-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
The process of fertilization is critically dependent on the mutual recognition of gametes and in Plasmodium, the male gamete surface protein P48/45 is vital to this process. This protein belongs to a family of 10 structurally related proteins, the so called 6-cys family. To identify the role of additional members of this family in Plasmodium fertilisation, we performed genetic and functional analysis on the five members of the 6-cys family that are transcribed during the gametocyte stage of P. berghei. This analysis revealed that in addition to P48/45, two members (P230 and P47) also play an essential role in the process of parasite fertilization. Mating studies between parasites lacking P230, P48/45 or P47 demonstrate that P230, like P48/45, is a male fertility factor, consistent with the previous demonstration of a protein complex containing both P48/45 and P230. In contrast, disruption of P47 results in a strong reduction of female fertility, while males remain unaffected. Further analysis revealed that gametes of mutants lacking expression of p48/45 or p230 or p47 are unable to either recognise or attach to each other. Disruption of the paralog of p230, p230p, also specifically expressed in gametocytes, had no observable effect on fertilization. These results indicate that the P. berghei 6-cys family contains a number of proteins that are either male or female specific ligands that play an important role in gamete recognition and/or attachment. The implications of low levels of fertilisation that exist even in the absence of these proteins, indicating alternative pathways of fertilisation, as well as positive selection acting on these proteins, are discussed in the context of targeting these proteins as transmission blocking vaccine candidates.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000853
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000853Summary
The process of fertilization is critically dependent on the mutual recognition of gametes and in Plasmodium, the male gamete surface protein P48/45 is vital to this process. This protein belongs to a family of 10 structurally related proteins, the so called 6-cys family. To identify the role of additional members of this family in Plasmodium fertilisation, we performed genetic and functional analysis on the five members of the 6-cys family that are transcribed during the gametocyte stage of P. berghei. This analysis revealed that in addition to P48/45, two members (P230 and P47) also play an essential role in the process of parasite fertilization. Mating studies between parasites lacking P230, P48/45 or P47 demonstrate that P230, like P48/45, is a male fertility factor, consistent with the previous demonstration of a protein complex containing both P48/45 and P230. In contrast, disruption of P47 results in a strong reduction of female fertility, while males remain unaffected. Further analysis revealed that gametes of mutants lacking expression of p48/45 or p230 or p47 are unable to either recognise or attach to each other. Disruption of the paralog of p230, p230p, also specifically expressed in gametocytes, had no observable effect on fertilization. These results indicate that the P. berghei 6-cys family contains a number of proteins that are either male or female specific ligands that play an important role in gamete recognition and/or attachment. The implications of low levels of fertilisation that exist even in the absence of these proteins, indicating alternative pathways of fertilisation, as well as positive selection acting on these proteins, are discussed in the context of targeting these proteins as transmission blocking vaccine candidates.
Introduction
Sexual reproduction is an obligate process in the Plasmodium life cycle and is required for transmission of the parasites between the vertebrate and mosquito hosts. The sexual phase is initiated by the formation of male and female cells (gametocytes) in the blood of the vertebrate host. Gametocytes are the precursors to the haploid male and female gametes that are produced in the mosquito midgut where fertilisation takes place. Successful fertilisation requires an ordered series of gamete-gamete interactions, specifically, the recognition of and adhesion to the female gamete by the motile male gamete, followed by a cascade of signalling events resulting from the fusion of the two gametes.
Despite their fundamental importance, relatively little is known about gamete receptors/ligands and their involvement in the process of gamete interactions of eukaryotes [1], [2], which is partly due to their rapid evolution and species-specific characteristics [3]. In Plasmodium the involvement of two gamete specific surface proteins P48/45 and HAP2/GCS1 has been demonstrated in male fertility and these proteins are to date the only known proteins with a demonstrable role in gamete-gamete interaction [4], [5], [6]. Parasites lacking P48/45 produce male gametes that fail to attach to fertile female gametes [4] while male gametes lacking of HAP2/GCS1 do attach to females, but they do not fuse due to an absence of membrane fusion between the two gametes [5]. P48/45 is one member of a family of proteins encoded within the genome of Plasmodium and this family is characterised by domains of roughly 120 amino acids in size that contain six positionally conserved cysteines (6-cys). The 6-cys family of proteins appears to be Apicomplexan specific and has a predicted relationship to the SAG proteins in Toxoplasma gondii [7], [8], [9], [10], [11]. Ten members of the 6-cys family have been identified. Most members are expressed in a discrete stage-specific manner in gametocytes, sporozoites or merozoites [8], [12], [13], [14], [15], [16]. The surface location of members of this family and their expression in gametes or in invasive stages (sporozoites and merozoites) suggests that they function in cell-cell interactions as has been shown for P48/45 in gamete adhesion. In addition to P48/45, five other 6-cys genes are transcribed in gametocytes, three of which (p230, p230p and p47) are exclusively expressed in the gamete stages of the malaria parasite [4], [8], [10], [12], [16], [17], [18], [19], indicating that these members of the gene family may also play a role in the process of gamete recognition and fertilisation. Indeed specific antibodies against the sexual stages of the human parasite Plasmodium falciparum, P48/45 and P230 can prevent zygote formation and thus block transmission of the parasite [19], [20], [21], [22], [23], [24], [25], [26]. Interestingly, P. falciparum mutants lacking P230 expression produce male gametes that fail to attach to erythrocytes resulting in a reduced formation of the characteristic ‘exflagellation centres’ and reduced oocyst formation in mosquitoes [27]. In order to investigate the role of the 6-cys proteins in parasite fertilisation we performed genetic and functional analysis on the five 6-cys proteins that are expressed in gametocytes. In this paper, we present evidence that in addition to P48/45, two 6-cys members (P230 and P47) also have an essential role in parasite fertilization. Interestingly, in P. falciparum evidence has been published that P48/45, P47 and P230 are under positive selection resulting in non-neutral sequence polymorphisms [28], [29], [30], [31]. By sequence analysis, we provide evidence that these three 6-cys proteins are undergoing strong but different rates of positive selection, either as a consequence sexual-selection driven by the competition between gametes or from natural selection exerted by the adaptive immune system of the host on proteins expressed in gametocytes.
Materials and Methods
Parasites
The gametocyte-producer clone cl15cy1 (HP) of P. berghei ANKA was used as the reference parasite line [32]. In addition, the following mutant lines of the ANKA strain were used: 2.33, a non-gametocyte producer (NP) line [33] and 137cl8 (RMgm-15, www.pberghei.eu), a mutant lacking expression of P48/45 [4].
Generation of mutants deficient in expressing 6-cys family members
To disrupt genes encoding different members of the 6-cys family, we constructed a number replacement constructs using plasmid pL0001 (www.mr4.com) which contains the pyrimethamine resistant Toxoplasma gondii (tg) dhfr/ts as a selectable-marker cassette (SC). Target sequences for homologous recombination were PCR amplified from P. berghei genomic DNA (ANKA, cl15cy1) using primers specific for the 5′ or 3′ end of the different 6-cys genes (see Table S1 for the sequence of the different primers). The PCR–amplified target sequences were cloned in plasmid pL0001 either upstream or downstream of the SC to allow for integration of the construct into the genomic target sequence by homologous recombination. DNA constructs used for transfection were obtained after digestion of the replacement constructs with the appropriate restriction enzymes (Table S1). Replacement constructs pL1138 (p47) and pL0123 (p36), were constructed using replacement plasmid pDB.DT∧H.DB [34] and plasmid pL0121 (p47&48/45) was constructed in the previously described replacement plasmid for disruption of pb48/45 (plasmid p54 is renamed here to pL1137; [4]). This plasmid was made by exchanging the 5′ pb48/45 targeting sequence with the 5′ targeting sequence of pb47. The p230pII replacement construct pL0120 is a derivative of plasmid pL0016 [35] containing the tgdhfr-ts SC, gfp (under control of the pbeef1aa promoter and 3′UTR of pbdhfr/ts) and p230p 5′ and 3′ targeting sequences [36]. Transfection, selection and cloning of mutant parasite lines were performed as described [32], [37] using P. berghei ANKA cl15cy1 as the parent reference line. For all mutants with an observable phenotype, mutants were generated and selected in two independent transfection experiments (Table S1). Of each transfection experiment we selected one cloned line for further genotype and phenotype analysis. Correct integration of the construct into the genome of mutant parasites was analysed by standard PCR analysis and Southern blot analysis of digested genomic DNA or of FIGE separated chromosomes [32]. PCR analysis on genomic DNA was performed using specific primers to amplify either part of the wild type locus (primers WT1 and 2) or the disrupted locus (primers INT1 and 2). See Table S2 for the sequence of these primers.
Analysis of expression by Northern and Western analysis
Total RNA was isolated from the different blood stage parasites of the gametocyte-producer clone cl15cy1 of P. berghei ANKA (HP), the non-gametocyte producer line 2.33 (NP) and the different mutant lines according to standard methods. To determine stage-specific transcription of the 6-cys family members, Northern blots containing RNA from different blood stages were hybridised with different gene specific probes, which were PCR-amplified using the primers shown in Table S2 (primer pairs WT1+ 2). To detect expression of the P48/45 protein we used polyclonal antiserum raised against recombinant P. berghei P48/45 as described [4]. For detection of P47 we generated the following polyclonal antiserum; a fragment of the Pb47 ORF (encoding amino acids 80–411) was PCR-amplified using primers L964 and L965 (Table S2) and cloned into the NdeI/BamHI sites of the expression vector pET-15b (Novagen) providing an N-terminal 6-Histidine tag. Polyclonal antiserum was raised in New Zealand rabbits by injection of 200 µg of gel-purified recombinant protein. Boosting was carried out subcutaneously with 3-weeks intervals using 200 µg protein in incomplete Freund's adjuvant. Serum (P47) obtained 2 weeks after the third boost was immuno-purified on immobilised purified recombinant P47. To detect P48/45 and P47 in the different mutant lines, total protein samples of purified gametocytes were fractionated on non-reducing 10% SDS polyacrylamide gels.
Phenotype analysis of parasite lines lacking expression of 6-cys gene family members
The fertility of wild type and mutant gamete populations was analysed by standard in vitro fertilisation and ookinete maturation assays [4], [17] from highly pure gametocyte populations [38]. The fertilisation rate of gametes is defined as the percentage of female gametes that develop into mature ookinetes determined by counting female gametes and mature ookinetes in Giemsa stained blood smears 16–18 hours after in vitro induction of gamete formation. Fertility of individual sexes (macro - and micro-gametes) was determined by in vitro cross-fertilisation studies in which gametes are cross-fertilised with gametes of lines that produce only fertile male (Δp47; 270cl1) or only fertile female gametes (Δp48/45; 137cl1 [4], [17], [39]. All fertilisation and ookinete maturation assays were done in triplicate on multiple occasions in independent experiments. In vivo ookinete, oocyst and salivary gland sporozoite production of the mutant parasites were determined by performing standard mosquito infections by feeding of Anopheles stephensi mosquitoes on infected mice [40]. Oocyst numbers and salivary gland sporozoites were counted at 7–10 days and 21–22 days respectively after mosquito infection. For counting sporozoites, salivary glands from 10 mosquitoes were dissected and homogenized in a homemade glass grinder in 1000µl of PBS pH 7.2 and sporozoites were counted in a Bürker-Türk counting chamber using phase-contrast microscopy [41]. Infectivity of sporozoites was determined by infecting mice through bites of 25–30 infected mosquitoes at day 21–25 after mosquito infection.
The formation of exflagellation centres (i.e. male gamete interactions with red blood cells) was determined by adding 10µl of infected tail blood to 100–300 µl of standard ookinete culture medium pH 8.2 to induce gamete formation. Ten minutes after induction of gamete formation a droplet of 5–10 µl was placed on a cover slip and analysed under a standard light microscope (40× magnification) as a hanging-drop using a well slide. When red blood cells were settled in a monolayer, the number of exflagellating male gametocytes was counted that form or did not form exflagellation centres. An exflagellation centre is defined as an exflaggelating male gametocyte with more than four tightly associated red blood cells [27]. The formation of exflagellation centres was performed using tail blood collected at day 6 or 7 from mice that were infected with 105 parasites without treatment with phenylhydrazine. For quantification of male-female interactions tail blood was collected from phenylhydrazine-treated mice with high numbers of gametocytes [42]. Tail blood (10µl) was collected at gametocytemias ranging between 4–8% and added to 100µl of standard ookinete culture medium pH 8.2 to induce gamete formation. Ten minutes after induction of gamete formation, the cell suspension was placed in a Bürker-Türk counting chamber and during a period of twenty minutes the male-female interactions were scored using a phase-contrast light microscope at a 40× magnification. Attachments of males to females were scored if the male had active (attachment-) interactions with the female for more than 3 seconds. Penetration of a female by the male gamete was scored as a fertilisation event.
Polymorphisms and sequence divergence of the Plasmodium 6-cys genes
Pairwise alignments were generated between the orthologous sequences of p48/45, p47 and p230 genes in P. berghei, P. yoelii and P. chabaudi; sequences were obtained from PlasmoDB (http://www.plasmodb.org version 6.1; see Table S3 for the accession numbers of the 6-cys gene family members). Complete gene sequences for a number of these genes were obtained from the Sanger Institute (A. Pain, personal communication). Maximum-likelihood estimates of rates of non-synonymous substitution (dN) and synonymous substitution (dS) between pairwise alignments were generated using the PAML algorithm (version 3.14; [43], [44]) using a codon-based model of sequence evolution [45], [46], with dN and dS as free parameters and average nucleotide frequencies estimated from the data at each codon position (F3×4 MG model [47]). For this analysis we assumed a transition/transversion bias (i.e. kappa value) that had been estimated previously and found to be similar in case of P. falciparum and P. yoelii, i.e. 1.53 [48]. A sliding window analysis of dN/dS ratios was performed of p230, p47 and p48/45 from the three rodent parasites. We analysed the dN/dS values of these genes across their length by analysing sequentially 300bp of the gene in 150bp steps. This analysis is essentially the same as the calculation of π (i.e. the number segregating or polymorphic sites) described for p48/45 in distinct P. falciparum isolates described by Escalante et al. [29]. We obtained the single nucleotide polymorphisms (SNPs) data identified from field and laboratory isolates of P. falciparum (excluding all P. reichenowi SNPs) from PlasmoDB (www.PlasmoDB.org). The alignment of these SNPs along the different genes (to scale) was extracted from the Genome Browser page of PlasmoDB. The locations of the SNPs were aligned onto the schematic representation of the 6-cys genes of the rodent parasites. It should be noted that the alignment of the p230 gene of the different Plasmodium species was only possible around 1008bp after the putative start site. In order to determine which residues of p230, p47 and p48/45 genes were under positive selection in the rodent malaria parasites, a Bayes Empirical Bayes (BEB) analysis was performed using sequences from the 3 rodent genomes and was calculated as described in Yang et al. [49]. To test which genes were undergoing positive selection the likelihood ratio test (LRT) was performed using a comparison of site specific models of evolution [50], [51]. This test compares a ‘nearly neutral’ model (without any residues under positive selection) and a ‘positive selection’ model (with residues under positive selection and therefore under adaptive evolution). Both models assume that there are different categories of codons, which evolve with different speeds. The ‘nearly neutral’ model assumes two categories of sites at which amino acid replacements are either neutral (dN/dS = 1) or deleterious (dN/dS<1). The ‘positive-selection’ model assumes an additional category of positively selected sites at which non-synonymous substitutions occur at a higher rate than synonymous ones (dN/dS>1). Likelihood values indicate how well a model fits to the analyzed alignment and answers the question if the ‘positive selection’ model fits better to the analyzed alignment than the ‘nearly neutral’ model.
Animal ethics statement
All animal experiments were performed after a positive recommendation of the Animal Experiments Committee of the LUMC (ADEC) was issued to the licensee. The Animal Experiment Committees are governed by section 18 of the Experiments on Animals Act and are registered by the Dutch Inspectorate for Health, Protection and Veterinary Public Health, which is part of the Ministry of Health, Welfare and Sport. The Dutch Experiments on Animal Act is established under European guidelines (EU directive no. 86/609/EEC regarding the Protection of Animals used for Experimental and Other Scientific Purposes).
Results
Four out of ten members of the 6-cys family of P. berghei are specifically transcribed in gametocytes
Ten members of the 6-cys family have been identified in Plasmodium and are found in all Plasmodium species (Table S3). We analysed the transcription profile of the 10 members during blood stage development of P. berghei by Northern blot analysis and combined this analysis with a search of publicly available literature, transcriptome and proteome datasets. This method established that multiple members are transcribed in gametocytes of which four members, p48/45, p47, p230, p230p, are transcribed exclusively in the gametocyte stage (Fig. 1A). The gametocyte specific expression of p48 and p230p has been shown before [4], [8]. Transcription of p38 occurs both in gametocytes and in asexual blood stages as has also been reported [8], whereas p12 is transcribed in all blood stages. The relative weak band observed in gametocytes might be due to low contamination of the gametocyte preparation with asexual blood stages (gametocyte samples always contain a small degree of contamination with schizonts when density gradients are used for gametocyte purification). Transcription of p41 and p12p show a complex pattern of multiple transcripts in all blood stages. The close paralogue pair p36 and p36p have quite different transcriptional profiles: p36p is not transcribed in blood stages but transcription is exclusive to sporozoites [14], [15] whereas p36 is transcribed both in gametocytes (Fig. 1B; [8], [52]) and in sporozoites [14], [15].
Fig. 1. Expression of the 10 members of the 6-cys family of Plasmodium. 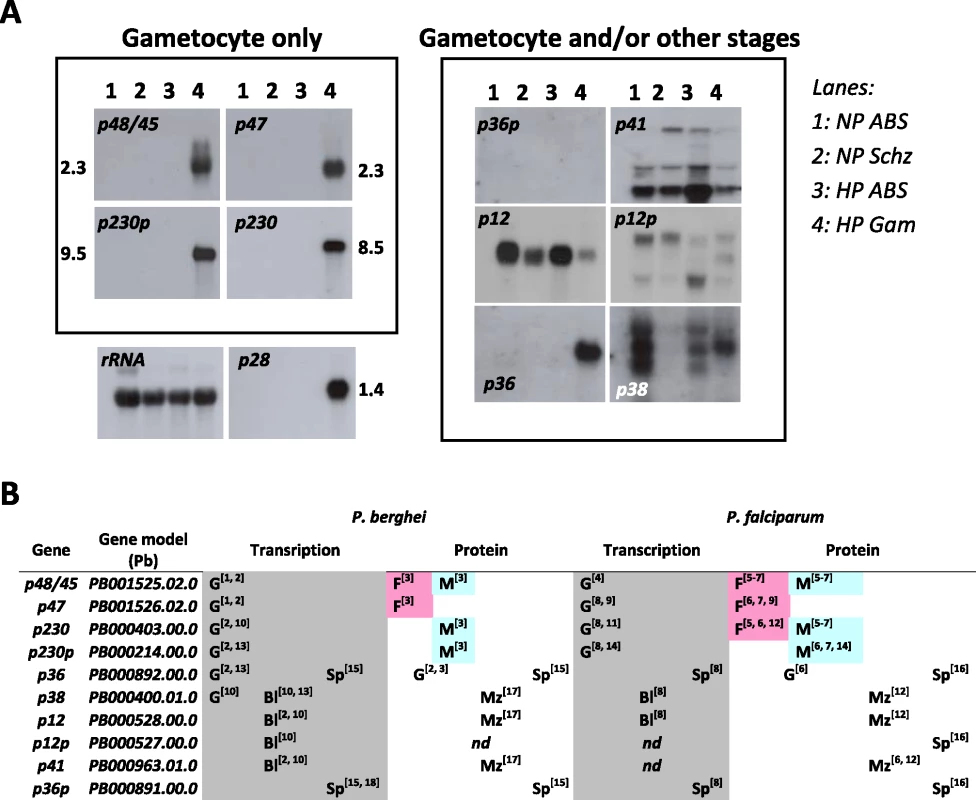
A. Northern blot analysis of transcription of the 10 P. berghei genes during blood stage development of a gametocyte non-producer (NP) and a high producer (HP) line. The left panel shows the four genes that are exclusively expressed in gametocytes. P36 and p36p are shown in the right panel since they are also expressed in the sporozoite stage (see B). As (loading) controls Northern blots were hybridized to probes recognising LSU rRNA (87R primer) and the gametocyte specific gene p28. Lanes: 1) NP asynchronous blood stages (ABS); 2) NP schizonts (Schz); 3) HP asynchronous blood stages; 4) HP purified gametocytes (Gam). B. Transcription and protein expression of the 10 genes determined by RNA and proteomic analyses (G = gametocyte; F = female gametocyte; M = Male gametocyte; Bl = blood stage; Mz = merozoite; Sp = sporozoite). References: 1 [4]; 2 [52]; 3 [17]; 4 [18]; 5 [71]; 6 [72]; 7 [73]; 8 [74]; 9 [12]; 10 [75]; 11 [10]; 12 [13]; 13 [8]; 14 [16]; 15 [14]; 16 [41]; 17 [76]; 18 [15]. Since no polyclonal or monoclonal antibodies exist for most of the 6-cys family members of P. berghei, except for P48/45 [4], P47 (this study) , P36 and P36p [14], data on expression of these proteins in different life cycle stages mainly comes from large-scale proteome analyses. For most members of the 6-cys family which have been detected by proteome analysis, the presence of the protein coincides with transcription of its gene (Fig. 1B). The exclusive presence of P48/45, P47, P230 and p230p in the proteomes of gametocytes corresponds to the transcription pattern of their respective genes. The presence of P48 and P47 in P. berghei gametocytes has been confirmed using polyclonal antibodies against these proteins (Fig. S1; [4]). P12, P38 and P41 have been detected in the proteome of merozoites which agrees with their transcription in the asexual blood stages and with their identification in the raft-like membrane proteome of the P. falciparum merozoite surface [13]. Also the presence of P36 in proteomes of both gametocytes and sporozoites [41], [52] and P36p in sporozoites [14], [41] fits with the transcription profile of these genes. Up to now only P12p has not been detected in any proteome of Plasmodium. Comparison of the transcription and expression patterns of the 10 conserved members of the 6-cys family of P. berghei with those of P. falciparum from large scale transcriptome and proteome analyses demonstrates that the expression patterns are conserved between the rodent and human parasite (Fig. 1B) and also confirms that four out of the 10 members are specific to the gametocyte stage.
Three out of 4 members of the 6-cys family of P. berghei that are specifically transcribed in gametocytes play a role in fertilisation
We previously reported the functional analysis of mutant P. berghei parasites that were deficient in expressing P48/45, generated by targeted disruption of p48/45 through a double crossover homologous recombination event [4]. Here we have used the same approach, schematically shown in Fig. 2A, to disrupt 5 other members of the 6-cys family that are transcribed in gametocytes. We excluded p12, p12p, p41 and p36p from this analysis since the results obtained from transcriptome and proteome analyses indicate a role for the first three of these genes during the asexual blood stage development (Fig. 1B). We have previously demonstrated in both, P. berghei and P. falciparum, that P36p is involved in liver-cell infection and disruption of its gene had no effect on development of gametes and fertilisation [15], [53]. Mutant parasite lines have been generated deficient in P47 (Δp47), P230 (Δp230), P230p (Δp230p), P38 (Δp38) or P36 (Δp36) and for each gene, mutants were selected from two independent transfection experiments (Table S1). Two different Δp230p mutant lines were generated, Δp230p-I and Δp230p-II, differing in which regions of 230p have been disrupted. In mutant Δp230-I a fragment is deleted from the second 6-cys domain (i.e. first 894aa still present) onwards whereas in mutant Δp230-II the deleted fragment includes part of the first 6-cys domain (i.e. first 492 amino acids still present). In addition we generated a mutant line deficient in the expression of both P48/45 and P47 (Δp48/45&Δp47). Correct disruption of the target-genes was verified by diagnostic PCR analysis (Fig. 2B) and Southern blot analysis of separated chromosomes and/or digested genomic DNA (data not shown). To demonstrate that the mutant parasite lines were deficient in expression of the targeted gene we analysed transcription of the corresponding genes by Northern blot analysis using mRNA collected from purified gametocytes (Fig. 2B). No transcripts of p47 and p38 could be detected in ΔP47 and Δp38 mutants, and no p48/45 and p47 transcripts are present in the DKO mutant Δp48/45&Δp47. Only small, truncated transcripts were detected for p230 and p230p in gametocytes of the Δp230 and Δp230p lines and also in Δp36 a truncated p36 transcript was found. Full length transcripts of wt p230 and p230p are 8.5 and 9.5 kb respectively, whereas truncated transcripts are approximately 2.5 kb in size. Since several of the disrupted genes are organised as pairs within the genome (i.e. p230&p230p and p48/45&p47), we analysed whether disruption of one member of a pair affected transcription of the other gene. For Δp48/45 parasites it has been shown before that disruption of p48/45 had no effect on expression of its paralog P47 [4]. In this study we similarly show for p47, p230 and p230p that disruption had no effect on transcription of its paralogous member (Fig. S1 A&B). In addition to the transcription analysis of the disrupted genes, we analysed the presence or absence of the proteins P47 and P48/45 in the mutant parasites by Western analysis using polyclonal antiserum (Fig. S1C). P47 is present in wt gametocytes and gametocytes of the Δp48/45 but is absent in Δp47 and Δp48/45&Δp47 gametocytes. P48/45 is present in wild type and absent in the Δp48/45&Δp47 gametocytes.
Fig. 2. Generation and analysis of mutants lacking expression of different members of the 6-cys family of genes. 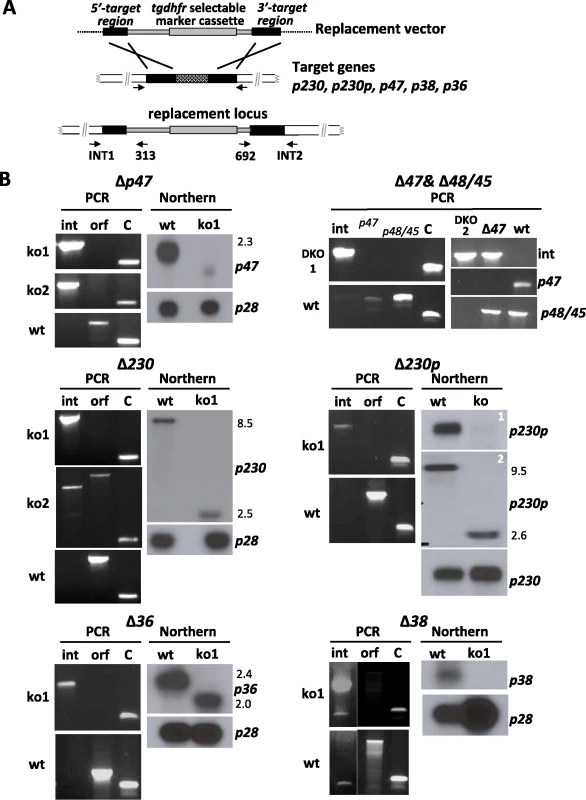
A. Schematic representation of the replacement construct used for disruption of the target genes by double cross-over homologous recombination. Correct integration of the construct results in disruption of target gene as shown (replacement locus) and is analysed by PCR (see B) using the primers INT1, 313, INT2 and 692 as shown in the figure and Table S1 and S2. Black boxes: the target regions of the 6-cys genes; grey box: the tgdhfr/ts selectable marker cassette. B. PCR analysis of correct disruption of the 6-cys genes and analysis of transcription of the genes in wild type and mutant (ko) parasite lines. PCRs were performed with primers that specifically amplify either the 5′ (INT1 and 313) or 3′ (INT2 and 692) regions of the disrupted locus (int). In addition PCR's to amplify the intact open reading frame (orf) were performed using genomic DNA of wild type parasites as a control (wt). The double knockout mutant Δp48/45&Δp47 was checked for both p47 and p48/45. Control PCR amplifying the gametocyte specific p28 gene (C). Northern blot analysis of transcription was performed using RNA extracted from gametocytes of wild type (wt) or mutant parasites. Blots were hybridised with 6-cys specific gene probes that were obtained by PCR amplification (see Table S2). As a control Northern blots were hybridized to a probe recognising the gametocyte specific gene p28. The sizes of transcripts (kb) are shown next to the Northern blots. We next analysed the phenotype of the different mutant lines during gametocyte and gamete development as well as during fertilisation, ookinete and oocyst formation using standard assays for phenotype analysis of the sexual - and mosquito stages of P. berghei. Surprisingly, three of the six mutants lacking expression of genes that are transcribed in gametocytes did not exhibit a phenotype that was different from wild type parasites during these stages of development. These mutants, Δp230p, Δp38 or Δp36, showed a normal growth of the asexual blood stage (data not shown), sexual development and development of the mosquito stages up to the mature oocysts (Table 1). All these mutant lines produced wild type numbers of gametocytes and gametes and showed normal fertilisation rates as measured by in vitro zygote/ookinete production (Table 1; Fig. 3). In contrast to the absence of a discernable fertilisation phenotype with the Δp230p, Δp38 and Δp36 mutants, we found that the capacity of fertilisation is severely affected in the other three mutants, (Fig. 3A). Specifically, Δp47, Δp230 and Δp48/45&Δp47 lines showed a fertilisation rate that was reduced by more than 99.9% compared to wt, as shown by the inhibition of zygote/ookinete production in vitro (Table 1; Fig. 3A). These mutants produced normal numbers of mature gametocytes during blood stage development. The analysis of in vitro gamete formation (exflagellation of males; emergence of female gametes from the erythrocyte) by light-microscopy also revealed that the process of gametocyte and gamete formation was not affected, resulting in the production of motile male gametes and female gametes, emerged from the host erythrocyte by more than 80% of the mature gametocytes (Table 1). At 16–18h after activation of gamete formation, the in vitro cultures of Δp47, Δp230 and Δp48/45&Δp47 lines contained many (clusters of) unfertilized, singly nucleated, female gametes. This phenotype of a strong reduction of fertilisation despite the formation of male and female gametes closely resembles the phenotype of Plasmodium parasites lacking P48/45 [4]. As had also been previously observed with the P48/45 deficient mutant, the fertilisation rate of gametes of the three mutant lines seems to be more efficient in the mosquito compared to in vitro fertilisation [4]. Compared to wild type parasites, the in vivo fertilisation of the mutants is reduced by 93–98% as calculated by ookinete and oocyst production in mosquitoes (Table 1), whereas the reduction of in vitro fertilisation rate is greater than 99.9%. Infections of naïve mice through bite of 20–30 mosquitoes infected with parasites of Δp47, Δp48/45&Δp47DKO and Δp230 parasites, resulted in blood stage infections containing only gene disruption mutants (i.e. mutant genotype and no ‘wild type’ parasites), as determined by PCR and Southern analysis of genomic DNA (results not shown). These results show that gametes of all three mutant lines still have a low capacity to fertilise, resulting in the production of viable and infective ookinetes, oocysts and sporozoites. Moreover, the results obtained with the double knock-out mutant Δp48/45&Δp47 indicate that the few fertilisation events in single knock-out mutants deficient in expression of either P47 or P48/P45 (this study and [4]) cannot be explained by a compensation effect due to its paralogous protein because the Δp48/45&Δp47 mutant still shows a comparable, albeit greatly reduced, ability to fertilise and to pass through the mosquito.
Fig. 3. Fertilisation rates and male and female fertility of mutants lacking expression of different members of the 6-cys family of proteins. 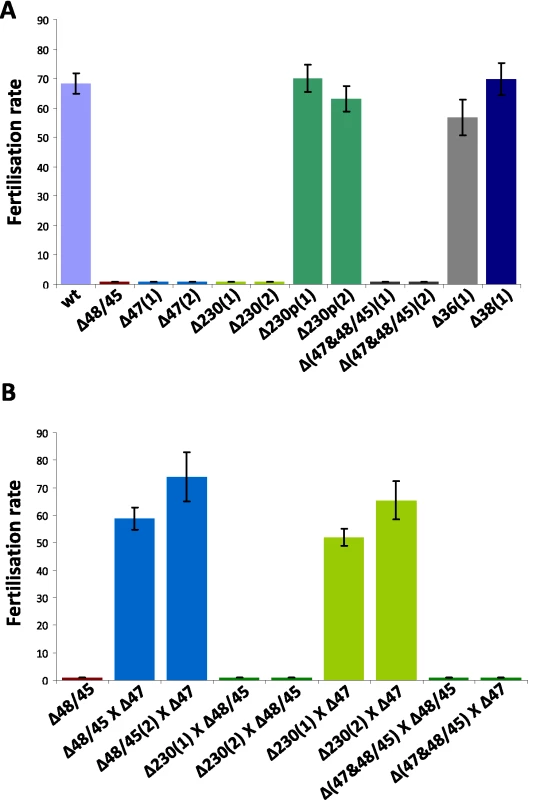
The fertilisation rate is defined as the percentage of female gametes that develop into mature ookinetes (ookinete conversion rates); 1 and 2 indicate mutants obtained from independent transfection experiments. A. Self-fertilisation rates of the different mutants, showing wild type fertilisation rates of mutants Δp230p, Δp36 and Δp38. B. Cross-fertilisation rates in assays in which gametes of the Δp47, Δp230 and Δp48/45& Δp47 mutants (that were affected in their fertilisation rate) were crossed with fertile females of Δp48/45. Δp47 males are fertile and fertilise Δp48/45 females at wild type rates whereas Δp230 males are infertile. Δp230 females are fertile and are fertilised by Δp47 males at wild type levels. Gametes of both sexes of the Δp48/45& Δp47 mutant are infertile. Tab. 1. Gametocyte/gamete production, fertilisation rate and development in mosquitoes of different mutants that lack expression of members of the 6-cys family of proteins. 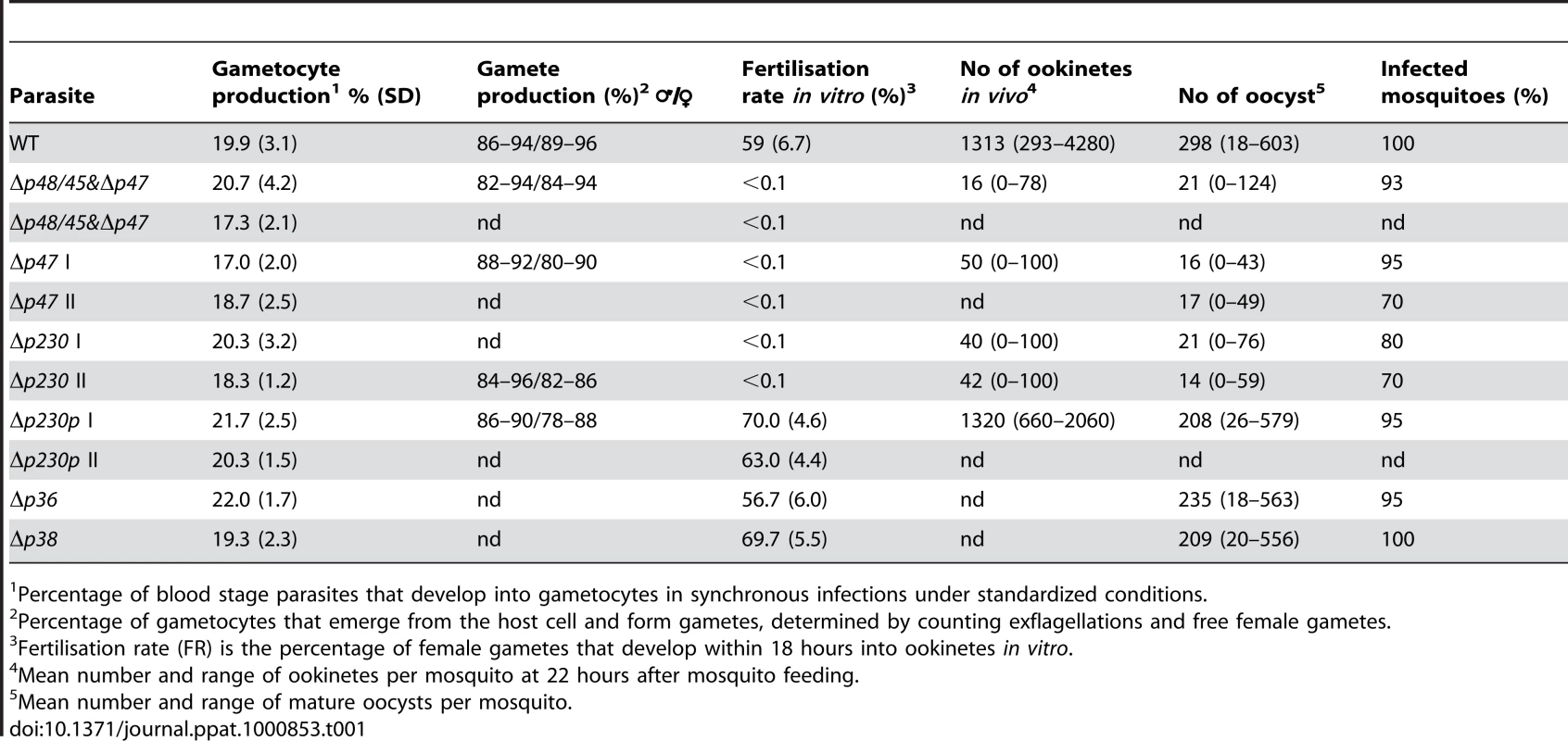
Percentage of blood stage parasites that develop into gametocytes in synchronous infections under standardized conditions. P230 plays a role in male gamete fertility and P47 in female gamete fertility
Fertility of the male and female gametes produced by the mutant lines can be determined by in vitro cross-fertilisation studies, where gametes are cross-fertilised with gametes of parasite lines that produce either only fertile male gametes or female gametes. Such an approach was used to establish that Δp48/45 parasites produced infertile male gametes, whereas the female gametes are completely fertile [4]. We performed different in vitro cross fertilisation experiments to determine whether the reduced fertilisation capacity of the Δp47 and Δp230 mutants was due to affected male gametes, female gametes or to both sexes. Gametes of both mutants were cross-fertilised with female gametes of Δp48/45 (males are infertile) to determine male fertility of Δp47 and Δp230. Male gametes of Δp47 were able to fertilise Δp48/45 females (at wild-type levels) whereas the males of Δp230 were unable to fertilise the Δp48/45 females (fertilisation rates <0.01%; Fig. 3B). These results demonstrate that male gametes of Δp47 are viable with wild type fertilisation capacity and therefore the fertilisation defect of Δp47 must be due to infertile females. The normal fertility of male gametes of Δp47 has also been shown in previous studies in which the males of this mutant have already been used in other cross-fertilisation studies [17], [39], [54], [55]. The lack of fertilisation in the crossing experiments of gametes of Δp230 with Δp48/45 shows that P230 plays a role in male fertility. In order to test the fertility of Δp230 females we crossed the gametes of this line with the fertile male gametes of Δp47 (as mentioned above the females are infertile). We find that Δp47 male gametes are able to fertilise Δp230 female gametes in a manner identical to their ability to fertilise Δp48/45 females (Fig. 3B). This demonstrates that female gametes of Δp230 have a fertility that is comparable to wild type female gametes and that the fertilisation defect is the result of infertile males. Crossing experiments performed with gametes of the double knockout mutant, Δp48/45&Δp47 with gametes of either Δp230, Δp47 or Δp48/45 did not result in increased fertilisation rates (<0.01%), demonstrating that gametes of both sexes are infertile in the double knock-out mutant (Fig. 3B).
Infertile Δp230 males form exflagellation centres but do not attach to females and fertile males do not attach to infertile Δ47 females
In P. falciparum it has been shown that male gametes lacking P230 expression have a reduced capacity to adhere to red blood cells, as measured by the formation of ‘exflagellation centres’ [27]. We therefore examined the ability of P. berghei male Δp230 gametes to attach to erythrocytes, by microscopic examination of exflagellation centre formation under standardized in vitro conditions. In these experiments 76–92% of exflagellating wt males and 72–90% exflagellating Δp230 male gametocytes, formed such centres (Table 2), indicating that in contrast to P. falciparum Δp230 in P. berghei both wt and Δp230 male gametes have a similar ability to interact with red blood cells. Gametocytes that did not form exflagellation centres were often floating on/above the red blood cell layer during exflagellation. Further analysis of single, free male gametes of Δp230 revealed that they were highly motile and often attach to red blood cells, producing characteristic red blood cell shape deformations due to the active interactions between the male gamete and the erythrocyte. Male gametes lacking expression of P48/45 do not attach to female gametes as has been previously shown by analysing male-female interactions by light microscopy [4], [5]. We therefore analysed the interactions between male and female gametes of Δp230 or Δp47, between 10 and 30 minutes after induction of gamete formation using phase-contrast microscopy. In wt parasites attachment of males to females was readily detected with a mean of over 25 attachments during a 20 minutes period of observation, with a mean of more than 6 confirmed fertilisations (i.e. male gamete penetrations; Table 2). In preparations of gametes of both Δp230 and Δp47 not a single fertilisation event was detected and the number of male and female gamete attachments was drastically reduced (Table 2). We observed that while male gametes of both mutants undergo active interactions with red blood cells and platelets, attachment of males to female gametes are hardly ever observed. These results show that P230 like P48/45 is a male fertility factor involved in recognition or attachment to females and that P47 is a female fertility factor involved in recognition or adherence by the male gamete. Whether P48/45 and P230 once on the surface of the male gamete directly interact with P47 on the surface of the female gamete is unknown. Unfortunately, repeated immuno-precipitation experiments with anti-P. berghei P48/45 antibodies and wt gamete preparations, in order to identify interacting partners, were unsuccessful (data not shown).
Tab. 2. The interactions of Δp230 and Δp47 male gametes with red blood cells (exflagellation centres) and female gametes (attachment and fertilisation). 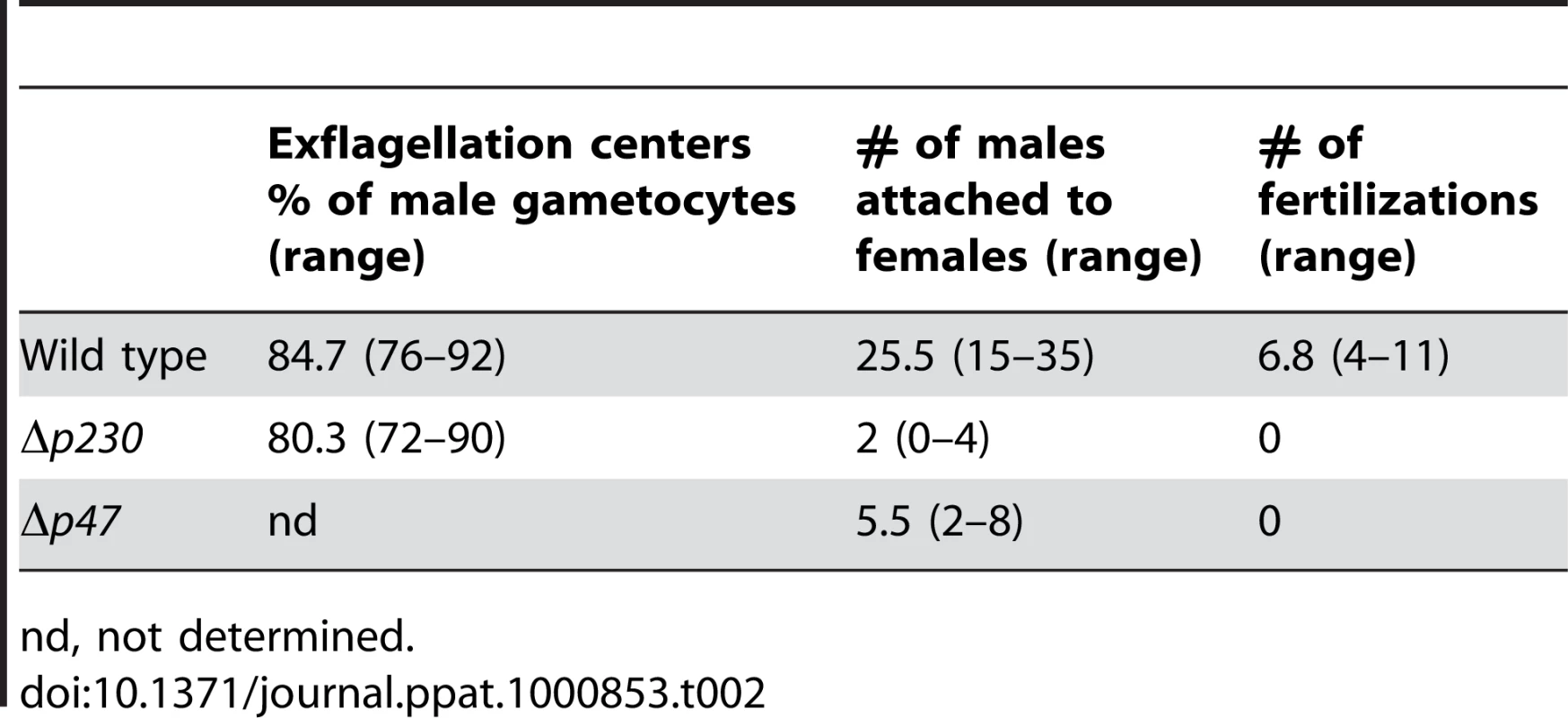
nd, not determined. Sequence polymorphism of Plasmodium proteins involved in fertilisation
Analyses of sequence polymorphisms of p48/45, p47 and p230 of laboratory and field isolates of P. falciparum has provided evidence that these proteins are under positive selection [28], [29], [30], [31]. We analysed synonymous (dN) and non-synonymous (dS) polymorphisms of p48/45, p47 and p230 by comparing these genes in three closely related rodent parasites P. berghei, P. yoelii and P. chabaudi by making use of the newly available gene sequences (www.PlasmoDB.org version 6.1). The updated dN/dS values for these genes obtained here, which is commonly used as an indicator of positive selection, were in all comparisons higher than the mean dN/dS value of all genes within the respective genomes (Table S4). However, only the dN/dS ratio of p47 in the P. berghei/P. yoelii comparison showed a significant difference with the mean dN/dS value (0.82 compared to the mean dN/dS of 0.26). Overall, P47 is in the top 4–6% of fastest evolving proteins in the rodent parasite genomes as compared to top 10–16% for P230 and 15–50% for P48/45 (Table S4). In addition, we have used the likelihood ratio test (LRT) to analyse if these genes were undergoing neutral or positive selection (see Materials and Methods). This test shows that p47 is indeed under positive selection (P = 0.006) when comparing the site/residue specific models of evolution.
We next examined sequence mutations in the same genes in more detail by performing a comparative dN/dS ratio analysis across these genes using small and corresponding regions of these genes using a ‘sliding window analysis’ (i.e. 300bp in 150bp intervals; Fig. 4; Table S4). This analysis showed that p47 has an exceptionally elevated dN/dS value (i.e. 1–2) in one area corresponding to the truncated B-type domain II as defined by [7]. Interestingly, although P230 had a relatively low overall dN/dS value (0.33–0.44), the sliding window analysis revealed that P230 contains several areas where the dN/dS ratio is higher than 1.0 with an increased ratio in all 3 species in particular around the B-type domain IV as defined by Gerloff et al. (2005). In order to analyse similarities in the location of sequence polymorphism between P. falciparum and the three rodent parasites, we aligned all known single nucleotide polymorphisms (SNPs) described for P230, P47 and P48/45 in P. falciparum (i.e. www.PlasmoDB.org; [56], [57], [58]) with the dN/dS ratios determined by the ‘sliding window analysis’ (for details see Materials and Methods; Fig. 4). Interestingly, the elevated dN/dS ratios of p47 domain II and domain IV of P230, both correspond with the location of high SNP densities in the orthologous P. falciparum genes. These findings would suggest that similar regions in the p47 and p230 genes of rodent parasites and P. falciparum are subject to positive selection. To predict which residues of the three P. berghei genes are under positive selection we performed a Bayes Empirical Bayes analysis (BEB; [49]). This analysis calculates dN/dS values (ω values) on each residue of a particular protein when the genes encoding these proteins are compared in least 3 similar species and an ω>1 indicates positive selection on a residue. For P47 ten residues were identified undergoing positive selection with ω values ranging between 4 and 7 (Table S5). Nine of these 10 residues are confined to the first two domains of P47 including the region B-type domain II. In P48/45 four residues were identified (ω values ranging between 1 and 2) and for P230 only one amino acid (ω = 1.3). Interestingly, this one residue in P230 (i.e. residue 845V) maps to the corresponding region of the P. falciparum P230, domain IV, where 6 of the 27 non-synonymous polymorphisms described by Gerloff et al. map (Table S4).
Fig. 4. Polymorphisms and sequence divergence across p230, p48/45 and p47. 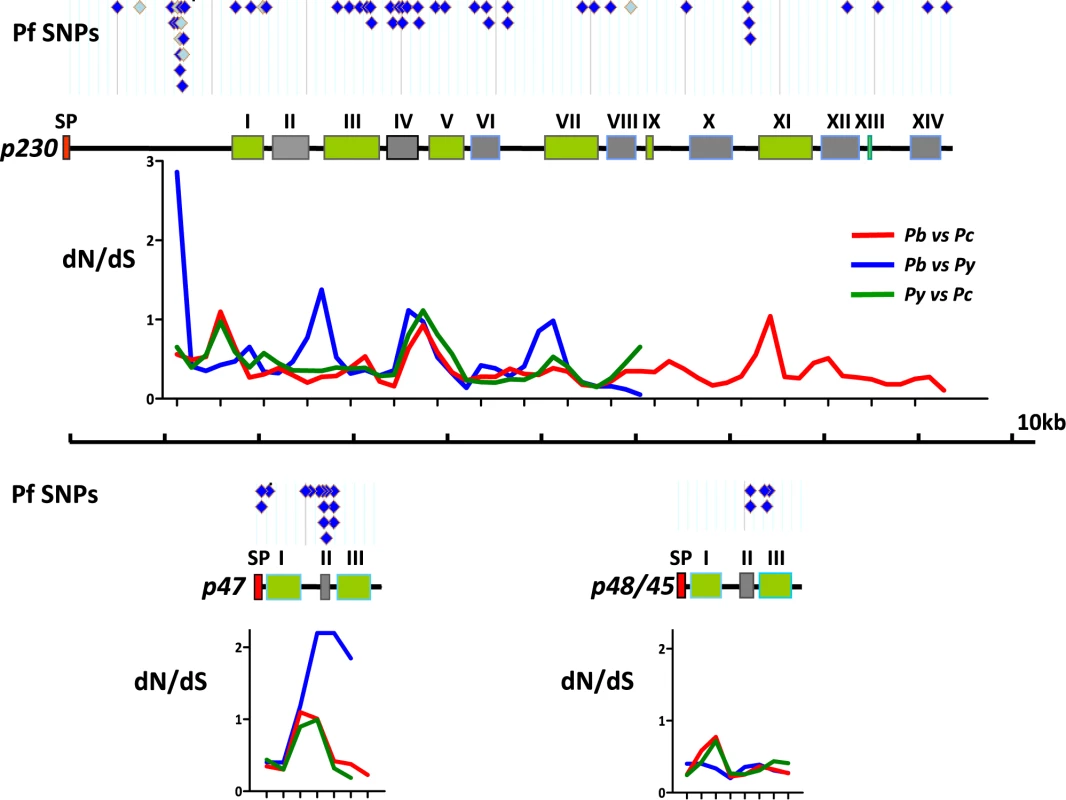
Schematic representation of p230, p47 and p48/45 (shown to scale). A- and B-type recurring domains (green and grey respectively; [7]) are shown and the numbering of domains (I–XIV) are shown as according to [7]. The putative Signal Peptide (SP) is indicated in red. Above each gene the locations of all single nucleotide polymorphisms (SNPs) are shown as identified in different P. falciparum strains in PlasmoDB (www.PlasmoDB.org; August 2009). Dark blue diamonds: non-synonymous polymorphisms; Light blue diamonds: synonymous polymorphisms. Below each gene the dN/dS ratios are shown across the length of the three rodent Plasmodium orthologs. This dN/dS analysis is performed using a ‘sliding-window’ analysis, where 300bp of corresponding DNA sequence was compared at 150bp intervals. The gene from each species has been compared to the same gene of the other species; Red: P. berghei against P. chabaudi; Blue: P. berghei against P. yoelii; Green: P. yoelii against P. chabaudi. The complete gene sequence is only available for P. berghei and P. chabaudi; The 5′ end of all three rodent parasite p230 genes is shorter than those of the P. falciparum p230 and therefore alignment of the P. falciparum to the rodent p230s is only possible ∼1kb after the start site. Discussion
Until recently the only protein proven to play a direct role in merging of the male and female gamete of Plasmodium gametes in Plasmodium was P48/45, a surface protein principally of male gametes shown to play an essential role in recognition of and attachment to females [4], [5]. Recently, two studies have identified a second protein, HAP2/GCS1 with a role early in fertilisation [5], [6]. Male gametes of mutant parasites lacking this protein can attach to female gametes but the subsequent fusion of the gametes is absent [5], a process which is clearly after the mutual recognition and attachment of gametes. Our studies provide evidence for the direct involvement of two additional proteins, P47 and P230, which like P48/45 play a key role in the initial phase of gamete-gamete recognition and attachment. The phenotype of mutants lacking P230 expression is identical to the phenotype of mutants lacking P48/45, i.e. male gametes do not recognize and attach to female gametes whereas the female gametes are fertile. These results show that the P230 protein, like P48/45, is a male fertility factor. A similar role of P48/45 and P230 in male fertility is perhaps not surprising since evidence has been reported that both proteins interact with each other. Unlike P48/45, P230 does not contain a glycosylphosphatidylinositol (GPI) anchor and in P. falciparum evidence has been found that P230 forms a complex with P48/45 at the surface of gametocytes and gametes [18], [27], [59], [60]. Indeed, analysis of P. falciparum mutants has shown that in the absence of P48/45 the P230 protein is not retained on the surface of gametes, a result which may indicate that tethering of P230 to the surface of the male gamete is mediated by P48/45 [27]. In contrast, in the absence of P230 the surface location of P48/45 is not affected in P. falciparum [27], [61]. If in P. berghei the same interaction occurs, and Δp48/45 gametes also lack surface expression of P230, then the failure of Δp48/45 and Δp230 males to attach to females might be solely due to the absence of P230 on the male gamete surface. This would imply that P230 and not P48/45 is the major male protein that is responsible for recognition of and attachment to the female. However, it has been shown that antibodies directed against P48/45 strongly reduce oocyst formation [19], [20], [24], [25], [26], indicating that either P48/45 antibodies disrupt the attachment of the translocated P230 to P48/45 after gamete formation or it may play a more direct role in fertilisation and that its function is not exclusively as a membrane anchor for P230.
Interestingly, in P. falciparum it has been shown that male gametes with a disrupted p230 gene are incapable of interacting with erythrocytes and do not form the characteristic exflagellation centres and these mutants show a strong reduction in oocyst formation [27]. These observations, in P. falciparum, indicate that P230 not only plays a role in gamete-gamete interactions but male gamete interactions with erythrocytes may be required for gamete maturation resulting in an optimal fertilisation capacity [27], [62]. Our analyses of Δp230 P. berghei male gametes in live preparations did not reveal any difference in their capacity to interact with red blood cells, suggesting that there are functional differences between P230 of P. berghei and P. falciparum. As the interaction between male and female gametes has not been analysed in the P. falciparum Δp230 mutants it is unknown whether the decreased oocyst formation results from the reduced gamete-erythrocyte interactions or is due to the lack of gamete recognition and attachment, as we have observed in P. berghei. Therefore, further research is needed to unravel whether P. falciparum P230 is also involved in gamete-gamete interactions like P. berghei P230. Moreover, additional research is required to identify the proteins at the surface of P. berghei male gametes that are responsible for the adherence of the male gametes to erythrocytes. Disruption of the close paralogue of p230, p230p, did not have any effect on fertilisation or on red blood cell attachment. The distinct phenotypes of Δp230 and Δp230p gametes demonstrate that the proteins encoded by these genes are not functional paralogues that are able to complement each others function as has been demonstrated for the paralogous protein pair P28 and P25 on the surface of zygotes [63]. The same is true for the paralogous proteins P48/45 and P47 (see below) or P36 and P36p [15], [64].
In addition to the important role of P230 in male fertility, our studies demonstrate that P47 plays a key role in P. berghei female gamete fertility. Both proteome analyses of P. berghei gametocytes [17] and IFA analysis of P. falciparum gametocytes using anti-P47 antibodies [12] have shown the female-specific expression of P47. In P. falciparum, P47 is located on the surface of the female gametes following emergence from the host erythrocyte. Our studies demonstrate that P. berghei females lacking P47 are not recognized by wild type males. These observations may suggest that P48/45 or P230 on the male gamete directly interact with P47 on the female for recognition and attachment. However, P48/45 and P230 may alternatively interact with additional, as yet unknown protein/s on the surface of the female that are dependent on the presence of P47, in an analogous manner to the interaction between P230 and P48/45 on the surface of the male gamete. Both P48/45 and P230 are also expressed in the female gametes of P. berghei and P. falciparum [17], [27]. The presence of these proteins on the female gamete surface does not result from male proteins that are released by the male during activation and subsequent binding to the female since ‘pre-activated’ female gametocytes also express these proteins (B van Schaijk, personal communication and [65]. However, an essential role for P48/45 and P230 in female gametocytes is not implicated in P. berghei since both Δp230 and Δp48/45 females demonstrate normal fertilisation, i.e. to wild-type levels, when incubated with wild type males.
Unexpectedly, the lack of expression of P47 in P. falciparum mutants appears not to have a role in fertilisation as determined by oocyst formation in mosquitoes [12]. This difference between P. berghei and P. falciparum suggests that the proposed model of the interactions between male P48/45 and/or P230 with female P47 (and/or P47-interacting proteins) being key for the recognition and attachment of gametes does not hold true for all Plasmodium species. However, these differences between P. falciparum and P. berghei might also be explained by the presence of an additional set of protein ligands in both species that mediate additional mechanisms of gamete recognition and attachment. Indeed by analysing P. berghei Δp48/45 mutants [4] and mutants lacking expression of P47 and P230 (this study) we found that low levels of fertilisation did occur. Surprisingly, in all mutants significant higher fertilisation rates were observed in mosquito midguts compared to in vitro rates of fertilisation. Even in the mutant lacking expression of both P48/45 and P47, the same low fertilisation rates are observed. Assuming that P. berghei Δp48/45 gametes lack P230 surface expression as has been shown for P. falciparum Δp48/45, then gametes of the double knock-out mutant can fertilise in the absence of essentially all three fertility factors of the 6-cys family, albeit at a reduced rate. These observations indicate the presence of additional proteins that secure fertilisation in the absence of the three members of the 6-cys family. For unidentified reasons this alternative fertilisation pathway appears to be much more efficient in vivo than in vitro, suggesting that mosquito factors influence this alternative route of fertilisation. The observed oocyst formation in Δp48/45 and Δp47 P. falciparum parasites [4], [12] might therefore also be explained by this route of fertilisation and the presence of relatively high numbers of oocysts might indicate that this alternative pathway is more efficient in P. falciparum in A. stephensi compared to P. berghei in A. stephensi. Such alternative pathways of fertilisation may have implications for development of transmission blocking vaccines that block fertilisation using antibodies directed against members of the 6-cys family of proteins and therefore it is important to identify the additional proteins involved in the process of recognition and attachment of gametes. It is possible that other members of the 6-cys family that are expressed in gametocytes (P230p, P38 and P36) may be involved in the alternative pathways of fertilisation. Although we found that gametes lacking expression of these proteins did not show a significant reduction in fertilisation, the effect of their absence on gamete fertility may only become evident in the absence of P48/45, P47 and P230. Further research using mutants lacking multiple 6-cys members is required to reveal whether other 6-cys family members or other unrelated proteins play a role in alternative routes of fertilisation.
For P48/45, P47 and P230 in P. falciparum evidence has been published that these proteins are under differing rates of positive selection resulting in non-neutral sequence polymorphisms [28], [29], [30], [31]. Polymorphisms in gamete proteins may be a consequence of sexual selection as is the case for gamete proteins of other organisms [3], [66]. However, sequence polymorphism in these Plasmodium genes may also result from natural selection exerted by the adaptive immune system of the host. These three proteins are expressed in mature gametocytes, and as only a very small percentage of gametocytes ever get passed on to a mosquito, the vast majority of gametocyte proteins (including these 6-cys members) are eventually released into the hosts circulation where they are exposed to the host immune system. Indeed it has been shown that P48/45 and P230 both elicit humoral responses in infected individuals that can mediate transmission blocking immunity [22], [24], [67], [68], [69], [70]. Our analyses on dN/dS values of the three rodent parasites provide additional evidence that directional selection pressures affect sequence polymorphisms of gamete surface proteins, especially evident for the female specific p47 which belongs to the top 4–6% fastest evolving genes in the rodent parasite genomes. Analysis of dN/dS variation across the genes by the sliding window approach on P230 identifies one region that is evolving rapidly in all the rodent parasites and, interestingly, this correlates with the same region in P. falciparum (B-type domain IV) that has the highest density of SNPs [7]. The correlation of the location of P. falciparum SNP's with increased dN/dS ratios in both P230 and P47 may indicate that similar selection pressures exists in different Plasmodium species. Whether this positive selection on these gamete proteins is driven by immune responses and/or mating interactions is presently unknown. However, insight into sequence polymorphisms in gamete surface proteins that are targets for TB vaccines and the influence of these polymorphisms on mating behaviour of parasites in natural populations of P. falciparum should help to improve TB vaccines development.
Supporting Information
Zdroje
1. ShurBD
RodehefferC
EnsslinMA
LyngR
RaymondA
2006 Identification of novel gamete receptors that mediate sperm adhesion to the egg coat. Mol Cell Endocrinol 250 137 148
2. RubinsteinE
ZiyyatA
WolfJP
Le NaourF
BoucheixC
2006 The molecular players of sperm-egg fusion in mammals. Semin Cell Dev Biol 17 254 263
3. SwansonWJ
VacquierVD
2002 The rapid evolution of reproductive proteins. Nat Rev Genet 3 137 144
4. van DijkMR
JanseCJ
ThompsonJ
WatersAP
BraksJA
2001 A central role for P48/45 in malaria parasite male gamete fertility. Cell 104 153 164
5. LiuY
TewariR
NingJ
BlagboroughAM
GarbomS
2008 The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22 1051 1068
6. HiraiM
AraiM
MoriT
MiyagishimaSY
KawaiS
2008 Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr Biol 18 607 613
7. GerloffDL
CreaseyA
MaslauS
CarterR
2005 Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc Natl Acad Sci U S A 102 13598 13603
8. ThompsonJ
JanseCJ
WatersAP
2001 Comparative genomics in Plasmodium: a tool for the identification of genes and functional analysis. Mol Biochem Parasitol 118 147 154
9. TempletonTJ
KaslowDC
1999 Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol Biochem Parasitol 101 223 227
10. WilliamsonKC
CriscioMD
KaslowDC
1993 Cloning and expression of the gene for Plasmodium falciparum transmission-blocking target antigen, Pfs230. Mol Biochem Parasitol 58 355 358
11. CarterR
CoulsonA
BhattiS
TaylorBJ
ElliottJF
1995 Predicted disulfide-bonded structures for three uniquely related proteins of Plasmodium falciparum, Pfs230, Pfs48/45 and Pf12. Mol Biochem Parasitol 71 203 210
12. van SchaijkBC
van DijkMR
van de Vegte-BolmerM
van GemertGJ
van DoorenMW
2006 Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol 149 216 222
13. SandersPR
GilsonPR
CantinGT
GreenbaumDC
NeblT
2005 Distinct protein classes including novel merozoite surface antigens in Raft-like membranes of Plasmodium falciparum. J Biol Chem 280 40169 40176
14. IshinoT
ChinzeiY
YudaM
2005 Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol Microbiol 58 1264 1275
15. van DijkMR
DouradinhaB
Franke-FayardB
HeusslerV
van DoorenMW
2005 Genetically attenuated, P36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc Natl Acad Sci U S A 102 12194 12199
16. EksiS
WilliamsonKC
2002 Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PfB0400w. Mol Biochem Parasitol 122 127 130
17. KhanSM
Franke-FayardB
MairGR
LasonderE
JanseCJ
2005 Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121 675 687
18. KockenCH
JansenJ
KaanAM
BeckersPJ
PonnuduraiT
1993 Cloning and expression of the gene coding for the transmission blocking target antigen Pfs48/45 of Plasmodium falciparum. Mol Biochem Parasitol 61 59 68
19. VermeulenAN
PonnuduraiT
BeckersPJ
VerhaveJP
SmitsMA
1985 Sequential expression of antigens on sexual stages of Plasmodium falciparum accessible to transmission-blocking antibodies in the mosquito. J Exp Med 162 1460 1476
20. CarterR
GravesPM
KeisterDB
QuakyiIA
1990 Properties of epitopes of Pfs 48/45, a target of transmission blocking monoclonal antibodies, on gametes of different isolates of Plasmodium falciparum. Parasite Immunol 12 587 603
21. WilliamsonKC
KeisterDB
MuratovaO
KaslowDC
1995 Recombinant Pfs230, a Plasmodium falciparum gametocyte protein, induces antisera that reduce the infectivity of Plasmodium falciparum to mosquitoes. Mol Biochem Parasitol 75 33 42
22. HealerJ
McGuinnessD
HopcroftP
HaleyS
CarterR
1997 Complement-mediated lysis of Plasmodium falciparum gametes by malaria-immune human sera is associated with antibodies to the gamete surface antigen Pfs230. Infect Immun 65 3017 3023
23. RoeffenW
GeeraedtsF
ElingW
BeckersP
WizelB
1995 Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230-specific antibodies is isotype dependent. Infect Immun 63 467 471
24. RoeffenW
MulderB
TeelenK
BolmerM
ElingW
1996 Association between anti-Pfs48/45 reactivity and P. falciparum transmission-blocking activity in sera from Cameroon. Parasite Immunol 18 103 109
25. TargettGA
HartePG
EidaS
RogersNC
OngCS
1990 Plasmodium falciparum sexual stage antigens: immunogenicity and cell-mediated responses. Immunol Lett 25 77 81
26. OutchkourovNS
RoeffenW
KaanA
JansenJ
LutyA
2008 Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci U S A 105 4301 4305
27. EksiS
CzesnyB
van GemertGJ
SauerweinRW
ElingW
2006 Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 61 991 998
28. EscalanteAA
LalAA
AyalaFJ
1998 Genetic polymorphism and natural selection in the malaria parasite Plasmodium falciparum. Genetics 149 189 202
29. EscalanteAA
GrebertHM
ChaiyarojSC
RiggioneF
BiswasS
2002 Polymorphism in the gene encoding the Pfs48/45 antigen of Plasmodium falciparum. XI. Asembo Bay Cohort Project. Mol Biochem Parasitol 119 17 22
30. ConwayDJ
MachadoRL
SinghB
DessertP
MikesZS
2001 Extreme geographical fixation of variation in the Plasmodium falciparum gamete surface protein gene Pfs48/45 compared with microsatellite loci. Mol Biochem Parasitol 115 145 156
31. AnthonyTG
PolleySD
VoglerAP
ConwayDJ
2007 Evidence of non-neutral polymorphism in Plasmodium falciparum gamete surface protein genes Pfs47 and Pfs48/45. Mol Biochem Parasitol 156 117 123
32. JanseCJ
RamesarJ
WatersAP
2006 High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1 346 356
33. BillkerO
DechampsS
TewariR
WenigG
Franke-FayardB
2004 Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117 503 514
34. WengelnikK
SpaccapeloR
NaitzaS
RobsonKJ
JanseCJ
1999 The A-domain and the thrombospondin-related motif of Plasmodium falciparum TRAP are implicated in the invasion process of mosquito salivary glands. Embo J 18 5195 5204
35. Franke-FayardB
TruemanH
RamesarJ
MendozaJ
van der KeurM
2004 A Plasmodium berghei reference line that constitutively expresses GFP at a high level throughout the complete life cycle. Mol Biochem Parasitol 137 23 33
36. JanseCJ
Franke-FayardB
MairGR
RamesarJ
ThielC
2006 High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145 60 70
37. MenardR
JanseC
1997 Gene targeting in malaria parasites. Methods 13 148 157
38. BeetsmaAL
van de WielTJ
SauerweinRW
ElingWM
1998 Plasmodium berghei ANKA: purification of large numbers of infectious gametocytes. Exp Parasitol 88 69 72
39. MairGR
BraksJA
GarverLS
WiegantJC
HallN
2006 Regulation of sexual development of Plasmodium by translational repression. Science 313 667 669
40. SindenRE
1997 Infection of mosquitoes with rodent malaria.
Crampton CBBJM
LouisC
The molecular biology of insect disease vectors; A Methods Manual London New York Chapman and Hall
41. LasonderE
JanseCJ
van GemertGJ
MairGR
VermuntAM
2008 Proteomic profiling of Plasmodium sporozoite maturation identifies new proteins essential for parasite development and infectivity. PLoS Pathog 4 e1000195 doi:10.1371/journal.ppat.1000195
42. JanseCJ
MonsB
RouwenhorstRJ
Van der KloosterPF
OverdulveJP
1985 In vitro formation of ookinetes and functional maturity of Plasmodium berghei gametocytes. Parasitology 91 ( Pt 1) 19 29
43. YangZ
1997 PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13 555 556
44. YangZ
2000 Maximum likelihood estimation on large phylogenies and analysis of adaptive evolution in human influenza virus A. J Mol Evol 51 423 432
45. GoldmanN
YangZ
1994 A codon-based model of nucleotide substitution for protein-coding DNA sequences. Mol Biol Evol 11 725 736
46. YangZ
NielsenR
GoldmanN
PedersenAM
2000 Codon-substitution models for heterogeneous selection pressure at amino acid sites. Genetics 155 431 449
47. MuseSV
GautBS
1994 A likelihood approach for comparing synonymous and nonsynonymous nucleotide substitution rates, with application to the chloroplast genome. Mol Biol Evol 11 715 724
48. Castillo-DavisCI
BedfordTB
HartlDL
2004 Accelerated rates of intron gain/loss and protein evolution in duplicate genes in human and mouse malaria parasites. Mol Biol Evol 21 1422 1427
49. YangZ
WongWS
NielsenR
2005 Bayes empirical bayes inference of amino acid sites under positive selection. Mol Biol Evol 22 1107 1118
50. ZhangJ
NielsenR
YangZ
2005 Evaluation of an improved branch-site likelihood method for detecting positive selection at the molecular level. Mol Biol Evol 22 2472 2479
51. YangZ
1998 Likelihood ratio tests for detecting positive selection and application to primate lysozyme evolution. Mol Biol Evol 15 568 573
52. HallN
KarrasM
RaineJD
CarltonJM
KooijTW
2005 A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science 307 82 86
53. van SchaijkBC
JanseCJ
van GemertGJ
van DijkMR
GegoA
2008 Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS ONE 3 e3549 doi:10.1371/journal.pone.0003549
54. RaineJD
EckerA
MendozaJ
TewariR
StanwayRR
2007 Female inheritance of malarial lap genes is essential for mosquito transmission. PLoS Pathog 3 e30 doi:10.1371/journal.ppat.0030030
55. BushellES
EckerA
SchlegelmilchT
GouldingD
DouganG
2009 Paternal effect of the nuclear formin-like protein MISFIT on Plasmodium development in the mosquito vector. PLoS Pathog 5 e1000539 doi:10.1371/journal.ppat.1000539
56. MuJ
AwadallaP
DuanJ
McGeeKM
KeeblerJ
2007 Genome-wide variation and identification of vaccine targets in the Plasmodium falciparum genome. Nat Genet 39 126 130
57. JeffaresDC
PainA
BerryA
CoxAV
StalkerJ
2007 Genome variation and evolution of the malaria parasite Plasmodium falciparum. Nat Genet 39 120 125
58. VolkmanSK
SabetiPC
DeCaprioD
NeafseyDE
SchaffnerSF
2007 A genome-wide map of diversity in Plasmodium falciparum. Nat Genet 39 113 119
59. KumarN
1987 Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol 9 321 335
60. KumarN
WizelB
1992 Further characterization of interactions between gamete surface antigens of Plasmodium falciparum. Mol Biochem Parasitol 53 113 120
61. EksiS
StumpA
FanningSL
ShenoudaMI
FujiokaH
2002 Targeting and sequestration of truncated Pfs230 in an intraerythrocytic compartment during Plasmodium falciparum gametocytogenesis. Mol Microbiol 44 1507 1516
62. TempletonTJ
KeisterDB
MuratovaO
ProcterJL
KaslowDC
1998 Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. J Exp Med 187 1599 1609
63. TomasAM
MargosG
DimopoulosG
van LinLH
de Koning-WardTF
2001 P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. Embo J 20 3975 3983
64. VanBuskirkKM
O'NeillMT
De La VegaP
MaierAG
KrzychU
2009 Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc Natl Acad Sci U S A 106 13004 13009
65. BustamantePJ
WoodruffDC
OhJ
KeisterDB
MuratovaO
2000 Differential ability of specific regions of Plasmodium falciparum sexual-stage antigen, Pfs230, to induce malaria transmission-blocking immunity. Parasite Immunol 22 373 380
66. VacquierVD
1998 Evolution of gamete recognition proteins. Science 281 1995 1998
67. SaeedM
RoeffenW
AlexanderN
DrakeleyCJ
TargettGA
2008 Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PLoS ONE 3 e2280 doi:10.1371/journal.pone.0002280
68. BousemaJT
DrakeleyCJ
SauerweinRW
2006 Sexual-stage antibody responses to P. falciparum in endemic populations. Curr Mol Med 6 223 229
69. DrakeleyCJ
ElingW
TeelenK
BousemaJT
SauerweinR
2004 Parasite infectivity and immunity to Plasmodium falciparum gametocytes in Gambian children. Parasite Immunol 26 159 165
70. HealerJ
McGuinnessD
CarterR
RileyE
1999 Transmission-blocking immunity to Plasmodium falciparum in malaria-immune individuals is associated with antibodies to the gamete surface protein Pfs230. Parasitology 119 ( Pt 5) 425 433
71. LoboCA
KumarN
1998 Sexual differentiation and development in the malaria parasite. Parasitol Today 14 146 150
72. FlorensL
WashburnMP
RaineJD
AnthonyRM
GraingerM
2002 A proteomic view of the Plasmodium falciparum life cycle. Nature 419 520 526
73. LasonderE
IshihamaY
AndersenJS
VermuntAM
PainA
2002 Analysis of the Plasmodium falciparum proteome by high-accuracy mass spectrometry. Nature 419 537 542
74. Le RochKG
ZhouY
BlairPL
GraingerM
MochJK
2003 Discovery of gene function by expression profiling of the malaria parasite life cycle. Science 301 1503 1508
75. Van DijkMR
2009 This paper.
76. KhanSM
2010 unpublished results
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Familiární středomořská horečka
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



