-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
Group B Streptococcus (GBS) is the leading cause of neonatal pneumonia, septicemia, and meningitis. We have previously shown that in adult mice GBS glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is an extracellular virulence factor that induces production of the immunosuppressive cytokine interleukin-10 (IL-10) by the host early upon bacterial infection. Here, we investigate whether immunity to neonatal GBS infection could be achieved through maternal vaccination against bacterial GAPDH. Female BALB/c mice were immunized with rGAPDH and the progeny was infected with a lethal inoculum of GBS strains. Neonatal mice born from mothers immunized with rGAPDH were protected against infection with GBS strains, including the ST-17 highly virulent clone. A similar protective effect was observed in newborns passively immunized with anti-rGAPDH IgG antibodies, or F(ab')2 fragments, indicating that protection achieved with rGAPDH vaccination is independent of opsonophagocytic killing of bacteria. Protection against lethal GBS infection through rGAPDH maternal vaccination was due to neutralization of IL-10 production soon after infection. Consequently, IL-10 deficient (IL-10−/−) mice pups were as resistant to GBS infection as pups born from vaccinated mothers. We observed that protection was correlated with increased neutrophil trafficking to infected organs. Thus, anti-rGAPDH or anti-IL-10R treatment of mice pups before GBS infection resulted in increased neutrophil numbers and lower bacterial load in infected organs, as compared to newborn mice treated with the respective control antibodies. We showed that mothers immunized with rGAPDH produce neutralizing antibodies that are sufficient to decrease IL-10 production and induce neutrophil recruitment into infected tissues in newborn mice. These results uncover a novel mechanism for GBS virulence in a neonatal host that could be neutralized by vaccination or immunotherapy. As GBS GAPDH is a structurally conserved enzyme that is metabolically essential for bacterial growth in media containing glucose as the sole carbon source (i.e., the blood), this protein constitutes a powerful candidate for the development of a human vaccine against this pathogen.
Published in the journal: . PLoS Pathog 7(11): e32767. doi:10.1371/journal.ppat.1002363
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002363Summary
Group B Streptococcus (GBS) is the leading cause of neonatal pneumonia, septicemia, and meningitis. We have previously shown that in adult mice GBS glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is an extracellular virulence factor that induces production of the immunosuppressive cytokine interleukin-10 (IL-10) by the host early upon bacterial infection. Here, we investigate whether immunity to neonatal GBS infection could be achieved through maternal vaccination against bacterial GAPDH. Female BALB/c mice were immunized with rGAPDH and the progeny was infected with a lethal inoculum of GBS strains. Neonatal mice born from mothers immunized with rGAPDH were protected against infection with GBS strains, including the ST-17 highly virulent clone. A similar protective effect was observed in newborns passively immunized with anti-rGAPDH IgG antibodies, or F(ab')2 fragments, indicating that protection achieved with rGAPDH vaccination is independent of opsonophagocytic killing of bacteria. Protection against lethal GBS infection through rGAPDH maternal vaccination was due to neutralization of IL-10 production soon after infection. Consequently, IL-10 deficient (IL-10−/−) mice pups were as resistant to GBS infection as pups born from vaccinated mothers. We observed that protection was correlated with increased neutrophil trafficking to infected organs. Thus, anti-rGAPDH or anti-IL-10R treatment of mice pups before GBS infection resulted in increased neutrophil numbers and lower bacterial load in infected organs, as compared to newborn mice treated with the respective control antibodies. We showed that mothers immunized with rGAPDH produce neutralizing antibodies that are sufficient to decrease IL-10 production and induce neutrophil recruitment into infected tissues in newborn mice. These results uncover a novel mechanism for GBS virulence in a neonatal host that could be neutralized by vaccination or immunotherapy. As GBS GAPDH is a structurally conserved enzyme that is metabolically essential for bacterial growth in media containing glucose as the sole carbon source (i.e., the blood), this protein constitutes a powerful candidate for the development of a human vaccine against this pathogen.
Introduction
Streptococcus agalactiae, also named Group B Streptococcus (GBS), is a Gram-positive encapsulated commensal bacterium of the human intestine that colonizes the vagina of up to 30% of healthy women. This bacterium is the leading cause of neonatal pneumonia, septicemia, and meningitis [1], [2], [3], [4]. Neonatal GBS infections are acquired through maternal transmission and may result in early-onset disease (EOD), which occurs within the first week of life, or in late-onset disease (LOD), that occurs after the first week and accounts for most meningitis cases and deaths [3], [5], [6]. Despite early antimicrobial treatment and improvement in neonatal intensive care, up to 10% of neonatal invasive GBS infections are lethal and 25 to 35% of surviving infants with meningitis experience permanent neurological sequelae [3]. Because recommendations for intrapartum antibiotic prophylaxis (IAP) for mothers in labor at risk for GBS infection have been widely implemented in many countries, the incidence of EOD has declined to <1/1,000 births, but the incidence of LOD has slowly increased in the last decade [7]. An unexpected burden of case fatalities among children aged less than 90 days caused by GBS infection was recently reported in different European countries [8], [9], [10]. Moreover, recent reports described the emergence of antibiotic-resistant GBS strains likely caused by the widespread use of IAP [11], [12].
Maternal vaccination is the best alternative to IAP to deal with GBS neonatal infections. Vaccines to prevent GBS disease have been initially developed by coupling capsular polysaccharide (CPS) antigens to immunogenic protein carriers. Glycoconjugate vaccines against nine GBS serotypes have been shown to be immunogenic in animals, but the existence of distinct epitope-specific capsular serotypes has hampered the development of a global GBS vaccine [5], [13]. Moreover, glycoconjugated vaccines directed against the ten known serotypes of GBS would not protect against infections by nontypeable GBS isolates that are increasingly being reported [14], [15], [16], [17].
The sequencing of numerous GBS genomes has accelerated advances in vaccine development and new protein antigens have been revealed using reverse vaccinology [5], [18], [19]. To avoid the selection of mutants that escape immune recognition, the ideal human GBS vaccine should be directed against structurally conserved antigens that are essential for GBS virulence and/or growth, but none of the hitherto described candidate antigens fulfills these requisites.
The causes for the neonatal susceptibility to GBS infections are still poorly understood. Newborn immune system is not completely developed at birth, and undergoes an age-dependent maturation until fully developed. Thus, invasive infections in the first days of life pose serious threats for the newborn due to accentuated deficiencies in both innate and adaptive arms of the immune responses. Cases of early-onset GBS sepsis are usually characterized by an unexpectedly low number of neutrophils in infected tissues [20], [21], [22], [23]. This is commonly explained by the reduced neutrophil chemotaxis and impaired granulocyte maturation observed in neonates [24], [25], [26]. Of interest, high concentration of plasma and cord blood IL-10 in preterm neonates evaluated for sepsis was associated with mortality and is considered as an early indicator of prognosis [27], [28].
We have previously shown that the essential housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) also acts as a GBS extracellular virulence factor that induces rapid production of interleukin-10 (IL-10) by the host [29]. Adult C57BL/6 mice (resistant to GBS infection) infected with a GBS mutant strain that over-express GAPDH (oeGAPDH) had increased bacterial colonization compared to mice infected with wild-type (WT) GBS. Increased bacterial burden in oeGAPDH infected C57BL/6 mice was accompanied by elevated serum levels of IL-10. Consequently, acquired susceptibility of C57BL/6 mice to oeGAPDH infection was completely reverted in IL-10-deficient animals [29]. This suggested that an exacerbated production of IL-10 during GBS infection might facilitate pathogen immune evasion. We demonstrate here that maternal immunization with rGAPDH confers protection against GBS infection in neonatal mice by abrogating the early IL-10 production detected upon the bacterial challenge. We also demonstrate that blocking GAPDH-induced early IL-10 production restores the recruitment of neutrophils in infected organs, which is essential for pathogen elimination and host protection against GBS infection.
Since GBS GAPDH is a structurally conserved enzyme that is metabolically essential for bacterial growth in blood, it constitutes an attractive target for the development of a human vaccine.
Results
GAPDH, a structurally conserved GBS protein
GAPDH, a key enzyme of the glycolytic pathway, is structurally conserved in all 8 published GBS genomes (identity>99.8%). Anti-rGAPDH IgG antibodies purified from sera of rGAPDH immunized mice or rabbits were thus used to demonstrate the presence of GAPDH in culture supernatants of ten unrelated GBS clinical isolates (Figure 1A) belonging to different serotypes and/or MLSTypes (Table S1). GBS GAPDH displays 44.7, 45.8 and 44.0% amino acid identity with rabbit, mice, and human GAPDH, respectively (Figure 1B). However, western blot and ELISA analysis revealed that rabbits and mice antibodies directed against GBS rGAPDH do not react with human, mouse, or rabbit GAPDH (Figure 2A and 2B). To favor the production of antibodies recognizing linear buried epitopes, mice were immunized with heat-denaturated rGAPDH (ΔT_rGAPDH). Anti-ΔT_rGAPDH antibodies purified from the sera of these animals did not show any cross-reactivity against mouse (self cross-reactivity) or human GAPDH when analyzed by western blot and ELISA (Figure 2C and 2E). These results are consistent with the fact that the longest identical stretches observed between eukaryotic and prokaryotic GADPH sequences are only 10-aminoacid long (Figure 1B).
Fig. 1. GAPDH is a conserved GBS protein. 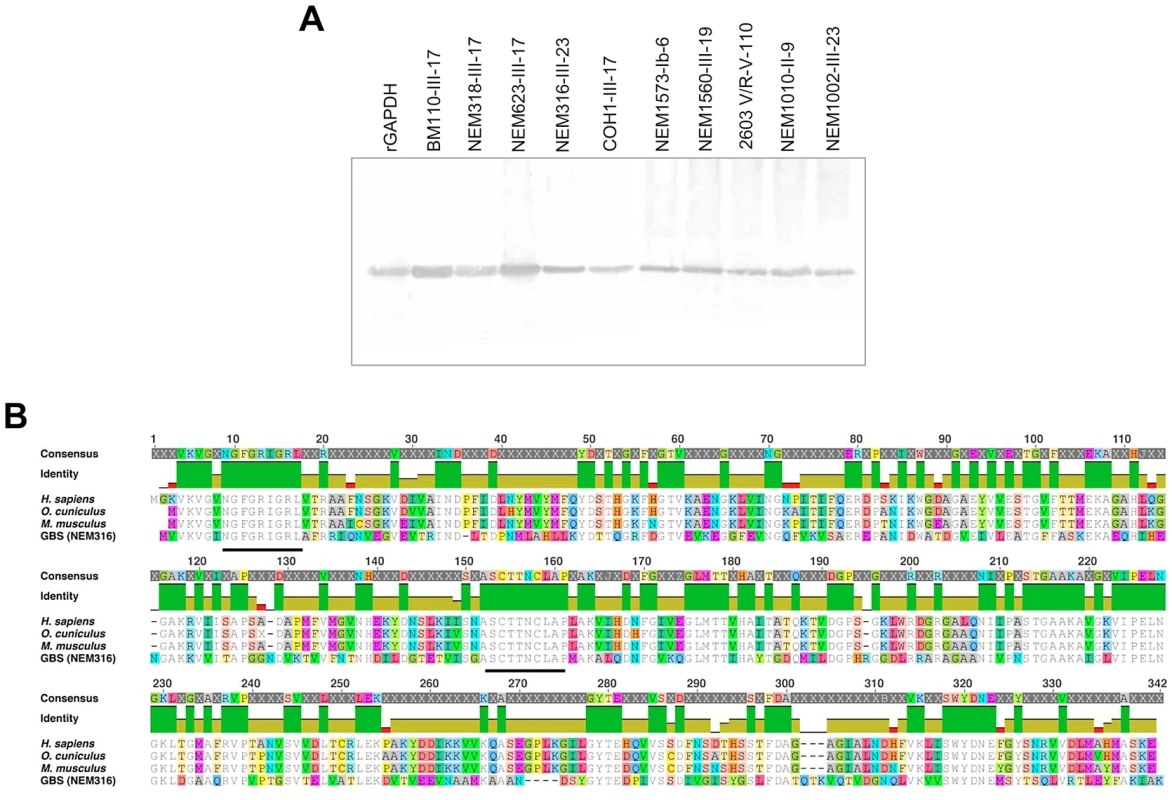
(A) Extracellular proteins from culture supernatants of different GBS clinical isolates were separated by SDS-page and analyzed by western-blot using anti-rGAPDH IgG obtained from rGAPDH-immunized rabbits. rGAPDH was used as a positive control. The data are representative of four independent experiments. (B) Multiple alignment of the aminoacid sequences of the human, rabbit, mice, and GBS GAPDH. The green vertical bars below the consensus sequences indicate identical aminoacid at the same position in all four sequences. The two heavy black lines delineate the two conserved 10-aminoacid stretches of identity. Fig. 2. Antibodies against GBS GAPDH do not cross-react with human, rabbit or mouse GAPDH. 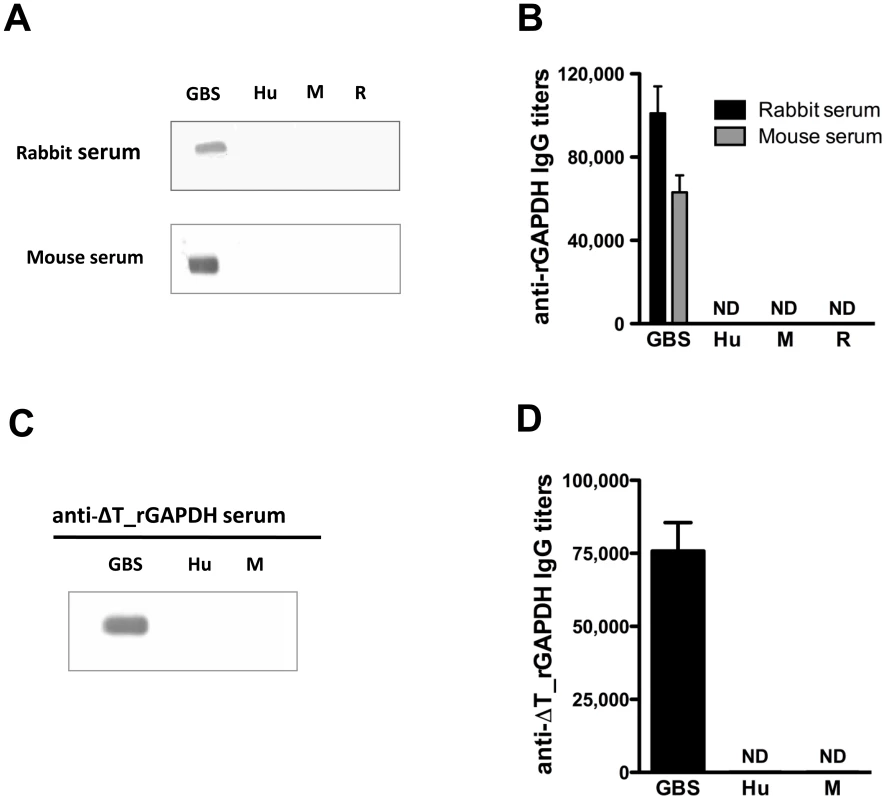
(A and B) Reactivity of rabbit and mouse anti-rGAPDH IgG against GBS, human (Hu), mouse (M) or rabbit (R) GAPDH were assessed by (A) western-blot or (B) ELISA analysis. (C and D) Reactivity of anti-rGAPDH IgG purified from the sera of mice immunized with heat-denaturated rGAPDH (ΔT_rGAPDH) against GBS, human or mouse GAPDH, were assessed by (C) western-blot or (D) ELISA analysis. ND, not detected. Maternal vaccination with rGAPDH protects neonates from GBS infection
To test whether maternal immunization with rGAPDH conferred protection to the offspring against GBS infection, female BALB/c mice were immunized with rGAPDH in alum adjuvant. Control mice were sham-immunized with the adjuvant alone.
Pups born from sham-immunized or rGAPDH-immunized females were infected intraperitoneally (i.p.) 48 h after birth with 5×106 colony-forming units (CFU) of serotype III virulent GBS strain NEM316. All but one mouse born from rGAPDH-immunized mothers survived the infection (95% survival) whereas 22 out of 27 infected pups succumbed to GBS challenge in the control group (18.5% survival) (Figure 3A). Most of the cases of GBS meningitis and LOD are caused by a serotype III hyper virulent clone, defined by multilocus sequence typing as ST-17 [30], [31], [32]. To better assess the effectiveness of maternal vaccination with rGAPDH, pups born from sham - or rGAPDH-immunized progenitors were i.p. infected 48 h after birth with 106 CFU of BM110, a serotype III GBS hyper virulent strain ST-17 (Table S1). All ST-17 GBS challenged neonates born from sham-immunized mothers died whereas mortality rate dropped to 21.4% in neonates born from rGAPDH-immunized mothers (Figure 3B). The protective effect conferred by rGAPDH maternal immunization was also observed in neonate mice infected by the subcutaneous (s.c.) route with 2.5×104 BM110 CFU. As shown in Figure 3C, none of the mice born from sham-immunized mothers survived this infectious challenge whereas only 23% of mice born from rGAPDH-immunized mothers died. Altogether, these results show that maternal vaccination with rGAPDH protected the offspring against GBS infections, including those caused by the hyper virulent strain BM110.
Fig. 3. Maternal immunization with rGAPDH protects newborn mice from GBS-induced death. 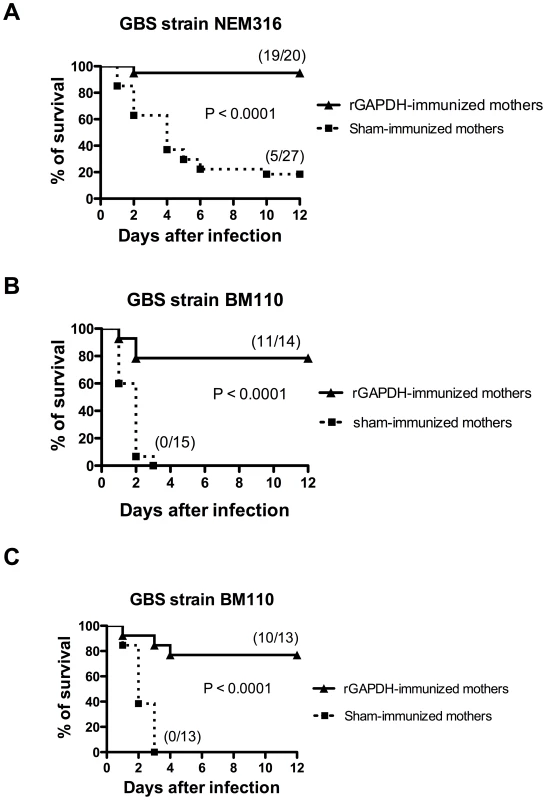
Pups born from sham- or rGAPDH-immunized mothers were infected i.p. 48 h after birth with (A) 5×106 NEM316 CFU or with (B) 106 CFU of the ST-17 hyper virulent strain BM110. (C) Mice pups were infected s.c. 48 h after birth with 2.5×104 CFU of BM110. The results represent data pooled from three independent experiments. In all figures depicting survival experiments, the numbers between parentheses represent the number of animals that survived the different infectious challenges versus the total number of infected animals. Statistical differences (P values) between immunized versus sham-immunized groups are indicated. Passive immunization with anti-rGAPDH F(ab')2-fragments protects newborns against GBS ST-17 challenge
Pups born from rGAPDH-immunized mothers presented increased serum titers of anti-rGAPDH IgG antibodies when compared with those born from sham-immunized mothers (Figure S1). To evaluate the importance of these maternal antibodies in the newborn protection against GBS infection, neonatal mice were passively immunized with purified anti-rGAPDH IgG antibodies 12 h prior to GBS challenge. The passive antibody transfer conferred protection against infection caused by the virulent NEM316 or hyper virulent BM110 strains (Figure 4A and 4B). Anti-rGAPDH IgG antibodies conferred a similar protection to neonate mice infected by the s.c. route (data not shown). As described by others [33], we observed that GAPDH is present at the cell surface of GBS strains (Figure S2) and, it was therefore conceivable that protection conferred by anti-rGAPDH antibodies could be due to an enhanced opsonophagocytosis-mediated killing of GBS. However, anti-rGAPDH IgG antibodies did not enhanced in vitro phagocytosis or complement-mediated killing of GBS BM110 cells (Figure 4C). This indicated that protection conferred by anti-rGAPDH antibodies was not mediated by these mechanisms. Furthermore, complete protection against GBS infection was observed in neonate mice treated with purified anti-rGAPDH F(ab')2 fragments 12 h before i.p. infection with BM110 strain. In contrast, all pups that received the same amount of control F(ab')2 fragments died within the first 3 days upon the infectious challenge (Figure 4D). Altogether, these results demonstrate that enhanced opsonophagocytic killing or complement activation did not mediate the observed protective effect of anti-rGAPDH antibodies.
Fig. 4. Passive immunization with purified anti-rGAPDH antibodies protects newborn mice from GBS-induced death. 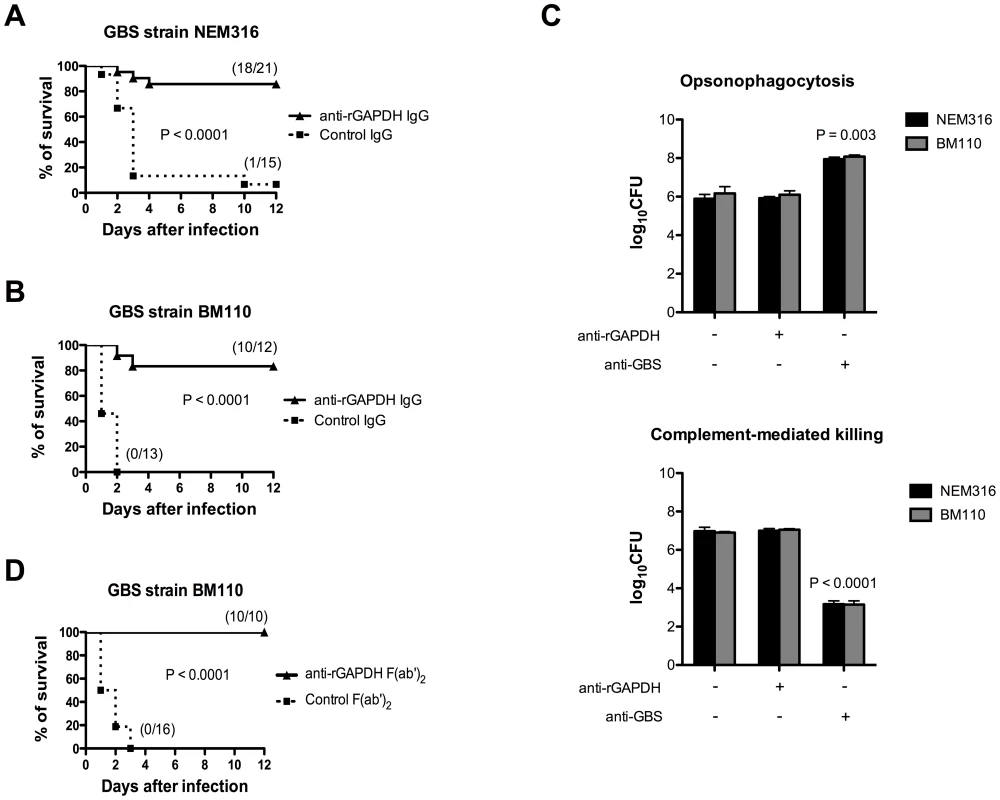
anti-rGAPDH IgG or control IgG (80 µg) were injected i.p. into mice pups and 12 h after the immunization they were infected i.p. with (A) 5×106 NEM316 CFU or with (B) 106 CFU of the ST-17 hyper virulent strain BM110. The results represent data pooled from two independent experiments. (C) Upper panel: BMM were stimulated in vitro with 106 CFU of GBS NEM316 (or BM110) plus 25 µg/mL of anti-rGAPDH IgG's, or 10% of serum containing anti-GBS antibodies, and incubated for 30 min at 37°C in 5% CO2. Data represent the mean + SEM. Results are representative of 3 independent experiments. Statistical differences (P values) are indicated. Lower panel: complement-mediated killing of GBS. Mice peripheral blood leukocytes were stimulated with 106 CFU of GBS NEM316 (or BM110) plus 25 µg/mL of anti-rGAPDH IgG's, or 10% of anti-GBS serum, and 5% of rabbit serum was added to the mixture as a source of complement. After 2 h of incubation period at 37°C, GBS CFU were evaluated on agar plates. Data represent the mean + SEM. Results are representative of 2 independent experiments. Statistical differences (P values) are indicated. Similar results were obtained when a higher concentration (100 µg/mL) of anti-rGAPDH IgG's was used. (D) Pups were passively immunized with 80 µg of anti-rGAPDH F(ab')2 fragments, or control (Fab')2 and infected 12 h later with 106 CFU of the ST-17 hyper virulent strain BM110. The results represent data pooled from two independent experiments. GBS GAPDH induces early IL-10 production in newborn mice
We have previously described a rise in IL-10 serum levels in adult mice treated with rGAPDH [29]. As shown in Figure S3, a similar increase in serum IL-10 levels was detected in newborn mice 1 h after i.p. injection of rGAPDH. Inactivation of rGAPDH enzymatic activity did not reduce this effect (Figure S3). This result indicates that IL-10 production induced by GBS GAPDH is independent of the dehydrogenase activity. We have also described that adult mice infected with GBS oeGAPDH mutant strain presented higher serum IL-10 levels than counterparts infected with WT GBS [29]. Thus, we also quantified the levels of serum IL-10 in mice pups at early times after GBS infection. As shown in Figure 5, infection of newborn mice with GBS WT strain NEM316 resulted in a rapid increase of serum IL-10 concentration. Maternal rGAPDH vaccination or treatment with anti-rGAPDH F(ab')2 fragments completely abrogated the elevated amount of IL-10 found in the sera of infected pups born from sham-immunized mothers or treated with control F(ab')2 (Figure 5A and 5B). Altogether, these results strongly suggest that the elevated IL-10 serum levels detected upon infection were due to GBS GAPDH.
Fig. 5. GAPDH neutralization abolishes IL-10 production observed in newborn mice early upon GBS infection. 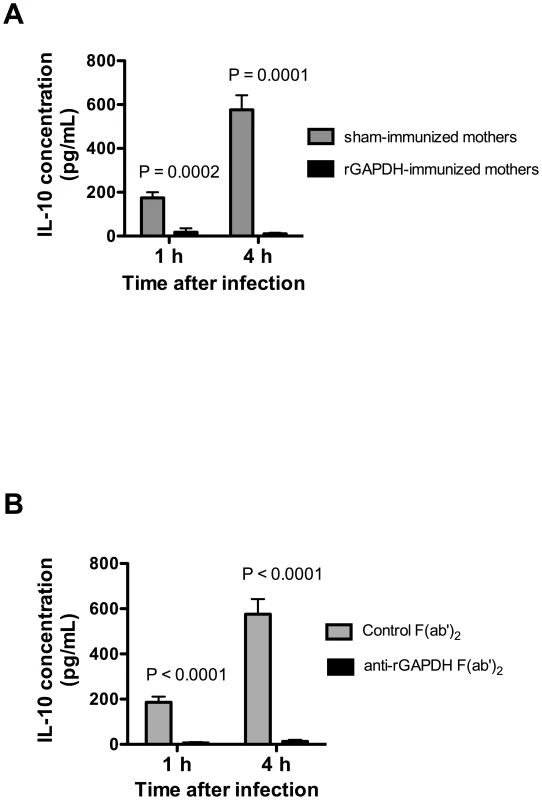
Serum IL-10 concentration in mice pups was measured 1 h and 4 h after i.p. infection with 5×106 NEM316 CFU (A) born from sham- or rGAPDH-immunized dams or (B) passively immunized with anti-rGAPDH F(ab')2 fragments or control F(ab')2. Data represent the mean + SEM. Results are a representative example out of 3 independent experiments. A minimum of 8 animals per group was used in each experiment. Statistical differences (P values) between immunized versus sham-immunized groups (A) or between pups treated with anti-rGAPDH F(ab')2 fragments versus controls (B) are indicated. Protection conferred by anti-rGAPDH antibodies is associated with inhibition of early IL-10 production in GBS-infected pups
The results presented above indicate that newborn susceptibility to GBS infection is most probably associated with early IL-10 production induced by GAPDH. To confirm this hypothesis, IL-10 deficient (IL-10−/−) pups and WT controls were infected with this bacterium. In agreement with our hypothesis, IL-10−/− pups were more resistant (78%) to GBS infection compared to WT controls (10%) (Figure 6A). To demonstrate further the essential role of IL-10 in neonatal susceptibility to GBS, newborn mice were treated with anti-IL-10 receptor (IL-10R) mAb 12 h before NEM316 or BM110 GBS challenge. As expected, most pups treated with anti-IL-10R mAb survived (86% or 82%, respectively) while all control pups died (Figure 6B and 6C). No additional protection was observed when newborn mice were treated simultaneously with anti-IL10R mAb and anti-rGAPDH IgG (Figure 7). Altogether, these results indicate that protection achieved using anti-GAPDH antibodies is due to inhibition of host IL-10 production. Several studies have shown that GBS can survive for prolonged periods within the phagolysosome of macrophages [34], [35], [36], [37]. Interestingly, we observed that the simultaneous addition of anti-rGAPDH IgG's, or anti-IL10R mAb, and GBS cells to bone marrow-derived macrophages (BMMφ) cultures inhibits the bacterial survival (Table 1). This result, combined with those shown in Figure S3, indicates that the inability of the macrophages to kill the intracellular GBS is due to IL-10 production induced by GBS GAPDH.
Fig. 6. Impairment of IL-10 signaling confers protection to newborn mice against GBS infection. 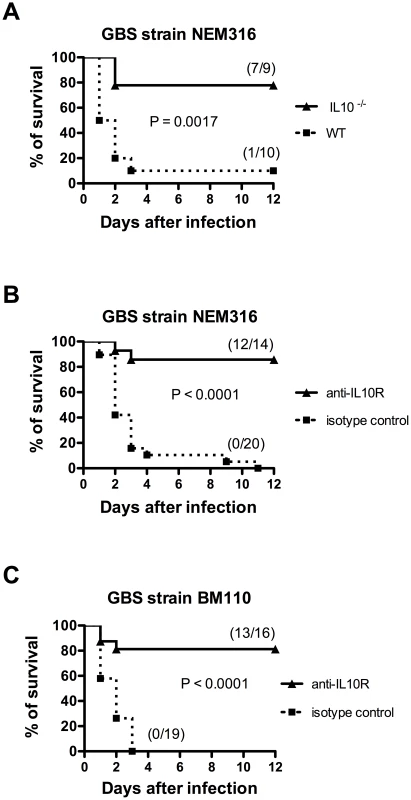
(A) IL10−/− and WT pups were infected i.p. with 5×106 NEM316 CFU (results represent data pooled from two independent experiments). (B) Newborn mice were injected i.p. with anti-IL10R mAb (anti-IL10R) or isotype control IgG (100 µg) and 12 h later were challenged i.p. with 5×106 NEM316 CFU (results represent data pooled from three independent experiments). (C) Newborn mice were injected i.p. with anti-IL10R mAb (anti-IL10R) or isotype control IgG (100 µg) and 12 h later were challenged s.c. with 2.5×104 BM110 CFU (results represent data pooled from four independent experiments). Statistical differences (P values) between IL-10−/− and WT pups (A) or between immunized versus sham-immunized groups (B and C) are indicated. Fig. 7. Simultaneous injection of anti-rGAPDH IgG's and anti-IL10R mAb does not increase survival of newborn mice infected with GBS BM110. 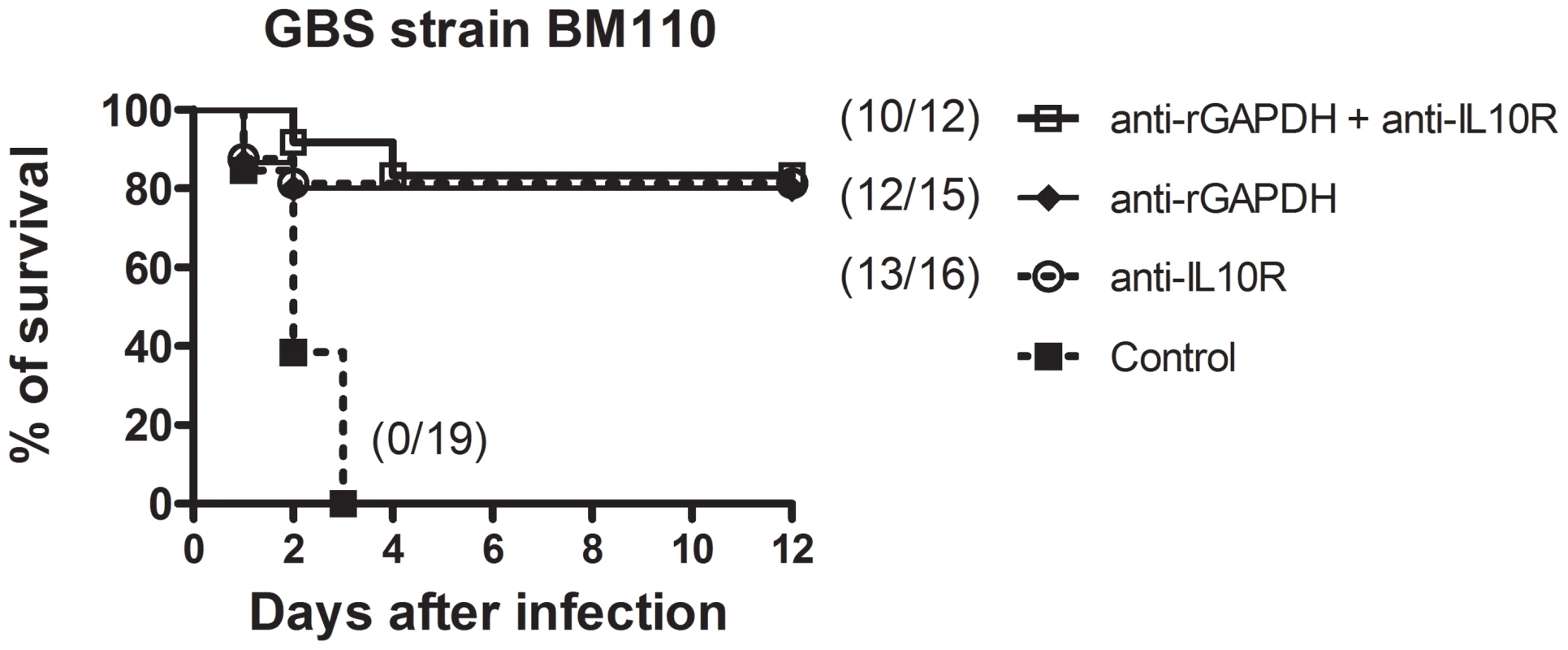
Newborn mice were treated i.p. with 100 µg of anti-IL10R mAb, anti-rGAPDH IgG's, or simultaneous with anti-IL10R mAb and anti-rGAPDH IgG's (anti-rGAPDH + anti-IL10R) 12 h before s.c. injection of 2.5×104 CFU of GBS BM110. Control pups received 100 µg of control IgG's. P<0.0001 between anti-IL10R-, anti-rGAPDH- and anti-rGAPDH+antiIL-10R-treated pups and controls. Tab. 1. Inhibition of GBS intracellular survival in cultured BMM. 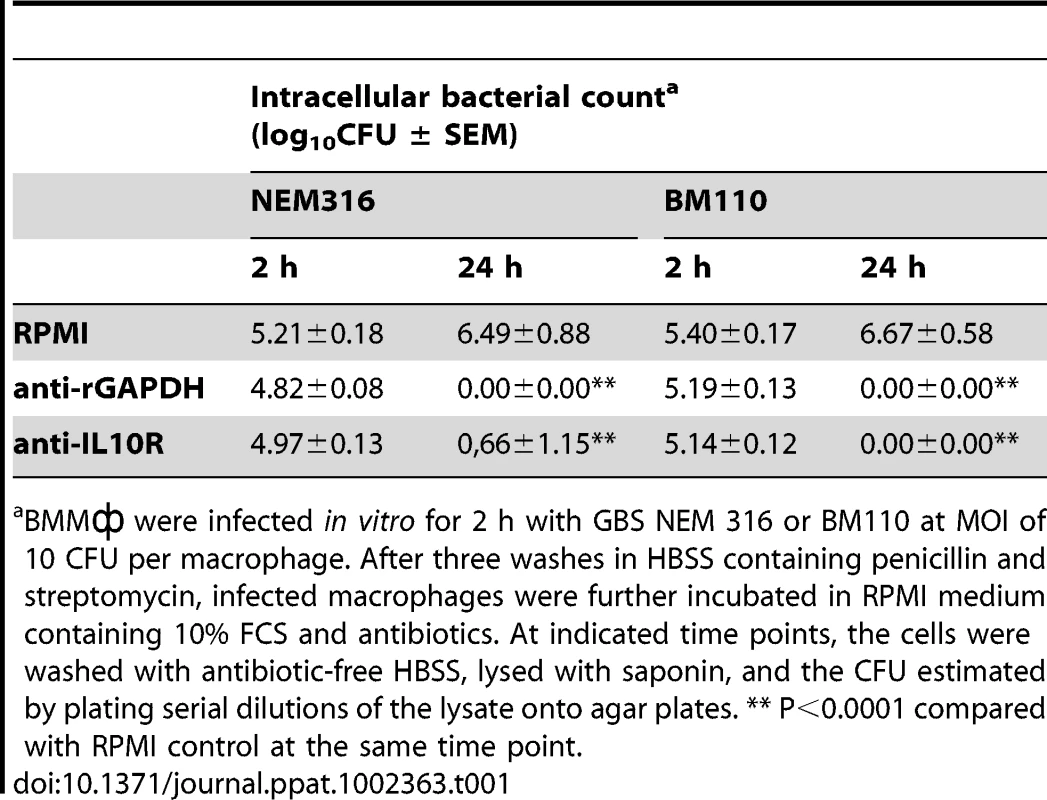
BMM were infected in vitro for 2 h with GBS NEM 316 or BM110 at MOI of 10 CFU per macrophage. After three washes in HBSS containing penicillin and streptomycin, infected macrophages were further incubated in RPMI medium containing 10% FCS and antibiotics. At indicated time points, the cells were washed with antibiotic-free HBSS, lysed with saponin, and the CFU estimated by plating serial dilutions of the lysate onto agar plates. ** P<0.0001 compared with RPMI control at the same time point. GAPDH blocks neutrophil recruitment in injured organs upon GBS infection
A plausible explanation for the observed protection induced by maternal vaccination with rGAPDH could be that GAPDH-mediated early IL-10 production, elicited upon the GBS challenge in newborns, inhibits the initiation of a host-protective inflammatory response. Neutrophil recruitment is an early event associated with protection against bacterial infection in neonates. Moreover, lack of neutrophil recruitment into infected organs has already been associated with neonatal susceptibility against GBS infections [21], [22], [23], [38]. To confirm that neutrophil recruitment into infected organs is an essential event for newborn protection against GBS infection, neutrophils of newborn mice were depleted by treatment with anti-Ly6G (1A8 clone) monoclonal antibodies (Figure S4). We observed that either blocking IL-10 signaling with neutralizing antibodies or anti-rGAPDH antibody treatment was not sufficient to protect neutropenic pups infected with a lethal inoculum of GBS (Figure 8).
Fig. 8. Neutrophils are essential for neonatal protection against GBS infection. 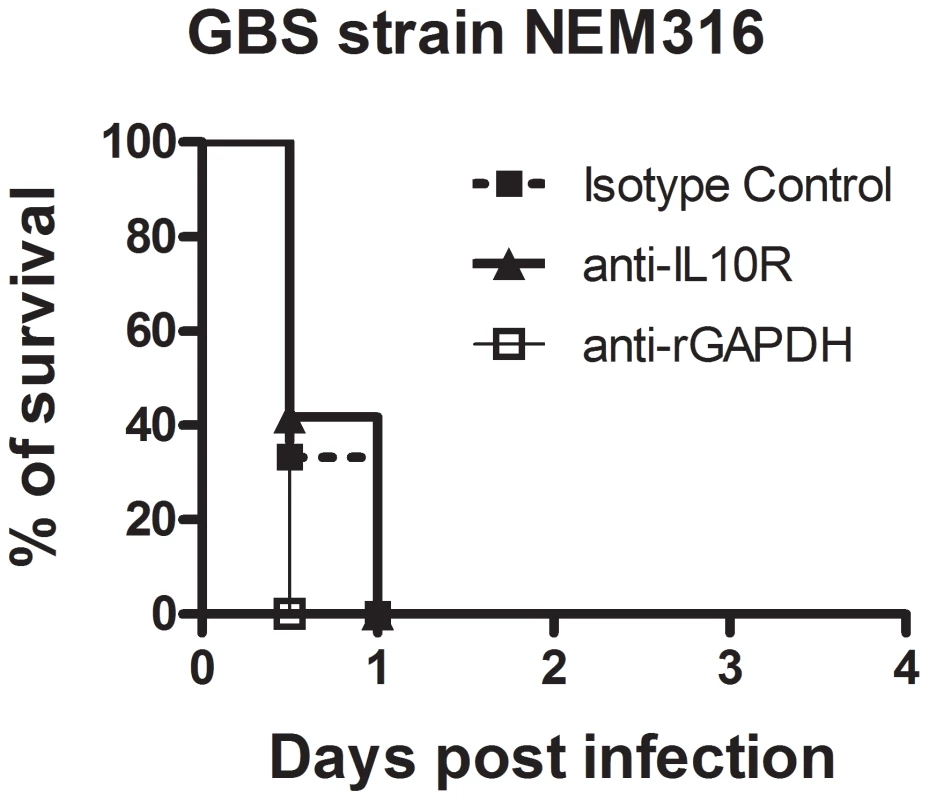
Neutropenic mice pups were injected i.p. with 100 µg of anti-rGAPDH antibodies (n = 11), anti-IL-10R mAb (n = 14) or control antibodies (n = 16) 12 h before i.p. infection with 5×106 CFU of NEM316. Results represent data pooled from three independent experiments. In addition, we assessed the numbers and frequency of neutrophils in the liver and lungs of GBS NEM316-infected pups previously treated with anti-rGAPDH IgG or anti-IL10R mAb. The frequency and the total number of neutrophils quantified 18 h after GBS challenge in the analyzed organs of infected pups treated with control IgG (or isotype control) was as low as with the non-infected pups (data not shown). Treatment with either anti-rGAPDH IgG or anti-IL-10R mAb prior to GBS infection significantly increased the neutrophil recruitment in organs (Figure 9A and 9B). Consistently, the organs of pups treated with anti-IL10R mAb or anti-rGAPDH IgG contained significantly less bacteria than those of untreated pups (Figure 9C). These results indicate that an efficient neutrophil recruitment into infected organs is crucial for neonatal protection against GBS infection whereas impaired neutrophil recruitment facilitates GBS colonization. Moreover, no bacterial colonization was detected three weeks after GBS infection in the brain or in any other organ of pups born from rGAPDH-immunized mothers and infected with NEM316 or with the ST-17 hyper-virulent strain BM110 (data not shown). These results indicate that rGAPDH-maternal vaccination is also effective in preventing LOD.
Fig. 9. GAPDH-induced IL-10 blocks neutrophil recruitment in injured organs. 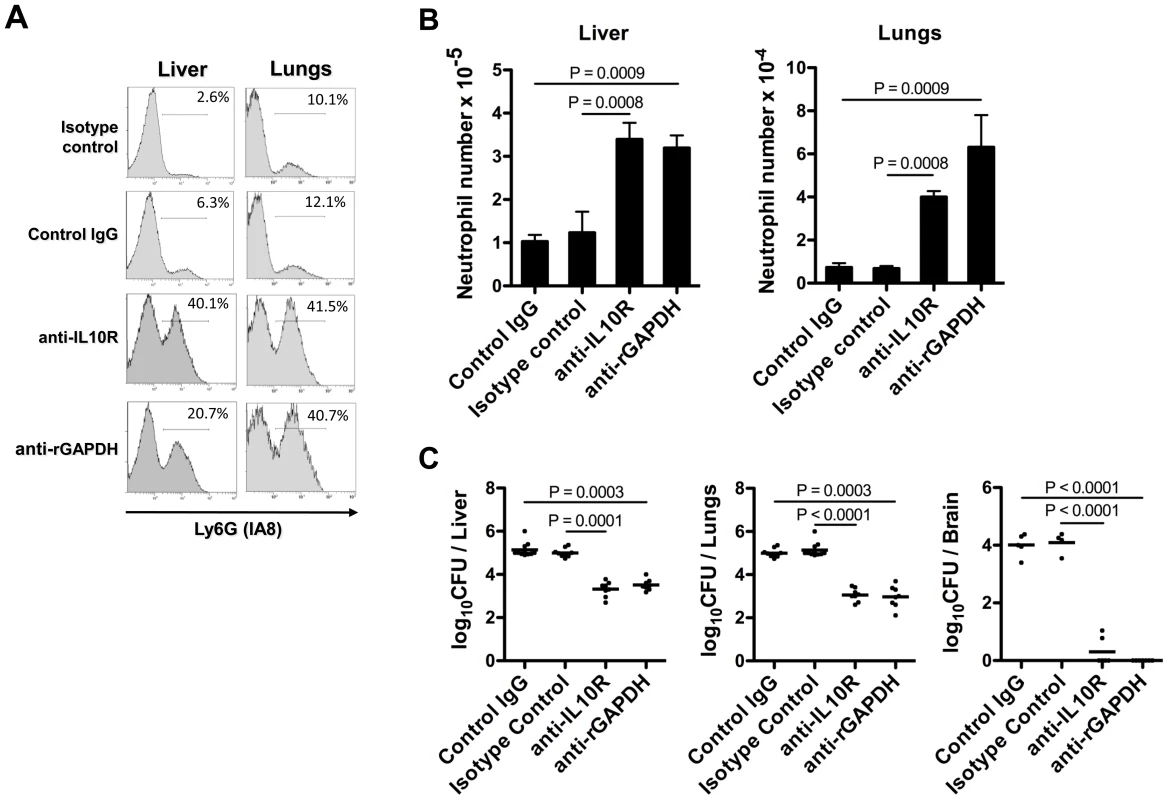
(A) Flow cytometric analysis of Ly6G expression on total lung or liver leukocyte cells from newborn mice treated i.p. with 100 µg of purified anti-rGAPDH IgG antibodies (anti-rGAPDH), of anti-IL10R mAb (anti-IL10R), or control antibodies (IgG and isotype control) and infected with 5×106 NEM316 CFU 12 h after antibody treatment. Pups were sacrificed 18 h after the bacterial challenge. The percentage of Ly6G+ cells is indicated. Results are representative of three independent experiments. (B) Total number of Ly6G+ cells (neutrophils) per organ observed in the different used groups. Data are the mean + SEM. Results are representative of 4 independent experiments and a minimal of 5 animals per group was used. Statistical differences (P values) between groups are indicated. (C) GBS CFU in the liver, lungs and brain of infected pups treated with control IgG (n = 11), isotype control IgG (n = 11), anti-IL10R (n = 8), or anti-rGAPDH (n = 8). Statistical differences (P values) between groups are indicated. Discussion
Newborns are highly susceptible to infectious disease and deficiencies of key components of the complement cascade combined with the inability to produce high amounts of antibodies against T-independent antigens greatly impairs their ability to respond to encapsulated bacteria [39]. Absence of previous interactions with environmental microbes also implies that no immunological memory exists against specific antigens, which means that the acquired immune protection of newborns relies mainly on antibodies passively transferred from their mothers [40]. In addition, previous reports indicate that neonatal innate immune cells are less efficient in producing Th1-type inflammatory cytokines, but more competent in producing the immunosuppressive cytokine IL-10 upon Toll-like receptor (TLR) engagement by microbial products [41], [42], [43], [44]. Moreover, newborn mice leukocytes are highly committed to produce increased amounts of IL-10 [44], [45], as also shown in human neonates [41], [43], [46]. As report in this work, high levels of serum IL-10 could be detected in the sera of GBS infected newborn mice early (1 h and 4 h) upon infection. This result is in agreement with a previous report by Cusumano et al. [47], showing that elevated levels of plasma IL-10 were detected in newborn mice 24 h and 48 h upon GBS challenge. These authors suggested a host protective role for IL-10 in the outcome of neonatal GBS sepsis as pre-treatment of newborn mice with recombinant IL-10 improved their survival upon a lethal s.c. GBS challenge [47]. Nevertheless, they also showed that a therapeutic administration of this cytokine (24 h after the bacterial challenge) did not improve survival. This would limit its use in human therapies because neonatal GBS infection is usually acquired before or during labor [3], [5], [6].
Our results revealed that blocking IL-10 signaling through anti-IL-10R mAb administration was sufficient to confer protection against a bacterial challenge using either the s.c. or i.p. routes. Thus, they contrastingly indicate that IL-10 has a deleterious effect in the newborn host resistance to GBS infection. The increased resistance of IL-10-deficient neonates to GBS infection reported here constitutes further support for a deleterious effect of IL-10 in host resistance to GBS. As shown here, the GBS GAPDH induced host IL-10 production detected early after bacterial infection.
IL-10 is produced by multiple cell types and inhibits leukocyte activation, pro-inflammatory cytokine production and down-regulates the expression of anti-microbial molecules on activated phagocytes [48], [49], [50], [51]. IL-10 also inhibits production of CC and CXC chemokines by activated monocytes [52], [53], [54]. Since these chemokines are implicated in the recruitment of leukocytes during inflammation, IL-10 production indirectly inhibits leukocyte trafficking to inflamed tissues [50], [55], [56].
IL-10 production was already associated with host susceptibility against different pathogens [24], [45], [57], [58], [59], [60], [61]. We show here that treatment of pups with either anti-IL10R mAb or anti-rGAPDH IgG prior to the GBS challenge increased the neutrophil recruitment in liver and lungs that is triggered upon infection. Neutrophil recruitment is a crucial event in the host effector immune response to GBS [21], [62], [63] and, consequently, lack of neutrophil infiltration in infected sites has been reported in cases of severe early-onset GBS sepsis [21], [22], [23], [38]. Thus, neutralization of GAPDH, and hence blockade of the induced IL-10 production, allowed an effective immune response at an early stage of infection that prevented death of pups. Moreover, pups protected by maternal immunization with rGAPDH presented no GBS CFU in the brain, lungs, and liver 3 weeks upon the infectious challenge. This indicates that protection achieved by this vaccination strategy might prevent LOD.
The recruitment of neutrophils into infected tissues is very important to restrain bacterial replication. Thus, qualitative and quantitative deficiencies in the neutrophils of newborns may explain the observed susceptibility to GBS infections. Indeed, newborn neutrophils have reduced adhesion capabilities due to reduced expression of adhesion molecules [24], [25] and they produced a limited number of microbicidal molecules. Moreover, the number of these cells is also reduced when compared to adults due to insufficiencies on neonatal granulocyte lineage development [26]. As a consequence, the intra-cellular and extracellular killing of pathogens is greatly impaired in neonates [64]. However, our results indicate that, despite these serious functional defects, the neutrophils of neonates can control the GBS infections as long as the inhibitory effect of IL-10 is blocked. Importantly, IL-10 blockade with a specific mAb did not significantly decreased the elevated serum TNF-α levels detected upon GBS infection in neonate mice [47]. This further suggests that impairment of neutrophil recruitment rather than inhibition of pro-inflammatory cytokines could be the prominent effect of IL-10 produced in the course of neonatal GBS infection.
Neonatal sepsis is a pathological condition associated with elevated levels of pro-inflammatory cytokines, including IL-1β, TNF-α, and IL-6. However, it has been previously described that cord blood or plasma IL-10 concentration is significantly increased in neonatal sepsis, constituting an early indicator of prognosis [27], [28]. Of interest, it was also reported that high IL-10 levels are found in children at initial phases of fulminant septic shock [65], [66]. This indicates that early IL-10 production, instead of being a physiological attempt to counterbalance the elevated levels of pro-inflammatory cytokines, could be a predisposing factor for disease. Our results are in accordance with this hypothesis and they provide the first evidence that the lack of neutrophil recruitment in infected organs combined with elevated cord blood IL-10 concentration may account for neonatal susceptibility to GBS infection. Hence, the discovery of GAPDH as an extracellular virulence factor of GBS that induces an early IL-10 production by the infected host could be a significant contribution to our understanding of the pathology of neonatal infections.
GAPDH is a promising candidate for a human GBS vaccine because it is an essential metabolic enzyme that also plays a critical role in virulence. Our results show that maternal vaccination with rGAPDH protects the offspring against GBS lethal infection, including those caused by the hyper virulent ST-17 clone, which is responsible for most cases of neonatal meningitis [31], [32], [38]. As a consequence, maternal rGAPDH vaccination might efficiently protect against both EOD and LOD [5], [67], [68]. We demonstrated that passive immunization of neonates with GAPDH-specific IgG antibodies is sufficient to confer protection against GBS infection. Importantly, rGAPDH maternal vaccination prevents the early production of IL-10 in GBS infected pups and similar protective effect was obtained when GAPDH-specific antibody F(ab')2 fragments were used instead of whole IgG. These results indicate that neutralization of GAPDH-mediated IL-10 production, rather than complement activation or bacterial opsonophagocytosis, accounts for the observed protection. The extracellular GAPDH was detected at the bacterial surface and in culture supernatants of GBS isolates, which suggests that neutralization of its biological activity by antibody binding should not be sterically impaired by surface capsular polysaccharides. Recently, Margarit et al. showed that pili proteins could be used as a human vaccine to prevent GBS infections but, due to sequence variability, a combination of 3 antigens was required to confer protection against 94% of contemporary GBS strains [19]. It is likely that under selective pressure this vaccine will select GBS variants expressing new pili antigens, as shown for Neisseria gonorrhoeae [69], [70], [71], [72], [73]. In contrast, since it is an essential and highly conserved metabolic enzyme, GAPDH is unlikely to accumulate rapidly escape mutations or rearrangements under such a selective immune pressure.
Taken together, our results demonstrate that extracellular GAPDH confers a selective advantage to GBS for survival in the infected host. In particular, GBS GAPDH acts on the host immune system to elicit IL-10 production thereby favoring bacterial colonization and survival. As we demonstrated that GBS GAPDH was still able to induce host IL-10 production upon exposure to an oxidative agent, this mechanism may still operate within the highly oxidative environment resulting from the host inflammatory response. Our data highlight the critical role played by this immunosuppressive cytokine in determining susceptibility to GBS infection at an early time after birth. Our results also show that GBS-associated pathology can be counteracted either by rGAPDH vaccination or IL-10 neutralization. In the future, it will be essential to explore the use of either strategy to induce protection towards other human neonatal pathogens.
Materials and Methods
Bacterial strains
Relevant characteristics of the GBS strains used in this study are summarized in Table S1. Escherichia coli BL21 (DE3) strain (Novagen) and the pET28a plasmid (Novagen) were used for production of recombinant GAPDH (rGAPDH) as described previously [29]. GBS was grown in Todd-Hewitt broth or agar (Difco Laboratories) containing 0.001 mg/mL of colistin sulphate and 0.5 µg/mL of oxalinic acid (Streptococcus Selective Supplement, Oxoid) and E. coli was cultured on Luria-Bertani medium. Bacteria were grown at 37°C.
Animals
Male and female BALB/c mice (6-8 weeks old) were purchased from Charles River. IL-10-deficient BALB/c (IL-10−/−) mice were kindly provided by Dr. A. O'Garra (National Institute for Medical Research, London, U.K.). New Zealand White rabbits were purchased from Charles River. Animals were kept at the animal facilities of the Institute Abel Salazar during the time of the experiments.
Ethics statement
This study was carried out in strict accordance with the recommendations of the European Convention for the Protection of Vertebrate Animals used for Experimental and Other Scientific Purposes (ETS 123) and 86/609/EEC Directive and Portuguese rules (DL 129/92). The animal experimental protocol was approved by the competent national authority Direcção Geral de Veterinária (DGV) (Protocol Permit Number: 0420/000/000/2008). All animal experiments were planned in order to minimize mice suffering.
Preparation of active and inactive recombinant GAPDH
Recombinant GAPDH (rGAPDH) was purified as described in detail previously [29]. Enzymatically inactive rGAPDH (inact-rGAPDH) was obtained by pretreatment of the enzyme with 500 µM H2O2. The lack of enzymatic activity upon inactivation was confirmed using a previously described enzymatic assay for GAPDH [29].
Maternal immunizations with rGAPDH
Recombinant GAPDH was used for maternal immunization assays. Female mice were injected intraperitoneally (i.p.) twice, with a 3-week intervening period, with 200 µL of a preparation containing 25 µg of rGAPDH in a 1 : 20 PBS/alum suspension (Aluminium hydroxide Gel; a kind gift of Dr Erik Lindblad, Biosector, Frederickssund, Denmark). The sham-immunized control animals received 200 µL of a 1 : 20 PBS/alum suspension. Immediately after the second injection, female and male mice were paired. Females were monitored closely during gestation and the day of delivery was recorded. Serum anti-rGAPDH antibody titers were determined by ELISA as previously described [29].
Antibody treatments
Antibody treatments were performed in newborn BALB/c mice 12 h prior to GBS infection. For passive immunizations, pups were injected i.p. with 100 µg of anti-rGAPDH IgG antibodies or anti-rGAPDH F(ab')2 fragments. Control animals received the same amount of control IgG's or F(ab')2 fragments issued from control IgG's. For IL-10 signaling blocking, 100 µg of anti-IL10R antibodies (1B1.3a, Schering-Plough Corporation) were administered i.p. and control animals received the same amount of matched isotype control antibody.
Challenging infections of newborn mice
Newborn mice were infected i.p. with 5×106 cells of GBS NEM316 or 106 cells of GBS BM110 (ST-17), 48 h after birth in a maximum volume of 40 µL. Subcutaneous (s.c.) infections were performed 48 h with 2.5×104 cells of GBS BM110 after birth in a total volume of 20 µL. Survival curves were determined in a 12-day experiment period and newborns were kept with their mothers during the entire time of the experiment. The liver, lungs, and brain of infected pups were aseptically removed at indicated time points and homogenized in PBS and serial dilutions of homogenized organs were plated on Todd-Hewitt agar to enumerate bacterial CFU.
Purification of anti-rGAPDH IgG antibodies
Adult mice or rabbit were immunized twice with 25 µg of rGAPDH in a PBS/alum suspension as described above and sera were collected 10 days after the second immunization. Pooled serum samples were applied to a Protein G HP affinity column (HiTrap, GE Healthcare Bio-Sciences AB) and purified IgG antibodies were then passed through an affinity column with immobilized rGAPDH (Hi-trap NHS-activated HP, GE Healthcare Bio-Sciences AB). Control IgGs were obtained from sera of mice or rabbits sham-immunized with a PBS/alum suspension and purified on a Protein G HP affinity column. Purified IgG antibody fractions were further equilibrated in PBS and stored at -80°C in frozen aliquots.
Preparation of anti-GBS serum
GBS-specific antiserum was obtained from mice immunized i.p. twice (with a 3-week interval) with isopropanol-fixed 105 GBS cells plus alum (total volume). Serum from immunized animals (Anti-GBS serum) was obtained from retro-orbital bleeding 10 days after the second immunization.
Preparation of F(ab')2 fragments
F(ab')2 fragments from anti-rGAPDH or control IgGs were obtained using IgG1 F(ab) and F(ab')2 Preparation Kit (Pierce) used according to manufacturer's instructions.
Opsonophagocytic assays
Bone marrow-derived macrophages (BMM) purified as described previously [74] were plated in 96-well plates (105 BMM/ well) and stimulated for 30 min at 37°C 5%CO2 with 106 CFU of GBS BM110 (or NEM316) in the medium alone (RPMI), the medium containing 25 µg/mL of anti-rGAPDH IgG's, or medium with 10% of serum containing anti-GBS IgG antibodies. After this incubation period, the plates were washed three times with HBSS to remove extracellular bacteria. To enumerate intracellular GBS CFU, 10% saponin (1∶100 dilution) was added to wells and serial dilutions of supernatant were plated onto agar plates.
Complement-mediated killing assay
Blood from adult mice was collected in heparinated tubes and diluted 1 : 1 in HBSS. 106 GBS NEM316 (or BM110) CFU with 25 µg/mL of anti-rGAPDH IgG's or 10% of serum containing anti-GBS IgG antibodies were then added. Rabbit serum (5%) was added to the mixture as a source of complement. After 2 h of incubation at 37°C, serial dilutions of the mixture were plated onto agar plates to evaluate complement-mediated GBS killing.
Intracellular survival of GBS in macrophages
The BMM, obtained as described previously [74], were infected with GBS strains NEM316 and BM110 at a macrophage:bacteria ratio of 1 : 10 in RPMI containing 10% FCS. Microplates were incubated for 2 h at 37°C in 5% CO2 for GBS phagocytosis. After this period, culture supernatants of infected macrophages were removed by aspiration and cells were washed three times (10 min for each wash) with HBSS containing penicillin (100 IU/mL) and streptomycin (50 µg/mL) to kill extracellular bacteria. Infected macrophages were further incubated in RPMI medium containing 10% FCS and the same concentrations of antibiotics. To quantify intracellular GBS, the supernatants containing antibiotics were removed, the cells were washed with antibiotic-free HBSS, lysed with saponin (0,1% final concentration), and the CFU were estimated by plating serial dilutions of the lysate onto agar plates.
Neutrophil recruitment
Neutrophil recruitment in liver and lungs of infected pups was evaluated by flow cytometry analysis. Briefly, 18 h after GBS infection, the organs were collected, gently homogenized in HBSS (Sigma), and passed through glass wool to remove cellular aggregates. PerCP/Cy5.5 anti-mouse Ly-6G antibody (clone 1A8; Biolegend) was used for neutrophil detection. Cells were analyzed by an Epics XL cytometer (Beckman Coulter).
Neutrophil depletion
Newborn mice were depleted of neutrophils by treatment with anti-Ly6G antibodies (clone 1A8, Biolegend). Antibody treatment was performed twice, 12 h before and immediately after GBS challenge. Each pup was injected with a total of 80 µg of anti-Ly-6G antibodies.
Interleukin-10 quantification
IL-10 was quantitated in the serum of newborn mice with an ELISA kit (eBioscience) used according to the manufacturer's instruction.
Detection of GAPDH
The presence of GAPDH in the culture supernatants of GBS strains was visualized by Western-blot analysis. Extracellular proteins were isolated as described previously [29]. The reactivity of purified anti-rGAPDH IgG antibodies obtained from the serum of rGAPDH immunized mice or rabbits against self or human GAPDH, was determined by Western-blot analysis or ELISA. Human, rabbit, and mouse GAPDH were purified from human erythrocytes, rabbit erythrocytes or mouse muscle as previously described [75], [76].
Statistical analysis
Student's T test was used to analyze the differences between groups. Survival studies were analyzed with the log-rank test. A P value<0.05 was considered statistically significant.
Supporting Information
Zdroje
1. BakerCJBarrettFFGordonRCYowMD 1973 Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr 82 724 729
2. BartonLLFeiginRDLinsR 1973 Group B beta hemolytic streptococcal meningitis in infants. J Pediatr 82 719 723
3. EdwardsMS 2006 Issues of antimicrobial resistance in group B streptococcus in the era of intrapartum antibiotic prophylaxis. Semin Pediatr Infect Dis 17 149 152
4. HeathPTSchuchatA 2007 Perinatal group B streptococcal disease. Best Pract Res Clin Obstet Gynaecol 21 411 424
5. JohriAKPaolettiLCGlaserPDuaMSharmaPK 2006 Group B Streptococcus: global incidence and vaccine development. Nat Rev Microbiol 4 932 942
6. ZangwillKMSchuchatAWengerJD 1992 Group B streptococcal disease in the United States, 1990: report from a multistate active surveillance system. MMWR CDC Surveill Summ 41 25 32
7. JordanHTFarleyMMCraigAMohle-BoetaniJHarrisonLH 2008 Revisiting the need for vaccine prevention of late-onset neonatal group B streptococcal disease: a multistate, population-based analysis. Pediatr Infect Dis J 27 1057 1064
8. FlueggeKSiedlerAHeinrichBSchulte-MoentingJMoennigMJ 2006 Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics 117 e1139 1145
9. HajduABlystadHHoibyEAKloumanESchimmerB 2006 Unexpected increase in case fatality of invasive group B streptococcal infections in infants in Norway, January-July 2006. Euro Surveill 11: E060727 060722
10. HeathPTBalfourGWeisnerAMEfstratiouALamagniTL 2004 Group B streptococcal disease in UK and Irish infants younger than 90 days. Lancet 363 292 294
11. BaltimoreRS 2007 Consequences of prophylaxis for group B streptococcal infections of the neonate. Semin Perinatol 31 33 38
12. CastorMLWhitneyCGComo-SabettiKFacklamRRFerrieriP 2008 Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol 2008 727505 727505
13. DoranKSNizetV 2004 Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol Microbiol 54 23 31
14. AmundsonNRFloresAEHillierSLBakerCJFerrieriP 2005 DNA macrorestriction analysis of nontypeable group B streptococcal isolates: clonal evolution of nontypeable and type V isolates. J Clin Microbiol 43 572 576
15. KongFLambertsenLMSlotvedHCKoDWangH 2008 Use of phenotypic and molecular serotype identification methods to characterize previously nonserotypeable group B streptococci. J Clin Microbiol 46 2745 2750
16. RamaswamySVFerrieriPFloresAEPaolettiLC 2006 Molecular characterization of nontypeable group B streptococcus. J Clin Microbiol 44 2398 2403
17. RamaswamySVFerrieriPMadoffLCFloresAEKumarN 2006 Identification of novel cps locus polymorphisms in nontypable group B Streptococcus. J Med Microbiol 55 775 783
18. MaioneDMargaritIRinaudoCDMasignaniVMoraM 2005 Identification of a universal Group B streptococcus vaccine by multiple genome screen. Science 309 148 150
19. MargaritIRinaudoCDGaleottiCLMaioneDGhezzoC 2009 Preventing bacterial infections with pilus-based vaccines: the group B streptococcus paradigm. J Infect Dis 199 108 115
20. FriedmanCARobbinsKKTempleDMMillerCJRawsonJE 1996 Survival and neutrophil kinetics in infants with severe group B streptococcal disease treated with gamma globulin. J Perinatol 16 439 442
21. HillHRBohnsackJFMorrisEZAugustineNHParkerCJ 1988 Group B streptococci inhibit the chemotactic activity of the fifth component of complement. J Immunol 141 3551 3556
22. HemmingVGMcCloskeyDWHillHR 1976 Pneumonia in the neonate associated with group B streptococcal septicemia. Am J Dis Child 130 1231 1233
23. QuiranteJCeballosRCassadyG 1974 Group B beta-hemolytic streptococcal infection in the newborn. I. Early onset infection. Am J Dis Child 128 659 665
24. AndersonDCHughesBJSmithCW 1981 Abnormal mobility of neonatal polymorphonuclear leukocytes. Relationship to impaired redistribution of surface adhesion sites by chemotactic factor or colchicine. J Clin Invest 68 863 874
25. BuhrerCGraulichJStibenzDDudenhausenJWObladenM 1994 L-selectin is down-regulated in umbilical cord blood granulocytes and monocytes of newborn infants with acute bacterial infection. Pediatr Res 36 799 804
26. KoenigJMYoderMC 2004 Neonatal neutrophils: the good, the bad, and the ugly. Clin Perinatol 31 39 51
27. CancelierACPetronilhoFReinkeAConstantinoLMachadoR 2009 Inflammatory and oxidative parameters in cord blood as diagnostic of early-onset neonatal sepsis: a case-control study. Pediatr Crit Care Med 10 467 471
28. RomagnoliCFrezzaSCingolaniADe LucaAPuopoloM 2001 Plasma levels of interleukin-6 and interleukin-10 in preterm neonates evaluated for sepsis. Eur J Pediatr 160 345 350
29. MadureiraPBaptistaMVieiraMMagalhaesVCameloA 2007 Streptococcus agalactiae GAPDH is a virulence-associated immunomodulatory protein. J Immunol 178 1379 1387
30. JonesNBohnsackJFTakahashiSOliverKAChanMS 2003 Multilocus sequence typing system for group B streptococcus. J Clin Microbiol 41 2530 2536
31. LamyMCDramsiSBilloetAReglier-PoupetHTaziA 2006 Rapid detection of the “highly virulent” group B Streptococcus ST-17 clone. Microbes Infect 8 1714 1722
32. PoyartCReglier-PoupetHTaziABilloetADmytrukN 2008 Invasive group B streptococcal infections in infants, France. Emerg Infect Dis 14 1647 1649
33. SeifertKNMcArthurWPBleiweisASBradyLJ 2003 Characterization of group B streptococcal glyceraldehyde-3-phosphate dehydrogenase: surface localization, enzymatic activity, and protein-protein interactions. Can J Microbiol 49 350 356
34. CornacchionePScaringiLFettucciariKRosatiESabatiniR 1998 Group B streptococci persist inside macrophages. Immunology 93 86 95
35. MonteiroGCHirataRJrAndradeAFMattos-GuaraldiALNagaoPE 2004 Surface carbohydrates as recognition determinants in non-opsonic interactions and intracellular viability of group B Streptococcus strains in murine macrophages. Int J Mol Med 13 175 180
36. QuachDvan SorgeNMKristianSABryanJDShelverDW 2009 The CiaR response regulator in group B Streptococcus promotes intracellular survival and resistance to innate immune defenses. J Bacteriol 191 2023 2032
37. Valenti-WeigandPBenkelPRohdeMChhatwalGS 1996 Entry and intracellular survival of group B streptococci in J774 macrophages. Infect Immun 64 2467 2473
38. LiuGYNizetV 2004 Extracellular virulence factors of group B Streptococci. Front Biosci 9 1794 1802
39. Marshall-ClarkeSReenDTaskerLHassanJ 2000 Neonatal immunity: how well has it grown up? Immunol Today 21 35 41
40. LemkeHCoutinhoALangeH 2004 Lamarckian inheritance by somatically acquired maternal IgG phenotypes. Trends Immunol 25 180 186
41. BelderbosMEvan BleekGMLevyOBlankenMOHoubenML 2009 Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin Immunol 133 228 237
42. ChelvarajanLPopaDLiuYGetchellTVStrombergAJ 2007 Molecular mechanisms underlying anti-inflammatory phenotype of neonatal splenic macrophages. J Leukoc Biol 82 403 416
43. KollmannTRCrabtreeJRein-WestonABlimkieDThommaiF 2009 Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 183 7150 7160
44. SunCMDeriaudELeclercCLo-ManR 2005 Upon TLR9 signaling, CD5+ B cells control the IL-12-dependent Th1-priming capacity of neonatal DCs. Immunity 22 467 477
45. GenoveseFMancusoGCuzzolaMBiondoCBeninatiC 1999 Role of IL-10 in a neonatal mouse listeriosis model. J Immunol 163 2777 2782
46. RainsfordEReenDJ 2002 Interleukin 10, produced in abundance by human newborn T cells, may be the regulator of increased tolerance associated with cord blood stem cell transplantation. Br J Haematol 116 702 709
47. CusumanoVGenoveseFMancusoGCarboneMFeraMT 1996 Interleukin-10 protects neonatal mice from lethal group B streptococcal infection. Infect Immun 64 2850 2852
48. FiorentinoDFZlotnikAMosmannTRHowardMO'GarraA 1991 IL-10 inhibits cytokine production by activated macrophages. J Immunol 147 3815 3822
49. FiorentinoDFZlotnikAVieiraPMosmannTRHowardM 1991 IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 146 3444 3451
50. MooreKWde Waal MalefytRCoffmanRLO'GarraA 2001 Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19 683 765
51. SaraivaMO'GarraA 2010 The regulation of IL-10 production by immune cells. Nat Rev Immunol 10 170 181
52. BerkmanNJohnMRoesemsGJosePJBarnesPJ 1995 Inhibition of macrophage inflammatory protein-1 alpha expression by IL-10. Differential sensitivities in human blood monocytes and alveolar macrophages. J Immunol 155 4412 4418
53. KopydlowskiKMSalkowskiCACodyMJvan RooijenNMajorJ 1999 Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J Immunol 163 1537 1544
54. Marfaing-KokaAMaravicMHumbertMGalanaudPEmilieD 1996 Contrasting effects of IL-4, IL-10 and corticosteroids on RANTES production by human monocytes. Int Immunol 8 1587 1594
55. Zuany-AmorimCCreminonCNeversMCNahoriMAVargaftigBB 1996 Modulation by IL-10 of antigen-induced IL-5 generation, and CD4+ T lymphocyte and eosinophil infiltration into the mouse peritoneal cavity. J Immunol 157 377 384
56. Zuany-AmorimCHaileSLeducDDumareyCHuerreM 1995 Interleukin-10 inhibits antigen-induced cellular recruitment into the airways of sensitized mice. J Clin Invest 95 2644 2651
57. BelkaidYHoffmannKFMendezSKamhawiSUdeyMC 2001 The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med 194 1497 1506
58. MurphyMLWilleUVillegasENHunterCAFarrellJP 2001 IL-10 mediates susceptibility to Leishmania donovani infection. Eur J Immunol 31 2848 2856
59. ReedSGBrownellCERussoDMSilvaJSGrabsteinKH 1994 IL-10 mediates susceptibility to Trypanosoma cruzi infection. J Immunol 153 3135 3140
60. RoqueSNobregaCAppelbergRCorreia-NevesM 2007 IL-10 underlies distinct susceptibility of BALB/c and C57BL/6 mice to Mycobacterium avium infection and influences efficacy of antibiotic therapy. J Immunol 178 8028 8035
61. SilvaRAAppelbergR 2001 Blocking the receptor for interleukin 10 protects mice from lethal listeriosis. Antimicrob Agents Chemother 45 1312 1314
62. CleatPHWellsCCoidCR 1984 Electron microscopic evidence of antibody entry into neutrophils after phagocytosis of highly virulent group B streptococci. J Gen Microbiol 130 3059 3061
63. SchuitKEDeBiasioR 1980 Kinetics of phagocyte response to group B streptococcal infections in newborn rats. Infect Immun 28 319 324
64. LevyOMartinSEichenwaldEGanzTValoreE 1999 Impaired innate immunity in the newborn: newborn neutrophils are deficient in bactericidal/permeability-increasing protein. Pediatrics 104 1327 1333
65. DerkxBMarchantAGoldmanMBijlmerRvan DeventerS 1995 High levels of interleukin-10 during the initial phase of fulminant meningococcal septic shock. J Infect Dis 171 229 232
66. LehmannAKHalstensenASornesSRokkeOWaageA 1995 High levels of interleukin 10 in serum are associated with fatality in meningococcal disease. Infect Immun 63 2109 2112
67. SchragSJZywickiSFarleyMMReingoldALHarrisonLH 2000 Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med 342 15 20
68. SchuchatA 1998 Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev 11 497 513
69. Abu-AsabMSLaassriMAmriH 2010 Algorithmic assessment of vaccine-induced selective pressure and its implications on future vaccine candidates. Adv Bioinformatics 2010 178069 178069
70. HaasRMeyerTF 1986 The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell 44 107 115
71. HelmRASeifertHS 2009 Pilin antigenic variation occurs independently of the RecBCD pathway in Neisseria gonorrhoeae. J Bacteriol 191 5613 5621
72. HillSADaviesJK 2009 Pilin gene variation in Neisseria gonorrhoeae: reassessing the old paradigms. FEMS Microbiol Rev 33 521 530
73. KlineKACrissAKWallaceASeifertHS 2007 Transposon mutagenesis identifies sites upstream of the Neisseria gonorrhoeae pilE gene that modulate pilin antigenic variation. J Bacteriol 189 3462 3470
74. TushinskiRJOliverITGuilbertLJTynanPWWarnerJR 1982 Survival of mononuclear phagocytes depends on a lineage-specific growth factor that the differentiated cells selectively destroy. Cell 28 71 81
75. HeRQYangMDZhengXZhouJX 1995 Isolation and some properties of glycated D-glyceraldehyde-3-phosphate dehydrogenase from rabbit muscle. Biochem J 309 Pt 1 133 139
76. MountassifDBaibaiTFourratLMoutaouakkilAIddarA 2009 Immunoaffinity purification and characterization of glyceraldehyde-3-phosphate dehydrogenase from human erythrocytes. Acta Biochim Biophys Sin (Shanghai) 41 399 406
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other SpeciesČlánek Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome ofČlánek The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 11- Jak souvisí postcovidový syndrom s poškozením mozku?
- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Microbial Spy Games and Host Response: Roles of a Small Molecule in Communication with Other Species
- Simple Rapid Near-Patient Diagnostics for Tuberculosis Remain Elusive—Is a “Treat-to-Test” Strategy More Realistic?
- Ultra-Efficient PrP Amplification Highlights Potentialities and Pitfalls of PMCA Technology
- Assessing Predicted HIV-1 Replicative Capacity in a Clinical Setting
- Inhibition of IL-10 Production by Maternal Antibodies against Group B Streptococcus GAPDH Confers Immunity to Offspring by Favoring Neutrophil Recruitment
- Anti-filarial Activity of Antibiotic Therapy Is Due to Extensive Apoptosis after Depletion from Filarial Nematodes
- West Nile Virus Experimental Evolution and the Trade-off Hypothesis
- Shedding Light on the Elusive Role of Endothelial Cells in Cytomegalovirus Dissemination
- Galactosaminogalactan, a New Immunosuppressive Polysaccharide of
- Fatal Prion Disease in a Mouse Model of Genetic E200K Creutzfeldt-Jakob Disease
- BST2/Tetherin Enhances Entry of Human Cytomegalovirus
- Metagenomic Analysis of Fever, Thrombocytopenia and Leukopenia Syndrome (FTLS) in Henan Province, China: Discovery of a New Bunyavirus
- Neurons are MHC Class I-Dependent Targets for CD8 T Cells upon Neurotropic Viral Infection
- Sap Transporter Mediated Import and Subsequent Degradation of Antimicrobial Peptides in
- A Molecular Mechanism for Bacterial Susceptibility to Zinc
- Genomic Transition to Pathogenicity in Chytrid Fungi
- Evolution of Multidrug Resistance during Infection Involves Mutation of the Essential Two Component Regulator WalKR
- ChemR23 Dampens Lung Inflammation and Enhances Anti-viral Immunity in a Mouse Model of Acute Viral Pneumonia
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
- A Gammaherpesvirus Cooperates with Interferon-alpha/beta-Induced IRF2 to Halt Viral Replication, Control Reactivation, and Minimize Host Lethality
- Early Secreted Antigen ESAT-6 of Promotes Protective T Helper 17 Cell Responses in a Toll-Like Receptor-2-dependent Manner
- CD4 T Cell Immunity Is Critical for the Control of Simian Varicella Virus Infection in a Nonhuman Primate Model of VZV Infection
- The Role of the P2X Receptor in Infectious Diseases
- Down-Regulation of Shadoo in Prion Infections Traces a Pre-Clinical Event Inversely Related to PrP Accumulation
- Cross-Reactive T Cells Are Involved in Rapid Clearance of 2009 Pandemic H1N1 Influenza Virus in Nonhuman Primates
- Single Molecule Analysis of Replicated DNA Reveals the Usage of Multiple KSHV Genome Regions for Latent Replication
- The Critical Role of Notch Ligand Delta-like 1 in the Pathogenesis of Influenza A Virus (H1N1) Infection
- Sequence-Based Analysis Uncovers an Abundance of Non-Coding RNA in the Total Transcriptome of
- Murine Gamma Herpesvirus 68 Hijacks MAVS and IKKβ to Abrogate NFκB Activation and Antiviral Cytokine Production
- EBV Tegument Protein BNRF1 Disrupts DAXX-ATRX to Activate Viral Early Gene Transcription
- SAG101 Forms a Ternary Complex with EDS1 and PAD4 and Is Required for Resistance Signaling against Turnip Crinkle Virus
- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- Rab7A Is Required for Efficient Production of Infectious HIV-1
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- Novel Anti-bacterial Activities of β-defensin 1 in Human Platelets: Suppression of Pathogen Growth and Signaling of Neutrophil Extracellular Trap Formation
- CD11b, Ly6G Cells Produce Type I Interferon and Exhibit Tissue Protective Properties Following Peripheral Virus Infection
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A Kinase Chaperones Hepatitis B Virus Capsid Assembly and Captures Capsid Dynamics
- Towards a Structural Comprehension of Bacterial Type VI Secretion Systems: Characterization of the TssJ-TssM Complex of an Pathovar
- Indirect DNA Readout by an H-NS Related Protein: Structure of the DNA Complex of the C-Terminal Domain of Ler
- The Pore-Forming Toxin Listeriolysin O Mediates a Novel Entry Pathway of into Human Hepatocytes
- The Human Herpesvirus-7 (HHV-7) U21 Immunoevasin Subverts NK-Mediated Cytoxicity through Modulation of MICA and MICB
- Avirulence Effector Avr3b is a Secreted NADH and ADP-ribose Pyrophosphorylase that Modulates Plant Immunity
- Murid Herpesvirus-4 Exploits Dendritic Cells to Infect B Cells
- Unique Type I Interferon Responses Determine the Functional Fate of Migratory Lung Dendritic Cells during Influenza Virus Infection
- Evolution of a Species-Specific Determinant within Human CRM1 that Regulates the Post-transcriptional Phases of HIV-1 Replication
- Transcriptome Analysis of Transgenic Mosquitoes with Altered Immunity
- Antibody Evasion by a Gammaherpesvirus O-Glycan Shield
- UDP-glucose 4, 6-dehydratase Activity Plays an Important Role in Maintaining Cell Wall Integrity and Virulence of
- Protease-Resistant Prions Selectively Decrease Shadoo Protein
- A LysM and SH3-Domain Containing Region of the p60 Protein Stimulates Accessory Cells to Promote Activation of Host NK Cells
- Deletion of AIF Ortholog Promotes Chromosome Aneuploidy and Fluconazole-Resistance in a Metacaspase-Independent Manner
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Multiple Candidate Effectors from the Oomycete Pathogen Suppress Host Plant Immunity
- The Splicing Factor Proline-Glutamine Rich (SFPQ/PSF) Is Involved in Influenza Virus Transcription
- A TNF-Regulated Recombinatorial Macrophage Immune Receptor Implicated in Granuloma Formation in Tuberculosis
- SH3 Domain-Mediated Recruitment of Host Cell Amphiphysins by Alphavirus nsP3 Promotes Viral RNA Replication
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



