-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInsecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
Many of the most dangerous human diseases are transmitted by insect vectors. After decades of repeated insecticide use, all of these vector species have demonstrated the capacity to evolve resistance to insecticides. Insecticide resistance is generally considered to undermine control of vector-transmitted diseases because it increases the number of vectors that survive the insecticide treatment. Disease control failure, however, need not follow from vector control failure. Here, we review evidence that insecticide resistance may have an impact on the quality of vectors and, specifically, on three key determinants of parasite transmission: vector longevity, competence, and behaviour. We argue that, in some instances, insecticide resistance is likely to result in a decrease in vector longevity, a decrease in infectiousness, or in a change in behaviour, all of which will reduce the vectorial capacity of the insect. If this effect is sufficiently large, the impact of insecticide resistance on disease management may not be as detrimental as previously thought. In other instances, however, insecticide resistance may have the opposite effect, increasing the insect's vectorial capacity, which may lead to a dramatic increase in the transmission of the disease and even to a higher prevalence than in the absence of insecticides. Either way—and there may be no simple generality—the consequence of the evolution of insecticide resistance for disease ecology deserves additional attention.
Published in the journal: . PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001000
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1001000Summary
Many of the most dangerous human diseases are transmitted by insect vectors. After decades of repeated insecticide use, all of these vector species have demonstrated the capacity to evolve resistance to insecticides. Insecticide resistance is generally considered to undermine control of vector-transmitted diseases because it increases the number of vectors that survive the insecticide treatment. Disease control failure, however, need not follow from vector control failure. Here, we review evidence that insecticide resistance may have an impact on the quality of vectors and, specifically, on three key determinants of parasite transmission: vector longevity, competence, and behaviour. We argue that, in some instances, insecticide resistance is likely to result in a decrease in vector longevity, a decrease in infectiousness, or in a change in behaviour, all of which will reduce the vectorial capacity of the insect. If this effect is sufficiently large, the impact of insecticide resistance on disease management may not be as detrimental as previously thought. In other instances, however, insecticide resistance may have the opposite effect, increasing the insect's vectorial capacity, which may lead to a dramatic increase in the transmission of the disease and even to a higher prevalence than in the absence of insecticides. Either way—and there may be no simple generality—the consequence of the evolution of insecticide resistance for disease ecology deserves additional attention.
Introduction
Vector-borne diseases are among the major causes of illness and death, particularly in tropical and subtropical countries. Vector control, through the use of insecticides, plays a key role in the prevention and control of infectious diseases such as malaria, dengue, and filariasis [1]. The widespread use of insecticides can, however, lead to the development of insecticide resistance, making insecticide use ineffective and limiting the available options for disease control [2]. Insecticide resistance, including resistance to multiple types of insecticides, has arisen in all the insect species that are the major vectors of human diseases (Table 1). Consequently, insecticide resistance is considered a serious public health challenge.
Tab. 1. Insecticide resistance mechanisms reported to date in natural populations of the main insect vectors of human diseases. 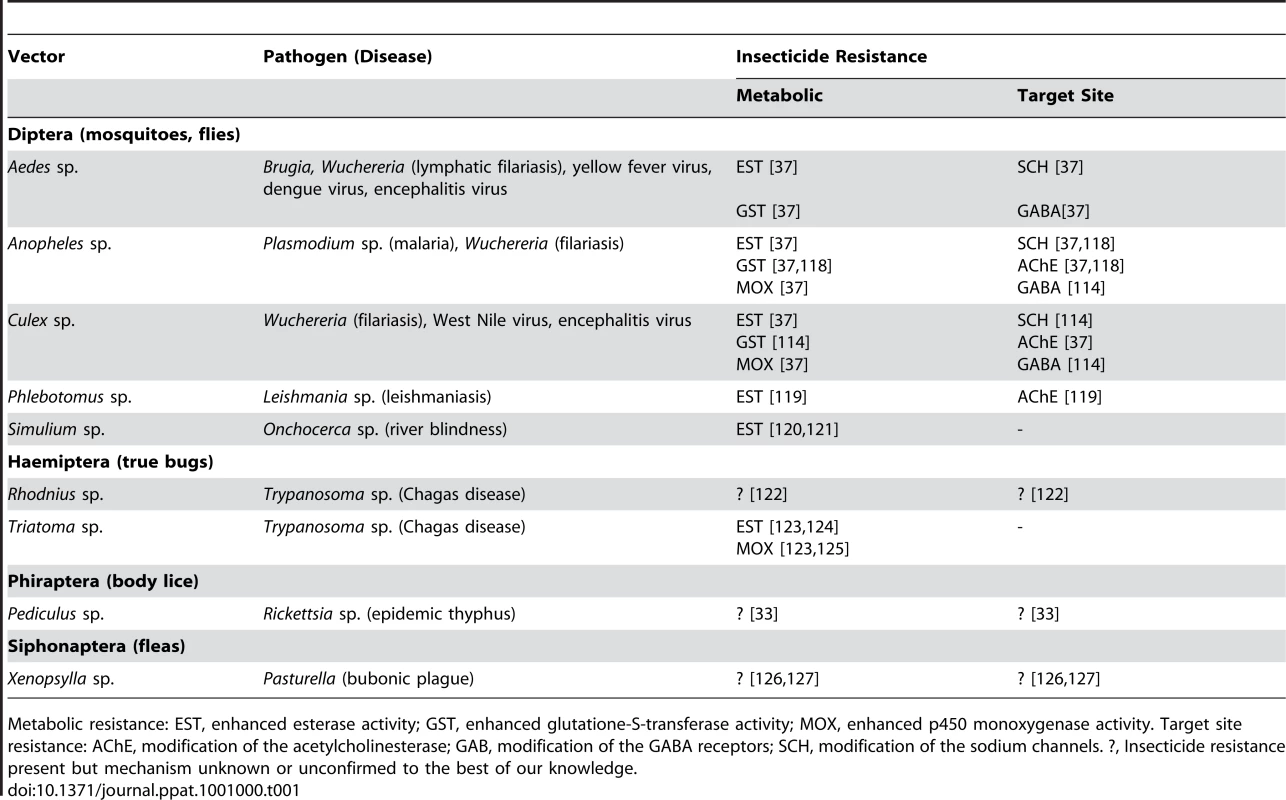
Metabolic resistance: EST, enhanced esterase activity; GST, enhanced glutatione-S-transferase activity; MOX, enhanced p450 monoxygenase activity. Target site resistance: AChE, modification of the acetylcholinesterase; GAB, modification of the GABA receptors; SCH, modification of the sodium channels. ?, Insecticide resistance present but mechanism unknown or unconfirmed to the best of our knowledge. What is the impact of insecticide resistance on the transmission of vector-borne diseases? This can be best explored using a fundamental concept describing the transmissibility of infectious diseases: the parasite's basic reproductive number, or (see Box 1). This quantity plays a central role in epidemiology because it provides a synthetic index of transmission intensity and establishes threshold criteria for disease establishment or eradication. In particular, the prevalence of a disease is expected to increase in a naive population only when is greater than one. A major aim of insecticide spraying is to reduce the number of vectors (and thus m in Box 1). The emergence of insecticide resistance, however, counters this control method by increasing the number of mosquitoes that survive the insecticide treatment (Figure 1, top). This can result in substantial increases in vector numbers, possibly to pre-treatment densities (or nearly so, if there are costs associated with insecticide resistance [3]–[9]). Concerns about rebounding vector populations have been sufficient to motivate the search for novel insecticides [10], [11], the development of continent-wide resistance surveillance networks [12], [13], and work on resistance management strategies aimed at retarding or preventing the spread of resistance [14]–[18].
Box 1. Basic Reproductive Number of Vector-Borne Diseases
In the following, we distinguish the vector (e.g., mosquito in malaria) from the host (e.g., mammalian host in malaria). A general expression for R0 can be readily derived for simple vector-borne diseases [41], [117], [118]. We present the expression of R0 when the vector population is heterogeneous, consisting of both susceptible and resistant (prime symbol) individuals:Where 1/r is the expected duration of the infection in the host (r is the rate of clearance of the infection in the host), m is the number of adult vectors per host, and IC is the individual vectorial capacity of the vector (modified from [119], [120]):The other parameters are defined as follows:
aU and aI: number of (uninfected and infected bites) on the focal host, per vector and per day, which depends on fU and fI, the vector's feeding rates, and on QU and QI, the proportion of those bites on the focal host (i.e., human versus other mammals in human malaria) such that aU = fUQU and aI = fIQI.
b: probability that a host becomes infected from a bite of an infected vector (i.e., host susceptibility and vector infectiousness).
c: probability that a vector becomes infected from a bite on an infected host (i.e., vector susceptibility).
g: death rate of the vector. In other words, 1/g is the expected lifespan of a vector, and e−g is the probability a mosquito survives one day.
n: incubation time of the parasite in the vector (i.e., number of days required for the vector to become infectious after biting an infected host).
Insecticide resistance can have an effect on vector abundance (m′) but may also alter the vector's individual vectorial capacity (IC′) by modifying the vector's longevity (1/g′), competence (b′, c′, n′), and behaviour ( and ).
Fig. 1. Effect of increasing insecticide coverage on (top) the frequency of insecticide resistance (IR, gray line), and, in the inset, the vector density with (full line) or without (dashed line) IR evolution; (bottom) the basic reproductive ratio of the infectious disease transmitted by the vector (see Box 1). 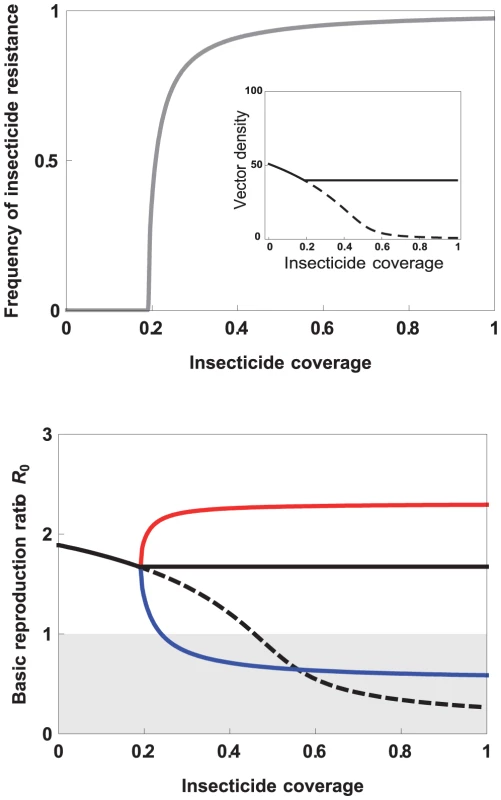
(Bottom) We consider different scenarios: in the absence of IR evolution in the vector (dashed black line), and after IR evolution when the IR insects are equally good vectors as the susceptible ones (full black line), better (red line), or worse (blue line). The gray area delimits the area where the parasite goes to extinction (R0<1). See Appendix S1 for the details of the model and parameter values. One factor that has been largely overlooked is the potential effects of insecticide resistance on the ability of the vectors themselves to transmit disease (the individual vectorial capacity, Box 1): are insecticide-resistant insects better or worse vectors of diseases than susceptible ones? Far from being mere flying syringes, vectors provide a very specific environment in which the parasites differentiate, proliferate, and migrate to the correct tissues to ensure transmission to the next host. Recent work suggests that this environment is drastically modified when insects become resistant to insecticides [19], [20]. McCarroll et al. [21], [22] have shown that insecticide-resistant Culex quinquefasciatus mosquitoes are less likely to transmit the filaria parasite Wuchereria bancrofti than their insecticide-susceptible counterparts, and insecticide resistance in Culex pipiens seems to interact in a complex way with microsporidian and bacterial organisms [23]–[25]. Thus, increasing numbers of resistant insects need not lead to proportionate increases in disease transmission: it depends on whether those insects are more or less permissive transmitters than their susceptible ancestors. In this review, we survey a range of possibilities. We conclude that if the few data that exist extend to other combinations of vector species, insecticide resistance mechanisms, and parasites, it is currently not possible to evaluate the public health significance of insecticide resistance.
Insecticide Resistance Mechanisms
Four classes of chemical insecticides are the mainstay of vector control programmes: organochlorines, organophosphates, carbamates, and pyrethroids [1]. More recently, two alternative insecticide types have been introduced, largely for the control of mosquito larvae: biopesticides (e.g., Bacillus thuringiensis, Bacillus sphaericus) and insect growth regulators, such as the juvenile hormone mimic, methoprene [1]. Cases of resistance to these alternative insecticides are still limited (but see [26]–[29]) and the underlying mechanisms are only beginning to be identified [30]–[32].
To date, four types of resistance mechanisms against the chemical insecticides mentioned above have been described: metabolic resistance, target site resistance, penetration resistance, and behavioural resistance. To illustrate our arguments, we focus on metabolic and target site resistance because they have been extensively investigated at both the genetic and molecular levels [33].
Metabolic resistance involves the sequestration, metabolism, and/or detoxification of the insecticide, largely through the overproduction of specific enzymes [34], [35]. Three main groups of enzymes have been identified (Table 1): carboxylesterases (efficient against organophosphate and carbamate insecticides), glutathione-S-transferases or GSTs (efficient against organophosphates, organochlorine, and pyrethroid insecticides) and cytochrome P450-dependent monoxygenases (efficient against most insecticide types, frequently in conjunction with other enzymes). The overproduction of these enzymes may be achieved via two non-exclusive mechanisms: gene amplification increasing the gene's copy number [35] and gene expression via modifications in the promoter region or mutations in trans-acting regulatory genes [35], [36]. In addition, in some mosquito species, carboxylesterase resistance to the insecticide malathion has been associated with a qualitative change in the enzyme (a few amino acid substitutions can increase the rate of hydrolysis of the enzyme [37]).
In contrast, target site resistance is achieved by point mutations that render the actual targets of an insecticide less sensitive to the active ingredient [33], [38]. Most insecticides developed to date are neurotoxic and aim for one of the following three targets: the acetylcholinesterase (whose role is the hydrolysis of the neurotransmitter acetylcholine), the γ-aminobutyric acid (GABA) receptors (chloride-ion neurotransmission channels in the insect's nervous system), or the sodium channels (responsible for raising the action potential in the neurons during the nerve impulses). The acetylcholinesterase is the target of organophosphorous and carbamate insecticides, the GABA receptors are the main targets of cyclodiene (organochlorine) insecticides, and the sodium channels are the targets of pyrethroid and organochlorine insecticides. Mutations in all three of these can confer resistance (Table 1).
What Effects on Parasite Transmission?
The evolution of insecticide resistance entails a battery of correlated life history changes in the insect, which are widely thought to be the result of pleiotropic effects of the insecticide resistance genes themselves, or of genes closely linked with them as a result of hitchhiking. These life history changes are often, though not always [39], [40], associated with fitness costs [3]–[9], that is, reduced fitness in the absence of insecticides. The question with which we are concerned here is how these changes interfere with the infection, development, and transmission of the parasite. Aside from the effect on mosquito population density, insecticide resistance can impact all of the main mosquito-related parameters in (given in Box 1). These include vector longevity, vector competence, and vector feeding behaviour. Below, we analyse each of them separately (see Table 2 for a summary).
Tab. 2. Potential effects of the different mechanisms of insecticide resistance (IR) on vector longevity, competence and behaviour, and expected effects on the parasite's R0. 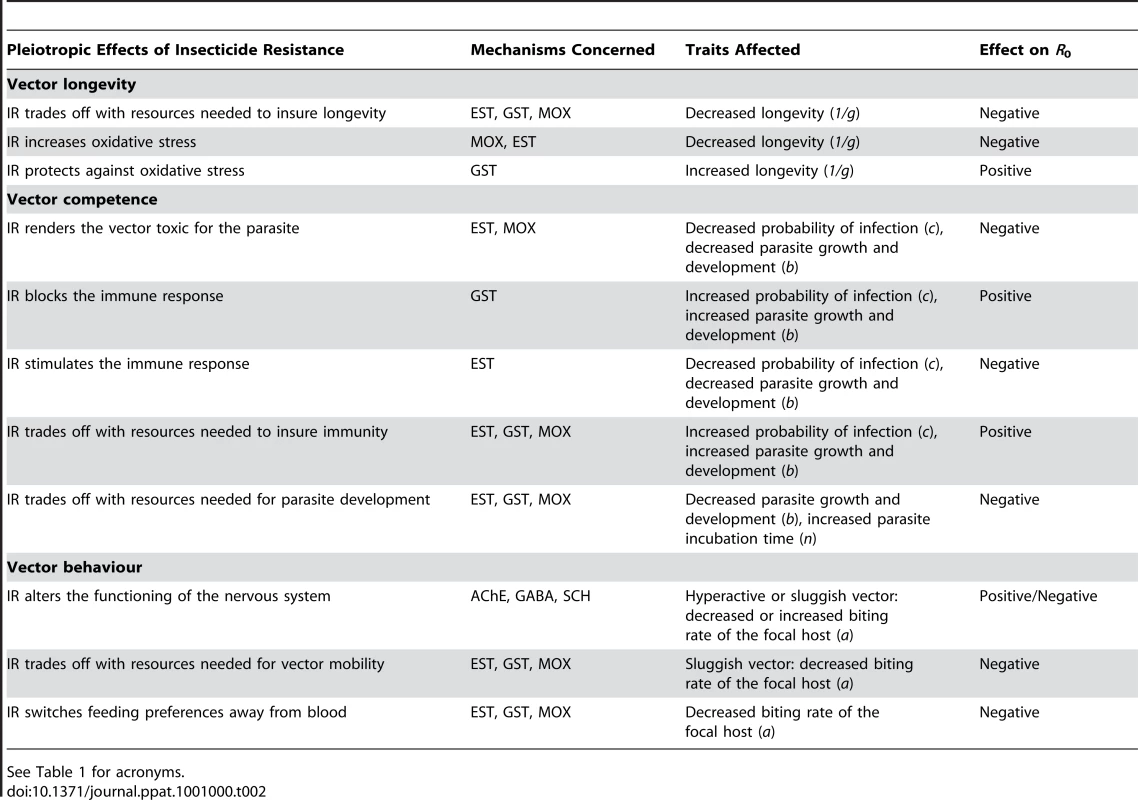
See Table 1 for acronyms. Insecticide Resistance and Vector Longevity
Vector longevity is an essential parameter in disease transmission, as it increases the potential for infective bites to hosts. As pointed out by MacDonald [41], the effect of longevity on disease transmission is particularly poignant for parasites like Plasmodium that need a minimum incubation period in the vector before being transmitted to a new host (Box 1). Yet, to our knowledge, there have been no thorough analyses on the effects of insecticide resistance on the longevity of Anopheles or indeed of any other vector of human disease, with one exception. In C. pipiens, insecticide resistance has been associated with a reduced longevity in the laboratory [24] and overwintering survival in the field [42], [43]. Similar effects of insecticide resistance on longevity have been obtained in other (non-vector) insect species [44]–[46]. Two main mechanisms may underlie this reduction in longevity: resource-based trade-offs and oxidative stress.
A well-known paradigm in evolutionary ecology is that diverting resources to one trait will, directly or indirectly, diminish the resources available for other traits [47]. This has been often put to the test using insect models, where it has been shown that, when resources are limited, an increased investment in certain fitness-associated traits such as fecundity is often coupled with a significant reduction in longevity [48], [49]. The deployment of insecticide resistance mechanisms, and in particular the overproduction of the detoxifying enzymes, likely requires substantial investment of resources. In the mosquito C. pipiens, for example, certain resistant genotypes can produce up to 50 times more esterases than their susceptible counterparts [50]. In other insects, these overproduced esterases can represent up to 3% of the total body proteins [51]. Lipids are likely victims of this large overinvestment in proteins, as they are an important source of the acetyl groups needed to synthesise the enzyme's constitutive amino acids [52]. Lipids are also the main fuel for insect survival [53], [54]. Unfortunately, so far as we are aware, no studies have quantified the level of lipids—or, indeed, any other energetic resource—in insecticide-resistant and -susceptible vectors.
Oxidative stress results from a mismatch between the production of damaging reactive oxygen species (ROS) and the production of protective antioxidants [55]. All organisms produce ROS as a result of the normal metabolic functioning of their cells [55]. The unwanted ROS produced in such reactions exert irreversible deleterious effects in the body [56] and have been widely proposed as a mechanism for ageing [55]–[57]. Blood feeding insects, in particular, face a considerable challenge from oxidative stress, because the digestion of haemoglobin results in a large production of ROS [58], [59]. In Anopheles, excess ROS production, though unrelated to insecticide resistance, has been recently shown to lead to a significant increase in mortality [60]. Two insecticide resistance mechanisms, in particular, may drastically alter ROS levels in insects, albeit in radically opposite ways: the p450 monoxygenases and the GSTs. The increased activity of p450 monoxygenases results in an excess production of harmful ROS because the stoichiometric demands of the enzymatic reaction are often not met [61]. This fact, previously known only from vertebrates [62], has been recently demonstrated in the house fly [63], and is thus likely to extend to other insect species. In contrast, GSTs have been shown to protect tissues against oxidative damage by increasing their solubility and aiding the excretion of free radicals [64]–[66]. A recent comparative study has found a clear association between GST expression and extended lifespan in fruit flies, nematodes, and mice [67]. Moreover, transgenic lines of Caenorhabditis elegans that produce 2.4 times more GSTs than controls show a 22% extension in their longevity [68]. The overexpression of GSTs in these transgenic worms is within the range found in insecticide-resistant vectors [69], [70]. Again, however, we are unaware of any studies addressing the longevity of vectors that are resistant to insecticides through the overproduction of GSTs.
Insecticide Resistance and Vector Competence
Vector competence, the successful invasion and subsequent development of the parasite in the vector, depends on the plethora of physiological and immunological factors that determine the insect's internal environment for the parasite. Insecticide resistance could interfere with parasite development in at least two ways. First, the physiological modifications that accompany the deployment of insecticide resistance mechanisms may render the vector toxic to parasites. In one of the few studies to have explicitly investigated the connection between insecticide resistance and disease transmission, McCarroll and collaborators showed that the development of the filaria W. bancrofti larvae was arrested in insecticide-resistant C. quinquefasciatus mosquitoes [21], [22] (but see [71]). Exactly what rendered the insecticide-resistant mosquito toxic to the parasite is not known, but it was hypothesised that the overproduction of carboxylesterases (see Table 1) in these mosquitoes resulted in a change in the redox potential of the tissues hosting the parasite, which led to the death of the larvae. Pending confirmation of a correlation between carboxylesterase and ROS production, these results could extend to other parasites whose vector stages have been shown to be highly susceptible to oxidative stress (such as Plasmodium [72] and Trypanosoma [73]), as well as to other insecticide resistance mechanisms (such as the p450 monoxygenases and GSTs) with a proven link with oxidative stress (see above).
Second, insecticide resistance could affect vector immunity. The combined complexity of the mode of action and the multiple substrate specificities of the enzymes involved in metabolic insecticide resistance (see Box 1) is such that these enzymes may have pleiotropic effects on one of the many steps of the immune cascade, from the recognition of the parasite as foreign, to the transduction of the signal and the deployment of the killing mechanism [74]. Yet, aside from a microarray study that showed upregulation of certain immune-related genes in insecticide-resistant strains of Anopheles gambiae [20], there are no studies that explicitly investigate the potential effects of insecticide resistance on insect immunity. Here we suggest two as yet unexplored possibilities.
The first concerns the protective role of GSTs (Table 1) against the effects of ROS on the parasites. Inducible ROS are a key component of the immune defence of Anopheles mosquitoes against Plasmodium [60], [75]. By neutralising the oxidative response of the mosquito to the parasite, overproduced GSTs could potentially increase the susceptibility of mosquitoes to the parasite.
The second concerns carboxylesterases (Table 1). Due to the overlapping substrate specificities existing between these enzymes and the serine proteases implicated in the melanization cascade, it has been suggested that carboxylesterases could have a positive effect on the formation of a melanin capsule around the parasite [76]. Two decades ago, an interesting association was found between an allele in an esterase locus and resistance by encapsulation in the G3 strain of A. gambiae infected with the B strain of Plasmodium cynomolgi [77]. The product of this gene was found to be a carboxylesterase with considerable sequence similarity to the carboxylesterase overproduced by insecticide-resistant Culex mosquitoes [78]. Subsequent (unpublished) studies, however, did not find any pattern of association between the carboxylesterase phenotype and Plasmodium susceptibility [79], but, to our knowledge, the question has not been investigated any further. More recently, carboxylesterases have been shown to be inducibly produced after bacterial [80] and viral [81] infections, suggesting that they may play a direct role in the invertebrate immune system. Thus, it is possible that upregulation of carboxylesterases as an adaptation against insecticides could, as an incidental side effect, make mosquitoes more resistant to pathogens.
Immunocompetence could also be affected through resource-based trade-offs. There is plenty of evidence that there are significant resource costs involved in the deployment and maintenance of the insect immune system [82]. Proteins seem to be the limiting resource for the encapsulation and antimicrobial responses in caterpillars [82], [83], and lipid metabolism has been shown to be implicated in the immune response of Aedes aegypti mosquitoes to a Plasmodium and a bacterial infection [84]. The production of large amounts of detoxifying enzymes, such as esterases or GSTs, is likely to deplete the resource pool, limiting the vector's ability to mount an immune response, therefore favouring the development of the parasite. It is worth noting, however, that resource limitation could also have the opposite effect if redirection of resources to insecticide resistance puts those resources beyond the reach of parasites: it could limit the development of parasites that depend on the host's energetic reserves to fulfil their own metabolic needs [85]. In vitro studies have, for example, shown that the mosquito gut stages of Plasmodium are greedy consumers of amino acids [86], lipids [87], and glucose [88]. There is also evidence that parasite production is positively correlated with resource availability in several invertebrates [89]–[92]. In these systems, the redirection of resources towards insecticide resistance is likely to impair the ability of the parasites to develop inside the vectors.
Insecticide Resistance and Vector Behaviour
Vector behaviour, particularly host choice, and biting rate have key effects on parasite transmission (Box 1). Mosquitoes with transmissible stages of Plasmodium persist at biting for longer than uninfected mosquitoes or mosquitoes infected with non-infectious stages [93]. Similar results have been obtained with Leishmania-infected sandflies [94]. In addition, recent work shows that uninfected mosquitoes are preferentially attracted to humans infected with transmissible gametocytes [95]. Because of its direct effect on the vector's neural system, target site resistance in particular has the potential for modifying the biting behaviour of uninfected and infected vectors alike.
Target-site resistance mutates key components of the vector's neural network, drastically modifying their performance and, thus, potentially also their response to external stimuli. In C. pipiens, for example, the single point mutation that renders the acetylcholinesterase insensitive to insecticides reduces the activity of the enzyme by up to 60% [96], which is likely to result in an excess of acetylcholine in the synapses and in a hyperactivity of the nervous system [6]. The most compelling examples of the effect of target site resistance on insect behaviour have not been carried out in vectors of diseases but on aphids and flies. In these insects, the kdr mutations alter the normal functioning of the sodium channels, causing a reduction in the excitability of the nervous system [97], [98]. Consequently, kdr-resistant aphids are less responsive to the presence of pheromone released by conspecifics [98], [99], increasing their vulnerability to parasitoid attack [100]. Furthemore, kdr-resistant flies are also less responsive to changes in temperature gradient than their insecticide-susceptible counterparts [98]. In mosquitoes, sodium channels are implicated in the transduction of the olfactory signal from the olfactory receptors to the central nervous system [101]. Target-site modifications, such as the kdr mutation, may render mosquitoes less responsive to the olfactory cues, such as lactic acid or ammonia [102], [103], that allow them to locate their hosts, thus reducing their efficiency as vectors. Rowland [104], [105] found that target-site resistance to organochlorine insecticides rendered A. gambiae and Anopheles stephensi mosquitoes less responsive to oviposition and predation-risk stimuli, but the effects on blood feeding behaviour have, to our knowledge, never been investigated.
Perhaps less intuitively, however, the behavioural side effects of insecticide resistance also extend to metabolic resistance. Foster et al. [98] showed that, in aphids, insecticide resistance through increased carboxylesterase titres were associated with a reduced ability to move away from senescing leaves. Berticat et al. [4] found that adults of C. pipiens that are resistant to insecticides through the overproduction of carboxylesterases suffered higher predation rates than susceptible ones, probably due to a decreased locomotive performance. This seemingly decreased mobility of insecticide-resistant insects is likely to be the result of resource depletion associated with the overproduction of carboxylesterases [98]. When applied to a blood-feeding vector, reduced motility may translate into reduced host-seeking efficiency and biting rates, although this has never been tested. A decrease in the energetic reserves may also switch the feeding preference of vectors away from hosts. In Ae. aegypti and Culex nigripalpus mosquitoes, resource deprivation, which is directly correlated with low energetic reserves, renders mosquitoes more responsive to sugar-rich odours like honey and less responsive to host odours [106].
Discussion
Whether a particular insect is a good vector, an occasional vector, or whether it presents an infection barrier for the parasite depends on a plethora of physiological, immunological, and behavioural variables. In this review, we have argued that any of these factors may potentially be altered by the evolution of insecticide resistance, with potentially drastic consequences for the epidemiology of disease (Figure 1). If insecticide resistance decreases the individual vectorial capacity of the vector (blue line in Figure 1), the transmission of the disease can decrease below the level attained in the absence of insecticide resistance evolution. In this case, insecticide resistance evolution may thus decrease the level of insecticide coverage needed to drive the parasite to extinction. An increase in the individual vectorial capacity (red line in Figure 1), on the other hand, may lead to a dramatic increase in the transmission of the disease and even to a higher prevalence than in the absence of insecticides. Moreover, even when local eradication does not occur (perhaps because initial is very high), the extent to which the very impressive disease control often achieved by insecticides is eroded as resistance spreads will depend not only on how vector densities recover but also on the vectorial capacity of individual vectors, which, as we have argued, can be dramatically altered by resistance. As is clear from our discussion above, surprisingly little work directly addresses this important issue. Below, we summarise what we consider to be the three main questions to be answered, and we outline some predictions that arise from the mode of action of the different insecticide resistance mechanisms.
The first question is whether insecticide-resistant vectors have a different lifespan than their susceptible counterparts. We expect that, in most cases, the effect of insecticide resistance will be to reduce vector longevity. This has been already shown in insects of agricultural interest as well as in Culex mosquitoes [24], [42]–[46], but it needs to be tested in the other vectors of diseases, most particularly those that transmit parasites with long incubation periods (e.g., the mosquitoes Anopheles and Aedes, and the kissing bugs Rhodnius and Triatoma) (Table 1). We further expect this longevity reduction to be especially drastic in insects with metabolic insecticide resistance as a result of resource-based trade-offs and/or increased oxidative stress. The one exception to this rule may be vectors overexpressing the GST, which has been shown to increase lifespan in organisms as diverse as Drosophila and nematodes [67], [68]. The longevity reduction in insecticide-resistant insects may, however, be offset by the parasite's influence on longevity. In C. pipiens mosquitoes infected with the microsporidia Vavraia culicis, the decrease in longevity associated with insecticide resistance is much larger for uninfected than for infected mosquitoes [24]. Indeed, parasites often have an effect on the longevity of their vectors, both positive and negative [107]. Thus, whenever possible, the potential interaction between insecticide resistance and parasite-mediated effects on the vector's lifespan needs to be investigated, ideally, using natural vector–parasite combinations [107], [108].
The second question is whether insecticide resistance alters the probability an insect becomes infected and/or the subsequent intensity of infection and production of transmission stages (or vector competence). McCarroll and co-workers [21], [22] have shown that insecticide-resistant mosquitoes have lower burdens of filaria parasites, possibly due to an increase in oxidative stress. Vontas et al. [109] failed to show differences in parasite burden between insecticide-resistant and -susceptible An. stephensi mosquitoes infected with Plasmodium yoellii, although the different geographic origin of the resistant and susceptible strains and the unnatural combination of an Asian vector with an African rodent parasite make these results difficult to interpret (see below). We expect the effects on parasite burden to be more drastic in vectors with metabolic resistance, as the production of large amounts of detoxification enzymes will likely render the physiological environment of the vector less than ideal for parasite development. Unfortunately, a mere reduction in parasite burden in insecticide-resistant insects is unlikely to have a drastic effect on disease transmission because, in most cases, a few parasites suffice to initiate a new infection in the host. As few as ten Plasmodium parasites are sufficient to establish a malaria infection [110]. One way in which parasite burden may influence transmission, however, is if it correlates with vector survival. There indeed is evidence, again from Plasmodium, that more heavily infected mosquitoes die faster [108], [111].
The third question is whether insecticide resistance modifies the biting rate or host choice of the uninfected and/or infected vector. We expect this effect to appear particularly in vectors that are resistant through modifications in the neural targets of the insecticide, because of the obvious connections between behaviour and the nervous system. Depending on the underlying mechanism, these modifications may result in either a hyperactive or a sluggish nervous system, but how this translates into feeding and host-choice behaviour remains to be investigated. Finally, of particular interest is whether insecticide resistance may be able to alter the parasite-mediated manipulation of vector feeding behaviour, even though, in most cases, this manipulation takes place through a physical interference with blood ingestion [107], without the involvement of the nervous system.
As illustrated above, the physiological mechanisms underlying insecticide resistance yield clear predictions as to how insecticide resistance may affect the different components of the parasite's (vector longevity, competence, and behaviour, Table 2). However, the same insecticide resistance mechanism may have opposite effects on each of these components by, for instance, increasing the vector's lifespan but interfering with the parasite's development (see GST, Table 2). It is therefore difficult to predict the overall effect of insecticide resistance on a parasite's . In addition, our predictions in Table 2 probably do not encompass all possible effects of insecticide resistance on disease transmission. The enzymes involved in the detoxification of insecticides belong to particularly complex families of enzymes whose substrate specificities and biological functions are not yet fully known [112]. Similarly, target-site mutations seem to have pleiotropic effects that go beyond the nervous system [113]. The problem of prediction gains additional intricacy from the fact that many insect vectors are now resistant to multiple insecticide types through a combination of different metabolic and target-site modification mechanisms [114], [115]. These different insecticide resistance mechanisms have been shown to interact with each other [6], but what consequences these interactions may have for parasite transmission will have to be resolved on a case-by-case basis.
Studies investigating the vectorial capacity of insecticide-resistant and -susceptible vectors are, in our view, urgently needed, but we note three experimental challenges that need to be overcome in order to reach strong conclusions. The first is that single comparisons of allopatric-resistant and -susceptible vector strains [19], [20], [109], [116] cannot disentangle the effects of insecticide resistance genes from other differences that inevitably arise during divergent evolutionary history. Much stronger inferences can be made if sympatric-resistant and -susceptible mosquitoes are compared, but in areas with a long and complex history of insecticide use, fully susceptible individuals can be very hard to find. If obtaining matched sympatric lines is not feasible, many unmatched resistant and sensitive lines are required. Another way forward is the comparison of laboratory-selected lines. But this raises a second experimental difficulty: the conclusions from laboratory-selected, insecticide-resistant strains may not be directly applicable to the conditions in the field. Curtis [21], [71] and McCarroll et al. [21] pointed out that McCarroll's [22] results with Culex and Wuchereria may have been the result of unnaturally high esterase levels in the laboratory-selected strains of the mosquito. In addition, while selecting for insecticide resistance in the laboratory, one may inadvertently select for other traits, such as developmental time, body size, immunocompetence, or longevity, which may have consequences for parasite transmission. The final experimental issue is that, whenever possible, studies should be carried out on natural vector–parasite combinations. Lessons from Plasmodium studies have taught us that results obtained using laboratory models, most notably concerning mosquito longevity [108] and immunity [117], are not necessarily applicable to natural vector–parasite combinations. We agree that overcoming all three of these pitfalls is not easy, but the logistic difficulties do not mean the problems can be ignored.
Thus far, we have concentrated our discussion on the short-term effects of insecticide resistance on parasite transmission through its impact on the parasite's (epidemiological time scale). However, the interaction between the parasite and the insecticide-resistant vector can also have long-term (evolutionary time scale) consequences. Insecticide resistance could exert a selective pressure for the evolution of the parasites by selecting for parasites with, for example, shorter incubation times (to compensate for the reduction in longevity), or faster multiplication rates (to compensate for higher parasite mortality). Conversely, if parasite burden is reduced in insecticide-resistant vectors, as McCarroll et al. [22] showed, this could facilitate the spread of insecticide resistance in vector populations submitted to a significant parasite pressure. Exploring these two evolutionary consequences is beyond the scope of this paper, but the interaction between insecticide resistance and parasitism clearly deserves further investigation.
Insecticide resistance is generally thought to undermine the control of vector-transmitted diseases. Consequently, there are ongoing efforts to develop resistance-breaking compounds [10], [11] and evolution-proof insecticidal strategies [14], [15], as well as improved resistance surveillance in the field [12], [13]. We suggest that another research problem be added to this agenda: the disease transmission capacity of resistant insects. In some instances, insecticide resistance may impair the ability of the vector to transmit diseases. If this effect is sufficiently large, the impact of insecticide resistance on disease management may not be as detrimental as previously thought. If so, current paradigms might be leading to a misallocation of research and control resources. We contend that there are surprisingly few well-documented cases of disease outbreaks in response to the evolution of insecticide resistance (in marked contrast to the well-documented public health problems caused by the evolution of drug resistance). Alternatively, insecticide resistance could improve the individual vectorial capacity of insects, further emphasising the urgent need for novel insecticides and resistance management strategies. Either way—and there may be no simple generality—the consequence of the evolution of insecticide resistance for disease ecology deserves additional attention.
Supporting Information
Zdroje
1. World Health Organisation 2006 Pesticides and their application for the control of vectors and pests of public health importance. WHO/CDS/NTD/WHOPES/GCDPP/2006.1
2. World Health Organisation 1998 Test procedures for insecticide resistance monitoring in malaria vectors; bio-efficacy and persistence of insecticides on treated surfaces. WHO/CDS/CPC/MAL/9812
3. HardstoneMC
LazzaroBP
ScottJG
2009 The effect of three environmental conditions on the fitness of cytochrome P450 monooxygenase-mediated permethrin resistance in Culex pipiens quinquefasciatus. BMC Evol Biol 9 42
4. BerticatC
DuronO
HeyseD
RaymondM
2004 Insecticide resistance genes confer a predation cost on mosquitoes, Culex pipiens. Genet Res 83 189 196
5. BourguetD
GuillemaudT
ChevillonC
RaymondM
2004 Fitness costs of insecticide resistance in natural breeding sites of the mosquito Culex pipiens. Evolution 58 128 135
6. BerticatC
BonnetJ
DuchonS
AgnewP
WeillM
2008 Costs and benefits of multiple resistance to insecticides for Culex quinquefasciatus mosquitoes. BMC Evol Biol 8 104
7. BerticatC
BoquienG
RaymondM
ChevillonC
2002 Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet Res 79 41 47
8. RoushRT
McKenzieJA
1987 Ecological genetics of insecticide and acaricide resistance. Annu Rev Entomol 32 361 380
9. SakyiKY
SarfoB
BrownCA
WilsonMD
BoakyeDA
2005 Investigation into the fitness cost of kdr insecticide resistance in Anopheles gambiae malaria vectors. Am J Trop Med Hyg 73 155 155
10. HemingwayJ
BeatyBJ
RowlandM
ScottTW
SharpBL
2006 The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol 22 308 312
11. ZaimM
GuilletP
2002 Alternative insecticides: an urgent need. Trends Parasitol 18 161 163
12. Kelly-HopeL
RansonH
HemingwayJ
2008 Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect Dis 8 387 389
13. ColemanM
SharpB
SeocharanI
HemingwayJ
2006 Developing an evidence-based decision support system for rational insecticide choice in the control of African malaria vectors. J Med Entomol 43 663 668
14. ReadAF
LynchPA
ThomasMB
2009 How to make evolution-proof insecticides for malaria control. PLoS Biol 7 e1000058 doi:10.1371/journal.pbio.1000058
15. KoellaJC
LynchPA
ThomasMB
ReadAF
2009 Towards evolution-proof malaria control with insecticides. Evolutionary Applications 2 469 480
16. LenormandT
RaymondM
1998 Resistance management: the stable zone strategy. Proc R Soc Lond B Biol Sci 265 1985 1990
17. CurtisCF
MillerJE
HodjatiMH
KolaczinskiJH
KasumbaI
1998 Can anything be done to maintain the effectiveness of pyrethroid-impregnated bednets against malaria vectors? Philos Trans R Soc Lond B Biol Sci 353 1769 1775
18. PenillaRP
RodriguezAD
HemingwayJ
TrejoA
LopezAD
2007 Cytochrome P-450-based resistance mechanism and pyrethroid resistance in the field Anopheles albimanus resistance management trial. Pestic Biochem Physiol 89 111 117
19. VontasJ
DavidJP
NikouD
HemingwayJ
ChristophidesGK
2007 Transcriptional analysis of insecticide resistance in Anopheles stephensi using cross-species microarray hybridization. Insect Mol Biol 16 315 324
20. VontasJ
BlassC
KoutsosAC
DavidJP
KafatosFC
2005 Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Mol Biol 14 509 521
21. McCarrollL
HemingwayJ
2002 Can insecticide resistance status affect parasite transmission in mosquitoes? Insect Biochem Mol Biol 32 1345 1351
22. McCarrollL
PatonMG
KarunaratneS
JayasuryiaHTR
KalpageKSP
2000 Insecticides and mosquito-borne disease. Nature 407 961 962
23. DuronO
LabbeP
BerticatC
RoussetF
GuillotS
2006 High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60 303 314
24. AgnewP
BerticatC
BedhommeS
SidobreC
MichalakisY
2004 Parasitism increases and decreases the costs of insecticide resistance in mosquitoes. Evolution 58 579 586
25. BerticatC
RoussetF
RaymondM
BerthomieuA
WeillM
2002 High Wolbachia density in insecticide-resistant mosquitoes. Proc R Soc Lond B Biol Sci 269 1413 1416
26. CornelAJ
StanichMA
McAbeeRD
MulliganFS
2002 High level methoprene resistance in the mosquito Ochlerotatus nigromaculis (Ludlow) in Central California. Pest Manag Sci 58 791 798
27. DameDA
WichtermanGJ
HornbyJA
1998 Mosquito (Aedes taeniorhynchus) resistance to methoprene in an isolated habitat. J Am Mosq Control Assoc 14 200 203
28. PaulA
HarringtonLC
ZhangL
ScottJG
2005 Insecticide resistance in Culex pipiens from New York. J Am Mosq Control Assoc 21 305 309
29. ChevillonC
BernardC
MarquineM
PasteurN
2001 Resistance to Bacillus sphaericus in Culex pipiens (Diptera : Culicidae) interaction between recessive mutants and evolution in southern France. J Med Entomol 38 657 664
30. ChalegreKDD
RomaoTP
AmorimLB
AnastacioDB
de BarrosRA
2009 Detection of an allele conferring resistance to Bacillus sphaericus binary toxin in Culex quinquefasciatus populations by molecular screening. Appl Environ Microbiol 75 1044 1049
31. DarbouxI
CharlesJF
PauchetY
WarotS
PauronD
2007 Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera : Culicidae). Cell Microbiol 9 2022 2029
32. OpotaO
CharlesJF
WarotS
PauronD
DarbouxI
2008 Identification and characterization of the receptor for the Bacillus sphaericus binary toxin in the malaria vector mosquito, Anopheles gambiae. Comp Biochem Physiol B Biochem Mol Biol 149 419 427
33. HemingwayJ
RansonH
2000 Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45 371 391
34. HemingwayJ
KarunaratneS
1998 Mosquito carboxylesterases: a review of the molecular biology and biochemistry of a major insecticide resistance mechanism. Med Vet Entomol 12 1 12
35. HemingwayJ
HawkesN
PrapanthadaraLA
JayawardenalKGI
RansonH
1998 The role of gene splicing, gene amplification and regulation in mosquito insecticide resistance. Philos Trans R Soc Lond B Biol Sci 353 1695 1699
36. RookerS
GuillemaudT
BergeJ
PasteurN
RaymondM
1996 Coamplification of esterase A and B genes as a single unit in Culex pipiens mosquitoes. Heredity 77 555 561
37. HemingwayJ
HawkesNJ
McCarrollL
RansonH
2004 The molecular basis of insecticide resistance in mosquitoes. Insect Biochem Mol Biol 34 653 665
38. WeillM
LutfallaG
MogensenK
ChandreF
BerthomieuA
2003 Insecticide resistance in mosquito vectors. Nature 423 136 137
39. ArnaudL
HaubrugeE
GageMJG
2005 The malathion-specific resistance gene confers a sperm competition advantage in Tribolium castaneum. Funct Ecol 19 1032 1039
40. McCartC
BucklingA
ffrench-ConstantRH
2005 DDT resistance in flies carries no cost. Curr Biol 15 R587 R589
41. MacDonaldG
1957 The epidemiology and control of malaria London Oxford University Press
42. GazaveE
ChevillonC
LenormandT
MarquineM
RaymondM
2001 Dissecting the cost of insecticide resistance genes during the overwintering period of the mosquito Culex pipiens. Heredity 87 441 448
43. ChevillonC
BourguetD
RoussetF
PasteurN
RaymondM
1997 Pleiotropy of adaptive changes in populations: comparisons among insecticide resistance genes in Culex pipiens. Genet Res 70 195 203
44. BoivinT
Chabert d'HieresC
BouvierJC
BeslayD
SauphanorB
2001 Pleiotropy of insecticide resistance in the codling moth, Cydia pomonella. Entomologia Experimentalis et Applicata 99 381 386
45. KonnoRH
OmotoC
2006 Fitness cost associated with carbosulfan resistance in Aphis gossypii Glover (Hemiptera : aphididae). Neotrop Entomol 35 246 250
46. YamamotoA
YonedaH
HatanoR
AsadaM
1995 Influence of hexythiazox resistance on life history parameters in the citrus red mite Panonychus citri (McGregor). J Pesticide Science 20 521 527
47. StearnsSC
1992 The evolution of life histories Oxford Oxford University Press 249
48. ReznickD
1992 Measuring the costs of reproduction. Trends Ecol Evol 7 42 45
49. KirkwoodTLB
RoseMR
1991 Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci 332 15 24
50. RaymondM
BerticatC
WeillM
PasteurN
ChevillonC
2001 Insecticide resistance in the mosquito Culex pipiens: what have we learned about adaptation? Genetica 112 287 296
51. DevonshireAL
MooresGD
1982 A carboxylesterase with broad substrate specificity causes organophosphorous, carbamate and pyrethroid resistance in peach potato aphids (Myzus persicae). Pestic Biochem Physiol 18 235 246
52. NijhoutHF
1994 Insect hormones Princeton (New Jersey) Princeton University Press 267
53. RiveroA
CasasJ
1999 Incorporating physiology into parasitoid behavioral ecology: the allocation of nutritional resources. Res Popul Ecol (Kyoto) 41 39 45
54. ClementsAN
1992 The biology of mosquitoes: development, nutrition and reproduction London Chapman & Hall 509
55. MonaghanP
MetcalfeNB
TorresR
2009 Oxidative stress as a mediator of life history trade-offs: mechanisms, measurements and interpretation. Ecol Lett 12 75 92
56. DowlingDK
SimmonsLW
2009 Reactive oxygen species as universal constraints in life-history evolution. Proc R Soc Lond B Biol Sci 276 1737 1745
57. RicklefsRE
2008 The evolution of senescence from a comparative perspective. Funct Ecol 22 379 392
58. RansonH
HemingwayJ
2005 Mosquito glutathione transferases. Methods Enzymol 401 226 241
59. Graca-SouzaAV
Maya-MonteiroC
Paiva-SilvaGO
BrazGRC
PaesMC
2006 Adaptations against heme toxicity in blood-feeding arthropods. Insect Biochem Mol Biol 36 322 335
60. Molina-CruzA
DejongRJ
CharlesB
GuptaL
KumarS
2008 Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J Biol Chem 283 3217 3223
61. Ortiz de MontellanoPR
de VossJJ
2005 Substrate oxidation by cytochrome p450 enzymes.
Ortiz de MontellanoPR
Cytochrome p450: Structure, mechanism and biochemistry New York Kluwer Academic 183 245
62. BastA
1986 Is formation of reactive oxygen by cytochrome p-450 perilous and predictable? Trends Pharmacol Sci 7 266 270
63. MuratalievMB
GuzovVM
WalkerFA
FeyereisenR
2008 P450 reductase and cytochrome b(5) interactions with cytochrome P450: effects on house fly CYP6A1 catalysis. Insect Biochem Mol Biol 38 1008 1015
64. HayesJD
McLellanLI
1999 Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic Res 31 273 300
65. VontasJG
SmallGJ
HemingwayJ
2001 Glutathione S-transferases as antioxidant defence agents confer pyrethroid resistance in Nilaparvata lugens. Biochem J 357 65 72
66. ParkesTL
HillikerAJ
PhillipsJP
1993 Genetic and biochemical analysis of gluthatione-S-transferase in the oxigen defense system of Drosophila melanogaster. Genome 36 1007 1014
67. McElweeJJ
SchusterE
BlancE
PiperMD
ThomasJH
2007 Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol 8 R132
68. AyyadevaraS
EngleMR
SinghSP
DandapatA
LichtiCF
2005 Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell 4 257 271
69. DingYC
HawkesN
MeredithJ
EgglestonP
HemingwayJ
2005 Characterization of the promoters of Epsilon glutathione transferases in the mosquito Anopheles gambiae and their response to oxidative stress. Biochem J 387 879 888
70. RansonH
RossiterL
OrtelliF
JensenB
WangXL
2001 Identification of a novel class of insect glutathione S-transferases involved in resistance to DDT in the malaria vector Anopheles gambiae. Biochem J 359 295 304
71. CurtisCF
2001 Insecticide resistance and mosquito-borne disease. Lancet 357 656 656
72. Vega-RodriguezJ
Franke-FayardB
DinglasanRR
JanseCJ
Pastrana-MenaR
2009 The glutathione biosynthetic pathway of Plasmodium is essential for mosquito transmission. PLoS Pathog 5 1000302 doi:10.1371/journal.ppat.1000302
73. MacLeodET
MaudlinI
DarbyAC
WelburnSC
2007 Antioxidants promote establishment of trypanosome infections in tsetse. Parasitology 134 827 831
74. DimopoulosG
2003 Insect immunity and its implication in mosquito-malaria interactions. Cell Microbiol 5 3 14
75. KumarS
ChristophidesGK
CanteraR
CharlesB
HanYS
2003 The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc Natl Acad Sci U S A 100 14139 14144
76. MyersM
RichmondRC
OakeshottJG
1988 On the origins of esterases. Mol Biol Evol 5 113 119
77. VernickKD
CollinsFH
1989 Association of a Plasmodium-refractory phenotype with an esterase locus in Anopheles gambiae. Am J Trop Med Hyg 40 593 597
78. CollinsFH
PaskewitzSM
CrewsoyenAE
1991 A genetic study of Plasmodium susceptibility in the African malaria vector Anopheles gambiae. Annales De La Societe Belge De Medecine Tropicale 71 225 232
79. Crews-OyenAE
KumarV
CollinsFH
1993 Association of two esterase genes, a chromosomal inversion, and susceptibility to Plasmodium cynomolgi in the African malaria vector Anopheles gambiae. Am J Trop Med Hyg 49 341 347
80. ShiotsukiT
KatoY
1999 Induction of carboxylesterase isozymes in Bombyx mori by E. coli infection. Insect Biochem Mol Biol 29 731 736
81. ChongsatjaPO
BourchookarnA
LoCF
ThongboonkerdV
KrittanaiC
2007 Proteomic analysis of differentially expressed proteins in Penaeus vannamei hemocytes upon Taura syndrome virus infection. Proteomics 7 3592 3601
82. PoveyS
CotterSC
SimpsonSJ
LeeKP
WilsonK
2009 Can the protein costs of bacterial resistance be offset by altered feeding behaviour? J Anim Ecol 78 437 446
83. LeeKP
CoryJS
WilsonK
RaubenheimerD
SimpsonSJ
2006 Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc R Soc Lond B Biol Sci 273 823 829
84. CheonHM
ShinSW
BianGW
ParkJH
RaikhelAS
2006 Regulation of lipid metabolism genes, lipid carrier protein lipophorin, and its receptor during immune challenge in the mosquito Aedes aegypti. J Biol Chem 281 8426 8435
85. HallSR
SimonisJL
NisbetRM
TessierAJ
CáceresCE
2009 Resouce ecology of virulence in a planktonic host-parasite system: an explanation using dynamic energy budgets. Am Nat 174 149 162
86. BallGH
ChaoJ
1976 Use of amino acids by Plasmodium relictum oocysts in vitro. Exp Parasitol 39 115 118
87. AtellaGC
Bittencourt-CunhaPR
NunesRD
ShahabuddinM
Silva-NetoMAC
2009 The major insect lipoprotein is a lipid source to mosquito stages of malaria parasite. Acta Trop 109 159 162
88. SchieferBA
WardRA
EldridgeBF
1977 Plasmodium cynomolgi: effects of malaria infection on laboratory flight performance of Anopheles stephensi mosquitoes. Exp Parasitol 41 397 404
89. BedhommeS
AgnewP
SidobreC
MichalakisY
2004 Virulence reaction norms across a food gradient. Proc R Soc Lond B Biol Sci 271 739 744
90. TsengM
2006 Interactions between the parasite's previous and current environment mediate the outcome of parasite infection. Am Nat 168 565 571
91. HallSR
KnightCJ
BeckerCR
DuffyMA
TessierAJ
2009 Quality matters: resource quality for hosts and the timing of epidemics. Ecol Lett 12 118 128
92. PulkkinenK
EbertD
2004 Host starvation decreases parasite load and mean host size in experimental populations. Ecology 85 823 833
93. AndersonRA
KoellaJC
HurdH
1999 The effect of Plasmodium yoelii nigeriensis infection on the feeding persistence of Anopheles stephensi Liston throughout the sporogonic cycle. Proc Biol Sci 266 1729 1733
94. RogersME
BatesPA
2007 Leishmania manipulation of sand fly feeding behavior results in enhanced transmission. PLoS Pathog 3 e91 doi:10.1371/journal.ppat.0030091
95. LacroixR
MukabanaWR
GouagnaLC
KoellaJC
2005 Malaria infection increases attractiveness of humans to mosquitoes. PLoS Biol 3 e298 doi:10.1371/journal.pbio.0030298
96. BourguetD
LenormandT
GuillemaudT
MarcelV
FournierD
1997 Variation of dominance of newly arisen adaptive genes. Genetics 147 1225 1234
97. LeeSH
SmithTJ
KnippleDC
SoderlundDM
1999 Mutations in the house fly Vssc1 sodium channel gene associated with super-kdr resistance abolish the pyrethroid sensitivity of Vssc1/tipE sodium channels expressed in Xenopus oocytes. Insect Biochem Mol Biol 29 185 194
98. FosterSP
YoungS
WilliamsonMS
DuceI
DenholmI
2003 Analogous pleiotropic effects of insecticide resistance genotypes in peach-potato aphids and houseflies. Heredity 91 98 106
99. FosterSP
WoodcockCM
WilliamsonMS
DevonshireAL
DenholmI
1999 Reduced alarm response by peach-potato aphids, Myzus persicae (Hemiptera : Aphididae), with knock-down resistance to insecticides (kdr) may impose a fitness cost through increased vulnerability to natural enemies. Bull Entomol Res 89 133 138
100. FosterSP
TomiczekM
ThompsonR
DenholmI
PoppyG
2007 Behavioural side-effects of insecticide resistance in aphids increase their vulnerability to parasitoid attack. Anim Behav 74 621 632
101. ZwiebelLJ
TakkenW
2004 Olfactory regulation of mosquito-host interactions. Insect Biochem Mol Biol 34 645 652
102. BraksMAH
MeijerinkJ
TakkenW
2001 The response of the malaria mosquito, Anopheles gambiae, to two components of human sweat, ammonia and L-lactic acid, in an olfactometer. Physiol Entomol 26 142 148
103. SteibBM
GeierM
BoeckhJ
2001 The effect of lactic acid on odour-related host preference of yellow fever mosquitoes. Chem Senses 26 523 528
104. RowlandM
1991 Activity and mating competitiveness of gamma-HCH/dieldrin resistant and susceptible male and virgin female Anopheles gambiae and An. stephensi mosquitos, with assessment of an insecticide rotation strategy. Med Vet Entomol 5 207 222
105. RowlandM
1991 Behavior and fitness of gamma-HCH/dieldrin resistant and susceptible female Anopheles gambiae and An. stephensi mosquitos in the absence of insecticide. Med Vet Entomol 5 193 206
106. ClementsAN
1999 The biology of mosquitoes: sensory reception and behaviour Wallingford CABI Publishing 740
107. LefevreT
ThomasF
2008 Behind the scene, something else is pulling the strings: Emphasizing parasitic manipulation in vector-borne diseases. Infect Genet Evol 8 504 519
108. FergusonHM
ReadAF
2002 Why is the effect of malaria parasites on mosquito survival still unresolved? Trends Parasitol 18 256 261
109. VontasJG
McCarrollL
KarunaratneS
LouisC
HurdH
2004 Does environmental stress affect insect-vectored parasite transmission? Physiol Entomol 29 210 213
110. SindenRE
DawesEJ
AlaviY
WaldockJ
FinneyO
2007 Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathogens 3 e195 doi:10.1371/journal.ppat.0030195
111. DawesEJ
ChurcherTS
ZhuangS
SindenRE
BasáñezMG
2009 Anopheles mortality is both age - and Plasmodium-density dependent: implications for malaria transmission. Malaria J 8 228
112. RansonH
ClaudianosC
OrtelliF
AbgrallC
HemingwayJ
2002 Evolution of supergene families associated with insecticide resistance. Science 298 179 181
113. LabbeP
BerticatC
BerthomieuA
UnalS
BernardC
2007 Forty years of erratic insecticide resistance evolution in the mosquito Culex pipiens. PLoS Genet 3 e205 doi:10.1371/journal.pgen.0030205
114. CorbelV
N'GuessanR
BrenguesC
ChandreF
DjogbenouL
2007 Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin (West Africa) and operational challenge for malaria vector control. Am J Trop Med Hyg 77 230
115. PereraMDB
HemingwayJ
KarunaratneSHPP
2008 Multiple insecticide resistance mechanisms involving metabolic changes and insensitive target sites selected in anopheline vectors of malaria in Sri Lanka. Malaria J 7 168
116. OkoyePN
BrookeBD
HuntRH
CoetzeeM
2007 Relative developmental and reproductive fitness associated with pyrethroid resistance in the major southern African malaria vector, Anopheles funestus. Bull Entomol Res 97 599 605
117. DongY
AguilarR
XiZ
WarrE
MonginE
2006 Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog 2 e52 doi:10.1371/journal.ppat.0020052
118. VectorBase 2010 AnoBase: the Anopheles database. Insecticide resistance database search. Available: http://anobase.vectorbase.org/ir/. Accessed 6 July 2010
119. SurendranSN
KarunaratneSHPP
AdamsZ
HemingwayJ
HawkesNJ
2005 Molecular and biochemical characterization of a sand fly population from Sri Lanka: evidence for insecticide resistance due to altered esterases and insensitive acetylcholinesterase. Bull Entomol Res 95 371 380
120. HemingwayJ
CallaghanA
KurtakDC
1989 Temephos resistance in Simulium damnosum Theobald (Diptera, Simuliidae): A comparative study between larvae and adults of the forest and savanna strains of this species complex. Bull Entomol Res 79 659 669
121. HemingwayJ
CallaghanA
KurtakDC
1991 Biochemical characterization of chlorphoxim resistance in adults and larvae of the Simulium damnosum complex (Diptera, Simulidae). Bull Entomol Res 81 401 406
122. VassenaCV
PicolloMI
ZerbaEN
2000 Insecticide resistance in Brazilian Triatoma infestans and Venezuelan Rhodnius prolixus. Med Vet Entomol 14 51 55
123. AudinoPG
VassenaC
BarriosS
ZerbaE
PicolloMI
2004 Role of enhanced detoxication in a deltamethrin-resistant population of Triatoma infestans (Hemiptera, reduviidae) from Argentina. Mem Inst Oswaldo Cruz 99 335 339
124. OrihuelaPLS
VassenaCV
ZerbaEN
PicolloMI
2008 Relative contribution of monooxygenase and esterase to pyrethroid resistance in Triatoma infestans (Hemiptera : Reduviidae) from Argentina and Bolivia. J Med Entomol 45 298 306
125. PicolloMI
VassenaC
OrihuelaPS
BarriosS
ZaidembergM
2005 High resistance to pyrethroid insecticides associated with ineffective field treatments in Triatoma infestans (Hemiptera : Reduviidae) from northern Argentina. J Med Entomol 42 637 642
126. KumarK
Jamil-Ur-RahmanS
SharmaSK
GillKS
KatyalR
1997 Entomological and rodent surveillance in plague-suspected areas during September 1994 and thereafter. Jpn J Med Sci Biol 50 97 111
127. KilonzoBS
1985 DDT resistance in Xenopsylla brasiliensis the common plague vector in Tanzania. Insect Science and Its Application 6 111 114
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



