-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaStructural and Functional Analysis of Viral siRNAs
A large amount of short interfering RNA (vsiRNA) is generated from plant viruses during infection, but the function, structure and biogenesis of these is not understood. We profiled vsiRNAs using two different high-throughput sequencing platforms and also developed a hybridisation based array approach. The profiles obtained through the Solexa platform and by hybridisation were very similar to each other but different from the 454 profile. Both deep sequencing techniques revealed a strong bias in vsiRNAs for the positive strand of the virus and identified regions on the viral genome that produced vsiRNA in much higher abundance than other regions. The hybridisation approach also showed that the position of highly abundant vsiRNAs was the same in different plant species and in the absence of RDR6. We used the Terminator 5′-Phosphate-Dependent Exonuclease to study the 5′ end of vsiRNAs and showed that a perfect control duplex was not digested by the enzyme without denaturation and that the efficiency of the Terminator was strongly affected by the concentration of the substrate. We found that most vsiRNAs have 5′ monophosphates, which was also confirmed by profiling short RNA libraries following either direct ligation of adapters to the 5′ end of short RNAs or after replacing any potential 5′ ends with monophosphates. The Terminator experiments also showed that vsiRNAs were not perfect duplexes. Using a sensor construct we also found that regions from the viral genome that were complementary to non-abundant vsiRNAs were targeted in planta just as efficiently as regions recognised by abundant vsiRNAs. Different high-throughput sequencing techniques have different reproducible sequence bias and generate different profiles of short RNAs. The Terminator exonuclease does not process double stranded RNA, and because short RNAs can quickly re-anneal at high concentration, this assay can be misleading if the substrate is not denatured and not analysed in a dilution series. The sequence profiles and Terminator digests suggest that CymRSV siRNAs are produced from the structured positive strand rather than from perfect double stranded RNA or by RNA dependent RNA polymerase.
Published in the journal: . PLoS Pathog 6(4): e32767. doi:10.1371/journal.ppat.1000838
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000838Summary
A large amount of short interfering RNA (vsiRNA) is generated from plant viruses during infection, but the function, structure and biogenesis of these is not understood. We profiled vsiRNAs using two different high-throughput sequencing platforms and also developed a hybridisation based array approach. The profiles obtained through the Solexa platform and by hybridisation were very similar to each other but different from the 454 profile. Both deep sequencing techniques revealed a strong bias in vsiRNAs for the positive strand of the virus and identified regions on the viral genome that produced vsiRNA in much higher abundance than other regions. The hybridisation approach also showed that the position of highly abundant vsiRNAs was the same in different plant species and in the absence of RDR6. We used the Terminator 5′-Phosphate-Dependent Exonuclease to study the 5′ end of vsiRNAs and showed that a perfect control duplex was not digested by the enzyme without denaturation and that the efficiency of the Terminator was strongly affected by the concentration of the substrate. We found that most vsiRNAs have 5′ monophosphates, which was also confirmed by profiling short RNA libraries following either direct ligation of adapters to the 5′ end of short RNAs or after replacing any potential 5′ ends with monophosphates. The Terminator experiments also showed that vsiRNAs were not perfect duplexes. Using a sensor construct we also found that regions from the viral genome that were complementary to non-abundant vsiRNAs were targeted in planta just as efficiently as regions recognised by abundant vsiRNAs. Different high-throughput sequencing techniques have different reproducible sequence bias and generate different profiles of short RNAs. The Terminator exonuclease does not process double stranded RNA, and because short RNAs can quickly re-anneal at high concentration, this assay can be misleading if the substrate is not denatured and not analysed in a dilution series. The sequence profiles and Terminator digests suggest that CymRSV siRNAs are produced from the structured positive strand rather than from perfect double stranded RNA or by RNA dependent RNA polymerase.
Introduction
The RNA silencing based antiviral plant response is one of the best studied antiviral strategies in plants. The key element of RNA silencing based antiviral strategies is the virus derived small interfering RNA (vsiRNA), which guides the RNA induced silencing complex (RISC) to target viral genomes in plants and invertebrates [1]. siRNAs are processed from double-stranded RNAs (dsRNA) or structured single-stranded RNAs (ssRNAs) by RNase III-like enzymes such as DICER [2],[3] (in plants there are several Dicer-like (DCL) genes). siRNAs guide the sequence-specific inactivation of target mRNAs by RISC [4].
Plant RNA viruses are strong inducers as well as targets of RNA silencing and high levels of vsiRNAs accumulate during the viral infection. However, despite of the extensive studies of siRNA biogenesis the origin of plant viral siRNA is still not understood. vsiRNAs are thought to be processed from ds viral RNA replication intermediates, local self-complementary ds regions of the viral genome or through the action of RNA-dependent RNA polymerases (RDRs) on viral RNA templates [1]. In plants two distinct classes of vsiRNAs have been identified: the primary siRNAs, which result from DCL mediated cleavage of an initial trigger RNA, and secondary siRNAs, whose biogenesis requires an RDR enzyme [5],[6].
DCL4 and DCL2 are the most important plant DICERs involved in virus induced RNA silencing and they can process ds or hairpin viral RNAs into vsiRNAs of 21 and 22 nt, respectively. Although DCL4 is the major player in vsiRNA production, in the absence of DCL4, DCL2 is also sufficient to produce 22 nt vsiRNA, which are biologically active in antiviral silencing response [7],[8]. siRNAs are associated with distinct Argonaute (AGO)-containing effector complexes to guide them to their RNA target molecules [1],[9],[10]. In plants, loading of siRNAs into a particular AGO complex is preferentially, but not exclusively, dictated by their 5′ terminal nucleotides [11]. AGO1 is the major slicer in plants but other AGO paralogs are likely to be involved, potentially also mediating translational repression [1],[11].
The accumulation of vsiRNAs may also depend on the presence of virus expressed silencing suppressor proteins. Several silencing suppressor proteins sequester the primary vsiRNAs thus inhibiting the accumulation of secondary vsiRNAs and the antiviral response [1],[12].
In Arabidopsis, RDR6 and RDR2 are required for virus (but not for all viruses) or sense transgene triggered RNA silencing, the spread of long-range cell-to-cell silencing, and the reception of the long-distance RNA silencing signal [1],[13],[14],[15]. RDR-dependent biogenesis of vsiRNAs was first demonstrated by Diaz-Pendon et al. [16]. Recently it was also shown that the production of Tobacco rattle virus (TRV) derived vsiRNAs and antiviral silencing are strongly dependent on the combined activity of the host-encoded RNA-dependent RNA polymerases such as RDR1, RDR2, and RDR6 suggesting that viral single-stranded RNAs, might be converted by RDR enzymes to dsRNAs, which could serve as a substrate for vsiRNA production [17]. However, this model is not supported by previous observations that the majority of vsiRNAs are derived from the plus (mRNA sense) viral strand [18],[19],[20]. In addition, it has been also shown that RDR6 is not required for virus-induced gene silencing when the endogenous phytoene desaturase (PDS) gene was silenced using the crucifer strain of tobacco mosaic virus (crTMV) and TRV based vectors [13]. These conflicting observations indicate that our knowledge of vsiRNA biogenesis is still far from complete.
Cymbidium ringspot virus (CymRSV) is a member of the Tombusvirus genus containing a positive single-stranded RNA genome with five open reading frames (ORFs) [21]. It is widely assumed that positive-strand RNA viruses replicate their genomes via dsRNA intermediates that may activate the siRNA generating machinery [22]. However, the accessibility of long viral dsRNA intermediates for DICER cleavage have not been proven experimentally. In addition, our previous studies suggested that highly structured viral RNAs might also be processed into vsiRNAs in virus-infected plants [18],[19],[23]. We have also shown that sensor RNAs with negative viral polarity are better targets of RISC mediated cleavage in CymRSV infected plants than those with positive polarity, which further suggests an excess of biologically active vsiRNAs with positive polarity [24].
In this work we analysed the composition and the molecular nature of vsiRNAs in virus infected plants in order to gain a better insight into the biogenesis of vsiRNAs using the high throughput 454 and Solexa sequencing strategies.
Results
Profile of viral short RNAs in CymRSV infected plants
To establish the profile of viral siRNAs, cDNA libraries of short RNAs were generated using total RNA extracted from the first systemically infected leaves of Nicotiana benthamiana infected with in vitro transcripts of either wild type CymRSV or with a mutant form of the virus that did not express the p19 silencing suppressor protein (Cym19stop). The cDNA libraries were sequenced on the 454 platform yielding around 100 000 sequences for each library (Table 1). The short RNA sequences were mapped to the viral genome and we found 65% of the reads on the positive strand of wild type CymRSV without mismatches. The mutant virus also produced vsiRNAs at a similar ratio from the positive strand (63.5%) indicating that the silencing suppressor did not affect strand preference and that the higher representation of plus strand is consistent. Next we assessed whether deep sequencing also identifies vsiRNA hot spots as small scale vsiRNA sequencing did [19]. The abundance profiles of vsiRNAs on the positive and negative strands of viral genomic RNA were very similar for wild type and mutant viruses (Figure S1A and B): vsiRNAs were produced from the entire genome with several hot spots on the positive strand and a few low abundance hot spots on the negative strand. The position of the hot spots were identical between wild type and mutant viruses indicating that p19 does not influence the generation of high abundance vsiRNAs and that the 454 platform yielded reproducible vsiRNA profiles.
Tab. 1. Summary of 454 sequencing. 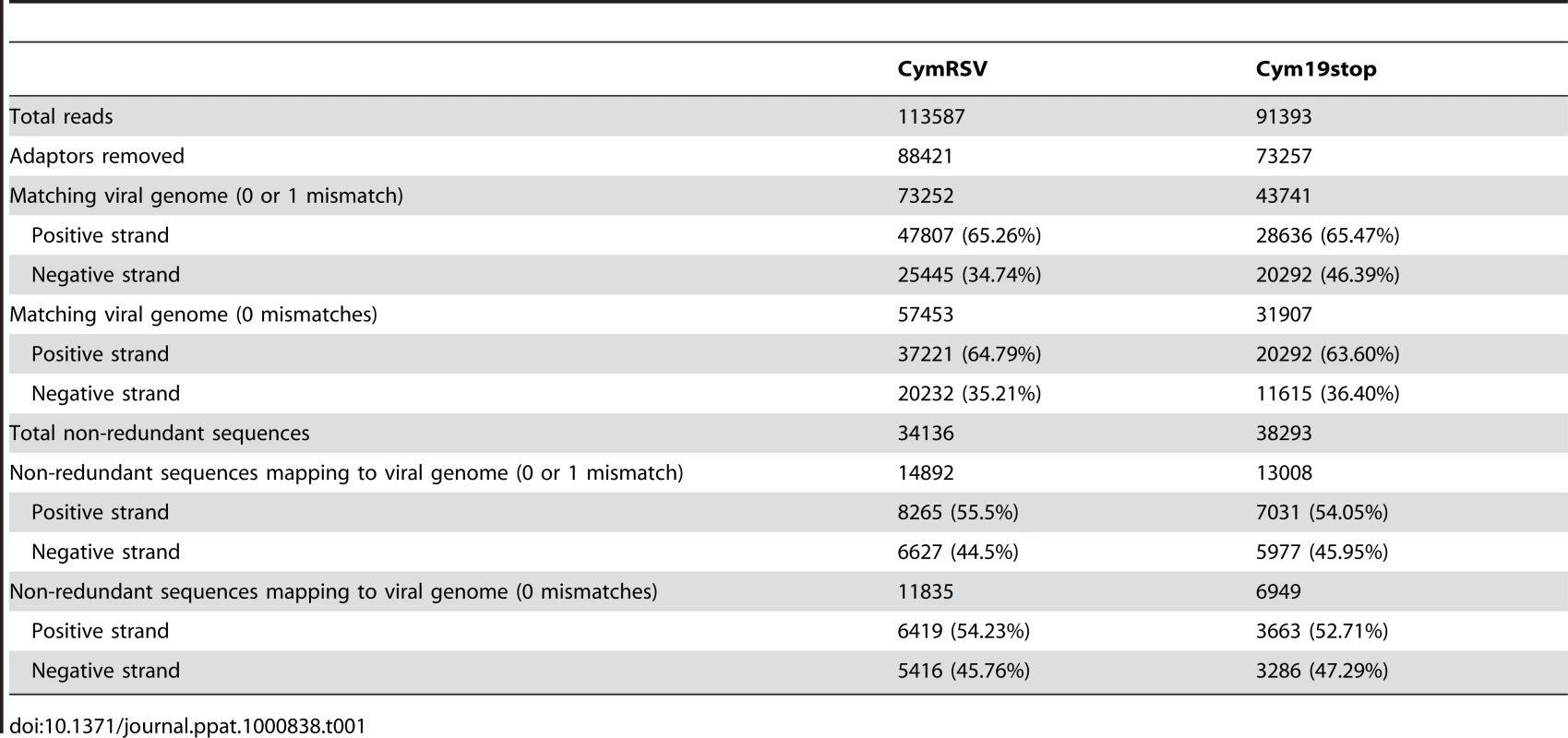
Next we wanted to experimentally validate the positions of the hot spots obtained by 454 sequencing. A 520 nt region (nucleotides 2650–3169 on the positive strand) containing one highly abundant and a few less abundant hot spots on the positive strand was selected for validation. Five hundred 21mer oligonucleotides (complementary to the plus strand of CymRSV) in a one nucleotide sliding window were designed to cover the entire selected region and spotted on a membrane. The short RNA fractions (19–24 nt) were isolated from first systemic leaves of plants infected with in vitro transcripts of wild type and mutant virus, labelled at the 5′ end and used as probes to hybridise to the membrane. The two dot blots gave very similar if not identical patterns (Figure 1A and B) providing experimental evidence that hot spots do exist and p19 does not influence the position of hot spots. The membranes were also hybridised to labelled short RNAs isolated from wild type CymRSV in vitro transcript infected Nicotiana clevelandii and a N. benthamiana where the RDR6 gene [13],[14] was silenced by an inverted repeat construct [15] (Figure 1C and D). These probes gave identical patterns to the previous two probes suggesting several conclusions. First, it indicated that viral siRNAs were generated from hot spots in the absence of RDR6 therefore this RNA dependent RNA polymerase was not absolutely essential for the increased production of CymRSV vsiRNAs from certain positions of the viral genome. Second, it showed that the positions of hot spots only depend on the viral genome and are not determined by the host since the hot spots mapped to the same regions in two different hosts. The fact that four experiments gave identical patterns of hot spots proved that the experimental approach is reproducible. Intensity of the dots was quantified and plotted along the selected region to obtain vsiRNA profiles. Comparison of vsiRNA profiles obtained by 454 and hybridisation, however, showed no correlation (Figure 1E and F), although both approaches were reproducible. We carried out another dot blot experiment covering a different region on the viral genome (4300–4505) and obtained similar result (Figure 1G, H and I). One of the differences between the two methods is that the short RNAs were de-phosphorylated before labelling the 5′ end while the 5′ 454 adapter was ligated directly without de-phosphorylation. If many short RNAs contain a 5′ end that cannot ligate directly to an adapter but can be labelled after de-phosphorylation that could explain the difference between the two sets of results. Alternatively, the two approaches have different preferences in detecting vsiRNA sequences. To distinguish between these possibilities first we analysed the 5′ end structure of vsiRNAs using the Terminator™ 5′-Phosphate-Dependent Exonuclease and then we profiled vsiRNAs using a different high-throughput sequencing platform after ligating the 5′ adapter either directly or following de - and re-phosphorylation to short RNAs extracted from plants inoculated with in vitro transcripts of either wild type or Cym19stop viruses.
Fig. 1. Dot blot and 454 profile of vsiRNAs. 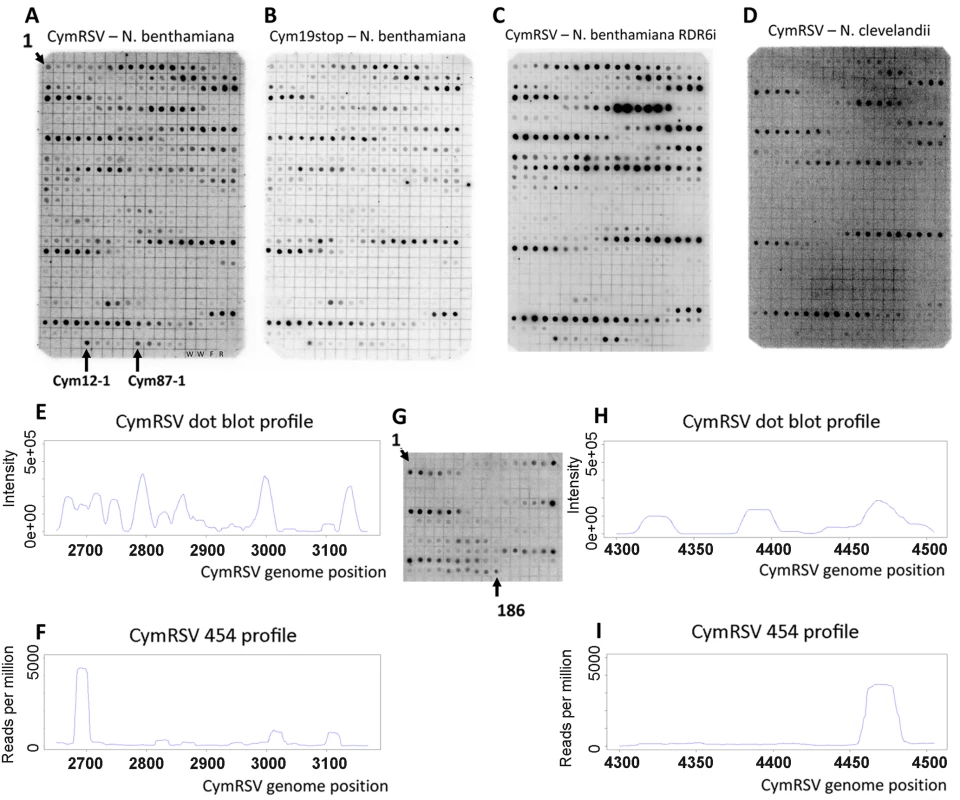
Short RNA fraction was purified from in vitro transcribed virus infected plants, dephosphorylated, 5′ labelled and hybridised to membranes containing 21mer oligonucleotides in a one nucleotide sliding window complementary to the plus strand of CymRSV in positions 2650–3169 (A–D) or 4300–4505 (G). Tissues of wild type N. benthamiana infected with either wild type CymRSV (A and G) or with p19 deficient mutant CymRSV (B) and either transgenic N. benthamiana with reduced RDR6 expression (C) or wild type N. clevelandii (D) infected with wild type CymRSV were used for RNA extraction. Signal intensity of dots on panel A was quantified and plotted along the viral genome (E) and compared with the read frequency for the same region obtained through 454 sequencing (F). Signal intensity of dots on panel G was quantified and plotted along the viral genome (H) and compared with the read frequency for the same region obtained through 454 sequencing (I). The layout of the membranes in panel A–D is the same since the same membrane was used after stripping. The position of the most 5′ oligonucleotides is indicated as 1 at the top left corner. The membranes contain two kinds of negative controls: two dots contain only water (W) and two non-specific oligonucleotides are M13 forward (F) and reverse (R). We monitored the specificity of hybridisation by dotting shorter oligonucleotides: an abundant probe (Cym12) was shortened from the 5′ end by 1, 2, 3, 4 and 5 nucleotides (Cym12-1 indicates the 20mer probe followed by the shorter probes). The same approach is used for another abundant probe Cym87. Analysis of the 5′ end of viral siRNAs
To decide whether a substantial percentage of vsiRNAs cannot be ligated directly to an adapter through the 5′ end we used the terminator exonuclease to study the 5′ end of vsiRNAs. This enzyme degrades RNA molecules in a 5′ to 3′ direction but it can only process molecules with a monophosphate at the 5′ end [25], however it is not known whether short dsRNA molecules can be processed by this enzyme. It was important to address this question since vsiRNAs are double stranded [19] therefore we digested a perfect duplex (with two nucleotide overhangs at the 3′ ends), which consisted of two 19mer in vitro synthesised and phosphorylated RNA oligonucleotides in decreasing concentration without or after denaturation and compared these with undigested samples (Figure 2A). The enzyme was not able to digest the non-denatured perfect duplex at all (Figure 2A left panel) indicating that these molecules are not substrates of this enzyme even if they have 5′ monophosphate end. Most of the RNA was digested by the exonuclease when only one of the strands from the duplex was digested, which demonstrates that the enzyme was functional and that the RNA oligonucleotides were efficiently phosphorylated. The enzyme was able to partially digest the duplex RNA if it was denatured before incubating with the enzyme (Figure 2A right panel). This experiment was repeated using two other perfect duplexes and the same result was obtained (Figure S3B).
Fig. 2. Terminator digest of synthetic siRNAs and vsiRNAs. 
A 19mer in vitro synthesised and phosphorylated siRNA was digested with Terminator™ 5′-Phosphate-Dependent Exonuclease in decreasing concentration without (left panel) or after denaturation (right panel)(A). The sense strand of the phosphorylated 19mer RNA oligonucleotide was digested in the highest concentration as a control for phosphorylation. The efficiency of the digestion was monitored by northern blot assay using a probe complementary to the sense strand of the synthetic siRNA. Total RNA from CymRSV infected N. benthamiana plants was mixed with the 19mer in vitro synthesised and phosphorylated siRNA and digested with Terminator™ 5′-Phosphate-Dependent Exonuclease in decreasing concentration without (left panel) or after denaturation (right panel) (B). The sense strand of the phosphorylated 19mer RNA was also mixed with total RNA from CymRSV infected N. benthamiana plants and digested in the highest concentration. The efficiency of the digestion was monitored by northern blot assay using probes complementary to the minus strand of the virus (Cym mix, a pool of five probes or Cym 3025), miR159, U6 snRNA or the sense strand of synthetic siRNA, respectively. RNA extracted from CymRSV in vitro transcript infected plants was treated with terminator exonuclease without or after denaturation and the level of different short RNAs was compared with an untreated sample by northern blotting. We used a miRNA probe as a positive control for the treatment because it is known that the 5′ end of miRNAs contain a monophosphate and so they are expected to be processed by terminator exonuclease. We also added the 19mer perfect duplex to the samples as an additional control. The miRNAs were indeed efficiently degraded by the terminator (Figure 2B miR159 probe) providing an internal control for the enzyme reaction. The perfect duplex gave the same result as previously: it was not digested at all without denaturation and was partially digested after denaturation (Figure 2B 19mer probe). Two different probes were used to analyse the viral siRNAs (Figure 2B Cym probes) and they gave identical results: vsiRNAs were partially digested with or without denaturation and the efficiency of the digestion was increased dramatically by diluting the sample (two more viral probes are shown on Figure S2). Comparing the digestion efficiency of the vsiRNAs and the perfect duplex after denaturation we can conclude that the vsiRNAs are even more efficiently digested than the 19mer duplex. This indicates that most vsiRNAs must have monophosphate at the 5′ end. The weak signal in the digested lanes of the diluted samples could be either due to renaturation or a small amount of 5′ triphosphate siRNAs. Based on this experiment we cannot rule out that a small amount of vsiRNAs have 5′ triphosphate but we can conclude that the majority of CymRSV vsiRNA possess 5′ monophosphate.
We also compared the digestion efficiency of vsiRNAs and the control perfect duplex without denaturation and found that vsiRNAs were much more efficiently digested without denaturation than the perfect duplex. This raises the possibility that the vsiRNAs are not perfect duplexes but imperfect dsRNA molecules with mismatches between the two strands as it was proposed previously [19]. To explore this we used synthetic imperfect duplexes with 2, 3 or 4 mismatches between the strands (Figure 3A and Figure S3C). Two kinds of duplexes were used with 4 mismatches that differed in the position of the mismatches (Figure 3A). First we digested all four imperfect duplexes at 2 µM concentration either without or after denaturation (Figure 3B). None of the imperfect duplexes were digested without denaturation but all of them were efficiently digested after denaturation. This showed that imperfect duplexes are digested more efficiently after denaturation than perfect duplexes because the perfect duplex was not digested at all at this concentration even after denaturation (compare right panels of Figure 2A and Figure 3B). Next we compared the digestion pattern of imperfect duplexes and vsiRNAs without denaturation by diluting each of the four imperfect duplexes and digesting them without denaturation (Figure 3C). The perfect duplex was used as a control and it was not digested even at the lowest concentration we tried (0.002 µM). The imperfect duplex with two mismatches showed a very small amount (if any) digestion at 0.002 µM but not at higher concentrations. The imperfect duplex with three mismatches was digested much more efficiently, showing big differences between digested and non-digested samples at concentrations 0.002 µM and even 0.004 µM. The imperfect duplex with four mismatches at the end of the duplex showed the most efficient digestion, detected even at 0.1 µM, while the same number of mismatches but at the middle of the duplex resulted efficient digestion at a slightly lower concentration (0.02 µM). Two more imperfect duplexes showed similar pattern (Figure S3C). This set of experiments demonstrated that perfect and imperfect duplexes show different digestion pattern by terminator exonuclease without denaturation and that vsiRNAs showed a similar pattern to the imperfect duplexes: they are both digested at the “right concentration”. The right concentration depends on the number and position of mismatches.
Fig. 3. Terminator digest of imperfect duplexes. 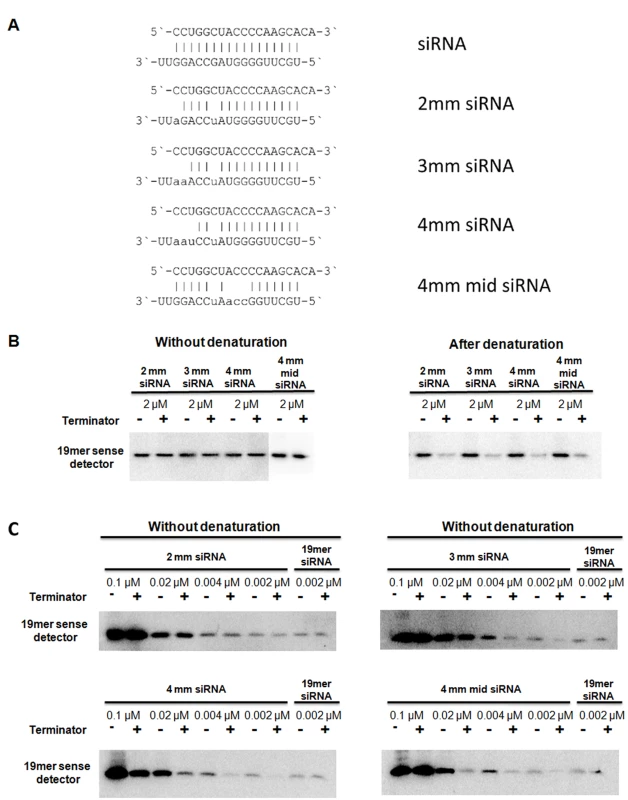
In vitro synthesised and phosphorylated imperfect RNA duplexes (A) were digested with Terminator™ 5′-Phosphate-Dependent Exonuclease at the concentration of 2 µM without (left panel) or after denaturation (right panel) (B). The efficiency of the digestion was monitored by northern blot assay using a probe complementary to the sense strand of the imperfect duplexes, which was identical in all duplexes. 2mm, 3mm and 4mm siRNAs contained 2, 3 or 4 mismatches, respectively. 4mm mid siRNA also contained 4 mismatches but at a more central location. The imperfect duplexes were digested at a decreasing concentration without denaturation (C). As a control, the perfect duplex was digested at the lowest concentration (0.002 µM). Profiling vsiRNAs in CymRSV infected plants by Solexa sequencing
We also studied the 5′ end of vsiRNAs by ligating another adapter molecule (different from the 454 5′ adapter) to the 5′ end of vsiRNAs. cDNA libraries of short RNAs were generated using total RNA extracted from early systemic leaves of N. benthamiana infected with in vitro transcripts of either wild type CymRSV or with a mutant virus, Cym19stop. The library generation includes an adaptor ligation to the 5′ end of the short RNAs and this can be done either after removing the phosphate(s) from the 5′ end or without modifying the 5′ end. When short RNAs are dephosphorylated the 5′ monophosphate, diphosphate or triphosphate is removed and then the short RNAs are re-phosphorylated ensuring that all short RNAs have a 5′ monophosphate.
Solexa sequencing of the four libraries resulted in over one and a half million reads for each library (Table 2). The ratio of vsiRNAs mapping to the positive and negative strands was identical for the wild type virus infected plants when the short RNAs were directly ligated to the adapter or after de - and re-phosphorylation. The high percentage of vsiRNAs derived from the positive strand (93%) confirmed once more that most vsiRNAs are not processed from the double stranded (ds) replicative form. This result also shows that the bias for positive strand sequences is not due to a modification at the 5′ end of the vsiRNAs because the two ligation approaches gave identical ratios. The ratio of positive/negative strand vsiRNAs and their distribution was also very similar in the libraries obtained by the two different ligation methods using RNA extracted from the mutant virus (Cym19stop) infected plants. However, the percentage of vsiRNAs generated from the negative strand of the mutant virus (Cym19stop) was higher than it was for the wild type virus (22–29% compared to 5–6%; Table 2). We hypothesised that this increase was due to higher ratio of negative strand genomic RNA to positive strand genomic RNA in the mutant virus infected plants compared to wild type virus infected plants and this was confirmed by Northern blot analysis (Figure S4).
Tab. 2. Summary of Solexa sequencing. 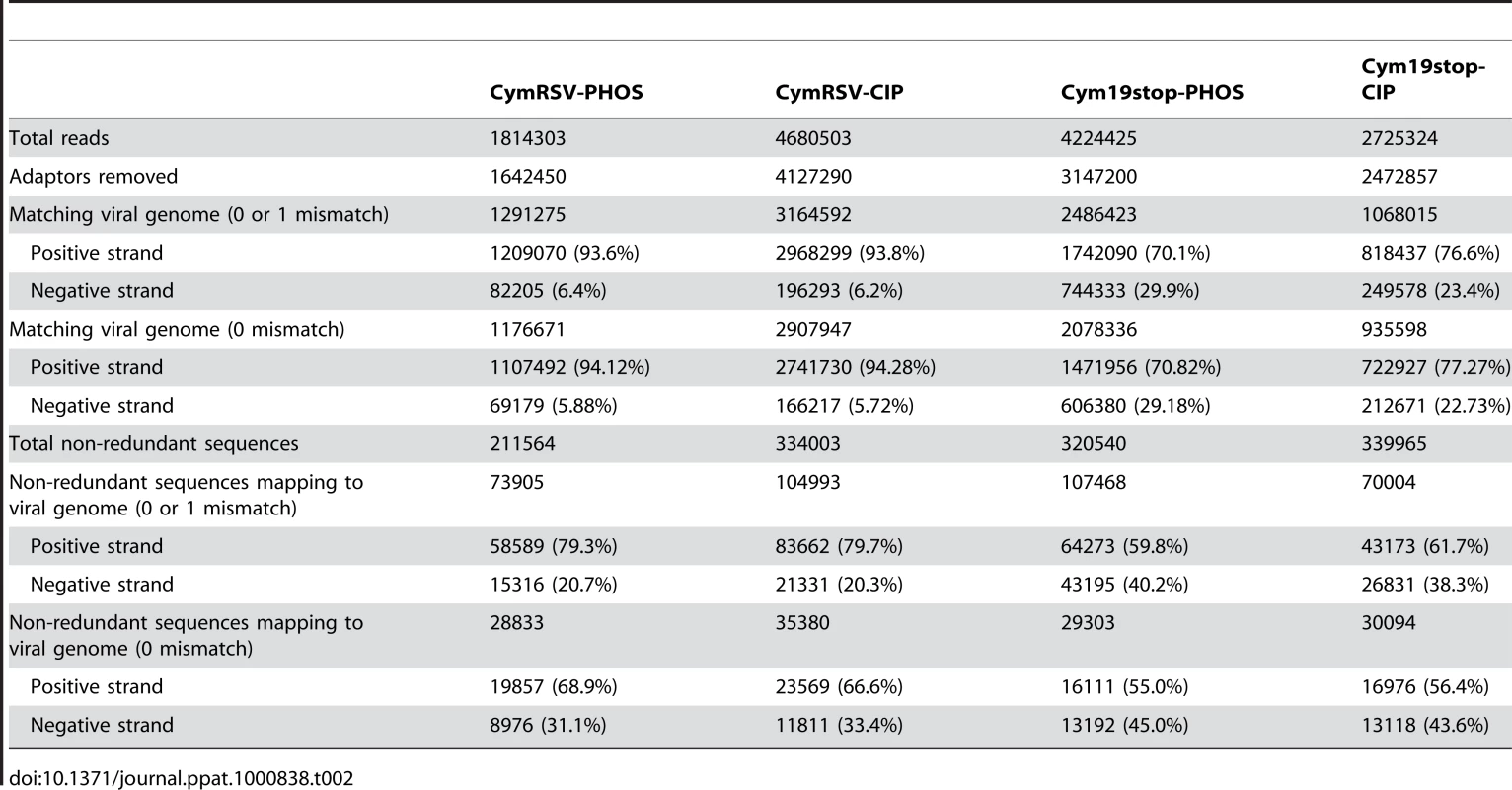
Although most redundant vsiRNAs matched perfectly the viral genome, we observed a higher ratio of not perfectly matching non-redundant reads/perfectly matching non-redundant reads for the positive strand vsiRNAs compared with the negative strand vsiRNAs (p<0.05). One possible explanation for this is the fact that a lot more sequences are derived from the positive strand therefore there is a higher probability to observe sequencing errors in positive strand vsiRNAs. In fact the difference is more pronounced for the wild type virus compared to the mutant virus most likely because the positive/negative strand vsiRNA ratio is higher for the wild type (15×) than for the mutant (2.7×) sample. We also simulated the effect of sequencing errors on positive and negative strand vsiRNAs. The perfectly matching positive and negative strand vsiRNAs were separated from the wild type data set and different rates of sequencing errors (1 error in 1000, 100 or 10 sequences) were simulated. The ratio of perfectly matching sequences to those matching with 0 or 1 mismatch was calculated. We found a similar effect on the positive and negative strand vsiRNAs when redundant reads were used but a much more pronounced effect on the positive strand vsiRNAs when the simulation was applied to non-redundant reads (Figure S5). This result confirms that the observed difference in ratios of not perfectly matching non-redundant reads/perfectly matching non-redundant reads for the positive strand vsiRNAs compared with the negative strand vsiRNAs can be due to sequencing errors. The other contributing factor could be if the not perfectly matching reads are not sequencing errors but vsiRNAs derived from mutated viral genomes. Positive strands can contain point mutations that are not present on the negative strand if no negative strand is produced from the mutant positive strand. However, it is less likely that the negative strand contains a mutation that is not present on the positive strand since the positive strands are produced from the negative strands.
The abundances of reads in all four libraries were plotted on the viral genome (Figure 4A, B, C and D) and hot spots were located. Comparison of the vsiRNA profiles obtained by Solexa and the dot blot approaches revealed a high similarity between the two profiles (Figure 4E and F). Since the Solexa platform gave more similar result to the dot blot than the 454 we analysed the Solexa profile further (direct comparison of the vsiRNA profiles obtained by 454 and solexa is shown on Figure S6).
Fig. 4. Solexa and dot plot profiles of vsiRNAs. 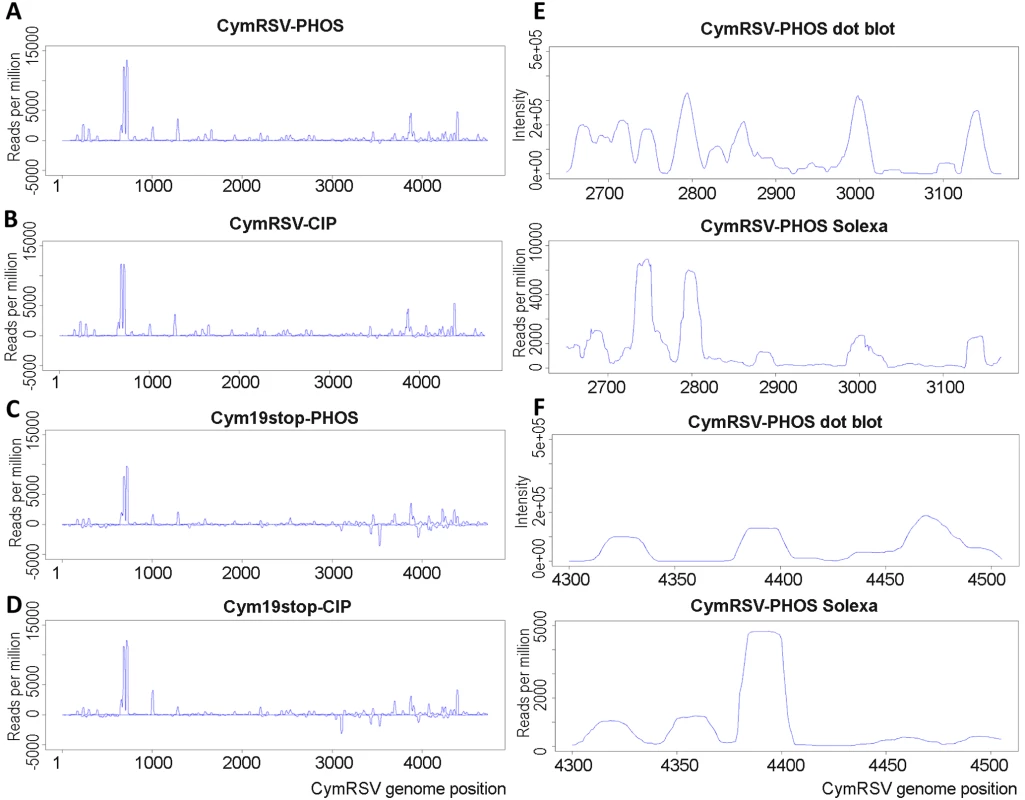
vsiRNA sequences obtained by Solexa sequencing were mapped to the CymRSV genome and normalised abundances were plotted for each sample (A–D). Short RNAs were sequenced from wild type (CymRSV) or silencing protein disabled (Cym19stop) in vitro transcript infected N. benthamiana following two different protocols. The 5′ adapter was either directly ligated to the short RNAs (PHOS) or first depohosphorylated and then re-phosphorylated before adapter ligation (CIP). Profiles obtained by dot blot and Solexa sequencing were compared for regions 2650–3169 (E) and 4300–4505 (F). Please note that the dot blot profiles are the same as those shown in Figure 1E and H. Size distribution and 5′ nucleotide bias was compared between the four libraries and specifically between vsiRNAs derived from hot spots (Figure S7). A slight bias was found for A and U at the 5′ position, especially in wild type virus infected plants but regardless of the ligation method. We also found a slight G bias in the first position of 22mer reads in the libraries from plants infected with the mutant virus. This bias was slightly more pronounced in the vsiRNAs derived from hot spots but there was no other significant difference in the size distribution and 5′ nucleotide bias between the total population of siRNAs and siRNAs generated from hot spot regions. The main difference revealed by this analysis was that the most abundant class of siRNAs in plants inoculated with wild type virus was the 21 nt sequences followed by the 22 nt class while in plants inoculated with the mutant virus the 22 nt siRNAs were more abundant than the class of 21 nt (the same was found by 454 sequencing; data not shown). To further investigate this difference we plotted the 21 and 22 nt sequences separately for all four libraries (Figure S8). The plots show a very similar pattern for 21 and 22 nt sequences in all four libraries and a very similar high positive/negative strand ratio.
Identifying potential vsiRNA duplexes
All of our results (including the presence of hot spots, the + strand bias, the independence of hot spots from RDR6, the similar pattern of libraries generated by direct ligation or after dephosphorylation and the similar profile of wild type and mutant viruses; please note that p19 blocks the generation of primer dependent secondary siRNAs) suggest that vsiRNAs are produced from structured + or − strands, rather than from the double-stranded replicative form or from host RDR generated double-stranded RNA. To explore this further one could predict the secondary structure of the viral genomic RNA or the hot spot regions. However, prediction of RNA secondary structure of long molecules is not reliable. It is also very likely that long RNA molecules have several possible conformations, which is difficult to model by using computational methods. Prediction of shorter regions is more reliable but this approach completely ignores that sequences far away from each other can pair with each other. Therefore secondary structures of hot spot regions are not informative either. We took a different approach and investigated whether the sequence reads derived from the + strand can potentially form duplexes with each other allowing up to four mismatches. We found a large number (8492) of such potential duplexes (Table S1) suggesting that vsiRNA duplexes indeed can be derived from the positive strand only. A similar approach identified 3617 imperfect duplexes that can derive from the − strand (Table S2).
Functional study of vsiRNAs
In order to understand the biological role of hot spots, we carried out a functional analysis of viral siRNAs. Different regions (200 nt length) of the viral genome in both plus and minus orientation were selected from hot spots and from non hotspots regions (Figure 5). These were cloned downstream of a green fluorescent protein (GFP) reporter gene and expressed from a binary vector. The reporter constructs were agroinfiltrated into systemically infected leaves of N. benthamiana plants inoculated with in vitro transcript of Cym19stop virus. In the absence of the silencing suppressor protein the viral siRNAs are incorporated into RISC and target the GFP mRNA if they are complementary to the viral fragment in the sensor construct [24],[26]. Some constructs contained fragments including hot spots and other constructs carried fragments that did not include hot spots. All sensor constructs were targeted by vsiRNAs, although with variable efficiency and without clear correlation with vsiRNA hotspots (Figure 5). In fact, some fragments not homologous to any hot spot (Figure 5, lanes F-) showed similar down-regulation to constructs with hot spot regions (Figure 5, lanes G-). This result indicates that vsiRNAs generated from hot spots are not more efficient than other vsiRNAs in spite of their much higher abundance.
Fig. 5. Functional analysis of vsiRNAs in planta. 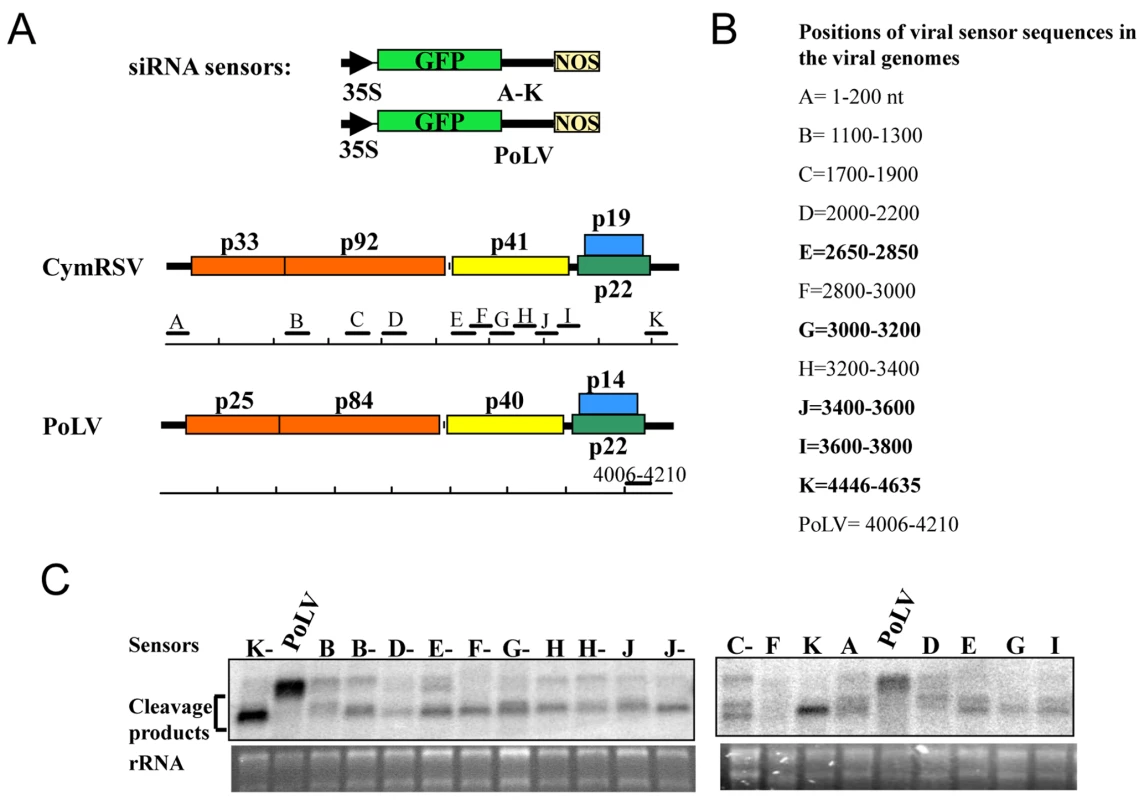
A: Schematic illustration of the GFP sensor sequences and their origins in the viral genomes. The GFP and viral coding sequences are indicated by coloured open boxes, and non coding regions are indicated by thick black lines. Viral target sequences, CymRSV: A to K and Photos Latent Virus (PoLV) [24] as a control, inserted downstream to the GFP ORF are scaled and positioned along the viral genomes. B: Length and position of viral sensor sequences are listed, and regions that contain hotspots are shown in bold. C: Northern blot analysis of viral sensor mRNAs agroinfiltrated into Cym19stop-infected N. benthamiana plants. RNA extracts at 3 days after agroinfiltration were analysed with a 32P-labeled RNA probe raised against the 3′-terminal part of the GFP ORF. PoLV indicates the negative uncleaved control, while the CymRSV viral sequences are indicated by the capital letters. The letter followed by “-” indicates the negative orientation of the sensor sequence, rRNA was used as loading control. Discussion
vsiRNA profiles in CymRSV infected plants
We used three approaches to profile vsiRNAs: 454 and Solexa high-throughput sequencing and hybridisation of 5′ labelled vsiRNAs to two arrays of DNA oligonucleotides covering a 520 and a 206 nt region of the viral genome. All three approaches gave reproducible results because total RNA extracted from wild type or suppressor deficient CymRSV infected plants gave very similar results for each technique. The hybridisation and Solexa sequencing showed good correlation with each other whereas the profile obtained using 454 sequencing was not in agreement. This suggests that ligation of the 454 adaptors have some sequence preference and not necessarily the most abundant molecules are present at high frequency in the cDNA library. This was also reflected by a bias to sequence reads starting with C nucleotide in the 454 library (data not shown) compared with a bias for U and A in the Solexa library (Figure S7). This however, does not mean that 454 is not appropriate to profile short or mRNAs in different samples. The 454 profile was reproducible demonstrating that the preference shown for certain sequences is the same in different samples therefore it is still a valid technique to compare the abundance of transcripts/sRNAs between samples. During revision of this manuscript it was reported that different high-throughput sequencing platforms and different library construction protocols led to different profiles within the same sample [27]. The reason for these differences is not known but is probably influenced by the sequence of the short RNAs to be profiled. Because of this it is possible that different platforms/protocols would give a more accurate profile in different systems. Clearly more studies are necessary that use different platforms in the same system, and in different systems in order to obtain a comprehensive picture about the reliability of the different platforms.
Both sequencing approaches identified more negative strand vsiRNAs in the p19 mutant virus infected plants, which mainly mapped to the last 1700 nt of the viral genome (downstream of nucleotide 3000). This shows a good correlation with the strongly increased level of subgenomic RNA 2 negative strand in the mutant virus infected plants compared with wild type virus infected plants, suggesting that the subgenomic RNAs also contribute to the vsiRNA pool.
The Solexa and array approaches both identified regions from which vsiRNAs were generated at a higher than average frequency. Although this was reported previously based on small scale sequencing of vsiRNAs it could not be ruled out that those observations were influenced by the small data size. This comprehensive study confirmed that there are hot spots on the viral genome that produce specific vsiRNAs at a very high abundance. We also showed that the positions of these hot spots are the same in at least two different hosts indicating that it is determined by the virus itself. The profile of vsiRNAs was also identical in wild type plants and in plants where RDR6 accumulation was suppressed. The symptoms of these two plants following CymRSV infection were also very similar (data not shown) demonstrating that RDR6 is not required for vsiRNA production from the CymRSV genome. Some viruses, such as cucumber mosaic virus [14] and potato virus X [15] cause more severe symptoms in the absence of RDR6 than on wild type plants but many other viruses including tobacco rattle virus, tobacco mosaic virus, turnip crinkle virus cause similar symptoms in the presence and absence of RDR6 [13]. This indicates that production of siRNAs from some viruses requires RDR6 but this enzyme is not involved in vsiRNA production from other viruses, including CymRSV. Since there are several other RDR family members in plants it is possible that one or more other RDRs are involved in CymRSV vsiRNA production through two potential mechanisms. For example it was reported recently that RDR1 is involved in vsiRNA production in TMV-Cg infected plants [28]. The first mechanism is similar to the generation of DCL-dependent (DCL-D) secondary siRNA production from transgenes [1] where primary siRNAs prime the synthesis of complementary RNA of the silenced RNA by RDR. The resulting dsRNA is processed by one of the DCL genes producing DCL-D secondary siRNAs. These DCL-D secondary siRNAs contain 5′ monophosphate like primary siRNAs that are also produced by one of the DCLs but independently of any RDR enzyme. The second possible mechanism was described in C. elegans where an RDR directly produces siRNAs without Dicer activity [25] (DCL-I; DCL independent). However, our data indicate that in the case of CymRSV none of these mechanisms play an important role as discussed next.
DCL-dependent secondary siRNAs are not abundant in CymRSV infected plants
The CymRSV encoded silencing suppressor (p19) was shown to bind primary siRNAs and suppress the accumulation of DCL-D secondary siRNAs by blocking the activity of the primary siRNAs [26]. This indicates that if DCL-D secondary siRNAs were produced at a high level in CymRSV infected plants than we should observe a different vsiRNA profile in the absence of the viral suppressor. In this scenario Cym19stop infected plants would contain more DCL-D secondary siRNAs than the wild type virus infected plant and the vsiRNA profile would be different in the two plants. However, we did not obtain different vsiRNA profiles in wild type and mutant virus infected plants indicating that most CymRSV siRNAs are primary siRNAs and not DCL-D secondary siRNAs. Moreover the vsiRNA profile of CymRSV infected RDR6 silenced plants was the same as the virus infected wild type plants further supporting our conclusion since RDR6 is involved in DCL-D secondary siRNAs biogenesis.
Dicer-independent viral siRNAs are not abundant in CymRSV infected plants
Although DCL-I secondary siRNAs have not been described in plants, plant virus derived siRNAs are good candidates for being direct products of an RDR. Most plant viruses are RNA viruses therefore vsiRNAs could be produced by the viral RDR or alternatively by one of the host RDRs. In addition, vsiRNAs were shown to be generated from hot spots on the viral genome that could be preferred sites of unprimed RNA synthesis. We took two different experimental approaches to analyse the 5′ structure of vsiRNAs at a genomic scale because DCL-I secondary siRNAs possess 5′ triphosphates (since the 5′ ends are not generated by Dicer cleavage but by RNA synthesis) and primary siRNAs have 5′ monophosphate. First we digested RNA extracted from virus infected plants with the terminator exonuclease and compared the level of vsiRNAs in treated and untreated samples by Northern blot. A similar level of vsiRNA in treated and untreated samples would suggest that most vsiRNAs carry 5′ triphosphates since the enzyme can only process molecules with 5′ monophosphate but not molecules with 5′ triphosphates. Our experiments revealed that most of the CymRSV vsiRNAs were processed by the enzyme when the samples were diluted thus we concluded that most vsiRNAs carry a 5′ monophosphate, although it cannot be ruled out that there is a small amount of vsiRNA with 5′ triphosphates.
We also used another approach to study the 5′ structure of vsiRNAs where we profiled the short RNAs after ligating an adaptor to the 5′ end either directly or after de - and re-phosphorylation. The direct ligation only reveals vsiRNAs with 5′ monophosphate while the other approach also identifies vsiRNAs with 5′ triphosphate. If the profiles are different that suggests that there are vsiRNAs with 5′ triphosphate or diphosphate ends because they would be included in the dephosphorylated library but not in the directly ligated library. However, if the sequence profiles of the two libraries are the same that would indicate that there are not vsiRNAs with 5′ triphosphate or diphosphate because removing the 5′ phosphates does not have any effect. The patterns of vsiRNA hot spots in plants infected with the wild type virus were identical when the short RNAs were ligated to the 5′ adapter directly or after de - and re-phosphorylation. This argues against the idea that vsiRNAs in hot spots are mainly siRNAs with triphosphate 5′ end because in that case we should have seen a shift between the patterns obtained in the two experiments. The mutant virus also generated a similar vsiRNA profile regardless of the ligation method.
Terminator exonuclease does not digest double stranded short RNA and its efficiency strongly depends on the concentration of the substrate
To investigate the 5′ end of vsiRNAs we also used another approach that is based on the ability of the Terminator™ 5′-Phosphate-Dependent Exonuclease to distinguish between 5′ monophosphate and 5′ triphosphate (i.e. only digests RNA with 5′ monophosphate). Since a negative result was possible in the terminator exonuclease experiments (i.e.: no digestion of vsiRNAs), it was important to monitor the enzyme activity. A possible internal control is to monitor the level of miRNAs before and after exonuclease treatment since this class of short RNAs has 5′ monophosphate and present in the RNA extracted from virus infected plants. However, we observed during our preliminary experiments that different samples showed different ratios of digested vsiRNAs while the miRNA was always completely digested (data not shown). We hypothesised that a big difference between vsiRNAs and miRNAs is their accumulation level. Small scale short RNA cloning and sequencing experiments found that the majority of the short RNA population in virus infected plants is virus derived [19]. Therefore we decided to use a synthetic siRNA duplex as an additional control. Another difference between miRNAs and vsiRNAs is that most miRNAs are incorporated into RISC quickly while most vsiRNAs are not co-purified with RISC [24]. As a consequence, most miRNAs are present as single stranded RNA and most vsiRNAs accumulate as dsRNA [19]. This raised the question whether the terminator exonuclease have lower affinity to dsRNA than ssRNA. We characterised the terminator exonuclease using a perfect duplex of two 19mer in vitro synthesised and phosphorylated RNA oligonucleotides. We found that the enzyme did not digest the non-denatured duplex at all. The duplex was partially digested following denaturation but only at lower concentration. The fact that the duplex was not digested at all at high concentration suggests that short RNAs can re-anneal very quickly but renaturation happens less frequently at lower concentrations therefore the enzyme activity increased dramatically. These results demonstrate that any RNA sample analysed by the terminator exonuclease has to be denatured and digested at different concentrations. This conclusion has an important implication beyond the plant virology field since the terminator exonuclease can be used to study the 5′ structure of RNA molecules in diverse systems, including endogenous siRNA biogenesis in animals.
Most CymRSV vsiRNAs are not generated from perfect dsRNAs
Interestingly, vsiRNAs were digested with a similar efficiency without or after denaturation, which is different from the efficiency of the digestion of a perfect duplex and very similar to the digestion pattern of imperfect duplexes. This suggests that vsiRNAs are not perfect duplexes but there are mismatches between the two strands. The most likely explanation is that most vsiRNAs are not generated from long perfectly matched double stranded RNAs but from precursor-miRNA like imperfect intramolecular structures. This is also supported by previous observation [19] and by the very strong strand bias of sequenced vsiRNAs: 93% of sequences obtained by the Solexa profiling derived from the positive strand. Theoretically, the imperfect duplex controls could be used to determine the number of mismatches in the vsiRNAs. However, one should know the concentration of vsiRNAs in order to compare with the concentrations of the imperfect duplexes. Unfortunately, this is very difficult since the vsiRNAs are part of the total RNA, which also contains many different plant short RNAs.
Identifying the precursor-miRNA like structures is not easy on long RNA molecules. Qi et al (2009) searched for stem-loop structures around hot spots of vsiRNAs on the TMV-Cg genome but did not find these structures [28]. However, this approach ignores the fact that distant regions of a long RNA can anneal to each other. Therefore instead of looking for local secondary structures, we identified imperfect duplexes in the sequenced library that can derive from the + or − strand. Although these imperfect duplexes do not prove that vsiRNAs derive from structured single stranded viral RNA, all our results support this model over other possibilities.
Hierarchical activity of DCL proteins on viral RNA
It was shown in TCV (turnip crinkle virus) and TRV infected Arabidopsis plants that DCL4 is the primary nuclease to produce vsiRNAs but if DCL4 is absent or suppressed DCL2 can also produce vsiRNAs [7]. Based on this we expected to find mainly 21 nt vsiRNAs produced by DCL4. In wild type virus infected plants indeed the 21 nt class was the dominant vsiRNA class followed by the 22 nt vsiRNAs. However, in the p19 mutant virus infected plants we found more 22 nt than 21 nt vsiRNAs. The first conclusion is that the viral structures are probably recognised by multiple DCLs. DCL4 predominantly produces 21 nt vsiRNAs and DCL2 mainly generates 22 nt vsiRNAs [7]. There are at least two possible explanations for the difference in the dominant size class of vsiRNAs between mutant and wild type virus infected plants. One possibility is that the 22 nt sequences are the consequence of primary vsiRNA activity, which is inhibited by p19 and therefore less 22 nt sequences are produced in wild type virus infected plants. If the 22 nt sequences are the products of primer-dependent secondary siRNA production, they would be expected to show a similar ratio of positive to negative strand since they would be produced from a perfect dsRNA consisting of the positive and negative strand of viral genome. Alternatively, 21 and 22 nt sequences are produced both in the presence and absence of p19 (i.e. independently of primary vsiRNA activity) from the same substrate RNA (i.e. mainly the positive viral RNA strand) but because p19 binds the 21 nt duplexes more strongly, these are preferentially stabilised by p19 in wild type virus infected plants. In this scenario, both 21 and 22 nt sequences would show a similar, high positive/negative strand ratio. By plotting the 21 and 22 nt sequences separately for all four solexa libraries we found that the profiles of 21 and 22 nt sequences were very similar to each other and both class displayed a high positive to negative strand ratio. This indicates that the 22 nt sequences are not the consequence of primary vsiRNA activity. In fact the plots suggest that more 22 nt sequences are generated from the viral RNA but because p19 binds to 21 nt duplexes more efficiently, the shorter duplexes are preferentially stabilised in wild type infected plants. The fact that in the absence of p19 the 22 nt sequences accumulate at a higher level than the 21 nt class raises the possibility that DCL2 is dominant over DCL4 in N. benthamiana at least in the case of processing CymRSV RNA. However, this hypothesis has to be tested using different dcl mutants but these do not exist in N. benthamiana at the moment.
Functional analysis of vsiRNAs
It was shown before that vsiRNAs do mediate cleavage of viral RNAs [24]. We asked the question whether viral sequences complementary to abundant vsiRNAs (i.e.: generated from hot spots) are targeted more efficiently than sequences that are recognised by vsiRNAs generated from non-hot spot regions. Interestingly, the abundance of vsiRNAs complementary to the sensor constructs did not show any correlation with targeting efficiency of the different sensors. There are several possible explanation for this. First, if hot spot regions form partially double stranded structures with other regions of the viral RNA it would mean that these regions are less efficiently targeted by vsiRNAs since it was reported that RNAs with strong local structures are less accessible for RISC mediated cleavages [18],[29]. The other potential explanation is that primary vsiRNAs are not good effectors of gene silencing. Indeed only small portion of vsiRNAs were found in high molecular weight complexes [24]. Moreover a recent report demonstrated that vsiRNAs produced in the absence of RDR6 are poor effectors of gene silencing [30]. We showed that most CymRSV vsiRNAs are primary siRNAs including the ones derived from hot spots, although, it is possible that the vsiRNAs, which efficiently target the viral RNA are the small amount of secondary siRNAs (DCL-D or DCL-I) that may be generated in CymRSV infected plants. However, we do not have evidence for this latter possibility.
Methods
Bioinformatic analysis
Adaptors were removed from both 454 and Solexa samples by searching for the last eight bases of the 5′ adaptor (in the case of 454 samples) and the first eight bases of the 3′ adaptor (for both 454 and Solexa samples) using the UEA sRNA tools adaptor removal application [31] The sequence after the 5′ adaptor match and before the 3′ adaptor match was retained for further analysis.
vsiRNA sequences were mapped to the CymRSV genome using PatMaN [32] allowing a single mismatch and zero gaps. vsiRNA abundances in each sample were normalised by dividing each count by the total number of trimmed reads for a given sample and then multiplying this value by one million. This gave a value of counts per million reads and ensured that the profiles were comparable even though the sample sizes varied. Profiles were plotted for each sample by taking the sum of the normalised abundance for each vsiRNA sequence covering a given nucleotide position on the viral genome.
Boundaries of hot spot regions were initially determined using a simple algorithm designed to detect peaks in the profile then checked manually on the graphical plots. Sequence logos (Figure S7) were drawn for vsiRNA size classes of 20, 21, and 22 nucleotides, respectively, using the program seqlogo from the WebLogo package version 2.8.2 [33].
A Perl script was written to identify vsiRNAs that could potentially base-pair with each other. Firstly sRNA sequences from the CymRSV PHOS Solexa sample were mapped to the plus strand of the CymRSV genome using PatMaN, allowing for one mismatch to the reference genome. All sequences matching to the plus strand of the CymRSV genome were then given as input to the script which used FASTA3 [34] to search each vsiRNA against the reverse complement of all plus strand genome matching sequences. Any resulting matches were then aligned to the query sequence using ClustalW [35] before finding the complement of the hit sequence to obtain the correct orientation. Any potential duplexes with four or fewer mismatches including a maximum of one bulge were accepted as potential pairs. This process was then repeated separately for the sequences matching to the minus strand of the CymRSV genome.
Agrobacterium tumefaciens infiltration, RNA isolation and hybridization analyses
A. tumefaciens infiltration was carried out according to Silhavy et al. (2002). For coinfiltration of N. benthamiana leaves, mixtures of strains carrying sensor constructs (OD600 = 0.15) and strains carrying suppressor constructs (OD600 = 0.3) were used. Total RNA from Agrobacterium-infiltrated N. benthamiana and virus infected plant leaves was isolated using Trizol reagent [36]. Denaturing RNA gel blot hybridisation and analyses were done as described previously [37].
Preparation of sensor constructs
All sensor constructs were prepared from the previously reported 35S-green fluorescent protein (GFP) binary plasmid [38]. First, a SmaI restriction site was inserted downstream of the GFP ORF by using the QuikChange site-directed mutagenesis kit (Stratagene) by following the instruction manual. The PCR fragment of 200 bp, corresponding to the indicated positions (Figure 5) of the CymRSV genome (accession no. NC 003532), was amplified by using appropriate 5′-phosphorylated oligonucleotides and placed into the unique SmaI site of the modified 35S-GFP plasmid. The plus and minus orientations of the inserts were selected, thus generating the different sensor constructs shown in Figure 5.
In vitro RNA transcription and plant inoculation
In vitro transcription of CymRSV and Cym19stop RNAs from linearized template plasmids and inoculation of RNA transcripts onto Nicotiana benthamiana, N. benthamiana GFP16c/RDR6i line [15] and N. clevelandii plants were performed as described previously [39].
RNA extraction from virus infected plants
Total RNA was extracted from 100 mg of systemicly infected leaf tissue [40]. Briefly, the homogenized plant materials were resuspended in 600 µL of extraction buffer (0.1 M glycine-NaOH, pH 9.0, 100 mM NaCl, 10 mM EDTA, 2% SDS, and 1% sodium lauroylsarcosine) and mixed with an equal volume of phenol. The aqueous phase was treated with equal volumes of phenol and chloroform, precipitated with ethanol, and resuspended in sterile water and used in subsequent reactions.
454 sequencing of small RNAs
Small RNA between 19–24nt were cloned from systemic leaves of N. benthamiana as described by Moxon et al. [41]. Briefly, the sRNA fraction was purified and ligated to adaptors without de-phosphorylating and re - phosphorylating the sRNA. The RNA was reverse transcribed, and amplified by PCR before being sent to 454 Life Sciences for pyrosequencing. The small RNA libraries are submitted to GEO and can be accessed through a super series number: GSE17278.
Solexa sequencing of small RNAs
Small RNA fraction (19–24 nt) of total RNA extracted from systemic leaves of N. benthamiana was isolated from 15% denaturing polyacrylamide gel. The eluted sRNA fraction was divided into two and one half was not treated (called CymRSV-PHOS and Cym19stop-PHOS) while the other half was de-phosphorylated with Shrimp Alkaline Phosphatase and re - phosphorylated with T4 Polynucleotide Kinase (called CymRSV-CIP and Cym19stop-CIP). The resulting sRNAs were ligated to adaptors in the following reaction. The purified, adaptor ligated short RNAs were converted to DNA by RT-PCR and the DNA was sequenced on a Solexa platform (Illumina). The small RNA libraries are submitted to GEO and can be accessed through a super series number: GSE17278.
Isolation, labeling and hybridization of small RNAs from virus infected plants
For preparation of 19–24 nt RNA fraction, 15 to 20 µg of total RNA from in vitro transcribed virus infected plants was subjected to electrophoresis through an 8% denaturing polyacrylamide gel followed by staining in 1× Tris-borate-EDTA and 0.5 µg/mL ethidium bromide solutions for 5 min. The 19–24 nt fraction was visualized by UV light and excised from the gel. The gel slice was crushed, covered with 2 volumes of elution buffer (80% formamide, 40 mM Pipes, pH 6.4, 1 mM EDTA, and 400 mM NaCl) and incubated overnight. The gel residues were pelleted by centrifugation and the supernatant was precipitated with ethanol. The RNA (∼100 ng) was dephosphorylated and then labeled in a 10-µl reaction in the presence of γ-32P-ATP and RNasin with 8 units of T4 polynucleotide kinase. The labeled 19–24 nt RNAs were used for hybridization either to membranes (Zeta-Probe GT, BioRad) containing five hundred 21mer DNA oligonucleotides in a one nucleotide sliding window covering a 520 nt region (nucleotides 2650–3169 on the positive strand, see oligonucleotide sequences in Table S3) or to a membrane containing hundred eighty-six 21mer DNA oligonucleotides in a one nucleotide sliding window covering a 206 nt region (nucleotides 4300–4505 on the positive strand, see oligonucleotide sequences in Table S3). As a control for cross hybridization two set of oligonucleotides, which were one to five nucleotides shorter at their 5′ ends were also spotted on the membranes (12-1, 12-2, 12-3, 12-4, 12-5, 87-1, 87-2, 87-3, 87-4 and 87-5). As a negative control to check background hybridization we spotted pUC/M13 Forward and Reverse primer onto the membranes or only water. Hybridization was performed in Ambion ULTRAhyb-Oligo Buffer at 37°C following the manifacturer's instruction. Signals were quantified with a Genius Image Analyzer (Syngene).
Terminator digest
19mer and 21 mer synthetic RNA molecules were phosphorylated and 30 µl of sense RNA (10 µM) and 30 µl of antisense RNA (10 µM) were incubated in 90 µl annealing buffer (30 mM HEPES-KOH pH 7.4, 100mM KCl, 2mM MgCl2, 50mM ammonium acetate) to form siRNA duplex (19 nt siRNA, siRNA171a and siRNA171b). We also prepared mismatch containing siRNA duplexes on the same way by incubating sense RNA with different mismatch containing antisense RNA (19mer antisense RNA 1mism_end, 19mer antisense RNA 2mism_end, 19mer antisense RNA 3mism_end, 19mer antisense RNA 3mism_middl, 2mm antisense siRNA171a and 3mm antisense siRNA171c). We digested the resulting siRNA duplexes for one hour at 30°C in decreasing concentration (2 µM, 0,4 µM, 0,2 µM and 0,04 µM or 0,1 µM, 0,02 µM, 0,004 µM and 0,002 µM and as a control 2 µM or 0,002 µM sense phosphorylated RNA oligo) in a 10 µl reaction in the presence of 0.25 U Terminator™ 5′-Phosphate-Dependent Exonuclease (Epicentre Biotechnologies) without or after 1 minute 90°C denaturation. Terminator treated and non-treated samples were loaded onto 0.5× TBE 15% UREA-Polyacrylamide gel. After electrophoresis the samples were transferred to Zeta-probe GT BioRad membranes by Semi-dry blotting. The membranes were hybridized with γ-32P-ATP labelled DNA oligonucleotide (19mer sense detector, siRNA171a sense detector and siRNA171b detector) complementary to the 19mer sense RNA or to the sense RNE of siRNA171a or to the sense RNA of siRNA171b respectively. Hybridization was performed in Ambion ULTRAhyb-Oligo Buffer at 37°C following the manifacturer′s instruction.
RNA extracted from CymRSV infected plants was mixed with 19mer siRNA duplex and was diluted containing decreasing concentration of total RNA and siRNA duplex (2,3 µg RNA+2 µM siRNA duplex, 0,46 µg RNA+0,4 µM siRNA duplex, 0,23 µg RNA+0,2 µM siRNA duplex, 0.046 µg RNA+0,04 µM siRNA duplex, and as a control 2,3 µg RNA+2 µM sense phosphorylated RNA oligo). We digested the resulting RNA and siRNA duplex mixture in a 10 µl reaction in the presence of 0.25 U Terminator™ 5′-Phosphate-Dependent Exonuclease without or after 1 minute 90°C denaturation for one hour at 30°C. Terminator treated and non-treated samples were loaded onto 0.5× TBE 15% UREA-Polyacrylamide gel. After electrophoresis the samples were transferred to Zeta-probe GT BioRad membranes by Semi-dry blotting. The membranes were hybridized with γ-32P-ATP labelled DNA oligonucleotides. Cym mix probe was a pool of five oligonucleotides (cym3125, cym3196, cym3420, cym3950 and cym4240) and complenetary to the negative strand of the virus genome. Cym3025 was also complementary to the negative strand of the virus genome. Cym minus probe was a pool of two oligonucleotides (cym1021 minus and cym3710 minus) and complementary to the positive strand of the virus genome. Cym 4632 LNA was also complementary to the positive strand of the virus genome. U6 detects the U6 snRNA while miR159 LNA detects the mature strand of miR159 and contains locked nucleic acid (LNA) nucleotides. The oligonucleotide sequences used for hybridisation are available in Table S3. Hybridization was performed in Ambion ULTRAhyb-Oligo Buffer at 37°C following the manifacturer's instruction.
Supporting Information
Zdroje
1. DingSW
VoinnetO
2007 Antiviral Immunity Directed by Small RNAs. Cell 130 413 426
2. BernsteinE
CaudyAA
HammondSM
HannonGJ
2001 Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409 363 366
3. NykanenA
HaleyB
ZamorePD
2001 ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell 107 309 321
4. HammondSM
BoettcherS
CaudyAA
KobayashiR
HannonGJ
2001 Argonaute2, a link between genetic and biochemical analyses of RNAi. Science 293 1146 1150
5. DunoyerP
HimberC
VoinnetO
2005 DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat Genet 37 1356 1360
6. WasseneggerM
KrczalG
2006 Nomenclature and functions of RNA-directed RNA polymerases. Trends Plant Sci 11 142 151
7. DelerisA
Gallego-BartolomeJ
BaoJ
KasschauKD
CarringtonJC
2006 Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313 68 71
8. FusaroAF
MatthewL
SmithNA
CurtinSJ
Dedic-HaganJ
2006 RNA interference-inducing hairpin RNAs in plants act through the viral defence pathway. EMBO Rep 7 1168 1175
9. VaucheretH
2008 Plant ARGONAUTES. Trends Plant Sci 13 350 358
10. HutvagnerG
SimardMJ
2008 Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9 22 32
11. BrodersenP
Sakvarelidze-AchardL
Bruun-RasmussenM
DunoyerP
YamamotoYY
2008 Widespread translational inhibition by plant miRNAs and siRNAs. Science 320 1185 1190
12. SilhavyD
BurgyanJ
2004 Effects and side-effects of viral RNA silencing suppressors on short RNAs. Trends Plant Sci 9 76 83
13. DalmayT
HamiltonA
RuddS
AngellS
BaulcombeDC
2000 An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell 101 543 553
14. MourrainP
BeclinC
ElmayanT
FeuerbachF
GodonC
2000 Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101 533 542
15. SchwachF
VaistijFE
JonesL
BaulcombeDC
2005 An RNA-Dependent RNA Polymerase Prevents Meristem Invasion by Potato Virus X and Is Required for the Activity But Not the Production of a Systemic Silencing Signal. Plant Physiol
16. Diaz-PendonJA
LiF
LiWX
DingSW
2007 Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell 19 2053 2063
17. DonaireL
BarajasD
Martinez-GarciaB
Martinez-PriegoL
PaganI
2008 Structural and genetic requirements for the biogenesis of tobacco rattle virus-derived small interfering RNAs. J Virol 82 5167 5177
18. SzittyaG
MolnarA
SilhavyD
HornyikC
BurgyanJ
2002 Short defective interfering RNAs of tombusviruses are not targeted but trigger post-transcriptional gene silencing against their helper virus. Plant Cell 14 359 372
19. MolnarA
CsorbaT
LakatosL
VarallyayE
LacommeC
2005 Plant virus-derived small interfering RNAs originate predominantly from highly structured single-stranded viral RNAs. J Virol 79 7812 7818
20. DuQS
DuanCG
ZhangZH
FangYY
FangRX
2007 DCL4 targets Cucumber mosaic virus satellite RNA at novel secondary structures. J Virol 81 9142 9151
21. RussoM
BurgyanJ
MartelliGP
1994 Molecular biology of tombusviridae. Adv Virus Res 44 381 428
22. AhlquistP
2002 RNA-dependent RNA polymerases, viruses, and RNA silencing. Science 296 1270 1273
23. HoT
PallettD
RusholmeR
DalmayT
WangH
2006 A simplified method for cloning of short interfering RNAs from Brassica juncea infected with Turnip mosaic potyvirus and Turnip crinkle carmovirus. J Virol Methods 136 217 223
24. PantaleoV
SzittyaG
BurgyanJ
2007 Molecular Bases of Viral RNA Targeting by Viral Small Interfering RNA-Programmed RISC. J Virol 81 3797 3806
25. PakJ
FireA
2007 Distinct populations of primary and secondary effectors during RNAi in C. elegans. Science 315 241 244
26. LakatosL
CsorbaT
PantaleoV
ChapmanEJ
CarringtonJC
2006 Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. Embo J 25 2768 2780
27. LinsenSE
de WitE
JanssensG
HeaterS
ChapmanL
2009 Limitations and possibilities of small RNA digital gene expression profiling. Nat Methods 6 474 476
28. QiX
BaoFS
XieZ
2009 Small RNA deep sequencing reveals role for Arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS ONE 4 e4971 doi:10.1371/journal.pone.0004971
29. AmeresSL
MartinezJ
SchroederR
2007 Molecular basis for target RNA recognition and cleavage by human RISC. Cell 130 101 112
30. VaistijFE
JonesL
2009 Compromised Virus-Induced Gene Silencing in RDR6-Deficient Plants. Plant Physiol 149 1399 1407
31. MoxonS
SchwachF
DalmayT
MacleanD
StudholmeDJ
2008 A toolkit for analysing large-scale plant small RNA datasets. Bioinformatics 24 2252 2253
32. PruferK
StenzelU
DannemannM
GreenRE
LachmannM
2008 PatMaN: rapid alignment of short sequences to large databases. Bioinformatics 24 1530 1531
33. CrooksGE
HonG
ChandoniaJM
BrennerSE
2004 WebLogo: a sequence logo generator. Genome Res 14 1188 1190
34. PearsonWR
2000 Flexible sequence similarity searching with the FASTA3 program package. Methods Mol Biol 132 185 219
35. ChennaR
SugawaraH
KoikeT
LopezR
GibsonTJ
2003 Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31 3497 3500
36. JohansenLK
CarringtonJC
2001 Silencing on the spot. Induction and suppression of RNA silencing in the Agrobacterium-mediated transient expression system. Plant Physiol 126 930 938
37. SilhavyD
MolnarA
LucioliA
SzittyaG
HornyikC
2002 A viral protein suppresses RNA silencing and binds silencing-generated, 21 - to 25-nucleotide double-stranded RNAs. Embo J 21 3070 3080
38. BrignetiG
VoinnetO
LiWX
JiLH
DingSW
1998 Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. Embo J 17 6739 6746
39. DalmayT
RubinoL
BurgyanJ
KollarA
RussoM
1993 Functional analysis of cymbidium ringspot virus genome. Virology 194 697 704
40. WhiteJL
KaperJM
1989 A simple method for detection of viral satellite RNAs in small plant tissue samples. J Virol Methods 23 83 93
41. MoxonS
JingR
SzittyaG
SchwachF
Rusholme PilcherRL
2008 Deep sequencing of tomato short RNAs identifies microRNAs targeting genes involved in fruit ripening. Genome Res 18 1602 1609
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument TurnoverČlánek Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic PathwaysČlánek The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-DependentČlánek Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 4- Stillova choroba: vzácné a závažné systémové onemocnění
- Perorální antivirotika jako vysoce efektivní nástroj prevence hospitalizací kvůli COVID-19 − otázky a odpovědi pro praxi
- Diagnostika virových hepatitid v kostce – zorientujte se (nejen) v sérologii
- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Choroby jater v ordinaci praktického lékaře – význam jaterních testů
-
Všechny články tohoto čísla
- Innate Recognition of Fungal Cell Walls
- Suppression of mRNAs Encoding Tegument Tetraspanins from Results in Impaired Tegument Turnover
- Junín Virus Infection of Human Hematopoietic Progenitors Impairs Proplatelet Formation and Platelet Release via a Bystander Effect Involving Type I IFN Signaling
- The Endosymbiotic Bacterium Induces Resistance to Dengue Virus in
- Natural Regulatory T Cells in Malaria: Host or Parasite Allies?
- Keratinocytes Determine Th1 Immunity during Early Experimental Leishmaniasis
- Spatial and Temporal Association of Outbreaks of H5N1 Influenza Virus Infection in Wild Birds with the 0°C Isotherm
- Novel Riboswitch Ligand Analogs as Selective Inhibitors of Guanine-Related Metabolic Pathways
- RNA Polymerase Activity and Specific RNA Structure Are Required for Efficient HCV Replication in Cultured Cells
- The Physical Relationship between Infectivity and Prion Protein Aggregates Is Strain-Dependent
- Inadequate Clearance of Translocated Bacterial Products in HIV-Infected Humanized Mice
- Topology and Organization of the Type III Secretion Needle Complex Components
- Temperature Modulates Plant Defense Responses through NB-LRR Proteins
- Peptide Inhibitors of Dengue-Virus Entry Target a Late-Stage Fusion Intermediate
- Identification of Host-Dependent Survival Factors for Intracellular through an siRNA Screen
- Exposure to HIV-1 Directly Impairs Mucosal Epithelial Barrier Integrity Allowing Microbial Translocation
- Increased Asymmetric Dimethylarginine in Severe Falciparum Malaria: Association with Impaired Nitric Oxide Bioavailability and Fatal Outcome
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Induces Brain Microvascular Endothelial Cell Detachment from the Matrix and Cleavage of Occludin: A Role for MMP-8
- Two Coregulated Efflux Transporters Modulate Intracellular Heme and Protoporphyrin IX Availability in
- The Type I NADH Dehydrogenase of Counters Phagosomal NOX2 Activity to Inhibit TNF-α-Mediated Host Cell Apoptosis
- Rhomboid 4 (ROM4) Affects the Processing of Surface Adhesins and Facilitates Host Cell Invasion by
- Increased Monocyte Turnover from Bone Marrow Correlates with Severity of SIV Encephalitis and CD163 Levels in Plasma
- The RING-CH Ligase K5 Antagonizes Restriction of KSHV and HIV-1 Particle Release by Mediating Ubiquitin-Dependent Endosomal Degradation of Tetherin
- Molecular Mechanisms of Ethanol-Induced Pathogenesis Revealed by RNA-Sequencing
- Highly Frequent Mutations in Negative Regulators of Multiple Virulence Genes in Group A Streptococcal Toxic Shock Syndrome Isolates
- Emergence and Pathogenicity of Highly Virulent Genotypes in the Northwest United States
- Structural and Functional Analysis of Viral siRNAs
- Prion Shedding from Olfactory Neurons into Nasal Secretions
- a GATA Transcription Factor That Directs Disparate Fates in Including Morphogenesis and Siderophore Biosynthesis
- Three Members of the 6-cys Protein Family of Play a Role in Gamete Fertility
- Complement as an Endogenous Adjuvant for Dendritic Cell-Mediated Induction of Retrovirus-Specific CTLs
- A Genomic Survey of Positive Selection in Provides Insights into the Evolution of Accidental Virulence
- Overcomes Stress of Azole Drugs by Formation of Disomy in Specific Multiple Chromosomes
- Blood Fluke Exploitation of Non-Cognate CD4 T Cell Help to Facilitate Parasite Development
- Antagonism of Tetherin Restriction of HIV-1 Release by Vpu Involves Binding and Sequestration of the Restriction Factor in a Perinuclear Compartment
- The Development of Therapeutic Antibodies That Neutralize Homologous and Heterologous Genotypes of Dengue Virus Type 1
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- Interactions with Bacteria in the Context of Human Health and Disease
- Viral Capsid Is a Pathogen-Associated Molecular Pattern in Adenovirus Keratitis
- Electron Tomography Reveals the Steps in Filovirus Budding
- Selective Condensation Drives Partitioning and Sequential Secretion of Cyst Wall Proteins in Differentiating
- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
- VacA Toxin/Subunit p34: Targeting of an Anion Channel to the Inner Mitochondrial Membrane
- Production of Extracellular Traps against and in Infected Lung Tissue Is Dependent on Invading Neutrophils and Influenced by Hydrophobin RodA
- A Differential Role for Macropinocytosis in Mediating Entry of the Two Forms of Vaccinia Virus into Dendritic Cells
- Impaired Innate Immunity in Mice but Preserved CD8 T Cell Responses against in -, -, - or -Deficient Mice
- SARS-CoV Pathogenesis Is Regulated by a STAT1 Dependent but a Type I, II and III Interferon Receptor Independent Mechanism
- Proteolysis of Human Thrombin Generates Novel Host Defense Peptides
- Multilayered Mechanism of CD4 Downregulation by HIV-1 Vpu Involving Distinct ER Retention and ERAD Targeting Steps
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Effect of Vaccination on the Evolution and Population Dynamics of Avian Paramyxovirus-1
- Reconstitution of SARS-Coronavirus mRNA Cap Methylation
- Deficiencies in Jasmonate-Mediated Plant Defense Reveal Quantitative Variation in Pathogenesis
- A Timescale for Evolution, Population Expansion, and Spatial Spread of an Emerging Clone of Methicillin-Resistant
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



