-
Články
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
- Kongresy
- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Threshold Haemoglobin Levels and the Prognosis of Stable Coronary Disease: Two New Cohorts and a Systematic Review and Meta-Analysis
Background:
Low haemoglobin concentration has been associated with adverse prognosis in patients with angina and myocardial infarction (MI), but the strength and shape of the association and the presence of any threshold has not been precisely evaluated.Methods and findings:
A retrospective cohort study was carried out using the UK General Practice Research Database. 20,131 people with a new diagnosis of stable angina and no previous acute coronary syndrome, and 14,171 people with first MI who survived for at least 7 days were followed up for a mean of 3.2 years. Using semi-parametric Cox regression and multiple adjustment, there was evidence of threshold haemoglobin values below which mortality increased in a graded continuous fashion. For men with MI, the threshold value was 13.5 g/dl (95% confidence interval [CI] 13.2–13.9); the 29.5% of patients with haemoglobin below this threshold had an associated hazard ratio for mortality of 2.00 (95% CI 1.76–2.29) compared to those with haemoglobin values in the lowest risk range. Women tended to have lower threshold haemoglobin values (e.g, for MI 12.8 g/dl; 95% CI 12.1–13.5) but the shape and strength of association did not differ between the genders, nor between patients with angina and MI. We did a systematic review and meta-analysis that identified ten previously published studies, reporting a total of only 1,127 endpoints, but none evaluated thresholds of risk.Conclusions:
There is an association between low haemoglobin concentration and increased mortality. A large proportion of patients with coronary disease have haemoglobin concentrations below the thresholds of risk defined here. Intervention trials would clarify whether increasing the haemoglobin concentration reduces mortality.
: Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 8(5): e32767. doi:10.1371/journal.pmed.1000439
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000439Summary
Background:
Low haemoglobin concentration has been associated with adverse prognosis in patients with angina and myocardial infarction (MI), but the strength and shape of the association and the presence of any threshold has not been precisely evaluated.Methods and findings:
A retrospective cohort study was carried out using the UK General Practice Research Database. 20,131 people with a new diagnosis of stable angina and no previous acute coronary syndrome, and 14,171 people with first MI who survived for at least 7 days were followed up for a mean of 3.2 years. Using semi-parametric Cox regression and multiple adjustment, there was evidence of threshold haemoglobin values below which mortality increased in a graded continuous fashion. For men with MI, the threshold value was 13.5 g/dl (95% confidence interval [CI] 13.2–13.9); the 29.5% of patients with haemoglobin below this threshold had an associated hazard ratio for mortality of 2.00 (95% CI 1.76–2.29) compared to those with haemoglobin values in the lowest risk range. Women tended to have lower threshold haemoglobin values (e.g, for MI 12.8 g/dl; 95% CI 12.1–13.5) but the shape and strength of association did not differ between the genders, nor between patients with angina and MI. We did a systematic review and meta-analysis that identified ten previously published studies, reporting a total of only 1,127 endpoints, but none evaluated thresholds of risk.Conclusions:
There is an association between low haemoglobin concentration and increased mortality. A large proportion of patients with coronary disease have haemoglobin concentrations below the thresholds of risk defined here. Intervention trials would clarify whether increasing the haemoglobin concentration reduces mortality.
: Please see later in the article for the Editors' SummaryIntroduction
Haemoglobin is a potentially useful prognostic biomarker among patients with stable coronary artery disease because of the pathological role of myocardial hypoxia in subsequent acute coronary events [1],[2] and the existence of interventions to increase haemoglobin concentration [3]–[5]. Measurement of haemoglobin is nearly universal in such patients, further underscoring the relevance of understanding its impact on prognosis. While previous studies suggest that low haemoglobin is associated with mortality in patients with coronary artery disease [6],[7], they have been limited in their ability to describe the shape of the relationship, to assess gender differences, and to compare patients with angina or prior myocardial infarction (MI). Furthermore there are concerns that the prognostic biomarker literature may be subject to reporting and other biases [8]. Thus several questions, relevant to clinical practice and the design of future trials, remain unanswered.
First, at what concentration of haemoglobin does risk increase; is there a threshold or is it a continuous graded relationship? This question is important in order to define optimal haemoglobin levels. While some studies have reported a continuous relationship between haemoglobin and prognosis among patients with coronary disease, others have reported a J-shaped relationship [6],[9]. No previous study has sought to evaluate whether and where there is a threshold. Second, how strong is the relationship in population-based samples, when accounting for coexisting conditions? This question is important in order to estimate excess attributable risks from nonoptimal haemoglobin concentrations. Third, how does the association between haemoglobin concentration and mortality differ between women and men, in whom haemoglobin homeostasis is known to differ? Fourth, do effects differ between patients with a history of angina only, and those with a history of MI? Many patients develop reduced left ventricular function after MI [10] and might be more sensitive to low haemoglobin concentration. Conversely, low haemoglobin may unmask symptoms of angina and bring patients to medical attention earlier, potentially reducing the apparent effect of low haemoglobin on mortality.
We evaluated prior knowledge on this topic by means of a systematic review and meta-analysis, and sought to extend this knowledge and address the above questions by carrying out a retrospective cohort study using the General Practice Research Database (GPRD).
Methods
Ethics Statement
The GPRD Division of the Medicines and Healthcare products Regulatory Agency has been granted Multi-Centre Research Ethics committee approval for all observational studies using GPRD data.
Study Populations
Over 95% of UK citizens are registered with a general practitioner and receive primary health care free at the point of use. The GPRD contains anonymised electronic clinical records of over 4 million such patients with details of consultations, diagnoses, referrals, prescriptions, and test results. Its use is supported by numerous validation studies on a wide range of diseases [11] and it has been shown to have accurate recording of MI [12].
Among GPRD patients aged 35 to 90, we defined two study populations on the basis of coded diagnoses between 2001–2006: firstly, patients with new onset stable angina and no previous acute coronary syndrome (ACS), and secondly, patients with a first MI. The date of the earliest stable angina or MI diagnosis for each patient was designated the “index date.” General practitioners routinely code medical diagnoses using the “Read” clinical terminology [13]; our codes are listed in Tables S1 (stable angina) and S2 (MI).
For the stable angina population, we included patients with a code for angioplasty or coronary artery bypass but not angina only if they had evidence of previous angina symptoms (e.g., a code for chest pain or a nitrate prescription; the date of the earliest such entry was taken as the index date). We excluded patients with ACS within 7 d after the index date because their initial presentation may have been unstable rather than stable angina. Further details are given in Text S1 (Additional Methods), Figure S1 (flowchart), and Text S2 (STROBE checklist).
From both populations we excluded patients who died within 7 d of the index date, patients with other cardiac conditions prior to the index date (myocarditis, endocarditis, pericarditis, valve disease, or congenital cardiac abnormalities), and patients who were pregnant less than 3 mo before the index date.
Risk Factors and Blood Parameters
We extracted all values of routinely recorded blood parameters (total cholesterol, haemoglobin, mean corpuscular volume [MCV], creatinine) for each patient, along with information on established cardiovascular risk factors (smoking, systolic blood pressure [BP], diabetes, and family history). For continuous variables, if values were recorded before and after the index date, we calculated a weighted average by linear interpolation to estimate the value at the index date. If only a single value was recorded, or all values were either before or after the index date, we used the single value nearest the index date.
Recording rates for established cardiovascular risk factors are high because they are included in the National Health Service (NHS) Quality and Outcomes Framework, which includes incentive payments for high quality management of chronic conditions [14]. GPRD smoking data has been validated against a population survey [15].
We estimated the glomerular filtration rate (eGFR) from serum creatinine using the four-variable Modification of Diet in Renal Disease (MDRD) formula, one of the methods recommended by international guidelines [16].
Comorbidity
Our adjusted analyses incorporated the Charlson comorbidity index, a numerical score based on 19 groups of medical conditions, which correlates well with mortality [17]. We regarded a comorbidity as being present if a Read code for the condition occurred prior to the index date (within the previous 5 y for cancers). The Charlson index incorporates the following cardiovascular comorbidities: congestive cardiac failure, MI, stroke, and peripheral vascular disease. The “MI” criterion was ignored for the purposes of this study, as patients in the stable angina cohort would not have had a previous MI, and patients in the MI cohort would all have an index MI. We dealt with age as a separate variable and not as part of the Charlson index, to ensure consistency across analyses with different levels of adjustment.
Follow-up and Endpoints
We limited follow-up to the time when the patient was registered and data from the practice fulfilled GPRD standards of data completeness. We excluded patients who were not registered with the practice for a minimum of 3 mo before and 3 mo after the index date (unless they died within that time). The primary endpoint was death from any cause, identified by a Read code denoting death or transfer out reason of “Death.” We investigated nonfatal strokes and ACS as secondary endpoints (“nonfatal” was defined as survival for more than 7 d after the event). We chose to investigate these diagnoses as secondary endpoints because they cause significant morbidity and share many common risk factors with angina and MI.
Statistical Analysis
Our statistical model aimed to identify upper and lower thresholds of an optimal haemoglobin range beyond which mortality risk increased (Figure 1A, Haemoglobin threshold model). Similar types of models have been used in other studies exploring prognosis and biomarkers (e.g., glycated haemoglobin, Selvin et al. [18]). In Text S1 and Figure S3, our model is reported fully, following recent recommendations [19].
Fig. 1. Adjusted HRs for death for patients with stable angina or MI, by haemoglobin concentration. 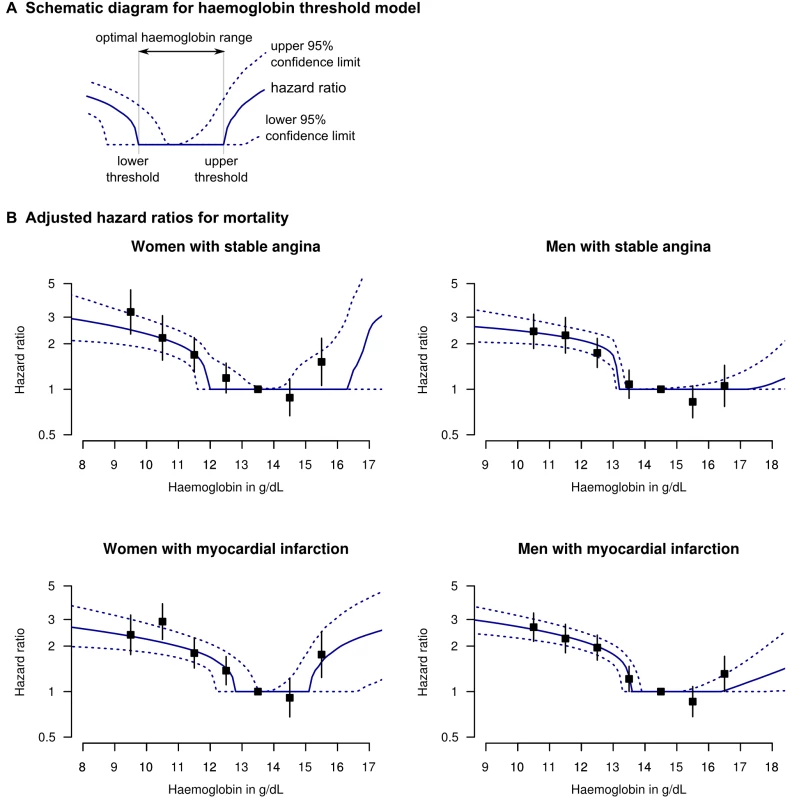
(A) Legend and diagram for haemoglobin threshold model; (B) HRs for death by gender and coronary diagnosis (new onset stable angina or first MI), adjusted for age, eGFR, systolic BP, total cholesterol, family history, diabetes, smoking, and comorbidity (Charlson index). The black squares denote HRs from the stratified haemoglobin model (with 95% CIs). In order to minimise bias arising from variable selection on statistical grounds [20], we decided a priori to include in our model only variables known to be relevant based on previous literature. We included age, systolic BP, total cholesterol, eGFR, and the Charlson comorbidity index as continuous variables with a log-linear risk profile; and smoking, gender, diabetes, and family history of coronary disease as binary variables. We used a semi-parametric Cox model with proportional hazards for all variables except haemoglobin, and tested the proportional hazards assumption by examining Schoenfeld residuals [21]. We analysed stable angina and MI patients separately, with no risk coefficients shared between the two analyses.
We also report hazard ratios (HRs) from a stratified model, similar to the approach of Sabatine et al. [6], in order to verify that our model was not distorted by any assumptions of its shape. For each gender, we stratified patients into groups at 1 g/dl intervals of haemoglobin, and used the interval with most people as the reference interval. We performed analyses using R Version 2.9.2 [22], Matlab (The Mathworks), and custom software in C++ (source code in Texts S4 and S5).
Systematic Review and Meta-analysis
We searched MEDLINE and EMBASE for prospective studies in which blood biomarkers were measured in patients with stable angina or at least 2 wk post ACS, and a relative risk for mortality or coronary events by haemoglobin concentration was reported with a p-value or confidence interval (CI). Our search results included studies focusing on biomarkers other than haemoglobin, but that reported a relative risk for haemoglobin in the results. Our search strategy, including a complete list of keywords, has been published as part of a systematic review of biomarkers in patients awaiting coronary revascularisation [23] and is also described in Text S1 (Additional Methods) and Figure S4 (MOOSE flow diagram). We excluded studies with less than 1 y of follow-up, but did not exclude studies based on methodological standards, sample size, or language. Two reviewers independently abstracted data from eligible articles, with disagreements resolved by consensus, or rarely by adjudication by a third reviewer.
We used the outcome of all-cause mortality, if available, for the meta-analysis. Since no studies evaluated thresholds, the only summary relative risk that could be obtained was linear. We converted the HR for each study to the equivalent risk for 1 g/dl lower haemoglobin, and calculated a summary relative risk using the random-effects method of DerSimonion and Laird [24], implemented in the “rmeta” package for R [25]. We weighted studies inversely by the log standard error of the relative risk. For studies which used an anaemia cutoff [26],[27], we divided the log relative risk by the difference in mean haemoglobin between the two groups. For the study by Reinecke et al., which used quintiles [9], we derived a linear relative risk by linear regression of the quintile-specific HRs on mean haemoglobin per quintile. We evaluated a similar model for our own cohort, with haemoglobin as a continuous linear variable, for comparison with the results of the meta-analysis. See Text S1 (Additional Methods) and Text S3 (MOOSE checklist) for more details.
Results
Baseline Characteristics
The number of eligible stable angina patients was 25,824 and the number of MI patients was 19,443. Women were more likely than men to have haemoglobin measured (88.3% versus 83.5%, p<0.001), but there was only a slight difference in the percentage of patients with any missing covariate data (23.0% for women and 25.1% for men, p<0.001). After excluding these patients, the final number of patients analysed was 20,131 for the stable angina population and 14,171 for MI. Of these, 861 patients developed MI after initially presenting with stable angina and were included in both cohorts, but with different index dates. The mean haemoglobin concentration was 13.0 g/dl in women and 14.2 g/dl in men (p<0.001).
Table 1 summarises the baseline characteristics for the angina patients (Table S3 for MI patients). Patients with lower haemoglobin tended to be older (p<0.001 for linear trend) and were more likely to have diabetes (p<0.001), renal impairment (p<0.001), and cancer (p<0.001). They also had lower MCV (p<0.001) and were more likely to be taking iron supplements (p<0.001) and nitrates (p<0.001). Among patients with MI and women with stable angina, those with higher haemoglobin were more likely to smoke (p<0.001), but there was no significant relationship for men with stable angina.
Tab. 1. Baseline characteristics of patients with new onset stable angina, by gender and haemoglobin category. 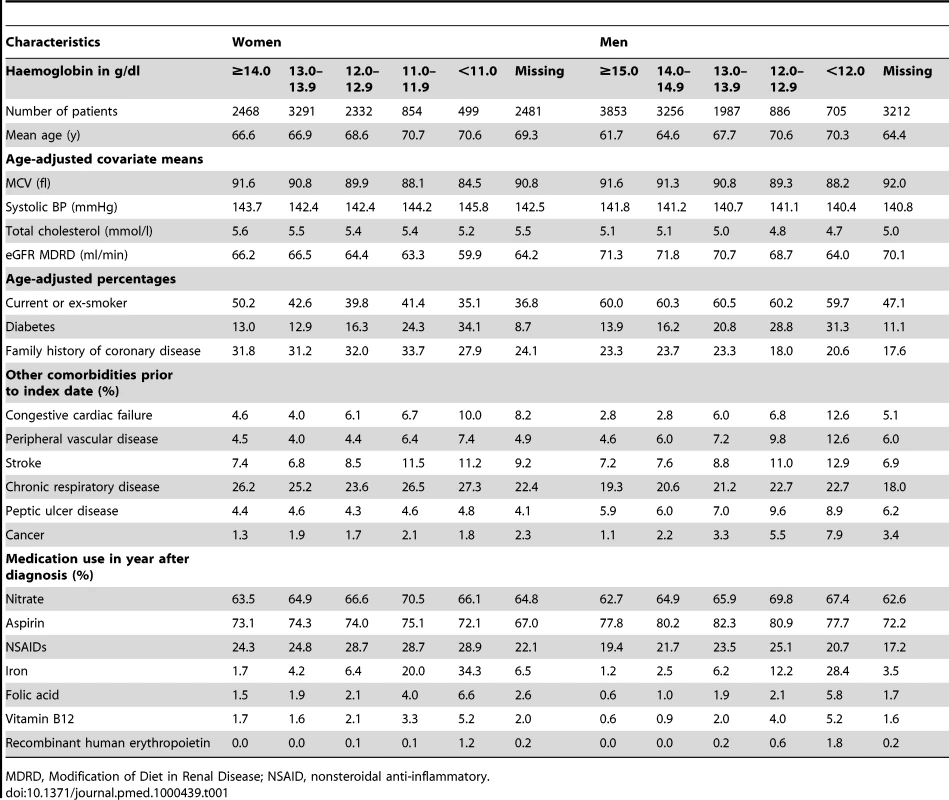
MDRD, Modification of Diet in Renal Disease; NSAID, nonsteroidal anti-inflammatory. Of the patients who were taking iron supplements, 47.8% had a laboratory record of iron status, 11.7% had microcytic red blood cells (MCV<80 fl), and 22.9% were iron deficient (ferritin <25 µg/l, transferrin saturation <16% or soluble transferrin receptor >2.5 mg/l).
Absolute Risks and Kaplan-Meier Curves
In patients with stable angina and haemoglobin less than 11 g/dl, the age adjusted mortality rate was 5.3% per year for women, compared to 9.8% for men. However for patients with stable angina and haemoglobin over 15 g/dl, the corresponding rates were 2.7% for women and 2.0% for men. The adjusted Kaplan-Meier curves show that lower haemoglobin was associated with a greater risk of death in both genders over 4 y of follow-up (Figures 2 and S2).
Fig. 2. Kaplan-Meier curves for patients with stable angina. 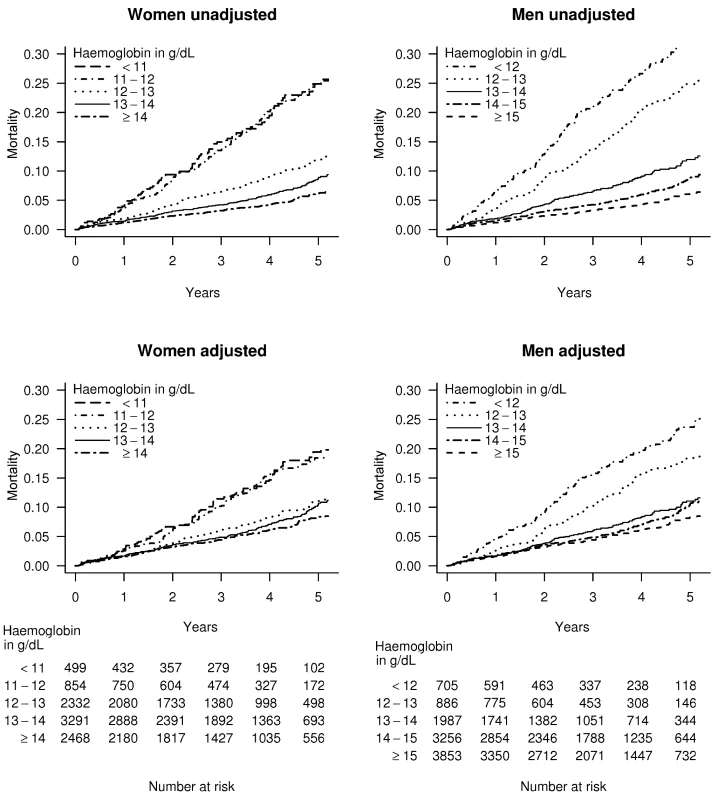
Unadjusted and multiply adjusted Kaplan-Meier curves showing the probability of death in men and women with new onset stable angina according to haemoglobin level. Adjustment was done by stratification on linear predictors from a Cox model, which included age, eGFR, systolic BP, total cholesterol, family history, diabetes, smoking, and comorbidity (Charlson index), but not sex or haemoglobin. Thresholds, Shape, and Strength of Association
There was a dose-dependent increase in mortality with decreasing haemoglobin below the optimal haemoglobin range in both genders and both study populations. For stable angina patients, the lower threshold was 11.9 g/dl (95% CI 11.5–13.5) for women and 13.1 (95% CI 13.0–13.4) for men. For the MI population the lower threshold was 12.8 (95% CI 12.1–13.5) for women and 13.5 (95% CI 13.2–13.9) for men (Table 2). Of men with MI, 29.5% had haemoglobin below the lower threshold and these patients had an adjusted HR for mortality of 2.00 (95% CI 1.76–2.29) compared to those with haemoglobin concentration in the lowest risk range. Mortality was higher at both extremes of haemoglobin concentration compared to the central range (Figure 1B), but there were very few patients with haemoglobin above the upper threshold, so there is less evidence for a dose-dependent effect at high haemoglobin levels.
Tab. 2. Upper and lower threshold haemoglobin concentrations (with 95% CIs), and excess population risk of mortality attributable to low or high haemoglobin levels. 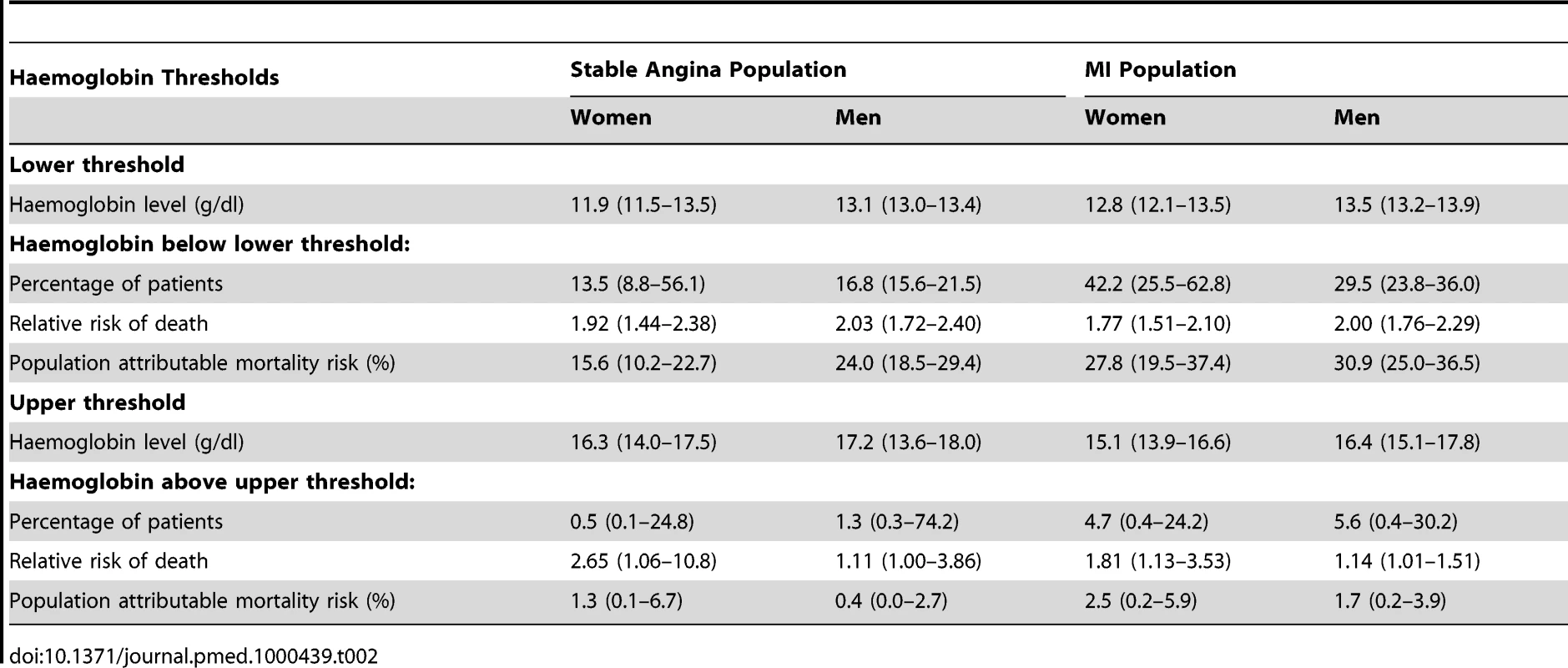
These findings were corroborated by the stratified haemoglobin model. For stable angina patients, the adjusted HR of death for women with haemoglobin 11–12 g/dl (compared to the reference group with haemoglobin 13–14) was 1.69; 95% CI 1.30–2.20 (Figure 1B). For men with Hb 12–13 g/dl (compared to 13–14) the HR was 1.74 (95% CI 1.39–2.17). Among MI patients, the corresponding HRs were 1.80 (95% CI 1.43–2.26) for women and 1.95 (95% CI 1.61–2.37) for men (Figure 1B; Table S4).
We defined the population attributable risk for low (or high) haemoglobin as the amount that the total modelled mortality risk for the entire cohort would change if all patients were assumed to have an optimal haemoglobin concentration. Among stable angina patients, the population risk attributable to low haemoglobin was 16% (95% CI 10–23%) for women and 24% (95% CI 19–29%) for men. For MI patients the corresponding risk was 28% (95% CI 20–37%) for women and 31% (95% CI 25–37%) for men (Table 2). The excess risk attributable to low haemoglobin was greater for MI patients compared to stable angina (ratio 1.44, 95% CI 1.10–1.88).
After standardising for differing baseline haemoglobin between the genders, the shape of the haemoglobin-prognosis relationship was similar in men and women.
Subgroup Analysis, Secondary Endpoints, and Mean Corpuscular Volume
In Table S5 we show that men and women in the highest MCV quintile (MCV greater than 95 fl) had a slightly increased risk of death compared to the middle quintile (HR 1.40 for women and 1.49 for men with stable angina; similar results for MI). Most of these patients had MCV values in the upper part of the “normal” range, as only 3.1% of stable angina patients and 3.5% of MI patients had red blood cells whose size exceeded the conventional threshold for macrocytosis (MCV>100 fl). Less than 1% of patients in the cohort had both macrocytosis and anaemia.
Subgroup analysis showed a similar association of low haemoglobin with mortality among patients with and without other comorbidities (Tables S6 and S7). There was no significant association between haemoglobin and risk of stroke. A post hoc analysis that included white cell count, congestive cardiac failure, and high density lipoprotein (HDL) cholesterol as additional independent variables yielded similar results to the main analysis.
Systematic Review and Meta-analysis
We identified ten eligible studies [7],[9],[26]–[33] that reported the relationship between haemoglobin and outcomes (1,127 events) in patients with stable coronary disease (Figure 3). None of these studies investigated evidence for the existence, or position, of any threshold haemoglobin value. According to a linear haemoglobin risk profile (a misleading model of the data), the summary relative risk from the meta-analysis was 1.29 (95% CI 1.20–1.38) for 1 g/dl lower haemoglobin. For comparison, the HR of death for 1 g/dl lower haemoglobin in our study was 1.18 (95% CI 1.14–1.21) for angina patients and 1.15 (95% CI 1.13–1.18) for MI patients.
Fig. 3. Systematic review and meta-analysis. 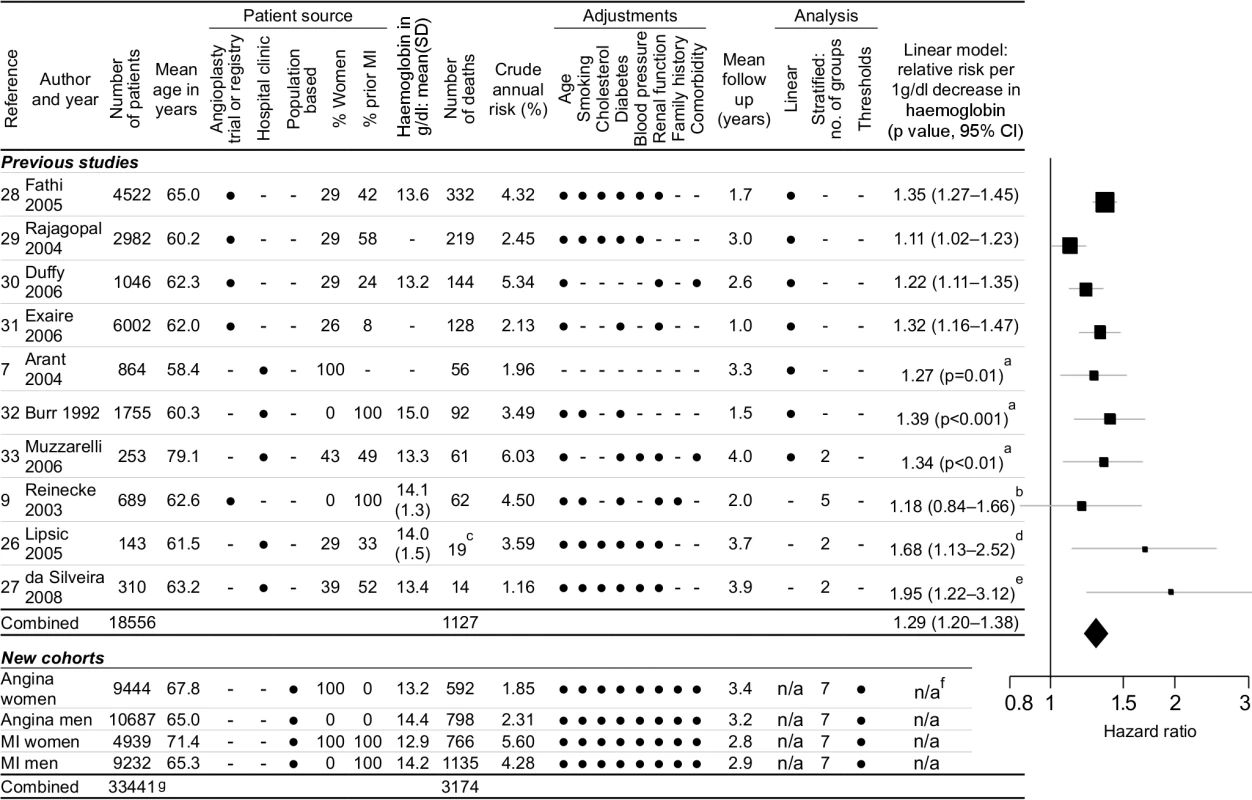
Systematic review and meta-analysis of studies investigating the relationship between haemoglobin concentration and prognosis in patients with stable coronary disease. Legend: aCI not reported in paper; standard error estimated from p-value. bReported by quintiles with fourth quintile as reference group; linear conversion by linear interpolation of relative risks across quintiles. cCardiovascular deaths and ACSs (all-cause mortality not reported in this study). dDerived from anaemia cutoff; HR 5.73 (95% CI 1.49–22.1) for anaemia (n = 14, mean haemoglobin = 11.0) versus nonanaemia (n = 129, mean haemoglobin = 14.4). eDerived from anaemia cutoff; HR 6.46 (95% CI 1.73–24.2) for anaemia (n = 71, mean haemoglobin = 11.2) versus nonanaemia (n = 239, mean haemoglobin = 14.0). fWe did not calculate risks from a linear model because it over-simplifies the shape of the relationship. gTotal number of patients is less than the sum of the individual cohort sizes because 861 patients were included in both angina and MI cohorts. Although the Egger test for publication bias was nonsignificant (p = 0.38) [34], there was significant heterogeneity between the studies (Cochran's Q = 17.96, p = 0.036).
Discussion
Summary of Main Findings
The new cohorts of patients with stable coronary disease reported here are substantially larger (3,174 deaths) than the combined previous literature (1,127 events), allowing reliable estimation for the first time of thresholds of haemoglobin concentration for excess risk. Our study demonstrates that thresholds exist (i.e., the risk is not linear, as has been presented in seven previous studies). Furthermore, the thresholds are higher than clinicians might anticipate, and thus a substantial proportion of patients (15%–30%) have a haemoglobin level that places them at markedly higher risk of death compared to patients with lowest risk haemoglobin levels. Our findings were consistent in women and men, among patients with angina alone, and those with a history of MI. Our results also suggest that the risk attributable to low haemoglobin was greater in patients with previous MI compared to those with stable angina, and the optimal (lowest risk) range of haemoglobin was narrower for patients with MI. Based on our attributable risk calculation, the association between haemoglobin and mortality might contribute to about a quarter of deaths after MI.
Our lower haemoglobin thresholds accord closely with the World Health Organisation (WHO) anaemia thresholds of 12 g/dl for women and 13 g/dl for men; this is noteworthy for two reasons. First, the WHO thresholds were defined to facilitate the detection of nutritional iron deficiency, and were not derived in relation to prognostic studies in any population [35],[36]. Second, prognostic associations often do not correlate with biomarker thresholds used to define other conditions. In the case of diabetes, there is increased risk with mildly elevated blood sugar levels that do not fulfil the criteria for diabetes [37], whereas for chronic kidney disease, risk increases only with moderate to severe renal impairment [38].
Like previous studies [6],[39],[40] we did find some evidence that a high haemoglobin is also associated with poor prognosis, giving a J-shaped relationship. However, there were few patients with high haemoglobin and they contributed little to the overall population risk (Table 2).
Gender Difference
The shape of the haemoglobin-prognosis relationship was similar in men and women, after adjusting for the higher baseline haemoglobin in men. Men have a physiologically higher haemoglobin concentration than women, because of an erythropoietin-independent effect of testosterone in stimulating erythrocyte production [41]. Anti-androgenic treatments can cause anaemia [42] and testosterone treatment can increase haemoglobin [43]. However, it is unclear why women tolerate a low haemoglobin better than men.
Stable Angina and Myocardial Infarction Compared
According to our model, the excess risk associated with low haemoglobin was greater for patients with MI than for those with stable angina (Table 2). If there is a causal relationship between low haemoglobin and worse prognosis, a possible mechanism is that the reduction in tissue oxygen delivery may lead to a persistent increase in cardiac output and left ventricular hypertrophy, further exacerbating myocardial ischaemia [44]. It is possible that these consequences are worse in patients who have already suffered a MI.
Limitations
Our prognostic cohort analysis has strengths compared to previous studies—size and statistical power, longitudinal recording of comorbidities, more complete adjustment for a priori confounders, direct derivation of clinical thresholds, and population base—but important limitations. First, and most important, as our study was observational we cannot conclude a causal relationship between haemoglobin concentration and mortality. It is possible that there was residual confounding, and the effect on mortality was due to the cause of the haemoglobin concentration, rather than the haemoglobin concentration itself. Our model included the Charlson comorbidity index to encode the presence of heart failure and other common chronic diseases, but it did not take into account the severity of the coronary artery disease or other medical conditions. Patients with more severe coronary disease or comorbidities may have lower haemoglobin, as is the case with chronic heart failure [45]. Hypoxia due to heart failure causes vasoconstriction of the afferent arterioles of the kidney, with consequent reduction in renal perfusion, activation of the renin–angiotensin–aldosterone system and production of antidiuretic hormone, causing plasma volume expansion and haemodilution. Erythropoietin secretion is reduced because of renal hypoxia, causing a further reduction in haemoglobin concentration, which exacerbates cardiac failure [46],[47]. This vicious circle of anaemia, heart failure, and chronic kidney disease has been called the “cardio renal anemia syndrome” [48]. However, significant confounding from comorbidity in our study is unlikely because we obtained similar results from the subset of patients without comorbidities, and from those who survived at least 1 y after the index date.
Second, bias might arise because of missing data and variable time intervals between measurement and disease onset. However in about half of patients we used an interpolated haemoglobin value (using at least one value before and one after the index date) and found similar results to the entire cohort.
Third, information on cause of death was not available. However, previous studies have shown that coronary disease is the most common cause of death among the patients studied [33], so any effect observed on mortality is likely to be due to coronary death.
Fourth, although the electronic recording of coronary disease diagnoses in primary care is reasonably accurate and complete [12],[49], the diagnosis of angina is less reliable than that of MI, and we were unable to further characterise either diagnosis in terms of angiographic results, electrocardiographic findings, or markers of myocardial necrosis.
Fifth, a shortcoming of our statistical model is that we only included variables considered to be relevant on the basis of prior knowledge. We did not consider interaction terms or nonlinear effects except for haemoglobin, with the result that our model may have been incompletely adjusted and may have missed some effect-modifying relationships. However, we did specifically investigate the most important interaction (haemoglobin and gender), and our approach had the advantage of being less susceptible to bias than variable selection and elimination on purely statistical grounds [20].
Sixth, we did not classify the cause of anaemia in all patients in our cohort, nor did we collect data on blood transfusions. Blood transfusions are recorded incompletely in the GPRD because they always occur in hospital but are rarely the primary reason for admission. However, we feel that these omissions would not have influenced or main results, because the majority of anaemic patients in our study had a haemoglobin level that was only slightly lower than the average, and may not have been “diagnosed” as anaemic in the sense that clinicians may not have considered the haemoglobin level as unremarkable.
Contribution of Systematic Review and Meta-analysis
The systematic review clarifies the limitations of previous studies; none had previously estimated the haemoglobin threshold values, or had assessed men and women, or angina and MI separately. There was significant heterogeneity between the studies, because they included populations with differing baseline risks and were analysed with varying levels of adjustment for other risk factors. In order to combine the relative risks we had to assume a log-linear relationship between haemoglobin and prognosis, and assume a normal distribution for haemoglobin levels in the population. Given the relatively small number of studies in the meta-analysis, it was not worthwhile to attempt a subgroup analysis or meta-regression.
As a crude summary measure of effect, ignoring the thresholds, the summary result from the meta-analysis (HR 1.52 per standard deviation [SD] haemoglobin) was somewhat larger than that from our cohort analysis (1.31). This finding likely reflects our more extensive adjustment for potential confounders. However, the relationship between haemoglobin and mortality that we observed was strong, comparable to that for low density lipoprotein cholesterol (1.38 per SD) [50] and fibrinogen (1.24 per SD) [23].
Clinical and Research Implications
Our findings provide observational evidence that contributes to the rationale for randomised controlled trials to evaluate management strategies aimed at increasing haemoglobin among suitable patients with stable coronary disease. Such experimental evidence would address whether clinicians should intervene, and whether haemoglobin levels are causal. These studies may include a trial of oral iron supplementation, or a more aggressive approach involving intravenous iron and erythropoiesis-stimulating agents. Outcome measures may include clinical events such as mortality and MI, as well as quality of life measures. We are not aware of any such trials that have been reported or are currently registered.
Clinical practice guidelines for the management of angina state that anaemia should be corrected [51], but do not specify investigations, interventions, or the target haemoglobin concentration. 94% of our patients had normocytic red blood cells (MCV 80–100 fl), and the extent to which causes of anaemia in such patients should be investigated is not known. Although our study shows that mild anaemia is associated with increased mortality, randomised trials using erythropoietin in other conditions have shown that complete correction of anaemia is not beneficial. In trials among patients with chronic kidney disease, a target haemoglobin above 13 g/dl has been associated with higher mortality (Correction of Hemoglobin and Outcomes in Renal Insufficiency [CHOIR]) [5] and a higher rate of stroke [52] than treatment to a lower haemoglobin target, and an increased risk of stroke compared to placebo (Trial to Reduce Cardiovascular Events With Aranesp Therapy [TREAT]) [4]. We found no relationship between haemoglobin and nonfatal stroke, but the overall number of strokes was small. In congestive cardiac failure, a meta-analysis of seven randomised controlled trials showed a reduction in hospitalisation with treatment of anaemia (relative risk 0.59, 95% CI 0.41–0.86), but no significant effect on mortality [3]. The ongoing phase III placebo-controlled trial of darbopoeitin alfa in congestive cardiac failure (Reduction of Events with Darbepoetin alfa in Heart Failure [RED-HF]) is aiming for a target haemoglobin of 13 g/dl and is due to complete in 2012 [53].
Irrespective of a possible causal, reversible relationship between haemoglobin concentration and mortality, further research is warranted to assess what incremental prognostic value haemoglobin might offer in risk stratifying patients with stable coronary disease.
Conclusions
There are threshold levels of haemoglobin below which women and men with stable coronary disease are at increased risk of death. However our study is observational and cannot be used to infer a causal relationship. Outcome trials of strategies to increase haemoglobin levels would be able to answer the question as to whether increasing haemoglobin among such patients is beneficial.
Supporting Information
Zdroje
1. WeissG
GoodnoughLT
2005 Anemia of chronic disease. N Engl J Med 352 1011 1023
2. AbramsJ
2005 Clinical practice. Chronic stable angina. N Engl J Med 352 2524 2533
3. van der MeerP
GroenveldH
JanuzziJL
van VeldhuisenD
2009 Erythropoietin treatment in patients with chronic heart failure: a meta-analysis. Heart 95 1309 1314
4. PfefferMA
BurdmannEA
ChenCY
CooperME
de ZeeuwD
2009 A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361 2019 2032
5. SinghAK
SzczechL
TangKL
BarnhartH
SappS
2006 Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355 2085 2098
6. SabatineMS
MorrowDA
GiuglianoRP
BurtonPB
MurphySA
2005 Association of hemoglobin levels with clinical outcomes in acute coronary syndromes. Circulation 111 2042 2049
7. ArantCB
WesselTR
OlsonMB
Bairey MerzCN
SopkoG
2004 Hemoglobin level is an independent predictor for adverse cardiovascular outcomes in women undergoing evaluation for chest pain: results from the National Heart, Lung, and Blood Institute Women's Ischemia Syndrome Evaluation Study. J Am Coll Cardiol 43 2009 2014
8. HemingwayH
RileyRD
AltmanDG
2009 Ten steps towards improving prognosis research. BMJ 339 b4184
9. ReineckeH
TreyT
WellmannJ
HeidrichJ
FobkerM
2003 Haemoglobin-related mortality in patients undergoing percutaneous coronary interventions. Eur Heart J 24 2142 2150
10. EnnezatPV
LamblinN
MouquetF
TricotO
QuandalleP
2008 The effect of ageing on cardiac remodelling and hospitalization for heart failure after an inaugural anterior myocardial infarction. Eur Heart J 29 1992 1999
11. HerrettE
ThomasSL
SchoonenWM
SmeethL
HallAJ
2010 Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 69 4 14
12. HammadTA
McAdamsMA
FeightA
IyasuS
Dal PanG
2008 Determining the predictive value of Read/OXMIS codes to identify incident acute myocardial infarction in the General Practice Research Database. Pharmacoepidemiol Drug Saf 17 1197 1201
13. NHS Information Authority 2009 The clinical terms version 3 (The Read Codes). Available: http://www.connectingforhealth.nhs.uk/systemsandservices/data/readcodes/index_html. Accessed 22 April 2010
14. NHS Information Centre 2007 The quality and outcomes framework. Available: http://www.ic.nhs.uk/statistics-and-data-collections/audits-and-performance/the-quality-and-outcomes-framework. Accessed 22 April 2010
15. LewisJD
BrensingerC
2004 Agreement between GPRD smoking data: a survey of general practitioners and a population-based survey. Pharmacoepidemiol Drug Saf 13 437 441
16. National Kidney Foundation 2002 K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39 S1 S266
17. SundararajanV
HendersonT
PerryC
MuggivanA
QuanH
2004 New ICD-10 version of the Charlson Comorbidity Index predicted in-hospital mortality. J Clin Epidemiol 57 1288 1294
18. SelvinE
SteffesMW
ZhuH
MatsushitaK
WagenknechtL
2010 Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362 800 811
19. SungL
HaydenJ
GreenbergML
KorenG
FeldmanBM
2005 Seven items were identified for inclusion when reporting a Bayesian analysis of a clinical study. J Clin Epidemiol 58 261 268
20. RobinsJM
GreenlandS
1986 The role of model selection in causal inference from nonexperimental data. Am J Epidemiol 123 392 402
21. GrambschP
TherneauT
1994 Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81 515 5
22. R Development Core Team 2009 R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 3-900051-07-0 Available: http://www.R-project.org. ISBN
23. HemingwayH
HenrikssonM
ChenR
DamantJ
FitzpatrickN
2010 The effectiveness and cost effectiveness of biomarkers for the prioritisation of patients awaiting coronary revascularisation: a systematic review and decision model. Health Technol Assess 14
24. DerSimonianR
LairdN
1986 Meta-analysis in clinical trials. Control Clin Trials 7 177 188
25. LumleyT
2009 The rmeta package: meta-analysis. Available: http://CRAN.R-project.org/package=rmeta. Accessed 22 April 2010
26. LipsicE
AsselbergsFW
van der MeerP
TioRA
VoorsAA
2005 Anaemia predicts cardiovascular events in patients with stable coronary artery disease. Neth Heart J 13 254
27. da SilveiraAD
RibeiroRA
RossiniAP
StellaSF
RittaHA
2008 Association of anemia with clinical outcomes in stable coronary artery disease. Coron Artery Dis 19 21 26
28. FathiRB
GurmHS
ChewDP
GuptaR
BhattDL
2005 The interaction of vascular inflammation and chronic kidney disease for the prediction of long-term death after percutaneous coronary intervention. Am Heart J 150 1190 1197
29. RajagopalV
GurmHS
BhattDL
LincoffAM
TchengJE
2004 Relation of an elevated white blood cell count after percutaneous coronary intervention to long-term mortality. Am J Cardiol 94 190 192
30. DuffyBK
GurmHS
RajagopalV
GuptaR
EllisSG
2006 Usefulness of an elevated neutrophil to lymphocyte ratio in predicting long-term mortality after percutaneous coronary intervention. Am J Cardiol 97 993 996
31. ExaireJE
ButmanSM
EbrahimiR
KleimanNS
HarringtonRA
2006 Provisional glycoprotein IIb/IIIa blockade in a randomized investigation of bivalirudin versus heparin plus planned glycoprotein IIb/IIIa inhibition during percutaneous coronary intervention: predictors and outcome in the Randomized Evaluation in Percutaneous coronary intervention Linking Angiomax to Reduced Clinical Events (REPLACE)-2 trial. Am Heart J 152 157 163
32. BurrML
HollidayRM
FehilyAM
WhiteheadP
1992 Haematological prognostic indices after myocardial infarction: evidence from the diet and reinfarction trial (DART). Eur Heart J 13 166 170
33. MuzzarelliS
PfistererM
2006 Anemia as independent predictor of major events in elderly patients with chronic angina. Am Heart J 152 991 996
34. EggerM
SmithGD
SchneiderM
MinderC
1997 Bias in meta-analysis detected by a simple, graphical test. BMJ 315 629 634
35. KilpatrickGS
HardistyRM
1961 The prevalence of anaemia in the community. a survey of a random sample of the population. Br Med J 1 778 782
36. World Health Organisation 1968 Nutritional anaemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser 405 5 37
37. StrandersI
DiamantM
van GelderRE
SpruijtHJ
TwiskJWR
2004 Admission blood glucose level as risk indicator of death after myocardial infarction in patients with and without diabetes mellitus. Arch Intern Med 164 982 988
38. Di AngelantonioE
DaneshJ
EiriksdottirG
GudnasonV
2007 Renal function and risk of coronary heart disease in general populations: new prospective study and systematic review. PLoS Med 4 e270 doi:10.1371/journal.pmed.0040270
39. GagnonDR
ZhangTJ
BrandFN
KannelW
1994 Hematocrit and the risk of cardiovascular disease–the Framingham study: a 34-year follow-up. Am Heart J 127 674 682
40. DaneshJ
CollinsR
PetoR
LoweG
2000 Haematocrit, viscosity, erythrocyte sedimentation rate: meta-analyses of prospective studies of coronary heart disease. Eur Heart J 21 515 520
41. FerrucciL
MaggioM
BandinelliS
BasariaS
LauretaniF
2006 Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 166 1380 1388
42. CurtisKK
PruthiRK
FonsecaR
GornetM
2007 Transfusion-dependent anemia after initiation of androgen deprivation therapy for metastatic prostate cancer. Urology 70 811
43. CovielloAD
KaplanB
LakshmanKM
ChenT
SinghAB
2008 Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93 914 919
44. MetivierF
MarchaisSJ
GuerinAP
PannierB
LondonG
2000 Pathophysiology of anaemia: focus on the heart and blood vessels. Nephrol Dial Transplant 15 Suppl 3 14 18
45. EzekowitzJA
McAlisterFA
ArmstrongPW
2003 Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation 107 223 225
46. TangYD
KatzSD
2006 Anemia in chronic heart failure: prevalence, etiology, clinical correlates, and treatment options. Circulation 113 2454 2461
47. GeislerB
2010 Treating anemia in heart failure patients: a review of erythropoiesis-stimulating agents. Expert Opin Biol Ther 10 1209 1216
48. SilverbergDS
WexlerD
IainaA
SteinbruchS
WollmanY
2006 Anemia, chronic renal disease and congestive heart failure–the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int Urol Nephrol 38 295 310
49. MealAG
PringleM
HammersleyV
2000 Time changes in new cases of ischaemic heart disease in general practice. Fam Pract 17 394 400
50. AngelantonioED
SarwarN
PerryP
KaptogeS
Emerging Risk Factors Collaboration 2009 Major lipids, apolipoproteins, and risk of vascular disease. JAMA 302 1993 2000
51. FoxK
GarciaMA
ArdissinoD
BuszmanP
CamiciPG
2006 Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J 27 1341 1381
52. ParfreyPS
FoleyRN
WittreichBH
SullivanDJ
ZagariMJ
2005 Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol 16 2180 2189
53. van VeldhuisenDJ
McMurrayJJ
2007 Are erythropoietin stimulating proteins safe and efficacious in heart failure? Why we need an adequately powered randomised outcome trial. Eur J Heart Fail 9 110 112
Štítky
Interní lékařství
Článek The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in AfricaČlánek If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2011 Číslo 5- Není statin jako statin aneb praktický přehled rozdílů jednotlivých molekul
- Magnosolv a jeho využití v neurologii
- Moje zkušenosti s Magnosolvem podávaným pacientům jako profylaxe migrény a u pacientů s diagnostikovanou spazmofilní tetanií i při normomagnezémii - MUDr. Dana Pecharová, neurolog
- Biomarker NT-proBNP má v praxi široké využití. Usnadněte si jeho vyšetření POCT analyzátorem Afias 1
- Antikoagulační léčba u pacientů před operačními výkony
-
Všechny články tohoto čísla
- Primary Prevention of Gestational Diabetes Mellitus and Large-for-Gestational-Age Newborns by Lifestyle Counseling: A Cluster-Randomized Controlled Trial
- Meta-analyses of Adverse Effects Data Derived from Randomised Controlled Trials as Compared to Observational Studies: Methodological Overview
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Characterizing the Epidemiology of the 2009 Influenza A/H1N1 Pandemic in Mexico
- The Joint Action and Learning Initiative: Towards a Global Agreement on National and Global Responsibilities for Health
- Let's Be Straight Up about the Alcohol Industry
- Advancing Cervical Cancer Prevention Initiatives in Resource-Constrained Settings: Insights from the Cervical Cancer Prevention Program in Zambia
- The Transit Phase of Migration: Circulation of Malaria and Its Multidrug-Resistant Forms in Africa
- Health Aspects of the Pre-Departure Phase of Migration
- Aripiprazole in the Maintenance Treatment of Bipolar Disorder: A Critical Review of the Evidence and Its Dissemination into the Scientific Literature
- Threshold Haemoglobin Levels and the Prognosis of Stable Coronary Disease: Two New Cohorts and a Systematic Review and Meta-Analysis
- If You Could Only Choose Five Psychotropic Medicines: Updating the Interagency Emergency Health Kit
- Migration and Health: A Framework for 21st Century Policy-Making
- Maternal Influenza Immunization and Reduced Likelihood of Prematurity and Small for Gestational Age Births: A Retrospective Cohort Study
- The Impact of Retail-Sector Delivery of Artemether–Lumefantrine on Malaria Treatment of Children under Five in Kenya: A Cluster Randomized Controlled Trial
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Low-Dose Adrenaline, Promethazine, and Hydrocortisone in the Prevention of Acute Adverse Reactions to Antivenom following Snakebite: A Randomised, Double-Blind, Placebo-Controlled Trial
- Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study
- Medical Students' Exposure to and Attitudes about the Pharmaceutical Industry: A Systematic Review
- Estimates of Outcomes Up to Ten Years after Stroke: Analysis from the Prospective South London Stroke Register
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Autoři: prof. MUDr. Vladimír Palička, CSc., Dr.h.c., doc. MUDr. Václav Vyskočil, Ph.D., MUDr. Petr Kasalický, CSc., MUDr. Jan Rosa, Ing. Pavel Havlík, Ing. Jan Adam, Hana Hejnová, DiS., Jana Křenková
Autoři: MUDr. Irena Krčmová, CSc.
Autoři: MDDr. Eleonóra Ivančová, PhD., MHA
Autoři: prof. MUDr. Eva Kubala Havrdová, DrSc.
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



