinum neurotoxin type G proteolyses the Ala81-Ala82 bond of rat synaptobrevin 2. Biochem Biophys Res Commun 200 829 835
16. YamasakiSBaumeisterABinzTBlasiJLinkE 1994 Cleavage of members of the synaptobrevin/VAMP family by types D and F botulinal neurotoxins and tetanus toxin. J Biol Chem 269 12764 12772
17. BlasiJChapmanERLinkEBinzTYamasakiS 1993 Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature 365 160 163
18. SchiavoGSantucciADasguptaBRMehtaPPJontesJ 1993 Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett 335 99 103
19. WilliamsonLCHalpernJLMontecuccoCBrownJENealeEA 1996 Clostridial neurotoxins and substrate proteolysis in intact neurons: botulinum neurotoxin C acts on synaptosomal-associated protein of 25 kDa. J Biol Chem 271 7694 7699
20. ForanPLawrenceGWShoneCCFosterKADollyJO 1996 Botulinum neurotoxin C1 cleaves both syntaxin and SNAP-25 in intact and permeabilized chromaffin cells: correlation with its blockade of catecholamine release. Biochemistry 35 2630 2636
21. BinzTBlasiJYamasakiSBaumeisterALinkE 1994 Proteolysis of SNAP-25 by types E and A botulinal neurotoxins. J Biol Chem 269 1617 1620
22. SchiavoGShoneCCBennettMKSchellerRHMontecuccoC 1995 Botulinum neurotoxin type C cleaves a single Lys-Ala bond within the carboxyl-terminal region of syntaxins. J Biol Chem 270 10566 10570
23. BlasiJChapmanERYamasakiSBinzTNiemannH 1993 Botulinum neurotoxin C1 blocks neurotransmitter release by means of cleaving HPC-1/syntaxin. EMBO J 12 4821 4828
24. SollnerTWhiteheartSWBrunnerMErdjument-BromageHGeromanosS 1993 SNAP receptors implicated in vesicle targeting and fusion. Nature 362 318 324
25. WeberTZemelmanBVMcNewJAWestermannBGmachlM 1998 SNAREpins: minimal machinery for membrane fusion. Cell 92 759 772
26. JahnRSchellerRH 2006 SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7 631 643
27. SuttonRBFasshauerDJahnRBrungerAT 1998 Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395 347 353
28. SudhofTCRothmanJE 2009 Membrane fusion: grappling with SNARE and SM proteins. Science 323 474 477
29. MontecuccoCRossettoOSchiavoG 2004 Presynaptic receptor arrays for clostridial neurotoxins. Trends Microbiol 12 442 446
30. BinzTRummelA 2009 Cell entry strategy of clostridial neurotoxins. J Neurochem 109 1584 1595
31. SimpsonLLRapportMM 1971 Ganglioside inactivation of botulinum toxin. J Neurochem 18 1341 1343
32. KitamuraMIwamoriMNagaiY 1980 Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim Biophys Acta 628 328 335
33. KamataYKozakiSSakaguchiGIwamoriMNagaiY 1986 Evidence for direct binding of Clostridium botulinum type E derivative toxin and its fragments to gangliosides and free fatty acids. Biochem Biophys Res Commun 140 1015 1019
34. KozakiSKamataYWataraiSNishikiTMochidaS 1998 Ganglioside GT1b as a complementary receptor component for Clostridium botulinum neurotoxins. Microb Pathog 25 91 99
35. RummelAMahrholdSBigalkeHBinzT 2004 The HCC-domain of botulinum neurotoxins A and B exhibits a singular ganglioside binding site displaying serotype specific carbohydrate interaction. Mol Microbiol 51 631 643
36. YowlerBCSchengrundCL 2004 Botulinum neurotoxin A changes conformation upon binding to ganglioside GT1b. Biochemistry 43 9725 9731
37. SwaminathanSEswaramoorthyS 2000 Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat Struct Biol 7 693 699
38. KohdaTIharaHSetoYTsutsukiHMukamotoM 2007 Differential contribution of the residues in C-terminal half of the heavy chain of botulinum neurotoxin type B to its binding to the ganglioside GT1b and the synaptotagmin 2/GT1b complex. Microb Pathog 42 72 79
39. StenmarkPDupuyJImamuraAKisoMStevensRC 2008 Crystal structure of botulinum neurotoxin type A in complex with the cell surface co-receptor GT1b-insight into the toxin-neuron interaction. PLoS Pathog 4 e1000129
40. StenmarkPDongMDupuyJChapmanERStevensRC 2010 Crystal structure of the botulinum neurotoxin type G binding domain: insight into cell surface binding. J Mol Biol 397 1287 1297
41. SchmittJKaralewitzABenefieldDAMushrushDJPruittRN 2010 Structural analysis of botulinum neurotoxin type G receptor binding. Biochemistry 49 5200 5205
42. StrotmeierJLeeKVolkerAKMahrholdSZongY 2010 Botulinum neurotoxin serotype D attacks neurons via two carbohydrate binding sites in a ganglioside dependent manner. Biochem J 431 207 216
43. RummelAHafnerKMahrholdSDarashchonakNHoltM 2009 Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J Neurochem 110 1942 1954
44. KaralewitzAPKrokenARFuZBaldwinMRKimJJ 2010 Identification of a Unique Ganglioside Binding Loop within Botulinum Neurotoxins C and D-SA. Biochemistry 49 8117 8126
45. FuZChenCBarbieriJTKimJJBaldwinMR 2009 Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F. Biochemistry 48 5631 5641
46. RummelAEichnerTWeilTKarnathTGutcaitsA 2007 Identification of the protein receptor binding site of botulinum neurotoxins B and G proves the double-receptor concept. Proc Natl Acad Sci U S A 104 359 364
47. YowlerBCKensingerRDSchengrundCL 2002 Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J Biol Chem 277 32815 32819
48. ChaiQArndtJWDongMTeppWHJohnsonEA 2006 Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature 444 1096 1100
49. DongMTeppWHLiuHJohnsonEAChapmanER 2007 Mechanism of botulinum neurotoxin B and G entry into hippocampal neurons. J Cell Biol 179 1511 1522
50. BullensRWO'HanlonGMWagnerEMolenaarPCFurukawaK 2002 Complex gangliosides at the neuromuscular junction are membrane receptors for autoantibodies and botulinum neurotoxin but redundant for normal synaptic function. J Neurosci 22 6876 6884
51. DongMLiuHTeppWHJohnsonEAJanzR 2008 Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol Biol Cell 19 5226 5237
52. KitamuraMTakamiyaKAizawaSFurukawaK 1999 Gangliosides are the binding substances in neural cells for tetanus and botulinum toxins in mice. Biochim Biophys Acta 1441 1 3
53. TsukamotoKKohdaTMukamotoMTakeuchiKIharaH 2005 Binding of Clostridium botulinum type C and D neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J Biol Chem 280 35164 35171
54. MontecuccoC 1986 How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem Sci 314 317
55. NishikiTKamataYNemotoYOmoriAItoT 1994 Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem 269 10498 10503
56. NishikiTTokuyamaYKamataYNemotoYYoshidaA 1996 The high-affinity binding of Clostridium botulinum type B neurotoxin to synaptotagmin II associated with gangliosides GT1b/GD1a. FEBS Lett 378 253 257
57. DongMRichardsDAGoodnoughMCTeppWHJohnsonEA 2003 Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J Cell Biol 162 1293 1303
58. RummelAKarnathTHenkeTBigalkeHBinzT 2004 Synaptotagmins I and II act as nerve cell receptors for botulinum neurotoxin G. J Biol Chem 279 30865 30870
59. JinRRummelABinzTBrungerAT 2006 Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature 444 1092 1095
60. DongMYehFTeppWHDeanCJohnsonEA 2006 SV2 is the protein receptor for botulinum neurotoxin A. Science 312 592 596
61. MahrholdSRummelABigalkeHDavletovBBinzT 2006 The synaptic vesicle protein 2C mediates the uptake of botulinum neurotoxin A into phrenic nerves. FEBS Lett 580 2011 2014
62. BajjaliehSMPetersonKShinghalRSchellerRH 1992 SV2, a brain synaptic vesicle protein homologous to bacterial transporters. Science 257 1271 1273
63. FeanyMBLeeSEdwardsRHBuckleyKM 1992 The synaptic vesicle protein SV2 is a novel type of transmembrane transporter. Cell 70 861 867
64. BajjaliehSMPetersonKLinialMSchellerRH 1993 Brain contains two forms of synaptic vesicle protein 2. Proc Natl Acad Sci U S A 90 2150 2154
65. JanzRSudhofTC 1999 SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neuroscience 94 1279 1290
66. LacyDBStevensRC 1999 Sequence homology and structural analysis of the clostridial neurotoxins. J Mol Biol 291 1091 1104
67. DollyJOBlackJWilliamsRSMellingJ 1984 Acceptors for botulinum neurotoxin reside on motor nerve terminals and mediate its internalization. Nature 307 457 460
68. SudhofTC 2004 The synaptic vesicle cycle. Annu Rev Neurosci 27 509 547
69. TakamoriSHoltMSteniusKLemkeEAGronborgM 2006 Molecular anatomy of a trafficking organelle. Cell 127 831 846
70. BennettMKCalakosNKreinerTSchellerRH 1992 Synaptic vesicle membrane proteins interact to form a multimeric complex. J Cell Biol 116 761 775
71. SchivellAEBatchelorRHBajjaliehSM 1996 Isoform-specific, calcium-regulated interaction of the synaptic vesicle proteins SV2 and synaptotagmin. J Biol Chem 271 27770 27775
72. LazzellDRBelizaireRThakurPSherryDMJanzR 2004 SV2B regulates synaptotagmin 1 by direct interaction. J Biol Chem 279 52124 52131
73. BaldwinMRBarbieriJT 2007 Association of botulinum neurotoxin serotypes a and B with synaptic vesicle protein complexes. Biochemistry 46 3200 3210
74. YaoJNowackAKensel-HammesPGardnerRGBajjaliehSM 2010 Cotrafficking of SV2 and synaptotagmin at the synapse. J Neurosci 30 5569 5578
75. YamashitaTHashiramotoAHaluzikMMizukamiHBeckS 2003 Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc Natl Acad Sci U S A 100 3445 3449
76. LiuYWadaRKawaiHSangoKDengC 1999 A genetic model of substrate deprivation therapy for a glycosphingolipid storage disorder. J Clin Invest 103 497 505
77. BoroffDAFleckU 1966 Statistical analysis of a rapid in vivo method for the titration of the toxin of Clostridium botulinum. J Bacteriol 92 1580 1581
78. LacyDBTeppWCohenACDasGuptaBRStevensRC 1998 Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol 5 898 902
79. KumaranDEswaramoorthySFureyWNavazaJSaxM 2009 Domain organization in Clostridium botulinum neurotoxin type E is unique: its implication in faster translocation. J Mol Biol 386 233 245
80. ChapmanER 2002 Synaptotagmin: a Ca(2+) sensor that triggers exocytosis? Nat Rev Mol Cell Biol 3 498 508
81. ZhangJZDavletovBASudhofTCAndersonRG 1994 Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell 78 751 760
82. HauckeVDe CamilliP 1999 AP-2 recruitment to synaptotagmin stimulated by tyrosine-based endocytic motifs. Science 285 1268 1271
83. Nicholson-TomishimaKRyanTA 2004 Kinetic efficiency of endocytosis at mammalian CNS synapses requires synaptotagmin I. Proc Natl Acad Sci U S A 101 16648 16652
84. PoskanzerKEMarekKWSweeneySTDavisGW 2003 Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature 426 559 563
85. WanQFZhouZYThakurPVilaASherryDM 2010 SV2 acts via presynaptic calcium to regulate neurotransmitter release. Neuron 66 884 895
86. DirilMKWienischMJungNKlingaufJHauckeV 2006 Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell 10 233 244
87. EleopraRTugnoliVRossettoOMontecuccoCDe GrandisD 1997 Botulinum neurotoxin serotype C: a novel effective botulinum toxin therapy in human. Neurosci Lett 224 91 94
88. EleopraRTugnoliVQuatraleRRossettoOMontecuccoC 2006 Clinical use of non-A botulinum toxins: botulinum toxin type C and botulinum toxin type F. Neurotox Res 9 127 131
89. WebbRPSmithTJWrightPMMontgomeryVAMeagherMM 2007 Protection with recombinant Clostridium botulinum C1 and D binding domain subunit (Hc) vaccines against C and D neurotoxins. Vaccine 25 4273 4282
90. JanzRGodaYGeppertMMisslerMSudhofTC 1999 SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron 24 1003 1016
91. JanzRSudhofTCHammerREUnniVSiegelbaumSA 1999 Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron 24 687 700
92. GasconSPaez-GomezJADiaz-GuerraMScheiffelePSchollFG 2008 Dual-promoter lentiviral vectors for constitutive and regulated gene expression in neurons. J Neurosci Methods 168 104 112
93. ChapmanERJahnR 1994 Calcium-dependent interaction of the cytoplasmic region of synaptotagmin with membranes. Autonomous function of a single C2-homologous domain. J Biol Chem 269 5735 5741
94. ChapmanERAnSEdwardsonJMJahnR 1996 A novel function for the second C2 domain of synaptotagmin. Ca2+-triggered dimerization. J Biol Chem 271 5844 5849
95. LewisJLDongMEarlesCAChapmanER 2001 The transmembrane domain of syntaxin 1A is critical for cytoplasmic domain protein-protein interactions. J Biol Chem 276 15458 15465
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in ChinaČlánek HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2Článek The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.Článek Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria ParasitesČlánek KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 MotifČlánek Viral EncephalomyelitisČlánek Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 3- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The Strain-Encoded Relationship between PrP Replication, Stability and Processing in Neurons is Predictive of the Incubation Period of Disease
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Human Macrophage Responses to Clinical Isolates from the Complex Discriminate between Ancient and Modern Lineages
- Dendritic Cells and Hepatocytes Use Distinct Pathways to Process Protective Antigen from
- Spatial Distribution and Risk Factors of Highly Pathogenic Avian Influenza (HPAI) H5N1 in China
- Rhesus TRIM5α Disrupts the HIV-1 Capsid at the InterHexamer Interfaces
- HIV Integration Targeting: A Pathway Involving Transportin-3 and the Nuclear Pore Protein RanBP2
- Antigenic Variation in Malaria Involves a Highly Structured Switching Pattern
- The Stealth Episome: Suppression of Gene Expression on the Excised Genomic Island PPHGI-1 from pv.
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Novel Escape Mutants Suggest an Extensive TRIM5α Binding Site Spanning the Entire Outer Surface of the Murine Leukemia Virus Capsid Protein
- Global Functional Analyses of Cellular Responses to Pore-Forming Toxins
- Sex and Death: The Effects of Innate Immune Factors on the Sexual Reproduction of Malaria Parasites
- Lung Adenocarcinoma Originates from Retrovirus Infection of Proliferating Type 2 Pneumocytes during Pulmonary Post-Natal Development or Tissue Repair
- Botulinum Neurotoxin D Uses Synaptic Vesicle Protein SV2 and Gangliosides as Receptors
- The Moving Junction Protein RON8 Facilitates Firm Attachment and Host Cell Invasion in
- KIR Polymorphisms Modulate Peptide-Dependent Binding to an MHC Class I Ligand with a Bw6 Motif
- The Coxsackievirus B 3C Protease Cleaves MAVS and TRIF to Attenuate Host Type I Interferon and Apoptotic Signaling
- Dissection of the Influenza A Virus Endocytic Routes Reveals Macropinocytosis as an Alternative Entry Pathway
- Viral Encephalomyelitis
- Sheep and Goat BSE Propagate More Efficiently than Cattle BSE in Human PrP Transgenic Mice
- Longistatin, a Plasminogen Activator, Is Key to the Availability of Blood-Meals for Ixodid Ticks
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Dynamic Imaging of the Effector Immune Response to Infection
- The Lectin Receptor Kinase LecRK-I.9 Is a Novel Resistance Component and a Potential Host Target for a RXLR Effector
- Host Iron Withholding Demands Siderophore Utilization for to Survive Macrophage Killing
- The Danger Signal S100B Integrates Pathogen– and Danger–Sensing Pathways to Restrain Inflammation
- The RNome and Its Commitment to Virulence
- A Novel Nuclear Factor TgNF3 Is a Dynamic Chromatin-Associated Component, Modulator of Nucleolar Architecture and Parasite Virulence
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Toxin that Hijacks the Host Ubiquitin Proteolytic System
- Invasive Extravillous Trophoblasts Restrict Intracellular Growth and Spread of
- Blood Meal-Derived Heme Decreases ROS Levels in the Midgut of and Allows Proliferation of Intestinal Microbiota
- Metabolite Cross-Feeding Enhances Virulence in a Model Polymicrobial Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



