-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
RNA Virus Replication Complexes
article has not abstract
Published in the journal: . PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000943
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1000943Summary
article has not abstract
The majority of viruses infecting animals and plants today are RNA viruses [1]. There are double-stranded (ds) RNA viruses with dsRNA genomes, as well as (+) and (−)RNA viruses whose genomes are single-stranded (ss) RNA of either positive or negative polarity. RNA viruses have small genomes that rarely exceed 30 kb in size, and a large portion of their genomes is used to encode proteins involved in viral RNA replication. Viral RNA synthesis is catalyzed by the virally encoded RNA-dependent RNA polymerase (RdRp), which lacks any proofreading activity. Although the details of RNA replication greatly vary among viruses, common principles clearly exist in the organization of the replication machinery of different RNA viruses.
(+)RNA Viruses Replicate Their Genomes on the Surface of Host Membranes
For many (+)RNA viruses, RNA replication requires viral enzymes such as RdRp, helicase, capping enzymes, and NTPase, as well as non-enzymatic proteins that participate in the assembly of the viral replication complex. Host cell proteins often play essential roles in (+)RNA virus replication as well. (+)RNA virus replication is asymmetric: the synthesis of (+)RNA, the one that is packaged into the progeny virus, is up to ∼100 times greater than that of (−)RNA [1]. Both the structure of the replicase itself and the cis-acting signals in the viral RNA template have been implicated in regulating the relative levels of genomic and antigenomic RNAs.
Host membranes play an important role in (+)RNA virus replication, as the RNA replication complexes released from membranes during purification generally lose the ability to catalyze true RNA replication, although a limited activity may be retained. In general, the intracellular membranes of cells infected with (+)RNA viruses rearrange to form anchor sites for viral RNA replication complexes [2]. Membrane tethering of the replication complexes is facilitated by non-structural proteins with hydrophobic sequences that enable membrane integration or interaction. In picornavirus-infected cells, viral RNA replication occurs on the cytoplasmic surfaces of double-membrane vesicles derived from the endoplasmic reticulum (ER) [3]. Two-dimensional (2D) arrays of polymerase-containing oligomeric structures have been observed on the surface of these vesicles, and the structural integrity of these 2D arrays correlates with the cooperative RNA binding and RNA elongation activities of the polymerase [4]. In cells infected with the flock house virus, outer mitochondrial membranes invaginate to create open-necked spherules of ∼50 nm in size [5]. On average, each spherule contains ∼100 membrane-spanning, self-interacting protein A molecules (i.e., the multifunctional RNA replication factor) and two to four genomic RNA replication intermediates [5]. Membranes may function to expedite the assembly of replication complexes, to protect/sequester viral RNAs, and also to help segregate the products from templates during replication.
Although little is known about the structure of the membrane-associated RNA replication complexes, (+)RNA virus RdRps have been well characterized to have an overall right-handed shape with three domains, the fingers, palm, and the thumb (reviewed in [6]) (Figure 1A). The palm domain contains an invariant, central, four-stranded β-sheet, with five highly conserved motifs named A to E. Motifs A and C, which contain the sequence –DxxxxD – and –GDD–, respectively, play important roles in metal ion coordination and nucleotide substrate selection during catalysis. The fingers and thumb domains are located on opposite sides of the active site canyon. Most (+)RNA virus RdRps can initiate RNA synthesis de novo, but a number of them use viral protein primers (VPgs) and produce protein-linked genomes. In the RdRp from the foot-and-mouth disease virus, uridylated VPg occupies a site similar to the typical RNA primer in the active site canyon, with the key residue Tyr3 and its associated uridylate (UMP) projected into the active site [7].
dsRNA Viruses Replicate and Transcribe Their Genomes in Intact Core Particles
Genome replication by dsRNA viruses occurs in subviral particles. These subviral particles, also called the cores, have an intact viral capsid that encloses the viral genome and RdRp molecules. For complex, multi-layered dsRNA viruses, the core is derived from the virion by removing outer capsid proteins during entry. Simple dsRNA viruses without extracellular life cycles often do not have additional capsid layers beyond the core. Progeny cores are assembled from mRNAs, which are then replicated inside the particle to generate the dsRNA genome. The number of gene segments can vary from one to 12 for different dsRNA viruses.
To date, all seven families of encapsidated dsRNA viruses have been analyzed by cryo-EM or crystallography (Figure 1B) (see [8]–[14] for crystal structures). Except for the birnavirus, dsRNA viruses share structural similarity by having a characteristic, 120-subunit T = 1 capsid surrounding their genome. In viruses with high genomic RNA densities, the genome is packed into liquid crystalline arrays that give rise to concentric shells of density, 25–30 Å apart, in the particle interiors. Due to the lack of icosahedral symmetry, viral polymerase is invisible in the structure of the viruses. Reoviruses have ∼12 copies of the polymerase in each virion, each associated with one of the ten to 12 dsRNA gene segments that make up the viral genome. The polymerases are likely to be tethered to the capsid near 5-fold symmetry axes through noncovalent interactions [15]. In addition to the central polymerase domain, dsRNA virus RdRps often have elaborate N - and C-terminal domains that keep the polymerase in the closed conformation (Figure 1A). In particular, the Reoviridae polymerase forms a rigid, caged structure with four well-defined tunnels that allow the inward and outward trafficking of the template, substrates, and products [16]. Capsid proteins play a key role in regulating polymerase function, as the polymerase alone is either inactive or exhibits only limited activities. This intra-particle mechanism of RNA synthesis coordinates genome packaging with replication during the infectious cycle.
Fig. 1. RNA virus replication machineries. 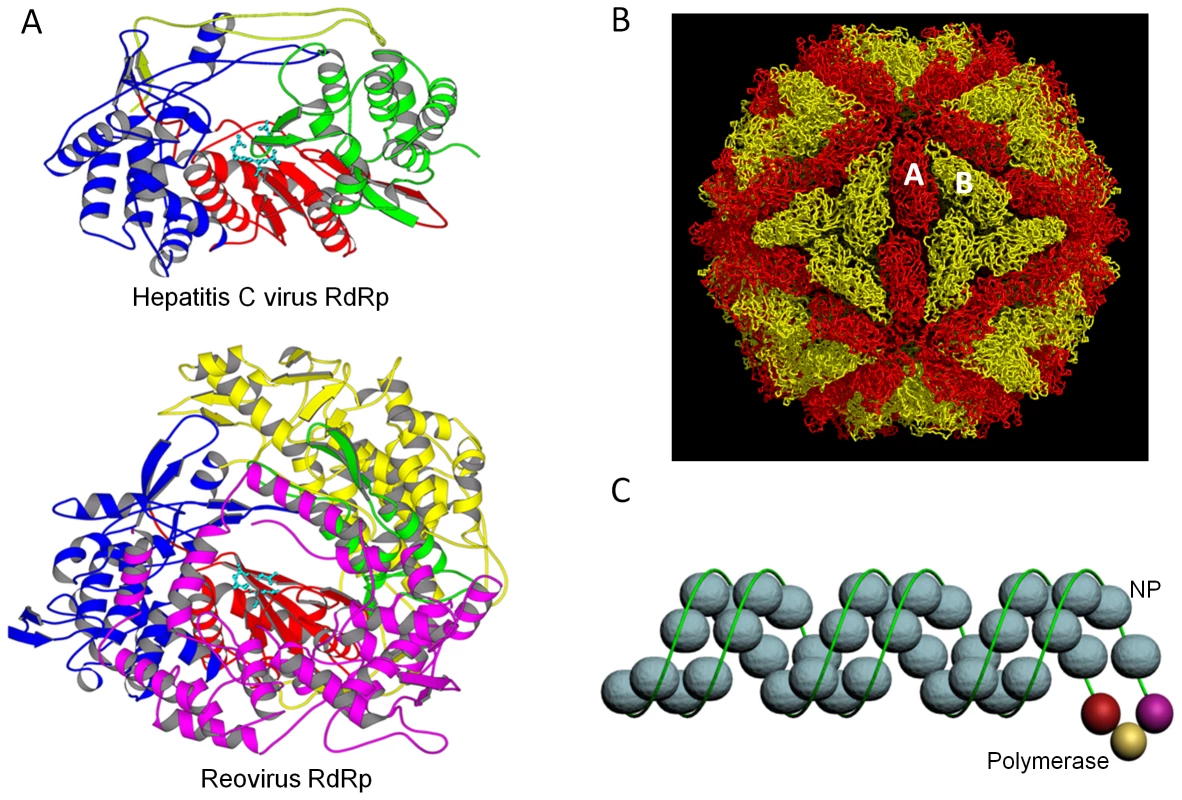
(A) RdRps of hepatitis C virus and reovirus. Hepatitis C virus is a (+)RNA virus from the Flaviviridae family, while reovirus is a dsRNA virus belonging to the Reoviridae family. In both structures, the fingers, palm, and the thumb of the polymerase are colored in blue, red, and green, respectively. Yellow and magenta highlight the N- and C-terminal domains, respectively. The three aspartic acid residues in the polymerase catalytic active site are shown in cyan. Reovirus RdRP has large N- and C-terminal domains with distinct functions: the N-terminal domain maintains the closed conformation of the active site, and the C-terminal domain serves as a processivity factor like a sliding clamp. (B) The yeast L-A virus (Totiviridae family). The two independent sets of capsid protein molecules, 60 copies each, are shown in red and yellow, respectively. The viral RdRP is likely to be tethered to the inner capsid near the 5-fold symmetry axis, and viral transcripts made inside the core are released through channels on or near the 5-fold axes. (C) An influenza A virus (Orthomyxoviridae family) RNP model. Blue spheres represent NP monomers, and the green line shows vRNA. A short duplex formed between the 5′ and the 3′ ends provides the binding site for the heterotrimeric RdRp. Overall, the RNP assumes a rod-shaped, double-helical structure that remains intact even after the vRNA is removed. A key question about dsRNA virus replication is how the (−)RNA template is selected from the dsRNA genome during transcription. A 5′-cap binding site, identified on the reovirus polymerase, provides a neat solution for some dsRNA viruses of high eukaryotic hosts [16]. Because only the (+)RNA strand is capped, cap binding keeps the polymerase proximal to the 3′-end of the (−)RNA strand, thus allowing efficient initiation of transcription. Negative charges on the inner surface of dsRNA viral capsids may help to facilitate viral genome movement during transcription. Viral transcripts are likely to exit the core through pores on or near the 5-fold symmetry axes.
(−)RNA Viruses Replicate Using Protein-Coated Templates and Generate Protein-Stabilized, Single-Stranded RNAs
(−)RNA viruses have a helical-shaped, protein-coated genome, also known as the ribonucloeprotein complex (RNP), which functions as the sole template for viral RNA replication and transcription (Figure 1C). Newly made viral RNA (vRNA) and the replication intermediate (cRNA) are packaged as RNPs and stabilized in a single-stranded form immediately after their synthesis.
The composition of the RNA replication machinery differs between segmented and non-segmented (−)RNA viruses. The influenza A virus, a segmented (−)RNA virus, contains eight vRNAs, each packaged as a double helical-shaped RNP with multiple nucleoproteins (NPs) and a heterotrimeric polymerase (PA, PB1, and PB2) that is bound to the conserved vRNA termini (Figure 1C). Each NP molecule binds to a 24-nt RNA at the outer periphery of the NP scaffold [17]. The influenza A virus polymerase has a compact structure [17] with at least four active sites: the polymerase active site and a 5′ vRNA binding site in PB1, an endonuclease active site in PA [18], [19], and a cap binding site in PB2 [20]. During transcription, the polymerase uses a short capped RNA fragment snatched from cellular mRNAs to initiate RNA synthesis. To switch from transcription to replication mode, it is necessary to change from capped RNA-primed initiation to unprimed initiation and to prevent pre-mature termination and polyadenylation. Free NP is essential for viral RNA replication, possibly because (1) NP interacts with the polymerase, thus altering its activity [21], or (2) NP prevents the degradation of the cRNA intermediate by forming RNP structures [22]. It has been proposed that viral RNA transcription is catalyzed by the polymerase associated with the vRNP template, whereas RNA replication is catalyzed by a newly made polymerase molecule [23]. The specific binding of the viral polymerase to the 5′-end of newly synthesized vRNAs presumably helps to recruit additional NPs, thus ensuring specific encapsidation of vRNA.
RNA replication of non-segmented (-)RNA viruses, such as the rabies virus and the vesicular stomatitis virus (VSV), requires an intricate interplay of the nucleoprotein N, the large protein L, a non-enzymatic P, and the N-enwrapped genome, also called the nucleocapsid [24]. In the nucleocapsid, each N protein has two lobes angled together to form a cavity that accommodates seven or nine bases of RNA [25]–[27]. In addition to nucleotide polymerization, L also possesses enzymatic activities for 5′ cap synthesis. During RNA synthesis, P delivers L to the active template through an N-P interaction that involves two adjacent N proteins in the nucleocapsid [28]. L-P binding to the N-RNA template triggers conformational changes in the nucleocapsid and allows the polymerase access to RNA.
Summary
Different replication strategies are used by (+), (−), and dsRNA viruses to limit dsRNA exposure, to regulate (+)RNA versus (−)RNA synthesis, and to modulate transcription versus replication throughout the infection cycle. The differences in the functionalities of the replication complexes are reflected in their structural composition, their subcellular localization, and in the interaction of the complex with viral RNA templates. It is expected that studies of RNA virus replication machineries will have a large impact on antiviral drug development due to their specific activities in virus replication.
Zdroje
1. BallLA
2007 Virus Replication Strategies.
KnipeD
HowleyP
Fields Virology Philadelphia Lippincott Williams & Wilkins 120 140 5 ed
2. SalonenA
AholaT
KaariainenL
2005 Viral RNA replication in association with cellular membranes. Curr Top Microbiol Immunol 285 139 173
3. EggerD
TeterinaN
EhrenfeldE
BienzK
2000 Formation of the poliovirus replication complex requires coupled viral translation, vesicle production, and viral RNA synthesis. J Virol 74 6570 6580
4. LyleJM
BullittE
BienzK
KirkegaardK
2002 Visualization and functional analysis of RNA-dependent RNA polymerase lattices. Science 296 2218 2222
5. KopekBG
PerkinsG
MillerDJ
EllismanMH
AhlquistP
2007 Three-dimensional analysis of a viral RNA replication complex reveals a virus-induced mini-organelle. PLoS Biol 5 e220 doi:10.1371/journal.pbio.0050220
6. NgKK
ArnoldJJ
CameronCE
2008 Structure-function relationships among RNA-dependent RNA polymerases. Curr Top Microbiol Immunol 320 137 156
7. Ferrer-OrtaC
AriasA
AgudoR
Perez-LuqueR
EscarmisC
2006 The structure of a protein primer-polymerase complex in the initiation of genome replication. Embo J 25 880 888
8. ReinischKM
NibertML
HarrisonSC
2000 Structure of the reovirus core at 3.6 A resolution. Nature 404 960 967
9. GrimesJM
BurroughsJN
GouetP
DiproseJM
MalbyR
1998 The atomic structure of the bluetongue virus core. Nature 395 470 478
10. NakagawaA
MiyazakiN
TakaJ
NaitowH
OgawaA
2003 The atomic structure of rice dwarf virus reveals the self-assembly mechanism of component proteins. Structure 11 1227 1238
11. CoulibalyF
ChevalierC
GutscheI
PousJ
NavazaJ
2005 The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell 120 761 772
12. NaitowH
TangJ
CanadyM
WicknerRB
JohnsonJE
2002 L-A virus at 3.4 A resolution reveals particle architecture and mRNA decapping mechanism. Nat Struct Biol 9 725 728
13. PanJ
DongL
LinL
OchoaWF
SinkovitsRS
2009 Atomic structure reveals the unique capsid organization of a dsRNA virus. Proc Natl Acad Sci U S A 106 4225 4230
14. DuquerroyS
Da CostaB
HenryC
VigourouxA
LibersouS
2009 The picobirnavirus crystal structure provides functional insights into virion assembly and cell entry. Embo J 28 1655 1665
15. ZhangX
WalkerSB
ChipmanPR
NibertML
BakerTS
2003 Reovirus polymerase lambda 3 localized by cryo-electron microscopy of virions at a resolution of 7.6 A. Nat Struct Biol 10 1011 1018
16. McDonaldSM
TaoYJ
PattonJT
2009 The ins and outs of four-tunneled Reoviridae RNA-dependent RNA polymerases. Curr Opin Struct Biol 19 775 782
17. ColomaR
ValpuestaJM
ArranzR
CarrascosaJL
OrtinJ
2009 The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog 5 e1000491 doi:10.1371/journal.ppat.1000491
18. YuanP
BartlamM
LouZ
ChenS
ZhouJ
2009 Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature 458 909 913
19. DiasA
BouvierD
CrepinT
McCarthyAA
HartDJ
2009 The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature 458 914 918
20. GuilligayD
TarendeauF
Resa-InfanteP
ColomaR
CrepinT
2008 The structural basis for cap binding by influenza virus polymerase subunit PB2. Nat Struct Mol Biol 15 500 506
21. NewcombLL
KuoRL
YeQ
JiangY
TaoYJ
2009 Interaction of the influenza a virus nucleocapsid protein with the viral RNA polymerase potentiates unprimed viral RNA replication. J Virol 83 29 36
22. VreedeFT
JungTE
BrownleeGG
2004 Model suggesting that replication of influenza virus is regulated by stabilization of replicative intermediates. J Virol 78 9568 9572
23. JorbaN
ColomaR
OrtinJ
2009 Genetic trans-complementation establishes a new model for influenza virus RNA transcription and replication. PLoS Pathog 5 e1000462 doi:10.1371/journal.ppat.1000462
24. WhelanSP
BarrJN
WertzGW
2004 Transcription and replication of nonsegmented negative-strand RNA viruses. Curr Top Microbiol Immunol 283 61 119
25. GreenTJ
ZhangX
WertzGW
LuoM
2006 Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science 313 357 360
26. AlbertiniAA
WernimontAK
MuziolT
RavelliRB
ClapierCR
2006 Crystal structure of the rabies virus nucleoprotein-RNA complex. Science 313 360 363
27. TawarRG
DuquerroyS
VonrheinC
VarelaPF
Damier-PiolleL
2009 Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 326 1279 1283
28. GreenTJ
LuoM
2009 Structure of the vesicular stomatitis virus nucleocapsid in complex with the nucleocapsid-binding domain of the small polymerase cofactor, P. Proc Natl Acad Sci U S A 106 11713 11718
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 7- Stillova choroba: vzácné a závažné systémové onemocnění
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
- Diagnostický algoritmus při podezření na syndrom periodické horečky
-
Všechny články tohoto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



