-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
The mosquito immune system is involved in pathogen-elicited defense responses. The NF-κB factors REL1 and REL2 are downstream transcription activators of Toll and IMD immune pathways, respectively. We have used genome-wide microarray analyses to characterize fat-body-specific gene transcript repertoires activated by either REL1 or REL2 in two transgenic strains of the mosquito Aedes aegypti. Vitellogenin gene promoter was used in each transgenic strain to ectopically express either REL1 (REL1+) or REL2 (REL2+) in a sex, tissue, and stage specific manner. There was a significant change in the transcript abundance of 297 (79 up - and 218 down-regulated) and 299 (123 up - and 176 down-regulated) genes in fat bodies of REL1+ and REL2+, respectively. Over half of the induced genes had predicted functions in immunity, and a large group of these was co-regulated by REL1 and REL2. By generating a hybrid transgenic strain, which ectopically expresses both REL1 and REL2, we have shown a synergistic action of these NF-κB factors in activating immune genes. The REL1+ immune transcriptome showed a significant overlap with that of cactus (RNAi)-depleted mosquitoes (50%). In contrast, the REL2+ -regulated transcriptome differed from the relatively small group of gene transcripts regulated by RNAi depletion of a putative inhibitor of the IMD pathway, caspar (35 up - and 140 down-regulated), suggesting that caspar contributes to regulation of a subset of IMD-pathway controlled genes. Infections of the wild type Ae. aegypti with Plasmodium gallinaceum elicited the transcription of a distinct subset of immune genes (76 up - and 25 down-regulated) relative to that observed in REL1+ and REL2+ mosquitoes. Considerable overlap was observed between the fat body transcriptome of Plasmodium-infected mosquitoes and that of mosquitoes with transiently depleted PIAS, an inhibitor of the JAK-STAT pathway. PIAS gene silencing reduced Plasmodium proliferation in Ae. aegypti, indicating the involvement of the JAK-STAT pathway in anti-Plasmodium defense in this infection model.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002394
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002394Summary
The mosquito immune system is involved in pathogen-elicited defense responses. The NF-κB factors REL1 and REL2 are downstream transcription activators of Toll and IMD immune pathways, respectively. We have used genome-wide microarray analyses to characterize fat-body-specific gene transcript repertoires activated by either REL1 or REL2 in two transgenic strains of the mosquito Aedes aegypti. Vitellogenin gene promoter was used in each transgenic strain to ectopically express either REL1 (REL1+) or REL2 (REL2+) in a sex, tissue, and stage specific manner. There was a significant change in the transcript abundance of 297 (79 up - and 218 down-regulated) and 299 (123 up - and 176 down-regulated) genes in fat bodies of REL1+ and REL2+, respectively. Over half of the induced genes had predicted functions in immunity, and a large group of these was co-regulated by REL1 and REL2. By generating a hybrid transgenic strain, which ectopically expresses both REL1 and REL2, we have shown a synergistic action of these NF-κB factors in activating immune genes. The REL1+ immune transcriptome showed a significant overlap with that of cactus (RNAi)-depleted mosquitoes (50%). In contrast, the REL2+ -regulated transcriptome differed from the relatively small group of gene transcripts regulated by RNAi depletion of a putative inhibitor of the IMD pathway, caspar (35 up - and 140 down-regulated), suggesting that caspar contributes to regulation of a subset of IMD-pathway controlled genes. Infections of the wild type Ae. aegypti with Plasmodium gallinaceum elicited the transcription of a distinct subset of immune genes (76 up - and 25 down-regulated) relative to that observed in REL1+ and REL2+ mosquitoes. Considerable overlap was observed between the fat body transcriptome of Plasmodium-infected mosquitoes and that of mosquitoes with transiently depleted PIAS, an inhibitor of the JAK-STAT pathway. PIAS gene silencing reduced Plasmodium proliferation in Ae. aegypti, indicating the involvement of the JAK-STAT pathway in anti-Plasmodium defense in this infection model.
Introduction
Mosquito-borne diseases cause tremendous morbidity and mortality worldwide [1]. New approaches to control vector-borne diseases include interruption of the association between pathogens and vectors by genetic manipulation of vectors and the development of transmission-blocking vaccines. Potential success of these approaches requires in-depth knowledge of the molecular interactions between vectors' defense mechanisms and the evolutionary established ability of a pathogen to overcome these defenses.
The yellow fever mosquito Aedes aegypti is the principal vector of Dengue fever and, due to a large body of knowledge amassed for this mosquito and readily available genetic and molecular tools, it also serves as an outstanding model for vector biology [2]. Sequencing and annotation of the genome of this mosquito have been critical in further advancing genomic and molecular approaches in studies of its immunity [3], [4]. Despite being five times larger than the genome of the malaria mosquito Anopheles gambiae, the Ae. aegypti genome consists of a similar number of protein-encoding genes, around 17,700 [3], [4]. Comparative genome analysis has indicated that 353 Aedes genes from 31 families are associated with immunity, compared with 285 and 338 immune genes in Drosophila melanogaster and An. gambiae, respectively, suggesting expansions of some immune gene groups in Ae. aegypti [4]. The key immune pathways are conserved between mosquitoes and the fruit fly; however, mosquitoes exhibit expansions of pattern recognition and effector molecules, likely due to their co-evolution with various pathogens [4]. Similar to Drosophila, mosquito Toll and IMD pathways constitute major immune pathways activating a battery of anti-microbial peptides and immune proteins in response to invasion by various microorganisms [4], [5]. The activation of genes encoding these immune effector molecules is accomplished by the action of the NF-κB transcription factors REL1, the orthologue of Drosophila Dorsal, and REL2, the Relish orthologue, respectively [6], [7], [8].
Another important defense mechanism in Arthropods is melanization, which mediates wound healing and parasite encapsulation [9]. The key enzyme of melanization, phenoloxidase (PO), is involved in the production of toxic melanin, which is deposited at the wound or around the parasite. A CLIP-domain serine protease cascade is responsible for amplification of signals, which are released upon infection, from wounded tissues or ruptured oenocytoids, and conversion of prophenoloxidase (PPO) into an active PO. (Reviewer 1, query 3) The activation of the melanization cascade is under strict regulation by serine protease inhibitors (serpins). The importance of melanization cascades in mosquitoes is indicated by major expansions in their melanization pathway gene families (10 PPOs, 25 Serpins, and 79 CLIPs in Ae. aegypti) [4], [10], [11].
The fat body of insects, such as Drosophila and mosquitoes, is the major metabolic tissue, and also serves as a powerful immune organ [5], [12]. Although the role of the fat body in immunity has been demonstrated for the model insect Drosophila [5], its precise function in immune responses in mosquitoes is still largely unknown. Deciphering the repertoire of immune genes expressed in the mosquito fat body is of particular importance because of the considerable expansion of immune-related genes in mosquitoes relative to that in Drosophila [4], [10], [11].
In previous studies, we have generated transgenic strains of Ae. aegypti, in which REL1 and REL2 were ectopically expressed under the control of the blood-meal-regulated promoter of the vitellogenin (Vg) gene in the fat body [13], [14]. In this current work, we took advantage of the availability of these transgenic strains and performed transcriptome analyses to characterize repertoires of fat body-specific genes controlled by Toll and IMD pathways in this vector. Using microarray-based genome-wide transcriptional analyses, we have characterized gene repertoires in two transgenic Ae. aegypti mosquito strains that ectopically express either REL1 (REL1+ strain) or REL2 (REL2+ strain). Moreover, we have shown a synergistic action of REL1 and REL2 in activating immune genes in the transgenic mosquito co-expressing both these NF-κB transcription factors. Infection of Ae. aegypti with Plasmodium gallinaceum resulted in the transcriptional modulation of a distinct subset of host immune genes. There was considerable overlap between the fat body transcriptome of Plasmodium-infected mosquitoes and the repertoire of genes regulated in mosquitoes transiently depleted of PIAS, an inhibitor of the JAK-STAT pathway. RNAi depletion of PIAS reduced Plasmodium proliferation in Ae. aegypti, indicating involvement of JAK-STAT in anti-parasite defense.
Results/Discussion
Fat body transcriptional responses in REL1+ and REL2+ transgenic Aedes aegypti female mosquitoes
Previously generated transgenic strains of the mosquito Ae. aegypti ectopically expressing either REL1 or REL2 [13], [14] have permitted us to decipher transcript repertoires of genes in the fat body controlled by the Toll and IMD pathways, respectively. We analyzed the transcriptional profiles of fat body-expressed genes using custom-made 60-mer oligonucleotide microarrays representing the approximately 17,700 Ae. aegypti genes [15]. The transgenic mosquitoes were constructed to ectopically express either recombinant REL1 or REL2 using the Vg promoter, which is a female - and fat-body-specific, blood meal-inducible gene [16]. The abundance of transcripts in REL1+ and REL2+ mosquitoes was compared with that in the non-transgenic wild type mosquitoes at 24 h post blood meal (PBM), and genes uniquely regulated by REL1+ and REL2+ mosquitoes were further analyzed. The time point of 24 h PBM was chosen for transcriptome analyses because it is the expression peak for the Vg gene, whose upstream regulatory region was used to drive the expression of both REL1 and REL2 transgenes. The REL1 transgene is maximally expressed in the fat body of the REL1+ transgenic strain at this PBM time [13]. We reexamined REL2 transgene expression profile in the REL2+ strain, reported in [14], by means of quantitative real time PCR (qRT-PCR) and found that its peak was at 24 h PBM (Figure S1).
The fat body transcriptome of REL1+ transgenic mosquitoes contained 297 gene transcripts, 79 of which were up-regulated and 218 down regulated (Figure 1A). Immune genes were the most predominant up-regulated group in the REL1+ fat body transcriptome, representing 66% of all up-regulated genes (Figure 1A and Table S1). Among the category of immune genes that were up-regulated in the REL1+ mosquito fat body were components of the Toll pathway, indicating the involvement of REL1 in the feedback regulation of its own pathway. These were genes encoding the Toll-specific pattern recognition receptor, the Gram Negative Binding Protein 1 (GNBP1), spätzle 3A, REL1, and the negative regulator of the Toll pathway, cactus (Table S1). Activation of effector molecule transcripts–the anti-microbial peptides (AMPs) defensins A, C and D, and lysozymes C10 and C11–was high (Table S1). Defensins represent the major antimicrobial peptides (AMP) in mosquitoes [4].
Fig. 1. Comparative transcriptome analysis of fat body genes in REL1+ and REL2+ transgenic mosquitoes. 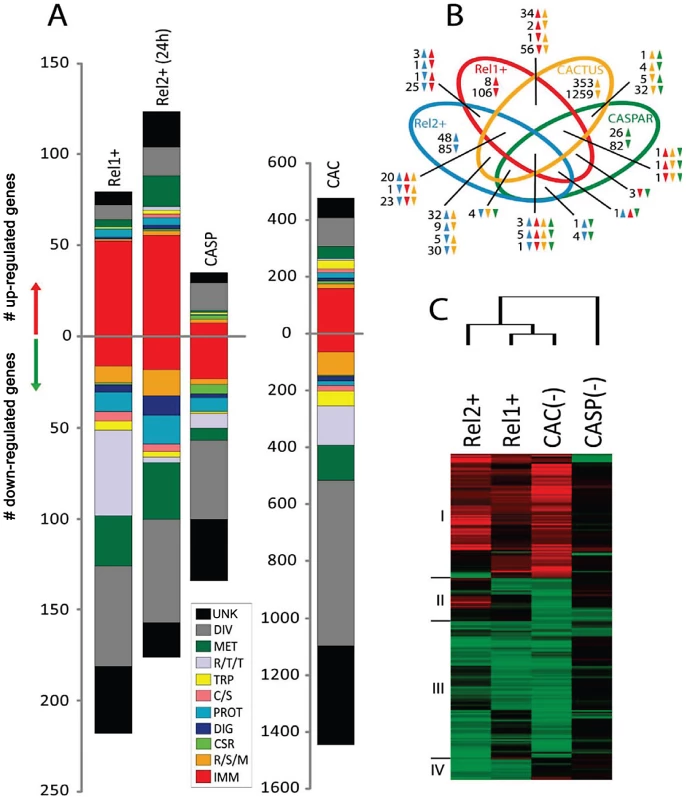
A) Functional classification of the REL1+ and REL2+ regulated transcriptome. Functional group abbreviations are: IMM, immunity; R/S/M, redox, stress and mitochondrion; CSR, chemosensory reception; DIG, digestive; C/S, cytoskeletal and structural; PROT, proteolysis; TRP, transport; R/T/T, replication, transcription, and translation; MET, metabolism; DIV, diverse functions; UNK, unknown functions. B) Venn diagram representing unique and shared transcriptome regulation in REL1+ and REL2+ transgenic mosquitoes, and cactus- and caspar-depleted mosquitoes. The overlapping regions represent genes that are concomitantly regulated in two, three, or four experimental conditions at the level of transcript abundance. The direction of gene transcript changes is indicated by upward-pointing and downward-pointing arrows. Green, Brown, Red, and Blue colors represent caspar-depleted, cactus-depleted, REL1+, and REL2+ transgenic mosquitoes, respectively. C) Hierarchical cluster analysis of fat body gene transcripts that were significantly regulated in at least two of the four experimental conditions; REL1+, REL2+, cactus- and caspar-depleted mosquitoes. Genes encoding opsonization factors, such as thio-ester proteins (TEP)–TEP2, 3, 20, 21, and 22–represented another predominant group of REL1-induced immune genes. Member of the TEP family have been identified in diverse animal species and play important roles in immune responses as components of the complement system [17]. In An. gambiae, hemocyte-specific TEP1 has been implicated as a key molecule involved in killing of midgut stages of Plasmodium [18]. It acts with two leucine-rich repeat (LRR) proteins, LRIM1 and APL1, as a complement system in parasite killing [19]. However, functions of most TEPs in insects, including mosquitoes, remain to be elucidated.
Transcripts of 6 genes, encoding galactose-specific C-type lectins were also elevated in the REL1+ transcriptome. Genes encoding proteolytic cascades and signaling modulators, CLIPs and serpins, were also represented in the immune repertoire of the REL1+-induced fat body transcriptome (Table S1). Previously, we have shown that some of these gene transcripts, TEP15, TEP20, defensin A, and CLIP13B, were activated by REL1, thus, providing additional confidence to our genome-wide transcriptome data set [7].
Transcript of the gene encoding an orthologue of the Drosophila JAK/STAT pathway receptor Domeless (Dome) was among the most highly increased upon REL1 activation in the fat body (AAEL012471), indicating the involvement of Toll-REL1 pathway in regulating JAK-STAT pathway (Table S1). Dome is the Drosophila homolog of the vertebrate transmembrane cytokine class I receptor, which serves as a signal transducer and mediates activation of totA in the fat body [20], [21]. Activation of Dome/JAK/STAT signaling requires hemocyte-specific cytokine Unpaired [21]. Fat body totA is also regulated by Relish, a Drosophila orthologue of mosquito REL2. Here, we provide evidence on the involvement of REL1 in up-regulation of Dome, the gene encoding a key component of the JAK/STAT pathway in the mosquito fat body.
Several recent studies in Drosophila have pointed out on a communication between immune tissues[21], [22], [23], [24]. In addition to hemocyte-specific cytokine mediated activation of the Dome/JAK/STAT in the fat body, blood cells are also required for the immune activation of the fat body [22], [24]. However, Rel proteins, Dif and Dorsal, also act in the fat body to produce factors that promote blood-cell number in Drosophila larvae [23]. The identity of these fat body factors remains undetermined. There is no evidence of possible involvement of Relish in similar fat body – blood cell communication. Utilization of fat body-specific, ectopically expressed REL1+ represents a unique opportunity to address the question about fat body – hemocyte communication in mosquitoes. Thus, although the ectopic expression of REL1 in the REL1+ transgenic mosquitoes is strictly fat body-specific, the fat body REL1-mediated production of blood cell stimulating factors could activate proliferation of blood cells adhered to fat body preparations. As a consequence, the overall transcriptome from REL+ mosquitoes could include genes from proliferating hemocytes. This aspect of fat body – blood cell communication will be studied further in the future.
The REL1+ controlled transcriptome contained a large number of genes (230) attributed to non-immune biological processes; 26.6% of these gene transcripts (REL1) were involved in ribosomal biogenesis, DNA replication and metabolism (Figure 1A; Table S1). 88% of these non-immune genes were down-regulated. Notably, 44 of down-regulated genes were related to ribosomal biogenesis and translation. This observation is in agreement with microarray analyses of ectopic expression of Rel proteins in Drosophila [25].
One of genes activated in the REL1+ fat body transcriptome encodes an orthologue of a vertebrate Grb2-associating protein (Gasp, AAEL002492; Table S1), which is a thymus-specific factor critical for T-cell differentiation [26]. Finding its function represents a potentially important aspect of immunity in the mosquito.
REL1 also up-regulates an orthologue of the cytosolic sulfotrnasferase, SULT (AAEL006334, Table S1). Members of SULT superfamily catalyze the sulfation of xenobiotics, hormones and neurotransmitters [27]. Considering multiple functions of these enzymes, it is difficult to predict the role of this REL1-dependent SULT in the mosquito fat body.
An interesting glimpse in the gene functional conservation is also provided by the Rel-mediated up-regulation of an orthologue of a vertebrate major facilitator superfamily domain-containing protein (Mfsd2a, AAEL009195; Table S1), which is expressed in brown adipose tissue and liver (the fat body is a functional analogue of these tissues combined) [28]. In vertebrates, Mfsd2a is highly expressed during thermogenesis and have been found to be a tumor suppressor [28], [29].
The fat body transcriptome of REL2+ transgenic mosquitoes contained 299 genes, 123 of which were up-regulated and 176 down regulated (Figure 1A and Table S2). Immune genes represented 44% of most highly up-regulated genes in the REL2+ fat body transcriptome. In particular, transcripts of AMPs and recognition molecules were enriched (Table S2). Genes encoding thio-ester proteins (TEP)–TEP20, 21, and 22–were also elevated among REL2-induced immune genes. Genes encoding factors of the IMD pathway–a peptidoglycan recognition protein (PGRP-S1) and REL2 - were up-regulated (Figure 1A and Table S2).
Two members of the APL1 family of leucine-rich (LRR) proteins, APL1B (AAEL012086) and APL1C (AAEL009520), were up-regulated in the REL2+ fat body transcriptome but not in the REL1+ one (Tables S1 and S2). LRR proteins play an important role in the innate responses against pathogens in plants, insects, and mammals [30], [31], [32]. APL1 (Anopheles Plasmodium-responsive leucine-rich repeat 1) was first identified in An. gambiae, in which it controls resistance to Plasmodium falciparum [33]. The APL1 family is comprised of paralogs APL1A, APL1B and APL1C [34]. APL1C is responsible for defense of An. gambiae against P. berghei, which is a rodent parasite. APL1C has been reported to function within the REL1-cactus immune signaling, which regulates APL1C at the transcriptional and translational levels [34]. However, further studies have revealed that protection of An. gambiae against its natural parasite P. falciparum is mediated by APL1A [35]. This protection correlates with the transcriptional control of APL1A by REL2, suggesting that REL2 anti-parasite phenotype results partially from its control of APL1A [35]. APL1C has been implicated in a complement-like pathway that mediates parasite killing interacting with LRIM1 and TEP1 [36], [37]. Our data indicate that Ae. aegypti APL1 proteins are controlled by REL2.
Up-regulation of a fibrinogen-related protein (AAEL004150) was also observed in the REL2+ fat body transcriptome. The fibrinogen-related gene family belongs to pattern recognition receptors and involved in innate immunity in both invertebrates and vertebrates [38], [39], [40]. In An. gambiae, fibrinogen-related proteins interact with Gram-positive, Gram-negative bacteria and co-localized with both P. berghei and P. falciparum [40]. It has been suggested that fibrinogen-related proteins expand pattern recognition capacity, thus, enhancing innate immunity against various pathogens.
227 genes in the REL2+ fat body transcriptome belonged to genes encoded factors of non-immune biological processes. Transcript levels of some genes related to non-immune functional categories, most notably stress and metabolism, were predominantly repressed (Figure 1A; Table S2). Thirty-three genes (6 induced and 27 repressed) in the REL2+ regulated transcriptome were related to proteolysis process. Interestingly, REL2+ transcriptome contained an up-regulated component of ribosome biogenesis, 20S rna accumulation protein 1 (AAEL004493), in contrast to overall down-regulation of genes related to ribosomal biogenesis and translation in the REL1 transcriptome. This difference points out on specificity of action of REL1 and REL2 not only in affecting immune, but also non-immune genes.
We also compared the fat body transcriptomes of REL1+ and REL2+ transgenic mosquito with those of mosquitoes in which either the negative regulator cactus or caspar had been depleted by RNAi silencing. The latter two transcriptomes have been previously reported and are represented here for comparative purposes only [15]. Cactus is a repressor of Drosophila Dorsal/Dif and mosquito REL1, which has been shown to directly interact with this NF-κB factor preventing the latter to translocate to the nucleus [5], [15], [41], [42]. In mosquitoes, cactus silencing results in activation of REL1 and its underlying immune responses [7], [10], [11], [15], [41]. Hierarchical clustering confirmed the close relationship between the immune transcriptomes regulated by transgene REL1 overexpression and cactus depletion (Figure 1C, Cluster I and Table S4). However, REL1+ affected transcript abundance of fewer genes in diverse functional classes compared to cactus depletion. Our analysis revealed the presence of the same 53 genes in transcriptomes from REL1+, REL2+ transgenic and cactus-depleted mosquitoes (Figure 1B). 30 of them belonged to immunity category.
In Drosophila and Anopheles, caspar has been shown to be an inhibitor of the IMD pathway, in which it has been suggested to prevent Dredd-dependent nuclear translocation of Relish and REL2 [43], [44]. In Ae aegypti, RNA depletion of caspar triggered up-regulation of only a small number of genes when compared with REL2 transgene ectopic expression ([15] and this report). Moreover, caspar-induced transcriptome only marginally overlapped with that of REL2+ (Figure 1C and Table S4). Further studies are required to clarify the role of caspar in the regulation of the IMD pathway.
Synergistic action of REL1 ad REL 2 in activating immune genes in the mosquito fat body
Importantly, 84 genes were present in both REL1+ and REL2+ fat body transcriptomes, suggesting co-regulation of these genes by the NF-kB factors REL1 and REL2 and their respective pathways (Figure 1B and Figure S2, Table S3). The majority of highly enriched gene transcripts (50%), which were common for both REL1+ and REL2+ fat body transcriptomes, belonged to the immunity category. The AMP genes defensins A, C, D and lysozyme C displayed increased mRNA abundance in response to either REL1 or REL2. However, REL2 appeared to be a more potent activator of these AMPs. A group of six galactose-specific C-type lectin transcripts was highly elevated in both transcriptomes. Gene transcripts encoding TEPs, TEP2, TEP20, TEP21 and TEP22, appeared to be equally upregulated by REL1 and REL2.
Only 22% of all non-immune genes were present in both REL1+ and REL2+ -regulated fat body transcriptomes in contrast of 50% of immune ones. Among non-immune genes that were induced in both transgenic mosquitoes was juvenile hormone esterase (JHE). Increased JHE activity has been linked with degradation of juvenile hormone during PBM development in Ae aegypti females [45]. However, modulation of juvenile hormone titer via immune factors has not been previously reported. The majority of non-immune down-regulated genes in both transcriptomes belonged to metabolism and cell cycle functional categories (Table S3).
To decipher whether genes represented in both REL1+ and REL2+ fat body transcriptomes were synergistically regulated by these NF-κB transcription factors, we generated a hybrid REL+/REL2+ transgenic mosquito strain by crossing REL1+ and REL2+ strains. Unlike parental RE11+ and REL2+ strains, the hybrid REL1/REL2 mosquitoes carried both Vg-REL1 and Vg-REL2 transgenes, which were ectopically expressed after a blood meal specifically in female fat bodies (Figure 2). We analyzed transcript abundance of selected genes representing the functional group of 33 immune genes upregulated in both REL1+ and REL2+ transcriptomes. REL1+, REL2+ and REL1+/REL2+ hybrid transgenic mosquitoes were blood fed, RNA was isolated from their fat bodies 24 h PBM and subjected to qRT-PCR analysis. This analysis revealed that the transcript levels of Defensin A, Defensin C, CLIPB39, and TEP20 genes in the REL1+/REL2+ hybrid transgenic female mosquitoes was considerably higher as compared to those in either REL1+ or REL2+ strains (Figure 2). Defensin A and Defensin C were particularly elevated in the REL1+/REL2+ hybrid mosquitoes (Figures 2A and 2B). A predominant concept in insect immunology is that Toll and IMD pathways act independently, with the Toll pathway responding to fungi and Gram-positive bacterium-derived Lys-type peptidoglycan and the IMD pathway to Gram-negative bacterium-derived diaminoacipimelic acid (DAP)-type peptidoglycan, with each pathway activating a separate set of effector genes [5], [42]. However, the transcriptome analyses of double Drosophila mutants of the Toll and IMD pathways have revealed some co-regulated antimicrobial peptides [46]. Regulation of Drosophila antifungal AMP Drosomycin mainly depends on Toll pathway and receives a modest input from IMD during a systemic immune response; though, the IMD pathway solely activates Drosomycin and Diptericin, respective target genes of the Toll and IMD pathways, in the local immune response.[47], [48]. Tanji et al. [49] have shown the synergistic action of Toll and IMD pathway in activating Drosomycin and Diptericin. Moreover, DIF and Relish form heterodimers to regulate antimicrobial peptides in Drosophila [50]. Our transgenic approach strongly suggests the synergistic action of REL1 (an orthologue of Dorsal) and REL2 (an orthologue of Relish) in activation of immune genes in the mosquito Ae. aegypti. The high level of up-regulation of immune genes in REL1+/REL2+ hybrid mosquitoes as co-expression of Vg-driven REL1 and REL2 clearly indicated the synergy of interaction between these NF-kB factors. Comparable levels of individual ectopic expression of each REL factor in a respective transgenic REL strain did not elicit similarly high up-regulation of immune factors, assayed in this experiment.
Fig. 2. Synergistic action of REL1 and REL2 in activating immune genes. 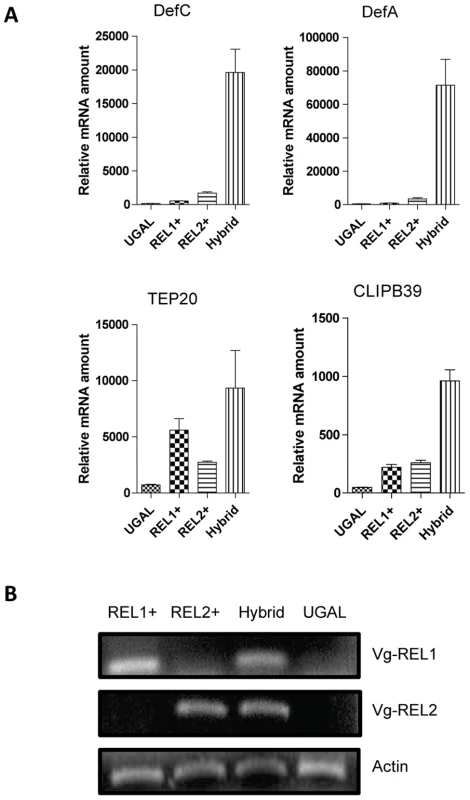
A) Transgenic mosquitoes with over-expressed REL1 or REL2 were used to generate hybrid transgenic mosquitoes over-expressing both of these factors. Fat bodies of REL1+, REL2+, and RE1+/REL2+ mosquitoes were dissected 24 h PBM and analyzed by means of quantitative RT-PCR for selected immune genes. A. Defensin A; B. Defensin C; C. TEP 20; D. CLIPB39. UGAL was used as a control. B) Both Vg-REL1 and Vg-REL2 transgenes were expressed in the hybrid mosquitoes. Total RNA isolated from females 24 h PBM were analyzed using specific primers for vitellogenin that recognize exclusively hybrid transgene mRNA. RT-PCR analysis indicated that both Vg-REL1 and Vg-REL2 transgenes were expressed in the hybrid mosquitoes. The same RNA samples were tested using actin specific primers as controls. Previously, we have shown that simultaneous ectopic expression of Defensin A and Cecropin A in Ae. aegypti leads to a complete elimination of the malaria parasite P. gallinaceum and interruption of its transmission in transgenic hybrid CecA/DefA mosquitoes [51]. Ectopic co-expression of REL1 and REL2 in a sex-, tissue - and stage-specific manner that elicits a strong synergistic effect on activation immune factors provides a potent method to study mosquito and pathogen interaction.
Control of melanization-related genes by REL1 and REL2
Microarray-based transcriptome analysis was used to study the effect of immune signal transduction pathways on melanization-related gene expression in the Ae. aegypti fat body. At 24 h PBM, 21 melanization-related genes were significantly upregulated in the fat body of transgenic REL1+ female mosquitoes, while 24 (22 up - and 2 down-) genes were controlled by REL2 ectopic expression in the same tissue (Figure S2, Table S5). CLIP-domain serine proteases can be separated into five subfamilies, among which CLIPA, B, and C are implicated in the activation of melanization. The CLIPA subfamily is composed of the non-catalytic clip domain serine protease homologues, which contain imperative PPO activation cofactors. Mosquito CLIPA14 and CLIPA6 are homologous to the PPO activation cofactors, Manduca sexta SPH1, SPH2 (serine protease homologue), and Holotrichia diomphalia PPAF2 (PPO activating factor) [52]. CLIPA1 and CLIPA11 were up-regulated in both REL1+ and REL2+. However, CLIPA5, CLIPA6, and CLIPA16 were induced only in the REL1+, while CLIPA14 was enriched in the REL2+. Two melanization proteases (CLIPB39 and CLIP40) [11] and CLIPB79 were induced in both REL1+ and REL2+ mosquitoes. Of all the CLIPC, D, and E subfamilies, only a single gene, CLIPE8, was induced in REL2+ (Table S5).
REL1 and REL2 differently regulated transcription of genes encoding Serpins. Serpins-9, −16, −4B, −4C were up-regulated only in the REL1+ mosquitoes, while Serpins-2, −11, and −23 mRNAs in REL2+ mosquitoes (Table S5). Serpins-1, −8, and, −16 mRNAs were elevated in both REL1+ and REL2+ mosquitoes (Table S5). In Ae. aegypti, Serpin 1 is involved in control of immune melanization, while Serpin-2 in tissue melanization, exemplified by the formation of melanotic tumors after RNAi Serpin-2 depletion [11]. PPO gene transcripts were not detected in either REL1+ or REL2+ fat-body-specific transcriptomes. This is in agreement with previous data showing that PPO genes are expressed in hemocytes in both Drosophila and mosquitoes [5], [53].
Transcriptional responses triggered by Plasmodium gallinaceum in Ae. aegypti
To assess the relationship of REL1+ and REL2+ transcriptomes with transcriptional responses induced by Plasmodium infection in the mosquito Ae. aegypti, we compared transcriptomes of mosquitoes fed on a Plasmodium-infected blood meal with those fed on a non-infectious blood meal. In both the midgut and fat body, the majority of genes regulated in the presence of Plasmodium belonged to diverse or unknown functional classes, defined as such because of insufficient information for assigning particular known functions. The number of genes that significantly changed their transcript levels after Plasmodium infection in the midgut was almost twice higher than that in the fat body; impressively, Plasmodium infection envoked the induction of 375 genes and repression of 724 genes in the midgut, while 513 genes were induced and 174 genes were repressed in the fat body (Figure 3, Tables S6 and S7). The quantitative RT-PCR used to verify transcripts levels for 23 genes (12 from PgFB and 11 from PgMD) showed a high degree of correlation (best-fit linear-regression R2 = 0.77) with the microarray transcriptome data (Figure S3). The immune-related genes were the third most-represented functional gene group in both the midgut (124 genes) and the fat body (99 genes) transcriptomes in Plasmodium-infected mosquitoes (Figure 3).
Fig. 3. Comparative analysis P. gallinaceum midgut- and fat body- responsive transcriptomes. 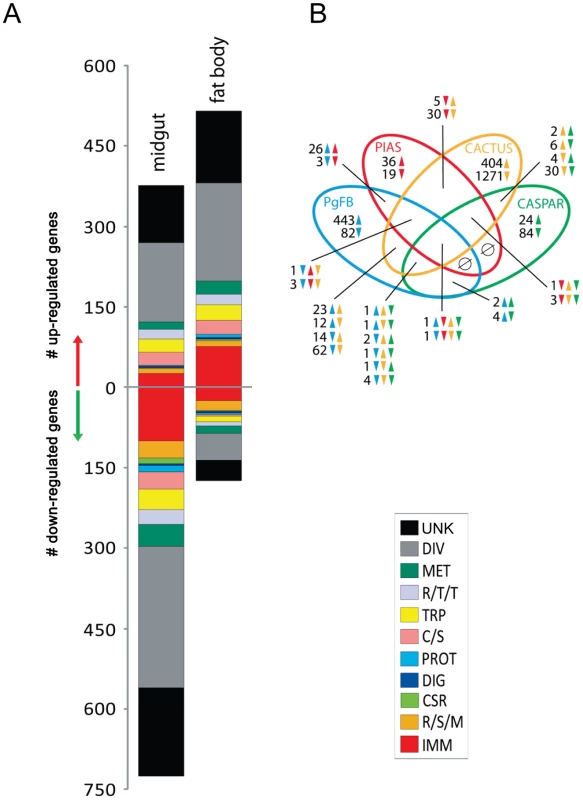
A) Functional classification of the PgMG- and PgFB-regulated transcriptomes. Functional group abbreviations are: IMM, immunity; R/S/M, redox, stress and mitochondrion; CSR, chemosensory reception; DIG, digestive; C/S, cytoskeletal and structural; PROT, proteolysis; TRP, transport; R/T/T, replication, transcription, and translation; MET, metabolism; DIV, diverse functions; UNK, unknown functions. B) Venn diagram representing unique and shared transcript regulation in PgMG, PgFB, and cactus- and caspar-depleted mosquitoes. The overlapping regions represent genes that are concomitantly regulated in two, three, or four experimental conditions at the level of transcript abundance. The direction of gene transcript regulation is indicated by upward-pointing and downward-pointing arrows. Green, Brown, Red, and Blue colors represent caspar-depleted, cactus-depleted, PIAS-depleted, and Plasmodium-infected mosquitoes, respectively. In the midgut, transcriptional responses affected by Plasmodium infection were marked by a significant down-regulation of immune gene transcripts (99 out of 124 regulated immune genes). Among these down-regulated immune genes were several serine proteases (SPs), CLIP-domain serine proteases, Serpins (serpins-4A, −7, −11, −17, −20 and −21), lysozyme C, various PRR molecules such as the PGRP proteins (PGRP-LA, PGRP-LD, and PGRP-SC2), GNBP1, fibrinogen-related proteins (FBN12, FBN12, FBN13, FBN18, FBN24, and FBN27), three AMPs (defensins A and D, cecropin D) (Table S6). Majority of genes putatively related to the melanization cascade were down-regulated in the midgut (26 down-regulated and only 4 up-regulated). The transcript abundance of CLIPA5, CLIPA6, CLIPB13A, CLIPB13B, CLIPB5 CLIPA3, CLIPB1, and Serpin-11 mRNAs were reduced in Plasmodium infected midgut (Table S6). CASPS18 was upregulated, while CASPS7 and CASPS20 were downregulated. 32 out of 40 genes related to oxidative stress were down-regulated, including eight cytochrome P450s, three carboxylesterase, and one glutathione peroxidase. Down-regulation of stress response gene expression suggested the existence of mechanism genes could potentially interfere with the proliferation of parasites in the midgut. Genes related to transport processes were differentially regulated in the Plasmodium-infected midgut transcriptome, with 38 repressed and 25 induced. A gene encoding oxidoreductase (AAEL003312) was significantly up-regulated, suggesting Plasmodium-mediated elevated activity of this enzyme (Table S6). Among potential functions of oxidoreductase is detoxification of reactive oxygen species (ROS), which play a pivotal role in anti-Plasmodium gut resistance [54].
A pronounced immune response was detected in the fat body of mosquitoes 24 h after a Plasmodium-infected blood meal (Figure 3 and Table S7). Significantly, Plasmodium infection resulted in the enrichment of 74 immune gene transcripts in the fat body (out of 99 regulated immune genes in this tissue). REL1 (AAEL007696) and REL2 (AAEL007624) were induced in the fat body transcriptome of the Plasmodium-infected mosquitoes, suggesting that infection with the parasite activated both the Toll and IMD pathways (Table S7). Components of the Toll pathway - GNBP3, TOLL8, TOLL11, and spätzle 6 - were also upregulated. The IMD receptors PGRP-LP and PGRP-S5 were elevated. However, IMD was down-regulated. Attacin C, Defensins C and D were among up-regulated AMPs.
A distinct immune response of the Aedes fat body to Plasmodium infection was the activation of two Down-syndrome adhesion molecules (Dscam, Table S7). Dscam is a member of the immunoglobulin superfamily, its gene comprises of multiple exons, alternative splicing of which generates 19,000 different extracellular domains and provides [55]. An. gambiae orthologue of Dscam contains 101 exons that can produce over 31,000 alternative splice forms [56]. Hemocyte-specific Dscam isoforms have been associated with phagocytotic uptake of bacteria [55], [57]. In An. gambiae, Dscam has been implicated in resistance to bacteria and Plasmodium [55]. Dscam is also expressed in Drosophila fat body, which is in agreement with our observation [55]. The role of fat body-specific Dscam isoforms remains to be elucidated.
Multiple TEPs (TEP13, TEP15, TEP20, TEP22, and TEP23) and leucine-rich (LRR) proteins were up-regulated in the fat body in response to Plasmodium infection as well; however, their functions are not clear. Plasmodium infection also caused changes in mRNA abundance in apoptosis related genes; IAP-2 (an inhibitor of apoptosis), CASPS18, and CASPS8 were induced, while CASPS19 was repressed in the fat body of the Plasmodium-infected mosquitoes. An interesting feature of Plasmodium-affected fat body transcriptome is the down-regulation of DOME, the JAK/STAT receptor, which was up-regulated in the REL1+ fat body transcriptome (Table S7). Another distinct feature of Plasmodium-affected fat body transcriptome was elevation transcriptional activity as evident by up-regulation of six zinc finger and forkhead transcription factors (Table S7).
We found that the fat body transcriptome, the gene encoding dual oxidase (DUOX, AAEL007563) was activated by Plasmodium infection (Table S7). DUOX enzyme is involved in production of reactive oxygen species (ROS), which have been implicated in anti-microbial immunity [58]. ROS has been implicated in innate immune responses in the gut and anti-Plasmodium defenses [54], [58], [59]. The anti-Plasmodium effect of ROS is mediated by bacterial flora [60]. The adverse effect of ROS is modulated by antioxidants, including Gpx [54]. Our finding of DUOX in the Plasmodium-induced fat body transcriptome adds a new aspect in immune function of ROS. A possibility of activation of these enzymes in hemocytes attached to the fat body could not be ruled out.
Availability of REL1+ - and REL2+-induced fat body transcriptomes permitted us to conduct a comparative analysis with the P. gallinaceum fat body (PgFB)-responsive transcriptome. The comparison of fat body of Plasmodium infection-responsive gene transcript repertoire with those of REL1+ and REL2+ mosquitoes showed an overlap between these transcriptomes (7 induced, 5 repressed) (Figure 4 and Table S8). Only three immune genes (CLIPB13B, CLIPB15, and Serpin-8) were found in the overlap of these transcriptomes, suggesting that immune responses elicited by the Plasmodium infection in the fat body were distinct from those regulated by either REL1+ - and REL2+ (Figure 4, Figure S2 and Table S8). Hierarchical clustering showed that cluster I consisted of a large group of down-regulated genes from PgFB-responsive and REL1+ and REL2+ gene repertoire, while cluster II represented genes, which were induced in by REL and repressed by Plasmodium challenge. Conversely, cluster IV was largely enriched by up-regulated immune genes (82%), which are putatively involved in melanization and signaling amplification (Serpins, CLIPs) and parasite recognition and killing (TEPs, Lectins) (Table S8).
Fig. 4. Comparative analysis of the P. gallinaceum fat body responsive to the REL1- and REL2-regulated transcriptomes. 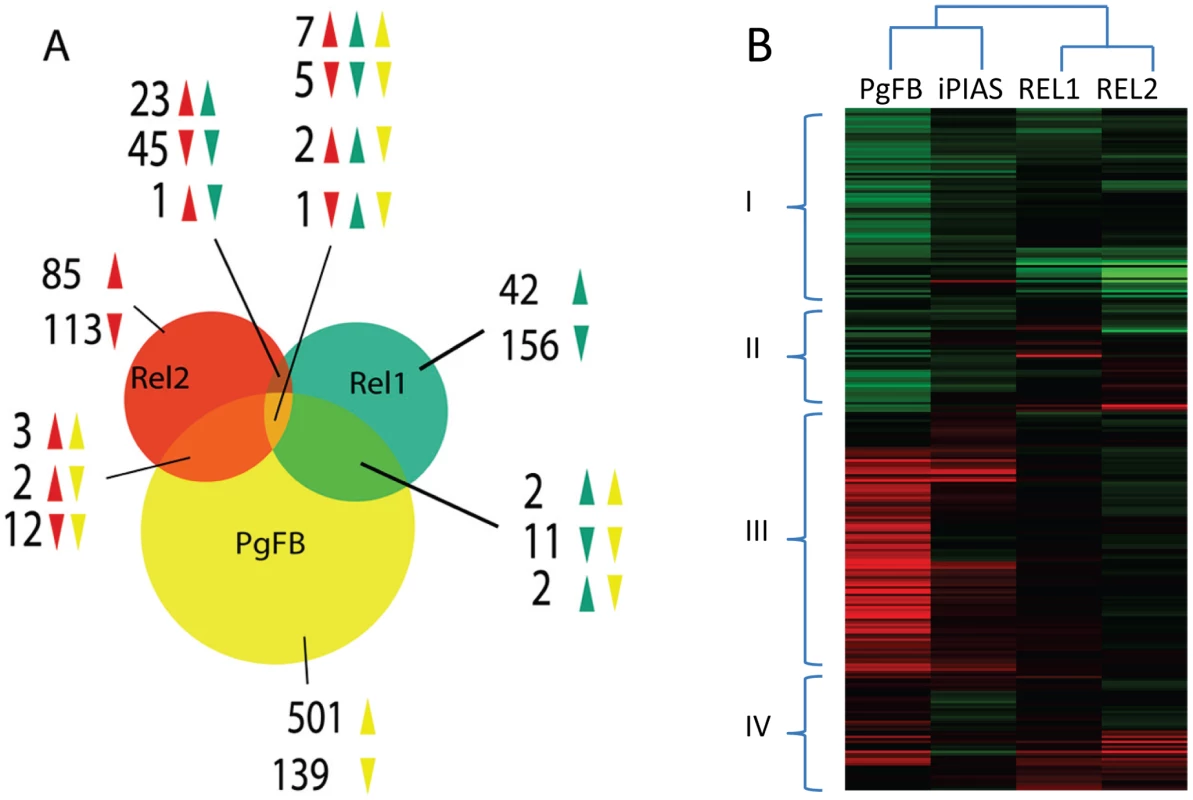
A) Venn diagram representing unique and shared gene transcript regulation in PgFB, REL1+, and REL2+ transgenic mosquitoes. The overlapping regions represent genes that are concomitantly regulated in two or three experimental conditions. The direction of gene regulation is represented by upward-pointing and downward-pointing arrows. B) Hierarchical cluster analysis of the genes that were regulated in REL1+, REL2+, PIAS-depleted, and P. gallinaceum challenged mosquitoes. The JAK-STAT pathway is involved in mosquito anti-Plasmodium defense
The core components of the JAK-STAT pathway are evolutionarily conserved from arthropods to mammals [61]. PIAS (Protein Inhibitor of Activated STAT) has been identified as a negative regulator of the JAK-STAT pathway in mammals, Drosophila and Aedes [62], [63]. A multiple polypeptide sequence alignment showed that insect PIAS had a domain structure similar to that from vertebrates (Figures S4A and S4B). The sequence of Aedes PIAS shared a high level of homology with Anopheles PIAS (identity 61%, similarity 71%) and Drosophila PIAS (identity 47%, similarity 57%). It also had a lower degree of homology with human PIAS (identity 32%, similarity 46%); but the invertebrate PIAS group formed its own independent clade (Figure S4C). Impressively, Ae aegypti PIAS gene contains ten alternative spliced isoforms, however, their respective roles are not known (Figure S4) [3]. We compared the Plasmodium infection-responsive fat body transcriptome with that of PIAS-gene silenced mosquitoes, which was reported earlier [63] and found a significant overlap between these transcriptomes [63] (29 gene transcripts: 26 induced and 3 repressed) (Figure 3). The transcript of the SOCS (suppressor of cytokine signaling) gene, which encodes another JAK-STAT pathway negative regulator, was highly enriched in the fat body in response to P. gallinaceum infection. Interestingly, not a single gene displaying transcript enrichment upon P. gallinaceum infection seemed to be co-regulated by cactus and PIAS silencing, suggesting that anti-Plasmodium responses mediated by Toll and JAK-STAT pathways were different. Hierarchical cluster analysis revealed several genes with mRNA abundance enriched in both PgFB, and PIAS-depleted fat body transcriptomes, but unaffected in REL1+ and REL2+ fat body transcriptomes (Figure 4, Cluster III; and Table S8). As stated, cluster IV is mainly composed by immune genes, which are involved in melanization, pattern recognition, and signaling amplification (Table S8). Additionally, overlap between PIAS-depleted and PgFB transcriptome revealed numerous important immune genes: a putative LRR, whose gene family has been linked to Plasmodium killing in An. gambiae [33], [64] , spätzle 6, and two dengue virus restriction factors (DVRF1 and DVRF2) [63] (Figure S2B).
We then evaluated the anti-P. gallinaceum activities of three major Ae aegypti immune pathways Toll, IMD, and JAK-STAT. One-day-old mosquitoes were injected with dsRNA for either one of the following negative regulator genes of these pathway: PIAS, caspar, cactus, or luc, as a control (Figure S5), and then fed on Plasmodium-infected blood 4 days later. Depletion of cactus, and hence activation of the Toll pathway REL1, resulted in the highest level of resistance to P. gallinaceum (Figure 5). Knockdown of PIAS, which resulted in the activation of the JAK-STAT pathway-regulated immune response, also increased mosquito resistance to parasite infection in the midgut by a six-fold (Figure 5). However, we observed no anti-P. gallinaceum effect upon activation of the IMD pathway REL2 factor through depletion of caspar (Figure 5). Depletions of the negative regulators of Toll, IMD, and JAK-STAT pathways – cactus, caspar and PIAS – demonstrated differential patterns of resistance in different mosquito-Plasmodium infection models. REL1 activation by depletion of cactus resulted in the strongest anti – P. berghei and anti-P. gallinaceum effects in An. gambiae and Ae. aegypti, respectively [7], [10], [11], [15], [41]. Depletion of caspar has shown that the IMD pathway is most effective against the human pathogen P. falciparum in An. gambiae and other anopheline species [44]. In the present study we have not observed any effect of caspar depletion on the resistance of Ae. aegypti to P. gallinaceum, while our previous study based on overexpression of REL2 in transgenic Ae. aegypti, has clearly shown involvement of IMD pathway in defense against this pathogen [14]. This discrepancy, taken together with the findings from our transcriptome studies of REL2+ and caspar-depleted mosquitoes, may suggest that caspar is likely to regulate a branch of the IMD pathway, involving a subset of effector genes. Moreover, we have also demonstrated that simultaneous overexpression of two anti-microbial peptides, Cecropin A and Defensin A, which are under the dual control of Toll and IMD pathways, lead to a complete elimination of P. gallinaceum and termination of transmission [51]. In this current study, we have implicated the JAK-STAT pathway in anti-Plasmodium defense in Ae. aegypti. The STAT pathway is involved in late-phase immunity against P. berghei and P. falciparum in An. gambiae [65].
Fig. 5. The role of PIAS in the defense against avian malaria parasite. 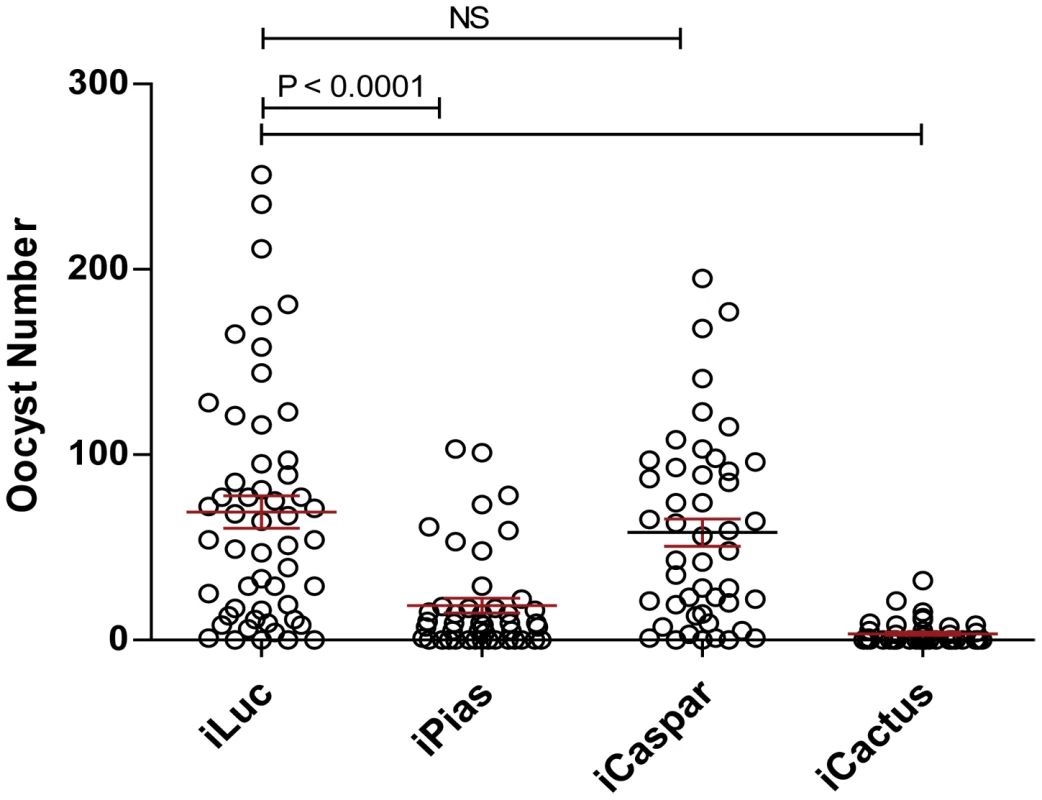
The effect of cactus, caspar, and PIAS in the defense against avian malaria parasite was compared. Depletion of PIAS and cactus significantly decreased the number of survival oocysts. However, the RNAi knock-down of caspar had no effect in the infection of avian malaria parasite to mosquito Ae. aegypti. Prefix i indicates the RNAi-mediated depletion of certain genes by direct injection of their corresponding dsRNAs. iLuc (luciferase dsRNA) was used as controls. The number of fully developed oocysts in each midgut was shown as a circle. The mean number of parasite oocysts for each group was indicated by a black bar. In conclusion, utilization of transgenic Ae. aegypti mosquitoes with altered immunity by means of ectopic expression of the NF-kB transcription factors REL1 and REL2 has permitted deciphering gene repertoires activated by Toll and IMD pathways in the fat body, the central tissue to mosquito immunity. Importantly, transgenic mosquitoes ectopically expressing both these factors exhibited strong synergistic activation of immune genes. A close correlation has been noted between REL1+ and cactus-depleted transcriptomes. In contrast, the REL2+ transcriptome was strikingly different from that of caspar-depleted mosquitoes, suggesting that caspar regulates a sub branch of the IMD pathway. Infections of the wild type Ae. aegypti with P. gallinaceum elicited enrichment of a distinct subset (76 up - and 25 down regulated) of immune gene transcripts relative to that observed in REL1+, REL2+, and cactus-depleted mosquitoes. Considerable overlap was observed between the fat body transcriptome of Plasmodium-infected mosquitoes and that of mosquitoes depleted of PIAS, the inhibitor of the JAK-STAT pathway. PIAS gene silencing reduced Plasmodium proliferation in Ae. aegypti, indicating the involvement of the JAK-STAT pathway in anti-Plasmodium defense in this infection model in addition to Toll and IMD pathways.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the University of California Riverside Institutional Animal Care and Use Committee (IACUC #A20100016; 05/27/2010) and all efforts were made to minimize suffering.
Experimental animals
Wild type and transgenic Ae. aegypti mosquitoes, of the UGAL/Rockefeller strain, REL1+ [13] and REL2+ [14], were maintained in laboratory culture under conditions of 27°C and 80% humidity. Female mosquitoes 3–5 days post-eclosion were fed on the blood of anesthetized white rats to initiate egg development.
Hybrid REL1+/REL2+ mosquitoes were generated by crossing REL1+ and REL2+. Selection of hybrids was performed as previously described [51]. To generate these hybrids, the two strains, REL1+ and REL2+, were maintained as homozygous for four generations before crossing. The hybrid strain was established by crossing REL1 females with REL2 males; the F1 hybrid females were used for the experiments. Adult mosquitoes were maintained on 10% sucrose solution and water [66]. The avian malaria P. gallinaceum was maintained under the natural transmission cycle between the mosquito and chickens. To determine the number of parasite oocysts in the midgut dissected at 8 days post-infection, the tissue was stained with 1% mercurochrome and oocysts were counted under Nikon E400 light microscopy. All dissections (fat body and midgut) were performed in Aedes physiological solution (APS) [66]. Abdominal walls with adhering fat body tissue and free from other internal tissues (thereafter called fat body) were washed in APS to removed hemolymph and blood cells before freezing in liquid nitrogen. Midgut preparations included the anterior and posterior (stomach) without Malpighian tubules and hindgut.
Computational analysis
PIAS sequences from different metazoan species, retrieved from NCBI, Vectorbase, and Ensembl, were analyzed in PROSITE and SMART to confirm conserved domain structures. Multiple sequences were aligned in ClustlX2.0 (Blosum matrixes, gap penalty 10, and extension penalty 0.1). A phylogenetic tree was constructed based on the neighbor-joining method and displayed by Treeview. Parasite oocyst data generated from three independent experiments were analyzed using the Kolmogorov-Smirnov goodness-of-fit test and pooled. The statistically significant difference between samples was calculated using the Mann-Whitney test (Graphpad 5.0).
RT-PCR, Real-time PCR, and Northern blot analysis
Total RNA was extracted from the fat body of eight mosquitoes using the Trizol method (Invitrogen), according to the manufacturer's protocol. Total RNA (5 µg) from each sample was separated on a formaldehyde gel, blotted and hybridized with the corresponding 32P-labeled DNA probe. Probes were generated using PCR and then following the High Prime (Roche) protocol. Actin was used as a loading control. For RT-PCR and Real-time PCR, cDNAs were synthesized from 2 µg total RNA using Omniscript Reverse Transcriptase kit (Qiagen). RNA was treated with DNase I (Invitrogen) before cDNA synthesis. PCR was performed using the Platinum High Fidelity Supermix (Invitrogen). Real-time PCR was performed on the iCycler iQ system (Bio-Rad, Hercules, CA) and we used an IQ SYBR green supermix (Bio-Rad). Quantitative measurements were performed in triplicate and normalized to the internal control of S7 ribosomal protein mRNA for each sample. Primers and probes are listed in Table S9. Real-time data were collected from the software iCycler v3.0. Raw data were exported to EXCEL for analysis.
Gene expression knockdown
Double-stranded RNA synthesis followed a method described previously [10], [15]. In brief, double-stranded RNA (dsRNA) of specific gene template was synthesized using the MEGAscript kit (Ambion). The luciferase gene was used to generate control iLuc dsRNA. After dsRNA synthesis, samples were treated by means of phenol/chloroform extraction and then ethanol precipitation. DsRNA was then suspended in Rnase-free water to reach a final concentration of 5 µg/µl. Naïve adult female mosquitoes were selected at 24 h post-emergence for dsRNA injection experiments. The Picospritzer II (General Valve, Fairfield, NJ) was used to introduce corresponding dsRNA into the thorax of CO2-anesthetized mosquito females, at one or two days post-emergence. DsRNA (300 nl) was injected into the thorax of each adult Ae. aegypti female mosquito. Primers used for dsRNA knockdowns are listed in Table S9.
Microarray assays
Transcription assays and analysis were conducted following standard protocols with a full genome Agilent-based microarray platform [15]. Relative mRNA abundance was compared between treated and control samples. In brief, 2–3 µg total RNA was used for probe synthesis of cy3 - and cy5-labeled dCTP. Hybridizations were conducted with an Agilent Technologies In Situ Hybridization kit at 60°C, according to the manufacturer's instructions. Hybridization intensities were determined with an Axon GenePix 4200AL scanner, and images were analyzed with Gene Pix software. The expression data were processed and analyzed as described previously [15]. In brief, the background-subtracted median fluorescent values were normalized according to a LOWESS normalization method, and Cy5/Cy3 ratios from replicate assays were subjected to t-tests at a significance level of p<0.05, using TIGR, MIDAS, and MeV software [67]. Expression data from all replicate assays were averaged with the GEPAS microarray preprocessing software prior to logarithm (base 2) transformation. Self–self hybridizations were used to determine the cut-off value for the significance of gene regulation on these types of microarrays to 0.8 in log2 scale, which corresponds to 1.74-fold regulation [68]. For genes with p<0.01, the average ratio was used as the final fold change; for genes with p>0.01, the inconsistent replicates (with distance to the median of replicate ratios larger than 0.8) were removed, and only the value from a gene with at least two replicates in the same direction of regulation were further averaged. Three independent biological replicate assays were performed. Numeric microarray gene expression data are presented in Tables S1, S2, S5, S6, S7; validation data by quantitative Real-time PCR - in Table S10 and Figure S3.
Supporting Information
Zdroje
1. BeatyBJPragerDJJamesAAJacobs-LorenaMMillerLH 2009 From tucson to genomics and transgenics: the vector biology network and the emergence of modern vector biology. PLoS Negl Trop Dis 3 e343
2. ClementsAN 1992 The biology of mosquitoes Volume 1: Development, Nutrition and Reproduction. London Chapman & Hall 511
3. NeneVWortmanJRLawsonDHaasBKodiraC 2007 Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316 1718 1723
4. WaterhouseRMKriventsevaEVMeisterSXiZAlvarezKS 2007 Evolutionary dynamics of immune-related genes and pathways in disease-vector mosquitoes. Science 316 1738 1743
5. LemaitreBHoffmannJ 2007 The host defense of Drosophila melanogaster. Annu Rev Immunol 25 697 743
6. ShinSWKokozaVLobkovIRaikhelAS 2003 Relish-mediated immune deficiency in the transgenic mosquito Aedes aegypti. Proc Natl Acad Sci USA 100 2616 2621
7. ShinSWKokozaVBianGCheonHMKimYJ 2005 REL1, a homologue of Drosophila dorsal, regulates toll antifungal immune pathway in the female mosquito Aedes aegypti. J Biol Chem 280 16499 16507
8. ShinSWKokozaVAhmedARaikhelAS 2002 Characterization of three alternatively spliced isoforms of the Rel/NF-kappa B transcription factor Relish from the mosquito Aedes aegypti. Proc Natl Acad Sci USA 99 9978 9983
9. KanostMRJiangHYuXQ 2004 Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev 198 97 105
10. ZouZShinSWAlvarezKSBianGKokozaV 2008 Mosquito RUNX4 in the immune regulation of PPO gene expression and its effect on avian malaria parasite infection. Proc Natl Acad Sci USA 105 18454 18459
11. ZouZShinSWAlvarezKSKokozaVRaikhelAS 2010 Distinct melanization pathways in the mosquito Aedes aegypti. Immunity 32 41 53
12. ArreseELSoulagesJL 2010 Insect fat body: energy, metabolism, and regulation. Annu Rev Entomol 55 207 225
13. BianGShinSWCheonHMKokozaVRaikhelAS 2005 Transgenic alteration of Toll immune pathway in the female mosquito Aedes aegypti. Proc Natl Acad Sci USA 102 13568 13573
14. AntonovaYAlvarezKSKimYJKokozaVRaikhelAS 2009 The role of NF-kappaB factor REL2 in the Aedes aegypti immune response. Insect Biochem Mol Biol 39 303 314
15. XiZRamirezJLDimopoulosG 2008 The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog 4 e1000098
16. KokozaVAhmedAChoWLJasinskieneNJamesAA 2000 Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti. Proc Natl Acad Sci USA 97 9144 9149
17. BlandinSLevashinaEA 2004 Thioester-containing proteins and insect immunity. Mol Immunol 40 903 908
18. BlandinSShiaoSHMoitaLFJanseCJWatersAP 2004 Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116 661 670
19. FraitureMBaxterRHSteinertSChelliahYFroletC 2009 Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe 5 273 284
20. BrownSHuNHombriaJC 2001 Identification of the first invertebrate interleukin JAK/STAT receptor, the Drosophila gene domeless. Curr Biol 11 1700 1705
21. AgaisseHPetersenUMBoutrosMMathey-PrevotBPerrimonN 2003 Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell 5 441 450
22. DijkersPFO'FarrellPH 2007 Drosophila calcineurin promotes induction of innate immune responses. Curr Biol 17 2087 2093
23. MatovaNAndersonKV 2010 Drosophila Rel proteins are central regulators of a robust, multi-organ immune network. J Cell Sci 123 627 633
24. BrennanCADelaneyJRSchneiderDSAndersonKV 2007 Psidin is required in Drosophila blood cells for both phagocytic degradation and immune activation of the fat body. Curr Biol 17 67 72
25. PalSWuJWuLP 2008 Microarray analyses reveal distinct roles for Rel proteins in the Drosophila immune response. Dev Comp Immunol 32 50 60
26. PatrickMSOdaHHayakawaKSatoYEshimaK 2009 Gasp, a Grb2-associating protein, is critical for positive selection of thymocytes. Proc Natl Acad Sci USA 106 16345 16350
27. CoughtrieMW 2002 Sulfation through the looking glass-recent advances in sulfotransferase research for the curious. Pharmacogenomics J 2 297 308
28. AngersMUldryMKongDGimbleJMJettenAM 2008 Mfsd2a encodes a novel major facilitator superfamily domain-containing protein highly induced in brown adipose tissue during fasting and adaptive thermogenesis. Biochem J 416 347 355
29. SpinolaMFalvellaFSColomboFSullivanJPShamesDS 2010 MFSD2A is a novel lung tumor suppressor gene modulating cell cycle and matrix attachment. Mol Cancer 9 62
30. BellJKMullenGELeiferCAMazzoniADaviesDR 2003 Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol 24 528 533
31. KuferTAFritzJHPhilpottDJ 2005 NACHT-LRR proteins (NLRs) in bacterial infection and immunity. Trends Microbiol 13 381 388
32. DolanJWalsheKAlsburySHokampKO'KeeffeS 2007 The extracellular leucine-rich repeat superfamily; a comparative survey and analysis of evolutionary relationships and expression patterns. BMC Genomics 8 320
33. RiehleMMMarkianosKNiareOXuJLiJ 2006 Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science 312 577 579
34. RiehleMMXuJLazzaroBPRottschaeferSMCoulibalyB 2008 Anopheles gambiae APL1 is a family of variable LRR proteins required for Rel1-mediated protection from the malaria parasite, Plasmodium berghei. PLoS One 3 e3672
35. MitriCJacquesJCThieryIRiehleMMXuJ 2009 Fine pathogen discrimination within the APL1 gene family protects Anopheles gambiae against human and rodent malaria species. PLoS Pathog 5 e1000576
36. PovelonesMWaterhouseRMKafatosFCChristophidesGK 2009 Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science 324 258 261
37. BaxterRHSteinertSChelliahYVolohonskyGLevashinaEA 2010 A heterodimeric complex of the LRR proteins LRIM1 and APL1C regulates complement-like immunity in Anopheles gambiae. Proc Natl Acad Sci U S A 107 16817 16822
38. FujitaT 2002 Evolution of the lectin-complement pathway and its role in innate immunity. Nat Rev Immunol 2 346 353
39. WangXZhaoQChristensenBM 2005 Identification and characterization of the fibrinogen-like domain of fibrinogen-related proteins in the mosquito, Anopheles gambiae, and the fruitfly, Drosophila melanogaster, genomes. BMC Genomics 6 114
40. DongYDimopoulosG 2009 Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J Biol Chem 284 9835 9844
41. FroletCThomaMBlandinSHoffmannJALevashinaEA 2006 Boosting NF-kappaB-dependent basal immunity of Anopheles gambiae aborts development of Plasmodium berghei. Immunity 25 677 685
42. HetruCHoffmannJA 2009 NF-kappaB in the immune response of Drosophila. Cold Spring Harb Perspect Biol 1 a000232
43. KimMLeeJHLeeSYKimEChungJ 2006 Caspar, a suppressor of antibacterial immunity in Drosophila. Proc Natl Acad Sci USA 103 16358 16363
44. GarverLSDongYDimopoulosG 2009 Caspar controls resistance to Plasmodium falciparum in diverse anopheline species. PLoS Pathog 5 e1000335
45. ShapiroABWheelockGDHagedornHHBakerFCTsaiLW 1986 Juvenile-Hormone And Juvenile-Hormone Esterase In Adult Females Of The Mosquito Aedes-Aegypti. J Insect Physiol 32 867 877
46. De GregorioESpellmanPTTzouPRubinGMLemaitreB 2002 The Toll and Imd pathways are the major regulators of the immune response in Drosophila. Embo J 21 2568 2579
47. LeulierFVidalSSaigoKUedaRLemaitreB 2002 Inducible expression of double-stranded RNA reveals a role for dFADD in the regulation of the antibacterial response in Drosophila adults. Curr Biol 12 996 1000
48. TzouPOhresserSFerrandonDCapovillaMReichhartJM 2000 Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13 737 748
49. TanjiTHuXWeberANIpYT 2007 Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol 27 4578 4588
50. TanjiTYunEYIpYT 2010 Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci U S A 107 14715 14720
51. KokozaVAhmedAWoon ShinSOkaforNZouZ 2010 Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA 107 8111 8116
52. YuXQJiangHWangYKanostMR 2003 Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol 33 197 208
53. BatonLARobertsonAWarrEStrandMRDimopoulosG 2009 Genome-wide transcriptomic profiling of Anopheles gambiae hemocytes reveals pathogen-specific signatures upon bacterial challenge and Plasmodium berghei infection. BMC Genomics 10 257
54. Jaramillo-GutierrezGMolina-CruzAKumarSBarillas-MuryC 2010 The Anopheles gambiae oxidation resistance 1 (OXR1) gene regulates expression of enzymes that detoxify reactive oxygen species. PLoS One 5 e11168
55. WatsonFLPuttmann-HolgadoRThomasFLamarDLHughesM 2005 Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science 309 1874 1878
56. DongYTaylorHEDimopoulosG 2006 AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol 4 e229
57. DongYTaylorHEDimopoulosG 2006 AgDscam, a hypervariable immunoglobulin domain-containing receptor of the Anopheles gambiae innate immune system. PLoS Biol 4 e229
58. RyuJHHaEMLeeWJ 2010 Innate immunity and gut-microbe mutualism in Drosophila. Dev Comp Immunol 34 369 376
59. SurachetpongWPakpourNCheungKWLuckhartS 2011 Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid Redox Signal 14 943 955
60. CirimotichCMDongYClaytonAMSandifordSLSouza-NetoJA 2011 Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332 855 858
61. ArbouzovaNIZeidlerMP 2006 JAK/STAT signalling in Drosophila: insights into conserved regulatory and cellular functions. Development 133 2605 2616
62. ShuaiKLiuB 2003 Regulation of JAK-STAT signalling in the immune system. Nat Rev Immunol 3 900 911
63. Souza-NetoJASimSDimopoulosG 2009 An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA 106 17841 17846
64. RiehleMMMarkianosKNiareOXuJLiJ 2006 Natural malaria infection in Anopheles gambiae is regulated by a single genomic control region. Science 312 577 579
65. GuptaLMolina-CruzAKumarSRodriguesJDixitR 2009 The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe 5 498 507
66. RoySGHansenIARaikhelAS 2007 Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol 37 1317 1326
67. DudoitSGentlemanRCQuackenbushJ 2003 Open source software for the analysis of microarray data. Biotechniques Suppl 45 51
68. YangIVChenEHassemanJPLiangWFrankBC 2002 Within the fold: assessing differential expression measures and reproducibility in microarray assays. Genome Biol 3 research0062
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



