-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
Introduction:
Improving maternal and newborn health in low-income settings requires both health service and community action. Previous community initiatives have been predominantly rural, but India is urbanizing. While working to improve health service quality, we tested an intervention in which urban slum-dweller women's groups worked to improve local perinatal health.Methods and Findings:
A cluster randomized controlled trial in 24 intervention and 24 control settlements covered a population of 283,000. In each intervention cluster, a facilitator supported women's groups through an action learning cycle in which they discussed perinatal experiences, improved their knowledge, and took local action. We monitored births, stillbirths, and neonatal deaths, and interviewed mothers at 6 weeks postpartum. The primary outcomes described perinatal care, maternal morbidity, and extended perinatal mortality. The analysis included 18,197 births over 3 years from 2006 to 2009. We found no differences between trial arms in uptake of antenatal care, reported work, rest, and diet in later pregnancy, institutional delivery, early and exclusive breastfeeding, or care-seeking. The stillbirth rate was non-significantly lower in the intervention arm (odds ratio 0.86, 95% CI 0.60–1.22), and the neonatal mortality rate higher (1.48, 1.06–2.08). The extended perinatal mortality rate did not differ between arms (1.19, 0.90–1.57). We have no evidence that these differences could be explained by the intervention.Conclusions:
Facilitating urban community groups was feasible, and there was evidence of behaviour change, but we did not see population-level effects on health care or mortality. In cities with multiple sources of health care, but inequitable access to services, community mobilization should be integrated with attempts to deliver services for the poorest and most vulnerable, and with initiatives to improve quality of care in both public and private sectors.Trial registration:
Current Controlled Trials ISRCTN96256793
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001257
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001257Summary
Introduction:
Improving maternal and newborn health in low-income settings requires both health service and community action. Previous community initiatives have been predominantly rural, but India is urbanizing. While working to improve health service quality, we tested an intervention in which urban slum-dweller women's groups worked to improve local perinatal health.Methods and Findings:
A cluster randomized controlled trial in 24 intervention and 24 control settlements covered a population of 283,000. In each intervention cluster, a facilitator supported women's groups through an action learning cycle in which they discussed perinatal experiences, improved their knowledge, and took local action. We monitored births, stillbirths, and neonatal deaths, and interviewed mothers at 6 weeks postpartum. The primary outcomes described perinatal care, maternal morbidity, and extended perinatal mortality. The analysis included 18,197 births over 3 years from 2006 to 2009. We found no differences between trial arms in uptake of antenatal care, reported work, rest, and diet in later pregnancy, institutional delivery, early and exclusive breastfeeding, or care-seeking. The stillbirth rate was non-significantly lower in the intervention arm (odds ratio 0.86, 95% CI 0.60–1.22), and the neonatal mortality rate higher (1.48, 1.06–2.08). The extended perinatal mortality rate did not differ between arms (1.19, 0.90–1.57). We have no evidence that these differences could be explained by the intervention.Conclusions:
Facilitating urban community groups was feasible, and there was evidence of behaviour change, but we did not see population-level effects on health care or mortality. In cities with multiple sources of health care, but inequitable access to services, community mobilization should be integrated with attempts to deliver services for the poorest and most vulnerable, and with initiatives to improve quality of care in both public and private sectors.Trial registration:
Current Controlled Trials ISRCTN96256793
: Please see later in the article for the Editors' SummaryIntroduction
The current consensus on improving maternal and newborn survival [1]–[3], particularly in low-income countries, is that perinatal care needs to involve both health service strengthening and community activities [4]–[6]. Recent advances include India's Janani Suraksha Yojana cash incentive for professional obstetric care [7], the development of home-based newborn care as a result of work in rural India [8]–[11], and the success of community mobilization around perinatal issues through women's groups [12],[13]. The balance of supply - and demand-side interventions remains uncertain: community mobilization seems likely to yield greater benefits when mortality rates are high, and a focus on health service quality when they are lower.
Community mobilization in this context has been almost exclusively rural, but urban health is a developing concern [14]. Currently, 30% of India's population is urban and 50 cities have populations of over 1 million [15]. Rural health inequalities are replicated in urban settings [16]–[19], with some important differences. Cities offer many potential sources of health care at a range of prices, and urban health care in India is provided by a burgeoning, largely unregulated private sector and a beleaguered public sector that caters mainly to poorer groups. In this context, both quality of care and choice of an appropriate provider are problematic [20],[21]. Access tends not to be limited by distance and scarcity, but by direct and indirect costs: urban life and available time are intensely monetized and 80% of health care expenditure is out of pocket [22]. Environmental determinants of health such as housing fabric, water, sanitation, and sewage dominate community perceptions of health needs in vulnerable urban communities. Socio-cultural heterogeneity hinders collective action, while an overburdened urban infrastructure struggles to meet growing demand.
Mumbai's City Initiative for Newborn Health began in 2004. A partnership between the Society for Nutrition, Education and Health Action (SNEHA), a non-government organisation (NGO) committed to improving the health of women and children in Mumbai's slums, the Municipal Corporation of Greater Mumbai (MCGM), and University College London aimed to improve maternal and newborn health in slum communities. Planning of India's National Urban Health Mission, analogue of the developing National Rural Health Mission, was underway. An Urban Social Health Activist (USHA) would work in vulnerable communities, but what she would do was undecided. The City Initiative was conceived as operationalizing a combination of supply - and demand-side interventions in the urban context. It combined improvement in municipal health care provision—reinstitution of antenatal clinics at health posts catering to the urban poor, clinical protocols for maternal and newborn care, improved communication between institutions, and consolidation of referral linkages—with community mobilization activities [23].
The community activities took as their model south Asian projects that have worked successfully with groups of rural women to improve perinatal health in their own communities, in a test of their transferability to urban slum populations [12],[13],[24]. The trial was a piece of operational research within the City Initiative. Our objective was to test an intervention in which slum-dweller women's groups discussed perinatal health, improved their knowledge through peer-learning, and developed and implemented local strategies. We examined a range of outcomes, including stillbirth and neonatal mortality rates and antenatal, intrapartum, and postpartum care (Text S1).
Methods
Ethics Statement
The activities of the City Initiative for Newborn Health were approved by MCGM Joint Municipal Commissioners in 2004. Given our interest in operational research at the interface between NGO activities and public health evaluation, the idea of a trial was in our minds. However, we were aware of no previous cluster randomised controlled trial (cRCT) involving urban slums, and there were serious doubts about whether it would be possible. Additionally, little was known about maternal and child health in Mumbai slums. For these reasons, in 2005, we set up a perinatal vital registration system in 48 slum communities as part of the City Initiative. The number was chosen because it was large enough to model scalability and would be sufficient if we proceeded to a trial. The surveillance was successful and it became clear that there was scope for achieving a cRCT design. Funding for a trial was received in 2007. Ethical approval was granted in the same year by the Independent Ethics Committee for Research on Human Subjects (Mumbai, reference IEC/06/31), the trial was registered (ISRCTN96256793), and its protocol was published in 2008 (Text S2) [25]. Cluster-level verbal consent for the study was given after community meetings with general practitioners, community-based organizations, NGOs, municipal representatives, political officers of major parties, and social workers.
Setting and Design
The capital of Maharashtra state, Mumbai has a provisional 2011 census population of 12.4 million, more than half of whom live in slums. About one-fifth of slum homes have a private toilet, 31% of residents have completed 10 y of education, and the total fertility rate is below the replacement threshold at 1.9 [26]. Public sector care is provided by the Municipal Corporation. Private health care is widely available and ranges from specialty hospitals to informal practitioners. A cRCT design was chosen because a group intervention was delivered in communities. Key participants were women who joined groups in 24 intervention clusters, to be compared with 24 control clusters. We implemented the trial in six municipal wards. Clusters included had at least 1,000 households, residents were aware of no plans for resettlement, and cluster separation was wide enough to minimise contamination. We excluded areas with transient communities—large construction gangs, pavement dwellings—and areas for which resettlement was being negotiated.
Intervention
All the human resources involved in community activities were employed by SNEHA. We recruited one full-time facilitator in each intervention cluster of about 1,000 households. This sakhi (friend) was a local woman with secondary education and leadership skills, preferably married with children. Her role was to conduct meetings with women, attend planning and supervision meetings, and support group action. After training, she began by profiling her settlement and building rapport with local stakeholders. She also attended regular training on a range of health care topics. Over about 6 mo, she set up ten women's groups, formative work having shown that women's mobility tended to be confined to their own alley. The groups met fortnightly and she met weekly with other sakhis and her supervisor. The intervention followed a 36-meeting cycle that was predetermined in general but developed iteratively in detail (Figure 1). There was no set point at which women had to join a group, and women of all ages, pregnant and non-pregnant, were welcome to participate. We took a participatory approach with an emphasis on sharing and peer-learning, rather than on the sakhi as an expert resource, and used the change methodology of Appreciative Inquiry to focus on the positive and to build energy for action through identification of the strengths of participants, their families, and neighbourhoods [27]. Appreciative approaches have gained traction as an alternative to problem-focused approaches [28],[29], although empirical evaluation in terms of health outcomes has been limited. Each step was simulated in sakhis' weekly meetings and supported by supervisors. The emphasis was on knowing what services were available, choosing appropriate perinatal health care, understanding best practice, and negotiating optimal care with family and providers.
Fig. 1. The intervention cycle. 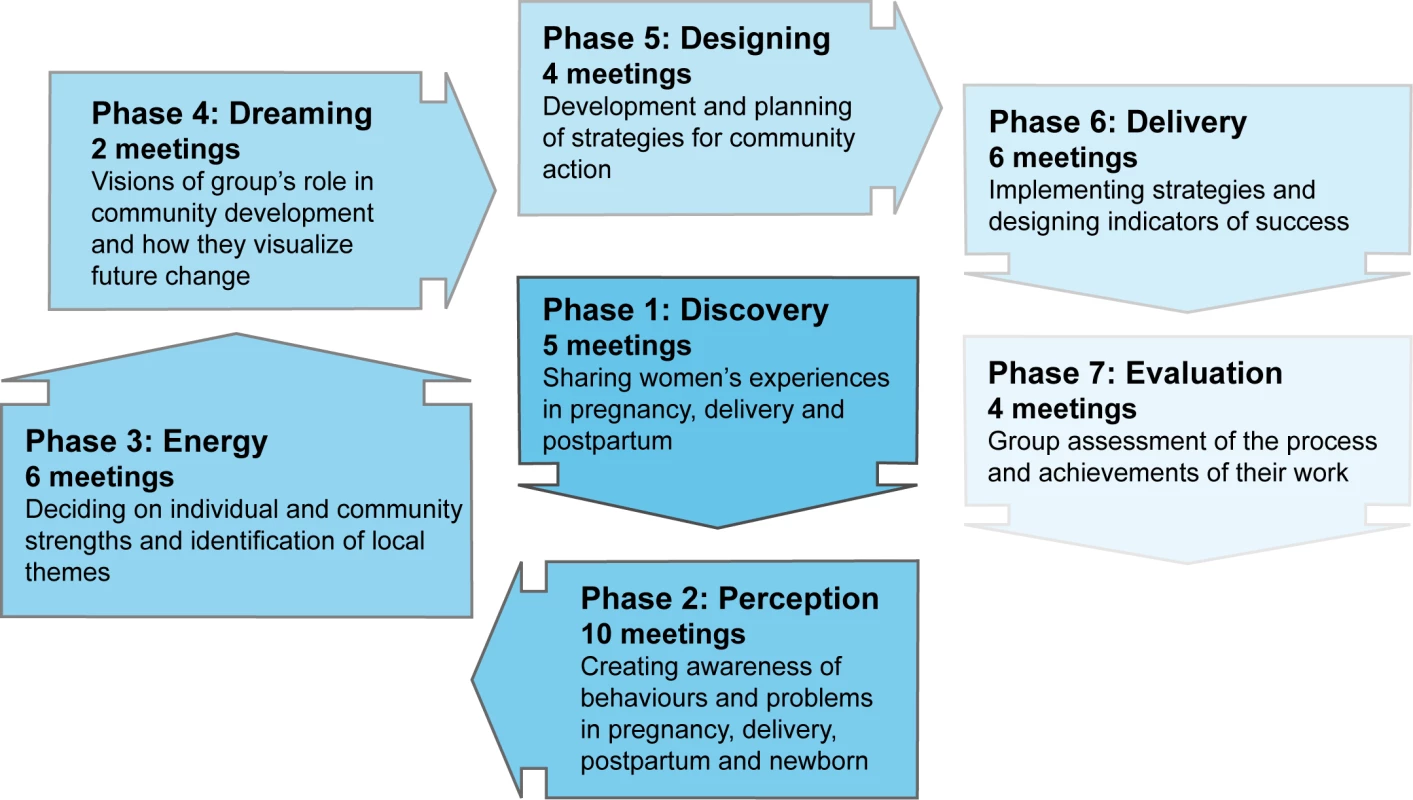
Outcomes
Routine and emergency antenatal, intrapartum, and postnatal care was documented, as well as illness in mother or baby. Previous trials in rural settings had shown no effects on stillbirth rates. In an urban setting with high levels of institutional delivery, we decided to present both stillbirth (death of an infant before or during delivery, per thousand births) and neonatal mortality (death of a live-born infant within the first 28 d of life, per thousand live births) rates.
Sample Size and Allocation
Using data collected in the pre-trial year, with a two-tailed 5% significance level, a conservative coefficient of variation between clusters (k) value of 0.3, and the recruitment numbers achieved, the trial had 80% power to detect a reduction in stillbirths from 12 to 7 per thousand, in neonatal deaths from 20 to 12 per thousand, and in extended perinatal mortality from 29 to 20 per thousand [30]. Through municipal documents, surveys, discussions with key informants, and site visits, we identified 92 slum clusters. In a transparent process, social workers external to the trial drew lots to select 48 in blocks of eight per ward, and then to allocate four clusters per block to the intervention. We chose this method because of our emphasis on participation and demystification of research. The nature of the intervention precluded allocation concealment.
Data Collection and Management
Sakhis recorded details of each meeting they facilitated, quantitative elements of which were entered into an electronic database. Supervisors recorded their observations of meetings and training and feedback sessions. A process evaluation officer attended and documented weekly meetings and interviewed sakhis, group members and non-members, and local stakeholders. We were able to draw on attendance and content data for 5,253 women's group meetings, 120 meetings observed by supervisors, summaries of 150 valuation meetings, ten focus group discussions, seven sets of questionnaires, seven role-play exercises, and interviews with 39 local stakeholders. Group attendance was documented by sakhis and supplemented with interview data collected by the process evaluation officer. We used the attendance data to identify group members in all 24 intervention clusters who had attended at least 15 meetings, and whose groups had reached the latter stages of the meeting cycle. We asked these women what they had done in terms of outreach, and subsequently categorized their responses.
The trial covered an estimated population of 283,000. The community-based vital surveillance system across the 48 clusters was based on previous models [12],[31]. Live births, stillbirths, neonatal deaths, and maternal deaths were identified by 99 locally resident women, each covering an average 600 households and receiving INR 50 (US$1.11) per identification. Every event was confirmed by one of 12 interviewers, who took verbal consent for a comprehensive interview at around 6 wk after delivery (Text S3). In the event of a death, one of six supervisors conducted verbal autopsy. Because of a fall in documented births later in the trial, we did a retrospective census of births and deaths in all 48 clusters. After the trial finished, we gave lists of all documented births and deaths in the preceding year to the field investigators, and to fieldworkers from other SNEHA projects. These agents checked house to house for any births or deaths that might not have been identified. The process identified 41 previously undocumented births, but no stillbirths or neonatal deaths.
We had an ethical responsibility to recommend that unwell mothers or infants visit a health facility, and to expedite care in emergencies. Records of events and completed questionnaires were subject to systematic and random checks for accuracy and completeness, both in the field and during entry into an electronic database (Access; Microsoft Corporation). Information provided by participants remained confidential and outputs did not include their names.
A steering group of external experts met in the first and second years and reviewed the design, intervention and recruitment. A Data Monitoring Committee met in April 2009, looked at data from the first 2 y, and recommended stopping the intervention after its cycles were complete and finishing the data collection as planned. Data collection continued until mid-2010 in order to pick up outcomes from all births to end-September 2009. It included a retrospective census to ensure data completeness. A further meeting in March 2011 considered the analyses and discussed presentation and publication.
Statistical Methods
The analysis used records of individual births and was by intention to treat at cluster level. Analysts were blind to allocation. For potential effects on health care, morbidity, and mortality, we did multivariable logistic regression with random effects grouped on cluster, comparing outcomes in intervention and control arms and adjusting for covariates by including them in the models. Quadrature checks confirmed the applicability of this approach. The Data Monitoring Committee recommended adjustment for socioeconomic status and for Muslim faith. Socioeconomic status was described by a household asset score based on standardised weights for the first component of a principal components analysis [32],[33]. This adjustment made no appreciable difference to the findings, and we present unadjusted odds ratios and 95% confidence intervals. There were differences in the mortality findings after adjustment, and we present unadjusted models, along with models adjusted for cluster mean household asset score at baseline, cluster proportion of Muslim respondents at baseline, and baseline cluster mortality rate [34].
The primary analysis compared records from intervention and control arms collected over 3 y. We did four pre-specified ancillary analyses using identical methods on successively filtered datasets: a comparison of outcomes in the latter 2 y of the trial, a comparison that included only women who had lived in a cluster for at least a year, a comparison of outcomes stratified by socioeconomic status, and a comparison of women who had been group members with women in intervention clusters who had not been members. Analyses were done in Stata 11. Causes of perinatal death based on verbal autopsies were assigned independently by two physicians, using an international classification [35]. Discordance was resolved by a pediatrician (DO).
Results
Process
The conceptual premise of the model was that facilitation of women's groups would lead to changes in members' knowledge and behaviour, with diffusion of influence to other local women. When the project began, women expressed a need for information on health and health care. Although slum women's groups (Mahila mandals) and action groups existed in some clusters, they tended to focus on single issues on an ad hoc basis. Sakhis formed 244 groups, a median ten per cluster. Groups were based in individual alleys because members were often reluctant to circulate, and the small size of homes restricted attendance. Other considerations included women's need to do piecework and to care for their families. A mean five women attended each meeting (range 2–20) and individual women attended 15 meetings each (range 1–50).
Group members were enthusiastic about acquiring new knowledge. They made substantial efforts to reach out to other local women: a sample of 235 members helped 1,372 other distinct women so that, on average, one woman reached out to six others, particularly by providing them with information on antenatal and newborn care, suggesting that they visit health providers, and occasionally accompanying them. Achieving collective action was, however, more challenging. Key activities undertaken by groups were attempts to create local awareness, extending support to other women, negotiating with civic authorities for amenities, and mobilizing and sharing resources. One group succeeded in getting the municipal corporation to cover local sewage channels, but efforts were generally more individual than collective and involved women's own families and neighbourhoods. Their desire for knowledge was tempered by time pressure and immediate concerns such as insecurity of tenure, which limited their inclination to get involved in wider action. There was attrition in the numbers of groups and membership in the later phases that attempted to broker collective strategizing. Membership was informal and withdrawal easy when participants felt that the commitment required would be onerous. 150 groups were sustained to the end of the cycle and member numbers dropped from an initial 2,948 to 656.
Recruitment
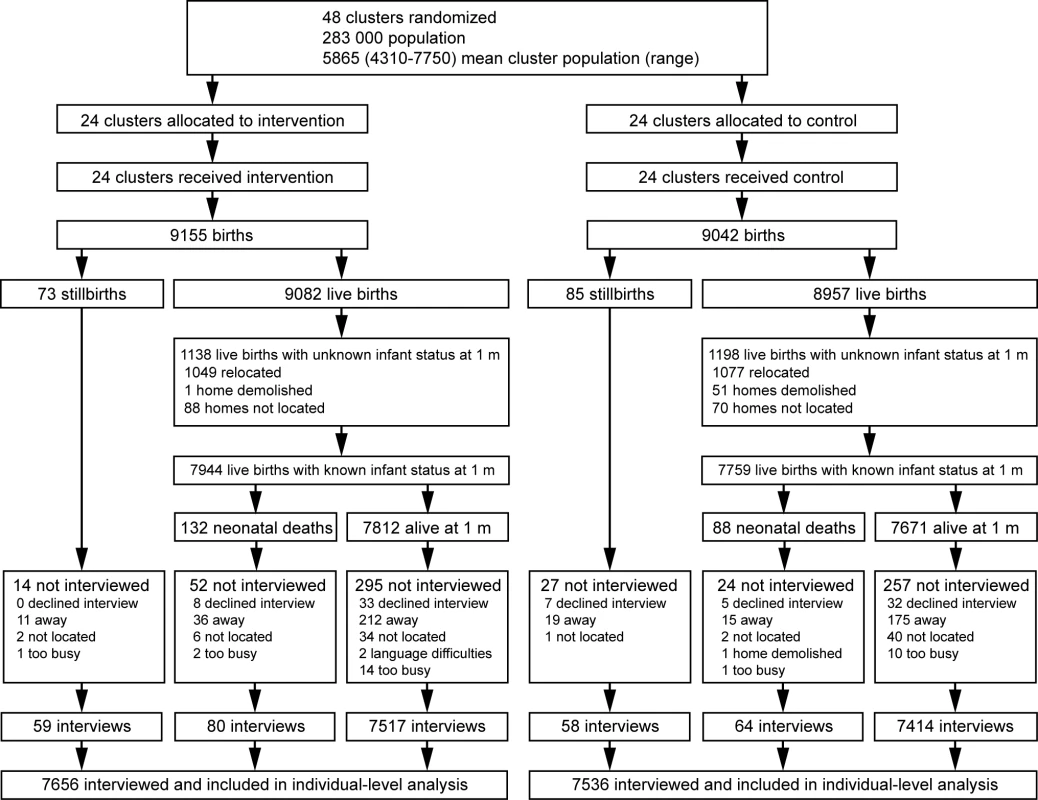
The trial ran from 1st October 2006 to 30th September 2009. We designated the pre-trial year of data collection a baseline period, leaving 3 complete years for the evaluation of effect. Figure 2 shows the trial profile.
Baseline Comparison
Table 1 summarizes cluster size and participant characteristics. There were insufficient births in nine clusters and we expanded their perimeters for subsequent years. Two clusters were reduced because of excess births. Numbers of households, population, and births were similar in intervention and control arms. The crude birth rate was 23 per 1,000. Mean maternal age was 24.2 y (standard deviation [SD] 4.05) in the intervention arm and 24.6 y (SD 4.33) in the control arm. A higher proportion of households in the control arm followed Islam (58%, compared with 33%). Control clusters had slightly more of the poorest quintile of women than intervention clusters (21% compared with 20%), but also of the least poor (23% compared with 17%). Health care practices were similar in intervention and control arms (unpublished data). Baseline stillbirth rates were 12.5 per thousand live births in both arms (42/3,347 in intervention and 42/3,338 in control). Neonatal mortality rates were 22.3 and 18.6 (63/2,845 in intervention and 50/2,712 in control).
Tab. 1. Cluster size and characteristics of women interviewed at 6 wk postpartum, comparing allocation groups in the 3 trial years. 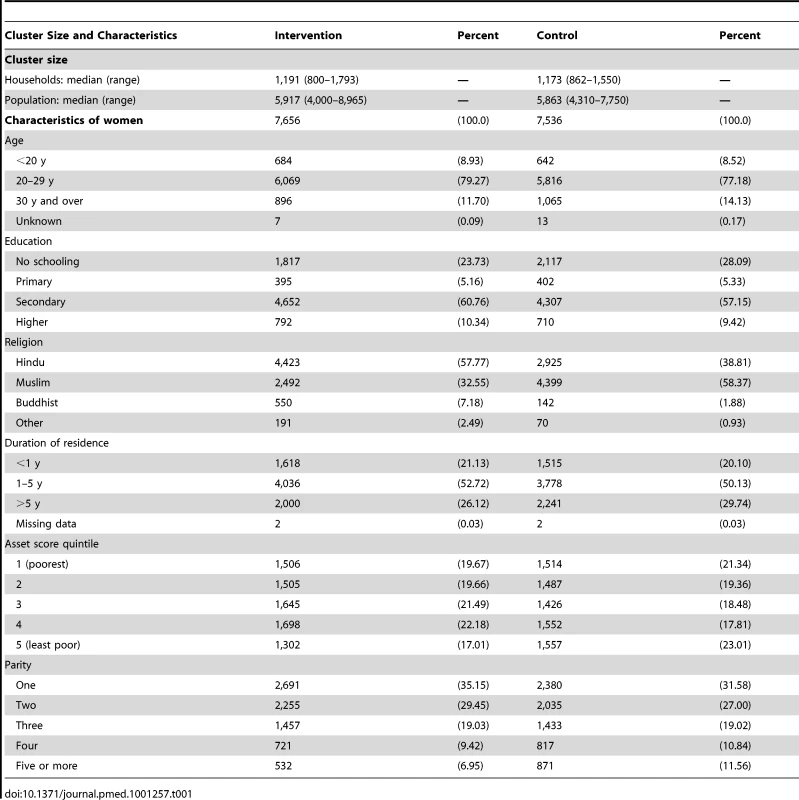
Impact Evaluation
The intention-to-treat analysis included 18,197 births over 3 y, a median 379 births per cluster (interquartile range [IQR] 263–480). For analysis of neonatal mortality rates, the vital status at 1 mo of 15,703 live-born infants was known. Analysis of other outcomes was based on detailed interviews with 15,192 women. We achieved interviews after 84% of births in the intervention and 83% in the control arm. Table 2 presents the findings for behavioural and morbidity outcomes, and Figure 3 time-series examples. We found no differences between intervention and control arms in uptake of antenatal care, reported work, rest and diet in later pregnancy, institutional delivery, early and exclusive breastfeeding, or care-seeking for maternal or neonatal problems. The occurrence of serious antenatal symptoms (premature rupture of membranes, antepartum hemorrhage, cessation of fetal movements, or maternal seizures) was less common in the intervention arm. Table 3 presents the mortality findings. The stillbirth rate was lower in the intervention arm, although this finding was only significant after adjustment for religion, socioeconomic status, and baseline cluster stillbirth rate (adjusted odds ratio 0.66, 95% CI 0.46–0.93). The neonatal mortality rate was higher in the intervention arm, but non-significantly after similar adjustment (1.42, 0.99–2.04). A combination of both these outcomes, the extended perinatal mortality rate, did not differ between arms (1.01, 0.78–1.31); Figure 4 shows an annual boxplot. There were 20 maternal deaths in the intervention and 24 in the control group, a combined maternal mortality ratio of 244 per 100,000 live births. We are aware of no harms associated with the intervention.
Fig. 3. Perinatal care practices, percentage in each allocation group, by trial year. 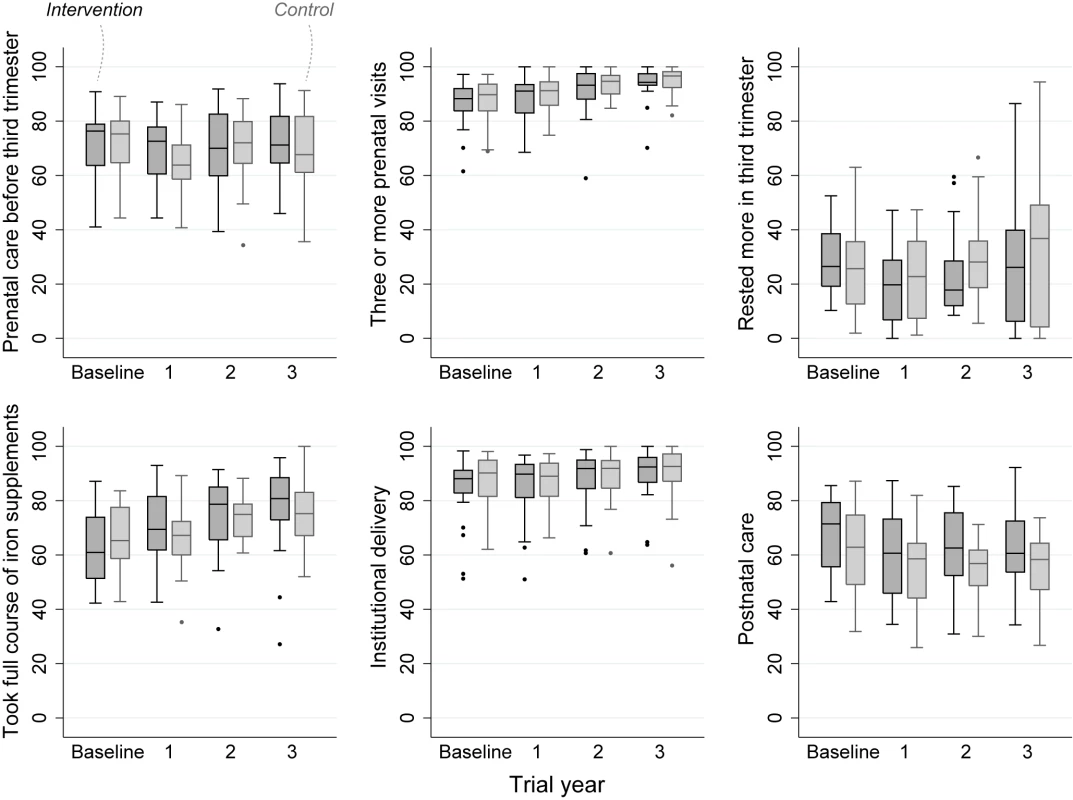
Fig. 4. Extended perinatal mortality rate in each allocation group, by trial year. 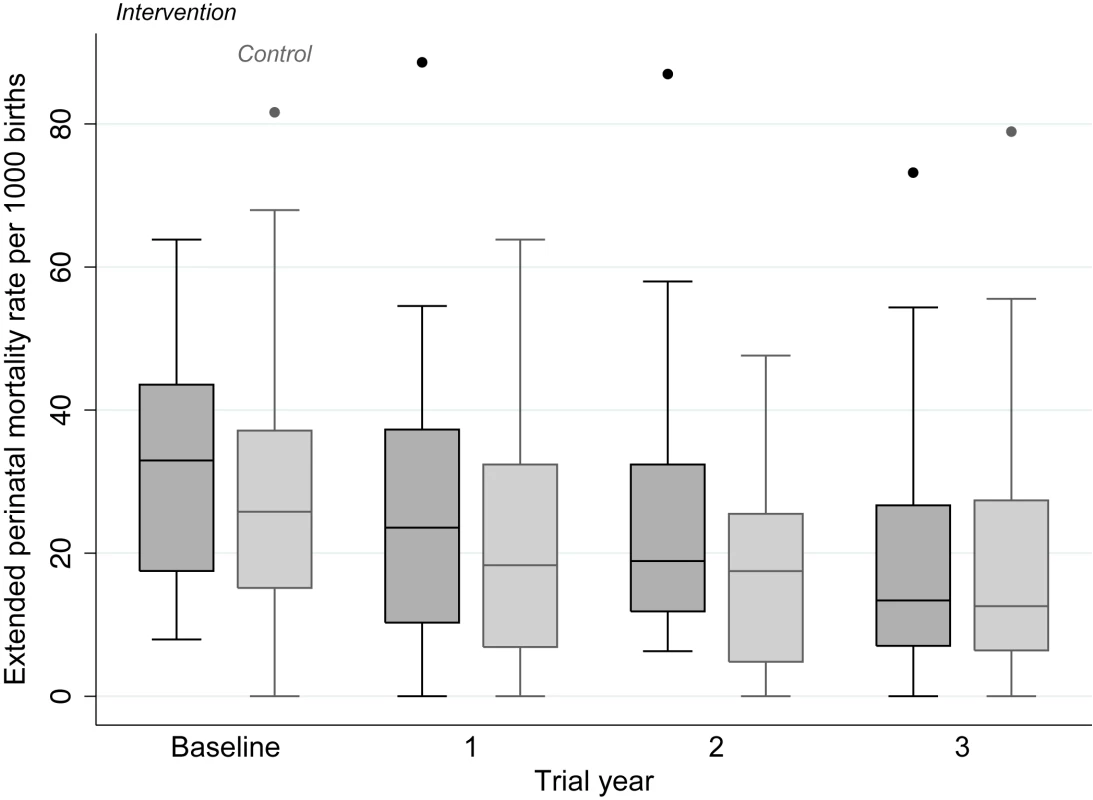
Tab. 2. Primary analysis of health care and morbidity outcomes over 3 y, comparing intervention and control arms. 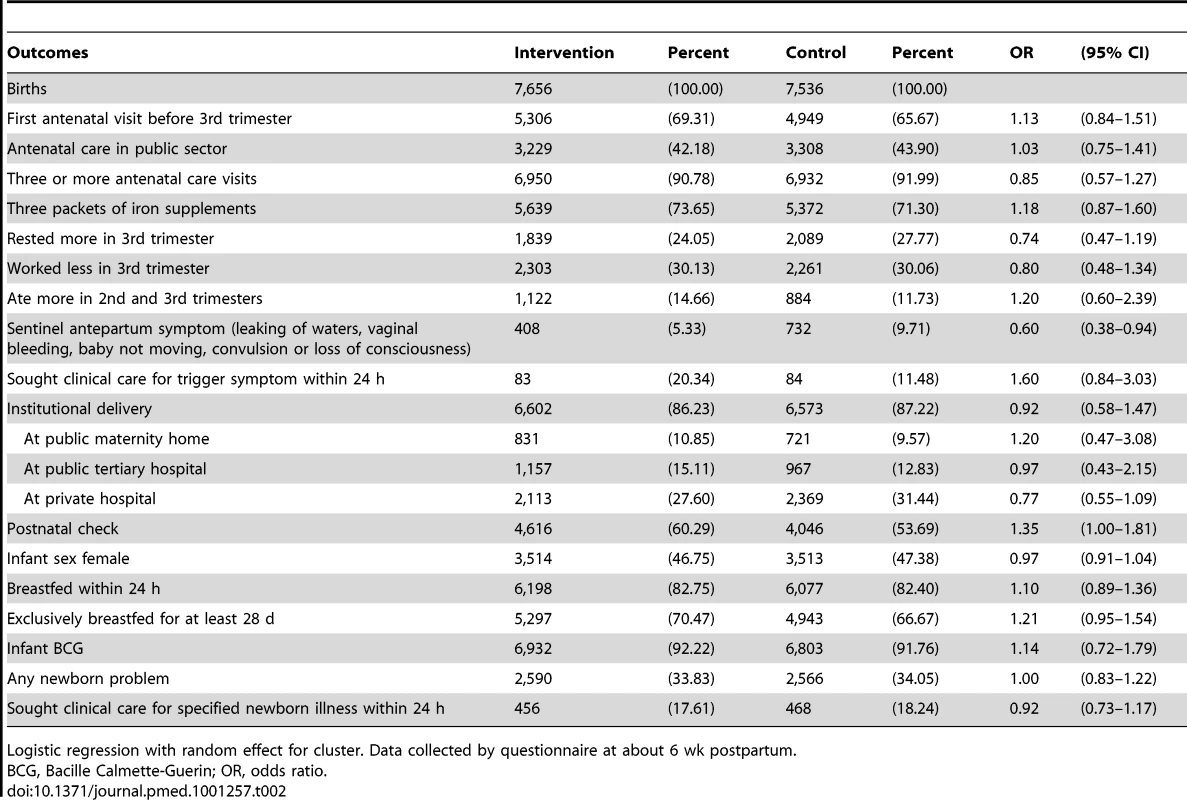
Logistic regression with random effect for cluster. Data collected by questionnaire at about 6 wk postpartum. Tab. 3. Primary analysis of mortality outcomes over 3 y, comparing intervention and control arms. 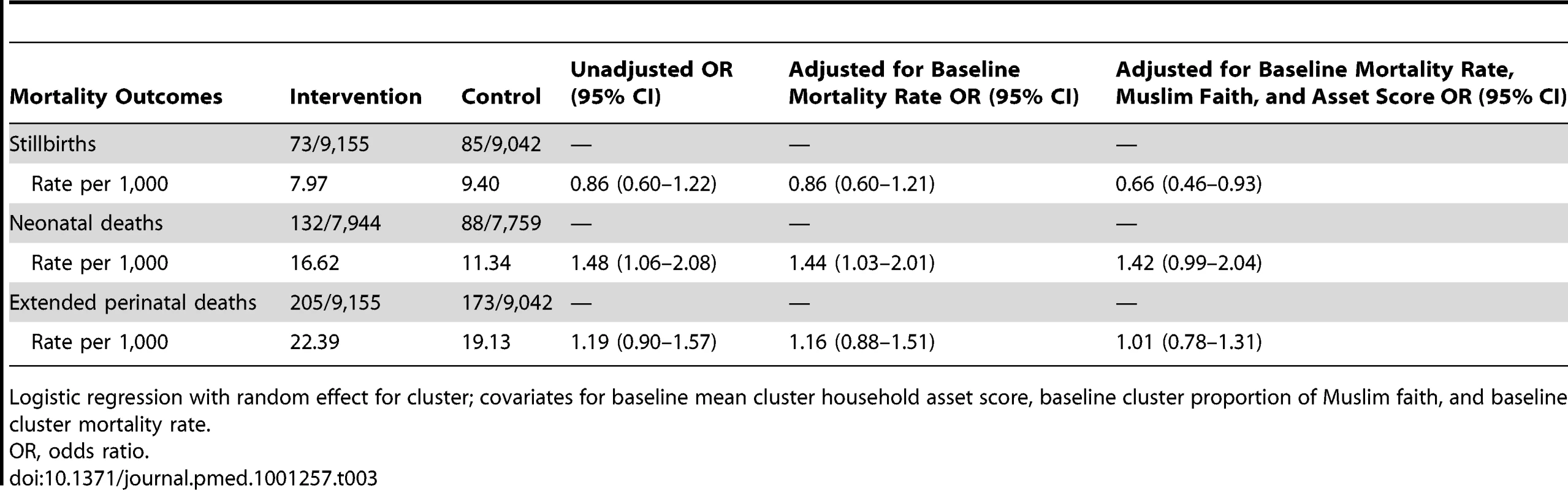
Logistic regression with random effect for cluster; covariates for baseline mean cluster household asset score, baseline cluster proportion of Muslim faith, and baseline cluster mortality rate. The findings did not differ substantially when we repeated the analysis to include only women who had lived in the trial clusters for at least a year, when we limited it to the last 2 y of the trial, or when we stratified by socioeconomic status (unpublished data). When we compared a sample of 191 group members with 10,053 non-members in the intervention arm, we found that they had a higher proportion of antenatal care in the public sector (odds ratio 1.52, 95% CI 1.06–2.20), that they were likelier to have rested more in the third trimester of pregnancy (1.74, 1.13–2.67), and that they were likelier to have had a postnatal check-up (1.58, 1.06–2.35). Verbal autopsies were available for 112 stillbirths and 145 neonatal deaths in the 3 y of the trial. The major causes of neonatal mortality were prematurity (40; 27%), intrapartum-related death (33; 23%), infection (33; 23%), and congenital anomalies (10; 7%). There were no obvious differences between allocation groups in the distribution of these causes.
Discussion
In a cRCT across 48 urban slum communities of Mumbai, it was possible to implement women's groups in challenging conditions. We did not, however, see substantial effects on health care. Women in intervention clusters reported fewer sentinel antenatal morbidities, but we lack a conceptual basis to explain this. The lower stillbirth rate and higher neonatal mortality rate in the intervention arm have, we think, three possible explanations. The first is chance, or residual confounding: it is possible that intervention and control groups differed systematically in ways unaccounted for by our analysis. Second, the intervention may have reduced stillbirths by encouraging timely care-seeking. It is conceivable that some of these infants did not survive subsequently, shifting the balance of mortality from stillbirth to neonatal death. If this was the case, we might have expected to see fewer fresh stillbirths and more early neonatal asphyxial deaths in the intervention arm, a finding not supported by the verbal autopsy data (unpublished data). Third, the data collection may have itself led to improvements in both trial arms. Given that it consisted of birth identification and an interview at 6 wk postpartum, we think this unlikely.
Municipal figures for the same period confirm a reduction in mortality across the city. The positive changes seen in both intervention and control groups are intriguing. We considered the possibility that external initiatives might have affected intervention and control groups, differentially or equally. Significant municipal initiatives in the trial period included some improvement in outreach services by community health volunteers, birth registration and pulse polio campaigns, and infectious disease surveillance. We have cluster-specific information on concurrent non-governmental initiatives. Two NGOs were working on general health in some clusters. We do not think that specific initiatives explain our findings. More likely, in our opinion, was a general improvement in environmental conditions accompanied by behaviour change. Conditions in slum areas improved manifestly over the trial period. Gutters were covered, sanitation block coverage increased, housing fabric became more durable, and there was widespread electricity supply. We think that health-related culture change is a natural accompaniment, all the more because of aspiration and the notions of modernity of Mumbai's residents.
The surveillance and intervention teams were separate. Each of the six municipal wards had a surveillance supervisor and two investigators, responsible for data collection in all eight clusters: a mix of intervention and control. The procedures were identical in intervention and control clusters, and the supervisors and investigators saw their work as unconcerned with the intervention. It is conceivable that in intervention areas sakhis could have told identifiers about births and deaths. We have discussed this with the field and data management teams, and we do not think that this happened. As local residents, the birth and death identifiers were aware that there was an intervention in their community, but were focused on their task and did not dwell on the comparative nature of the trial.
The trial demonstrated the value of a counterfactual control group and the potential weakness of ecological evaluation, the commonest example being a before-after comparison. If we had based our assessment on the trends in the intervention arm seen in Figures 3 and 4 (a design used by many programs), it would have appeared an unqualified success [36]. A fall in documented births in the third year of the trial was partly explained by demolition of some settlements, and by difficulties in follow-up. This illustrates a key limitation of urban initiatives: population mobility and the fact that, the poorer the target group, the more transient are their homes. Public health trials would benefit from census data and better registries. Good registration would certainly help when outcome numbers are small and a single missed stillbirth has a substantial effect on a rate per thousand. Our subsequent trial will use censuses rather than prospective ascertainment.
We think that the trial raises three general issues: coverage, target group, and the emergent pattern of health care in cities in the South. Achieving sufficient intervention coverage has been a challenge in other settings [6],[24]. In a situation of space and time constraint, with a lack of social cohesion despite high population density, we did not manage to trigger diffusion of innovation. Using population estimates for Mumbai slum areas from the most recent National Family Health Survey (4.7 members per household; women aged 15–49 constituting 26.7% of the population) [26], our women's groups at their peak involved 8%, and at their nadir 2%, of women of reproductive age (although, as mentioned earlier, outreach communication may have multiplied these figures by up to six times).
Convening community groups was feasible and learning and behaviour change possible, but achieving the impetus necessary for wider change was challenging: group members helped others individually but balked at collective strategizing. There was attrition in group numbers over the course of the intervention, suggesting that women stopped attending when they felt that they had either learned enough or were required to invest more time and energy. Collective action was clearly a big step for groups to take, possible challenges being time-poverty and restrictions on movement, concerns about tenure, lack of confidence, and perhaps a lack of conviction in perinatal health as a major issue. Our target group were slum dwellers, but not exclusively the poorest among them. In a city in which more than half of the population live in slums, slum households themselves encompass a spectrum of socioeconomic realities [16],[37]. For potential replicability, the model included municipal wards with a range of infant mortality rates and, although restricted to slum populations, the intervention may not have succeeded in mobilizing the poorest and most at risk, who tend to be hardest to reach. Although socioeconomic status was not associated with differences in trial outcomes between intervention and control arms, our other research in the same population has demonstrated associations between dimensions of vulnerability and maternal and newborn health risks [38] and we have described inequities in access to routine maternal health services [16],[39] and morbidity care [40]. On the basis of these findings, our strategy has changed: subsequent interventions will target the most vulnerable families and we will intensify our efforts to improve quality of care in private and public facilities.
The third issue was the complexity of urban health care. Antenatal care was the norm and the nadir for institutional delivery in trial clusters was 75%. Around 57% of antenatal care and 30% of deliveries were in the private sector (this in a slum-dwelling population). Open access to private providers, and to institutions at all levels of the public sector hierarchy, is a challenge to systematic health care delivery. Our findings confirmed the tendency to bypass public maternity homes, which should handle uncomplicated deliveries, in favour of tertiary institutions. Women's group discussions included clarification of appropriate sites of consultation and considerations of price and quality, reflected in higher use of public sector services by group members. The study underlined the need to work on quality of care in both public and private sectors. Although regulatory insufficiencies make intervention difficult, quality control in the private sector needs to feature more in debates about health care in low-income countries.
The wider implications of our findings include a tipping of the balance of perinatal intervention in cities toward improvement in service quality, with an emphasis on intrapartum vigilance and resuscitation. Indeed, there have been concerns about the utility of cash incentives for institutional delivery in the urban context, the argument being that access is not the primary issue. Our question was not whether women's groups were beneficial to their members. Members valued the groups and their opportunities for peer learning, showed behaviour change, and helped other women in their communities. Exchange of knowledge about health and health services, rights, social networks, and increased confidence are public goods, although there are challenges in quantifying such outcomes in public health terms. Rather, the question was about the added value of women's groups—over and above activities to improve health care quality—in terms of measurable changes in perinatal health at population level. While acknowledging the possibility that others might be able to achieve this through more intensive community activities in higher mortality settings, our own programme did not show effect. Community groups will feature in our subsequent interventions, as they must in any participatory initiative. We will, however, attempt to integrate them more strongly with pro-poorest targeting, service provision at household level, strengthening of links between communities and service providers, and partnerships with public and private sector providers to improve quality of care.
Supporting Information
Zdroje
1. FilippiVRonsmansCCampbellOMGrahamWJMillsA 2006 Maternal health in poor countries: the broader context and a call for action. Lancet 368 1535 1541
2. JohnsBSigurbjornsdottirKFogstadHZupanJMathaiM 2007 Estimated global resources needed to attain universal coverage of maternal and newborn health services. Bull World Health Organ 85 256 263
3. PrataNSreenivasAVahidniaFPottsM 2009 Saving maternal lives in resource-poor settings: facing reality. Health Policy 89 131 148
4. BhuttaZAChopraMAxelsonHBermanPBoermaT 2010 Countdown to 2015 decade report (2000–10): taking stock of maternal, newborn, and child survival. Lancet 375 2032 2044
5. NairNTripathyPProstACostelloAOsrinD 2010 Improving newborn survival in low-income countries: community-based approaches and lessons from South Asia. PLoS Med 7 e1000246 doi:10.1371/journal.pmed.1000246
6. OsrinDProstA 2010 Perinatal interventions and survival in resource-poor settings: which work, which don't, which have the jury out? Arch Dis Child 95 1039 1046
7. LimSSDandonaLHoisingtonJAJamesSLHoganMC 2010 India's Janani Suraksha Yojana, a conditional cash transfer programme to increase births in health facilities: an impact evaluation. Lancet 375 2009 2023
8. BangATBangRABaituleSBReddyMHDeshmukhMD 1999 Effect of home-based neonatal care and management of sepsis on neonatal mortality: field trial in rural India. Lancet 354 1955 1961
9. BangATReddyHMDeshmukhMDBaituleSBBangRA 2005 Neonatal and infant mortality in the ten years (1993 to 2003) of the Gadchiroli field trial: effect of home-based neonatal care. J Perinatol 25 S92 107
10. WHO UNICEF 2009 WHO/UNICEF Joint Statement: home visits for the newborn child: a strategy to improve survival. WHO/FCH/CAH/09.02 Geneva and New York World Health Organization and United Nations Children's Fund
11. GoudarSSDhadedSMMcClureEMDermanRJPatilVD 2011 ENC training reduces perinatal mortality in Karnataka, India. J Matern Fetal Neonatal Med 27 27
12. ManandharDSOsrinDShresthaBPMeskoNMorrisonJ 2004 Effect of a participatory intervention with women's groups on birth outcomes in Nepal: cluster randomized controlled trial. Lancet 364 970 979
13. TripathyPNairNBarnettSMahapatraRBorghiJ 2010 Effect of a participatory intervention with women's groups on birth outcomes and maternal depression in Jharkhand and Orissa, India: a cluster-randomised controlled trial. Lancet 375 1182 1192
14. PaulVKSachdevHSMavalankarDRamachandranPSankarMJ 2011 Reproductive health, and child health and nutrition in India: meeting the challenge. Lancet 377 332 349
15. HPEC 2011 Report on Indian urban infrastructure and services New Delhi High Powered Expert Committee (HPEC) for estimating the investment requirements for urban infrastructure services
16. Shah MoreNBapatUDasSBarnettSCostelloA 2009 Inequalities in maternity care and newborn outcomes: one-year surveillance of births in vulnerable slum communities in Mumbai. Int J Equity Health 8 21
17. BaruRAcharyaAAcharyaSShiva KumarAKNagarajK 2010 Inequities in access to health services in India: caste, class and region. Economic & Political Weekly 45 49 58
18. MatthewsZChannonANealSOsrinDMadiseN 2010 Examining the “urban advantage” in maternal health care in developing countries. PLoS Med 7 e1000327 doi:10.1371/journal.pmed.1000327
19. AgarwalS 2011 The state of urban health in India; comparing the poorest quartile to the rest of the urban population in selected states and cities. Environment & Urbanization 23 13 28
20. DasJHammerJ 2007 Money for nothing: the dire straits of medical practice in Delhi, India. J Dev Econ 83 1 36
21. Skordis-WorrallJPaceNBapatUDasSMoreNS 2011 Maternal and neonatal health expenditure in Mumbai slums (India): a cross sectional study. BMC Public Health 11 150
22. Shiva KumarAKChenLCChoudhuryMGanjuSMahajanV 2011 Financing health care for all: challenges and opportunities. Lancet 377 668 679
23. FernandezAOsrinD 2006 The city initiative for newborn health. PLoS Med 3 e339 doi:310.1371/journal.pmed.0030339
24. AzadKBarnettSBanerjeeBShahaSKhanKS 2010 Effect of scaling up women's groups on birth outcomes in three rural districts in Bangladesh: a cluster-randomised controlled trial. Lancet 375 1193 1202
25. MoreNSBapatUDasSPatilSPorelM 2008 Cluster-randomised controlled trial of community mobilisation in Mumbai slums to improve care during pregnancy, delivery, postpartum and for the newborn. Trials 9 7
26. IIPS and Macro International 2008 National Family Health Survey (NFHS-3), India, 2005–06: Maharashtra Mumbai International Institute for Population Sciences and Macro International
27. CooperriderDLSrivastavaS 1987 Appreciative inquiry in organizational life. Pasmore W, Woodman R, editors. Research in organization change and development Greenwich (Connecticut) JAI Press 129 169
28. Trosten-BloomAWhitneyD 1999 Appreciative Inquiry: the path to positive change. Key MK, editor. Managing change in healthcare: innovative solutions for people-based organizations New York McGraw-Hill Companies 113 128
29. RuheMCBobiakSNLitakerDCarterCAWuL 2011 Appreciative Inquiry for quality improvement in primary care practices. Qual Manag Health Care 20 37 48
30. HayesRBennettS 1999 Simple sample size calculation for cluster-randomized trials. Int J Epidemiol 28 319 326
31. BarnettSNairNTripathyPBorghiJRathS 2008 A prospective key informant surveillance system to measure maternal mortality - findings from indigenous populations in Jharkhand and Orissa. BMC Pregnancy Childbirth 8 6
32. FilmerDPritchettL 2001 Estimating wealth effects without expenditure data - or tears: an application to educational enrollments in states of India. Demography 38 115 132
33. VyasSKumaranayakeL 2006 Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan 21 459 468
34. HayesRMoultonL 2009 Cluster randomised trials Boca Raton (Florida) Chapman & Hall/CRC
35. WinboIGSereniusFHDahlquistGGKallenBA 1998 NICE, a new cause of death classification for stillbirths and neonatal deaths. Neonatal and intrauterine death classification according to etiology. Int J Epidemiol 27 499 504
36. RonsmansCVannesteAMChakrabortyJvan GinnekenJ 1997 Decline in maternal mortality in Matlab, Bangladesh: a cautionary tale. Lancet 350 1810 1814
37. AgarwalSTanejaS 2005 All slums are not equal: child health conditions among the urban poor. Indian Pediatrics 42 233 244
38. OsrinDDasSBapatUAlcockGAJoshiW 2011 A rapid assessment scorecard to identify informal settlements at higher maternal and child health risk in Mumbai. J Urban Health Epub ahead of print
39. Shah MoreNAlcockGBapatUDasSJoshiW 2009 Tracing pathways from antenatal to delivery care for women in Mumbai, India: cross-sectional study of maternity in low-income areas. International Health 1 71 77
40. Shah MoreNAlcockGDasSBapatUJoshiW 2010 Spoilt for choice? Cross-sectional study of care-seeking for health problems during pregnancy in Mumbai slums. Glob Public Health 6 746 759
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání