-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
Prions are unconventional infectious agents thought to be primarily composed of PrPSc, a multimeric misfolded conformer of the ubiquitously expressed host-encoded prion protein (PrPC). They cause fatal neurodegenerative diseases in both animals and humans. The disease phenotype is not uniform within species, and stable, self-propagating variations in PrPSc conformation could encode this ‘strain’ diversity. However, much remains to be learned about the physical relationship between the infectious agent and PrPSc aggregation state, and how this varies according to the strain. We applied a sedimentation velocity technique to a panel of natural, biologically cloned strains obtained by propagation of classical and atypical sheep scrapie and BSE infectious sources in transgenic mice expressing ovine PrP. Detergent-solubilized, infected brain homogenates were used as starting material. Solubilization conditions were optimized to separate PrPSc aggregates from PrPC. The distribution of PrPSc and infectivity in the gradient was determined by immunoblotting and mouse bioassay, respectively. As a general feature, a major proteinase K-resistant PrPSc peak was observed in the middle part of the gradient. This population approximately corresponds to multimers of 12–30 PrP molecules, if constituted of PrP only. For two strains, infectivity peaked in a markedly different region of the gradient. This most infectious component sedimented very slowly, suggesting small size oligomers and/or low density PrPSc aggregates. Extending this study to hamster prions passaged in hamster PrP transgenic mice revealed that the highly infectious, slowly sedimenting particles could be a feature of strains able to induce a rapidly lethal disease. Our findings suggest that prion infectious particles are subjected to marked strain-dependent variations, which in turn could influence the strain biological phenotype, in particular the replication dynamics.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000859
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000859Summary
Prions are unconventional infectious agents thought to be primarily composed of PrPSc, a multimeric misfolded conformer of the ubiquitously expressed host-encoded prion protein (PrPC). They cause fatal neurodegenerative diseases in both animals and humans. The disease phenotype is not uniform within species, and stable, self-propagating variations in PrPSc conformation could encode this ‘strain’ diversity. However, much remains to be learned about the physical relationship between the infectious agent and PrPSc aggregation state, and how this varies according to the strain. We applied a sedimentation velocity technique to a panel of natural, biologically cloned strains obtained by propagation of classical and atypical sheep scrapie and BSE infectious sources in transgenic mice expressing ovine PrP. Detergent-solubilized, infected brain homogenates were used as starting material. Solubilization conditions were optimized to separate PrPSc aggregates from PrPC. The distribution of PrPSc and infectivity in the gradient was determined by immunoblotting and mouse bioassay, respectively. As a general feature, a major proteinase K-resistant PrPSc peak was observed in the middle part of the gradient. This population approximately corresponds to multimers of 12–30 PrP molecules, if constituted of PrP only. For two strains, infectivity peaked in a markedly different region of the gradient. This most infectious component sedimented very slowly, suggesting small size oligomers and/or low density PrPSc aggregates. Extending this study to hamster prions passaged in hamster PrP transgenic mice revealed that the highly infectious, slowly sedimenting particles could be a feature of strains able to induce a rapidly lethal disease. Our findings suggest that prion infectious particles are subjected to marked strain-dependent variations, which in turn could influence the strain biological phenotype, in particular the replication dynamics.
Introduction
Transmissible spongiform encephalopathies (TSE), such as human Creutzfeldt-Jakob disease, sheep scrapie, bovine spongiform encephalopathy (BSE) and chronic wasting disease of cervidae, are infectious, fatal, neurodegenerative disorders caused by prions [1]. Prions are unconventional pathogens primarily composed of PrPSc, a rearranged conformer of the ubiquitously expressed prion protein (PrPC), whose precise physiological function is largely unknown. Upon infection, PrPSc dictates the self-perpetuating conformational conversion of PrPC into nascent PrPSc. This conversion involves – without any apparent post-translational modification – the refolding of soluble, alpha-helix-rich PrPC molecules into beta-sheet enriched PrPSc polymers that form deposits in TSE-infected brains [2], [3] and are assumed to be responsible for the observed neurodegenerative disorders [4]. The conversion reaction may proceed through a nucleated polymerization mechanism in which PrPSc multimers recruit PrPC molecules and trigger their conformational conversion into PrPSc (for review [5]). The refolding/multimerisation process confers distinct physico-chemical properties to PrPSc, such as insolubility in non-denaturing detergents and partial resistance to proteolysis [6].
Distinct prion entities, referred to as strains, are known to self-propagate in the same host and exhibit distinguishable phenotypic traits that are heritable, such as incubation time, neuropathological and biochemical properties (for reviews: [7], [8], [9]). Accumulating experimental evidence indicates that strain-specified properties are encoded within structural differences in the conformation of the PrPSc molecules, which are faithfully imparted to host PrPC during the conversion process [10], [11], [12], [13], [14], [15], [16], [17]. However, the extent to which the aggregation state varies between different stains, and participates to strain-specific prion biology is unknown. The various fractionation methods and preparative procedures previously employed to estimate the size of the infectious particles [18], [19], [20], [21], [22], [23], [24], [25] have led to a vast range of measured sizes, making it difficult to relate any variation to potential strain differences. Of note, almost all of these studies used substantially purified PrPSc as a starting material.
In this study, we developed a specific protocol to fractionate PrP particles according to their sedimentation velocity properties in a viscous medium, characterized their relative levels of infectivity and looked for strain-specific variations. In contrast to previous reports, experiments were performed on crude brain homogenates, which a priori contain all TSE infectivity. We worked with a panel of strains that were biologically cloned on homogeneous genetic backgrounds, obtained after transmission of either classical and atypical (Nor98) sheep scrapie and BSE, or hamster scrapie infectious sources in transgenic mice expressing ovine PrP (VRQ allele; tg338 mice) and hamster PrP (tg7 line), respectively. We demonstrate that the sedimentation profile of the infectious component dramatically varies with the strain. We further show that the predominance of slowly sedimenting infectious particles that segregate from the bulk of proteinase K-resistant PrPSc particles may be a distinctive feature of strains able to induce a rapidly lethal disease.
Results
Optimizing the conditions to analyze non-denatured PrPSc polymers by sedimentation velocity
PrPSc aggregates present in detergent-solubilised brain tissue homogenates were fractionated by sedimentation velocity centrifugation in an iodixanol gradient (Optiprep). The experimental conditions were established with brain material from tg338 mice that were infected or not with LA21K fast strain (referred to as LA21K), a prototypal, rapid strain that kills the mice within ∼2 months (see Table 1 for information on the strains used in this study). As a first step, we tested a variety of detergents for solubilization, which showed variable efficacy in terms of partition of PrPC and PrPSc species. For example, the use of standard solubilization buffers containing Triton X-100 and sodium deoxycholate or sarkosyl led to sedimentation of both isoforms throughout the gradient (Figure S1), indicating an incomplete release of total PrP from cellular constituents. In contrast, the sequential use of dodecyl maltoside and sarkosyl resulted in more efficient separation of the two PrP isoforms. Thus, in the conditions eventually employed (see Figure 1 for a summarizing flow diagram), the bulk of PrPC molecules remained in the upper fractions 1–4 (Figure 2A and D, green line), while both PrPSc (Figure 2B) and proteinase K (PK) resistant PrPSc species (Figure 2C–D, black line) were mainly detected in fractions 6–20 of the gradient. Importantly, no pelleted PrP material was observed in the selected conditions. Increasing the ultracentrifugation time caused the majority of PrPSc to sediment toward the heaviest fractions of the gradient, indicating that this material had not reached its density equilibrium (data not shown). Both dodecyl maltoside and sarkosyl are known to efficiently solubilize membrane structures, including rafts [26], [27], [28], yet PrPSc could be attached to abnormal, prion-induced structures. To address this point, brain homogenates were solubilized using these detergents in more stringent conditions, i.e. at 37°C instead of 4°C [29], however the sedimentation profile of PrPSc was affected only marginally (Figure S2A).
Fig. 1. Flow diagram describing the sedimentation velocity protocol and the analysis of prion particles infectivity with regard to PrP<sup>Sc</sup> content. 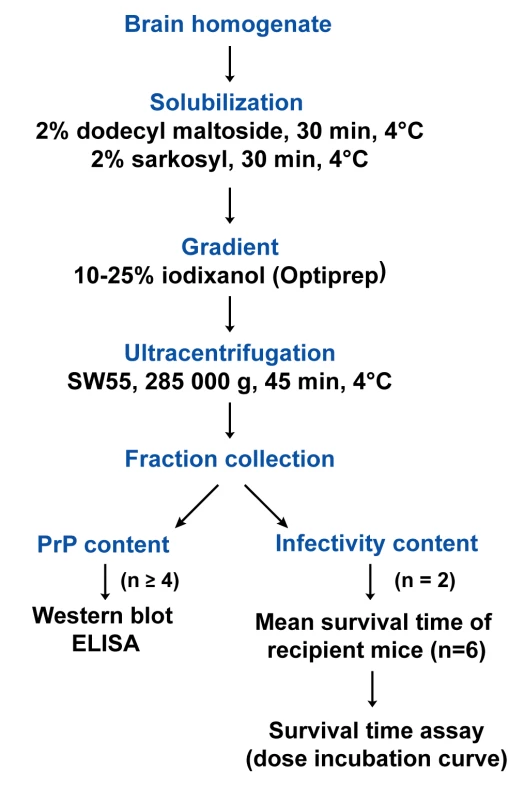
Fig. 2. Immunoblot analysis of velocity sedimented PrP material from tg338 mouse brain. 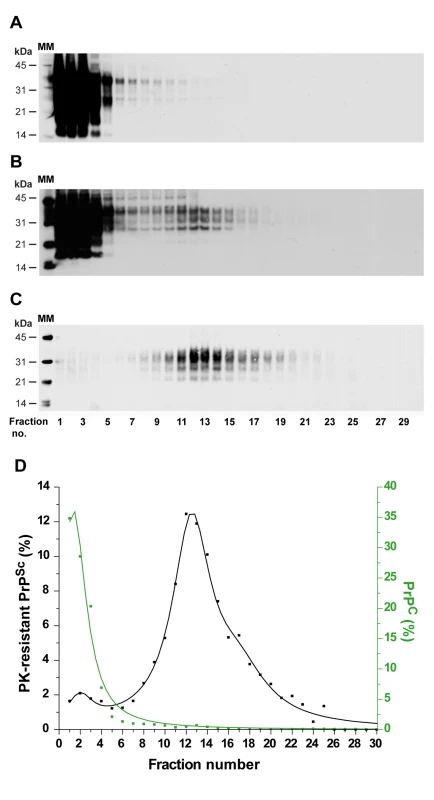
Brain homogenates from uninfected (A) or scrapie-infected (B–C; LA21K strain) tg338 mice were solubilized and fractionated by sedimentation velocity. The collected fractions (numbered from top to bottom of the gradient) were analyzed for PrP content by immunoblot without (A, B) or after PK digestion (C). (D) Graph showing the relative amount of PrPC (green line) and PK-resistant PrPSc (black line) per fraction. MM: molecular markers. Tab. 1. Phenotypic traits of the ovine and hamster prion strains used in the study. 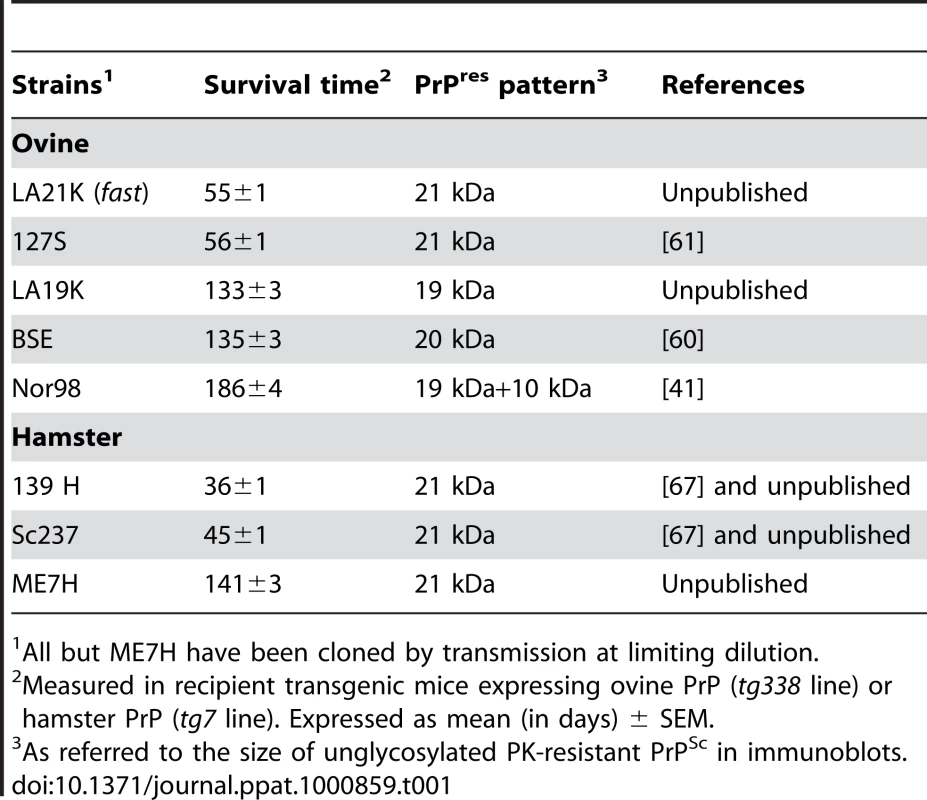
All but ME7H have been cloned by transmission at limiting dilution. In order to assess the reproducibility of the partition and to enable quantitative analysis of the data, 7 independent fractionations were performed using different pooled or individual brains and the resulting data fitted (Figure 3A, black line). This revealed that ∼80% of the PK-resistant PrPSc material sedimented as one major peak (maximum in fractions 10–12) with a Gaussian-like distribution. Standard globular macromolecules and ovine recombinant PrP oligomers [30] loaded on gradients run in parallel enabled estimation of the approximate molecular mass of the PK-resistant PrPSc aggregates forming the peak in fraction 10–12: between 200 and 500 kDa (by reference to the marker proteins, the sedimentation profile of which was affected only marginally in the presence of detergents), and ∼850 kDa based on the position of the 36-mer PrP oligomer (Figure 3A).
Fig. 3. Distinct PK-resistant PrPSc and infectivity sedimentation profiles of ovine prion strains. 
Brain homogenates from tg338 mice infected with LA21K (A), 127S (B), LA19K (C), Nor98 (D) and sheep BSE (E) were solubilized and fractionated by sedimentation velocity. Fractions collected from the gradient were analyzed for PK-resistant PrPSc content (black line) and for infectivity (red line). The mean levels of PK-resistant PrPSc per fraction have been obtained from the immunoblot or ELISA analysis of n = 4 to 7 (as indicated on each graph) independent fractionations. Since the replicates gave consistent results, these data were combined and fit. For each fraction, the percentage of total PK-resistant PrPSc detected on the immunoblot is presented (left axis). For each fraction of each strain, infectivity was determined by measuring mean survival times in reporter tg338 mice (mean ± SEM; right, red axis) and by applying these values to standard dose response curves [34], established by inoculation of serial tenfold dilutions of mouse brain homogenates infected with the same strain (see Figure S5 and material and methods). In these titration experiments, animals inoculated with 2 mg of infectious brain tissue were assigned a relative infectious dose of 0. The right, blue logarithmic scale provides the strain-specific reciprocal relation between survival time and relative infectious dose. For all but 127S strain, the data presented are the mean of n = 2 independent titrations. The sedimentation peaks of standard molecular mass markers (MM markers) aldolase (158 kDa), catalase (232 kDa), ferritin (440 kDa), thyroglobulin (669 kDa) and of 12-, 24-, and 36-mers of ovine recombinant PrP (recPrP) oligomers are indicated on the top of the graph. When solubilized brain material was PK-treated prior to ultracentrifugation, the PrPres sedimentation profile resembled that observed with intact brain material (Figure S2B). However, when semi-purified PrPSc in the form of scrapie-associated fibrils [31], [32] was resolubilized and centrifuged, a markedly different profile was obtained, with peaks in fractions 22 and 30 (bottom fraction) (Figure S2C). Interestingly, fast sedimenting PrPSc material was also observed with Italian scrapie agent (referred to as SSit), which in tg338 mice produces very long incubation times and abundant plaque-like PrPSc deposits in the brain [33], in contrast to the LA21K agent. These plaques can be stained by thioflavin S (Figure S3A–B), indicating the presence of amyloid fibrils. When SSit-infected brain material was fractionated, the majority of PrPSc multimers peaked in fractions 24 to 30 of the gradient (Figure S3C).
These results suggest that the experimental conditions employed preserve potential differences in the aggregation state of PrPSc thereby enabling the comparative analysis of sedimentation properties of “close to natural” PrPSc aggregates and of associated infectivity.
Fast ovine prion strains have distinct infectivity and PK-resistant PrPSc sedimentation profiles
The distribution of prion infectivity throughout the gradients was determined by an incubation time bioassay [34]. tg338 mice were inoculated intracerebrally with diluted aliquots from the different fractions. In terminally diseased mice, the PrPSc electrophoretic profile and regional distribution in the brain observed for representative fractions were both consistent and similar to that with the original brain material, indicating a conservation of the strain biological phenotype (Figure S4A and data not shown). The mean survival time values resulting from the analysis of 2 independent gradients are shown in Figure 3A (red line). Typically, the mice inoculated with the PK-resistant PrPSc-richest fractions (6–20) succumbed to disease in more than 80 days, whereas those inoculated with fractions 1–3 died in a markedly shorter time, ∼60–70 days. The correlation between the mean survival time values and infectivity was established by using a standard infectious dose/survival time curve previously established for this strain (Figure S5). This analysis indicated that fractions 1–2 were between 100 - and 1000-fold more infectious than fractions 6–20 (Figure 3A, blue scale). These upper fractions - within the sedimentation peak of aldolase (158 kDa) and upstream of 12-mer PrP oligomer – totaled <10% of PK-resistant PrPSc molecules (Figure 3A).
There is substantial evidence to indicate that a fraction of PrPSc can exhibit low sedimenting properties and be PK-sensitive [28], [35], [36]. Recently, thermolysin has been used as a means to isolate PK-sensitive forms of PrPSc, while degrading PrPC [37]. When the upper fractions from LA21K gradients were thermolysin-digested, no enrichment in thermolysin-resistant species was observed by immunoblot as compared to unfractionated brain material (Figure S6A–C). To further analyze the forms of PrPSc present in the upper fractions, aliquots were centrifuged at 100 000 g for 1 h to produce soluble (supernatant) and insoluble (pellet) fractions, before immunoblot analysis. The ratio of soluble and insoluble PrP species in LA21K versus uninfected fractions was determined based on signal intensities. As a result, the top two LA21K fractions were reproducibly shown to contain equivalent amounts of soluble material and about 2-fold more sedimentable material as compared to the corresponding uninfected fractions (Figure S6D).
Detergents and lipids have been proposed to increase the apparent infectious titer of PrPSc preparations non-specifically [38], [39] and such compounds are relatively abundant in the upper fractions of the gradient. To test whether such an effect was responsible for the comparatively high infectivity levels of the top fractions, the PK-resistant PrPSc-enriched fractions 10 to 12 were mixed, incubated with either the top fractions 1 to 3 of a gradient made with uninfected tg338 brain or with dodecyl maltoside alone, and inoculated to mice. As a result, in either condition, the relative titer of these fractions was not significantly modified (Figure 4).
Fig. 4. Effect of cellular components or detergent present in the gradient top fractions on infectivity of the LA21K PK-resistant PrPSc–richest fractions. 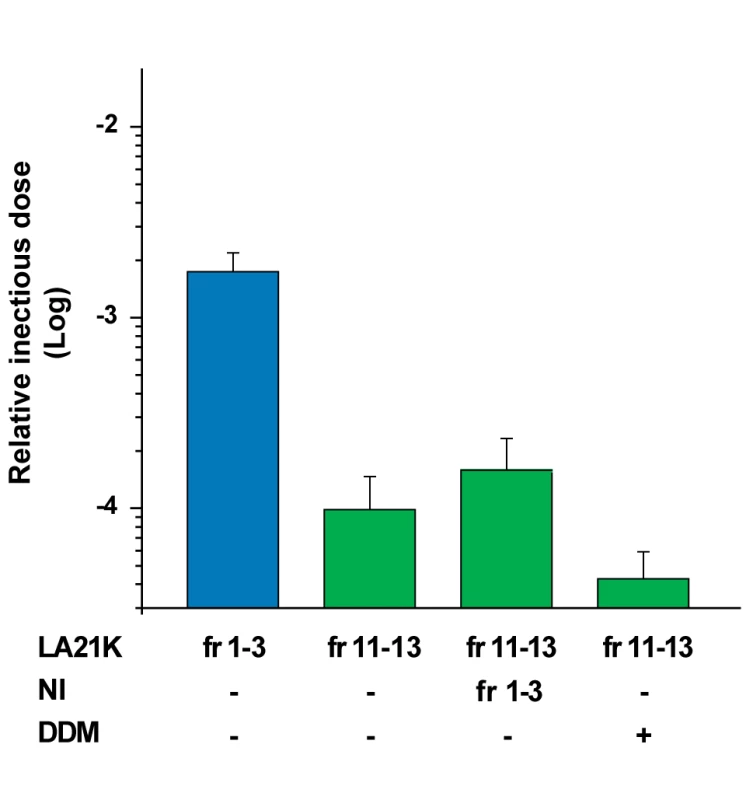
LA21K PK-resistant PrPSc-richest fractions 11 to 13 (fr11–13) were mixed together and incubated (vol∶vol) with either the upper fractions 1 to 3 (fr1–3) of a gradient made with uninfected tg338 mouse brain (NI) or with dodecyl maltoside (DDM) at 2% (wt/vol.) final concentration. The specific influence of sarkosyl was not tested since the gradients were prepared in a solution containing this detergent (see methods). The resulting mixture was inoculated to recipient mice. Their relative infectious titer was estimated by an incubation time bioassay, and compared to that of the fractions 1 to 3 of the same gradient pooled and diluted in 5% glucose (vol∶vol). The infectious titers are expressed as relative infectious doses as in Figure 3. To confirm that the differences in survival times observed between mice inoculated with the various fractions were correlated with differences in infectivity content, a mouse-free, cell bioassay was used. The distribution and level of LA21K infectivity in the gradient was measured using Rov cells [40] that were exposed in parallel to fraction aliquots and to serial tenfold dilutions of a LA21K brain homogenate prepared in the same conditions. Consistent with the bioassay data, the most infectious fractions were found at the top of the gradient and were ≥100-fold more infectious than the middle fractions (Figure S7).
Overall these data indicate that the upper fractions were intrinsically highly infectious. The fact that the cumulated infectivity in the gradient fractions did not differ significantly from that present in the loaded material prior solubilization also supports the conclusion that the detergents used did not alter infectivity estimates.
Brains of tg338 mice infected by another fast ovine strain named 127S (Table 1) were also fractionated and analyzed for PrP and infectivity content. 127S PK-resistant PrPSc peaked in fractions 10–12 (Figure 3B), as in the case of LA21K agent, despite some variation of the sedimentation profile in the bottom part of the gradient. Strikingly, the sedimentation profile of infectivity again largely segregated from that of PK-resistant PrPSc as assessed by mouse bioassay. The top two fractions were at least 50–100-fold more infectious than all the other fractions, including the major PK-resistant PrPSc peak (Figure 3B).
Infectivity and PK-resistant PrPSc sedimentation profiles of “slow” ovine strains
We next examined whether the decoupling of PK-resistant PrPSc and infectivity sedimentation profiles was a general feature of ovine strains. Three more strains were studied of which the incubation time in tg338 mice is at least twice that of LA21K and 127S: LA19K, Nor98 and sheep BSE (see Table 1). Four to five independent fractionations with different pooled or individual brains were performed for each strain. The combined curves resulting from the replicate analysis of PrP content indicated that a majority of PK-resistant PrPSc peaked in fractions 10–12, similar to that seen with the two fast strains. However, faster sedimenting species were also observed, notably in fractions 16, 20 for LA19K and fractions 22–24 for Nor98 (Figure 3C–D). Remarkably, the infectivity sedimentation profile of these 3 strains, as established from bioassay of two independent gradients, tended to overlap PK-resistant PrPSc distribution, with a very small proportion of the total infectivity in the top fractions. LA19K most infectious fractions ranged from fractions 8 to 24 with a peak in fraction 20 (range of mean survival time: 152 to 163 days), while the top and bottom fractions were ∼100-fold less infectious (mean survival time ∼185 to 210 days; Figure 3C). Nor98 infectivity peaked in fraction 11 and to a lesser degree in fraction 17 and 22 (mean survival time 222, 240 and 245 days, respectively; Figure 3D). Fractions in the immediate vicinity of these peaks were among the most infectious, (except fraction 13). In contrast, the upper fractions were ∼100-fold less infectious (survival time prolonged by >40 days). The most infectious sheep BSE fractions were found in fractions 6–12, 16 and 20 (mean survival times of 155–160, 164 and 163 days) while the top and bottom fractions were about 50-fold and 100-fold less infectious, respectively (survival time of ∼175 days and >180 days; Figure 3E).
Sedimentation properties of hamster prions
To further explore the possibility that slow sedimenting infectivity could be a specific feature of fast prion strains, we applied the same sedimentation velocity protocol to three hamster strains passaged on tg7 transgenic mice expressing hamster PrP (Table 1). For fast strains 139H and Sc237, the infectivity peaked in the top two fractions, which contained ∼10% of the total PK-resistant PrPSc material present in the gradient. The two 139H PK-resistant PrPSc peaks in fractions 11–12 and 16–18 and the Sc237 PK-resistant PrPSc peak in fraction 11–12 were ∼50-fold and <10-fold less infectious, respectively (n = 2 independent experiments made with different individual brains; Figure 5A–B). ME7H strain, characterized by a longer incubation time, produced a different picture since the mice inoculated with the PK-resistant PrPSc peak in fraction 11 were the fastest to succumb to disease, i.e. ∼180 days, whereas those inoculated with the top 3 fractions had mean survival times significantly prolonged by 20 to 40 days (Figure 5C). Therefore, much less infectivity was present in the upper region of the gradient than in the PK-resistant PrPSc containing fractions (about 50–100-fold, based on the available results of the endpoint titration of ME7H, still ongoing). Collectively, the contrasted sedimentation properties of fast and slow hamster strains were reminiscent of the results obtained with the ovine strains.
Fig. 5. Distinct PK-resistant PrPSc and infectivity sedimentation profiles of hamster prion strains. 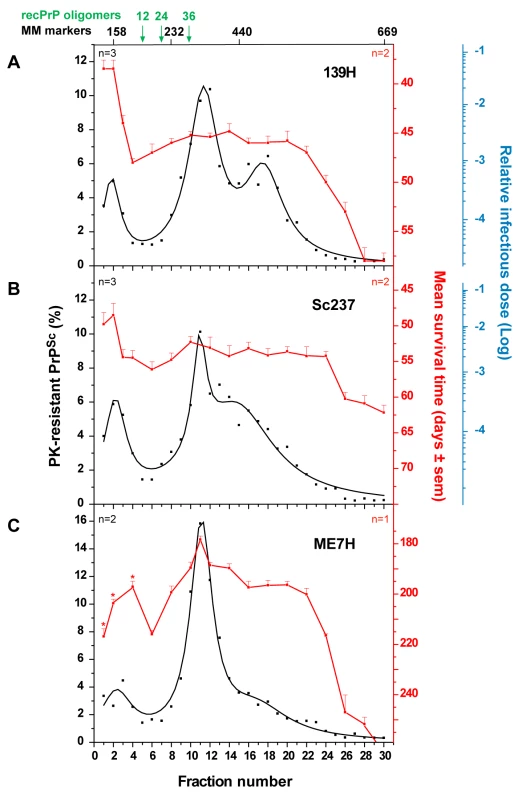
Brain homogenates from tg7 mice infected with 139H (A), Sc237 (B) and ME7H (C) strains were solubilized and fractionated by sedimentation velocity. Fractions collected from the gradients were analyzed for PK-resistant PrPSc content by immunoblot (black line) and for infectivity by an incubation time bioassay (A–B; red line). The mean levels of PK-resistant PrPSc per fraction have been obtained from the immunoblot or ELISA analysis of n = 2 to 3 (as indicated on each graph) independent fractionations. Since the replicates gave consistent results, these data were combined and fit. For each fraction, the percentage of total PK-resistant PrPSc detected on the immunoblot is presented (left axis). For all but ME7H strain, infectivity per fraction was determined by measuring mean survival times in reporter tg7 mice (mean ± SEM; right, red axis) and by applying these values to standard dose response curves, established by inoculation of serial tenfold dilutions of mouse brain homogenates infected with the same strain (see Figure S5 and material and methods). In these titration experiments, animals inoculated with 2 mg of infectious brain tissue were assigned a relative infectious dose of 0. The right, blue logarithmic scale provides the strain-specific reciprocal relation between survival time and relative infectious dose. For all but ME7H strain, the data presented are the mean of n = 2 independent titrations. For ME7H, the mean survival time (C) is shown (no titration curve available yet). *The mean survival times of mice inoculated with ME7H fraction 1, 2 and 4 were significantly prolonged compared to that of mice inoculated with fraction 11 (p<0.05, Kruskall-Wallis test). The sedimentation peaks of standard molecular mass markers (MM markers) aldolase (158 kDa), catalase (232 kDa), ferritin (440 kDa), thyroglobulin (669 kDa) and of 12-, 24-, and 36-mers of ovine recombinant PrP (recPrP) oligomers are indicated on the top of the graph. Discussion
Here we compared the sedimentation velocity properties of the infectivity and of abnormal PrP amongst several prion strains, using experimental conditions aimed at preserving as much as possible the “natural” multimerization state of the prion particles while minimizing artifacts due to improper membrane solubilization. To our knowledge this is the first study that allows a rigorous comparison of phenotypically distinct strains, cloned and propagated on the same genetic background. We found striking, strain-specific differences in the sedimentation profile of the infectious prion particles, which are not reflected in the sedimentation properties of the bulk of PrPSc.
Fractionation of five tg338 mouse-passaged ovine prions revealed a major PK-resistant PrPSc population, which peaked at the same position of the gradient regardless of the strain. By comparison with standard molecular mass markers and recombinant ovine PrP oligomers [30], we estimated that this population might correspond to approximately 12–30 PrP monomers averaging ∼30 kDa each, if constituted of PrP only, with the caveat that great caution must be exercised when attributing a size to a polymer by comparing its velocity to that of molecular mass markers. This result suggests that PrPSc is not a collection of multimers with a regular continuum of size. However, the overall sedimentation profiles were not uniform among the strains, indicating that the size distribution of PrPSc aggregates is strain-dependent. tg7 mouse-passaged hamster prions showed PrPSc sedimentation characteristics resembling that of ovine strains, with the same position of the major peak and limited variation. Greater differences in the size distribution of PrPSc aggregates may however exist depending on strain and/or PrP sequence, since one amyloid-forming ovine prion (Italian scrapie; Figure S3) showed a clear shift of PrPSc toward heavier fractions of the gradient.
In two studies, larger polymers were shown to be more PK-resistant than smaller ones [28], [35], indicating that the resistance to proteolysis of PrPSc largely depends on its quaternary structure. The PrPSc associated with Nor98 agent, a newly discovered strain responsible for an atypical form of field scrapie, is highly sensitive to PK digestion, when compared to the other ovine strains studied here [41], [42]. Notwithstanding, Nor98 PrPSc exhibited not only the same predominant peak as the other strains, but also the highest proportion of faster sedimenting PrPSc species. This argues that the pronounced PK sensitivity of Nor98 PrPSc is not due to low size aggregates, but rather to its tertiary structure. Supporting this view, its C-terminal region is accessible to PK, in contrast to classical scrapie agents [43], [44].
The infectivity sedimentation profiles unexpectedly contrast with that of the PrPSc aggregates. While infectivity and PK-resistant PrPSc roughly co-fractionated for LA19K, Nor98 and sheep BSE, their respective distribution was mostly decoupled for LA21K and 127S: with these two fast strains, infectivity essentially partitioned in the upper fractions, which were 2–3 logs more infectious than the PK-resistant PrPSc–richest fractions. LA21K, 127S and Nor98 are three strains exhibiting similarly high infectious titers in tg338 mice, making the observed differences particularly striking. Remarkably, a comparable situation was observed for hamster strains. Thus, ME7H infectivity and PK-resistant PrPSc sedimentation velocity profiles were broadly congruent, whereas for the fast strains 139H and Sc237 the infectivity peaked in the upper region of the gradient. Altogether, these findings lend support to the view that the predominance of slow sedimenting particles may be a common feature of prion strains with short incubation time.
Such a decoupling between infectivity and PK-resistant PrPSc with respect to the size of the particles is to our knowledge unprecedented in the literature. Two earlier studies performed with fast hamster prions [18], [23] also reported a relatively low PrPres content of the most infectious fractions. However, the level of infectivity in these fractions did not exceed that of the PrPres-richest fractions. The preparations used were fibrillar PrPres material under the form of SAF or Rods, disaggregated by sonication in the presence of anionic detergents [18], [23]. Such a procedure is likely to destroy discrete subpopulations of infectious particles, which may explain the observed discrepancy.
Which form(s) of PrPSc could support the high infectivity of the fast strains' slow sedimenting component? One possibility is that it consists essentially of PK-resistant aggregates, with high specific infectivity [24], [45], [46], [47]. If so, then ≥99% of LA21K or 127S infectivity would be supported by ≤10% of PK-resistant PrPSc molecules. Alternatively, infectivity could be mostly associated with a form of PrPSc with low resistance to PK. While a variable, strain-dependent proportion of abnormal PrP seems fairly PK-sensitive [28], [35], [36], [48], [49], little is known about its specific infectivity, with two recent studies suggesting that it could be minimal [37], [50]. However, a PK-sensitive and soluble form of PrPSc has been shown to support a substantial fraction of infectivity [51] and to have a good in vitro converting activity [35]. The abundance of both PrPC and other components in the upper fractions impeded further characterization of the most infectious PrPSc particles in the present study. Classical approaches based on PrPSc conformation-dependent assay [36], [49], [52] are unhelpful here; as already shown by others, low sedimenting, PrPC-rich fractions contain little or no conformation-dependent immunoreactive material [28], [35]. Also, we failed to detect soluble or thermolysin-resistant PrPSc material that might be indicative of the presence of PK-sensitive molecules [48], [51] in these fractions. Additional experiments including the titration of PK-treated and then fractionated infectious material are ongoing to further assess the protease-resistance of the slow sedimenting component.
What physical properties could account for slow sedimentation of fast strains infectious component? The detergents employed are known to produce a high degree of membrane solubilization [27], [28], [53], [54], [55], [56], including for GPI-anchored proteins of detergent-resistant microdomains [26], and they led indeed to an efficient solubilization of PrPC (Figure S6E). Dodecyl maltoside is also known to preserve activity of protein complexes in the detergent-solubilized state [27]. It is still possible that a tightly bound cellular component of low density, such as lipid molecule [45], or low-density lipoprotein [57], remains part of the prion particles in the most infectious fractions. This would imply that the tightness of such an interaction specifically differs between strains. Alternatively, the low sedimenting infectivity component could involve truly small size PrPSc particles. In this hypothesis, the data would indicate a size smaller than a PrP pentamer, which is compatible with that reported for PK-sensitive PrPSc aggregates [28], [35]. As a means to distinguish between small size and lipid associated PrPSc aggregates, LA21K gradient centrifugation time was doubled. As a result, infectivity was found to peak in fraction 4 instead of fraction 2 (data not shown). While arguing against lipid floatation of the most infectious component, this does not exclude the presence of tightly associated lipids. Additional experiments will be needed to address this issue.
The neuropathology induced in mice by the fast and slow sedimenting particles did not differ for a given prion, therefore suggestive of structurally related multimers (Figure S4). The slow sedimenting infectious particles could reflect a stronger tendency of large PrPSc polymers to fragment. In the case of yeast prions [PSI+], it has been proposed that the fittest strains are those whose large fibers break more easily into smaller oligomers that in turn act as new seeds for conversion [58], a concept that was then extended to mammalian prions [59]. In this regard, our preliminary results indicate that LA21K and 127S PrPSc aggregates exhibit the lowest ‘stability’ among the ovine strains, as assayed by conformational stability assay.
In addition to providing another measurable criterion of prion strain-related phenotypic variation, this study revealed the diversity of their infectious component. Further biochemical and biophysical investigations will be crucial for a mechanistic understanding of the replication dynamics of mammalian prions, in relation with the disease phenotype.
Materials and Methods
Ethics statement
All the experiments involving animals were approved by the INRA Jouy-en-Josas ethics committee in accordance with the European Community Council Directive 86/609/EEC.
Prion strains
The ovine prion strains used in this study have been obtained through serial transmission and subsequent biological cloning by limiting dilutions of classical and atypical field scrapie and experimental sheep BSE sources to tg338 transgenic mice expressing the VRQ allele of ovine PrP. The characterization of their phenotype in tg338 mice was performed as previously reported [41], [60], [61]. Pooled or individual tg338 mouse brain homogenates (20% wt/vol. in 5% glucose) were used in centrifugation analyses. Three hamster strains, 139H, Sc237 and ME7H, were also studied. These strains (kindly provided by R. Carp, Staten Island, NY, USA) were serially passaged on tg7 transgenic mice expressing hamster PrP (kindly provided by CSL-Behring (Marburg); [48], [62]). Both 139H and Sc237 were subsequently cloned by limiting dilution on this genetic background. Individual tg7 infected brains (20% wt/vol.) were used in centrifugation analyses. Non-infected brain tissue homogenates served as controls.
Sedimentation velocity in iodixanol gradients
The entire procedure was performed at 4°C. Mouse brain homogenates were solubilized by adding an equal volume of solubilization buffer (50 mM HEPES pH 7.4, 300 mM NaCl, 10 mM EDTA, 2 mM DTT, 4% (wt/vol.) dodecyl-β-D-maltoside (Sigma)) and incubated for 30 min on ice. Sarkosyl (N-lauryl sarcosine; Fluka) was added to a final concentration of 2% (wt/vol.) and the incubation continued for a further 30 min on ice. A volume of 150 µl was loaded on a 4.8 ml continuous 10–25% iodixanol gradient (Optiprep, Axys-shield), unless specified otherwise, with a final concentration of 25 mM HEPES pH 7.4, 150 mM NaCl, 2 mM EDTA, 1 mM DTT, 0.5% Sarkosyl. Gradient linearity was verified by refractometry. In standard experiments (Figure 1), the gradients were centrifuged at 285 000 g for 45 min in a swinging-bucket SW-55 rotor using an Optima LE-80K ultracentrifuge (Beckman Coulter). Gradients were then manually segregated into 30 equal fractions of 170 µl from the bottom using a peristaltic pump. Fractions were aliquoted for immunoblot or bioassay analyses. The strains were fractionated in parallel to preserve as much as possible identical experimental conditions. To avoid any cross-contamination, each piece of the equipment was thoroughly decontaminated with 5 M NaOH followed by several rinses in deionised water after each gradient collection. Standard markers (GE Healthcare, Little Chalfont, UK) of aldolase (158 kDa), catalase (232 kDa), ferritin (440 kDa) and thyroglobulin (669 kDa) were run in parallel.
Sedimentation velocity of PK treated brain homogenates
The protocol used was as described above except that PK (100 µg/ml final concentration; Euromedex, Mundolsheim, France) was added during the solubilization phase in sarkosyl (1 h at 37°C).
Sedimentation velocity of semi-purified PrPSc (SAF)
Brain homogenates were treated with 20 µg/ml of PK for 1 h at 37°C. The digestion was stopped by the addition of 5 mM phenylmethylsulfonyl fluoride. The solution was added to 10% sarkosyl and 10 mM Tris-HCl pH 7.4 and then centrifuged at 175 000 g for 30 min at 20°C on a 10% (wt/vol.) sucrose cushion in a Beckmann TL100 ultracentrifuge. Pellets were resuspended in 2% (wt/vol.) dodecyl maltoside and the above solubilization/fractionation protocol was followed.
Sedimentation velocity of recombinant PrP oligomers
Monomeric and oligomeric forms of purified ovine recombinant PrP [30] were resuspended at a final concentration of 7–10 µM in 20 mM citrate buffer. An aliquot (150 µl) was loaded onto a 10–25% iodixanol gradient in citrate buffer and centrifuged at 285 000 g for 45 min in a SW-55 rotor. Gradients were segregated as described above. Fraction aliquots (20 µl) were analyzed for PrP content by immunoblot (see below). In these conditions, recombinant, monomeric PrP was found in the upper fractions 1–3 (not shown).
Analysis of recombinant PrP, PrPC, PrPSc and PK-resistant PrPSc contents by immunoblot
Aliquots of the collected fractions were treated or not with 50 µg/ml PK before methanol precipitation. The pellet was resuspended in Laemmli buffer and denatured at 100°C for 5 min. The samples (15 µl) were run on 4–12% NuPAGE gels (Invitrogen, Cergy Pontoise, France), electrotransferred onto nitrocellulose membranes, and probed with 0.1 µg/ml biotinylated anti-PrP monoclonal antibody Sha31 as previously described [60]. Immunoreactivity was visualized by chemiluminescence (GE Healthcare). The amount of PrP present in each fraction was determined by the GeneTools software after acquisition of chemiluminescent signals with a GeneGnome digital imager (Syngene, Frederick, Maryland, United States).
Analysis of PK-resistant PrPSc content by Sandwich ELISA
All Bio-Rad TeSeE detection kit reagents were kindly provided by S. Simon (CEA, France; [63]). Briefly, aliquots (75 µl) of the collected fractions were digested with PK (50 µg/ml final concentration) for 1 h at 37°C before B buffer precipitation and centrifugation at 28 000 g for 15 min. The pellet was resuspended in 25 µl of 5 M urea before denaturation at 100°C for 10 min. R6 buffer (200 µl) was subsequently added to the samples and duplicates were analyzed in microtiter plates coated with anti-PrP antibody 11C6. The plates were left at room temperature for 2 h. After 3 washes in R2 buffer, 100 µl/well of the enzyme conjugate (Bar224 anti-PrP antibody) was added for 2 h. The substrate (100 µl) was added for 30 min and incubated in the dark. The absorbance was read at 450 nm. A dilution range of ovine, monomeric recombinant PrP was used for quantification of relative PK-resistant PrPSc levels.
Decomposition of PrPSc sedimentation profiles
The PK-resistant PrPSc sedimentation profiles obtained by either immunoblot or ELISA were normalized to units and decomposed using multiple Gaussians fits procedures with a maximum entropy minimization approach.
Analysis of thermolysin-resistant PrPSc by immunoblot
Fractions were methanol-precipitated. The pellet was resuspended in lysis buffer (2% sodium deoxycholate, 2% Triton X-100, 200 mM Tris-HCl pH 7.4) and mixed with an equal volume of thermolysin diluted in lysis buffer to yield a final concentration of 125 µg/ml (unless indicated otherwise) for 1 h at 70°C. The samples were analyzed by electrophoresis (4–12% gels) and immunoblotted as above. Blots were probed with either Sha31b or anti-octarepeat specific Pc248 anti-PrP antibody [64] at a final concentration of 0.1 µg/ml, before acquisition of chemiluminescent signals with a GeneGnome digital imager and analysis by the GeneTools software (Syngene, Frederick, Maryland, United States).
Sedimentation assay of PrP species present in the fractions
Aliquots (20 µl) of the fractions were added to 80 µl of 5% glucose before centrifugation at 100 000 g for 1h at 4°C in a Beckmann TL100 ultracentrifuge to generate soluble (supernatant) and insoluble (pellet) fractions. Proteins in the supernatant were precipitated with 400 µl of cold methanol, centrifuged at 16 000 g for 30 min before denaturation in 100 µl of sample buffer. The insoluble pellet was resuspended in 20 µl of Laemmli buffer before denaturation. Samples (20 µl) were analyzed by immunoblot as described above.
Mouse bioassay for infectivity titration
Fractions 1 to 4 and then every other two fractions (unless specified otherwise) were diluted extemporarily in 5% glucose (1∶5). This procedure was performed in a class II microbiological cabinet according to a strict protocol to avoid any cross-contamination. Individually identified 6 - to 10-week old tg338 or tg7 recipient mice (n = 6 mice per fraction) were inoculated intracerebrally with 20 µl of the solution. Recipient mice inoculated with fractionated uninfected mouse brain were euthanized while still healthy at >400 days post-infection. Their brain was negative for PrPres content. Mice showing TSE neurological signs were monitored daily and euthanized in extremis. Brains were removed and analyzed for PrPres content by either immunoblot or histoblot (see below) as a confirmatory test. The survival time was defined as the number of days from inoculation to euthanasia.
The survival times of tg338 or tg7 reporter mice was measured for each tenfold dilution tested during endpoint titration experiments performed with all but ME7H strains. Animals inoculated with 2 mg of infectious brain tissue were assigned a relative infectious dose of 0. From these data, curves representing the relative infectious dose to survival time were established ([41] and Figure S5). The different patterns in survival time distribution among the gradients can thus be looked at as a function of relative infectious dose so as to estimate what difference in survival times between inoculated fractions means in terms of infectivity.
Rov cell assay for infectivity titration
The scrapie cell assay technique will be fully described elsewhere. Briefly, LA21K gradient fractions aliquots (typically 20–30 µL) were methanol precipitated before resuspension in culture medium (alpha minimal essential medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 10 µg/ml streptomycin). We verified that methanol precipitation did not affect the overall level of infectivity. Rov cell [40] monolayers established in a 96 well plate were exposed to the fractions for one week. After several washes, the cells were further cultivated for two weeks before fixation and PrPSc detection by immunofluorescence as previously described [65]. Immunofluorescence signals were acquired with an inverted fluorescence microscope (Zeiss Axiovert). A program in NIH Image J software was designed to quantify the levels of PrPSc signal per cell in each well. Serial tenfold dilutions of LA21K infected brain homogenates were prepared in the same conditions and run in parallel experiments to establish a tissue culture infectious doses curve that directly relates to the percentage of PrPSc content.
Histopathology
Brains were rapidly removed from euthanized mice and frozen on dry ice. Cryosections were cut at 8–10 µm, transferred onto Superfrost slides and kept at −20°C until use. Histoblot analyses were performed on 3 brains per infection, using the 12F10 anti-PrP antibody as described [60]. For thioflavin-S binding, formalin - or methanol-fixed sections were incubated with 0.01% thioflavin-S for 1 hour as previously described [66]. Sections were then incubated with nuclear marker 4′, 6-diamidino-2-phenylindole (Sigma), mounted in fluoromount-G (Interchim) before acquisition with an inverted fluorescence microscope (Zeiss Axiovert) and analysis with the Metamorph software.
Accession numbers
The Swiss-Prot accession numbers for the proteins mentioned in the text are sheep (P23907) and hamster PrP (P04273).
Supporting Information
Zdroje
1. CollingeJ
2001 Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24 519 550
2. PrusinerSB
1998 Prions. Proc Natl Acad Sci U S A 95 13363 13383
3. WeissmannC
2004 The state of the prion. Nat Rev Microbiol 2 861 871
4. MallucciG
CollingeJ
2005 Rational targeting for prion therapeutics. Nat Rev Neurosci 6 23 34
5. CaugheyB
LansburyPT
2003 Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26 267 298
6. BoltonDC
McKinleyMP
PrusinerSB
1982 Identification of a protein that purifies with the scrapie prion. Science 218 1309 1311
7. BeringueV
VilotteJL
LaudeH
2008 Prion agent diversity and species barrier. Vet Res 39 47
8. BruceME
2003 TSE strain variation. Br Med Bull 66 99 108
9. CollingeJ
ClarkeAR
2007 A general model of prion strains and their pathogenicity. Science 318 930 936
10. TellingGC
ParchiP
DeArmondSJ
CortelliP
MontagnaP
1996 Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science 274 2079 2082
11. BessenRA
MarshRF
1994 Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J Virol 68 7859 7868
12. CaugheyB
RaymondGJ
BessenRA
1998 Strain-dependent differences in beta-sheet conformations of abnormal prion protein. J Biol Chem 273 32230 32235
13. ThomzigA
SpassovS
FriedrichM
NaumannD
BeekesM
2004 Discriminating scrapie and bovine spongiform encephalopathy isolates by infrared spectroscopy of pathological prion protein. J Biol Chem 279 33847 33854
14. CollingeJ
SidleKC
MeadsJ
IronsideJ
HillAF
1996 Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 383 685 690
15. SimVL
CaugheyB
2008 Ultrastructures and strain comparison of under-glycosylated scrapie prion fibrils. Neurobiol Aging
16. BessenRA
KociskoDA
RaymondGJ
NandanS
LansburyPT
1995 Non-genetic propagation of strain-specific properties of scrapie prion protein. Nature 375 698 700
17. CastillaJ
MoralesR
SaaP
BarriaM
GambettiP
2008 Cell-free propagation of prion strains. Embo J 27 2557 2566
18. RiesnerD
KellingsK
PostK
WilleH
SerbanH
1996 Disruption of prion rods generates 10-nm spherical particles having high alpha-helical content and lacking scrapie infectivity. J Virol 70 1714 1722
19. SafarJ
WangW
PadgettMP
CeroniM
PiccardoP
1990 Molecular mass, biochemical composition, and physicochemical behavior of the infectious form of the scrapie precursor protein monomer. Proc Natl Acad Sci U S A 87 6373 6377
20. SklaviadisTK
ManuelidisL
ManuelidisEE
1989 Physical properties of the Creutzfeldt-Jakob disease agent. J Virol 63 1212 1222
21. TateishiJ
KitamotoT
MohriS
SatohS
SatoT
2001 Scrapie removal using Planova virus removal filters. Biologicals 29 17 25
22. SunR
LiuY
ZhangH
ManuelidisL
2008 Quantitative recovery of scrapie agent with minimal protein from highly infectious cultures. Viral Immunol 21 293 302
23. SilveiraJR
RaymondGJ
HughsonAG
RaceRE
SimVL
2005 The most infectious prion protein particles. Nature 437 257 261
24. SomervilleRA
DunnAJ
1996 The association between PrP and infectivity in scrapie and BSE infected mouse brain. Arch Virol 141 275 289
25. SklaviadisT
DreyerR
ManuelidisL
1992 Analysis of Creutzfeldt-Jakob disease infectious fractions by gel permeation chromatography and sedimentation field flow fractionation. Virus Res 26 241 254
26. BrownDA
RoseJK
1992 Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68 533 544
27. le MaireM
ChampeilP
MollerJV
2000 Interaction of membrane proteins and lipids with solubilizing detergents. Biochim Biophys Acta 1508 86 111
28. TzabanS
FriedlanderG
SchonbergerO
HoronchikL
YedidiaY
2002 Protease-sensitive scrapie prion protein in aggregates of heterogeneous sizes. Biochemistry 41 12868 12875
29. LondonE
BrownDA
2000 Insolubility of lipids in triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim Biophys Acta 1508 182 195
30. EghiaianF
DaubenfeldT
QuenetY
van AudenhaegeM
BouinAP
2007 Diversity in prion protein oligomerization pathways results from domain expansion as revealed by hydrogen/deuterium exchange and disulfide linkage. Proc Natl Acad Sci U S A 104 7414 7419
31. MerzPA
SomervilleRA
WisniewskiHM
ManuelidisL
ManuelidisEE
1983 Scrapie-associated fibrils in Creutzfeldt-Jakob disease. Nature 306 474 476
32. LasmezasCI
DeslysJP
RobainO
JaeglyA
BeringueV
1997 Transmission of the BSE agent to mice in the absence of detectable abnormal prion protein. Science 275 402 405
33. BeringueV
BencsikA
Le DurA
ReineF
LaiTL
2006 Isolation from cattle of a prion strain distinct from that causing bovine spongiform encephalopathy. PLoS Pathog 2 e112 doi:10.1371/journal.ppat.0020112
34. PrusinerSB
CochranSP
GrothDF
DowneyDE
BowmanKA
1982 Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol 11 353 358
35. PastranaMA
SajnaniG
OniskoB
CastillaJ
MoralesR
2006 Isolation and characterization of a proteinase K-sensitive PrPSc fraction. Biochemistry 45 15710 15717
36. SafarJG
GeschwindMD
DeeringC
DidorenkoS
SattavatM
2005 Diagnosis of human prion disease. Proc Natl Acad Sci U S A 102 3501 3506
37. CronierS
GrosN
TattumMH
JacksonGS
ClarkeAR
2008 Detection and characterization of proteinase K-sensitive disease-related prion protein with thermolysin. Biochem J 416 297 305
38. SomervilleRA
CarpRI
1983 Altered scrapie infectivity estimates by titration and incubation period in the presence of detergents. J Gen Virol 64(Pt 9) 2045 2050
39. GabizonR
McKinleyMP
PrusinerSB
1987 Purified prion proteins and scrapie infectivity copartition into liposomes. Proc Natl Acad Sci U S A 84 4017 4021
40. ViletteD
AndreolettiO
ArcherF
MadelaineMF
VilotteJL
2001 Ex vivo propagation of infectious sheep scrapie agent in heterologous epithelial cells expressing ovine prion protein. Proc Natl Acad Sci U S A 98 4055 4059
41. Le DurA
BeringueV
AndreolettiO
ReineF
LaiTL
2005 A newly identified type of scrapie agent can naturally infect sheep with resistant PrP genotypes. Proc Natl Acad Sci U S A 102 16031 16036
42. BenestadSL
ArsacJN
GoldmannW
NoremarkM
2008 Atypical/Nor98 scrapie: properties of the agent, genetics, and epidemiology. Vet Res 39 19
43. ArsacJN
AndreolettiO
BilheudeJM
LacrouxC
BenestadSL
2007 Similar biochemical signatures and prion protein genotypes in atypical scrapie and Nor98 cases, France and Norway. Emerg Infect Dis 13 58 65
44. KlingebornM
WikL
SimonssonM
RenstromLH
OttingerT
2006 Characterization of proteinase K-resistant N - and C-terminally truncated PrP in Nor98 atypical scrapie. J Gen Virol 87 1751 1760
45. KleinTR
KirschD
KaufmannR
RiesnerD
1998 Prion rods contain small amounts of two host sphingolipids as revealed by thin-layer chromatography and mass spectrometry. Biol Chem 379 655 666
46. SafarJG
KellingsK
SerbanA
GrothD
CleaverJE
2005 Search for a prion-specific nucleic acid. J Virol 79 10796 10806
47. BarronRM
CampbellSL
KingD
BellonA
ChapmanKE
2007 High titers of transmissible spongiform encephalopathy infectivity associated with extremely low levels of PrPSc in vivo. J Biol Chem 282 35878 35886
48. CronierS
BeringueV
BellonA
PeyrinJM
LaudeH
2007 Prion strain - and species-dependent effects of antiprion molecules in primary neuronal cultures. J Virol 81 13794 13800
49. ThackrayAM
HopkinsL
BujdosoR
2007 Proteinase K-sensitive disease-associated ovine prion protein revealed by conformation-dependent immunoassay. Biochem J 401 475 483
50. DeleaultAM
DeleaultNR
HarrisBT
ReesJR
SupattaponeS
2008 The effects of prion protein proteolysis and disaggregation on the strain properties of hamster scrapie. J Gen Virol 89 2642 2650
51. BerardiVA
CardoneF
ValanzanoA
LuM
PocchiariM
2006 Preparation of soluble infectious samples from scrapie-infected brain: a new tool to study the clearance of transmissible spongiform encephalopathy agents during plasma fractionation. Transfusion 46 652 658
52. SafarJ
WilleH
ItriV
GrothD
SerbanH
1998 Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4 1157 1165
53. de la MazaA
ParraJL
1997 Solubilizing effects caused by the nonionic surfactant dodecylmaltoside in phosphatidylcholine liposomes. Biophys J 72 1668 1675
54. LambengN
GrossmannM
ChatelainP
FuksB
2006 Solubilization and immunopurification of rat brain synaptic vesicle protein 2A with maintained binding properties. Neurosci Lett 398 107 112
55. LucheS
SantoniV
RabilloudT
2003 Evaluation of nonionic and zwitterionic detergents as membrane protein solubilizers in two-dimensional electrophoresis. Proteomics 3 249 253
56. RibosaII
Sanchez-LealJ
ComellesF
GarciaMT
1997 Solubilization of Large Unilamellar Liposomes by Alkyl Glycosides. J Colloid Interface Sci 187 443 446
57. SafarJG
WilleH
GeschwindMD
DeeringC
LatawiecD
2006 Human prions and plasma lipoproteins. Proc Natl Acad Sci U S A 103 11312 11317
58. TanakaM
CollinsSR
ToyamaBH
WeissmanJS
2006 The physical basis of how prion conformations determine strain phenotypes. Nature 442 585 589
59. LegnameG
NguyenHO
PeretzD
CohenFE
DeArmondSJ
2006 Continuum of prion protein structures enciphers a multitude of prion isolate-specified phenotypes. Proc Natl Acad Sci U S A 103 19105 19110
60. BeringueV
AndreolettiO
Le DurA
EssalmaniR
VilotteJL
2007 A bovine prion acquires an epidemic bovine spongiform encephalopathy strain-like phenotype on interspecies transmission. J Neurosci 27 6965 6971
61. VilotteJL
SoulierS
EssalmaniR
StinnakreMG
VaimanD
2001 Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine prp. J Virol 75 5977 5984
62. PrusinerSB
ScottM
FosterD
PanKM
GrothD
1990 Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell 63 673 686
63. SimonS
NugierJ
MorelN
BoutalH
CreminonC
2008 Rapid typing of transmissible spongiform encephalopathy strains with differential ELISA. Emerg Infect Dis 14 608 616
64. MoudjouM
TreguerE
RezaeiH
SabuncuE
NeuendorfE
2004 Glycan-controlled epitopes of prion protein include a major determinant of susceptibility to sheep scrapie. J Virol 78 9270 9276
65. PaquetS
DaudeN
CourageotMP
ChapuisJ
LaudeH
2007 PrPc does not mediate internalization of PrPSc but is required at an early stage for de novo prion infection of Rov cells. J Virol 81 10786 10791
66. BeringueV
Le DurA
TixadorP
ReineF
LepourryL
2008 Prominent and persistent extraneural infection in human PrP transgenic mice infected with variant CJD. PLoS ONE 3 e1419 doi:10.1371/journal.pone.0001419
67. HeckerR
TaraboulosA
ScottM
PanKM
YangSL
1992 Replication of distinct scrapie prion isolates is region specific in brains of transgenic mice and hamsters. Genes Dev 6 1213 1228
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



