-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
Plasmodium falciparum malaria causes 500 million clinical cases with approximately one million deaths each year. After many years of exposure, individuals living in endemic areas develop a form of clinical immunity to disease known as premunition, which is characterised by low parasite burdens rather than sterilising immunity. The reason why malaria parasites persist under a state of premunition is unknown but it has been suggested that suppression of protective immunity might be a mechanism leading to parasite persistence. Although acquired immunity limits the clinical impact of infection and provides protection against parasite replication, experimental evidence indicates that cell-mediated immune responses also result in detrimental inflammation and contribute to the aetiology of severe disease. Thus, an appropriate regulatory balance between protective immune responses and immune-mediated pathology is required for a favourable outcome of infection. As natural regulatory T (Treg) cells are identified as an immunosuppressive lineage able to modulate the magnitude of effector responses, several studies have investigated whether this cell population plays a role in balancing protective immunity and pathogenesis during malaria. The main findings to date are summarised in this review and the implication for the induction of pathogenesis and immunity to malaria is discussed.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000771
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1000771Summary
Plasmodium falciparum malaria causes 500 million clinical cases with approximately one million deaths each year. After many years of exposure, individuals living in endemic areas develop a form of clinical immunity to disease known as premunition, which is characterised by low parasite burdens rather than sterilising immunity. The reason why malaria parasites persist under a state of premunition is unknown but it has been suggested that suppression of protective immunity might be a mechanism leading to parasite persistence. Although acquired immunity limits the clinical impact of infection and provides protection against parasite replication, experimental evidence indicates that cell-mediated immune responses also result in detrimental inflammation and contribute to the aetiology of severe disease. Thus, an appropriate regulatory balance between protective immune responses and immune-mediated pathology is required for a favourable outcome of infection. As natural regulatory T (Treg) cells are identified as an immunosuppressive lineage able to modulate the magnitude of effector responses, several studies have investigated whether this cell population plays a role in balancing protective immunity and pathogenesis during malaria. The main findings to date are summarised in this review and the implication for the induction of pathogenesis and immunity to malaria is discussed.
Natural Regulatory T Cells
Control of infection is the main function of the immune system. Some pathogens are difficult to eradicate and require a potent immune response involving destructive and cytotoxic mediators, which can result in adverse effects and immunopathology. Lymphoid populations exerting immunosuppressive activity, such as natural regulatory T (Treg) cells, have been shown to play a critical role in balancing protective immune responses and immune-mediated pathology [1]–[4]. This suppressor T cell linage is characterised by expression of high levels of CD25 and CD4 [5], while also expressing low levels of CD127 [6]. Natural Treg cells constitutively express high levels of the T cell inhibitory receptor cytotoxic T lymphocyte–associated molecule 4 (CTLA-4) [7] and the glucocortcoid-inducible tumour necrosis factor receptor (GITR) [8]. Forkhead box P3 (Foxp3) is the only transcription factor required for generation of natural Treg cells [9] and it constitutes the most specific marker for this cell population. Natural Treg cells, initially identified by their capacity to inhibit the development of autoimmune gastritis [10], are now known to prevent other autoimmune conditions such as diabetes [7] and inflammatory bowel disease [2], [11], as well as inhibit anti-tumour immunity [12]. They have been shown to inhibit both IL-2 production and cell proliferation of conventional CD4+ T lymphocytes [13] by contact-dependent mechanisms and/or through the production of anti-inflammatory cytokines such as TGF-β [14] and IL-10 [15]. Although they have limited proliferative capacity and are unable to secrete IL-2, natural Treg cells exert their suppressive function after stimulation through the T cell receptor, a process that appears to require antigen-presenting cells [13]. After activation, natural Treg cells do no require further stimulation and are able to suppress T cell responses in a non-specific manner.
In addition to natural Treg cells, induced Treg cells constitute another subset of suppressor lymphocytes [16]. Unlike naturally occurring Treg cells, which develop in the thymus, induced Treg cells are derived from conventional CD4+ T cells and acquire suppressive activity upon activation and exposure to signals such as immunosuppressive cytokines or immature dendritic cells (DCs).
Why Are Treg Cells Expected to Play a Role in Malaria Infection?
Malaria is one the most serious infectious diseases of humans, infecting 5%–10% of the world's population, with approximately 500 million clinical cases annually. This infection is transmitted to vertebrate hosts by the bite of female Anopheles mosquitos that are infected with protozoan parasites of the genus Plasmodium. Although there are several different species of Plasmodium parasites, most cases of severe disease and fatalities are caused by the blood-stage cycle of Plasmodium falciparum, which is endemic in sub-Saharan Africa and throughout the tropics. The fatalities are associated with a spectrum of discrete and overlapping disease syndromes including acute respiratory distress, coagulopathy, shock, metabolic acidosis, hypoglycaemia, renal failure, pulmonary oedema, and cerebral involvement [17]. In endemic areas, the most susceptible population to symptoms associated with severe malaria are children under the age of 5 who have experienced few parasitic infections. After many years of exposure, individuals living in endemic areas develop clinical immunity to disease, which is characterised by low parasitemia levels rather than sterilising immunity [18], [19]. However, this clinical immunity is known as “premunition”, as it requires ongoing exposure to the pathogen, and is lost quite rapidly in the absence of transmission. The reason why malaria parasites persist under the state of premunition is unknown, but it has been suggested that immunosuppressive mechanisms able to modulate protective immunity might lead to the persistence of parasites. In support of this hypothesis, several immunosuppressive processes have been described accompanying both human and experimental malaria infections, including reduced T cell proliferative and IFN-γ responses to parasite antigens [20], [21] and production of immunosuppressive cytokines such as IL-10 [15], [22] and TGF-β [23]. As natural Treg cells have been shown to mediate their suppressive effects through the production of these cytokines, it has been tempting to postulate that this immunoregulatory lineage could impede induction of immunity to malaria.
Although acquired host immunity limits the clinical impact of malaria infection and provides protection against parasite replication, experimental evidence indicates that cell-mediated immune responses also result in detrimental inflammation and contribute to induction of severe disease. In both P. falciparum infections and rodent malaria models, severe disease syndromes arise in diverse organs (e.g., brain, lungs, placenta) and appear to result from the combined effect of cytoadherence of parasitised red blood cells (pRBCs) in vascular beds and a strong host pro-inflammatory response mediated by cytokines such as TNF-α [24], LT-α [25], and IFN-γ [26], and effector cells such as CD4 [27], [28] and CD8 T cells [29], [30], natural killer (NK) T cells [31], [32], and NK cells [33]. As pro-inflammatory cytokines produced by these cells upregulate the expression of adhesion molecules such as intercellular cell adhesion molecule 1 (ICAM-1) [34], which is involved in the recognition of parasitic proteins expressed on pRBCs [35], it has been proposed that local and/or systemic inflammatory cascades exacerbate parasite sequestration. Furthermore, emerging evidence from both human malaria and experimental animal models [29], [30], [36], [37] has revealed the presence of leukocytes in blood vessels of inflamed tissue, suggesting that in addition to their systemic effects, intravascular infiltration of these cells might result in local inflammation and could also contribute to disease induction. Thus, cell-mediated immune responses appear to play a dual role in malaria: mediating protective immunity on the one hand, and contributing to severe inflammation and pathogenesis on the other. Whether natural Treg cells are involved in promoting a balance between these processes has been the subject of several studies. In this review we have summarised the main findings to date investigating the role of natural Treg cells during malaria, including experimental infection using a variety of murine infection models as well as human field studies. The implication of those findings for the induction of pathogenesis and immunity to malaria is discussed.
Natural Treg Cells and the Control of Parasite Clearance in Rodent Malaria Infection Models
Most of the studies using rodent models of infection have relied on the use of anti-CD25 antibodies for in vivo depletion of natural Treg cells (Table 1). Although this appears to be a standard procedure to effectively deplete natural Treg cells in vivo, one disadvantage of this approach is that CD25 is upregulated in activated T cells, thus making it difficult to specifically target natural Treg cells in experimental models in which conventional lymphocytes are expected to become activated. This potential confounder should be addressed experimentally or taken into consideration for interpretation of experimental results.
Tab. 1. Effect of T<sub>reg</sub> cell depletion in the outcome of malaria in rodent infection models. 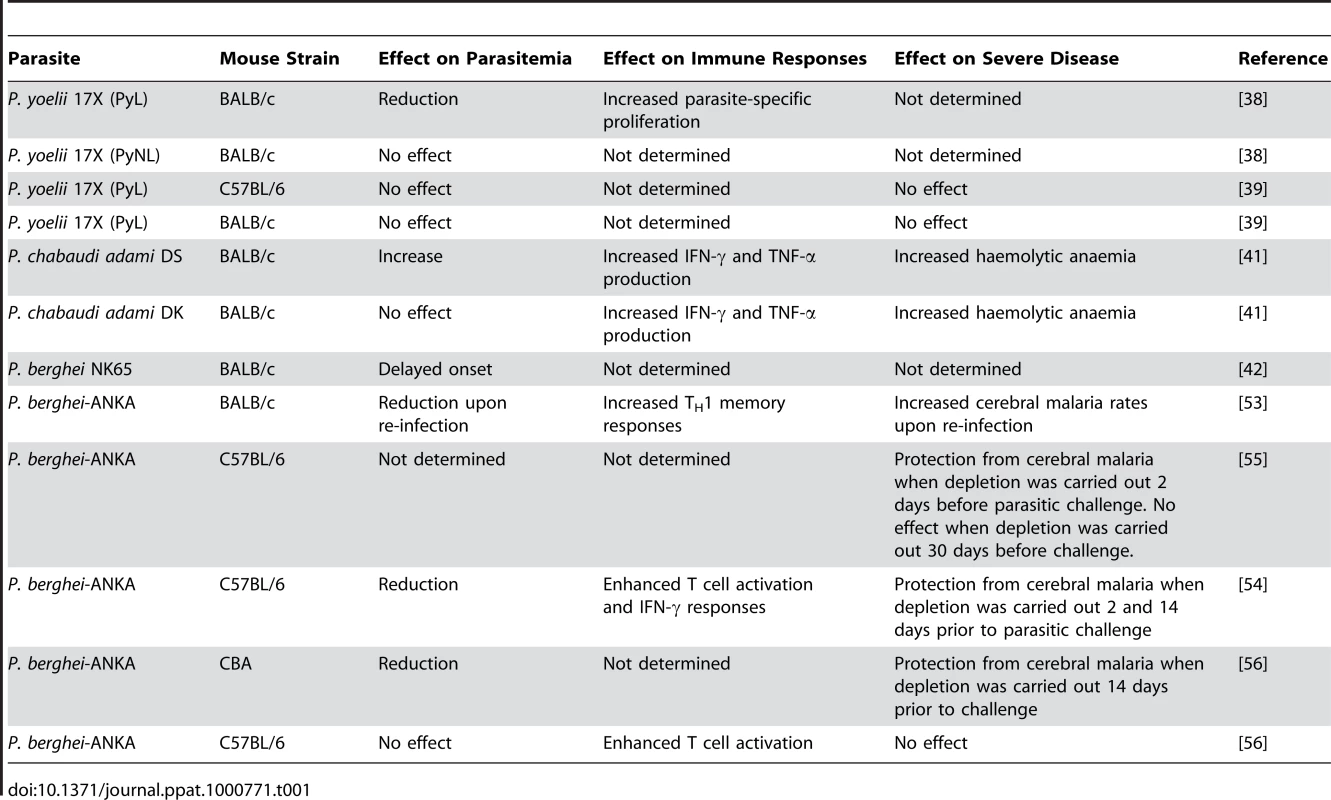
Several studies have investigated whether natural Treg cells modulate the induction of immune mechanisms responsible for the control of parasite burden. Experimental infections with malaria species such as Plasmodium chabaudi and Plasmodium yoelii are particularly useful for addressing this question since they result in high parasitemia levels and do not induce T cell–mediated organ-specific disease syndromes (Table 2). There are two sub-strains of P. yoelii 17X. One of them is highly virulent (PyL) and induces lethal infections, whereas the other one (PyNL) results in a self-resolving non-lethal infection. This difference in outcome suggested that while infection with non-lethal parasites induces adaptive immune responses able to limit parasite replication, immunosuppressive mechanisms might operate when mice are infected with the lethal sub-strain, preventing the induction of protective immunity. Two independent studies have investigated whether natural Treg cells play a role in the inhibition of immune responses against P. yoelii 17X. One found that depletion of natural Treg cells protects BALB/c mice from overwhelming parasitemia and death after infection with PyL parasites, raising the possibility that natural Treg cell function provides an essential escape mechanism used by malaria parasites to evade host-mediated immunity [38]. A subsequent study found that Foxp3+ regulatory T cells expand and become activated in response to infection with both PyL and PyNL parasites. However, in vivo depletion experiments failed to recapitulate the initial observations supporting a role for natural Treg cells in the control of parasitemia levels [39]. Moreover, CD4+ CD25− Foxp3−CD127− adaptive T cells and not natural Treg cells were found to be the source of IL-10 responsible for downregulation of protective immune responses during P. yoelii lethal infections [39]. The reasons for this inconsistency are unclear. The composition of intestinal flora has been shown to influence the development of natural Treg cells [40]. It is therefore possible that Treg cell depletion in genetically identical mouse strains housed in various animal facilities could lead to different infection outcomes if such mice differ in the composition of bacteria in their gastrointestinal tract.
Tab. 2. Malaria rodent models used to investigate T<sub>reg</sub> cell function. 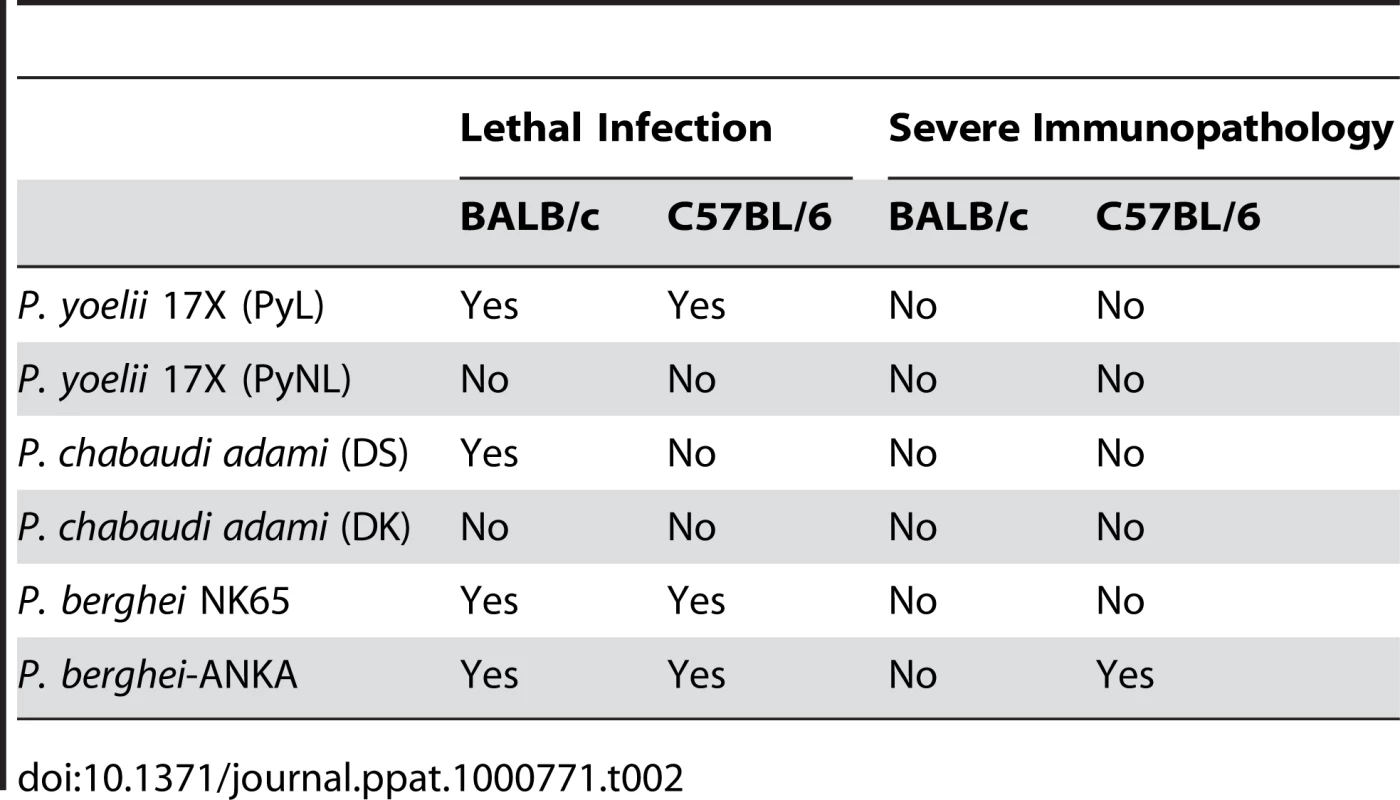
The impact of natural Treg cell depletion was also evaluated in infection of mice with lethal (DS) and non-lethal (DK) strains of P. chabaudi adami. Treg cell depletion resulted in increased production of IFN-γ and TNF-α in response to both lethal and non-lethal P. chabaudi adami infection. In spite of the enhancement of pro-inflammatory responses, Treg cell depletion did not alter the course of infection in animals challenged with non-lethal parasites [41]. Curiously, Treg cell depletion was found to exacerbate parasite burden and accelerate death in mice infected with the lethal DS strain. The haemolytic anaemia that results from hyperparasitemia was also enhanced in the absence of natural Treg cells. These studies accord with the notion that in experimental models resulting in parasitemia of high density, T helper cell 1 (TH1) responses are not sufficient to control parasite burden, and consistently the Treg cell–dependent modulation of pro-inflammatory responses is not critical in determining infection outcome (Figure 1B).
Fig. 1. Treg cells in malaria: Lessons learnt from experimental rodent models. 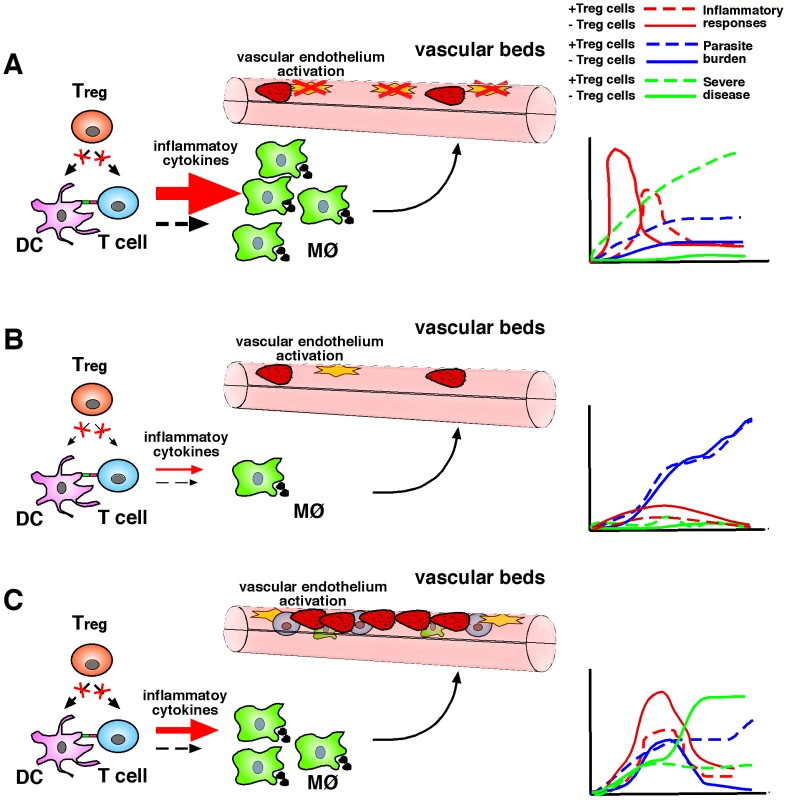
(A) In murine models characterised by high pro-inflammatory responses to infection, Treg cell depletion may result in a strong and rapid cellular immune response, which could rapidly control parasitemia, in turn reducing parasite-mediated pathology. MØ, macrophage. (B) In an infection setting resulting in poor inflammatory responses to malaria, a lack of Treg cells may improve the induction of such responses but at levels that are not sufficient to control parasite burden or induce immunopathology. (C) In re-infection models, TH1 responses generated in the absence of Treg cells during primary exposure appear to be robust enough to facilitate control of parasitemia in a secondary challenge at the expense of increased immune-mediated severe disease induction. The effect of natural Treg cell depletion with anti-CD25 antibody was also evaluated in immunised as well as naïve BALB/c mice after infection with P. berghei NK65. Treg cell depletion resulted in a delay in the onset of parasitemia compared to non-depleted controls [42]. Whether Treg cell depletion influenced the development of immune responses involved in the control of parasitemia was not investigated.
The Role of Natural Treg Cells in the Control of Experimental Cerebral Malaria
Although infection of mice with P. yoelii, P. berghei NK65, and P. chabaudi adami results in hyperparasitemia and death of susceptible mice due to haemolytic anaemia, it does not induce T cell–mediated organ-specific immunopathology. Therefore, a contribution of Treg cells to the control of pathogenic immune responses cannot be accurately assessed in those infection models. In contrast, infection of susceptible mouse strains with Plasmodium berghei-ANKA induces cerebral malaria (Table 2). This murine infection has many features in common with the human disease and is thus an accepted model for certain important aspects of clinical malaria [43]–[46]. It manifests an inflammatory cytokine–dependent encephalopathy associated with upregulation of adhesion molecules on the cerebral microvascular endothelium, which facilitates sequestration of pRBCs and the resulting recruitment of inflammatory CXCR3+ leukocytes [33], [47], [48]. C57BL/6 mice, genetically predisposed towards TH1-dominated responses [49], are susceptible to the murine cerebral malaria syndrome, whereas BALB/c mice, with a genetically determined bias towards TH2 responses, are resistant [50]. Moreover, C57BL/6 and BALB/c mouse strains differ in the expression of molecules encoded by a polymorphic genetic region called the natural killer complex (NKC) [51], and it has been shown that the differential expression of these receptors in NK cells and CD1d-restricted NKT cells influences their immunological behaviour in response to malaria infection and accounts for the degree of susceptibility to severe malaria [31], [32]. C57BL/6 NKC alleles are associated with disease susceptibility and the increased severity to cerebral malaria is associated with a differential immune response to infection, characterised by a significantly enhanced IFN-γ production [32], [52]. Interestingly, anti-inflammatory cytokines such as TGF-β and IL-10 have been found to regulate type-1 responses during infection [23] and to play a protective role against P. berghei–mediated cerebral malaria [22], respectively, suggesting that resistance to disease could be associated with the development of suppressive mechanisms aimed at directly inhibiting pathogenic inflammatory responses. Specifically to test that proposition, we investigated the induction of T cell responses in cerebral malaria–resistant mouse strains. Our main results revealed that CD4+ T cells from cerebral disease–resistant BALB/c mice display a strong inhibition of cell proliferation and IL-2 secretion in response to both parasite-specific and polyclonal stimuli and that natural Treg cells appear to play a role in the inhibition of T cell function occurring during infection [53]. Moreover, anti-CD25 depletion experiments revealed that Treg cells also prevent the development of parasite-specific TH1 memory cells involved in the induction of cerebral malaria during a secondary parasitic challenge, demonstrating a regulatory role for this cell population in the control of pathogenic responses leading to fatal disease [53]. Interestingly, although the lack of natural Treg cells during primary exposure to malaria enhanced TH1 memory responses and increased disease severity during re-infection, it also resulted in improved control of parasite burden. Thus, the results support the notion that although TH1 responses could be beneficial to control malaria re-infection, they should be carefully regulated in order to prevent harmful pathogenesis (Figure 1C).
Two independent studies investigated the effect of natural Treg cell depletion with anti-CD25 antibody in the development of cerebral malaria using the disease-susceptible C57BL/6 mouse strain. Because both CD4 and CD8 T cells have been shown to contribute to the induction of severe malaria in this mouse strain, the use of anti-CD25 antibodies in Treg cell depletion protocols requires careful experimental design to avoid targeting activated T cells, which could confound interpretation of the results. Both studies evaluated susceptibility rates of C57BL/6 mice that had been depleted of natural Treg a day prior to infection with P. berghei-ANKA [54], [55]. Under these experimental conditions, anti-CD25 injection resulted in complete protection from cerebral malaria. To minimize depletion of parasite-specific activated T cells that could be potentially involved in cerebral malaria induction, both studies performed Treg cell depletion several weeks prior to parasitic challenge. Whereas Treg cell depletion 30 days before challenge did not alter parasite burden and susceptibility rates of C57BL/6 mice to cerebral malaria [55], anti-CD25 treatment carried out 14 days before parasitic challenge appeared to protect susceptible mice from fatality [54]. Similar results were obtained when natural Treg cells were depleted under similar conditions in cerebral malaria–susceptible CBA mice [56]. Protection from disease was associated with reduced parasite burden and reduced sequestration of pRBCs in brains of infected mice [54]. The increased resistance to infection correlated with enhanced levels of T cell activation and IFN-γ production in spleens of P. berghei-ANKA-infected mice. These latter data suggest that administration of anti-CD25 antibody does not lead to significant depletion of activated antigen-specific T cells in these experiments. However, a recent study shows that Treg cell depletion in DEREG mice, which are transgenic for a bacterial chromosome expressing a diphtheria toxin (DT) receptor–enhanced GFP fusion protein under the control of the foxp3 locus that allows for selective depletion of Foxp3+ Treg cells by DT injection, did not alter the incidence of cerebral malaria after acute P. bergei-ANKA infection [57].
Thus, the contribution of Treg cells to the induction of cerebral malaria in the C57BL/6 background remains controversial. If a role in acute responses is proven, it might be the case that strong early IFN-γ responses produced in the absence of Treg cells in disease-susceptible C57BL/6 mice facilitate control of parasite burden, resulting in reduced sequestration and protection from cerebral malaria (Figure 1A). The magnitude of inflammatory responses obtained after Treg cell depletion in disease-resistant BALB/c animals (genetically predisposed to produce poor IFN-γ responses to malaria [52]) results in a more modest effect, which only exacerbates disease severity but is not sufficient to control parasitemia or increase fatality rates in a primary infection. In cerebral malaria–resistant animals, the impact of Treg cell depletion becomes evident after re-infection, as TH1 memory responses generated in the absence of Treg cells during initial exposure appear to be robust enough to control re-infection despite increased cerebral malaria induction rates. These considerations imply that natural Treg cell function could be either beneficial or detrimental depending on the timing and magnitude of pro-inflammatory responses to malaria and the genetic background of the host.
Human Studies
A few studies have investigated the role of natural Treg cells in human malaria (Table 3). The implementation of such human studies is quite complex. A variety of different factors, including study design, number of individuals recruited for the study cohort, and use of fresh or cryopreserved samples may all have an impact on the results. These variables should be taken into account when assessing the relevance of conclusions arising from human disease association studies.
Tab. 3. Association between natural T<sub>reg</sub> cells and the outcome of human malaria infections. 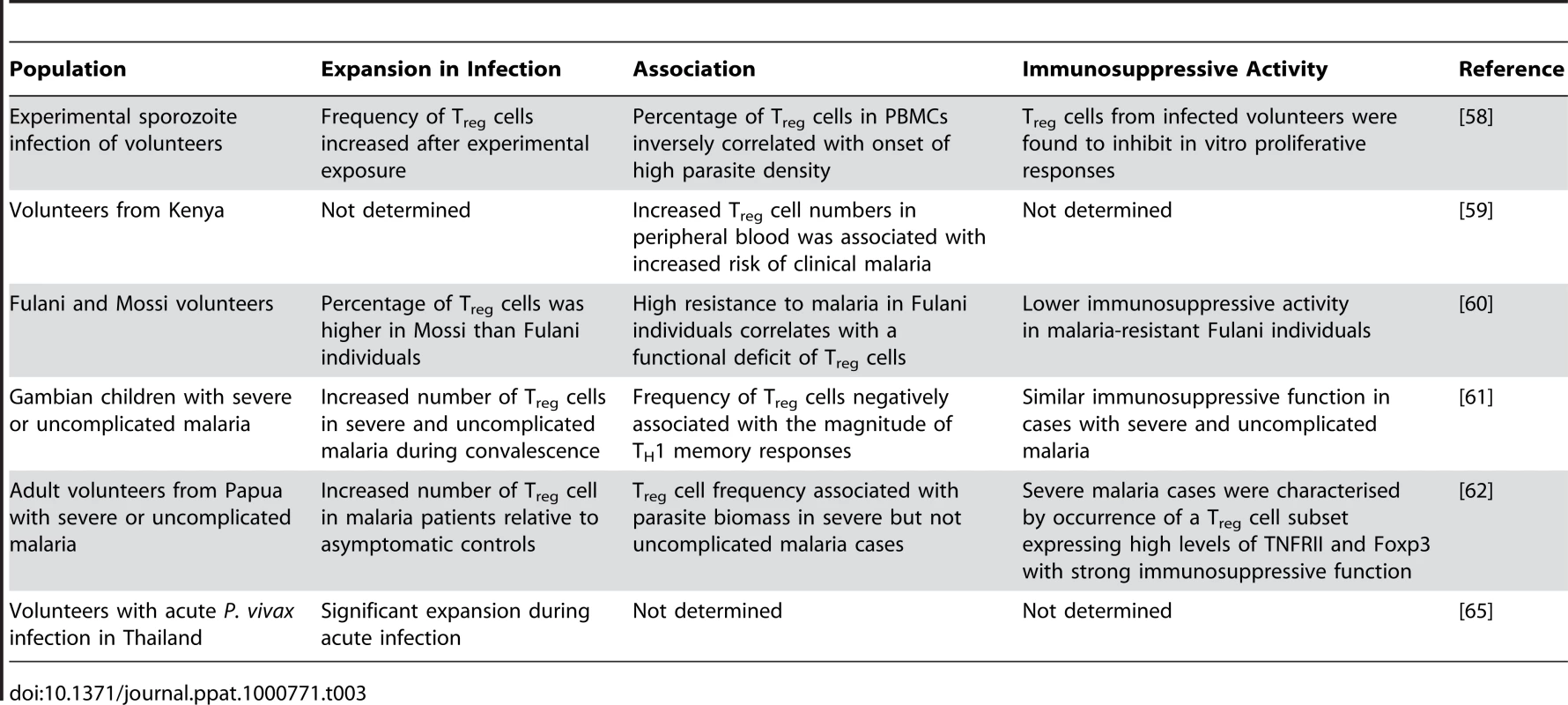
Foxp3 mRNA expression levels as well as CD3+CD4+CD25hiCD69− cell numbers were found to become highly upregulated after experimental sporozoite infection of volunteers via mosquito bites. Interestingly, individuals with a high percentage of CD4+ Treg cells amongst their peripheral blood mononuclear cells (PBMCs) were found to reach high parasite densities earlier than those with low Treg cell frequencies, suggesting that the presence of Treg cells in peripheral blood from infected individuals might facilitate blood-stage parasite replication. Reduced production of the pro-inflammatory cytokines IFN-γ, IL6, and IL-12 as well as significantly enhanced production of TGF-β levels were also found to be associated with high parasite burden [58]. Interestingly, monocytes and not Treg cells were found to be the cellular source of TGF-β produced in response to infection. The authors proposed that monocyte-derived TGF-β might favour Treg cell differentiation by inducing Foxp3 expression in naïve peripheral CD4+ T lymphocytes. Increased numbers of natural Treg cells would in turn downregulate pro-inflammatory responses, which could result in enhanced parasite growth [58].
The frequency of natural Treg cells was also investigated in naturally infected individuals in a malaria-endemic region of coastal Kenya. Multivariate analysis showed a significant association between increased CD4+ CD25high numbers in peripheral blood and increased risk of clinical malaria [59]. The majority of CD4+ CD25high cells were found to express Foxp3, suggesting that natural Treg cells may negatively affect natural acquired immunity to malaria.
Interethnic comparative approaches on the susceptibility to malaria performed in West Africa revealed that despite similar levels of exposure, Fulani individuals appear to be more resistant to P. falciparum malaria, as they display reduced parasitemia levels and lower incidence of clinical episodes than other ethnic groups. When the expression of genes involved in immune responses was analysed in PBMCs of Fulani and sympatric Mossi individuals, increased expression of TH1 (IFN-γ, IL-18, TBX21) as well as TH2-related genes (IL-4, IL-9, GATA3) was detected in the more resistant Fulani individuals [60]. Interestingly, the upregulation of immunity-associated genes correlated with reduced frequencies of CD4+ CD25+ Foxp3+ cells in peripheral blood in Fulani compared to Mossi individuals and reduced expression of Treg cell–related products such as CTLA4 and TGF-β. Moreover, whereas in vitro depletion of CD25+ cells enhanced proliferative responses to P. falciparum antigens in PBMCs from Mossi individuals, Treg cell depletion did not alter parasite-specific proliferation rates of the Fulani, suggesting that a functional deficit of natural Treg cells could be involved in the lower susceptibility to malaria of this ethnic group [60].
The role of natural Treg cells in clinical malaria was investigated in children with either severe or uncomplicated malaria [61]. TH1 responses were found to be more elevated in severe compared to uncomplicated malaria cases. However, no significant differences in the absolute number or the frequency of natural Treg cells were detected between these two groups. Moreover, in vitro depletion studies with anti-CD25 resulted in similarly increased cell proliferation to schizont antigen in both severe and uncomplicated malaria cases, suggesting that Treg cells from both groups of patients have similar activity and implying that natural Treg cell function is not sufficient to downregulate the strong TH1 response to infection occurring in children with severe malaria. Interestingly, high FOXP3 mRNA levels at base line were found to be associated with reduced parasite-specific IFN-γ memory responses amongst PBMCs collected form convalescent malaria patients [61]. This result resembles findings obtained in the P. berghei-ANKA mouse model of severe malaria [47]. Whether Treg cells acquired during a primary infection in human malaria are able to modulate TH1 memory responses involved in parasite clearance without inducing immunopathology requires further investigation.
The relationship between Treg cell function and parasite biomass was evaluated in adults with uncomplicated or severe malaria in an endemic area of West Papua [62]. Treg cell numbers increased in malaria cases compared to non-infected controls, indicating that these cells expand in response to infection. In patients with severe but not uncomplicated malaria, Treg cell frequency was correlated with parasite biomass. Phenotypic characterization revealed that Treg cells from severe malaria cases express high levels of TNFRII and constitute a subset of regulatory cells that express high levels of Foxp3 and appear to exert strong immunosuppressive function [62].
Recent in vitro studies using PBMCs from healthy donors stimulated with P. falciparum–infected RBCs have provided interesting insights into the mechanism by which Treg cells are induced by malaria [63]. Stimulation with parasitised RBCs was found to induce expansion of two Treg cell populations expressing different levels of Foxp3. Only cells expressing intermediate (Foxp3int) but not high (Foxp3hi) Foxp3 levels were found to secrete large amounts of effector cytokines. Moreover, high proportions of Foxp3hi cells in the co-culture systems correlated with reduced production of pro-inflammatory cytokines, which suggests that this subset plays a role in inhibition of parasite-induced effector responses. Both malaria-elicited Treg subsets appeared to be derived from conventional CD4+CD25−Foxp3− T cells. The parasite-driven upregulation of Foxp3 levels required activated monocytes. However, soluble factors rather than direct contact were found to be responsible for this process. These results suggest a T cell receptor–independent mechanism for bystander activation of CD4+CD25+Foxp3+ T cells during malaria.
Phenotypic characterization of Treg cells in PBMCs of healthy Gambian donors ex vivo revealed a very heterogenous population of Foxp3+ cells in this cohort study, with cells displaying different expression levels of CD25 [64]. In vitro stimulation with crude parasite extracts increased CD25 expression levels. The memory phenotype of CD4+Foxp3+ T cells was also investigated using expression of the chemokine receptors CCR7 and CCR4 to identify central and effector memory cells, respectively [64]. Ex vivo analysis indicated that antigen-experienced (CD45RO+) Foxp3+ cells were predominantly effector memory cells. Moreover, these cells were also found to express high levels of CD95 consistent with a highly pro-apoptotic phenotype.
Only one study has investigated whether Treg cells become activated in response to Plasmodium vivax infection. Similar to association studies in P. falciparum malaria, the percentage of Foxp3+ Treg cells was found to increase in acute P. vivax infection. In vitro elicitation assays confirmed this observation, as the number of Foxp3+ cells amongst PBMCs significantly increases in response to P. vivax antigens [65]. The relevance of these findings in the context of P. vivax malaria needs to be further investigated.
Concluding Remarks
The current evidence from rodent infection models as well as human studies suggests that the natural Treg cell pool becomes activated and expands in response to malaria infection [39], [41], [55], [61], [62], [66]. Whether such expansion results from direct proliferation, cytokine-driven differentiation, and/or recruitment of natural Treg cells to secondary lymphoid organs is not completely understood. In vitro studies provided evidence that Treg cells proliferate in response to malarial antigens [62], [65]. The precise mechanism by which such antigens stimulate natural Treg cells is still unclear. It has been shown that Treg cells express toll-like receptors (TLRs) and proliferate in response to lipopolysaccharide [67], raising the possibility that putative malarial TLR agonists such as GPI [68], [69], [70] or hemozoin-bound DNA [71] might stimulate Treg function during infection. Although it is not known if these molecules can specifically stimulate proliferation upon binding to natural Treg cell–expressing TLRs, a recent study demonstrated that TLR9 engagement in DCs is required for natural Treg cell activation by malaria parasites [72].
Although the available literature does not support a unified model for the role of natural Treg cells in malaria, a common feature emerging from data in experimental models and human association studies appears to be the ability of this suppressive T cell population to modulate acute pro-inflammatory and/or TH1 memory responses to infection. In animal models, the increased inflammatory response resulting from natural Treg cell depletion was not always sufficient to control parasitemia levels, particularly in models of high parasite density in which antibody-mediated responses are known to play an important role in protective immunity. In low parasite density models, though, pro-inflammatory responses generated in the absence of natural Treg cells appeared to be more efficient in controlling acute infection, leading to reduced organ-specific parasite sequestration. Consistent with an immunoregulatory role in chronic conditions [73], [74], evidence arising from rodent re-infection models [53] as well as severe malaria association studies in humans [61] supports the notion that natural Treg cells modulate parasite-specific TH1 memory rather than acute responses involved in disease induction, suggesting that this cell population may limit the risk of immune-mediated pathogenesis upon re-infection. On the other hand, human studies available to date reveal associations between Treg cell frequency and increased parasite densities, which in some cases were found to correlate with increased severity of disease. Overall, the literature suggests that whereas in some settings Treg cells might modulate detrimental inflammation involved in disease induction, in other cases Treg cell–mediated inhibition of immune responses results in increased parasite density, which is also known to be a key determinant of disease severity. As often in malaria, the picture seems to be more complex than initially envisaged, and whether natural Treg cell activation results in a favourable outcome for the parasite or the host depends on a variety of factors, including the timing and magnitude of such pro-inflammatory responses to infection, the level of exposure to the parasite, and the genetic background of the host.
The findings concerning the role of Treg cells in relation to malaria infection thus appear consistent with the growing literature on the role of Treg cells in other infectious diseases, supporting the general view that these cells maintain homeostasis by controlling the magnitude of immune responses. Broadly speaking, in the context of other infectious diseases, Treg cells curtail potent and sometimes over-vigorous effector responses. The selective basis of this activity appears to be the need to limit the significant tissue damage that can arise as a side-consequence of antimicrobial immune responses. For example, reactive oxygen species, while a powerful weapon against microbes, can cause significant damage to the host. In certain circumstances, the need to reduce or prevent serious tissue damage can compromise the ability to control infection. Examples of the role of Treg in reducing immunopathology can be found in the original observation of their role in control of colitis [75], reduction of pulmonary inflammation in Pneumocystis [76], control of hepatic pathology in Schistosoma infections [77] and control of immunopathological leasions in viral infections [78], etc. The evidence for Treg cell–dependent control of immune responses, leading sometimes to failure of the effector arm, comes most clearly from studies on chronic infections e.g., Leishmania major [79]. One outcome of the counter-regulatory activity against the effector response appears to be the promotion of pathogen persistence, as exemplified by Litomosoides [80]. Treg cells may thus play a crucial role in maintaining long-term, balanced host/pathogen relationships, and hence in the promotion of commensalism.
Future Directions
Future directions in Treg cell research of relevance to malaria can be envisaged at several levels. Firstly, the activation of Treg cell or their induction in the periphery appears to result from a wide range of signals, including direct stimulation of TLR or other pattern-recognition receptors, the maturation and activation of DCs and other accessory cells by pathogens through similar pathways, the cellular cytokine milieu and the potential contribution of parasite ligands with direct regulatory roles. For mechanistic studies, much can be attempted with human and murine cells in vitro, and in murine models in vivo, to analyse the molecular signals of parasite origin leading to Treg cell activation, and the other contributory cells of the immune system. Secondly, as exemplified here, the contribution of Treg cells depends upon the kinetics and dynamics of the infection, and much more needs to be done in whole animal models of chronic or repeated infection malaria infection, to test experimentally the contribution of Treg to infection and disease processes during re-exposure. Finally, activation of Treg cells urgently needs to be assessed in the context of longitudinal cohort studies in human populations to further test the association of Treg activation parameters with respect to parasitological and pathophysiological outcome variables. Put together, these three avenues may be required to test the proposition that malaria actively engages in Treg cell activation in order to promote the survival of the pathogen.
Zdroje
1. KullbergMC
JankovicD
GorelickPL
CasparP
LetterioJJ
2002 Bacteria-triggered CD4+ T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med 196 505 515
2. MaloyKJ
SalaunL
CahillR
DouganG
SaundersNJ
2003 CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp Med 197 111 119
3. HoriS
CarvalhoTL
DemengeotJ
2002 CD4+CD25+ regulatory T cells suppress CD4+ T cell-mediated pullmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol 32 1282 1291
4. MontagnoliC
BacciA
BozzaS
GazianoR
MosciP
2002 B7/CD28-dependent CD4+CD25+ regulatory T cells are essential components of the memory-protective immunity to Candida albicans. J Immunol 169 6298 6308
5. SakaguchiS
SakaguchiN
AsanoM
ItohM
TodaM
1995 Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol 155 1151 1164
6. LiuW
PutnamAL
Xu-YuZ
SzotGL
LeeMR
2006 CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203 1701 1711
7. SalomonB
LenschowDJ
RheeL
AshourianN
SinghB
2000 B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregualtory T cells that control autoimmune diabetes. Immunity 12 431 440
8. McHughRS
WhittersMJ
PiccirilloCA
YoungDA
ShevachEM
2002 CD4+CD25+ immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity 16 311 323
9. HoriS
NomuraT
SakaguchiS
2003 Control of regulatory T cell development by the transcription factor Foxp3. Science 299 1057 1061
10. AsanoM
TodaM
SakaguchiN
SakaguchiS
1996 Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med 184 387 396
11. MottetC
UhligHH
PowrieF
2003 Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 170 3939 3943
12. ShimizuJ
YamazakiS
SakaguchiS
1999 Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol 163 5211 5218
13. ThorntonAM
ShevachEM
1998 CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med 188 287 296
14. NakamuraK
KitaniA
StroberW
2001 Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med 194 629 644
15. AssemanC
MauzeS
LeachMW
CoffmanRL
PowrieF
1999 An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190 995 1004
16. BluestoneJA
AbbasAK
2003 Natural versus adaptive regulatory T cells. Nat Rev Immunol 3 253 257
17. WhiteNJ
HoM
1992 The pathophysiology of malaria. Adv Parasitol 31 83 173
18. BairdJK
1995 Host age as a determinant of naturally acquired immunity to Plasmodium falciparum. Parasitol Today 11 105 111
19. MarshK
KinyanjuiS
2006 Immune effector mechanisms in malaria. Parasite Immunol 28 51 60
20. BejonP
MwacharoJ
KaiO
TodrykS
KeatingS
2007 The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol 179 4193 4201
21. PlebanskiM
FlanaganKL
LeeEA
ReeceWH
HartK
1999 Interleukin 10-mediated immunosuppression by a variant CD4 T cell epitope of Plasmodium falciparum. Immunity 10 651 660
22. KossodoS
MonsoC
JuillardP
VeluT
GoldmanM
1997 Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology 91 536 540
23. OmerFM
RileyEM
1998 Transforming growth factor beta production is inversely correlated with severity of murine malaria infection. J Exp Med 188 39 48
24. GrauGE
FajardoLF
PiguetPF
AlletB
LambertPH
1987 Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 237 1210 1212
25. EngwerdaCR
MynottTL
SawhneyS
De SouzaJB
BickleQD
2002 Locally up-regulated lymphotoxin alpha, not systemic tumor necrosis factor alpha, is the principle mediator of murine cerebral malaria. J Exp Med 195 1371 1377
26. GrauGE
HeremansH
PiguetPF
PointaireP
LambertPH
1989 Monoclonal antibody against interferon g can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci USA 86 5572 5574
27. GrauGE
PiguetPF
EngersHD
LouisJA
VassalliP
1986 L3T4+ T lymphocytes play a major role in the pathogenesis of murine cerebral malaria. J Immunol 137 2348 2354
28. YanezDM
ManningDD
CooleyAJ
WeidanzWP
van der HeydeHC
1996 Participation of lymphocyte subpopulations in the pathogenesis of experimental murine cerebral malaria. J Immunol 157 1620 1624
29. NitcheuJ
BonduelleO
CombadiereC
TefitM
SeilheanD
2003 Perforin-dependent brain-infiltrating cytotoxic CD8+ T lymphocytes mediate experimental cerebral malaria pathogenesis. J Immunol 170 2221 2228
30. BelnoueE
KayibandaM
VigarioAM
DescheminJC
van RooijenN
2002 On the pathogenic role of brain-sequestered alphabeta CD8+ T cells in experimental cerebral malaria. J Immunol 169 6369 6375
31. HansenDS
SchofieldL
2004 Regulation of immunity and pathogenesis in infectious diseases by CD1d-restricted NKT cells. Int J Parasitol 34 15 25
32. HansenDS
SiomosMA
BuckinghamL
ScalzoAA
SchofieldL
2003 Regulation of murine cerebral malaria pathogenesis by CD1d-restricted NKT cells and the natural killer complex. Immunity 18 391 402
33. HansenDS
BernardNJ
NieCQ
SchofieldL
2007 NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. J Immunol 178 5779 5788
34. BauerPR
Van Der HeydeHC
SunG
SpecianRD
GrangerDN
2002 Regulation of endothelial cell adhesion molecule expression in an experimental model of cerebral malaria. Microcirculation 9 463 470
35. ChakravortySJ
CraigA
2005 The role of ICAM-1 in Plasmodium falciparum cytoadherence. Eur J Cell Biol 84 15 27
36. TaylorTE
FuWJ
CarrRA
WhittenRO
MuellerJS
2004 Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10 143 145
37. HearnJ
RaymentN
LandonDN
KatzDR
de SouzaJB
2000 Immunopathology of cerebral malaria: morphological evidence of parasite sequestration in murine brain microvasculature. Infect Immun 68 5364 5376
38. HisaedaH
MaekawaY
IwakawaD
OkadaH
HimenoK
2004 Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med 10 29 30
39. CouperKN
BlountDG
WilsonMS
HafallaJC
BelkaidY
2008 IL-10 from CD4CD25Foxp3CD127 adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathog 4 e1000004 doi:10.1371/journal.ppat.1000004
40. StrauchUG
ObermeierF
GrunwaldN
GursterS
DungerN
2005 Influence of intestinal bacteria on induction of regulatory T cells: lessons from a transfer model of colitis. Gut 54 1546 1552
41. CambosM
BelangerB
JacquesA
RouletA
ScorzaT
2008 Natural regulatory (CD4+CD25+FOXP+) T cells control the production of pro-inflammatory cytokines during Plasmodium chabaudi adami infection and do not contribute to immune evasion. Int J Parasitol 38 229 238
42. LongTT
NakazawaS
OnizukaS
HuamanMC
KanbaraH
2003 Influence of CD4+CD25+ T cells on Plasmodium berghei NK65 infection in BALB/c mice. Int J Parasitol 33 175 183
43. Brian de SouzaJ
RileyEM
2002 Cerebral malaria: the contribution of studies in animal models to our understanding of immunopathogenesis. Microbes Infect 4 291 300
44. MillerLH
BaruchDI
MarshK
DoumboOK
2002 The pathogenic basis of malaria. Nature 415 673 679
45. SchofieldL
GrauGE
2005 Immunological processes in malaria pathogenesis. Nat Rev Immunol 5 722 735
46. ClarkIA
SchofieldL
2000 Pathogenesis of malaria. Parasitol Today 16 451 454
47. NieCQ
BernardNJ
NormanMU
AmanteFH
LundieRJ
2009 IP-10-mediated T cell homing promotes cerebral inflammation over splenic immunity to malaria infection. PLoS Pathog 5 e1000369 doi:10.1371/journal.ppat.1000369
48. MiuJ
MitchellAJ
MullerM
CarterSL
MandersPM
2008 Chemokine gene expression during fatal murine cerebral malaria and protection due to CXCR3 deficiency. J Immunol 180 1217 1230
49. ScottP
PearceE
CheeverAW
CoffmanRL
SherA
1989 Role of cytokines and CD4+ T-cell subsets in the regulation of parasite immunity and disease. Immunol Rev 112 161 182
50. KossodoS
GrauGE
1993 Profiles of cytokine production in relation with susceptibility to cerebral malaria. J Immunol 151 4811 4820
51. ScalzoAA
BrownMG
ChuDT
HeuselJW
YokoyamaWM
1999 Development of intra-natural killer complex (NKC) recombinant and congenic mouse strains for mapping and functional analysis of NK cell regulatory loci. Immunogenetics 49 238 241
52. HansenDS
EvansKJ
D'OmbrainMC
BernardNJ
SextonAC
2005 The natural killer complex regulates severe malarial pathogenesis and influences acquired immune responses to Plasmodium berghei ANKA. Infect Immun 73 2288 2297
53. NieCQ
BernardNJ
SchofieldL
HansenDS
2007 CD4+ CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infect Immun 75 2275 2282
54. AmanteFH
StanleyAC
RandallLM
ZhouY
HaqueA
2007 A role for natural regulatory T cells in the pathogenesis of experimental cerebral malaria. Am J Pathol 171 548 559
55. VigarioAM
GorgetteO
DujardinHC
CruzT
CazenavePA
2007 Regulatory CD4+ CD25+ Foxp3+ T cells expand during experimental Plasmodium infection but do not prevent cerebral malaria. Int J Parasitol 37 963 973
56. RandallLM
AmanteFH
McSweeneyKA
ZhouY
StanleyAC
2008 Common strategies to prevent and modulate experimental cerebral malaria in mouse strains with different susceptibilities. Infect Immun 76 3312 3320
57. SteegC
AdlerG
SparwasserT
FleischerB
JacobsT
2009 Limited Role of CD4+Foxp3+ Regulatory T Cells in the Control of Experimental Cerebral Malaria. J Immunol
58. WaltherM
TongrenJE
AndrewsL
KorbelD
KingE
2005 Upregulation of TGF-beta, FOXP3, and CD4+CD25+ regulatory T cells correlates with more rapid parasite growth in human malaria infection. Immunity 23 287 296
59. TodrykSM
BejonP
MwangiT
PlebanskiM
UrbanB
2008 Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE 3 e2027 doi:10.1371/journal.pone.0002027
60. TorciaMG
SantarlasciV
CosmiL
ClementeA
MaggiL
2008 Functional deficit of T regulatory cells in Fulani, an ethnic group with low susceptibility to Plasmodium falciparum malaria. Proc Natl Acad Sci U S A 105 646 651
61. WaltherM
JeffriesD
FinneyOC
NjieM
EbonyiA
2009 Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog 5 e1000364 doi:10.1371/journal.ppat.1000364
62. MinigoG
WoodberryT
PieraKA
SalwatiE
TjitraE
2009 Parasite-dependent expansion of TNF receptor II-positive regulatory T cells with enhanced suppressive activity in adults with severe malaria. PLoS Pathog 5 e1000402 doi:10.1371/journal.ppat.1000402
63. ScholzenA
MittagD
RogersonSJ
CookeBM
PlebanskiM
2009 Plasmodium falciparum-mediated induction of human CD25Foxp3 CD4 T cells is independent of direct TCR stimulation and requires IL-2, IL-10 and TGFbeta. PLoS Pathog 5 e1000543 doi:10.1371/journal.ppat.1000543
64. FinneyOC
NwakanmaD
ConwayDJ
WaltherM
RileyEM
2009 Homeostatic regulation of T effector to Treg ratios in an area of seasonal malaria transmission. Eur J Immunol 39 1288 1300
65. JangpatarapongsaK
ChootongP
SattabongkotJ
ChotivanichK
SirichaisinthopJ
2008 Plasmodium vivax parasites alter the balance of myeloid and plasmacytoid dendritic cells and the induction of regulatory T cells. Eur J Immunol 38 2697 2705
66. HisaedaH
HamanoS
Mitoma-ObataC
TetsutaniK
ImaiT
2005 Resistance of regulatory T cells to glucocorticoid-induced TNFR family-related protein (GITR) during Plasmodium yoelii infection. Eur J Immunol 35 3516 3524
67. CaramalhoI
Lopes-CarvalhoT
OstlerD
ZelenayS
HauryM
2003 Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med 197 403 411
68. NeblT
De VeerMJ
SchofieldL
2005 Stimulation of innate immune responses by malarial glycosylphosphatidylinositol via pattern recognition receptors. Parasitology 130 Suppl S45 62
69. SchofieldL
TachadoSD
1996 Regulation of host cell function by glycosylphosphatidylinositols of the parasitic protozoa. Immunol Cell Biol 74 555 563
70. TachadoSD
Mazhari-TabriziR
SchofieldL
1999 Specificity in signal transduction among glycosylphosphatidylinositols of Plasmodium falciparum, Trypanosoma brucei, Trypanosoma cruzi and Leishmania spp. Parasite Immunol 21 609 617
71. ParrocheP
LauwFN
GoutagnyN
LatzE
MonksBG
2007 Malaria hemozoin is immunologically inert but radically enhances innate responses by presenting malaria DNA to Toll-like receptor 9. Proc Natl Acad Sci U S A 104 1919 1924
72. HisaedaH
TetsutaniK
ImaiT
MoriyaC
TuL
2008 Malaria parasites require TLR9 signaling for immune evasion by activating regulatory T cells. J Immunol 180 2496 2503
73. MittruckerHW
KaufmannSH
2004 Mini-review: regulatory T cells and infection: suppression revisited. Eur J Immunol 34 306 312
74. BelkaidY
RouseBT
2005 Natural regulatory T cells in infectious disease. Nat Immunol 6 353 360
75. PowrieF
LeachMW
MauzeS
MenonS
CaddleLB
1994 Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1 553 562
76. McKinleyL
LogarAJ
McAllisterF
ZhengM
SteeleC
2006 Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of pneumocystis pneumonia. J Immunol 177 6215 6226
77. HesseM
PiccirilloCA
BelkaidY
PruferJ
Mentink-KaneM
2004 The pathogenesis of schistosomiasis is controlled by cooperating IL-10-producing innate effector and regulatory T cells. J Immunol 172 3157 3166
78. SuvasS
AzkurAK
KimBS
KumaraguruU
RouseBT
2004 CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol 172 4123 4132
79. BelkaidY
PiccirilloCA
MendezS
ShevachEM
SacksDL
2002 CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420 502 507
80. TaylorMD
LeGoffL
HarrisA
MaloneE
AllenJE
2005 Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J Immunol 174 4924 4933
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Jak souvisí postcovidový syndrom s poškozením mozku?
- Infekční komplikace virových respiračních infekcí – sekundární bakteriální a aspergilové pneumonie
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



