-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
article has not abstract
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002207
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1002207Summary
article has not abstract
An accurate, simple, and direct test for tuberculosis (TB) has been a priority for many years, but to date no such test has become available. Easily detectable sensitive and specific biomarkers are elusive and may remain so. In parallel to the essential extensive efforts in biomarker discovery performed in this field, we suggest it is also worthwhile to evaluate the utility of biomarkers of TB infection in a “treat-to-test” strategy. Biomarkers of TB infection already identified or detected in ongoing systematic studies that are not useful for simple near-patient tests may nonetheless be suitable in a treat-to-test strategy. Thus, we call for the investigation of the kinetics of these biomarkers during the early phase of treatment.
Background
To date no simple, rapid, accurate test for TB has been developed. An effective immunologic lateral flow assay able to test blood, urine, or conceivably sputum would be a major step forwards. However, assays based on antibody detection, although highly effective for other diseases, have a dismal record for TB diagnostics in spite of decades of dedicated research. In recent years, a selection of the then available antibody-based assays were tested and shown to have no value for the diagnosis of active TB [1]. The situation with antigen-based assays may be more promising, but even with these assays progress has been limited. Antigens with acceptable specificity have been identified and tests are available to detect them, but the sensitivity is generally low. Notably, the detection of mycobacterially derived lipoarabinomannan (LAM) in urine has been shown to be specific, but, except possibly in specific groups of patients (HIV-infected with low CD4 counts), the sensitivity is currently far too low to replace microscopy [2]. As recently reviewed by McNerney and Daley (2011) [3], there is not only a need for new biomarkers, but also new detection technologies.
Because of these obstacles, molecular methods based on DNA amplification have been increasingly developed and applied. This work has resulted in a number of highly effective and innovative assays with excellent performance [4]. Unfortunately, DNA amplification technologies are fundamentally more complex than, for example, a lateral flow-based immunological assay. Thus, providing and maintaining molecular testing where it is most needed will be a huge logistical and financial challenge.
For this reason, there remains considerable effort invested in identifying suitable human and mycobacterial biomarkers for use in a simple and rapid immunological assay. Indeed, state of the art detection and bioinformatics techniques are now being applied systematically for this purpose. Recently, Berry et al. (2010) [5] published a detailed transcriptional analysis of the human response to mycobacterial infection and identified transcriptional signatures that appear to be associated with TB infection and different stages of infection. An analysis of the dominant proteins present during different phases in a Mycobacterium tuberculosis infection model in guinea pigs was recently published by Kruh et al. (2010) [6], and proteomic analysis revealed that highly immunogenic TB antigens were released in exosomes of TB-infected macrophages [7]. Kunnath-Velayudhan et al. (2010) [8] studied the antibody response of patients at different stages of the disease and documented marked antibody target preferences between patients, as well as a correlation of the response with disease burden.
These efforts to characterize antibodies and other indicators of TB disease are essential, but the failure to date to identify a diagnostic biomarker, admittedly with less sophisticated tools, suggests to us that discovery of suitable biomarkers and the development of a useful test for near-patient diagnostic use in the immediate future is far from certain. That is why we would like to take this opportunity to call for the consideration of the investigation of a parallel pragmatic strategy that may allow the development of a clinically useful assay in a shorter period.
Discussion
We would like to suggest that the use of immunological assays be considered in a treat-to-test strategy. In this approach, TB suspects would be started on treatment empirically, and after a number of days, a test would be performed to measure mycobacterial or host biomarkers.
There is evidence available supporting this proposition. A proportion of patients beginning TB treatment suffer a so-called paradoxical response, which is presumably an immunological reaction to the burst of mycobacterial antigens released when treatment starts [9], [10].
Mattos et al. (2010) [11] recently investigated the levels of specific antibodies in patients with active TB or after 3 or 6 months of treatment. They identified an increase in serum levels of antibodies against an intracellular antigen (the 16-KDa alpha crystallin) during therapy in many patients. This antibody response presumably followed the release of intracellular mycobacterial antigens as a result of the initial wave of bacterial killing. Indeed, it has been established that upon initiation of appropriate TB therapy, the majority of the mycobacteria present are killed in the first few days of therapy [12].
Measurement of antigens during the peak of bacterial killing (Figure 1), and thus antigen release, would in principle reduce the analytical sensitivity required for direct testing at presentation.
Fig. 1. Schematic representation of the expected release of TB antigens and consequential host antibody response upon initiation of treatment. 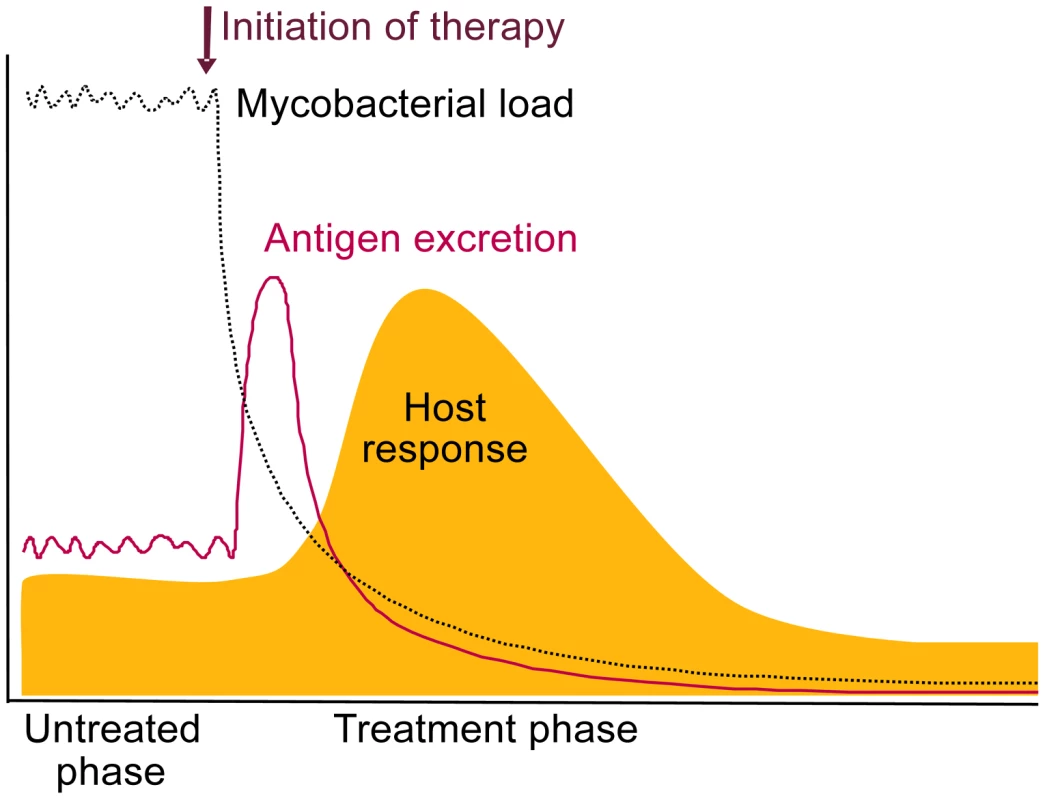
We propose that measurement of these increased levels can be useful for diagnosing TB in a treat-to-test strategy. We also expect antibody titers to rapidly increase upon release of TB antigens, as the immune system will have been primed during initial infection (Figure 1). A relative increase in anti-TB antibody levels occurring after a short treatment period would also be indicative of true infection and effective therapy. The shift towards antibodies against intracellular TB antigens shown by Mattos et al. (2010) [11] during successful therapy strongly supports this. The timing of this response is yet to be determined and would be most interesting diagnostically if changes are detectable within 1 or 2 weeks. As antibody profiles of TB patients have been shown to vary considerably between patients [8], antibody testing may not be straightforward and may require multiplex testing.
Currently, due to the lack of better diagnostic tools, TB suspects are often empirically prescribed a short course of broad spectrum antibiotics in an attempt to rule out TB. A presumptive diagnosis of TB is made only if the symptoms remain. This “rule-out” strategy causes further delay for TB patients requiring anti-TB treatment. Also, due to the frequent inappropriate prescription of fluoroquinolones as broad spectrum antibiotics, there is a serious danger of increased resistance to fluoroquinolones, which are valuable second-line TB drugs [13]. Increased fluoroquinolone resistance has already been detected in India [14] and other countries. Our suggested “rule-in” strategy would avoid treatment delay and avoid possible mono-therapy with potential second-line drugs but would result in a number of non-TB patients receiving a few doses of multidrug TB treatment for testing.
In many locations, a significant proportion of patients are started on a full course of TB therapy on the basis of clinical suspicion without bacteriological confirmation. This occurs both in high-income and low-income settings [15]–[18], and only in some cases after a long period is therapy reevaluated.
We are aware that our suggested treat-to-test strategy is not the ideal “rapid” test, and, for example, would likely fail in patients with multidrug-resistant (MDR) or monoresistant TB strains, as these may not be differentiated from non-TB patients. Additionally, in some situations patients may be infected with multiple genotypes [19], one of which is MDR, and in this case initial killing of the sensitive isolate may result in a positive signal for this type of test. However, it should be noted that this type of mixed infection is also not easily identified using molecular methods. It was recently reported that the Xpert MTB/RIF assay requires between 65% and 100% of the DNA present to be derived from a resistant isolate to detect rifampicin resistance [20].
Nonetheless, a test that could detect treatment response, if applied in a different way, is in fact highly desirable, as patients known to be infected with TB but receiving ineffective therapy could be identified. It could also be envisioned that the extent of killing is reflected in the biomarker response, and susceptibility to only one or two drugs in the cocktail results in an intermediate response. This would ultimately mean that a biomarker response could be used to classify patients as either having susceptible TB, (M)DR-TB, or no TB. Thus, a negative result or weak response in a treat-to-test strategy could also be used to initiate further investigation for other diseases as well as testing for MDR-TB.
As a treat-to-test strategy would involve an additional visit to a diagnostic centre, as opposed to one-stop microscopy [21] or a true rapid near-patient test, there remains a risk of dropout during the diagnostic procedure. This is also true for culture and microscopy performed on multiple samples. And it should be noted that the use of broad spectrum antibiotics—particularly as agents are often inappropriately selected with activity against M. tuberculosis—has been shown to result in a delay in starting multidrug treatment [22], as well as inevitably some loss to follow up.
In summary, although a true direct test for TB remains a priority, biomarkers identified in ongoing systematic studies may not in the short term lead to assays with the required sensitivity and specificity. Nonetheless, we believe some of these biomarkers may be suitable in a treat-to-test strategy, so we encourage measurement of their kinetics during the early phase of treatment.
Zdroje
1. SteingartKRHenryMLaalSHopewellPCRamsayA 2007 Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med 4 e202 doi:10.1371/journal.pmed.0040202
2. DhedaKDavidsVLendersLRobertsTMeldauR 2010 Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS ONE 5 e9848 doi:10.1371/journal.pone.0009848
3. McNerneyRDaleyP 2011 Towards a point-of-care test for active tuberculosis: obstacles and opportunities. Nat Rev Microbiol 9 204 213
4. BoehmeCCNabetaPHillemannDNicolMPShenaiS 2010 Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363 1005 1015
5. BerryMPGrahamCMMcNabFWXuZBlochSA 2010 An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 466 973 977
6. KruhNATroudtJIzzoAPrenniJDobosKM 2010 Portrait of a pathogen: the Mycobacterium tuberculosis proteome in vivo. PLoS ONE 5 e13938 doi:10.1371/journal.pone.0013938
7. GiriPKKruhNADobosKMSchoreyJS 2010 Proteomic analysis identifies highly antigenic proteins in exosomes from M. tuberculosis-infected and culture filtrate protein-treated macrophages. Proteomics 10 3190 3202
8. Kunnath-VelayudhanSSalamonHWangHYDavidowALMolinaDM 2010 Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc Natl Acad Sci U S A 107 14703 14708
9. ChengVCHoPLLeeRAChanKSChanKK 2002 Clinical spectrum of paradoxical deterioration during antituberculosis therapy in non-HIV-infected patients. Eur J Clin Microbiol Infect Dis 21 803 809
10. BreenRASmithCJBettinsonHDartSBannisterB 2004 Paradoxical reactions during tuberculosis treatment in patients with and without HIV co-infection. Thorax 59 704 707
11. MattosAMAlmeida CdeSFrankenKLAlvesCCAbramoC 2010 Increased IgG1, IFN-gamma, TNF-alpha and IL-6 responses to Mycobacterium tuberculosis antigens in patients with tuberculosis are lower after chemotherapy. Int Immunol 22 775 782
12. JindaniADoréCJMitchisonDA 2003 Bactericidal and sterilizing activities of anti-tuberculosis drugs during the first 14 days. Am J Respir Crit Care Med 167 1348 1354
13. SterlingTR 2004 The WHO/IUATLD diagnostic algorithm for tuberculosis and empiric fluoroquinolone use: potential pitfalls. Int J Tuberc Lung Dis 8 1396 1400 Review
14. AgrawalDUdwadiaZFRodriguezCMehtaA 2009 Increasing incidence of fluoroquinolone-resistant Mycobacterium tuberculosis in Mumbai, India. Int J Tuberc Lung Dis 13 79 83
15. TariqSMTariqS 2004 Empirical treatment for tuberculosis: survey of cases treated over 2 years in a London area. J Pak Med Assoc 54 88 95
16. LawnSDAylesHEgwagaSWilliamsBMukadiYD 2011 Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis 15 287 295
17. PekWYCheeCBWangYT 2002 Bacteriologically-negative pulmonary tuberculosis–the Singapore tuberculosis control unit experience. Ann Acad Med Singapore 31 92 96
18. LeeCHKimWJYooCGKimYWHanSK 2005 Response to empirical anti-tuberculosis treatment in patients with sputum smear-negative presumptive pulmonary tuberculosis. Respiration 72 369 374
19. MallardKMcNerneyRCrampinACHoubenRNdlovuR 2010 Molecular detection of mixed infections of Mycobacterium tuberculosis strains in sputum samples from patients in Karonga District, Malawi. J Clin Microbiol 48 4512 4518
20. BlakemoreRStoryEHelbDKopJBanadaP 2010 Evaluation of the analytical performance of the Xpert MTB/RIF assay. J Clin Microbiol 48 2495 2501
21. RamsayAYassinMACambanisAHiraoSAlmotawaA 2009 Front-loading sputum microscopy services: an opportunity to optimise smear-based case detection of tuberculosis in high prevalence countries. J Trop Med 2009 398767
22. DooleyKEGolubJGoesFSMerzWGSterlingTR 2002 Empiric treatment of community-acquired pneumonia with fluoroquinolones, and delays in the treatment of tuberculosis. Clin Infect Dis 34 1607 1612
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



