-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Radiation-attenuated Plasmodium sporozoites (RAS) are the only vaccine shown to induce sterilizing protection against malaria in both humans and rodents. Importantly, these “whole-parasite” vaccines are currently under evaluation in human clinical trials. Studies with inbred mice reveal that RAS-induced CD8 T cells targeting liver-stage parasites are critical for protection. However, the paucity of defined T cell epitopes for these parasites has precluded precise understanding of the specific characteristics of RAS-induced protective CD8 T cell responses. Thus, it is not known whether quantitative or qualitative differences in RAS-induced CD8 T cell responses underlie the relative resistance or susceptibility of immune inbred mice to sporozoite challenge. Moreover, whether extraordinarily large CD8 T cell responses are generated and required for protection following RAS immunization, as has been described for CD8 T cell responses following single-antigen subunit vaccination, remains unknown. Here, we used surrogate T cell activation markers to identify and track whole-parasite, RAS-vaccine-induced effector and memory CD8 T cell responses. Our data show that the differential susceptibility of RAS-immune inbred mouse strains to Plasmodium berghei or P. yoelii sporozoite challenge does not result from host - or parasite-specific decreases in the CD8 T cell response. Moreover, the surrogate activation marker approach allowed us for the first time to evaluate CD8 T cell responses and protective immunity following RAS-immunization in outbred hosts. Importantly, we show that compared to a protective subunit vaccine that elicits a CD8 T cell response to a single epitope, diversifying the targeted antigens through whole-parasite RAS immunization only minimally, if at all, reduced the numerical requirements for memory CD8 T cell-mediated protection. Thus, our studies reveal that extremely high frequencies of RAS-induced memory CD8 T cells are required, but may not suffice, for sterilizing anti-Plasmodial immunity. These data provide new insights into protective CD8 T cell responses elicited by RAS-immunization in genetically diverse hosts, information with relevance to developing attenuated whole-parasite vaccines.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000998
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000998Summary
Radiation-attenuated Plasmodium sporozoites (RAS) are the only vaccine shown to induce sterilizing protection against malaria in both humans and rodents. Importantly, these “whole-parasite” vaccines are currently under evaluation in human clinical trials. Studies with inbred mice reveal that RAS-induced CD8 T cells targeting liver-stage parasites are critical for protection. However, the paucity of defined T cell epitopes for these parasites has precluded precise understanding of the specific characteristics of RAS-induced protective CD8 T cell responses. Thus, it is not known whether quantitative or qualitative differences in RAS-induced CD8 T cell responses underlie the relative resistance or susceptibility of immune inbred mice to sporozoite challenge. Moreover, whether extraordinarily large CD8 T cell responses are generated and required for protection following RAS immunization, as has been described for CD8 T cell responses following single-antigen subunit vaccination, remains unknown. Here, we used surrogate T cell activation markers to identify and track whole-parasite, RAS-vaccine-induced effector and memory CD8 T cell responses. Our data show that the differential susceptibility of RAS-immune inbred mouse strains to Plasmodium berghei or P. yoelii sporozoite challenge does not result from host - or parasite-specific decreases in the CD8 T cell response. Moreover, the surrogate activation marker approach allowed us for the first time to evaluate CD8 T cell responses and protective immunity following RAS-immunization in outbred hosts. Importantly, we show that compared to a protective subunit vaccine that elicits a CD8 T cell response to a single epitope, diversifying the targeted antigens through whole-parasite RAS immunization only minimally, if at all, reduced the numerical requirements for memory CD8 T cell-mediated protection. Thus, our studies reveal that extremely high frequencies of RAS-induced memory CD8 T cells are required, but may not suffice, for sterilizing anti-Plasmodial immunity. These data provide new insights into protective CD8 T cell responses elicited by RAS-immunization in genetically diverse hosts, information with relevance to developing attenuated whole-parasite vaccines.
Introduction
Plasmodium infections are a global health crisis resulting in ∼300 million cases of malaria each year and ∼1 million deaths [1], [2], [3], [4], [5]. At present, there are no effective licensed anti-malarial vaccines. Most vaccines under clinical evaluation are only partially protective and, for unknown reasons, immunity rapidly wanes [6]. Thus, development of an effective malaria vaccine that provides long-term protection remains an important goal to improve global health.
Immunization with radiation-attenuated sporozoites (RAS) is the only documented means to induce sterilizing protection in both humans [7], [8] and rodents [9] and, importantly this approach is under evaluation in clinical trials [10]. Studies with inbred mouse strains reveal a prominent and often essential role for CD8 T cells in RAS-induced protection [11]. However, RAS-immune inbred mice also exhibit substantial differences in resistance to challenge with Plasmodium berghei (Pb) or P. yoelii (Py) sporozoites, two major models of experimental malaria that are thought to differ in virulence. Despite decades of research, the precise characteristics of protective memory CD8 T responses following RAS-vaccination remain poorly understood. One reason for this relates to the limited number of defined CD8 T cell epitopes derived from rodent species of Plasmodia.
BALB/c mice mount H-2Kd-restricted CD8 T cell responses against single defined circumsporozoite (CS) protein-derived epitopes from either Pb or Py and these epitopes can be targets of protective CD8 T cells [12], [13]. However, despite evidence that non-CS antigens can also be targets of protective immunity [14], [15], there are few additional Plasmodium-specific epitopes identified from antigens other than CS in BALB/c mice, and no identified protective epitopes in H-2b C57BL/6 (B6) mice. Thus, the paucity of epitope information for these parasites has contributed to our incomplete understanding of the specific quantitative and qualitative characteristics of RAS-induced CD8 T cell responses in inbred mice that are relatively easy (BALB/c) or difficult (B6) to protect against Plasmodium sporozoite challenge [11]. Moreover, we recently showed that the threshold of memory CD8 T cell responses to the Pb-CS epitope (monospecific responses) required for sterilizing immunity against sporozoite challenge was extremely large [16]. Importantly, it is unknown whether a more diverse memory CD8 T cell response generated by whole parasite based RAS vaccination will decrease the threshold number of memory cells required for protection. This issue is of great relevance to translation of the attenuated whole parasite vaccines to humans.
The identification and characterization of infection - or vaccination-induced, antigen-specific CD8 T cell populations has historically required defined antigenic peptide determinants with known MHC restriction. However, specific activation markers can be used to track effector, but not memory, CD8 T cell responses to viral vaccines in humans in the absence of defined antigenic determinants or known MHC-restriction [17]. We recently described an alternative surrogate actiation marker approach, relying on concurrent downregulation of surface CD8α and upregulation of CD11a (α-chain of LFA-1) on effector and memory antigen-specific CD8 T cells responding to bacterial and viral-infections in mice [18]. Herein, we apply this surrogate marker approach to identify and longitudinally track the total CD8 T cell response following RAS-immunization in rodents. This surrogate marker approach allowed us for the first time to evaluate CD8 T cell responses and protective immunity to RAS-immunization in both inbred and outbred hosts. Collectively, our data show that despite broadening the number of antigenic targets through whole-parasite vaccination, extraordinarily large numbers of memory CD8 T cells are required, but not always sufficient, to protect the host against liver-stage Plasmodium infection. These data provide fundamentally new insight into protective CD8 T cell responses elicited by RAS-immunization in genetically diverse hosts, information with relevance to developing attenuated whole-parasite vaccines to protect humans.
Results
The CD8αloCD11ahi T cell phenotype specifically identifies RAS vaccine-induced effector and memory CD8 T cells
Relative resistance after RAS-vaccination of both rodents and humans is commonly studied by sporozoite challenge 1–2 weeks following the last immunization [8], [11], [19], [20] and thus evaluates immunity mediated by recently stimulated T cell populations. Herein, we wished to examine RAS-induced protection only after stable memory immune responses have been generated. Thus, we challenged RAS-vaccinated mice >60 days post-immunization, when numerically and phenotypically stable memory CD8 T cell populations are established following acute infections [21]. At this memory time point, a single Pb-RAS vaccination protected 100% of BALB/c mice, but failed to protect any B6 mice against homologous Pb sporozoite challenge, whereas one Py-RAS vaccination had minimal (BALB/c, 10%) or no (B6, 0%) protective efficacy against homologous Py sporozoite challenge (Figure 1A). These data demonstrate both mouse strain and Plasmodium species-dependent protection after single RAS-immunization of mice challenged at a bona fide memory time point.
Fig. 1. Sensitivity and specificity of the CD8αloCD11ahi surrogate activation marker approach to identify RAS vaccination-induced CD8 T cell responses. 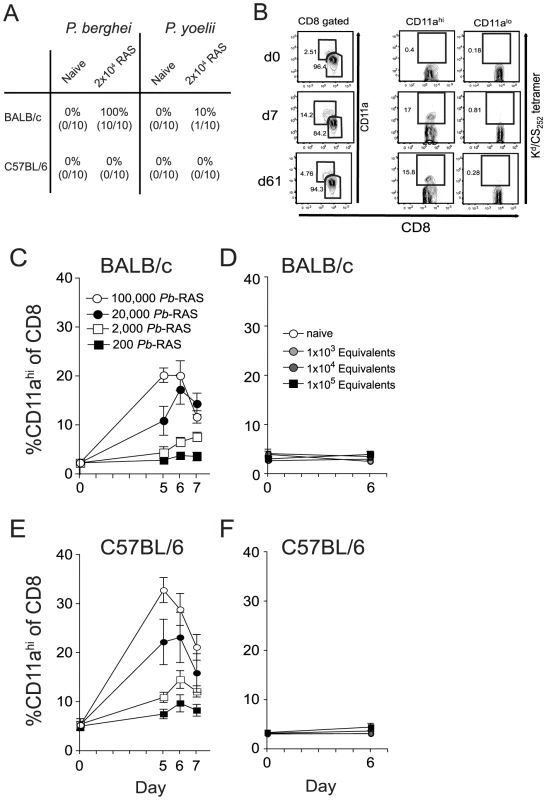
(A) Protection against P. berghei (Pb) or P. yoelii (Py) sporozoite challenge in BALB/c and B6 mice singly vaccinated with either 2×104 Pb- or Py-RAS and challenged with 1000 Pb or Py sporozoites >80 days later. Numbers indicate % protected (no. protected/no. challenged×100). (B) Peripheral blood mononuclear cells from a single BALB/c mouse collected before (day 0) and after 2×104 Pb-RAS vaccination (day 7, 61) were stained for CD8α and CD11a. Left column of dot plots shows the fraction of circulating CD8 T cells exhibiting an antigen-experienced phenotype (CD8αloCD11ahi) at each time point following Pb-RAS vaccination. Right columns of dot plots show the fraction of cells within the CD11ahi and CD11alo gates that stain with Kd/CS252–260 tetramer. (C) BALB/c mice were vaccinated with the indicated number of Pb-RAS and CD8αloCD11ahi responses were tracked in the peripheral blood of individual mice on day 0, 5, 6 and 7. N = 10 mice/dose, except for 1×105 Pb-RAS group (N = 5). Data are the mean ± S.D. (D) Irradiated salivary gland homogenate from non-infected mosquitoes was injected into naïve BALB/c mice i.v. The CD8αloCD11ahi response was evaluated in the peripheral blood of individual mice before (day 0) and after (day 6) injection. ‘Equivalent’ refers to the final dilution of salivary gland homogenate injected into mice within each group. Dilutions were made based on an average recovery of ∼15,000 sporozoites per mosquito, calculated over >15 independent mosquito dissections. Data are mean ± S.D. for 3 mice per group. (E,F) similar to C,D except C57BL/6 mice were analyzed. To examine the protective CD8 T cell response elicited by RAS-vaccination, we applied our recently described surrogate activation marker approach, based on downregulation of CD8α and upregulation of CD11a (CD8αloCD11ahi) [18], to identify RAS-induced CD8 T cells. We chose to focus our initial analyses on peripheral blood (PBL) so that individual mice could be analyzed longitudinally. Importantly, long-term longitudinal analyses of naïve mice in our colony reveal that the circulating CD8αloCD11ahi T cell pool remains low (2–3% of all circulating CD8 T cells) and stable for >250 days (data not shown). For vaccinated mice, the fraction of CD8αloCD11ahi T cells in the PBL was determined prior to, and at various intervals after, immunization with 2×104 Pb-RAS in individual animals. We detected substantial increases in the frequency of CD8αloCD11ahi T cells in the blood of vaccinated mice at 7 and 61 days (effector and memory time points, respectively) post-immunization (Figure 1B, left column as an example). Interestingly, only 16±3% of Pb-RAS-induced effector (day 7) CD8 T cells in BALB/c mice are specific for the known H-2Kd-restricted CS252–260 epitope and, importantly, all of these defined antigen-specific CD8 T cells are found in the CD8αloCD11ahi population (Figure 1B, right columns). Moreover, the fraction of CS252–260-specific CD8 T cells among the CD8αloCD11ahi population consistently remains ∼16% throughout the memory phase of the response (day 61) (Figure 1B, right columns). Based on previous studies showing that T cell responses against diverse epitopes are coordinately regulated [22], these data further support that the surrogate activation marker approach identifies true RAS-induced, Plasmodium-specific CD8 T cells. Thus, ∼85% of Pb-RAS-induced CD8 T cells in BALB/c mice are reactive against epitopes from undefined antigens. Similar results were obtained for the CS280–288 epitope after single immunization of BALB/c mice with Py-RAS, although the fraction of CS280–288-specific memory CD8 T cells in the circulating CD8αloCD11ahi compartment was only ∼7% (data not shown).
To further demonstrate specificity of the surrogate activation marker approach, we determined that the increase over baseline (PBL analyzed before immunization) in the fraction of circulating CD8αloCD11ahi T cells 5–7 days after immunization depended on the immunizing dose of Pb-RAS in both BALB/c and B6 mice (Figure 1C and E) and was not observed in mice immunized with an equivalent suspension of irradiated salivary gland homogenates from non-infected mosquitoes (Figure 1D and F). Thus, the CD8αloCD11ahi T cell response is specific for Plasmodium-antigens and not mosquito salivary gland antigens. Moreover, CD8 T cell responses in the blood of RAS-immune B6 and BALB/c mice were representative of CD8αloCD11ahi responses in the spleen and liver, both in terms of frequency (Figure 2A) and total number (Figure 2B). Finally, we addressed specificity at the memory stage by transferring sort purified CD8αhiCD11alo (naïve) or CD8αloCD11ahi (memory) cells from day 78 RAS-immune B6 mice (CD45.2) into CD45.1 hosts. Only the population of transferred CD8αloCD11ahi T cells underwent secondary expansion after RAS-immunization of the recipient mice (Figure S1). Thus, the CD8αloCD11ahi phenotype cells present at memory time points after RAS-immunization are Plasmodium-specific (Figure S1). As a composite, these data demonstrate that the changes in frequency of circulating CD8αloCD11ahi T cells in individual RAS-immunized mice reflects the distribution of parasite-specific effector and memory CD8 T cells in peripheral tissues and can be used to evaluate the total CD8 T cell response to RAS-immunization prior to sporozoite challenge.
Fig. 2. RAS vaccination of BALB/c and C57BL/6 mice increases the frequency and total number of CD8αloCD11ahi cells in spleen, liver and peripheral blood. 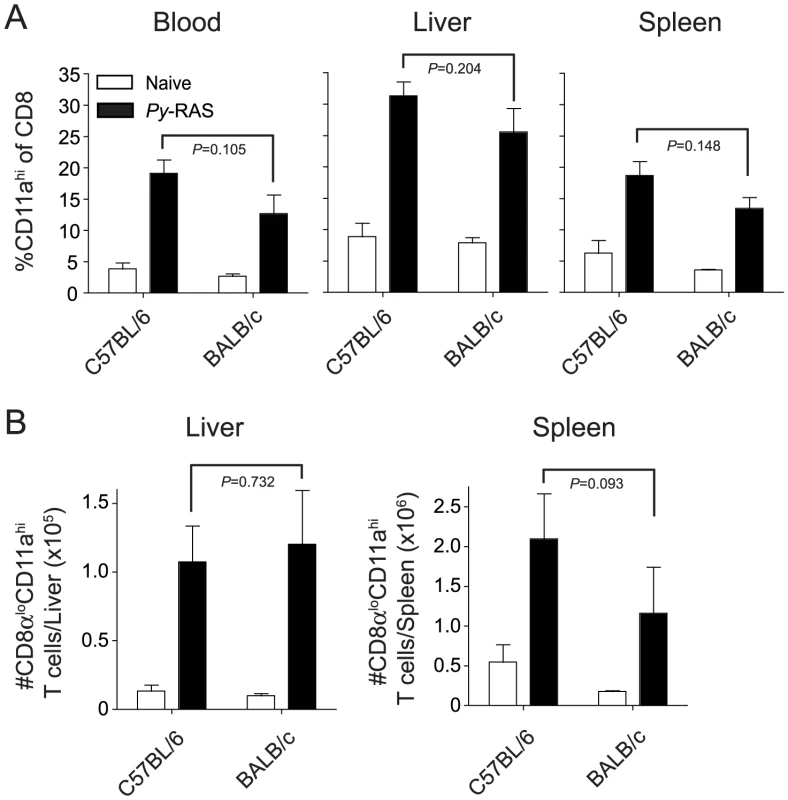
Naïve BALB/c and C57BL/6 mice were vaccinated with 2×104 Py-RAS. Seven days later, mononuclear cells were isolated from the spleen, liver and blood of immune (N = 3/group) and naïve (N = 3) mice and stained for CD8α and CD11a. Data (mean ± S.D.) are expressed as the frequency (A) or total number (B) of CD8 T cells exhibiting the antigen-experienced phenotype (CD8αloCD11ahi) in each tissue. Statistics were determined by unpaired, two-tailed t-tests. Longitudinal analyses of RAS-induced effector and memory CD8 T cell responses
We next examined the magnitude and kinetics of total CD8 T cell responses in the PBL of BALB/c and B6 mice following Pb - or Py-RAS vaccination (Figure 3A and B, respectively), using an immunizing dose of RAS (2×104 sporozoites) that fell within the linear range of the CD8 T cell response in both inbred mouse strains (Figure 1C,E). We observed substantial increases in the frequency of CD8αloCD11ahi T cells in the PBL of all groups, which peaked 6 days after RAS-immunization, followed by contraction and the formation of numerically stable primary (1°) memory populations (Figure 3A,B). Importantly, although B6 mice are more susceptible to sporozoite challenge following a single Pb - or Py-RAS immunization compared to BALB/c mice [11] (Figure 1A), and CD8 T cells are necessary to mediate protection in Pb-RAS immune mice (Figure 3C), Pb - or Py-RAS vaccination of B6 mice induced 1° effector and memory CD8 T cell responses that were ∼2-fold higher (p<0.0001) than observed in BALB/c mice (Figure 3A,B). Thus, our surrogate activation marker approach revealed that the susceptibility of single RAS-immunized B6 mice to homologous Pb or Py challenge is not due to a diminished total anti-Plasmodial CD8 T cell response.
Fig. 3. The magnitude and kinetics of RAS-vaccination-induced CD8 T cell responses in BALB/c and C57BL/6 inbred mice. 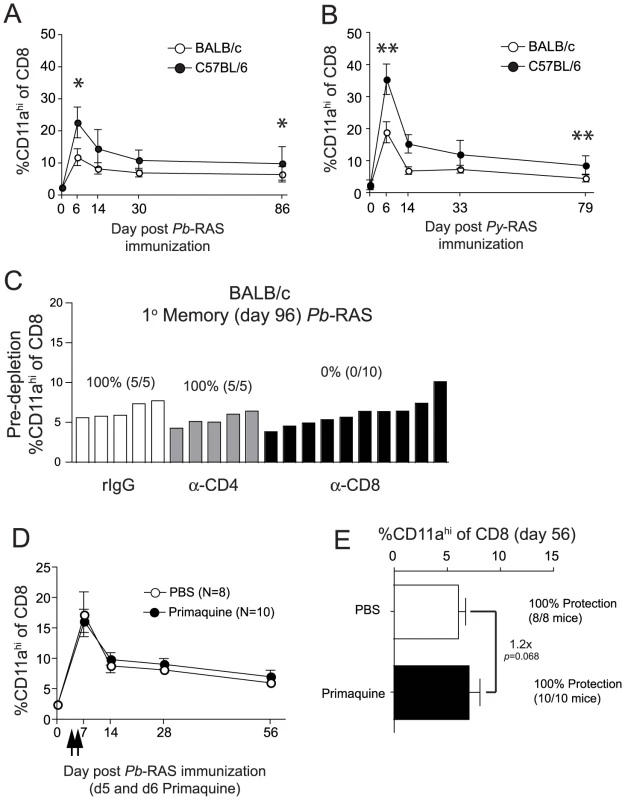
(A and B) Kinetics and magnitude of the total CD8 T cell response in the blood of BALB/c and C57BL/6 mice following 2×104 Pb-RAS (A) or Py-RAS (B) vaccination. Data (mean±S.D.) are from 30 mice/strain. *P<0.0001, **P<0.0001. (C) BALB/c mice (N = 20) were vaccinated with 2×104 Pb-RAS. Ninety-six days later, the frequency of CD8αloCD11ahi T cells in the peripheral blood was evaluated and individual mice were ranked according to the magnitude of the memory CD8 T cell response. Mice were treated with rat IgG, anti-CD4 (GK1.5) or anti-CD8 (2.43) 3 days and 1 day prior to challenge with 1000 Pb sporozoites. Numbers above refer to percent of T cell-depleted mice protected (no. protected/no. challenged ×100) following sporozoite challenge. (D) BALB/c mice were vaccinated with 2×104 Pb-RAS. On days 5 and 6 post-vaccination, mice were injected i.p. with PBS or 60 mg/kg primaquine/PBS solution (arrows). Circulating CD8 T cell responses were evaluated in mice at the indicated time points. (E) Fifty-six days following Pb-RAS vaccination, CD8 T cell responses in the blood were evaluated. Mice were challenged 3 days later (day 59) with 1000 Pb sporozoites. Numbers refer to % protected (no. protected/no. challenged×100). Data in D,E are mean±S.D. from 8–10 mice/group. Statistics in A, B and E were determined by unpaired, two-tailed t-tests. RAS remain infectious to host hepatocytes, but are unable to undergo differentiation into blood stage merozoites [23], [24]. Interestingly, persistence (up to 6 months) of radiation-attenuated parasites was reported in the livers of RAS-vaccinated rats [25] and persistence of attenuated parasites has been hypothesized to underlie the long-term protective capacity of RAS-induced memory CD8 T cells [25], [26]. To address this hypothesis, we treated BALB/c mice with 60 mg/kg primaquine on days 5 and 6 following Pb-RAS-vaccination to eliminate persisting parasites. In contrast to previous studies [25], [26], we found that primaquine treatment did not decrease protection against sporozoite challenge at a memory time point (Figure 3E). Consistent with this result, primaquine treatment at these time points did not reduce RAS-specific circulating CD8 T cell frequencies (Figure 3D,E). In parallel, we verified the efficacy of primaquine (route, dose, schedule) by treating naive BALB/c mice 24 and 48 hrs following challenge with 1000 infectious sporozoites. Primaquine treatment effectively stopped the development of blood stage infection in 100% (5/5) mice, whereas 5/5 vehicle-treated mice developed patent blood stage parasitemia. Thus, following the induction of CD8 T cell responses via Pb-RAS-vaccination of BALB/c mice, the persistence of attenuated parasites in the liver does not regulate the stability or protective capacity of the RAS-induced memory CD8 T cell populations.
Homologous boosting markedly expands RAS-induced memory CD8 T cell populations but fails to confer protective immunity to C57BL/6 mice
Short-interval (every 2–3 weeks) booster RAS-immunizations improve protection against sporozoite challenge of mice [11], [27], [28] although the impact on the Plasmodium-specific CD8 T cell compartment is unknown. Additionally, the impact of long-interval boosting, as generally employed in human vaccines, on RAS-induced protection at a secondary memory time point is unknown. Thus, we examined the effect of homologous RAS-boosting on bona fide memory CD8 T cell populations. Booster immunization at memory time points (60–80 days after initial priming) with Pb-RAS in B6 mice or Py-RAS in BALB/c mice induced secondary expansion of CD8αloCD11ahi T cells (Figure 4A,C). Surprisingly, the peak secondary response did not exceed the magnitude of the peak primary response to initial priming. Still, booster immunization resulted in a doubling of the secondary (2°) memory CD8 T cell populations in both mouse strains (Figure 4B,D). Importantly, we observed 100% protection in Pb-RAS-vaccinated B6 mice and Py-RAS-vaccinated BALB/c mice following sporozoite challenge at 2° memory time points after boosting (days 168 and 154, respectively) (Figure 4B,D), which remained wholly CD8 T cell-dependent (Figure S2). Of note, homologous Pb - or Py-RAS-boosting enriched the fraction of CS252–260 - or CS280–288-specific CD8 T cells (to ∼30% and 15%, respectively) within the CD8αloCD11ahi compartment compared to single immunized BALB/c mice (Figure 1B and Figure S3). Importantly, these secondary CS252–260 - or CS280–288-specific memory CD8 T cells are also found exclusively in the CD8αloCD11ahi compartment (Figure S3). Thus, homologous Pb-RAS or Py-RAS boosting of B6 or BALB/c mice, respectively, doubles the frequency of circulating RAS-specific 2° memory CD8 T cells and affords CD8 T cell-dependent sterilizing immunity against a stringent sporozoite challenge. Moreover, enrichment of the CS-specific responses in RAS-boosted BALB/c mice suggests that although ∼85–95% of the total initial CD8 T cell response targets antigens of undefined specificity, the CS-specific response in BALB/c mice dominates the recall response. These data for the first time reveal the effect of homologous RAS boosting on bona fide memory CD8 T cell responses, and further demonstrate that antigen-specific 2° memory CD8 T cell populations are also accurately identified using the CD8αloCD11ahi surrogate activation marker approach.
Fig. 4. Homologous Pb- or Py-RAS boosting of mice elicits robust memory CD8 T cell responses but does not enhance protection of C57BL/6 mice against Py sporozoite challenge. 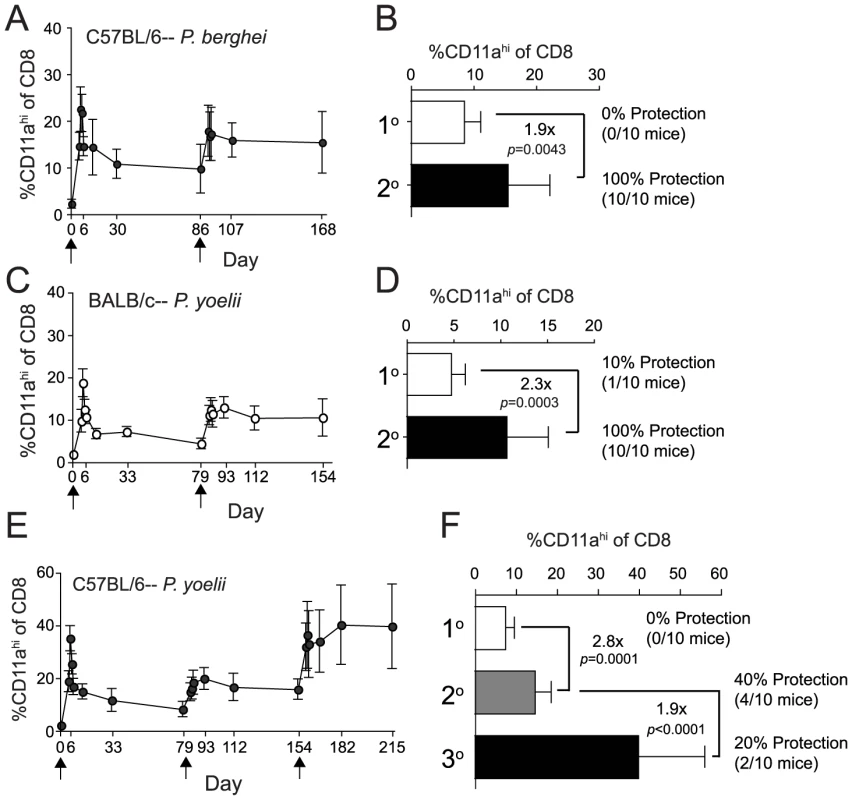
(A) The kinetics and magnitude of the circulating RAS vaccine-induced CD8 T cell response in C57BL/6 mice primed and boosted (arrows) with 2×104 Pb-RAS. (B) Fold increase in secondary memory (d168) CD8 T cell responses in the blood of C57BL/6 mice following homologous Pb-RAS boost. Numbers to the right indicate the fraction of primary (1°) or secondary (2°) memory C57BL/6 mice that were protected following Pb sporozoite challenge (no. protected/no. challenged ×100). (C,D) Similar to A,B, except BALB/c mice were prime-boosted (arrows) with 2×104 Py-RAS and challenged with P. yoelii sportozoites. Data in A–D are mean±S.D. from 20 mice. (E,F) Similar to C,D, except C57BL/6 mice were primed and boosted twice (arrows) with 2×104 Py-RAS. Data in E,F are mean±S.D. from 30 mice. Statistics were determined by unpaired, two-tailed t-tests. For B,D and F, one hundred percent (10/10) of strain- and age-matched, naïve mice challenged in parallel were parasitized. Consistent with the results described above, homologous boosting of Py-RAS immune B6 mice also doubled (on average) the frequency of RAS-induced 2° memory CD8 T cells (Figure 4E,F). However, these mice exhibited only modest (40%) protection against sporozoite challenge at a 2° memory time point (day 154) (Figure 4F). Interestingly, a second booster immunization with Py-RAS resulted in a sustained increase in the frequency of CD8αloCD11ahi T cells, which now represented on average ∼40% of the CD8 T cell compartment of the PBL at day 215 (Figure 4E,F). However, even this extreme commitment of Py-RAS-induced tertiary (3°) memory CD8 T cells did not improve protection when these mice were challenged at a 3° memory time point (day 215) (Figure 4F). Thus, we could not achieve substantial levels (>70–80%) of protection against Py sporozoite challenge in B6 mice boosted every 60–70 days and challenged 60 days after the last boost. This contrasts sharply with reports that examine protective immunity following short interval boosting (every 2–3 weeks) followed by challenge ∼14 days after the last boost [11], [29]. One clear difference between these two immunization regimens is the substantial role for CD4 T cells in protection after short-interval boost and challenge approaches in B6 mice [11], [29], whereas we could detect no role for CD4 T cells in protection against Pb sporozoite challenge of B6 mice, or against Py challenge of BALB/c mice in our long-interval prime-boost approach (Figure S2). These disparate results strongly suggest that the timing of RAS-immunization and sporozoite challenge significantly influences both the composition and protective capacity of the RAS-induced cellular response. Indeed, we are currently evaluating quantitative and qualitative characteristics of the total CD8 T cell response and protection following short-interval, prime-boost RAS vaccination and challenge, as well as evaluating surrogate activation marker approaches to specifically identify antigen-experienced CD4 T cells.
Longitudinal analyses of RAS vaccine-induced CD8 T cell responses within individuals of an outbred population
We show that BALB/c and B6 mice fall on opposite ends of the spectrum regarding their ability to resist sporozoite challenges at memory time points following either Pb - or Py-RAS long-interval prime-boost vaccination. Indeed, many studies [14], [30], [31], [32], [33], [34], [35], [36], [37] employ BALB/c mice to evaluate whole-attenuated parasite vaccine-induced protective immunity, and it is unclear how these data model CD8 T cell responsiveness and protective immunity in outbred populations, such as humans, following RAS-vaccination. Thus, we next turned our attention toward analyses of the CD8 T cell response in outbred Swiss Webster mice. Due to the lack of information on MHC alleles and antigens in outbred populations, this analysis was only made possible through development of the CD8αloCD11ahi surrogate activation marker approach [18]. On the population level (N = 30 mice), the kinetics and magnitude of Py-RAS-induced CD8 T cell responses of outbred mice mirrored those observed in inbred mice (Figure 5A). However, and in striking contrast to the inbred mice, the initial CD8 T cell response following Py-RAS-vaccination in outbred mice was not uniform and varied widely, both in magnitude and day of the peak (Figure 5C–F). Consistent with this, outbred mice also exhibited more variability in the magnitude of the 1° memory (Figure 5G) and 2° memory (Figure 5H) CD8 T cell response, compared to inbred mice. Similar to what was observed in both BALB/c and B6 mice singly vaccinated with Py-RAS, Swiss Webster mice challenged with Py sporozoites at a 1° memory time point (day 79) were not efficiently protected (Figure 5B). However, boosting Swiss Webster mice with Py-RAS resulted in a doubling (on average) of sporozoite-specific 2° memory CD8 T cells, and 80% of these mice were protected against a sporozoite challenge on day 154 (Figure 5B). Thus, CD8αloCD11ahi surrogate markers can be used to identify and longitudinally track protective CD8 T cell responses in outbred mice following RAS prime-boost vaccination. In addition, these data show that despite the variability in magnitude of initial RAS-induced CD8 T cell response of outbred hosts, homologous boosting increases the secondary memory CD8 T cell population and protective immunity against sporozoite challenge.
Fig. 5. Py-RAS-specific CD8 T cell responses and protective immunity are markedly enhanced following prime-boost vaccination of an outbred mouse population. 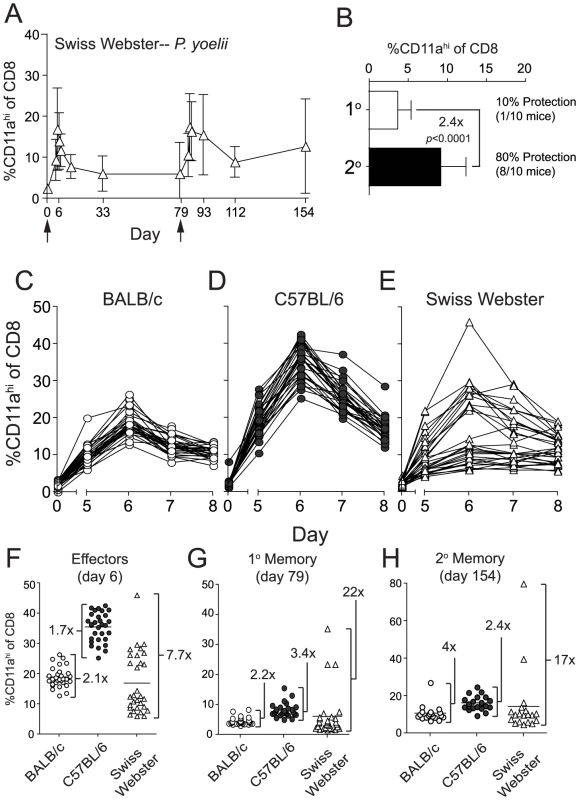
(A) The kinetics and magnitude of CD8 T cell responses in the blood of individual Swiss Webster mice following priming and boosting (arrows) with 2×104 Py-RAS. (B) Fold expansion of Py-RAS-induced primary (1°, d79) and secondary (2°, d154) memory CD8 T cell responses in the blood of Swiss Webster mice. Numbers to the right indicate the fraction of 1° or 2° memory Swiss Webster mice that were protected following Py sporozoite challenge (no. protected/no. challenged×100). One hundred percent (10/10) of age-matched, naïve Swiss Webster mice challenged in parallel were parasitized. Data in B are mean±S.D. from 20 mice. Statistics were determined by unpaired, two-tailed t-tests. BALB/c (C), C57BL/6 (D) and Swiss Webster mice (E) (N = 30 each) were vaccinated with 2×104 Py-RAS. Peripheral blood was collected before (day 0) and on days 5–8 following vaccination. CD8αloCD11ahi T cell responses were measured in individual BALB/c, C57BL/6 and Swiss Webster mice at the initial effector stage (F), primary memory (G) or secondary memory (H) following priming and homologous boost with 2×104 Py-RAS as in A. Each symbol represents an individual mouse. Numbers in F-H refer to the fold difference between the highest and lowest responders within each mouse strain at each time point. An effector memory (TEM) CD8 T cell phenotype, but not anti-sporozoite antibody titers, correlates with protective immunity following Py-RAS vaccination of inbred and outbred mice
While protection of RAS-vaccinated mice using the long-interval (>60 days), prime-boost scenario described above is CD8 T cell dependent (Figure S2), RAS-immunization also elicits a strong sporozoite-specific antibody response [29], [38]. To determine whether differences in the Py-RAS-induced sporozoite-specific antibody response correlated with relative resistance or susceptibility to Py sporozoite challenge, we analyzed serum from individual BALB/c, B6 and Swiss Webster mice for sporozoite-specific IgG titers at each memory time-point. Importantly, an examination of anti-sporozoite titers at the secondary memory time point (day 154), where significant protection was achieved in BALB/c and Swiss Webster but not B6 mice (Figures 4D and 5B, respectively), revealed no clear correlation between IgG titers and protection from sporozoite challenge (Figure S4A). Moreover, antibody titer was not significantly different between 2° memory BALB/c mice (100% protected) and 3° memory B6 mice (20% protected) (P = 0.0789, Figure S4B), or between individual protected and non-protected B6 mice (P = 0.4484, Figure S4C). Thus, reduced anti-sporozoite IgG antibody titers do not appear to explain the enhanced susceptibility of RAS-vaccinated B6 mice to sporozoite challenge.
We next examined potential qualitative differences in phenotype and specific functional attributes of protective and non-protective memory CD8 T cells. The nature of our longitudinal analyses precluded the collection of large quantities of blood from individual immunized mice. Thus, the small clinical sample limited our initial analyses to a key subset of markers that distinguish “central memory” (TCM; CD62Lhi, CD27hi) from “effector memory” (TEM; CD62Llo, CD27lo) CD8 T cell populations [39], [40], and a marker associated with memory CD8 T cell survival (IL7 receptor α chain, CD127) [41]. At 60 days post-immunization most RAS-induced memory CD8 T cells in each mouse strain, vaccinated with either Pb - or Py-RAS, expressed a TEM phenotype (CD62Llo, CD27lo, CD127lo, data not shown). However, to more directly address potential relationships between RAS-induced memory CD8 T cell phenotype and protection we evaluated expression of the same markers after Py-RAS boosting of BALB/c and Swiss Webster mice (both protected) and B6 mice (not protected). The most striking difference between non-protective 2° memory CD8 T cells in B6 mice and protective memory CD8 T cells in BALB/c and Swiss Webster mice was the differential expression of CD62L (Figure 6A). Forty percent (on average) of Py-RAS-induced 2° memory cells in B6 mice expressed the CD62Lhi TCM phenotype (Figure 6A). In contrast, representation of the CD62Lhi TCM phenotype among Py-RAS-induced 2° memory cells in BALB/c and Swiss Webster mice was reduced 3-fold or 4-fold, respectively (Figure 6A). A similar trend was observed for the CD27hi phenotype (Figure 6B), while no correlation between CD127 expression and protective capacity was observed (Figure 6C). Thus, non-protective 2° memory CD8 T cells in Py-RAS boosted B6 mice exhibit a more TCM phenotype, expressing significantly higher levels of CD62L and CD27 relative to protective 2° memory CD8 T cells in BALB/c or Swiss Webster mice (Figure 6A,B).
Fig. 6. Protection correlates with an effector memory (TEM) phenotype on circulating Py-RAS-induced secondary memory CD8 T cells in inbred and outbred mice. 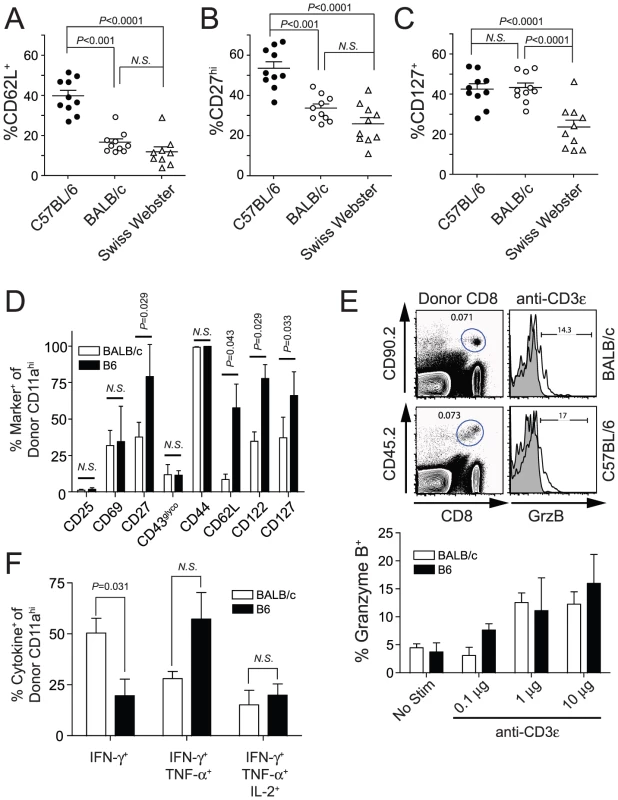
(A–C) C57BL/6, BALB/c and Swiss Webster mice (N = 10/strain) were vaccinated with 2×104 Py-RAS. Seventy-nine days later, mice received a homologous boost of 2×104 Py-RAS. Seventy-five days after boost (day 154), circulating T cells were stained for CD8α, CD11a, CD62L, CD27 and CD127. (D–F) Py-RAS-specific, secondary memory BALB/c and C57BL/6 CD8 T cells were generated following adoptive transfer as described in Materials and Methods. (D) Donor-derived cells were analyzed directly ex vivo for surface expression of the indicated activation- or survival-associated marker. (E) Cells were stimulated ex vivo for 5 hrs using titrations of plate-bound anti-CD3ε and subsequently analyzed for the intracellular expression of granzyme B. Representative dot plots and histograms (top) and summary graph (bottom) are shown. (F) Cells were stimulated with 1µg/mL plate-bound anti-CD3ε for 5.5 hrs prior to staining for intracellular co-expression of IFN-γ, TNF-α and IL-2. In A–D, data represent the fraction of CD8αloCD11ahi T cells within each group expressing the indicated marker. Statistics were determined by unpaired, two-tailed t-tests. Data in D–F are mean±S.D. and represent analyses from 5 individual BALB/c and B6 recipient mice. As a complimentary approach, we next performed a series of adoptive transfer studies in order to more clearly identify and directly compare RAS-induced 2° memory CD8 T cells in BALB/c and B6 mice. We transferred 8×104 Py-RAS-induced, CD8αloCD11ahi 1° memory (d78) CD8 T cells into allelically disparate BALB/c and B6 recipients, which were subsequently immunized with Py-RAS to generate populations of endogenous 1° memory and allelically marked 2° memory parasite-specific CD8 T cells. One month after the booster immunization, we performed extensive phenotypic and functional analyses of the donor-derived, 2° memory CD8 T cells in each B6 and BALB/c recipient mouse. Surface expression of many markers, such as CD25, CD69, CD43glyco (Figure 6D and Figure S5) were indistinguishable between these populations. In addition, we found no statistically significant differences in expression of integrins (β1, β2, β7, αM, αX, αE, α4, α5 or α6) or inhibitory receptors (PD-1, LAG-3, 2B4, CD160, KLRG-1 or CTLA-4) on RAS-induced, 2° memory CD8 T cells in B6 and BALB/c mice (data not shown). However, in line with our initial observations, RAS-induced, non-protective 2° memory CD8 T cells in B6 mice exhibit a more TCM-like phenotype, relative to BALB/c mice, characterized by relatively higher proportions of CD27 and CD62L expressing cells (Figure 6D and Figure S5). We also observed significantly higher CD122 and CD127 expression on RAS-induced, 2° memory CD8 T cells in B6 mice (Figure 6D and Figure S5). Collectively, our phenotypic analyses support the notion that a TEM phenotype among RAS-induced, parasite-specific CD8 T cells strongly correlates with protection against liver stage Plasmodium infection.
To examine specific functional attributes of RAS-induced memory CD8 T cells in BALB/c and B6 mice we relied on polyclonal TCR cross-linking to trigger the ex vivo induction of Granzyme B and inflammatory cytokine expression by allelically marked, 2° memory CD8 T cells. We found that similar fractions of BALB/c and B6 2° memory CD8 T cells expressed Granzyme B in response to dose-titrations of plate-bound anti-CD3ε (Figure 6E). Moreover, we observed equivalent IFN-γ production (% positive and MFI) by RAS-induced CD8αloCD11ahi 2° memory CD8 T cells in BALB/c and B6 mice (data not shown). Interestingly, however, a significantly higher fraction of B6 2° memory CD8 T cells co-expressed TNF-α and IFN-γ relative to BALB/c 2° memory CD8 T cells, the majority of which expressed IFN-γ alone (Figure 6G). Collectively, these data show that a TEM phenotype, but neither Granzyme B nor polyfunctional cytokine expression, correlates with protective anti-Plasmodial liver stage immunity mediated by RAS-induced memory CD8 T cells.
Extreme numerical requirements for CD8 T cell-mediated protective immunity to sporozoite challenge
We previously reported that the numerical threshold for protection of BALB/c mice against Pb-sporozoite challenge mediated solely by memory CS252–260-specific CD8 T cells is exceedingly high (>1% of PBL (refs [16], [42]), or >8% of CD8 T cells, Figure 7). One explanation for the enormously high threshold for sterilizing protection in that scenario is that protective memory CD8 T cells recognize only a single antigenic determinant from P. berghei. On the other hand, RAS-vaccination has been shown to elicit protective CD8 T cells targeting non-CS antigens [14], [15] and our data are consistent with the majority of RAS-induced CD8 T cells targeting non-CS antigens (Figure 1B and Figure S3). Thus, broadening the number of antigens (i.e. additional parasite-derived proteins that may be more efficiently processed or presented compared to CS) through whole parasite RAS-vaccination may lower the numerical requirements for protective immunity mediated by memory CD8 T cells. However, when we tabulated memory CD8 T cell responses in groups of RAS-vaccinated inbred and outbred mice that resisted sporozoite challenge, we identified similarly extreme numerical relationships between circulating memory CD8 T cell responses and protective anti-sporozoite immunity (Table 1). This is most evident for RAS-induced protective immunity against P. yoelii, which replicates faster in the liver [43] and exhibits a lower ID50 [32] compared to P. berghei, and thus, may better mimic the virulence of P. falciparum in humans. For example, anti-Py memory CD8 T cell responses representing ∼9% of CD8 T cells in BALB/c mice and ∼19% of CD8 T cells in Swiss Webster mice confer protection against a Py-sporozoite challenge, and responses exceeding 40% of CD8 T cells failed to efficiently protect B6 mice. Thus, the numerical requirements for sterilizing immunity following Plasmodium RAS-vaccination are extraordinarily high, regardless of whether the protective pool of memory CD8 T cells react to a single antigenic determinant after subunit vaccination, or whether the CD8 T cell response is directed against a broader set of antigenic determinants after whole-parasite immunization.
Fig. 7. Sterilizing anti-P. berghei sporozoite immunity in BALB/c mice is associated with memory CD8 T cell responses of single antigenic-specificity that exceed 8% of all circulating CD8 T cells. 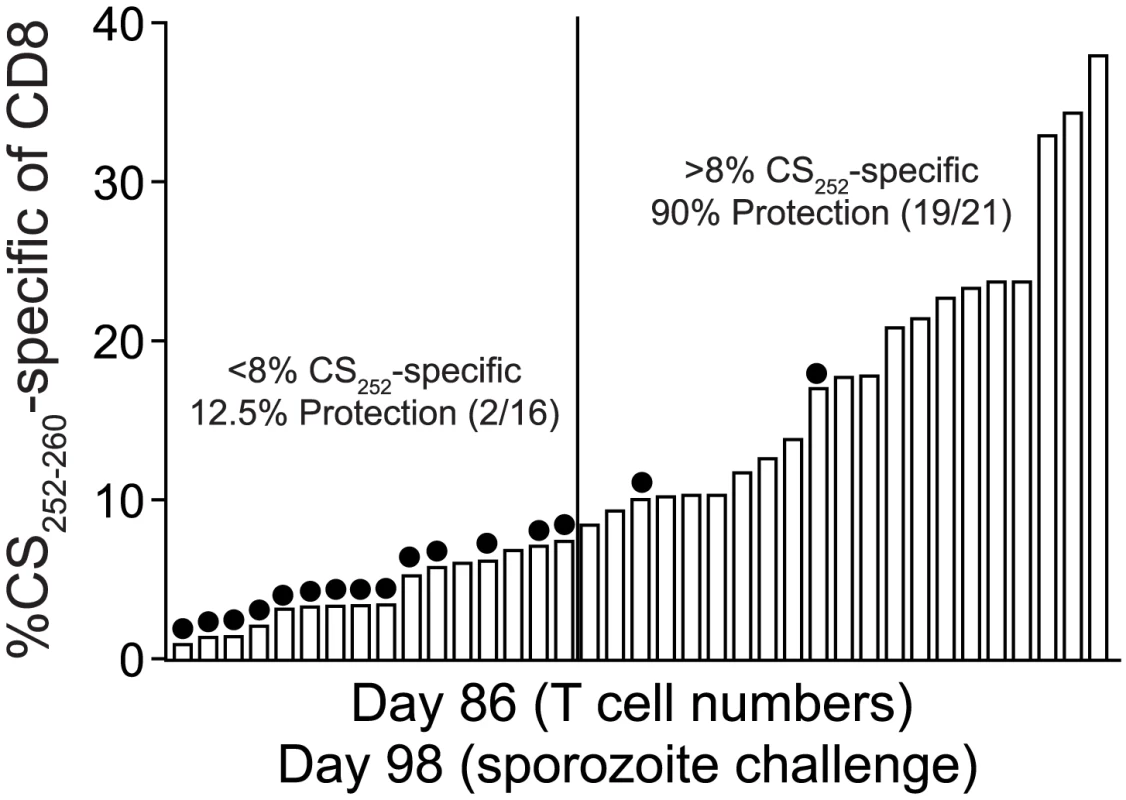
BALB/c mice were DC-CS252–260 prime, LM-CS252–260 boost immunized as previously described [16]. Eighty-six days following immunization, peripheral blood from individual mice was assayed for the frequency of CS252–260-specific memory CD8 T cells using intracellular IFN-γ cytokine staining. Mice are ranked according to the magnitude of the CS252–260-specific memory CD8 T cell response. Three days after T cell analyses, mice were challenged with 1000 Pb sporozoites and blood stage parasitemia was evaluated in individual mice with Giemsa stain. Non-protected mice are indicated with filled circles. Numbers above the graph refer to percent protection among mice scoring above or below the 8% circulating CS252–260-specific memory CD8 T cell threshold, which is based on the clearest visual break-point between non-protected and protected mice each ranked according to the magnitude of the CD8 T cell response. Ninety-one percent (20/22) of age-matched, naïve BALB/c mice challenged in parallel were parasitized. Tab. 1. Extreme commitment of the CD8 T cell compartment is associated with protection against Plasmodium sporozoite challenge. 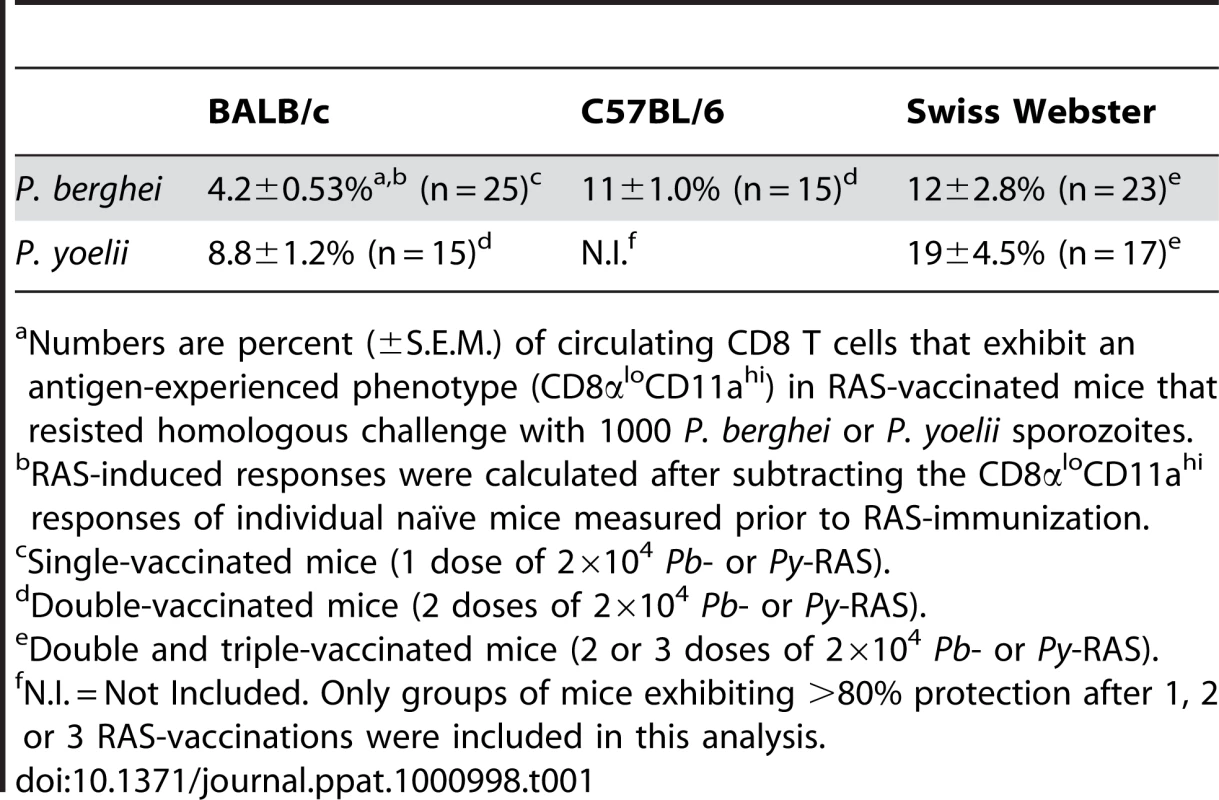
Numbers are percent (±S.E.M.) of circulating CD8 T cells that exhibit an antigen-experienced phenotype (CD8αloCD11ahi) in RAS-vaccinated mice that resisted homologous challenge with 1000 P. berghei or P. yoelii sporozoites. Discussion
Although a critical protective role for CD8 T cells in RAS-immune mice was established more than 25 years ago, the characteristics of the protective CD8 T cell response remained essentially undefined due to the lack of defined Plasmodium epitopes. Here, we used surrogate activation markers to identify and longitudinally track RAS-induced CD8 T cell populations in the blood of individual hosts. This approach enabled us to describe specific quantitative and qualitative characteristics of memory CD8 T cell populations that mediate protection against sporozoite challenge. Moreover, the surrogate activation marker approach allowed us to monitor RAS vaccine-induced CD8 T cell responses in individuals within outbred populations of mice, without a priori knowledge of MHC alleles or parasite-specific antigenic determinants. These latter analyses revealed that, despite variability to the initial immunization, prime-boost RAS-vaccination effectively enhances parasite-specific memory CD8 T cell responses and affords sterilizing protective immunity among individuals of an outbred population. Collectively our studies show that independent of genetic background, extremely high frequencies of RAS-induced memory CD8 T cells are required, but may not always suffice for sterilizing anti-Plasmodial immunity, information directly relevant to ongoing efforts to translate attenuated whole-malaria parasite vaccines to humans.
We previously reported that an extraordinarily large frequency of circulating CS-specific memory CD8 T cells generated by subunit vaccination is required to protect BALB/c mice against a stringent Pb sporozoite challenge [16]. In that study, memory CD8 T cell populations were generated such that they only targeted a single antigenic determinant derived from the parasite CS protein, CS252–260. Herein we report the striking observation that diversifying the targets of the CD8 T cell response through whole-attenuated-parasite vaccination only minimally (if at all) reduces the numerical requirements for memory CD8 T cell-mediated protective immunity. For example, we show that following Pb-RAS vaccination of BALB/c mice (the scenario in which protection is easiest to achieve) resistance to sporozoite challenge at a memory time point is associated with ≥4% of the CD8 T cell compartment exhibiting the antigen-experienced phenotype (CD8αloCD11ahi). Thus, the magnitude of the RAS-induced, poly-specific memory CD8 T cell response associated with protection against P. berghei challenge is only ∼2-fold lower than the mono - (CS252–260)-specific memory CD8 T cell response (∼8% of the CD8 T cell compartment). Additionally, protection against Pb is associated with even larger memory CD8 T cell responses in B6 and outbred Swiss Webster mice (11% and 12%, respectively). Further, resistance to P. yoelii has even more extreme requirements, with protection associated with RAS-induced memory CD8 T cell responses exceeding ∼9 or ∼19% of the CD8 T cell compartment in BALB/c and Swiss Webster mice, respectively. Thus, our data demonstrate that poly-specific memory CD8 T cell-mediated sterilizing immunity to sporozoite challenge, regardless of the relative virulence of the Plasmodium species, requires commitment of a substantial fraction of the entire CD8 T cell compartment.
This extreme numerical requirement is perhaps not surprising given the extraordinarily low ratio of Plasmodium-infected cells to total hepatocytes in the mammalian host following challenge with physiological numbers of sporozoites (∼1000). Indeed, the gold standard readout for protection against sporozoite challenge is sterilizing immunity, or the prevention of blood stage infection. From a conservative perspective, this level of protection requires that each of a maximum of 1000 infected hepatocytes (among >108 or >1011 total hepatocytes in the mouse or human liver, respectively) be targeted through direct CTL activity or indirectly via the release of cytokines by parasite-specific memory CD8 T cells in order to prevent the development of blood stage infection. Thus, each RAS-induced memory CD8 T cell must surveil an extremely large number of hepatocytes in order to identify all cells that harbor parasites. The exceedingly low number of infected cells among the whole liver (needle in the haystack [16]), coupled with the fact that every single infected cell must be successfully targeted to prevent blood stage infection, is the likely explanation for why the numerical requirements for memory CD8 T cell-mediated, anti-Plasmodial liver stage immunity are so high.
While it is unclear why commitment of nearly 40% of the CD8 T cell compartment to the anti-Plasmodial memory CD8 T cell response is insufficient to effectively protect B6 mice, our studies extend the literature [11], [20] by showing that host genetics play a significant role in determining the outcome of sporozoite challenge following RAS-vaccination at bona fide memory time points. We were unable to detect CS-specific CD8 T cell responses in RAS-vaccinated B6 mice (data not shown), which could account for reduced protection. However, experiments have consistently shown that RAS-immune B10.D2 mice are equally as difficult to protect as B6 and B10 mice [11], [20]. Importantly, B10.D2 mice express the same MHC genes as BALB/c mice and thus are able to mount CD8 T cell responses against the defined CS epitopes. Thus, vaccine-induced CD8 T cell responses against the defined immunodominant CS determinant are not sufficient for protection, underscoring the role of non-MHC-linked genes in regulating RAS-induced, anti-liver stage immunity.
Another hypothesis to explain the dramatic susceptibility of hyper-Py-RAS-immune B6 mice is that critical phenotypic or functional attributes of the memory CD8 T cell response, that differ in B6 and BALB/c, regulate protective liver stage immunity. Although we found no differences in granzyme B, IFN-γ, TNF-α or IL-2 production by BALB/c - compared to B6-derived, RAS-induced memory CD8 T cells we did observe differential expression of key molecules that differentiate TCM from TEM populations. RAS-induced memory CD8 T cell populations in B6 mice consistently exhibited elevated proportions of CD62LhiCD27hi populations (TCM phenotype) compared to the predominantly TEM populations found in BALB/c and Swiss Webster mice. These data demonstrate a clear correlation between the expression of the TEM (CD27loCD62Llo) phenotype of secondary memory CD8 T cells and the ability of RAS-immune BALB/c and Swiss Webster mice to resist sporozoite challenge. The reason(s) for the difference in memory phenotype between RAS-immune B6 and BALB/c or/Swiss Webster mice are unknown. Interestingly, a recent report using a sensitive in vivo assay suggests that CS antigen persists for long periods of time after RAS immunization [44]. These studies were carried out only in BALB/c mice due to reagent availability. Given the potential of prolonged antigen encounter to influence memory T cell phenotype, it will be of interest to determine if antigen fails to persist in B6 mice and accounts for the altered T cell phenotype and reduced protection after RAS-immunization. Finally, it should be noted that, due to the enormous numbers of memory CD8 T cells required for sterilizing immunity, adoptive transfer studies to compare the per cell protective capacity of CD8αloCD11ahi memory populations from RAS-immune BALB/c and B6 have not succeeded Although it may be possible to measure reductions in parasite liver burden in CD8 T cell-recipient mice using quantitative PCR, this generally requires challenging mice with supraphysiological doses of sporozoites, a scenario that we wished to avoid. In addition, our interests are focused on the properties of memory CD8 T cells that result in sterilizing immunity and it is currently unclear how reduction in parasite burden after high dose challenge models complete elimination of infected hepatocytes. Clearly many other characteristics of the RAS-induced memory CD8 T cell response may contribute to protective immunity and warrant further investigation. Importantly, our surrogate activation marker approach should allow for detailed, prospective characterization of RAS-induced memory CD8 T cell responses in individual hosts, so that potential links between specific memory CD8 T cell attributes and protection can be evaluated. The identification of additional factors that correlate with or determine CD8 T cell-mediated protective immunity following RAS-vaccination should provide key insight into the pathways of protective CD8 T cell-mediated immunity elicited through whole-parasite vaccination.
Our data highlight the utility of the surrogate activation marker approach for understanding Plasmodium-induced CD8 T cell responses in genetically diverse populations by providing a framework with which the field can begin to address several additional critical knowledge gaps. First, use of outbred rodents to evaluate vaccine-induced responsiveness and protective immunity is much more likely to mimic responses in genetically diverse humans or non-human primates. Identifying and characterizing individual-to-individual variability in response to vaccination should provide additional critical information that will complement data obtained through studying the highly overlapping responses in genetically identical, inbred rodent populations. Second, our studies provide a framework with which to optimize whole parasite immunization. Identifying ways to enhance potentially suboptimal delivery routes, vaccine doses or schedules, based on a quantitative and qualitative assessment of the total CD8 T cell response, will significantly improve efforts to optimize RAS-vaccination, or any other candidate vaccine delivery approaches. Lastly, the surrogate activation marker approach now permits direct comparisons between RAS and the genetically attenuated parasite (GAP) vaccines. Recent work has shown that such genetically attenuated Plasmodium parasites, harboring defined mutations in one or more key genes required for full liver stage differentiation, afford CD8 T cell-dependent protective immunity in rodents [33], [45]. Moreover, it has been shown that the targeted gene(s) precisely control the point during liver stage development that the GAP arrests [31], [45], [46], [47]. Whether early arresting or late arresting GAP-vaccine candidates differentially impact the protective characteristics of the CD8 T cell response is unknown. Given the potential safety advantage of GAP vaccination, these will be critically important questions that can now be directly addressed using surrogate activation markers to identify vaccination-induced effector and memory CD8 T cell responses.
Materials and Methods
Ethics statement
All animal studies and procedures were approved by the University of Iowa Animal Care and Use Committee, under PHS assurance, Office of Laboratory Animal Welfare guidelines.
Mice
Specific pathogen-free BALB/c, C57BL/6, and Swiss Webster mice were purchased from the National Cancer Institute (NCI) and housed at the University of Iowa animal care unit at the appropriate biosafety level.
Parasites
Female Anopheles stephensi mosquitoes infected with either P. berghei (NK65) or P. yoelii (17XNL) were purchased from the New York University insectary.
Immunizations
P. berghei and P. yoelii sporozoites were isolated from the salivary glands of infected A. stephensi mosquitoes. Sporozoites were attenuated by exposure to 200 Gy (20,000 rads). Mice were immunized with 200 to 100,000 RAS i.v. Boosted mice received 20,000 RAS no less than 60 days apart. In some experiments mice were injected with 60 mg/kg primaquine (Sigma-Aldrich, St. Louis, MO) i.p. on days 5 and 6 following RAS immunization. Subunit immunizations were performed as previously described [16]. Briefly, BALB/c mice were primed via tail vein injection of 1×106 splenic dendritic cells coated with peptides corresponding to CS252–260 of P. berghei (DC-CS252–260). Seven days later, mice were boosted with 2×107 CFU of recombinant Listeria monocytogenes expressing the CS252–260 determinant as a secreted minigene (LM-CS252–260).
Sporozoite challenges
P. berghei and P. yoelii sporozoites were isolated from the salivary glands of infected A. stephensi mosquitoes. Immunized and naïve age-matched mice were challenged with 1000 sporozoites i.v. Thin blood smears were performed 10 days after sporozoite challenge. Parasitized red blood cells were identified by Giemsa stain and oil-immersion (1000×) light microscopy. Protection is defined as the absence of blood stage parasites. At least 10 fields (∼10–15,000 red blood cells) were examined for each mouse designated as protected. Protected mice were subsequently rechallenged following T cell depletion to verify that protection was CD8 T cell-dependent.
Quantification and characterization of antigen-specific CD8 T cells
RAS vaccine-induced CD8 T cell populations were identified by staining spleen or liver single cell suspensions, or peripheral blood following lysis of red blood cells, with anti-CD8α clone 53–6.7 (eBioscence, San Diego, CA) and anti-CD11a (LFA-1α) clone M17/4 (eBioscience) antibodies. Sporozoite-specific CD8 T cells were phenotyped by staining cells with anti-CD27 clone LG.7F9, anti-CD43 clone 1B11, anti CD62L clone MEL-14, anti-CD127 clone A7R34, anti-CD25 clone PC61, anti-CD69 clone H1.2F3, anti-CD44 clone IM7, anti-CD122 clone 5H4 antibodies, all from eBioscience. In some experiments, 8×104 Py-RAS-induced primary memory cells from CD90.2+ BALB/c or CD45.2+ B6 mice were adoptively transferred to naïve, congenic (CD90.1+ or CD45.1+) recipients. One day following transfer, recipients were boosted with 1×105 Py-RAS. Thirty-three days later, splenocytes were stimulated ex vivo in anti-CD3ε-coated wells. BALB/c and B6 donor cells were identified by CD11ahiCD8αloCD90.2+ or CD11ahiCD8αloCD45.2+ surface staining and further characterized by intracellular staining for IFN-γ, TNF-α and IL-2, or Granzyme B. CS252–260 - and CS280–288-specific CD8 T cells were identified by incubating peripheral blood leukocytes with Kd/CS252–260-APC labeled tetramers or Kd/CS280–288-APC labeled tetramers, respectively. Cells were then stained with anti-CD8α, anti-CD90.2 and anti-CD11a. Following subunit immunization, the frequency of circulating CS252–260-specific CD8 T cells was determined by ex vivo intracellular cytokine staining for IFN-γ following a 5.5 hour incubation with brefeldin A in the presence or absence of CS252–260 peptide-coated P815 cells were used as antigen presenting cells. Cells were analyzed using a BD FACSCanto and data was analyzed using FLOWJO Software (Tree Star, Inc, Ashland, OR). All animals were pre-bled prior to RAS vaccination to establish individual background circulating CD8αloCD11ahi T cell frequencies.
T cell depletions
Immunized mice were injected with 0.4 mg i.p. rat IgG, anti-CD4 (clone GK1.5), or anti-CD8 (clone 2.43) antibodies on day −3 and day −1 prior to challenge with sporozoites. Depletion was verified by analyzing CD4 (clone RM4-5) and CD8 (clone 53-6.7) T cell populations in the blood of individual mice prior to challenge. In each case, the relevant population represented <0.5% of the PBL.
Sporozoite-specific antibody titer
The serum sporozoite-specific antibody titer from immunized mice was determined by the indirect fluorescent antibody test (IFAT). Sporozoites were air dried on a multiwell microscope slide (Cel-Line Thermo Scientific) and blocked with 1% BSA/PBS. Sporozoite-specific IgG antibodies were detected by incubating with Cy3-conjugated goat anti-mouse IgG (Jackson Immunoresearch Laboratories). Titers are expressed as the inverse of the lowest dilution of serum that retained immunoreactivity against air-dried sporozoites.
Supporting Information
Zdroje
1. BryceJ
Boschi-PintoC
ShibuyaK
BlackRE
2005
WHO estimates of the causes of death in children.
Lancet
365
1147
1152
2. HaySI
SnowRW
2006
The Malaria Atlas Project: Developing Global Maps of Malaria Risk.
PLoS Med
3
e473
3. SachsJ
MalaneyP
2002
The economic and social burden of malaria.
Nature
415
680
685
4. SnowRW
GuerraCA
NoorAM
MyintHY
HaySI
2005
The global distribution of clinical episodes of Plasmodium falciparum malaria.
Nature
434
214
217
5. GuerraCA
SnowRW
HaySI
2006
Mapping the global extent of malaria in 2005.
Trends Parasitol
22
353
358
6. TodrykSM
HillAV
2007
Malaria vaccines: the stage we are at.
Nat Rev Microbiol
5
487
489
7. ClydeDF
1975
Immunization of man against falciparum and vivax malaria by use of attenuated sporozoites.
Am J Trop Med Hyg
24
397
401
8. HoffmanSL
GohLM
LukeTC
SchneiderI
LeTP
2002
Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites.
J Infect Dis
185
1155
1164
9. NussenzweigRS
VanderbergJ
MostH
OrtonC
1967
Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei.
Nature
216
160
162
10. ButlerD
2009
Initiative targets malaria eradication.
Nature
462
19
11. DoolanDL
HoffmanSL
2000
The complexity of protective immunity against liver-stage malaria.
J Immunol
165
1453
1462
12. WeissWR
BerzofskyJA
HoughtenRA
SedegahM
HollindaleM
1992
A T cell clone directed at the circumsporozoite protein which protects mice against both Plasmodium yoelii and Plasmodium berghei.
J Immunol
149
2103
2109
13. RomeroP
MaryanskiJL
CorradinG
NussenzweigRS
NussenzweigV
1989
Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria.
Nature
341
323
326
14. GrunerAC
MauduitM
TewariR
RomeroJF
DepinayN
2007
Sterile protection against malaria is independent of immune responses to the circumsporozoite protein.
PLoS One
2
e1371
15. KumarKA
SanoG
BoscardinS
NussenzweigRS
NussenzweigMC
2006
The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites.
Nature
444
937
940
16. SchmidtNW
PodyminoginRL
ButlerNS
BadovinacVP
TuckerBJ
2008
Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria.
Proc Natl Acad Sci U S A
105
14017
14022
17. MillerJD
van der MostRG
AkondyRS
GlidewellJT
AlbottS
2008
Human effector and memory CD8+ T cell responses to smallpox and yellow fever vaccines.
Immunity
28
710
722
18. RaiD
PhamNL
HartyJT
BadovinacVP
2009
Tracking the Total CD8 T Cell Response to Infection Reveals Substantial Discordance in Magnitude and Kinetics between Inbred and Outbred Hosts.
J Immunol
183
7672
7681
19. RieckmannKH
1990
Human immunization with attenuated sporozoites.
Bull World Health Organ
68
Suppl
13
16
20. WeissWR
GoodMF
HollingdaleMR
MillerLH
BerzofskyJA
1989
Genetic control of immunity to Plasmodium yoelii sporozoites.
J Immunol
143
4263
4266
21. KaechSM
HembyS
KershE
AhmedR
2002
Molecular and functional profiling of memory CD8 T cell differentiation.
Cell
111
837
851
22. BuschDH
PilipIM
VijhS
PamerEG
1998
Coordinate regulation of complex T cell populations responding to bacterial infection.
Immunity
8
353
362
23. VanderbergJP
NussenzweigRS
MostH
1968
Further studies on the Plasmodium berghei-Anopheles stephensi–rodent system of mammalian malaria.
J Parasitol
54
1009
1016
24. ZechiniB
CordierL
NgonseuE
D'AlessandroU
WeryM
1999
Plasmodium berghei development in irradiated sporozoite-immunized C57BL6 mice.
Parasitology
118 ( Pt 4)
335
338
25. SchellerLF
AzadAF
1995
Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites.
Proc Natl Acad Sci U S A
92
4066
4068
26. BerenzonD
SchwenkRJ
LetellierL
Guebre-XabierM
WilliamsJ
2003
Protracted protection to Plasmodium berghei malaria is linked to functionally and phenotypically heterogeneous liver memory CD8+ T cells.
J Immunol
171
2024
2034
27. NussenzweigR
VanderbergJ
MostH
1969
Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. IV. Dose response, specificity and humoral immunity.
Mil Med
134
1176
1182
28. NussenzweigRS
VanderbergJP
MostH
OrtonC
1969
Specificity of protective immunity produced by x-irradiated Plasmodium berghei sporozoites.
Nature
222
488
489
29. OliveiraGA
KumarKA
Calvo-CalleJM
OthoroC
AltszulerD
2008
Class II-restricted protective immunity induced by malaria sporozoites.
Infect Immun
76
1200
1206
30. DoolanDL
HoffmanSL
1999
IL-12 and NK cells are required for antigen-specific adaptive immunity against malaria initiated by CD8+ T cells in the Plasmodium yoelii model.
J Immunol
163
884
892
31. LabaiedM
HarupaA
DumpitRF
CoppensI
MikolajczakSA
2007
Plasmodium yoelii sporozoites with simultaneous deletion of P52 and P36 are completely attenuated and confer sterile immunity against infection.
Infect Immun
75
3758
3768
32. SedegahM
WeissWW
HoffmanSL
2007
Cross-protection between attenuated Plasmodium berghei and P. yoelii sporozoites.
Parasite Immunol
29
559
565
33. TarunAS
DumpitRF
CamargoN
LabaiedM
LiuP
2007
Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells.
J Infect Dis
196
608
616
34. KhanZM
VanderbergJP
1992
Specific inflammatory cell infiltration of hepatic schizonts in BALB/c mice immunized with attenuated Plasmodium yoelii sporozoites.
Int Immunol
4
711
718
35. KumarKA
BaxterP
TarunAS
KappeSH
NussenzweigV
2009
Conserved protective mechanisms in radiation and genetically attenuated uis3(-) and uis4(-) Plasmodium sporozoites.
PLoS One
4
e4480
36. ChattopadhyayR
ContehS
LiM
JamesER
EpsteinJE
2009
The Effects of radiation on the safety and protective efficacy of an attenuated Plasmodium yoelii sporozoite malaria vaccine.
Vaccine
27
3675
3680
37. TrimnellA
TakagiA
GuptaM
RichieTL
KappeSH
2009
Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes.
J Immunol
183
5870
5878
38. SchofieldL
VillaquiranJ
FerreiraA
SchellekensH
NussenzweigR
1987
Gamma interferon, CD8+ T cells and antibodies required for immunity to malaria sporozoites.
Nature
330
664
666
39. WherryEJ
TeichgraberV
BeckerTC
MasopustD
KaechSM
2003
Lineage relationship and protective immunity of memory CD8 T cell subsets.
Nat Immunol
4
225
234
40. TomiyamaH
TakataH
MatsudaT
TakiguchiM
2004
Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function.
Eur J Immunol
34
999
1010
41. KuCC
MurakamiM
SakamotoA
KapplerJ
MarrackP
2000
Control of homeostasis of CD8+ memory T cells by opposing cytokines.
Science
288
675
678
42. SchmidtNW
ButlerNS
HartyJT
2009
CD8 T cell immunity to Plasmodium permits generation of protective antibodies after repeated sporozoite challenge.
Vaccine
27
6103
6106
43. BrionesMR
TsujiM
NussenzweigV
1996
The large difference in infectivity for mice of Plasmodium berghei and Plasmodium yoelii sporozoites cannot be correlated with their ability to enter into hepatocytes.
Mol Biochem Parasitol
77
7
17
44. CockburnIA
ChenYC
OverstreetMG
LeesJR
van RooijenN
2010
Prolonged antigen presentation is required for optimal CD8+ T cell responses against malaria liver stage parasites.
PLoS Pathog
6
e1000877
45. JobeO
LumsdenJ
MuellerAK
WilliamsJ
Silva-RiveraH
2007
Genetically attenuated Plasmodium berghei liver stages induce sterile protracted protection that is mediated by major histocompatibility complex Class I-dependent interferon-gamma-producing CD8+ T cells.
J Infect Dis
196
599
607
46. AlyAS
MikolajczakSA
RiveraHS
CamargoN
Jacobs-LorenaV
2008
Targeted deletion of SAP1 abolishes the expression of infectivity factors necessary for successful malaria parasite liver infection.
Mol Microbiol
69
152
163
47. VaughanAM
O'NeillMT
TarunAS
CamargoN
PhuongTM
2009
Type II fatty acid synthesis is essential only for malaria parasite late liver stage development.
Cell Microbiol
11
506
520
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



