-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Quasispecies Theory and the Behavior of RNA Viruses
A large number of medically important viruses, including HIV, hepatitis C virus, and influenza, have RNA genomes. These viruses replicate with extremely high mutation rates and exhibit significant genetic diversity. This diversity allows a viral population to rapidly adapt to dynamic environments and evolve resistance to vaccines and antiviral drugs. For the last 30 years, quasispecies theory has provided a population-based framework for understanding RNA viral evolution. A quasispecies is a cloud of diverse variants that are genetically linked through mutation, interact cooperatively on a functional level, and collectively contribute to the characteristics of the population. Many predictions of quasispecies theory run counter to traditional views of microbial behavior and evolution and have profound implications for our understanding of viral disease. Here, we discuss basic principles of quasispecies theory and describe its relevance for our understanding of viral fitness, virulence, and antiviral therapeutic strategy.
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1001005
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1001005Summary
A large number of medically important viruses, including HIV, hepatitis C virus, and influenza, have RNA genomes. These viruses replicate with extremely high mutation rates and exhibit significant genetic diversity. This diversity allows a viral population to rapidly adapt to dynamic environments and evolve resistance to vaccines and antiviral drugs. For the last 30 years, quasispecies theory has provided a population-based framework for understanding RNA viral evolution. A quasispecies is a cloud of diverse variants that are genetically linked through mutation, interact cooperatively on a functional level, and collectively contribute to the characteristics of the population. Many predictions of quasispecies theory run counter to traditional views of microbial behavior and evolution and have profound implications for our understanding of viral disease. Here, we discuss basic principles of quasispecies theory and describe its relevance for our understanding of viral fitness, virulence, and antiviral therapeutic strategy.
Introduction
The rapid evolution of RNA viruses complicates the management of chronic infections and the control of emerging infectious agents [1]–[3]. The ongoing global AIDS pandemic and the resurgence of influenza highlight the difficulties associated with these genetically labile pathogens [4]–[6]. RNA viruses have also been responsible for recent sporadic epidemics of emerging and reemerging viral diseases including dengue, West Nile fever, and Ebola [7], [8]. Because of their high mutation rates, these viruses are moving targets for therapeutic intervention and frequently develop resistance to vaccines and antiviral drugs [9]. A clearer understanding of viral evolutionary dynamics and its relationship to virulence and drug resistance may facilitate the development of more effective therapeutics.
The evolutionary dynamics of RNA viruses are complex and their high mutation rates, rapid replication kinetics, and large population sizes present a challenge to traditional population genetics [10]. Quasispecies theory is a mathematical framework that was initially formulated to explain the evolution of life in the “precellular RNA world [11].” It builds on classical population genetics, but seeks to explore the consequences of error-prone replication and near-infinite population sizes for genome evolution [12], [13]. More recently, quasispecies theory has been used to describe the evolutionary dynamics of RNA viruses, and many of its predictions have been validated experimentally in model systems [2], [14], [15]. Some of these observations challenge more traditional views of evolution and have profound implications for the control and treatment of viral diseases.
Here we explain basic aspects of quasispecies theory, describe key experiments that define “quasispecies effects,” and highlight how these results may shape our view of viral pathogenesis, antiviral drug development, and vaccine design. We will stress three clinically relevant principles. First, the fitness of a particular virus sequence may be determined more by its freedom to mutate into related sequences than by its own replicative capacity. Second, many viruses operate near a threshold of “error catastrophe” and may be combated by increasing their replication error rates. Third, increasing the fidelity of genome replication may paradoxically attenuate viruses.
Error-Prone Replication and Viral Quasispecies
Most viruses encode enzymes responsible for replicating their DNA or RNA genomes. The intrinsic error rate, or fidelity, of the replicase determines the mutation rate for that virus and the range of genetic variation upon which natural selection can act. Viral RNA polymerases exhibit characteristically low fidelity with measured mutation rates of roughly 10−4 mutations per nucleotide copied, which is orders of magnitude greater than those of nearly all DNA-based viruses and organisms [10], [15], [16]. Given the large population sizes observed in both experimental and natural infections, it is estimated that every possible point mutation and many double mutations are generated with each viral replication cycle and may be present within the population at any time [17]. Because RNA viruses exist as swarms of similar variants that are continuously regenerated by mutation of related sequences, our ability to predict the outcome of an infection or a therapeutic intervention from studies of isolated clones is limited. Even a defined molecular clone will quickly transform into a collection of related sequences when introduced into cells. This collection is the quasispecies and is organized around a master sequence [12].
The genetic organization of populations is often depicted using the concept of sequence space, a geometric representation of all possible sequences where physical distance reflects genetic similarity. For example, a given viral genome will undergo replication and generate hundreds of progeny that differ at roughly one position (Figure 1). Subsequent rounds of replication will generate a more complex mutant distribution with variants lying farther away from each other in sequence space. This ensemble of mutants forms a “cloud” of variants, or quasispecies, in which mutation generates a swarm of candidate genomes that is pruned by natural selection. According to population genetics, the frequency of a given variant in a population is closely approximated by its ability to survive and reproduce—its fitness. In quasispecies formulations, where mutation rates are elevated, frequency is also subject to the probability that the variant will be generated de novo by mutation of its neighbors in sequence space [12]. In RNA viruses, the contribution of mutation to genotype frequency is significant, and variants are “coupled” in sequence space [18]. That is, a low fitness variant can be maintained at a higher than expected frequency because it is coupled to a well-represented, higher fitness genotype in sequence space. The phenomenon of mutational coupling is one of the defining characteristics of a quasispecies, as it places individual mutants within a functional network of variants [2].
Fig. 1. RNA viruses exist as a quasispecies. 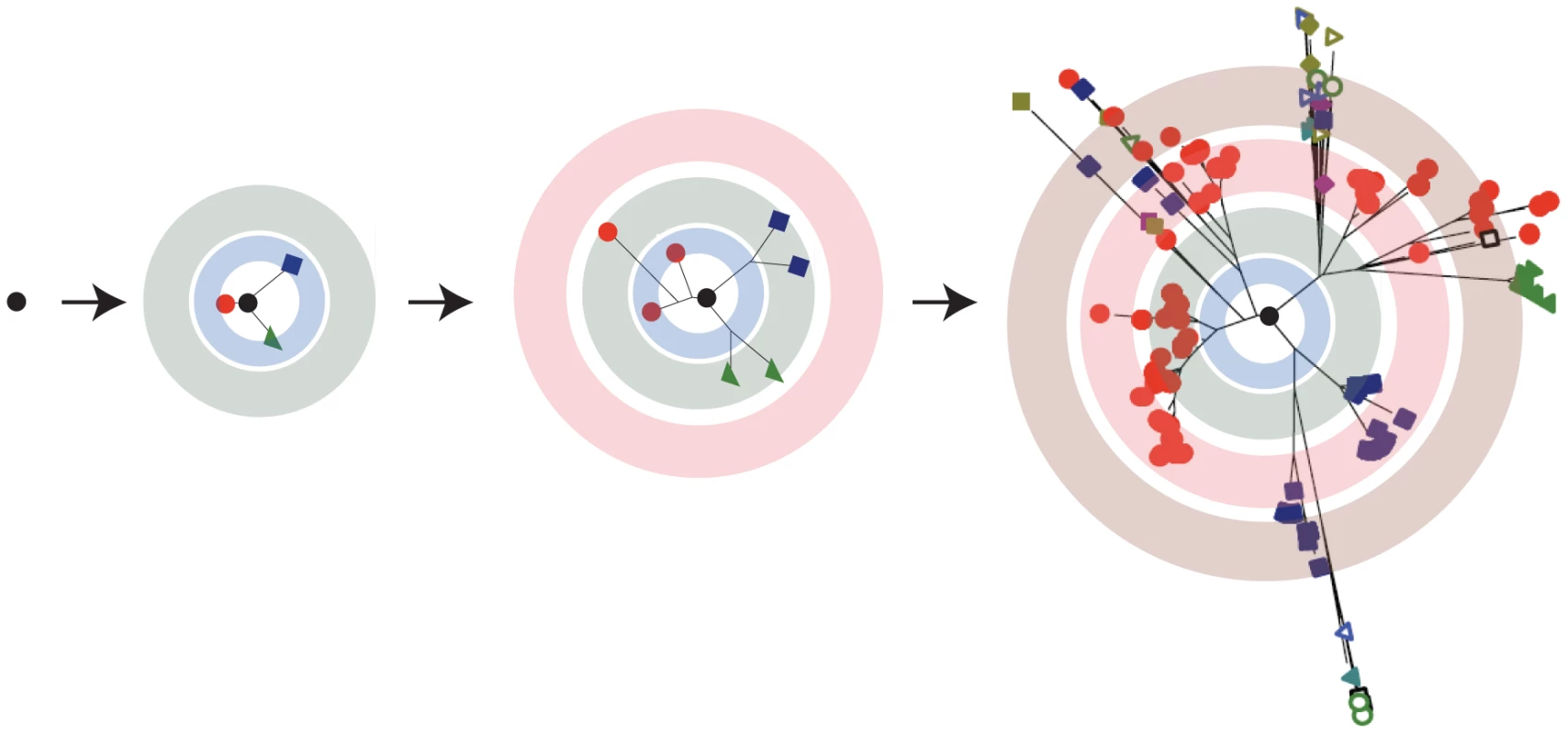
A virus replicating with a high mutation rate will generate a diverse mutant repertoire over the course of a few generations. In these trees, each branch indicates two variants linked by a point mutation and the concentric circles represent serial replication cycles. The resulting distribution is often represented as a cloud centered on a master sequence. This two dimensional schematic is a vast oversimplification of the intraquasispecies connectivity. In the mathematical formulations of quasispecies theory, sequence space is multidimensional, with numerous branches between variants. Viral populations evolve within a fitness landscape where the “ground level” is a representation of the range of genotypes in sequence space. The “altitude” at any given location is the fitness associated with that particular genotype. The environment and its selective pressures determine the contours of the corresponding landscape, and adaptation to an environment involves a mutational walk from one point in the fitness landscape to another (Figure 2A). In quasispecies theory, a network of mutationally coupled variants will span the corresponding peaks and valleys of the fitness landscape. A fast replicating population well suited to a given environment will inhabit a high and narrow peak in the fitness landscape, while a less fit but more genetically diverse population will occupy a lower, broader one.
Fig. 2. The fitness landscape and survival of the flattest. 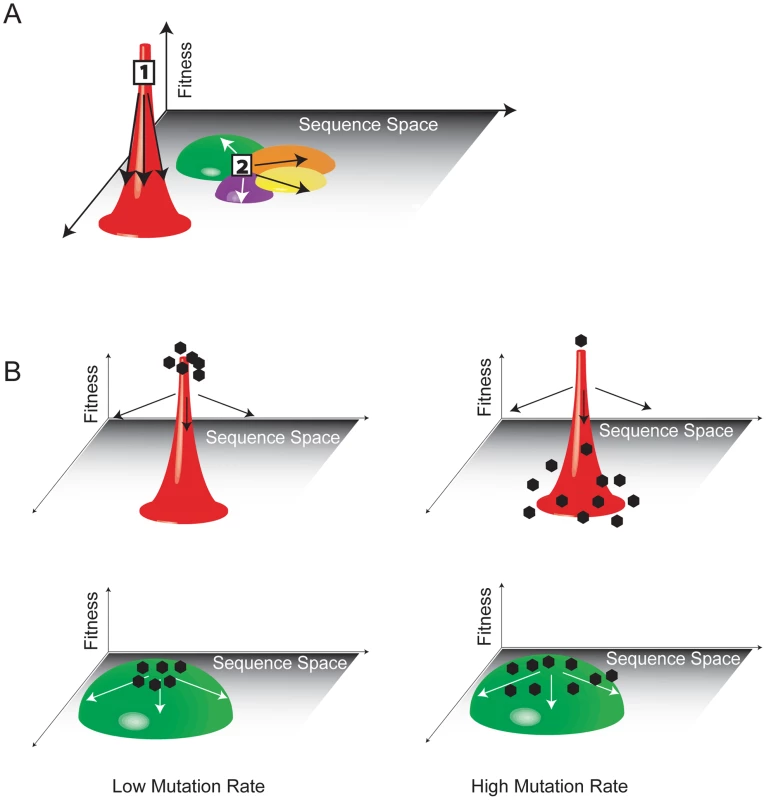
(A) Population 1 has high fitness but is trapped in sequence space because mutation leads to a dramatic loss of fitness. Population 2 is more mutationally robust because mutation leads to minor fitness losses. The flatter population is ideally situated to move through sequence and access other local peaks through neighboring mutational networks (indicated in different colors). (B) At low mutation rates, variants will be genotypically stable and cluster at the top of the fitness peak. The variant with the highest fitness will easily outcompete all others. At high mutation rates, variants spread out over the corresponding peaks. Variants on the flatter peak (green) remain near their fitness optimum and have a higher mean fitness than the population located on the steeper peak (red). The flatter population will prevail. A viral quasispecies, then, is a cloud of diverse variants that are genetically linked through mutation, interact cooperatively on a functional level, and collectively contribute to the characteristics of the population. The unit of selection is the population as a whole, and the nature of the functional interactions among genetically distinct variants is therefore of critical importance to pathogenesis in infected hosts. These effects and their biomedical implications are described below.
The Problem of Fitness and Survival of the Flattest
Mutation and selection are the most fundamental processes in evolution. In Darwinian evolution, natural selection acts on existing genetic variation to optimize fitness. Conceptually, fitness refers to how well a given organism “fits” into its environment, often reflected in how well it survives and reproduces [19]. In experimental settings, precise fitness measurements are paramount, and virologists typically use replicative capacity as a surrogate for fitness [20]. While replication is useful as a first approximation, other factors such as immune escape, transmissibility, and cellular tropism are important components of fitness in the dynamic host environment [21]. Furthermore, because quasispecies theory adds the complexity of mutant networks, we must incorporate a population-based model into our fitness definition.
Measuring the fitness of individual variants within a population may misrepresent the fitness of a quasispecies. Early experiments with vesicular stomatitis virus (VSV) established that high fitness variants could be suppressed to low levels within a complex population [22]. Conversely, longitudinal studies of dengue virus isolates have identified defective clones that are stably maintained at a high frequency in the population [23]. Further consideration of the fitness landscape model may explain these paradoxical results (Figure 2B). Consider two populations, generated from either a fast replicator or a slower one. At low mutation rates, the fast replicator will triumph because its progeny are genetically identical and generated more quickly. In the fitness landscape, this population occupies a tall, narrow peak, where there is little genotypic diversity and maximal fitness. In RNA viruses, elevated mutation rates mean that a fast replicator will give rise to genetically diverse progeny, many of which are significantly less fit than the parent. Quasispecies theory predicts that slower replicators will be favored if they give rise to progeny that are on average more fit; these populations occupy short, flat regions of the fitness landscape [18]. This effect, termed survival of the flattest, has been observed in digital organisms, bacteriophages, and VSV [24]–[28]. Flat quasispecies accept mutation without a corresponding effect on fitness, and these mutationally robust populations form a large, selectively neutral network of variants in sequence space. While neutral mutations do not change the corresponding phenotype, they may have epistatic effects on subsequent mutations and redraw the genotype–phenotype map [29].
A flat quasispecies with an expansive mutant repertoire can explore vast regions of sequence space without consequence and is poised to adapt to rapid environmental change. This framework may explain many observed phenomena with direct clinical relevance. Arboviruses must successfully adapt to insect and mammalian hosts and their associated fitness landscapes. A quasispecies that occupies a broad, flat region of sequence space could access neutral networks and local fitness peaks in either environment. Similarly, retrospective studies of primary HIV isolates suggest that HIV could be moving to a flatter and less fit region of sequence space [20], [30]–[32]. In the case of influenza, detailed antigenic mapping of the hemagglutinin protein suggests that interpandemic strains remain antigenically stable for years despite genetic drift and evolve over a neutral region of sequence space. This steady accumulation of genetic diversity is punctuated by epochal shifts in antigenicity [33]. Though this adaptive process occurs on the interhost level over a long time scale, it highlights the importance of “flatness” in viral evolution.
Error Catastrophe
Given their high mutation rates, it is not surprising that many discussions of RNA virus evolution focus on the relationship between genetic diversity and adaptability. While it is clear that RNA viruses have the capacity to quickly explore large regions of sequence space, genome size and selective constraints place significant limitations on the amount of diversity that is actually expressed [34]. Most RNA virus genomes are relatively small and contain either overlapping reading frames or sequences that serve both coding and structural functions. Similarly, coding mutations that mediate escape from host immune surveillance may compromise protein function. In VSV, for example, approximately 60% of spontaneous mutations are deleterious [35]. Evolutionary theory would suggest that the short-term cost of mutation is balanced by the long-term benefit of adaptability in the face of a dynamic environment [36], [37].
Quasispecies theory accepts this trade-off, and proposes an upper limit to mutation rate, the “error threshold” (Figure 3A). According to Eigen's original formulations, a quasispecies can remain at equilibrium despite a high mutation rate [38], [39]. Small increases in mutation rate will upset this balance as the master sequence itself disappears and meaningful genetic information is lost in an avalanche of errors. Because Eigen compared this process to chemical phase transitions, it has been termed “mutational meltdown.” It is now clear that many RNA viruses replicate near the error threshold. Early studies with VSV showed that chemical mutagens generally reduced viral infectivity, and studies with poliovirus clearly demonstrated that mutagenic nucleoside analogs push viral populations to extinction [40]–[43]. The effect is dramatic—a 4-fold increase in mutation rate resulted in a 95% reduction in viral titer. Others have found similar results with lymphocytic choriomeningitis virus (LCMV) and foot and mouth disease virus (FMDV) [44]–[48]. The lethal mutagenesis of viral populations is perhaps the most important implication of the error threshold.
Fig. 3. Mutant distributions and the error threshold. 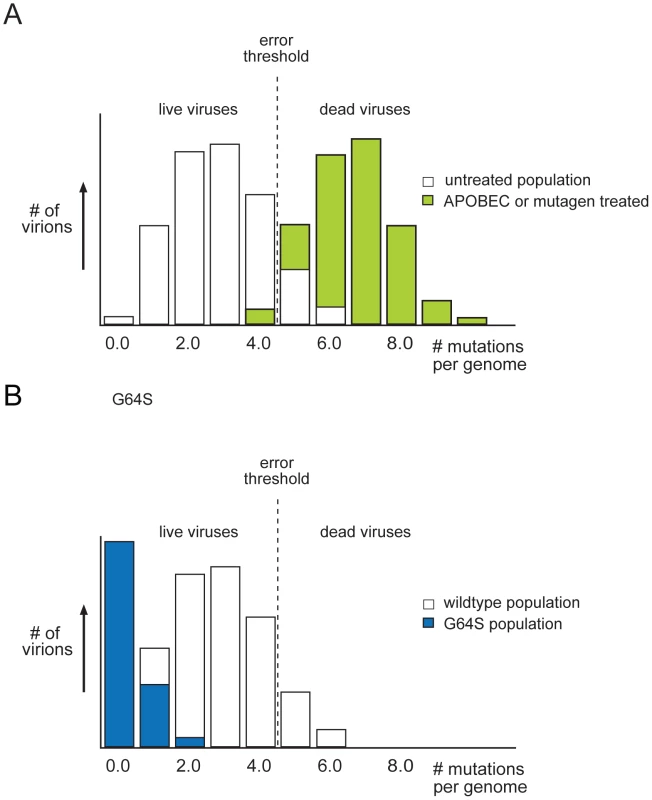
(A) The majority of viruses in a wild-type population has few mutations and is viable Some viruses, bearing a higher mutational load, are nonviable and considered beyond the threshold of error catastrophe (shown in green). Small increases in mutation frequency, mediated by host APOBEC proteins or exogenous mutagen, push the distribution to the right, past the error threshold. The number of errors per genome is sufficiently high to lethally mutate a majority of the population. (B) A high fidelity polymerase results results in a narrower quasispecies situated farther from the error threshold. This population is more resistant to the effect of mutagen, because it does not accumulate as many mutations, as the wild type does not cross the error threshold. Figure adapted from Crotty et al. [42]. Recent work on host cell restriction of retroviral infection suggests that lethal mutagenesis is a natural form of antiviral defense [49]. The cytidine deaminase APOBEC3G was initially identified as a protein targeted by the HIV Vif protein during replication [50]. Humans have 11 APOBEC proteins that edit cellular messages by converting cytosine bases to uracil. Subsequent work demonstrated that APOBEC proteins could induce lethal mutagenesis of HIV through widespread deamination of the HIV genome during reverse transcription, and highly mutagenized HIV genomes with signatures of deamination have been observed in patients [51]–[56]. This mechanism appears to be evolutionarily conserved and active against hepatitis B virus, simple retroviruses, and endogenous retroelements [57]. While mutation-independent activities have also been identified, it is clear that APOBEC-mediated lethal mutagenesis is a critical cellular defense against RNA viruses. The fact that these pathogens replicate close to the error threshold makes them particularly sensitive to slight increases in mutational load (Figure 3A).
These observations suggest that lethal mutagenesis could be an effective therapeutic strategy for RNA virus infections [58]. Ribavirin, a nucleoside with broad antiviral activity, has attracted considerable interest, and can induce lethal mutagenesis of Hantaan virus and poliovirus [42], [43], [59]. While ribavirin is used clinically for the treatment of both respiratory syncytial virus and hepatitis C virus, it has pleiotropic effects, and its mechanism of action in these infections is unclear [60]. Another mutagen, 5-fluorouracil, is licensed as a chemotherapeutic agent, and its antiviral activity against LCMV in animal models may predict efficacy for other arenaviruses, such as Lassa fever [61], [62]. Spurred by these results, Loeb and colleagues have identified a number of nucleoside analogs capable of inducing the lethal mutagenesis of HIV [63]–[65].
While these results are encouraging, much work needs to be done before lethal mutagenesis can be considered as a quasispecies-inspired therapeutic strategy. Some have argued for a distinction between lethal mutagenesis and error catastrophe, and have suggested, on theoretical grounds, that the above experiments do not show a true phase transition with loss of a master sequence. Indeed, careful studies of lethal mutagenesis by Lowenstein and colleagues have shown an imperfect correlation between mutational load and population extinction [46]. Pre-extinction populations exhibit marked heterogeneity in both the location and number of mutations per genome. The dynamics of extinction are further complicated by the observation that highly mutagenized genomes can accelerate extinction by interfering with the replication of their less mutated brethren [47], [66]. In that case, a mutagenized population would collapse without the genetic melting of an error catastrophe. While these discrepancies may reflect the gap between mathematical theory and biological complexity, the distinction could have “real world” implications. Mutation is a double-edged sword, and adaptive evolution can be accelerated at high mutation rates [67]. Absent a true mutational meltdown, a large pre-extinction population could serve as a reservoir for novel mutants that mediate antiviral resistance or immune escape [68]–[70].
The potential for mutagen resistance is another important concern. In principle, a virus could evolve biochemical resistance to nucleosides either by excluding the drug from its active site or by lowering its intrinsic error rate (see below). Quasispecies theory suggests that viruses could also achieve resistance by moving to flatter regions of the fitness landscape, where the density of neutral mutations is higher. Combination therapy with mutagens and traditional antivirals may minimize the probability of mutagenic escape, and work in the FMDV system is encouraging [71], [72]. The safety of nucleoside analogs is a major concern that is largely unexplored. Drug concentrations in the above studies were often in the millimolar range, and mutagenic nucleosides with a wider therapeutic index are clearly needed. Given the potential for off-target effects on host cell polymerases, therapy would likely need to be short term—a problem for HIV, where eradication of the latent reservoir is critical.
Mutation Rate, Virulence, and Attenuation
Evolutionary theory predicts that high mutation rates are favored in a dynamic environment, and viral error rates may have been optimized by natural selection [36], [37]. For RNA viruses, low replicative fidelity generates a diverse population of variants. While these variants are generally less fit, they may quickly dominate if a sudden change in environment, such as immune pressure, shifts the corresponding fitness landscape. Conversely, a homogeneous population, generated by high replicative fidelity, would lack this flexibility and might be less successful in the dynamic host environment.
Recent work by two groups in the poliovirus system provides experimental support for this model. Drawing on experience with ribavirin and lethal mutagenesis, they hypothesized that a mutant with a low mutation rate would be less sensitive to lethal mutagenesis and resistant to ribavirin. Both groups sought to isolate ribavirin resistant mutants from a poliovirus quasispecies and recovered a variant with a single amino acid substitution in the viral polymerase (3DpolG64S) [73], [74]. This mutant was relatively resistant to lethal mutagenesis, and assays for selectable markers indicated that the G64S population had a lower mutation rate and exhibited less genetic diversity (Figure 3B) [73], [75], [76]. While wild-type and G64S quasispecies replicated with similar kinetics, the former was more “fit” in direct competition assays [75], [76]. Together, these data suggested that high mutation rates confer an evolutionary advantage to RNA viruses.
The G64S quasispecies was markedly attenuated in a transgenic mouse model for poliovirus infection; the high fidelity variants were less successful at accessing the central nervous system (CNS), the site of disease [76]. Importantly, virulence was determined in large part by the diversity of the coinfecting population (Figure 4). When brain-derived polioviruses were introduced as members of the genetically homogeneous G64S population, they were not able to invade the CNS. In contrast, brain-derived viruses readily accessed the CNS when the population inoculum contained a wild-type distribution of variants. These results suggest that quasispecies diversity, rather than the selection of individual variants, correlates with enhanced virulence [76]. The demonstration of cooperative interactions among mutants in a population established the relevance of quasispecies theory to studies of viral pathogenesis. Vignuzzi and colleagues extended this model and hypothesized that the observed attenuation of the high fidelity variants could be exploited for vaccine design [77]. Focusing on the G64 position, they engineered several high fidelity mutants that were markedly attenuated in mice. These viruses stimulated high titers of neutralizing antibody in immunized mice, and provided lasting protection against subsequent lethal challenge with wild-type virus.
Fig. 4. Population diversity is a virulence determinant. 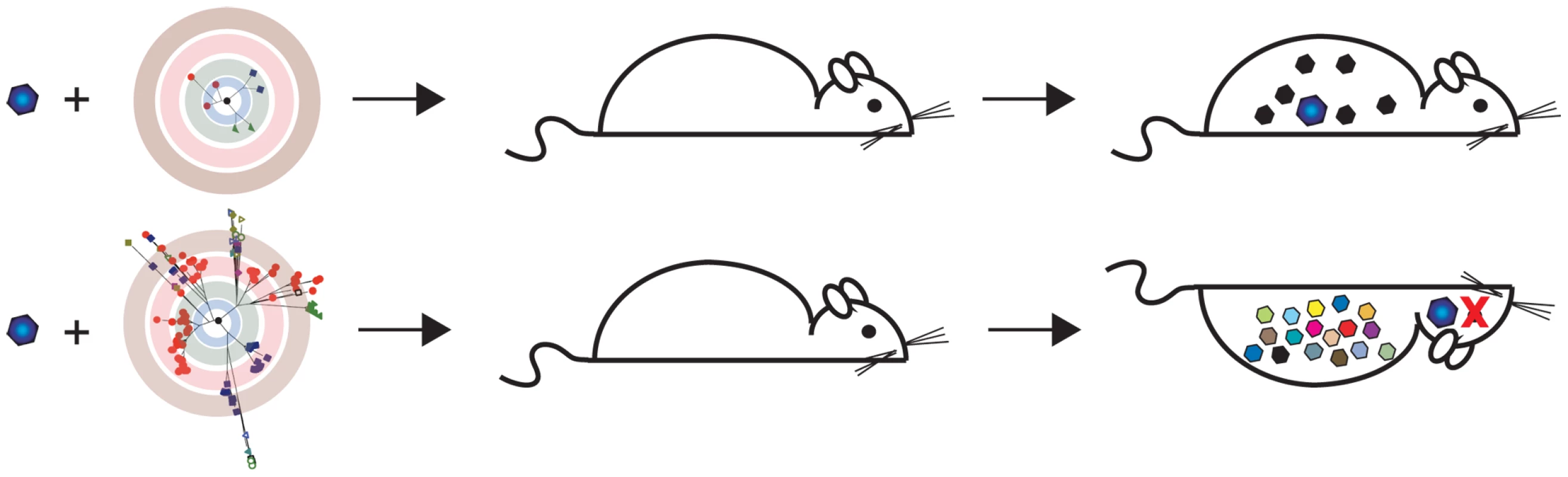
Results of experiments described in Vignuzzi et al. [76]. A neurovirulent clone of poliovirus was isolated from the brains of mice that had been infected with a wild-type strain. Naive mice were then reinfected with this clone as part of either a genetically constrained (top) or diverse population (bottom). Although all mice received the neurovirulent clone, only those infected with a diverse quasispecies developed disease. Subpopulations within the diverse quasispecies cooperated with the neurovirulent clone to facilitate its entry into the CNS. This work shows how quasispecies theory and its predictions can lead to the rational development of live, attenuated vaccines. Because variants with low mutation rates would be less likely to revert to wild type in a vaccinee, attenuation should be a stable phenotype and increase the safety of candidate vaccines. Caution is warranted, however, since the error rates of the high fidelity variants are still orders of magnitude greater than those of DNA viruses. It is also unclear whether reducing mutation rate will similarly affect the adaptability and virulence of other RNA viruses. Further work in other systems is clearly needed before this work can be translated into new vaccines [78], [79]. As in the case of lethal mutagenesis, the modulation of fidelity offers an opportunity to use mutation rate as a therapeutic strategy for the control of viral disease.
Perspectives on the Future
Quasispecies theory has had a profound influence on virology, and experiments with model RNA viruses have validated many of its predictions. Considerably less is known about how its population-based models apply to the evolutionary behavior of RNA viruses in infected hosts, and it will be challenging to translate them to the complex reality of viral infection in patients. The initial studies pose as many questions as they answer. What is the best measure of viral fitness in a dynamic population? How can we use a fitness landscape model to understand selection pressure in vivo, where many such landscapes coexist? Do the principles of mutational robustness and survival of the flattest determine the behavior of populations in the host ecosystem? How does population diversity influence pathogenesis? What are the mechanisms by which variants or subpopulations cooperate, and does this have implications for coinfection?
While early forays into complex experimental systems have provided tantalizing results, much more work is needed in this area if quasispecies theory is to be translated from bench to bedside. Improved assays for characterizing viral populations are critical, and the advent of ultra high throughput, or “deep” sequencing, is particularly exciting. Several groups have used this technology to explore the complexity of mutant spectra in clinical samples from HIV - and HBV-infected patients, although the sequencing error rate presents certain limitations for studies of RNA as opposed to DNA viruses [80]–[82]. Discerning quasispecies structure from the wealth of sequencing data presents significant computational challenges as well, since current techniques are underdeveloped and do not permit us to determine which mutations are linked on the same genome. Complementary approaches, such as molecular barcoding, will also be required if we are to understand how the mutant spectrum changes temporally or spatially within an infected host [83]. Finally, future drug and vaccine studies will need to be carried out in well-defined animal models, as subtle differences can have a significant impact on experimental outcome. Despite these obstacles, we are confident that quasispecies theory will soon move out of the laboratory and begin to influence the control and treatment of viral disease.
Zdroje
1. DomingoE
BaranowskiE
Ruiz-JaraboCM
Martin-HernandezAM
SaizJC
1998 Quasispecies structure and persistence of RNA viruses. Emerg Infect Dis 4 521 527
2. DomingoE
MartinV
PeralesC
Grande-PerezA
Garcia-ArriazaJ
2006 Viruses as quasispecies: biological implications. Curr Top Microbiol Immunol 299 51 82
3. DuffyS
ShackeltonLA
HolmesEC
2008 Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet 9 267 276
4. BeigelJH
FarrarJ
HanAM
HaydenFG
HyerR
2005 Avian influenza A (H5N1) infection in humans. N Engl J Med 353 1374 1385
5. DawoodFS
JainS
FinelliL
ShawMW
LindstromS
2009 Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med 360 2605 2615
6. RambautA
PosadaD
CrandallKA
HolmesEC
2004 The causes and consequences of HIV evolution. Nat Rev Genet 5 52 61
7. FieldsBN
KnipeDM
HowleyPM
2007 Fields' virology Philadelphia Wolters Kluwer Health/Lippincott Williams & Wilkins2 v. (xix, 3091, I-3086 p.)
8. GeisbertTW
JahrlingPB
2004 Exotic emerging viral diseases: progress and challenges. Nat Med 10 S110 121
9. GerrishPJ
Garcia-LermaJG
2003 Mutation rate and the efficacy of antimicrobial drug treatment. Lancet Infect Dis 3 28 32
10. HollandJ
SpindlerK
HorodyskiF
GrabauE
NicholS
1982 Rapid evolution of RNA genomes. Science 215 1577 1585
11. EigenM
1971 Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften 58 465 523
12. BiebricherCK
EigenM
2006 What is a quasispecies? Curr Top Microbiol Immunol 299 1 31
13. EigenM
1993 Viral quasispecies. Sci Am 269 42 49
14. DomingoE
SaboD
TaniguchiT
WeissmannC
1978 Nucleotide sequence heterogeneity of an RNA phage population. Cell 13 735 744
15. BatscheletE
DomingoE
WeissmannC
1976 The proportion of revertant and mutant phage in a growing population, as a function of mutation and growth rate. Gene 1 27 32
16. SteinhauerDA
HollandJJ
1987 Rapid evolution of RNA viruses. Annu Rev Microbiol 41 409 433
17. VignuzziM
StoneJK
AndinoR
2005 Ribavirin and lethal mutagenesis of poliovirus: molecular mechanisms, resistance and biological implications. Virus Res 107 173 181
18. WilkeCO
2005 Quasispecies theory in the context of population genetics. BMC Evol Biol 5 44
19. OrrHA
2009 Fitness and its role in evolutionary genetics. Nat Rev Genet 10 531 539
20. Quinones-MateuME
ArtsEJ
2006 Virus fitness: concept, quantification, and application to HIV population dynamics. Curr Top Microbiol Immunol 299 83 140
21. DomingoE
HollandJJ
1997 RNA virus mutations and fitness for survival. Annu Rev Microbiol 51 151 178
22. de la TorreJC
HollandJJ
1990 RNA virus quasispecies populations can suppress vastly superior mutant progeny. J Virol 64 6278 6281
23. AaskovJ
BuzacottK
ThuHM
LowryK
HolmesEC
2006 Long-term transmission of defective RNA viruses in humans and Aedes mosquitoes. Science 311 236 238
24. WilkeCO
WangJL
OfriaC
LenskiRE
AdamiC
2001 Evolution of digital organisms at high mutation rates leads to survival of the flattest. Nature 412 331 333
25. BurchCL
ChaoL
2000 Evolvability of an RNA virus is determined by its mutational neighbourhood. Nature 406 625 628
26. CodoñerFM
DarósJ-A
SoléRV
ElenaSF
2006 The fittest versus the flattest: experimental confirmation of the quasispecies effect with subviral pathogens. PLoS Pathog 2 e136 doi:10.1371/journal.ppat.0020136
27. SanjuanR
CuevasJM
FurioV
HolmesEC
MoyaA
2007 Selection for Robustness in Mutagenized RNA Viruses. PLoS Genet 3 e93 doi:10.1371/journal.pgen.0030093
28. QuerJ
HersheyCL
DomingoE
HollandJJ
NovellaIS
2001 Contingent neutrality in competing viral populations. J Virol 75 7315 7320
29. DraghiJA
ParsonsTL
WagnerGP
PlotkinJB
2010 Mutational robustness can facilitate adaptation. Nature 463 353 355
30. AriënKK
TroyerRM
GaliY
ColebundersRL
ArtsEJ
2005 Replicative fitness of historical and recent HIV-1 isolates suggests HIV-1 attenuation over time. AIDS 19 1555 1564
31. RichmanDD
2006 Antiviral drug resistance. Antiviral Res 71 117 121
32. RollandM
BranderC
NickleDC
HerbeckJT
GottliebGS
2007 HIV-1 over time: fitness loss or robustness gain? Nat Rev Microbiol 5 1 2
33. KoelleK
CobeyS
GrenfellB
PascualM
2006 Epochal evolution shapes the phylodynamics of interpandemic influenza A (H3N2) in humans. Science 314 1898 1903
34. OverbaughJ
BanghamCR
2001 Selection forces and constraints on retroviral sequence variation. Science 292 1106 1109
35. SanjuánR
MoyaA
ElenaSF
2004 The distribution of fitness effects caused by single-nucleotide substitutions in an RNA virus. Proc Natl Acad Sci USA 101 8396 8401
36. ElenaSF
SanjuánR
2005 Adaptive value of high mutation rates of RNA viruses: separating causes from consequences. J Virol 79 11555 11558
37. HolmesEC
2003 Error thresholds and the constraints to RNA virus evolution. Trends Microbiol 11 543 546
38. BiebricherCK
EigenM
2005 The error threshold. Virus Res 107 117 127
39. EigenM
2002 Error catastrophe and antiviral strategy. Proc Natl Acad Sci USA 99 13374 13376
40. HollandJJ
DomingoE
de la TorreJC
SteinhauerDA
1990 Mutation frequencies at defined single codon sites in vesicular stomatitis virus and poliovirus can be increased only slightly by chemical mutagenesis. J Virol 64 3960 3962
41. LeeCH
GilbertsonDL
NovellaIS
HuertaR
DomingoE
1997 Negative effects of chemical mutagenesis on the adaptive behavior of vesicular stomatitis virus. J Virol 71 3636 3640
42. CrottyS
CameronCE
AndinoR
2001 RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci U S A 98 6895 6900
43. CrottyS
MaagD
ArnoldJJ
ZhongW
LauJY
2000 The broad-spectrum antiviral ribonucleoside ribavirin is an RNA virus mutagen. Nat Med 6 1375 1379
44. DomingoE
ParienteN
AiraksinenA
Gonzaĺez-LopezC
SierraS
2005 Foot-and-mouth disease virus evolution: exploring pathways towards virus extinction. Curr Top Microbiol Immunol 288 149 173
45. Grande-PerezA
Gomez-MarianoG
LowensteinPR
DomingoE
2005 Mutagenesis-induced, large fitness variations with an invariant arenavirus consensus genomic nucleotide sequence. J Virol 79 10451 10459
46. Grande-PerezA
LazaroE
LowensteinP
DomingoE
ManrubiaSC
2005 Suppression of viral infectivity through lethal defection. Proc Natl Acad Sci U S A 102 4448 4452
47. Grande-PerezA
SierraS
CastroMG
DomingoE
LowensteinPR
2002 Molecular indetermination in the transition to error catastrophe: systematic elimination of lymphocytic choriomeningitis virus through mutagenesis does not correlate linearly with large increases in mutant spectrum complexity. Proc Natl Acad Sci U S A 99 12938 12943
48. SierraS
DávilaM
LowensteinPR
DomingoE
2000 Response of foot-and-mouth disease virus to increased mutagenesis: influence of viral load and fitness in loss of infectivity. J Virol 74 8316 8323
49. MalimMH
2009 APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond, B, Biol Sci 364 675 687
50. SheehyAM
GaddisNC
ChoiJD
MalimMH
2002 Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature 418 646 650
51. HarrisRS
BishopKN
SheehyAM
CraigHM
Petersen-MahrtSK
2003 DNA deamination mediates innate immunity to retroviral infection. Cell 113 803 809
52. MangeatB
TurelliP
CaronG
FriedliM
PerrinL
2003 Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424 99 103
53. ZhangH
YangB
PomerantzRJ
ZhangC
ArunachalamSC
2003 The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424 94 98
54. VartanianJP
MeyerhansA
SalaM
Wain-HobsonS
1994 G→A hypermutation of the human immunodeficiency virus type 1 genome: evidence for dCTP pool imbalance during reverse transcription. Proc Natl Acad Sci U S A 91 3092 3096
55. KiefferTL
KwonP
NettlesRE
HanY
RaySC
2005 G→A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J Virol 79 1975 1980
56. JaniniM
RogersM
BirxDR
McCutchanFE
2001 Human immunodeficiency virus type 1 DNA sequences genetically damaged by hypermutation are often abundant in patient peripheral blood mononuclear cells and may be generated during near-simultaneous infection and activation of CD4(+) T cells. J Virol 75 7973 7986
57. HolmesRK
MalimMH
BishopKN
2007 APOBEC-mediated viral restriction: not simply editing? Trends Biochem Sci 32 118 128
58. AndersonJP
DaifukuR
LoebLA
2004 Viral error catastrophe by mutagenic nucleosides. Annu Rev Microbiol 58 183 205
59. SeversonWE
SchmaljohnCS
JavadianA
JonssonCB
2003 Ribavirin causes error catastrophe during Hantaan virus replication. J Virol 77 481 488
60. HofmannWP
HerrmannE
SarrazinC
ZeuzemS
2008 Ribavirin mode of action in chronic hepatitis C: from clinical use back to molecular mechanisms. Liver Int 28 1332 1343
61. Ruiz-JaraboCM
LyC
DomingoE
de la TorreJC
2003 Lethal mutagenesis of the prototypic arenavirus lymphocytic choriomeningitis virus (LCMV). Virology 308 37 47
62. McCormickJB
KingIJ
WebbPA
ScribnerCL
CravenRB
1986 Lassa fever. Effective therapy with ribavirin. N Engl J Med 314 20 26
63. LoebLA
EssigmannJM
KazaziF
ZhangJ
RoseKD
1999 Lethal mutagenesis of HIV with mutagenic nucleoside analogs. Proc Natl Acad Sci USA 96 1492 1497
64. LoebLA
MullinsJI
2000 Lethal mutagenesis of HIV by mutagenic ribonucleoside analogs. AIDS Res Hum Retroviruses 16 1 3
65. HarrisKS
BrabantW
StyrchakS
GallA
DaifukuR
2005 KP-1212/1461, a nucleoside designed for the treatment of HIV by viral mutagenesis. Antiviral Res 67 1 9
66. Gonzalez-LopezC
AriasA
ParienteN
Gomez-MarianoG
DomingoE
2004 Preextinction viral RNA can interfere with infectivity. J Virol 78 3319 3324
67. SpringmanR
KellerT
MolineuxI
BullJ
2009 Evolution at a High Imposed Mutation Rate: Adaptation Obscures the Load in Phage T7. Genetics
68. GiffordRJ
RheeS-Y
ErikssonN
LiuTF
KiuchiM
2008 Sequence editing by Apolipoprotein B RNA-editing catalytic component [corrected] and epidemiological surveillance of transmitted HIV-1 drug resistance. AIDS 22 717 725
69. PillaiSK
WongJK
BarbourJD
2008 Turning up the volume on mutational pressure: is more of a good thing always better? (A case study of HIV-1 Vif and APOBEC3). Retrovirology 5 26
70. MulderLCF
HarariA
SimonV
2008 Cytidine deamination induced HIV-1 drug resistance. Proc Natl Acad Sci USA 105 5501 5506
71. ParienteN
SierraS
AiraksinenA
2005 Action of mutagenic agents and antiviral inhibitors on foot-and-mouth disease virus. Virus Res 107 183 193
72. ParienteN
AiraksinenA
DomingoE
2003 Mutagenesis versus inhibition in the efficiency of extinction of foot-and-mouth disease virus. J Virol 77 7131 7138
73. ArnoldJJ
VignuzziM
StoneJK
AndinoR
CameronCE
2005 Remote site control of an active site fidelity checkpoint in a viral RNA-dependent RNA polymerase. J Biol Chem 280 25706 25716
74. PfeifferJK
KirkegaardK
2003 A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc Natl Acad Sci U S A 100 7289 7294
75. PfeifferJK
KirkegaardK
2005 Increased fidelity reduces poliovirus fitness and virulence under selective pressure in mice. PLoS Pathog 1 e11 doi:10.1371/journal.ppat.0010011
76. VignuzziM
StoneJK
ArnoldJJ
CameronCE
AndinoR
2006 Quasispecies diversity determines pathogenesis through cooperative interactions in a viral population. Nature 439 344 348
77. VignuzziM
WendtE
AndinoR
2008 Engineering attenuated virus vaccines by controlling replication fidelity. Nat Med 14 154 161
78. RacanielloVR
2006 One hundred years of poliovirus pathogenesis. Virology 344 9 16
79. RenRB
CostantiniF
GorgaczEJ
LeeJJ
RacanielloVR
1990 Transgenic mice expressing a human poliovirus receptor: a new model for poliomyelitis. Cell 63 353 362
80. BushmanFD
HoffmannC
RonenK
MalaniN
MinkahN
2008 Massively parallel pyrosequencing in HIV research. Aids 22 1411 1415
81. ErikssonN
PachterL
MitsuyaY
RheeSY
WangC
2008 Viral population estimation using pyrosequencing. PLoS Comput Biol 4 e1000074 doi:10.1371/journal.pcbi.1000074
82. Margeridon-ThermetS
ShulmanNS
AhmedA
ShahriarR
LiuT
2009 Ultra-deep pyrosequencing of hepatitis B virus quasispecies from nucleoside and nucleotide reverse-transcriptase inhibitor (NRTI)-treated patients and NRTI-naive patients. J Infect Dis 199 1275 1285
83. MazurkiewiczP
TangCM
BooneC
HoldenDW
2006 Signature-tagged mutagenesis: barcoding mutants for genome-wide screens. Nat Rev Genet 7 929 939
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



