-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
Results from several observational studies of HIV-discordant couples and a randomized controlled trial (HIV Prevention Trials Network 052) show that antiretroviral therapy (ART) can greatly reduce heterosexual HIV transmission in stable HIV-discordant couples. However, such data do not prove that ART will reduce HIV incidence at the population level. Observational investigations using ecological measures have been used to support the implementation of HIV treatment for the specific purpose of preventing transmission at the population level. Many of these studies note ecological associations between measures of increased ART uptake and decreased HIV transmission. Given the urgency of implementing HIV prevention measures, ecological studies must de facto be used to inform current strategies. However, the hypothesis that widespread ART can eliminate HIV infection may have raised expectations beyond what we may be able to achieve. Here we review and discuss the construct of the exposure and outcome measures and analysis methods used in ecological studies. By examining the strengths and weaknesses of ecological analyses, we aim to aid understanding of the findings from these studies to inform future policy decisions regarding the use of ART for HIV prevention.
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001260
Category: Review
doi: https://doi.org/10.1371/journal.pmed.1001260Summary
Results from several observational studies of HIV-discordant couples and a randomized controlled trial (HIV Prevention Trials Network 052) show that antiretroviral therapy (ART) can greatly reduce heterosexual HIV transmission in stable HIV-discordant couples. However, such data do not prove that ART will reduce HIV incidence at the population level. Observational investigations using ecological measures have been used to support the implementation of HIV treatment for the specific purpose of preventing transmission at the population level. Many of these studies note ecological associations between measures of increased ART uptake and decreased HIV transmission. Given the urgency of implementing HIV prevention measures, ecological studies must de facto be used to inform current strategies. However, the hypothesis that widespread ART can eliminate HIV infection may have raised expectations beyond what we may be able to achieve. Here we review and discuss the construct of the exposure and outcome measures and analysis methods used in ecological studies. By examining the strengths and weaknesses of ecological analyses, we aim to aid understanding of the findings from these studies to inform future policy decisions regarding the use of ART for HIV prevention.
Introduction
Ecological studies use observational data to examine relationships between exposures and outcomes at the level of groups rather than individuals [1]. When individual-level data are unavailable, ecological studies can provide important insight into population-level trends [2],[3]. Ecological studies appeal to researchers and policy-makers because they are inexpensive, use existing data, and are applicable to a broad range of issues. However, statistical models using only group-level data cannot evaluate person-level details and are therefore unable to test etiological hypotheses [2],[4]–[6]. Further, because ecological studies often use separate data sources to measure exposures and their potential effects, the link between exposures and outcomes cannot be determined at the individual level.
Concern over these limitations has focused on “ecological fallacy,” in which associations detected at the population level are mistakenly interpreted to reflect the experience of individuals in that population [1]. The first study describing ecological fallacy presented an analysis of literacy and immigration in the US, in which states with higher proportions of immigrants were shown to have higher average literacy rates [7]. An “ecologically fallacious” interpretation of this association would be that immigrants have higher literacy rates than native-born individuals; in fact, individual-level analysis shows lower literacy rates among immigrants. The best explanation for this particular population-level observation is that immigrants tend to settle in sites where the native-born individuals have higher literacy levels [7].
Despite their limitations, ecological studies play an important role in generating hypotheses that can be tested in experimental or individual-level observational studies [2],[8]. For instance, ecological analyses were successfully applied during the exploratory phases of research on male circumcision to prevent HIV, in which geographical associations between circumcision rates and HIV prevalence [9]–[11] provided the foundation for two decades of observational research [12],[13] on the topic. All three randomized clinical trials that followed were halted because of a readily demonstrable reduction of HIV acquisition in circumcised men [14],[15]. A Cochrane review published in 2009 concluded that male circumcision is a clinically viable HIV prevention strategy [16].
Here we describe an illustrative set of observational studies that use ecological measures to examine the population-level effects of antiretroviral therapy (ART) on HIV transmission. We critically review what these studies measure, how they measure it, and how their findings are interpreted. These results are used to provide insight into the strengths and limitations of this approach.
Ecological Studies of ART for HIV Prevention
The narrative of exploring the effects of treatment on prevention shares similarities with the narrative for male circumcision, though with a somewhat different chronology. The hypothesis that antiretroviral agents can prevent sexual HIV transmission was suggested in 1988 shortly after the development of azidothymidine, which was found to effectively penetrate the genital tract [17]. This report was followed by more intensive study of the effect of newly developed antiretroviral agents on HIV replication in the male and female genital tract [18]–[21]. In 1994, Musicco et al. observed that azidothymidine could reduce transmission of HIV in a cohort of discordant couples by 50% [22]. Several clinical trials in the late 1990s showing that ART stopped mother-to-child transmission lent further credibility to the potential use of ART to prevent sexual transmission [23]. In 2000, a randomized clinical trial, HIV Prevention Trials Network 052 (HPTN 052), was launched to determine the magnitude and durability of the effect of combination antiretroviral agents on the prevention of sexual transmission of HIV [24]. After this trial was launched, several [25]–[28], but not all [29], individual-level observational studies reported a protective effect of ART against HIV transmission in serodiscordant couples. In addition, many modeling exercises suggested varying degrees of population-level prevention benefit from broader use of ART [30], the most widely discussed of which predicted elimination of HIV within five years under ideal conditions [31]. These models are discussed in a review by Eaton et al. [32] in the July 2012 PLoS Medicine Collection, “Investigating the Impact of Treatment on New HIV Infections.” A third group of eight ecological studies examined the population-level effects of widespread ART on HIV incidence using ecological measures, and reported significant effects [33]–[38] in all but two cases [39],[40]. These eight studies are the focus of this review. Finally, in mid-2011, the HPTN 052 investigators reported a 96% reduction of HIV transmission in heterosexual couples over the 1.7 years of follow-up [41].
The promise of ART to control—and perhaps even eliminate [31]—HIV has mobilized calls from public health leaders to integrate preventive and clinical applications of ART [42]–[45]. In light of several trials showing markedly improved survival for those initiating ART earlier in the course of disease, such initiatives often emphasize the clinical benefits that early treatment can bring HIV-infected persons [46],[47]. However, numerous behavioral, epidemiological, and programmatic challenges may well limit the ability to translate the individual-level prevention benefits of ART to a larger population [48]–[52]. As such, demonstration of a minimally biased population-level benefit is critical. Not surprisingly, there is a credible tension between the need for more randomized individual - and community-level trials (also called cluster randomized controlled trials), and the immediate scale-up of HIV treatment to prevent the spread of HIV [53]–[55]. The arguments for immediate and broader roll-out of ART for the sake of prevention are based on the HPTN 052 study [41], observational studies of transmission within HIV-discordant couples [25]–[29], ecological reports [33]–, and modeling exercises [31],[56]–[59].
In this report we examine eight influential ecological studies that assess the population-level effects of ART on HIV transmission (Table 1). Most of the studies are from North America [33],[34],[36],[38]–[40], with one set in Taiwan [35] and one in Australia [37]. Each study uses an ecological measure of the exposure, such as access to ART, or the outcome, such as HIV incidence, or both (summarized in Table 1; further considerations detailed in Table 2).
Tab. 1. Summary of exposure and outcome measures in studies using ecological measures to assess population-level effects of ART on HIV transmission. 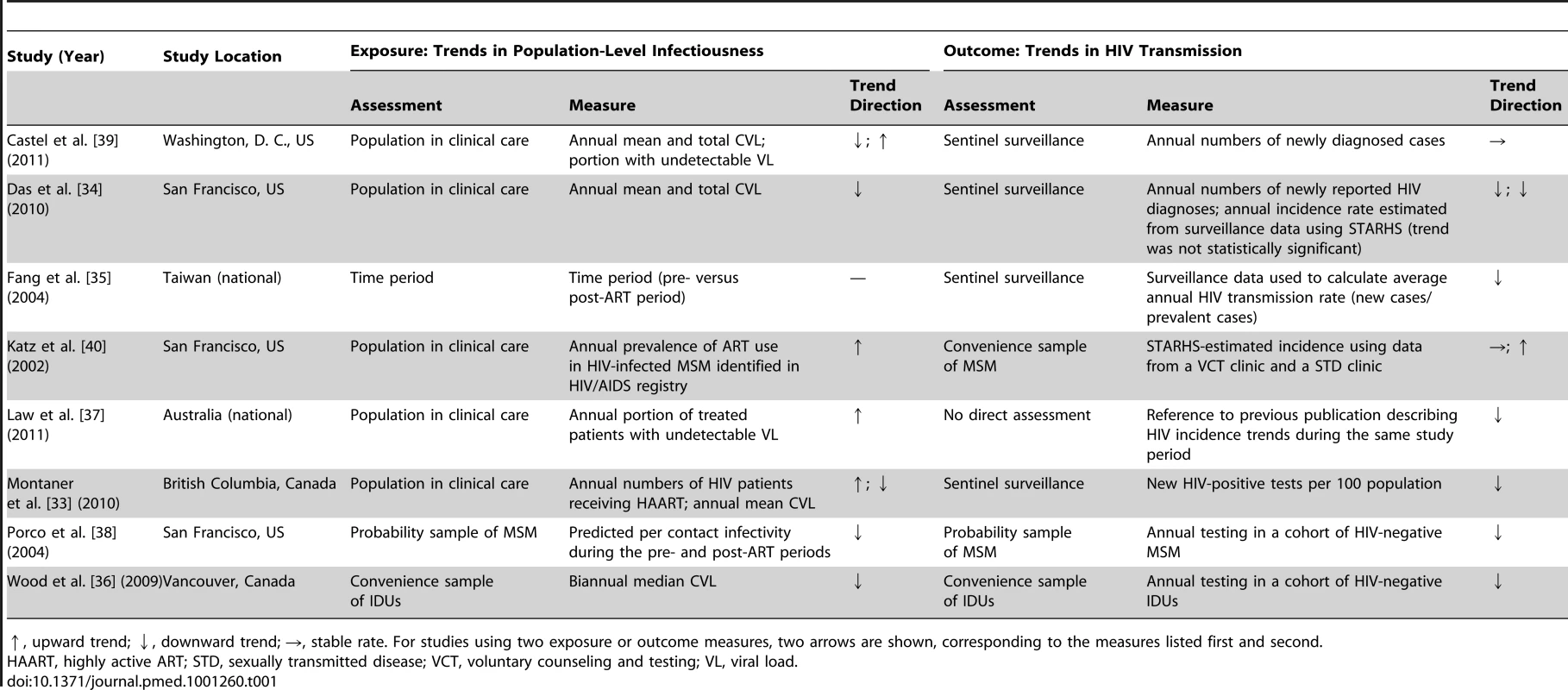
↑, upward trend; ↓, downward trend; →, stable rate. For studies using two exposure or outcome measures, two arrows are shown, corresponding to the measures listed first and second. Tab. 2. Summary of measures used and considerations for their use. 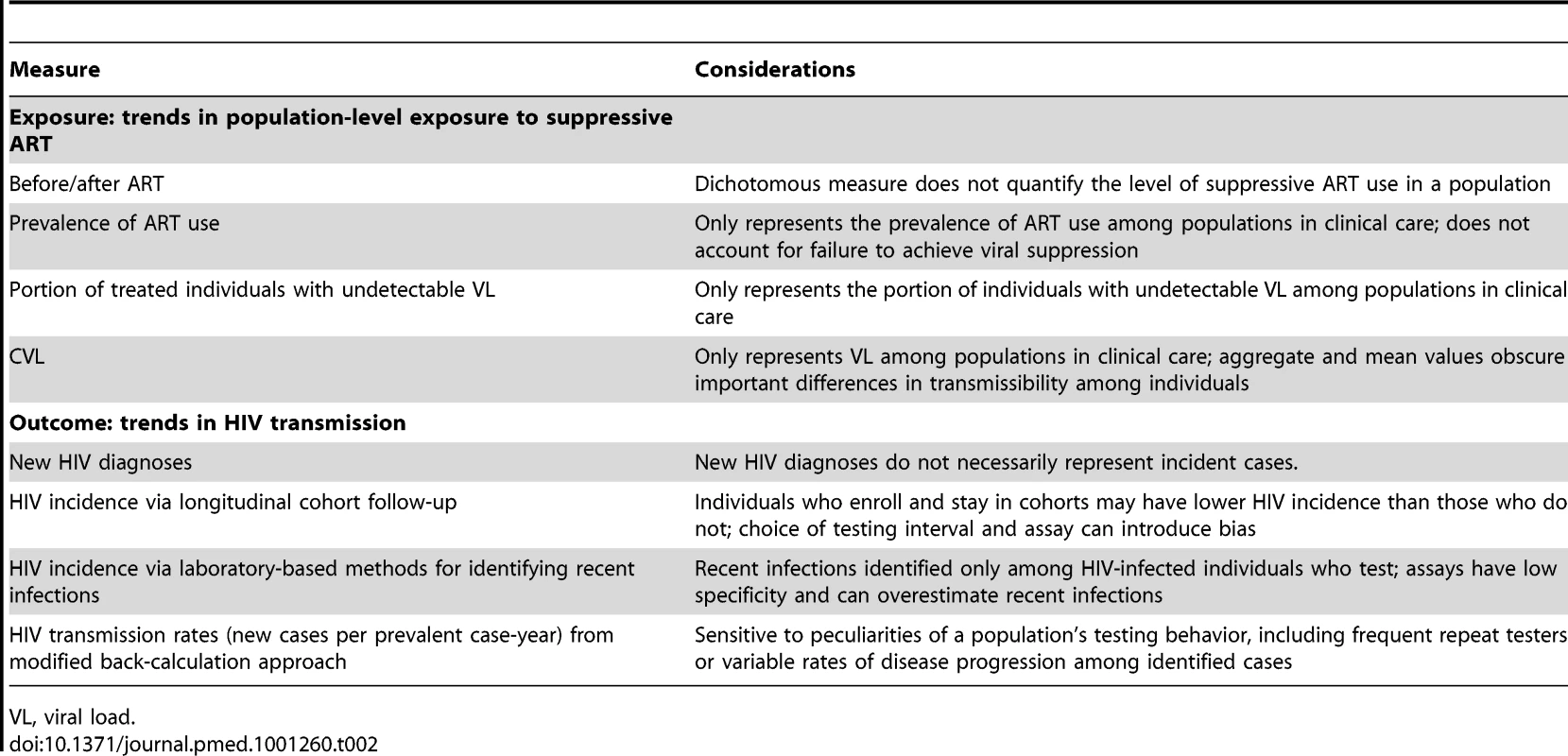
VL, viral load. Measuring Population Exposure to ART
The simplest way investigators have characterized ART exposure in a population of HIV-infected persons is to use a dichotomous “before/after” measure, as in the case of Fang et al. [35] and Porco et al. [38], based on the time at which scale-up of local HIV treatment policies improved access to ART. Other investigators have used more detailed measures of ART exposure, including Montaner et al. [33], who estimated the number of HIV-infected persons known to be receiving ART in a population, or Katz et al. [40], who used prevalence of ART use among all identified HIV patients. How well these measures reflect actual ART exposure of an entire HIV-infected population depends on the extent to which some subpopulations remain “hidden” to investigators. ART exposure of the entire HIV-infected population can only be measured if every person with HIV infection can be identified and their treatment status assessed.
The hypothesis that population ART usage will decrease HIV incidence relies on the assumption that ongoing HIV care will sustain viral suppression, which is essential to transmission prevention [60]. However, large numbers of HIV-infected persons are lost to follow-up along the path from testing to suppressive treatment [61],[62]; the US Centers for Disease Control and Prevention recently estimated that only about 24% of the 1.2 million people in the US with HIV infection in 2010 were virally suppressed (Figure 1) [61]–[63]. Even once in care, rates of treatment refusal by eligible individuals can be substantial [64].
Fig. 1. Estimated numbers of HIV-infected individuals in the US retained (and corresponding percentages lost) at various stages of the test, link, and treat cascade. 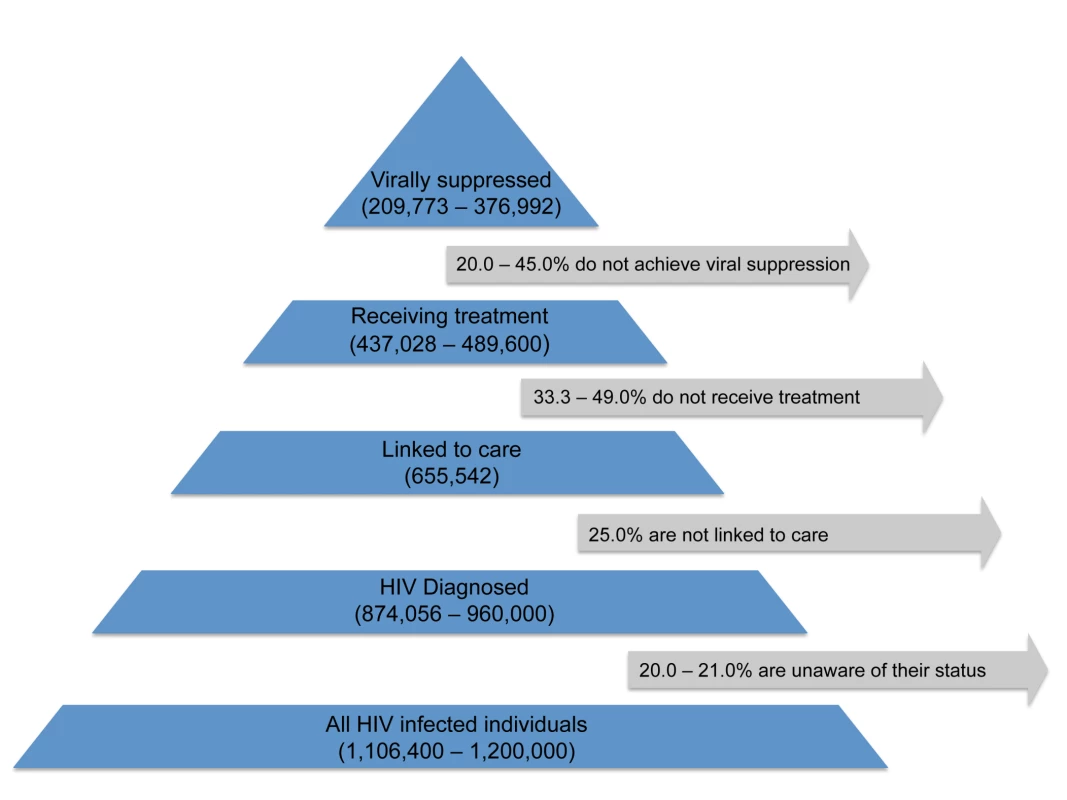
This figure is based on data from [61],[62]. To address the shortcomings of measures that do not fully reflect suppressive ART use, alternative metrics incorporate viral load information, based on the well-understood relationship between sexual transmission of HIV from infected individuals and viral concentrations in their blood [65] and genital fluids [66]. One such measure uses the proportion (or absolute number) of treated individuals in a study population with undetectable viral load, usually defined as having fewer than 400 copies/ml [33],[37]–[39].
A related measure, community viral load (CVL), is used by Das et al. [34], Montaner et al. [33], and Wood et al. [36], and is defined as the total, mean [33],[34], or median [36] viral load for a particular group or geographic region in a given period of time (usually a year). CVL may be a useful biomarker for describing population-level treatment outcomes over time, particularly in cases in which geospatial information about the patients' primary residence or point of medical care is available, allowing investigators to compare geographic disparities in CVL with other predictive factors such as socioeconomic status or proximity to health care programs [34],[39]. However, because most CVL measures rely on public health surveillance data [33],[34],[39], these exposure measures reflect the treatment outcomes only for the subset of the HIV-infected population who get tested for HIV, link to care, and remain in care long enough to contribute such measurements. Patients with acute infection unidentified by serological testing are de facto not considered in the calculation of CVL, but may well be expected to contribute disproportionately to onward HIV transmission [67],[68]. Additionally, the use of an aggregate measure of viral load in a community cannot capture other important drivers of HIV transmission, such as the distribution of viral loads within the population, sexual and drug-using behaviors, and the sexual or drug-use networks through which these behaviors spread HIV.
Outcome Assessment: Tracking Population HIV Transmission
Accurate assessment of HIV incidence is critical for evaluating the population-level effect of interventions, but such assessment is challenging. The simplest approach is taken by Law et al. [37], who simply refer to HIV incidence trends cited in past publications [37]. Another approach, taken by Montaner et al. [33], Das et al. [34], and Castel et al. [39], estimates population-based incidence from information on newly identified cases, using new HIV diagnoses as a direct proxy for new infections. Obviously, newly diagnosed patients acquired HIV at some unknown earlier time, and so they are not “incident” in the traditional use of the word. Using new diagnoses as a proxy for incidence also misses populations that do not seek testing and that may have lower access to health care and a corresponding higher risk for acquiring HIV [69],[70]. Changes in the number of new HIV diagnoses may reflect actual changes in incidence, but will also be affected by changes in availability of services and testing behaviors [71].
A second population-based incidence estimation method, used by Fang et al. [35], back-calculates past incidence from new diagnoses [35]. This method relies in part on assumptions of uniform parameters for disease progression markers such as the onset of AIDS symptoms or the proportion of newly diagnosed individuals with CD4 cell count less than 200 cells/µl, although variation in the rate of decline of CD4 cell count over time and across gender, ethnicity, and HIV subtypes undermines the validity of this method [72]–[74].
Laboratory assays to identify persons with recent HIV infection can be applied to stored biospecimens collected in the course of routine surveillance or epidemiological research studies and may provide a more rigorous method to determine current HIV incidence from new diagnoses. The serologic testing algorithm for recent HIV seroconversion (STARHS) [75] derives current HIV incidence from the prevalence of recent infections, based on the assay window period, delineated by the seroconversion dates as detected by the original HIV-1 antibody test and the STARHS method, and adjusting for the estimated prevalence among non-testers and the probability that HIV-infected individuals will test, receive treatment, and/or have missing specimens. Although the investigations that use this method take advantage of existing surveillance data, as in the case of Das et al. [34] and Katz et al. [40], logistical challenges in storing and tracking remnant blood can affect the completeness of data. Furthermore, even relatively new laboratory methods such as the detuned enzyme-linked immunosorbent assay or the newer BED capture enzyme immunoassay have been known to misclassify established infections as incident infections; therefore, results must be interpreted with some caution [76]. The use of this approach has generally fallen out of favor pending development of better laboratory-based tests or algorithms [77].
In contrast to population-based methods, longitudinal cohort follow-up data have also been used to define population incidence, as in the analyses carried out by Wood et al. [36] and Porco et al. [38]. Although long considered the gold standard of HIV incidence estimation, cohort follow-up is not immune to bias, such as that which can result from the choice of testing intervals and HIV assay [78]. Moreover, cohort participants may be a poor proxy for the rest of the population, especially if the individuals who enroll and remain in the cohort have fewer risk behaviors than their unobserved counterparts.
Identifying the Effects of ART on HIV Transmission
The ultimate aim of these investigations is to determine whether population-level ART exposure has affected HIV transmission. Some investigators used inductive reasoning to synthesize either their own results [38],[40] or a combination of their own results and other published reports [37]. The remaining studies quantify the association by comparing transmission rates, defined as the ratio of new cases to prevalent cases in an interval of time, before and after introduction of ART [35], or by using time series regression modeling [33],[34],[36],[39]. Although these methods of analysis differ considerably, it is worth consideration that nearly every study arrives at the same conclusion: that increased population exposure to ART leads to lower HIV transmission (Table 3).
Tab. 3. Analysis methods and conclusions regarding effects. 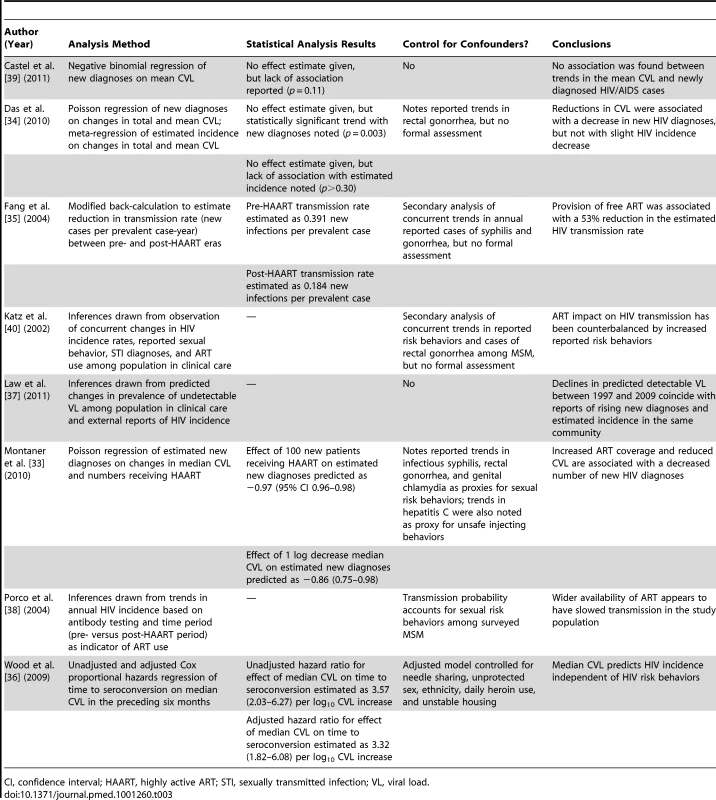
CI, confidence interval; HAART, highly active ART; STI, sexually transmitted infection; VL, viral load. However, inaccurate assessment of exposures or outcomes can generate bias [51]. Overestimating the decline in incidence, for instance—perhaps because of an unrecognized change in testing behaviors—could produce an upward bias in the estimated impact of ART on HIV transmission. Additionally, statistical associations do not show causation, and observed trends in HIV diagnoses may be due to factors other than population-level exposure to ART. For example, declines in HIV incidence in settings worldwide—most of which started to occur before ART was available or could be expected to have had an effect—have been ascribed to various phenomena, including the saturation of HIV in high-risk groups [79] and changes in sexual behavior in response to the HIV pandemic [71],[78]. Although the potential confounding effects of changes in HIV-related risk behaviors have been widely acknowledged, only one report, from Vancouver [36], formally controls for them in a regression model (Table 3). By comparison, another study from British Columbia attributes large numbers of averted HIV infections among injection drug users (IDUs) to broader uptake of ART in the community, but some have suggested that the analysis underestimates the potential protective effects of other HIV prevention measures directed at the same community [80]. Indeed, the protective effects of Vancouver's safer injection sites have been documented in the past [81],[82]. Also, consistent ART adherence may be difficult to sustain in IDUs [83], further suggesting that factors beyond viral suppression may have contributed to the reduction in HIV incidence in this population.
The ability of ART to visibly reduce the number of newly diagnosed cases of HIV takes time, because most new diagnoses are made years after infection occurs, and many patients present with a reduced CD4 count, reflecting substantial progression of HIV disease. But in some ecological studies, the effect of ART is presumed to be almost immediate. In the report from British Columbia [33], where combination ART was introduced in 1996, the largest decrease in documented new HIV diagnoses took place between 1997 and 2000, but it is reasonable to question whether enough suppressive combination ART was immediately available to most patients to explain this decline.
Alternative Results and Other Considerations
The comparative lack of reports investigating the ecological effects of population-level ART in settings where rising incidence rates have been detected [84],[85] suggests potential publication bias. It is also noteworthy that ecological studies of ART for HIV prevention are almost exclusively from developed western settings, likely because of the limited availability of surveillance data, viral load measurements, or registry data in resource-constrained settings.
Stable or rising HIV incidence among certain population subgroups with ready access to ART suggests the possibility that identified relationships between ART access and declines in HIV diagnoses in the studies reviewed here may be overstated. For example, HIV incidence (estimated by STARHS) increased and then stabilized among voluntary testers in San Francisco between 1999 and 2006 [85], and model-estimated numbers of new HIV infections among men who have sex with men (MSM) in British Columbia increased by 13% from 2005 to 2008 [86]. In parts of Australia, the number of HIV diagnoses among MSM between 2000 and 2006 doubled, although cohort data suggest that this observation may be largely driven by new infections among older MSM [87],[88]. In Denmark [89] and the UK [90], incidence rates among MSM have reportedly increased. In Canada, some subgroups of IDUs have experienced rising HIV incidence, including Aboriginals [91], women [92], and youth [93], prompting a call for renewed prevention efforts [94].
More than 50 experimental studies of treatment as prevention are in some stage of development, and more can be anticipated [95],[96]. Policy-makers often do not have the luxury of waiting years for trial data, and all decisions take place under a certain degree of uncertainty. To this end, several studies, including some considered in this review, have successfully applied novel tools of geospatial mapping and phylogenetic analysis to aid interpretation of observational data. A study in the UK [97] used viral molecular phylogeny to determine the single most likely transmitter among MSM, allowing the investigators to account for the higher transmission probability of individuals with acute and early infection. Other studies, including those from San Francisco [34] and Washington, D. C. [39], used geospatial analysis to illustrate the spatial distribution of HIV-infected individuals in communities. Most recently, investigators at the Africa Centre for Health and Population Studies in South Africa have been able to identify a relationship between the density of ART use and HIV acquisition risk within a community by studying HIV incidence in a longitudinal cohort of more than 16,000 individuals (personal communication, F. Tanser). Additional strengths of this study include the use of information about the patients' primary residence and attempts to control for at least some possible confounders of the relationship between ART uptake and HIV incidence in the same community.
Several large cluster randomized controlled trials are being developed [98]. A team from the Harvard School of Public Health AIDS Initiative, working with partners in Botswana, will target individuals with sustained, high plasma viral loads for immediate treatment, a strategy that could have exponential public health benefits [73]. A second group from the London School of Hygiene & Tropical Medicine and Imperial College London plans to test the feasibility and impact of a universal test-and-treat strategy along with other combination prevention measures, including male circumcision [99]. But clinical trials have their own limitations, including time, cost, ethical challenges, and perturbations to the underlying community that can cause bias. And there is never a guarantee that approaches employed in a trial will prove effective outside of the trial setting.
Conclusions
Suppressive ART prevents HIV transmission in stable, monogamous, heterosexual couples. While ART seems to hold great promise as a public health tool, its population-level benefits have not been proven. Although ecological studies can play an important role in the development of new HIV prevention strategies, they are methodologically limited to building justification of further formal scientific inquiry into population-level effects of the potential policies in question. They are therefore the first of many steps in the path from science to policy, beginning with the establishment of biological plausibility, and progressing to assessment of an individual-level effect and then a group-level effect. Though most policy decisions must be made under conditions of uncertainty, the hypothesis that widespread ART can eliminate HIV infection [31],[100] may have raised expectations beyond what can actually be achieved. Additionally, implementation of treatment as prevention is not without its risks, including the rise of population-level drug resistance with the rapid uptake of ART in the face of continued limited infrastructure, and increased risk compensation by treated individuals who believe that treatment alone may justify forgoing other forms of protection [101]–[103].
Although we expect an impact of ART at the population level, the magnitude of the effect may not be as great as some hope; measuring the impact of ART roll-out on HIV spread, as in several planned cluster randomized controlled trials, therefore remains a critical step. Much as combination prevention methods are believed to be better than single interventions for HIV prevention [104], all the methods available to determine the benefits of prevention interventions, including ecological studies, should be deployed. The results must be weighed and used with a full understanding of the methods used to define the outcomes of treatment of HIV infection for prevention of transmission.
Key Points
-
Several strong observational studies and one randomized controlled trial, HPTN 052, demonstrate that ART reduces the transmission of HIV in stable, heterosexual HIV-discordant couples.
-
A number of ecological studies, which use observational data to examine relationships between exposures and outcomes at the level of groups instead of individuals, have found associations between the broader use of antiretroviral agents and reductions in new HIV diagnoses in at-risk populations or in the general population. Ecological studies may generate hypotheses that can be explored using other experimental or observational methods.
-
A better understanding of the strengths and limitations of the various exposure and outcome measures used in ecological studies that examine the population-level effects of ART on HIV transmission, and the methods used to analyze them, is essential for the effective application of the findings in policy-making processes.
-
Methodological challenges such as measurement error, selection bias, confounding, and assumptions about the time lag of effects must be taken into account when interpreting the results of these studies.
-
Prospective measurement of the population-level impact of ART can be approached through cluster randomized controlled trials, but the cost, time, and degree of difficulty of designing and conducting these studies are appreciable, and the potential for the intervention itself to introduce bias can threaten the validity of the results.
-
Measuring the population-level benefits of ART is critical to HIV prevention efforts, and consideration of results of all methods may be used to inform ongoing research and public health policy. A firm understanding of the strengths and weaknesses of each approach is crucial to the interpretation of results and allocation of resources.
Zdroje
1. RothmanKJGreenlandSLashTL 2008 Modern epidemiology Philadelphia Lippincott Williams & Wilkins
2. PiantadosiSByarDPGreenSB 1988 The ecological fallacy. Am J Epidemiol 127 893 904
3. HallettTBWhitePJGarnettGP 2007 Appropriate evaluation of HIV prevention interventions: from experiment to full-scale implementation. Sex Transm Infect 83 Suppl 1 i55 i60 doi:10.1136/sti.2006.023663
4. MorgensternH 1995 Ecologic studies in epidemiology: concepts, principles, and methods. Annu Rev Public Health 16 61 81 doi:10.1146/annurev.pu.16.050195.000425.bv
5. GreenlandSRobinsJ 1994 Invited commentary: ecologic studies—biases, misconceptions, and counterexamples. Am J Epidemiol 139 747 760
6. SchwartzS 1994 The fallacy of the ecological fallacy: the potential misuse of a concept and the consequences. Am J Public Health 84 819 824 doi:10.2105/AJPH.84.5.819
7. RobinsonW 1950 Ecological correlations and the behavior of individuals. Am Sociol Rev 15 351 357 doi:10.1093/ije/dyn357
8. DurrheimDOgunbanjoG 2004 Making sense of statistics for the family practitioner: “what are ecological studies?” S Afr Fam Pract 46 48
9. MosesSBradleyJENagelkerkeNJRonaldARNdinya-AcholaJO 1990 Geographical patterns of male circumcision practices in Africa: association with HIV seroprevalence. Int J Epidemiol 19 693 697
10. BongaartsJReiningPWayPConantF 1989 The relationship between male circumcision and HIV infection in African populations. AIDS 3 373 377
11. CaldwellJCCaldwellP 1996 The African AIDS epidemic. Sci Am 274 62-63, 66-68
12. WeissHAQuigleyMAHayesRJ 2000 Male circumcision and risk of HIV infection in sub-Saharan Africa: a systematic review and meta-analysis. AIDS 14 2361 2370
13. SiegfriedNMullerMDeeksJVolminkJEggerM 2005 HIV and male circumcision—a systematic review with assessment of the quality of studies. Lancet Infect Dis 5 165 173 doi:10.1016/S1473-3099(05)01309-5
14. BaileyRCPlummerFAMosesS 2001 Male circumcision and HIV prevention: current knowledge and future research directions. Lancet Infect Dis 1 223 231 doi:10.1016/S1473-3099(01)00117-7
15. GrayRHKigoziGSerwaddaDMakumbiFWatyaS 2007 Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 369 657 666 doi:10.1016/S0140-6736(07)60313-4
16. SiegfriedNMullerMDeeksJJVolminkJ 2009 Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev 2009 CD003362 doi:10.1002/14651858.CD003362.pub2
17. HenryKChinnockBJQuinnRPFletcherCVde MirandaP 1988 Concurrent zidovudine levels in semen and serum determined by radioimmunoassay in patients with AIDS or AIDS-related complex. JAMA 259 3023 3026
18. DumondJBYehRFPattersonKBCorbettAHJungBH 2007 Antiretroviral drug exposure in the female genital tract: implications for oral pre - and post-exposure prophylaxis. AIDS 21 1899 1907 doi:10.1097/QAD.0b013e328270385a
19. NicolMRKashubaADM 2010 Pharmacologic opportunities for HIV prevention. Clin Pharmacol Ther 88 598 609 doi:10.1038/clpt.2010.189
20. PereiraASKashubaADFiscusSAHallJETidwellRR 1999 Nucleoside analogues achieve high concentrations in seminal plasma: relationship between drug concentration and virus burden. J Infect Dis 180 2039 2043 doi:10.1086/315149
21. KashubaADDyerJRKramerLMRaaschRHEronJJ 1999 Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob Agents Chemother 43 1817 1826
22. MusiccoMLazzarinANicolosiAGaspariniMCostigliolaP 1994 Antiretroviral treatment of men infected with human immunodeficiency virus type 1 reduces the incidence of heterosexual transmission. Italian Study Group on HIV Heterosexual Transmission. Arch Intern Med 154 1971 1976
23. SiegfriedNvan der MerweLBrocklehurstPSintTT 2011 Antiretrovirals for reducing the risk of mother-to-child transmission of HIV infection. Cochrane Database Syst Rev 2011 CD003510 doi:10.1002/14651858.CD003510.pub3
24. CohenMSMcCauleyMGambleTR 2012 HIV treatment as prevention and HPTN 052. Curr Opin HIV AIDS 7 99 105 doi:10.1097/COH.0b013e32834f5cf2
25. DonnellDBaetenJMKiarieJThomasKKStevensW 2010 Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet 375 2092 2098 doi:10.1016/S0140-6736(10)60705-2
26. BunnellREkwaruJPSolbergPWamaiNBikaako-KajuraW 2006 Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS 20 85 92
27. SullivanPKayitenkoreKChombaEKaritaEMwananyandaL 2010 Reduction of HIV transmission risk while prescribed anti-retroviral therapy (ARVT): Misclassification of ARVT status as a methodological issue [abstract]. AIDS Vaccine 2010; 28 Sept–1 Oct 2010; Atlanta, Georgia, US. AIDS Res Hum Retroviruses 26 A-1 A-184 doi:10.1089/aid.2010.9998
28. Del RomeroJCastillaJHernandoVRodriguezCGarciaS 2010 Combined antiretroviral treatment and heterosexual transmission of HIV-1: cross sectional and prospective cohort study. BMJ 340 c2205 c2205 doi:10.1136/bmj.c2205
29. WangLGeZLuoJShanDGaoX 2010 HIV transmission risk among serodiscordant couples: a retrospective study of former plasma donors in Henan, China. J Acquir Immune Defic Syndr 55 232 238 doi:10.1097/QAI.0b013e3181e9b6b7
30. GayCLCohenMS 2008 Antiretrovirals to prevent HIV infection: pre - and postexposure prophylaxis. Curr Infect Dis Rep 10 323 331
31. GranichRMGilksCFDyeCDe CockKMWilliamsBG 2009 Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373 48 57 doi:10.1016/S0140-6736(08)61697-9
32. EatonJWJohnsonLFSalomonJABärnighausenTBendavidE 2012 HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 9 e1001245 doi:10.1371/journal.pmed.1001245
33. MontanerJSLimaVDBarriosRYipBWoodE 2010 Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 376 532 539 doi:10.1016/S0140-6736(10)60936-1
34. DasMChuPLSantosG-MScheerSVittinghoffE 2010 Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE 5 e11068 doi:10.1371/journal.pone.0011068
35. FangC-THsuH-MTwuS-JChenM-YChangY-Y 2004 Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis 190 879 885 doi:10.1086/422601
36. WoodEKerrTMarshallBDLLiKZhangR 2009 Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ 338 b1649 doi:10.1136/bmj.b1649
37. LawMGWoolleyITempletonDJRothNChuahJ 2011 Trends in detectable viral load by calendar year in the Australian HIV observational database. J Int AIDS Soc 14 10 doi:10.1186/1758-2652-14-10
38. PorcoTCMartinJNPage-ShaferKAChengACharleboisE 2004 Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS 18 81 88
39. CastelADBefusMWillisSGriffinAWestT 2011 Use of the community viral load as a population-based biomarker of HIV burden, Washington, DC. AIDS 26 345 353 doi:10.1097/QAD.0b013e32834de5fe
40. KatzMHSchwarczSKKelloggTAKlausnerJDDilleyJW 2002 Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health 92 388 394
41. CohenMSChenYQMcCauleyMGambleTHosseinipourMC 2011 Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 493 505 doi:10.1056/NEJMoa1105243
42. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention Conference Programme 2011 Fauci: looking to Rome, with optimism. Available: http://blog.ias2011.org/post/2011/07/10/Looking-to-Rome-with-Optimism.aspx. Accessed 26 December 2011
43. del RioC 2011 Treatment is prevention: the results of HPTN 052. Journal Watch. Available: http://aids-clinical-care.jwatch.org/cgi/content/full/2011/719/1. Accessed 25 November 2011
44. SquiresKEHughesJM 2011 [Letter to the President of the United States on behalf of the Infectious Diseases Society of America and the HIV Medicine Association.] Available: http://www.hivma.org/uploadedFiles/HIVMA/Policy_and_Advocacy/Policy_Priorities/Evidence_Based_Prevention/Comments/HPTN_052_Letter.pdf#search=%22squireshughes%22. Accessed 3 June 2012
45. MaughTH2nd 2011 May 13 Anti-HIV drugs prove highly effective in preventing transmission of virus. Los Angeles Times Available: http://articles.latimes.com/2011/may/13/health/la-he-aids-prevention-20110513. Accessed 29 May 2012
46. Panel on Antiretroviral Guidelines for Adults and Adolescents 2012 Guidelines for the use of antiretroviral agents in HIV-1 infected adults and adolescents. US Department of Health and Human Services Available: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed 29 May 2012
47. World Health Organization 2011 The strategic use of antiretrovirals for treatment and prevention of HIV Geneva World Health Organization Available: http://www.who.int/hiv/pub/meetingreports/consultation_20111116/en/index.html. Accessed 29 May 2012
48. EpsteinHMorrisM 2011 HPTN 052 and the future of HIV treatment and prevention. Lancet 378 225 doi:10.1016/S0140-6736(11)61117-3
49. CatesW 2011 HPTN 052 and the future of HIV treatment and prevention. Lancet 378 224 225 doi:10.1016/S0140-6736(11)61116-1
50. AnglemyerARutherfordGWEggerMSiegfriedN 2011 Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev 2011 CD009153 doi:10.1002/14651858.CD009153
51. SchwartländerBStoverJHallettTAtunRAvilaC 2011 Towards an improved investment approach for an effective response to HIV/AIDS. Lancet 377 2031 2041 doi:10.1016/S0140-6736(11)60702-2
52. GeffenN 2011 When to start antiretroviral therapy in adults: the results of HPTN 052 move us closer to a “test-and-treat” policy. South Afr J HIV Med 12 9 11 Available: http://sajhivmed.org.za/index.php/sajhivmed/article/view/733/586. Accessed 29 May 2012
53. MontanerJSG 2011 Treatment as prevention—a double hat-trick. Lancet 378 208 209 doi:10.1016/S0140-6736(11)60821-0
54. 2011 HIV treatment as prevention—it works. Lancet 377 1719 doi:10.1016/S0140-6736(11)60713-7
55. SealeALazarusJVGrubbIFakoyaAAtunR 2011 HPTN 052 and the future of HIV treatment and prevention. Lancet 378 226 225 doi:10.1016/S0140-6736(11)61118-5
56. LawMGPrestageGGrulichAVan de VenPKippaxS 2001 Modelling the effect of combination antiretroviral treatments on HIV incidence. AIDS 15 1287 1294
57. BlowerSBodineEKahnJMcFarlandW 2005 The antiretroviral rollout and drug-resistant HIV in Africa: insights from empirical data and theoretical models. AIDS 19 1 14
58. LimaVDJohnstonKHoggRSLevyARHarriganPR 2008 Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis 198 59 67 doi:10.1086/588673
59. Meyer-RathGOverM 2012 HIV treatment as prevention: modelling the cost of antiretroviral treatment—state of the art and future directions. PLoS Med 9 e1001247 doi:10.1371/journal.pmed.1001247
60. CohenMSGayCL 2010 Treatment to prevent transmission of HIV-1. Clin Infect Dis 50 S85 S95 doi:10.1086/651478
61. GardnerEMMcLeesMPSteinerJFdel RioCBurmanWJ 2011 The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 52 793 800 doi:10.1093/cid/ciq243
62. BurnsDNDieffenbachCWVermundSH 2010 Rethinking prevention of HIV type 1 infection. Clin Infect Dis 51 725 731 doi:10.1086/655889
63. US Centers for Disease Control and Prevention 2011 Nov 29 Only one quarter of Americans with HIV have virus under control. Available: http://www.cdc.gov/nchhstp/newsroom/WAD2011PressRelease.html. Accessed 29 May 2012
64. KatzITEssienTMarindaETGrayGEBangsbergDR 2011 Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS 25 2177 2181 doi:10.1097/QAD.0b013e32834b6464
65. QuinnTCWawerMJSewankamboNSerwaddaDLiC 2000 Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med 342 921 929 doi:10.1056/NEJM200003303421303
66. BaetenJMKahleELingappaJRCoombsRWDelany-MoretlweS 2011 Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med 3 77ra29 doi:10.1126/scitranslmed.3001888
67. PowersKAGhaniACMillerWCHoffmanIFPettiforAE 2011 The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 378 256 268 doi:10.1016/S0140-6736(11)60842-8
68. BrennerBGRogerMRoutyJPMoisiDNtemgwaM 2007 High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis 195 951 959 doi:10.1086/512088
69. Lopez-QuinteroCShtarkshallRNeumarkYD 2005 Barriers to HIV-testing among Hispanics in the United States: analysis of the National Health Interview Survey, 2000. AIDS Patient Care STDS 19 672 683 doi:10.1089/apc.2005.19.672
70. SpielbergFBransonBMGoldbaumGMLockhartDKurthA 2003 Overcoming barriers to HIV testing: preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr 32 318 327
71. SheltonJDCohenMBarnhartMHallettT 2010 Is antiretroviral therapy modifying the HIV epidemic? Lancet 376 1824 1825 doi:10.1016/S0140-6736(10)62163-0
72. WandHWilsonDYanPGonnermannAMcDonaldA 2009 Characterizing trends in HIV infection among men who have sex with men in Australia by birth cohorts: results from a modified back-projection method. J Int AIDS Soc 12 19 doi:10.1186/1758-2652-12-19
73. NovitskyVWangRBussmannHLockmanSBaumM 2010 HIV-1 subtype C-infected individuals maintaining high viral load as potential targets for the “test-and-treat” approach to reduce HIV transmission. PLoS ONE 5 e10148 doi:10.1371/journal.pone.0010148
74. WolbersMBabikerASabinCYoungJDorrucciM 2010 Pretreatment CD4 cell slope and progression to AIDS or death in HIV-infected patients initiating antiretroviral therapy—the CASCADE collaboration: a collaboration of 23 cohort studies. PLoS Med 7 e1000239 doi:10.1371/journal.pmed.1000239
75. JanssenRSSattenGAStramerSLRawalBDO'BrienTR 1998 New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 280 42 48
76. Le VuSPillonelJSemailleCBernillonPLe StratY 2008 Principles and uses of HIV incidence estimation from recent infection testing—a review. Euro Surveill 13 18969
77. BuschMPPilcherCDMastroTDKaldorJVercauterenG 2010 Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS 24 2763 2771 doi:10.1097/QAD.0b013e32833f1142
78. Joint United Nations Programme on HIV/AIDS 2010 Methods for estimating HIV incidence. Epi Alert, UNAIDS Quarterly Update on HIV Epidemiology Available: http://www.unaids.org/en/media/unaids/contentassets/documents/dataanalysis/epi_alert_2010Q1_en.pdf. Accessed 3 June 2012
79. SheltonJDHalperinDTWilsonD 2006 Has global HIV incidence peaked? Lancet 367 1120 1122 doi:10.1016/S0140-6736(06)68436-5
80. GrulichAEWilsonDP 2010 Is antiretroviral therapy modifying the HIV epidemic? Lancet 376 1824 doi:10.1016/S0140-6736(10)62162-9
81. KerrTSmallWBuchnerCZhangRLiK 2010 Syringe sharing and HIV incidence among injection drug users and increased access to sterile syringes. Am J Public Health 100 1449 1453 doi:10.2105/AJPH.2009.178467
82. StoltzJ-AWoodESmallWLiKTyndallM 2007 Changes in injecting practices associated with the use of a medically supervised safer injection facility. J Public Health (Oxf) 29 35 39 doi:10.1093/pubmed/fdl090
83. WoodEMontanerJSGYipBTyndallMWSchechterMT 2003 Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ 169 656 661
84. MurrayJMPrestageGGriersonJMiddletonMMcDonaldA 2011 Increasing HIV diagnoses in Australia among men who have sex with men correlated with the growing number not taking antiretroviral therapy. Sex Health 8 304 310 doi:10.1071/SH10114
85. TruongHMKelloggTKlausnerJDKatzMHDilleyJ 2006 Increases in sexually transmitted infections and sexual risk behavior without a concurrent increase in HIV incidence among men who have sex with men in San Francisco: a suggestion of HIV serosorting? Sex Transm Infect 82 461 466 doi:10.1136/sti.2006.019950
86. British Columbia Centre for Disease Control 2011 HIV and sexually transmitted infections 2010. Available: http://www.bccdc.ca/NR/rdonlyres/2035512C-DBEC-495B-A332-C410EE9520C7/0/CPS_Report_STI_HIV_2010_annual_report_FINAL_20111122.pdf. Accessed 29 May 2012
87. GuyRJMcDonaldAMBartlettMJMurrayJCGieleCM 2007 HIV diagnoses in Australia: diverging epidemics within a low-prevalence country. Med J Aust 187 437 440
88. GrulichAEKaldorJM 2008 Trends in HIV incidence in homosexual men in developed countries. Sex Health 5 113 118 doi:10.1071/SH07075
89. DukersNHSpaargarenJGeskusRBBeijnenJCoutinhoRA 2002 HIV incidence on the increase among homosexual men attending an Amsterdam sexually transmitted disease clinic: using a novel approach for detecting recent infections. AIDS 16 F19 F24
90. DouganSElfordJChadbornTRBrownAERoyK 2006 Does the recent increase in HIV diagnoses among men who have sex with men in the UK reflect a rise in HIV incidence or increased uptake of HIV testing? Sex Transm Infect 83 120 125 doi:10.1136/sti.2006.021428
91. DuncanKCReadingCBorweinAMMurrayMCPalmerA 2011 HIV incidence and prevalence among aboriginal peoples in Canada. AIDS Behav 15 214 227 doi:10.1007/s10461-010-9792-y
92. SpittalPMCraibKJPWoodELalibertéNLiK 2002 Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ 166 894 899
93. MillerCLWoodESpittalPMLiKFrankishJC 2004 The future face of coinfection: prevalence and incidence of HIV and hepatitis C virus coinfection among young injection drug users. J Acquir Immune Defic Syndr 36 743 749
94. BruneauJDanielMAbrahamowiczMZangGLamotheF 2011 Trends in human immunodeficiency virus incidence and risk behavior among injection drug users in montreal, Canada: a 16-year longitudinal study. Am J Epidemiol 173 1049 1058 doi:10.1093/aje/kwq479
95. GranichRGuptaSSutharABSmythCHoosD 2011 Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res 9 446 469
96. BoilyMCBuvéABaggaleyRF 2010 HIV transmission in serodiscordant heterosexual couples. BMJ 340 c2449 doi:10.1136/bmj.c2449
97. FisherMPaoDBrownAESudarshiDGillON 2010 Determinants of HIV-1 transmission in men who have sex with men: a combined clinical, epidemiological and phylogenetic approach. AIDS 24 1739 1747 doi:10.1097/QAD.0b013e32833ac9e6
98. BoilyMCMâsseBAlsallaqRPadianNSEatonJW 2012 HIV treatment as prevention: considerations in the design, conduct, and analysis of cluster randomized controlled trials of combination HIV prevention. PLoS Med 9 e1001250 doi:10.1371/journal.pmed.1001250
99. Imperial College London Faculty of Medicine 2011 About PopART. Available: http://www1.imperial.ac.uk/medicine/research/researchthemes/infection/infectious_diseases/hiv_trials/hiv_prevention_technologies/popart/about/. Accessed 3 June 2012
100. 2011 Jun 2 The end of AIDS? The Economist Available: http://www.economist.com/node/18774722. Accessed 29 May 2012
101. CrepazNLylesCMWolitskiRJPassinWFRamaSM 2006 Do prevention interventions reduce HIV risk behaviors among people living with HIV? A meta-analytic review of controlled trials. AIDS 20 143 157 doi:10.1097/01.aids.0000196166.48518.a0
102. HasseBLedergerberBHirschelBVernazzaPGlassTR 2010 Frequency and determinants of unprotected sex among HIV-infected persons: the Swiss HIV Cohort Study. Clin Infect Dis 51 1314 1322 doi:10.1086/656809
103. CohenMS 2010 HIV treatment as prevention and “the Swiss statement”: in for a dime, in for a dollar? Clin Infect Dis 51 1323 1324 doi:10.1086/656810
104. CoatesTJRichterLCaceresC 2008 Behavioral strategies to reduce HIV transmission: how to make them work better. Lancet 372 669 684 doi:10.1016/S0140-6736(08)60886-7
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



