-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaHIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
There is growing enthusiasm for increasing coverage of antiretroviral treatment among HIV-infected people for the purposes of preventing ongoing transmission. Treatment as prevention will face a number of barriers when implemented in real world populations, which will likely lead to the effectiveness of this strategy being lower than proposed by optimistic modelling scenarios or ideal clinical trial settings. Some settings, as part of their prevention and treatment strategies, have already attained rates of HIV testing and use of antiretroviral therapy—with high levels of viral suppression—that many countries would aspire to as targets for a treatment-as-prevention strategy. This review examines a number of these “natural experiments”, namely, British Columbia, San Francisco, France, and Australia, to provide commentary on whether treatment as prevention has worked in real world populations. This review suggests that the population-level impact of this strategy is likely to be considerably less than as inferred from ideal conditions.
Published in the journal: . PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001231
Category: Review
doi: https://doi.org/10.1371/journal.pmed.1001231Summary
There is growing enthusiasm for increasing coverage of antiretroviral treatment among HIV-infected people for the purposes of preventing ongoing transmission. Treatment as prevention will face a number of barriers when implemented in real world populations, which will likely lead to the effectiveness of this strategy being lower than proposed by optimistic modelling scenarios or ideal clinical trial settings. Some settings, as part of their prevention and treatment strategies, have already attained rates of HIV testing and use of antiretroviral therapy—with high levels of viral suppression—that many countries would aspire to as targets for a treatment-as-prevention strategy. This review examines a number of these “natural experiments”, namely, British Columbia, San Francisco, France, and Australia, to provide commentary on whether treatment as prevention has worked in real world populations. This review suggests that the population-level impact of this strategy is likely to be considerably less than as inferred from ideal conditions.
Introduction
HIV prevention decision-makers across the world are considering the expansion of antiretroviral therapy (ART) for HIV-infected people in order to reduce their infectiousness and thus prevent onward transmission. This approach, called treatment as prevention, is a paradigm shift from using ART for the sole purpose of improving the health and longevity of patients with HIV. We are now in an era where the secondary benefit of ART is being considered as potentially the primary public health approach to controlling HIV epidemics.
Several findings suggest that treatment might be effective as prevention: the HPTN 052 study demonstrated that ART reduces sexual transmission between discordant couples in a trial setting [1]; various ecological studies from community settings have shown an association between ART programs and reduced markers of incidence [2]–[5]; associations have been demonstrated between reduced viral load and lower infectiousness [6]–[8]; and some theoretical models even suggest that under idealised conditions, elimination might be possible [9],[10]. However, these findings do not imply that widespread scale-up of ART programs under real world conditions will reduce HIV incidence at a population level to the degree that some people are expecting (i.e., towards elimination). Cluster-randomised trials are currently underway in Africa to investigate the impact of high coverage of ART at the population level. In the meantime, models are projecting potential epidemic trajectories associated with treatment-as-prevention strategies under less ideal conditions [11], and various national and international organisations are already discussing operational issues about how to implement treatment as prevention [12].
We do not need to wait for trials of increased ART coverage to be completed, or speculate through the use of mathematical models, to have some understanding of the likely population-level impact of this strategy. Treatment as prevention has essentially been implemented in some settings already for a considerable time. Planned treatment-as-prevention approaches involve frequent universal testing and initiation of ART early post-diagnosis, but increasing treatment coverage at any stage of infection—and reaching high degrees of viral suppression across a population of people living with HIV—is de facto treatment as prevention. Some settings have achieved these objectives as part of their independent prevention and treatment responses: these settings can be considered as natural experiments for treatment as prevention at the population level.
Natural Experiment Case Studies
British Columbia, Canada
A study by Montaner et al. [3] has been widely promoted as demonstrating treatment as prevention in a community setting, namely, among people who inject drugs (PWID) in British Columbia, Canada. In British Columbia, there is universal access to free rapid HIV testing (though it is not known what proportion of PWID get tested for HIV each year). Guidelines for ART in British Columbia indicate that any HIV-positive patient may commence treatment, regardless of CD4 count, and ART is recommended for all symptomatic patients with established disease, and for asymptomatic individuals with CD4 cell count ≤500 cells/µl [13]. Estimates for ART coverage are difficult to quantify precisely, but coverage is considered to be relatively high and has certainly increased over time.
Montaner et al. found that there was an association between declining rates of HIV diagnoses and increasing rates of testing, ART coverage, and viral suppression. However, it is not clear to what extent the reduction in incidence is attributable to ART versus other interventions. As discussed by Smith et al. [14], also in the July 2012 PLoS Medicine Collection, “Investigating the Impact of Treatment on New HIV Infections”, analyses conducted for British Columbia have been ecological, and declines in incidence could be attributed to other prevention programs specifically targeting this population group over the same period [15].
San Francisco, United States
With high, and increasing, rates of HIV testing and ART coverage and effectiveness, San Francisco is an obvious case study for evaluating the role of treatment as prevention. It is estimated that rates of HIV testing have been increasing in San Francisco, such that ∼72% of the core group at risk of infection, namely, men who have sex with men (MSM), received an HIV test in the past 12 months, and only 15%–20% of HIV cases are undiagnosed [4]. An increasing proportion of HIV-infected patients are enrolled in care (∼71% in 2004 and 78% in 2008) [16], and mean levels of community viral load have significantly decreased (from ∼25,000 copies/ml in 2004/2005 to 15,000 copies/ml in 2008) [4].
Das et al. [4] used San Francisco's HIV/AIDS surveillance system to examine trends in community viral load and new HIV diagnoses, as a surrogate marker for incidence. They found that reductions in community viral load were associated with decreased HIV diagnoses since 2004. As a purely ecological study, causation cannot be attributed to ART, but their results suggest that high coverage of ART could have reduced HIV transmission at the population level. However, although the number of newly diagnosed and reported HIV cases has been declining in San Francisco, the rate of new infections is still relatively high [4], possibly because of the substantial HIV prevalence (∼25%) among MSM [17],[18]. As such, even if the average per individual infectiousness is reduced, there is still likely to be a significant number of new HIV infections occurring at the population level each year.
France
A “treatment as prevention” statement has been released by the French National AIDS Council [19], which takes a less assertive approach to “test and treat” but still strongly promotes testing and treatment. The level of undiagnosed infections in France is approximately 25%–30%, comparable to levels in other resource-rich settings [20]–[28]. ART guidelines in France in 2007 indicated that ART should be started as early as possible for symptomatic patients and those with high viral loads (>100,000 copies/ml), and for asymptomatic patients when the CD4 count reached 350 cells/µl [29]. There have been significant increases in the uptake of ART among eligible people in France (to ∼85%), and ∼92% of treated patients achieve plasma viral suppression [30]. However, treatment-as-prevention strategies cannot be said to have been fully implemented in France, as many patients initiate ART too late [29].
The outcomes of the natural experiment in France suggest that there may be differences between at-risk groups in the population-level effectiveness of ART for reducing incidence: HIV incidence has declined or remained stable in all major population groups, except MSM, where incidence has been high and increasing [30]. Data from behavioural studies indicate that unprotected anal sex and numbers of sexual partners among MSM have increased [31] (also coinciding with increases in syphilis transmission [32]), raising the possibility that disinhibition or independent sociobehavioural changes could undercut the effectiveness of treatment as prevention. It is also possible that the increased HIV incidence among MSM could be due to higher risk behaviours among those who are not on ART and do not have suppressed virus.
Australia
Australia could also be considered a setting where a natural experiment for treatment as prevention has taken place. First, the HIV epidemic is highly concentrated, with the majority (∼80%) of all HIV cases being among MSM [33], a population generally well educated and actively engaged with respect to HIV. Second, HIV testing is routinely carried out by most MSM, with approximately 60%–75% of men self-reporting an annual test [34] and just 10% of men reporting having never been tested [35]. Third, all regimen lines and combinations of ART are publicly funded and freely available to all HIV-infected patients. Australian guidelines for treatment advise considering ART when CD4 cell count is <500 cells/µl, and definitely treating when CD4 cell count is <350 cells/µl. There are increased numbers of people receiving ART in Australia (at about 70% of all individuals living with HIV) [36]; however, about 20% of individuals commence ART when CD4 cell count is <200 cells/µl, because of late presentation [33]. Fourth, the proportion of people on ART with undetectable viral load has increased from 65% to 90% (at 400 copies/ml sensitivity; 40% to 85% at 50 copies/ml sensitivity) [37]. Further information and analyses on these data are provided elsewhere [38].
It is likely that many countries would aspire to the conditions in Australia as a target for treatment as prevention, as this is a real world population with high coverage of effective treatment. However, new HIV diagnoses, which can be interpreted as reflecting HIV incidence [39], have increased from around 700 cases in 1999 (a nadir of national diagnoses) to around 1,000 new cases annually [33]. This suggests that implementation of treatment as prevention may have less impact on reducing population incidence than previously expected.
Limits to Treatment as Prevention
Treatment as prevention possibly has the greatest chance to succeed now in resource-rich countries with concentrated HIV epidemics, where there is generally universal access to ART, adequate infrastructure, and guidelines that enable early initiation of treatment. However, it is in these very settings that HIV incidence, or surrogate markers thereof, has been increasing [40]–[45], as in Australia and France. Indeed, at the latest Annecy Group meeting (consisting of representatives from national HIV/AIDS surveillance organisations from developed countries in North America, Australia, Western Europe, and the UK) in Rome in January 2011, it was ascertained that despite differences in epidemiological profiles, surveillance systems, and programmatic responses, HIV epidemics among MSM were generally stable or increasing in almost all of these developed-country settings, despite widespread and increasing availability and effectiveness of ART. Outbreaks of HIV among PWID have also recently been observed in some of these countries [46],[47].
There are numerous possible reasons for the apparent ineffectiveness of increased treatment in reducing HIV incidence in “natural experiments”. One potential explanation is changes in risk behaviour (shifts in cultural practices, condom fatigue, or risk compensation), as observed in numerous settings including France and Australia [48]. Another possible explanation is the influence of migration from higher prevalence regions, which leads to greater numbers of detected cases in the country of question—sometimes used as a measure of incidence—as well as greater background prevalence. There is also the potential emergence of marginalised groups that experience additional barriers to accessing services. These marginalised groups often include migrant and other populations that experience relatively high levels of stigma and discrimination but that are also at greatest risk of HIV infection. Increases in prevalence of other sexually transmitted diseases can also increase HIV incidence, since some sexually transmitted diseases act as a biological cofactor for increasing both HIV infectiousness and susceptibility [49],[50]. Another possibility is that treatment is not as effective in reducing infectiousness for riskier modes of transmission as it is for heterosexual transmission (the only mode of transmission considered in the HPTN 052 study) [4],[51]–[53]. Currently, there is little evidence that treatment as prevention is as effective for MSM and PWID [54],[55]. These factors may help explain the observed increases in HIV incidence in the era of expanded ART.
One way to consider the problem is that there is a series of barriers to overcome for treatment to be effective in reducing infectiousness (Figure 1). As indicated by Gardner et al. [56], treatment can have a population-level effect in prevention if a high proportion of all HIV-infected people (i) are tested for HIV, (ii) are linked to clinical care in a timely manner, (iii) are retained in care, (iv) receive effective ART, and (v) are adherent to treatment and regularly monitored. It is not uncommon for people to drop out at any of these barriers. Idealised conditions for a treatment-as-prevention strategy may involve setting targets of 90% of all people at each barrier progressing to the next stage. However, as pointed out by Gardner et al., this would result in a maximum of just 66% of HIV-infected people in the population having suppressed virus. Populations of people on ART may have reduced transmission potential, but transmission events are still likely to occur from individuals on ART, as well as from the remaining HIV-infected population without suppressed virus [57].
Fig. 1. Series of steps required in order to reduce onward transmission from someone infected with HIV. 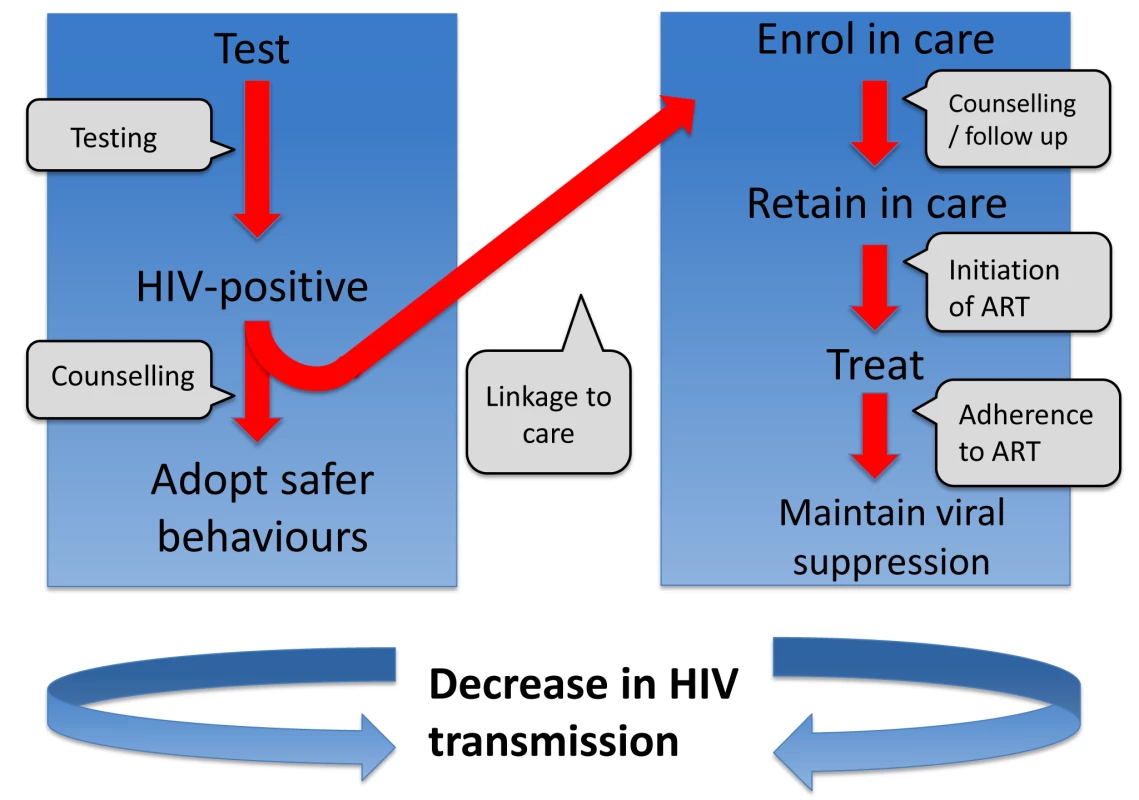
A related problem of treatment as prevention is that the significant advances in the effectiveness of ART in reducing viral replication have decreased HIV/AIDS-associated mortality [58],[59], thereby resulting in a growing pool of HIV-infected people. There is a balance between ART reducing infectiousness and increasing prevalence. This is demonstrated in the natural experiment case of Australia. As shown in Figure 2, the estimated average number of onward HIV infections resulting from each HIV-infected person per year has decreased substantially, but has levelled off at a value above zero. At the same time, the prevalence of HIV has been steadily increasing in Australia because of increased survival due to effective ART (the trend is not altered considerably when adjusted for population size). Correspondingly, overall population incidence has increased over this period. Also, acute HIV infection, with high viremia and high infectiousness, is likely to be an important contributing factor to ongoing transmission [60]–[63], particularly as most of these cases are usually unrecognised.
Fig. 2. Estimated number of people living with HIV in Australia and per capita transmission rate over time. 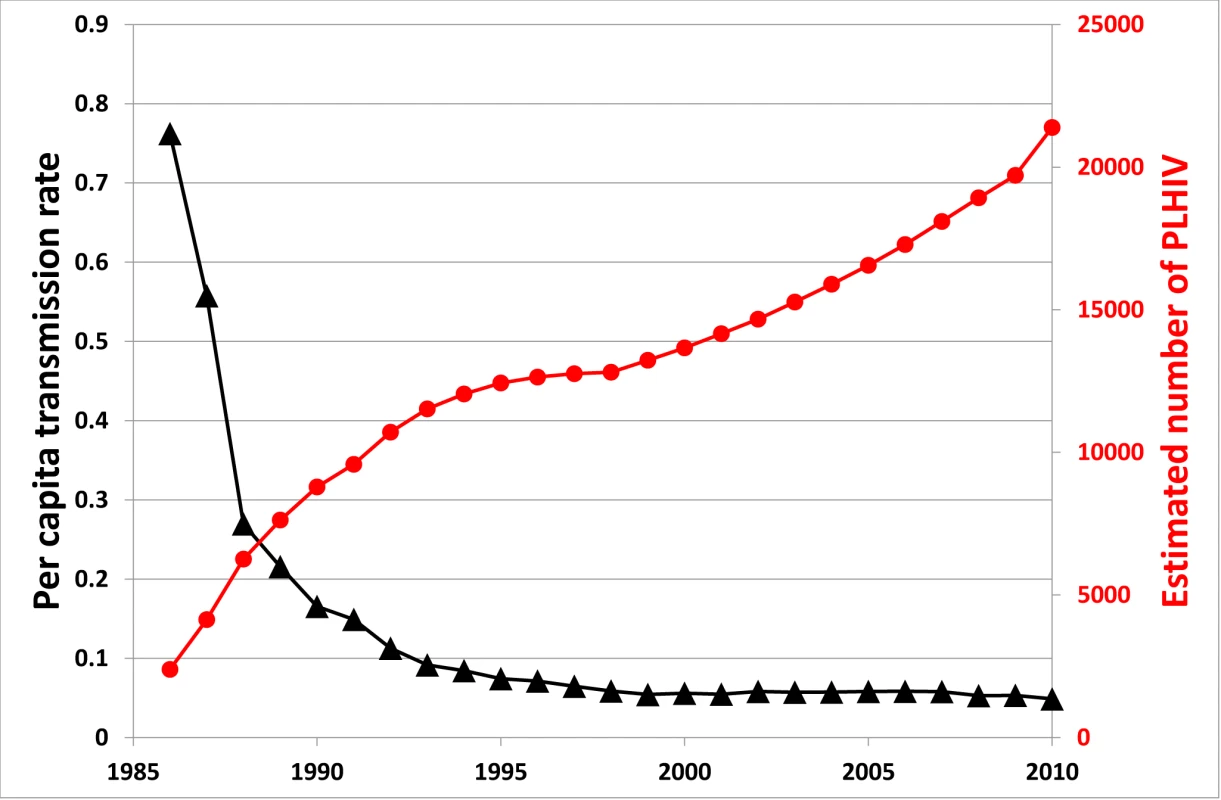
The per capita transmission rate is defined as the average number of new onward HIV infections resulting from each HIV-infected person per year; this is calculated as the number of new diagnoses in a given year (as a surrogate marker for incidence) divided by the estimated number of people living with HIV (PLHIV). On the positive side, the potential problem of there being an increased pool of potential transmitters produced by successful ART may turn out to be minor. People's sexual-transmission-related behaviours generally decrease as they age. Therefore, the average transmission rate per infected person may not be reflective of transmission from the majority of people living with HIV. It may be possible to reduce the average reproduction number, R, to below the elimination threshold, R<1, even with higher prevalence. Future studies may be able to assess whether this is feasible under realistic conditions.
It is important to note that over the last 5–10 years there have been substantial increases in ART programs across low - and middle-income countries. There is now clear evidence of decreasing HIV prevalence across eastern and southern Africa, which is undoubtedly multifactorial but may reflect some impact of ART on transmission (as assessed by Johnson et al. [64] for South Africa). There have also been large reductions in mortality and corresponding reductions in prevalence throughout Asia associated with ART programs (e.g., [65]–[67]). Although such benefits are to be celebrated, and there is little doubt that ART programs have likely had an impact in reducing incidence, the levels of undiagnosed infections and treatment coverage make it unlikely that treatment as prevention can lead towards elimination at this stage.
Conclusions
The efficacy of treatment in reducing transmission has been demonstrated for heterosexual transmission in the HPTN 052 trial, with supporting evidence from other types of studies. However, this does not imply that increased ART coverage will result in substantial declines in incidence in real world populations. The average per person rate of transmission will decrease because of ART, but it will likely saturate at a level above zero. Due to increased prevalence of potential transmitters, and other limitations, it may be difficult to decrease overall population incidence without other prevention approaches. While trial results are obtained under optimised conditions, where regular counselling and condoms are provided and where there are relatively low rates of sexually transmitted infections, this is often not the situation in the real world. There are also other external factors that may limit the impact of treatment as prevention, including adherence to treatment and shifts in sexual behaviours.
Justifiably, there is large enthusiasm for treatment as prevention. But current planning is based on expected outcomes informed by clinical trials and models—with supporting evidence from ecological and observational studies—that may be overly optimistic. Natural experiments suggest that there are limitations to the overall benefit that can be achieved with this strategy. Before large portions of HIV/AIDS budgets [68] are shifted to treatment as prevention in place of traditional prevention approaches, these limitations need to be given appropriate consideration. It must be acknowledged that ART is cost-effective with respect to clinical benefits [69],[70] and is likely to be even more so if prevention benefits are included. But combination prevention using other approaches proven to be effective, feasible, and cost-effective is also essential to reduce incidence among all major groups at risk of infection.
Key Points
-
The real world effectiveness of treatment as prevention is likely to be less than the efficacy measured in trials or calculated in optimistic model scenarios.
-
Some settings have attained rates of testing and effective ART coverage that many countries would aspire to as targets for treatment-as-prevention strategies.
-
Examination of data from treatment-as-prevention “natural experiments” suggests that there are limitations to reductions in population incidence.
-
Limitations might stem from behaviour changes, difficulties linking patients with and retaining them in clinical care, differences in the effectiveness of ART for different modes of HIV transmission, and the increasing pool of potential transmitters produced by successful ART.
Zdroje
1. CohenMSChenYQMcCauleyMGambleTHosseinipourMC 2011 Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 493 505
2. MontanerJSHoggRWoodEKerrTTyndallM 2006 The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 368 531 536
3. MontanerJSLimaVDBarriosRYipBWoodE 2010 Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 376 532 539
4. DasMChuPLSantosGMScheerSVittinghoffE 2010 Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS ONE 5 e11068 doi:10.1371/journal.pone.0011068
5. MiddelkoopKBekkerLGMyerLJohnsonLFKloosM 2011 Antiretroviral therapy and TB notification rates in a high HIV prevalence South African community. J Acquir Immune Defic Syndr 56 263 269
6. AnglemyerARutherfordGWBaggaleyRCEggerMSiegfriedN 2011 Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. Cochrane Database Syst Rev 2011 CD009153
7. AttiaSEggerMMullerMZwahlenMLowN 2009 Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS 23 1397 1404
8. QuinnTCWawerMJSewankamboNSerwaddaDLiC 2000 Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med 342 921 929
9. GranichRMGilksCFDyeCDe CockKMWilliamsBG 2009 Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373 48 57
10. HoltgraveDR 2010 Is the elimination of HIV infection within reach in the United States? Lessons from an epidemiologic transmission model. Public Health Rep 125 372 376
11. EatonJWJohnsonLFSalomonJABärnighausenTBendavidE 2012 HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 9 e1001245 doi:10.1371/journal.pmed.1001245
12. GranichRGuptaSSutharABSmythCHoosD 2011 Antiretroviral therapy in prevention of HIV and TB: update on current research efforts. Curr HIV Res 9 446 469
13. British Columbia Centre for Excellence in HIV/AIDS 2011 Therapeutic guidelines: antiretroviral treatment (ARV) of adult HIV infection—as of January 2011. Available: http://www.cfenet.ubc.ca/sites/default/files/uploads/BCCfE%20Adult%20Therapeutic%20Guidelines_Jan2011.pdf. Accessed 7 December 2011
14. SmithMKPowersKAMuessigKEMillerWCCohenMS 2012 HIV treatment as prevention: the utility and limitations of ecological observation. PLoS Med 9 e1001260 doi:10.1371/journal.pmed.1001260
15. KerrTSmallWBuchnerCZhangRLiK 2010 Syringe sharing and HIV incidence among injection drug users and increased access to sterile syringes. Am J Public Health 100 1449 1453
16. San Francisco Department of Public Health HIV/AIDS Statistics and Epidemiology Section 2009 HIV/AIDS epidemiology annual report 2008 San Francisco San Francisco Department of Public Health
17. ScheerSKelloggTKlausnerJDSchwarczSColfaxG 2008 HIV is hyperendemic among men who have sex with men in San Francisco. Sex Transm Infect 84 493 498
18. San Francisco Department of Public Health HIV/AIDS Statistics, Epidemiology, and Intervention Research Section 2005 HIV/AIDS epidemiology annual report: 2005. Available: http://sfhiv.org/files/data_reports/hiv_aids_annual_rpt/HIVAIDAnnlRpt2005.pdf. Accessed 10 May 2012
19. BourdillonFCadoreBDozonJ-PGaudinPKapusta-PalmerC 2009 April 9 Statement followed by recommendations on the appropriateness of treatment as an innovative tool for fighting the epidemic of HIV infections Paris Conseil National du SIDA
20. Joint United Nations Programme on HIV/AIDS 2010 Epidemiological factsheet: France Geneva Joint United Nations Programme on HIV/AIDS
21. Haute Autorité de Santé 2009 HIV infection screening in France: screening strategies Saint-Denis La Plaine (France) Haute Autorité de Santé
22. WilsonDPHoareAReganDGWandHLawM 2008 Mathematical models to investigate recent trends in HIV notifications among men who have sex with men in Australia Sydney National Centre in HIV Epidemiology and Clinical Research
23. JinFPrestageGPMcDonaldARamacciottiTImrieJC 2008 Trend in HIV incidence in a cohort of homosexual men in Sydney: data from the Health in Men Study. Sex Health 5 109 112
24. GlynnMKRhodesP 2005 Estimated HIV prevalence in the United States at the end of 2003 [abstract]. National HIV Prevention Conference; Atlanta, Georgia; 12–15 June 2005
25. BransonB 2007 Current HIV epidemiology and revised recommendations for HIV testing in health-care settings. J Med Virol 79 Suppl 1 S6 S10
26. BransonBMHandsfieldHHLampeMAJanssenRSTaylorAW 2006 Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 55 1 17
27. SteklerJDGoldenMR 2009 Learning from the missed opportunities for HIV testing. Sex Transm Infect 85 2 3
28. DoddsJPJohnsonAMParryJVMerceyDE 2007 A tale of three cities: persisting high HIV prevalence, risk behaviour and undiagnosed infection in community samples of men who have sex with men. Sex Transm Infect 83 392 396
29. LatthaphasavangVBouldouyreMARachlineAPonscarmeDRozenbaumW 2012 Antiretroviral therapy initiation in france: adherence to national guidelines and outcome. J Int Assoc Physicians AIDS Care (Chic) 11 40 46
30. Le VuSLe StratYBarinFillonelJCazeinF PSurveillance de la syphilis en France, 2000–2006: recrudescence des diagnostics en 2006 2010 Population-based HIV-1 incidence in France, 2003–08: a modelling analysis. Lancet Infect Dis 10 682 687
31. VelterABouyssou-MichelAde BusscherPOJauff ret-RoustideMSemailleC 2007 Enquête presse gay 2004 Saint-Maurice Institut de Veille Sanitaire
32. Bouyssou MichelAGallayAJanierMDupinNHaliouaB 2008 Surveillance de la syphilis en France, 2000–2006: recrudescence des diagnostics en 2006. Bull Epidemiol Hebdo 5–6 39 42
33. The Kirby Institute 2011 HIV, viral hepatitis and sexually transmissible infections in Australia: annual surveillance report 2011 Sydney University of New South Wales
34. FogartyAMaoLZablotskaISalterMPrestageG 2006 The Health in Men and Positive Health cohorts: a comparison of trends in the health and sexual behaviour of HIV-negative and HIV-positive gay men, 2002–2005 Sydney University of New South Wales, National Centre in HIV Social Research
35. National Centre in HIV Research 2011 HIV/AIDS, hepatitis and sexually transmissible infections in Australia: annual report of trends in behaviour 2011 Sydney University of New South Wales
36. FalsterKGelgorLShaikAZablotskaIPrestageG 2008 Trends in antiretroviral treatment use and treatment response in three Australian states in the first decade of combination antiretroviral treatment. Sex Health 5 141 154
37. The Kirby Institute 2011 September Australian HIV Observational Database: annual report Sydney University of New South Wales
38. LawMGWoolleyITempletonDJRothNChuahJ 2011 Trends in detectable viral load by calendar year in the Australian HIV observational database. J Int AIDS Soc 14 10
39. MallittKAWilsonDPMcDonaldAWandH 2011 HIV incidence trends vary between jurisdictions in Australia: an extended back-projection analysis of men who have sex with men. Sex Health 9 138 143
40. GrulichAEKaldorJM 2008 Trends in HIV incidence in homosexual men in developed countries. Sex Health 5 113 118
41. SullivanPSHamoudaODelpechVGeduldJEPrejeanJ 2009 Reemergence of the HIV epidemic among men who have sex with men in North America, Western Europe, and Australia, 1996–2005. Ann Epidemiol 19 423 431
42. MorrisSRLittleSJ 2011 MSM: resurgent epidemics. Curr Opin HIV AIDS 6 326 332
43. BezemerDde WolfFBoerlijstMCvan SighemAHollingsworthTD 2008 A resurgent HIV-1 epidemic among men who have sex with men in the era of potent antiretroviral therapy. AIDS 22 1071 1077
44. JansenIAGeskusRBDavidovichUJurriaansSCoutinhoRA 2011 Ongoing HIV-1 transmission among men who have sex with men in Amsterdam: a 25-year prospective cohort study. AIDS 25 493 501
45. European Centre for Disease Prevention and Control, World Health Organization Regional Office for Europe 2011 HIV/AIDS surveillance in Europe: 2010 Stockholm European Centre for Disease Prevention and Control Available: http://ecdc.europa.eu/en/publications/Publications/101129_SUR_HIV_2009.pdf. Accessed 3 May 2012
46. ParaskevisDNikolopoulosGTsiaraCParaskevaDAntoniadouA 2011 HIV-1 outbreak among injecting drug users in Greece, 2011: a preliminary report. Euro Surveill 16. Available: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19962. Accessed 3 May 2012
47. European Monitoring Centre for Drugs and Drug Addiction 2011 Annual report 2011: the state of the drugs problem in Europe Lisbon European Monitoring Centre for Drugs and Drug Addiction Available: http://www.emcdda.europa.eu/attachements.cfm/att_143743_EN_EMCDDA_AR2011_EN.pdf. Accessed 3 May 2012
48. ZablotskaIBCrawfordJImrieJPrestageGJinF 2009 Increases in unprotected anal intercourse with serodiscordant casual partners among HIV-negative gay men in Sydney. AIDS Behav 13 638 644
49. FlemingDTWasserheitJN 1999 From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect 75 3 17
50. GalvinSRCohenMS 2004 The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2 33 42
51. FisherMPaoDBrownAESudarshiDGillON 2010 Determinants of HIV-1 transmission in men who have sex with men: a combined clinical, epidemiological and phylogenetic approach. AIDS 24 1739 1747
52. PorcoTCMartinJNPage-ShaferKAChengACharleboisE 2004 Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS 18 81 88
53. JinFJanssonJLawMPrestageGPZablotskaI 2010 Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS 24 907 913
54. KelleyCFHaalandREPatelPEvans-StrickfadenTFarshyC 2011 HIV-1 RNA rectal shedding is reduced in men with low plasma HIV-1 RNA viral loads and is not enhanced by sexually transmitted bacterial infections of the rectum. J Infect Dis 204 761 767
55. WilsonDP 2010 Evidence is still required for treatment as prevention for riskier routes of HIV transmission. AIDS 24 2891 2893
56. GardnerEMMcLeesMPSteinerJFDel RioCBurmanWJ 2011 The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 52 793 800
57. WilsonDPLawMGGrulichAECooperDAKaldorJM 2008 Relation between HIV viral load and infectiousness: a model-based analysis. Lancet 372 314 320
58. HammerSMSaagMSSchechterMMontanerJSSchooleyRT 2006 Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA 296 827 843
59. PalellaFJJrDelaneyKMMoormanACLovelessMOFuhrerJ 1998 Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338 853 860
60. HayesRJWhiteRG 2011 Role of acute infection in HIV transmission. Lancet 378 1913 1914 author reply 1914-1915
61. WilliamsBGGranichRDyeC 2011 Role of acute infection in HIV transmission. Lancet 378 1913 author reply 1914-1915
62. PowersKAGhaniACMillerWCHoffmanIFPettiforAE 2011 The role of acute and early HIV infection in the spread of HIV and implications for transmission prevention strategies in Lilongwe, Malawi: a modelling study. Lancet 378 256 268
63. MillerWCRosenbergNERutsteinSEPowersKA 2010 Role of acute and early HIV infection in the sexual transmission of HIV. Curr Opin HIV AIDS 5 277 282
64. JohnsonLFHallettTBRehleTMDorringtonRE 2012 The effect of changes in condom usage and antiretroviral treatment coverage on human immunodeficiency virus incidence in South Africa: a model-based analysis. J R Soc Interface E-pub ahead of print. doi:10.1098/rsif.2011.0826
65. ZhangFDouZMaYZhangYZhaoY 2011 Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis 11 516 524
66. ZhangFDouZMaYZhaoYLiuZ 2009 Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med 151 241 251 W-252
67. YangCHHuangYFHsiaoCFYehYLLiouHR 2008 Trends of mortality and causes of death among HIV-infected patients in Taiwan, 1984–2005. HIV Med 9 535 543
68. Kaiser Family Foundation 2011 International AIDS assistance from donor governments: commitments & disbursements, 2002–2010. Available: http://facts.kff.org/chart.aspx?ch=946. Accessed 3 May 2012
69. FreedbergKALosinaEWeinsteinMCPaltielADCohenCJ 2001 The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 344 824 831
70. BadriMMaartensGMandaliaSBekkerLGPenrodJR 2006 Cost-effectiveness of highly active antiretroviral therapy in South Africa. PLoS Med 3 e4 doi:10.1371/journal.pmed.0030004
Štítky
Interní lékařství
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2012 Číslo 7- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



