-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSpliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
Trans-splicing of leader sequences onto the 5′ends of mRNAs is a widespread phenomenon in protozoa, nematodes and some chordates. Using parallel sequencing we have developed a method to simultaneously map 5′splice sites and analyze the corresponding gene expression profile, that we term spliced leader trapping (SLT). The method can be applied to any organism with a sequenced genome and trans-splicing of a conserved leader sequence. We analyzed the expression profiles and splicing patterns of bloodstream and insect forms of the parasite Trypanosoma brucei. We detected the 5′ splice sites of 85% of the annotated protein-coding genes and, contrary to previous reports, found up to 40% of transcripts to be differentially expressed. Furthermore, we discovered more than 2500 alternative splicing events, many of which appear to be stage-regulated. Based on our findings we hypothesize that alternatively spliced transcripts present a new means of regulating gene expression and could potentially contribute to protein diversity in the parasite. The entire dataset can be accessed online at TriTrypDB or through: http://splicer.unibe.ch/.
Published in the journal: . PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001037
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001037Summary
Trans-splicing of leader sequences onto the 5′ends of mRNAs is a widespread phenomenon in protozoa, nematodes and some chordates. Using parallel sequencing we have developed a method to simultaneously map 5′splice sites and analyze the corresponding gene expression profile, that we term spliced leader trapping (SLT). The method can be applied to any organism with a sequenced genome and trans-splicing of a conserved leader sequence. We analyzed the expression profiles and splicing patterns of bloodstream and insect forms of the parasite Trypanosoma brucei. We detected the 5′ splice sites of 85% of the annotated protein-coding genes and, contrary to previous reports, found up to 40% of transcripts to be differentially expressed. Furthermore, we discovered more than 2500 alternative splicing events, many of which appear to be stage-regulated. Based on our findings we hypothesize that alternatively spliced transcripts present a new means of regulating gene expression and could potentially contribute to protein diversity in the parasite. The entire dataset can be accessed online at TriTrypDB or through: http://splicer.unibe.ch/.
Introduction
Trypanosoma brucei is a unicellular eukaryotic parasite with a digenetic life cycle alternating between the tsetse fly and a variety of mammalian hosts. Besides its importance as a human and veterinary pathogen it has been key to the discovery and understanding of general biological principles such as RNA editing, antigenic variation, GPI anchoring and trans-splicing [1], [2], [3], [4], [5]. The genome sequence of the 11 megabase-sized chromosomes of T. brucei revealed a compact structure containing about 9000 predicted genes, including 900 pseudogenes and 1700 genes specific to T. brucei [6]. The majority of protein coding genes in trypanosomes is organized in polycistronic units that are transcribed by RNA polymerase II (Pol II) [7]. Polycistronic RNA precursors are processed into mature monocistronic mRNAs by trans-splicing of a 39 nt leader sequence to the 5′ end and polyadenylation of the 3′ end [5]. With the exception of the actin promoter, no Pol II promoters for protein-coding genes have been identified, reviewed in [8]. Recently, an elegant study showed correlation between the position of histone marks and putative transcription start sites, suggesting that chromatin structure plays a major role in directing Pol II to its promoter sites [9]. While promoter structures are still elusive, it has long been known that transcription itself is regulated very little, if at all. The regulation of gene expression occurs mainly at the level of RNA stability, translation and protein stability (for review see [8]). Four microarray analyses have shown the transcriptome of the organism to be rather static, with only 2–10% of the transcripts being stage-regulated [10], [11], [12], [13]. This limited degree of regulation at the level of transcript abundance is surprising, given the fundamental changes in morphology and metabolism that occur during the development of the parasite, and especially in the light of the large differences that occur in the closely related species Trypanosoma cruzi, where approximately half of the genome is regulated at the level of transcript abundance [14]. To date, very little is known about the way in which trypanosomes regulate translation, but in many other eukaryotes the 5′ untranslated regions (UTRs) contain sequence elements that are responsible for regulating protein synthesis. In order to analyze the T. brucei genome for such elements it is crucial to delineate the 5′ UTR of each expressed gene. In the past, bioinformatics approaches have been used to predict 5′ splice sites in T. brucei, but few of these have been confirmed experimentally [15]. Using a novel high throughput parallel sequencing approach that we term spliced leader trapping (SLT), we have now mapped the vast majority of 5′ UTRs and analyzed the developmental regulation of transcript abundance in bloodstream and insect form trypanosomes. SLT also provides a means of selectively analyzing the transcriptome of parasites without having to purify them from host tissue. Furthermore, since trans-splicing of a spliced leader has been identified not only in protozoa but also in cnidarians, nematodes, flatworms and ascidians, SLT could potentially be applied to a wide variety of organisms [5], [16], [17], [18], [19], [20].
Results
Libraries, sequencing and mapping of splice acceptor sites
Long slender and short stumpy bloodstream form trypanosomes are the proliferative and quiescent life cycle stages, respectively, in the mammalian host, while the procyclic form is a proliferative form in the midgut of the insect host. In order to map the 5′splice sites and quantify abundance of the corresponding transcripts in these three life cycle stages, poly(A)-RNA was purified and first strand cDNA was synthesized using random hexamers (Figure S1). In order to process multiple samples in one sequencing channel a four-nucleotide barcode was added to the 3′end of the cDNAs. After amplification and size fractionation to 120–160 base pairs the cDNA library was sequenced on a Genome Analyzer (GA-II, Illumina) using the Chrysalis 36 cycles v 3.0 sequencing kit and 76 cycles. Base calling was performed using the Genome Analyzer Pipeline and linker sequences were removed while separating reads according to identified barcodes. The estimated error probability of the retained bases was 1.1%. Inserts containing a barcode were up to 70 base pairs (bp) with an average insert size of 42 bp (median 45 bp, σ 17 bp; Figure 1).
Fig. 1. Insert sizes and gene discovery rate. 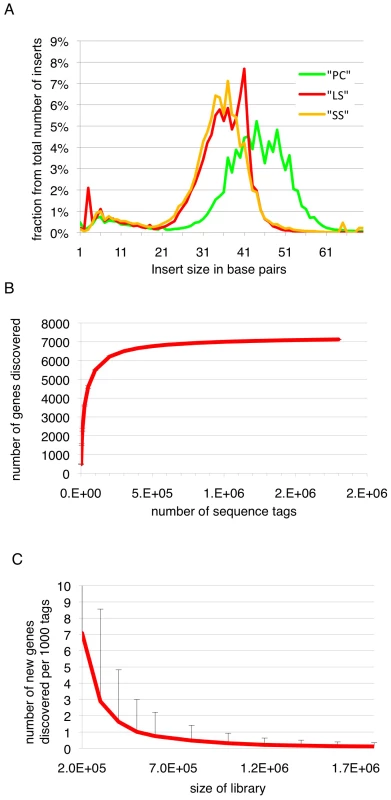
(A) Insert sizes of the sequence tags of the three libraries in base pairs (bp); long slender bloodstream form (LS, red), short stumpy bloodstream form (SS, orange) and procyclic form (PC, green). (B) Simulation of gene coverage from the long slender bloodstream form library; considered are all tags that lead to UTRs ≤2 kb. Error bars depict 99.99% confidence interval from five simulations assuming normal distribution. (C) Number of new genes discovered per 1000 tags depending on the total number of tags, error bars depict 95% confidence interval from five simulations, assuming normal distribution. We obtained 4.6 million sequence tags that could be attributed to one of the libraries (Table 1). Of these 4.5 million tags (98%) could be aligned to one of the 11 megabase-sized chromosomes of T. brucei, the Antat 1.1 variant surface glycoprotein (VSG; 227,063 reads) or the telomeric expression sites (40,333 reads). Depending on the library, 77% to 79% of all genes had at least one tag associated with their 5′ UTR or annotated coding sequence. The median number of tags per gene ranged from 12 in the long slender bloodstream form to 24 in the procyclic form (Table 1). The dynamic range of the SLT method is best described in the bloodstream library where it spanned more than 5 orders of magnitude from 1 tag for an individual gene to 205,410 tags or 11% of all tags in that library for the Antat1.1 VSG. The majority of genes without any tags were hypothetical unlikely (316), hypothetical (118), expression site associated genes (116) and hypothetical conserved genes (103).
Tab. 1. Library statistics. 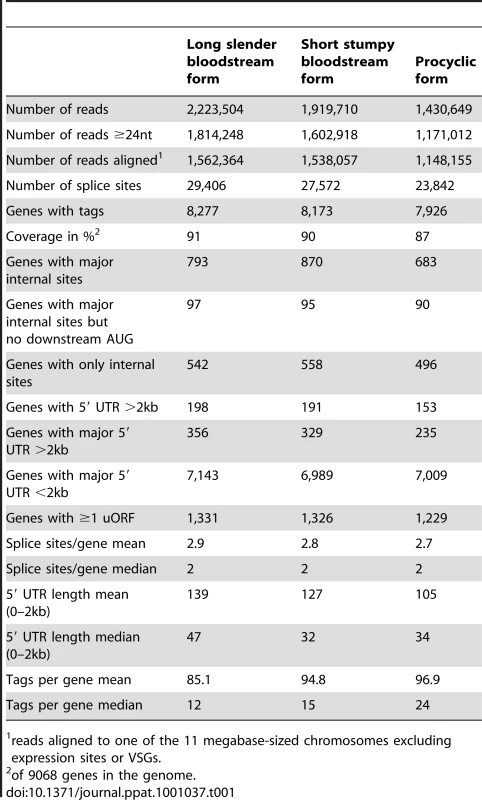
reads aligned to one of the 11 megabase-sized chromosomes excluding expression sites or VSGs. Using information for the positions of snoRNAs, rRNAs and tRNAs, together with the direction of transcription, we estimated the number of polycistronic transcription units to be approximately 200. Transcription profile analysis of the majority of individual transcription units containing five or more protein coding genes (excluding VSG genes) indicated large variations in transcript abundance between adjacent genes. Of 270 monocistronic mRNA transcription units that have been annotated, 76 had major splice sites less than 2kb upstream of the ATG and eight were differentially expressed.
It has recently been shown that procyclin-associated genes (PAGs) are located preferentially at a subset of strand-switch regions, or between polycistrons that are transcribed by different polymerases, and that a region within PAG1 could silence transcription by Pol I [21]. Based on SLT analysis, mature transcripts from genes downstream of PAGs were not observed for five of the six annotated sites. In one case transcripts were absent from the procyclic form, while the bloodstream form produced spliced transcripts downstream of two PAG-like genes (Tb11.01.6210-6220). It has been documented that overlapping sense and antisense transcription can occur at a single locus, giving rise to processed mRNAs from both strands [22]. We identified 140 unique antisense spliced leader addition sites with ≥1 tag per million (TPM), 60 of which were detected in more than one life cycle stage. Thirty-eight of the unique antisense splice leader addition sites were found on the reverse strand of a hypothetical gene at the end of a transcription unit and twenty-three were in strand-switch regions.
Expression profiling and comparison with previous transcriptome studies
In order to evaluate if the expression profiles were consistent with previously published data we first analyzed 20 well-studied genes and found good agreement between the direction and magnitude of change in transcript abundance between life cycle stages (Table S1). These included the phosphoglycerate kinases PGKB and PGKC [23], the nuclear-encoded cytochrome oxidase complex subunits (IV–X) [24], the terminal alternative oxidase [25], invariant surface glycoproteins (ISGs; [26]) and the major surface glycoproteins, VSG and procyclins [27]. In total, 3554 genes or ∼40% of the genome significantly changed expression levels in at least one of the three life cycle stages (statistical significance of <10−5; [28]; Table 2). More than 2000 differentially expressed transcripts could be observed between the bloodstream and procyclic forms, while 1226 changes in expression level could be detected between the proliferative long slender and quiescent short stumpy bloodstream stages. (Figure 2; Table 2). Of the entire transcriptome 5472 transcripts (60%) did not change abundance between life cycle stages; 919 of these transcripts were represented by ≥25 TPM in all stages.
Fig. 2. Whole genome comparisons of gene expression levels. 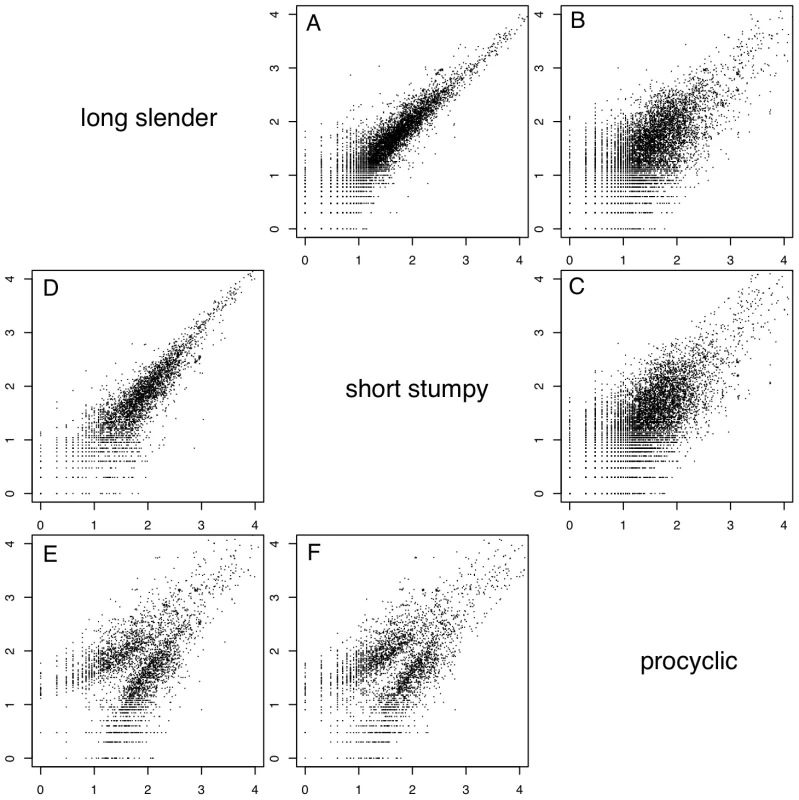
Each subfigure (A–F) shows a scatter plot of mRNA expression levels (log10 tags per million) between two life-cycle stages. Each dot represents one gene. (A) long slender/short stumpy, (B) long slender/procyclic, (C) short stumpy/procyclic. In panels D–F only genes with statistically significant difference in expression levels between any two life stages of the parasite are shown (Audic-Claverie P<105). Tab. 2. Differentially expressed and spliced transcripts. 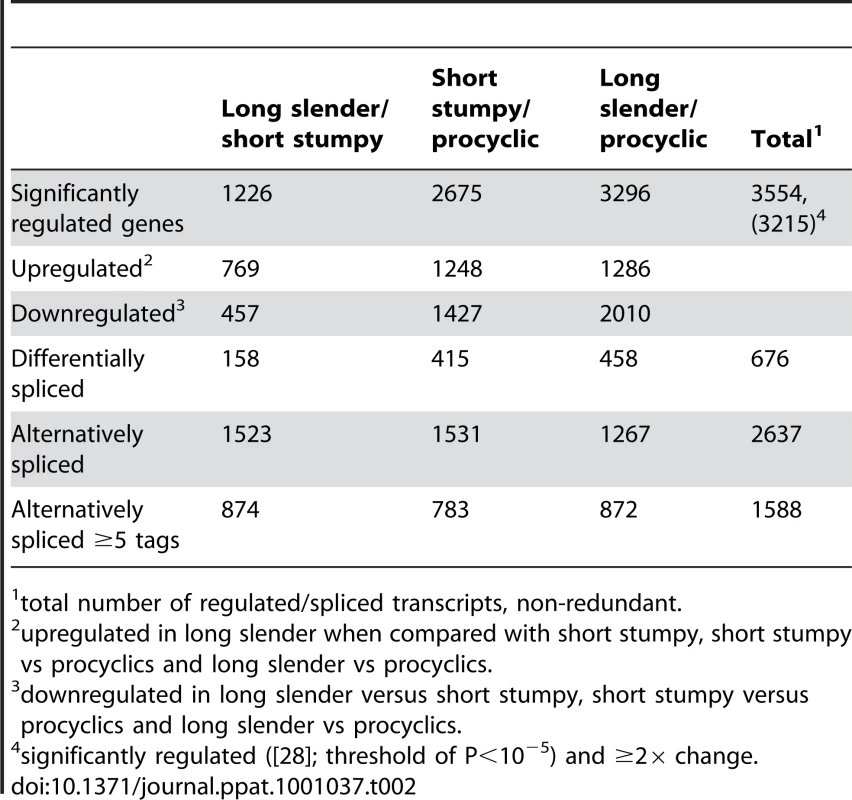
total number of regulated/spliced transcripts, non-redundant. We further evaluated the SLT approach by comparing the data to a previously published tiling array study [11]. Transcripts more abundant in the procyclic form correlated with a coefficient of 0.77 or 0.93 to the SLT approach, depending on the statistics used by Koumandou et al. (Figure S2, [11]). Transcripts more abundant in the bloodstream form showed a correlation coefficient of 0.23 or 0.43 (Figure S3). When we compared our data to the most recent microarray study by Jensen et al. [12], which identified 234 transcripts are being less abundant in the procyclic than the bloodstream form, 172 showed the same direction of change while 62 did not agree with our study. In addition, from the 317 transcripts that are increased in the procyclic form, 270 are in agreement with our study. Of the 551 transcripts that were significantly changed ≥2-fold between the two life cycle stages in the study by Jensen et al. ∼80% showed the same pattern in our study. Using the two data sets, we have compiled a list of 442 genes that show a robust change in expression pattern (Table S2).
When we analyzed the metabolic pathways as annotated in KEGG we found the majority to be down regulated in the stumpy form when compared to the long slender form (Figure S4, S5, S6, Table S3). In the glycolytic pathway for example, 8 of 11 transcripts are decreased in abundance in the short stumpy form, which is in good agreement with previous studies of the metabolic pathways in quiescent cells in general [29] and trypanosome short stumpy form in particular (for review see [30]). We furthermore compared the expression profile of ten genes between bloodstream long slender and procyclic cells using SLT and RT qPCR and found positive correlation between the two methods (Pearson correlation r = 0.97; p-value of p<10−5; Figure S7, Table S4). Additionally, we performed two RNAi experiments (Alba1 and Alba 3/4) in procyclic forms (Figure S8). The efficiency of the knockdown was then measured by Northern blot analysis and SLT. For Alba1 both techniques showed a knockdown in message level to 6% compared to the uninduced cell line. For Alba 3/4 Northern blot analysis indicated a knockdown to 8% while the value measured by SLT was 13%. The overall correlation between the uninduced and the induced libraries was between 0.97 (Alba1) and 0.94 (Alba 3/4; Spearman rank correlation coefficient). Lastly we compared the expression profile from SLT with one run of regular RNAseq from poly(A)-RNA from procyclic forms (24 million sequence tags) and found a positive correlation between the two techniques (Spearman ρ = 0.69, Figure S9).
Analysis of a VSG expression site
In addition to the different life cycle stages of Antat 1.1, we also analyzed the widely used monomorphic bloodstream form MITat 1.2 (221) and mapped the sequence tags to the active VSG expression site (Figure 3; [31]). The most highly expressed gene was VSG 221 with more than 69,000 TPM. The second most highly expressed gene in the expression site was the ESAG 12 gene (761 tags) followed by a hypothetical gene that has not been annotated previously, now designated ESAG13 (138 tags) and ESAG 6 and 7, which encode the two subunits of the transferrin receptor (80 and 43 tags; for review see [32]).
Fig. 3. Expression levels of genes in the active VSG expression site. 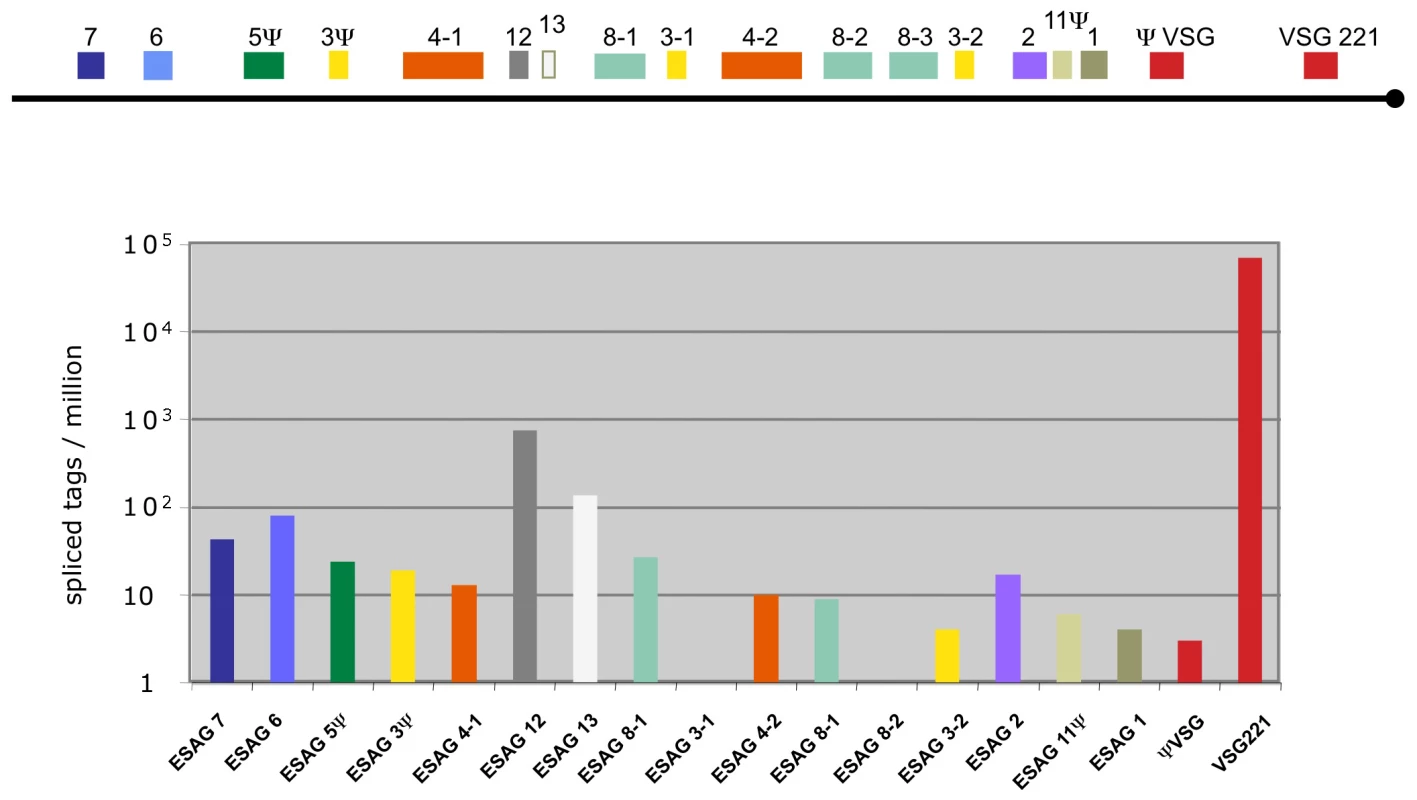
Schematic representation of the tag frequency distribution in the bloodstream expression site 1 (BES1/TAR40), as annotated by Hertz-Fowler et al. [31]. The hypothetical gene between ESAG 12 and ESAG 8-1 encodes a protein of 96 amino acids and is renamed ESAG 13. Splice site detection and changes in alternative splicing patterns during development
We identified 29,406 splice sites in the T. brucei genome with a median of two splice sites per gene (bloodstream form AnTat1.1; Table 1). The major splice site was strictly conserved with 94% of the splice acceptor dinucleotides being AG preceded by an upstream polypyrimidine tract (−14 to −43, relative to the splice site; Figure 4A). Twenty percent of the minor splice acceptor sites contained a dinucleotide other than AG. GG occurred in 7% of these while TG, AA, GA and AC were found in 2% of the minor splice sites (Figure 4B–C). The least abundant dinucleotide was CC. When we compared the major splice sites from this study to a previous genome-wide prediction we found that about 40% of the major sites and 6% of the minor sites had correctly been predicted by the model [15]. Using the position of the major splice sites we determined the mean length of all 5′ UTRs to be 104–138 nucleotides and the median length to be 32–47 nucleotides (Figure 4D–E, Table 1). In the procyclic life cycle stage we identified 588 transcripts with splice sites that would allow N-terminal extension of the annotated protein coding gene and, for 11 of these, peptides corresponding to the N-terminal region were recently detected by mass spectrometry (Table S5; [33]). About 500 of the currently annotated genes only had internal splice sites 3′ of the predicted AUG start codon, and depending on the life cycle stage, 683–870 genes had major internal splice sites (Table 1). Of the transcripts with major internal sites ∼90% had a downstream AUG in the same reading frame within 400 nucleotides of the splice site, leaving about 10% of the transcripts without a likely translation start. In each life cycle stage >1200 transcripts exhibited an alternative splicing pattern (Figure 5–6 Table 3). A transcript was termed alternatively spliced if the major splice site contained less than 60% of all sequence tags. We identified four different types of alternative splicing, based on the potential functional consequences (Figure 7). Splicing type A renders the transcript potentially untranslatable since the transcript does not contain an in-frame AUG downstream of the alternative splice site (Figure 7, Figure S10). Splicing type B leads to a 5′ truncation of the original open reading frame, with a potential downstream AUG start site (Figure S11). Splice Type C does not change the open reading frame, but it includes or excludes potential regulatory elements such as upstream open reading frames (uORFs) in the 5′ UTR (Figure S12). Splice type D potentially allows for the use of a novel open reading frame due to the inclusion of an AUG start codon in a different reading frame (Figure S13). We then analyzed if the major splice sites for a particular transcript changed between the life cycle stages and termed this differential splicing (Figure 6). Depending on the two life stages we compared, 158–458 differential splicing events affecting a total of 676 genes were identified (Table 2). We additionally verified the differential splicing patterns for three transcripts (Tb927.1.790, Tb927.6.4240, Tb11.02.2700) by RT qPCR and found positive correlation between the SLT and the RT qPCR data (Pearson r = 0.85; 95% confidence interval; Table S6).
Fig. 4. Splice site conservation and 5′ UTR length distribution. 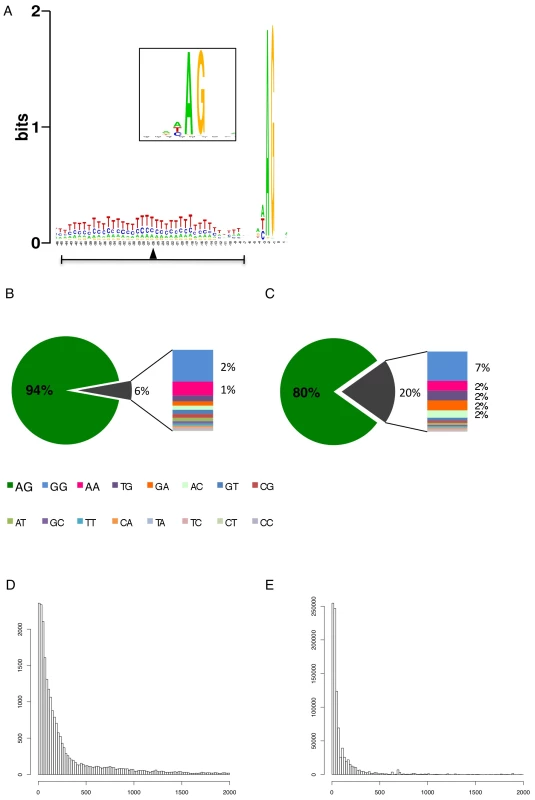
(A) Consensus of the major splice sites from procyclic form T. brucei. The inset shows a close up of the conserved AG at the splice site. The upstream polypyrimidine tract is dominated by T residues starting at an average distance of 26 nucleotides (black triangle; ±19) upstream of the splice site. (B–C) Splice site comparison. Abundance of dinucleotides at the splice acceptor sites for (B) major splice sites and (C) minor splice sites in T. brucei procyclic forms. (D–E) Untranslated region (5′ UTR) length distribution in procyclic forms using the major splice site on a per gene basis (D). 5′ UTR length distribution of the major splice site on a per transcript basis (E). Fig. 5. Example of an alternatively spliced transcript (Tb11.02.2700). 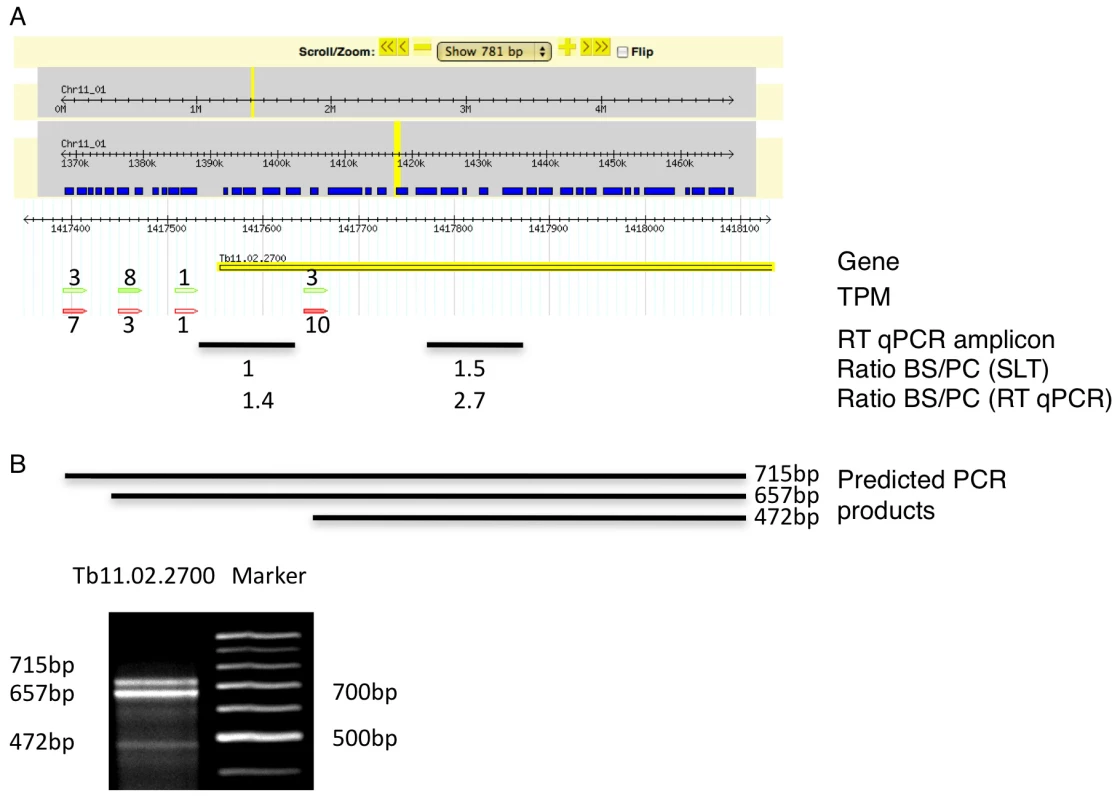
(A) Display of the splicing profile for Tb11.02.2700 using Gbrowse. Spliced leader tags from bloodstream form (red) and procyclic form (green) are shown; numbers indicate tags per million (TPM). The position and size of the RT qPCR amplicon is depicted by a black bar (RT qPCR amplicon). Below the bar the mRNA abundance ratios as determined by SLT and qPCR respectively, are shown. (B) Expected size of PCR product using a spliced leader-specific oligonucleotide together with a gene specific oligonucleotide are depicted. Gel shows PCR products from procyclic cDNA. Fig. 6. Example of a differentially spliced transcript (Tb927.6.4240). 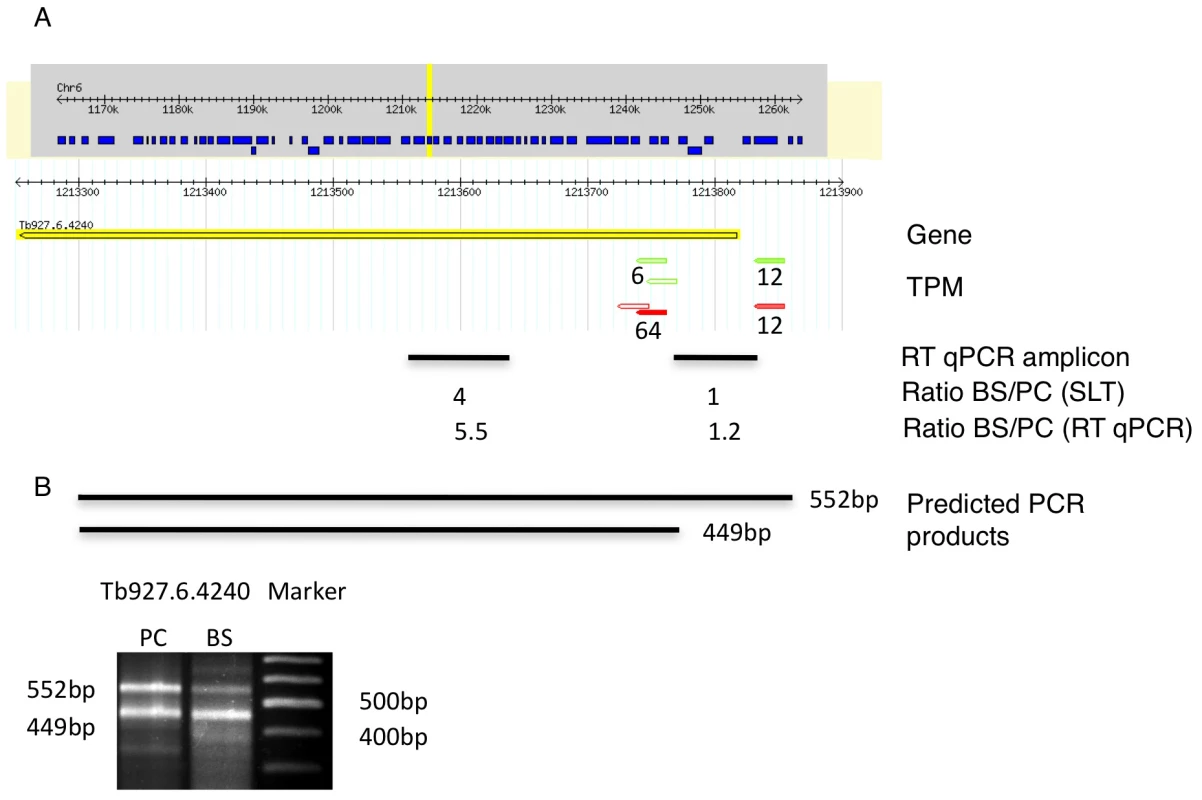
(A) Display of the splicing profile for Tb927.6.4240 using Gbrowse. Spliced leader tags from bloodstream form (red) and procyclic form (green) are shown. Numbers indicate tags per million. The position and size of the RT qPCR amplicon is depicted by a black bar (RT qPCR amplicon). Below the bar the ratios of mRNA abundance as determined by SLT and qPCR respectively are shown. (B) Expected size of PCR products using a spliced leader-specific oligonucleotide together with a gene specific oligonucleotide are depicted. Gel shows PCR products from procyclic (PC) and bloodstream (BS, long slender) cDNA. Fig. 7. Proposed functions for alternative splicing variants. 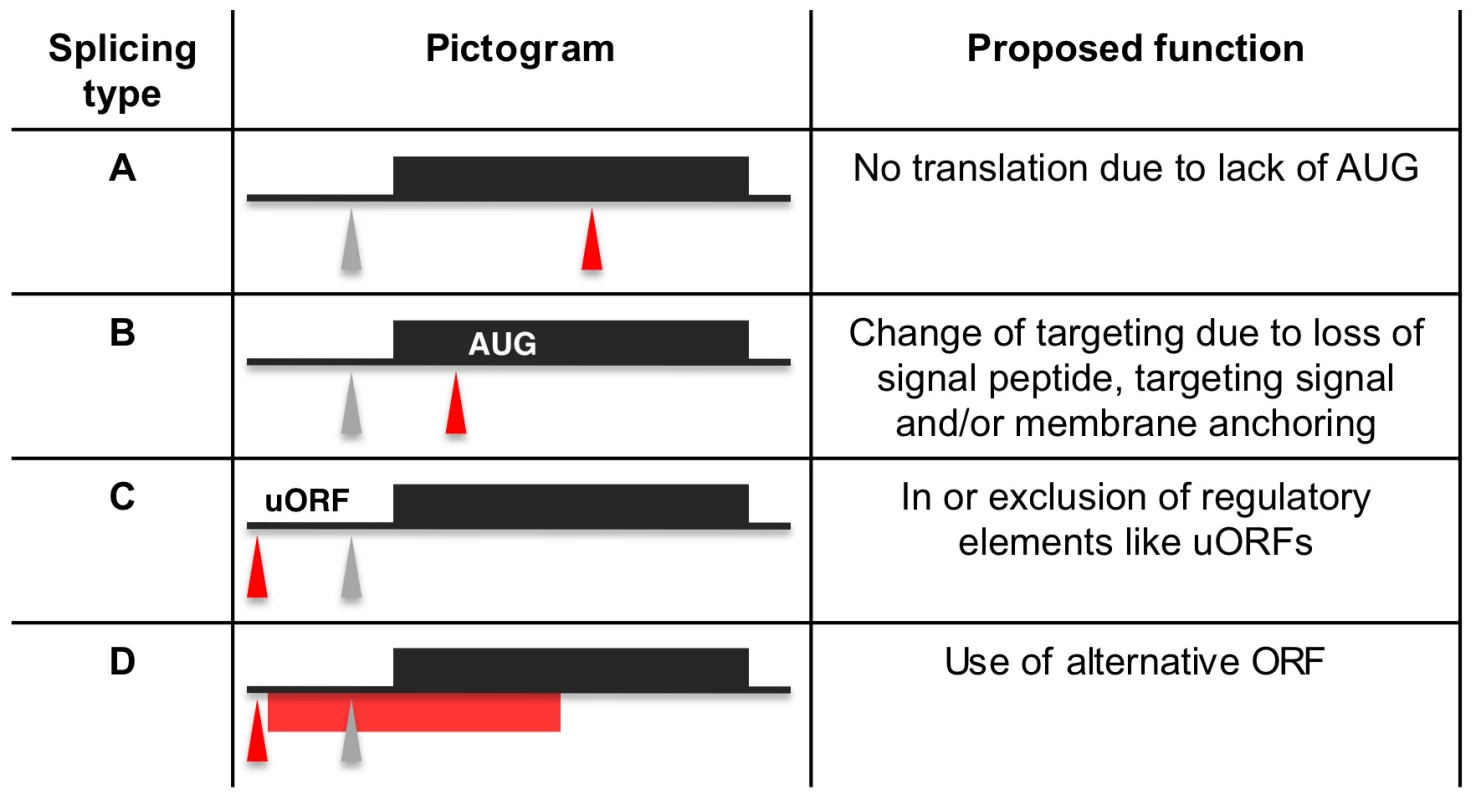
Regular splice sites (grey triangles) and alternative splice sites (red triangles) are depicted on a pre-processed mRNA (black line) containing an open reading frame (ORF; black box). The red bar depicts an alternative ORF. AUG depicts an alternative translation start site. Upstream open reading frames are denoted uORF. Tab. 3. Alternatively spliced transcripts. 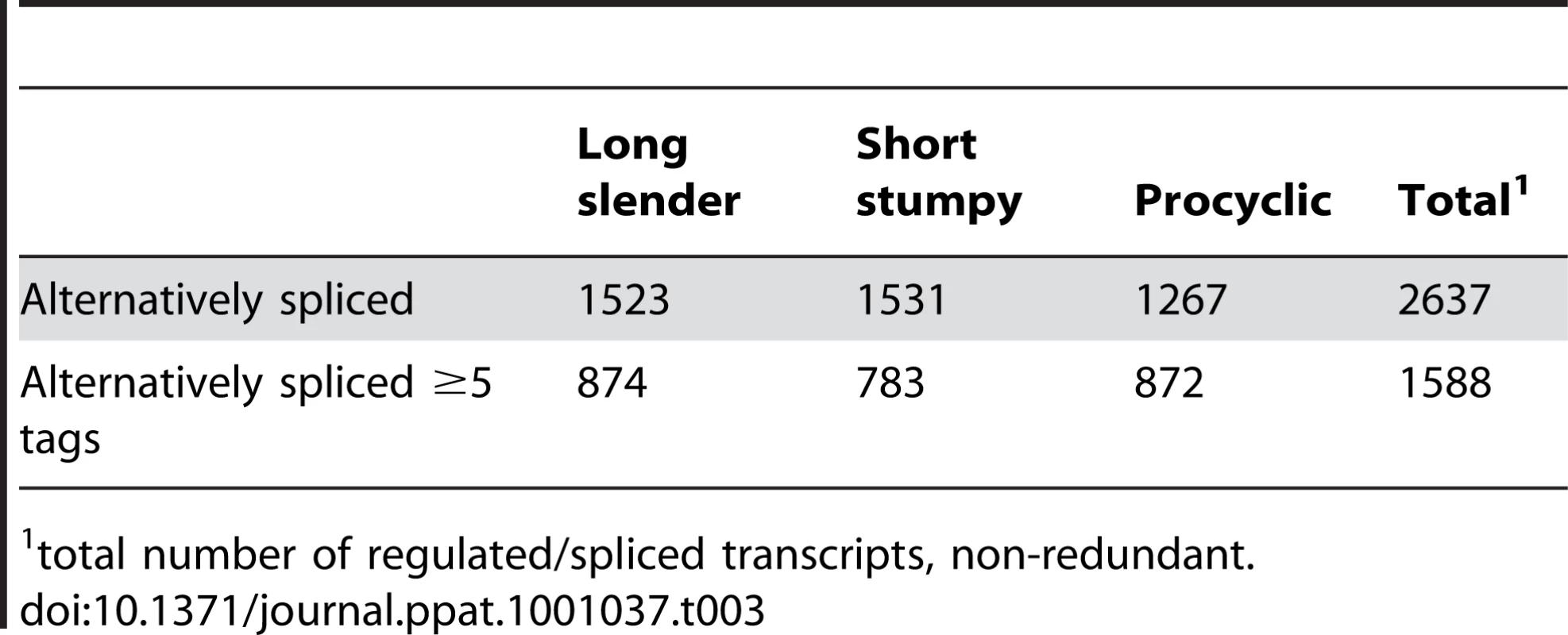
total number of regulated/spliced transcripts, non-redundant. Alternative splicing of mRNAs encoding aminoacyl tRNA synthetases (AARS)
Of the 23 currently annotated distinct AARS the Asp-RS, Lys-RS, Trp-RS and Tyr-RS are each encoded by two genes, one of which contains a mitochondrial targeting signal (MTS) at the N-terminus as determined by MITOPROT (Table S7; [34]. Recently, Charrière and coworkers verified the cytosolic and mitochondrial localization of the Trp-RS and Asp-RS experimentally [35], [36]. Five additional AARS (Asn-RS, Pro-RS, Glu-RS, Gln-RS, Ser-RS) contain an N-terminal MTS; for these AARS, however, there is no cytosolic isoform encoded in the genome. When we analyzed their expression and splicing patterns we found that the major 5′ splice site is internal to the currently annotated AUG start codon leaving only a small fraction of the splice sites upstream of the first AUG (Figure 8, Table S7). Thus the major translation product would exclude the MTS. Additionally, 4 AARS (Arg-RS, Cys-RS, His-RS and Leu-RS) contain an internal MTS that is masked by 48–101 amino acids at the N-terminus (Table S7). However these MTS could become N-terminal if a downstream start codon would be used as the translation start site.
Fig. 8. Alternatively spliced aminoacyl tRNA synthetase mRNAs (AARS). 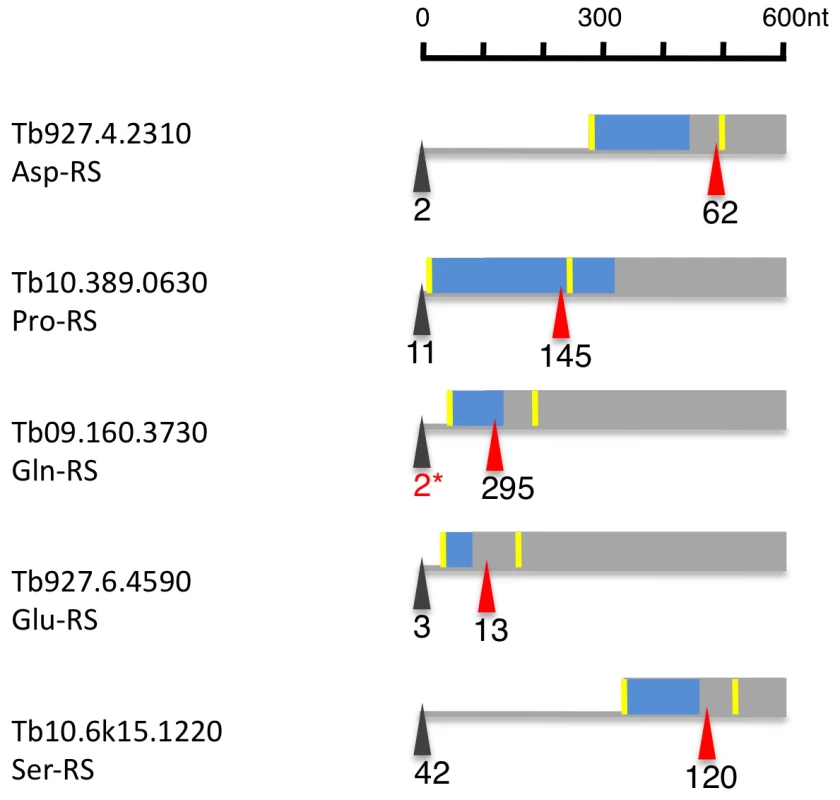
Depicted are 600 nucleotides from the 5′ region of six alternatively spliced AARS transcripts. The major spliced site (red triangle) and minor splice site (black triangle) are indicated in tags per million in procyclic forms. The yellow bar indicates the AUG downstream of the minor and major splice sites. The blue box represents the N-terminal mitochondrial targeting signal as determined by MITOPROT. The 2* marks tags, that were only seen in larger procyclic libraries (e.g. Alba1 and 3/4). Discussion
We have developed a cost effective method to analyze whole genome expression and splicing profiles from organisms employing leader trans-splicing. This was tested on T. brucei, where we obtained 85% coverage of all genes with fewer than 1 million sequence tags. A major improvement over traditional microarray technology is the possibility of sequencing directly from the spliced leader, which allows selective analysis of the transcriptome of intracellular parasites like the amastigote forms of Trypanosoma cruzi or forms that are closely associated with their host and extremely difficult to purify like the epimastigote form of T. brucei in tsetse salivary glands.
Using the SLT approach we sequenced more than 1 million splice site tags from poly(A)-mRNA from each of three life cycle stages of T. brucei. Each sequence tag covered at least 24 nucleotides 3′ of the spliced leader/5′ UTR junction. Even though the analyzed strains (Antat 1.1 and Mitat 1.2) were not identical to the genome strain (TREU 927) mapping of the 24mer sequence tags onto the TREU 927 genome was very successful, given that the majority (98%) could be aligned with high statistical significance using a combination of mismatch and sequence quality scores (see materials and methods). In the process, this method revealed the existence of a new expression site associated gene in an active VSG expression site.
In general, our data are in good agreement with the current genome annotation, and strongly support both the number and positions of putative transcription start sites identified by binding of specific histones [8]. In addition, 98% of the sequence tags from the procyclic form map in the sense orientation of annotated transcription units, and only 2% in the zones between transcription units, where the direction of transcription is not immediately obvious from the annotations. The splicing patterns indicated that transcription overlaps in several of the converging strand switch regions as has been suggested previously for converging transcription units transcribed by Pol I and Pol II, respectively [22]. Interestingly, bloodstream form cells show twice the number of sequence tags mapping to the zones between the transcription units compared to the procyclic form, indicating that the control of transcription initiation may be less specific, or degradation less efficient in this life stage. It is also possible that splice site recognition is different, or not equally efficient between the stages, leading to the increased number of processed RNAs from the regions between the transcription units.
The large variation in transcript abundance that we detected within transcription units was expected and is in good agreement with previous studies, strengthening the notion that steady state RNA levels in trypanosomes are regulated mainly post-transcriptionally at the level of RNA processing and/or RNA stability. When we analyzed the monocistronic transcription units we found >70% unlikely to be expressed in the life cycle stages analyzed because we could not detect any splice sites within 2 kb upstream of the start codon. Of the remaining 76 transcripts, only 8 showed differential expression.
Previous microarray studies of T. brucei using genomic arrays or single probe arrays have indicated a rather static transcriptome with relatively few changes between the bloodstream and procyclic forms. Estimates ranged from 2–6% of the genome being regulated at the transcript abundance level [9], [10], [11], [12], [13]. A more recent study by Jensen et al. using eight oligonucleotides per gene on a Nimblegen microarray found that up to 700 transcripts or (8%) change expression between the two life cycle stages [12]. Considering these studies it would appear that a multiprobe array is more likely to detect a larger set of regulated genes. Furthermore, using arrays with only one probe in the 5′ region of the genes is likely to be affected by the misannotation of the start sites of open reading frames and/or by alternative splicing. Using SLT we found 30% of the transcripts from protein-coding genes to be significantly regulated between the long slender bloodstream form and the procyclic form (Table 2). The number of changes increased to 40% of all genes when extended to include the short stumpy form of the parasite. Even at a more conservative threshold (≥2-fold, significantly changed), 35% of all genes exhibited changes in transcript abundance. In conclusion, we have found that a much larger cohort of genes changes expression levels during development than was previously thought to be the case. This is in line with recent findings that about 50% of the genome of T. cruzi is regulated at the level of transcript abundance [14]. When we compared our results with the previous microarray studies we find different levels of correlation in gene expression depending on the study. While some part of the differences might be due to the higher sensitivity of the SLT approach we have to keep in mind that the low level of correlation might also be due to strains and culture conditions that vary considerably between the different studies. We also employed three different approaches to verify the results obtained by SLT. (i) Results from RT qPCR showed strong positive correlation with the SLT approach for the expression level changes between life stages and the differential splicing events (Figure S7, Table S4, Table S6). (ii) We also performed two RNAi experiments indicating that the abundance of the RNAi target transcript as quantified by SLT is in excellent agreement with quantification by Northern blots. The overall correlation between the induced and uninduced transcriptomes was on a par with the technical reproducibility of RNAseq, further supporting that SLT tag counts serve as a sufficient proxy for comparisons between different life cycle stages or cell lines of the same organism (Figure S8). (iii) We included the correlation to one run of regular RNAseq of poly(A)-mRNA (24 million sequence tags, Figure S9). While not perfect, the correlation between SLT and RNAseq (Spearman ρ = 0.69) is nearly on a par with that between a technical comparison of RNAseq and microarrays (Spearman ρ = 0.75 e.g. [37]).
During the revision of this manuscript Siegel et al. published a study describing the expression profile in bloodstream and procyclic forms of T. brucei using RNAseq [38]. Although the approaches used in both studies are different (RNAseq vs. SLT) many features found in both studies are well correlated; the mean number of splice sites per gene (2.6 versus 2.7–2.9), the mean lengths of the 5′ UTRs (184 versus 105–135), the number of genes with internal splice sites (488 versus 496–558) and the large dynamic range (105 to 106). However, there are also a number of features that do not correlate so well, most striking of which is the difference in expression profile. We identified almost 40% of the genes as being regulated significantly while Siegel et al. found only about 10%. There are several reasons that could explain the differences observed: (i) strain differences and the number of life stages analyzed, (ii) growth conditions for bloodstream forms (in vitro versus in vivo), (iii) cDNA preparation, with RNAseq being more likely to capture precursors and breakdown intermediates (e.g. intron sequences), and (iv) scaling of the data (tags/million versus constant median count/gene). The major advantage of SLT when compared to conventional RNAseq is the cost effective mapping of 5′ splice sites in splice leader bearing organisms, especially intracellular parasites where the purification of RNA is very difficult.
According to our study, VSG transcripts account for 7–11% of spliced mRNAs in bloodstream forms trypanosomes, which is in excellent agreement with the value obtained from hybridization data [39]. The levels of most other transcripts, however, are at least two to three orders of magnitude lower. Assuming there to be approximately 40,000 mRNA molecules in a procyclic trypanosome, and approximately half that number in a bloodstream form (Haanstra and co-workers and our calculations), a large number (>5000, 65%) would be present at <1 mRNA per cell [40]. Similar results have been reported for yeast where more than 80% of all transcripts are present at ≤2 copies per cell [41], [42]. The question if the small number of transcripts is distributed evenly across the population of trypanosomes, or accumulates in very few cells during a transcriptional burst, remains to be investigated. Recently it has been suggested that transcription in yeast is much more steady, with fewer transcriptional bursts than seem to occur in mammalian cells [43], [44], [45].
The data presented here describes the splice sites for 85% of the annotated genes in the T. brucei genome (Table 1). Spliced leader addition sites were very well conserved within and between life cycle stages. AG was the predominant splice acceptor dinucleotide of the major splice sites, however 20% of the minor splice sites used other dinucleotides, predominantly GG (Figure 4A–C). The least abundant acceptor dinucleotide was CC followed by any pyrimidine combination. The major splice sites contained a 15 (±6) nucleotide polypyrimidine stretch at an average distance of 31 nucleotides (±19) upstream of the actual splice acceptor site. Both these findings are in very good agreement with previously published experimental data on individual splice sites [46]. The UTR length distribution indicated 5′ UTRs with a median length between 32 to 47 nucleotides, which is similar to yeast (50 nt; [47]; Figure 4 D–E). Interestingly we detected a shift towards longer UTRs in the long slender bloodstream form when compared to the procyclic form. This is at least in part due to the differential use of splice sites in the two life forms and might indicate differential regulation of translation. About half of the major splice sites in the different life stages could not be predicted using our current splice site recognition model, although the majority contained the signals that conform to our current understanding of splice sites. An obvious consequence of alternative splicing would be the change of N-terminal targeting sequences as has been shown for T. cruzi (LYT1) and for alternative cis-splicing in other systems [48], [49], [50], [51]. Our analysis indicated this likely to be the case for several AARS that are essential in the cytosol and mitochondrion [35], [36] thus providing evidence that alternative splicing is a potential mechanism for dual localization of proteins similar to what has been reported for the LYT1 gene in T. cruzi [48].
More than 500 transcripts with splice sites exclusively 3′ of the annotated start site were identified by SLT. While we cannot exclude that a very small fraction is still spliced upstream of the annotated AUG, we consider it much more likely that the bona fide start codon is within the open reading frame. A second set of transcripts (>600) indicated the possibility of 5′ extensions to the currently annotated open reading frames, which would effect changes in the N-terminus of the corresponding protein. The evaluation of these N-terminal extensions is much more difficult and will require additional experiments. However, by screening the recently published proteomics dataset from Panigrahi and co-workers, we were able to identify 11 candidates where peptides corresponding to a region upstream of the annotated start codon are expressed in the procyclic form trypanosomes [33]. Depending in the life cycle stage, we also identified 90–97 transcripts in which alternative splicing ablated the start codon, suggesting this form of splicing plays a minor but significant role in regulating gene expression.
Most surprising was that a large number of transcripts showed differential abundance of alternative splice variants in the three life stages analyzed. A previous report indicated that T. brucei used different splice sites on an artificial construct, but it remained unclear if, and how frequently, this might occur in the T. brucei transcriptome [52]. We found more than 600 differentially spliced transcripts between the life stages, supporting the idea that alternative splicing has functional consequences for the regulation of parasite development. One open question is if the actual splicing event is regulated or the differential abundance is a result of altered stability of the RNA transcripts. So far, we have been unable to detect sequence elements in the vicinity of the alternative splice sites that would explain the differential regulation of splicing itself. It is worth noting, however, that transcripts encoding several of the core components of the spliceosome, such as SMD1, SMD3 and SMG, are themselves differentially regulated during development; this may reflect an adaptation of the splicing machinery to differences in the major splicing targets, such as the VSG and procyclin transcripts, or to the subtleties of alternative splicing. These hypotheses should now be testable on a genome-wide scale, using the SLT approach in combination with RNA knockdown of specific splicing components.
Materials and Methods
Cell lines
T. brucei brucei AnTat 1.1 and MITat 1.2 (221) were used in this study. Late procyclic forms of AnTat 1.1 were cultivated in SDM79 supplemented with 10% FBS. Bloodstream forms were grown in mice. For bloodstream forms of AnTat 1.1, mice were immunosuppressed with 260mg/kg cyclophosphoamide (Sigma) 24 hours prior to intraperitoneal injection of 106 parasites. Short stumpy parasites were harvested 5 days post-infection at a density of 2–4×108/ml blood. 75–80% of the cells showed a short stumpy phenotype as determined by light microscopy of blood smears after methanol fixation. Long slender forms were harvested from untreated mice at day three post-infection at a density of <5×107 cells/ml blood. MITat 1.2 bloodstream forms were grown in mice as described above for long slender bloodstream forms of AnTat 1.1. Parasites were purified from whole blood using DE-52 anion exchange resin equilibrated to pH 8 with a bicine glucose buffer. After purification cells were centrifuged and resuspended in TriPure RNA isolation reagent (Roche, Switzerland).
RNA extraction and library construction
RNA was extracted using TriPure (Roche, Switzerland) according to the manufacturer.
Poly(A) mRNA was purified from 7.5 µg total RNA using Dynabeads oligo-(dT) beads according to the manufacturer (Invitrogen, USA). First strand cDNA was synthesized from poly(A) RNA using random hexamers and Superscript II reverse transcriptase (Invitrogen, USA) in a final volume of 20 µl for 1 hour at 42°C. Half of the first strand mix was used for second strand synthesis (10 µl 1st strand mix, 1 µl RNaseH, 15 minutes at 37°C). Second strand synthesis was done using 2 µl 10× Thermopol buffer (New England BioLabs), 1 µl dNTPs 10 mM, 1 µl 2nd strand primer 10 µM ([BIOT]5′ - AATGATACGGCGACCACCGAGATCTACAGTTTCTGTACTATATTG -3′), 2 units Taq polymerase (New England BioLabs, USA) and 4.5 µl H2O by incubation for 5 minutes at 50°C and then 5 minutes at 72°C. The cDNA was purified on a Qiagen MinElute column (Qiagen, USA) and eluted in 10ul TE buffer. Adapter ligation to the purified dsDNA was done using (10.0 µl DNA, 2.5 µl 10× Ligase Buffer (New England BioLabs), 10.0 µl H2O, 1.0 µl Fasteris customized bar-coded paired-end Illumina adapter, 600 units T4 DNA Ligase (New England BioLabs, USA) for 1 hour at room temperature. The ligation mix was purified from unligated linker with streptavidin beads (Dynabeads). After incubation at room temperature for 15 minutes beads were separated on a magnetic stand, and washed twice and then resuspended in 20 µl 10 mM Tris buffer. 5 µl was used for PCR amplification with primers (5′ - AATGATACGGCGACCACCGA -3′/5′ - CAAGCAGAAGACGGCATACGAGATCGGTCTCGGCATTCCTGCTGAAC -3′) following the standard Illumina mRNA-SEQ library amplification protocol. Fragments in the range 120–160 bp were separated on a 2% agarose-TBE gel and subsequently purified on Qiagen Gel Extraction MinElute columns (Qiagen, USA). Quality control for insert size was performed by TOPO cloning and subsequent ABI sequencing. Sequencing on the Illumina Genome Analyzer was carried out using the following sequencing primer (5′ - ACCGAGATCTACAGTTTCTGTACTATATTG -3′).
In total we sequenced three libraries from bloodstream form mRNA, (long slender and short stumpy, both Antat1.1 and monomorphic Lister 427), one from procyclic form mRNA (Antat1.1), 2×2 RNAi libraries (uninduced and induced), where each pair could be considered a biological replicate. Lastly we prepared and sequenced one conventional RNAseq library from procyclic mRNA (Antat1.1).
Bioinformatic analysis
Base calling was performed using the Genome Analyzer Pipeline (Illumina). Linker sequences were removed while separating reads according to identified barcodes. Only sequence reads of inserts with a length of at least 24 nucleotides were retained. A pipeline was set up using languages with open source interpreters (bash, perl and R) to automate the following analysis. The reads were mapped to the genome sequence of T. brucei TREU927 using maq ([53] http://maq.sourceforge.net) with n = 3 and an effective first read length of 24. Single mapping reads were separated from multi mapping reads by an alignment quality threshold of 30. Tags ending in the same position were joined together, and their counts were added (using bioperl, [54]). Tag counts were normalized to the library size (number of reads of length 24 or more) and scaled linearly to reflect counts of tags per million (TPM). Mapped tags were assigned to the annotated protein coding gene 3′ of the tag. Tags mapping internally to a coding sequence (CDS) were assigned to it as internal splice sites. Data was exported in tabular and GFF format (http://www.sanger.ac.uk/Software/formats/GFF/) and then visualized using Gbrowse [55]. Alternative spliced leader addition sites were cataloged for each gene. Genes with ≤60% tags in the assigned major splice site were designated alternatively spliced. The 5′ UTR lengths were calculated and visualized using R [56]. Upstream open reading frames (uORFs) were detected on the mapped 5′ UTR, counted and assigned a note describing their length, whether they were terminated on the 5′ UTR, or overlapped the bona fide (CDS) start codon. Sequences surrounding the splice sites were extracted using bioperl and visualized as sequence logos using WebLogo [57], [58]. Differences in expression levels of a gene in two stages was tested for significance according to Audic and Claverie with a threshold of P<10−5. Scatterplots of the differential gene expression levels of all libraries were produced using R [28]. When a gene had different major splice sites in two stages, the normalized counts of these sites were tested for a statistically significant difference (Fisher two-tailed test, P<10−5). Significant differences were termed differential trans-splicing events. Expression levels were pooled over T. brucei specific KEGG pathways and visualized as heatmaps after log2 transform and hierarchical average linkage clustering of euclidian distances using MeV ([59]; http://www.genome.jp/kegg; http://www.tm4.org/mev.html).
Anti-sense splice sites were detected using bioperl. In order to simulate gene coverage a subset of reads was drawn randomly without replacement from one library and the mapping and analysis pipeline executed for each subset. This was repeated five times for each subset size. Saturation curves were drawn for several parameters, with error bars given as confidence intervals assuming normal distribution. Comparison to splice model predictions were made according to Benz et al. with the mapped splice sites substituted for the previous small mapping EST set [15]. All bioinformatics tools, programs, pipelines used in this study will be provided upon request. All sequence data including the regular RNAseq data is available through our website and TriTrypDB.
cDNA and PCR and RT qPCR
PCR was used to confirm the splice sites detected by SLT. 3 µg total RNA was used as a template for reverse transcription in 50 µl AMV reverse transcriptase buffer (Promega, USA) in the presence of 1mM dNTPs, 360 ng random hexamers, 80 U RNasIn (Promega, USA) and 60 U AMV reverse transcriptase (Promega, USA). Subsequently 1µl of this reaction was used for PCR. For PCR the splice leader primer CGCTATTATTAGAACAGTTTCTGTAC-3′ (Tm 55°C), and reverse primers 5′ - GTTGCATCCGGTGTTCTTTT -3′ (Tb11.02.2700) and 5′-AGCAGTCATCAATTCTTCCT-3′ (Tb927.6.4240) were used. The reaction was performed in 25µl, (2mM MgCl2, 2.5µl PCR 10× buffer, 400nM primer, 1 unit of Taq DNA polymerase (Qiagen, USA), 0.2mM dNTP and 30ng/ul cDNA) for 30 cycles. RT qPCR was done essentially as described previously. cDNA for RT-qPCR was prepared as described above. The primers were designed such they would amplify regions of 80 to 150 nucleotides using the online software tool for real time PCR from Genescript (Table S8). Real-time PCR was run on the GeneAmp 7000 (Applied Biosystems) using 30ng of cDNA, 400nM oligonucleotides in 25µl of the MESA GREEN qPCR Master mix for SYBR assay (Eurogentec). Values were normalized to beta tubulin and the amplification efficiency was derived from a cDNA dilution series covering five logs. Average values and standard deviations of 3 RT qPCRs from one cDNA sample are shown.
Sequence data
The sequence data can be accesed through GEO (http://www.ncbi.nlm.nih.gov/geo/) with the accession number GSE22571 or through our website http://splicer.unibe.ch/ or in future also from TriTrypDB (http://tritrypdb.org/tritrypdb/).
Ethics statement
All animal work has been conducted in accordance with the regulations for the production of bloodstream form trypanosomes of the cantonal animal protection agency in Bern, Switzerland. Approved by the cantonal animal protection agency in Bern Switzerland (form B, 14/07).
Supporting Information
Zdroje
1. BenneR
Van den BurgJ
BrakenhoffJP
SloofP
Van BoomJH
1986 Major transcript of the frameshifted coxII gene from trypanosome mitochondria contains four nucleotides that are not encoded in the DNA. Cell 46 819 826
2. BorstP
CrossGA
1982 Molecular basis for trypanosome antigenic variation. Cell 29 291 303
3. FergusonMA
CrossGA
1984 Myristylation of the membrane form of a Trypanosoma brucei variant surface glycoprotein. J Biol Chem 259 3011 3015
4. de AlmeidaML
TurnerMJ
StambukBB
SchenkmanS
1988 Identification of an acid-lipase in human serum which is capable of solubilizing glycophosphatidylinositol-anchored proteins. Biochem Biophys Res Commun 150 476 482
5. SuttonRE
BoothroydJC
1986 Evidence for trans splicing in trypanosomes. Cell 47 527 535
6. BerrimanM
GhedinE
Hertz-FowlerC
BlandinG
RenauldH
2005 The genome of the African trypanosome Trypanosoma brucei. Science 309 416 422
7. Martinez-CalvilloS
YanS
NguyenD
FoxM
StuartK
2003 Transcription of Leishmania major Friedlin chromosome 1 initiates in both directions within a single region. Mol Cell 11 1291 1299
8. ClaytonCE
2002 Life without transcriptional control? From fly to man and back again. Embo J 21 1881 1888
9. SiegelTN
HekstraDR
KempLE
FigueiredoLM
LowellJE
2009 Four histone variants mark the boundaries of polycistronic transcription units in Trypanosoma brucei. Genes Dev 23 1063 1076
10. BremsS
GuilbrideDL
Gundlesdodjir-PlanckD
BusoldC
LuuVD
2005 The transcriptomes of Trypanosoma brucei Lister 427 and TREU927 bloodstream and procyclic trypomastigotes. Mol Biochem Parasitol 139 163 172
11. KoumandouVL
NatesanSK
SergeenkoT
FieldMC
2008 The trypanosome transcriptome is remodelled during differentiation but displays limited responsiveness within life stages. BMC Genomics 9 298
12. JensenBC
SivamD
KiferCT
MylerPJ
ParsonsM
2009 Widespread variation in transcript abundance within and across developmental stages of Trypanosoma brucei. BMC Genomics 10 482
13. KabaniS
FennK
RossA
IvensA
SmithTK
2009 Genome-wide expression profiling of in vivo-derived bloodstream parasite stages and dynamic analysis of mRNA alterations during synchronous differentiation in Trypanosoma brucei. BMC Genomics 10 427
14. MinningTA
WeatherlyDB
AtwoodJ3rd
OrlandoR
TarletonRL
2009 The steady-state transcriptome of the four major life-cycle stages of Trypanosoma cruzi. BMC Genomics 10 370
15. BenzC
NilssonD
AnderssonB
ClaytonC
GuilbrideDL
2005 Messenger RNA processing sites in Trypanosoma brucei. Mol Biochem Parasitol 143 125 134
16. KrauseM
HirshD
1987 A trans-spliced leader sequence on actin mRNA in C. elegans. Cell 49 753 761
17. RajkovicA
DavisRE
SimonsenJN
RottmanFM
1990 A spliced leader is present on a subset of mRNAs from the human parasite Schistosoma mansoni. Proc Natl Acad Sci U S A 87 8879 8883
18. TessierLH
KellerM
ChanRL
FournierR
WeilJH
1991 Short leader sequences may be transferred from small RNAs to pre-mature mRNAs by trans-splicing in Euglena. EMBO J 10 2621 2625
19. VandenbergheAE
MeedelTH
HastingsKE
2001 mRNA 5′-leader trans-splicing in the chordates. Genes Dev 15 294 303
20. ZhangH
HouY
MirandaL
CampbellDA
SturmNR
2007 Spliced leader RNA trans-splicing in dinoflagellates. Proc Natl Acad Sci U S A 104 4618 4623
21. HaenniS
StuderE
BurkardGS
RoditiI
2009 Bidirectional silencing of RNA polymerase I transcription by a strand switch region in Trypanosoma brucei. Nucleic Acids Res 37 5007 5018
22. LinigerM
BodenmullerK
PaysE
GallatiS
RoditiI
2001 Overlapping sense and antisense transcription units in Trypanosoma brucei. Mol Microbiol 40 869 878
23. ColasanteC
RoblesA
LiCH
SchwedeA
BenzC
2007 Regulated expression of glycosomal phosphoglycerate kinase in Trypanosoma brucei. Mol Biochem Parasitol 151 193 204
24. MayhoM
FennK
CraddyP
CrosthwaiteS
MatthewsK
2006 Post-transcriptional control of nuclear-encoded cytochrome oxidase subunits in Trypanosoma brucei: evidence for genome-wide conservation of life-cycle stage-specific regulatory elements. Nucleic Acids Res 34 5312 5324
25. ChaudhuriM
SharanR
HillGC
2002 Trypanosome alternative oxidase is regulated post-transcriptionally at the level of RNA stability. J Eukaryot Microbiol 49 263 269
26. ZiegelbauerK
OverathP
1992 Identification of invariant surface glycoproteins in the bloodstream stage of Trypanosoma brucei. J Biol Chem 267 10791 10796
27. RoditiI
CarringtonM
TurnerM
1987 Expression of a polypeptide containing a dipeptide repeat is confined to the insect stage of Trypanosoma brucei. Nature 325 272 274
28. AudicS
ClaverieJM
1997 The significance of digital gene expression profiles. Genome Res 7 986 995
29. BrechtM
ParsonsM
1998 Changes in polysome profiles accompany trypanosome development. Mol Biochem Parasitol 97 189 198
30. GrayJV
PetskoGA
JohnstonGC
RingeD
SingerRA
2004 “Sleeping beauty”: quiescence in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 68 187 206
31. Hertz-FowlerC
FigueiredoLM
QuailMA
BeckerM
JacksonA
2008 Telomeric expression sites are highly conserved in Trypanosoma brucei. PLoS One 3 e3527
32. BorstP
FairlambAH
1998 Surface receptors and transporters of Trypanosoma brucei. Annu Rev Microbiol 52 745 778
33. PanigrahiAK
OgataY
ZikovaA
AnupamaA
DalleyRA
2009 A comprehensive analysis of Trypanosoma brucei mitochondrial proteome. Proteomics 9 434 450
34. ClarosMG
VincensP
1996 Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241 779 786
35. CharriereF
HelgadottirS
HornEK
SollD
SchneiderA
2006 Dual targeting of a single tRNA(Trp) requires two different tryptophanyl-tRNA synthetases in Trypanosoma brucei. Proc Natl Acad Sci U S A 103 6847 6852
36. CharriereF
O'DonoghueP
HelgadottirS
Marechal-DrouardL
CristoderoM
2009 Dual targeting of a tRNAAsp requires two different aspartyl-tRNA synthetases in Trypanosoma brucei. J Biol Chem 284 16210 16217
37. MarioniJC
MasonCE
ManeSM
StephensM
GiladY
2008 RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 18 1509 1517
38. SiegelTN
HekstraDR
WangX
DewellS
CrossGA
Genome-wide analysis of mRNA abundance in two life-cycle stages of Trypanosoma brucei and identification of splicing and polyadenylation sites. Nucleic Acids Res
39. De LangeT
MichelsPA
VeermanHJ
CornelissenAW
BorstP
1984 Many trypanosome messenger RNAs share a common 5′ terminal sequence. Nucleic Acids Res 12 3777 3790
40. HaanstraJR
StewartM
LuuVD
van TuijlA
WesterhoffHV
2008 Control and regulation of gene expression: quantitative analysis of the expression of phosphoglycerate kinase in bloodstream form Trypanosoma brucei. J Biol Chem 283 2495 2507
41. YassourM
KaplanT
FraserHB
LevinJZ
PfiffnerJ
2009 Ab initio construction of a eukaryotic transcriptome by massively parallel mRNA sequencing. Proc Natl Acad Sci U S A 106 3264 3269
42. HolstegeFC
JenningsEG
WyrickJJ
LeeTI
HengartnerCJ
1998 Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95 717 728
43. ZenklusenD
LarsonDR
SingerRH
2008 Single-RNA counting reveals alternative modes of gene expression in yeast. Nat Struct Mol Biol 15 1263 1271
44. NewlandsS
LevittLK
RobinsonCS
KarpfAB
HodgsonVR
1998 Transcription occurs in pulses in muscle fibers. Genes Dev 12 2748 2758
45. FeminoAM
FayFS
FogartyK
SingerRH
1998 Visualization of single RNA transcripts in situ. Science 280 585 590
46. SiegelTN
TanKS
CrossGA
2005 Systematic study of sequence motifs for RNA trans splicing in Trypanosoma brucei. Mol Cell Biol 25 9586 9594
47. VassellaE
BraunR
RoditiI
1994 Control of polyadenylation and alternative splicing of transcripts from adjacent genes in a procyclin expression site: a dual role for polypyrimidine tracts in trypanosomes? Nucleic Acids Res 22 1359 1364
48. BenabdellahK
Gonzalez-ReyE
GonzalezA
2007 Alternative trans-splicing of the Trypanosoma cruzi LYT1 gene transcript results in compartmental and functional switch for the encoded protein. Mol Microbiol 65 1559 1567
49. LopezV
KelleherSL
2009 Zinc transporter-2 (ZnT2) variants are localized to distinct subcellular compartments and functionally transport zinc. Biochem J 422 43 52
50. JuneauK
NislowC
DavisRW
2009 Alternative splicing of PTC7 in Saccharomyces cerevisiae determines protein localization. Genetics 183 185 194
51. AshibeB
HiraiT
HigashiK
SekimizuK
MotojimaK
2007 Dual subcellular localization in the endoplasmic reticulum and peroxisomes and a vital role in protecting against oxidative stress of fatty aldehyde dehydrogenase are achieved by alternative splicing. J Biol Chem 282 20763 20773
52. HelmJR
WilsonME
DonelsonJE
2008 Different trans RNA splicing events in bloodstream and procyclic Trypanosoma brucei. Mol Biochem Parasitol 159 134 137
53. LiH
RuanJ
DurbinR
2008 Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res 18 1851 1858
54. StajichJE
BlockD
BoulezK
BrennerSE
ChervitzSA
2002 The Bioperl toolkit: Perl modules for the life sciences. Genome Res 12 1611 1618
55. SteinLD
MungallC
ShuS
CaudyM
MangoneM
2002 The generic genome browser: a building block for a model organism system database. Genome Res 12 1599 1610
56. R-Development-Core-Team 2009 R: A Language and Environment for Statistical Computing Vienna, Austria.
57. SchneiderTD
StephensRM
1990 Sequence logos: a new way to display consensus sequences. Nucleic Acids Res 18 6097 6100
58. CrooksGE
HonG
ChandoniaJM
BrennerSE
2004 WebLogo: a sequence logo generator. Genome Res 14 1188 1190
59. KanehisaM
GotoS
2000 KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28 27 30
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 8- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



