-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Lessons from Agriculture for the Sustainable Management of Malaria Vectors
article has not abstract
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001262
Category: Policy Forum
doi: https://doi.org/10.1371/journal.pmed.1001262Summary
article has not abstract
Summary Points
-
The effectiveness of insecticide-treated bed nets and indoor insecticide sprays to control adult mosquito vectors is being threatened by the spread of insecticide resistance.
-
We argue for expanding beyond “insecticide monotherapy” to more sustainable integrated vector management strategies that use optimal suites of control tactics.
-
Experience in agriculture suggests that such integrated approaches can provide more effective and durable pest management.
-
This shift will require increased investment in research and translational science.
-
Failure to act risks a resurgence of malaria and erosion of community support and donor commitment.
Vector Control and the Emerging Insecticide Resistance Crisis
The 2011 World Malaria Report [1] showed welcome progress in the fight against the world's most important vector-borne disease. In the last 10 years, the estimated incidence of malaria has fallen by 17% globally, with malaria-specific mortality rates reduced by 25%. Central to these gains, especially in Africa, has been the massive scale-up of chemical insecticide interventions against malaria mosquito vectors. Current malaria vector control relies almost exclusively on killing adult mosquitoes with chemical insecticides deployed as either insecticide-treated nets (ITNs) or indoor residual sprays (IRS). However, these technologies use a limited arsenal of insecticides originally developed for agriculture, and their efficacy is threatened by the spread of insecticide resistance [1]–[3]. In 2010, 27 countries in sub-Saharan Africa reported mosquitoes resistant to pyrethroids [1]. Such resistance is alarming because pyrethroids are the only class of insecticides approved for use on ITNs and account for two-thirds of the total product (by area) used in IRS for malaria control [4]. Evidence suggests that resistance is beginning to reduce control [5],[6]. Implementation of alternative management strategies is needed to slow and reverse this trend.
Parallels with Agriculture
In the middle of the last century, the development of cheap and effective synthetic chemical insecticides revolutionized crop protection. Widespread use of broad-spectrum insecticides reduced pest damage substantially in many systems, prompting discussion of pest eradication, similar to some current discussions of eradication ofmalaria. However, rapid evolution of insecticide resistance, pest resurgence due to disruption of biological control, and harmful environmental side effects quickly revealed the limitations of “pesticide monotherapy” [7]–[9].
The search to find new chemical insecticides continued, stimulated by the transient efficacy of products in use and increased restrictions on available insecticides because of their toxicity to people and other non-target organisms. Meanwhile, academic and government researchers explored ways to reduce reliance on insecticides. In crop systems where insecticide use was actually exacerbating pest problems, researchers combined diverse tools such as pest monitoring and forecasting, conservation of natural pest control, habitat manipulation, and resistant host plants, and thereby limited pesticide use to situations where it was necessary [10]. This approach, called integrated pest management (IPM) [10], reduces the risk of insecticide resistance. IPM is knowledge intensive, relying heavily on farmers' understanding and monitoring of local conditions. Its development therefore engendered a culture of farmer participation and decision-making, providing a balance to the former top-down, technology-driven approach. While not a panacea, IPM is now a cornerstone of many production systems in both developed and developing countries [10]–[14]. Even new technologies, such as genetically engineered crops, can be more effective and sustainable when used with other tactics in IPM [12],[15].
Current malaria vector control has more in common with the agricultural practices of the 1950s than contemporary IPM (Figure 1). There is a reliance locally on single technologies associated with fast-acting insecticides used in ways that impose intense selection pressure for resistance.
Fig. 1. Features of current vector control strategies compared with potential integrated vector management (IVM). 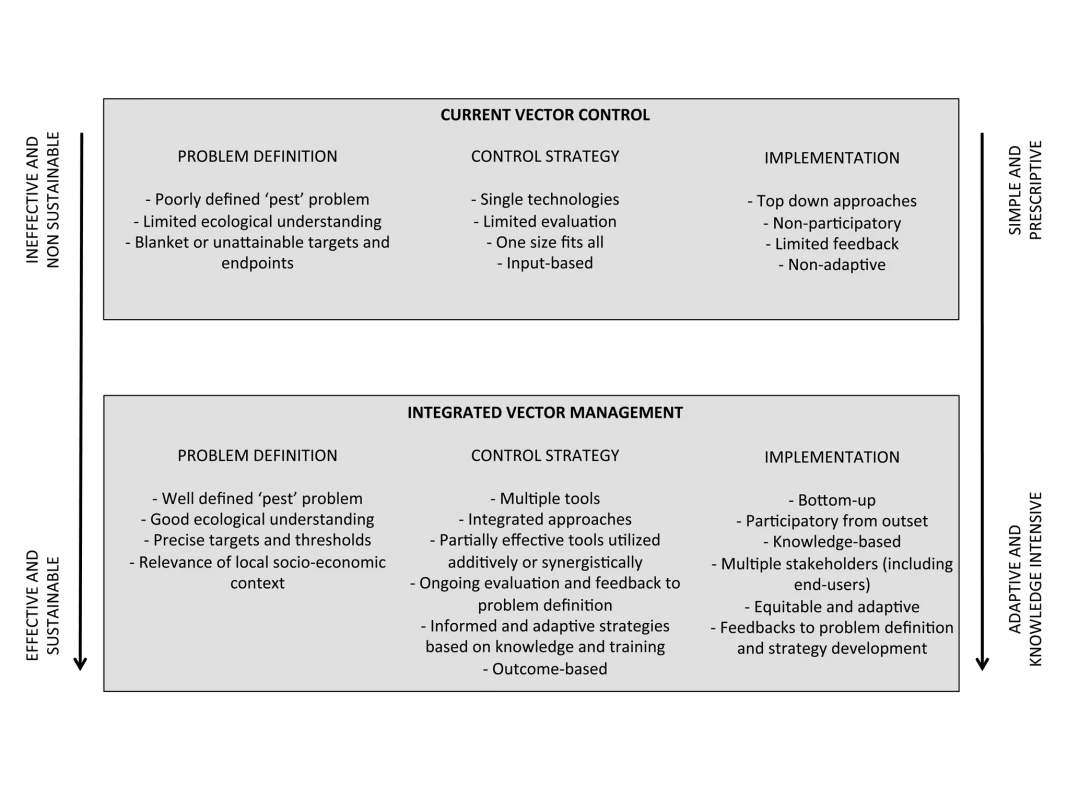
The arrows indicate trends representative of the contrasting strategies. Progression towards IVM has the potential to increase the effectiveness and sustainabilty of control, but requires more diverse and knowledge-intensive approaches. The pending resistance crisis creates an urgent need to develop and implement integrated, multi-tactic strategies for vector control that parallel IPM in agriculture. We call this “integrated vector management” (IVM), which we define as the optimal use of diverse tools, tactics, and resources to reduce transmission of disease by vectors. The potential of IVM has been discussed previously (e.g., [16],[17]), and tacit recognition of the approach already exists in World Health Organization (WHO) policy [18],[19]. The transition to more sustainable IVM will, however, require increased efforts in several key areas.
Quantifying the Problem
One of the foundations of IPM and thus IVM is to quantify the “pest” or “vector” problem and define the targets for control. For malaria this might seem straightforward—“control mosquitoes and reduce disease as much as possible”. Yet, it is surprising how little is understood about how local vector ecology contributes to infection. A typical list of unknowns could include the temporal and spatial distribution of biting, rate of parasite development, local variation in vector competence, sites where mosquitoes rest, the causes and rate of adult mosquito mortality, the nature of density-dependent regulation, and sometimes even which vector species is most important [20],[21]. Equally little is understood regarding the impact of insecticide resistance on vectorial capacity and malaria epidemiology [22],[23]. These unknown factors influence the approaches and strategies required to reduce malaria transmission in a particular setting. For example, while a 30% reduction in infectious bites might substantially reduce disease prevalence in a low transmission environment, even a 90% reduction might not be sufficient in a high transmission environment [24]. Effective IVM requires a better understanding of local vector and transmission ecology with appropriate targets for control defined in ways analogous to economic thresholds of pest density used widely to guide pest control decisions in agriculture.
Conventional Chemicals
Highly lethal insecticides like pyrethroids knock down and kill mosquitoes rapidly after contact. This lethality can provide excellent disease control, yet it also selects intensely for resistance. Development of replacement insecticides is one recognized strategy to address this problem [25]. However, the insecticide target product profiles prescribed by the WHO Pesticide Evaluation Scheme (WHOPES) set a high bar with respect to rapid killing, high persistence, and low mammalian toxicity. This, together with protracted regulatory procedures, means new insecticides are still many years off [3]. Moreover, novel chemistry will not prevent resistance evolution [26]. Resistance management strategies used in agriculture such as insecticide combinations and rotations require two or more insecticides with diverse modes of action to avoid cross-resistance [27], yet this diversity is not commonly available for vector control [28]. This problem is compounded when the same insecticide active ingredients are used in both agriculture and vector control [29],[30]. In the only controlled trial of resistance management strategies for malaria mosquito vectors we know of, rotations or mosaics did not delay pyrethroid resistance [26],[31].
In addition, ITNs and IRS only target mosquitoes inside domestic dwellings, leaving potentially significant fractions of the vector community untouched. While outdoor biting tends to be less epidemiologically important than indoor biting, it still contributes to transmission [32],[33]. Thus, even in the absence of resistance, it is unlikely that ITNs and IRS will be sufficiently effective to meet the goal of long-term malaria suppression in intense transmission settings.
Additional Tools
Current vector control relies on killing mosquitoes quickly with neurotoxins. However, more subtle approaches, such as slow-acting insecticides that shorten adult mosquito longevity, could also reduce transmission while imposing less intense selection for resistance [24],[34]. Alternative modes of action that impair olfaction, flight, energy metabolism, or immunity could further contribute to reduced vectorial capacity (e.g., see [35]). Such “sub-lethal insecticides” would represent genuinely new additions to the mosquito control tool kit that extend beyond the current fast-acting insecticide paradigm [36].
In addition, chemical insecticides that act against the adult vectors are not the only available tools. Physical barriers such as house screens [37], habitat management to reduce vector breeding site quality [38], microbial larvicides [39], and manipulation of nectar sources [40] could contribute to reduced disease transmission. Other tools in development such as fungal biopesticides [41], odor-baited traps [42], manipulation or release of parasites [43], and genetically modified [44],[45] or transinfected mosquitoes [46] could add to the list.
Individually, many of these technologies face today the same constraints that alternatives to insecticides faced in crop protection: marketing and regulatory systems for new products favored broad spectrum, fast-acting, lethal insecticides that provided stand alone, albeit unsustainable, solutions to pest problems. Against this model, subtler alternative methods cannot compete, except in an IPM/IVM context, where the benefit comes from the sum of the parts. It is important that regulatory frameworks are amenable to IVM to encourage research and development (R&D) and prevent barriers to ultimate commercialization.
Integrated Strategies and Sustainable Implementation
Developing effective IVM will require better understanding of the impact of control tactics individually and in various combinations [39],[47]–[49]. Again, there is surprisingly little relevant research. Yet, different combinations of tools could deliver the same end points with strategies optimized over time and space.
Development of IVM will also require substantial money and effort. It has been estimated that effective delivery of ITN or IRS measures will require 40%–61% of projected national malaria control program budgets [50]. This is in sharp contrast to the 4% of the global malaria R&D budget that is currently spent on vector control [51]. Given the historic and contemporary significance of vector control in reducing malaria [52], this level of funding is inadequate. Experience from agriculture suggests that with appropriate engagement and education, even complex knowledge-intensive practices can be successfully implemented. Extensive IPM programs in many developing countries indicate that such strategies are best developed and implemented via bottom-up approaches engaging end users from the outset in research and development [53],[54]. Embracing this philosophy can bolster vector control and move it away from top-down prescriptions towards adaptive, surveillance-, and evidence-based strategies that vary in space and time depending on local conditions. As with IPM, IVM can be best advanced by engaging the end users and working in partnerships to generate shared knowledge and solutions relevant to the local context. This strategy is necessary not only to develop effective solutions, but also to avert the risks of donor and community fatigue. There is no “quick fix” for sustainable vector control, or for eradication of malaria.
Conclusions
Ensuring continued advance in malaria control requires rethinking how we manage vector populations. Current strategies rely heavily on repeated application of single neurotoxic insecticides that quickly kill adult mosquitoes. This narrow paradigm is beginning to fail, as it did in agriculture, as well as in previous malaria eradication campaigns of the '50s and '60s. We should not abandon ITNs and IRS; these can be useful in IVM just as insecticides are in IPM. But experience with IPM in agriculture suggests that integrated approaches have the potential to provide more effective and durable pest management. To achieve the equivalent for malaria control requires additional tools in the armory, a better understanding of the impact of individual tools and their interactions, appropriate training for end users, and design of novel integrated strategies that maximize impact and fit the local ecological and socioeconomic context. Given the current lack of any clear alternative to the current insecticide paradigm, researchers, policy makers, and funding agencies need to act now to support this more diverse and adaptive ap proach. It is unlikely that any single tactic or combination of tactics will provide a permanent solution. Vector control programs must proactively and continuously innovate to optimize and sustain impact.
Zdroje
1. WHO 2011 World malaria report 2011 Geneva World Health Organization
2. YewhalawDWassieFSteurbautWSpanoghePVan BortelW 2011 Multiple insecticide resistance: an impediment to insecticide-based malaria vector control program. PLoS ONE 6 e16066 doi:10.1371/journal.pone.0016066
3. RansonHN'GuessanRLInesJMoirouxNNkuniZ 2011 Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol 27 91 98
4. Van den BergHZaimMYadavRSSoaresAAmeneshewaB 2012 Global trends in the use of insecticides to control vector-borne diseases. Environ Health Perspect 120 577 582
5. ChandaEHemingwayJKleinschmidtIRehmanAMRamdeenV 2011 Insecticide resistance and the future of malaria control in Zambia. PLoS ONE 6 e24336 doi:10.1371/journal.pone.0024336
6. TrapeJFTallADiagneNNdiathOLyAB 2011 Malaria morbidity and pyrethroid resistance after the introduction of insecticide-treated bednets and artemisinin-based combination therapies: a longitudinal study. Lancet Infect Dis 11 925 932
7. PimentelDAndowDA 1984 Pest management and pesticide impacts. Insect Sci Applic 5 141 149
8. Van LenterenJC 1991 Insects, man and environment: who will survive? HansenJA Environmental concerns. An interdisciplinary exercise London Elsevier Sci. Publ 191 210
9. CouncilNR 1986 Pesticide resistance: strategies and tactics for management Washington (D.C.) National Academy Press
10. KoganF 1998 Integrated pest management: historical perspectives and contemporary developments. Annu Rev Entomol 42 243 270
11. LewisWJLenterenJCvPhatakSCTumlinsonJH 1998 A total system approach to sustainable pest management. Proc Natl Acad Sci U S A 94 12243 12248
12. NaranjoSEEllsworthPC 2009 Fifty years of the integrated control concept: moving the model and implementation forward in Arizona. Pest Manag Sci 65 1267 1286
13. GomieroTPimentelDPaolettiMG 2011 Is there a need for a more sustainable agriculture? CRC Crit Rev Plant Sci 30 6 23
14. WeddlePWWelterSCThomsonD 2009 History of IPM in California pears-50 years of pesticide use and the transition to biologically intensive IPM. Pest Manag Sci 65 1287 1292
15. TabashnikBESistersonMSEllsworthPCDennehyTJAntillaL 2010 Suppressing resistance to Bt cotton with sterile insect releases. Nat Biotechnol 28 1304 1307
16. BeierJCKeatingJGithureJIMacDonaldMBImpoinvilDE 2008 Integrated vector management for malaria control. Malar J 7 Suppl 1
17. Van den BergHTakkenW 2007 A framework for decision-making in integrated vector management to prevent disease. Trop Med Int Health 12 1230 1238
18. WHO 2008 WHO position statement on integrated vector management. Wkly Epidemiol Rec 83 177 181
19. WHO 2010 Handbook on integrated vector management Geneva World Health Organization 78
20. The malERA Consultative Group on Vector Control 2011 A research agenda for malaria eradication: vector control. PLoS Med 8 e1000401 doi:10.1371/journal.pmed.1000401
21. FergusonHMDornhausABeecheABorgemeisterCGottliebM 2010 Ecology: a prerequisite for malaria elimination and eradication. PLoS Med 7 e1000303 doi:10.1371/journal.pmed.1000303
22. RiveroAVezilierJWeillMReadAFGandonS 2010 ) Insecticide control of vector-borne diseases: when is insecticide resistance a problem? PLoS Pathog 6 e1001000 doi:10.1371/journal.ppat.1001000
23. JonesCSanouAGuelbeogoWSagnonNFJohnsonP 2012 Aging partially restores the efficacy of malaria vector control in insecticide-resistant populations of Anopheles gambiae s.l. from Burkina Faso. Malar J 11 24
24. KoellaJCLynchPAThomasMBReadAF 2009 Towards evolution-proof malaria control with insecticides. Evol Appl 2 469 480
25. HemingwayJBeatyBJRowlandMScottTWSharpBL 2006 The innovative vector control consortium: improved control of mosquito-borne diseases. Trends Parasitol 22 308 312
26. ReadAFLynchPAThomasMB 2009 How to make evolution-proof insecticides for malaria control. PLoS Biol 7 e1000058 doi:10.1371/journal.pbio.1000058
27. DenholmIRowlandMW 1992 Tactics for managing pesticide resistance in arthropods - theory and practice. Annu Rev Entomol 37 91 112
28. NauenR 2007 Insecticide resistance in disease vectors of public health importance. Pest Manag Sci 63 628 633
29. BaletaA 2009 Insecticide resistance threatens malaria control in Africa. Lancet 374 1581 1582
30. YadouletonAAsidiADjouakaRBaraimaJAgossouC 2009 Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J 14 103
31. PenillaRPRodriguezADHemingwayJTorresJLArredondo-JimenezJI 1998 Resistance management strategies in malaria vector mosquito control. Baseline data for a large scale field trial against Anopheles albimanus in Mexico. Med Vet Entomol 12 217 233
32. ReddyMROvergaardHJAbagaSReddyVPCacconeA 2011 Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J 10 184
33. KilleenGFMooreSJ 2012 Target product profiles for protecting against outdoor malaria transmission. Malar J 11 17
34. ThomasMReadA 2007 Can fungal biopesticides control malaria? Nat Rev Microbiol 5 377 383
35. BlanfordSShiWPChristianRMardenJHKoekemoerLL 2011 Lethal and pre-lethal effects of a fungal biopesticide contribute to substantial and rapid control of malaria vectors. PLoS ONE 6 e23591 doi:10.1371/journal.pone.0023591
36. TakkenWKnolsBG 2009 Malaria vector control: current and future strategies. Trends Parasitol 25 101 104
37. KirbyMJNjieMDilgerELindsaySW 2009 Importance of eaves to house entry by anopheline, but not culicine, mosquitoes. J Med Entomol 46 505 510
38. ImbahaleSMweresaCTakkenWMukabanaW 2011 Development of environmental tools for anopheline larval control. Parasit Vectors 4 130
39. FillingerUNdengaBGithekoALindsaySW 2009 Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: a controlled trial. Bull World Health Org 87 655 665
40. MullerGCBeierJCTraoreSFToureMBTraoreMM 2010 Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali, West Africa. Malar J 9 210
41. ScholteEJNg'habiKKihondaJTakkenWPaaijmansK 2005 An entomopathogenic fungus for control of adult African malaria mosquitoes. Science 308 1641 1642
42. OkumuFOKilleenGFOgomaSBiswaroLSmallegangeRC 2010 Development and field evaluation of a synthetic mosquito lure that is more attractive than humans. PLoS ONE 5 e8951 doi:10.1371/journal.pone.0008951
43. CirimotichCMDongYMClaytonAMSandifordSLSouza-NetoJA 2011 Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 332 855 858
44. WindbichlerNMenichelliMPapathanosPAThymeSBLiH 2011 A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473 212 215
45. IsaacsATLiFWJasinskieneNChenXGNirmalaX 2011 Engineered resistance to Plasmodium falciparum development in transgenic Anopheles stephensi. PLoS Pathog 7 e1002017 doi:10.1371/journal.ppat.1002017
46. HughesGLKogaRXuePFukatsuTRasgonJL 2011 Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog 7 e1002043 doi:10.1371/journal.ppat.1002043
47. KleinschmidtISchwabeCShivaMSeguraJLSimaV 2009 Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg 81 519 524
48. HancockPA 2009 Combining fungal biopesticides and insecticide-treated bednets to enhance malaria control. PLoS Comput Biol 5 e1000525 doi:10.1371/journal.pcbi.1000525
49. Van den BergHTakkenW 2009 Evaluation of integrated vector management. Trends Parasitol 25 71 76
50. KiszewskiAJohnsBSchapiraADelacolletteCCrowellV 2007 Estimated global resources needed to attain international malaria control goals. Bull World Health Org 85 623 630
51. PATH 2011 Staying the Course? Malaria research and development in a time of economic uncertainty Seattle PATH (Program for Appropriate Technology in Health) 98
52. AlonsoPLBrownGArevalo-HerreraMBinkaFChitnisC 2011 A research agenda to underpin malaria eradication. PLoS Med 8 doi:10.1371/journal.pmed.1000406 e1000406
53. Van den BergHJigginsJ 2007 Investing in farmers: the impacts of farmer field schools in relation to integrated pest management. World Development 35 663 686
54. BrooksSLoevinsohnM 2011 Shaping agricultural innovation systems responsive to food insecurity and climate change. Nat Resour Forum 35 185 200
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- S prof. Vladimírem Paličkou o racionální suplementaci kalcia a vitaminu D v každodenní praxi
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



