-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
Salmonella enterica serovar Typhimurium is a common food-borne pathogen that induces inflammatory diarrhea and invades intestinal epithelial cells using a type three secretion system (T3SS) encoded within Salmonella pathogenicity island 1 (SPI1). The genes encoding the SPI1 T3SS are tightly regulated by a network of interacting transcriptional regulators involving three coupled positive feedback loops. While the core architecture of the SPI1 gene circuit has been determined, the relative roles of these interacting regulators and associated feedback loops are still unknown. To determine the function of this circuit, we measured gene expression dynamics at both population and single-cell resolution in a number of SPI1 regulatory mutants. Using these data, we constructed a mathematical model of the SPI1 gene circuit. Analysis of the model predicted that the circuit serves two functions. The first is to place a threshold on SPI1 activation, ensuring that the genes encoding the T3SS are expressed only in response to the appropriate combination of environmental and cellular cues. The second is to amplify SPI1 gene expression. To experimentally test these predictions, we rewired the SPI1 genetic circuit by changing its regulatory architecture. This enabled us to directly test our predictions regarding the function of the circuit by varying the strength and dynamics of the activating signal. Collectively, our experimental and computational results enable us to deconstruct this complex circuit and determine the role of its individual components in regulating SPI1 gene expression dynamics.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1001025
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001025Summary
Salmonella enterica serovar Typhimurium is a common food-borne pathogen that induces inflammatory diarrhea and invades intestinal epithelial cells using a type three secretion system (T3SS) encoded within Salmonella pathogenicity island 1 (SPI1). The genes encoding the SPI1 T3SS are tightly regulated by a network of interacting transcriptional regulators involving three coupled positive feedback loops. While the core architecture of the SPI1 gene circuit has been determined, the relative roles of these interacting regulators and associated feedback loops are still unknown. To determine the function of this circuit, we measured gene expression dynamics at both population and single-cell resolution in a number of SPI1 regulatory mutants. Using these data, we constructed a mathematical model of the SPI1 gene circuit. Analysis of the model predicted that the circuit serves two functions. The first is to place a threshold on SPI1 activation, ensuring that the genes encoding the T3SS are expressed only in response to the appropriate combination of environmental and cellular cues. The second is to amplify SPI1 gene expression. To experimentally test these predictions, we rewired the SPI1 genetic circuit by changing its regulatory architecture. This enabled us to directly test our predictions regarding the function of the circuit by varying the strength and dynamics of the activating signal. Collectively, our experimental and computational results enable us to deconstruct this complex circuit and determine the role of its individual components in regulating SPI1 gene expression dynamics.
Introduction
Salmonella enterica is a common food-borne pathogen that causes an array of diseases in humans, ranging from self-limiting gastroenteritis to life-threatening systemic infections [1], [2]. The bacterium initiates infection by invading intestinal epithelial cells using a type three secretion system (T3SS) encoded within a forty kilobase region of the chromosome called Salmonella Pathogenicity Island 1 (SPI1) [3], [4], [5], [6], [7], [8]. The bacterium uses this T3SS to inject proteins into the cytoplasm of host cells [9], [10], [11]. The injected proteins commandeer the host cell actin-cytoskeleton machinery and promote the uptake of the bacterium into these otherwise non-phagocytic cells [12], [13], [14], [15]. The genes encoding the SPI1 T3SS are tightly regulated by a network of interacting transcriptional regulators that are responsive to a combination of environmental and intracellular signals [16], [17], [18]. These signals are presumably used by Salmonella as anatomical cues for initiating invasion and also for coordinating SPI1 gene expression with other cellular processes, most notably adhesion and motility [19], [20], [21], [22], [23], [24].
The master regulator for the SPI1 gene circuit is HilA, a transcription factor that contains a DNA-binding motif belonging to the OmpR/ToxR family [4] and a large C-terminal domain of unknown function [25]. HilA activates the expression of the genes encoding the structural components of the SPI1 T3SS [4], [26], [27], [28]. HilA also activates the expression of an AraC-like transcription factor, InvF, involved in regulating the expression of the SPI1 secreted effector proteins and their cognate chaperones [29], [30]. HilA expression, in turn, is regulated by three AraC-like transcription factors - HilC, HilD, and RtsA – with homologous DNA binding domains [22], [31], [32]. Both hilC and hilD are encoded within SPI1 whereas rtsA is encoded elsewhere on the chromosome. These three transcription factors can independently activate HilA expression. They can also activate each others' and their own expression [16]. Specifically, HilC, HilD, and RtsA are all capable of individually activating the PhilA PhilC, PhilD, and PrtsA promoters. These auto-regulatory interactions result in three coupled positive feedback loops comprising HilC, HilD, and RtsA, the output of each capable of activating HilA expression (Figure 1A). Of the three, HilD is dominant, as there is no HilA expression in its absence [33]. This reflects the fact that many activating signals, both environmental and intracellular, affect SPI1 gene expression by modifying the activity of HilD protein [16], [18], [19], [23], [34], [35]. In addition to positive regulation, SPI1 gene expression is also subject to negative regulation. HilE, a protein of unknown structure encoded outside SPI1, binds HilD [34] and prevents it from activating its target promoters.
Fig. 1. SPI1 gene expression is hierarchical and exhibits a switch-like transition from the “off” to the “on” state. 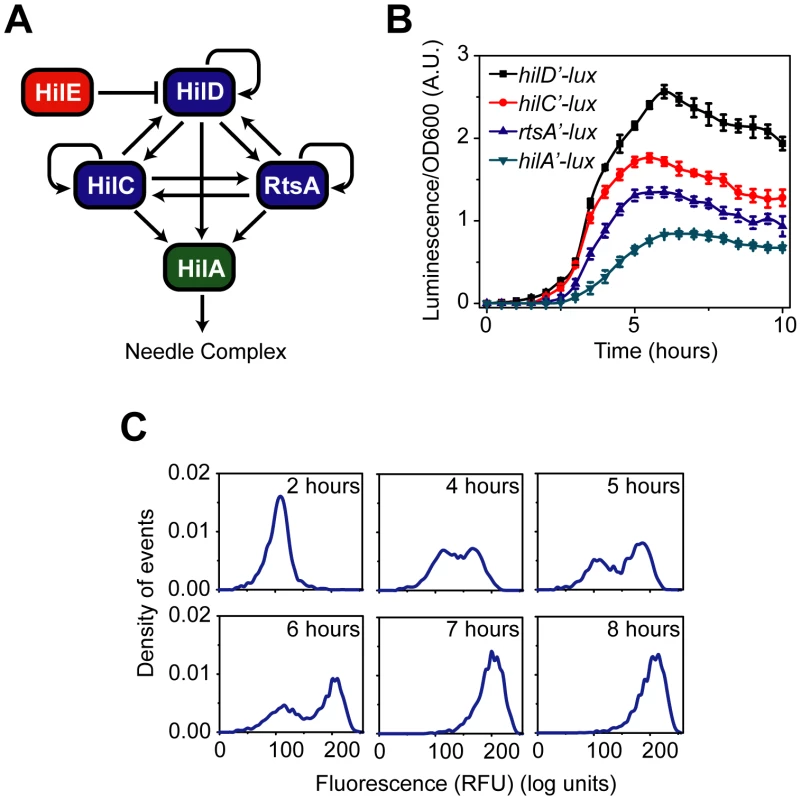
(A) Diagram of the SPI1 gene circuit. HilA is the master SPI1 regulator as it activates the expression of the genes encoding the T3SS. HilA, in turn, is regulated by HilC, HilD, and RtsA. These three regulators can independently activate HilA expression. They can also activate their own expression and that of each other's. HilE represses the activity of HilD by binding to it and preventing it from activating its target promoters. (B) Time-course dynamics of PhilD (pSS074), PhilC (pSS075), PrtsA (pSS076), and PhilA (pSS077) promoter activities in wild-type cells as determined using luciferase transcriptional reporters. To induce SPI1 gene expression, cells were first grown overnight in LB/no salt and then sub-cultured into fresh LB/1% NaCl conditions to an OD of 0.05 and grown statically. Luminescence values were normalized with the OD600 absorbance to account for cell density. Average promoter activities from three independent experiments on separate days are reported. For each experiment, six samples were tested. Error-bars indicate standard deviation. (C) Dynamics of PhilA (pSS055) promoter activity in wild-type cells as determined using green fluorescent protein (GFP) transcriptional fusions and flow cytometry. The SPI1 gene expression was induced as described above. Samples were collected at the indicated times and arrested in their respective state by adding chloramphenicol. Approximately 30,000 cell measurements were used to construct each histogram. As a control, we expressed GFP from a constitutive promoter and observed continuous, rheostatic-like expression dynamics and a homogenous response in the population (Figure S1E). Strain genotypes and plasmid descriptions are provided in Tables 1 and 2. While the core architecture of the SPI1 gene circuit has been determined (Figure 1A), the functions of these interacting regulators and associated feedback loops are still unknown. Therefore, to deconstruct this circuit, we measured gene expression dynamics at both population and single-cell resolution in a number of SPI1 regulatory mutants. Based on these experimental results, we constructed a simple mathematical model of the SPI1 gene circuit. Using the model, we demonstrate that the circuit serves two functions. The first is to place a threshold on SPI1 activation, ensuring that the genes encoding the T3SS are expressed only in response to the appropriate combination of environmental and cellular cues. The second is to amplify SPI1 gene expression. To experimentally test these two predictions, we rewired the SPI1 network by changing its regulatory architecture. The resulting experimental and computational analyses underpin an integrated model for the regulation of SPI1 gene expression.
Results
Dynamics of SPI1 gene expression
To investigate the dynamics of SPI1 gene expression, we grew cells statically in Luria-Bertani (LB) medium using 1% NaCl as the inducing signal. Growth in low-oxygen and high-salt conditions has previously been shown to induce SPI1 gene expression in vitro [4], [28]. In these experiments, we grew the cells overnight in LB/no salt and then sub-cultured them into fresh LB/1% NaCl medium, thus inducing a transition from SPI1-repressing to SPI1-inducing conditions. We employed two different reporter systems to measure gene expression. In our bulk, population-level experiments, we measured gene expression using plasmid-based promoter fusions to the luciferase operon, luxCDABE, from Photorhabdus luminescens [36], [37]. In our single-cell experiments, we employed promoter fusions to the green fluorescent protein (GFP) using an otherwise identical plasmid-based system [38].
The advantage of using the luciferase reporter system is that it is sensitive to dynamic changes in promoter activity, particularly at low levels of expression [39]. However, bacterial luciferase produces insufficient light for single-cell studies, hence the need for fluorescent reporters. We also note that the bacterial luciferase reporter system imposes a metabolic burden due to the production of the luciferase substrate, tetradecanal, by LuxC, LuxD, and LuxE [40]. To account for any potential biases associated with bacterial luciferase, we repeated a number of population-level experiments using the GFP reporters with similar results (results not shown).
We measured gene expression dynamics in wild-type cells using the luciferase reporter system. After a brief lag following subculture, we found that the PhilD and PhilA promoters were activated in a sequential manner, consistent with HilD being necessary for HilA expression (Figure 1B). In the case of the PhilC and PrtsA promoters, we found that they were activated at roughly the same time as the PhilD promoter. This hierarchy can also be seen when the expression values are normalized with respect to their maximal value (Figure S1A). These results indicate that there is a temporal hierarchy in SPI1 gene expression, with HilC, HilD, and RtsA at the top of the transcriptional cascade and HilA at the bottom. A similar hierarchy has also been observed in the activation of the downstream promoters regulating the expression of the genes encoding the T3SS and secreted effector proteins [41].
We also measured wild-type gene expression dynamics using flow cytometry in order to determine how individual cells within the population behave during SPI1 induction. In the case of the PhilA promoter, the dynamics were not continuous; rather, individual cells transitioned from an “off” to the “on” state in a switch-like manner (Figure 1C). By switch-like, we mean that the individual cells exist in one of two expression states. At intermediate times, transient heterogeneity in the population is observed, with most cells existing in either the “off” or “on” state. Similar switch-like dynamics were also observed for the PhilC, PhilD, and PrtsA promoters, with a comparable hierarchy in activation times as observed in the population data (Figure S1B–D). We note that heterogeneity in SPI1 gene expression has been previously observed by others [42]. As the SPI1 gene circuit involves multiple interacting positive feedback loops, these results are not surprising. In particular, positive feedback is known to be an integral element in many cellular switches [43]. To identify the genesis of this behavior, we further investigated the regulation of SPI1 gene expression.
Induction of the SPI1 gene circuit begins with a step increase in PhilD promoter activity
HilD is necessary for HilA expression. Even though HilC and RtsA can independently activate HilA expression when constitutively expressed from ectopic promoters, these two regulators are incapable of doing so in the absence of HilD when expressed from their native promoters [16]. Therefore, to understand the role of HilD, we measured gene expression dynamics in a ΔhilD mutant using the luciferase reporters. In the case of the PhilA promoter, we observed no activity in the absence of HilD (data not shown), consistent with previous reports [16], [33]. In the case of the PhilD promoter, we observed a weak, step-like increase in activity in the absence of HilD (Figure 2A). When we performed identical experiments using flow cytometry, we found that the PhilD promoter again transitions from an “off” to the “on” state in a switch-like manner (Figure 2B). These results are identical to what is observed in wild-type cells, the only difference being that the magnitude of expression is significantly reduced when HilD is not present. We also performed identical experiments in a ΔSPI1 ΔrtsA mutant and observed the same response (Figure S2A), indicating that the transient switch in PhilD promoter activity is not due to any SPI1 regulator but rather factors external to SPI1.
Fig. 2. SPI1 gene expression is induced by a step increase in PhilD promoter activity. 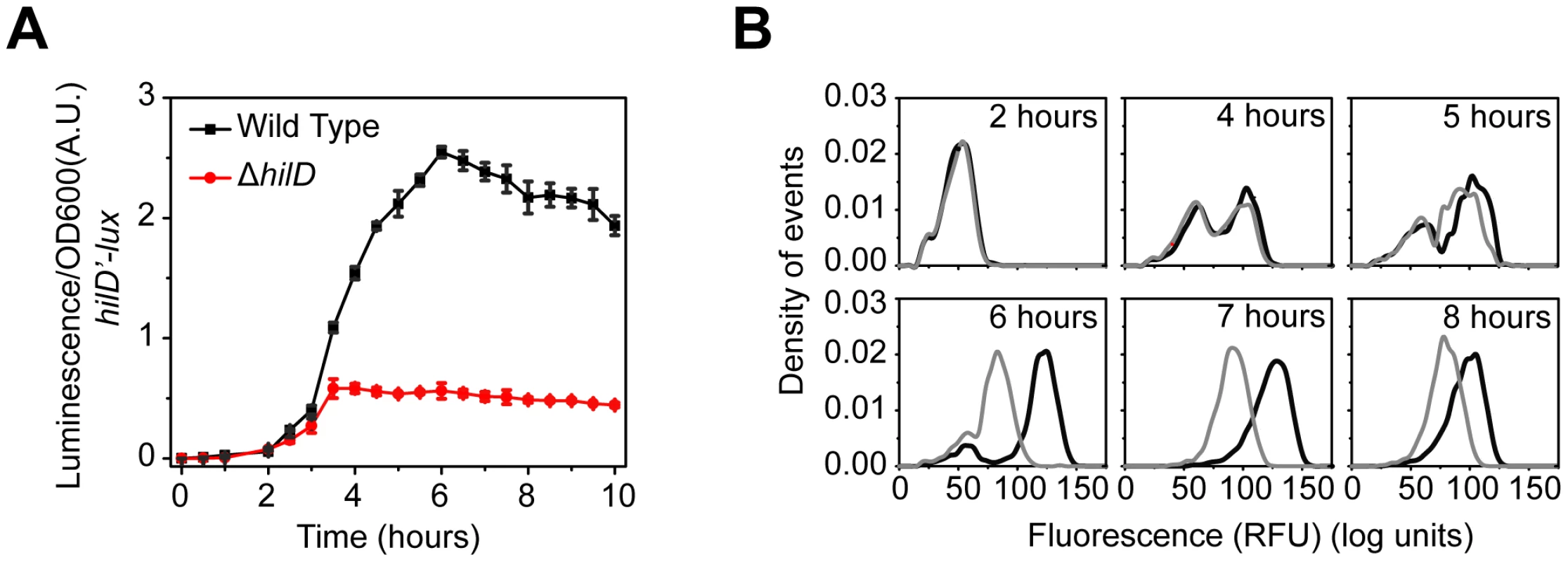
(A) Comparison of time-course dynamics for PhilD (pSS074) promoter activities in wild type (black) and a ΔhilD mutant (JS253, red) as determined using luciferase transcriptional reporters. (B) Comparison of PhilD (pSS072) promoter activity in wild type (black) and a ΔhilD mutant (JS253, grey) as determined using GFP transcriptional reporters and flow cytometry. Note that the activation of the PhilD promoter is switch-like both in wild type and the ΔhilD mutant. Experiments were performed as described in Figure 1. These results demonstrate that the SPI1 gene circuit is activated by a step increase in PhilD promoter activity. This signal is then amplified by a positive feedback loop involving HilD. As discussed below, HilC and RtsA serve to further amplify this signal. Interestingly, the heterogeneity in SPI1 activation is not due to the interacting positive feedback loops within the circuit but rather is intrinsic to the activating signal. The signals activating the PhilD promoter, however, are unknown. While multiple global regulators are known to affect SPI1 gene expression [44], these regulators appear to affect the activity of the HilD protein and not its promoter [16], [18], [19], [23], [35].
With regards to HilC and RtsA, we found that the PhilC promoter was active in absence of HilD, though at a reduced level, whereas the PrtsA promoter was effectively off (Figure S2B). However, even though the PhilC promoter is active in the absence of HilD, HilA is not expressed. These results suggest that activation of the PhilD promoter is the trigger mechanism for induction of SPI1 gene expression. Interestingly, when we assayed PhilC promoter activity in a ΔhilD mutant using flow cytometry, we found that the dynamics were not switch-like but rather continuous and rheostatic (Figure S2C). This homogeneity within the population indicates that the signal activating the PhilC promoter is fundamentally different than the one activating the PhilD promoter.
HilC and RtsA function as transcriptional amplifiers and accelerators
Unlike HilD, the HilC and RtsA proteins are not absolutely required for HilA expression. Yet, these two proteins can independently induce transcription from the PhilA promoter when constitutively expressed from an ectopic promoter [16]. To understand the role of these two proteins in regulating SPI1, we compared gene expression in wild type and a ΔhilC ΔrtsA mutant using the luciferase reporters (Figure 3A). Deleting these two regulators decreases the activity of the PhilD and PhilA promoters. Moreover, in the ΔhilC ΔrtsA mutant, there is also a delay in the induction of the PhilA promoter. This delay becomes more apparent when we normalize the luminescence measurements with respect to their maximal values (Figure S3A). When we measured gene expression at single-cell resolution using flow cytometry, we again observed a switch-like response in the ΔhilC ΔrtsA mutant (Figure 3B). The main difference relative to wild type was that the transition from the “off” to “on” state occurred more slowly in the absence of HilC and RtsA. Also, the activity of the PhilA promoter in the “on” state was lower in the ΔhilC ΔrtsA mutant than in wild type. With the PhilD promoter, we did not observe any change in the timing of promoter activation in the ΔhilC ΔrtsA mutant relative to wild type (Figure 3C and S3A). Rather, we observed only a decrease in the level of PhilD promoter activity associated with the “on” state. Similar results for both promoters are observed in the single deletion mutants, though the overall effect is small, indicating that HilC and RtsA additively contribute to SPI1 gene expression (Figure S3B–E). Based on these results, we conclude that HilC and RtsA serve two functions in the SPI1 circuit. First, HilC and RtsA amplify HilA and HilD expression, in the sense that HilA and HilD expression is reduced the absence of HilC and RtsA. Second, HilC and RtsA accelerate the transition of HilA expression from the “off” to the “on” state.
Fig. 3. HilC and RtsA amplify SPI1 gene expression. 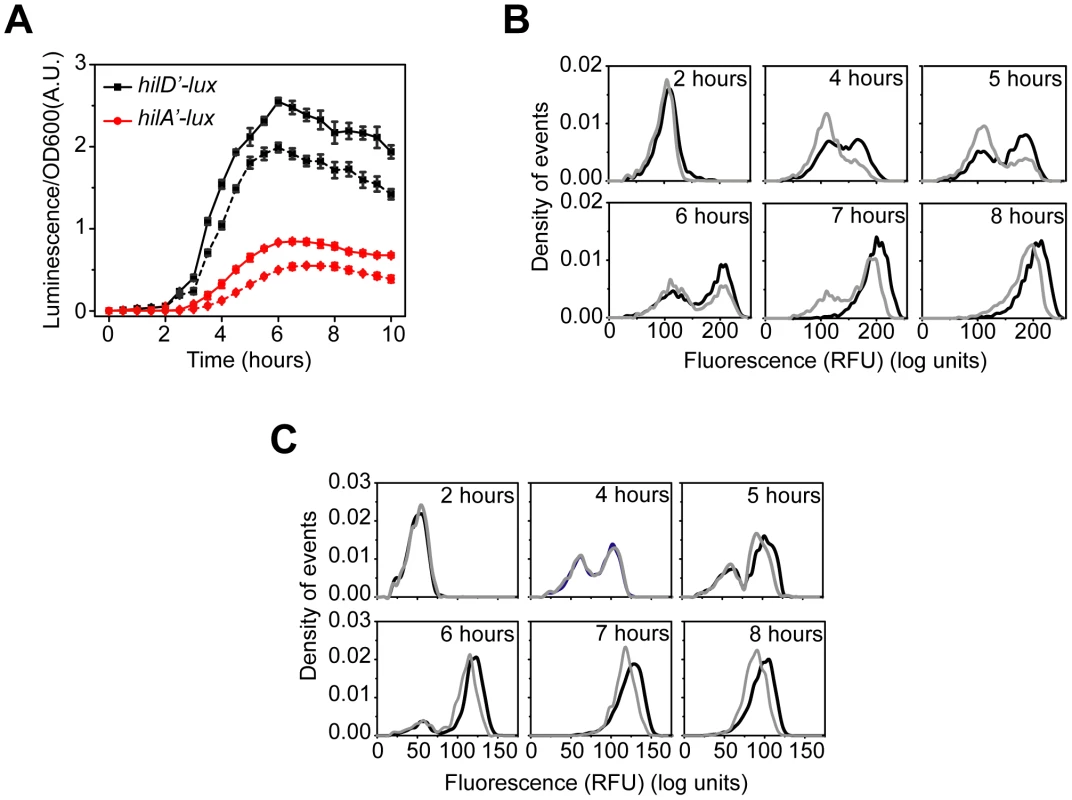
(A) Comparison of time-course dynamics for PhilD (pSS074, black) and PhilA (pSS077, red) promoter activities in wild type (solid lines) and a ΔhilC ΔrtsA mutant (CR350, dashed lines) as determined using luciferase transcriptional reporters. (B and C) Comparison of PhilA (pSS055, B) and PhilD (pSS072, C) promoter activities in wild type (black) and a ΔhilC ΔrtsA mutant (CR350, grey) as determined using GFP transcriptional reporters and flow cytometry. Note that the loss of HilC and RtsA causes both a delay and decrease in PhilA promoter activity whereas it causes only a decrease in activity in the case of the PhilD promoter. Experiments were performed as described in Figure 1. HilE dampens SPI1 gene expression
We next investigated the role of HilE in the SPI1 gene circuit. HilE binds to HilD and prevents it from activating the PhilD, PhilC, PrtsA, and PhilA promoters [34]. As HilD is at the top of the SPI1 transcriptional cascade, HilE is able to repress the expression of all SPI1 genes. However, unlike the other regulators, HilE does not participate in a feedback loop, as its expression is not regulated by any SPI1 gene (data not shown). Rather, its expression is regulated by exogenous factors. For example, the type I fimbrial regulator, FimZ, increases HilE expression whereas the phosphoenolpyruvate phosphotransferase system (PTS) regulator, Mlc, represses it [23], [24], [35].
We compared gene expression using the luciferase assay in wild type and a ΔhilE mutant (Figure 4A). In the case of both the PhilD and PhilA promoters, we observed a roughly two-fold increase in promoter activity in the absence of HilE. However, we found that HilE did not affect the timing of activation for these two promoters (Figure S4A). Similar results were observed in the flow cytometry experiments for the PhilD and PhilA promoters (Figure 4B and S4B) and the PhilC and PrtsA promoters (data not shown). These data suggest that HilE serves to dampen SPI1 gene expression by reducing the maximal level of promoter activity.
Fig. 4. HilE dampens SPI1 gene expression. 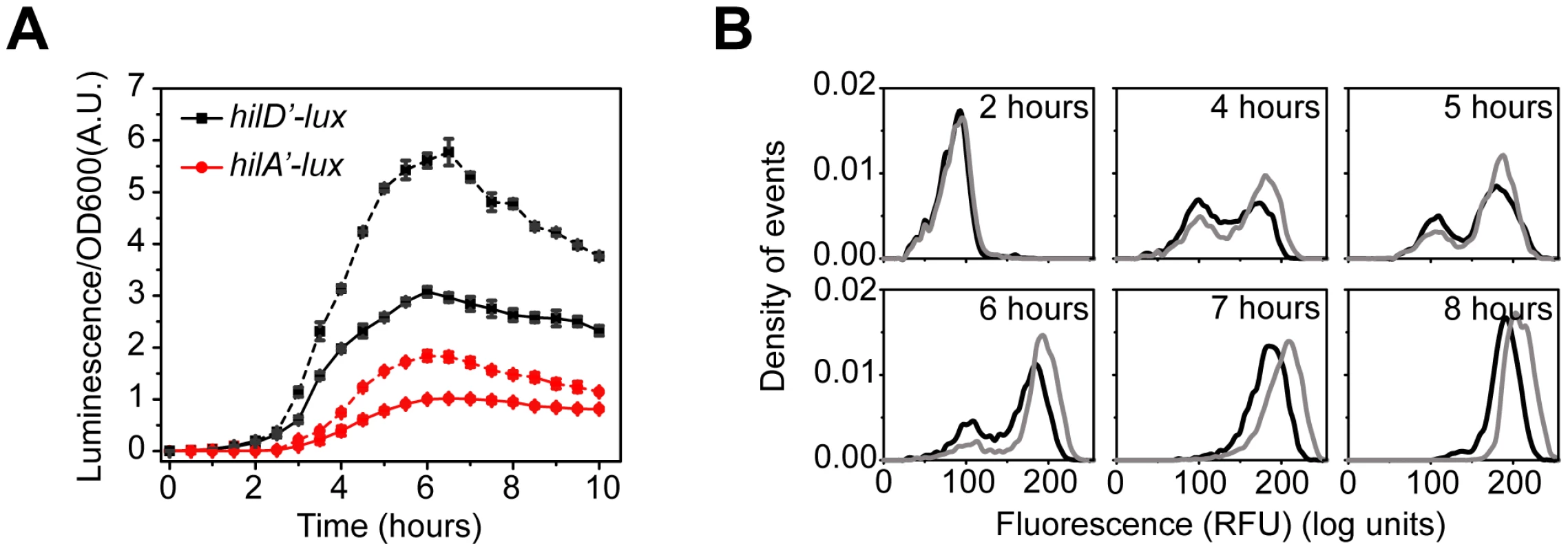
(A) Comparison of time-course dynamics for PhilD (pSS074, black) and PhilA (pSS077, red) promoter activities in wild type (solid lines) and a ΔhilE mutant (CR361, dashed lines) as determined using luciferase transcriptional reporters. (B) Comparison of PhilA (pSS055) promoter activities in wild type (black) and a ΔhilE mutant (CR361, grey) as determined using GFP transcriptional reporters and flow cytometry. Similar results are also observed with the PhilD promoter, though the phenotypic effect is much larger (Figure S4). Experiments were performed as described in Figure 1. Computational analysis of SPI1 gene circuit
The defining feature of the SPI1 gene circuit is the presence of three coupled positive feedback loops. An immediate question then is why are multiple loops present when most bacterial circuits employing feedback have just one. To explore this question in more detail, we constructed a simple mathematical model of the SPI1 gene circuit based on our understanding of how it functions (details provided in the Materials and Methods section). The model is qualitatively consistent with our experimental results, both with respect to the dynamics of HilD, HilC, RtsA, and HilA expression (Figure 5A–C) as well as the effects of mutations on HilD and HilA expression (Figure 5D–E) at both population and single-cell resolution.
Fig. 5. Mathematical model is able to accurately capture SPI1 gene expression dynamics both for wild type and key mutants. 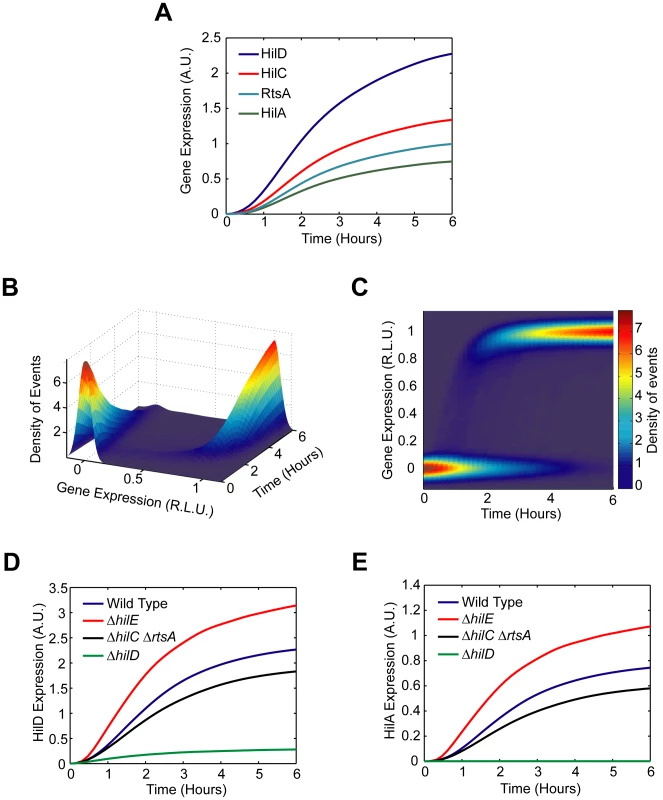
(A) Time-course simulation of HilD, HilC, RtsA, and HilA expression dynamics in wild-type cells. These results are the average of 1000 simulations. These simulations are meant to capture the population-level behavior of the circuit. (B) Time-course simulation of HilA expression at single-cell resolution. The expression values are normalized to one and plotted on a log scale. The expression values are given in relative log units (R.L.U.). Similar expression dynamics are also seen for HilD, HilC, and RtsA (see Matlab code provided as supplementary material). (C) Same results provided as a two-dimension heat plot, where the color intensity denotes the density of events. Note that the model captures the transient heterogeneity observed in our flow cytometry data where cells in both the “off” and “on” states are found at intermediate times. Panels A–C were generated from the same set of simulation runs. (D and E) Time-course simulation of HilD (D) and HilA (E) expression dynamics in wild type and ΔhilD, ΔhilC ΔrtsA, and ΔhilE mutants at population resolution. The results for each mutant were obtained from the average of 1000 simulations. Similar behavior is also seen at single-cell resolution. Mutants were simulated by setting the activity of the respective gene to zero in the model. A detailed description of the model is provided in the Materials and Methods. In constructing this model, we assumed that asynchronous activation of the PhilD promoter in individual cells causes the transient heterogeneity observed in SPI1 gene expression. We specifically assumed that the PhilD promoter is activated at random times in individual cells, where the times are exponentially distributed. Otherwise, the model is entirely deterministic. To capture the heterogeneous response, we also needed to assume that the switch from the “off” to “on” state occurs rapidly in individual cells, more rapidly than what is observed in the population (Figure 1B). Otherwise, the cells will respond homogenously as differences in the timing of the activating signal in individual cells would be smoothed out due to the slow kinetics of the circuit. As our results demonstrate, this mechanism is sufficient for generating transient heterogeneity. In fact, if the PhilD promoter is activated in all cells at the same time or the kinetics of the switch are too slow, then the population behaves homogenously (Figure S5A–B). In the case of the PhilC promoter, we assumed that it was activated at the same time in all cells. While transient heterogeneity is observed in wild type cells (Figure S1C), the PhilC promoter behaves homogenously in a ΔhilD mutant (Figure S2C). Our model is also able to capture this behavior (Figure S5C–D).
Our goal in constructing this model was not simply to recapitulate our experimental results but rather to explore the behavior of the circuit by simulating it over a range of different parameter values. In particular, we employed the model to explore the roles of coupled positive feedback and HilE in regulating SPI1 gene expression. When performing this parametric analysis, we found it most informative to focus on the steady-state behavior of the SPI1 gene circuit. This enabled us to explore the effect of a limited number of model parameters two at a time and also bypass the issue of stochasticity. As a consequence, our analysis is confined to the parameters characterizing the regulatory topology of the circuit and not those defining the dynamics (e.g. degradation and protein-protein association/disassociation rates).
We first considered the role of positive feedback on HilD expression, given the central role of this SPI1 regulator. To perform this analysis, we varied the degree by which the SPI1 regulators - HilC, HilD, and RtsA - could activate HilD expression by simulating the model at different values for the parameter . When interpreting these results, we found it informative to also vary the strength of the activating signal in our simulations, given by the parameter in the model. As shown in Figure 6A, HilD expression increases as the value of the parameter increases, equivalent to increasing the strength of the feedback on HilD expression. When this feedback is sufficiently strong, the response to the activating signal becomes discontinuous and switch-like. These results suggest that, in addition to amplifying the response, positive feedback may serve, along with HilE as described below, to endow the SPI1 circuit with an activation threshold. This threshold would ensure that SPI1 gene expression occurs only when a sufficiently strong activating signal is present. Moreover, the threshold decreases as the strength of the feedback increases, indicating that there is a tradeoff between the degree of amplification and the size of the threshold.
Fig. 6. Parametric analysis of model predicts that SPI1 gene circuit functions as an amplifier and encodes an activation threshold. 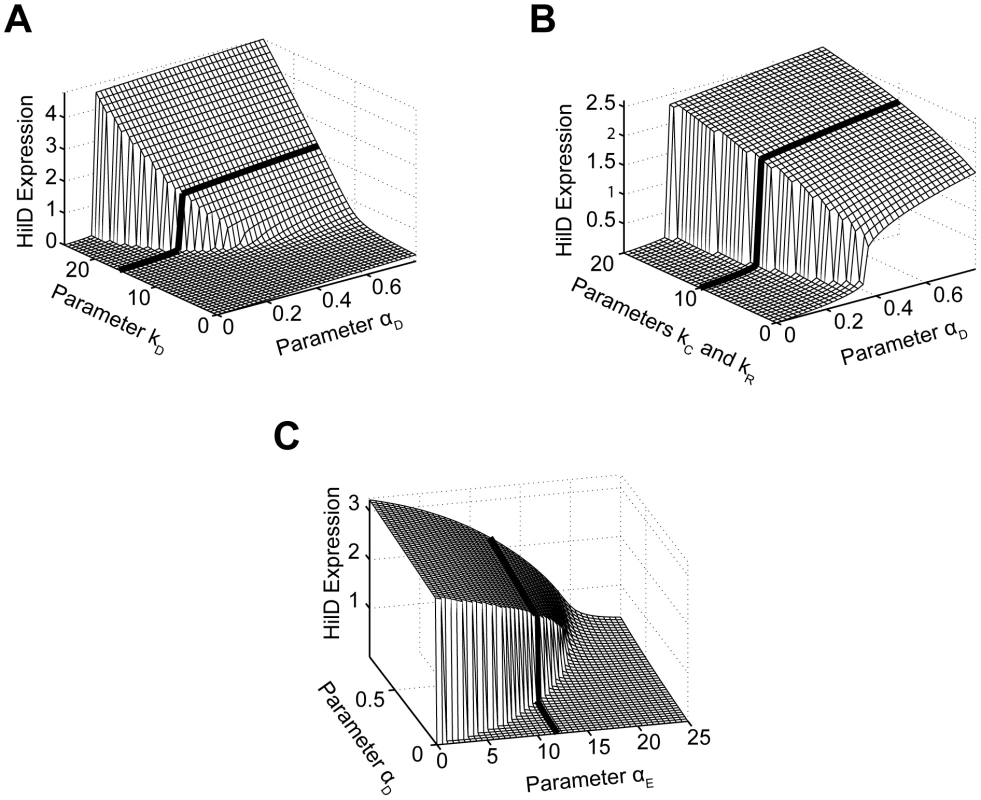
(A) Effect of positive feedback on HilD expression. Plot shows steady-state concentration of HilD as a function of the parameters and . The parameter specifies the degree by which the SPI1 regulators - HilC, HilD, and RtsA - can activate HilD expression, effectively the strength of positive feedback on HilD expression. The parameter specifies the strength of the signal activating HilD expression. (B) Effect of HilC and RtsA on HilD expression. Plot shows the steady-state concentration of HilD as a function of the parameters , , and . The parameters and specify the degree by which the SPI1 regulators - HilC, HilD, and RtsA - can activate HilC and RtsA expression, respectively. In other words, these parameters set the strength of feedback on HilC and RtsA expression. In these simulations, the parameters and were both varied in tandem: the numerical values for the two are the same. (C) Effect of HilE on HilD expression. Plot shows the steady-state concentration of HilD as a function of the parameters and . The parameter specifies the rate of HilE expression. Results for HilA are shown in Figures S6A–C. The black lines in the plots are used to denote the results obtained using the nominal parameters (aside from ). A detailed description of the model is provided in the Materials and Methods. Next, we explored the effect of HilC and RtsA on SPI1 gene expression by varying the strength of their connectivity within the circuit. Specifically, we varied the degree by which the SPI1 regulators – HilD, HilC and RtsA - could enhance both HilC and RtsA gene expression, given respectively by the parameters and in the model. As HilC and RtsA both have a weaker effect on SPI1 gene expression than HilD, the degree of amplification is also less strong though the overall trend is the same (Figure 6B). Similar results are also obtained when the expression of only one protein is varied, though the effect then is even weaker (data not shown). These results suggest that HilC and RtsA serve to fine tune SPI1 gene expression. A useful analogy here is to consider the fine and coarse focusing knobs on a microscope, where HilC and RtsA provide the fine-tune control and HilD the coarse control. This may explain why HilC and RtsA have a significantly weaker effect on SPI1 gene expression than HilD as the circuit is more robust than one with three strong regulators in the sense that only a single regulator defines the behavior of the circuit whereas the others simply tune the output.
Last, we explored the effect of HilE on SPI1 gene expression. Unlike the other SPI1 regulators, HilE is not known to be involved in any feedback loops with the other SPI1 regulators. Rather, its expression is controlled by exogenous factors. In our simulations, we varied the rate of HilE expression, given by the parameter in the model. Consistent with its role as a negative regulator, HilE decreased both HilD and HilA expression in a dose-dependent manner (Figures 6C and S6C). In addition, when expressed at a sufficiently high rate, HilE effectively shuts off the expression of HilD and HilA, a result that we also observe experimentally (data not shown). Most notably, our model predicts that HilE sets the threshold for SPI1 activation - as the rate of HilE expression increases so does the threshold for activation and vice versa. The exogenous factors regulating HilE expression, therefore, may serve to tune this activation threshold. However, we note that HilE alone does not endow the SPI1 circuit with a threshold. Rather, the threshold results from the complex interplay between HilE and the HilD positive feedback loop (Figure S6D).
Taken together, these results allow us to assign putative function to the interacting regulators and associated feedback loops comprising the SPI1 gene circuit. When viewed as a whole, the circuit appears to serve two functions. The first is to place a threshold on SPI1 activation, ensuring that the assembly of the needle complex is initiated only in response to the appropriate combination of environmental and cellular cues. The second is to amplify SPI1 gene expression.
Rewiring the SPI1 gene circuit
Our computational analysis predicts that the SPI1 gene circuit functions as a gene expression amplifier with a variable activation threshold. While our experimental results directly support the conclusion regarding gene amplification (Figures 2 and 3), the one concerning the activation threshold is not evident from our experimental results, and thus derives solely from analysis of the model. Therefore, to test this prediction regarding the threshold experimentally, we rewired the SPI1 gene circuit by replacing the PhilD promoter with the weaker PhilC promoter at its native chromosomal locus in an otherwise ΔhilC background. In this mutant, (ΔPhilD::PhilC ΔhilC), hilD is transcriptionally regulated in a manner similar to hilC. If the circuit does indeed function to place a threshold on activation, then we expect that this mutant will be unable to induce HilA expression if the activating signal for the PhilC promoter is too weak to overcome the threshold.
We found the PhilA promoter is not active in this strain (Figure 7A), suggesting that the PhilC activating signal is too weak to overcome the threshold as hypothesized. If true, then according to our model, removing HilE should enable HilA expression as it sets the activation threshold. In agreement with our model predictions, we found that if the hilE gene is removed, then the PhilA promoter is active in a related strain (ΔPhilD::PhilC ΔhilC ΔhilE) (Figure 7A). In other words, by removing the threshold set by HilE, HilD is capable of inducing HilA expression when expressed from the weaker PhilC promoter. However, when the threshold is present, HilD is not expressed at sufficiently high levels to overcome this threshold.
Fig. 7. Rewiring SPI1 gene circuits demonstrates that HilE imposes threshold on activation. 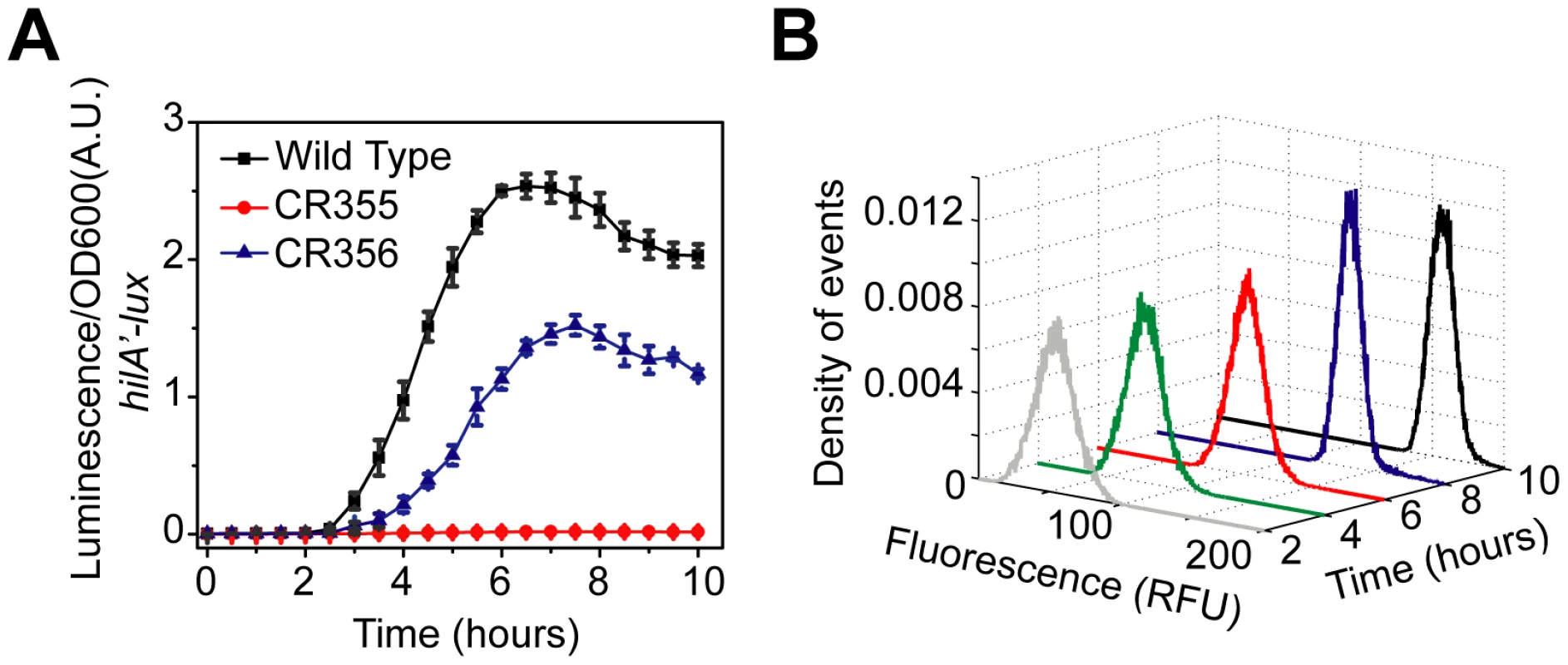
(A) Comparison of time-course dynamics for PhilA (pSS077) promoter activities in wild type (black), CR355 (ΔPhilD::PhilC ΔhilC, red) and CR356 (ΔPhilD::PhilC ΔhilC ΔhilE, blue) as determined using luciferase transcriptional reporters. In strain CR355, the PhilD promoter was replaced with the PhilC promoter in an otherwise ΔhilC background. In this strain, hilD is transcriptionally regulated in a manner identical to hilC. Strain CR356 is the same as CR355 except that it lacks HilE. (B) Dynamics of PhilA promoter activity in CR356 as determined using green fluorescent protein (GFP) transcriptional fusions and flow cytometry. Note that the activation of the PhilA promoter in CR356 is no longer switch-like but rather rheostatic in nature. Similar dynamics are seen with the PhilC promoter (Figure S2C). Experiments were performed as described in Figure 1. When we measured gene expression in this strain (ΔPhilD::PhilC ΔhilC ΔhilE) using flow cytometry, we no longer observed the transient heterogeneity found in wild type. Rather, we found that the population responded homogeneously (Figure 7B). As we have previously noted, the input signal to the PhilC promoter is not switch-like but instead is homogenous and rheostatic in nature (Figure S2C). These results further support our hypothesis that the switch-like dynamics observed in wild type is due to asynchronous activation of the PhilD promoter (Figure 2B) and not intrinsic to the circuit. In particular, when hilD is expressed from the PhilC promoter then hilA expression is also not switch-like but instead homogenous and rheostatic. Thus, the characteristics of the output of the circuit match the input. In other words, the qualitative dynamics of the input driving hilD expression are also observed in the network output, namely hilA expression. Collectively, these results support our conclusion that the SPI1 gene circuit functions as a genetic amplifier with an activation threshold, where the circuit magnifies the activating signal only if this signal exceeds a defined threshold.
A remaining question concerns the uniqueness of the SPI1 regulators given their similarity to one another. Namely, to what degree are HilC, RtsA, and HilD interchangeable? Of the three, HilD is the most important as HilA is not expressed in its absence. In formulating the model, we needed to assume that HilD was dominant in the sense that it was required for activating HilA expression. We also needed to assume that HilD was necessary for establishing connectivity within the network, where it was again required for HilC - and RtsA-dependent activation of the PhilC, PhilD, and PrtsA promoters (see Materials and Methods for further details). HilC and RtsA, on the other hand, appear to play an ancillary role in regulating SPI1 gene expression. These two proteins simply tune gene expression in a HilD-dependent manner. One specific question then is whether this behavior is intrinsic to these proteins, as assumed in the model, or simply due to these proteins not being expressed at sufficiently high levels (as HilC and RtsA can independently activate SPI1 gene expression when over expressed).
To explore this issue in more detail, we rewired the SPI1 gene circuit by placing hilC under the control of the PhilD promoter. In this reciprocal design, we replaced the PhilC promoter with the PhilD promoter at its native chromosomal locus in an otherwise ΔhilD background (ΔPhilC::PhilD ΔhilD). The rationale behind this promoter replacement experiment was to see whether HilC alone could induce HilA expression if expressed from the PhilD promoter. As HilD is capable of inducing HilA expression in absence of HilC or RtsA, we reasoned that HilC may be able to do the same in the absence of HilD if it is transcribed in a manner similar to hilD. However, despite trying designs where different sections of the promoter region were replaced, we were unable to engineer a strain where the PhilA promoter was active in the absence of HilD (data not shown). These results lend credence to our hypothesis regarding HilD dominance used in formulating the model, namely that HilD is necessary for activating the SPI1 promoter under physiological conditions.
Discussion
Using a combination of experimental and computational approaches, we found that the SPI1 gene circuit functions as a signal amplifier with an activation threshold. This virulence switch likely ensures that the SPI1 T3SS is assembled only when the bacterium has reached its target site for invasion, the distal small intestine [45]. Salmonella is thought to be able to determine its location within the host by sensing a number of environmental factors, key among them oxygen and osmolarity [28]. In addition to these environmental signals, SPI1 gene expression is also coordinated with other cellular processes such as motility and adhesion [19], [20], [21], [22], [23], [24]. The accumulated evidence to date, including the results from this study, indicates that HilD is the primary site for signal integration. According to our model, these activating signals, both intracellular cellular and environmental, initiate SPI1 gene expression by inducing the expression and activation of HilD through still unknown mechanisms. HilE, however, binds to HilD and inhibits its activity. Only when the activating signals are sufficiently strong is HilD expressed at a high enough level to overcome sequestration by HilE and activate the expression of the other SPI1 regulators – HilC, RtsA, and HilA - and also further induce its own expression. Once induced, HilC and RtsA serve to further amplify and also accelerate SPI1 gene expression. The result is a two-state switch with a defined activation threshold, defined in the sense that the threshold is set by the level of HilE expression and possibly other systems that function through HilD protein [44].
A notable feature of the SPI1 gene circuit is the presence of three, coupled positive feedback loops. At the most fundamental level, positive feedback amplifies the response to an external signal [46], [47]. It is also capable of effectively transforming a continuous input into a digital output when the feedback is sufficiently strong. In the context of bacterial gene circuits, positive feedback has most often been associated with multi-stable switches and cell population heterogeneity [43], [48]. What makes the SPI1 gene circuit particularly intriguing is that most bacterial systems utilizing positive feedback, at least those documented so far in the literature, possess only a single loop.
We first note that these additional feedback loops, namely the ones regulating the expression of HilC and RtsA, do not add redundancy to the circuit, as the loss of HilD effectively shuts off SPI1 gene expression. Rather, they serve to further amplify and accelerate SPI1 gene expression. In vivo, loss of either HilC or RtsA does not significantly attenuate intestinal invasion. Yet, loss of both does [16], indicating that the amplification or acceleration provided by these loops plays an important physiological role. Whether this role is simply to ensure that the SPI1 structural genes are expressed at sufficiently high levels or to provide a sharp activation threshold is still unknown.
Only a few studies to date, mostly focused on eukaryotic systems where this regulation is more common, have explored systems employing coupled positive feedback [49], [50], [51], [52]. In one notable theoretical study, the coupling of a slow and fast positive feedback loop was shown to yield a “dual-time” switch that is capable of being rapidly induced yet still is robust to fluctuations in the activating signal [49]. However, these properties are not obtained when two loops of the same type are coupled. While rapid induction is observed in SPI1 gene expression, there is no evidence to suggest that some loops are fast whereas others are slow. Furthermore, these loops do not operate synergistically in the sense that coupling in the SPI1 gene circuit does not engender new functions unattainable with just a single loop.
As the loops involving HilC and RtsA only additively contribute to the response, we imagine that the coupling in SPI1 may result instead from the piecewise evolution of the circuit. According to this model, HilC and RtsA were acquired to compensate for the inability of HilD alone to mediate a robust response. The motivation for this model comes from a recent study where a synthetic gene circuit coupling two weak positive feedback loops was engineered [53]. The authors found that their coupled circuit yielded a bistable response that, in the case of a single loop circuit, could be obtained only with an ultrasensitive activator even though the individual regulators in the coupled circuit lacked this behavior. Based on these results, the authors speculated that natural circuits could evolve using a similar approach - rather than evolve a circuit with a single regulator requiring precise biochemical properties, a more robust and facile solution may be obtained by simply linking together multiple regulators that alone lack the requisite properties. Similarly, others have shown that by changing the regulatory architecture of a circuit one can affect is behavior without commensurate changes in the underlying proteins [54], [55], [56]. We hypothesize that a similar process may have occurred with the SPI1 gene circuit. As such, this model provides one possible explanation as to why the circuit involves multiple feedback loops when one alone would suffice.
In a related study, we found that the gene circuit controlling the expression of type I fimbriae in Salmonella utilizes two coupled positive feedback loops [24]. In this system, the expression of the genes encoding the type I fimbriae is controlled by two regulators, FimY and FimZ. These two proteins form two coupled positive feedback loops and encode a logical AND gate or, alternatively, a coincidence circuit. A similar logic may also be also encoded within the SPI1 gene circuit. In particular, HilC is expressed in the absence of HilD. Moreover, the signals activating the PhilC promoter appear to be different than the ones activating the PhilD promoter, given their dissimilar dynamics. We are tempted therefore to speculate that, in addition to being an amplifier, the SPI1 gene circuit may also function as some sort of coincidence circuit, optimally expressing SPI1 genes only when the activating signals for both the PhilC and PhilD promoter are present. Coupled feedback in this case would reinforce the effect of these signals and further link the two. While such a model alone would not explain why multiple feedbacks loops are present in the SPI1 gene circuit, it may nonetheless provide one possible advantage for such a design.
In conclusion, we have been able to propose an integrated model for the regulation of SPI1 gene expression. While this system has been studied extensively, an integrated model of its regulation was previously lacking. Using a combination of experimental and computational analyses, we have been able to deconstruct this complex circuit and determine how the individual components contribute towards its integrated function. A key element in our analysis involved rewiring the SPI1 genetic circuit. As the kinetic parameters are unavailable and difficult to perturb, direct validation of our model remains an elusive challenge. However, by rewiring the circuit, we were nonetheless able to test a number of predictions from our mathematical model. Such an approach provides a powerful framework for integrating models with experimental data, particularly when parameters values are lacking or difficult to perturb. Finally, our results provide a detailed examination of a natural system employing coupled positive feedback, a mechanism of control that to date has primarily been investigated in eukaryotes.
Materials and Methods
Growth conditions
All experiments were performed in Luria-Bertani broth (LB) (10 g/L tryptone, 5 g/L yeast extract, and 10 g/L NaCl) unless otherwise specified. Bacterial strains were grown at 37°C except for strains carrying the temperature sensitive plasmids, pKD46 or pCP20, which were grown at 30°C as described previously [57]. Antibiotics were used at the following concentrations: ampicillin at 100 µg/mL; chloramphenicol at 34 µg/mL; kanamycin at 40 µg/mL, and tetracycline at 25 µg/mL.
Bacterial strains and plasmids
All Salmonella enterica serovar Typhimurium strains used in this study are isogenic derivatives of strain ATCC 14028 (American Type Culture Collection) and are listed in Table 1. The strain CR349 (ΔSPI1::FRT ΔrtsA5) was made by first transducing the Δ(invH-avrA)2916::cm (called ΔSPI1::cm) allele from JS481 into the strain JS248 (ΔrtsA5) using P22HTint [58]. The chloramphenicol antibiotic resistance gene was then removed by introducing pCP20. To make the strain CR350 (ΔhilC::FRT ΔrtsA5), we first transduced the ΔhilC::cm allele from JS252 (ΔhilC::cm) into the strain JS248 (ΔrtsA5). The antibiotic resistance marker was then removed using pCR20. The strain CR351 (ΔhilE::kan) was made by replacing the hilE gene (genomic region 4763554–4764087) with the kanamycin resistance gene from pKD4 using λ-Red mediated homologous recombination [57].
Tab. 1. List of strains used in this study. 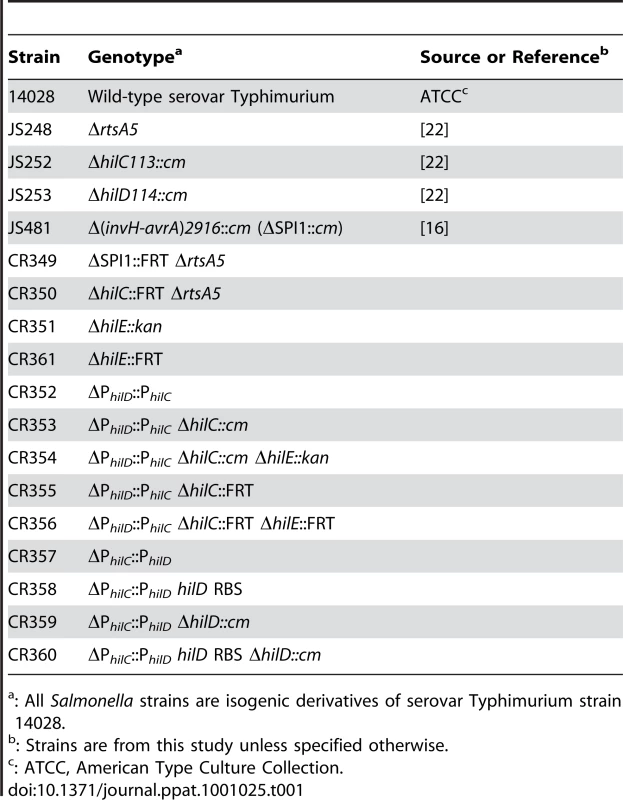
: All Salmonella strains are isogenic derivatives of serovar Typhimurium strain 14028. The strain CR352 (ΔPhilD::PhilC) was made using a two-step counter selection procedure involving the tetRA element from transposon Tn10 [59]. In the first step, the PhilD promoter (genomic region 3017694–3017820) was replaced with the tetRA element using λ-Red mediated homologous recombination. The tetRA marker was then moved into a clean wild-type background (14028) by P22 transduction. Next, the tetRA element was replaced by the PhilC promoter (genomic region 3013780–3013010) using λ-Red mediated homologous recombination and a fusaric acid counter selection, as described previously [59]. The resulting strain, CR352 (ΔPhilD::PhilC), has the hilD gene with its native ribosome binding site under the control of PhilC promoter. The strain CR354 (ΔPhilD::PhilC ΔhilC::FRT ΔhilE::FRT) was made by P22 transduction, using the strains JS252 (ΔhilC::cm) and CR351 (ΔhilE::kan) as donors. The antibiotic resistance markers were removed by introducing pCP20. Similarly, we also constructed two strains, CR357 and CR358, where the PhilC promoter was replaced by the PhilD promoter. In the first design, CR357 (ΔPhilC::PhilD), the PhilC promoter (genomic region 3013780–3013010) was replaced with the PhilD promoter (genomic region 3017694–3017820) leaving the hilC ribosome binding site intact. In second design, CR358 (ΔPhilC::PhilD hilD RBS), the upstream region of the hilC gene (genomic region 3013780–3013000) was replaced by the upstream region of the hilD gene (genomic region 3017694–3017830). All mutants were subsequently checked using primers that bound outside the region deleted. All chromosomal promoter replacements were verified by amplifying and sequencing the mutated regions.
All plasmids used in the study are listed in Table 2. Transcriptional fusions to the SPI1 promoters were made by cloning the promoter of interest upstream of either the green fluorescent protein (GFP) or the luxCDABE operon from Photorhabdus luminescens on a medium-copy plasmid [36], [37]. To construct the plasmid pSS098, pPROBE-gfp was digested with EcoRI and NheI and pPROTet.E was digested with EcoRI and AvrII. The gfp gene fragment from the digested pPROBE-gfp was then cloned into the digested pPROTet.E resulting in the plasmid pSS098. All constructs were sequenced prior to transformation in the wild-type and mutant strains.
Tab. 2. List of plasmids used in this study. 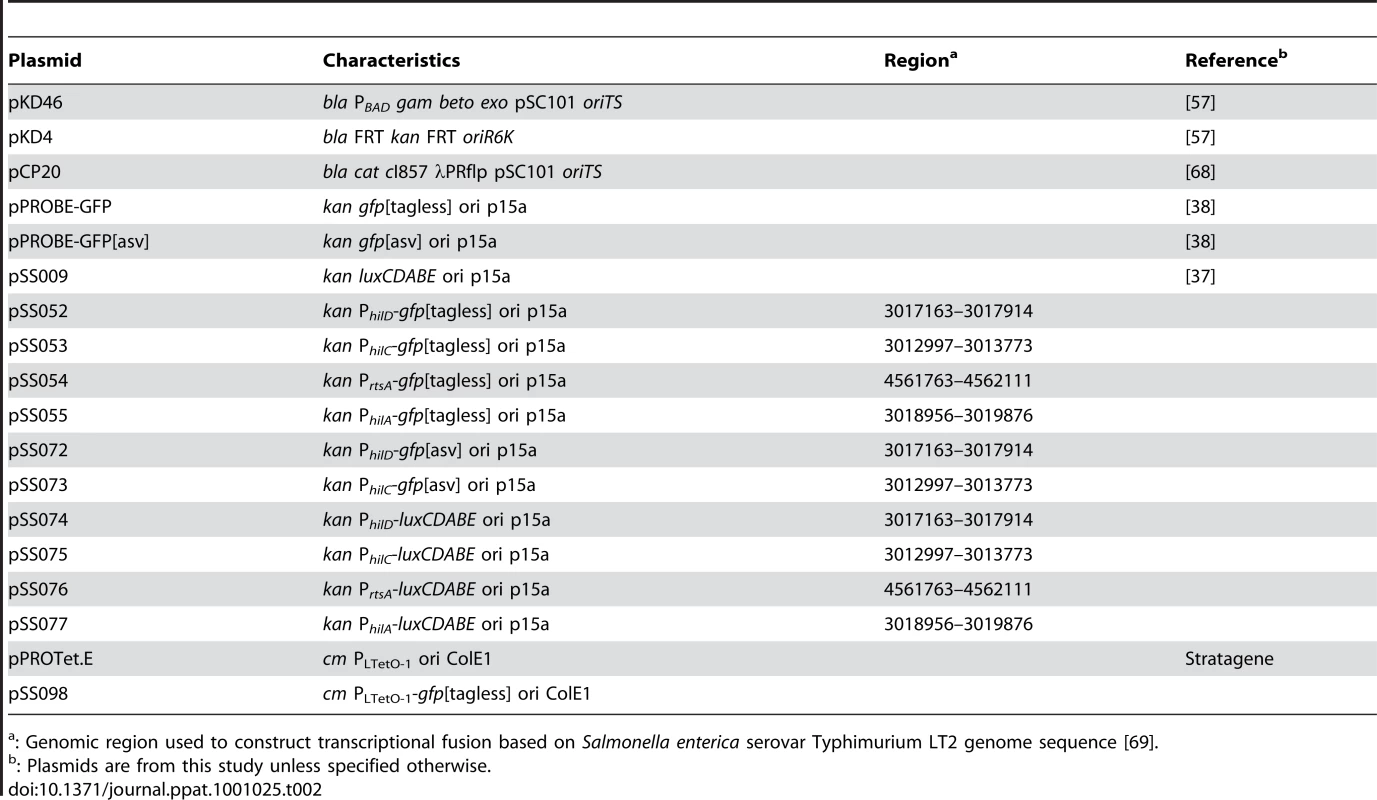
: Genomic region used to construct transcriptional fusion based on Salmonella enterica serovar Typhimurium LT2 genome sequence [69]. Fluorescence measurements
Cultures were first grown overnight in LB medium lacking salt under vigorous shaking at 37°C (SPI1 repressing conditions) and then sub-cultured 1∶1000 into fresh LB medium (with salt) and grown statically in test tubes at 37°C for 12 hours [34], [60]. A 100 µL aliquot of each culture was then transferred to a 96-well microplate, and fluorescence and absorbance (OD600) were measured using a Tecan Safire2 microplate reader. The fluorescence readings, given in terms of relative fluorescence units (RFU), were normalized to the OD600 absorbance to account for cell density.
For single-cell fluorescence measurements, overnight cultures were first grown under SPI1-repressing conditions at 37°C. The cells were then sub-cultured to an OD of 0.05 into fresh LB medium (with salt) and grown statically at 37°C. Samples were collected at different time points by resuspending them in phosphate buffered saline (PBS) with 34 µg/mL chloramphenicol in order to arrest translation and then storing on ice. All fluorescent-activated cell sorting (FACS) experiments were performed on a BD LRS II system from BD Biosciences. Data extraction and analysis for the FACS experiments was done using FCS Express Version 3 (De Novo Software). For all FACS experiments, fluorescence values of 30,000 events were recorded and reported as a histogram.
In the flow cytometry experiments involving the PhilC and PhilD promoters, we used destabilized GFP transcriptional fusions where the sequence AANDENYAASV was appended to the C-terminus of the protein. This tag reduces the half life of GFP from approximately 24 hours to 110 minutes [38], [61]. The reason that we needed to employ destabilized GFP is that PhilC and PhilD promoters are partially active even when the cells are grown in SPI1-repressing conditions. As a consequence, we were unable to observe the “off” to the “on” transition using “tagless” GFP. We did not run into similar problems with the PhilA and PrtsA promoters and consequently used transcriptional fusions to “tagless” GFP. Qualitatively similar results are obtained when using destabilized GFP transcriptional fusions to these promoters (data not shown).
Luminescence measurements
For time-course luminescence experiments, cultures were grown overnight at 37°C in SPI1-repressing conditions. The overnight cultures were then sub-cultured to an OD of 0.05 into fresh LB medium (with salt). A 100 µL aliquot of each culture was then transferred to a 96-well microplate. This is denoted by time 0 h in our kinetic luminescence experiments. In addition, 20 µL of mineral oil was also added to the well to prevent evaporation [62]. The cells were then grown statically at 37°C, and luminescence and absorbance (OD600) readings were taken every 5 minutes using a Tecan Safire2 microplate reader. The luminescence readings, given in terms of relative light units (RLU), were normalized to the OD600 absorbance to account for cell density. Three independent experiments were performed on separate days. For each experiment, six samples were tested. The average values and standard deviations are reported.
Model description
The major assumptions used in formulating the model are enumerated below.
-
In formulating the model, we focused solely on the interacting SPI1 regulators - HilC, HilD, HilE, and RtsA - and their role in regulating hilA expression. In particular, we ignored the effects of additional external regulators [17], [44]. These external factors were accounted for implicitly in the model through our choice of the kinetic parameters. In other words, we assumed that there are no additional feedback loops beyond those detailed in Figure 1A. As a consequence, we treated these external regulators as constant inputs into the model. The validity of this hypothesis is debatable, though there is insufficient evidence at this time to consider any reasonable alternatives. We also did not include the downstream SPI1 regulators – InvF and SicA – in the model. These downstream regulators do not appear to affect HilA expression. Rather, they are thought to regulate the timing of expression of the proteins comprising the SPI1 needle complex and the secreted effectors [29], [41], [63]. In these regards, the model focuses only on initiation and ignores assembly and secretion. It also does not account for the decrease in SPI1 gene expression when cells enter stationary phase (Figure 1B).
-
The model does not account for negative regulation by HilA and SprB. HilA, in particular, negatively regulates its own expression by apparently binding to the PhilA promoter and repressing transcription [64]. Likewise, SprB, a transcription factor from the LuxR/UhaP family that is positively regulated by HilA, appears to bind to the HilD promoter and weakly repress its activity [65]. Inclusion of these negative feedback loops does not substantively affect the results from our model and, for simplicity, we chose to ignore them in the model.
-
The model does not distinguish between transcription and translation. Both are lumped together in a single step. As a consequence, the rate of protein synthesis is assumed to be linearly proportional to the concentration of mRNA within the cell. Our justification for this assumption is that, based on a number of unpublished observations, we believe that the regulation of HilD occurs primarily either at the transcriptional or the post-translational level (i.e. the level of HilD protein).
-
HilC, HilD, and RtsA are all AraC-like transcription factors and likely function only in the dimeric form. In the model, we assume for simplicity that the dimers form spontaneously and are stable (i.e. the dimerization reaction is irreversible). As a consequence, the model does not distinguish between the monomeric and dimeric forms; all protein is assumed to be in the dimeric form. We also do not account for the possible formation of heterodimers.
-
HilC and RtsA can independently induce HilA expression [16]. Yet, in the absence of HilD, HilA is not expressed even though hilC is transcribed (albeit at reduced levels). To account for HilD dominance (or rather dominant epistasis) in the model, we needed to assume that the SPI1 promoters have two binding sites with occupancy of both required for transcription. We specifically assumed that one site is highly specific for HilD with only weak affinity for HilC and RtsA. This first binding site establishes dominance as it effectively probes for whether HilD is present in the cell. Moreover, because of its high affinity, HilD will occupy this site even when expressed at low levels. Due to their weak affinity, neither HilC nor RtsA will occupy this site under physiological conditions. However, when over expressed, the elevated concentrations of these proteins will compensate for their weak affinity for this site, allowing them to bind. The second site, on the other hand, has moderate affinity for all three regulators (with the affinity for HilD still the highest) and serves to tune expression in proportion to their aggregate concentration. Other alternatives are possible, though this model for promoter regulation offers perhaps the simplest mechanism to explain HilD dominance consistent with what we already know about SPI1 gene expression. Moreover, others have found that the SPI1 promoters contain multiple binding sites for the HilC, HilD, and RtsA [31], [32], so this assumption is not entirely implausible. Lastly, we note that while HilD dominance has been documented previously only in the case of the PhilA promoter, our data suggests that it also extends to the PhilC, PhilD, and PrtsA promoters as detailed below.
-
The most speculative aspect of the model concerns the mechanism for activation of the SPI1 promoters – PhilA, PhilC, PhilD, and PrtsA - by HilC, HilD, and RtsA. In the model, we assume that all four promoters have the same two binding sites, one highly specific for HilD and the other much less so (see Assumption 5). While there is no mechanistic data to support this hypothesis, we have found that the promoter activities are linearly proportional to one another when we compared them at varying levels of NaCl induction and in different genetic backgrounds (Figure S7). The simplest explanation for this linear correlation is that all four promoters have the same two binding sites. As a consequence, we used the same mathematical expressions and parameters to model occupancy of the PhilA, PhilC, PhilD, and PrtsA promoters by the SPI1 regulators. Aside from our supporting data, we significantly reduce the number of free parameters in the model by invoking this assumption.
-
The model assumes that HilE not only binds and inhibits HilD but also promotes its degradation. While there is no experimental data to support such a mechanism, we found it necessary to match our experimental results for the ΔhilE mutant. In the absence of such a mechanism, we found that the steady-state concentrations of HilD and HilA were not affected by HilE, a result contrary to experimental observations.
-
The model assumes that the transient heterogeneity observed in the gene expression data is due solely to asynchrony in the timing of the activation signal. To model this behavior, we assumed that the PhilD promoter is activated at random times in individual cells, where the activation times are exponentially distributed. In the case of the PhilC promoter, we assumed that it is activated in a deterministic manner. For simplicity, we assumed that both promoters have, on average, the same activation kinetics. Beyond asynchrony in the timing of activation, we do not believe that noise arising from any number of possible sources plays a critical role in SPI1 gene expression beyond introducing variability in the gene expression measurements (see below).
-
To qualitatively compare the simulation results with our flow cytometry data, we employed density estimation using a Gaussian kernel with fixed bandwidth. This method replaces each data point with a Gaussian basis function of constant variance. While this method is typically used to smooth data, namely to approximate a discrete histogram with a continuous function, we employed it to artificially introduce noise into our model. Our motivation was simply to obtain a better qualitative fit to the flow cytometry data where, aside from the heterogeneity, we observed variable gene expression in individual cells. While we do not believe this variability is significant for understanding how the circuit functions, we nonetheless attempted to capture it in our model. As we do not know the origins of this variability (e.g. stochastic gene expression, measurement error, etc), we simply assumed that there was an additive Gaussian noise term in the model, effectively what density estimation does.
We note that Mande and coworkers previously published a mathematical model of the SPI1 gene circuit [66], [67]. While there is substantial overlap between their model and ours, the Mande model does not account for the critical role of positive feedback on HilD expression, a key finding in our experimental investigations. More significantly, their model does not include HilE. As a consequence, the major conclusion drawn from the analysis of our model regarding the activation threshold cannot be obtained from theirs.
Model equations
The governing equations for the model are the following:(1)(2)(3)(4)(5)(6)(7)where denotes time and the state variable denotes the concentration of HilD, the concentration of HilE, the concentration of HilC, the concentration of RtsA, the concentration of HilA, the concentration of the HilE-HilD complex, and the concentration of the luciferase reporter for the PhilD promoter. We included this last state variable, , to better match the model to our experimental data. Otherwise, we needed to account for the fraction of HilD bound to HilE () and the associated differences in the stabilities of the respective moieties. The variable is used to denote an exponentially distributed random variable with a rate parameter and the function is used to denote the Heaviside step function. The occupancy state of the two respective binding sites within the SPI1 promoters are given by the following equilibrium expressions(8)and(9)The parameter definitions and nominal values are given in Table 3.
Tab. 3. Parameter definitions and nominal values. 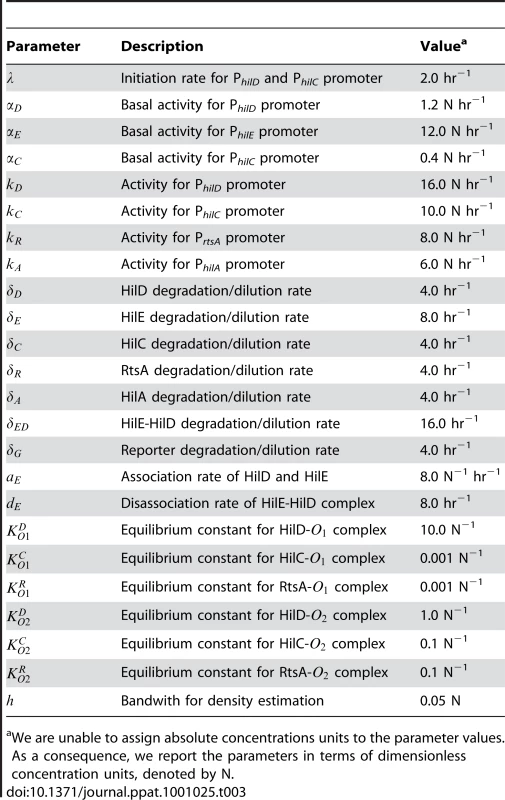
We are unable to assign absolute concentrations units to the parameter values. As a consequence, we report the parameters in terms of dimensionless concentration units, denoted by N. In our simulations, we first generated the pseudo random variable and then simulated the model using this value. The value for denotes the time when the PhilD promoter is activated in an individual cell. To model this transition, we employed the Heaviside step function, which has a value zero when the argument is negative and one when positive. Thus, when induced, the PhilD promoter undergoes a step-like increase in activity. We then repeated this procedure multiple times in order to gather statistics for an ensemble of cells.
With regards to the model parameters, insufficient data are available to accurately and uniquely estimate them. However, as our goal was simply to construct a model that captured the general trends observed in the data, we simply choose numerical values for the parameters that provided a good qualitative fit. In these regards, the model is only semi-quantitative given the subjective basis of our parameterization. That said, the model captures our current understanding of the SPI1 gene circuit and provides a reasonable fit to the data as documented in the main text.
Numerical solution
The set of coupled ordinary differential equations comprising the model were solved in Matlab 7.2 (The Mathworks, http://www.mathworks.com) using the ode15s routine where the initial conditions of all state variables where set to zero. To account for random initiation times, the model was simulated 1000 times using the built-in random number generator. The Matlab m-file used to generate the figures is provided as supplemental information.
Supporting Information
Zdroje
1. EllermeierCD
SlauchJM
2006 The genus Salmonella.
DworkinM
FalkowS
RosenbergE
SchleiferK-H
StackebrandtE
The prokaryotes, 3rd ed New York, NY. Springer 123 158
2. MillerSI
PeguesPA
2000 Salmonella species, including Salmonella typhi.
BennettJE
DolinR
Principles of infectious diseases Philadelphia PA Churchill Livingstone 2344 2363
3. MillsDM
BajajV
LeeCA
1995 A 40 kb chromosomal fragment encoding Salmonella typhimurium invasion genes is absent from the corresponding region of the Escherichia coli K-12 chromosome. Mol Microbiol 15 749 759
4. LeeCA
JonesBD
FalkowS
1992 Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci U S A 89 1847 1851
5. KimbroughTG
MillerSI
2000 Contribution of Salmonella typhimurium type III secretion components to needle complex formation. Proc Natl Acad Sci U S A 97 11008 11013
6. KuboriT
MatsushimaY
NakamuraD
UralilJ
Lara-TejeroM
1998 Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280 602 605
7. KimbroughTG
MillerSI
2002 Assembly of the type III secretion needle complex of Salmonella typhimurium. Microbes Infect 4 75 82
8. SukhanA
KuboriT
WilsonJ
GalanJE
2001 Genetic analysis of assembly of the Salmonella enterica serovar Typhimurium type III secretion-associated needle complex. J Bacteriol 183 1159 1167
9. CollazoCM
GalanJE
1997 The invasion-associated type III system of Salmonella typhimurium directs the translocation of Sip proteins into the host cell. Mol Microbiol 24 747 756
10. CollazoCM
GalanJE
1996 Requirement for exported proteins in secretion through the invasion-associated type III system of Salmonella typhimurium. Infect Immun 64 3524 3531
11. CornelisGR
2006 The type III secretion injectisome. Nat Rev Microbiol 4 811 825
12. GinocchioCC
OlmstedSB
WellsCL
GalanJE
1994 Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell 76 717 724
13. FrancisCL
RyanTA
JonesBD
SmithSJ
FalkowS
1993 Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature 364 639 642
14. HaywardRD
KoronakisV
2002 Direct modulation of the host cell cytoskeleton by Salmonella actin-binding proteins. Trends Cell Biol 12 15 20
15. ZhouD
GalanJ
2001 Salmonella entry into host cells: the work in concert of type III secreted effector proteins. Microbes Infect 3 1293 1298
16. EllermeierCD
EllermeierJR
SlauchJM
2005 HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol Microbiol 57 691 705
17. AltierC
2005 Genetic and environmental control of salmonella invasion. J Microbiol 43 Spec No 85 92
18. EllermeierJR
SlauchJM
2008 Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190 476 486
19. LinD
RaoCV
SlauchJM
2008 The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J Bacteriol 190 87 97
20. LucasRL
LostrohCP
DiRussoCC
SpectorMP
WannerBL
2000 Multiple factors independently regulate hilA and invasion gene expression in Salmonella enterica serovar typhimurium. J Bacteriol 182 1872 1882
21. IyodaS
KamidoiT
HiroseK
KutsukakeK
WatanabeH
2001 A flagellar gene fliZ regulates the expression of invasion genes and virulence phenotype in Salmonella enterica serovar Typhimurium. Microb Pathog 30 81 90
22. EllermeierCD
SlauchJM
2003 RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J Bacteriol 185 5096 5108
23. BaxterMA
JonesBD
2005 The fimYZ genes regulate Salmonella enterica Serovar Typhimurium invasion in addition to type 1 fimbrial expression and bacterial motility. Infect Immun 73 1377 1385
24. SainiS
PearlJA
RaoCV
2009 Role of FimW, FimY, and FimZ in regulating the expression of type i fimbriae in Salmonella enterica serovar Typhimurium. J Bacteriol 191 3003 3010
25. DalyRA
LostrohCP
2008 Genetic analysis of the Salmonella transcription factor HilA. Can J Microbiol 54 854 860
26. AhmerBM
van ReeuwijkJ
WatsonPR
WallisTS
HeffronF
1999 Salmonella SirA is a global regulator of genes mediating enteropathogenesis. Mol Microbiol 31 971 982
27. BajajV
HwangC
LeeCA
1995 hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol 18 715 727
28. BajajV
LucasRL
HwangC
LeeCA
1996 Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol Microbiol 22 703 714
29. DarwinKH
MillerVL
2000 The putative invasion protein chaperone SicA acts together with InvF to activate the expression of Salmonella typhimurium virulence genes. Mol Microbiol 35 949 960
30. DarwinKH
MillerVL
2001 Type III secretion chaperone-dependent regulation: activation of virulence genes by SicA and InvF in Salmonella typhimurium. Embo J 20 1850 1862
31. SchechterLM
LeeCA
2001 AraC/XylS family members, HilC and HilD, directly bind and derepress the Salmonella typhimurium hilA promoter. Mol Microbiol 40 1289 1299
32. OlekhnovichIN
KadnerRJ
2002 DNA-binding activities of the HilC and HilD virulence regulatory proteins of Salmonella enterica serovar Typhimurium. J Bacteriol 184 4148 4160
33. SchechterLM
DamrauerSM
LeeCA
1999 Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol 32 629 642
34. BaxterMA
FahlenTF
WilsonRL
JonesBD
2003 HilE interacts with HilD and negatively regulates hilA transcription and expression of the Salmonella enterica serovar Typhimurium invasive phenotype. Infect Immun 71 1295 1305
35. LimS
YunJ
YoonH
ParkC
KimB
2007 Mlc regulation of Salmonella pathogenicity island I gene expression via hilE repression. Nucleic Acids Res 35 1822 1832
36. WinsonMK
SwiftS
HillPJ
SimsCM
GriesmayrG
1998 Engineering the luxCDABE genes from Photorhabdus luminescens to provide a bioluminescent reporter for constitutive and promoter probe plasmids and mini-Tn5 constructs. FEMS Microbiol Lett 163 193 202
37. SainiS
BrownJD
AldridgePD
RaoCV
2008 FliZ Is a posttranslational activator of FlhD4C2-dependent flagellar gene expression. J Bacteriol 190 4979 4988
38. MillerWG
LindowSE
1997 An improved GFP cloning cassette designed for prokaryotic transcriptional fusions. Gene 191 149 153
39. HakkilaK
MaksimowM
KarpM
VirtaM
2002 Reporter genes lucFF, luxCDABE, gfp, and dsred have different characteristics in whole-cell bacterial sensors. Anal Biochem 301 235 242
40. MeighenEA
1991 Molecular biology of bacterial bioluminescence. Microbiol Rev 55 123 142
41. TemmeK
SalisH
Tullman-ErcekD
LevskayaA
HongSH
2008 Induction and relaxation dynamics of the regulatory network controlling the type III secretion system encoded within Salmonella pathogenicity island 1. J Mol Biol 377 47 61
42. HautefortI
ProencaMJ
HintonJC
2003 Single-copy green fluorescent protein gene fusions allow accurate measurement of Salmonella gene expression in vitro and during infection of mammalian cells. Appl Environ Microbiol 69 7480 7491
43. MitrophanovAY
GroismanEA
2008 Positive feedback in cellular control systems. Bioessays 30 542 555
44. EllermeierJR
SlauchJM
2007 Adaptation to the host environment: regulation of the SPI1 type III secretion system in Salmonella enterica serovar Typhimurium. Curr Opin Microbiol 10 24 29
45. CarterPB
CollinsFM
1974 The route of enteric infection in normal mice. J Exp Med 139 1189 1203
46. BecskeiA
SeraphinB
SerranoL
2001 Positive feedback in eukaryotic gene networks: cell differentiation by graded to binary response conversion. Embo J 20 2528 2535
47. MaedaYT
SanoM
2006 Regulatory dynamics of synthetic gene networks with positive feedback. J Mol Biol 359 1107 1124
48. DubnauD
LosickR
2006 Bistability in bacteria. Mol Microbiol 61 564 572
49. BrandmanO
FerrellJEJr
LiR
MeyerT
2005 Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science 310 496 498
50. CuiJ
ChenC
LuH
SunT
ShenP
2008 Two independent positive feedbacks and bistability in the Bcl-2 apoptotic switch. PLoS One 3 e1469
51. ThomasR
ThieffryD
KaufmanM
1995 Dynamical behaviour of biological regulatory networks–I. Biological role of feedback loops and practical use of the concept of the loop-characteristic state. Bull Math Biol 57 247 276
52. TianXJ
ZhangXP
LiuF
WangW
2009 Interlinking positive and negative feedback loops creates a tunable motif in gene regulatory networks. Phys Rev E Stat Nonlin Soft Matter Phys 80 011926
53. ChangDE
LeungS
AtkinsonMR
ReiflerA
ForgerD
2009 Building biological memory by linking positive feedback loops. Proc Natl Acad Sci U S A
54. WuK
RaoCV
2010 The role of configuration and coupling in autoregulatory gene circuits. Mol Microbiol 75 513 527
55. MitrophanovAY
JewettMW
HadleyTJ
GroismanEA
2008 Evolution and dynamics of regulatory architectures controlling polymyxin B resistance in enteric bacteria. PLoS Genet 4 e1000233
56. KatoA
MitrophanovAY
GroismanEA
2007 A connector of two-component regulatory systems promotes signal amplification and persistence of expression. Proc Natl Acad Sci U S A 104 12063 12068
57. DatsenkoKA
WannerBL
2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 6640 6645
58. DavisR
BosteinD
RothJR
1980 Advanced bacterial genetics: a manual for genetic engineering NY Cold Spring Harbor Laboratory Press
59. KarlinseyJE
2007 lambda-Red genetic engineering in Salmonella enterica serovar Typhimurium. Methods Enzymol 421 199 209
60. FahlenTF
MathurN
JonesBD
2000 Identification and characterization of mutants with increased expression of hilA, the invasion gene transcriptional activator of Salmonella typhimurium. FEMS Immunol Med Microbiol 28 25 35
61. AndersenJB
SternbergC
PoulsenLK
BjornSP
GivskovM
1998 New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl Environ Microbiol 64 2240 2246
62. KalirS
McClureJ
PabbarajuK
SouthwardC
RonenM
2001 Ordering genes in a flagella pathway by analysis of expression kinetics from living bacteria. Science 292 2080 2083
63. DarwinKH
MillerVL
1999 InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J Bacteriol 181 4949 4954
64. De KeersmaeckerSC
MarchalK
VerhoevenTL
EngelenK
VanderleydenJ
2005 Microarray analysis and motif detection reveal new targets of the Salmonella enterica serovar Typhimurium HilA regulatory protein, including hilA itself. J Bacteriol 187 4381 4391
65. SainiS
RaoCV
2010 SprB is the molecular link between Salmonella pathogenicity island 1 (SPI1) and SPI4. J Bacteriol 192 2459 2462
66. MaithreyeR
MandeSS
2007 Modelling of the regulation of the hilA promoter of type three secretion system of Salmonella enterica serovar Typhimurium. Syst Synth Biol 1 129 137
67. GaneshAB
RajasinghH
MandeSS
2009 Mathematical modeling of regulation of type III secretion system in Salmonella enterica serovar Typhimurium by SirA. In Silico Biol 9 S57 72
68. CherepanovPP
WackernagelW
1995 Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene 158 9 14
69. McClellandM
SandersonKE
SpiethJ
CliftonSW
LatreilleP
2001 Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413 852 856
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



