-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
article has not abstract
Published in the journal: . PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1000949
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1000949Summary
article has not abstract
Iron is a vital nutrient for virtually all forms of life. The requirement for iron is based on its role in cellular processes ranging from energy generation and DNA replication to oxygen transport and protection against oxidative stress. Bacterial pathogens are not exempt from this iron requirement, as these organisms must acquire iron within their vertebrate hosts in order to replicate and cause disease.
Vertebrates Sequester Iron from Invading Pathogens
One of the first lines of defense against bacterial infection is the withholding of nutrients to prevent bacterial outgrowth in a process termed nutritional immunity. The most significant form of nutritional immunity is the sequestration of nutrient iron [1]. The vast majority of vertebrate iron is intracellular, sequestered within the iron storage protein ferritin or complexed within the porphyrin ring of heme as a cofactor of hemoglobin or myoglobin. Further, the aerobic environment and neutral pH of serum ensures that extracellular iron is insoluble and hence difficult to access by invading pathogens. This difficulty is enhanced by the serum protein transferrin, which binds iron with an association constant of approximately 1036 [2]. Taken together, these factors ensure that the amount of free iron available to invading bacteria is vastly less than what is required to replicate and cause disease (Figure 1A).
Fig. 1. A representative battle during the war for iron. 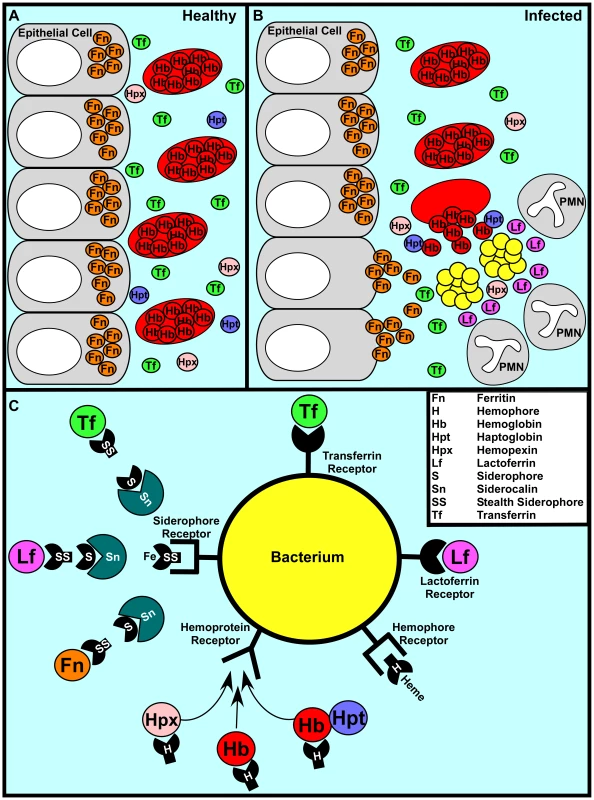
(A) In a healthy individual iron is largely intracellular, sequestered within ferritin or as a cofactor of heme complexed to hemoglobin within erythrocytes. Any extracellular free iron is rapidly bound by circulating transferrin. Hemoglobin or heme that is released as a result of natural erythrocyte lysis is captured by haptoglobin and hemopexin, respectively. Taken together, these factors ensure that vertebrate tissue is virtually devoid of free iron. (B) During infection, bacterial pathogens are capable of altering the battlefield to increase the abundance of potential iron sources. Bacterial cytotoxins damage host cells, leading to the release of ferritin while hemolytic toxins lyse erythrocytes, liberating hemoglobin. The resulting inflammatory response includes the release of lactoferrin from secondary granules contained with polymorphonuclear leukocytes (PMNs). Bacterial pathogens are capable of exploiting these diverse iron sources through the elaboration of a variety of iron acquisition systems. (C) Bacterial pathogens can acquire iron through receptor-mediated recognition of transferrin, lactoferrin, hemopexin, hemoglobin, or hemoglobin–haptoglobin complexes. Alternatively, secreted siderophores can remove iron from transferrin, lactoferrin, or ferritin, whereupon siderophore–iron complexes are recognized by cognate receptors at the bacterial surface. Analogously, secreted hemophores can remove heme from hemoglobin or hemopexin and deliver heme to bacterial cells through binding with hemophore receptors. Siderophore-mediated iron acquisition is inhibited by the innate immune protein siderocalin, which binds siderophores and prevents receptor recognition. This host defense is circumvented through the production of stealth siderophores that are modified in such a way as to prevent siderocalin binding. The importance of nutritional immunity as it pertains to iron is exemplified by the increased susceptibility to infection of individuals with iron overload due to thalassemia and primary hemochromatosis, two of the most common genetic diseases of humans [3]. The degree to which transferrin is iron saturated can vary from 25% to 30% in a healthy individual to 100% in patients with hemochromatosis, negating the antimicrobial properties of transferrin-mediated iron sequestration [2]. The impact of this iron overload is perhaps best demonstrated by the enhanced susceptibility of hemochromatosis patients to Vibrio vulnificus infections [4]. Whereas V. vulnificus is killed by normal blood and rarely causes infection in healthy individuals, it grows rapidly in blood from patients with hemochromatosis, leading to a high risk of fatal infections in this cohort [4]. Moreover, the administration of excess iron increases the virulence of numerous pathogens in animal models, further highlighting the protection provided by nutritional immunity [2], [5].
Many Bacterial Pathogens Sense Iron Depletion as a Signal That They Are within a Vertebrate Host
Vertebrates are devoid of free iron, ensuring that all bacterial pathogens encounter a period of iron starvation upon entering their hosts. In keeping with this, bacterial pathogens have evolved to sense iron depletion as a marker of vertebrate tissue. This sensing typically involves transcriptional control mediated by the iron-dependent repressor known as Fur (ferric uptake regulator) [6]. Fur binds to target sequences in the promoters of iron-regulated genes and represses their expression in the presence of iron. In the absence of iron, Fur-mediated repression is lifted and the genes are transcribed. Fur orthologs have been identified in numerous genera from both Gram-negative and Gram-positive bacteria and contribute to the virulence of both animal and plant pathogens [7].
A number of genes encoding for proteins involved in iron utilization have been reported to be positively regulated by Fur during iron-replete conditions [8]. This positive regulation occurs through Fur-mediated repression of a small RNA that represses genes encoding iron utilization proteins. This second level of regulation prevents the use of iron by non-essential enzymes during times of iron starvation. RNA-dependent regulation of iron utilization is a conserved process that has been identified in multiple bacterial pathogens, including Vibrio sp., Pseudomonas aeruginosa, Escherichia coli, Shigella flexneri, and Bacillus subtilis [8].
Many high G+C content Gram-positive bacteria express an additional iron-dependent repressor belonging to the DtxR family. The DtxR family was named for its founding member, the diphtheria toxin repressor. In fact, one of the first iron-dependent virulence factors described was diphtheria toxin produced by Corynebacterium diphtheria [2]. DtxR family members negatively regulate genes involved in processes ranging from iron acquisition to virulence factor expression [5].
In addition to sensing alterations in iron levels, bacterial pathogens can also sense heme as a marker of vertebrate tissue. Heme-responsive activators have been identified in Serratia marcescens, the pathogenic Bordetella, C. diphtheriae, Bacillus anthracis, and Staphylococcus aureus [5], [9], [10], [11]. Heme-sensing systems presumably alert bacterial pathogens when they are in contact with vertebrate tissues rich in heme, triggering the expression of systems involved in heme-iron acquisition and metabolism.
All Bacterial Pathogens Can Circumvent Iron Withholding
In order to thrive within vertebrates, bacteria must possess mechanisms to evade nutritional immunity. Perhaps the most elegant mechanism to circumvent iron withholding is employed by Borrelia burgdorferi, the causative agent of Lyme disease. B. burgdorferi has evolved to not require iron for growth by substituting manganese in its metal-requiring enzymes [12]. Most pathogens have not evolved this simple defense strategy and instead circumvent iron withholding through high-affinity iron uptake mechanisms that compete against host-mediated sequestration. These uptake systems can be divided into three main categories: siderophore-based systems, heme acquisition systems, and transferrin/lactoferrin receptors (Figure 1C).
Siderophores are low molecular weight iron-binding complexes that are secreted from bacteria. Siderophores bind iron with an association constant that can exceed 1050, enabling bacteria to compete with iron sequestration by transferrin and lactoferrin [2]. Upon removing iron from host proteins, iron-loaded siderophores are bound by cognate receptors expressed at the bacterial surface. The siderophore–iron complex is then internalized into the bacterium and the iron is released for use as a nutrient source. The importance of siderophores to bacterial virulence is demonstrated by the decreased fitness of siderophore-defective strains in animal models of infection [2], [7].
Heme acquisition systems typically involve surface receptors that recognize either heme or heme bound to hemoproteins such as hemoglobin or hemopexin. Heme is then removed from hemoproteins and transported through the envelope of bacteria into the cytoplasm. Once inside the cytoplasm, the iron is released from heme through the action of heme oxygenases or reverse ferrochelatase activity [13]–[15]. Bacterial pathogens can also elaborate secreted heme-scavenging molecules that remove heme from host hemoproteins. These molecules, known as hemophores, are functionally analogous to siderophores but are proteins that target heme, whereas siderophores are small molecules that target iron atoms [16]. As is the case with siderophore transport systems, genetic defects in heme acquisition systems reduce bacterial fitness in many animal models of infection [2], [7].
In addition to acquiring iron from transferrin and lactoferrin through siderophore-based mechanisms, some bacteria are capable of direct recognition of these host proteins. The most well-studied transferrin and lactoferrin receptors are present in pathogenic members of the Neisseriaceae and Pasteurellaceae [7]. These proteins are modeled to recognize human transferrin or lactoferrin, leading to iron removal and subsequent transport into the bacterial cytoplasm. Human challenge models with Neisseria gonorrhoeae suggest that gonococci expressing both lactoferrin and transferrin receptors exhibit a selective advantage within the host, underscoring the importance of this iron acquisition strategy to these organisms [5], [17].
Targeting Bacterial Iron Acquisition as a Second Layer of Defense against Infection
A second layer of nutritional immunity employed by vertebrates is to combat siderophore-mediated iron acquisition through the production of siderocalin [18]. Siderocalin, also referred to as lipocalin-2 or neutrophil gelatinase-associated lipocalin (NGAL), is a protein that is secreted by neutrophils in response to infection. Siderocalin binds enterobactin, the primary siderophore of many enteric bacteria, and sequesters the siderophore–iron complex, preventing bacterial uptake. Mice lacking siderocalin exhibit increased sensitivity to enterobactin-expressing bacteria, demonstrating the pathophysiological relevance of this anti-siderophore defense system [19].
The requirement for iron by bacterial pathogens ensures that iron acquisition systems are expressed and surface exposed during infection. This fact has established surface-exposed iron receptors as viable vaccine candidates for the prevention of bacterial infection. The enterobactin receptor FetA from Neisseria meningitidis [20], the siderophore receptor IroN from Escherichia coli [21], the hemoglobin receptor HgbA from Haemophilus ducreyi [22], surface proteins of the S. aureus Isd heme uptake machinery [23], and a combination of E. coli iron acquisition proteins [24] are examples of iron utilization systems that have been proposed as candidate vaccines.
Bacterial Pathogens Are Leading the Arms Race for Nutrient Iron
Resistance to siderocalin is a conserved strategy across multiple pathogenic microbes. A primary bacterial defense against siderocalin involves the production of stealth siderophores. These molecules represent structurally modified enterobactin-type siderophores that are resistant to siderocalin binding. The Gram-positive pathogen B. anthracis produces the siderophore petrobactin, which incorporates a 3,4-dihydroxybenzoyl chelating subunit that prevents siderocalin binding [25]. Similarly, Salmonella Typhimurium produces salmochelin, a glycoslyated derivative of enterochelin that is not targeted by siderocalin [26]. The production of stealth siderophores is the most recently uncovered layer in the arms race for nutrient iron during host–pathogen interactions. Undoubtedly, we have not yet discovered the complete armamentarium in this battle that has tremendous implications for the outcome of bacterial infections.
Zdroje
1. Kehl-FieTE
SkaarEP
2009 Nutritional immunity beyond iron: a role for manganese and zinc. Curr Opin Chem Biol 14 218 224
2. BullenJJ
GriffithsE
1999 Iron and infection: molecular, physiological and clinical aspects New York John Wiley and Sons
3. GanzT
NemethE
2006 Regulation of iron acquisition and iron distribution in mammals. Biochim Biophys Acta 1763 690 699
4. BullenJJ
SpaldingPB
WardCG
GutteridgeJM
1991 Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch Intern Med 151 1606 1609
5. CrosaJH
MeyAR
PayneSM
2004 Iron transport in bacteria Washington (D.C.) ASM Press 499
6. HantkeK
1981 Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet 182 288 292
7. RatledgeC
DoverLG
2000 Iron metabolism in pathogenic bacteria. Annu Rev Microbiol 54 881 941
8. MasseE
SalvailH
DesnoyersG
ArguinM
2007 Small RNAs controlling iron metabolism. Curr Opin Microbiol 10 140 145
9. VanderpoolCK
ArmstrongSK
2003 Heme-responsive transcriptional activation of Bordetella bhu genes. J Bacteriol 185 909 917
10. BibbLA
KunkleCA
SchmittMP
2007 The ChrA-ChrS and HrrA-HrrS Signal Transduction Systems Are Required for Activation of the hmuO Promoter and Repression of the hemA Promoter in Corynebacterium diphtheriae. Infect Immun 75 2421 2431
11. StauffDL
SkaarEP
2009 The heme sensor system (HssRS) of Staphylococcus aureus. Contrib Microbiol 16 120 135
12. PoseyJE
GherardiniFC
2000 Lack of a role for iron in the Lyme disease pathogen. Science 288 1651 1653
13. WilksA
2002 Heme oxygenase: evolution, structure, and mechanism. Antioxid Redox Signal 4 603 614
14. LetoffeS
HeuckG
DelepelaireP
LangeN
WandersmanC
2009 Bacteria capture iron from heme by keeping tetrapyrrol skeleton intact. Proc Natl Acad Sci U S A 106 11719 11724
15. ReniereML
TorresVJ
SkaarEP
2007 Intracellular metalloporphyrin metabolism in Staphylococcus aureus. Biometals 20 333 345
16. WandersmanC
DelepelaireP
2004 Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58 611 647
17. CornelissenCN
KelleyM
HobbsMM
AndersonJE
CannonJG
1998 The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol 27 611 616
18. GoetzDH
HolmesMA
BorregaardN
BluhmME
RaymondKN
2002 The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell 10 1033 1043
19. FloTH
SmithKD
SatoS
RodriguezDJ
HolmesMA
2004 Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432 917 921
20. ThompsonEA
FeaversIM
MaidenMC
2003 Antigenic diversity of meningococcal enterobactin receptor FetA, a vaccine component. Microbiology 149 1849 1858
21. RussoTA
McFaddenCD
Carlino-MacDonaldUB
BeananJM
OlsonR
2003 The siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect Immun 71 7164 7169
22. AfoninaG
LeducI
NepluevI
JeterC
RouthP
2006 Immunization with the Haemophilus ducreyi hemoglobin receptor HgbA protects against infection in the swine model of chancroid. Infect Immun 74 2224 2232
23. Stranger-JonesYK
BaeT
SchneewindO
2006 Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A 103 16942 16947
24. AlteriCJ
HaganEC
SivickKE
SmithSN
MobleyHL
2009 Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog 5 e1000586 doi:10.1371/journal.ppat.1000586
25. AbergelRJ
WilsonMK
ArceneauxJE
HoetteTM
StrongRK
2006 Anthrax pathogen evades the mammalian immune system through stealth siderophore production. Proc Natl Acad Sci U S A 103 18499 18503
26. HantkeK
NicholsonG
RabschW
WinkelmannG
2003 Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A 100 3677 3682
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



