-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
Since 2007, many cases of fever, thrombocytopenia and leukopenia syndrome (FTLS) have emerged in Henan Province, China. Patient reports of tick bites suggested that infection could contribute to FTLS. Many tick-transmitted microbial pathogens were tested for by PCR/RT-PCR and/or indirect immunofluorescence assay (IFA). However, only 8% (24/285) of samples collected from 2007 to 2010 tested positive for human granulocytic anaplasmosis (HGA), suggesting that other pathogens could be involved. Here, we used an unbiased metagenomic approach to screen and survey for microbes possibly associated with FTLS. BLASTx analysis of deduced protein sequences revealed that a novel bunyavirus (36% identity to Tehran virus, accession: HQ412604) was present only in sera from FTLS patients. A phylogenetic analysis further showed that, although closely related to Uukuniemi virus of the Phlebovirus genus, this virus was distinct. The candidate virus was examined for association with FTLS among samples collected from Henan province during 2007–2010. RT-PCR, viral cultures, and a seroepidemiologic survey were undertaken. RT-PCR results showed that 223 of 285 (78.24%) acute-phase serum samples contained viral RNA. Of 95 patients for whom paired acute and convalescent sera were available, 73 had serologic evidence of infection, with 52 seroconversions and 21 exhibiting a 4-fold increase in antibody titer to the virus. The new virus was isolated from patient acute-phase serum samples and named Henan Fever Virus (HNF virus). Whole-genome sequencing confirmed that the virus was a novel bunyavirus with genetic similarity to known bunyaviruses, and was most closely related to the Uukuniemi virus (34%, 24%, and 29% of maximum identity, respectively, for segment L, M, S at maximum query coverage). After the release of the GenBank sequences of SFTSV, we found that they were nearly identical (>99% identity). These results show that the novel bunyavirus (HNF virus) is strongly correlated with FTLS.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002369
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002369Summary
Since 2007, many cases of fever, thrombocytopenia and leukopenia syndrome (FTLS) have emerged in Henan Province, China. Patient reports of tick bites suggested that infection could contribute to FTLS. Many tick-transmitted microbial pathogens were tested for by PCR/RT-PCR and/or indirect immunofluorescence assay (IFA). However, only 8% (24/285) of samples collected from 2007 to 2010 tested positive for human granulocytic anaplasmosis (HGA), suggesting that other pathogens could be involved. Here, we used an unbiased metagenomic approach to screen and survey for microbes possibly associated with FTLS. BLASTx analysis of deduced protein sequences revealed that a novel bunyavirus (36% identity to Tehran virus, accession: HQ412604) was present only in sera from FTLS patients. A phylogenetic analysis further showed that, although closely related to Uukuniemi virus of the Phlebovirus genus, this virus was distinct. The candidate virus was examined for association with FTLS among samples collected from Henan province during 2007–2010. RT-PCR, viral cultures, and a seroepidemiologic survey were undertaken. RT-PCR results showed that 223 of 285 (78.24%) acute-phase serum samples contained viral RNA. Of 95 patients for whom paired acute and convalescent sera were available, 73 had serologic evidence of infection, with 52 seroconversions and 21 exhibiting a 4-fold increase in antibody titer to the virus. The new virus was isolated from patient acute-phase serum samples and named Henan Fever Virus (HNF virus). Whole-genome sequencing confirmed that the virus was a novel bunyavirus with genetic similarity to known bunyaviruses, and was most closely related to the Uukuniemi virus (34%, 24%, and 29% of maximum identity, respectively, for segment L, M, S at maximum query coverage). After the release of the GenBank sequences of SFTSV, we found that they were nearly identical (>99% identity). These results show that the novel bunyavirus (HNF virus) is strongly correlated with FTLS.
Introduction
In May 2007, a county hospital in Xinyang City, Henan Province treated three patients with fever, abdominal pain, bloating, nausea, vomiting, gastrointestinal bleeding, and elevated aminotransferases. The local hospital diagnosed the disease as acute gastroenteritis. A family member of one patient reported the disease to the Henan Center for Disease Control and Prevention (CDC), which sent a team to investigate. The investigation revealed that the disease had the following characteristics: (1) acute onset with fever; (2) low white blood cell and platelet counts; (3) high levels of alanine and aspartate transaminases; (4) positive urine protein. On the basis of these features, the Henan CDC excluded the possibility of gastrointestinal disorders. In order to identify the disease etiology, the Henan CDC team used the above clinical characteristics as the case definition to search for similar cases in local hospitals in this and neighboring counties, while establishing a disease surveillance system that required all medical institutions to report cases that met the above case definition. Altogether, 79 cases were found in 2007 in Henan, with 10 fatalities (case fatality rate, 12.7%). All patients were farmers and resided in mountainous or hilly villages, and many had reported tick bites 7–9 days before illness, further suggesting an infectious etiology. In recent years, patients with similar clinical symptoms were reported with human granulocytic anaplasmosis (HGA; Anaplasma phagocytophilum) in neighboring Anhui province [1]. In 2005, there was an epidemic of Tsutsugamushi (scrub typhus/Orientia tsutsugamushi) in this area [2]. Clinical investigations, epidemiological analyses, and laboratory testing prompted consideration of rickettsial diseases as possible causes, including HGA, human monocytic ehrlichiosis (HME; Ehrlichia chaffeensis), and Tsutsugamushi disease. Specific methods such as polymerase chain reaction (PCR) and immunofluorescence assays (IFAs) for these pathogens were then used to determine if these cases were attributable to HGA or HME [3], [4]. However, only 18 of 79 (22.7%) patients were positive for A. phagocytophilum based on serology and DNA testing. Thus, the disease was initially considered at least partly caused by A. phagocytophilum, and cases were provisionally diagnosed as suspected HGA based on clinical and epidemiological data [1]–[3], [5]–[7]. In the 3 years since 2007, 206 suspected cases have been discovered in Henan, but there was only a very low positive rate of A. phagocytophilum confirmation (6 of 206 patients) and no pathogen was isolated. Similar cases were also reported in the mountainous and hilly areas of nearby Shandong, Jiangsu, Hubei and Anhui provinces, indicating that the disease already existed for some time and was widely distributed [7]. We decided to address the possible causative pathogen underlying this infection.
On the basis of epidemiological and clinical characteristics, we considered two types of diseases to be possible: rickettsial and arthropod-borne viral disease. Because of the low rates of A. phagocytophilum (rickettsial disease) detection, the research team intensified its virus search to take into account arthropod-borne viruses, including Flaviviridae (Dengue viruses [DENV], Japanese encephalitis virus [JEV]), Togaviridae (Chikungunya virus, Eastern equine encephalitis virus [EEEV], Western equine encephalitis virus), and Bunyaviridae (Crimean-Congo hemorrhagic fever virus, Hantaan virus, and Rift valley fever virus) [8]. Specific PCR assays for these viruses were used [9]–[16]. However, none of the patients from 2007 to 2010 was positive for these viruses, suggesting a new infectious agent, possibly a virus, remained to be discovered. Thus, the syndrome was considered an emerging infectious disease and was named fever, thrombocytopenia and leukopenia syndrome (FTLS). To identify the etiology, the research team adopted the following strategy: 1) sequencing of randomly amplified cDNA/DNA from FTLS patient samples using high-throughput Illumina sequencing to specifically explore viral communities present in patients suffering from FTLS, 2) PCR detection of target DNA directly from clinical specimens, 3) viral culture, 4) immunodetection methods, and 5) electron microscopic study of the morphology of the cultured virus.
Culture followed by serological and molecular tests is a standard approach for identifying an unknown virus. However, culture of an unknown virus is time-consuming, even taking several years to confirm a novel infection like HIV [17]. Otherwise, virus culture often fails because of the lack of cell lines capable of supporting propagation of viruses (e.g., hepatitis B and C virus). Methods for cloning nucleic acids of microbial pathogens directly from clinical samples offer opportunities for pathogen discovery, thereby laying the foundation for future studies aimed at assessing whether novel or unexpected viruses play a role in disease etiology. Random PCR and subtractive cloning sequencing have identified previously unknown pathogens as etiological agents of several acute and chronic infectious diseases [18], [19]. Recently, high-throughput sequencing approaches have been used for pathogen detection and discovery in clinical samples [20]–[22]. We also developed a method for exploring viruses, both known and novel, using high-throughput Illumina sequencing. In this study, high-throughput Illumina sequencing was applied to specifically explore the viral communities in patients with FTLS, using healthy subjects as controls. Here, we provide evidence for the discovery of a novel bunyavirus associated with FTLS through high-throughput sequencing. Subsequent culture of the virus and PCR detection of the specific virus in patient specimens confirmed these findings.
Materials and Methods
Ethics statement
This research was approved by the Review Board of the Center for Disease Control and Prevention of Henan Province, the Review Board of the Center for Disease Control and Prevention of Xinyang city, the Review Board of Beijing Institute of Genomics, the Review Board of Beijing Genomics Institute in Shenzhen, and the Review Board of the Institute of Microbiology and Epidemiology. All participants gave written informed consent for use of their samples in research.
Biosafety
Given the serious nature of FTLS, it was decided to handle all clinical specimens and perform all experiments involving live virus in a biosafety level-3 (BSL-3) facility.
Clinical samples
We studied 285 Henan province patients with FTLS whose samples were submitted to the Henan Province CDC between May 2007 and July 2010. Acute-phase serum samples from all patients were collected. Paired convalescent sera were available from 95 patients.
Diagnostic testing of sera for microbial agents possibly related to FTLS
Sera were tested by reverse transcription (RT)-PCR, PCR, and/or indirect IFA serological assays for a number of microbial agents, including A. phagocytophilum, Ehrlichia chaffeensis, Dengue fever virus, Japanese encephalitis virus, Chikungunya virus, Eastern equine encephalitis virus, Western equine encephalitis virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, Sandfly fever Naples Sabin virus, and Hantavirus [3]–[6], [9]–[16], [23]. Antigen slides for diagnosis of HGA (A. phagocytophilum) were purchased from Focus Diagnostics (IF1450G, CA, USA). Antigen slides for diagnosis of other pathogens were prepared by our laboratories. Fluorescein isothiocyanate (FITC)-conjugated goat anti-human IgG (Fc) was purchased from Sihuan Sci-Technics Company (Beijing, China).
Sample processing for Illumina sequencing
Equal quantities (100 µL) of acute-phase sera from 10 FTLS patients who had a history of tick bite were pooled and centrifuged at 1000 x g for 10 minutes. The supernatant was collected for DNA and RNA extraction. The same was done for 10 sera from healthy subjects (control).
DNA and RNA extraction
DNA was extracted from 140 µL of each sample using the QIAamp DNA mini Kit (Qiagen, 51304) according to the manufacturer's instructions. DNA was eluted from the columns with 50 µL water containing 20 µg/mL RNaseA. After incubation at 37°C for 15 minutes to eliminate RNA, DNA was used immediately or stored at −80°C.
Total RNA was extracted from 140 µL of each sample using the QIAamp viral RNA mini Kit (Qiagen, 52904) according to the manufacturer's instructions. RNA was eluted from the columns with 50 µL of diethyl pyrocarbonate (DEPC)-treated water containing 1 U DNaseI. Samples were incubated at 37°C for 15 minutes to eliminate human DNA, followed by DNase inactivation at 95°C for 10 minutes. RNA was used immediately or stored at −80°C.
Reverse transcription
cDNA was synthesized from 6 µL of RNA by reverse transcription at 45°C for 50 minutes in a 20-µL solution containing 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, 100 ng of random hexamer primers, 200 U of Superscript II (Invitrogen, 18064–014), 25 U of RNasin (Promega, N2511), and 0.5 mM dNTPs.
Random hexamer PCR
Random hexamer PCR was carried out in a 25-µL mixture containing 4 µL of cDNA or DNA, 10 mM Tris-HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 100 µM dNTPs, 1 U Taq DNA Polymerase (Promega, M1661) and 100 ng of random hexamer primers containing a linker (5'-GCCGGAGCTCTGCAGAATTCNNNNNN-3'). After denaturing at 95°C for 5 minutes, targets were amplified by 45 cycles of 95°C for 30 seconds, 40°C for 30 seconds, 50°C for 30 seconds, and 72°C for 90 seconds. The amplified products were detected by agarose gel electrophoresis. Pure water was used as a negative control.
High-throughput sequencing by the Illumina method
FTLS patient and control genomic DNA/cDNA libraries were constructed according to the manufacturer's instructions (Illumina). In brief, RT-PCR and PCR products were roughly quantified by UV absorption and equal amounts of each sample were mixed. Nucleic acids within the mixtures were sheared by sonication, and fragments in the 150–180-bp range were collected by cutting bands from an agarose gel after electrophoresis. The sheared DNA and cDNA ends were repaired using Klenow DNA polymerase, after which 5' termini were phosphorylated and 3' termini were polyadenylated. The adaptors were added, PCR enrichment was performed, and 150–180-bp fragments were collected for sequencing by the Illumina method.
The sequencing procedure was performed according to the manufacturer's instructions (Illumina). In this process, template library DNA was hybridized to the surface of the flow cells and multiple copies of DNA were made to form clusters using the Illumina cluster station. Workflow steps included template hybridization, isothermal amplification, linearization, and final denaturation and hybridization of sequencing primers. Paired-end sequencing (100 cycles) was performed using a four-color DNA Sequencing-By-Synthesis (SBS) technology following the manufacturer's instructions.
Short Oligonucleotide Analysis Package (SOAP) was used to handle the large amounts of short reads generated by parallel sequencing [24]. Briefly, after filtering out highly repetitive sequences and adaptor sequences, the overlapping datasets between FTLS and healthy subjects were analyzed by subtracting fragments that mapped to both host genomic-plus-transcript and bacteria databases. The non-redundant reads were mapped onto a virus database downloaded from NCBI (ftp://ftp.ncbi.nih.gov/genbank/). The resulting alignments were filtered to identify unique sequences by examining alignment (identity ≥80%) and E-value scores (e≤10−2). Filtered unique alignments were examined in the taxonomy database (NCBI) using a custom software application written in Perl (BioPerl version 5.8.5). Unmapped reads were examined in GenBank nucleic acid and protein databases using BLASTn and BLASTx, respectively [25]–[27]. Unique alignments were examined in the taxonomy database (NCBI). Sequences without hits were placed in the ‘‘unassigned’’ category. Sequences were phylotyped as human, bacterial, phage, viral, or other based on the identity of the best BLAST hit. Considering misannotation and low-complexity for Illumina short reads, sequences assigned to the same virus family were further assembled into contigs with Velvet 1.1.04 (K-mer length = 21; coverage cutoff: default 0; Insert length: PE only; minor contig length: 42) for direct comparison with GenBank nucleic acid databases using BLASTn [28]. Contigs were also blasted with GenBank protein databases using BLASTx [28]. An E-value cutoff of 1×10−5 was applied to both BLASTn and BLASTx analyses. Sequences phylotyped as viral were placed in the “viral” category.
RT-PCR detection of the bocavirus
Our mass sequencing data revealed that some sequences showed possible infection with human bocavirus, which belongs to the Parvoviridae family. PCR performed to detect human bocavirus tentatively identified in sera from FTLS patient samples amplified a 291-bp fragment of the NS1 gene, as described previously [29]. Amplified products were detected by agarose gel electrophoresis and sequenced using an ABI 3730 DNA Sequencer.
RT-PCR detection of the novel bunyavirus
Our mass sequencing data revealed a 168-bp sequence (C361, Accession: HQ412604) indicating the possible presence of a novel virus with closest identity (i.e., lowest E-value) to Tehran virus which belongs to the Phlebovirus genus of the Bunyaviridae family. We developed a PCR strategy based on the 168-bp sequence identified in sera from FTLS patient samples to detect the novel bunyavirus using forward (PF: 5'-GAC ACG CTC CTC AAG GCT CT-3') and reverse (PR: 5'-GCC CAG TAG CCC TGA GTT TC-3') primers designed with Primer3 (Supplementary Figure S1). PCR was carried out in a 25-µL mixture containing 4 µL of cDNA, 10 mM Tris–HCl (pH 8.4), 50 mM KCl, 2.5 mM MgCl2, 100 µM dNTPs, 1 U Taq Pol (Promega, M1661), 0.25 µM forward primer, and 0.25 µM reverse primer. Thermocycling conditions were as follows: 95°C for 4 minutes (denaturation), followed by 35 cycles of 94°C for 30 seconds, 54°C for 30 seconds, and 72°C for 30 seconds. Amplified products were detected by agarose gel electrophoresis and sequenced using an ABI 3730 DNA Sequencer.
Virus isolation
The Vero E6 cell line (African green monkey kidney cell) was selected for isolation of the novel bunyavirus associated with FTLS because it supports the growth of many bunyaviruses [30], [31]. Vero E6 cell lines were inoculated with six serum samples that contained novel bunyavirus RNA. Each sample underwent at least three cell culture passages in Vero E6 cell line before being considered negative. Medium was replenished on day 7, and cultures were terminated 14 days after inoculation. All cultures were observed daily for cytopathic effect (CPE). Virus-infected cells and uninfected cells were also examined for the novel bunyavirus by RT-PCR at each passage. Vero cell cultures with obvious CPE and containing novel bunyavirus RNA were further analyzed by morphology, genome sequencing, and serology.
Transmission electron microscopy
Cells showing CPE and containing novel bunyavirus RNA were collected for thin-section electron microscopy. After discarding the culture supernatant, virus-infected cells (50 mL) were mixed 1∶1 with 4% glutaraldehyde (paraformaldehyde), placed onto Formvar-carbon-coated grids, and stained with 1% methylamine tungstate. Specimens for thin-section electron microscopy were prepared by dehydrating washed cell pellets with serial dilutions of acetone and embedding in epoxy resin. Ultrathin sections were cut on an Ultracut LKBV ultramicrotome, stained with uranyl acetate and lead citrate, and examined under a transmission electron microscope (JEM-1400).
Genome sequencing
The medium from 20 mL of novel bunyavirus-infected Vero E6 cells was centrifuged at 1,000 x g for 10 minutes and then at 4,000 x g for 10 minutes, after which the supernatant was collected. PEG8000 was added to the supernatant at a final concentration of 10% (w/v) followed by centrifugation at 20,000 x g for 2 hours. The pellet was resuspended in 2 mL 1× phosphate-buffered saline (PBS) for RNA extraction. Random RT-PCR was performed, and the products (500–1500 bps) were collected and ligated into the pGEM-T vector (Promega, A3600) by incubating overnight at 16°C. Escherichia coli JM109 were transformed with the ligation mixture and cultured on LB agar containing X-gal. White clones were sequenced using an ABI 3730 DNA Sequencer. Contaminating human and extraneous sequences were eliminated using CrossMatch, and the complete sequence was assembled using Phred-Phrap-Consed [32]. Bridge RT-PCR was employed for gap-closure.
Phylogenetic analysis
Phylogenetic analyses were performed using the neighbor-joining method in the MEGA software package, version 4.0.2 [33]. Available nucleotide or protein sequences from known viruses were obtained from GenBank for inclusion in the phylogenetic trees. Selected sequences from GenBank included those with the greatest similarity to the sequence read in question based on BLAST alignments as well as representative sequences from all major taxa within the relevant Bunyaviridae family. To further establish the relationships between the new virus and the members of the Phleboviruses genus, we included all sequences for phleboviruses available in GenBank. Branching orders of the phylograms were verified statistically by resampling the data 1,000 times in a bootstrap analysis with the branch-and-bound algorithm, as applied in MEGA.
Serologic detection by indirect IFA
After successful isolation of the novel bunyavirus, we developed an indirect IFA to detect specific antibodies in patient serum specimens, as previously described [4]. In brief, monolayers of virus-infected Vero E6 cells showing CPE and containing novel bunyavirus RNA were harvested, and one volume of infected cells was mixed with 0.5 volumes of non-infected cells. The mixture was centrifuged at 1,000 x g for 10 minutes, after which cells were resuspended in 1× PBS, spotted onto 12-well glass slides, and fixed with acetone for 10 minutes. Sera from patients with FTLS (including 285 acute-phase samples and 95 paired sera), patients with respiratory diseases (80 serum samples), and healthy subjects (50 serum samples) were applied to the cells. Samples (diluted 1∶20 in PBS) were screened by first spotting 50 µL of each serum sample per well and incubating for 30 minutes at 37°C. After washing for 10 minutes in PBS, 20 µL of FITC-conjugated goat anti-human IgG (Sihuan Sci-Technics Company, Beijing, China) diluted 1∶40 in buffer containing Evans blue was added to each well and incubated for 30 minutes. After washing, slides were mounted in glycerin and examined by immunofluorescence microscopy. A titer of 1∶20 was considered positive.
Results
Epidemiology and clinical features of the disease
All 285 patients with FTLS were from the Henan Province of China and were provisionally diagnosed as suspected HGA on the basis of similar clinical manifestations [5], [7]. They represented four different epidemiologically linked sporadic cases and a few clusters of cases including 79 patients in 2007, seven patients in 2008, 47 patients in 2009, and 152 patients in 2010. The patients presented mainly between April and October, peaking in April-May during the tea-picking season in Henan. All patients resided in mountainous and hilly rural areas. In our study, 238 of 285 patients tested positive for novel bunyavirus infection by RT-PCR and/or IFA.
Epidemiology and clinical features of the disease in laboratory-confirmed patients
The median age of patients was 57.2 years (range, 23–88) and the male-to-female ratio was 1 to 2.27; 219 patients (92.02%) were farmers and 19 (7.98%) were workers or students. Among patients, 52 (21.85%) reported a tick bite within 2 weeks (5–14 days) before the onset of clinical manifestations; the remaining patients did not recall receiving a tick bite.
The main clinical features in confirmed patients included sudden onset of fever (>37.5°C −40°C) lasting up to 10 days, fatigue, anorexia, headache, myalgia, arthralgia, dizziness, enlarged lymph nodes, muscle aches, vomiting and diarrhea, upper abdominal pain, and relative bradycardia (Table 1). A small number of cases suffered more severe complications, including hypotension, mental status alterations, ecchymosis, gastrointestinal hemorrhage, pulmonary hemorrhage, respiratory failure, disseminated intravascular coagulation, multiple organ failure, and/or death. Most patients had a good outcome, but elderly patients and those with underlying diseases, neurological manifestations, coagulopathy, or hyponatremia tended to have a poorer outcome.
Tab. 1. Symptoms of 238 patients with FTLS at presentation. 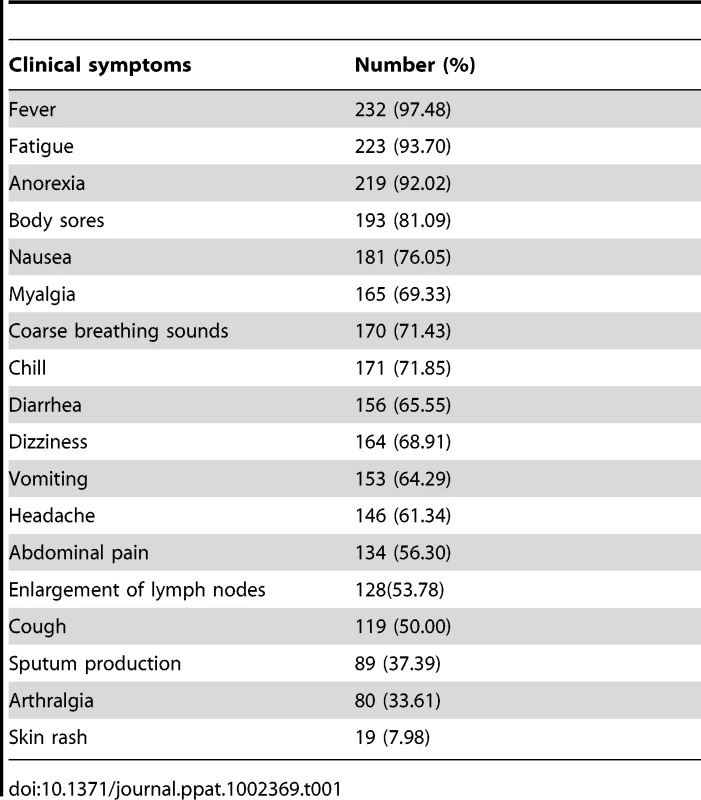
Laboratory tests showed that confirmed patients characteristically developed thrombocytopenia, leukopenia, proteinuria, and elevated serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (Table 2). Biochemical tests revealed generally higher levels of lactate dehydrogenase, creatine kinase, AST and ALT enzymes, especially AST.
Tab. 2. Initial laboratory findings of 238 patients with FTLS. 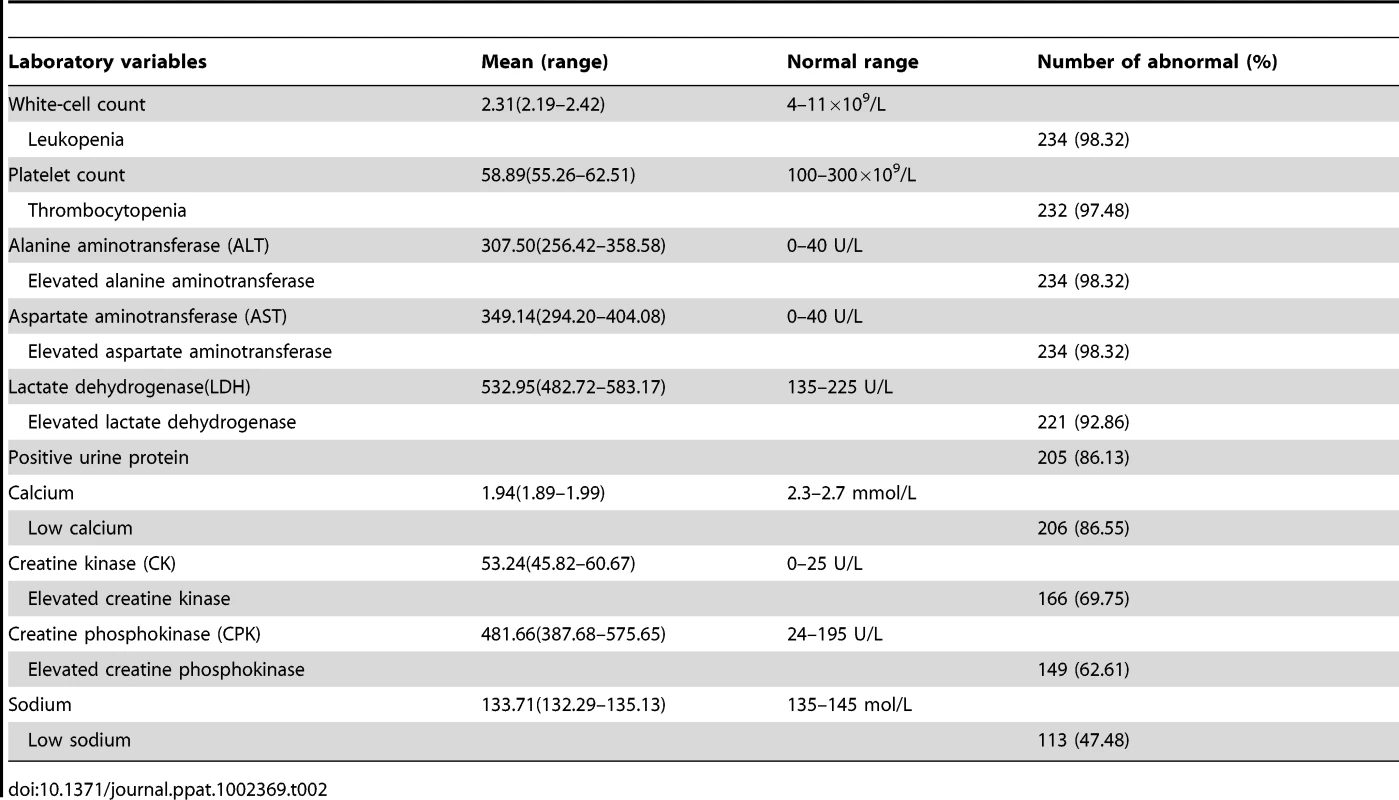
Metagenomic analysis and identification of unknown pathogen
One lane for each of the two sample pools (FTLS and healthy controls), each consisting of 10 samples, was sequenced. The proportions of high-quality sequences in FTLS and control pools were 98.08% (10,198,407/10,397,161) and 97.88% (10,250,809/10,472,315), respectively. Unique, high-quality sequence reads were then classified into broad taxonomic groups based on the taxonomy of the most frequent top-scoring BLAST matches for each sequence. Virus sequences constituted 0.0065% (67,969) and 0.0048% (50,677) of the reads in FTLS and control libraries, respectively (Table 3). After contig assembly, the number of sequences phylotyped as “viral” decreased to 3,163 and 2,412, respectively, in the two pools (Table 4). To screen for possible viruses, we focused exclusively on viruses that were present in patient sera.
Tab. 3. Categorization of sequence reads based on SOAP criteria. 
Criteria for SOAP including ID≥80%, E-value≤1e-2 and Match query≥0.7. For detection of known viruses, non-redundant reads were directly aligned with the GenBank database of nucleic acids using BLASTn software. Some sequences from Adenoviridae (human adenovirus), Herpesviridae (herpesvirus), Papillomaviridae (papillomavirus) and Retroviridae (human endogenous retrovirus) were detected in both FTLS patients and healthy subject samples. Some hepatitis B virus (HBV) and human bocavirus sequences were detected only in patient sera, indicating the presence of HBV and human bocavirus infections among these patients. To detect novel viruses, we examined sequence data against the GenBank protein database using BLASTx. An analysis of the deduced protein sequences revealed four different virus families in sera from FTLS patients (Table 4). Among these were viruses from Hepadnaviridae, which are not known to cause FTLS; Torque teno virus (TTV) from Anelloviridae, which has been reported to be associated with certain inflammatory states [34], but is not known to be transmitted by arthropods; and viruses from the Parvoviridae family, including human bocavirus, which could cause febrile illness and signs of FTLS. Although human bocaviruses are not known to be transmitted by arthropods, feline panleukopenia virus, a parvovirus, is strongly suspected to be transmitted by arthropods [35]. All family Parvoviridae sequences detected in FTLS samples were also assembled and their protein sequences deduced. Included among these samples were four fragments, all of which were found to be highly homologous to human bocavirus; one fragment showed the greatest similarity to human bocavirus 2 isolate 53044 (identity = 86%) with the lowest E-value (8×10−17). Human bocavirus was further detected by PCR in the pool of 10 serum samples, but only one individual sample within the pool tested positive for bocavirus. The final virus family detected in sera from FTLS patients was the Bunyaviridae family, which contains viruses known to cause FTLS after tick bites [8], [30]. All family Bunyaviridae sequences detected in FTLS samples, including 11 fragments (1 S-segment, 2 M-segments, and 8 L-segment fragments), were assembled and their protein sequences were deduced. Among these 11 novel virus fragments was a 168-bp fragment (C361, Accession: HQ412604) of the polymerase gene that showed the greatest similarity to Tehran virus (identity = 36%) with the lowest E-value (3×10−7). This suggested the presence of a novel virus or a known virus whose genome had not yet been sequenced.
Tab. 4. sequence reads with limited BLASTx identity to known viruses. 
*Bold indicates virus families only found in FTLS patient sera. An E-value cutoff of 1e-5 for BLASTx was applied. To more accurately assess the genetic relationships to known viruses, we constructed a phylogenetic tree using a neighbor-joining method [33]. The result showed that the potentially novel virus clustered with Toscana virus, Uukuniemi virus, and Rift Valley fever virus of the Phlebovirus genus (Figure 1A). The pools of 10 serum samples from patients and 10 serum samples from healthy subjects were also screened by PCR for the presence of the novel bunyavirus. All 10 samples from patients tested positive for the novel bunyavirus; however, all 10 samples from healthy subjects tested negative. Thus, we further focused on the virus from the family Bunyaviridae.
Fig. 1. Phylogenetic analysis of novel bunyavirus proteins. 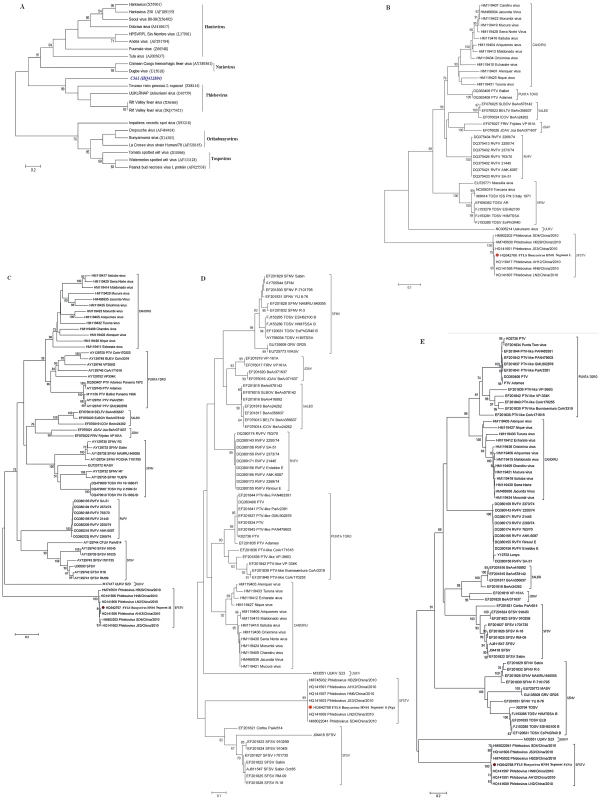
Phylogenetic trees were generated by comparing the translated amino acid sequences of individual sequence reads to the corresponding sequences from known bunyaviruses using a neighbor-joining method. The horizontal-line distance represents the number of sites at which the two compared sequences are different. Bootstrap values deduced from 1000 replicates. A: C361 fragment (Accession number HQ412604). B: FTLS bunyavirus (Henan isolate), Segment L (Accession number HQ642766). C: FTLS bunyavirus (Henan isolate), Segment M (Accession number HQ642767). D: FTLS bunyavirus (Henan isolate), Segment S (Np) (Accession number HQ642768). E: FTLS bunyavirus (Henan isolate), Segment S (Ns) (Accession number HQ642768). Confirmation of the presence of the novel virus in patients' sera
Using specifically designed RT-PCR primers, we detected viral RNA in 223 of the 285 acute serum samples tested (Table 5). The specificity of the RT-PCR was confirmed by sequencing selected PCR products. None of the 80 sera from patients with respiratory diseases or the 50 sera from healthy subjects was positive using the novel virus-specific RT-PCR.
Tab. 5. Detection of novel FTLS bunyavirus-specific IgG antibody and viral RNA by IFA and RT-PCR. 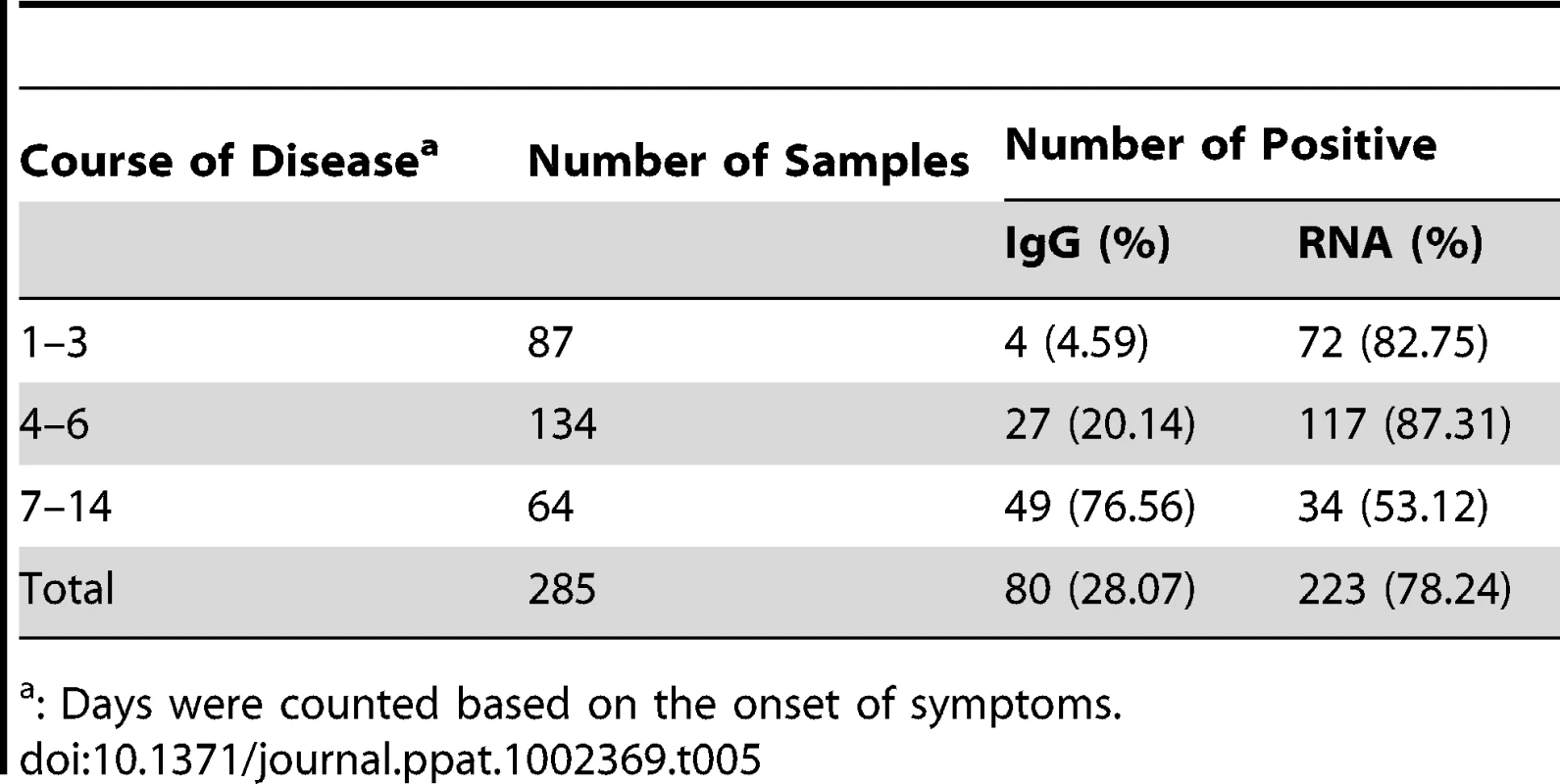
: Days were counted based on the onset of symptoms. Isolation of the novel virus
Six acute serum samples that tested positive for the novel bunyavirus by specific RT-PCR were inoculated onto Vero E6 cells, and four virus strains were isolated. The initial CPE analysis showed rounded refractile cells 2–4 days after inoculation. CPE did not progress in the initial cultures, but appeared slightly at 24 hours in subsequent passages (Figure 2A). RT-PCR revealed the presence of RNA for the novel bunyavirus in all four virus strains, and all isolates reacted with the serum of a convalescent patient in IFA (Figure 2C). In addition, electron microscopy showed the presence of virus particles approximately 80–90 nm in diameter—a size compatible with a bunyavirus (Figure 3). Virus particles were presumably localized to the Golgi apparatus (Figure 3).
Fig. 2. Vero E6 cells inoculated with acute serum specimens from patients with FTLS. 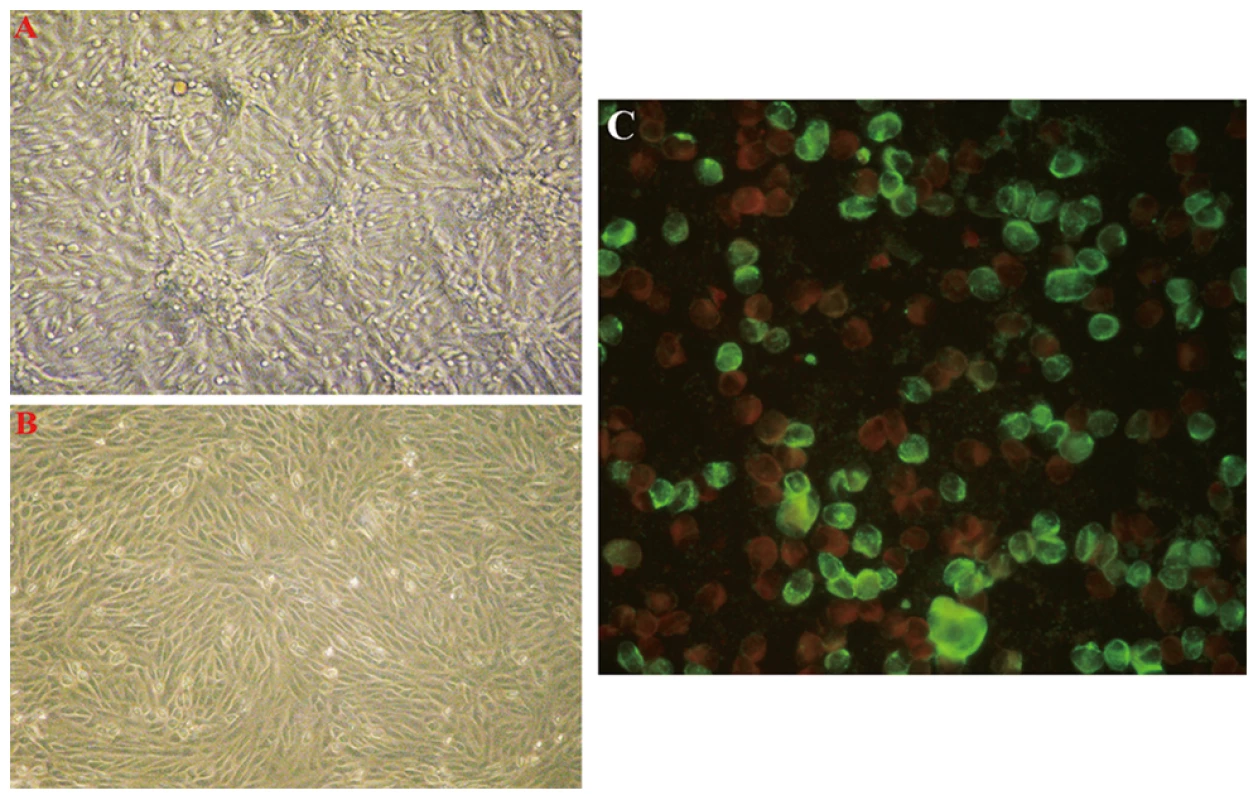
The typical early CPE seen with novel bunyavirus isolates from patients with FTLS is shown in part A (×40). Mock-inoculated Vero cells are shown in part B (×40). Infected Vero cells reacted with the serum of a convalescent FTLS patient in an indirect IFA in part C (×400). Fig. 3. Thin-section electron microscopy of novel FTLS-associated bunyavirus Grown in Vero E6 Cells. 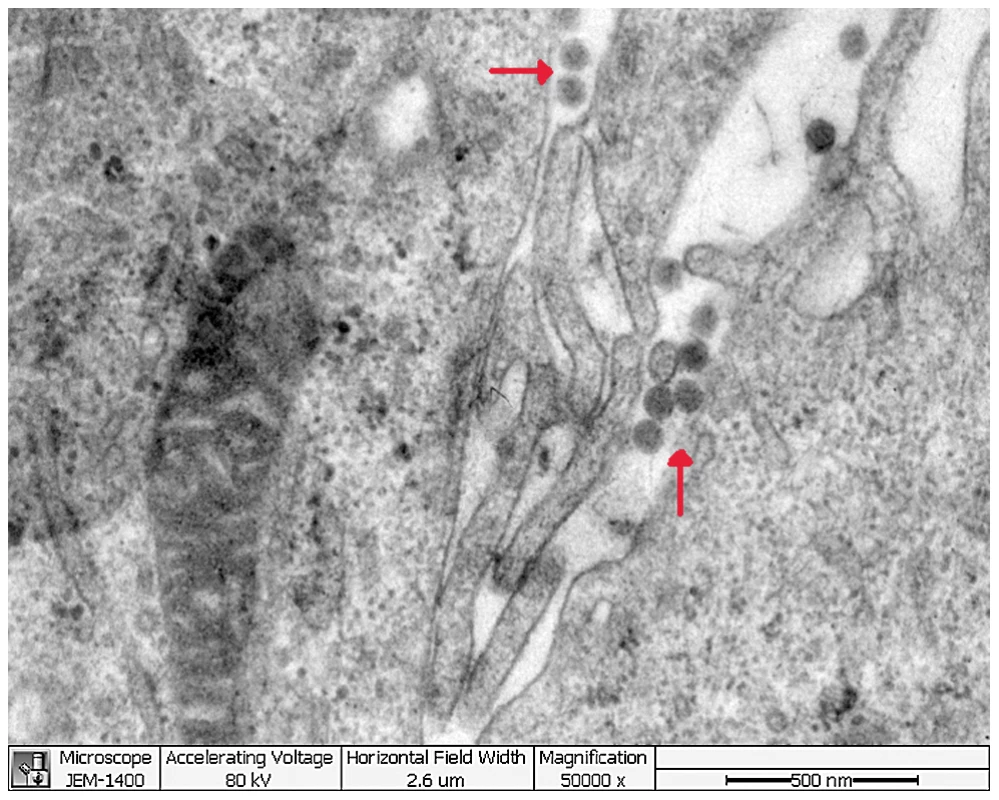
Confirmation of the isolated virus by direct sequencing
Genome sequencing of one isolate (HN01) revealed three segments of negative polarity, single-stranded RNA, including a large segment (L; GenBank HQ642766), a medium-sized segment (M; GenBank HQ642767), and a small segment (S; GenBank HQ642768). The deduced amino acid sequence of the L segment had the highest homology (34%, E value = 3×10−163) to RNA polymerase of the Uukuniemi virus of the Phlebovirus genus, whereas the M segment had the highest homology (26%, E value = 8×10−55) to glycoprotein genes of the Punta Toro virus in the Phlebovirus genus. Of the two proteins encoded by the ambisense S segment, one had the highest homology to nucleocapsid protein (39%, E value = 3×10−40) of the Rift Valley Fever virus, and the other had the highest homology to nonstructural protein genes (24%, E value = 0.049) of the Punique Virus, both of which belong to the Phlebovirus genus. Collectively, these findings confirm that this virus belongs to the Phlebovirus genus of Bunyaviridae.
During the revision of this manuscript, some new sequences of SFTSV (severe fever with thrombocytopenia syndrome) were released. A comparison of these new SFTSV sequences with the sequence of this novel virus showed that they were highly homologous (>99% identity). Using sequences of Phlebovirus available in GenBank, a phylogenetic analysis showed that, although most closely related to the Uukuniemi virus of the Phlebovirus genus (34%, 24%, and 29% of maximum identity, respectively, for segment L, M, S at maximum query coverage), the three genomic segments of the novel virus, along with the SFTSV sequences, were highly divergent (Figure 1B–1E).
Antibody response to the bunyavirus
IgG antibodies to the novel bunyavirus were detected in 80 of 285 acute-phase serum samples from patients with FTLS (Table 5). Of 95 patients from whom paired acute - and convalescent-phase sera were available, 52 had seroconversions and 21 had greater than 4-fold increases in antibody titer to the virus. Six had less than a 4-fold increase in antibody titer to the virus, but all paired sera tested positive. Sixteen patients tested negative to the virus, suggesting that some non-FTLS patients with similar symptoms were included in this study, a situation that is not surprising given that FTLS is a newly emerging disease. The acute-phase sera of four patients from whom the virus was isolated tested negative for IgG antibody to the virus. All convalescent sera obtained 2 months later from the same four patients contained IgG antibody to the virus. None of the 130 sera from patients with respiratory diseases or healthy subjects had detectable antibody.
Discussion
Since 2007, there has been an increase in reported cases of FTLS in Xinyang City, Henan Province. These patients were tentatively diagnosed as having A. phagocytophilum infection. However, only a few (8.4%, 24/285) such patients had evidence for A. phagocytophilum infection, and none of the 285 patients tested positive for the many other pathogens capable of causing similar clinical and laboratory manifestations that were also investigated. These findings suggested novel infectious agents, including viruses.
Traditionally, virus culture is very important for identifying an unknown viral infection. Before performing the Illumina sequencing strategy, we attempted viral and rickettsial culture with DH82 and BHK cell lines, but the lack of an obvious CPE led us to initially abandon this approach. Here, mass sequence data obtained by Illumina sequencing revealed four virus families that appeared only in FTLS patient sera. Among these four virus families, viruses from the Parvoviridae and Bunyaviridae families reportedly can cause signs of FTLS and be transmitted by arthropods. However, only one sample from a pool of ten samples tested positive for bocavirus by PCR, suggesting that bocavirus from the Parvoviridae is not likely involved in FTLS. For viruses in the Bunyaviridae family, the incidence of infection is closely linked to vector activity. For example, tick-borne viruses are more common in the late spring and late summer when tick activity peaks. Human infections with certain Bunyaviridae, such as Crimean-Congo hemorrhagic fever virus, are associated with high levels of morbidity and mortality [30]. Considering the tick-bite history of many FTLS patients, we focused on Bunyaviridae family viruses.
The entire Bunyaviridae family contains more than 300 members arranged in four genera of arthropod-borne viruses (Orthobunyavirus, Nairovirus, Phlebovirus and Tospovirus) and one genus (Hantavirus) of rodent-borne viruses [30], [36]. The Phlebovirus genus currently comprises 68 antigenically distinct serotypes, only a few of which have been studied. The 68 known serotypes are divided into two groups: the Phlebotomus fever group (the sandfly group, transmitted by Phlebotominae sandflies) comprises 55 members, and the Uukuniemi group (transmitted by ticks) comprises the remaining 13 members. Of these 68 serotypes, eight are linked to disease in humans, including the Alenquer, Candiru, Chagres, Naples, Punta Toro, Rift Valley fever, Sicilian, and Toscana viruses [30]. Phleboviruses have tripartite genomes consisting of a large (L), medium (M), and small (S) RNA segment.
In screening for unknown viruses, species hits alone likely carry little weight. Thus, we used all sequences in the family Bunyaviridae for our analysis. A 168-bp fragment of the polymerase gene with the lowest E-value and high sequence identity was used as the sequence of the unknown virus. This virus sequence was detected in all 10 pooled samples, indicating that the virus is involved in FTLS. After detecting a possible novel bunyavirus through high-throughput Illumina sequencing, we inoculated Vero cell lines, which are known to be sensitive to phleboviruses, with sera from six positive patients and were subsequently able to detect the virus by RT-PCR [30], [31]. Although the CPE was modest, RT-PCR confirmed the infection. Genome sequencing was performed and a phylogenetic analysis of the genome sequence showed that this virus clustered into the Phlebovirus branch, but was divergent from other known phleboviruses. These results confirm the novelty of this virus within the Phlebovirus genus of the family Bunyaviridae [36]. Furthermore, virus size and propagation in cells were similar to that of the bunyaviruses.
PCR and serological tests were performed to further test the causal link between the new virus and FTLS. Although we have not completely fulfilled Koch's postulates, evidence implicating this new bunyavirus in the outbreak of the disease among patients with FTLS is compelling.
In view of the fact that the disease is caused by a novel bunyavirus, and taking into account that the disease was first discovered in Henan (HN), we propose the name "Henan Fever" for the FTLS disease cause by the novel virus (proposed name “Henan Fever Virus” [HNF virus]). Since the submission of this manuscript, a bunyavirus was identified as the cause of FTLS in Chinese patients from other regions of China, and the authors have named this virus “SFTSV” to indicate that it is the cause of severe fever with thrombocytopenia syndrome [37]. After release of the GenBank sequences referred to in the Yu paper, we compared the sequences of SFTSV with those of FTLSV and found that they were nearly identical (>99% identity). As we first identified the syndrome in 2007 and described the presence of the virus in patients between 2007 and 2010, we suggest that the name “HNF virus” should take precedence. The most distinctive feature of the current work includes the use of an unbiased metagenomic approach for viral pathogen discovery that facilitated the rapid creation and implementation of standard culture, serological, and molecular diagnostic approaches. However, there are other differences between the results described here and those reported by Yu et al; notably, we observed slight, but distinctive, CPE in Vero cells. The reason for the failure to observe CPE in Vero cells infected with the “SFTSV” bunyavirus [37], whose genome is nearly identical to that of bunyavirus isolated from our FTLS patients, is unclear. Perhaps this reflects the fact that the ensuing CPE is not dramatic. Alternately, this could indicate the existence of distinct viral strains that vary in pathogenicity, virulence, and possibly even disease manifestations. This is an area of active study in our laboratories.
The discovery of this new virus will assist in the rapid diagnosis of this disease and help to distinguish it from other diseases caused by pathogens such as A. phagocytophilum, E. chaffeensis, Crimean-Congo hemorrhagic fever virus, Hantavirus, dengue virus, Japanese encephalitis virus, and Chikungunya virus. Furthermore, the availability of the new virus will facilitate the future development of new therapeutic interventions, such as vaccines and drugs.
Supporting Information
Zdroje
1. HeJGChengZXWuJBYangXXLiQ 2006 The Epidemiological Investigation on a human to human anaplasmosis infection in South Anhui Province in 2006. World J Infect 5 343 347
2. XuBLChenHMZhuQZhangJXiaSL 2006 The Epidemiological Investigation on the First Outbreak of Tsutsugamushi Disease in Henan Province. Henan J Prev Med 17 3
3. WenBJianRZhangYChenR 2002 Simultaneous Detection of Anaplasma marginale and a New Ehrlichia Species Closely Related to Ehrlichia chaffeensis by Sequence Analyses of 16S Ribosomal DNA in Boophilus microplus Ticks from Tibet. J Clin Microbiol 40 3286 3290
4. DuP 1982 Medical experimental virology. Shanghai Shanghai Association of Microbiology 211 212
5. DumlerJSChoiKSGarcia-GarciaJCBaratNSScorpioDG 2005 Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg Infect Dis 11 1828 1834
6. EngTRHarkessJRFishbeinDBDawsonJEGreeneCN 1990 Epidemiologic, clinical, and laboratory findings of human ehrlichiosis in the United States, 1988. JAMA 264 2251 2258
7. MOH of the people's republic of China 2008 Guide for Human granulocytic anaplasmosis control. http://www.moh.gov.cn/publicfiles/business/htmlfiles/mohbgt/pw10805/200804/31429.htm
8. PaulSDianaS 2001 Dictionary of Microbiology and Molecular Biology. Chichester John Wiley & Sons, Ltd 1 4 3rd edition.
9. MeiyuFHuoshengCCuihuaCXiaodongTLianhuaJ 1997 Detection of flaviviruses by reverse transcriptase polymerase chain reaction with the universal primer set. Microbiol Immunol 41 209 213
10. BaiZLiuLTuZYaoLLiuJ 2008 Real-time PCR for detecting circulating dengue virus in the Guangdong Province of China in 2006. J Med Microbiol 57 1547 1552
11. XieRHXuFZhuHPChengYKFuGM 2009 Development and evaluation of TaqMan-based one-step reverse transcription-polymerase chain reaction assay for the detection of Japanese encephalitis virus. Zhonghua Liu Xing Bing Xue Za Zhi 30 277 280
12. SanthoshSRParidaMMDashPKPateriyaAPattnaikB 2007 Development and evaluation of SYBR Green I-based one-step real-time RT-PCR assay for detection and quantification of Chikungunya virus. J Clin Virol 39 188 193
13. KangXLiYLiuHLinFCaiX 2010 A duplex real-time reverse transcriptase polymerase chain reaction assay for the detection of Western Equine Encephalitis virus and Eastern Equine Encephalitis virus. Virol J 7 284
14. ChinikarSGoyaMMShirzadiMRGhiasiSMMirahmadiR 2008 Surveillance and laboratory detection system of Crimean-Congo haemorrhagic fever in Iran. Transbound Emerg Dis 55 200 204
15. SuzukiABisordiILevisSGarciaJPereiraLE 2004 Identifying rodent hantavirus reservoirs, Brazil. Emerg Infect Dis 10 2127 2134
16. GarciaSCranceJMBillecocqAPeinnequinAJouanA 2001 Quantitative Real-Time PCR detection of Rift Valley Fever virus and its application to evaluation of antiviral compounds. J Clin Microbiol 39 4456 4461
17. JinQ 2001 Medical Molecular Virology. Beijing Science Press 1st edition. 659
18. PeirisJSMLaiSTPoonLLGuanYYamLY 2003 Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361 1319 1325
19. FranchiniGCollaltiEAryaSKFenyöEMBiberfeldG 1987 Genetic analysis of a new subgroup of human and simian T-lymphotropic retroviruses: HTLV-IV, LAV-2, SBL-6669, and STLV-IIIAGM. AIDS Res Hum Retroviruses 3 11 17
20. Cox-FosterDLConlanSHolmesECPalaciosGEvansJD 2007 A Metagenomic Survey of Microbes in Honey Bee Colony Collapse Disorder. Science 318 283
21. FinkbeinerSRAllredAFTarrPIKleinEJKirkwoodCD 2008 Finkbeiner Metagenomic Analysis of Human Diarrhea: Viral Detection and Discovery. PLoS Pathog 4 e1000011
22. PalaciosGDruceJDuLTranTBirchC 2008 A New Arenavirus in a Cluster of Fatal Transplant-Associated Diseases. N Engl J Med 358 991 998
23. Woods Lt ColJB 2005 USAMRIID's Medical Management of Biological Casualties Handbook. Fort Detrick, Maryland U.S. Army Medical Institute of Infectious Diseases 143 144 6th edition.
24. LiRLiYKarstenKWangJ 2008 Sequence analysis SOAP: short oligonucleotide alignment program. BIOINFORMATICS 24 713 714
25. BohlanderSKEspinosaR3rdLe BeauMMRowleyJDDíazMO 1992 A method for the rapid sequence-independent amplification of microdissected chromosomal material. Genomics 13 1322 1324
26. AltschulSFMaddenTLSchäfferAAZhangJZhangZ 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389 3402
27. LiWGodzikA 2006 Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22 1658 1659
28. HuangXMadanA 1999 CAP3: a DNA sequence assembly program. Genome Res 9 868 877
29. SlootsTPMcErleanPSpeicherDJArdenKENissenMD 2006 Evidence of human coronavirus HKU1 and human bocavirus in Australian children. J Clin Virol 35 99 102
30. BishopDHCalisherCHCasalsJChumakovMPGaidamovichSY 1980 Bunyaviridae. Intervirology 14 125 143
31. JinQ 2001 Medical Molecular Virology. Beijing Science Press 1st edition. 528
32. EwingBHillierLWendlMCGreenP 1998 Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8 175 185
33. SwoffordDL 1998 PAUP*.Phylogenetic Analysis Using Parsimony (*and Other Methods). Sunderland, Massachusettes Sinauer Associates
34. WoottonSCKimDSKondohYChenELeeJS 2011 Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 183 1698 1702
35. GreeneCEAddieDD 2006 Feline parvovirus infections. In: Greene CE (ed). Infectious Diseases of the Dog and Cat. St. Louis: Saunders Elsevier 78-88
36. MayoMA 2002 Virus taxonomy-Houston 2002. Arch Virol 147 1071 1076
37. YuXJLiangMFZhangSYLiuYLiJD 2011 Fever with Thrombocytopenia Associated with a Novel Bunyavirus in China. N Engl J Med 364 1523 1532
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



